ABSTRACT
The significance of Bifidobacterium to human health can be appreciated from its early colonization of the neonatal gut, where Bifidobacterium longum represents the most abundant species. While its relative abundance declines with age, it is further reduced in several diseases. Research into the beneficial properties of B. longum has unveiled a range of mechanisms, including the production of bioactive molecules, such as short-chain fatty acids, polysaccharides, and serine protease inhibitors. From its intestinal niche, B. longum can have far-reaching effects in the body influencing immune responses in the lungs and even skin, as well as influencing brain activity. In this review, we present the biological and clinical impacts of this species on a range of human conditions beginning in neonatal life and beyond. The available scientific evidence reveals a strong rationale for continued research and further clinical trials that investigate the ability of B. longum to treat or prevent a range of diseases across the human lifespan.
KEYWORDS: Bifidobacterium, Bifidobacterium longum ssp. infantis, Bifidobacterium longum ssp. longum, Health, Neonate, Probiotic, Gut microbiota, Necrotizing enterocolitis, Cardiovascular disease, Immunity, Cognitive impairment
Introduction
The human colon is recognized as one of the most densely populated ecosystems on earth with the bacterial component reported to reach 1014 cells.1 These microorganisms are intricately linked to host physiology and health since they perform several essential functions with consequences not only for the gastrointestinal environment but also for remote organs of the body. Such functions include the development of the host immune system from birth and maintaining immune homeostasis throughout life,2 protection from pathogen invasion in the gut via colonization resistance,3 energy regulation,4 and production of bioactive metabolites and nutrients.5 These also influence the many bidirectional interactions between the gut microbiota and other organs/systems of the body including the nervous system,6 lungs,7 and skin.8
Throughout life, several diseases and conditions have been linked with imbalanced gut microbiota profiles. In the preterm neonate, abnormal microbial colonization of the infant intestine is recognized as a risk factor for necrotizing enterocolitis (NEC).9,10 Gut microbiome dysbiosis has been associated with gastrointestinal, cardiovascular, metabolic, and neurological diseases, along with autoimmune diseases and allergies across all stages of life.11,12
Bifidobacterium longum is a commensal gastrointestinal tract (GIT) inhabitant that is recognized as a significant member of the human gut microbiota and is the most abundant species in the infant gut.13 It exerts numerous beneficial health effects.14 These range from the production of bioactive substances to bifidobacterial surface-associated molecules that interact with the host.15 Several B. longum strains have thus been developed as probiotics – “live microorganisms which when administered in adequate amounts, confer a health benefit on the host”16
The efficacies of this species have been demonstrated in preclinical models and in clinical studies in early human life and beyond. Therefore, in this review, we provide a comprehensive overview of clinical efficacies and biological observations following B. longum administration to infants, adults, and elderly in terms of gastrointestinal, cardiovascular, immune, neurological, and respiratory health and disease, as well as host skin based on the results of randomized, double-blind, placebo-controlled trials (RDBPCTs). The relevant literature was obtained following a search in PubMed using the search terms ‘Bifidobacterium longum; double-blind’ with the PubMed filters ‘Randomized Controlled Trial; Clinical Trial.’
Some of the diseases can be categorized as non-communicable diseases (NCDs), linked with genetic, environmental, and lifestyle factors. Yet, research has revealed altered gut microbiota profiles in individuals with NCDs.17 The trials have been performed with B. longum strains alone or in combination with other strains and/or prebiotics, the latter of which is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit.”18
In most cases, the strains were administered orally. The results reveal that B. longum alone or in combination with other strains and prebiotics can impact various systems in the body and may alleviate disease symptoms or prevent the onset of illness indicating a role for B. longum in the prevention and management of several diseases from early life and throughout the human lifespan. However, further clinical trials are warranted before these results can be generalized to appropriate consumers/patients.
B. longum and overview of its beneficial mechanisms of action
B. longum is composed to date of four subspecies, B. longum ssp. infantis, B. longum ssp. longum, B. longum ssp. suis, and B. longum ssp. suillum. Until recently, the latter two had only been isolated from pigs and calves,19,20 however, B. longum ssp. suillum has since been isolated from the infant gut (unpublished data). Notably, the name B. longum ssp. infantis is often shortened to B. infantis in the literature and B. longum ssp. longum to B. longum. In this review, we refer to the names provided in the literature.
Bifidobacteriales has been identified as the most abundant bacterial class in the infant gut (present at 80.6%) with B. longum representing 56.2% of the species.13 In breastfed infants, B. longum ssp. infantis is the most prevalent subspecies21 possibly contributed to by its capacity to digest human milk oligosaccharides (HMOs).22 In adults, levels of bifidobacteria reduce to 2–14% of relative abundance23 and include species such as B. longum ssp. longum,24 B. adolescentis, B. catenulatum,25,26 B. pseudolongum, B. bifidum, and B. breve.27 While in the elderly, bifidobacteria levels have been reported to markedly decrease in abundance,28–30 and a significant negative correlation has been reported between B. longum relative abundance and host age.31
B. longum is an excellent colonizer of the human gut. For example, following a single oral administration, the strain B. longum ssp. longum AH1206 persisted in the human gut of 30% of trial subjects for the six-month duration of the study.32–34 In contrast, other supplemented strains, such as Lactobacillus plantarum (now Lactiplantibacillus plantarum), Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB12 are generally detected in feces in decreasing amounts for a few days after ingestion and rarely beyond 1 week.35,36 A longitudinal study investigating the persistence of B. longum ssp. longum strains in the human gut from infancy revealed that strains confirmed to colonize and persist as early as 90 d after birth were still present at 6 y of age.37 Successful colonization of the human gut is partly attributed to the ability of B. longum to metabolize host- and diet-derived carbohydrates, such as HMOs and plant polysaccharides that cannot be digested by the host.15 Over 13% of the clusters of orthologous gene (COG) families within the pangenome of the Bifidobacterium genus are devoted to carbohydrate metabolism.38 Among human infantB. longum ssp. longum strains, Arboleya et al.32 identified 22 glycosyl hydrolase families via pan-genome analysis. Interestingly, B. longum ssp. infantis is specialized for HMO utilization22, while B. longum ssp. longum can also utilize plant-derived polysaccharides, supportive of its ability to colonize both infants and adults.32–34
In the gut, B. longum metabolizes carbohydrates to short-chain fatty acids (SCFAs), acetate and lactate. In a mouse model, acetate produced in the gut by bifidobacteria has been shown to improve intestinal defenses and protect against infection.39 Acetate and lactate reduce the pH in the gut, which is believed to prevent microbiota imbalances and prevent pathogen growth.40,41 Indeed, Henrick et al.42 reported a trend for increasing fecal pH in breast-fed infants over the past century (from 5.0 to 6.5) that is associated with loss of specialized Bifidobacterium species and may pose increased risk for microbiota dysbiosis. In mice, acetate has been shown to promote intestinal antibody immunoglobulin (Ig)A responses in the gut via the G-protein coupled receptor GPR43.43 Acetate can be used by butyrate-producing bacteria in the gut, such as Faecalibacterium prausnitzii, to produce butyrate.44 Butyrate is used as an energy source by gut epithelial cells and is involved in several physiological functions including intestinal barrier function,45 immunity,46 and brain function.47 Lactate can also cross the blood–brain barrier and behave as a neuromodulator in the brain.48 Another metabolite produced by B. longum ssp. infantis following growth on HMOs is indole-3-lactic acid, a tryptophan metabolite that has been shown to significantly decrease inflammation in gut epithelial cells.49 Indole-3-lactic acid was identified as the anti-inflammatory molecule in B. infantis secretions that prevents transcription of the inflammatory cytokine interleukin (IL)-8 in immature, but not mature, intestinal enterocytes.50 Indeed, indole-3-lactic acid exerts different anti-inflammatory, anti-viral, and cell development effects on immature and mature enterocytes and has been proposed as a potential therapeutic in the prevention and treatment of NEC in premature infants.51
Many bifidobacteria produce polysaccharides including capsular polysaccharides (CPSs) that are bound to the cell surface and exopolysaccharides (EPSs) that are loosely attached to the bacterial cell or secreted into the surrounding environment.52 These molecules serve to protect bacterial cells against harsh environments encountered in the gastrointestinal tract but can also be involved in crosstalk with the gut environment. Bifidobacterial EPSs have been shown to enhance adhesion to eukaryotic cell lines and depending on chemical and physical properties have been shown to elicit or reduce an immune response.53 Surface-associated EPSs have been shown to reduce pathogen colonization in mice.54 More recently, Yan et al.55 reported that a ropy-EPS-producing B. longum ssp. longum strain alleviated the symptoms of dextran sodium sulfate- (DSS-)induced colitis in mice and reduced inflammation by decreasing pro-inflammatory cytokines. However, a non-ropy-EPS-producing B. longum ssp. longum strain failed to decrease pro-inflammatory cytokine levels. Furthermore, the ropy EPS-producing strain maintained the expression of genes involved in mucosal barrier function after DSS challenge but a non-EPS producing strain failed to maintain such gene expression.
Certain Bifidobacterium species including B. longum have been shown to produce serine protease inhibitors (serpins) and harbor serpin-encoding genes.56,57 Serpins serve to promote bifidobacterial colonization as they protect bacterial cells from host-derived proteases. Indeed, Ivanov et al.56 reported that serpin from B. longum effectively inhibited eukaryotic elastase-like proteases, leading them to speculate on its role in immunomodulation given that elastase is released at sites of intestinal inflammation by activated neutrophils. The serpin-producing B. longum strain NCC 2705 was capable of attenuating gliadin-induced immunopathology in a mouse model of gluten sensitivity, while its serpin-knockout counterpart failed to elicit such an effect.58 Most recently, the concept of the ‘gut serpinome’ has been introduced – serpins produced by the gut microbiota – given the capacities of these serpins to inhibit proteases involved in the pathogenesis of inflammatory bowel disease (IBD) and their potential for innovative therapies.59
Interestingly, not all B. longum strains can alleviate disease symptoms as already noted.55 Chen et al.60 investigated the impact of three conjugated linoleic acid- (CLA)-producing B. longum strains on DSS-induced colitis in mice. Only one strain, B. longum CCFM681, proved capable of alleviating colitis by inhibiting pro-inflammatory pathways, protecting the intestinal mechanical barrier, and modulating the gut microbiota. These beneficial effects are correlated with CLA production and while all three strains were confirmed CLA producers in vitro, B. longum CCFM681 produced significantly more CLA in the colon than the other two strains, suggesting that CLA production, in this case, was responsible for relieving colitis. Another study reported that B. longum strains with different genotypes in the arginine biosynthesis pathway had different abilities for protecting a d-galactose-induced aging mouse model against host aging – proposed to be associated with their differing abilities to alter the gut microbiota metabolome.31
B. longum effects in infants and children
Gastrointestinal health and disease
Necrotizing enterocolitis and late-onset sepsis
Preterm infants are at increased risk of developing the intestinal inflammatory disease, NEC, due to the underdeveloped gastrointestinal environment and preterm microbiome signatures.61,62 NEC is estimated to affect 5–12% of preterm infants (<1500 g at birth) with mortality rates as high as 20–30%.61,63 Several prenatal, perinatal, and neonatal risk factors have been identified,63 including intestinal immaturity and abnormal microbial colonization of the infant intestine.9,10 Prior to the onset of NEC in preterm infants, the intestinal microbiome has been characterized by reduced microbial diversity,61,64,65 increased relative abundance of Proteobacteria and decreased relative abundances of Firmicutes and Bacteroidetes,66 including lower levels of commensals such as bifidobacteria.67 A recent study using mice revealed that NEC microbiota (from patients with NEC) causes intestinal injury in germ-free mice following fecal microbiota transplantation.68 Thus, the use of beneficial bacterial strains to prevent and treat NEC is an area of continued and growing interest.61,69–71
In a prospective multicentre RDBPCT (ProPrems trial), Jacobs et al.72 investigated the impact of a combination of bacterial strains on the occurrence of late-onset sepsis in preterm infants (born before 32 weeks’ gestation). The formulation included B. longum ssp. infantis BB02, Streptococcus thermophilus TH-4, and B. animalis ssp. lactis BB-12 (in maltodextrin powder) and the placebo group received maltodextrin. Bacterial strains were associated with a 54% reduction in NEC of Bell stage 2 or more in very preterm infants; however, they did not reduce definite late-onset sepsis or mortality. A follow-on study revealed that the bacterial formulation was associated with increased Bifidobacterium in the gut microbiota of the very preterm infants (p < 0.001) and decreased Enterococcus levels (p = 0.02), suggesting that Bifidobacterium may have a protective effect against NEC.73 The bacterial formulations of B. longum, L. acidophilus, Lactobacillus rhamnosus (now Lacticaseibacillus rhamnosus), and Saccharomyces boulardii showed a trend toward lowering NEC (4% versus 12%) in very low birth weight neonates in a randomized, double-blinded controlled trial where breast milk served as the control (no placebo included).74 However, the authors suggest that the use of breast milk in the control group may have narrowed the differences between the two groups given the beneficial properties associated with breast milk. Cross-contamination between the two groups in the hospital setting could also have impacted the results where the control group may have acquired strains from the formulation, although this was not assessed in the study. Furthermore, 73% of infants in the control group were born by cesarean delivery versus 52% in the test group; however, cesarean-delivered infants may have benefited more from the intervention given that colonization of these infants with beneficial microbes, such as Bifidobacterium is delayed.75
Gastroschisis
Gastroschisis describes a ventral body wall defect where the bowel exits the infant’s body in utero.76 Infants born with this condition undergo long periods of gastric suctioning and hospital stays. In a randomized, placebo-controlled, blinded pilot study, the administration of B. longum ssp. infantis to infants with gastroschisis partially attenuated the significant gut dysbiosis observed in these infants; however, there was no impact on the length of hospital stay.77 Specifically, the gut microbiota of infants born with gastroschisis was dominated by Enterobacteriaceae, Staphylococcaceae, Streptococcaceae, and Enterococcaceae. Long-term studies of infants born with this condition are limited but increased prevalence of obesity and hypercholesterolemia in later childhood and teen years have been documented in this group,78 which could be linked to the early gut microbiota dysbiosis.79 B. longum ssp. infantis exposure was associated with colonization with moderate numbers of Bifidobacteriaceae but the effect was most pronounced after gastric suction had ended and the strain was fed orally (as opposed to the twice-daily 1-h exposure of the gastric mucosa to the strain). The authors suggest that more pilot studies with more frequent and/or higher doses of strains are needed to decipher whether administering a bacterial formulation during gastric suctioning has any impact. Future studies would also benefit from a larger sample size (given that only 24 infants were enrolled in the study) and better coordination in the timing of sample collection and number of samples collected per infant. However, the results of this pilot trial suggest that B. longum ssp. infantis could have a role to play in the therapy of gastroschisis. Further studies are warranted that address the most appropriate strains for infants with gastroschisis, the precise method of treatment, dosage, and frequency, and the long-term benefits of such for health and disease evasion.
Childhood diarrhea
In developing countries, childhood diarrhea is the second leading cause of infant mortality (respiratory diseases being the first)80 and rotavirus is the leading cause of acute diarrhea-related deaths worldwide in children under the age of five.81 The strain B. longum ssp. infantis CECT7210 (B. infantis IM1) isolated from the feces of a breast-fed infant has been shown to inhibit rotavirus infection of cell lines and provide preliminary protection against virus infection in a mouse model.82 Using this strain in supplemented infant formula, Escribano et al.83 investigated its effectiveness in reducing diarrhea incidence in healthy term infants during 12 weeks of intervention in a multicentre RDBPCT. In the overall study period, the median diarrhea events per infant were recorded as 0.29 ± 1.07 for the control group and 0.05 ± 0.28 for the B. infantis IM1 group (p = 0.059), and this reached significance by week 8 (p = 0.047). However, it should be pointed out that the incidence of diarrhea among the whole sample was small overall, which could be due to the young age of the participants (<3 months) who would harbor protective maternal antibodies. The strains B. longum BORI and L. acidophilus AD031 were associated with a significant reduction in diarrhea duration (by 1.2 d) in infants hospitalized with rotavirus infection (p = 0.001) in a RDBPCT that lasted for 3 d, while fever duration, diarrhea, and vomiting frequencies tended to be reduced by the strains.84 The short duration of the trial suggests that a longer treatment period could result in better outcomes for the parameters tested.
Irritable bowel syndrome
Formulations containing B. longum have also generated promising results in improving symptoms of irritable bowel syndrome (IBS) and ulcerative colitis (UC) in children. IBS symptoms include abdominal pain and alterations in bowel habits, but the exact pathogenesis is unclear.85 A multicentre, crossover RDBPCT reported that administration of a mixture of B. infantis M-63, B. breve M-16 V, and B. longum BB536 for 6 weeks to children with IBS resulted in a complete resolution of abdominal pain in a significantly higher number of children compared with placebo (p = 0.006), and significantly improved abdominal pain frequency (p = 0.02).86 Moreover, 48% of children with IBS reported an improvement in quality-of-life following treatment versus 17% in the placebo group (p = 0.001). However, it is not known if the washout period of 2 weeks was sufficient to prevent a “carryover” effect between treatments, which can be a limitation of crossover trials.
Inflammatory bowel disease
UC describes a recurring inflammation of the colon and rectum with symptoms of abdominal pain, bloody diarrhea, fecal urgency, and tenesmus.87 Along with Crohn’s disease (CD) – an inflammatory disease that can affect any part of the intestine, it is classified as an IBD. In a 1-y-long, RDBPCT, consumption of the probiotic blend VSL#3® (consisting of four strains of Lactobacillus, three strains of Bifidobacterium including B. longum and B. infantis, and one strain of Streptococcus salivarius subsp. thermophilus, see Table 1 for details) by children with newly diagnosed UC in conjunction with IBD therapy demonstrated significant efficacy for inducing and maintaining remission (p < 0.001) compared with placebo and IBD therapy.88 A significantly lower rate of relapse was recorded in the VSL#3® group (p = 0.014). In this case, the authors concluded that the high bacterial counts of 3 × 1011 cells/g contributed to the success of the formulation along with the large number of different strains. However, the small sample size (n = 29) used in this trial suggests that confirmatory trials should be conducted with higher patient numbers.
