Abstract
Cancer treatment protocols depend on tumor type, localization, grade, and patient. Despite aggressive treatments, median survival of patients with Glioblastoma (GBM), the most common primary brain tumor in adults, does not exceed 18 months, and all patients eventually relapse. Thus, novel therapeutic approaches are urgently needed.
Radiotherapy (RT) induces a multitude of alterations within the tumor ecosystem, ultimately modifying the degree of tumor immunogenicity at GBM relapse. The present manuscript reviews the diverse effects of RT radiotherapy on tumors, with a special focus on its immunomodulatory impact to finally discuss how RT could be exploited in GBM treatment through immunotherapy targeting. Indeed, while further experimental and clinical studies are definitively required to successfully translate preclinical results in clinical trials, current studies highlight the therapeutic potential of immunotherapy to uncover novel avenues to fight GBM.
Keywords: glioblastoma, immunomodulation, radiotherapy
Cancer treatment protocols mostly involve surgery, radiotherapy (RT), and chemotherapy depending on the patient, tumor type, localization, and grade. Glioblastoma (GBM) is the most common primary brain tumor in adults. Since the establishment of the Stupp protocol in 2005, including RT,1 median survival of GBM patients has not evolved and does not exceed 18 months. Thus, novel approaches to limit GBM relapse are urgently needed. Importantly, RT induces a multitude of alterations within the tumor ecosystem, ultimately modifying the degree of tumor immunogenicity at relapse. In fact, accumulating preclinical and clinical evidence indicates that RT has systemic antitumor effects in several solid tumors and can be redefined as a partner for cancer immunotherapy.2 The present manuscript will review the diverse immunomodulatory responses related to cytostatic and toxic effects of RT, to finally discuss how RT could be exploited in GBM treatment through immunotherapy targeting.
The Limits of Radiotherapy, the Gold-standard Cancer Treatment
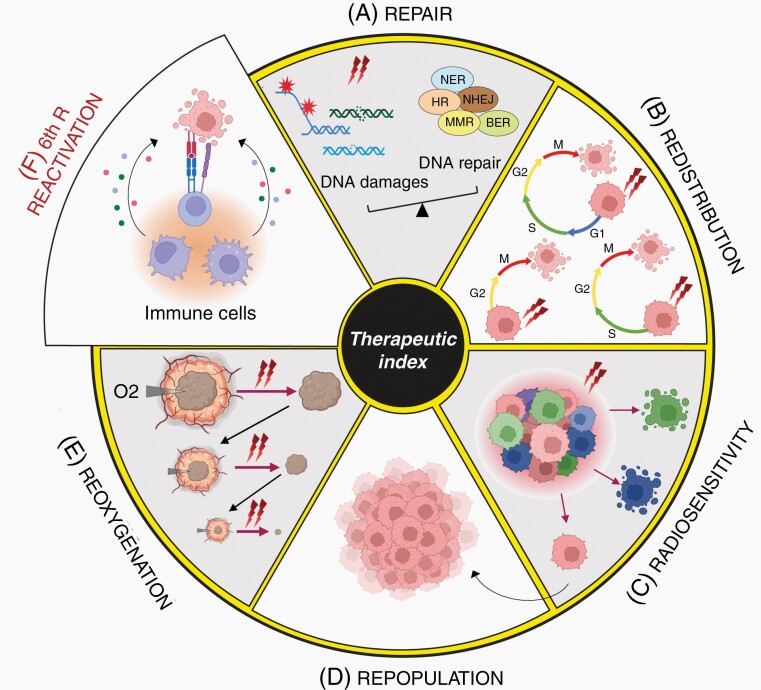
RT is based on tumor exposure to photons or particles radiation to cause irreversible damages resulting in cancer cell death. Its efficacy relies on its ability to penetrate tissues and break chemical bonds within the cells. Since the first description of X-rays by Roentgen in 1895, clinical use of ionizing radiation has undergone huge technical developments boosted by advances in physics, imaging, treatments planning as well as improved understanding of radiobiology. RT can be given with the intent of cure but also as a palliative treatment to relieve patients, alone, or in combination with other treatments. Common protocols deliver external doses of radiation to patients through external beam radiation therapy. Radiation sources might also be placed within the patient’s body during brachytherapy or vectorized with antibodies, nanobodies, or others.3 Clinical settings might be adjusted depending on the patient, tumor location, and size to reach an optimal therapeutic index, to maximize tumor growth control while limiting radiotoxicity to adjacent normal tissues. Following the oldest rules in radiobiology, cells are most sensitive to RT when they have a high division rate and grow in an oxygen-rich environment.4 Actually, several biological factors, referred as the “five Rs”, have been identified to describe how cells respond to RT5: Repair, Redistribution, Radiosensitivity, Repopulation, andReoxygenation (Figure 1). As the number of cancer survivors increases, improving RT therapeutic index is a priority and requires a better understanding of mechanisms involved in tumor radiation response and escape.
Figure 1.
The 6Rs of radiation biology define RT therapeutic index: (A) Repair. Radiation exposure can lead to different DNA damages (SSB, DSB, base mismatches, and bulky adducts) leading to activation of different mechanisms (MMR, NER, BER, HR, and NHEJ) depending on the type of DNA damages. The cell ability to repair DNA damages is a key determinant of their fate. If the level of DNA damage exceeds DNA repair abilities of cancer cells, the cell will either die, enter senescence, or undergo cell transformation through accumulation of mutations. (B) Redistribution. Cell redistribution within the cell cycle phases is a major determinant of radiation sensitivity with cells being most radiosensitive during the G2-M transition, less sensitive in the G1 phase, and least sensitive in the late S phase. With multiple doses of radiation, cells in G2-M are preferentially killed. The time between fractions will allow tumor cells to progress to a sensitive phase of the cell cycle. Sensitization due to this “re-assortment” leads to therapeutic gain. (C) Intrinsic Radiosensitivity. Tumors are very heterogeneous with differential response to radiation. Some tumors are highly radioresistant after high radiation doses while others respond to low doses of radiation. Tumor intrinsic and individual radiosensitivity varies with time and during treatment. (D) Repopulation. Most radioresistant cells can evade RT killing and contribute to tumor repopulation promoting recurrence and metastasis. (E) Reoxygenation. Reoxygenation plays a key role in radiation sensitivity owing to oxygen ability to fix DNA damages. Aerated cells are more radiosensitive than hypoxic cells. Fractionated radiation will allow sufficient time for reoxygenation of previous hypoxic areas for a better radiosensitization and tumor clearance. (F) Immune reactivation. RT efficacy relies on the activation of the immune system, with the release of different factors that can ensure tumor cells killing in primary or distant sites. MMR, Mismatch repair; NER, nucleotide-excision repair; BER, Base-excision repair; HR, Homologous Recombination; NHEJ, Non-Homologous End Joining.
Radiotherapy Kills Tumor Cells through DNA Damage
Radiation exposure causes various biological effects depending on the linear energy transfer that penetrates and deposits energy into the cell. It also depends on the total dose, the fractionation rate, and the inner radiosensitivity of the targeted cells. Radiation primarily hit biological molecules (DNA, protein, lipids) directly and indirectly through water radiolysis generating free radicals. Because of its conservative role in genome integrity, DNA is considered as the main target of radiation exposure, leading to accumulation of single- (SSB) or double-strand breaks (DSB), DNA base damage, DNA-DNA and DNA-protein crosslinks with chromosomal rearrangements. Of note, it is commonly admitted that 1 Gy of X-ray dose produces about 1000 SSB and about 50–100 DSB in a mammalian cell. Phosphorylation at Ser-139 of H2AX, the minor histone H2A variant, is a common reliable method to detect DNA damage.6 Unrepair or misrepair of DNA damages results in the accumulation of gene mutations, blocking the cell ability to divide and proliferate further, and ultimately leading to cell death or transformation.7
Radiation also affect organelles such as endoplasmic reticulum (ER), mitochondria, lysosomes, and ribosomes. For example, radiation can cause an ER stress response leading to autophagic cell death or apoptosis. Radiation can also significantly impact mitochondria function through mitochondrial membrane depolarization, ROS generation, and cytochrome c release, ultimately leading to apoptosis.8 Radiation may also directly destabilize cell membrane through alteration of its composition or indirectly through ROS generation, and lipid peroxidation.8
DNA Damages Activate the DNA Repair Machinery
In response to DNA damages, cells can activate different stress-response pathways, collectively termed DNA Damage Response (DDR), leading to cell cycle arrest to facilitate DNA repair, maintenance of genomic stability, and ultimately determine the fate of irradiated cells (Figure 2). DDR is primarily coordinated by the interconnected ATM-Chk2 and ATR-Chk1 signaling cascades, along with DNA-dependent protein kinase (DNA-PK).9 DDR is initiated by different sensor protein complexes, which will recruit and activate the 3 kinases signaling pathways at the DNA damage sites. Both ATM and DNA-PK are recruited and activated primarily at DSB while ATR is activated at SSB, in association with its partner ATRIP (Figure 2).
