Abstract
In nonsulfur purple bacteria, redox homeostasis is achieved by the coordinate control of various oxidation-reduction balancing mechanisms during phototrophic anaerobic respiration. In this study, the ability of Rhodobacter capsulatus to maintain a balanced intracellular oxidation-reduction potential was considered; in addition, interrelationships between the control of known redox-balancing systems, the Calvin-Benson-Bassham, dinitrogenase and dimethyl sulfoxide reductase systems, were probed in strains grown under both photoheterotrophic and photoautotrophic growth conditions. By using cbbI (cbb form I operon)-, cbbII-, nifH-, and dorC-reporter gene fusions, it was demonstrated that each redox-balancing system responds to specific metabolic circumstances under phototrophic growth conditions. In specific mutant strains of R. capsulatus, expression of both the Calvin-Benson-Bassham and dinitrogenase systems was influenced by dimethyl sulfoxide respiration. Under photoheterotrophic growth conditions, coordinate control of redox-balancing systems was further manifested in ribulose 1,5-bisphosphate carboxylase/oxygenase and phosphoribulokinase deletion strains. These findings demonstrated the existence of interactive control mechanisms that govern the diverse means by which R. capsulatus maintains redox poise during photoheterotrophic and photoautotrophic growth.
Rhodobacter capsulatus is a nonsulfur purple photosynthetic bacterium that exhibits diverse respiratory abilities, allowing this organism to grow under a variety of environmental conditions. Branched respiratory electron transport pathways allow R. capsulatus to grow aerobically in the dark, either chemoautotrophically or chemoheterotrophically, by using O2 as the terminal electron acceptor. Indeed, its high capacity for aerobic chemoautotrophic growth distinguishes it from other well-studied nonsulfur purple bacteria, such as R. sphaeroides and Rhodospirillum rubrum (26). Like other organisms of this group, R. capsulatus can also grow anaerobically in the light, either photoautotrophically or photoheterotrophically, using cyclic photosynthetic electron transport to generate a proton motive force. These organisms can grow fermentatively as well. Due to such metabolic versatility, R. capsulatus provides an excellent system with which to gain insight into the control of redox homeostasis. Yet, compared to the thorough and well-characterized redox control studies of Escherichia coli (for a review, see reference 17 or 51 and references therein), knowledge of the control of redox homeostasis in R. capsulatus is somewhat limited.
During phototrophic growth, various electron acceptors are employed, in a hierarchical manner, to maintain a balanced redox state in R. capsulatus (50). In the presence of organic carbon under light anaerobic growth conditions (photoheterotrophic growth), the redox-balancing mechanism(s) consists primarily of the Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway (CBB system). Under some growth conditions, the dinitrogenase enzyme complex (dinitrogenase system), the dimethyl sulfoxide (DMSO) reductase (DMSOR) system, or other systems yet to be identified or implicated in redox control are employed. Specific reactions of the CBB pathway allow CO2 to function as a sink for excess reducing equivalents generated by the metabolism of carbon substrates such as l-malate and succinate. Thus, the predominant role of the CBB pathway during photoheterotrophic growth is to balance the oxidation-reduction potential of the cell (13, 24, 55). The capacity for CO2-dependent growth under photoautotrophic growth conditions is accomplished primarily by the CBB system, where the chief role of the CBB pathway is to provide the cell with carbon via the assimilation of CO2. The duality of roles of the CBB system leads to an interplay between the maintenance of redox poise and the control of carbon metabolism under photoheterotrophic and photoautotrophic growth conditions. The dinitrogenase system is synthesized in most phototrophs when the organism is placed in an ammonia-free environment. This system enables Rhodobacter to grow under conditions in which dinitrogen is the sole source of nitrogen (N2-dependent growth); i.e., the cells catalyze the reduction and assimilation of atmospheric dinitrogen to ammonia, accompanied by the reduction of protons to molecular hydrogen. The process of dinitrogen fixation requires much reducing power and is an energy-intensive process (5). Not only does the dinitrogenase system play a primary role in nitrogen metabolism (23), but it has also been shown to be involved in redox homeostasis in Rhodobacter and Rhodospirillum rubrum (22, 50). Photoheterotrophic growth with a poor nitrogen source such as glutamate signals the cell to synthesize the dinitrogenase system (for a review, see reference 25 and references therein). Under such growth conditions, the excess reducing equivalents generated by the oxidation of carbon substrates, such as malate, are consumed by the reduction of protons and consequent evolution of molecular hydrogen by a hydrogenase-like activity of the dinitrogenase system. This allows the cell to balance its intracellular redox potential (20). Physiological studies have shown that a link between carbon metabolism and nitrogen metabolism exists that is intimately associated with the control of intracellular redox poise in R. capsulatus (50), R. sphaeroides (22, 43), and R. rubrum (22). Specifically, in the absence of a functional CBB system (achieved through the inactivation of genes encoding key and unique enzymes of the CBB pathway), spontaneous variants of strains with photoheterotrophic competency (PHC) and CBB deficiency dissipate excess reducing equivalents as H2 gas by derepressing the dinitrogenase system (22, 50). Respiration of the auxiliary oxidant DMSO or trimethylamine-N-oxide (TMAO) through the DMSOR system has also been shown to play an important role in the maintenance of redox poise during phototrophic growth of R. capsulatus (27, 44). Indeed, DMSO respiration allows growth of CBB-deficient strains of R. sphaeroides (11, 18, 19, 55) and R. capsulatus (40, 50) under photoheterotrophic growth conditions in the presence of a fixed nitrogen source. Thus, the reduction of DMSO or TMAO serves as an additional mechanism by which to dissipate excess reducing equivalents generated by carbon metabolism.
In this study, reporter-gene promoter fusions were employed to examine the expression of the CBB, dinitrogenase, and DMSOR systems in response to different environmental and metabolic signals. CBB-deficient strains and a dinitrogenase-derepressing strain of R. capsulatus were used in these studies. Contributions of the different redox systems to photoautotrophic carbon metabolism and the interaction with nitrogen metabolism were explored, and the results of these studies reflect on the overall control of redox homeostasis in R. capsulatus.
MATERIALS AND METHODS
Bacterial strains and reporter gene fusion plasmids.
The genotypes and phenotypes of the R. capsulatus strains and reporter-gene fusion plasmids utilized in this study are summarized in Tables 1 and 2. CBB-deficient strains SBI/II and SBP and subsequent spontaneous variants derived from them, strains RCP and SBP-PHC, respectively, were previously described, and their growth potential in various media has been reported (37, 40, 50). Strain SBI/II lacks form I and form II ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) due to the introduction, respectively, of a spectinomycin resistance cartridge in cbbL and a kanamycin resistance cartridge in cbbM, while strain SBP lacks phosphoribulokinase due to the introduction of the Ω-spectinomycin cassette into cbbP. In order to achieve photoheterotrophic growth comparable to that of wild-type strain SB1003, an exogenous electron acceptor, such as DMSO, must be provided during photoheterotrophic growth with a fixed nitrogen source (ammonia). R. capsulatus strains that have acquired PHC in the absence of external electron acceptors like DMSO and can use ammonia as a fixed nitrogen source include strains RCP and SBP-PHC. Strain RCP (R. capsulatus photoheterotrophically competent) is a spontaneous variant of strain SBI/II that maintains the cbbLS cbbM phenotype, while strain SBP-PHC is a spontaneous variant of strain SBP that maintains the cbbP phenotype. The redox-balancing mechanism that allows photoheterotrophic competency in the absence of a functional CBB cycle remains to be established in strain RCP, while derepression of the dinitrogenase enzyme complex has been shown to be essential to allow strain SBP-PHC to maintain photoheterotrophic competency (50).