Table 1.
An overview of clinical trials investigating the impact of B. longum on infants and children.
| Condition/Disease/Biological Parameter | Participants; Age | Formulation | CFU; Dose; Duration | Clinical Effects and Biological Observations of Intervention Group Compared with Placebo Group | Reference; Trial ID |
|---|---|---|---|---|---|
|
Gastrointestinal Conditions | |||||
| Late onset sepsis | 1099; Very preterm infants (<1500 g) |
B. longum ssp. infantis BB02, S. thermophilus TH-4, B. animalis ssp. lactis BB-12 |
109; 2 daily doses until hospital discharge or term corrected age | Significant reduction in NEC of Bell stage 2 or more; No reduction in definite late-onset sepsis or all-cause mortality |
Jacobs et al.72 ACTRN012607000144415 (Multicentre) |
| Time to reach full enteral feeds in very low birth-weight newborns | 104; Very Low Birth Weight (75–1499 g) |
B. longum, L. acidophilus, L. rhamnosus, S. boulardii |
1.25 x 109; 1 daily dose, From initiation of enteral feeds till hospital discharge |
Trend towards reduced NEC; No impact on feed tolerance |
Shashidhar et al.74 CTRI/2012/08/002853 |
| Gastroschisis | 24; Gestational age at birth > 34 weeks | B. longum ssp. infantis ATCC 15,697 | 109; 2 daily doses for 6 weeks or until hospital discharge | Higher Bifidobacteriaceae, lower Clostridiaceae; Trend towards lower Enterobacteriaceae, Enterococcaceae, Staphylococcaceae, & Streptococcaceae; No impact on length of hospital stay |
Powell et al.77
NCT01316510 |
| Diarrhea | 151; Term infants (< 3 months) | B. longum ssp. infantis CECT7210 | 107; daily, 12 weeks | Significantly reduced diarrhoea episodes at week 8 | Escribano et al.83
NCT02096302 (Multicentre) |
| Rotavirus disease | 57; 9–16 months |
B. longum BORI, L. acidophilus AD031 |
2 x 1010 B. longum, 2 x 109 L. acidophilus; 2 daily doses, 3 d | Significantly reduced duration of diarrhoea (by 1.2 d); Tended to ameliorate duration of fever, frequencies of diarrhoea & vomiting |
Park et al.84 |
| Irritable bowel syndrome | 48; 8–17.9 y |
B. infantis M-63, B. longum BB536, B. breve M-16V |
3 x 109
B. longum, 1 x 109 B. infantis,1 x 109 B. breve; 6 weeks |
Resolution of abdominal pain in significantly higher number of children; Significantly improved frequency of abdominal pain; Improvement in Quality of Life significantly higher |
Giannetti et al.86
NCT02566876 (Multicentre) |
| Ulcerative colitis | 29; 1.7–16.1 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus |
4.5 x 1011−1.8 x 1012 (age-dependent); 1 year | Significant efficacy for inducing & maintaining remission | Miele et al.88 |
| Immunity | |||||
| Immunity & Gut microbiota composition | 264; healthy term newborns | B. longum BB536 | 1 x 107; 12 months | Significantly elevated levels of IF-γ secretion cells; Ratio of IF-γ/IL-4 secretion cells significantly higher; Faecal bifidobacteria counts & bifidobacteria/Enterobacteriaceae ratio significantly higher |
Wu et al.89 |
| Severe sepsis & levels of pro- & anti-inflammatory cytokines | 100; 3 months to 12 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus |
4.5 x 1011; 7 d | Significant decrease in pro-inflammatory cytokines; Significant increase in anti-inflammatory cytokines; Significant reduction in Sequential Organ Failure Assessment score; Lower incidence of healthcare-associated infections; Reduced duration of stay in intensive care unit |
Angurana et al.90 |
| Cardiovascular Health | |||||
| Dyslipidaemia | 38; 10.8 ± 2.1 y |
B. longum ssp. longum BL04, B. animalis ssp. lactis MB 2409, B. bifidum MB 109B |
3 x 109, daily; 3 months | Significantly reduced total cholesterol & LDL-cholesterol | Guardamagna et al.91 |
| Developmental Disorders | |||||
| Autism spectrum disorder | 26; 4–5 y |
B. infantis Bi-26, L. rhamnosus HN001, B. lactis BL-04, L. paracassei LPC-37, & FOS |
1010 daily; 108 d |
Significantly increased beneficial bacteria when compared with baseline; Significantly reduced levels of suspected pathogens; Significantly increased SCFA & homovanillic acid; Significantly reduced serotonin; Improved gastrointestinal autism severity |
Wang et al.92 |
| Respiratory Health and Seasonal Allergies | |||||
| Common winter diseases | 135; 3–7 y |
B. infantis R0033, B. bifidum R0071, L. helveticus R0052, FOS |
3 x 109 750 mg; once daily; 3 months |
Reduced the number of children who suffered at least one winter disease by 25% & limited the number of school days lost | Cazzola et al.93 |
| Upper respiratory illnesses | 219; 2–6 y | B. longum BB536 | 5 x 109; 5 d/week for 10 months | Significantly reduced duration of sore throat; Numerically reduced duration of runny nose & cough; Increased Faecalibacterium in gut microbiota |
Lau et al.94 NCT02434042 |
| Seasonal allergic rhinitis & intermittent asthma | 40; 9 ± 2.2 y |
B. longum BB536, B. infantis M-63, B. breve M-16V |
3 x 109 1 x 109 1 x 109; once daily; 8 weeks |
Significantly relieved nasal symptoms of allergic rhinitis & improved quality of life | Miraglia Del Giudice et al.95 NCT02807064. |
| Skin Health | |||||
| Eczema development in at-risk infants | 241 pregnant women. Supplementation began 2 months before delivery and during first 2 months of breast feeding. 205 infants assessed |
B. longum BL999, L. rhamnosus LPR or B. longum BL999, L. paracasei ST11 |
1 x 109 daily; 4 months | Both formulations significantly reduced the risk of developing eczema in at-risk infants | Rautava et al.96 |
| Eczema development in at-risk infants | 245 Infants; newborn |
B. longum BL999, L. rhamnosus LPR |
~9 x 107 ~ 2 x 108; daily; 6 months |
No impact | Soh et al.97 |
| Moderate atopic dermatitis | 50; 4–17 y |
B. longum CECT 7347, B. lactis CECT 8145, L. casei CECT 9104 |
1 x 109 daily; 12 weeks | Significantly reduced SCORAD & reduced eczema spread & intensity | Navarro-López et al.98 NCT02585986 |
Immunity
Probiotics have been defined as immunostimulatory – for example, they act against infection by inducing production of the pro-inflammatory cytokine IL-12 that activates natural killer (NK) cells and develops T helper (Th)1 cells; or immunoregulatory – they promote production of the anti-inflammatory cytokine IL-10 and T regulatory cells (T-regs).99 Bifidobacteria have been shown to modulate specific immune cells and pathways in both animals and humans. The mechanisms involved are not yet fully understood and vary from strain to strain but they can induce pro- or anti-inflammatory effects. For the adaptive immune system, the balance of T-cell subsets Th1, Th2, Th17, and T regulatory cells [Tregs]) is critical to homeostasis.100,101
During pregnancy, a bias toward Th2 cells protects the fetus.102 Th2-type cytokines tend to produce an anti-inflammatory response.103 After birth, the development of the Th1 immune response (Th1-type cytokines tend to be pro-inflammatory)103 can reset the Th1/Th2 balance, and it is suggested that exposure to environmental microbes plays a critical role in this.89 In infants, high levels of circulating Th2-associated chemokines and low levels of Th1-chemokines have been associated with allergic disease and sensitization.104 Wu et al.89 investigated the impact of administering B. longum BB536 to healthy newborn infants on the immune response and intestinal microbiota over a 12-month period where interferon-γ (IF-γ) secretion cells were used to represent Th1 cytokines, and IL-4 secretion cells were used to represent Th2 cytokines. At 7 months of age, infants in the BB536 group had significantly elevated levels of IF-γ secretion cells compared with the control group (p = 0.007), and the ratio of IF-γ/IL-4 secretion cells was significantly higher in the supplemented group (p = 0.044). By 2 and 4 months of age, the fecal bifidobacteria counts and the bifidobacteria/Enterobacteriaceae ratio were significantly higher in the BB536 group (p < 0.05). However, B. longum BB536 had no impact on serum antibody titers following vaccination with vaccines for hepatitis B, poliomyelitis, and Diphtheria tetanus toxoid and pertussis when compared with the control group. It is possible that the healthy term infants in this RDBPCT already had adequate antibody responses to the vaccines, thus BB536 did not exert a further effect. This has been reported in other studies following the administration of beneficial bacteria.105,106
In children with severe sepsis, 7 d of supplementation with VSL#3® was associated with significant reductions in the pro-inflammatory cytokines IL-6 (p = 0.001), IL-12p70 (p = 0.001), IL-17 (p = 0.01), and tumor necrosis factor-α (TNF- α) (p = 0.01) compared with the placebo group.90 The anti-inflammatory cytokine IL-10 and transforming growth factor-β1 were significantly increased (p = 0.02 and p = 0.01, respectively). However, caution should be exerted when interpreting these results since cytokine profiling was not performed in duplicate. The Sequential Organ Failure Assessment score was significantly lower in the VSL#3® group compared with placebo on day 7 (1 versus 3). Duration of intensive care unit (ICU) stay was also reduced in the VSL#3® group compared with placebo (6.5 d versus 9). There was also a non-significant trend toward a lower incidence of healthcare-associated infections in the VSL#3® group compared with placebo (14% v 20%). Despite these promising findings, caution is warranted in their interpretation given that the percentage of patients with septic shock in the placebo was greater than that in the test group (60% versus 48%, respectively) which could have influenced these results. Thus, further trials with better randomization are required to confirm these findings. For further details of these trials, see Table 1.
Cardiovascular health
Cardiovascular diseases (CVDs) affect the heart and blood vessels and include a range of complications and conditions from abnormal heart rhythms to heart failure, heart attack, and stroke, as examples. The World Health Organization (WHO) estimates that CVDs are responsible for approximately 17.9 million deaths annually with more than 4 out of 5 deaths due to heart attacks and strokes.107 High blood pressure and high cholesterol are risk factors for CVDs. Statins are generally prescribed to lower low-density lipoprotein (LDL) cholesterol and thus reduce cardiovascular events and mortality.108 However, some patients report side effects from statin therapy such as muscle pain, and while a recent systematic review reported that only a small minority of symptoms are due to statins, the development of new-onset diabetes mellitus was significantly higher when taking statins.109 Thus, there is a need for alternative treatments that lower cholesterol without subsequent side effects.
Blood lipid profiles
Lipoprotein disorders can be inherited and can lead to the early development of atherosclerosis in children,110 which can manifest as CVDs in adulthood.111 International guidelines recommend good nutrition as the primary approach to reducing excess cholesterol in children, particularly LDL cholesterol, while the use of drug treatment is a last-resort option when dietary treatment and recommended supplements (e.g., plant sterols) prove insufficient.112 Thus, cholesterol-lowering bacterial strains could offer a viable strategy, in conjunction with healthy nutrition, to control cholesterol levels in children. Guardamagna et al.91 investigated the impact of a three-strain formulation on lipid profiles in children (10.8 ± 2.1 y) affected by primary dyslipidemia. Enrolled children had to have serum total cholesterol levels greater than their age- and sex-specific 90th percentile.113 Exclusion criteria included secondary dyslipidemia, obesity, or overweight, disorders of the renal or endocrine systems or liver and chronic diseases that required treatment. The formulation consisted of three different Bifidobacterium species, namely B. longum ssp. longum BL04, B. animalis ssp. lactis MB 2409, and B. bifidum MB 109B. The mixture was capable of cholesterol assimilation, bile salt hydrolase activity, and conversion of linoleic acid to CLA. Assimilation refers to the ability of bacteria to assimilate cholesterol into the bacterial cell membrane, thus reducing cholesterol reabsorption in the gut.114 Bacterial bile salt hydrolase deconjugates bile salts into bile acids that are then excreted from the body in the feces.115 CLA has demonstrated a host of beneficial activities in animal studies and human cell lines, including protection against obesity and atherosclerosis.116 In the RDBPC crossover study, 3 months of treatment significantly reduced total cholesterol by 3.4% and LDL-cholesterol by 3.8% compared with placebo (p = 0.001).91 Despite this, LDL and total cholesterol values of the participants remained above the acceptable values of <110 mg/dl and<170 mg/dl, respectively, for children117 following treatment (at 135 and 212 mg/dl, respectively), bringing into question the physiological relevance of the results and whether the duration of the trial was adequate. Furthermore, all participants in the study were given a dietary regimen (STEP 1 diet) by a trained dietitian 4 weeks prior to commencement of the trial, which itself resulted in statistically significant reductions in total and LDL cholesterol in the placebo group compared with baseline values. Indeed, while the bacterial formulation resulted in 4.6% (p = 0.0001) and 8.2% (p = 0.0001) reductions in total and LDL cholesterol from the baseline, respectively, the placebo resulted in reductions of 3.5% (p = 0.001) and 6.3% (p = 0.0007%), respectively. The crossover nature of the study could be a contributing factor if the 4-week washout period was too short to prevent potential carryover effects in the formulation. Thus, further studies are warranted in animals and humans to decipher the bacterial formulations, duration, and dosage regimens that generate physiologically meaningful reductions in LDL and total cholesterol. For further details of these trials, see Table 1.
Developmental disorders
The microbiota-gut-brain axis describes the bi-directional communication pathways between the gut microbiota and its metabolites, the central, enteric, and autonomic nervous systems, and the hypothalamic-pituitary-adrenal axis.118 Certain members of the gut microbiota, including B. longum, have been shown to produce neurochemicals such as the major inhibitory neurotransmitter gamma amino butyric acid,119 or are involved in the regulation of host serotonin biosynthesis.120,121 Furthermore, bacterially produced SCFAs are involved in the microbiota-gut-brain axis with the potential to influence mood, cognition, and brain disorder etiology, directly or indirectly.122
Autism spectrum disorders
In 2012, it was estimated that 1 in 160 children globally had a pervasive developmental disorder including an autism spectrum disorder (ASD).123 But the reported prevalence has since increased, and in Ireland alone, the prevalence rate for ASD in children was estimated at 1.5%.124 Comorbidities include gastrointestinal issues such as abdominal pain, constipation, and diarrhea.125 Wang et al.92 performed a RDBPCT in children with ASD to investigate the impact of a synbiotic formulation on ASD symptoms as well as gut microbiota composition, SCFA concentrations, and levels of neurotransmitters. A synbiotic is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host.”126 In this case, the synbiotic consisted of four bacterial strains with the prebiotic fructo-oligosaccharides (FOS). However, before the intervention took place, differences in fecal microbiota composition, SCFA production, and plasma neurotransmitters were investigated between children diagnosed with ASD and normal children. Interestingly, children with ASD had significantly lower levels of beneficial bacteria, B. longum and Bifidobacteriales in terms of relative abundance (p < 0.05) and significantly higher levels of Ruminococcus and Clostridium (p < 0.01). Levels of the SCFAs butyrate, propionate, and acetic acid were also significantly lower in children with ASD (p < 0.05). Furthermore, these children were found to be in a hyper-serotonergic state and had significantly decreased levels of homovanillic acid (p < 0.001), an indicator of dopaminergic activity in the central nervous system.127 The synbiotics, which consisted of B. infantis Bi-26, L. rhamnosus HN001, B. lactis BL-04, Lactobacillus paracasei (now Lacticaseibacillus paracasei) LPC-37, and the prebiotic FOS, was administered for 108 d in total, and the analysis of parameters was performed at 30, 60 and 108 d. The synbiotic was associated with significantly increased beneficial bacteria when compared with baseline (day 0) including B. longum (days 30 and 60, p < 0.05; day 108 p < 0.001) and reduced levels of suspected pathogens such as Clostridium (day 108, p < 0.05) compared with the placebo group (children with ASD receiving placebo). The synbiotic also resulted in significant elevations in individual SCFA levels, significantly increased homovanillic acid, and significantly reduced serotonin, which were not observed in the placebo group. However, as the authors point out, the synbiotic failed to modulate a number of neurotransmitters and metabolites including glutamine, glutamic acid, acetylcholine, gamma amino butyric acid, arginine, histidine, and histamine. But the synbiotic did improve gastrointestinal symptoms in participants and reduced autism severity as assessed by the Autism Treatment Evaluation Checklist (ATEC). Further studies are warranted with larger sample sizes and to determine if FOS is also necessary for the observed effects.
Respiratory health and seasonal allergies
Upper respiratory tract infections describe viral or bacterial infections of the nose, pharynx, larynx, sinuses, and large airways, and have been identified as one of the top three diagnoses in outpatient settings.128 In 2003, it was estimated that non-influenza-related, viral respiratory tract infections in the United States posed an annual economic burden above $22 billion.129 Seasonal allergies, including hay fever or allergic rhinitis, occur at a certain time of the year when pollen counts are high with symptoms of runny nose, watery eyes, coughing, and sneezing. In allergic 2-year-old children, the gut microbiota has been characterized by lower bifidobacteria, lactobacilli, and Bacteroides and higher levels of aerobic microorganisms including Staphylococcus aureus compared with non-allergic children. Thus, interventions with beneficial bacteria have the potential to prevent or reduce the severity of respiratory illnesses and seasonal allergies through modulation of the gut microbiota, its functionality, and host immunity.
In a multicentre RDBPC pilot study, children who had previously suffered at least three episodes of common winter diseases including ear, nose, and throat, respiratory tract, or gastrointestinal illnesses were administered a synbiotic formulation for 3 months to determine its efficacy at preventing common winter diseases.93 The synbiotic consisted of B. infantis R0033, B. bifidum R0071, L. helveticus R0052, and FOS. Compared with placebo, the synbiotic resulted in a 25% relative risk reduction in the percentage of children who suffered at least one winter disease during the treatment period (p = 0.045) and limited the number of school days lost (p = 0.043). However, a potential limitation of the study is the unbalanced number of children in each group (n = 73, placebo; n = 62, test) due to enrollment difficulties that could have resulted in a study bias. Despite this, the results are promising and should help in the strategic design of a larger clinical trial.