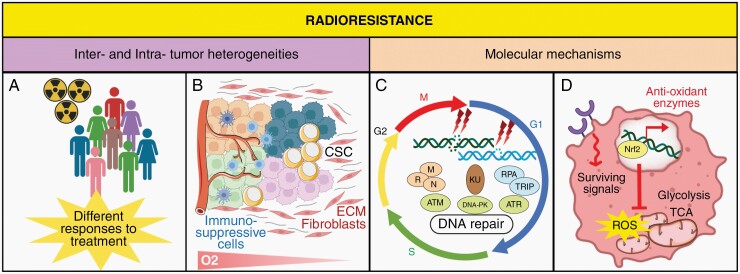
Figure 2.
Different aspects of Radioresistance. (A) Radioresistance is mediated by inter-tumoral heterogeneity that refers to differences between patients, due to environmental and etiological factors, and leading to differential response to RT. (B) Multiple subclones resulting from genomic and biological variations underlie the intratumoral heterogeneity. Microenvironmental features, including hypoxia and the production of multiple factors by stromal cells, may lead to radioresistance. These factors favor the dominant immuno-suppressive TME, and the presence of CSC that contribute to tumor recurrence by their unlimited growth and differentiation potential. (C) The distribution of cells in the cell cycle, and their DNA repair capabilities in response to radiation-induced DNA damages contribute to radioresistance. DSB and SSB are sensed by the MRN complex, Ku or RPA/ATRIP, which activate kinases including ATM, ATR, and DNA-PK that mediate DNA damage response. (D) Radioresistance occurs through constitutive activation of survival pathways, alteration of glucose metabolism triggered by hypoxia and increased protection against oxidative stress by Nrf2 expression and subsequent antioxidant enzymes. CSC, Cancer Stem Cells; SSB, Single-Strand Breaks; DSB, Double-Strand Breaks; DNA-PK, DNA-dependent protein Kinase; ATM, Ataxia telangiectasia mutated; ATR, ATM/RAD3-related; ECM, Extracellular Matrix; ROS, Reactive Oxygen Species; TCA, Tricarboxylic Acid Cycle; TME, Tumor Microenvironment.
Once activated, ATM, ATR, and DNA-PK phosphorylate a large number of overlapping substrates, including H2AX, the checkpoint control kinases CHK1 and CHK2, as well as the tumor-suppressor protein p53, to promote and coordinate efficient DNA repair and other DNA metabolic events (eg, transcription, replication, and mitosis).9,10 Following its phosphorylation, p53 accumulates in the nucleus, where it mainly acts as a transcription factor, leading to cell cycle arrest. DNA damages are then repaired, either through non-homologous end joining (NHEJ) and homologous recombination (HR) for DSB, or through base-excision repair (BER) and nucleotide-excision repair (NER) mechanisms for SSB.10 However, while SSB and base damages are usually efficiently repaired, misrepair of DSB may occur resulting in genetic information loss, translocation, chromosomal abnormalities or rearrangements, and ultimately genomic instability and aneuploidy.10
The degree of DNA damages combined with the extent of p53 modifications (phosphorylation, acetylation, methylation) will determine subsequent cell fate. If the DNA damages can be repaired completely, the cell will continue to cycle while improper DNA repair will lead to cell death, either by apoptosis, mitotic catastrophe, or senescence.
Alteration of the DNA Repair Machinery in Cancer Cells
In normal cells, DDR is meant to repair DNA and maintain genome integrity. However, altered DDR, as observed in most cancer cells, may result in cell survival at the cost of genomic instability, cancer predisposition, and therapeutic resistance.11 For example, DDR pathway genes are frequently altered in human gliomas.12 Accordingly, alterations of the ATM/Chk2/p53 cascade accelerate tumor formation in a glioma mouse model.12 As a crucial tumor suppressor gene, mutation or deletion in p53 gene prevents its anti-tumoral function and alters RT efficacy.
Beside genetic mutations, tumor cells usually display less efficient DNA repair machinery as compared to normal cells. Precancerous lesions must inactivate p53 or other DDR actors to proceed to a more aggressive status.13,14 Reduced DDR efficiency results in high rate of mutational events and sustained genomic instability, which is the prime mechanism involved in tumor uncontrolled proliferation and resistance to RT. Indeed, while radiation-induced or constitutively-activated stress signaling pathways such as EGFR/PI3K/Akt, JAK/STAT, and RAS/Raf/MAPK pathways, exert key survival and proliferation activities involved in treatment resistance, attenuated radiation-induced p53 also enhances radioresistance. Furthermore, low expression of 53BP1, a DDR protein involved in NHEJ, has been associated with higher local recurrence in triple negative breast cancer patients treated with surgery and RT.15
Epigenetic modulations also affect the DNA repair machinery leading to different tumor cell sensitivity or tolerability to radiation. For example, trimethylation of lysine 27 of histone H3 (H3K27me3) was associated with increased RT sensitivity in gliomas and low-grade breast cancer tumors.16,17 Global remodulation of the DNA methylation landscape, as observed in IDH1/2 mutated tumors, was also associated with increased radiosensitivity through decreased anti-oxidant defenses.18,19 Some studies also reported that elevated activity of histone deacetylases (HDAC) and subsequent altered histone acetylation increased radiation resistance in breast cancer cells.20 Notably, epigenetic changes can occur after RT treatment leading to enhanced epigenetic heterogeneity and acquired radioresistance.
Tumor Heterogeneity, an Extra Weapon to Escape RT
Tumors are highly heterogeneous with distinct molecular, phenotypical, and functional signatures, which determine different levels of sensitivity to anticancer therapies. Inter-tumoral heterogeneity can be in part pinpointed through (epi)genetic profiling in order to predict patient response to RT (Figure 2). In contrast, intra-tumoral heterogeneity illustrates the spatial and the temporal heterogeneity between the primary tumor and its subsequent recurrence.
The small subpopulation of cancer stem-like cells (CSCs) plays a key role in intra-tumoral heterogeneity. These cells, described as the cardinal reason of therapeutic resistances and tumor recurrences, display a huge tumorigenic potential, and inherent resistance to radiation through increased DDR and ROS scavenging mechanisms21,22 (Figure 2). Accordingly, an increased number of CSCs has been observed in nonresponding tumors to RT. Furthermore, CSCs display a wide ability to bear DSBs accumulation, increasing tumor genomic instability, and subsequent heterogeneity. RT also favors the conversion of non-CSCs to CSCs, as observed in breast cancer and GBM.23 Single cell transcriptomic analyses identified other molecular subpopulations displaying different level of radiosensitivity. Importantly, RT directly enhances tumor heterogeneity through radio-induced genomic instability and the emergence of resistant clonogenic tumor cells.24 Numerous preclinical data support that RT selects out radiation-resistant tumor clones over the dominant but more sensitive ones. However, the innate radiosensitivity of tumor cell varies with time and treatments, independently of its genetic and molecular profiling.