TABLE 1.
Bacterial strains and plasmids utilized in this study
| Strain or plasmid | Genotype and phenotypea or relevant characteristic(s) | Reference(s) |
|---|---|---|
| R. capsulatus | ||
| SB1003 | cbbLS+cbbM+cbbP+ PHa+ PHg+ RubisCO+ PRK+ | 58 |
| SBI/II | cbbLS cbbM cbbP+ PHa− PHg+ RubisCO− PRK+ | 40 |
| RCP | cbbLS cbbM cbbP+ PHa+ PHg+ RubisCO− PRK+ | 37, 50 |
| SBP | cbbLS+cbbM+cbbP PHa+/− PHg+ RubisCO− PRK− | 40 |
| SBP-PHC | cbbLS+cbbM+cbbP PHa+ PHg+ RubisCO− PRK−; derepresses nitrogenase system | 37, 50 |
| Plasmids | ||
| pRK2013 | Helper plasmid for triparental conjugation | 14 |
| PXLB | Translational fusion; cbbI::lacZ; Tetr | 40 |
| PXFB | Translational fusion; cbbII::lacZ; Tetr | 40 |
| pALS53 | Translational fusion; dorC::lacZ; Tetr | A. Shaw and A. G. McEwan unpublished data |
| pHU316 | Translational fusion; nifH::lacZ; Tetr | 21 |
| pVKD1 | R. sphaeroides cbbI translational promoter fusion to lacZ; Tetr | 6 |
PHa, photoheterotrophic growth with ammonia as the nitrogen source; PHg, photoheterotrophic growth with glutamate as the nitrogen source; PHa−, lack of photoheterotrophic growth with ammonia as the nitrogen source and requirement of an alternative electron acceptor (DMSO); PHa+/−, gradual photoheterotrophic growth (barely grows; slow doubling time compared to wild type).
TABLE 2.
Redox-balancing systems for photoheterotrophic growth conditions in strains of R. capsulatus
| Strain | Redox-balancing system under following growth conditiona
|
|||
|---|---|---|---|---|
| M-A | M-A-D | M-G | M-G-D | |
| SB1003 | CBBb | CBB; DMSORc | CBB; Nitrogenased | CBB; nitrogenase; DMSOR |
| SBI/II | No growthe | DMSOR | Nitrogenase | Nitrogenase; DMSOR |
| RCP | Unknownf | Unknown; DMSOR | Unknown; Nitrogenase | Unknown; nitrogenase; DMSOR |
| SBP | Poor growth | DMSOR | Nitrogenase | Nitrogenase; DMSOR |
| SBP-PHC | Nitrogenase | Nitrogenase; DMSOR | Nitrogenase | Nitrogenase; DMSOR |
M-A, malate-ammonia; M-A-D, malate-ammonia-DMSO; M-G, malate-glutamate; M-G-D, malate-glutamate-DMSO.
CBB, CBB system.
DMSOR, DMSOR system.
Nitrogenase, dinitrogenase enzyme complex.
No growth in the absence of an exogenous oxidant, such as DMSO.
Unknown, redox-balancing mechanism remains to be established.
Media and growth conditions.
E. coli strain JM109 (57) containing reporter gene fusion plasmids was grown aerobically on LB medium (1) at 37°C with appropriate antibiotic selection. Photoheterotrophic cultures of R. capsulatus were grown anaerobically in Ormerod's medium (36) supplemented with 0.4% dl-malate, as required, and 1 μg of thiamine/ml. The nitrogen source was provided as either 30 mM ammonia or 6.8 mM l-glutamate. Photoheterotrophic and photoautotrophic growth of all cultures was monitored by measuring the optical density at 600 nm (OD660) of cultures with a Beckman spectrophotometer. The concentrations of antibiotics used for selection of the R. capsulatus strains were as follows: rifampin, 50 μg/ml; kanamycin, 5 μg/ml; spectinomycin, 10 μg/ml; tetracycline, 3 μg/ml for stock cultures or 0.5 μg/ml for plasmid maintenance under phototrophic growth conditions. For E. coli, the antibiotic concentrations for plasmid maintenance were as follows: kanamycin, 20 μg/ml; tetracycline, 6 μg/ml. DMSO and TMAO were each utilized at a concentration of 30 mM.
Conjugation techniques.
E. coli strain JM107 (57), containing mobilizable helper plasmid pRK2013 (14), was used in triparental matings in order to independently conjugate the respective reporter gene fusion plasmids into recipient R. capsulatus strains. Donor and recipient cultures were grown to a high turbidity (late log phase) in peptone-yeast extract (PYE) medium (56) containing appropriate antibiotics and washed three times with PYE medium prior to mating in order to eliminate any interference from the presence of antibiotics. Following conjugation, exconjugants were grown at 30°C on PYE agar plates containing rifampin and tetracycline and (if appropriate) spectinomycin and/or kanamycin as counterselective agents against E. coli; tetracycline was provided for plasmid maintenance.
Cell extracts and enzyme assays.
Culture samples (10 to 20 ml) were harvested in late log phase (OD660 = 0.9 to 1.2), washed in buffer (25 mM Tris-Cl, 1 mM EDTA [pH 8.0]), and disrupted by sonication. The resultant cell debris was removed by centrifugation at 18,000 × g for 15 min at 4°C. β-Galactosidase activity was measured as previously reported (40); the production of o-nitrophenol (31) was continuously measured over a time period of 10 min by monitoring the increase in A405 on a Spectronic GENESYS 2 spectrophotometer. Specific activities were calculated by using the change in steady-state A405 per minute and the extinction coefficient for o-nitrophenol of 3.1 × 103 cm2/mmol (54). Levels of activity were checked for cells taken at different stages of growth, with no significant change in the enzyme activity patterns noted. However, to ensure reproducibility of all comparisons, all samples were taken from cultures at close to the same OD660 and it was ensured that this never exceeded 1.2. Protein concentrations were determined by the Bio-Rad protein assay dye binding reagent (Bio-Rad Laboratories, Hercules, Calif.) using bovine serum albumin as the standard.
RESULTS
Interplay of the CBB, dinitrogenase, and DMSOR systems under photoheterotrophic growth conditions.
Various systems are employed by R. capsulatus to balance the intracellular oxidation-reduction potential when the organism is exposed to specific environmental conditions (Table 2). During photoheterotrophic growth on carbon substrates such as l-malate, the CBB system is expressed to allow CO2, produced as a result of the oxidation of the organic substrate, to function as an electron sink for the excess reducing equivalents generated during metabolism. The dinitrogenase system, in conjunction with the CBB cycle, serves as a redox-balancing tool during photoheterotrophic growth with a poor nitrogen source (such as glutamate); growth with ammonia represses the synthesis of this system. Furthermore, the DMSOR system contributes to redox poise under phototrophic growth conditions in the presence of the auxiliary oxidants DMSO and TMAO. No additional mechanism(s) employed to remove reducing equivalents is known. Specific reporter gene promoter fusions were used to monitor the expression of the CBB, dinitrogenase, and DMSOR systems in CBB-deficient strains and photoheterotrophically competent CBB-deficient strains (which included a dinitrogenase-derepressing strain) of R. capsulatus (Table 2). The responses of the selected redox-balancing systems to different environmental and metabolic signals under photoheterotrophic growth conditions were established by using each of these strains.