Consuming B. longum BB536 for 10 months alleviated the symptoms of upper respiratory illnesses in children aged 2–6 y old in a parallel RDBPCT.94 Specifically, the strain was associated with a reduced duration of sore throat by 46% (p = 0.018), runny nose by 15% (p = 0.087), and cough by 16% (p = 0.087) compared with the placebo. Interestingly, the analysis of the gut microbiota revealed an increase in the genus Faecalibacterium in the BB536 group between 0 and 10 months, which was not observed in the placebo group (p < 0.05). In the previous section on Immunity, the same strain was shown to increase IF-γ secretion cells in healthy-term newborns.89 The same strain combined with B. infantis M-63 and B. breve M-16 V proved effective for significantly relieving nasal symptoms of allergic rhinitis (nasal itching, nasal obstruction, sneezing, rhinorrhea, and itchy eyes; p < 0.005) and improving quality of life (p < 0.001) in children suffering from seasonal allergic rhinitis and intermittent asthma due to pollen.95 However, the small sample size used in this trial (n = 40) suggests that further trials with larger participant numbers are needed to confirm the findings.
Skin health
While the large intestine is estimated to carry 1014 bacterial cells, the skin microbiome is said to harbor 1011.1 Common skin disorders have been associated with imbalances in the skin microbiome such as acne vulgaris, which has been associated with Cutibacterium acnes type 1A130,131 and atopic dermatitis, associated with increased S. aureus abundance132,133 as examples. For extensive reviews on this topic, the reader is referred to De Pessemier et al.8 and O’ Sullivan et al.134.
The gut – skin axis describes the bidirectional communication between the gut ecosystem and skin and is generally mediated via the host’s immune system.8 Indeed, many skin disorders have been associated with altered gut microbiota (reviewed extensively by De Pessemier et al.8. For example, atopic dermatitis has been associated with reduced gut levels of Bacteroidetes, Akkermansia, and Bifidobacterium, and higher levels of F. prausnitzii, Clostridium, and Escherichia coli.135–141 Following a review of the evidence, the World Allergy Organization recommended probiotic supplementation for prevention of allergy in infants, albeit the evidence was described as ‘very low quality’.142
A small number of RDBPCTs have been performed with B. longum-containing formulations, particularly in infants and children, with promising results.
Atopic dermatitis
The inflammatory skin disorder, atopic dermatitis, is said to be the most common inflammatory skin disease.143 With symptoms of dry, itchy, cracked, and sore skin, atopic dermatitis can significantly impact quality of life. Paller et al.144 reported that atopic dermatitis also puts patients at risk of other non-allergic conditions, such as anxiety, attention deficit hyperactivity disorder (ADHD), and depression and it is also associated with bacterial and viral cutaneous and extra-cutaneous infections. While moisturizers are considered standard therapy, a recent review of over-the-counter therapies revealed that not all are beneficial with some being deleterious.143 Thus, microbial interventions that can modulate the inflammatory status of the skin pose a highly attractive option.
Rautava et al.96 investigated the impact of maternal supplementation with different bacterial strains during the last 2 months of pregnancy and the first 2 months of breastfeeding on reducing the risk of eczema development in high-risk infants in a parallel RDBPCT. Infants were considered high risk if the mother presented with atopic sensitization and had a history of or active allergic disease. Two formulations were assessed: B. longum BL999 with L. rhamnosus LPR and BL999 with L. paracasei ST11, and the infants were assessed for 24 months. Both formulations were deemed safe and significantly reduced the risk of developing eczema (p < 0.001 for both). More specifically, while 71% of infants in the placebo group developed eczema, only 29% in each intervention group were recorded as having eczema. Chronically persistent eczema was reported in 26% of the placebo group but only in 10% of the BL999 + LPR group (p = 0.016) and 6% of the BL999 + ST11 group (p = 0.003). The bacterial strains had no impact on the risk of atopic sensitization in infants. Interestingly, supplementing at-risk infants with B. longum BL999 and L. rhamnosus LPR in commercially available cow’s milk formula for the first 6 months of life had no impact on eczema incidence or atopic sensitization during the first year of life.97 Rautava et al.96 suggest that prenatal probiotic supplementation may alter the maternal intestinal and vaginal microbiota, which provide important colonizing inocula to the newborn infant.62 The authors also suggested that maternal administration of bacterial strains may alter the immuno-physiology of the foetoplacental unit. Indeed, the same research group reported such an observation in humans following a RDBPCT whereby strains of B. lactis or B. lactis with L. rhamnosus GG significantly altered toll-like receptor (TLR)-related gene expression in both the placenta and fetal gut.145 Furthermore, evidence suggests that maternal intestinal microbes can be transferred to breast milk via the enteromammary pathway146 and orally ingested probiotic strains have been identified in mother’s breast milk,147,148 which could provide a means of transferring the strains directly to the breastfed infant. Indeed, for a single breastfeeding mother-infant pair of cesarean delivery, Kordy et al.149 identified a distinct B. breve strain in the infant stool, maternal breast milk, and maternal rectum suggesting transfer of maternal gut bacteria to the mammary gland and then to the infant. Furthermore, beneficial bacteria in breast milk may be supported by HMOs.150
In a group of young participants (aged 4–17 y), 12 weeks of supplementation with the formulation B. longum CECT 7347, B. lactis CECT 8145, and L. casei CECT 9104 reduced the SCORAD (Scoring Atopic Dermatitis) index during the supplementation period, and reduced eczema spread and intensity.98 After 12 weeks, the mean reduction in the SCORAD index for the intervention group was 19.2 points greater than in the placebo group (p < 0.001). Furthermore, the proportion of days of topical steroid use was also significantly less in the test group (p < 0.003). However, the dose of topical corticosteroid treatment was not recorded. For further details of these trials, see Table 1.
B. longum effects in adults
Gastrointestinal health and disease
Inflammatory bowel disease
UC has a prevalence of 156 to 291 cases per 100,000 persons per year and is more prevalent in adults than children.151 A synbiotic that consisted of B. longum 536 isolated from healthy rectal epithelium combined with a FOS-inulin prebiotic (Synergy 1; Orafti, Tienen, Belgium) was associated with a significant reduction in mucosal inflammatory markers, improved appearance of chronic inflammation and regeneration of epithelial tissue following 4 weeks of treatment in patients with active UC in a RDBPCT.152 Total bifidobacteria numbers on the mucosal surface of patients also increased, although it was not possible to determine if these were the administered bacteria or host bifidobacteria that benefited from the growth-promoting properties of the prebiotic. However, the authors concluded that a longer treatment period could lead to better clinical outcomes. Therefore, the same formulation was assessed in a RDBPCT where CD patients consumed the synbiotic for up to 6 months.153 Specifically, significant reductions in CD activity indices (p = 0.02) and histological scores (p = 0.018) were recorded for the synbiotic group. As for the previous study, mucosal-associated bifidobacteria increased in the test group. However, the authors noted that the synbiotic was most effective in patients with colon-related CD. The strain was later assessed alone for induction of remission in patients with active UC following 8 weeks of treatment in a multicentre RDBPCT.154 While 63% of patients in the test group showed remission, 52% in the placebo group also showed remission (p = 0.395) which is a very high percentage for a placebo group, as pointed out by the authors. This could be due to several trial design features, including ‘definition of remission’ but is most likely due to the standard medical treatments that all participants received. Despite this, a significant decrease was observed for UC disease activity index scores in the B. longum 536 group from baseline to week 8 (p < 0.01), but no significant decrease was observed in the placebo group (p = 0.88). Likewise, significant differences were observed in the 536 group from baseline to week 8 for the Rachmilewitz endoscopic index and the Mayo subscore but not in the placebo group. Mucosal healing rate was greater for the 536 group, but the difference between B. longum 536 and placebo groups was not significant.
The efficacy of VSL#3® for the treatment of UC has been investigated in several clinical trials. In combination with a low-dose prodrug of a conventional IBD treatment (balsalazide), VSL#3® proved significantly superior to conventional treatments alone (balsalazide or 5-aminosalicylic acid [5-ASA]) for obtaining remission in patients with active mild-to-moderate UC following 8 weeks of treatment.155 In patients with relapsing UC and receiving conventional 5-ASA treatment and/or immunosuppressants, VSL#3® treatment for 8 weeks was associated with significantly improved UC disease activity index scores compared with placebo (p = 0.01), improved rectal bleeding, and the formulation tended to induce remission in relapsing patients.156 As a sole treatment for mild-to-moderate active UC, VSL#3® resulted in significantly higher patient numbers with improved UC disease activity index scores following 6 weeks of administration compared with placebo (32.5 versus 10%, respectively, p = 0.001), and after 12 weeks of treatment, 42.9% of patients in receipt of VSL#3® achieved remission compared with 15.7% in the placebo group (p < 0.001).157 The formulation proved less effective for preventing CD recurrence, however, in patients following surgery in a multicentre RDBPCT following 90 d of treatment.158 However, the low rate of recurrence in the placebo group rendered this trial underpowered to observe statistical differences. In the second phase of the trial (an open-label study; days 91 to 365), patients receiving VSL#3® for the entire 365 d exhibited a lower rate of recurrence and lower levels of inflammatory cytokines though statistical significance was not observed.
Irritable bowel syndrome
Microbial formulations containing B. longum have also proven clinically effective for providing relief from IBS symptoms in adults (Table 2). Ki Cha et al.159 investigated the impact of a seven-strain species mix on diarrhea-dominant IBS that consisted of B. longum, L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, and S. thermophilus following daily treatment for 8 weeks. Throughout the study period, the proportion of participants in the intervention group reporting adequate relief from IBS symptoms was significantly higher than that in the placebo group (p < 0.05); however, the formulation failed to induce “superior effects” on individual symptoms including abdominal pain. The intervention significantly improved stool consistency and IBS quality-of-life improvement tended to be higher for the intervention group. Denaturing gradient gel electrophoresis profiles of the fecal microbiota revealed that the intervention was associated with stabilization of the intestinal microbiota. It should be noted that the follow-up period of 2 weeks is substantially less than the recommended follow-up for IBS trials of 6–12 months.
Table 2.
An overview of clinical trials investigating the impact of B. longum on adults.
| Condition/Disease/Biological Parameter | Participants; Age | Formulation | CFU; Dose; Duration | Clinical Effects and Biological Observations of Intervention Group Compared with Placebo Group | Reference; Trial ID |
|---|---|---|---|---|---|
| Gastrointestinal Conditions | |||||
| Ulcerative colitis | 16; > 18 y |
B. longum 536, Synergy 1 prebiotic (FOS & inulin) |
2 x 1011, 6 g prebiotic; 2 daily doses; 4 weeks | Sigmoidal scores significantly reduced; β-defensins mRNA significantly reduced; TNF-α & IL1α significantly reduced; Reduced inflammation in biopsies; Regeneration of epithelial tissue |
Furrie et al.152 |
| Crohn’s Disease | 35; > 18 y |
B. longum 536, Synergy 1 prebiotic (FOS & inulin) |
2 x 1011, 6 g prebiotic; 2 daily doses; 6 months | Significant reductions in CD activity indices & histological scores; TNF-α significantly reduced; Mucosal bifidobacteria increased |
Steed et al.153 |
| Ulcerative colitis | 56; > 18 y | B. longum 536 | 2–3 x 1011; 3 daily doses; 8 weeks | Significant decrease in UC disease activity index scores from baseline to week 8; Significant decrease in Rachmilewitz score from baseline to week 8; Significant decrease in Mayo subscore from baseline to week 8; Increased mucosal healing rate |
Tamaki et al.154 (Multicentre) |
| Ulcerative colitis | 90; > 18 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus, Balsalazide |
9 x 1011, 2.25 g daily; 8 weeks | Significantly increased number of patients in remission; Achieved remission faster |
Tursi et al.155 |
| Ulcerative colitis | 131; > 18 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus |
3.6 x 1014, daily; 8 weeks | Significantly higher proportion of patients experienced improvement in UCDAI score of at least 50%; Achieved remission faster; Significantly improved rectal bleeding |
Tursi et al.156 (Multicentre) |
| Ulcerative colitis | 147; > 18 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus |
3.6 x 1012; 2 daily doses; 12 weeks | Significantly higher proportion of patients experienced improvement in UCDAI score of > 50%; Significantly increased number of patients in remission |
Sood et al.157 (Multicentre) |
| Crohn’s Disease | 119; > 16 y | VSL#3: B. longum, B. breve, B. infantis, L. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, S. salivarius subsp. thermophilus |
9 x 1011; 2 daily doses; 365 d or beginning day 91 until day 365 | Reduced mucosal inflammatory cytokine levels at days 90 & 365 for patients receiving probiotic for 365 d; Lower rate of recurrence among patients receiving probiotic for 365 d |
Fedorak et al.158
NCT00175292 (Multicentre) |
| Irritable bowel syndrome | 50; 18–65 y |
B. longum, L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, S. thermophilus |
1 x 1010; daily; 8 weeks | Adequate relief significantly higher; Stool consistency significantly improved; IBS quality of life tended to be higher; Stabilisation of intestinal microbiota |
Ki Cha et al.159 |
| Irritable bowel syndrome | 25; > 18 y |
B. longum BB536, L. rhamnosus HN001, Vitamin B6 |
5 x 109, 1.4 mg; daily; 1 month | Significantly improved abdominal pain, bloating & disease severity; Significantly improved colonic permeability; Increased lactic acid bacteria & bifidobacteria |
Bonfrate et al.160
NCT03815617 |
| Irritable bowel syndrome (diarrhea-dominant) | 80; > 18 y |
B. longum DSMZ 32,946, B. bifidum DSMZ 32,403, B. lactis DSMZ 32,269, L. acidophilus DSMZ 32,418, L. rhamnosus FloraActive™ 19070–2 FOS |
5 x 109, 947 mg; 2 daily doses; 8 weeks | Significant improvement in symptom scores | Skrzydlo-Radomańska et al.161 NCT04206410 |
| Irritable bowel syndrome (IBS-C, IBS-D, IBS-M) | 248; > 18 y | B. longum R0175 | 1 x 1010; daily; 8 weeks | Improved quality of life in emotional wellbeing & social functioning; Increased energy levels that impacted willingness & ability to perform everyday tasks |
Lewis et al.162 NCT02213172 |
| Irritable bowel syndrome | 362; > 18 y | B. infantis 35624 | 1 x 106 or 1 x 108 or 1 x 1010; 4 weeks |
1 x 108 CFU significantly improved abdominal pain, bloating, bowel dysfunction, incomplete evacuation, straining, & flatulence | Whorwell et al.163 |
| Irritable bowel syndrome | 77; > 18 y | B. infantis 35624 | 1 x 1010; 8 weeks | Significant reduction in symptom scores for abdominal pain/discomfort, bloating/distension, & bowel movement difficulty; Normalisation of abnormal IL-10/IL-12 ratio |
O’ Mahony et al.164 |
| Acute-radiation induced diarrhea | 246; > 18 y |
B. longum BB-536, L. acidophilus LAC-361 |
1.3 x 109, 2 daily doses or 1 x 1010, 3 daily doses |
No effect during radiation treatment in non-surgery patients but 1.3 x 109 CFU reduced number of patients with moderate to severe diarrhea after radiation therapy; In surgery patients (before radiation therapy), 1.3 x 109 CFU increased proportion of patients without very severe diarrhea during treatment. |
Demers et al.165
NCT01839721 |
| Constipation | 94; > 18 y |
B. longum UABI-14, B. animalis ssp. lactis UABIa-12, B. bifidum UABb-10, L. acidophilus DDS-1 |
1.5 x 1010, 1 daily dose | Faster normalisation of stool frequency & consistency after 1 week of treatment; Higher relative abundance of Ruminococcaceae & lower relative abundance of Erysipelotrichaceae |
Martoni et al.166 NCT02418507 |
| Lactose intolerance | 23; > 18 y |
B. longum BB536, L. rhamnosus HN001, Vitamin B6 |
5 x 109, 1.4 mg; daily; 1 month | Significantly decreased bloating & improved constipation; Enriched genera involved in lactose digestion; Increased acetic acid, 2-methylpropanoic acid, nonenal, indolizine 3-methyl, decreased phenol |
Vitellio et al.167 NCT03815617 |
| Stress-induced gastrointestinal symptoms | 75; > 18 y | B. longum Rosell-175, L. acidophilus Rosell-52 | 3 x 109; daily; 3 weeks | Significantly reduced abdominal pain & nausea/vomiting | Diop et al.168 |
| Immunity | |||||
| Blood anti-oxidative activity in asymptomatic H. pylori colonised subjects | 53; 20–60 y |
B. longum 46, L. paracasei 8700:2, L. fermentum ME-3, FOS |
3 x 109, 6.6 g, 2 doses daily; 3 weeks | Significantly increased total antioxidative status; Significantly decreased ratio between oxidised & reduced glutathione |
Hütt et al.169 |
| Ulcerative colitis/Inflammatory biomarkers | 22; 18–75 y | B. infantis 35624 | 1 x 1010, daily, 6 weeks | Significantly reduced C-reactive protein Numerically reduced IL-6 |
Groeger et al.170 |
| Chronic fatigue syndrome/Inflammatory biomarkers | 48; 18–65 y | B. infantis 35624 | 1 x 1010, daily, 8 weeks | Significantly reduced C-reactive protein & TNF-α Numerically reduced IL-6 |
Groeger et al.170 |
| Psoriasis/Inflammatory biomarkers | 26; 18–60 y | B. infantis 35624 | 1 x 1010, daily, 8 weeks | Significantly reduced C-reactive protein & TNF-α | Groeger et al.170 |
| Healthy subjects | 35; 18–65 y | B. infantis 35624 | 1 x 1010, daily, 8 weeks | No impact on pro-inflammatory markers | Groeger et al.170 |
| Peritoneal dialysis patients/Inflammatory biomarkers & endotoxin | 39; ≥ 18 y |
B. longum A101, B. bifidum A218, B. catenulatum A302, L. plantarum A87 |
4 x 109, daily; 6 months | Significantly reduced IL-6, TNF-α, IL-5, & endotoxin; Significantly increased IL-10 levels; Preserved residual renal function which significantly declined in the placebo group after 6 months. |
Wang et al.171 |
| Hemodialysis patients/Inflammatory biomarkers, endotoxin, & anti-heat shock protein 70 antibodies | 75; 30–65 y |
B. longum LAF-5, B. lactis BIA 6, B. bifidum BIA 6, L. acidophilus T16 FOS GOS Inulin |
1.35 x 108, 15 g, 4 times daily; 12 weeks |