The Immunosuppressive Properties of RT
Beside cancer cells, tumors include non-transformed cells such as stromal cells, immune cells, cancer-associated fibroblasts (CAF), vascular endothelial cells, as well as secreted molecules assembling the extracellular matrix (ECM). Underestimated for long, interactions between neoplastic cells and components of the TME have a major impact on tumor initiation, progression, metastasis, and treatment response (Figure 2).25–27
Immune cells, in particular lymphoid cells, are among the most radiosensitive cells of the TME. The first step to prime an adaptive T cell-mediated immune response is antigen uptake by an antigen-presenting cell (APC) such as macrophages or dendritic cells (DC). DC are the most potent APC and their activation is usually mediated by interactions between their surface receptors with danger signals, also called DAMPs for damage associated molecular patterns.28 Once activated, DC are characterized by high levels of MHC, costimulatory molecules, and IL-12 production, and migrate to lymph nodes to activate T cells. Because of their huge production in anti-oxidative molecules, macrophages, including tumor-associated macrophages (TAM), are one of the most radioresistant cells in humans. However, RT can also affect their pro-tumoral M2 or anti-tumoral M1 polarization.29
Radiosensitivity of T cells generally depends on their activation state. Resting lymphocytes are more sensitive to irradiation than their activated counterparts. For example, regulatory T cells (Tregs, CD25+, FoxP3+) are more radioresistant than T or B lymphocytes.30 Furthermore, irradiated Tregs display a better ability to proliferate, regenerate and/or infiltrate.30 Importantly, their recruitment in the irradiated TME contributes to RT immunosuppressive properties through the secretion of various immunosuppressive cytokines, including IL-10, TGF-β, and IL-35. Interestingly, TGF-β signaling can be induced by RT and stimulate DDR while its inhibition impairs ATM activity, increases genome instability, and renders cells more sensitive to radiation.31 Moreover, Tregs express several inhibitory molecules such as CTLA-4, that binds to costimulatory molecules (CD80/CD86) expressed by DC or macrophages reducing their immune function and their ability to activate cytotoxic T cells.32 Through indoleamine 2,3 dioxygenase (IDO) activity, Tregs also catalyze the conversion of the critical amino acid tryptophan into kynurenine, leading to T cell starvation. Tregs are also able to degrade immunogenic released ATP into adenosine by the surface ectonucleotidases CD39 and CD73.33 Finally, the immunosuppressive TME created by radio-induced activation of Tregs can be reinforced by increased expression of inhibitory immune receptor ligands on tumor cells, such as PD-1.34
Myeloid-derived suppressor cells (MDSCs) also participate to the radio-induced immunosuppressive TME and radiation resistance. Within few hours following radiation, the secretion of pro-inflammatory factors including colony stimulating factor 1 (CSF1), type-I IFN (IFN-I), DAMPs, and chemokines (CCL2, CCL12, and CCL7) induces the differentiation and migration of MDSCs to the inflamed tumor site.35 MDSCs will then secrete immunosuppressive cytokines, express IDO and the surface ectonucleotidases. MDSCs also produce high levels of intracellular arginase-1 (ARG1), leading to L-arginine depletion and T cell arrest. Finally, MDSCs also inhibit T cell proliferation by limiting the presence of cysteine, or by direct engagement with T cells inhibitory receptors.36
Apart from these immunosuppressive effects on the immune compartment, RT is also an emerging and significant contributor to anti-tumor response.
The Immune Stimulatory Effects of Radiotherapy
On first sight, RT does not appear as a natural ally for the immune system since it eliminates immune cells in the irradiated areas. However, this effect is somehow restricted in space and time and depends on RT modalities. RT immunostimulatory effect relies on its ability to reactivate anti-tumor immune responses, and as such is considered as the 6th R of the therapeutic index (Figure 1F).
RT Induces an Immune Abscopal Effect
RT definitively requires an immune response to reach an optimal efficacy. The best example is the abscopal effect described in a few number of patients where radiation shrinks the targeted tumor but also tumors located in distant sites from the irradiated areas.37 Initial cases were reported for melanoma and papillary adenocarcinoma, but an abscopal effect has also been observed in other tumor sites.38–40
Several preclinical studies reported a direct connection between the abscopal effect and the immune system.41–44 First, similar doses of radiation reduced tumor growth more efficiently in immunocompetent mice than in immunodeficient mice.45 Second, in mice implanted with either Lewis lung carcinoma (LLC) or fibrosarcoma, progression of tumors implanted in a distant site was reduced when legs were irradiated.41 Third, the use of immunotherapy could also boost abscopal effect of RT.42,44 For example, in a bilateral syngeneic mouse model of breast cancer, the combination of RT on one flank with the systemic delivery of an immunoadjuvant, led to a significant growth delay of both the irradiated and the non-irradiated tumors.44 Further preclinical studies suggest that hypofractionation favors an antitumor immune response in mice. In this regard, a wider use of stereotactic radiosurgery (SRS), a non-invasive RT that uses highly focused radiation beams, and stereotactic body RT may favor the abscopal effect occurrence. Tumor size and heterogeneity also affect the degree of abscopal effect: immunogenic tumors promote an abscopal response while larger tumors containing hypoxic immunosuppressive areas tend to be more resistant to treatment.46
Thus, while the frequency, durability, and potency of RT abscopal effects have been formerly investigated in clinical trial settings, many open questions remain regarding RT modalities required to achieve these rare responses.
RT Induces Immunogenic Cell Death
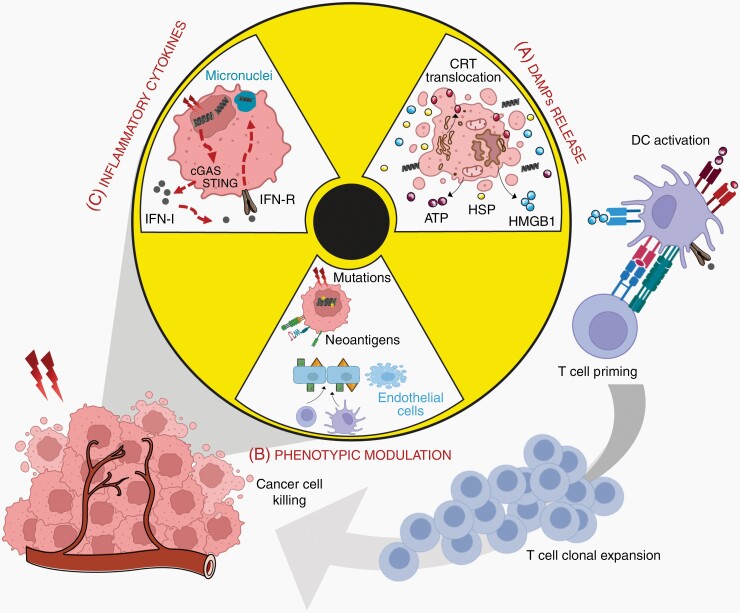
Radiation are the main inducers of immunogenic cell death (ICD), a peculiar form of apoptosis favoring effector T cell killing and APC priming. Dying cells release waves of tumor-associated antigens (TAA) in a phenomenon called “epitope spreading”. These signals favor cancer cell recognition by immune cells, in particular with DC activation eliciting the recruitment of primed T specific lymphocytes in the tumor site (Figure 3).
Figure 3.
The immunostimulatory effects of Radiotherapy. (A) RT triggers immunogenic cell death with the translocation of CRT, the release of HMGB1, ATP and HSP. (B) Cancer cell exposure to radiation generates DNA mutations and the expression of neoantigens; irradiation also affects endothelial cells inducing their apoptosis, enhanced expression of selectins (triangles) and integrins (squares), and subsequent permeability. (C) DNA leakage and micronuclei formation in irradiated cells are sensed by the cGAS-STING pathway, resulting in the production of IFN-I that interacts with its receptor to mediate the release of inflammatory cytokines. When exposed or secreted, DAMPs bind to APC receptors to mediate their maturation and activation, and the subsequent priming of adaptive anti-tumoral immune response and the activation and expansion of cytotoxic T cells. Attracted to the tumor inflammatory site, these cells contribute to treatment-resistant cell killing. IFN-I, Type I Interferon; CRT, Calreticulin; HSP, Heat Shock Protein; HMGB1, High Mobility Group Box 1; ATP, Adenosine triphosphate; DAMPs, Damage Associated Molecular Patterns.
Radiation promote ICD through three distinct mechanisms. First, cell surface exposure of calreticulin determines the immunogenicity of cancer cell death (Figure 3).47 Indeed, radiation trigger a rapid and pro-apoptotic translocation of calreticulin from the ER to the plasma membrane, resulting in caspases cascade and subsequent Bax-Bak activation. Once exposed at the cell membrane, calreticulin provides an “eat-me” signal leading to the uptake of dying cancer cells by DC, tumor antigen presentation to T cells, and their concomitant clonal expansion.48 The exposure of Heat Shock Proteins (HSP70 and HSP90) on the cell surface cooperates with DC activation to generate an anti-tumoral T cell response. Fractionated RT is the main stimulus for cell death induction and Hsp70 release, in particular when tumor cells are mutated for p53 or not expressing MGMT.49 Importantly, this signal is counteracted by CD47, widely expressed in various cancers including GBM, by inhibiting macrophage phagocytic activity through its interaction with SIRP-α receptors.50 Interestingly, in human papillomavirus–positive head and neck tumors, RT reduced CD47 expression in a dose-dependent manner.51
Second, RT induces the release of HMGB1, a histone chromatin-binding protein with potent immunomodulatory effects on DC and macrophages (Figure 3). Through its interaction with various receptors, including Toll-like receptors (TLR) and receptor for advanced glycation end products (RAGE), HMGB1 induces the activation of NFκB and downstream inflammatory pathways, leading to the production of different pro-inflammatory cytokines.52,53 HMGB1 is also released during necrosis, a feature of malignant GBM. Third, RT contributes to anti-tumoral immunity through the release of ATP that promotes monocytes recruitment and activation. Indeed, its binding to purinergic receptors, P2RX7 and PR2Y2, on monocytes leads to the activation of NOD-like receptor family pyrin domain-containing-3 (NLRP3) inflammasome, which in turn induces IL-1β production and CD8 + priming.54,55
RT Turns “Cold” into “Hot” Tumors
As described above, the immune TME greatly differs from one tumor to another. Accordingly, they are defined as “cold” (non-T cell inflamed) or “hot” (T cell inflamed). RT may convert “cold” into “hot” tumor through several mechanisms (Figure 3). First, RT can unmask tumor antigens rendering them more visible to the innate and adaptive immune system.56 Radiation-induced DNA damages also increase tumor mutational load, which is further increased when DDR is defective, favoring immune recognition, and clearance.57Radiation-induced mutagenesis is a stochastic cell-specific process, where a given mutation is generated in one particular radiation-escaping cell. Thus, while an increase in mutational burden can enhance tumor aggressiveness, it also generates neoantigens that can be recognized by the immune system. Several studies also demonstrate that RT induces novel peptide synthesis in tumor cells and enhances antigen presentation by MHC-I molecules.58 RT also contributes to the generation of broadened TCR repertoire with increased CD8/CD4 ratio, mainly through an upregulation of the CD80 costimulatory signal.59 Furthermore, as any stress, RT upregulates the expression of NKG2D ligands involved in NK cells recruitment and activation. Note that the mechanisms involved in tumor mutational burden seem to directly affect subsequent response to immunotherapy. In particular, deficiency in mismatch repair genes has recently emerged as an indicator of response to PD-1 blockade in patients with cancer. However, a subset of patients displaying hypermutated glioma due to defective DDR lacks both recognition of tumor cells by the immune system and the response to PD-1 blockade.60
Finally, radiation also induce the expression of a wide variety of neoantigens expressed by endothelial cells, including ICAM-1, VCAM-1, integrins, selectins, and cadherins. These molecules favor immune cells trafficking to the tumor site where they exert their cytotoxic effects via Fas, Fas-L, and TRAIL whose expression is upregulated by RT.
RT Activates the cGAS-STING Pathway
While DNA is usually confined within the nucleus and mitochondria, RT causes DNA leakage into the cytosol, which will be sensed by STING leading to the activation of innate and adaptive immune responses through the production of IFN-I (Figure 3).61 The breakdown of the micronuclear envelope significantly contributes to cytosolic DNA release, which may occur for several days after recovery from DNA damages.62 Cytosolic DNA triggers conformational changes in cGAS enzyme leading to the generation of cyclic GMP-AMP (cGAMP), which activates STING. Once activated, STING dimers translocate from the ER to the Golgi apparatus and perinuclear microsomes. During this process, STING recruits and activates the kinases TBK1 and IKK, which in turn phosphorylate the transcription factor IRF3 and the IκB family leading to NFκB activation. The combined action of activated IRF3 and NFκB induces a robust immune response including the production of IFN-I and activation of the inflammasome.62 IFN-I plays an important role in this process through the regulation of DC function, activation of T and B cells, and generation of long-lived memory cells (Figure 3). IFN-I can also increase NK cell cytotoxicity by modulating the surface expression of activating and inhibitory receptors.63 STING-induced IFN-I also promotes immune suppression by recruiting MDSC into the TME,35 increasing PD-L1 expression,64 or activating the DNA exonuclease TREX1, which will degrade cytosolic DNA and prevent cGAS-STING activation.34 The importance of the cGAS/STING pathway in antitumor therapy has led to several studies investigating the therapeutic use of STING agonists, either as a monotherapy or in combination with radiation.
Exploiting Radiation Immunostimulatory Effects against GBM
RT, a Key Player in GBM Treatment
GBM, defined as grade IV glioma according to the WHO classification, is the most frequent and aggressive primary brain tumor in adults. The current standard-of-care treatment, the Stupp protocol, consists of a surgical resection followed by RT with concomitant and adjuvant chemotherapy with TMZ.1 The cumulative dose of RT for GBM is 60 Gy delivered over 30 daily fractions. However, GBM patient median survival is only 18 months with less than 5% of patients surviving over 5 years due to systematic relapse.1
Given this dismal prognosis, alternatives to the Stupp protocol have been tested through higher doses and reduced numbers of fractions (hypofractionated RT), in particular in elderly patients or patients with poor clinical factors.65 Hypofractionated RT is generally considered to be effective, safe and associated with limited morbidity. In fact, a recent meta-analysis of eleven comparative trials of hypofractionated RT as first-line treatment in GBM patients compared to standard RT protocol reported comparable survival outcome with the benefit of a shortened duration in all patients.66 Other RT-based strategies are currently being explored. Brachytherapy based on 125Iodine has been used as a salvage strategy for inoperable GBM, such as recurrent tumors.67 However, radiation leakage into the surrounding brain is currently limiting this clinical approach. Consequently, this radioactive emitting source has been progressively replaced by safer isotopes. This approach led to the development of the GammaTile, a treatment recently approved by the FDA for GBM treatment, which involves inserting encapsulated radioactive 131Cesium seeds into the surgical cavity.68 Proton therapy, which uses proton particles rather than photons, has also been investigated to reduce radiation exposure to the surrounding brain. This approach significantly improved the quality of life of recurrent GBM patients as well as provided a slight survival benefit, despite some reported toxicities.69 FLASH-RT is a novel modality of irradiation delivered at ultra-high dose rate.70 Delivered as hypofractionated regimens, FLASH-RT significantly reduces radiation-induced toxicities in the normal brain without compromising tumor cure. Finally, the delivery of high radiation doses to the tumor can also be achieved via Gamma Knife radiosurgery, which is a noninvasive SRS. This approach seems to improve survival rates in recurrent GBM patients with minimal burden.71
GBM Heterogeneity, a Challenge for RT
In addition to their diffuse and infiltrative nature limiting resection, a characteristic feature of GBM is a high degree and dynamic intra-tumoral heterogeneity. First, GBM exhibit diverse genetic alterations including mutation and/or deletion of EGFR, PTEN, NF1, and p53 genes. The epigenetic hypermethylation of the MGMT promoter also affects tumor ability to fix DNA damage induced by alkylating agents such as TMZ.72 At the transcriptomic level, two main GBM subtypes, the proneural and the mesenchymal ones, coexist within a single tumor. A proneural subtype enrichment has been associated to a more favorable outcome whereas the mesenchymal subtype was associated to poorer survival.73–75 This molecular identity is dynamic and a radiation-induced molecular conversion from proneural-to-mesenchymal subtype has been reported.76–78 Importantly, molecular simplicity correlated with better outcome while high degree of heterogeneity correlates with worst outcome.75 At the cellular level, GBM contain GBM stem-like cells (GSCs), which are intrinsically highly resistant to DNA damage.22,79In fact, several genes involved in HR, such as RAD51, BRCA1, and BRCA2, are overexpressed in GSC. Several recent findings uncover a dynamic plasticity between bulk tumor cells and GSCs, in particular upon radiation.80,81 Thus, GBM heterogeneity becomes even more complex after irradiation.
The Promises of Combining Radiotherapy to Immunotherapy in GBM
Clinical trials of immunotherapy alone have been disappointing in GBM despite multiple approaches including antibodies-based therapy, tumor vaccines, gene therapy, or adoptive cell therapy. However, since RT may act synergistically with immunotherapy to generate both a robust anti-tumor immune response and overcome intrinsic and adaptive resistance, several combined protocols are currently under investigation.
GBM and immunogenicity.
—The accessibility of the brain to immunotherapy has been a debate for long with the central nervous system (CNS) historically considered as an immune privileged area thanks to the selective permeability of the blood–brain barrier (BBB), the paucity of specialized APC, and the low expression of MHC-I in brain parenchyma limiting antigen presentation. Nevertheless, recent data have redefined the immunological activities in the CNS. Brain diseases such as autoimmune diseases or infections by neurotropic viruses generate inflammation showing that a robust immune response can occur in the brain. CNS immune surveillance is ensured by resident APC, called microglia, that can migrate to inflammatory sites for antigen presentation. Recent works have also identified a lymphatic network, draining the cerebrospinal fluid and meningeal leukocytes, where CNS-derived antigens are captured by APCs and presented to patrolling T cells.82 The secretion of different pro-inflammatory chemokines and cytokines will compromise the BBB integrity and allow immune cells infiltration.83 The presence of T infiltrating lymphocytes (TIL) after brain lesions is another argument for cerebral anti-tumor responses.
However, the complexity of GBM immunosuppressive microenvironment does facilitate tumor evasion. GBM are characterized by a low tumor mutational burden (TMB) and few TIL, most of them being Tregs or with exhausted immune profiles.84 Furthermore, TMB may evolve with GBM relapse. In particular, recurrent GBM exhibit significant TMB following the Stupp protocol. Another study reported an inverse relationship between GBM TMB and enrichment of inflammatory gene signatures in tumor relapse but not in newly diagnosed GBM.85 Thus, given their substantial heterogeneity, whether molecular biomarkers may reliably predict the development of hypermutation or response to immunotherapy remain to be determined.
The hypoxic GBM microenvironment, characteristic of these tumors, favors this immunosuppressive TME through STAT3 pathway, HIF-1α upregulation and subsequent activation of Tregs, and production of VEGF. Microglia, TAM, and MDSC also contribute to this immunosuppression through the secretion of PGE-2, TGF-β, IL-10, and IDO.84 Recent evidence even suggests that GBM can induce systemic immunosuppression by T cell sequestration in the bone marrow through the downregulation of the sphingosine 1-phosphate receptor-1 (S1PR1).86 Finally, TMZ or corticosteroids lead to lymphopenia through bone marrow sequestration of T cells and depression of effective anti-tumor immune response, with Treg population being the less sensitive to this cytotoxic effect.83
Synergy between RT and immunotherapy in preclinical models.
—Several studies using preclinical GBM models have reported synergistic effects of RT and immunotherapy, most of them evaluating immune checkpoint inhibitors (ICI) (Table 1). Immune checkpoints, such as CTLA-4 and PD-1, are ligands binding to inhibitory T cell receptors allowing tumor escape from immunosurveillance. In most studies, the combination of RT with successive concomitant and adjuvant administrations of specific monoclonal antibody increased mice survival and reduced tumor growth.87–90 However, the best efficacy was observed in triple therapy, when a third anti-tumor antibody was added to the combination of RT and ICI. Indeed, in mice treated with RT, CTLA4 blockade, and a monoclonal antibody activating the costimulatory molecule 4-1BB increased their survival compared to monotherapy or dual therapy.89 Similar results were observed when RT and anti-PD-1 were combined with anti-TIM3 antibodies, the latter activating lymphocyte function and survival.88 Both triple therapies resulted in more than 50% of mice surviving over 100 days, while mice treated with RT alone died in 25 days.88,89 Furthermore, these treatments conferred a long-term protection since long-surviving mice did not develop tumor when a subsequent sub-cutaneous tumor cell injection was performed. Ectopic VEGF can also potentiate anti-PD1 ICI therapy by increasing lymphatic drainage in the CNS as well as T cell priming involved in GBM clearance.91 Similarly, a long-lasting anti-tumor memory response was observed.
Table 1.
Combination of RT with Immunotherapy in Preclinical GBM Models
| Immunotherapy Protocol | Preclinical Model | Outcome | Reference | |
|---|---|---|---|---|
| Combination of stereotactic RT protocol of 1 × 10 Gy on day 10 after tumor implantation with immune checkpoint antibodies | ||||
| - mAb anti-PD1 (10 mg/kg) | - Orthotopic | - Increased mice survival | (Zeng et al. 2013) | |
| - Concomittant then adjuvant | C57Bl/6 | - Reduced tumor growth | ||
| - 4 doses, every 2 days | - 1.3 × 106 GL261luc | - 25% long survival | ||
| - No tumor growth after rechallenge | ||||
| - mAb anti-GITR (10 mg/kg) | - Orthotopic C57Bl/6 | - No survival advantage | (Patel et al. 2016) | |
| - concomittant then adjuvant | - 1.3 × 106 GL261-luc | |||
| - 3 doses, every 3 days | ||||
| - mAb anti-PD1 (10 mg/kg) | - Orthotopic C57Bl/6 | - Increased mice survival | (Kim et al. 2017) | |
| - Concomittant then adjuvant | - 1.3 × 106 GL261-luc | - Reduced tumor growth | ||
| - 3 doses, every 2 days | - 60% long survival | |||
| - Ab anti-TIM3 (10 mg/kg) | - Orthotopic C57Bl/6 | - Increased mice survival | (Kim et al. 2017) | |
| - 3 doses on day 7, 11, 15 | - 1.3 × 106 GL261-luc | - Reduced tumor growth | ||
| - 50% long survival | ||||
| - Combination mAb anti-PD1 & Ab anti-TIM3 (both 10 mg/kg) | - Orthotopic C57Bl/6 | - Increased mice survival | (Kim et al. 2017) | |
| - Concomittant then adjuvant | - 1.3 × 106 GL261-luc | - Reduced tumor growth | ||
| - 100% long-survival | ||||
| - Humanized Ab anti-CD47 | - Orthotopic NSG | - Increased mice survival | (Gholamin et al. 2020) | |
| - Concomitant | - human primary cells | - Reduced tumor growth | ||
| - Doses not indicated | ||||
| - mAb anti-CTLA4 (8 mg/kg) | - Orthotopic C57Bl/6 | - Increased mice survival | (Belcaid et al. 2014) | |
| - 3 doses (i.p.), day 11, 14, 17 | - 1.3 × 106 GL261-luc | - 20% long survival | ||
| - Ab anti-4-1BB (24 mg/kg) | - Orthotopic C57Bl/6 | - No survival advantage | (Belcaid et al. 2014) | |
| - 3 doses (i.p.), day 14, 17, 23 | - 1.3 × 106 GL261-luc | |||
| - Combination mAb anti-CTLA4 (8 mg/kg) & anti-4-1BB (24 mg/kg) | - Orthotopic C57Bl/6 | - Increased mice survival | (Belcaid et al. 2014) | |
| - 3 doses each (i.p.) on day 11, 14, 17, and 14, 17, 23 respectively | - 1.3 × 106 GL261-luc | - 50% long survival | ||
| Combination of stereotactic RT protocol of 1 × 4 Gy on day 7 after tumor implantation with CAR T cells | ||||
| - NKG2D-CAR T cells (5 × 106) | - Orthotopic C57Bl/6 | - Increased mice survival | (Weiss et al. 2018) | |
| - 3 i.v. doses (day 5, 7, 10) | - 20 000 GL261-luc | - Decreased tumor growth | ||
| - 20% long survival | ||||
| - No tumor after tumor rechallenge | ||||
| Combination of stereotactic RT protocol of 2 × 5 Gy on days 9 and 16 after tumor implantation with targeted nonviral nanoparticles | ||||
| - Targeted nanoparticles containing PD-L1/EGFR si-RNAs (75ug/dose) | - Orthotopic C57Bl/6 | - Increased mice survival | (Erel-Akbaba et al. 2019) | |
| - Retro-orbital injection | - 50 000 GL261-luc | |||
| - 6 doses (day 12, 13, 15, 19, 20, 22) |
The design of chimeric antigen receptors (CAR) allows T cell engineering and improves anti-tumor targeting. CAR-T cell therapy, providing encouraging clinical responses in hematological malignancies, can also be applied to GBM. In this context, ligands of NK receptors, such as NKG2D ligands, are interesting since they are overexpressed in stressed or transformed cells.92 In fact, a recent study reported a synergistic effect of RT and NKG2D-CAR T cells in an orthotopic GBM model.93 This protocol also conferred a long-term protection since surviving mice did not develop tumor after re-challenge, mainly due to the local persistence of NKG2D-CAR T cells.
Therefore, RT-based immunotherapy for GBM patients is worthy of further exploration, even if clinical translation turns out to be more challenging than anticipated. Indeed, preclinical models present several limitations since they relied either on syngeneic models involving immunocompetent mice harboring tumors with artificial immunogenicity and lacking human GBM heterogeneity, or on xenograft models retaining some tumor heterogeneity but requiring immunodeficient mice.
Clinical trials with immunotherapy as a 2d line treatment.
—To date, the majority of immunotherapies mainly included ICI, vaccines, and adoptive cell transfer, involved patients with GBM relapse and were administrated as monotherapy. These clinical trials mainly investigated the safety rather than the therapeutic impact on overall survival and progression-free survival of immunotherapy. Despite numerous studies, immunotherapy protocols targeted a limited number of antigens including IL-13Rα2, EGFRvIII, and Her2 either through adoptive cell therapy using CAR T cells, targeted monoclonal antibodies or tumor vaccines.94 The majority of these studies have been deeply disappointing due to progressive antigen loss. However, phase II/III trials remain essential to appreciate the therapeutic benefits and associated toxicities of such therapies.
Recently, a systematic comparison of newly diagnosed GBM treated either with the Stupp protocol alone or followed by immunotherapy has been published with the inclusion of 1239 patients, half of them receiving the Stupp while the other half received the Stupp protocol followed by immunotherapy.95 This meta-analysis revealed that RT followed by immunotherapy was neither associated with a significant improvement in 1-year overall survival, a significant improvement of 1-year progression-free survival, nor a significant incidence of adverse effects. While these results were disappointing, this analysis presents several significant limitations, including the diversity of immunotherapy in terms of agents, administration routes, doses, and treatment length.
RT with concomitant immunotherapy in clinical trials.
—Few clinical trials tested concomitant treatments of RT and immunotherapy. The safety and efficacy of concomitant administration of bevacizumab with RT was initially investigated in a prospective study where 25 patients with recurrent GBM, were treated with hypofractionated RT (5 × 6 Gy) combined with anti-VEGF immunotherapy96 (Table 2). This study was further completed with additional and concomitant administration of pembrolizumab.97 Another concomitant strategy, including newly diagnosed GBM patients, tested the safety and immunogenicity of IMA950 vaccine, composed of nine glioma-associated CD8 peptides and two tumor-associated CD4 peptides, in addition to standard treatments.98 Both studies reported that these combinations were safe and well tolerated, with promising efficacy response on patient median survival or primary immunogenicity. Further studies are now required to establish robust results.
Table 2.
Clinical Trials Including GBM Patients with Concomitant RT and Immunotherapy
| Study design | Patients | Reference | Outcome | |
|---|---|---|---|---|
| Published trials | ||||
| - Phase I, single arm, to test the safety of pembrolizumab (anti-PD1) | - 32 patients with recurrent GBM under hypofractionated RT (5 × 6 Gy) + bevacizumab (10 mg/kg) | (Sahebjam et al. 2020) | - No toxicity of pembrolizumab - Encouraging results of patient median overall survival and progression-free survival |
|
| - Phase I, single arm, to test the safety of therapeutic vaccine including 11 tumor-associated peptides | - 45 Newly diagnosed patients undergoing standard RT + TMZ protocol | (Rampling et al. 2016) | - Benign toxicity, mainly grade 1 IRS - Increased targeted immunogenicity in 30% of patients |
|
| - Phase I, single arm, to test the safety of bevacizumab (anti-VEGF) | - 25 patients with recurrent GBM under hypofractionated RT (5 × 6 Gy) + | (Gutin et al. 2009) | - No toxicity of bevacizumab - Increased median survival of 50% |
|
| - Phase I/II, single arm, to test the safety of cetuximab (anti-EGFR) | - 15 patients with recurrent GBM undergoing standard RT + TMZ protocol | (Combs et al. 2006) | - No published results | |
| On-going clinical trials | ||||
| - Phase III, single arm, to test the efficacy of Nivolumab (anti-PD1) | - Newly diagnosed patients with standard RT + TMZ protocol |
NCT02617589 : active, not recruiting NCT02667587 : active, not recruiting |
||
| - Phase II, single arm, to test the efficacy of pembrolizumab (anti-PD1) | - Newly diagnosed patients with standard RT + TMZ protocol |
NCT03197506 : active, not recruiting NCT03899857 : active, not recruiting |
||
| - Phase I/II, single arm, to test the safety and efficacy of Durvalumab (anti-PD-L1) | - Patients with recurrent GBM under hypofractionated RT (3 × 8 Gy) | NCT02866747 : recruiting | ||
| - Phase I/II, single arm, to test the efficacy of Atezolizumab (anti-PD-L1) | - Newly diagnosed patients with standard RT + TMZ protocol |
NCT03174197 : active, not recruiting |
||
| - Phase II, single arm, to test the efficacy of Avelumab (anti-PD-L1) | - Newly diagnosed patients with standard RT + TMZ protocol |
NCT02968940 : active, not recruiting NCT03047473 : active, not recruiting NCT04729959 : not yet recruiting |
With the development of various RT protocols, several on-going clinical trials are investigating immunotherapy efficacy based on RT modalities (Table 2). Indeed, RT-induced pro-immunogenic effects are observed following conventional RT with radiation doses ranging from 2 Gy up to more than 30 Gy. However, the optimal RT protocol to induce a clinically relevant anti-tumor immunity remains to be defined. For example, the best activation of cGAS/STING pathway occurs between 2 and 8 Gy while higher radiation doses do not confer immunogenicity due to a dose-dependent upregulation of TREX1.34 Thus, future research will also require the identification of predictive biomarkers that can help in scheduling combination modalities.
Promising immunotherapy exploiting RT immunostimulatory effects.
—Therapeutic monoclonal antibodies are one of the most successful cancer immunotherapies since they can be used to target specific tumor antigen, to generate memory immune responses but also as ICI. Durvalumab (MEDI4736), a humanized PD-L1 monoclonal antibody, is currently being tested in a multicenter phase II trial combining RT with bevacizumab in GBM patients (NCT02336165).99 Strikingly, and in contrast to monotherapy using PD-1/PD-L1 inhibitors, one patient survived longer. Notably, bevacizumab can help in overcoming neurological complications of radiation-induced brain necrosis after SRS treatment for GBM and brain metastases.100 Thus, such combinatorial therapy with RT appears as a promising strategy against GBM.
The recognition of DNA mechanism to initiate rapid innate immune response through activation of the cGAS-STING pathway also opens new strategy for GBM patients after RT. Indeed, STING agonists can increase T cell infiltration into gliomas through proinflammatory activation of suppressive tumor stroma, and can reverse the suppressive phenotype of MDSCs.101 Furthermore, orthotopic gliomas grew faster in STING knockout mice or following STING agonists administration.102,103 Injection of STING agonists not only reduced tumor progression in mice, but also generated strong systemic immune responses rejecting distant metastases and providing long-lived immunologic memory. Thus, the STING pathway plays a critical role in limiting GBM progression and STING agonists display strong translational potential. The common clinical schedule of hyperfractionated RT delivered to GBM patients104 induces an IFN-I response in tumor cells characterized by an increased expression of interferon stimulated genes. The response peaks within 3 weeks of treatment and coincides with a convergence to a plateau in clonogenic survival in vitro and treatment resistance in vivo. Using relevant and diverse in vitro and in vivo models, we recently demonstrated a radio-induced micronuclei formation in diverse GBM cells.25 Interestingly, senescent endothelial cells, which have been detected within the TME of recurrent GBM, exacerbate this process.25,105 Altogether, these results suggest a potential clinical benefit of targeting STING, IFN-I, or specific IFN-I response genes during fractionated low-dose RT. Of note, a recent study reported a significant toxicity of a direct combination of RT and IFN-I agonists highlighting the need to optimize both RT dose-scheduling and IFN-I targeting.106
Another recent study reported that combination of RT with CXCR4-CXCL12 inhibition, through synthetic nanoparticles loaded with the CXCR4 inhibitor AMD3100, sensitized GBM cells to radiation-induced ICD and reprogrammed the immunosuppressive microenvironment, in particular through reduced infiltration of CXCR4 + immunosuppressive MDSCs into the TME.107 This antitumor adaptative immune response also led to long-term survival and immunological memory response preventing tumor growth in mice rechallenged with GBM cells in the contralateral hemisphere.
The fact that intratumoral administration of NKG2D-CAR T cells confers long-term immune memory is of particular interest. As stress-induced ligands, expression of NKG2D ligands by tumor cells is upregulated following irradiation.108 Besides being involved in NK cell-mediated killing of tumor cells, NKG2D receptors are also potent costimulatory receptors involved in Vγ9Vδ2 T cell activation, a restricted immune subpopulation at the interface between innate and adaptative immunity.109 Interestingly, GBM cells from the mesenchymal subtype display strong expression of several NKG2D ligands, including MICA/B and ULBP2/5/6, allowing their spontaneous recognition and killing by Vγ9Vδ2 T cells, both in vitro and in vivo.92 This natural and selective eradication of GBM cells represents a particularly attractive strategy though adoptive transfer of Vγ9Vδ2 T cells, which can be further improved by genetic engineering. These effector cells can also be administrated through an autologous or an allogeneic adoptive cell transfer since their immunoreactivity is independent of MHC expression, which could be of particular interest given the dramatic decrease of immune cells in GBM patients following treatments.110
Conclusion
In conclusion, the distinct CNS immunological profile and the cold milieu of GBM offer challenging opportunities to implement immunotherapies in their therapeutic treatments. Since RT promotes immunostimulatory effects, we are convinced that immunotherapies will uncover novel avenues to fight GBM. However, efforts to optimize immunotherapies are definitively required to overcome BBB penetration, GBM dynamic heterogeneities, and the interplay between GBM immunosuppressive TME, RT protocols, and immunotherapy targeting. In such combinatorial approaches, timing and scheduling are of huge importance, to benefit from RT acute immunostimulatory effect while avoiding late immunosuppressive consequences. This could be relatively ensured by a better exploitation of immunotherapy as first-line treatment. Finally, efforts are required to generate relevant preclinical models integrating the human cerebral environment to successfully translate preclinical results in clinical trials.
Contributor Information
Hala Awada, Nantes Université, CRCI2NA, INSERM, CNRS, F-44000 Nantes, France; Anti-Tumor Therapeutic Targeting Laboratory, Faculty of Sciences, Lebanese University, Hadath, Beirut, Lebanon.
François Paris, Nantes Université, CRCI2NA, INSERM, CNRS, F-44000 Nantes, France; Institut de Cancérologie de l’Ouest, Saint-Herblain, France.
Claire Pecqueur, Nantes Université, CRCI2NA, INSERM, CNRS, F-44000 Nantes, France.
Funding
Hala Awada was funded by the ERASMUS and SAFAR program. This work was supported by funding from ARC Foundation and Ligue contre le Cancer from Comité de Vendée et Comité de Loire Atlantique.
Conflict of interest statement. All authors declare no conflict of interest.
Authorship statement. HA, CP analyzed the literature, wrote, revised and proofread the manuscript. FP proofread the manuscript. All authors have read and approved the final version.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Sato H, Demaria S, Ohno T. The role of radiotherapy in the age of immunotherapy. Jpn J Clin Oncol. 2021; 51(4):513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerber DE, Chan TA. Recent advances in radiation therapy. AFP. 2008; 78(11):1254–1262. [PubMed] [Google Scholar]
- 4. Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004; 4(9):737–747. [DOI] [PubMed] [Google Scholar]
- 5. Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol. 1989; 56(6):1045–1048. [DOI] [PubMed] [Google Scholar]
- 6. Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002; 158(4):486–492. [DOI] [PubMed] [Google Scholar]
- 7. Asaithamby A, Hu B, Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc Natl Acad Sci. 2011; 108(20):8293–8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Wang H, Qian H. Biological effects of radiation on cancer cells. Mil Med Res. 2018; 5(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010; 108:73–112. [DOI] [PubMed] [Google Scholar]
- 10. Maier P, Hartmann L, Wenz F, Herskind C. Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int J Mol Sci . 2016;17(1):102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FMG. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15(3):166–180. [DOI] [PubMed] [Google Scholar]
- 12. Squatrito M, Brennan CW, Helmy K, et al. . Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 2010; 18(6):6191–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartkova J, Horejsí Z, Koed K, et al. . DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005; 434(7035):864–870. [DOI] [PubMed] [Google Scholar]
- 14. Gorgoulis VG, Vassiliou LVF, Karakaidos P, et al. . Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005; 434(7035):907–913. [DOI] [PubMed] [Google Scholar]
- 15. Neboori HJR, Haffty BG, Wu H, et al. . Low p53 binding protein 1 (53BP1) expression is associated with increased local recurrence in breast cancer patients treated with breast-conserving surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 83(5):e677–e683. [DOI] [PubMed] [Google Scholar]
- 16. Gabriel N, Balaji K, Jayachandran K, et al. . Loss of H3K27 trimethylation promotes radiotherapy resistance in medulloblastoma and induces an actionable vulnerability to BET Inhibition. Cancer Res. 2022; 82(10):2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Healey MA, Hu R, Beck AH, et al. . Association of H3K9me3 and H3K27me3 repressive histone marks with breast cancer subtypes in the Nurses’ Health Study. Breast Cancer Res Treat. 2014; 147(3):639–651. [DOI] [PubMed] [Google Scholar]
- 18. Molenaar RJ, Radivoyevitch T, Nagata Y, et al. . IDH1/2 mutations sensitize acute myeloid leukemia to PARP inhibition and this is reversed by IDH1/2-mutant inhibitors. Clin Cancer Res. 2018; 24(7):1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garnier D, Renoult O, Alves-Guerra MC, Paris F, Pecqueur C. Glioblastoma stem-like cells, metabolic strategy to kill a challenging target. Front Oncol. 2019; 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharda A, Rashid M, Shah SG, et al. . Elevated HDAC activity and altered histone phospho-acetylation confer acquired radio-resistant phenotype to breast cancer cells. Clin Epigenet. 2020; 12(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diehn M, Cho RW, Lobo NA, et al. . Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009; 458(7239):780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bao S, Wu Q, McLendon RE, et al. . Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006; 444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 23. Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer stem cells and radioresistance: DNA repair and beyond. Cancers (Basel). 2019; 11(6):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alfonso JCL, Berk L. Modeling the effect of intratumoral heterogeneity of radiosensitivity on tumor response over the course of fractionated radiation therapy. Radiat Oncol. 2019; 14(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Degorre C, Renoult O, Parplys AC, et al. . Mechanistic insights of radiation-induced endothelial senescence impelling glioblastoma genomic instability at relapse. bioRxiv. 2021. doi: 10.1101/2021.12.13.472364. [DOI] [Google Scholar]
- 26. Fletcher-Sananikone E, Kanji S, Tomimatsu N, et al. . Elimination of radiation-induced senescence in the brain tumor microenvironment attenuates glioblastoma recurrence. Cancer Res. 2021;81(23):5935– 5947. doi: 10.1158/0008-5472.CAN-21-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Olson IE, Carlstrom LP, et al. . Impact of the radiated brain microenvironment on a panel of human patient-derived xenografts. doi: 10.1101/2020.06.03.132365. [DOI]
- 28. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015; 16(13):e498–e509. [DOI] [PubMed] [Google Scholar]
- 29. Chen Y, Song Y, Du W, et al. . Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019; 26(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as A “Game Changer” in cancer radiotherapy. Int J Mol Sci. 2019;20(13):3212. doi: 10.3390/ijms20133212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Liu Y, Chiang YJ, et al. . DNA damage activates TGF-β signaling via ATM-c-Cbl-mediated stabilization of the type II receptor TβRII. Cell Rep. 2019; 28(3):735–745.e4. [DOI] [PubMed] [Google Scholar]
- 32. Guo Q, Huang F, Goncalves C, Del Rincón SV, Miller WH. Translation of cancer immunotherapy from the bench to the bedside. Adv Cancer Res. 2019; 143:1–62. doi: 10.1016/bs.acr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Li XL, Mo YZ, et al. . Effects of tumor metabolic microenvironment on regulatory T cells. Mol Cancer. 2018; 17(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. . DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017; 8(1):15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liang H, Deng L, Hou Y, et al. . Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017; 8(1):1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang C, Jeong SY, Song SY, Choi EK. The emerging role of myeloid-derived suppressor cells in radiotherapy. Radiat Oncol J. 2020; 38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016; 40(1):25–37. [DOI] [PubMed] [Google Scholar]
- 38. Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975; 48(574):863–866. [DOI] [PubMed] [Google Scholar]
- 39. Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. 1973; 46(543):220–222. [DOI] [PubMed] [Google Scholar]
- 40. Ngwa W, Irabor OC, Schoenfeld JD, et al. . Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018; 18(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Camphausen K, Moses MA, Ménard C, et al. . Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003; 63(8):1990–1993. [PubMed] [Google Scholar]
- 42. Demaria S, Kawashima N, Yang AM, et al. . Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005; 11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 43. Demaria S, Ng B, Devitt ML, et al. . Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004; 58(3):862–870. [DOI] [PubMed] [Google Scholar]
- 44. Chakravarty PK, Alfieri A, Thomas EK, et al. . Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999; 59(24):6028–6032. [PubMed] [Google Scholar]
- 45. Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008; 68(11):4026–4030. [DOI] [PubMed] [Google Scholar]
- 46. Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017; 9(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiersma VR, Michalak M, Abdullah TM, Bremer E, Eggleton P. Mechanisms of translocation of ER chaperones to the cell surface and immunomodulatory roles in cancer and autoimmunity. Front Oncol. 2015; 5:7. doi: 10.3389/fonc.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Panaretakis T, Kepp O, Brockmeier U, et al. . Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009; 28(5):578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rubner Y, Muth C, Strnad A, et al. . Fractionated radiotherapy is the main stimulus for the induction of cell death and of Hsp70 release of p53 mutated glioblastoma cell lines. Radiat Oncol. 2014; 9(1):89. doi: 10.1186/1748-717X-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lumniczky K, Sáfrány G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett. 2015; 356(1):114–125. [DOI] [PubMed] [Google Scholar]
- 51. Vermeer DW, Spanos WC, Vermeer PD, et al. . Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013; 133(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamazaki T, Hannani D, Poirier-Colame V, et al. . Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014; 21(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serrano-del Valle A, Anel A, Naval J, Marzo I. Immunogenic cell death and immunotherapy of multiple myeloma. Front Cell Dev Biol. 2019; 7: 50. doi: 10.3389/fcell.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ko A, Kanehisa A, Martins I, et al. . Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014; 21(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015; 25(1):11–17. [DOI] [PubMed] [Google Scholar]
- 56. Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune priming of the tumor microenvironment by radiation. Trends Cancer. 2016; 2(11):638–645. [DOI] [PubMed] [Google Scholar]
- 57. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015; 348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 58. Reits EA, Hodge JW, Herberts CA, et al. . Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006; 203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rudqvist NP, Pilones KA, Lhuillier C, et al. . Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res. 2018; 6(2):139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bouffet E, Larouche V, Campbell BB, et al. . Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016; 34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 61. Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014; 54(2):289–296. [DOI] [PubMed] [Google Scholar]
- 62. Khoo LT, Chen LY. Role of the cGAS-STING pathway in cancer development and oncotherapeutic approaches. EMBO Rep. 2018; 19(12):e46935. doi: 10.15252/embr.201846935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fuertes MB, Domaica CI, Zwirner NW. Leveraging NKG2D ligands in immuno-oncology. Front Immunol. 2021; 12:713158. doi: 10.3389/fimmu.2021.713158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sato H, Jeggo PA, Shibata A. Regulation of programmed death‐ligand 1 expression in response to DNA damage in cancer cells: implications for precision medicine. Cancer Sci. 2019; 110(11):3415–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jablonska PA, Diez-Valle R, Pérez-Larraya JG, et al. . Hypofractionated radiation therapy and temozolomide in patients with glioblastoma and poor prognostic factors. A prospective, single-institution experience. PLoS One. 2019; 14(6):e0217881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Trone JC, Vallard A, Sotton S, et al. . Survival after hypofractionation in glioblastoma: a systematic review and meta-analysis. Radiat Oncol. 2020; 15(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kickingereder P, Hamisch C, Suchorska B, et al. . Low-dose rate stereotactic iodine-125 brachytherapy for the treatment of inoperable primary and recurrent glioblastoma: single-center experience with 201 cases. J Neurooncol. 2014; 120(3):615–623. [DOI] [PubMed] [Google Scholar]
- 68. Gessler DJ, Ferreira C, Dusenbery K, Chen CC. GammaTile®: surgically targeted radiation therapy for glioblastomas. Future Oncol. 2020; 16(30):2445–2455. [DOI] [PubMed] [Google Scholar]
- 69. Scartoni D, Amelio D, Palumbo P, Giacomelli I, Amichetti M. Proton therapy re-irradiation preserves health-related quality of life in large recurrent glioblastoma. J Cancer Res Clin Oncol. 2020; 146(6):1615–1622. [DOI] [PubMed] [Google Scholar]
- 70. Montay-Gruel P, Acharya MM, Gonçalves Jorge P, et al. . Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 2021; 27(3):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sadik ZHA, Hanssens PEJ, Verheul JB, et al. . Gamma knife radiosurgery for recurrent gliomas. J Neurooncol. 2018; 140(3):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10): 997–1003. [DOI] [PubMed] [Google Scholar]
- 73. Phillips HS, Kharbanda S, Chen R, et al. . Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006; 9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 74. Verhaak RGW, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010; 17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang Q, Hu B, Hu X, et al. . Tumor evolution of glioma intrinsic gene expression subtype associates with immunological changes in the microenvironment. Cancer Cell. 2017; 32(1):42–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Halliday J, Helmy K, Pattwell SS, et al. . In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci USA. 2014; 111(14):5248–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bhat KPL, Balasubramaniyan V, Vaillant B, et al. . Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013; 24(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schmitt MJ, Dramaretska Y, Barozzi I, et al. . Phenotypic mapping of pathological crosstalk between glioblastoma and innate immune cells by synthetic genetic tracing. Cancer Discov. 2021; 11(3):754–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lan X, Jörg DJ, Cavalli FMG, et al. . Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017; 549(7671):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mao P, Joshi K, Li J, et al. . Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA. 2013; 110(21):8644–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Minata M, Audia A, Shi J, et al. . Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 2019; 26(7):1893–1905.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rustenhoven J, Drieu A, Mamuladze T, et al. . Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021; 184(4):1000–1016.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Choi BD, Maus MV, June CH, Sampson JH. Immunotherapy for glioblastoma: adoptive T-cell Strategies. Clin Cancer Res. 2019; 25(7):2042–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020; 20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gromeier M, Brown MC, Zhang G, et al. . Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun. 2021; 12(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chongsathidkiet P, Jackson C, Koyama S, et al. . Sequestration of T-cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018; 24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zeng J, See AP, Phallen J, et al. . Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013; 86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim JE, Patel MA, Mangraviti A, et al. . Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017; 23(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Belcaid Z, Phallen JA, Zeng J, et al. . Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014; 9(7):e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Patel MA, Kim JE, Theodros D, et al. . Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J ImmunoTher Cancer. 2016; 4:28. doi: 10.1186/s40425-016-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Song E, Mao T, Dong H, et al. . VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020; 577(7792):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chauvin C, Joalland N, Perroteau J, et al. . NKG2D controls natural reactivity of Vγ9Vδ2 T lymphocytes against mesenchymal glioblastoma cells. Clin Cancer Res. 2019; 25(23):7218–7228. [DOI] [PubMed] [Google Scholar]
- 93. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018; 78(4):1031–1043. [DOI] [PubMed] [Google Scholar]
- 94. Vanderbeek AM, Rahman R, Fell G, et al. . The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro-Oncology. 2018; 20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lara-Velazquez M, Shireman JM, Lehrer EJ, et al. . A comparison between chemo-radiotherapy combined with immunotherapy and chemo-radiotherapy alone for the treatment of newly diagnosed glioblastoma: a systematic review and meta-analysis. Front Oncol. 2021; 11:662302. doi: 10.3389/fonc.2021.662302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gutin PH, Iwamoto FM, Beal K, et al. . Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009; 75(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sahebjam S, Forsyth PA, Tran ND, et al. . Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: results from a phase I study. Neuro Oncol. 2020; 23(4):677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rampling R, Peoples S, Mulholland PJ, et al. . A cancer research UK first time in human phase i trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016; 22(19):4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Reardon DA, Kaley TJ, Dietrich J, et al. . Phase 2 study to evaluate safety and efficacy of MEDI4736 (durvalumab [DUR]) in glioblastoma (GBM) patients: An update. J Clin Oncol. 2017; 35(15_suppl):2042–2042. [Google Scholar]
- 100. Boothe D, Young R, Yamada Y, et al. . Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013; 15(9):1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ager CR, Reilley MJ, Nicholas C, et al. . Intratumoral STING activation with T-cell checkpoint modulation generates systemic antitumor immunity. Cancer Immunol Res. 2017; 5(8):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Corrales L, Glickman LH, McWhirter SM, et al. . Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015; 11(7):1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ager CR, Zhang H, Wei Z, et al. . Discovery of IACS-8803 and IACS-8779, potent agonists of stimulator of interferon genes (STING) with robust systemic antitumor efficacy. Bioorg Med Chem Lett. 2019; 29(20):126640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Goedegebuure RSA, Kleibeuker EA, Buffa FM, et al. . Interferon- and STING-independent induction of type I interferon stimulated genes during fractionated irradiation. J Exp Clin Cancer Res. 2021; 40(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Borovski T, Bek e P, van Tellingen O, et al. . Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene. 2013; 32(12):1539–1548. [DOI] [PubMed] [Google Scholar]
- 106. Goedegebuure RSA, Vonk C, Kooij LP, Derks S, Thijssen VLJL. Combining radiation therapy with interferons: back to the future. Int J Radiat Oncol Biol Phys. 2020; 108(1):56–69. [DOI] [PubMed] [Google Scholar]
- 107. Alghamri MS, Banerjee K, Mujeeb AA, et al. . Systemic delivery of an adjuvant CXCR4–CXCL12 signaling inhibitor encapsulated in synthetic protein nanoparticles for glioma immunotherapy. ACS Nano. 2022; 16(6):8729–8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim JY, Son YO, Park SW, et al. . Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006; 38(5):474–484. [DOI] [PubMed] [Google Scholar]
- 109. Rincon-Orozco B, Kunzmann V, Wrobel P, et al. . Activation of Vγ9Vδ2 T Cells by NKG2D. J Immunol. 2005; 175(4):2144–2151. [DOI] [PubMed] [Google Scholar]
- 110. Bryant NL, Suarez-Cuervo C, Gillespie GY, et al. . Characterization and immunotherapeutic potential of γδ T-cells in patients with glioblastoma. Neuro Oncol. 2009; 11(4):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]