Abstract
The neuroinflammatory response that is elicited after spinal cord injury contributes to both tissue damage and reparative processes. The complex and dynamic cellular and molecular changes within the spinal cord microenvironment result in a functional imbalance of immune cells and their modulatory factors. To facilitate wound healing and repair, it is necessary to manipulate the immunological pathways during neuroinflammation to achieve successful therapeutic interventions. In this review, recent advancements and fresh perspectives on the consequences of neuroinflammation after SCI and modulation of the inflammatory responses through the use of molecular‐, cellular‐, and biomaterial‐based therapies to promote tissue regeneration and functional recovery will be discussed.
Keywords: central nervous system, inflammatory response, nerve regeneration, neural tissue engineering, tissue engineering scaffolds
1. INTRODUCTION
Spinal cord injury (SCI) is one of the leading causes of long‐term physical impairment, with an increase in global prevalence of approximately 368 per 100,000 patients over the last 30 years. 1 According to epidemiological studies, the most common causes of SCI across all populations result from falls, sports‐related injuries, and traffic accidents. 2 , 3 , 4 , 5 Patients with SCI usually suffer from temporary or permanent disabilities including loss of motor, sensory, and autonomic functions, and at times experience psychological stress, such as depression or a change in personality. 6 While these effects are debilitating for the patients, the consequences of SCI also extend to the families in the form of caretaking assistance and financial dependency.
Self‐recovery of neural functions after complete SCI is rare, in part due to a lack of plasticity and limited regenerative capacity of the neural tissues. 7 Following an initial mechanical injury that leads to tears, compression, and distortion to the spinal cord, vascular changes characterized by vasodilation, hyperemia, and petechial hemorrhages occur. 8 Progressively, the spinal cord undergoes a series of cellular and molecular changes, including edema, gliosis hyperplasia, formation of an intrinsic inhibitory environment, scarring, and neuroinflammation, which would hinder axonal regeneration. 8 , 9 , 10 Current SCI treatments remain palliative in the form of stabilization, surgical decompression, medication, and rehabilitation. 5 The complex and dynamic pathophysiological events after SCI, especially a cascade of immunological responses resulting from neuroinflammation, pose as a major challenge for many therapeutic interventions, including cell‐ and biomaterial‐based therapies. 11 Thus, a comprehensive review of the recent advancements in the cellular and molecular mechanisms involved in neuroinflammation after SCI is crucial to develop strategic interventions against this debilitating condition.
2. NEUROINFLAMMATION AFTER SCI
Neuroinflammation is defined as an inflammatory response that occurs within the brain or spinal cord. Upon damage to the blood‐spinal cord barrier (BSCB) after a physical trauma, neuroinflammation is one of the key components during the primary phase, which persists towards the secondary phase of injury. 8 , 12 The acute period of neuroinflammation is characterized by an infiltration of neutrophils and monocytes to the site of injury, 13 whereas in the chronic phase, the progressive tissue degeneration that takes place across a period of months is primarily driven by lymphocytes. 14 Inflammatory responses play a central role in regulating the pathophysiology after SCI, which greatly contributes to the repair of damaged tissues. 15 , 16 However, excessive inflammation may also lead to apoptosis of neurons and oligodendrocytes, resulting in a decline in neuronal functions. 16 Inevitably, changes within the spinal cord microenvironment during neuroinflammation may aggravate and accelerate the course of SCI.
3. MICROENVIRONMENT CHANGES DURING NEUROINFLAMMATION
During neuroinflammation, a cascade of cellular and molecular inflammatory pathways is activated, which includes the influx of circulating immune cells (neutrophils, monocytes, and lymphocytes), activation and proliferation of resident microglia and astrocytes, and the production of several mediators such as cytokines, chemokines, and reactive oxygen species by immune cells that reside in the central nervous system (CNS; Figure 1). 8 , 12 , 14 , 17 Paradoxically, while these secreted molecules are important in re‐establishing tissue homeostasis and assisting in wound healing and repair, 18 , 19 there are also collateral effects of secondary damage by inhibiting axonal regeneration or causing neuronal hypersensitivity, leading to neuropathic pain. 20 , 21 , 22 Together, this imbalance may impair regenerative capacity and functional recovery.
FIGURE 1.
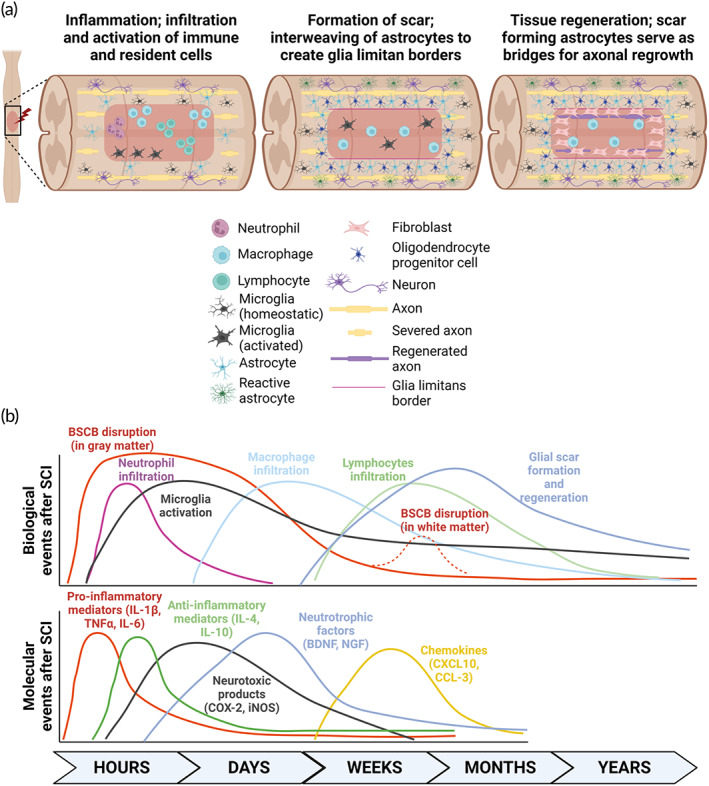
Schematic of the spinal cord microenvironment after spinal cord injury (SCI). (a) Within the first few hours after injury, inflammation occurs when peripheral immune cells begin infiltrating the lesion site, and resident immune cells become activated. Progressively, peri‐lesion perimeters with multicellular components including astrocytes, neurons, macrophages, microglia, oligodendrocyte progenitor cells, fibroblast, and activated astrocytes start to form a compact astrocyte core, regulating the formation of a glial scar to restrict inflammation and protect the surrounding of the injured tissue. These scar‐forming astrocytes serve as bridges for axonal regrowth, and structural tissue regeneration occurs weeks to months after SCI. (b) Timeline of both biological and molecular events following SCI. Illustrations are adapted from Donnelly and Popovich 41 and created with BioRender.com.
3.1. Cellular imbalance
3.1.1. Peripheral immune cells
Within a few hours after SCI, the first immune cell type to arrive at the site of injury is the neutrophils. 12 They secrete oxidative and proteolytic enzymes to sterilize the lesion and prepare the tissue for subsequent repair. 12 However, their presence is short‐lived approximately 3–5 days, plausibly due to their neurotoxic nature as neutrophils release potent free radicals. 8 , 12 A few days after neutrophils infiltration, monocyte‐derived macrophages are recruited to phagocytose dead cells including apoptotic neutrophils from the lesion. 23 Interestingly, these macrophages have been reported to also secrete factors such as resolvins and protectins to prevent further recruitment of neutrophils to the damaged tissue. 24 Unlike neutrophils, macrophages reside in the SCI lesions for as long as a year in humans. 12 , 25 While the recruitment of these innate immune cells serves to promote neuronal regeneration, wound healing, and tissue repair, both cell types instinctively produce proteases including matrix metalloproteinases (MMP), and oxidative metabolites that would compromise the BSCB. 12 , 26 , 27
On the other hand, adaptive immune cells such as the T‐ and B‐lymphocytes also infiltrate the lesion site, albeit only after weeks to months later. 12 , 14 SCI‐induced T‐lymphocytes typically have a life span of 1–2 months and are also involved in the recovery and regeneration of the spinal cord tissues. 28 , 29 Reportedly, T‐lymphocytes elicit their neuroprotective capability through the recognition of specific neural antigens, such as myelin basic protein (MBP), whereby a drastic improvement in the rate of neuronal survival was observed. 30 , 31 However, even though T‐lymphocytes are relatively lower in numbers than macrophages, they are also capable in inflicting tissue damage, albeit controversially, through the recognition of the same MBP antigen. 32 These opposing outcomes arise, depending on the spinal cord microenvironment at the time of injury, which would drive the equilibrium towards either a pathogenic Th1 or immunoregulatory Th2 lymphocytes expansion. 33 For instance, in the event of more regulatory T‐lymphocytes recruitment to the lesion, there could be a more robust expression of neurotrophins, which would ameliorate the tissue damage induced by the secreted pro‐inflammatory cytokines. 34 In association to an increase in T‐lymphocytes infiltration, there is an acute upregulation of cell death‐related genes and potassium voltage‐gated channel‐related (Kv) genes. 35 , 36 The high expression of Kv genes such as contactin‐2 (CNTN2) typically occurs in response to early demyelination in rats. 36 Furthermore, chronic T‐cell activation is shown to be involved in pathological tissue fibrosis and scarring. 37
Since neural gene‐specific proteins such as anti‐MBP antibodies are detected after SCI, B‐lymphocytes are also involved during neuroinflammation. 32 Mice deficient in B‐lymphocytes exhibited an improved locomotor function and reduced spinal pathology, indicating a pathogenic role of these cells in spinal cord tissue repair. 38 The antibodies produced by SCI‐induced B‐lymphocytes are shown to be neurotoxic as the passive transfer of sera from SCI animals induced glial reactivity that is accompanied by prominent neuron loss. 14 Interestingly, concomitant tissue injury may induce anti‐CNS antibodies that are able to promote axonal regeneration and remyelination. 14 , 39 For instance, antibodies targeting myelin may cause spinal cord demyelination, however, some antibodies prevent the binding by other myelin proteins that are inhibitory to axon growth and remyelination. 14 , 40 Together, there is a significant and long‐term contribution of peripheral immune cells during neuroinflammation within the spinal cord microenvironment.