Significantly decreased pro-inflammatory markers (C-reactive protein & IL-6), endotoxin levels, & anti-heat shock protein 70 antibodies | Haghighat et al.172 IRCT2017041233393N1 |
| Cardiovascular Health | |||||
| Normal or moderately elevated cholesterol | 34; 18–65 y |
B. longum BB536, L. acidophilus 145 in fermented milk drink |
2.7x107 −1x108 (CFU/g) 1.4–2.1x108 (CFU/g) 375 g daily; 4 weeks |
Significantly decreased LDL-cholesterol in those with baseline level of total cholesterol > 190 mg/dl Significantly reduced HDL-cholesterol |
Andrade and Borges,173 |
| Type 2 diabetes | 54; 35–70 y |
B. longum, L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. breve, S. thermophilus |
7 x 109 2 x 109 7 x 109 1.5 x 109 2 x 108 2 x 1010 1.5 x 109, daily; 8 weeks |
Prevented increase in fasting plasma glucose, Significantly reduced serum hs-CRP, Increased plasma levels of glutathione |
Asemi et al.174 |
| Depression, Anxiety, and Cognitive Functioning | |||||
| Healthy/Psychological impact | 55; 30–60 y |
B. longum R0175, L. helveticus R0052 |
3 x 109 daily; 30 d |
Significantly alleviated psychological distress; Significantly reduced urinary cortisol |
Messaoudi et al.175 |
| Low mood for at least 2 y | 79; ≥ 16 y |
B. longum R0175, L. helveticus R0052 |
3 x 109 daily; 8 weeks |
No effect on low mood; No impact on inflammatory biomarkers |
Romijn et al.176
ACTRN12613000438752 |
| Major depressive disorder but on antidepressant drugs | 81; 36.5 ± 8.03 y |
B. longum R0175, L. helveticus R0052 |
≥1010 daily; 8 weeks |
Significant decrease in the Beck Depression Inventory; Significant decrease in kynurenine/tryptophan ratio; Improved appetite |
Kazemi et al.177 (IRCT2015092924271N1) Kazemi et al.178 |
| Healthy/Cognition, Mood, Sleep quality | 38; 18–35 y |
B. longum BL04, L. fermentum LF16, L. rhamnosus LR06, L. plantarum LP01 |
4 x 109 daily; 6 weeks | Significantly improved mood & sleep quality; Reduced depressive mood state, anger, & fatigue |
Marotta et al.179 NCT03539263 |
| IBS/Mild to moderate depression & anxiety | 44; 26–58 y | B. longum NCC3001 | 1 x 1010 daily; 6 weeks | Significantly reduced depression scores; Significantly improved general physical health; Decreased brain responses to negative emotional stimuli based on fMRI analysis |
Pinto-Sanchez et al.180 NCT01276626 |
| Alzheimer’s disease/Cognitive functions & Metabolic status | 79; 68–84.3 y |
B. longum, B. bifidum, L. acidophilus & selenium |
2 x 109 2 x 109 2 x 109 200 μg daily; 12 weeks |
Significantly improved cognitive function; Significantly reduced hs-CRP, insulin levels, homeostasis model of assessment-insulin resistance, serum triglycerides; Significantly increased total antioxidant capacity, the quantitative insulin sensitivity check index; Significantly improved cholesterol profiles |
Tamtaji et al.181 IRCT20170612034497N5 |
| Respiratory Health and Seasonal Allergies | |||||
| Japanese cedar pollinosis during pollen season | 44; 22–57 y | B. longum BB536 | 5 x 1010; twice daily; 13 weeks | Significantly relieved symptoms; Significantly modulated Th2-skewed immune response; Suppressed Bacteroides fragilis levels in faecal microbiota |
Xiao et al.182 Odamaki et al.183 |
| Japanese cedar pollen exposure in environmental exposure unit | 24; 25–56 y | B. longum BB536 | 5 x 1010; twice daily; 4 weeks | Significantly reduced ocular symptom scores; Reduced disruption of normal activities following exposure; Significantly reduced total medications to alleviate symptoms |
Xiao et al.184 |
| Seasonal allergies during allergy season | 173; 27 ± 1 year |
B. longum MM2, B. bifidum G9–1, L. gasseri KS-13 |
3 x 109; daily; 8 weeks | Significantly improved rhinoconjunctivitis-specific quality of life | Dennis-Wall et al.185 NCT02349711 |
| Perennial allergic rhinitis | 95; 19–65 y |
B. longum IM55, L. plantarum IM76 |
1 x 1010; daily; 4 weeks | Significantly reduced total nasal symptom scores & rhinorrhea Numerically reduced sneezing & nasal congestion Significantly improved anti-allergic immunological profiles |
Kang et al.186 KCT0003760 |
| Common cold | 454; adults |
B. longum SP 07/3, B. bifidum MF 20/5, L. gasseri PA 16–8 |
5 x 107; daily; 3 months & 5.5 months (two different study groups) | Shortened common cold episodes by at least two 2 d & reduced severity of symptoms; After 14 d of supplementation, CD4+ T helper cells increased & cytotoxic plus T suppressor cells (CD8+) significantly increased |
De Vrese et al.187 |
| Skin | |||||
| Reactive skin | 66; healthy females | B. longum lysate | 10% lysate applied to skin twice daily; 2 months | Significantly decreased skin sensitivity; Reduced skin dryness |
Guéniche et al.188 |
In the crossover RDBPCT, consumption of B. longum BB536 and L. rhamnosus HN001 with vitamin B6 for 1 month significantly improved IBS symptoms (abdominal pain and bloating) and disease severity compared with placebo (p < 0.0001).160 The formulation was also associated with improved colonic permeability as measured by sucralose recovery in urine – but had no impact on small intestinal permeability. Presumptive lactic acid bacteria and bifidobacteria increased during treatment, as did volatile organic compounds including butanoic, pentanoic, and propanoic acids, and hydrocarbons, while phenol decreased. However, the sample size of this crossover study was small at n = 25, and the washout period was 15 d, so a carryover effect of treatment cannot be ruled out.
A synbiotic preparation consisting of FOS and five bacterial strains including B. longum DSMZ 32,946, B. bifidum DSMZ 32,403, B. lactis DSMZ 32,269, L. acidophilus DSMZ 32,418, and L. rhamnosus FloraActive™ 19070–2 significantly improved diarrhea-associated IBS (IBS-D) symptoms in patients in an 8-week treatment period.161 Specifically, compared with the placebo, the synbiotic was associated with significant improvements on the IBS-Global Improvement Scale (−GIS) (p = 0.043) and the IBS-Symptom Severity Scale (−SSS) (p = 0.042) following 4 and 8 weeks of consumption. However, while 80 patients were enrolled in the study, only 68 completed it, thus the sample size was relatively small such that the statistical power necessary to determine statistically significant differences between treatments could be limited. Also, the authors cannot rule out if the maltodextrin administered to the placebo group (but not the synbiotic group) had an impact given that IBS severity significantly decreased from baseline in the placebo group after weeks 4 and 8 of the trial. Thus, further trials are warranted with larger sample sizes and a better placebo treatment to enable a more accurate comparison of placebo and intervention.
The impact of B. longum R0175 alone on the gastrointestinal symptoms and psychiatric comorbidities of IBS was investigated by Lewis et al.162 in a 3-arm RDBPCT involving 251 adults with either constipation-associated IBS (IBS-C), IBS-D, or mixed pattern IBS (IBS-M). The other strain that was assessed was L. paracasei HA-196, and the treatment period lasted for 8 weeks. Questionnaires were used to assess IBS symptoms, stool consistency, frequency, and quality of life. L. paracasei proved to be the most effective strain for improving IBS symptoms, particularly for IBS-C and IBS-D patients. IBS patients are also at increased risk of depression, anxiety, bipolar, and sleep disorders.189 Both strains improved emotional well-being and social functioning, but participants consuming B. longum R0175 also reported increased energy levels that positively impacted their willingness and ability to perform everyday tasks. A placebo effect of 33% was recorded in this study. A meta-analysis conducted by Patel et al.190 reported that the placebo response in participants with IBS can range from 16% to 71% for pharmaceutical interventions or natural health products. Lewis et al.162 suggested that prior to the intervention, a longer period examining bowel habits of participants may help to mitigate the placebo effect.
Whorwell et al.163 reported that administered bacterial dose can significantly impact efficacy. In a study involving 362 primary care IBS patients, B. infantis 35624 failed to provide relief from symptoms at doses of 106 and 1010 colony forming units (CFU)/ml for 4 weeks but at 108 CFU/ml the strain significantly relieved many IBS symptoms compared with placebo (and other doses) including abdominal pain, as well as bloating, bowel dysfunction, flatulence, straining, and incomplete evacuation. In this case, the strain was prepared as a freeze-dried powder and packed into capsules with an excipient. A previous study had demonstrated the efficacy of 1010 CFU/ml of B. infantis 35624 for relieving IBS symptoms in patients when administered in a milk drink – a response that was associated with a normalization of the anti-inflammatory to pro-inflammatory cytokine ratio.164 In capsule form, the 1010 CFU/ml of B. infantis 35624 ‘coagulated’ into a ‘glue-like mass’ which the authors state is due to the intense hygroscopic nature of the strain that presumably impacted its growth characteristics in the gut; but with time the higher formulation should lead to noticeable benefits as the bacterium replicates to the concentration required to achieve efficacy.
Diarrhea
Diarrhea is a common side effect of pelvic radiation therapy with up to 80% of patients reported suffering from acute radiation-induced diarrhea.191 B. longum has proven effective in the treatment of diarrhea when used in combination with other strains. FloraActive™ is a formulation containing B. longum BB-536 and L. acidophilus LAC-361. In a RDBPCT, 246 pelvic radiation patients were randomized to receive a placebo or one of the two doses of FloraActive™; a standard dose twice daily (1.3 × 109 CFU) or a high dose, thrice daily (1 × 1010 CFU).165 Patients began taking the formulation in capsule form on the first day of therapy and continued until treatment ended with time to first appearance of grade≥2-3-4 diarrhea using Kaplan–Meier curves. The formulation did not prevent moderate-to-severe diarrhea during treatment. However, at the end of treatment or during the 2 weeks after treatment, 35% of the standard dose group experienced less of the moderate-to-severe diarrhea compared with only 17% in the placebo group (p = 0.04). In patients who had surgery before radiation commenced, the standard dose group had a higher proportion of patients without very severe (grade 4) diarrhea (97%) versus placebo (74%) (p = 0.03) during treatment. The higher dose formulation proved less effective than the standard dose, highlighting again the importance of clinical data at different dosage levels. It should be noted that a dietary intervention was included in this study for all participants based on individualized recommendations by a dietitian that generally involved reducing total intake of lipids and advice on intake of dietary fibers and simple or complex carbohydrates; this may have reduced digestive symptomatology in all participants.
Constipation
The prevalence of functional constipation amongst adults in the community has been estimated to be 14%.192 In a RDBPCT, a formulation with B. longum and three other strains (B. animalis ssp. lactis, B. bifidum, L. acidophilus) failed to achieve improvements in symptomology (based on patient assessment of constipation – symptom [PAC-SYM] and patient assessment of constipation – quality of life [PAC-QoL]) compared with the placebo group.166 The authors state that this was possibly due to a high placebo response, which can be the case for participants with bowel disorders but may be controlled with a placebo run-in period that would enable the exclusion of high responders. Also, the PAC-SYM score was not included in the initial inclusion criteria, which again may have impacted the ability to differentiate between groups at the end of the trial. Despite this, the formulation was associated with faster normalization of stool frequency and consistency following 1 week of treatment in the 4-week intervention period. The study assessed fecal microbiota at baseline and endpoint of the study and reported a significantly higher relative abundance of Ruminococcaceae and lower relative abundance of Erysipelotrichaceae in the formulation group. Interestingly, the abundance of the Ruminococcaceae family has been shown to positively correlate with faster intestinal transit and improved Bristol Stool Scale scores.193
Lactose intolerance
It has already been demonstrated that the formulation B. longum BB536 and L. rhamnosus HN001 with vitamin B6 proved effective for the treatment of IBS.160 The same formulation also alleviated the symptoms of lactose intolerance in lactose-intolerant subjects following 30 d of treatment in a crossover RDBPCT.167 The formulation was associated with increased fecal abundance of lactose-digesting genera including Bifidobacterium that positively correlated with increased acetic acid and 2-methylpropanoic acid, while decreased phenol positively correlated with relative amounts of genera from Proteobacteria. A previous study found that growing B. longum B6 in lactose prior to treatment increased its ability to improve lactose digestion in sufferers as it induces higher β-galactosidase activity in the strain.194
Stress-induced gastrointestinal symptoms
Psychological stress is known to contribute to several gastrointestinal dysfunctions through the brain-gut axis.195 The formulation Probio-Stick which contains B. longum Rosell-175 and L. acidophilus Rosell-52 proved effective in relieving stress-induced gastrointestinal symptoms in volunteers in a RDBPCT following 3 weeks of intervention.168 Specifically, the formulation significantly reduced abdominal pain (p = 0.004) and nausea/vomiting (p = 0.009) in the volunteers who had been selected for the trial based on suffering from daily stress with at least two stress-induced symptoms in the previous month. However, the sample size was small at n = 23 suggesting that further trials are warranted with greater participant numbers to confirm these results. For further details of these trials, see Table 2.
Immunity
Helicobacter pylori infection of the stomach causes persistent oxidative stress in the stomach and induces chronic inflammation that can lead to peptic ulcers, gastritis, and gastric cancer.196,197 Hütt et al.169 investigated the impact of a synbiotic product on antioxidative activity in asymptomatic subjects, 53% of which were colonized with H. pylori in a cross-over RDBPCT. Blood sera samples were analyzed for total antioxidative status (TAS) and de-proteinated whole blood, plasma, and erythrocyte lysate were analyzed for oxidized glutathione (GSSG) and reduced glutathione (GSH). The synbiotic consisted of Raftilose P95® (oligofructose) and three bacterial strains, namely B. longum 46, L. fermentum (now Limosilactobacillus fermentum) ME-3, and L. paracasei 8700:2. The L. fermentum strain had previously demonstrated high TAS while all three strains exhibited moderate antagonistic activity against H. pylori.198 The H. pylori-colonized subjects had significantly reduced sera levels of TAS compared with H. pylori-negative subjects. Following 3 weeks of synbiotic administration, TAS values in H. pylori-colonized subjects significantly increased compared with baseline (p = 0.004) and the ratio between oxidized and reduced glutathione decreased (p = 0.016). There was no impact on H. pylori colonization, but the authors suggest that the enterocoated synbiotic capsules were only soluble in the small intestine and thus the bacterial strains did not encounter H. pylori in the stomach.169
In three separate RDBPCTs, the strain B. infantis 35624 was evaluated for its impact on inflammatory biomarkers in patients with UC, chronic fatigue syndrome, and psoriasis, the latter two being examples of extra-intestinal inflammatory diseases.170 The impact of bacterial strain on inflammatory biomarkers of healthy subjects was also assessed. Inflammatory biomarkers included C-reactive protein (CRP), and the pro-inflammatory cytokines IL-6 and TNF-α. The serum or plasma level of the acute phase protein, CRP, is a useful indicator of systemic pro-inflammatory activity in several inflammatory conditions.199 Compared with healthy volunteers, all patients exhibited significantly increased levels of CRP (p < 0.001), IL-6 (p < 0.05), and TNF-α (p < 0.001) at baseline. In UC patients, consumption of the strain for 6 weeks was associated with significantly reduced CRP levels compared with the placebo group (p = 0.0327), while the reduction in IL-6 levels approached significance (p = 0.057). The strain had no impact on TNF-α levels in UC patients. For those suffering from chronic fatigue syndrome, 8 weeks of oral administration of the strain significantly reduced CRP (p = 0.0285) and TNF-α (p = 0.0214) levels compared with the placebo group, while the reduction in IL-6 levels approached significance (p = 0.054). Psoriasis patients also consumed the strain for 8 weeks where it was associated with significant reductions in CRP (p = 0.0425) and TNF-α (p = 0.0405) levels but had no impact on IL-6. The strain had no impact on the pro-inflammatory biomarkers in healthy individuals following 8 weeks of administration compared with placebo. However, lipopolysaccharide-stimulated peripheral blood mononuclear cells from B. infantis 35624-treated healthy subjects demonstrated significantly reduced in vitro secretion of TNF-α and IL-6. The authors state that the reductions in inflammatory markers observed in this trial would be indicative of clinical remission and a lower risk of relapse but larger patient numbers would be necessary to demonstrate clinical efficacy.
Chronic kidney disease is associated with chronic inflammation and thus elevated levels of IL-6 and TNF-α.171 Endotoxin, from the outer membrane of gram negative bacteria, is both a source and indicator of inflammation in this disease.200,201 Furthermore, the gut microbiota of these patients is disrupted by dramatic reductions in commensal species, such as bifidobacteria and lactobacilli.202 In a RDBPCT, Wang et al.171 investigated the impact of a formulation on cytokine and endotoxin levels in peritoneal dialysis patients. The formulation contained B. longum A101, B. bifidum A218, B. catenulatum A302, and L. plantarum A87 and was administered to patients for 6 months. Patients in the intervention group displayed significantly reduced IL-6 (p < 0.001), TNF-α (p = 0.019), IL-5 (p = 0.002), and endotoxin (p = 0.007) by the end of treatment and significantly increased IL-10 levels (p = 0.027) compared with placebo. While the residual renal function significantly declined in the placebo group (p = 0.008) after 6 months, residual renal function was preserved in the intervention group after the same time period (p = 0.176). However, there were no significant differences between groups for clinical outcomes, such as cardiovascular events and peritonitis. This study was conducted on a small patient number (n = 39). Also, the lack of long-term follow-up is a limitation of the study given the chronic nature of the disease. Therefore, trials with larger sample sizes and longer-term follow-ups that focus on other parameters, such as cardiovascular events – given that CVDs are responsible for approximately 50% of all deaths at end-stage renal disease – are warranted to confirm the clinical usefulness of this formulation for chronic kidney disease patients.