The CBB system.
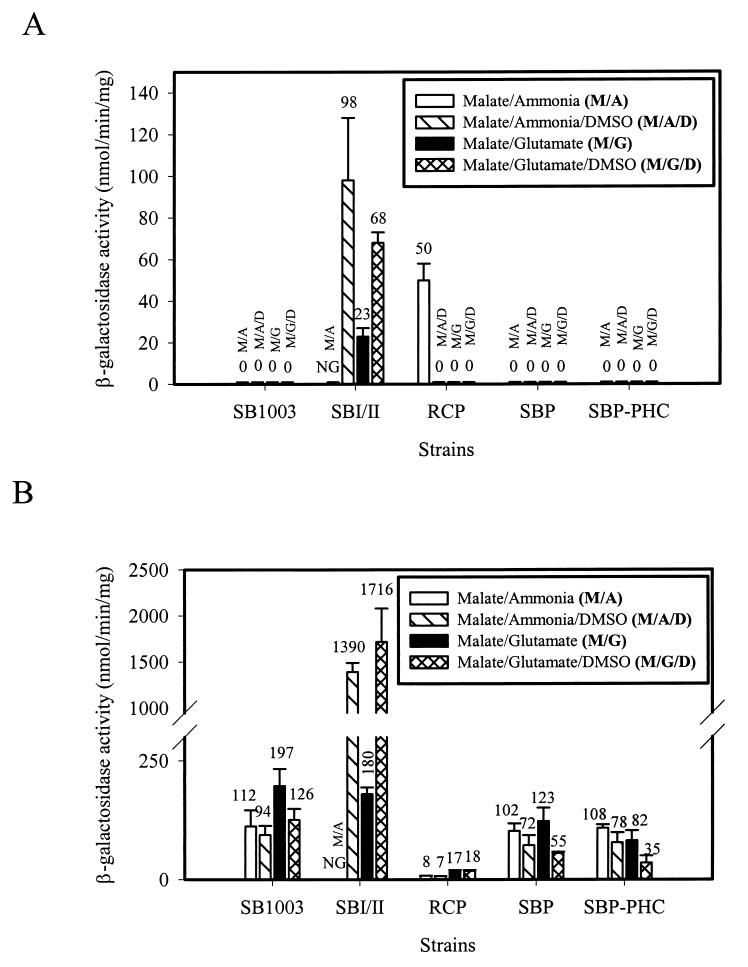
In R. capsulatus, form I RubisCO is not synthesized during photoheterotrophic growth with malate as the carbon source and ammonia as the nitrogen source (39, 48). However, form I RubisCO synthesis is observed during growth with reduced carbon substrates, such as butyrate, or during photoautotrophic (1.5% CO2–98.5% H2) growth conditions (16, 39, 48). Consistent with the established regulation of cbbI (the cbb form I operon), wild-type strain SB1003 did not exhibit cbbI promoter activity under photoheterotrophic growth conditions with ammonia as the fixed nitrogen source in the absence or presence of the exogenous electron acceptor DMSO (Fig. 1A). In addition, no cbbI promoter activity was detected during growth with glutamate (which also serves as an ancillary carbon source to l-malate) in the absence or presence of DMSO (Fig. 1A). Thus, normal regulation of the cbbI promoter was maintained in wild-type strain SB1003 regardless of the nitrogen source (ammonia or glutamate) or the presence or absence of an auxiliary oxidant (DMSO). Additionally, strains SBP and SBP-PHC exhibited wild-type regulation of the cbbI promoter for all of the photoheterotrophic growth conditions tested (Fig. 1A). By contrast, strains SBI/II and RCP showed definitive cbbI promoter activity during photoheterotrophic growth (Fig. 1A). cbbI promoter activity was observed in strain SBI/II with either ammonia or glutamate as the nitrogen source in the presence of DMSO or in the absence of DMSO when glutamate was used as the nitrogen source. Strain RCP exhibited cbbI promoter activity only during growth with ammonia as the nitrogen source in the absence of DMSO (Fig. 1A). Interestingly, strain SBI/II exhibited threefold higher cbbI promoter activity during photoheterotrophic growth in the presence of glutamate when supplemented with DMSO (Fig. 1A); strain SBI/II does not grow in the absence of DMSO with ammonia as the nitrogen source due to the lack of an expressed redox-balancing mechanism (40, 50).
FIG. 1.
cbbI::lacZ (A) and cbbII::lacZ (B) promoter activities during photoheterotrophic growth of R. capsulatus CBB-deficient strains. β-Galactosidase activities were determined in three or four independent cultures assayed in duplicate. NG indicates no growth under photoheterotrophic growth conditions with ammonia as the nitrogen source in the absence of DMSO.
In contrast to cbbI, cbbII was expressed under all growth conditions that require the CBB system (39, 40); for photoheterotrophic conditions with l-malate as the carbon source, the primary role of CO2 fixation via the CBB cycle (using only cbbII) is to maintain the redox poise of the cell. Wild-type strain SB1003 exhibited cbbII promoter activity under photoheterotrophic growth conditions with either ammonia or glutamate as the nitrogen source, in the absence or presence DMSO (Fig. 1B). Strain SBI/II, however, exhibited a 10-fold induction of cbbII promoter activity when cultures were supplemented with DMSO while retaining wild-type control during growth in the absence of DMSO (Fig. 1B). Strain RCP expressed low basal levels of cbbII promoter activity under all of the photoheterotrophic growth conditions tested (Fig. 1B). Similar to strain RCP (Fig. 1B), additional PHC strains that were derived from RubisCO-deficient strain SBI/II (M. A. Tichi and F. R. Tabita, unpublished data) were also shown to exhibit basal, uninduced levels of cbbII expression under the four growth conditions depicted in Fig. 2B. Thus, upon developing photoheterotrophic competency, RubisCO-deficient strains such as strain RCP regained the normal low basal levels of cbbII promoter activity during photoheterotrophic growth; however, the absolute promoter activity levels were, for unknown reasons, considerably reduced and were similar to those observed with cbbRII strains (53). Strains SBP and SBP-PHC exhibit comparable wild-type (strain SB1003) control of cbbII promoter activity during photoheterotrophic growth in the presence of ammonia, although somewhat less activity was observed with malate-glutamate-DMSO (Fig. 1B). Among all of the CBB-deficient strains, it appears from these results that only strain SBI/II showed substantial activation of both cbbI and cbbII promoter activities in the presence of DMSO. Apparently, basic regulatory mechanisms are altered in this strain.