3.1.2. Resident immune cells of the CNS
Apart from the peripheral circulating innate and adaptive immune cells, resident cells of the CNS, such as microglia and astrocytes, also play crucial roles during neuroinflammation after SCI. Having the same progenitor as tissue macrophages, the microglia comprise 10% of the population in the CNS. 42 These cells perform primary immunosurveillance functions of the tissue microenvironment, where they become elevated on the first day after SCI, and rapidly induce the production of cytokines and chemokines to recruit peripheral macrophages to the site of injury. 43 , 44 , 45 Trophic factors secreted by microglia are necessary for the survival and proliferation of infiltrating cells, as well as the growth and regeneration of axons in the spinal cord lesion. 46 , 47 At the same time, microglia may also help to prevent further expansion of the lesion site. 48 While the microglia responding to the damage after SCI is associated with tissue reorganization, it was reported to impede functional recovery of the neural tissue through the production of MMP‐9, which has been widely reported to amplify pro‐inflammatory cytokine secretion and affect the BSCB integrity, thereby interfering with plasticity and recovery. 49 , 50
Astrocytes are found in two areas of SCI lesion: (1) tissues that are spared by injury and (2) scar borders. The phenotype and functions of the astrocytes are distinct in both compartments. 51 Astrocytes that reside in spared tissues are reactive, non‐proliferative, and hypertrophic, and they primarily intermingle with neurons and synapses. 51 These hypertrophic astrocytes interact closely with neurons to promote axon sprouting and synapse plasticity through regulating the expression of neurocan, tenascin‐C, or directly producing thrombospondin‐1. 52 , 53 , 54 On the other hand, scar‐forming astrocytes are majority spontaneously proliferated upon damage, where they interweave to create glia limitans borders that restrict inflammation and keep non‐neural lesion core apart from adjacent functioning spinal cord tissue. 55 , 56 Surprisingly, axonal regeneration is not impeded by the presence of astrocyte scar formation as these scar‐forming astrocytes may serve as bridges for axonal growth. 57 Instead, the disruption of the scar tissues, shown through the use of loss‐of‐function transgenic mice that selectively kill proliferating scar‐forming astrocytes, led to an attenuation of axon growth after SCI. 58
Astrocyte scar borders are intertwined with reactive oligodendrocyte progenitor cells that express neuron glial antigen 2 (NG2‐OPCs). Similarly, NG2‐OPCs are also present in both the spared tissues and scar borders. However, there have been several conflicting studies on axonal regrowth by these hypertrophic NG2‐OPCs within the scar borders, 59 , 60 , 61 , 62 , 63 which warrant further investigations to understand the roles of these cells during neuroinflammation. Overall, the roles of these spinal cord neural cells play an important role in regulating tissue damage after SCI.
3.2. Molecular imbalance
3.2.1. Cytokines and chemokines
Cytokines are regulatory mediators that contribute immensely during neuroinflammation, neurodegeneration, and neuropathic pain through intricate cross‐talks and interplays. 64 They are usually classified into pro‐inflammatory or anti‐inflammatory proteins, 64 although some cytokines may exhibit pro‐inflammatory and anti‐inflammatory properties under various circumstances. 65 Endogenous cells in the spinal cord, mainly the neurons, microglia, and astrocytes, support the early production of key inflammatory mediators, such as interleukin (IL)‐1β, IL‐6, and tumor necrosis factor‐alpha (TNFα). 66 , 67 , 68 , 69 These pro‐inflammatory cytokines, along with others including granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and leukocyte inhibitory factor (LIF), contribute to the dynamic imbalance within the spinal cord microenvironment. 66 , 67 , 70 At low concentrations, these cytokines elicit protective functions by inducing neurotrophic factors and adhesion molecules on the cell surface, which assist in leukocyte recruitment to the injury site. 71 However, at a higher concentration, their pro‐inflammatory nature typically causes neuronal damage and destruction through the activation of transcription factors that stimulate the expression of neurotoxic genes such as cyclooxygenase 2 (COX‐2) and inducible nitric oxide synthase (iNOS). 72 , 73 High amounts of IL‐1 within the spinal cord microenvironment result in increased vascular permeability and lymphocyte recruitment, while IL‐6 promotes the activation and infiltration of peripheral immune cells and microglia. 74 Blockade of IL‐6 signaling was reported to enhance SCI recovery as it abrogates damaging inflammatory activity and reduces the severity of connective tissue scar formation. 74 , 75 TNFα is involved in several aspects of SCI neuroinflammation. Upon secretion, TNFα promotes the extravasation of neutrophils to the damaged tissue through adhesion molecules including vascular cell adhesion molecule‐1 (VCAM‐1) and intracellular adhesion molecule‐1 (ICAM‐1). 76 TNFα also induces changes to the permeability of endothelial cells, thereby compromising the integrity of the BSCB. 76 In addition, this pro‐inflammatory cytokine exerts cytotoxic effects on oligodendrocytes, resulting in demyelination. 76 Furthermore, TNFα also contributes to fibrotic scarring by stimulating the proliferation and hypertrophy of astrocytes. 77
Anti‐inflammatory cytokines including IL‐4 and IL‐10 are also produced to regulate and aid in functional recovery after SCI. 78 IL‐4 is secreted by activated T‐lymphocytes and is involved in the Th2 immunoregulatory pathway where it regulates the activation of acute macrophages and restrict secondary cavity formation after SCI. 79 In addition, IL‐4 also drives microglia and macrophages toward an anti‐inflammatory phenotype that reduces tissue damage, thereby leading to an improved functional recovery. 80 The production of IL‐10 by monocytes/macrophages, astrocytes, and microglia functions to suppress the inflammatory responses through the reduction of TNFα, IL‐1β, S100β, and iNOS. 15 , 78 , 81 IL‐10 is involved in regulating the influx and efflux of macrophages out of the injured nerve, reducing the production of pro‐inflammatory chemokines and cytokines, and it is necessary for myelin‐phagocytosis‐induced shift of macrophages from pro‐inflammatory to anti‐inflammatory. 82 Furthermore, the loss of IL‐10 affects axon regeneration, resulting in a poor recovery of motor and sensory functions. 82 More recently, a scaffold that comprise photocrosslinked gelatin hydrogel that was incorporated with polyamidoamine and IL‐10 enhanced tissue remodeling and promoted axonal regeneration. 83
On the other hand, chemokines are small, secreted molecules that stimulate specific functions during inflammation. The kinetics of chemokine production usually parallel the infiltration of immune cells after SCI. 45 Chemokines that belong to the α family (CXC) primarily participate in chemotaxis functions, whereas those in the β family (CC) provide priming signal for immune cells. 76 For instance, CXCL10 is involved in T‐lymphocyte recruitment after SCI, which contributes to post‐traumatic tissue loss, 84 while CCL3 enhances the production of other pro‐inflammatory cytokines through the G‐protein coupled receptors CCR1, CCR4, and CCR5, leading to an exacerbation of inflammation that contributes to secondary tissue damage after SCI. 85 Taken together, the unregulated production of inflammatory mediators, albeit molecularly small, can lead to disastrous consequences toward functional recovery after SCI.
3.2.2. Neurotrophic factors
The levels of growth promoting and inhibiting factors become disproportionate after SCI, resulting in an inhibitory environment within the spinal cord tissue. Neurotrophic molecules have been reported to enhance the survivability and proliferation capacity of neural cells and axonal regeneration within the spinal cord. 86 As such, an imbalance in these factors can lead to oligodendrocyte and neuronal death, as well as axonal degeneration. The most common neurotrophic factors include brain‐derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin‐3 (NT‐3). 86 , 87 These neurotrophic mediators are synthesized as pro‐peptides, which are cleaved intracellularly into mature neurotrophic proteins. 88
BDNF is a key molecule that plays a neuroprotective role by regulating synaptic plasticity and contributing to synaptic transmission. 89 However, its expression level is reduced drastically after SCI, and the overexpression of BDNF alleviates neuroinflammation through the induction of tyrosine kinase receptor B and phosphorylated p38. 90 NGF expression after SCI demonstrated improved behavioral outcomes by promoting axonal sprouting of the sensory afferents. 91 However, NGF has also been associated with neuropathic pain after nerve injury, where the binding of NGF to its receptors activates several downstream signaling pathways including the MAPK pathway. 92 This in turn led to the activation of NF‐kB p65, which promotes the production of pro‐inflammatory cytokines such as TNF‐α and IL‐1β, resulting in the development and maintenance of pain. 93 , 94 Interestingly, the pro‐peptide of NGF, which is secreted in abundance after traumatic injuries, has been shown to reduce the number of oligodendrocytes through p75. 87 , 95 In addition, the complex formed between the precursor of NGF with Sortilin and p75 also triggers an apoptotic cascade. 96 Hence, the imbalance between neurotrophic factors and their precursors may also affect neural cell survival and death.
3.2.3. Ionic imbalance
It is understood that biochemical events associated with secondary tissue damage include the disruptions of ionic homeostasis of K+, Na+, and Ca2+ ion channels. 97 Following SCI, these channels are dysregulated due to damage to the cell membrane, as well as the release of pro‐inflammatory mediators by immune cells. 98 Disrupting the myelin sheath of axons within the spinal cord tissue causes the imbalance of K+ channels, which leads to further demyelination. 99 At the same time, the concentration of Na+ becomes upregulated intracellularly, while K+ and Mg2+ become upregulated extracellularly, which eventually results in cellular edema. 100 This ionic imbalance further triggers intracellular phospholipase activity and acidosis. 101 Specifically, damaged neurons after SCI release high concentrations of glutamate neurotransmitter, causing Ca2+ dysregulation, which compromises cellular machinery while increasing neural cell death. 102 , 103 , 104 Overall, ion imbalance plays a vital role in regulating the pathophysiology changes after SCI.
4. MANIPULATING NEUROINFLAMMATION TO TREAT SCI
Extensive attempts have been made in modulating neuroinflammation to improve recovery after SCI, either through blockade of detrimental immune cell functions and neurotoxic pathways or enhancing the production of reparative and restorative cells and molecules. These approaches range from molecular‐, cell‐ or biomaterial‐based therapies that target different aspects of neuroinflammation after SCI.
4.1. Molecular‐ and cell‐based therapies to improve SCI recovery
4.1.1. Depletion of immune cells and mediators
Therapeutic interventions that target specific cell types or intracellular signaling pathways have demonstrated positive prognosis in treating SCI. Neuroprotection can be achieved through the attenuation of peripheral immune cells infiltration by targeting adhesion molecules that are expressed on the surface of monocytes and/or neutrophils, which can rescue the capacity of donor cell populations to promote locomotor improvement after SCI. 105 , 106 , 107 , 108 For instance, antibodies that target CD11d/CD18 or α4β1 integrins expressed on monocyte, macrophages, and CD11d expressing microglia disrupt monocyte‐endothelial cell interactions and reduce both microglia and macrophage accumulation within the lesion site, leading to a reduction in tissue loss and increased functional recovery after SCI in rodent models. 109 , 110 , 111 , 112 The use of anti‐Ly6G antibodies to deplete neutrophils has also led to improved recovery outcomes. 108 Depletion of both neutrophils and monocytes showed an early reduction in oxidative stress, nonheme iron, and expression of MMP‐9 and stabilization of the BSCB, and thus greatly promoting neurological healing. 107 However, due to the double‐edged nature of neuroinflammation, some studies have also shown a negative impact on wound healing and neurological outcomes when neutrophils are depleted. 113 , 114
Depletion of B‐lymphocyte with therapeutic CD20 antibodies, such as rituximab or obinutuzumab, has also been used in modulating neuroinflammation and immunological events associated with SCI by reducing cell death and nitric oxide level. 115 These monoclonal antibodies also inhibit constitutive NF‐kB signaling pathways by reducing the phosphorylation of components involved in the NF‐kB pathway. 116 This is crucial as NF‐kB is one of the pivotal mediators of pro‐inflammatory gene expression, as well as the transcription of pro‐inflammatory cytokines, chemokines, and adhesion molecules. 117 In addition, therapeutic CD20 antibodies also led to lower expressions of TNFα and IL‐1β, which are associated with damage after SCI. 76 , 77 , 115 As B‐lymphocytes have a role in trafficking T‐cells into the CNS, 118 earlier findings have indicated that treatment with CD20 antibodies also affected T‐lymphocyte activation, plausibly due to a decrease in antigen presentation by B‐lymphocytes after depletion. 119 Meanwhile, directly depleting T‐lymphocytes by split‐dose gamma radiation after thymectomy in 4‐week‐old rats may also enhance neuronal survival after SCI. 120
Other than depleting immune cells or their adhesion factors, inhibition of cytokines or chemokines is another approach for limiting leukocyte infiltration and alleviating neuroinflammation. For instance, treatment with a broad‐spectrum chemokine receptor antagonist, vMIP‐II, reduces leukocyte influx and astrogliosis, while increasing axon and myelin sparing, and neuronal survival. 121 , 122 In addition, blocking the pro‐inflammatory cytokine, IL‐6, that promotes macrophages activation may also improve SCI recovery. Specifically, the monoclonal antibody, MR16‐1, that targets IL‐6 cytokine leads to the reduction of iNOS‐ and CD16/32+ macrophages, while promoting arginase‐1‐ and CD206+ macrophages. 74 Interestingly, the effects of IL‐6 inhibition are not only limited to macrophage or microglia, as it also alters astrocyte activation and ameliorates functional recovery after SCI. 75 , 123 Antagonizing CXCL10, the chemokine that is responsible for T‐lymphocyte recruitment, has led to reduced neuronal death, an increase in axonal regeneration, and improve functional recovery after SCI. 121 Furthermore, anti‐CXCL10 treatment also decreases the number of macrophages and B‐lymphocytes. 124 The use of infliximab, which targets the pro‐inflammatory cytokine, TNFα, as well as the genetic deletion of TNFα receptors drastically reduce neuroinflammation and oxidative injury while ameliorating neuropathic pain after SCI. 125 , 126 Exogenous administration of IL‐1 receptor antagonist also led to a reduction in apoptosis and blocks p38 mitogen‐activated protein kinase pathway. 127 Collectively, these findings suggest that targeting the inflammatory pathways is an alternative to improve neuroprotection and recovery after SCI.
4.1.2. Promoting or transplanting cells with reparative and restorative functions
Another approach to improve functions after SCI focuses on immunomodulation and promotion of reparative immune cells such as the anti‐inflammatory macrophages, either by pharmacological or transplantation therapies.
Pharmacological agents have been widely used to promote SCI recovery by reducing inflammation and redirecting immune cells toward the reparative pathway. One commonly used macrolide antibiotic, Azithromycin, has been reported to promote anti‐inflammatory macrophage activation, which limits the secondary injury process after SCI, leading to improved tissue recovery. 128 , 129 Another anti‐inflammatory drug, minocycline, when administered acutely in a SCI rodent model has efficiently modulated the resident microglia to reduce its pro‐inflammatory response while maintaining a pro‐regenerative environment. 130 Exogeneous administration of Maresin 1, a highly conserved specialized pro‐resolving mediator, has been demonstrated to resolve inflammatory responses by downregulating pro‐inflammatory cytokines such as CXCL1, CXCL2, CCL3, CCL4, IL‐6, and CSF3, silencing major inflammatory intracellular signaling pathways such as STAT1, STAT3, STAT5, p38, and ERK1/2, as well as altering macrophage activation toward the anti‐inflammatory phenotype. 131 A more recent and comprehensive review on other immunomodulatory agents in spinal cord injury can be found in Wu et al. 132
Stem cell therapies have recently garnered attention for SCI treatment due to their capability to differentiate and replace degenerated neural cells. 133 Transplanted stem cells have been shown to promote neuro‐ and vascular‐protective outcomes at different phases of SCI. 134 In addition to reorganizing the neuronal network, these cells also reduce local and systemic inflammation, support axonal regeneration and synaptic sprouting, and reduce glial scars. 134 The mechanisms of stem cell therapy are categorized into three distinct roles: (1) cell replacement, where transplanted cells differentiate into neuronal or vascular cells to compensate for the lost functions 135 , 136 ; (2) functional multipotency, where the secretion of trophic factors from transplanted cells contribute to new neuronal circuit regeneration 137 and (3) stem cell regeneration, where the transplanted stem cells activate regeneration of host neuronal stem cells. 138 Many stem cell types including mesenchymal stem cells, neuronal stem cells, olfactory ensheathing cells, and Schwann cells, have been extensively shown as promising cell sources for transplantation due to their capacity to ameliorate tissue damage and assist in functional recovery through immunomodulation, pro‐angiogenic signaling and neural differentiation. 134 , 139 , 140 In addition, these stem cells secrete mediators and cell adhesion molecules that play fundamental roles in improving tissue repair and regeneration, involving the activation of endogenous anti‐inflammatory macrophages and microglia. 133 , 141 , 142 , 143 However, the inflammatory microenvironment of the injured spinal cord can limit the regenerative capacity of endogenous or transplanted cells and lead to allograft rejection. 144 , 145 Hence, the exogenous administration of drugs to diminish the detrimental functions of immune cells have greatly facilitated the efficacy of cell‐based therapies against SCI. 146
One example is methylprednisolone (MP), which is widely known as a potent corticosteroid. MP has significant neuroprotective and immunosuppressive functions by triggering immune cells apoptosis and reducing inflammatory events. 147 , 148 , 149 Furthermore, it was documented that MP can inhibit the lipid peroxidation process and protect oligodendrocytes from apoptotic‐mediated neuronal death after SCI. 150 More importantly, through clinical trials and meta‐analysis, the use of MP has significantly improved motor scores in SCI patients. 151 , 152 However, according to the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS), MP is only recommended as an option for acute spinal injury treatment, and should only be taken with the prior knowledge that the evidence suggesting harmful side effects is more consistent than any suggestion of clinical benefit. 153
Cyclosporine A (CsA), a calcineurin inhibitor, is a potent inhibitor of T‐lymphocyte activation that is commonly used to prevent allograft rejection and graft‐versus‐host disease. 154 However, contrasting findings on the effectiveness of CsA on the survival of grafted stem cells and improve functional recovery have arisen. 155 , 156 The difference in outcomes could be attributed to the source of stem cells and the type of animal models. Another calcineurin inhibitor, tacrolimus (FK506), also exhibits potent immunosuppressive properties that reduce the extent of secondary injury after SCI. 157 Similar to CsA, FK506 also targets the T‐lymphocytes and inhibits their proliferation. A handful of studies on transplantation to treat SCI using various stem cells have reported the safety and efficacy of FK506 and its potency in promoting graft survival and improving motor functions. 158 , 159 , 160 The benefits of these calcineurin inhibitors are further enhanced when used in combination. 161 , 162
However, there remain several challenges in drug delivery to ameliorate neuroinflammation. For instance, the majority of the noninvasive route of drug delivery is less efficient in accessing the CNS, including the spinal cord, due to the presence of a BSCB. 163 In addition, most of the bioactive compounds that can pass through the BSCB are lipophilic, which may have reduced stability and half‐lives under physiological conditions, resulting in difficulties to maintain an optimal dosage. 164 More importantly, drug diffusion within the host may lead to off‐target effects, which has been reported with corticosteroids, where patients experienced severe side effects such as seizure, pneumonia, and haematemesis. 165 As clinical trials of corticosteroids in SCI have been relatively small, with an emphasis on subgroup effects, the use of corticosteroids in SCI should remain an area of controversy. 165 Thus, the involvement of biomaterial‐based approaches may help overcome some of the challenges faced during drug delivery.
4.2. Biomaterial‐based therapies to modulate neuroinflammation and treat SCI
4.2.1. Localized drug delivery
To tackle the challenges in drug delivery to the injured spinal cord, noninvasive strategies utilizing drug‐loaded nanoparticles have been developed to overcome the BSCB. 166 , 167 , 168 In recent years, nanoparticles with neuroinflammation‐targeting designs allowed more targeted delivery and had led to better recovery. 169 , 170
On the other hand, although it is more invasive, delivering the drugs in situ can bypass the BSCB and reach the injured site directly. Combined with a controlled‐release mechanism, localized drug delivery can reduce the potential side effects of the immunomodulation drugs. For instance, loading anti‐inflammation drugs in scaffolds or combining drug‐loaded micro/nanoparticles with a hydrogel had demonstrated a reduction in microglia/macrophages activation and pro‐inflammatory interleukins by ensuring that the local concentration of the drug is high enough to have a therapeutic effect (Table 2). 171 , 172 , 175 , 176 , 177 , 178 , 180 , 187 , 189 , 194 , 196 More importantly, the particles can be designed to selectively target the microglia/macrophages and control uptake kinetics by changing surface charge. 176 , 197 Other than low molecular weight anti‐inflammatory drugs, scaffolds loaded with growth factors, microRNAs, and anti‐inflammatory cytokine‐encoding lentivirus also showed promising effects in reducing macrophage/microglial activation and improving functional recovery. 185 , 190 , 191 These growth factors and microRNAs also have a direct effect on stimulating nerve regeneration, which makes them ideal candidates that could have a synergistic effect in both anti‐inflammation and nerve regeneration.
TABLE 2.
Selected drug‐loaded scaffold‐based approaches with immunomodulation features after spinal cord injury
| Scaffold material | Drug | Cell | Model | Results based on immune response | References |
|---|---|---|---|---|---|
| Small molecule drugs | |||||
| Alginate/PLGA microspheres | Minocycline and paclitaxel | – | Rat T8–T10 hemisection | Decreased ED1+ cells | 171 |
| Dextran sulfate | Minocycline hydrochloride | – | Rat C5 contusion | Reduced CD68+ cells, the percentage of M1 cells (%M1), M1/M2 ratio but no significant change of %M2 | 172, 173 |
| 3D‐biodegradable porous hybrid nanoscaffolds (Chitosan‐manganese dioxide) | Methylprednisolone | – |
In vitro THP1 monocytes, Mouse T8 hemisection |
Reduced expression of pro‐inflammatory cytokine genes (TNF, IL1b, IL6, CCL2, and CCL5) in vitro and in vivo; Reduced CD11b+ macrophage infiltration and glial scar in vivo |
174 |
| Agarose/PLGA‐nanoparticles | Methylprednisolone | – | Rat T9–T10 contusion |
Reduced number of ED1+ cells is correlated with the diffused drug; Diminished the expression of pro‐inflammatory related proteins including Calpain and iNOS |
175 |
| AC/PMMA‐nanoparticles | Mimetic‐drug compounds (To‐Pro3) | – | Mouse T11 compression | Selective uptake of the PMMA‐NPs by the activated CD11b+ microglia/macrophages | 176 |
| Glycol chitosan‐oxidized HA | Tauroursodeoxycholic acid | – | Rat T9 contusion | Decreased pro‐inflammatory cytokines (TNF α, IL‐1 β, IL‐6) and GFAP | 177 |
| PLGA–PEG–PLGA | Baricitinib | – | Rat SCI | Inhibited the phosphorylation of JAK2, STAT3 and suppressed the production of inflammatory cytokines; Inhibited M1 polarization in microglia | 178 |
| RADA16‐FGL | Taxol | – | Rat T9 contusion | Reduced CD68+ and GFAP+ cells | 179 |
| Acellular spinal cord scaffold | Bisperoxovanadium | – | Rat T9–T10 hemisection | Enhanced M2 polarization and decreased M1 polarization | 180 |
| Injectable PEG‐diacerein/Graphene oxide | Diacerein | – | In vitro BV‐2 microglia and astrocytes, Rat T9 compression | Decreased the microglial LPS‐induced inflammation and astrocytes hyperactivation; Decreased astrocytic scar area in vivo | 181 |
| Hybrid Fmoc‐grafted chitosan/Fmoc‐IKVAV hydrogel | Curcumin | – | Rat T9 transection | Increased Arg1+ cells and percentage of Arg1+/CD68+ | 182 |
| Electrospun PLLA | Ibuprofen and triiodothyronine (T3) | – | Rat T8 contusion | Decreased glutamate release and percentage of astrocytes | 183 |
| Glycol chitosan and oxidized hyaluronate | Gold nanoparticles conjugated with ursodeoxycholic acid | – | In vitro bone‐marrow‐derived macrophages, Rat T9 compression |
Combined with NIR, increase in local temperature decreased NO, TNF α, IL‐1 β Decreased CD68, CD86, TNF‐α, IL‐1 β through MAPKs in vivo |
184 |
| Proteins/peptides | |||||
| Collagen | NT3 | – | Rat T9–T10 hemisection | Reduced macrophage/microglial activation (Iba1, NG2) | 185 |
| HA/MC | Anti‐inflammatory peptide KAFAKLAARLYRKALARQLGVAA (KAFAK) and BDNF | – | Rat T10 compression |
Reduced pro‐inflammatory cytokines expression (TNF‐α, IL‐1β, IL‐6) and glial scar formation; Increased IL‐10 expression |
186 |
| Chitosan–Collagen | Serp‐1 | – | Rat T10 dorsal column crush injury | Reduced CD3+ T Cell Infiltration, no effect on F4/80 macrophages | 187 |
| Functionalized peptides: RADA16‐IKVAV/ RADA16‐RGD | Cocktail of growth factors | – | Rat T9 transection | Induced the populating CD68+/IBA1+ macrophages/microglia cells into M2 phenotypes, producing anti‐inflammatory factors. | 188 |
| PLGA–PEG–PLGA | Milk fat globule–epidermal growth factor 8 | – | Rat T9–T10 crush injury |
Promoted microglia conversion to M2 type; Decreased CD68+ macrophages and iNOS+ cells; Increased CD206+ cells |
189 |
| Nucleic acids | |||||
| Aligned PCLEEP fiber‐collagen | miR‐219/miR‐338 | – | In vitro rat microglia, Rat C5 hemisection |
Decreased activation of microglia and astrocytes in vitro; Decreased expressions of TNF‐α and GFAP in vivo. |
190 |
| PLG multichannel bridge | IL‐10 and IL‐4 encoding lentiviral vectors | – | Mouse T9–T10 hemisection | Induced polarization of macrophages to M2 phenotype | 191 |
| Extracellular vesicles | |||||
| Injectable F127–polycitrate–polyethyleneimine hydrogel | hMSC‐extracellular vesicles | – | Rat T10 transection | Reduced fibrotic scar, CD68+, and Iba1+ cells | 192 |
| PPFLMLLKGSTR‐HA | hMSC‐exosomes | – | Rat T9–T10 transection | Decreased expression of iNOS, damage 4‐hydroxynonenal (HNE) and 8‐hydroxy‐2′‐deoxyguanosine (OHdG) | 193 |
| Combinations of small molecules/proteins/nucleic acids | |||||
| Microenvironment‐responsive microsol electrospun fiber scaffold | IL‐4 plasmid‐loaded liposomes, NGF | – | Rat T9 hemisection | Sequential release of plasmids and NGF shifted immune cells subtype to down‐regulate the acute inflammation response, promoted the polarization of local microglia/macrophages to M2 phenotype, reduced scar tissue formation | 194 |
| Self‐assembling RGD‐PEG‐maleimide hydrogel depot | Methylprednisolone sodium succinate, bFGF, BDNF, and VEGF | – | Rat T10–T11 contusion |
Reduced Iba1 and CD68 expression RNA‐Seq shows reduced expressions of macrophages, monocytes, neutrophils, T‐lymphocytes, B‐lymphocytes, microglia markers |
195 |
Note: The phenotypes of macrophages and microglia are presented as reported by the respective studies. In these studies, M1 typically refers to the pro‐inflammatory phenotypes whereas M2 typically refers to the anti‐inflammatory phenotypes.
4.2.2. Scaffolds for cell delivery
In addition to drug delivery, tissue engineering scaffolds have emerged as a powerful platform in combination with cell‐based therapies as a form of regenerative intervention. A central component of tissue engineering is the use of biomaterials as a vehicle for cell transplantation by providing mechanical stability and support for cell adhesion and migration or recruiting endogenous progenitor cells from the surrounding tissues. 198 When the scaffolds are used to deliver cells, biomaterial scaffolds and cells synergistically controlled immune response and tissue regeneration (Table 3). 199 , 203 , 204 , 205 , 207 , 208 , 209 , 210 , 211 , 212 , 213 Notably, mesenchymal stem cells secrete immunomodulating substances such as exosomes and CCL‐2 to convert the macrophages/microglia into anti‐inflammatory phenotypes. 193 , 200 , 212 However, some implanted materials can evoke the host inflammatory response as they are regarded as foreign bodies that have been introduced to the site of lesion. 214 Hence, it would be highly beneficial to design the SCI scaffolds to be immunomodulatory through manipulating material chemistry and mechanical properties before combining with cells and drugs to achieve better recovery outcomes.
TABLE 3.
Selected scaffolds for cell delivery with immunomodulation features after spinal cord injury
| Scaffold material | Drug | Cell | Model | Results based on immune response | References |
|---|---|---|---|---|---|
| Scaffolds with cells | |||||
| Cell‐adaptable neurogenic hydrogel | – | ADSCs | Rat T9–T10 transection |
Recruited macrophages toward M2 phenotype with M2 macrophages containing exosome and increased expression of CD206; Reduced IL‐6, pAkt, and IL‐6/PI3K/Akt signaling |
199 |
| PLGA scaffold | – | hMSCs | Rat T9–T10 hemisection |
hMSCs survived well with PLGA scaffold; Diminished presence of CD3+ T‐cells; Mitigated invasion of iNOS‐carrying mononuclear leukocytes; Reduced number of CD68+ microglia/macrophage |
200 |
| PLGA scaffold | – | hMSCs | Rat T9–T10 hemisection | Soft scaffold with hMSCs reduced neural inflammatory markers of CD11b, nitrotyrosine (a marker of oxidative protein nitration), and GFAP | 201 |
| Fibrous PGA scaffold | – | hNPCs | Rat T10–T11 hemisection |
Reduced microglia/macrophage infiltration; Polarized microglia/macrophage from M1 to M2 type |
202 |
| Acellular spinal cord scaffold | – | bMSC | Rat T9–T10 hemisection | Decreased numbers of CD68+ macrophages (microglia) and CD6+ T cells | 203 |
| Recombinant spider silk protein (spidroin) and HA hydrogel | – | hNPC | In vitro human peripheral blood mononuclear cells | Spidroins but not with HA hydrogel increased the proportion of activated CD69+ CD4+ T cells, CD8+ T cells, B‐cells, and NK cells | 204 |
| Agarose/Carbomer/PEG+RGD + ECM | – | hMSC | Mouse T12 compression |
Increased amount of recruited macrophages; 10‐fold increase of Arginase I transcript |
205 |
| GelMa | – | miNSCs | Mouse T9–T10 transection | Reduced CD68+ reactive macrophages/microglia at the lesion site and at the rostral and caudal regions; inhibited astrocytic scar formation | 206 |
| Spinal cord‐derived ECM hydrogel | – | Stem cells from human apical papilla | In vitro mouse microglia |
Increased Arg1 expression; Decreased Nos2/Arg1 ratio |
207 |
| Decellularized spinal cord/electrospun PLGA shell | – | Rat NSC | Rat T10 transection |
Induced macrophage/microglia polarization toward M2 phenotype; Increased CD206+ cells and CD206/CD86 ratio |
208 |
| Nerve‐guide collagen scaffold | – | Rat MSC | Rat T9 hemisection |
No infiltrated neutrophils and lymphocytes; Induced M2 polarization (reduced CD68 and iNOS, increased CD206) |
209 |
| Scaffolds with both drugs and cells | |||||
| Fibrin hydrogel | Lycium barbarum oligosaccharide (LBO) | Nasal mucosa‐derived MSCs (EMSCs) | Rat T10 transection | Promoted microglia M2 polarization through PI3K–Akt–mTOR pathway | 210 |
| Pluronic F‐127, heparin | bFGF | Dental pulp stem cells | Rat T9 crush injury | Decreased microglia/macrophages activation and pro‐inflammatory cytokine (IL‐6, NF‐κB, TNF‐α) | 211 |
| Agarose/Carbomer/PEG+RGD + ECM | human chemokine (C—C motif) ligand 2 (hCCL2) | hMSC | Mouse T12 compression | Increased macrophages recruitment and conversion to M2 phenotype | 212 |
Note: The phenotypes of macrophages and microglia are presented as reported by the respective studies. In these studies, M1 typically refers to the pro‐inflammatory phenotypes whereas M2 typically refers to the anti‐inflammatory phenotypes.
4.2.3. Material chemistry
Traditionally, implantable biomaterials have been designed to be biocompatible by evading the immune system and minimizing foreign body responses. Earlier studies on implants in the CNS found that many of the materials and coatings might be pro‐inflammatory and have low biocompatibility. 215 To improve material biocompatibility, low protein‐binding coatings such as alginate could be useful in reducing microglial attachment. 215 However, such an approach also limits the attachment of other neural cells that are essential for regeneration. Consequently, the focus has shifted toward exploiting the properties of the biomaterials to modulate the immune response and immune cell phenotypes to achieve the desired outcomes such as better regeneration. 216
While anti‐inflammatory effects were evaluated in most scaffolds in the form of reduced macrophage/microglial activation, more recent materials and scaffolds designed for SCI were increasingly assessing pro‐ and anti‐inflammatory phenotypic switching as a feature of immunomodulation. Thus far, the majority of the natural materials used including decellularized extracellular matrices (ECM), collagen, laminin, chitosan, hyaluronic acid (HA), gelatin, and fibrin have well‐documented biocompatibility and anti‐inflammatory effects (Table 1). 230 , 231 , 232 Furthermore, some of these materials such as collagen, chitosan fragments, high molecular weight HA can reduce activation of macrophages, microglia, and astrocytes while polarizing macrophages toward the anti‐inflammatory phenotypes. 209 , 217 , 221 , 233 , 234 Likewise, scaffolds developed based on decellularized tissue are rich with ECM proteins and hence can promote anti‐inflammatory macrophage polarization and recruit CD4+ Th2 T‐lymphocytes to provide a pro‐regenerative environment. 208 , 219 , 235 , 236 , 237 This is particularly crucial for cell delivery where small molecules produced by activated T‐lymphocytes might be cytotoxic to the grafted cells. 238
TABLE 1.
Selected scaffold‐based approaches with immunomodulation features after spinal cord injury
| Scaffold material | Drug | Cell | Model | Results based on immune response | References |
|---|---|---|---|---|---|
| Natural materials | |||||
| Fragmented chitosan hydrogel suspension (Chitosan–FPHS) | – | – | In vitro mouse monocytes, Rat T8–T9 hemisection |
Polarized macrophage polarization towards anti‐inflammatory phenotypes with decreasing degree of acetylation (DA) and increasing chitosan concentration; Decreased M1 macrophages with low DA chitosan‐FPHS implant in vivo |
217 |
| Chitosan–FPHS | – | – | Rat T8–T9 hemisection | Increased levels of M2 marker protein CD206 and Arg1 | 218 |
| Porcine brain‐derived decellularized extracellular matrix | – | – | In vitro rat macrophages, Rat T9 contusion |
Increased Arg1+ M2 macrophages and IL‐10 expression; Decreased CD86+ macrophages and increased Arg1+ M2 macrophages in vivo |
219 |
| Injectable optimized acellular nerve graft | – | – | Rat C4–C5 contusion | Increased the number of CD206+ M2 macrophages and expressions of CD206, arginase‐1 and IL‐10 | 220 |
| Methacrylated high molecular weight HA | – | – | Rat T7–T8 hemisection |
Decreased ED1+ macrophages; Limited astrocyte activation and CSPG deposition |
221 |
| Acetylated dextran microspheres | – | – | Rat T10 contusion |
Reduced GFAP+ astrocytes and CD68+ microglia; Reduced neuron death by sequestering glutamate and calcium ions |
222 |
| Synthetic/hybrid materials | |||||
| Imidazole‐polyorganophosphazenes (I‐5) hydrogel | – | – | Rat T10 contusion |
Decreased Iba1+ cells; Hydrogel interacted with macrophages and activated macrophage‐mediated wound healing responses |
223 |
| Hyaluronan/poly(ethylene glycol) diacrylate (HA/PEGDA) | – | – | Rat T9–T10 hemisection | Decreased Iba1+ cells and reactive astrocytes | 224 |
| Hyaluronan/methyl cellulose (HA/MC) | – | – | Rat T7 post‐traumatic syringomyelia (compression followed by subarachnoid injection of kaolin) | Decreased CSPG deposition and IL‐1α cytokine level but did not decrease neutrophil or macrophage/microglial activation | 225 |
| Graphene oxide | – | – | Rat C6 hemisection | Decreased ED1+ and Iba1+ cells with the presence of M2 macrophages | 226 |
| Poly(hydroxybutyrate‐co‐hydroxyvalerate)/polylactic acid/collagen (PHBV/PLA/Col) membrane duraplasty | – | – | Rat T10 contusion |
Decreased the expression of NLRP3, ASC, cleaved‐caspase‐1, IL‐1β, TNF‐α, and CD86 but increased the expression of CD206; Reduced the infiltration of CD86+ macrophages to the lesion site |
227 |
| PCL‐HA nanofiber‐hydrogel composite | – | – | Rat T9 contusion | Polarized Infiltrated macrophages towards M2 phenotype; M2 macrophages congregated in nanofiber‐rich areas | 228 |
| Aligned PEG tubes in fibrin | – | – | Mouse T9–T10 hemisection |
No difference in number of CD45+ leukocytes, Arg1+ M2 macrophages, Lyg6+ neutrophils, CD4+ T cells; Increased CD11c+dendritic cells, F4/80+ macrophages |
229 |
Note: The phenotypes of macrophages and microglia are presented as reported by the respective studies. In these studies, M1 typically refers to the pro‐inflammatory phenotypes whereas M2 typically refers to the anti‐inflammatory phenotypes.
Synthetic materials such as polyurethane (PU), polylactic acid (PLA), polylactic‐co‐glycolic acid (PLGA), polycaprolactone (PCL), graphene oxide, and imidazole‐polyorganophosphazenes, which have been used as scaffold materials for SCI regeneration, have also been assessed to reduce inflammation. 191 , 194 , 223 , 226 , 239 Although the anti‐inflammatory macrophages were observed in some of these scaffolds, the mechanism of how the materials polarize the macrophages is less clear. 223 , 226 Long‐term evaluation is also needed to confirm that the products from polymers degradation do not elicit an additional inflammatory response. Furthermore, caution should be exercised regarding the hydrophilicity of the polymer surfaces as monocytes/macrophages adhere better onto hydrophobic surfaces. 240 , 241 Therefore, it is desired to use coatings or additives to better control the immune response towards the polymer surfaces. In particular, ECM proteins or ECM‐derived peptides, which are effective in modulating macrophages, T lymphocytes, and B lymphocytes towards the anti‐inflammatory phenotypes, could be used to modify polymer surfaces. 232 Similarly, L1 cell adhesion molecules, which are natively found on cell surfaces, could reduce inflammatory microglial encapsulation in vivo when it was utilized as a coating. 242
4.2.4. Stiffness
Similar to the material chemistry of the SCI scaffolds, evaluations of the effect of scaffold mechanical properties on peripheral immune cell responses have been mainly performed on macrophages but are limited to other peripheral immune cells, such as neutrophils and lymphocytes. Nevertheless, the relationship between these immune cells and the mechanosensing of substrate stiffness is well‐established (Table 2), which could be referenced for SCI scaffold designs. 237 Depending on the range of substrate modulus tested, stiffer substrates generally stimulate higher activation and secretion of pro‐inflammatory cytokines from macrophages (130–840 kPa), dendritic cells (2–50 kPa), and neutrophils (0.2–128 kPa from two studies). 243 , 244 , 245 , 246 On the other hand, substrate stiffness had contrasting effects on different characteristics of T‐ and B‐lymphocytes. For example, human CD4+ and CD8+ T‐lymphocytes were activated and produced more cytokines on a substrate with stiffness at around 100 kPa as compared to substrates with stiffnesses of 0.5 kPa, 6.4 kPa, or 2 MPa. 247 , 248 For B‐lymphocytes, antigens on the stiffer substrates stimulated stronger activation responses in the range of substrate modulus tested (2.6–1100 kPa from two studies). However, the stiffer substrate (1100 kPa) had weaker B‐lymphocyte proliferation responses and in vivo antibody responses as compared to the softer substrate (20 kPa). 249 , 250
Substrate stiffness is a major contributing factor besides materials chemistry in triggering gliosis from astrocytes and microglia around implants in the CNS. A stiff substrate with a modulus of 30 kPa could activate both astrocytes and microglia into pro‐inflammatory phenotypes and secreted more TLR4, PPARγ, Caspase‐1, and IL‐1β, as compared to the more compliant substrate (100 Pa). 251 , 252 Likewise, increased astrogliosis and upregulation of inflammatory proteins were found in astrocytes on stiff substrates with moduli of 8 or 30 kPa as compared to the compliant 100–200 Pa soft substates. 251 , 253 Interestingly, A1 type reactive astrocytes with increased expression of IL‐1β and GFAP were observed in 3D soft hydrogel (43 Pa as compared to 991 Pa) instead, 254 suggesting the differences in modulus range and model dimension could lead to contrasting astrocyte response toward substrate stiffness. As the glial scar is also softer than the healthy spinal cord tissue and is correlated with astrocyte reactivity, 255 it is important for the scaffold to have a stiffness that matches the native tissue. In addition, regenerative approaches that involve glia scar digestion should also be cautious of the effect of matrix softening on astrocyte activation.
In general, softer or physiologically compliant scaffolds appear to induce less immune cells activation and pro‐inflammatory cytokines secretion. The future scaffold design could also explore manipulating the invading peripheral immune cells through scaffold stiffness.
4.2.5. Porosity and surface topography
Apart from having a tissue‐compliant stiffness, for better integration with host tissue and to provide contact guidance, scaffolds are usually designed to allow efficient cell infiltration, in which pore size was also found to regulate macrophage phenotypes. 256 , 257 , 258 , 259 Otherwise, the scaffolds may elicit FBR, which in turn leads to larger glial scar or cyst formation. In addition to the macroarchitecture of the scaffolds, the microarchitecture of the scaffolds is also crucial in modulating the immune response through the surface topography of the implants. 231 The responses of neural cells toward surface topography are frequently exploited for neural tissue engineering but less consideration has been placed on the inflammatory response post‐SCI. 260
Macrophage phenotype can be modulated by regulating cell shape through micro or nanopattern topographical cues. 261 Specifically, the elongated macrophages on the 400–500 nm wide nanopatterned grooves were driven toward an anti‐inflammatory phenotype. 262 Similarly, electrospun nanofiber scaffold has served as an alternative to providing topographical stimuli. In particular, a reduced number of macrophages, macrophage activation, and secretion of pro‐inflammatory molecules were found on PLA nanofiber (ø 600 nm) scaffolds as compared to films and microfibrous (ø 1.6 μm) scaffolds. 263 Similar results were also observed with PCL scaffolds. As compared to PCL films and random nanofibers, the aligned nanofibers (ø 506 nm) scaffolds had reduced monocyte/macrophage adhesion and a thinner fibrous capsule in vivo. 264 Recently, in a transplanted nanofiber‐hydrogel composite scaffold for SCI treatment, anti‐inflammatory macrophages were found to be present predominantly in the areas with the nanofibers, suggesting the possible role of nanofibers directly modulating immune cells phenotype. 228 On the other hand, while less is known about regulating lymphocytes and neutrophils through surface topography, lymphocytes and neutrophils found on implants with rough surfaces, created through sandblasting followed by acid‐etching or physical scratching, secreted less pro‐inflammatory cytokines. 265 , 266 , 267 In particular, rough and hydrophilic surfaces polarized the adaptive immune system toward the pro‐regenerative Th2 phenotype mediated by macrophages. 267
Similar to macrophages, nanofiber topography has a positive effect on astrocytes as nanofiber topography promoted astrocyte adhesion with downregulated GFAP expression, leading to reduced astrocytes activity. 239 Aligned electrospun fiber topography (ø 2.4 μm) also directed astrocytic migration and increased the rates of glutamate uptake as a readout for neuroprotective effect. 268 Conversely, aligned PLA microfibers (ø 1.8 μm) mildly induced cytotoxic A1 phenotype, which could be alleviated by the presence of transforming growth factor β3 (TGFβ3). 269 For microglia, a higher concentration of the pro‐inflammatory cytokine TNF‐α was detected in culture media on fibers (ø 1.1 μm) than on films. 270 This suggests that while microglia and macrophage are performing similar functions in phagocytosis, the response of these cells to the surface topography is different.
5. FUTURE PERSPECTIVES AND CONCLUSIONS
SCI elicits an inflammatory cascade that exerts a complex and dynamic microenvironment within the spinal cord tissue. Although substantial advances have been made to identify the cellular and molecular pathways that shape the immunological responses after SCI, appropriate interventions that involve the use of stem cells and/or biomaterials are necessary to avoid enhanced neuroinflammatory events that may derail tissue regeneration and recovery. While there remain limitations and challenges to current SCI therapies including the route of drug delivery to alleviate the immune responses, there are currently alternative approaches that increase the permeability into the BSCB through microbubble‐assisted focused ultrasound. 271 However, evaluation of the safety of such a strategy in human is underway, and clinical usage would require precise control over parameters to reduce inflammatory responses, glial cell activation, and tissue damage. 272
On the other hand, the future scaffold for treating SCI should include immunomodulation design to work synergistically with the strategies that promote nerve regeneration through neurite outgrowth, remyelination, and reduced glial scarring (Figure 2). Physical and chemical characteristics of the material for better immunomodulation outcomes should be included in future scaffold designs. Specifically, the combination of material chemistry (biocompatible), scaffold macroarchitecture (porous), surface topography (nanofibrous), surface coating (with favorable cell adhesion sites), stiffness (tissue stiffness‐matching), will likely give a favorable control for the immune response. 231 , 273 , 274 We will also expect to see more systemic anti‐inflammation or immunomodulation drug administration to synergistically enhance nerve regeneration with existing neural tissue engineering therapies. 275 , 276 Furthermore, other newer immunomodulation drugs (parthenolide, 277 14‐3‐3t, 278 miR‐194 279 ) and cell transplantations (olfactory ensheathing cells, 280 T‐lymphocytes 281 , 282 ) can be further explored and incorporated in the future strategies. In particular, thiazolidinediones and miR‐124 283 , 284 , 285 , 286 , 287 have demonstrated the ability to target both inflammatory response and neuronal differentiation making them promising candidates to be combined with scaffold‐mediated delivery approaches for treating SCI. Since the inflammation and regeneration processes involve different stages and different cell populations, scaffolds with a sequential delivery mechanism of drugs or physical signals targeting different stages could be more effective in promoting nerve regeneration and motor recovery after SCI. 194 , 288
FIGURE 2.
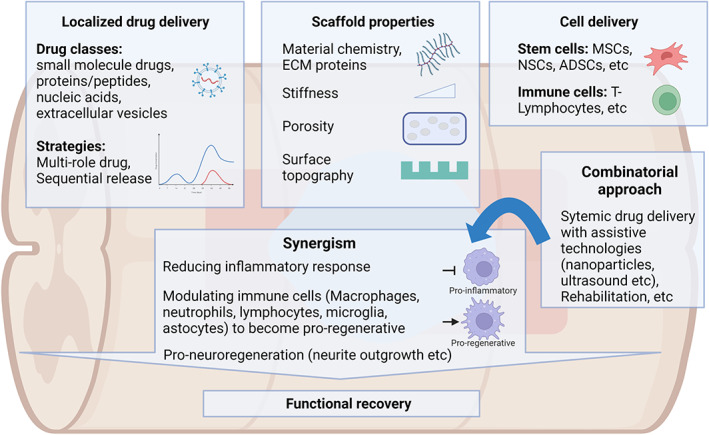
Biomaterial‐based therapies to modulate neuroinflammation and treat SCI. The combination of biomaterial design, drug delivery, cell therapy, and rehabilitation can be utilized to target neuroinflammation and neuroregeneration to achieve a synergistic effect in promoting functional recovery after SCI. Illustrations are created with BioRender.com.
Current immunomodulation approaches for treating SCI are mainly through immune response reduction and macrophage phenotypic shift. 289 , 290 , 291 , 292 It will be valuable to assess other immune cells and responses as well as target these mediators for better nerve regeneration. As discussed earlier, future scaffold designs may benefit from referring to the biomaterial approaches in targeting autoimmune diseases, graft rejection, and inflammation in other tissues. 216 , 282 , 293 , 294 , 295 , 296 , 297
Finally, including a rehabilitation regimen would also be beneficial as rehabilitation and scaffold implantation was found to synergistically promote the skewing of macrophage phenotype toward anti‐inflammatory phenotypes and better functional recovery. 298 , 299 A combinatorial approach will increase the likelihood of more successful immunomodulation and consequently functional recovery after SCI.
AUTHOR CONTRIBUTIONS
Cheryl Lee: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Wai Hon Chooi: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Shi Yan Ng: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Sing Yan Chew: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing – review and editing (equal). All authors approved this manuscript for publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10389.
ACKNOWLEDGMENTS
This work is supported by the National Research Foundation, Singapore, under its Intra‐CREATE Thematic Grant (Award number: NRF2019‐THE002‐0001).
Lee CY‐P, Chooi WH, Ng S‐Y, Chew SY. Modulating neuroinflammation through molecular, cellular and biomaterial‐based approaches to treat spinal cord injury. Bioeng Transl Med. 2023;8(2):e10389. doi: 10.1002/btm2.10389
Cheryl Yi‐Pin Lee and Wai Hon Chooi contributed equally to this study.
Funding information National Research Foundation, Singapore, Grant/Award Number: NRF2019‐THE002‐0001
Contributor Information
Shi‐Yan Ng, Email: syng@imcb.a-star.edu.sg.
Sing Yian Chew, Email: sychew@ntu.edu.sg.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18(1):24‐25. [DOI] [PubMed] [Google Scholar]
- 2. Wu Q, Li YL, Ning GZ, et al. Epidemiology of traumatic cervical spinal cord injury in Tianjin. China Spinal Cord. 2012;50(10):740‐744. [DOI] [PubMed] [Google Scholar]
- 3. Lenehan B, Street J, Kwon BK, et al. The epidemiology of traumatic spinal cord injury in British Columbia. Canada Spine. 2012;37(4):321‐329. [DOI] [PubMed] [Google Scholar]
- 4. DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011;92(3):332‐338. [DOI] [PubMed] [Google Scholar]
- 5. Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3(1):17018. [DOI] [PubMed] [Google Scholar]
- 6. O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259‐3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagappan PG, Chen H, Wang D‐Y. Neuroregeneration and plasticity: a review of the physiological mechanisms for achieving functional recovery postinjury. Mil Med Res. 2020;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21(4):429‐440. [DOI] [PubMed] [Google Scholar]
- 9. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 10. Banjara M, Ghosh C. Sterile Neuroinflammation and strategies for therapeutic intervention. Int J Inflam. 2017;2017:8385961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boisserand LSB, Kodama T, Papassin J, et al. Biomaterial applications in cell‐based therapy in experimental stroke. Stem Cells Int. 2016;2016:6810562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129(Pt 12):3249‐3269. [DOI] [PubMed] [Google Scholar]
- 13. Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL‐1 receptor/MyD88‐dependent fashion. Brain Behav Immun. 2010;24(4):540‐553. [DOI] [PubMed] [Google Scholar]
- 14. Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus‐like autoantibody synthesis. J Neurochem. 2006;99(4):1073‐1087. [DOI] [PubMed] [Google Scholar]
- 15. Genovese T, Esposito E, Mazzon E, et al. Absence of endogenous interleukin‐10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem. 2009;108(6):1360‐1372. [DOI] [PubMed] [Google Scholar]
- 16. Shen LF, Cheng H, Tsai MC, Kuo HS, Chak KF. PAL31 may play an important role as inflammatory modulator in the repair process of the spinal cord injury rat. J Neurochem. 2009;108(5):1187‐1197. [DOI] [PubMed] [Google Scholar]
- 17. Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462(2):223‐240. [DOI] [PubMed] [Google Scholar]
- 18. Okada S, Nakamura M, Katoh H, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829‐834. [DOI] [PubMed] [Google Scholar]
- 19. Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36(2):137‐144. [DOI] [PubMed] [Google Scholar]
- 20. Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brambilla R, Bracchi‐Ricard V, Hu WH, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo W, Wang H, Watanabe M, et al. Glial‐cytokine‐neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27(22):6006‐6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res. 2008;86(9):1944‐1958. [DOI] [PubMed] [Google Scholar]
- 24. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173‐182. [DOI] [PubMed] [Google Scholar]
- 25. Chang HT. Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal Cord. 2007;45(2):174‐182. [DOI] [PubMed] [Google Scholar]
- 26. Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526‐7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scholz M, Cinatl J, Schädel‐Höpfner M, Windolf J. Neutrophils and the blood‐brain barrier dysfunction after trauma. Med Res Rev. 2007;27(3):401‐416. [DOI] [PubMed] [Google Scholar]
- 28. Velardo MJ, Burger C, Williams PR, et al. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J Neurosci. 2004;24(39):8562‐8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartz M, Hauben E. T cell‐based therapeutic vaccination for spinal cord injury. Prog Brain Res. 2002;137:401‐406. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz M, Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7(6):252‐258. [DOI] [PubMed] [Google Scholar]
- 31. Evans FL, Dittmer M, de la Fuente AG, Fitzgerald DC. Protective and regenerative roles of T cells in central nervous system disorders. Front Immunol. 2019;10:2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ankeny DP, Popovich PG. B cells and autoantibodies: complex roles in CNS injury. Trends Immunol. 2010;31(9):332‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10(12):542‐550. [DOI] [PubMed] [Google Scholar]
- 34. Hammarberg H, Lidman O, Lundberg C, et al. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS‐infiltrating T and natural killer cells. J Neurosci. 2000;20(14):5283‐5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45(4):349‐363. [DOI] [PubMed] [Google Scholar]
- 36. Satzer D, Miller C, Maxon J, et al. T cell deficiency in spinal cord injury: altered locomotor recovery and whole‐genome transcriptional analysis. BMC Neurosci. 2015;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119(10):2990‐2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24(3):639‐647. [DOI] [PubMed] [Google Scholar]
- 40. Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008:209(2):378‐388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed]
- 42. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314‐1318. [DOI] [PubMed] [Google Scholar]
- 44. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388‐399. [DOI] [PubMed] [Google Scholar]
- 45. Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague‐Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443‐464. [DOI] [PubMed] [Google Scholar]
- 46. Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S. Inflammation‐induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16(2):99‐102. [DOI] [PubMed] [Google Scholar]
- 47. Shaked I, Tchoresh D, Gersner R, et al. Protective autoimmunity: interferon‐gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2005;92(5):997‐1009. [DOI] [PubMed] [Google Scholar]
- 48. Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia. 2009;57(15):1610‐1618. [DOI] [PubMed] [Google Scholar]
- 49. Hansen CN, Fisher LC, Deibert RJ, et al. Elevated MMP‐9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 2013;33(32):13101‐13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early‐ and late‐phase development of neuropathic pain. Nat Med. 2008;14(3):331‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18(7):942‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deller T, Haas CA, Naumann T, Joester A, Faissner A, Frotscher M. Up‐regulation of astrocyte‐derived tenascin‐C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience. 1997;81(3):829‐846. [DOI] [PubMed] [Google Scholar]
- 53. Haas CA, Rauch U, Thon N, Merten T, Deller T. Entorhinal cortex lesion in adult rats induces the expression of the neuronal chondroitin sulfate proteoglycan neurocan in reactive astrocytes. J Neurosci. 1999;19(22):9953‐9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tyzack GE, Sitnikov S, Barson D, et al. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3‐regulated TSP‐1 expression. Nat Commun. 2014;5:4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wanner IB, Anderson MA, Song B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3‐dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870‐12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang T, Dai Y, Chen G, Cui S. Dissecting the dual role of the glial scar and scar‐forming astrocytes in spinal cord injury. Front Cell Neurosci. 2020;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Busch SA, Horn KP, Cuascut FX, et al. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage‐induced axonal dieback after spinal cord injury. J Neurosci. 2010;30(1):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hackett AR, Lee JK. Understanding the NG2 glial scar after spinal cord injury. Front Neurol. 2016;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Filous AR, Tran A, Howell CJ, et al. Entrapment via synaptic‐like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci. 2014;34(49):16369‐16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26(14):3829‐3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203(1):8‐21. [DOI] [PubMed] [Google Scholar]