In hemodialysis patients, a synbiotic formulation proved even more effective than the bacterial formulation alone for significantly decreasing pro-inflammatory markers (C-reactive protein and IL-6), endotoxin levels, and anti-heat shock protein 70 antibodies compared with the placebo following 12 weeks of intervention in a RDBPCT.172 Heat shock protein 70 protects cells from urea-induced damage.203 The synbiotics consisted of FOS, galactooligosaccharides (GOS), and inulin with B. longum LAF-5, B. lactis BIA 6, B. bifidum BIA 6, and L. acidophilus T16, while the bacterial formulation alone consisted of the same four strains. The authors state that the short timeline of the study (12 weeks) did limit the statistical power for detection of parameter changes in the two groups.
For further details of these trials, see Table 2.
Cardiovascular health
Blood lipid profiles
In a crossover RDBPCT, daily consumption of a fermented milk drink containing B. longum BB536 (known to have high bile salt hydrolase activity204) and L. acidophilus 145 by women with normal or moderately elevated cholesterol significantly decreased LDL-cholesterol (p = 0.014) in those with a baseline level of total cholesterol above 190 mg/dl.173 The authors suggest that this result may be due to the genotype of apolipoprotein E of participants, which is known to impact the response to dietary lipids. High-density lipoprotein (HDL) cholesterol was also significantly reduced (p < 0.01) by the BB536-containing fermented milk, which may increase the risk for CVDs, thus this formulation requires further testing and specific mechanisms of action need to be unraveled.
Type 2 diabetes
Type 2 diabetes is a known risk factor for CVD with patients presenting with dyslipidaemia along with insulin resistance. Increased levels of inflammation and oxidative stress have also been reported in type 2 diabetic patients.205 Asemi et al.174 presented the first trial investigating the impact of a bacterial formulation on metabolic profiles, high-sensitivity- (hs-) CRP, and oxidative stress in type 2 diabetic patients. The inclusion criteria included fasting plasma glucose ≥126 mg/dl, blood sugar (2 h postprandial) ≥200 mg/dl, and hemoglobin A1 C (HbA1 C) ≥6.5%. The multispecies mix consisted of seven different strains including B. longum, L. acidophilus, L. casei (now Lacticaseibacillus casei), L. rhamnosus, L. bulgaricus, B. breve, and S. thermophilus. After 8 weeks of supplementation, the formulation prevented an increase in fasting plasma glucose compared with the placebo (p = 0.01). It was also associated with significantly reduced serum hs-CRP (p = 0.02) and increased plasma levels of the antioxidant glutathione (p = 0.03). However, it had no impact on lipid profiles, serum insulin, uric acid, and total antioxidant capacity. It would be interesting to determine if higher doses of the formulation could impact these parameters.
For further details of these trials, see Table 2.
Depression, anxiety, and cognitive functioning
Anxiety and depression
According to the WHO, over 300 million people globally suffer from depression and an estimated 264 million people suffer from anxiety disorders.206 Existing pharmacological treatments tend to vary in terms of efficacy with many exerting only modest effects.207 An increasing number of studies are investigating the efficacy of beneficial bacteria as treatments for anxiety and depressive disorders.
The formulation B. longum R0175 and L. helveticus R0052 was shown to significantly reduce anxiety-like behavior in rats (p < 0.05) following 2 weeks of treatment based on a screening model for anti-anxiety agents.175 In the same study, the formulation was assessed for its psychological impact on normal volunteers following 30 d of administration in a RDBPCT. In humans, the bacterial formulation alleviated psychological distress with volunteers showing lower scores for somatization (p < 0.05), depression (p < 0.05), and anger-hostility as evaluated via the Hopkins Symptom Checklist (HSCL-90), and lower scores for the Hospital Anxiety and Depression Scale (HADS global score, p < 0.05; HADS anxiety score, p < 0.06). Test subjects also performed better at problem-solving as measured via the Coping Checklist CCL) (p < 0.05). While urinary cortisol levels remained stable in the placebo group, levels in the intervention group decreased over time (p < 0.05). However, the same formulation had no impact on participants with low mood in a RDBPCT following 8 weeks of treatment when used as a primary treatment.176 The formulation also had no impact on inflammatory biomarkers. The participants in this trial reported continuous low mood for at least 2 y, thus the authors suggest that the bacterial strains may be more effective for treating patients reporting shorter terms of low mood or less severe low mood, or that its use as a primary treatment may take longer than 8 weeks to exert its effects. In line with this, a more recent trial investigating the same strains in patients with major depressive disorder in receipt of antidepressant drugs for at least 3 months before the trial reported a significant decrease in the Beck Depression Inventory (BDI) score following 8 weeks of treatment compared with placebo (p = 0.042).177 The formulation was also associated with a significant decrease in the kynurenine/tryptophan ratio (p = 0.048) thus impacting serotonin levels by driving tryptophan along the serotonin pathway as opposed to its conversion to kynurenine.208 In a post hoc analysis of the same clinical trial, Kazemi et al.178 reported that the bacterial strains were associated with improved appetite in major depressive disorder patients, an important finding given that poor appetite and weight loss can be features of depression. However, the study has limitations including the fact that due to the lengthy recruitment phase, the trial was conducted at different times/seasons of the year, which could have an impact on depression.209 The patients were not all taking the same antidepressant drug. Other differences between groups, including lifestyle, diet, and vitamin D status, may have diluted the effect of the formulation. Thus, confirmatory trials without these limitations are needed. It would also be of interest to determine if the bacterial strains in the formulation exert pharmacological effects on the antidepressant drugs.
The bacterial strains B. longum BL04, L. fermentum LF16, L. rhamnosus LR06, and L. plantarum LP01 were reported to significantly improve mood and sleep quality, and reduce depressive mood state, anger, and fatigue in healthy volunteers in a RDBPCT.179 However, the trial consisted of 38 participants, thus larger scale trials are warranted to confirm these results. Furthermore, the results of the study are based on a self-reporting questionnaire, thus, as the authors suggest, future confirmatory trials would benefit from “categorical diagnostic tools” and clinical assessments of participants.
Patients suffering from IBS often report comorbidities such as depression, anxiety, and somatization, amongst others.210 In this respect, B. longum NCC3001 was assessed for its efficacy to treat depression and anxiety in IBS patients following 6 weeks of administration in a RDBPCT.180 The strain was previously shown to normalize anxiety-like behavior in a mouse model of chemical colitis via the vagal pathway and it reduced excitability of enteric neurons.211 It was also shown to normalize the Brain-Derived Neurotrophic Factor (BDNF) in mice with mild-to-moderate colonic inflammation.212 BDNF has been identified as a link in the gut-brain axis,213 and reduced expression has been associated with gut dysbiosis and the onset of anxiety-like behavior in germ-free animals.214 By week 6, 14/22 patients in the NCC3001 group exhibited reduced depression scores of at least 2 points on the HAD scale, compared with 7/22 patients in the placebo group (p = 0.04).180 At week 10, depression scores were still reduced in the intervention group. Quality of Life in the physical subdomain also significantly increased for the intervention group (p = 0.03) with patients reporting improvements in general physical health. Functional magnetic resonance imaging (fMRI) analysis revealed that the bacterial strain was associated with decreased responses to negative emotional stimuli in various brain areas including the amygdala and fronto-limbic regions compared with placebo. Despite the promising results, the authors suggest that clinician-administered rating scales may be superior to the HAD scale used in this study. Furthermore, the placebo group showed lower baseline depression scores than the test group although the authors confirmed that after adjusting for baseline differences, the statistically significant result in favor of NCC3001 remained, as it did when only a subset of patients with baseline scores indicating depression (HAD-D ≥ 8) was analyzed. Thus, strategically designed confirmatory trials without these limitations are warranted to verify the data.
Alzheimer’s disease
Alzheimer’s disease is a progressive disease of the brain and is believed to contribute to 60–70% of dementia cases worldwide.215 Disease pathology is associated with the presence of β-amyloid plaques in the brain.216 However, several other factors have also been linked to disease pathogenesis, including the gut microbiota.217,218 Physiological features identified in the blood of Alzheimer’s disease patients include reduced antioxidant capacity and increased levels of reactive oxygen species (ROS), as well as increased markers of inflammation.219 Based on these observations, Tamtaji et al.181 performed a RDBPCT investigating the impact of three bacterial strains and the trace element selenium on cognitive function and metabolic status of patients with Alzheimer’s disease. Selenium has been shown to attenuate the pathology of Alzheimer’s disease and protect against cognitive decline.220 The bacterial strains included B. longum, B. bifidum, and L. acidophilus and patients were administered selenium alone or selenium co-supplemented with the bacteria, or placebo for 12 weeks.181 The combination of strains and selenium proved most effective as it was associated with improved cognitive function with a significant increase in the mini-mental state examination score in patients receiving co-supplementation compared with those on selenium alone or placebo (p < 0.001). Co-supplementation was also associated with an improvement in some metabolic profiles including significantly reduced hs-CRP (p < 0.001) and significantly increased total antioxidant capacity (p < 0.001), significantly lower insulin levels (p < 0.001), significantly lower homeostasis model of assessment-insulin resistance (HOMA-IR) (p < 0.001) and increased quantitative insulin sensitivity check index (QUICKI) (p < 0.006); it was associated with significantly decreased serum triglycerides (p = 0.02) and significantly improved (p < 0.05) cholesterol profiles. These findings are particularly relevant given the link between insulin resistance and Alzheimer’s disease,221 and the potential role of high triglyceride levels in cognitive impairment.222 It would be interesting to determine if the bacterial formulation alone is capable of exerting similar effects to the co-supplemented product, particularly since selenium supplementation has recently been touted as a “good alternative” to relieve some symptoms of mild cognitive impairment and Alzheimer’s disease.223 For further details of these trials, see Table 2.
Respiratory illness
Seasonal allergy
In adults, B. longum BB536 has proven to be effective in the treatment of Japanese cedar pollinosis, an IgE-mediated type 1 allergy,182,184 which has been described as one of the most common allergic diseases in Japan.224 During 13 weeks of treatment with B. longum BB536 in subjects with a clinical history of Japanese cedar pollinosis (and during high pollen season), nine subjects out of 22 in the placebo group had to leave the trial prematurely to take prescribed medication as opposed to only 2 subjects out of 22 in the intervention group.182 Significant decreases in symptom scores for rhinorrhea (p = 0.0167) and nasal blockage (p = 0.0118) were recorded for the BB536 group compared with placebo and composite scores of the weekly scores for sneezing, rhinorrhea, nasal blockage, nasal itching, eye symptoms, and throat symptoms were significantly lower in the BB536 group (p = 0.0339). Pollen dispersion was associated with a Th2-skewed immune response. However, BB536 tended to suppress Japanese cedar pollinosis-specific IgE, and significantly suppressed elevations in plasma thymus- and activation-regulated chemokine (TARC) at weeks 4 (p = 0.038) and 8 (p = 0.031) compared with placebo. TARC has been used in several studies to measure disease severity in atopic dermatitis.225 In a follow-on study, fecal samples of the trial participants were investigated for changes to the gut microbiota.183 Subjects suffering from Japanese cedar pollinosis had increased levels of Bacteroides fragilis compared with healthy subjects, but this increase was suppressed by B. longum BB536 treatment. The same research group examined the impact of 4 weeks of BB536 intake on Japanese cedar pollen exposure in an environmental exposure unit (EEU) in a crossover RDBPCT.184 Ocular symptom scores were significantly reduced for the BB536 group compared with placebo during the 4 h of pollen exposure (p < 0.1), and scores for disruption of normal activities following exposure were also lower in the BB536 group. Furthermore, the BB536 group used significantly fewer total medications to alleviate symptoms compared with the placebo group (p = 0.041). However, in both trials, sample sizes were low with only 24 participants in the latter trial. Furthermore, in the latter trial, BB536 failed to impact other symptoms including nasal and throat symptoms. The authors suggest that differences in exposure patterns between the pollen season and the EEU could account for this where the latter involved a heavy and concentrated exposure for 4 h which may have been too aggressive for BB536 to alleviate symptoms. Thus, the EEU may not be a suitable model to verify treatment efficacy for seasonal allergies.
In adult participants who self-identified as having seasonal allergies, the bacterial formulation B. longum MM2, B. bifidum G9–1, and L. gasseri KS-13 was associated with a significant improvement in rhinoconjunctivitis-specific quality of life during allergy season when compared with the placebo group (p = 0.0092).185 Changes in the immune parameters tested (serum total IgE and Treg percentage), from baseline to week 6 were similar for both the intervention and placebo groups such that the mechanism of action remains unclear. The authors suggest that administration of the bacterial formulation before commencement of allergy season may have generated different results by allowing the gut microbiota and immune system time to respond to the intervention before allergen exposure. Further trials are warranted to determine if prophylactic administration of the formulation could improve its efficacy.
For sufferers of perennial allergic rhinitis, 4 weeks of consuming the formulation NVP-1703 composed of B. longum IM55 and L. plantarum IM76 significantly reduced total nasal symptom scores at weeks 1, 3, and 4.186 It also significantly reduced rhinorrhea at weeks 1, 3, and 4, and numerically, but not statistically, reduced sneezing and nasal congestion over the 4 weeks. In terms of immunity, NVP-1703 was associated with improved anti-allergic immunological profiles of participants by significantly increasing IL-10 levels (p = 0.047), and significantly increasing the ratios of IL-10/IL-14 (p = 0.046) and IL-10/IL-13 (p = 0.018). NVP-1703 was also associated with significantly reduced allergy-specific IgE levels (p = 0.033). The study also reported that NVP-1703 reduced urinary levels of prostaglandin F2α and leukotriene E4, though not significantly. These molecules are useful biomarkers of eosinophil and mast cell activation – involved in allergic inflammation. The participants in this study suffered mild-to-moderate persistent rhinitis, capable of enduring symptoms without medication. Thus, it would be worthwhile investigating the efficacy of NVP-1703 in patients with more severe allergy and determine if the formulation is as effective as or better than conventional treatments.
Common cold
In terms of the common cold, consumption of the strains B. longum SP 07/3, B. bifidum MF 20/5, and L. gasseri PA 16–8 by two different cohorts of people in a RDBPCT during two winter/spring periods for at least 3 months resulted in a shortening of common cold episodes by at least 2 days and reduced severity of symptoms compared with the placebo group (p = 0.045).187 Fourteen days after supplementation, the strains were associated with increased CD4+ T-helper cells and significantly increased cytotoxic plus T-suppressor cells (CD8+) compared with placebo (p = 0.035) in a randomly tested subset of participants. Both CD4+ and CD8+ are involved in cell-mediated immunity, thus, they destroy infected cells.
For further details of these trials, see Table 2.
Skin health
Reactive skin
A B. longum lysate (an ultrasound-inactivated suspension) applied to face, arm, and leg skin as a topical cream by healthy female volunteers with reactive skin for a 2-month intervention period was associated with a significant decrease in skin sensitivity by day 57 (p = 0.0024), whereby the skin exhibited increased resistance to physical and chemical aggression.188 Volunteers in the test group also reported a reduction in skin dryness after 29 d of applying the cream (p = 0.03). However, the lysate failed to improve skin barrier recovery rate. Based on an ex vivo human skin model, the lysate was found to decrease vasodilation, edema, mast cell degranulation, and TNF-α release, and in nerve cells, it reduced neuron reactivity and accessibility.
For further details of this trial, see Table 2.
B. longum effects in elderly
Gastrointestinal health and disease
Aging significantly impacts the composition and functionality of the gut microbiome,226 which has been attributed to several factors including lifestyle changes, ill health, and medication. A reduction in SCFA producers, including bifidobacteria, increased abundance of pathogens and facultative anaerobes, and a reduction in microbial diversity are recognized features of the elderly microbiome.227–229
Constipation
Constipation can be a common issue for elderly people living in nursing homes. To investigate the efficacy of Bifidobacterium to improve bowel movement regularity in nursing home residents, Pitkala et al.230 enrolled 209 subjects in a RDBPCT where residents were randomized to receive either a fermented oat drink with 1 × 109 CFU of two B. longum strains (46 and 2c) or the oat drink with B. lactis BB12 (1 × 109 CFU) or the placebo oat drink without viable bacteria, daily for 7 months. Residents receiving bacterial strains had significantly more frequent bowel movements (without causing diarrhea) than those in the placebo group. The administration of these strains on elderly nursing home residents suffering from constipation should help improve quality of life. However, there were several limitations within this study due to the challenging nature of the study population. Indeed, a number of participants refused to cooperate during study follow-up, and a number of participants died during the study. Of the initial 209 participants, 179 completed the study. Indeed, the authors state that randomized intervention studies on frail nursing home residents are rare as a consequence of such challenges.
The administration of B. longum BB536 to elderly patients receiving enteral feeding for 16 weeks significantly normalized defecation frequency in subjects with low and high-frequency defecation.231 However, the authors state that the effects were mild when compared with other therapies, such as laxatives and prokinetics. Also, the authors caution against extrapolating the outcome to individuals with severe and/or chronic gastrointestinal issues.
Immunity
As we age, organ function changes, a process referred to as senescence.239 Immunosenescence describes the immune dysfunction that occurs with aging240 and is associated with many factors, one of which is chronic inflammation.239 Indeed, pro-inflammatory cytokines have been shown to increase with aging.241,242 Given that the thymus degenerates with age and T cell production decreases, strategies that restore T cell production have been proposed as effective interventions for immunosenescence.239 The immune-modulating capabilities of bacteria, such as B. longum potentially render them ideal candidates to stimulate the immune system of older people. Thus, a number of RDBPCTs have investigated various commensal bacteria for their ability to beneficially modulate the immune response.
In the previous section on Constipation, the RDBPCT conducted by Pitkala et al.230 revealed that consumption of a fermented oat drink with B. longum strains 46 and 2c or B. lactis BB12 by elderly institutionalized residents significantly improved the frequency of bowel movements. A follow-on study investigated fecal bifidobacteria and two anti-inflammatory and one pro-inflammatory cytokine (TGF-β1, IL-10, TNF-α, respectively) in 55 of these subjects.232 While the study subjects were found to have relatively high levels of Bifidobacterium (1010 cells/g feces), consumption of the B. longum strains resulted in increases in B. adolescentis and B. catenulatum along with a modest increase in B. longum. The intervention did not significantly impact the serum cytokine levels, but significant associations were found between cytokine levels and the presence of specific Bifidobacterium species, which was observed for the two intervention groups (two B. longum strains 46 and 2c, or B. lactis BB12) and the placebo. Interestingly, lower levels of IL-10 were observed in the presence of members of the B. longum group, while the presence of B. breve correlated with higher levels of TGF-β1, although the changes were small, and the biological relevance of the results is uncertain.
Twelve weeks of supplementation with B. longum BB536 in hospitalized elderly patients receiving enteral tube feeding was associated with significantly increased fecal bifidobacteria.233 At weeks 4 and 16, serum IgA was increased in the intervention group compared with the placebo, though this was not significant. Interestingly, the significant decline in NK cell activity observed in the placebo group throughout the study was not observed in the BB536 group where NK cell activities were maintained at a stable level. Furthermore, the study found that a subgroup of participants with low initial NK cell activity particularly benefited from the bacterial strains, which increased NK cell activity from baseline throughout the study and was significantly different from the placebo at weeks 8 and 12. Other studies have reported that probiotics can improve innate immunity in participants with low NK cell activities.243–245 Furthermore, the BB536 group showed a tendency for increased number of bowel movements and lower body temperature.
In a RDBPC-crossover study involving healthy elderly subjects mainly recruited from the community, consumption of a synbiotic consisting of B. longum and the prebiotic Synergy 1 (FOS, inulin) for 4 weeks was associated with significantly increased fecal bifidobacteria counts (p < 0.0001), and Actinobacteria members (Atopobium group as well as bifidobacteria) (p = 0.0004) and Firmicutes (p < 0.0001) and significantly reduced Proteobacteria members (p < 0.0001).234 Butyrate production was also increased in the synbiotic group (p < 0.04). In terms of immunity, a number of cytokines were assessed, but of them all, the synbiotic only significantly impacted the levels of the pro-inflammatory cytokine TNF-α, which were reduced at weeks 2 (p = 0.02) and 4 (p = 0.0406). The synbiotic did not impact any of the measured clinical parameters including C-reactive protein, full blood counts, blood lipids, and levels of glucose, insulin, and immunoglobulins.
Three weeks of consuming a formulation consisting of B. longum MM2, B. bifidum G9–1, and L. gasseri KS-13 maintained CD4+ lymphocytes in elderly subjects and was associated with significantly increased IL-10 levels (p < 0.0001) compared with placebo, resulting in a less inflammatory cytokine profile in a crossover RDPCT.235 However, the crossover nature of the study was associated with limitations. First, during the second stage of the study, the time of year (start of cold and flu season) impacted the cytokine analysis as concentrations were highest at the final time point independent of the intervention or placebo. The washout period of 5 weeks was deemed too short since a carryover effect was observed for the cytokines in participants who had initially received the formulation but were then in the placebo group in period 2. The intervention was found to modify the fecal microbiota with test subjects having significantly increasing bifidobacteria (p < 0.05), lactic acid bacteria (p < 0.05), and decreasing E. coli levels (p < 0.05). In a more recent RBDPCT, consumption of the formulation B. longum Bar33 and L. helveticus Bar13 by elderly subjects for 30 d was associated with significantly improved immune function via an increase in naïve, activated memory, regulatory T cells, B cells, and NK cells and a decrease in memory T cells (p < 0.05) compared with the placebo.236 In the same study, the authors studied the two strains in mice and revealed that they significantly increased regulatory T cells while decreasing γδ T cells, and increased B cells compared with control mice. For further details of these trials, see Table 3.
Table 3.
An overview of clinical trials investigating the impact of B. longum on elderly.
| Condition/Disease/Biological Parameter | Participants; Age | Formulation | CFU; Dose; Duration | Clinical Effects and Biological Observations of Intervention Group Compared with Placebo Group | Reference; Trial ID |
|---|---|---|---|---|---|
|
Gastrointestinal Conditions | |||||
| Regularity of bowel movements in elderly nursing home residents | 209; Elderly | B. longum 46, B. longum 2c | 1 x 109; daily; 7 months | Significantly increased bowel movements without causing diarrhoea | Pitkala et al.230 |
| Defecation frequency in elderly patients receiving enteral feeding | Trial 1–83; Elderly Trial 2–123; Elderly |
B. longum BB536 | T1: 5 x 1010; daily; 16 weeks T2: 5 x 1010 or 2.5 x 1010; 16 weeks |
Significantly normalised defecation frequency in subjects with low & high frequency defecation | Kondo et al.231 |
| Immunity | |||||
| Faecal microbiota & Immune parameters | 55; > 84.3 ± 0.98 y |
B. longum 46, B. longum 2c |
1 x 109, daily; 7 months | Increased B. adolescentis & B. catenulatum No significant impact on immune parameters |
Ouwehand et al.232 |
| Faecal microbiota & Immune parameters | 45; ≥ 65 y receiving enteral tube feeding | B. longum BB536 | 5 x 1010; twice daily, 12 weeks; | Numerical increased IgA Maintained NK cell activity |
Akatsu et al.233 |
| Faecal microbiota & function, & Immune parameters | 43; 65–90 y |
B. longum Synergy 1 prebiotic (FOS & inulin) |
2 x 1011, 6 g; twice daily, 4 weeks; | Beneficially modulated the gut microbiota; Increased butyrate production; Significantly reduced TNF-α |
Macfarlane et al.234
NCT01226212 |
| Faecal microbiota & Immune parameters | 32; 70 ± 1 y |
B. longum MM2, B. bifidum G9–1, L. gasseri KS-13 |
3 x 109, daily; 3 weeks | Maintained CD4+ lymphocytes; Significantly increased IL-10; Significantly increased bifidobacteria, lactic acid bacteria, decreased E. coli |
Spaiser et al.235 NCT01662206 |
| Physiological status & Immune parameters | 98; 84 ± 7.8 y |
B. longum Bar33, L. helveticus Bar13 |
1 x 109, daily; 30 d | Significantly increased naïve, activated memory, regulatory T cells, B cells, & NK cells & decreased memory T cells | Finamore et al.236 |
| Mood and Cognition | |||||
| Healthy elderly/Cognitive function, Body composition, & Bowel habits | 38; 66–78 y |
B. longum ssp. longum BB536, B. longum ssp. infantis M-63, B. breve M-16V B. breve B-3 & resistance training |
1.25 x 1010 1.25 x 1010 1.25 x 1010 1.25 x 1010 daily; 12 weeks |
Improved cognitive functioning; Significantly decreased depression-anxiety scores; Significantly improved body mass index scores; Significantly increased frequency of defecation |
Inoue et al.237 UMIN000021749 |
| Healthy elderly/Cognition, Mood, & Intestinal health | 63; ≥ 65 y |
B. longum BORI, B. bifidum BGN4 |
1 x 109 daily; 12 weeks |
Significantly reduced inflammation-associated gut bacteria; Significantly increased serum levels of BDNF; Significantly improved mental attention & executive function; Significantly reduced stress |
Kim et al.238 KCT0003929 |
Mood and cognition
Cognitive aging in older adults has been described as following three different developmental patterns: successful aging, normal aging, and cognitive aging.246 During successful aging, cognitive function remains relatively stable. Normal aging is associated with a slight decline in cognitive functioning, while cognitive aging is defined by a steady decline of cognitive ability. Subjective cognitive decline, described as a “self-experienced decline in cognitive ability” has been proposed as the first notable indicator of preclinical Alzheimer’s disease.247 In a recent epidemiological study involving 16 cohorts of people from 15 countries (39,387 cognitively unimpaired individuals >60 y of age), subjective cognitive decline was estimated to affect approximately a quarter of the individuals.248 Thus, strategies that aid in the management of cognitive decline in elderly subjects could play a significant role in improving quality of life and reducing the risks for dementia and Alzheimer’s disease.
In healthy elderly subjects, a Bifidobacterium formulation, containing two B. longum strains (B. longum ssp. longum BB536, B. longum ssp. infantis M-63) and two B. breve strains (B. breve M-16 V, B. breve B-3) combined with moderate resistance training improved cognitive functioning as measured via the Japanese version of the Montreal Cognitive assessment instrument.237 The strains were associated with a significant decrease in depression-anxiety scores compared with the placebo that included resistance training (p = 0.012), based on self-reporting questionnaires. The strains were also associated with improved body mass index scores (p < 0.001) and increased frequency of defecation (p = 0.023). However, the authors state that the 12-week intervention period was relatively short and represents a limitation of this study. In addition, the sample size of n = 38 limited the statistical power.
In the first well-controlled, multicentre RDBPCT, Kim et al.238 investigated the impact of the strains B. longum BORI and B. bifidum BGN4 on healthy, community-dwelling older adults in terms of cognition, mood, and intestinal health. Twelve weeks of supplementation significantly reduced the abundance of inflammation-associated bacteria including Eubacterium, Clostridiales and Prevotellaceae. The genus Allisonella was also significantly reduced, which is known to produce the biogenic amine histamine that can provoke inflammation.249 The formulation also significantly increased serum levels of BDNF compared with the placebo (p < 0.05) and this was negatively correlated with strain-associated shifts in Eubacterium and Clostridiales. Furthermore, the experimental group exhibited significantly improved mental attention and executive function (p < 0.05) and reduced stress (p < 0.05). However, the 12-week intervention period is potentially too short – a longer period may reveal changes in some of the cognitive functions for which no significant improvements were noted. The study also lacks direct evidence of improvement in peripheral and cerebral inflammation following consumption of the formulation, thus, further mechanistic studies are required. While neuropsychological assessments were performed by a professionally trained panel, self-reporting was used by participants to assess mood status, which carries the risk of recall bias. Thus, further studies are required to confirm these results. For further details of these trials, see Table 3.
Conclusion
Sixty-four clinical trials have been included in this review with participants ranging from infants to elderly (Figure 1, Tables 1–3). Twenty trials investigated the efficacy of B. longum alone, while the remaining 44 trials investigated B. longum with other bacterial strains, and/or prebiotics. Significantly, many of the diseases investigated are classified as NCDs, e.g., CVD, diabetes, IBD, or mental health issues. According to the NCD Alliance, the ‘catastrophic expenses’ due to NCD treatment threaten to push 100 million people into poverty each year250. Thus, NCDs are recognized as a significant challenge by the 2030 Agenda for Sustainable Development Goals,251 and a commitment has been made to reduce a third of premature mortality from NCDs through prevention and treatment.251 The trials presented suggest that B. longum administration alone or in combination with other bacterial species and prebiotics may have the potential to reduce severity of or prevent certain diseases, including NCDs, in early life, across adulthood and into old age.
Figure 1.
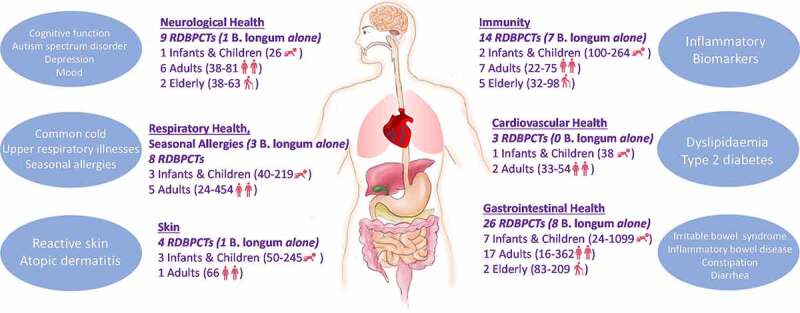
Overview of 64 clinical trials conducted with B. longum alone or with other species, and prebiotics. Trials have been categorised according to region/organ of body under investigation. Total number of trials conducted in each category is provided, as well as number of trials conducted in infants and children, adults, and the elderly. The lowest and highest numbers of participants in these trials are included. The most common trial endpoints are provided for each category.
However, the trials presented in this review are not without their limitations and in this respect, confirmatory trials are warranted before results can be extrapolated to the appropriate population groups. Many of the trials used insufficient sample sizes, thus diminishing power to detect clinically/biologically significant effects, or randomization was inadequate with prognostic factors unequally distributed across placebo or intervention groups resulting in the over/under estimation of intervention effects. Several authors reported that longer intervention times may have yielded more clinical/biological effects from intervention, thus adequate consideration should be given to the trial duration. Trials that used self-reporting assessments would also benefit from definitive diagnostic tools. Furthermore, crossover trials should be avoided if possible unless the precise washout time is known. But they can also be limited by the fact that they are often performed over different seasons of the year, which can impact host immunity or depression, for example. Thus, these limitations should be avoided. In the future, greater standardization across clinical trials performed with B. longum strains alone and in formulations could help provide stronger rationale for more widespread use in the treatment and prevention of disease. This will require greater collaboration between research groups, design of gold-standard, standardized clinical studies that address the same endpoints and biomarkers of health and disease with a focus on dosage and duration, notwithstanding standardized procedures for measuring gut microbiota and metabolome changes to help identify causal mechanisms. However, the evidence to date suggests that continued research and investment into the beneficial properties of B. longum is worthwhile given that this species could serve to significantly improve several aspects of human health from birth and beyond.
Funding Statement
This work was supported by Science Foundation Ireland under grant number 12RC2273-P2.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Sender R, Fuchs S, Milo R.. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan I, Bai Y, Zha L, Ullah N, Ullah H, Shah SRH, Sun H, Zhang C. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front Cell Infect Microbiol. 2021;11:716299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J Innate Immun. 2018;10:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 2014;73:477–489. [DOI] [PubMed] [Google Scholar]
- 6.Cryan JF, O’ Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 7.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. [DOI] [PubMed] [Google Scholar]
- 8.De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut-skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranowski JR, Claud EC. Necrotizing enterocolitis and the preterm infant microbiome In: Internet. In: Guandalini S, Indrio F, editors. Probiotics and Child Gastrointestinal Health. Cham: Springer International Publishing;2019. [cited 2022 May 16]. 25–36.Available from. https://link.springer.com/10.1007/5584_2018_313 [Google Scholar]
- 10.Duchon J, Barbian ME, Denning PW. Necrotizing enterocolitis. Clin Perinatol. 2021;48:229–250. [DOI] [PubMed] [Google Scholar]
- 11.Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascal M, Perez-Gordo M, Caballero T, Escribese MM, Lopez Longo MN, Luengo O, Manso L, Matheu V, Seoane E, Zamorano M, et al. Microbiome and allergic diseases. Front Immunol. 2018;9:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, et al. Diversity of bifidobacteria within the infant gut microbiota. PloS One. 2012;7:e36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arboleya S, Watkins C, Stanton C, Ross RP. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Yu Z, Zhao J, Zhang H, Zhai Q, Chen W. Colonization and probiotic function of Bifidobacterium longum. J Funct Foods. 2019;53:157–165. [Google Scholar]
- 16.FAO/WHO. Food and Agriculture Organization and World Health Organization Expert Consultation . Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. [Internet]. Available from: http://www.fao.org/tempref/docrep/fao/meeting/009/y6398e.pdf
- 17.Finlay BB. CIFAR Humans, the Microbiome. Are noncommunicable diseases communicable? Science. 2020;367:250–251. [DOI] [PubMed] [Google Scholar]
- 18.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 19.Kelly WJ, Cookson AL, Altermann E, Lambie SC, Perry R, Teh KH, Otter DE, Shapiro N, Woyke T, Leahy SC. Genomic analysis of three Bifidobacterium species isolated from the calf gastrointestinal tract. Sci Rep. 2016;6:30768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattarelli P, Bonaparte C, Pot B, Biavati B. Proposal to reclassify the three biotypes of Bifidobacterium longum as three subspecies: bifidobacterium longum subsp. longum subsp. nov., Bifidobacterium longum subsp. infantis comb. nov. and Bifidobacterium longum subsp. Suis Comb Nov Int J Syst Evol Microbiol. 2008;58:767–772. [DOI] [PubMed] [Google Scholar]
- 21.Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105:18964–18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J-Z, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odamaki T, Bottacini F, Kato K, Mitsuyama E, Yoshida K, Horigome A, Xiao J-Z, van Sinderen D. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci Rep. 2018;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaplin AV, Brzhozovskii AG, Parfenova TV, Kafarskaia LI, Volodin NN, Shkoporov AN, Ilina EN, Efimov BA. Species diversity of bifidobacteria in the intestinal microbiota studied using MALDI-TOF Mass-Spectrometry. Vestn Ross Akad Med Nauk. 2015;435–440. [PubMed] [Google Scholar]
- 26.Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turroni F, Foroni E, Pizzetti P, Giubellini V, Ribbera A, Merusi P, Cagnasso P, Bizzarri B, De’angelis GL, Shanahan F, et al. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl Environ Microbiol. 2009;75:1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PloS One. 2010;5:e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claesson MJ, Cusack S, O’ Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. 2021;18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Yang C, Yu L, Tian F, Wu Y, Zhao J, Zhang H, Yang R, Chen W, Hill C, et al. Human gut-derived B. longum subsp. longum strains protect against aging in a d-galactose-induced aging mouse model. Microbiome. 2021;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arboleya S, Bottacini F, O’ Connell-Motherway M, Ryan CA, Ross RP, van Sinderen D, Stanton C. Gene-trait matching across the Bifidobacterium longum pan-genome reveals considerable diversity in carbohydrate catabolism among human infant strains. BMC Genomics. 2018;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido D, Ruiz-Moyano S, Kirmiz N, Davis JC, Totten SM, Lemay DG, Ugalde JA, German JB, Lebrilla CB, Mills DA. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. Longum SC596 Sci Rep. 2016;6:35045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly SM, Munoz-Munoz J, van Sinderen D. Plant glycan metabolism by bifidobacteria. Front Microbiol. 2021;12:609418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matijašić B B, Obermajer T, Lipoglavšek L, Sernel T, Locatelli I, Kos M, Šmid A, Rogelj I. Effects of synbiotic fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double-blind, placebo-controlled trial. J Dairy Sci. 2016;99:5008–5021. [DOI] [PubMed] [Google Scholar]
- 36.Costa GN, Marcelino-Guimarães FC, Vilas-Bôas GT, Matsuo T, Miglioranza LHS. Potential fate of ingested Lactobacillus plantarum and its occurrence in human feces. Appl Environ Microbiol. 2014;80:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oki K, Akiyama T, Matsuda K, Gawad A, Makino H, Ishikawa E, Oishi K, Kushiro A, Fujimoto J. Long-term colonization exceeding six years from early infancy of Bifidobacterium longum subsp. longum in human gut. BMC Microbiol. 2018;18:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milani C, Lugli GA, Duranti S, Turroni F, Bottacini F, Mangifesta M, Sanchez B, Viappiani A, Mancabelli L, Taminiau B, et al. Genomic encyclopedia of type strains of the genus Bifidobacterium. Appl Environ Microbiol. 2014;80:6290–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. [DOI] [PubMed] [Google Scholar]
- 40.Chichlowski M, Shah N, Wampler JL, Wu SS, Vanderhoof JA. Bifidobacterium longum subspecies infantis (B. infantis) in pediatric nutrition: current state of knowledge. Nutrients. 2020;12:1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Z-T Y, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;23:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henrick BM, Hutton AA, Palumbo MC, Casaburi G, Mitchell RD, Underwood MA, Smilowitz JT, Frese SA. Elevated fecal pH Indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century. mSphere. 2018;3:18–e00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915–923. [DOI] [PubMed] [Google Scholar]
- 45.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol-Gastrointest Liver Physiol. 2012;302:G1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. [DOI] [PubMed] [Google Scholar]
- 48.Bozzo L, Puyal J, Chatton J-Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PloS One. 2013;8:e71721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrlich AM, Pacheco AR, Henrick BM, Taft D, Xu G, Huda MN, Mishchuk D, Goodson ML, Slupsky C, Barile D, et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020;20:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng D, Sommella E, Salviati E, Campiglia P, Ganguli K, Djebali K, Zhu W, Walker WA. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res. 2020;88:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang W, Cho KY, Meng D, Walker WA. The impact of indole-3-lactic acid on immature intestinal innate immunity and development: a transcriptomic analysis. Sci Rep. 2021;11:8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pyclik M, Srutkova D, Schwarzer M, Górska S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins – their chemical structure and biological attributes. Int J Biol Macromol. 2020;147:333–349. [DOI] [PubMed] [Google Scholar]
- 53.López P, Monteserín DC, Gueimonde M, de Los Reyes-Gavilán CG, Margolles A, Suárez A, Ruas-Madiedo P. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res Int. 2012;46:99–107. [Google Scholar]
- 54.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, O’ Connell-Motherway M, Shanahan F, Nally K, Dougan G, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci. 2012;109:2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan S, Yang B, Zhao J, Zhao J, Stanton C, Ross RP, Zhang H, Chen W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019;10:1595–1608. [DOI] [PubMed] [Google Scholar]
- 56.Ivanov D, Emonet C, Foata F, Affolter M, Delley M, Fisseha M, Blum-Sperisen S, Kochhar S, Arigoni F. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J Biol Chem. 2006;281:17246–17252. [DOI] [PubMed] [Google Scholar]
- 57.Turroni F, Foroni E, O’ Connell-Motherway M, Bottacini F, Giubellini V, Zomer A, Ferrarini A, Delledonne M, Zhang Z, van Sinderen D, et al. Characterization of the serpin-encoding gene of Bifidobacterium breve 210b. Appl Environ Microbiol. 2010;76:3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarville JL, Dong J, Caminero A, Bermudez-Brito M, Jury J, Murray JA, Duboux S, Steinmann M, Delley M, Tangyu M, et al. A commensal Bifidobacterium longum strain prevents gluten-related immunopathology in mice through expression of a serine protease inhibitor. Appl Environ Microbiol. 2017;83:17–e01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mkaouar H, Mariaule V, Rhimi S, Hernandez J, Kriaa A, Jablaoui A, Akermi N, Maguin E, Lesner A, Korkmaz B, et al. Gut serpinome: emerging evidence in ibd. Int J Mol Sci. 2021;22:6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Chen H, Ding J, Stanton C, Ross RP, Zhao J, Zhang H, Yang B, Chen W. Bifidobacterium longum ameliorates dextran sulfate sodium-induced colitis by producing conjugated linoleic acid, protecting intestinal mechanical barrier, restoring unbalanced gut microbiota, and regulating the toll-like receptor-4/nuclear factor-κb signaling pathway. J Agric Food Chem. 2021;69:14593–14608. [DOI] [PubMed] [Google Scholar]
- 61.Healy DB, Ryan CA, Ross RP, Stanton C, Dempsey EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. 2022;7:22–33. [DOI] [PubMed] [Google Scholar]
- 62.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’ Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meister AL, Doheny KK, Travagli RA. Necrotizing enterocolitis: it’s not all in the gut. Exp Biol Med Maywood NJ. 2020;245:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMurtry VE, Gupta RW, Tran L, Blanchard EE, Penn D, Taylor CM, Ferris MJ. Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet Lond Engl. 2016;387:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torrazza RM, Ukhanova M, Wang X, Sharma R, Hudak ML, Neu J, Mai V. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PloS One. 2013;8:e83304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Y, Du W, Xiao S, Zeng B, She X, Liu D, Du H, Li L, Li F, Ai Q, et al. Colonization of fecal microbiota from patients with neonatal necrotizing enterocolitis exacerbates intestinal injury in germfree mice subjected to necrotizing enterocolitis-induction protocol via alterations in butyrate and regulatory T cells. J Transl Med. 2021;19:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuna A, Yu W, Menden HL, Feng L, Srinivasan P, Chavez-Bueno S, Ahmed I, Umar S, Sampath V. NEC-like intestinal injury is ameliorated by Lactobacillus rhamnosus GG in parallel with SIGIRR and A20 induction in neonatal mice. Pediatr Res. 2020;88:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy K, Ross RP, Ryan CA, Dempsey EM, Stanton C. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Front Nutr. 2021;8:667188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang K, Zhang X, Lv A, Fan S, Zhang J. Saccharomyces boulardii modulates necrotizing enterocolitis in neonatal mice by regulating the sirtuin 1/NF‑κB pathway and the intestinal microbiota. Mol Med Rep. 2020;22:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, Morley CJ, Garland SM, ProPrems Study Group . Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. 2013;132:1055–1062. [DOI] [PubMed] [Google Scholar]
- 73.Plummer EL, Bulach DM, Murray GL, Jacobs SE, Tabrizi SN, Garland SM, ProPrems Study Group . Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiol. 2018;18:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shashidhar A, Suman Rao PN, Nesargi S, Bhat S, Chandrakala BS. Probiotics for promoting feed tolerance in very low birth weight neonates - a randomized controlled trial. Indian Pediatr. 2017;54:363–367. [DOI] [PubMed] [Google Scholar]
- 75.Hoang DM, Levy EI, Vandenplas Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021;110:60–67. [DOI] [PubMed] [Google Scholar]
- 76.Feldkamp ML, Carey JC, Sadler TW. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet A. 2007;143A:639–652. [DOI] [PubMed] [Google Scholar]
- 77.Powell WT, Borghese RA, Kalanetra KM, Mirmiran M, Mills DA, Underwood MA. Probiotic administration in infants with gastroschisis: a pilot randomized placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2016;62:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris EL, Minutillo C, Hart S, Warner TM, Ravikumara M, Nathan EA, Dickinson JE. The long term physical consequences of gastroschisis. J Pediatr Surg. 2014;49:1466–1470. [DOI] [PubMed] [Google Scholar]
- 79.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73:32–40. [DOI] [PubMed] [Google Scholar]
- 80.Ugboko HU, Nwinyi OC, Oranusi SU, Oyewale JO. Childhood diarrhoeal diseases in developing countries. Heliyon. 2020;6:e03690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim AH, Hogarty MP, Harris VC, Baldridge MT. The complex interactions between rotavirus and the gut microbiota. Front Cell Infect Microbiol. 2020;10:586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muñoz JAM, Chenoll E, Casinos B, Bataller E, Ramón D, Genovés S, Montava R, Ribes JM, Buesa J, Fàbrega J, et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl Environ Microbiol. 2011;77:8775–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Escribano J, Ferré N, Gispert-Llaurado M, Luque V, Rubio-Torrents C, Zaragoza-Jordana M, Polanco I, Codoñer FM, Chenoll E, Morera M, et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: a randomized controlled trial. Pediatr Res. 2018;83:1120–1128. [DOI] [PubMed] [Google Scholar]
- 84.Park MS, Kwon B, Ku S, Ji GE. The efficacy of Bifidobacterium longum BORI and Lactobacillus acidophilus AD031 probiotic treatment in infants with rotavirus infection. Nutrients. 2017;9:E887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adriani A, Ribaldone DG, Astegiano M, Durazzo M, Saracco GM, Pellicano R. Irritable bowel syndrome: the clinical approach. Panminerva Med InternetAvailable from 2018;60 [[cited 2022 May 16]]. https://www.minervamedica.it/index2.php?show=R41Y2018N04A0213. [DOI] [PubMed] [Google Scholar]
- 86.Giannetti E, Maglione M, Alessandrella A, Strisciuglio C, De Giovanni D, Campanozzi A, Miele E, Staiano A. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J Clin Gastroenterol. 2017;51:e5–10. [DOI] [PubMed] [Google Scholar]
- 87.Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc. 2019;94:1357–1373. [DOI] [PubMed] [Google Scholar]
- 88.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. [DOI] [PubMed] [Google Scholar]
- 89.Wu B, Yang Y, Xu X, Wang W-P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr WJP. 2016;12:177–182. [DOI] [PubMed] [Google Scholar]
- 90.Angurana SK, Bansal A, Singhi S, Aggarwal R, Jayashree M, Salaria M, Mangat NK. Evaluation of effect of probiotics on cytokine levels in critically ill children with severe sepsis: a double-blind, placebo-controlled trial. Crit Care Med. 2018;46:1656–1664. [DOI] [PubMed] [Google Scholar]
- 91.Guardamagna O, Amaretti A, Puddu PE, Raimondi S, Abello F, Cagliero P, Rossi M. Bifidobacteria supplementation: effects on plasma lipid profiles in dyslipidemic children. Nutr Burbank Los Angel Cty Calif. 2014;30:831–836. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Li N, Yang J-J, Zhao D-M, Chen B, Zhang G-Q, Chen S, Cao R-F, Yu H, Zhao C-Y, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020;157:104784. [DOI] [PubMed] [Google Scholar]
- 93.Cazzola M, Pham-Thi N, Kerihuel J-C, Durand H, Bohbot S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: a randomized, double-blind, placebo-controlled pilot study. Ther Adv Respir Dis. 2010;4:271–278. [DOI] [PubMed] [Google Scholar]
- 94.Lau A-Y, Yanagisawa N, Hor Y-Y, Lew L-C, Ong J-S, Chuah L-O, Lee Y-Y, Choi S-B, Rashid F, Wahid N, et al. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Benef Microbes. 2018;9:61–70. [DOI] [PubMed] [Google Scholar]
- 95.Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130:1355–1360. [DOI] [PubMed] [Google Scholar]
- 97.Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YPM, Wong HB, Pai N, Lee BW, Shek L-C. Probiotic supplementation in the first 6 months of life in at risk Asian infants–effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2009;39:571–578. [DOI] [PubMed] [Google Scholar]
- 98.Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, Ruzafa-Costas B, Genovés-Martínez S, Chenoll-Cuadros E, Carrión-Gutiérrez M, Horga de la Parte J, Prieto-Merino D, Codoñer-Cortés FM. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MdAk A, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int. 2018;2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jameson SC. Maintaining the norm: t-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. [DOI] [PubMed] [Google Scholar]
- 101.O’ Neill I, Schofield Z, Hall LJ. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg Top Life Sci. 2017;1:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sykes L, MacIntyre DA, Yap XJ, Ponnampalam S, Teoh TG, Bennett PR. Changes in the Th1: th2 cytokine bias in pregnancy and the effects of the anti-inflammatory cyclopentenone prostaglandin 15-deoxy-Δ(12,14)-prostaglandin J2. Mediators Inflamm. 2012;2012:416739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berger A. Science commentary: th1 and Th2 responses: what are they? BMJ. 2000;321:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abrahamsson TR, Sandberg Abelius M, Forsberg A, Björkstén B, Jenmalm MC. A Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization: th1/Th2-associated chemokine imbalance. Clin Exp Allergy. 2011;41:1729–1739. [DOI] [PubMed] [Google Scholar]
- 105.Pérez N, Iannicelli JC, Girard-Bosch C, González S, Varea A, Disalvo L, Apezteguia M, Pernas J, Vicentin D, Cravero R. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur J Nutr. 2010;49:173–179. [DOI] [PubMed] [Google Scholar]
- 106.Youngster I, Kozer E, Lazarovitch Z, Broide E, Goldman M. Probiotics and the immunological response to infant vaccinations: a prospective, placebo controlled pilot study. Arch Dis Child. 2011;96:345–349. [DOI] [PubMed] [Google Scholar]
- 107.WHO. Cardiovascular diseases [Internet]. 2022. Available from: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
- 108.Cholesterol Treatment Trialists’ Collaboration CTT), Fulcher J, O’ Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet Lond Engl. 2015;385: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 109.Finegold JA, Manisty CH, Goldacre B, Barron AJ, Francis DP. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol. 2014;21:464–474. [DOI] [PubMed] [Google Scholar]
- 110.Wilson DP Is atherosclerosis a pediatric disease? [Internet]. In: Comprehensive Free Endocrinology Book. 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395576
- 111.Pac-Kozuchowska E, Krawiec P, Grywalska E. Selected risk factors for atherosclerosis in children and their parents with positive family history of premature cardiovascular diseases: a prospective study. BMC Pediatr. 2018;18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents . National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(5):S213–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martino F, Puddu PE, Pannarale G, Colantoni C, Zanoni C, Martino E, Barillà F. Arterial blood pressure and serum lipids in a population of children and adolescents from Southern Italy: the Calabrian Sierras Community Study (CSCS). Int J Cardiol. 2013;168:1108–1114. [DOI] [PubMed] [Google Scholar]
- 114.Tomaro-Duchesneau C, Jones ML, Shah D, Jain P, Saha S, Prakash S. Cholesterol assimilation by Lactobacillus probiotic bacteria: an in vitro investigation. Biomed Res Int. 2014;2014:380316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S, Rossi M. Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol. 2013;97:8273–8281. [DOI] [PubMed] [Google Scholar]
- 116.Yang B, Chen H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen W. Review of the roles of conjugated linoleic acid in health and disease. J Funct Foods. 2015;15:314–325. [Google Scholar]
- 117.Kwiterovich PO. Recognition and Management of Dyslipidemia in Children and Adolescents. J Clin Endocrinol Metab. 2008;93:4200–4209. [DOI] [PubMed] [Google Scholar]
- 118.Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, La Fata G, Miani M, Patterson E, Pot B, Shortt C, et al. The microbiota–gut–brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol Life Sci. 2022;79:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barrett E, Ross RP, O’ Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. [DOI] [PubMed] [Google Scholar]
- 120.Cao Y-N, Feng L-J, Wang B-M, Jiang K, Li S, Xu X, Wang W-Q, Zhao J-W, Wang Y-M. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J Gastroenterol. 2018;24:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res off J Int Soc Autism Res. 2012;5:160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Department of Health . Estimating prevalence of Autism Spectrum Disorders (ASD) in the Irish population: a review of data sources and epidemiological studies [Internet]. 2018. Available from: https://assets.gov.ie/10707/ce1ca48714424c0ba4bb4c0ae2e510b2.pdf
- 125.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. [DOI] [PubMed] [Google Scholar]
- 126.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lambert GW, Eisenhofer G, Cox HS, Horne M, Kalff V, Kelly M, Jennings GL, Esler MD. Direct determination of homovanillic acid release from the human brain, an indicator of central dopaminergic activity. Life Sci. 1991;49:1061–1072. [DOI] [PubMed] [Google Scholar]
- 128.Thomas M, Bomar PA. Upper respiratory tract infection [Internet]. In: StatPearls [Internet]. Treasure Island (FL). 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532961/ [PubMed]
- 129.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. [DOI] [PubMed] [Google Scholar]
- 130.Fitz-Gibbon S, Tomida S, Chiu B-H, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pécastaings S, Roques C, Nocera T, Peraud C, Mengeaud V, Khammari A, Dréno B. Characterisation of Cutibacterium acnes phylotypes in acne and in vivo exploratory evaluation of Myrtacine®. J Eur Acad Dermatol Venereol JEADV. 2018;32 Suppl 2:15–23. [DOI] [PubMed] [Google Scholar]
- 132.NISC Comparative Sequence Program, Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22: 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun Z, Huang S, Zhu P, Yue F, Zhao H, Yang M, Niu Y, Jing G, Su X, Li H, et al. A microbiome-based index for assessing skin health and treatment effects for atopic dermatitis in children. mSystems. 2019;4:19–e00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Sullivan JN, Rea MC, Hill C, Ross RP. Protecting the outside: biological tools to manipulate the skin microbiota. FEMS Microbiol Ecol. 2020;96:fiaa085. [DOI] [PubMed] [Google Scholar]
- 135.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. [DOI] [PubMed] [Google Scholar]
- 137.Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee E, Lee S-Y, Kang M-J, Kim K, Won S, Kim B-J, Choi KY, Kim B-S, Cho H-J, Kim Y, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol off Publ Am Coll Allergy Asthma Immunol. 2016;117:91–92.e1. [DOI] [PubMed] [Google Scholar]
- 139.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song H, Yoo Y, Hwang J, Na Y-C, Kim HS. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852–860. [DOI] [PubMed] [Google Scholar]
- 141.Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, Kumemura M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–591. [DOI] [PubMed] [Google Scholar]
- 142.Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, Beyer K, Burks W, Canonica GW, Ebisawa M, et al. World allergy organization-McMaster University guidelines for allergic disease prevention (GLAD-P): probiotics. World Allergy Organ J. 2015;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elias PM. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann Allergy Asthma Immunol off Publ Am Coll Allergy Asthma Immunol. 2022;128:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paller A, Jaworski JC, Simpson EL, Boguniewicz M, Russell JJ, Block JK, Tofte S, Dunn JD, Feldman SR, Clark AR, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821–838. [DOI] [PubMed] [Google Scholar]
- 145.Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102:178–184. [DOI] [PubMed] [Google Scholar]
- 146.Rodríguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr Bethesda Md. 2014;5:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr. 2009;49:349–354. [DOI] [PubMed] [Google Scholar]
- 148.Simpson MR, Avershina E, Storrø O, Johnsen R, Rudi K, Øien T. Breastfeeding-associated microbiota in human milk following supplementation with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12. J Dairy Sci. 2018;101:889–899. [DOI] [PubMed] [Google Scholar]
- 149.Kordy K, Gaufin T, Mwangi M, Li F, Cerini C, Lee DJ, Adisetiyo H, Woodward C, Pannaraj PS, Tobin NH, et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PloS One. 2020;15:e0219633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salli K, Hirvonen J, Siitonen J, Ahonen I, Anglenius H, Maukonen J. Selective utilization of the human milk oligosaccharides 2’-fucosyllactose, 3-fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria. J Agric Food Chem. 2021;69:170–182. [DOI] [PubMed] [Google Scholar]
- 151.Lynch WD, Hsu R. Ulcerative colitis [Internet. StatPearls. Treasure Island (FL): StatPearls Publishing;2022. [cited 2022 May 16]. Available from. http://www.ncbi.nlm.nih.gov/books/NBK459282/ [Google Scholar]
- 152.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’ Neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, Cummings JH, Macfarlane S. Clinical trial: the microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther. 2010;32:872–883. [DOI] [PubMed] [Google Scholar]
- 154.Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, Kusaka T, Uose S, Hisatsune H, Tojo M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc off J Jpn Gastroenterol Endosc Soc. 2016;28:67–74. [DOI] [PubMed] [Google Scholar]
- 155.Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit Int Med J Exp Clin Res. 2004;10:I126–131. [PubMed] [Google Scholar]
- 156.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol off Clin Pract J Am Gastroenterol Assoc. 2009;7:1202–9, 1209.e1. [DOI] [PubMed] [Google Scholar]
- 158.Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, Enns R, Bitton A, Chiba N, Paré P, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastroenterol Hepatol off Clin Pract J Am Gastroenterol Assoc. 2015;13:928–935.e2. [DOI] [PubMed] [Google Scholar]
- 159.Ki Cha B, Mun Jung S, Hwan Choi C, Song I-D, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung W-S, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. [DOI] [PubMed] [Google Scholar]
- 160.Bonfrate L, Di Palo DM, Celano G, Albert A, Vitellio P, De Angelis M, Gobbetti M, Portincasa P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur J Clin Invest [Internet]. [cited 2022 May 16]; 2020; 50. https://onlinelibrary.wiley.com/doi/10.1111/eci.13201. Available from [DOI] [PubMed] [Google Scholar]
- 161.Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kosikowski W, Szczerbiński M, Gantzel J, Cukrowska B. The effectiveness of synbiotic preparation containing Lactobacillus and Bifidobacterium probiotic strains and short chain fructooligosaccharides in patients with diarrhea predominant irritable bowel syndrome-a randomized double-blind, placebo-controlled study. Nutrients. 2020;12:E1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lewis ED, Antony JM, Crowley DC, Piano A, Bhardwaj R, Tompkins TA, Evans M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in alleviating symptoms of irritable bowel syndrome (IBS): a randomized, placebo-controlled study. Nutrients. 2020;12:E1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’ Mahony L, Kiely B, Shanahan F, Quigley EMM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. [DOI] [PubMed] [Google Scholar]
- 164.O’ Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’ Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. [DOI] [PubMed] [Google Scholar]
- 165.Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr Edinb Scotl. 2014;33:761–767. [DOI] [PubMed] [Google Scholar]
- 166.Martoni CJ, Evans M, Chow CE, Chan LS, Leyer G. Impact of a probiotic product on bowel habits and microbial profile in participants with functional constipation: a randomized controlled trial. J Dig Dis. 2019;20:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vitellio P, Celano G, Bonfrate L, Gobbetti M, Portincasa P, De Angelis M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: a randomised, double-blind, cross-over study. Nutrients. 2019;11:E886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res N Y N. 2008;28:1–5. [DOI] [PubMed] [Google Scholar]
- 169.Hütt P, Andreson H, Kullisaar T, Vihalemm T, Unt E, Kals J, Kampus P, Zilmer M, Mikelsaar M. Effects of a synbiotic product on blood antioxidative activity in subjects colonized with Helicobacter pylori. Lett Appl Microbiol. 2009;48:797–800. [DOI] [PubMed] [Google Scholar]
- 170.Groeger D, O’ Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EMM. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang I-K, Y-Y W, Yang Y-F, Ting I-W, Lin C-C, Yen T-H, Chen J-H, Wang C-H, Huang C-C, Lin H-C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2015;6:423–430. [DOI] [PubMed] [Google Scholar]
- 172.Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effects of synbiotics and probiotics supplementation on serum levels of endotoxin, heat shock protein 70 antibodies and inflammatory markers in hemodialysis patients: a randomized double-blinded controlled trial. Probiotics Antimicrob Proteins. 2020;12:144–151. [DOI] [PubMed] [Google Scholar]
- 173.Andrade S, Borges N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J Dairy Res. 2009;76:469–474. [DOI] [PubMed] [Google Scholar]
- 174.Asemi Z, Zare Z, Shakeri H, Sabihi S, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1–9. [DOI] [PubMed] [Google Scholar]
- 175.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson J-F, Rougeot C, Pichelin M, Cazaubiel M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. [DOI] [PubMed] [Google Scholar]
- 176.Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51:810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38:522–528. [DOI] [PubMed] [Google Scholar]
- 178.Kazemi A, Noorbala AA, Djafarian K. Effect of probiotic and prebiotic versus placebo on appetite in patients with major depressive disorder: post hoc analysis of a randomised clinical trial. J Hum Nutr Diet. 2020;33:56–65. [DOI] [PubMed] [Google Scholar]
- 179.Marotta A, Sarno E, Del Casale A, Pane M, Mogna L, Amoruso A, Felis GE, Fiorio M. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psychiatry. 2019;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin F-P, Cominetti O, Welsh C, Rieder A, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153:448–459.e8. [DOI] [PubMed] [Google Scholar]
- 181.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M, Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr Edinb Scotl. 2019;38:2569–2575. [DOI] [PubMed] [Google Scholar]
- 182.Xiao J-Z, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, Miyaji K, Iwatsuki K, Togashi H, Enomoto K, et al. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2006;36:1425–1435. [DOI] [PubMed] [Google Scholar]
- 183.Odamaki T, Xiao J-Z, Iwabuchi N, Sakamoto M, Takahashi N, Kondo S, Miyaji K, Iwatsuki K, Togashi H, Enomoto T, et al. Influence of Bifidobacterium longum BB536 intake on faecal microbiota in individuals with Japanese cedar pollinosis during the pollen season. J Med Microbiol. 2007;56:1301–1308. [DOI] [PubMed] [Google Scholar]
- 184.Xiao J, Kondo S, Yanagisawa N, Miyaji K, Enomoto K, Sakoda T, Iwatsuki K, Enomoto T. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int off J Jpn Soc Allergol. 2007;56:67–75. [DOI] [PubMed] [Google Scholar]
- 185.Dennis-Wall JC, Culpepper T, Nieves C, Rowe CC, Burns AM, Rusch CT, Federico A, Ukhanova M, Waugh S, Mai V, et al. Probiotics (Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2) improve rhinoconjunctivitis-specific quality of life in individuals with seasonal allergies: a double-blind, placebo-controlled, randomized trial. Am J Clin Nutr. 2017;105:758–767. [DOI] [PubMed] [Google Scholar]
- 186.Kang M-G, Han S-W, Kang H-R, Hong S-J, Kim D-H, Choi J-H. Probiotic NVP-1703 alleviates allergic rhinitis by inducing IL-10 Expression: a four-week clinical trial. Nutrients. 2020;12:E1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr Edinb Scotl. 2005;24:481–491. [DOI] [PubMed] [Google Scholar]
- 188.Guéniche A, Bastien P, Ovigne JM, Kermici M, Courchay G, Chevalier V, Breton L, Castiel-Higounenc I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp Dermatol. 2010;19:e1–8. [DOI] [PubMed] [Google Scholar]
- 189.Yeh H-W, Chien W-C, Chung C-H, J-M H, Tzeng N-S. Risk of psychiatric disorders in irritable bowel syndrome-A nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. [DOI] [PubMed] [Google Scholar]
- 190.Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis1. Neurogastroenterol Motil. 2005;17:332–340. [DOI] [PubMed] [Google Scholar]
- 191.Visich KL, Yeo TP. The prophylactic use of probiotics in the prevention of radiation therapy-induced diarrhea. Clin J Oncol Nurs. 2010;14:467–473. [DOI] [PubMed] [Google Scholar]
- 192.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 193.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jiang T, Mustapha A, Savaiano DA. Improvement of lactose digestion in humans by ingestion of unfermented milk containing Bifidobacterium longum. J Dairy Sci. 1996;79:750–757. [DOI] [PubMed] [Google Scholar]
- 195.Labanski A, Langhorst J, Engler H, Elsenbruch S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: a transdisciplinary challenge. Psychoneuroendocrinology. 2020;111:104501. [DOI] [PubMed] [Google Scholar]
- 196.Jain U, Saxena K, Chauhan N. Helicobacter pylori induced reactive oxygen Species: a new and developing platform for detection. Helicobacter [Internet]. [cited 2022 Jun 21]; 2021; 26. https://onlinelibrary.wiley.com/doi/10.1111/hel.12796. Available from [DOI] [PubMed] [Google Scholar]
- 197.Qadri Q, Rasool R, Gulzar GM, Naqash S, Shah ZA. H. pylori infection, inflammation and gastric cancer. J Gastrointest Cancer. 2014;45:126–132. [DOI] [PubMed] [Google Scholar]
- 198.Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006;100:1324–1332. [DOI] [PubMed] [Google Scholar]
- 199.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hauser AB, Stinghen AEM, Gonçalves SM, Bucharles S, Pecoits-Filho R. A gut feeling on endotoxemia: causes and consequences in chronic kidney disease. Nephron Clin Pract. 2011;118:c165–172. discussion c172. [DOI] [PubMed] [Google Scholar]
- 201.Sun PP, Perianayagam MC, Jaber BL. Endotoxin-binding affinity of sevelamer: a potential novel anti-inflammatory mechanism. Kidney Int Suppl. 2009;76:S20–25. [DOI] [PubMed] [Google Scholar]
- 202.Wang I-K, Lai H-C, C-J Y, Liang C-C, Chang C-T, Kuo H-L, Yang Y-F, Lin C-C, Lin H-H, Liu Y-L, et al. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol. 2012;78:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lebherz-Eichinger D, Krenn CG, Roth GA. Keratin 18 and heat-shock protein in chronic kidney disease. Adv Clin Chem. 2013;62:123–149. [DOI] [PubMed] [Google Scholar]
- 204.Grill J, Schneider F, Crociani J, Ballongue J. Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum bb536. Appl Environ Microbiol. 1995;61:2577–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 206.WHO . Depression and other common mental disorders: global health estimates. Geneva: World Health Organization; 2017. [Google Scholar]
- 207.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet. 2018;391:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oxenkrug G. Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets. 2013;14:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wirz-Justice A, Ajdacic V, Rössler W, Steinhausen H-C, Angst J. Prevalence of seasonal depression in a prospective cohort study. Eur Arch Psychiatry Clin Neurosci. 2019;269:833–839. [DOI] [PubMed] [Google Scholar]
- 210.Shiha MG, Aziz I. Review article: physical and psychological comorbidities associated with irritable bowel syndrome. Aliment Pharmacol Ther [Internet]. [cited 2022 Jun 27]; 2021; 54. https://onlinelibrary.wiley.com/doi/10.1111/apt.16589. Available from [DOI] [PubMed] [Google Scholar]
- 211.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. [DOI] [PubMed] [Google Scholar]
- 213.Maqsood R, Stone TW. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41:2819–2835. [DOI] [PubMed] [Google Scholar]
- 214.Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.WHO. Dementia [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
- 216.Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–318. [DOI] [PubMed] [Google Scholar]
- 217.Guo L, Xu J, Du Y, Wu W, Nie W, Zhang D, Luo Y, Lu H, Lei M, Xiao S, et al. Effects of gut microbiota and probiotics on Alzheimer’s disease. Transl Neurosci. 2021;12:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15. [DOI] [PubMed] [Google Scholar]
- 219.Gubandru M, Margina D, Tsitsimpikou C, Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM, Kouretas D. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem Toxicol. 2013;61:209–214. [DOI] [PubMed] [Google Scholar]
- 220.Aaseth J, Skalny AV, Roos PM, Alexander J, Aschner M, Tinkov AA. Copper, iron, selenium and lipo-glycemic dysmetabolism in Alzheimer’s disease. Int J Mol Sci. 2021;22:9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wei Z, Koya J, Reznik SE. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Front Neurosci. 2021;15:687157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dimache AM, Șalaru DL, Sascău R, Stătescu C. The role of high triglycerides level in predicting cognitive impairment: a review of current evidence. Nutrients. 2021;13:2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pereira ME, Souza JV, Galiciolli MEA, Sare F, Vieira GS, Kruk IL, Oliveira CS. Effects of Selenium supplementation in patients with mild cognitive impairment or alzheimer’s disease: a systematic review and meta-analysis. Nutrients. 2022;14:3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Enomoto T. Reevaluation of hygiene theory. Arerugi Allergy. 2004;53:465–468. [PubMed] [Google Scholar]
- 225.Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, Guttman-Yassky E. Biomarkers in atopic dermatitis—a review on behalf of the International Eczema Council. J Allergy Clin Immunol. 2021;147:1174–1190.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Salazar N, González S, Nogacka AM, Rios-Covián D, Arboleya S, Gueimonde M, de L RGC. Microbiome: effects of ageing and diet. Curr Issues Mol Biol. 2020;36:33–62. [DOI] [PubMed] [Google Scholar]
- 227.Biagi E, Rampelli S, Turroni S, Quercia S, Candela M, Brigidi P. The gut microbiota of centenarians: signatures of longevity in the gut microbiota profile. Mech Ageing Dev. 2017;165:180–184. [DOI] [PubMed] [Google Scholar]
- 228.Salazar N, López P, Valdés L, Margolles A, Suárez A, Patterson ÁM, Cuervo A, de Los RGC, Ruas-Madiedo P, Gonzalez S, et al. Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J Am Coll Nutr. 2013;32:399–406. [DOI] [PubMed] [Google Scholar]
- 229.Salazar N, Arboleya S, Valdã©s L, Stanton C, Ross P, Ruiz L, Gueimonde M, de Los Reyes-Gavilã¡n Cg. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front Genet [Internet] Available from 2014;5 [[cited 2022 May 16]]. http://journal.frontiersin.org/article/10.3389/fgene.2014.00406/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pitkala KH, Strandberg TE, Finne Soveri UH, Ouwehand AC, Poussa T, Salminen S. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J Nutr Health Aging. 2007;11:305–311. [PubMed] [Google Scholar]
- 231.Kondo J. Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World J Gastroenterol. 2013;19:2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ouwehand AC, Bergsma N, Parhiala R, Lahtinen S, Gueimonde M, Finne-Soveri H, Strandberg T, Pitkälä K, Salminen S. Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol Med Microbiol. 2008;53:18–25. [DOI] [PubMed] [Google Scholar]
- 233.Akatsu H, Iwabuchi N, Xiao J, Matsuyama Z, Kurihara R, Okuda K, Yamamoto T, Maruyama M. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J Parenter Enter Nutr. 2013;37:631–640. [DOI] [PubMed] [Google Scholar]
- 234.Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther. 2013;38:804–816. [DOI] [PubMed] [Google Scholar]
- 235.Spaiser SJ, Culpepper T, Nieves C, Ukhanova M, Mai V, Percival SS, Christman MC, Langkamp-Henken B. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J Am Coll Nutr. 2015;34:459–469. [DOI] [PubMed] [Google Scholar]
- 236.Finamore A, Roselli M, Donini L, Brasili DE, Rami R, Carnevali P, Mistura L, Pinto A, Giusti A, Mengheri E. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutr Burbank Los Angel Cty Calif. 2019;63–64:184–192. [DOI] [PubMed] [Google Scholar]
- 237.Inoue T, Kobayashi Y, Mori N, Sakagawa M, Xiao J-Z, Moritani T, Sakane N, Nagai N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9:843–853. [DOI] [PubMed] [Google Scholar]
- 238.Kim C-S, Cha L, Sim M, Jung S, Chun WY, Baik HW, Shin D-M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol Ser A. 2021;76:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: a key player in cancer development. J Hematol OncolJ Hematol Oncol. 2020;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Walford RL. The immunologic theory of aging. The Gerontologist. 1964;4:195–197. [DOI] [PubMed] [Google Scholar]
- 241.Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. [DOI] [PubMed] [Google Scholar]
- 242.Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. [DOI] [PubMed] [Google Scholar]
- 243.Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-Cell activity. J Nutr. 2007;137:791S–793S. [DOI] [PubMed] [Google Scholar]
- 244.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. [DOI] [PubMed] [Google Scholar]
- 245.Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12 ® and Lactobacillus paracasei ssp. paracasei, L. casei 431 ® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107:876–884. [DOI] [PubMed] [Google Scholar]
- 246.Cai L, Chan JSY, Yan JH, Peng K. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci [Internet] Available from 2014;6 [[cited 2022 Sep 6]]. http://journal.frontiersin.org/article/10.3389/fnagi.2014.00031/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-benarous F, Hampel H, Kochan NA, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non–alzheimer’s disease dementia. Alzheimers Dement. 2019;15:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.for Cohort Studies of Memory in an International Consortium, Cosmic) RS, Pabst A, Riedel-Heller SG, Jessen F, Turana Y, Handajani YS, Brayne C, Matthews FE, Stephan BCM, et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res Ther. 2020;12: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.NCD Alliance . The financial burden of NCDs [Internet]. 2017. Available from: https://ncdalliance.org/why-ncds/the-financial-burden-of-ncds#:~:text=In%20total%20the%20five%20leading,US%24%202%20trillion%20per%20year
- 251.WHO. Noncommunicable diseases [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases