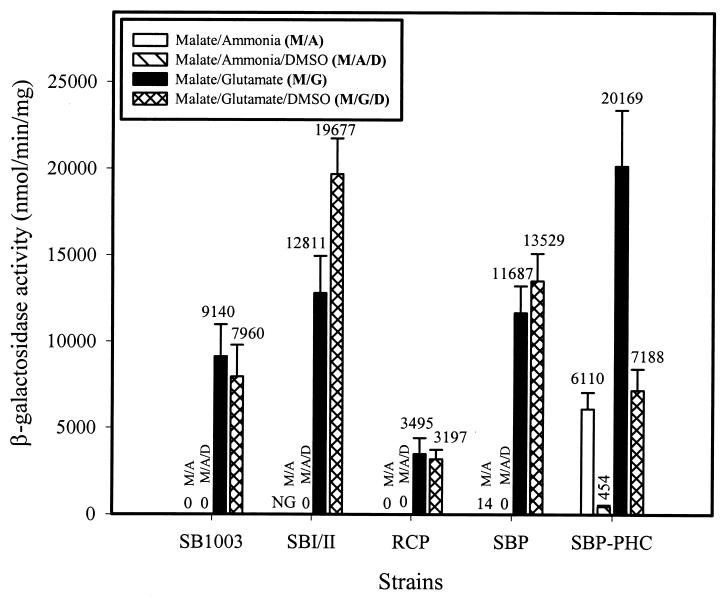
FIG. 2.
nifH::lacZ promoter activity during photoheterotrophic growth of R. capsulatus with either ammonia or glutamate as the nitrogen source. β-Galactosidase activities were determined in four or five independent cultures assayed in duplicate. NG indicates no growth under photoheterotrophic growth conditions with ammonia as the nitrogen source in the absence of an ancillary electron acceptor (DMSO).
The dinitrogenase system.
Previous studies indicated that the conventional molybdenum dinitrogenase system of R. capsulatus (encoded by the nifHDK genes) provides a sufficient compensatory electron sink in the absence of an operational CBB pathway under photoheterotrophic growth conditions with glutamate as the nitrogen source. Redox poise is also accomplished in the presence of ammonia in PHC strains of R. capsulatus. Under these conditions, the organism derepresses the synthesis of an active and unmodified dinitrogenase enzyme complex (50). Consistent with this regulatory scheme, strain SBP-PHC exhibited nifH promoter activity when grown photoheterotrophically in the presence of ammonia, while the wild-type strain and additional CBB-deficient strains repressed nifH promoter activity (Fig. 2). Also consistent with previous findings (50), the nifH promoter activity of strain SBP-PHC was decreased 13.4-fold and 2.8-fold under DMSO-supplemented growth conditions when either ammonia or glutamate, respectively, was used as the nitrogen source; nifH promoter activity of parent strain SBP was hardly affected by the addition of DMSO under growth conditions permissive for dinitrogenase expression (Fig. 2). Even though strain RCP exhibited a 2.5-fold decrease in nifH promoter activity compared to wild-type strain SB1003, the promoter activities observed during malate-glutamate growth for both strains were not appreciably altered by the addition of DMSO (Fig. 2). nifH promoter activity increased 2.5-fold in strain SBI/II during photoheterotrophic growth with glutamate supplemented with DMSO compared to that in wild-type strain SB1003.
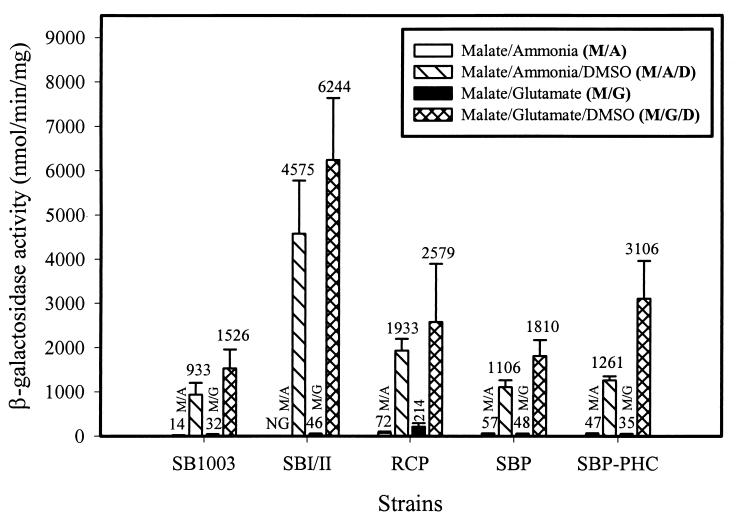
The DMSOR system.
Excess reductant generated by photosynthetic electron transport is transferred via the ubiquinone pool to the periplasmic terminal electron acceptor DMSOR during phototrophic growth in the presence of DMSO or TMAO (27–29). Thus, DMSO respiration via the DMSOR system (encoded by the genes dorCDA) contributes to the maintenance of redox homeostasis. Accordingly, dorC promoter activity was examined in wild-type strain SB1003 and the CBB-deficient strains of R. capsulatus in order to assess dor expression during photoheterotrophic growth. During growth in the presence of DMSO, dorC promoter activity was induced in all R. capsulatus strains regardless of the supplied nitrogen source (Fig. 3). Compared to wild-type strain SB1003, strain SBI/II exhibited enhanced dorC promoter activity (4.1 to 4.9-fold) during photoheterotrophic growth with DMSO when either glutamate or ammonia, respectively, was used as the nitrogen source (Fig. 3). These results were consistent with the enhanced cbbI, cbbII, and nifH promoter activities obtained in strain SBI/II under DMSO-supplemented growth conditions.
FIG. 3.
dorC::lacZ promoter activity of R. capsulatus under photoheterotrophic growth conditions with or without DMSO. β-Galactosidase activities were determined in four or five independent cultures assayed in duplicate. NG indicates no growth in the absence of DMSO under conditions with ammonia as the nitrogen source.
PHC and coordinate regulation of redox poise and carbon metabolism.
The first indication that the PHC phenotype and the control of cbb expression might be linked in R. capsulatus came from studies with strain RCP, where low basal levels of cbbII promoter activity were obtained under all of the photoheterotrophic growth conditions tested. In addition, unlike all of the other strains, including the wild type, strain RCP expressed cbbI promoter activity only during growth with ammonia in the absence of DMSO (a condition under which the PHC phenotype is obligatory for growth) (Fig. 1A and B).
The hierarchical use of electron acceptors and control mechanisms involved in phototrophic redox homeostasis in R. capsulatus differs from the situation found in R. sphaeroides and R. rubrum (50). Specifically, coordinate control of the DMSOR and dinitrogenase systems in PHC strains appears to be unique to R. capsulatus. The development of a PHC phenotype and the control of cbb expression also differed in R. capsulatus and R. sphaeroides. This was particularly evident when heterologous expression experiments were performed with an R. sphaeroides translational cbbI::lacZ promoter fusion vector to examine the effect of the PHC phenotype on R. sphaeroides cbb promoter activities in the R. capsulatus wild-type and PHC strain backgrounds (Table 3). Plasmid pVKD1 contains, in addition to the cbbI promoter of R. sphaeroides, the upstream and divergently transcribed R. sphaeroides cbbR gene (6, 7). Endogenous R. capsulatus CbbR proteins do not recognize R. sphaeroides cbb promoters; thus, transcription of this cbbI promoter in the R. capsulatus background is dependent on its cognate CbbR protein. It is clear that the R. capsulatus cbbI promoter is not expressed in photoheterotophically grown wild-type R. capsulatus (Fig. 1A). However, when an R. sphaeroides cbbR-cbbI promoter plasmid (pVKD1) was used, β-galactosidase activity was obtained under all of the photoheterotrophic growth conditions tested in an R. capsulatus strain RCP background and very low, but demonstrable, basal levels of activity were detected in wild-type R. capsulatus (Table 3). R. sphaeroides cbbI promoter activity in R. capsulatus strain RCP was highest when the PHC growth phenotype was obligatory (malate-ammonia medium) and decreased up to fourfold as additional redox-balancing systems were used by the organism (Table 3), i.e., when glutamate was used as a nitrogen source (and the dinitrogenase system was synthesized) or when DMSO was added to cultures (when the DMSOR system was synthesized). The high level of cbbI expression obtained in a malate-ammonia medium is very similar to what occurs when R. capsulatus cbbI expression is monitored in strain RCP (Fig. 1A), suggesting that the basic environment in R. capsulatus RCP is responsible for regulating CbbR-dependent transcription, no matter whether transcription is directed by the R. sphaeroides or R. capsulatus CbbR protein and the cognate cbbI promoter. The fact that very low levels of R. sphaeroides CbbR-dependent cbbI promoter activity was observed in a wild-type R. capsulatus environment is compatible with the finding that R. capsulatus CbbR-dependent cbbI promoter activity was not even detected under these growth conditions. Perhaps the low-level expression of the R. sphaeroides cbbI promoter, compared to the R. capsulatus cbbI promoter, was due to the presence of the unique upstream activator region in the promoter-distal region of the R. sphaeroides cbbI promoter (6, 7).
TABLE 3.
Promoter activity using an R. sphaeroides cbbI::lacZ promoter fusion in R. capsulatus wild-type strain SB1003 and strain RCP under photoheterotrophic growth conditions
| Photoheterotrophic growth condition | β-Galactosidase activitya of strain:
|
|
|---|---|---|
| SB1003 | RCP | |
| Malate-ammonia | 15 ± 6 | 1,233 ± 166 |
| Malate-ammonia-DMSO | 13 ± 6 | 734 ± 263 |
| Malate-glutamate | 20 ± 5 | 422 ± 157 |
| Malate-glutamate-DMSO | 8 ± 2 | 364 ± 90 |
β-Galactosidase activities (mean ± standard deviation) are expressed as nanomoles per minute per milligram and were determined in three independent cultures of each strain (assayed in duplicate) containing plasmid pVKD1 from R. sphaeroides.
Integrative control of the DMSOR redox-balancing system with photoautotrophic carbon metabolism.
To assess the integration of the DMSOR and CBB systems with photoautotrophic metabolism, dorC and cbb promoter activities were examined in wild-type strain SB1003. dorC promoter activity in wild-type strain SB1003 was not significantly altered under photoautotrophic (CO2-H2) growth conditions when cultures were supplemented with DMSO in the presence of ammonia or glutamate (Table 4). Likewise, cbbII promoter activity in wild-type strain SB1003 was not significantly changed when the exogenous electron acceptor DMSO was added under photoautotrophic growth conditions, no matter whether ammonia or glutamate was used as the nitrogen source (Table 4). These results were analogous to cbbII promoter activities obtained for wild-type strain SB1003 during photoheterotrophic growth with malate, where the addition of DMSO did not affect activity (Fig. 1B). In wild-type strain SB1003, cbbI promoter activity, however, decreased sixfold during photoautotrophic growth in the presence of DMSO compared to growth in the absence of DMSO (Table 4). These data suggested that the presence of DMSO has a selective effect on expression of the cbbI promoter in R. capsulatus during photoautotrophic growth. No cbbI promoter activity was observed in the wild-type strain under photoautotrophic growth conditions when glutamate was added as a potential carbon and nitrogen source in the absence or presence of DMSO (Table 4). It is apparent from these results that the DMSOR and cbbI systems are reciprocally regulated under photoautotrophic conditions, suggesting that electron flow through the DMSOR system negatively impacts cbbI expression.
TABLE 4.
Levels of CBB and DMSOR promoter systems in wild-type strain SB1003 during photoautotrophic growth in the presence or absence of DMSO with ammonia G glutamate as the nitrogen source
| Phototrophic growth condition | β-Galactosidase activity (nmol/min/mg)a
|
||
|---|---|---|---|
| cbbI::lacZ | cbbII::lacZ | dorC::lacZ | |
| CO2-H2-ammonia | 162 ± 8 | 794 ± 59 | 9 ± <1 |
| CO2-H2-ammonia-DMSO | 27 ± 14 | 1,192 ± 206 | 795 ± 171 |
| CO2-H2-glutamate | 0 | 510 ± 28 | 12 ± 1 |
| CO2-H2-glutamate-DMSO | 0 | 602 ± 108 | 1,283 ± 184 |
β-Galactosidase activities were determined in three independent cultures assayed in duplicate.
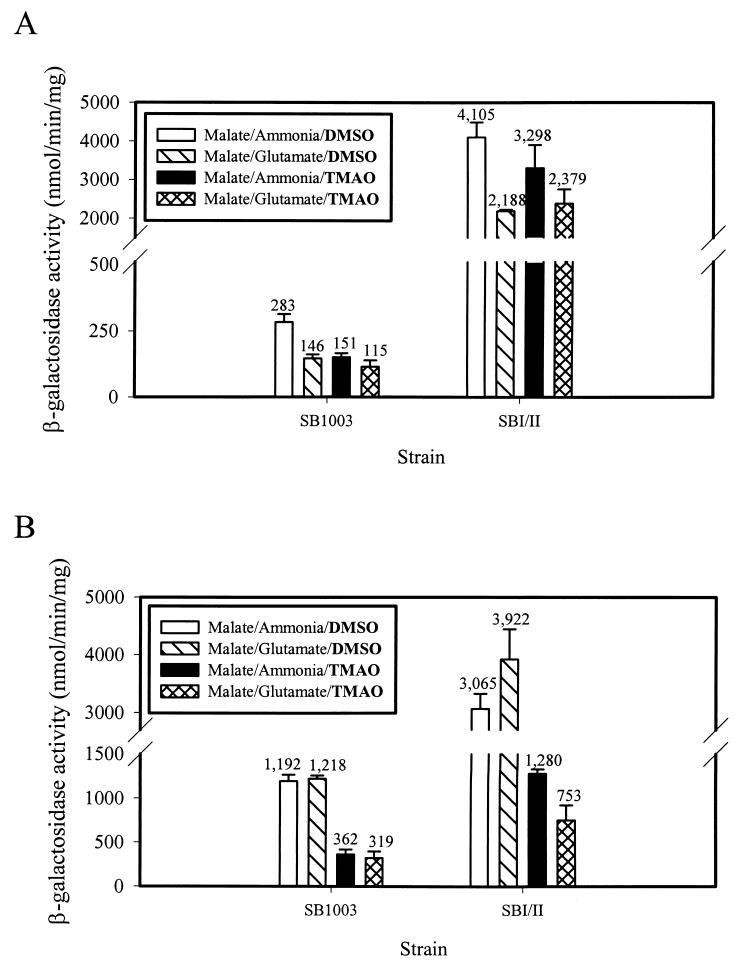
To confirm that the control of the cbbII system is independent of controls on the DMSOR system, an experiment was designed to take advantage of the fact that the DMSOR system catalyzes both DMSO and TMAO respiration; despite this, dorC promoter activity decreases during photoheterotrophic growth when TMAO is used as an exogenous electron acceptor instead of DMSO (47). Using either DMSO or TMAO as the electron acceptor for the DMSOR system did not significantly affect cbbII promoter expression in either wild-type strain SB1003 or strain SBI/II under photoheterotrophic growth conditions (Fig. 4A and B). In addition, strain SBI/II maintained up-regulated expression of the cbbII system in the presence of either TMAO or DMSO. By contrast, however, the addition of TMAO to photoheterotrophic cultures of strains SB1003 and SBI/II resulted in a three- to fivefold decrease in dorC promoter activity compared to the activities obtained in the presence of DMSO (Fig. 4B).
FIG. 4.
cbbII::lacZ (A) and dorC::lacZ (B) promoter activities in wild-type R. capsulatus strain SB1003 and strain SBI/II during photoheterotrophic growth with either DMSO or TMAO as the supplied exogenous electron acceptor in the presence of either ammonia or glutamate as the nitrogen source.
DISCUSSION
Nonsulfur purple bacteria couple their ability to assimilate carbon dioxide and dinitrogen to photosynthetic energy generation and the production of required reducing equivalents (15). However, knowledge of how various redox-balancing systems interact and contribute to successful photoheterotrophic or photoautotrophic metabolism is limited. In the present study, the expression of three important redox-balancing mechanisms, the CBB, dinitrogenase, and DMSOR systems, was shown to be either coordinately regulated or influenced by the presence of one system or the other. This is necessary to ensure balance in the use of reducing equivalents generated by phototrophic metabolism (Fig. 5). The control of anaerobic respiratory pathway gene expression in R. capsulatus is comparable to the situation in E. coli, where there is also coordinate and integrative control over the redox-balancing systems (for a review, see reference 17 and references therein). However, in the present study, evidence for linkage in the control of key redox-balancing systems (i.e., those important for CO2 fixation, nitrogen fixation, and DMSO respiration) is presented for the first time in both the photoheterotrophic and photoautotrophic growth modes.
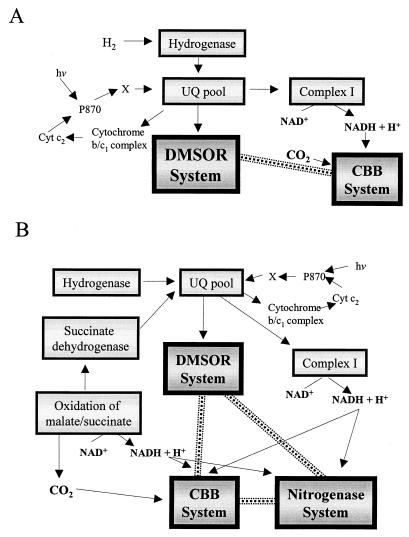
FIG. 5.
Mechanisms involved in adaptation of the efficiency of energy conservation to intracellular requirements under photoautotrophic (A) and photoheterotrophic (B) growth conditions. Cyclic photosynthetic electron transport and the specific redox-balancing mechanisms of the CBB, DMSOR, and nitrogenase systems contribute to redox homeostasis in R. capsulatus. NADH generated by the oxidation of carbon substrates or reverse electron flow via complex I is dissipated by the CBB system (A and B) and perhaps the nitrogenase system (B). Flux of reductant from the ubiquinone pool is transduced to the DMSOR system under phototrophic growth conditions (A and B). The dotted line indicates the influence of DMSO reduction on specific redox-balancing mechanisms under phototrophic environmental conditions (A and B).
The interplay between the dual roles (maintenance of redox poise and carbon metabolism) of the CBB system has been shown to correlate with the expression of the DMSOR system. The integration of the CBB system and the DMSOR system in phototrophic metabolism is not unprecedented. Phototrophic growth on highly reduced substrates such as butyrate and propionate is known to depend upon the addition of exogenous CO2 as an electron acceptor (52). Under these conditions, the CBB system is obligately required for growth (11, 12, 39). An auxiliary oxidant, DMSO or TMAO, can substitute for CO2 under these phototrophic growth conditions (44). Moreover, during phototrophic growth on less-reduced carbon substrates (e.g., l-malate), the DMSOR system can also replace the need for a functional CBB system in R. capsulatus (40, 50). The results of the present investigation indicated that cbbI of the CBB system of R. capsulatus was responsive to activation of the DMSOR system under photoautotrophic growth conditions, while cbbII was unaffected by the DMSOR system under either photoheterotrophic or photoautotrophic growth conditions. By contrast, RubisCO-deficient strain SBI/II exhibited a different response in that both cbbI and cbbII promoter activities were raised to photoautotrophic (1.5% CO2–98.5% H2) wild-type levels under photoheterotrophic growth conditions in the presence of DMSO. In fact, all of the redox systems, as exemplified by the respective promoter fusions, were up-regulated in strain SBI/II.
In Rhodobacter, redox homeostasis is achieved through the interplay of cyclic photosynthetic electron transport and specific redox-balancing mechanisms of anaerobic metabolism during phototrophic growth (30). It has been suggested that the electron acceptors involved in photosynthetic metabolism function as a sink for excess reducing equivalents or prevent the overreduction of the cyclic electron transport system. This interaction between redox poise and electron transport occurs at the level of the ubiquinone pool (13; Fig. 5). Respiratory electron flow to the DMSOR system has been shown to branch from cyclic electron transport at the level of the ubiquinone pool (27, 28); thus, activation of the DMSOR system under phototrophic growth conditions may siphon reductant from the ubiquinone pool. Studies of the related organism R. sphaeroides indicated that flux from the ubiquinone pool is transduced through a pathway involving cbb3-type cytochrome c oxidase, while a signal involved in the flow of reductant is conveyed to the PrrBA (RegBA) signal transduction pathway (33–35). In R. capsulatus, the two-component signal transduction system RegBA (PrrBA) has been shown to be involved in the regulation of operons important for photosynthetic gene expression (46), CO2 fixation (53), and nitrogen fixation and H2 oxidation (8, 50). Indeed, the current model suggests that the RegBA system responds to the overall intracellular redox state (22, 49), although more-detailed studies are required to elucidate the specific redox-sensing mechanisms that influence the Reg system of R. capsulatus (4, 8). Under photoautotrophic growth conditions in R. capsulatus, the RegBA global regulatory system was shown to be involved in activation of cbbI promoter expression, as well as maximal expression of the cbbII promoter (53). It is possible that by activating the DMSOR system, which alters the oxidation-reduction potential of the ubiquinone pool, a redox signal is transmitted to a regulatory system that, in turn, controls the expression of key operons involved in phototrophic metabolism. In R. capsulatus, the RegBA system plays more of a critical role in regulating cbbI since this operon is up-regulated only during photoautotrophic metabolism while cbbII is expressed under a variety of conditions. This could explain the sensitivity of cbbI to activation of the DMSOR system under photoautotrophic growth conditions. Alternatively, additional, unknown factors that have been postulated to be involved in expression of the CBB system in R. sphaeroides (6, 7) and R. capsulatus (53) could play a critical role in transmitting a redox signal to control key operons involved in redox homeostasis.
During photoheterotrophic metabolism, redox poise is also achieved by the coordinate integration of the DMSOR and CBB systems, as well as the dinitrogenase system. Indeed, in the absence of an operational CBB system, spontaneous variants of R. capsulatus derepress the dinitrogenase system, resulting in photoheterotrophic competency (50). Dinitrogenase-catalyzed proton reduction and the consequent evolution of H2 gas are important for maintenance of redox poise in R. capsulatus (20). The current study monitored the interplay between the CBB and dinitrogenase systems, as well as the DMSOR system, in CBB-deficient and PHC strains of R. capsulatus. Although the specific regulatory mechanism(s) involved in the derepression of dinitrogenase in PHC mutant strains of R. capsulatus remains to be established, it should be noted that the PrrBA (RegBA) two-component regulatory system is involved in the maintenance of the PHC phenotype of an R. sphaeroides dinitrogenase-derepressing strain (22). Additionally, the Reg system was shown to be involved in the control of nitrogen fixation in wild-type R. capsulatus (8) and Bradyrhizobium japonicum (3).
The specific integration of redox mechanisms with the derepression of the dinitrogenase system in R. capsulatus differs from the situation in R. sphaeroides (50). For example, activation of the DMSOR system under photoheterotrophic growth conditions diminishes nif expression in a dinitrogenase-derepressing strain of R. capsulatus while exhibiting no effect in R. sphaeroides (22, 42). We have also observed differences in R. sphaeroides and R. capsulatus cbbI promoter expression in an R. capsulatus PHC strain background. This could be due to differences in general redox response between cbbIs of the two organisms, the differential effects of specific metabolic signals on the cognate CbbR proteins, or a combination of both possibilities. With different ecological niches in aquatic ecosystems (41), it is not unexpected that R. capsulatus and R. sphaeroides differentially regulate processes involved in the control of redox homeostasis in response to the environmental milieu. An indication of this possibility was previously suggested by the demonstration of differences in the roles of the global regulatory systems of FnrL (47, 59, 60) and RegBA (PrrBA) (2, 9, 10, 32, 46) during phototrophic growth in R. sphaeroides and R. capsulatus.
A question that must be addressed concerns the potential role and coordinate control of specific metabolic signals with redox homeostasis in response to environmental factors. In R. sphaeroides (7) and Rhodopseudomonas palustris, whose genomic sequence was recently completed (http://www.jgi.doe.gov/tempweb/JGImicrobial/html/index.html), it is apparent that a single cbbR gene controls the transcription of the two major cbb operons. A separate upstream and divergently transcribed cbbR gene, however, controls each cbb operon in R. capsulatus (38, 39, 53). LysR-type transcriptional regulators, such as CbbR, generally utilize a metabolite or coinducer produced by the pathway they regulate (45). Clearly, the current study has demonstrated that a complex interrelationship of specific redox-balancing systems exists in R. capsulatus and probably other nonsulfur purple bacteria. Since activation of the DMSOR system affects control of the CBB system under photoautotrophic environmental conditions and in some instances may also cause up-regulation of promoter sequences important for redox balancing under photoheterotrophic growth conditions, it is important to determine if these observed regulatory events are coordinated with the appearance of and subsequent interaction with a specific metabolic signal metabolite(s). Perhaps strain SBI/II can be effectively used in such investigations since there is dramatic up-regulation of operons important for redox control in this strain. Continued studies of the nature of the signal(s) that influences both CbbR and the more global redox-sensing pathways required for photoheterotrophic and photoautotrophic growth are warranted.
ACKNOWLEDGMENTS
We thank A. Shaw and A. G. McEwan for the R. capsulatus translational dorC-lacZ plasmid. We also thank A. G. McEwan and Janet Gibson for helpful advice and insightful discussions concerning this work and Janet Gibson for helpful editing.
This work was supported by NIH grant GM45404 from the U.S. Public Health Service.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- 2.Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. doi: 10.1016/s0092-8674(00)81074-0. [DOI] [PubMed] [Google Scholar]
- 3.Bauer E, Thomas K, Fischer H M, Hennecke H. Expression of the fixR-nifA operon in Bradyrhizobium japonicum depends on a new response regulator, RegR. J Bacteriol. 1998;180:3853–3863. doi: 10.1128/jb.180.15.3853-3863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buggy J, Bauer C E. Cloning and characterization of senC, a gene involved in both aerobic respiration and photosynthesis gene expression in Rhodobacter capsulatus. J Bacteriol. 1995;177:6958–6965. doi: 10.1128/jb.177.23.6958-6965.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burris R H. Nitrogenases. J Biol Chem. 1991;266:9339–9342. [PubMed] [Google Scholar]
- 6.Dubbs J M, Tabita F R. Two functionally distinct regions upstream of the cbbI operon of Rhodobacter sphaeroides regulate gene expression. J Bacteriol. 1998;180:4903–4911. doi: 10.1128/jb.180.18.4903-4911.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubbs J M, Bird T H, Bauer C E, Tabita F R. Interaction of CbbR and RegA* transcription regulators with the Rhodobacter sphaeroides cbbI promoter-operator region. J Biol Chem. 2000;275:19224–19230. doi: 10.1074/jbc.M002125200. [DOI] [PubMed] [Google Scholar]
- 8.Elsen S, Dischert W, Colbeau A, Bauer C E. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J Bacteriol. 2000;182:2831–2837. doi: 10.1128/jb.182.10.2831-2837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falcone D L, Tabita F R. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J Bacteriol. 1991;173:2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcone D L, Tabita F R. Complementation analysis and regulation of CO2 fixation gene expression in a ribulose 1,5-bisphosphate carboxylase/oxygenase deletion strain of Rhodospirillum rubrum. J Bacteriol. 1993;175:5066–5077. doi: 10.1128/jb.175.16.5066-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson S J, Jackson J B, McEwan A G. Anaerobic respiration in the Rhodospirillaceae: characterisation of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol Rev. 1987;46:117–143. [Google Scholar]
- 14.Figurski D, Helinski D R. Replication of an origin-containing derivative of the plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gest H. Energy conversion and generation of reducing power in bacterial photosynthesis. Adv Microb Physiol. 1972;7:243–282. [Google Scholar]
- 16.Gibson J L, Tabita F R. Isolation and preliminary characterization of two forms of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas capsulata. J Bacteriol. 1977;132:818–823. doi: 10.1128/jb.132.3.818-823.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunsalus R P. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol. 1992;174:7069–7074. doi: 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallenbeck P L, Lerchen R, Hessler P, Kaplan S. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase expression in Rhodobacter sphaeroides. J Bacteriol. 1990;172:1736–1748. doi: 10.1128/jb.172.4.1736-1748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallenbeck P L, Lerchen R, Hessler P, Kaplan S. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of R. sphaeroides. J Bacteriol. 1990;172:1749–1761. doi: 10.1128/jb.172.4.1749-1761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillmer P, Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol. 1977;129:724–731. doi: 10.1128/jb.129.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hübner P, Masepohl B, Klipp W, Bickle T A. nif gene expression studies in Rhodobacter capsulatus: ntrC-independent repression by high ammonium concentrations. Mol Microbiol. 1993;10:123–132. doi: 10.1111/j.1365-2958.1993.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 22.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern M, Kamp P B, Paschen A, Masepohl B, Klipp W. Evidence for a regulatory link of nitrogen fixation and photosynthesis in Rhodobacter capsulatus via HvrA. J Bacteriol. 1998;180:1965–1969. doi: 10.1128/jb.180.7.1965-1969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lascelles J. The formation of ribulose 1,5-diphosphate carboxylase by growing cultures of Athiorhodaceae. J Gen Microbiol. 1960;23:449–510. doi: 10.1099/00221287-23-3-499. [DOI] [PubMed] [Google Scholar]
- 25.Ludden P W, Roberts G P. The biochemistry and genetics of nitrogen fixation by photosynthetic bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 929–947. [Google Scholar]
- 26.Madigan M T, Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979;137:524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwan A G, Cotton N P J, Ferguson S J, Jackson J B. The role of auxiliary oxidants in the maintenance of a balanced redox poise for photosynthesis in bacteria. Biochim Biophys Acta. 1985;810:140–147. [Google Scholar]
- 28.McEwan A G, Wetzstein H G, Jackson J B, Ferguson S J. Periplasmic location of the terminal reductase of trimethylamine-N-oxide and dimethylsulphoxide respiration in the photosynthetic bacterium Rhodopseudomonas capsulata. Biochim Biophys Acta. 1985;806:410–417. [Google Scholar]
- 29.McEwan A G, Richardson D J, Hudig H, Ferguson S J, Jackson J B. Identification of cytochromes involved in trimethylamine-N-oxide and dimethylsulphoxide respiration in Rhodobacter capsulatus. Biochim Biophys Acta. 1989;810:308–314. [Google Scholar]
- 30.McEwan A G. Photosynthetic electron transport and anaerobic metabolism in purple non-sulfur bacteria. Antonie van Leeuwenhoek. 1994;66:151–164. doi: 10.1007/BF00871637. [DOI] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 32.Mosley C S, Suzuki J Y, Bauer C E. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J Bacteriol. 1994;176:7566–7573. doi: 10.1128/jb.176.24.7566-7573.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Gara J P, Eraso J M, Kaplan S. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1998;180:4044–4050. doi: 10.1128/jb.180.16.4044-4050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh J-I, Kaplan S. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 35.Oh J-I, Eraso J M, Kaplan S. Interacting regulatory circuits involved in orderly control of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 2000;182:3081–3087. doi: 10.1128/jb.182.11.3081-3087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ormerod J G, Ormerod K S, Gest H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by the photosynthetic bacteria: relationships with nitrogen metabolism. Arch Biochem Biophys. 1961;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- 37.Paoli G C. Ph.D. thesis. Columbus: The Ohio State University; 1997. [Google Scholar]
- 38.Paoli G C, Soyer F, Shively J M, Tabita F R. Rhodobacter capsulatus genes encoding form I ribulose-1,5-bisphosphate carboxylase/oxygenase (cbbLS) and neighboring genes were acquired by a horizontal gene transfer. Microbiology. 1998;144:219–227. doi: 10.1099/00221287-144-1-219. [DOI] [PubMed] [Google Scholar]
- 39.Paoli G C, Strom-Morgan N, Shively J M, Tabita F R. Expression of the cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch Microbiol. 1995;164:396–405. [PubMed] [Google Scholar]
- 40.Paoli G C, Vichivanives P, Tabita F R. Physiological control and regulation of the Rhodobacter capsulatus cbb operons. J Bacteriol. 1998;180:4258–4269. doi: 10.1128/jb.180.16.4258-4269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfennig N. General physiology and ecology of photosynthetic bacteria. In: Clayton R K, Sistrom W R, editors. The photosynthetic bacteria. New York, N.Y: Plenum Press; 1978. pp. 3–18. [Google Scholar]
- 42.Qian Y. Ph.D. thesis. Columbus: The Ohio State University; 1997. [Google Scholar]
- 43.Qian Y, Tabita F R. Expression of glnB and a glnB-like gene (glnK) in a ribulose bisphosphate carboxylase/oxygenase-deficient mutant of Rhodobacter sphaeroides. J Bacteriol. 1998;180:4644–4649. doi: 10.1128/jb.180.17.4644-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson D J, King G F, Kelly D J, McEwan A G, Ferguson S J, Jackson J B. The role of auxiliary oxidants in maintaining redox balance during growth of Rhodobacter capsulatus on propionate and butyrate. Arch Microbiol. 1988;150:131–137. [Google Scholar]
- 45.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 46.Sganga M W, Bauer C E. Regulatory factors controlling photosynthetic reaction center and light harvesting gene expression in Rhodobacter capsulatus. Cell. 1992;68:945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 47.Shaw A L, Leimkuhler S, Klipp W, Hanson G R, McEwan A G. Mutational analysis of the dimethylsulfoxide respiratory (dor) operon of Rhodobacter capsulatus. Microbiology. 1999;145:1409–1420. doi: 10.1099/13500872-145-6-1409. [DOI] [PubMed] [Google Scholar]
- 48.Shively J M, Davidson E, Marrs B L. Derepression of the synthesis of the intermediate and large forms of ribulose-1,5-bisphosphate carboxylase/oxygenase in Rhodopseudomonas capsulatus. Arch Microbiol. 1984;138:233–236. doi: 10.1007/BF00402127. [DOI] [PubMed] [Google Scholar]
- 49.Tabita F R. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a different perspective. Photosynth Res. 1999;60:1–28. [Google Scholar]
- 50.Tichi M A, Tabita F R. Maintenance and control of redox poise in Calvin-Benson-Bassham pathway deficient strains of Rhodobacter capsulatus. Arch Microbiol. 2000;174:322–333. doi: 10.1007/s002030000209. [DOI] [PubMed] [Google Scholar]
- 51.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 52.van Niel C B. The culture, general physiology, morphology, and classification of the non-sulfur purple and brown bacteria. Bacteriol Rev. 1944;8:1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vichivanives P, Bird T H, Bauer C E, Tabita F R. Multiple regulators and their interaction in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J Mol Biol. 2000;300:1079–1099. doi: 10.1006/jmbi.2000.3914. [DOI] [PubMed] [Google Scholar]
- 54.Wallenfels K. β-Galactosidase (crystalline) Methods Enzymol. 1962;5:212–219. [Google Scholar]
- 55.Wang X, Falcone D L, Tabita F R. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain redox balance of the cell. J Bacteriol. 1993;175:3372–3379. doi: 10.1128/jb.175.11.3372-3379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weaver K E, Tabita F R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983;156:507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 58.Yen H C, Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulatus. J Bacteriol. 1976;126:619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation of Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeilstra-Ryalls J H, Gabbert K, Mouncey N J, Kaplan S, Kranz R G. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J Bacteriol. 1997;179:7264–7273. doi: 10.1128/jb.179.23.7264-7273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]