- 64. Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang J‐M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin‐1beta, interleukin‐6, and tumor necrosis factor‐alpha in human traumatic spinal cord injury. Spine. 2004;29(9):966‐971. [DOI] [PubMed] [Google Scholar]
- 67. Xia M, Zhu Y. The regulation of Sox2 and Sox9 stimulated by ATP in spinal cord astrocytes. J Mol Neurosci. 2015;55(1):131‐140. [DOI] [PubMed] [Google Scholar]
- 68. Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba‐1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mortazavi MM, Verma K, Harmon OA, et al. The microanatomy of spinal cord injury: a review. Clin Anat. 2015;28(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 70. Jin X, Yamashita T. Microglia in central nervous system repair after injury. J Biochem. 2016;159(5):491‐496. [DOI] [PubMed] [Google Scholar]
- 71. Ellison JA, Velier JJ, Spera P, et al. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29(8):1698‐1706. discussion 1707. [DOI] [PubMed] [Google Scholar]
- 72. Liu N‐K, Xu X‐M. Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regen Res. 2012;7(26):2051‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Y, Liu L, Barger SW, Mrak RE, Griffin WS. Vitamin E suppression of microglial activation is neuroprotective. J Neurosci Res. 2001;66(2):163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guerrero AR, Uchida K, Nakajima H, et al. Blockade of interleukin‐6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mukaino M, Nakamura M, Yamada O, et al. Anti‐IL‐6‐receptor antibody promotes repair of spinal cord injury by inducing microglia‐dominant inflammation. Exp Neurol. 2010;224(2):403‐414. [DOI] [PubMed] [Google Scholar]
- 76. Garcia E, Aguilar‐Cevallos J, Silva‐Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016;2016:9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Camand E, Morel MP, Faissner A, Sotelo C, Dusart I. Long‐term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur J Neurosci. 2004;20(5):1161‐1176. [DOI] [PubMed] [Google Scholar]
- 78. Ren H, Chen X, Tian M, Zhou J, Ouyang H, Zhang Z. Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agents. Adv Sci. 2018;5(11):1800529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee SI, Jeong SR, Kang YM, et al. Endogenous expression of interleukin‐4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010;88(11):2409‐2419. [DOI] [PubMed] [Google Scholar]
- 80. Francos‐Quijorna I, Amo‐Aparicio J, Martinez‐Muriana A, López‐Vales R. IL‐4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64(12):2079‐2092. [DOI] [PubMed] [Google Scholar]
- 81. Lau D, Harte SE, Morrow TJ, Wang S, Mata M, Fink DJ. Herpes simplex virus vector‐mediated expression of interleukin‐10 reduces below‐level central neuropathic pain after spinal cord injury. Neurorehabil Neural Repair. 2012;26(7):889‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Siqueira Mietto B, Kroner A, Girolami EI, Santos‐Nogueira E, Zhang J, David S. Role of IL‐10 in resolution of inflammation and functional recovery after peripheral nerve injury. J Neurosci. 2015;35(50):16431‐16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shen H, Xu B, Yang C, et al. A DAMP‐scavenging, IL‐10‐releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials. 2022;280:121279. [DOI] [PubMed] [Google Scholar]
- 84. Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184(1):456‐463. [DOI] [PubMed] [Google Scholar]
- 85. Pelisch N, Rosas Almanza J, Stehlik KE, Aperi BV, Kroner A. CCL3 contributes to secondary damage after spinal cord injury. J Neuroinflammation. 2020;17(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Keefe KM, Sheikh IS, Smith GM. Targeting Neurotrophins to specific populations of neurons: NGF, BDNF, and NT‐3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18(3):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Harrington AW, Leiner B, Blechschmitt C, et al. Secreted proNGF is a pathophysiological death‐inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101(16):6226‐6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33(1):9‐12. [DOI] [PubMed] [Google Scholar]
- 89. Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. 2001;14(11):1800‐1808. [DOI] [PubMed] [Google Scholar]
- 90. Liang J, Deng G, Huang H. The activation of BDNF reduced inflammation in a spinal cord injury model by TrkB/p38 MAPK signaling. Exp Ther Med. 2019;17(3):1688‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20(12):4435‐4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Molloy NH, Read DE, Gorman AM. Nerve growth factor in cancer cell death and survival. Cancers (Basel). 2011;3(1):510‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dai W‐L, Yan B, Bao Y‐N, Fan J‐F, Liu J‐H. Suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1‐MAPK/NF‐κB signaling pathways. Cell Commun Signal. 2020;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beattie MS, Harrington AW, Lee R, et al. ProNGF induces p75‐mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36(3):375‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF‐induced neuronal cell death. Nature. 2004;427(6977):843‐848. [DOI] [PubMed] [Google Scholar]
- 97. O'Hare Doig RL, Santhakumar S, Fehily B, et al. Acute cellular and functional changes with a combinatorial treatment of Ion Channel inhibitors following spinal cord injury. Front Mol Neurosci. 2020;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Garcia VB, Abbinanti MD, Harris‐Warrick RM, Schulz DJ. Effects of chronic spinal cord injury on relationships among Ion Channel and receptor mRNAs in mouse lumbar spinal cord. Neuroscience. 2018;393:42‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Alizadeh A, Dyck SM, Karimi‐Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lemke M, Demediuk P, McIntosh TK, Vink R, Faden AI. Alterations in tissue Mg++, Na+ and spinal cord edema following impact trauma in rats. Biochem Biophys Res Commun. 1987;147(3):1170‐1175. [DOI] [PubMed] [Google Scholar]
- 101. Liu WM, Wu JY, Li FC, Chen QX. Ion channel blockers and spinal cord injury. J Neurosci Res. 2011;89(6):791‐801. [DOI] [PubMed] [Google Scholar]
- 102. Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754‐774. [DOI] [PubMed] [Google Scholar]
- 103. Herrero‐Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C‐Cdh1. Nat Cell Biol. 2009;11(6):747‐752. [DOI] [PubMed] [Google Scholar]
- 104. Duchen MR. Mitochondria, calcium‐dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012;464(1):111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti‐CD 11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5(4):343‐352. [DOI] [PubMed] [Google Scholar]
- 106. Bao F, Bailey CS, Gurr KR, et al. Human spinal cord injury causes specific increases in surface expression of β integrins on leukocytes. J Neurotrauma. 2011;28(2):269‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee SM, Rosen S, Weinstein P, van Rooijen N, Noble‐Haeusslein LJ. Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J Neurotrauma. 2011;28(9):1893‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nguyen HX, Hooshmand MJ, Saiwai H, et al. Systemic neutrophil depletion modulates the migration and fate of transplanted human neural stem cells to rescue functional repair. J Neurosci. 2017;37(38):9269‐9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti‐inflammatory treatment. Exp Neurol. 2000;166(1):52‐64. [DOI] [PubMed] [Google Scholar]
- 110. Geremia NM, Bao F, Rosenzweig TE, et al. CD11d antibody treatment improves recovery in spinal cord‐injured mice. J Neurotrauma. 2012;29(3):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Saville LR, Pospisil CH, Mawhinney LA, et al. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156(1–2):42‐57. [DOI] [PubMed] [Google Scholar]
- 112. Bao F, Omana V, Brown A, Weaver LC. The systemic inflammatory response after spinal cord injury in the rat is decreased by α4β1 integrin blockade. J Neurotrauma. 2012;29(8):1626‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/gr‐1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29(3):753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation. 2014;11(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Casili G, Impellizzeri D, Cordaro M, Esposito E, Cuzzocrea S. B‐cell depletion with CD20 antibodies as new approach in the treatment of inflammatory and immunological events associated with spinal cord injury. Neurotherapeutics. 2016;13(4):880‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Baritaki S, Militello L, Malaponte G, Spandidos DA, Salcedo M, Bonavida B. The anti‐CD20 mAb LFB‐R603 interrupts the dysregulated NF‐κB/snail/RKIP/PTEN resistance loop in B‐NHL cells: role in sensitization to TRAIL apoptosis. Int J Oncol. 2011;38(6):1683‐1694. [DOI] [PubMed] [Google Scholar]
- 117. Liu T, Zhang L, Joo D, Sun S‐C. NF‐κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Anthony DC, Dickens AM, Seneca N, et al. Anti‐CD20 inhibits T cell‐mediated pathology and microgliosis in the rat brain. Ann Clin Transl Neurol. 2014;1(9):659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Merli M, Ferrario A, Maffioli M, Arcaini L, Passamonti F. