ABSTRACT
Hosts can initiate macroautophagy/autophagy as an antiviral defense response, while viruses have developed multiple ways to evade the host autophagic degradation. However, little is known as to whether viruses can target lipids to subvert autophagic degradation. Here, we show that a low abundant signaling lipid, phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2), is required for rice black-streaked dwarf virus (RBSDV) to evade the autophagic degradation in the insect vector Laodelphax striatellus. RBSDV binds to PtdIns(3,5)P2 and elevates its level through its main capsid protein P10, leading to inhibited autophagy and promoted virus propagation. Furthermore, we show that PtdIns(3,5)P2 inhibits the autophagy pathway by preventing the fusion of autophagosomes and lysosomes through activation of Trpml (transient receptor potential cation channel, mucolipin), an effector of PtdIns(3,5)P2. These findings uncover a strategy whereby a plant virus hijacks PtdIns(3,5)P2 via its viral capsid protein to evade autophagic degradation and promote its survival in insects.
KEYWORDS: Autophagy; lysosome-autophagosome fusion; PtdIns(3,5)P2; RBSDV; trpml
Introduction
Glycerophospholipids are important components of biological membranes. Among them, phosphatidylinositol (PtdIns) and phosphorylated derivatives (phosphoinositides, PIPs) are signaling molecules mediating many cellular events [1,2]. PIPs are generated by phosphorylation of PtdIns at positions 3, 4, and 5 of the inositol ring. Phosphatidylinositol-3,5-bisphosphate (PtdIns(3,5)P2) is the least abundant of the PIPs, contributing 0.05%-0.1% to the total PIPs pool, but is crucial for diverse cellular functions, such as regulation of endolysosome morphology, trafficking, and acidification of the vacuole 3,4,5]. PIKFYVE (phosphoinositide kinase, FYVE-type zinc finger containing) in mammals and fab1 (fab1 kinase) in insects is the sole upstream kinase that exclusively converts PtdIns3P to PtdIns(3,5)P2 [6,7]. PtdIns(3,5)P2 has been reported to regulate animal virus entry and release [8,9]. However, the understanding of the PtdIns(3,5)P2 function in virus-host interaction remains rudimentary.
Macroautophagy/autophagy is a conserved intracellular degradation pathway that can function as an intrinsic antiviral mechanism to eliminate virus infection. During long-term co-evolution, viruses have developed counterdefense strategies to subvert the autophagic response for their benefit [10]. The viral proteins of human cytomegalovirus (HCMV), herpes simplex virus 1, and Kaposi sarcoma-associated herpesvirus can inhibit autophagosome formation by interacting with Atg6/BECN1 [11–14]. Barley stripe mosaic virus γb inhibits phagophore formation by competing for the Atg7-Atg8 interaction [15]. Turnip mosaic virus 6K2 and VPg disrupt recognition of the autophagy cargo receptor NBR1 with viral-encoded RNA silencing suppressor HCpro and limit the antiviral capacity of autophagy [16]. Overall, viral protein interaction with host autophagy-related proteins is a common strategy for both plant and animal viruses to inhibit autophagy. Although lipids have been reported to be involved in autophagy [17], whether the virus can use them to escape the autophagic degradation is unknown.
Rice black-streaked dwarf virus (RBSDV) is a double-stranded RNA (dsRNA) reovirus in the genus Fijivirus of the Reoviridae family. It is transmitted by the insect vector Laodelphax striatellus Fallén (small brown planthopper, SBPH) in a persistent and propagative manner and causes severe agricultural losses [18]. Its genome contains ten dsRNA segments (S1-S10). The abundance of the main capsid protein P10 is used as the indicator of RBSDV accumulation in both insects and plants [19,20]. Autophagy can be induced by RBSDV P10 at the initial stage of viral infection in L. striatellus to suppress virus invasion and accumulation [21]. Although autophagy is induced to degrade RBSDV, the virus accumulates in L. striatellus over time [22]. How RBSDV suppresses autophagy during the process of infection is still mysterious.
In this study, we show that RBSDV binds to and elevates the cellular level of PtdIns(3,5)P2 in infected L. striatellus cells. The virus-induced PtdIns(3,5)P2 blocks the formation of autolysosomes and prevents the virus from autophagic degradation. Moreover, we show that inhibition of PtdIns(3,5)P2 causes a significant decrease of two other plant viruses in their insect vectors. These results reveal a strategy for plant viruses to counteract autophagy in insect vectors and suggest that the PtdIns(3,5)P2 synthesis pathway can be targeted pharmaceutically to inhibit virus propagation.
Results
RBSDV P10 protein binds to PtdIns(3,5)P2
The viral capsid protein plays critical roles in the viral life cycle, such as interaction with the host at the initial infection step, participation in virion assembly, and encapsidation of the viral genome. To analyze the function of RBSDV capsid protein P10, its protein sequence was analyzed by the Robetta server (https://robetta.bakerlab.org) with an ab initio modeling algorithm using the default setting, and P10 was predicted as a potential lipid-binding protein (Figure S1A). We first determined whether membrane lipids interact with RBSDV. The binding between virus particles and 15 glycerophospholipids was analyzed by a lipid-binding assay. The results showed that crude extract of RBSDV bound to PtdIns(3,5)P2 strongly (Figure 1A). Next, we analyzed the binding between P10 protein and the glycerophospholipids. The purified His-P10 protein was incubated with lipid strips containing different amounts of phospholipids. The P10 protein could bind to all 7 PIPs, with the highest affinity to PtdIns(3,5)P2 (Figure 1B). To further determine the binding affinity between P10 and PIPs, we incubated the lipid strip with His-P10 at different concentrations. Similar to the results observed in Figure 1B, P10 preferentially bound to PtdIns(3,5)P2, as compared with other lipids (Figure 1C).
Figure 1.
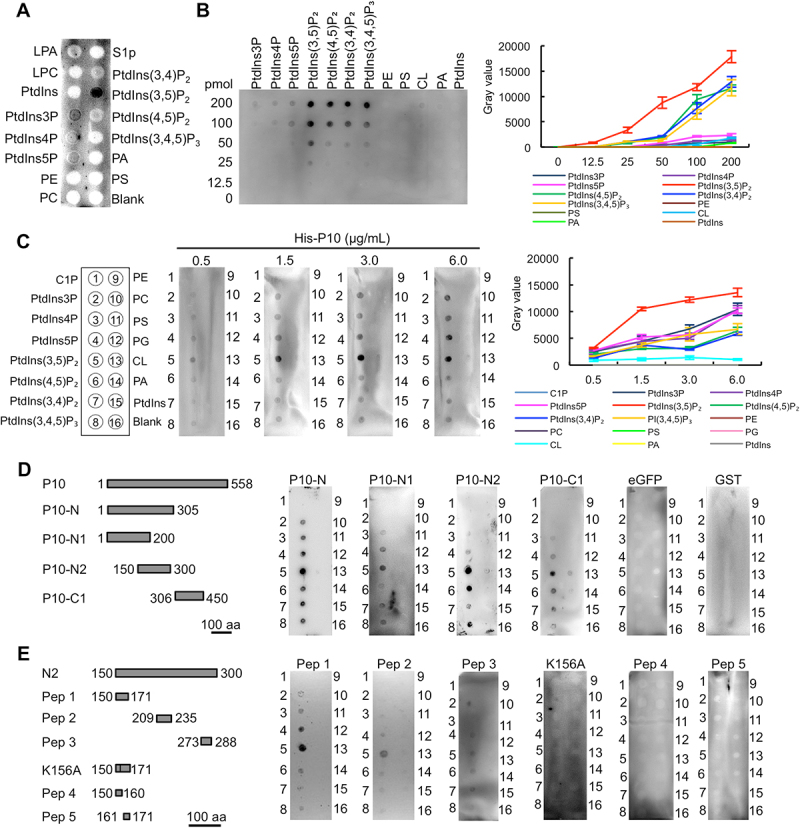
RBSDV P10 protein binds to PtdIns(3,5)P2. (A) The binding between RBSDV crude extract and phospholipids. (B) The binding between different phospholipids and P10 protein (1.5 μg/mL). (C) The binding between P10 protein at a gradient concentration and phospholipids. (D) The binding between P10 truncated proteins (50 μg/mL) and phospholipids. (E) The binding between P10 peptides (50 μg/mL) and phospholipids. The binding was analyzed by lipid binding assay. LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; S1P, sphingosine-1-phosphate; C1P, ceramide-1-phosphate; PtdIns3P, phosphatidylinositol-3-phosphate; PtdIns4P, phosphatidylinositol-4-phosphate; PtdIns5P, phosphatidylinositol-5-phosphate; PtdIns(3,5)P2, phosphatidylinositol-3,5-bisphosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; PtdIns(3,4)P2, phosphatidylinositol-3,4-bisphosphate; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-triphosphate; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PS, phosphatidylserine; PG, phosphatidylglycerol; CL, cardiolipin; PA, phosphatidic acid; PtdIns, phosphatidylinositol.
To identify the binding region of P10 with PtdIns(3,5)P2, four truncated P10 proteins fused with an enhanced green fluorescent protein (eGFP) tag, namely P10-N (1–305 amino acids, aa), P10-N1 (1–200 aa), P10-N2 (150–300 aa), and P10-C1 (306–450 aa), were expressed in Escherichia coli cells and purified by Ni-NTA beads (Figure S1B-E). All four recombinant proteins could bind to PIPs, albeit with varying specificity, while eGFP and GST proteins could not bind to any of the lipids tested (Figure 1D).
The conserved regions in P10 were analyzed by aligning the protein sequences of RBSDV P10 with six homologs in the Fijivirus genus (Figure S2). Three conserved polypeptide regions were selected and subsequently synthesized with additional biotinylation modification. The binding to PtdIns(3,5)P2 was identified with P10 peptide 1 (Pep 1), Pep 2, and Pep 3 (Figure 1E). Pep 4 and Pep 5, the two truncated polypeptides of Pep 1, failed to bind any PIPs. In addition, the K156A mutation on Pep 1 completely abolished its binding to all PIPs (Figure 1E). Taken together, these results suggest that RBSDV binds PIPs through its capsid protein P10. The binding between PtdIns(3,5)P2 and P10 requires multiple regions and positively-charged lysine.
Both RBSDV and P10 protein elevates PtdIns(3,5)P2 level
To analyze whether virus infection affects the PtdIns(3,5)P2 level in L. striatellus, we compared the relative contents of PtdIns(3,5)P2 in RBSDV-uninfected and -infected L. striatellus by dot-ELISA. The nymphs of L. striatellus were allowed a 2-day acquisition access period (AAP) on RBSDV-infected plants and then transferred to healthy rice seedlings. After rearing on healthy rice seedling for 8 days (8 d after AAP), the nymphs were collected as RBSDV-infected L. striatellus. The nymphs reared on healthy rice seedlings for all the time were collected as RBSDV-uninfected control. The dot-ELISA assay showed that the PtdIns(3,5)P2 abundance increased after RBSDV infection, while the level of phosphatidylserine (PS), used as a control since PS does not bind to RBSDV, was unchanged (Figure 2A). We also investigate the PtdIns(3,5)P2 level at different infection times. The RBSDV-infected nymphs were collected at 2 d, 6 d, and 10 d after the AAP, and the virus-uninfected nymphs reared on healthy rice plants were collected as control. The dot-ELISA showed that the relative PtdIns(3,5)P2 level did not change in the uninfected control at different time points, whereas it was increased with the virus infection time in the RBSDV-infected nymphs (Figure 2B). To analyze the subcellular localization of PtdIns(3,5)P2 and RBSDV in L. striatellus, PtdIns(3,5)P2 and RBSDV were detected by immunofluorescence assay using the antibody against PtdIns(3,5)P2 and RBSDV P10, respectively. The results showed that PtdIns(3,5)P2 and RBSDV P10 mainly distributed and colocalized in the cytoplasm in virus-infected cells. The PtdIns(3,5)P2 intensity in RBSDV-infected cells was significantly higher than that in RBSDV-uninfected cells (Figure 2C). These data indicate that RBSDV colocalizes with PtdIns(3,5)P2 and can increase the level of PtdIns(3,5)P2 in L. striatellus.
Figure 2.
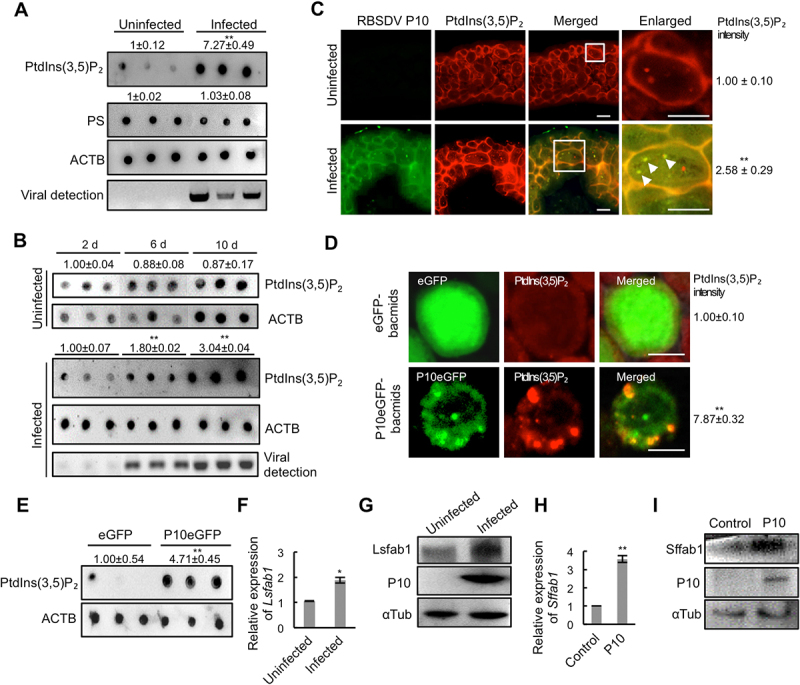
RBSDV colocalizes with and elevates PtdIns(3,5)P2 in L. striatellus. (A) Analysis of the abundance of PtdIns(3,5)P2 or PS in RBSDV -uninfected and -infected L. striatellus at 4 d after acquisition access period (aap) by dot-ELISA assay. Each dot represents a sample containing 16 nymphs. Three dots in each group represent three biological replications. (B) The relative PtdIns(3,5)P2 level at different virus infection time points. The relative PtdIns(3,5)P2 level in RBSDV-uninfected or -infected L. striatellus was analyzed at 2 d, 6 d, and 10 d after AAP by dot-ELISA. (C) The localization of RBSDV and PtdIns(3,5)P2 in the RBSDV-uninfected or -infected intestines of L. striatellus was analyzed by immunofluorescence at 8 d after AAP. RBSDV and PtdIns(3,5)P2 were labeled with RBSDV monoclonal antibody conjugated FITC (green) and PtdIns(3,5)P2 antibody conjugated rhodamine (red), respectively. The white arrowhead in the enlarged images indicated the colocalized RBSDV P10 and PtdIns(3,5)P2. The numbers of red fluorescent dots in three areas of 50 × 50 μm2 of each L. striatellus intestine sample were counted. The average number was calculated, and three biological replications were carried out. The number of red fluorescent dots in RBSDV-uninfected L. striatellus was normalized to 1.00, and the relative intensity of PtdIns(3,5)P2 in RBSDV-infected sample was calculated. (D) The localization of P10 and PtdIns(3,5)P2 were analyzed in eGFP- or P10eGFP- bacmids infected Sf9 cells at 48 h post-infection by immunofluorescence analysis. PtdIns(3,5)P2-rhodamine (red) was used to label PtdIns(3,5)P2. (E) The relative abundance of PtdIns(3,5)P2 in eGFP- or P10eGFP- bacmids infected Sf9 cells were analyzed at 72 h post-infection by dot-ELISA assay. (F-G) The expression of Lsfab1 was analyzed in RBSDV -uninfected or -infected L. striatellus by RT-qPCR at 4 d after AAP (F) and western blotting at 8 d after AAP (G). (H-I) The expression of Sffab1 in Sf9 cells expressing RBSDV P10 was analyzed by RT-qPCR at 48 h post-transfection (H) and by western blotting at 96 h post-transfection (I). Scale bars, 10 μm. Error bars, means ± SE. The data was analyzed by Student’s t-test. *, p <0.05; **, p <0.01.
As P10 bound to and colocalized with PtdIns(3,5)P2, we investigated whether P10 alone can modulate the PtdIns(3,5)P2 level. The P10eGFP and eGFP proteins were expressed in Spodoptera frugiperda (Sf9) cells. The relative PtdIns(3,5)P2 level was analyzed by immunofluorescence analysis and dot-ELISA assay. In the cells infected with P10eGFP-bacmids, P10eGFP protein was colocalized with PtdIns(3,5)P2 on punctuate structures, and the fluorescence intensity of PtdIns(3,5)P2 was significantly higher than that in the cells expressed eGFP (Figure 2D). Dot-ELISA assay also showed that PtdIns(3,5)P2 level significantly increased upon P10eGFP expression (Figure 2E). Collectively, these results demonstrate that RBSDV P10 protein colocalizes with PtdIns(3,5)P2 and elevates its level in Sf9 cells.
To reveal the possible reasons for the increase of PtdIns(3,5)P2 abundance, the expression of Lsfab1, a phosphoinositide 5-kinase required for PtdIns(3,5)P2 generation, was analyzed in RBSDV-uninfected and -infected L. striatellus. RBSDV infection significantly increased the expression of Lsfab1 at both transcript and protein levels (Figure 2F-G). In addition, the expression of Sffab1 was increased at transcript and protein levels in Sf9 cells expressing P10 protein (Figure 2H-I). Co-immunoprecipitation (Co-IP) assay showed that P10 could interact with Lsfab1 (Figure S3). These results indicate that RBSDV P10 elevates PtdIns(3,5)P2 level by inducing the expression of fab1.
PtdIns(3,5)P2 modulates RBSDV propagation in L. striatellus
To address the role of PtdIns(3,5)P2 in RBSDV infection in L. striatellus, the dsLsfab1 was injected into RBSDV-infected L. striatellus nymphs to reduce PtdIns(3,5)P2 and dseGFP was injected as a control. In dsLsfab1-treated L. striatellus, the expression of Lsfab1 decreased at both transcript and protein levels (Figure S4A-B). Dot-ELISA assay showed that the PtdIns(3,5)P2 level significantly decreased in the L. striatellus injected with dsLsfab1 (Figure 3A). The relative virus abundance was examined by analyzing RBSDV P10 transcript levels using RT-qPCR in dseGFP and dsLsfab1 -treated L. striatellus. RBSDV P10 protein levels were also analyzed using western blotting and immunofluorescence. The results showed that P10 transcript and protein levels decreased in dsLsfab1 -treated L. striatellus (Figure 3B-D). In addition, the virus abundance in RBSDV-infected L. striatellus was analyzed after treatment with the fab1 inhibitor YM201636. YM201636 treatment resulted in a significantly decrease of PtdIns(3,5)P2 level and RBSDV P10 expression (Figure 3E-H). Interestingly, RBSDV in the YM201636-treated or dsLsfab1-injected intestines of L. striatellus formed less abundant but enlarged aggregates, which colocalized with PtdIns(3,5)P2 (Figure 3D,3H). These results demonstrate that reduction of the PtdIns(3,5)P2 level inhibits RBSDV propagation in L. striatellus.
Figure 3.
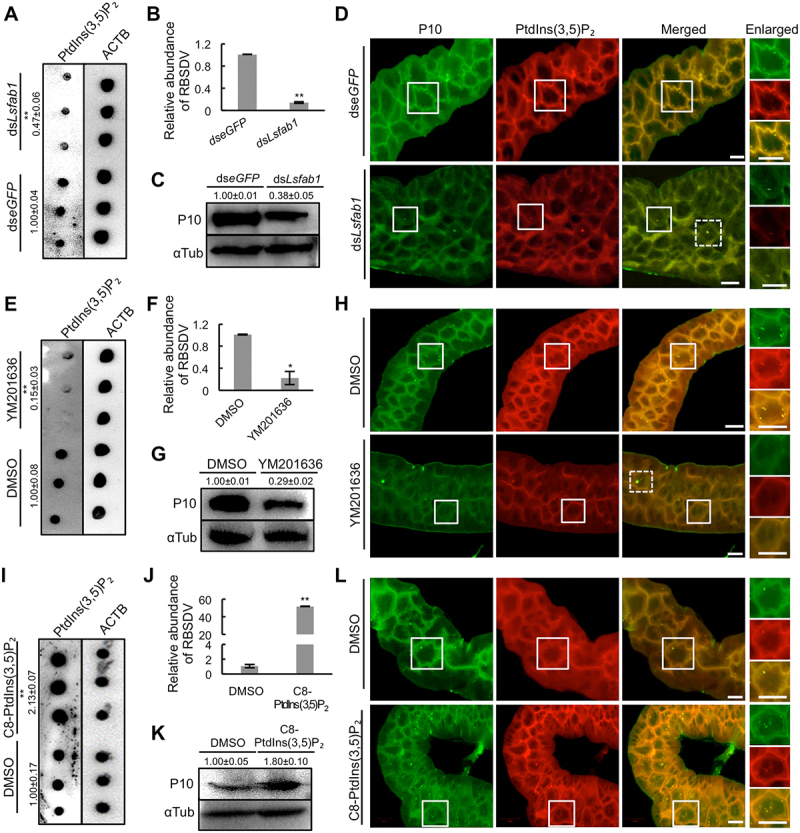
PtdIns(3,5)P2 is required for RBSDV infection. (A) The relative abundance of PtdIns(3,5)P2 was analyzed in RBSDV-L. striatellus injected with dseGFP or dsLsfab1 by dot-ELISA at 4 d after AAP. (B-D) RBSDV abundance in L. striatellus knocked down of Lsfab1 was analyzed by RT-qPCR (B), western blotting (C), and immunofluorescence analysis (D) at 4, 10, and 8 d post microinjection, respectively. The areas in the white box were presented in enlarged images. The dashed box showed the cells having enlarged aggregates. (E) YM201636 treatment decreased the relative abundance of PtdIns(3,5)P2 in L. striatellus. After acquiring the virus for 2 days, L. striatellus was injected with DMSO or YM201636 (20 μM), reared for another 4 days, and collected for dot-ELISA assay. (F-H) The expression of RBSDV P10 was analyzed in L. striatellus by RT-qPCR (F), western blotting (G), and immunofluorescence analysis (H) at 4, 10, and 8 d post YM201636 treatment, respectively. (I) Application of C8-PtdIns(3,5)P2 increased the relative abundance of PtdIns(3,5)P2 in L. striatellus. After acquiring the virus for 2 days, L. striatellus was injected with DMSO or C8-PtdIns(3,5)P2 (0.5 μM), reared for another 4 days, and collected for dot-ELISA assay. (J-L) The RBSDV abundance was analyzed in L. striatellus by RT-qPCR (J), western blotting (K), and immunofluorescence analysis (L) at 4, 10, and 8 d post-C8-PtdIns (3,5)P2 treatment, respectively. Virus-infected intestines were labeled with RBSDV monoclonal antibody-FITC and PtdIns(3,5)P2-rhodamine. Scale bars, 10 μm. Error bars, mean ± SE. The quantification was analyzed by Student’s t-test. *, p <0.05; **, p <0.01.
Conversely, to analyze the effect of an increased PtdIns(3,5)P2 level on RBSDV propagation, C8-PtdIns(3,5)P2 (phosphatidylinositol 3,5-bisphosphate diC8) was injected into the RBSDV-infected L. striatellus nymphs, and DMSO was injected as a control. The PtdIns(3,5)P2 level was increased after injection of C8-PtdIns(3,5)P2 (Figure 3H). RT-qPCR, western blotting, and immunofluorescence assays showed that injection of C8-PtdIns(3,5)P2 significantly increased the viral abundance in L. striatellus (Figure 3J-L). These data indicate that RBSDV infection elevates PtdIns(3,5)P2 level to facilitate virus propagation in L. striatellus.
To investigate whether C8-PtdIns(3,5)P2 could rescue the virus accumulation in Lsfab1-deficient L. striatellus, L. striatellus nymphs were injected with dsLsfab1 and then immediately transferred to RBSDV-infected rice plants. After 3 days, C8-PtdIns(3,5)P2 was injected into the L. striatellus nymphs, and DMSO was injected as a control. Both RT-qPCR and western blotting showed that the virus accumulation increased after C8-PtdIns(3,5)P2 treatment (Figure S4C-D). However, the viruliferous rate of L. striatellus was not affected by YM201636 and C8-PtdIns(3,5)P2 treatment (Figure S4E).
We next investigated whether the regulation role of PtdIns(3,5)P2 on virus propagation is conserved. PtdIns(3,5)P2 production was inhibited by YM201636 treatment, and its effects on the propagation of rice stripe virus (RSV) and southern rice black-streaked dwarf virus (SRBSDV) were analyzed in their insect vectors. RSV in the genus Tenuivirus and family Phenuiviridae is also transmitted by L. striatellus [23], while SRBSDV is another virus in the genus Fijivirus in the family Reoviridae transmitted by Sogatella furcifera [24,25]. To test the binding of RSV or SRBSDV capsid protein with lipids, the RSV nucleocapsid protein (NP) or SRBSDV P10 were expressed in E. coli cells and purified (Figure S1 G-H). The dot-ELISA assay showed that both the nucleocapsid protein of RSV and SRBSDV P10 could bind to PtdIns(3,5)P2 (Figure S5A-B). RT-qPCR results showed that when RSV-infected L. striatellus or SRBSDV-infected S. furcifera was treated with YM201636, the virus abundance in the insect significantly decreased (Figure S5C-D). The reduction of virus abundance in YM201636-treated L. striatellus and S. furcifera was also confirmed by western blotting (Figure S5E-F). Altogether, these results demonstrate that PtdIns(3,5)P2 is a pro-viral host factor for these viruses in insect vectors.
Inhibition of PtdIns(3,5)P2 leads to reduction of RBSDV in endocytic vesicles
Next, we determine how PtdIns(3,5)P2 regulates virus abundance in insects. Deficiency in cellular PtdIns(3,5)P2 production leads to enlarged endosomes [4,17,26]. Since the endocytic pathway is required for reovirus to establish effective infections [27,28], and the enlarged endocytic vesicles containing RBSDV also appeared after inhibition of PtdIns(3,5)P2 production in L. striatellus (Figure 3D,3H), we hypothesized that PtdIns(3,5)P2 likely affects virus infection in insects by regulating the endocytic pathway. In addition, YM201636 treatment causes the accumulation of autophagosomes during the autophagy processes [29,30], while autophagy negatively regulates RBSDV infection in L. striatellus [21]. We hypothesized that the decrease of RBSDV after inhibition of PtdIns(3,5)P2 production is due to the inhibition of viral endocytosis and the promotion of viral autophagy degradation.
To confirm these hypotheses, the distribution of RBSDV on endosomes and autophagosomes in RBSDV-infected L. striatellus was analyzed after the YM201636 treatment. RBSDV was labeled with antibodies against RBSDV P10, while early endosomes, late endosomes, lysosomes, and autophagosomes were labeled with marker proteins Rbsn-5/EEA1 (Rabenosyn-5), Rab7, Lamp1, and Atg8 in the intestines of L. striatellus, respectively. As shown in Figure 4A (upper panel), RBSDV colocalized with Rbsn-5, Rab7, and Lamp1, and only a small number of viruses colocalized with Atg8 in the control experiment. After YM201636 treatment, the relative abundance of RBSDV that colocalized with early endosomes, late endosomes, and lysosomes significantly decreased, while it increased in the autophagosome (Figure 4A and 4B lower panel,). In YM201636-treated L. striatellus, the colocalized punctate structures with a diameter ≥3 μm were higher in amount, while the structures with a diameter <3 μm were less than that in the control experiment (Figure 4C-D). These data indicated that the deficiency of PtdIns(3,5)P2 reduced the virus abundance in the endocytic vesicles in cells of L. striatellus and caused the formation of the enlarged vesicles that originated from early endosomes, late endosomes, lysosomes, and autophagosomes. The colocalization of P10 and Atg8 on the enlarged vesicles in YM201636-treated L. striatellus also suggested that the deficiency of PtdIns(3,5)P2 caused the decrease of virus abundance via the autophagy pathway.
Figure 4.
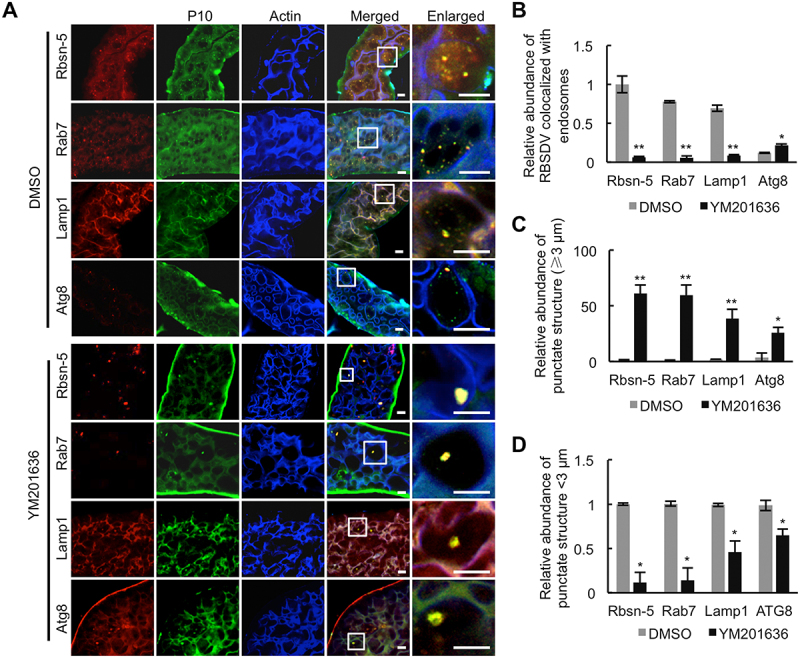
Inhibition of PtdIns(3,5)P2 reduces RBSDV abundance in endocytic vesicles. (A) The colocalization of RBSDV with early endosome, late endosome, lysosome, or autophagosome was analyzed in the intestines of RBSDV-infected L. striatellus treated with DMSO or YM201636. RBSDV, early endosomes, late endosomes, lysosomes, and autophagosomes were labeled by immunofluorescence analysis with conjugated antibodies RBSDV-FITC, Rbsn-5-rhodamine, Rab7-rhodamine, Lamp1-rhodamine, Atg8–rhodamine, and phalloidin-350 after treatment with DMSO or YM201636 (20 μM) for one day. (B) The relative abundance of fluorescence punctate structures that could be labeled with RBSDV and early endosome, late endosome, lysosome, or autophagosome was calculated in the intestines of DMSO or YM201636 -treated L. striatellus. (C) The relative abundance of the fluorescence punctate structures with diameter <3 μm in DMSO and YM201636 -treated L. striatellus was analyzed. (D) The relative abundance of the enlarged punctate structures with diameter ≥3 μm was calculated in DMSO and YM201636 -treated L. striatellus. The areas in the white box were presented in enlarged images. Scale bars, 10 μm. Error bars, mean ± SE. The quantification was analyzed by Student’s t-test. *, p <0.05; **, p <0.01.
PtdIns(3,5)P2 regulates RBSDV abundance via the autophagy pathway
A previous study reported that Atg8-II increased in RBSDV-infected L. striatellus at the early infection stage, and autophagy negatively regulates virus accumulation in L. striatellus [21]. In the present study, we found that the Atg8-II protein level and the expression of the autophagy-related genes Atg3, Atg8, and Atg6 at transcript level increased in RBSDV-infected L. striatellus at 10 d after AAP (Figure S6A-B). Silencing the autophagy-related protein genes Atg3, Atg8, or Atg6 increased the viral abundance (Figure S6C-E). The RBSDV abundance at both RNA and protein levels decreased in L. striatellus treated with the autophagy inducer rapamycin and increased with the autophagy inhibitor 3-methyladenine (3-MA) treatment (Figure S6F-G). Consistent with previous results [21], these data confirmed that inhibition of the autophagy pathway promotes RBSDV propagation in L. striatellus.
To test whether PtdIns(3,5)P2 facilitates virus infection by inhibiting autophagy defense in L. striatellus, the regulation of PtdIns(3,5)P2 on autophagy were analyzed. After acquiring RBSDV on virus-infected plants for 2 days, L. striatellus were injected with DMSO, C8-PtdIns(3,5)P2, or YM201636, and then reared on healthy rice seedlings for 10 days. The expressions of RBSDV P10, Atg8, and ref(2)P/SQSTM1 (refractory to sigma P) at the protein level were examined by western blotting. P10 abundance, Atg8-I:Atg8-II ratio, and ref(2)P/SQSTM1 level all increased after C8-PtdIns(3,5)P2 treatment (Figure 5A, left panel), while YM201636 treatment caused the opposite results (Figure 5A, right panel). The ratio of the Atg8-I to Atg8-II level is regarded as an accurate indicator of the formation or the degradation of autophagosome [31], while ref(2)P/SQSTM1 is regarded as an indicator of the component degradation in autolysosomes by the autophagy pathway [32]. The results indicate that higher PtdIns(3,5)P2 levels promote RBSDV propagation in L. striatellus by inhibiting the autophagy pathway. In contrast, lower PtdIns(3,5)P2 levels inhibit virus propagation by promoting the autophagic degradation of the virus. Taken together, these data suggest that PtdIns(3,5)P2 is beneficial for RBSDV infection in insects by inhibiting autophagic degradation.
Figure 5.
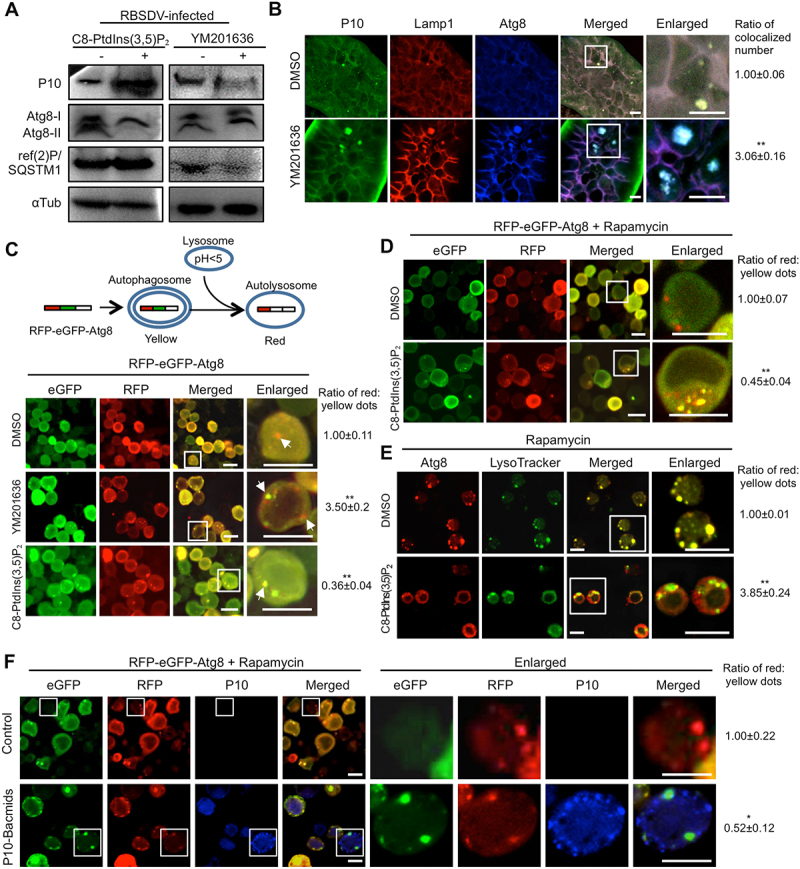
PtdIns(3,5)P2 inhibits autophagy by blocking lysosome-autophagosome fusion. (A) PtdIns(3,5)P2 regulated the autophagy level in L. striatellus. After acquiring the virus for 2 days, the nymphs of L. striatellus were injected with DMSO, YM201636 (20 μM), or C8-PtdIns(3,5)P2 (0.5 μM). The relative abundances of P10, Atg8, and ref(2)P/SQSTM1 in L. striatellus were detected by western blotting at 10 d post microinjection. (B) YM201636 treatment caused the colocalization of RBSDV, lysosomes, and autophagosomes. After acquiring the virus for 6 days, the nymphs of L. striatellus were injected with DMSO or YM201636 (20 μM) and collected at 2 d post microinjection. The colocalization of RBSDV, lysosome, and autophagosome in the intestines of L. striatellus was analyzed by immunofluorescence analysis with conjugated antibodies RBSDV-FITC, Lamp1-rhodamine, and Atg8-Alexa Fluor 647. (C) PtdIns(3,5)P2 regulated the lysosome-autophagosome fusion in Sf9 cells. Sf9 cells were infected with RFP-eGFP-Atg8 bacmids for 6 h, and then incubated with DMSO, YM201636 (100 nM), or C8-PtdIns(3,5)P2 (50 nM) for 6 h. The cells were viewed by confocal microscopy at 36 h post reagents treatment. The white arrows indicated the red and yellow dots in the cells. (D-E) PtdIns(3,5)P2 inhibited rapamycin-induced lysosome-autophagosome fusion in Sf9 cells. The Sf9 cells were transfected with RFP-eGFP-Atg8 bacmids for 6 h and treated with rapamycin (20 μM) for another 6 h. The cells were rinsed with PBS buffer and then incubated with DMSO or C8-PtdIns(3,5)P2 (50 nM) for 12 h. The expression of RFP-eGFP-Atg8 in the Sf9 cells was analyzed by a confocal microscopy (D). Sf9 cells were treated with rapamycin for 6 h, and then incubated with DMSO or C8-PtdIns(3,5)P2 (50 nM) for 6 h. The Atg8 and lysosomes in Sf9 cells were labeled by immunofluorescence analysis with Atg8-rhodamine antibody and LysoTracker (E). (F) P10 protein blocks the fusion of autophagosome and lysosome. The Sf9 cells were infected with RFP-eGFP-Atg8 bacmids and P10 bacmids together for 6 h, and then treated with 20 μM rapamycin for 6 h. After 24 h culture in complete medium, the expression of GFP, RFP, and P10 was detected by immunofluorescence analysis. RBSDV monoclonal antibody conjugated Alexa Fluor 647 was used to detect P10 protein. The numbers of yellow dots and red dots were counted in 150 cells with distinct cell contours and containing punctate fluorescent dots in each treatment, and then the numbers of red:yellow dots was analyzed. The ratio of red:yellow dot numbers in the cells treated with YM201636 or C8-PtdIns(3,5)P2 was normalized to that in the DMSO control. Scale bars, 10 μm. Error bars, mean ± SE. The quantification was analyzed by Student’s t-test. *, p <0.05; **, p <0.01.
PtdIns(3,5)P2 manipulates the autophagy pathway by regulating the lysosome-autophagosome fusion
Next, we studied how PtdIns(3,5)P2 regulates the autophagy pathway. During the autophagic process, the autophagosome and lysosome fuse together to form the autolysosome and then degrade the contents within it [10,33]. The localization of RBSDV P10, Lamp1, and Atg8 on the enlarged vesicles suggested that RBSDV, lysosomes, and autophagosomes were colocalized together after YM201636 treatment in L. striatellus. To confirm whether YM201636 treatment promotes the fusion of lysosomes and autophagosomes to degrade RBSDV, RBSDV-infected L. striatellus was treated with YM201636 or DMSO. Two days later, the colocalization of RBSDV, lysosomes, and autophagosomes was analyzed. As shown in Figure 5B, RBSDV P10, lysosome marker Lamp1, and autophagosome marker Atg8 were colocalized on the enlarged vesicles in YM201636-treated L. striatellus, while the virus particles mainly colocalized with Lamp1, but not with Atg8 in the DMSO-treated L. striatellus. The results suggest that YM201636 treatment promotes the fusion of autophagosomes and lysosomes to form autolysosomes.
To confirm the function of PtdIns(3,5)P2 in regulating the fusion of autophagosomes and lysosomes, a tandem reporter construct pFastBac-RFP-eGFP-Atg8 was used to track the fusion during the process of autolysosome formation. The GFP fluorescence is sensitive to acidic and/or proteolytic conditions, whereas the RFP is not. The autophagosomes are labeled in yellow, and autolysosomes formed from autophagosomes fusing with lysosomes are labeled in red in the cells expressing tandem RFP and eGFP -tagged Atg8 [32,34]. RFP-eGFP-Atg8 was expressed in Sf9 cells for 6 h using a Bac-Bac system, and the cells were subsequently incubated with 100 nM YM201636 or 50 nM C8-PtdIns(3,5)P2 for another 6 h. The ratio of red:yellow dot numbers dramatically increased in Sf9 cells after YM201636 treatment (Figure 5C, middle panel vs. upper panel), whereas decreased when supplemented with C8-PtdIns(3,5)P2 (Figure 5C, lower panel vs. upper panel). The data demonstrate that PtdIns(3,5)P2 regulates the fusion of lysosomes and autophagosomes.
We also tested whether PtdIns(3,5)P2 could inhibit the autophagosome-lysosome fusion induced by rapamycin. The Sf9 cells expressing RFP-eGFP-Atg8 were treated with 20 μM rapamycin for 6 h, and then incubated with 50 nM C8-PtdIns(3,5)P2 for 12 h. Compared with the DMSO-treated control, the C8-PtdIns(3,5)P2 treatment significantly reduced the ratio of red:yellow fluorescent dot number (Figure 5D). Moreover, the Sf9 cells were treated with rapamycin directly, followed by C8-PtdIns(3,5)P2 or DMSO treatment. The colocalization of lysosomes and autophagosomes were detected as yellow dots in rapamycin-treated Sf9 cells with DMSO treatment, indicating that lysosomes and autophagosomes fused together after rapamycin treatment. When C8-PtdIns(3,5)P2 was added to the rapamycin-treated Sf9 cells, the ratio of red:yellow dot numbers dramatically increased (Figure 5E). These results suggest that PtdIns(3,5)P2 could inhibit the autophagosome-lysosome fusion induced by rapamycin treatment in Sf9 cells.
P10 protein alone can increase the PtdIns(3,5)P2 level, and a-higher level of PtdIns(3,5)P2 inhibits the fusion of autophagosome and lysosome. We further investigated whether P10 protein could block the fusion of autophagosome and lysosome. The Sf9 cells were infected with RFP-eGFP-Atg8 bacmids and P10 bacmids together for 6 h, and the cells infected with RFP-eGFP-Atg8 bacmids only were used as control. The cells were then treated with rapamycin for 6 h and the expression of RFP-eGFP-Atg8 and P10 were detected by immunofluorescence analysis. The ratio of red:yellow dot numbers in the cells expressing RFP-eGFP-Atg8 and P10 was significantly lower than that in the control cells (Figure 5F), indicating that P10 inhibits the autophagosome-lysosome fusion.
Trpml is required for PtdIns(3,5)P2 to regulate virus propagation via the autophagy pathway
PtdIns(3,5)P2 recruits effector proteins to regulate intracellular signaling and membrane dynamics. Several effectors of PtdIns(3,5)P2 have been identified, including voltage-gated Ca2+ channel (CACNA1C/CaV1.2) [35], the ENTH-containing proteins Ent3 and Ent5 [36], CHMP3/mVps24 [37], Atg18 [38], and Trpml [39]. As Trpml is a reported effector of PtdIns(3,5)P2 that acts on autophagosome-lysosome fusion [40], we hypothesized that PtdIns(3,5)P2 could regulate RBDSV-induced autophagy through its effector Trpml.
To test this hypothesis, we analyzed the effect of RBSDV infection on the expression of LsTrpml in L. striatellus. RT-qPCR and western blotting assay showed that both transcript and protein levels of LsTrpml increased after RBSDV infection (Figure 6A-B). We then analyzed whether P10 could induce the expression of Trpml. When RBSDV P10 protein was expressed in Sf9 cells, the SfTrpml transcript and protein levels were higher than that in the control (Figure 6C-D). As P10 protein could elevate PtdIns(3,5)P2 level, we investigated whether the Trpml expression was affected by PtdIns(3,5)P2 level. The L. striatellus was injected with C8-PtdIns(3,5)P2 or DMSO and then transferred to healthy rice seedlings for 4 days. RT-qPCR results showed that the mRNA level of LsTrpml increased in C8-PtdIns(3,5)P2-injected L. striatellus (Figure 6E). In contrast, when the insects were subjected to YM201636 treatment or by dsRNA-mediated Lsfab1 knockdown, the LsTrpml mRNA accumulation decreased (Figure 6E). These data suggest that RBSDV P10 and PtdIns(3,5)P2 regulates the expression of LsTrpml.
Figure 6.
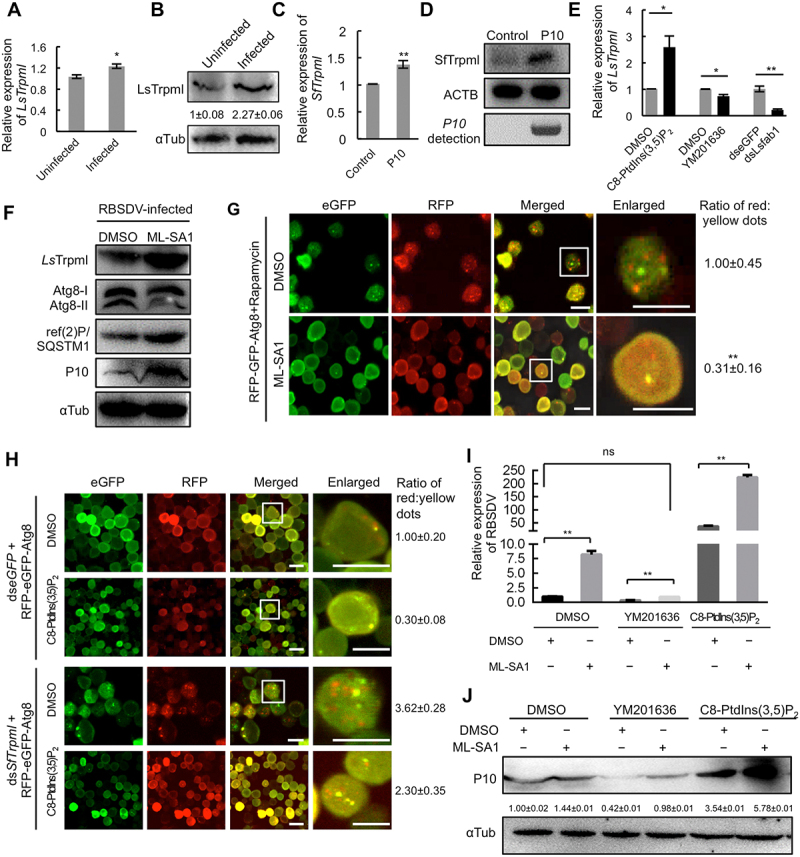
PtdIns(3,5)P2 inhibits autophagosome-lysosome fusion by activating Trpml. (A-B) The LsTrpml expression was analyzed in RBSDV -uninfected and -infected L. striatellus by RT-qPCR at 4 d after AAP (A) and western blotting at 8 d after AAP (B). (C-D) The SfTrpml expression was analyzed in RBSDV P10 expressing Sf9 cells by RT-qPCR at 48 h and western blotting at 96 h after bacmids infection. (E) The LsTrpml expression was analyzed by RT-qPCR at 4 d after DMSO, C8-PtdIns(3,5)P2 (0.5 μM), YM201636 (20 μM) or dseGFP/dsLsfab1 microinjection. (F) Activation of Trpml promoted RBSDV propagation and inhibited autophagy in L. striatellus. After acquiring the virus for 2 days, the nymphs of L. striatellus were treated with DMSO or ML-SA1 (20 nM). The abundance of RBSDV, Atg8, and ref(2)P/SQTSM1 was analyzed by western blotting at 10 d after the treatment. (G) ML-SA1 inhibited the lysosome-autophagosome fusion in Sf9 cells. Sf9 cells were infected with RFP-eGFP-Atg8 bacmids for 6 h, treated with rapamycin (20 μM) for 6 h, and then incubated with DMSO or ML-SA1 (10 nM) for 24 h. The cells were analyzed by confocal microscopy. The ratio of red:yellow dot numbers was obtained by calculating the average number of dots in 150 cells. (H) Knockdown of SfTrpml promoted the lysosome-autophagosome fusion in Sf9 cells. Sf9 cells were transfected with dsSfTrpml or dseGFP for 12 h, rinsed, and incubated with RFP-eGFP-Atg8 bacmids/C8-PtdIns(3,5)P2 mixture or RFP-eGFP-Atg8 bacmids/DMSO mixture for 36 h. The expression of RFP-eGFP-Atg8 was analyzed by confocal microscopy. The ratio of red:yellow dot numbers was obtained by calculating the average number of red/yellow dots in 150 cells. (I-J) Trpml is required for PtdIns(3,5)P2 to promote virus propagation. The nymphs of L. striatellus were treated with DMSO, YM201636 (20 μM), or C8-PtdIns(3,5)P2 (0.5 μM), respectively, and reared on RBSDV-infected rice plant for 4 days. Then the L. striatellus was injected with DMSO or ML-SA1 (20 nM). The virus abundance in L. striatellus was analyzed by RT-qPCR (I) and western blotting assay (J) at 4 d and 8 d after ML-SA1 treatment, respectively. Scale bars, 10 μm. Error bars, mean ± SE. The quantification was analyzed by Student’s t-test. *, p <0.05; **, p <0.01
Next, we investigated whether LsTrpml affects the autophagy process and RBSDV propagation in L. striatellus. RBSDV-infected L. striatellus was injected with ML-SA1, a specific Trpml activator, to activate Trpml. DMSO was injected as a control. The L. striatellus were then reared on healthy rice seedlings for 10 days, and the expression of LsTrpml, RBSDV P10, and Atg8 was analyzed by western blotting. We found that ML-SA1 treatment reduced Atg8-II level and significantly increased LsTrpml, ref(2)P/SQSTM1, and RBSDV P10 abundance (Figure 6F), suggesting that LsTrpml can promote RBSDV propagation by inhibiting the autophagy pathway.
Furthermore, we analyzed whether LsTrpml regulates the fusion of autophagosomes and lysosomes. Sf9 cells expressing RFP-eGFP-Atg8 were treated with rapamycin for 6 h to induce the formation of autophagosomes. Then, the cells were incubated with ML-SA1 or DMSO for 24 h. As shown in Figure 6G, the ratio of red:yellow fluorescent dot numbers decreased when ML-SA1 was applied, indicating that activation of LsTrpml by ML-SA1 blocks the fusion of autophagosomes and lysosomes.
To investigate whether Trpml was required for PtdIns(3,5)P2 to regulate the fusion of autophagosome and lysosome, the Sf9 cells were transfected with dsRNA of SfTrpml, and dseGFP was transfected as a control. The knockdown of SfTrpml was confirmed by RT-qPCR and western blotting in the cells treated with dsSfTrpml. After transfecting dsRNAs for 36 h, the cells were incubated with RFP-eGFP-Atg8 bacmids/C8-PtdIns(3,5)P2 mixture. As a control, the cells were incubated with RFP-eGFP-Atg8 bacmids/DMSO. Compared with the dseGFP-transfected Sf9 cells that were incubated with RFP-eGFP-Atg8 bacmids/DMSO, the ratio of red:yellow dot numbers increased in dsSfTrpml-transfected Sf9 cells that incubated with RFP-eGFP-Atg8 bacmids/DMSO (Figure 6H, the third row vs the first row). In the cells expressing RFP-eGFP-Atg8 and treated with dsSfTrpml, the ratio of red:yellow dot numbers decreased after C8-PtdIns(3,5)P2 treatment (Figure 6H, the fourth row vs the third row). These results reveal that Trpml is required for PtdIns(3,5)P2 to regulate autophagosome-lysosome fusion.
Next, we investigated whether LsTrpml can reverse the decrease of virus abundance caused by the reduction of PtdIns(3,5)P2. After the PtdIns(3,5)P2 level was reduced in RBSDV-infected L. striatellus by YM201636 treatment, the L. striatellus was transferred to RBSDV-infected rice plants for 4 days to acquire virus and then injected with ML-SA1 or DMSO. The virus abundance was evaluated by RT-qPCR and western blotting at 4 days and 8 days after ML-SA1 treatment. We found that the application of ML-SA1 increased the virus abundance in YM201636-treated L. striatellus to a level similar to that in the DMSO-treated samples without ML-SA1 treatment (Figure 6I-J). On the contrary, to confirm whether the elevated PtdIns(3,5)P2 promotes virus propagation through LsTrpml, L. striatellus was treated with C8-PtdIns(3,5)P2 and reared on RBSDV-infected plants to acquire virus for 4 days. Then, the L. striatellus was injected with ML-SA1 or DMSO. When ML-SA1 was applied to L. striatellus treated with C8-PtdIns(3,5)P2, ML-SA1 further dramatically stimulated RBSDV accumulation (Figure 6I-J). These data suggest that Trpml rescues the reduction of virus abundance caused by PtdIns(3,5)P2 inhibition.
Discussion
Several viral proteins were reported to bind to PIPs directly, such as vaccinia virus H7 protein which binds to PtdIns3P and PtdIns4P [41], and human immunodeficiency type 1 Gag, which binds to PtdIns(4,5)P2 [42]. RBSDV P10 is the first reported viral protein that preferentially binds to PtdIns(3,5)P2 directly. The binding between PtdIns(3,5)P2 and truncated proteins suggested that there were multiple binding sites on P10 protein. No canonical PIP binding domains were identified in Vph1, a subunit isoform of the vacuolar-type H+-translocating ATPase (V-ATPase) in yeast, which interacts with PtdIns(3,5)P2 directly [43]. Whether there are conserved binding residues in P10 still needs to be investigated. Lysine (K) has been reported to mediate the conformational change of the binding protein TPCN1/TPC1 and activation of PtdIns(3,5)P2 [44]. K156A mutant in Pep1 completely abolished the binding (Figure 1 (f)1), confirming that K is critical for the binding. YM201636 treatment interferes with the release of equine infectious anemia virus virus-like particles formed by Gag protein. The K49 mutation of Gag in the PI-binding pocket severely inhibited virus-like particle release and the S100A mutant exhibited a different localization pattern [8]. The binding and colocalization of RBSDV P10 and PtdIns(3,5)P2 may contribute to the endosome localization and release of RBSDV in the host cells.
In yeast, plant, and mammalian cells, PtdIns(3,5)P2 level could be elevated under hyperosmotic stress, insulin, and growth factor stimuli [45–48]. However, the mechanism of how these biological or abiotic stresses induce the PtdIns(3,5)P2 is still unknown. Among the PIPs, the mechanism of PtdIns4P synthesis induced by a viral protein has been elucidated. The picornaviral 3 CD protein induces PtdIns4P by activating the PtdIns4P biogenesis pathway, which requires GBF1 (golgi brefeldin A resistant guanine nucleotide exchange factor 1) and ARF1 (ADP ribosylation factor 1) [49]. GBF1 activates its substrate ARF1 by converting the ARF1-ADP to ARF1-GTP, and the activated ARF1 recruits and activates PI4KB/PI4KIIIβ (phosphatidylinositol 4-kinase beta) for PtdIns4P production [50,51]. Nevertheless, some viral proteins recruit PI4KB independent of GBF1 and ARF1 [52,53]. In our study, P10 protein is sufficient to induce PtdIns(3,5)P2 and the expression of fab1. We analyzed the interaction between P10 and Lsfab1 by Co-IP assay and found that P10 interacted with Lsfab1. The results indicate that P10 may active fab1 directly. AMP-activated protein kinase (AMPK) is a key activator of PIKFYVE/fab1, which phosphorylates PIKFYVE at Ser307 and facilitates PIKFYVE translocation to vesicular structures [54]. RBSDV P10 can promote the phosphorylation of AMPK in L. striatellus [21]. Another possible mechanism for upregulation of PtdIns(3,5)P2 by P10 protein is that P10 phosphorylates AMPK, and then the phosphorylated AMPK promotes the translocation of fab1 to vesicle structures and the activation of fab1 to synthesize PtdIns(3,5)P2.
Autophagy is a conserved degradation pathway responsible for the clearance of damaged organelles or dysfunctional proteins in eukaryotes. The autophagic process plays a dual role in virus infection. Hosts activate the autophagy pathway as an immune response to degrade viruses, while viruses take advantage of autophagy to facilitate virus infection [55–57]. For some viruses, autophagy can be activated or inhibited at different infection stages of virus infection. In response to HCMV infection, autophagy is triggered during the early stage of infection but is inhibited at later stages by the terminal repeat sequence 1 protein of HCMV through interaction with the BECN1 [58]. RBSDV transiently induces autophagy at 2–4 days after feeding of the insect vectors on virus-infected plants, an early time point for RBSDV infection [21]. As RBSDV is a persistent and propagative virus, the intriguing question is how RBSDV reverses and inhibits the established autophagy after the early stage of infection. Our study demonstrates a strategy that RBSDV P10 binds to PtdIns(3,5)P2 and induces its production, which inhibits the autophagy pathway to favor virus infection in insects. We show that PtdIns(3,5)P2, a low abundant host signaling lipid other than host proteins shown by previous studies, can be targeted by a viral protein for autophagic escaping.
PtdIns(3,5)P2 deficiency or reduction caused the enlargement of vacuoles or endosomes, which could be labeled with the endosomal marker protein of early endosomes, late endosomes, or lysosomes in many eukaryotic cells [4,17,26,59,60]. In addition to early endosomes, late endosomes, and lysosomes, we also showed that autophagosome marker Atg8 could be labeled on the enlarged vacuoles (Figure 4). It was further confirmed that PtdIns(3,5)P2 regulates autophagy levels by manipulating autophagosome-lysosome fusion (Figure 5C-F). Similarly, decreased lysosomal PtdIns(3,5)P2 level in neuronal cells due to INPP5E (inositol polyphosphate-5-phosphatase E) expression leads to activated cortactin that binds and stabilizes actin filaments, thus facilitating the fusion of autophagosomes and lysosomes [61,62]. These studies indicate that PtdIns(3,5)P2 plays an essential role in the fusion of lysosomes and autophagosomes.
The PIP lipids regulate diverse downstream cellular pathways by recruiting various effector proteins. Trpml, a PtdIns(3,5)P2 effector protein, also known as a mucolipin, is a ubiquitously-expressed lysosomal calcium channel. Activation of Trpml through ML-SA1 degrades dengue virus 2 and Zika virus by promoting lysosome acidification and protease activity, independent of autophagy induction [63]. There are three Trpml proteins in mammals, Trpml1 to Trpml3 [64], but only one in insects [65]. Compared with Trpml in mammals, the role of Trpml in insects is largely unknown. Our data show that L. striatellus Trpml is required by PtdIns(3,5)P2 to modulate the lysosome and autophagosome fusion and virus degradation. The mechanism of how Trpml regulates the fusion of lysosomes and autophagosomes needs further investigation. One possible mechanism is that Trpml regulates the Ca2+-dependent centripetal movement of lysosomes toward autophagosomes through the Apoptosis-linked gene-2, an EF-hand-containing protein in lysosomes [39,66].
In summary, our study revealed that RBSDV P10 binds to and elevates PtdIns(3,5)P2 level, which activates Trpml to block the fusion of lysosome and autophagosome and facilitate the virus escape from autophagic degradation in L. striatellus (Figure 7). RBSDV P10 protein interacts with glyceraldehyde-3-phosphate dehydrogenase, which is required for the activation of autophagy to suppress RBSDV accumulation in L. striatellus [21]. These studies suggest that virus protein plays a dual role in the autophagy process. PtdIns(3,5)P2 was required for RSV, RBSDV and SRBSDV propagation. The findings demonstrate that PtdIns(3,5)P2 plays a critical role in regulating virus infection. It can be considered as a promising drug target for controlling plant viruses in insect vectors.
Figure 7.
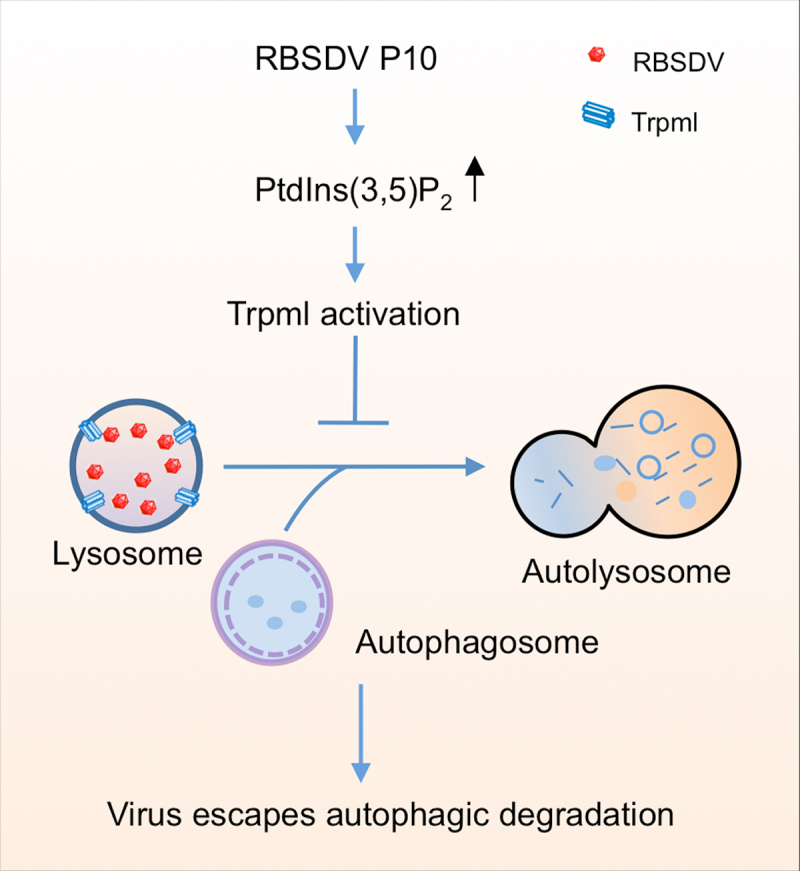
Schematic model for RBSDV escaping autophagic degradation by regulating PtdIns(3,5)P2 in L. striatellus. RBSDV infection increased PtdIns(3,5)P2 level via its capsid protein P10. The high level of PtdIns(3,5)P2 blocks the lysosome – autophagosome fusion through its effector protein Trpml, inhibits the autophagic degradation, and finally facilitates the virus survival in its insect vector.
Materials and methods
Insects and virus source
The L. striatellus (RBSDV and RSV -free), RSV-infected L. striatellus, and S. furcifera were originally collected from rice fields in Jiangsu province and maintained in the laboratory. The insects were reared on rice seedlings in a growth chamber at 26°C with 55 ± 5% humidity and a 16 h light/8 h dark cycle. RBSDV-infected or RSV-infected rice plants were collected from rice fields and propagated via insect transmission. SRBSDV-infected rice plants were provided by Dr. Tong Zhang (South China Agricultural University, PRC).
Preparation of RBSDV crude extract
RBSDV crude extract was prepared as follows. The RBSDV-infected rice plants were cut into small pieces and added to the precooled extraction buffer (0.05 M Na2HPO4, 0.005 M disodium EDTA, 0.01 M Na2SO3, 1 mM PMSF, pH 6.5). The mixture was homogenized in an ice-water bath using a handheld high-speed blender and then filtered with cotton gauze. Each 30-mL filtered homogenate was mixed with 10 mL 1,1,2-trichlorotrifluoroethane (Mucklin, T819019) and shaken in an ice box at 170 rpm for 10 min followed by centrifugation at 5000 × g at 4°C for 60 min. The upper aqueous layer was filtered through the 0.45-µm syringe filter (Merck Millipore, SLHP033RS) and collected as the RBSDV crude extract.
Plasmid construction
The nucleotide sequence of RBSDV P10, P10-N, -N1, -N2, and -C1 were amplified by PCR with high fidelity DNA polymerase (Takara, R045A) and the primers listed in Table S1. RBSDV P10 or eGFP was cloned into pET-15b (Merck, 69661). P10-N, -N1, -N2, and -C1 fragments were inserted into pET15b-eGFP plasmid, respectively. RSV NP gene and SRBSDV main capsid protein P10 gene were cloned into pGEX-4T-1 (Amersham, 28954549) and pET32a (+) (Merck, 69015), respectively.
The eGFP, RBSDV P10, and LsAtg8 genes were amplified with their specific primers (Table S1), respectively. P10eGFP and RFP-eGFP-Atg8 fragments were constructed by overlapping PCR and cloned into pFastBac1 vector. The pFastbac-eGFP, pFastbac-P10eGFP, and RFP-eGFP-Atg8 plasmids were expressed in Sf9 cells (provided by Professor Bin Li from Nanjing Normal University) using Baculovirus Expression System.
Protein expression and purification
The E. coli BL21 (DE3) Rosetta cells (ComWin Biotechnology, CW0811S) containing expression plasmids were grown at 37°C to an optical density at 600 nm (OD600) of 0.6–0.8 and induced with 0.1–0.4 mM IPTG (Beyotime Biotechnology, ST1416-25 g) at 16°C for 16 h. The induced cells were sonicated in lysis buffer (50 mM Tris-Cl, 200 mM NaCl, 5% glycerol, 5 mM DTT, 1% Triton X-100 [Sigma-Aldrich, X100-100 mL], pH 8.0) and then centrifuged at 15,000 × g, 4°C for 30 min. The supernatant was then incubated with Ni-NTA beads (Sangon Biotechnology, C600033–0025) for His-tagged protein purification or GST-beads (Beyotime Biotechnology, P2251) for GST-tagged protein for 1 h at 4°C. His-tagged proteins were washed with 0.02 M imidazole (Sigma-Aldrich, I0250) and eluted with 0.3 M imidazole. GST-tagged proteins were eluted with 15 mM reduced glutathione (Beyotime Biotechnology, S0073). RBSDV His-P10 was purified as previously described [67] and stored at −20°C. Purified proteins were concentrated with concentrators (Millipore, UFC905096) and stored at −20°C for further use.
RBSDV P10 peptides synthesis
Main capsid protein sequences of seven different reoviruses, namely, RBSDV (NCBI: AHK06880.1), maize rough dwarf virus (GenBank No: AEA35043.1), SRBSDV (GenBank No: AFM97295.1), Mal de Rio Cuarto virus (GenBank No: YP_956849.1), oat sterile dwarf virus (GenBank No: BAA25150.1), Nilaparvata lugens reovirus (GenBank No: NP_619775.1) and Fiji disease virus (GenBank No: YP_249765.1) were multi-aligned by SnapGene (version: 4.2.4). Conserved positively charged lysine (K) sites in P10 protein were identified by sequence alignment. The N-terminal-biotinylated peptides (150–171, 209–235, 273–288, 150–160, 161–171, 150–171 and the mutant peptide K156A) of RBSDV P10 were synthesized by Shanghai Youlong Biotechnology Company. The information of the peptides is shown in Table S2.
Lipid binding assay and dot-ELISA
Lipids were dissolved in chloroform and then spotted onto the nitrocellulose membrane (PALL, 66485) strips at settled concentrations. All the lipids used in this study were purchased from Avanti Polar Lipids, lnc, including PtdIns3P (850150P), PtdIns4P (850151P), PtdIns5P (850152P), PtdIns(3,4)P2 (850153P), PtdIns(4,5)P2 (850155P), PtdIns(3,5)P2 (850154P), PtdIns(3,4,5)P3 (850156P), PS (840035), C1P (860599), PE (840024P), PC (840054P), PG (841148P), CL (710332), PA (840074P) and PtdIns (840044P). PIP strips membrane (Molecular Probes, P23750) was used to analyze the binding between the lipids and RBSDV crude extract.
In the lipid binding assay, the PIP strips were blocked with 3% fatty acid-free bovine serum albumin (BSA; Sangon Biotechnology, A602448) in 1×TBS (20 mM Tris, 150 mM NaCl, pH 8.0) for 1 h and then incubated with RBSDV crude, purified His-tagged protein (P10, P10-N, N1, N2, P10-C1, eGFP, or SRBSDV P10), or purified GST-tagged RSV NP protein, GST protein (Abcam, ab81793) for 3 h at room temperature. After washing, the strips were incubated with the mouse monoclonal anti-RBSDV, anti-6×His tag (Abmart, M20001L), anti-GST tag (Beyotime Biotechnology, AF5063) antibody for 2 h, followed by incubating with goat anti-mouse IgG-HRP antibody (Bioss incorporated, bs-0296G-HRP) for 1 h at room temperature. Then, the strips were analyzed by a chemiluminescence gel imaging system (Tanon 5200 Multi). To analyze the bindings between the peptides and lipids, the biotin-labeled peptides were synthesized by Yonglong Biotechnology and detected using HRP-conjugated streptavidin antibody (Solarbio Life Science, SE068). Each lipid-binding assay was repeated at least three times, and the intensity of the signal (gray value) was calculated by ImageJ (version: 1.53c).
The relative abundance of PtdIns(3,5)P2 in L. striatellus or Sf9 cells was examined by dot-ELISA assay. The relative abundance of PS, which did not bind to P10, was analyzed as a control. Briefly, insects or cells were ground in carbonate buffer solution (CBS; 68.6 mM sodium bicarbonate, 31.4 mM sodium carbonate, pH 9.6) and centrifuged at 15,294×g for 10 min at 4°C. The supernatant was diluted with CBS 50-fold and then dot-blotted onto PVDF membrane (Merck Millipore, ISEQ00010), respectively. After blocking with 3% BSA in 1× TBS for 1 h, the membrane was incubated with mouse monoclonal anti-PtdIns(3,5)P2 (Echelon Biosciences, Z-P035), rabbit polyclonal anti-PS (Cloud Clone Crop, PAB881Ge01), or mouse monoclonal anti-ACTB/β-actin (Beyotime Biotechnology, AF0003) antibodies, respectively, followed by incubating with goat anti-rabbit IgG-HRP antibody (Bioss incorporated, bs-0295 G-HRP) or goat anti-mouse IgG-HRP (Bioss incorporated, bs-0296G-HRP) antibody, and then developed with chemiluminescence according to the manufacturer’s protocol (ECL; Beyotime Biotechnology, P0018FM). The ACTB was detected as the loading control. At least three biological replicates were carried out. Each insect sample contained a pool of 16 L. striatellus and about 2.5 × 105 cells grown in one well in 6-well culture plate were collected as a cell sample. The relative abundance of PtdIns(3,5)P2 and PS was calculated according to the average gray value measured by ImageJ and normalized to ACTB. The significance was analyzed using the Student’s t-test.
RNA extraction and RT-qPCR
Total RNA of the L. striatellus or Sf9 cells was extracted by RNAiso (Takara, 9109) according to the manufacturer’s protocol. The RNA was quantified by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) and reverse transcribed using PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, RR047A) following the manufacturer’s instructions. Subsequently, RT-qPCR was conducted on IQTM 5 multicolor real-time PCR detection system (BIO-RAD) with TB Green Premix Ex Taq reagent (Takara, RR4204). The relative expression of P10 was calculated as the relative abundance of RBSDV at the RNA level. Relative gene expression levels were normalized to the housekeeping gene LsRpL5 (ribosomal protein L5, GenBank: HQ385973) in L. striatellus, Sfactin 1 (GenBank: KP735520.1) in S. furcifera, SfRpL32 (ribosomal protein L32, GenBank: XM_035597771.1) in Sf9, and calculated by 2−ΔΔCT (cycle threshold). Each experiment contained three independent biological and three technical replications.
SDS-PAGE and western blotting assay
Total proteins were extracted with RIPA lysis buffer (Beyotime Biotechnology, P0013B), and the protein concentrations were determined by the BCA protein assay kit (Sangon Biotechnology, C503021–0500). The total proteins were then mixed with a 5×protein loading buffer and boiled at 99°C for 10 min. After SDS-PAGE, the proteins were transferred to PVDF membranes by eBlotTM L1 (GenScript) and incubated with specific primary antibodies, followed with HRP-conjugated goat anti-rabbit or anti-mouse IgG. The blotted membranes were developed using a chemiluminescence gel imaging system, and the gray value of each sample was calculated using ImageJ. The αTub served as the loading control.
To detect the fab1, ACTB, and αTub protein, the anti-PIKFYVE antibody (Solarbio Life Science, K004715P), anti-ACTB antibody (Beyotime Biotechnology, AF0003), and anti-αTub antibody (Beyotime Biotechnology, AF0001) were used respectively. Rabbit polyclonal anti-Atg8, mouse monoclonal anti-RSV, and mouse monoclonal anti-SRBSDV were used to detect Atg8, RSV NP, and SRBSDV P10 protein, respectively. Anti-RSV and anti-SRBSDV monoclonal antibodies were provided by Prof. Jianxiang Wu from Zhejiang University, and rabbit polyclonal anti-Atg8 was provided by Prof. Lili Zhang from Chinese Academy of Sciences. The RBSDV abundance at protein level was analyzed after the L. striatellus was treated with 3-MA (Aladdin, M129496), rapamycin (MedChemExpress, HY -10219), dseGFP, dsLsfab1, DMSO (Solarbio Life Science, D8371), YM201636 (TargetMol Chemicals Incorporated, TM-T6110), C8-PtdIns(3,5)P2 (Echelon Biosciences, P-3508), or ML-SA1 (MedChemExpress, HY-108462) by western blotting using RBSDV P10 specific rabbit polyclonal antibody. RBSDV P10 specific rabbit polyclonal antibody was produced by Yonglong Biotechnology (Shanghai, China) using purified His-P10 protein. To prepare the RBSDV P10 specific rabbit polyclonal antibody, the pET15b-P10 plasmid was transformed into E. coli BL21 (DE3) Rosetta cells. Recombinant His-P10 protein was purified and used for immunization. Nilaparvata lugens Trpml gene sequence (GenBank: KX249700) and Recilia dorsalis ref(2)P/SQSTM1 gene sequence (GenBank: MF038048) [55] were subjected to BLAST comparison with the L. striatellus genome (GenBank: GCA_017141395.1). The homolog cDNA sequences of Trpml and ref(2)P/SQSTM1 in L. striatellus were obtained and deposited in GenBank with the accession number MZ476564 and MZ476565, respectively. The corresponding protein sequences of Trpml (GenBank: UIA40723) and ref(2)P/SQSTM1 (GenBank: UIA40724) in L. striatellus were also deposited in NCBI. Rabbit polyclonal anti-Trpml antibody against LsTrpml peptide KGWDPTREVSSYPPC and polyclonal anti-ref(2)P/SQSTM1 antibody against ref(2)P/SQSTM1 peptide VIEIGKLGSKESPDC were produced by GenScript (Nanjing, China).
dsRNA synthesis and RNAi assays
The gene fragments of fab1, Atg3, Atg8, Atg6, and Trpml in L. striatellus were amplified with the primers listed in Table S1. The PCR products were purified, and the dsRNAs were synthesized by TranscriptAid T7 high yield transcription kit (Thermo Fisher Scientific, K0441) according to the manufacturer’s instructions. The dsRNA of eGFP was used as the control. After the nymphs of L. striatellus acquired RBSDV on the virus-infected plants for 2 days, the dsRNA (500 ng/μL) was injected into the conjunction between prothorax and mesothorax of the nymphs of L. striatellus by FemtoJet microinjector (Eppendorf, Germany). The RNAi efficiency was assessed in the RBSDV-uninfected L. striatellus by RT-qPCR. Each RNAi assay was represented with three biological replicates.
Cell culture, transfection, and baculovirus infection
Sf9 cells were cultured in Sf900TM III SFM (1×; Thermo Fisher Scientific, 12658019) with 2% bovine serum at 28°C. A Bac-to-Bac Baculovirus Expression System (Gibco, 10359016) was used in this study according to the manufacturer’s instructions. Briefly, the recombinant bacmids were prepared by transformation of recombinant plasmids, pFastBac-eGFP, pFastBac-P10eGFP, or pFastBac-RFP-eGFP-Atg8 into E. coli DH10 Bac cells (Thermo Fisher Scientific, 10361012). The recombinant bacmid DNA was transfected into Sf9 cells using Lipofectamine 3000 reagent (Thermo Fisher Scientific, L3000015) diluted with Opti-MEMTM I reduced serum medium (Thermo Fisher Scientific, 31985070) according to manufacturer’s instructions. The culture supernatants were collected at 3 days after transfection and stored at 4°C for use.
Immunofluorescence analysis
The dsRNAs, YM201636, DMSO, or C8-PtdIns(3,5)P2 injected L. striatellus intestines were carefully dissected in ice-cold phosphate-buffered saline (PBS; Sangon Biotechnology, C500626–0001) buffer (pH 7.4), fixed with 4% (w:v) paraformaldehyde (PFA; Thermo Fisher Scientific, R37814) in 1× PBS for 2 h, and then permeabilized with 2% (v:v) Triton X-100 for 30 min. The samples were blocked with 3% BSA for 30 min, immunolabeled with specific fluorescence-conjugated antibodies for 1 h at 4°C, and then analyzed with LSM 710 confocal laser-scanning microscope (ZEISS, Germany). RBSDV P10, PtdIns(3,5)P2, Rbsn-5, Rab7, Lamp1, Atg8 and actin filaments in L. striatellus intestines were detected with RBSDV monoclonal antibody (provided by Prof. Jianxiang Wu from Zhejiang University) conjugated Alexa FluorTM 488 (FITC; Thermo Fisher Scientific, A33077), PtdIns(3,5)P2 antibody (Echelon Biosciences, Z-P035) conjugated Alexa FluorTM 555 (rhodamine; Thermo Fisher Scientific, A33080), Rbsn-5 antibody (Novus Biologicals, NBP2–36568) conjugated rhodamine, Rab7 antibody (Cell Signaling Technology, 9367S) conjugated rhodamine, Lamp1 antibody (Thermo Fisher Scientific, PA1-654A) conjugated rhodamine, Atg8 antibody conjugated Alexa FluorTM 647 (Thermo Fisher Scientific, A33084), and phalloidin CruzFluor™ 350 (Santa Cruz Biotechnology, sc -363789).
To detect PtdIns(3,5)P2 in Sf9 cells, the cells were rinsed with PBS 3 times, fixed with 2% PFA for 30 min, permeabilized with 0.2% Triton X-100 for 15 min, immunolabeled with PtdIns(3,5)P2-rhodamine antibody for 1 h at 4°C, and then analyzed with LSM 710 for confocal imaging. To detect SfAtg8 in Sf9 cells, the cells were rinsed with PBS for 3 times, incubated with LysoTrackerTM Deep Red (Thermo Fisher Scientific, L12492) for 15 min at 28°C in a dark environment, then the cells were fixed with 2% PFA for 30 min, permeabilized with 0.2% Triton X-100 for 15 min, immunolabeled with Atg8-rhodamine antibody for 1 h at 4°C. The cells were analyzed with LSM 710 microscope. To detect the fluorescence in Sf9 cells expressed with RFP-eGFP-Atg8, the rapamycin, ML-SA1, DMSO, or dsRNAs treated cells were fixed with 2% PFA and then viewed under LSM 710. The fluorescent intensity was analyzed by ImageJ.
Co-IP assay
Co-IP assay was performed using a kit (Yeasen Biotechnology, 36403ES08) according to the manufacturer’s instructions. Briefly, RBSDV P10 antibody was added to the resin in the kit for immobilization for 1h at room temperature. RBSDV-uninfected or -infected L. striatellus were lysed. The supernatants were incubated with the purified P10-immobilized resin for 1 h at 4°C, respectively. The Co-IP fraction was collected for subsequent SDS-PAGE and western blotting analysis using PIKFYVE antibodies (Solarbio Life Science, K004715P).
Quantification and statistical analysis
All the statistical analyses were performed using IBM SPSS Statistics (version: 19.0) software (IBM Corp.). Data are shown as the means ±SE. Determination of statistical significance was conducted using Student’s t-test. Asterisks indicate significant differences, *, p<0.05; **, p<0.01.
Supplementary Material
Acknowledgment
We thank Prof. Jiangxiang Wu from the Institute of Biotechnology, Zhejiang University for providing the mouse monoclonal antibodies against RBSDV and SRBSDV, Prof. Lili Zhang from the Institute of Microbiology, Chinese Academy of Sciences for providing the L. striatellus Atg8 antibody, Prof. Bin Li from Nanjing Normal University for providing the Sf9 cell, and Dr. Tong Zhang from College of Agriculture, South China Agricultural University for providing the SRBSDV-infected rice plants. We thank Paul Daly in Jiangsu Academy of Agricultural Sciences for language editing.
Funding Statement
The work was supported by the National Natural Science Foundation of China [31872639; 32001871; 31770164; 32102211]; Natural Science Foundation of Jiangsu Province [BK20200283; BK20190268]; Jiangsu Province’s Innovation Program [JSSCTD202142].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15548627.2022.2116676
References
- [1].Balla T., and Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. [DOI] [PubMed] [Google Scholar]
- [3].Hirano T, Sato, MH. Diverse physiological functions of FAB1 and phosphatidylinositol 3,5-bisphosphate in plants. Front Plant Sci. 2019;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jin N, Lang MJ, Weisman, LS. Phosphatidylinositol 3,5-bisphosphate: regulation of cellular events in space and time. Biochem Soc Trans. 2016;44:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCartney AJ, Zhang Y, Weisman LS. Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays. 2014;36:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gary JD, Sato TK, Stefan CJ, et al. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell. 2002;13:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zolov SN, Bridges D, Zhang YL, et al. Pikfyve generates PtdIns(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci USA. 2012;109:17472–17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fernandes F, Chen K, Ehrlich LS, et al. Phosphoinositides direct equine infectious anemia virus gag trafficking and release. Traffic. 2011;12:438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qiu S, Leung A, Bo YX, et al. Ebola virus requires phosphatidylinositol (3,5) bisphosphate production for efficient viral entry. Virology. 2018;513:17–28. [DOI] [PubMed] [Google Scholar]
- [10].Choi Y, Bowman JW, Jung JU, et al. utophagy during viral infection - a double-edged sword. Nat Rev Microbiol. 2018;16:340–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liang C, E X, Jung JU, et al. Downregulation of autophagy by herpesvirus Bcl-2 homologs. Autophagy. 2008;4:268–272. [DOI] [PubMed] [Google Scholar]
- [12].Lussignol M, Queval C, Bernet-Camard MF, et al. The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J Virol. 2013;87:859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mouna L, Hernandez E, Bonte D, et al. Analysis of the role of autophagy inhibition by two complementary human cytomegalovirus BECN1/Beclin 1-binding proteins. Autophagy. 2016;12:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Orvedahl A, Alexander D, Talloczy Z, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host & Microbe. 2007;1:23–35. [DOI] [PubMed] [Google Scholar]
- [15].Yang M, Zhang YL, Xie XL, et al. Barley stripe mosaic virus γb protein subverts autophagy to promote viral infection by disrupting the ATG7-ATG8 interaction. Plant Cell. 2018;30:1582–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hafren A, Ustun S, Hochmuth A, et al. Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor HCpro. Plant Physiol. 2018;176:649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hasegawa J, Strunk BS, Weisman LS, et al. PI5P and PtdIns(3,5)P2: minor, but essential phosphoinositides. Cell Struct Funct. 2017;42:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu N, Zhang L, Ren YD, et al. Rice black-streaked dwarf virus: From multiparty interactions among plant-virus-vector to intermittent epidemics. Philos Trans R Soc Lond B Biol Sci. 2020;21:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lu LN, Wang Q, Huang DQ, et al. Rice black-streaked dwarf virus P10 suppresses protein kinase C in insect vector through changing the subcellular localization of LsRACK1. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang ZY, Chen DY, Sun F, et al. ARGONAUTE 2 increases rice susceptibility to rice black- streaked dwarf virus infection by epigenetically regulating HEXOKINASE 1 expression. Mol Plant Pathol. 2021;22:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Q, Lu LN, Zeng M, et al. Rice black-streaked dwarf virus P10 promotes phosphorylation of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) to induce autophagy in Laodelphax striatellus. Autophagy. 2022;18(4):745–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hajano JUD, Wang B, Ren YD, et al. . Quantification of southern rice black streaked dwarf virus and rice black streaked dwarf virus in the organs of their vector and nonvector insect over time. Virus Research. 2015;208:145–155. [DOI] [PubMed] [Google Scholar]
- [23].Xu Y, Fu S, Tao X, et al. Rice stripe virus: exploring molecular weapons in the arsenal of a negative-sense RNA virus. Annu Rev Phytopathol. 2021;59:351–371. [DOI] [PubMed] [Google Scholar]
- [24].Zhang HM, Yang J, Chen JP, et al. A black-streaked dwarf disease on rice in China is caused by a novel fijivirus. Arch Virol. 2008;153:1893–1898. [DOI] [PubMed] [Google Scholar]
- [25].Zhou GH, Wen JJ, Cai DJ, et al. Southern rice black-streaked dwarf virus: a new proposed Fijivirus species in the family Reoviridae. Chinese Sci Bull. 2008;53:3677–3685. [Google Scholar]
- [26].Jefferies HBJ, Cooke FT, Jat P, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P2 production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mainou BA, Dermody TS. Transport to late endosomes is required for efficient Reovirus infection. J Virol. 2012;86:8346–8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schulz WL, Haj AK, Schiff LA, et al. Reovirus uses multiple endocytic pathways for cell entry. J Virol. 2012;86:12665–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Lartigue J, Polson H, Feldman M, et al. PIKfyve regulation of endosome-linked pathways. Traffic. 2009;10:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martin S, Harper CB, May LM, et al. Inhibition of PIKfyve by YM-201636 dysregulates autophagy and leads to apoptosis-independent neuronal cell death. PLoS One. 2013;8:e60152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol. 2009;19:359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang XQ, Chen SP, Yang XR, et al. Friend or enemy: a dual role of autophagy in plant virus infection. Front Microbiol. 2020;11:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ding BB, Zhang GY, Yang XD, et al. Phosphoprotein of human parainfluenza virus type 3 blocks autophagosome-lysosome fusion to increase virus production. Cell Host & Microbe. 2014;15:564–577. [DOI] [PubMed] [Google Scholar]
- [35].Touchberry CD, Bales IK, Stone JK, et al. Phosphatidylinositol 3,5-Bisphosphate (PtdIns(3,5)P2) potentiates cardiac contractility via activation of the ryanodine receptor. J Biol Chem. 2010;285:40312–40321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Friant S, Pecheur EI, Eugster A, et al. Ent3p is a PtdIns(3,5)P2 effector required for protein sorting to the multivesicular body. Dev Cell. 2003;5:499–511. [DOI] [PubMed] [Google Scholar]
- [37].Whitley P, Reaves BJ, Hashimoto M, et al. . Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. B Biol Chem. 2003;278:38786–38795. [DOI] [PubMed] [Google Scholar]
- [38].Dove SK, Piper RC, McEwen RK, et al. Svp1p defines a family of phosphatidylinositol 3,5- bisphosphate effectors. EMBO J. 2004;23:1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dong XP, Shen DB, Wang X, et al. PtdIns(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang P, Xu M, Wu Y, et al. Multiple facets of TRPML1 in autophagy. Cell Calcium. 2020;88:102196. [DOI] [PubMed] [Google Scholar]
- [41].Kolli S, Meng XZ, Wu X, et al. Structure-function analysis of vaccinia virus H7 protein reveals a novel phosphoinositide binding fold essential for poxvirus replication. J Virol. 2015;89:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wen Y, Feigenson GW, Vogt VM, et al. Mechanisms of PI(4,5)P2 enrichment in HIV-1 viral membranes. J Mol Bio. 2020;432:5543–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Banerjee S, Clapp K, Tarsio M, et al. Interaction of the late endo-lysosomal lipid PtdIns(3,5)P2 with the Vph1 isoform of yeast V-ATPase increases its activity and cellular stress tolerance. J Biol Chem. 2019;294:9161–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].She J, Guo JT, Chen QF, et al. . Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bridges D, Ma JT, Park S, et al. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol Biol Cell. 2012;23:2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ikonomov NC, Sbrissa D, Dondapati R, et al. ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp Cell Res. 2007;313:2404–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meijer HJG, Divecha N, vandenEnde H, et al. Hyperosmotic stress induces rapid synthesis of phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta. 1999;208:294–298. [Google Scholar]
- [48].Tsujita K, Itoh T, Ijuin T, et al. Myotubularin regulates the function of the late endosome through the GRAM domain-phosphatidylinositol 3,5-bisphosphate interaction. J Biol Chem. 2004;279():13817–13824. [DOI] [PubMed] [Google Scholar]
- [49].Banerjee S, Aponte-Diaz D, Yeage C, et al. Hijacking of multiple phospholipid biosynthetic pathways and induction of membrane biogenesis by a picornaviral 3CD protein. PLoS Pathog. 2018;14(5):e1007086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu Y, Kahn RA, Prestegard JH, et al. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol. 2010;17(7):876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Godi A, Pertile P, Meyers R, et al. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1(5):280–287. [DOI] [PubMed] [Google Scholar]
- [52].Dorobantu CM, Ford-Siltz LA, Sittig SP, et al. GBF1- and ACBD3-independent recruitment of PI4KIIIβ to replication sites by rhinovirus 3A proteins. J Virol. 2015;89:1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dorobantu CM, vanderSchaar HM, Ford LA, et al. Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1. J Virol. 2014;88:2725–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu Y, Lai YC, Hill EV, et al. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction stimulated glucose uptake in skeletal muscle. Biochem J. 2013;455(2):195–206. [DOI] [PubMed] [Google Scholar]
- [55].Chen Y, Chen Q, Li MM, et al. Autophagy pathway induced by a plant virus facilitates viral spread and transmission by its insect vector. PLoS Pathog. 2017;13:e1006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang LL, Wang XR, Wei XM, et al. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy. 2016;12:1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang M, Ismayil A, Liu Y, et al. Autophagy in plant-virus interactions. Annu Rev Virol. 2020;7:403–419. [DOI] [PubMed] [Google Scholar]
- [58].Chaumorcel M, Lussignol M, Mouna L, et al. The human cytomegalovirus protein TRS1 inhibits autophagy via its interaction with Beclin 1. J Virol. 2012;86:2571–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nicot AS, Fares H, Payrastre B, et al. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell. 2006;17:3062–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rutherford AC, Traer C, Wassmer T, et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hasegawa J, Iwamoto R, Otomo T, et al. Autophagosome-lysosome fusion in neurons requires INPP5E, a protein associated with Joubertsyndrome. EMBO J. 2016;35:1853–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nakamura S, Hasegawa J, Yoshimori T, et al. Regulation of lysosomal phosphoinositide balance by INPP5E is essential for autophagosome-lysosome fusion. Autophagy. 2016;12:2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xia ZQ, Wang LY, Li SY, et al. ML-SA1, a selective TRPML agonist, inhibits DENV2 and ZIKV by promoting lysosomal acidification and protease activity. Antiviral Res. 2020;182:104922. [DOI] [PubMed] [Google Scholar]
- [64].Santoni G, Morelli MB, Amantini C, et al. Involvement of the TRPML mucolipin channels in viral infections and anti-viral innate immune responses. Front Immunol. 2020;11:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Feng XH, Huang Y, Lu YG, et al. Drosophila TRPML forms PtdIns(3,5)P2-activated cation channels in both endolysosomes and plasma membrane. J Biol Chem. 2014;289:4262–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li XR, Rydzewski N, Hider A, et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18:404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu HQ, Zhou YJ, Xu QF, et al. Selection of DNA aptamers for subcellular localization of RBSDV P10 protein in the midgut of small brown planthoppers by emulsion PCR-based SELEX. Viruses 2020. 2012;12:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


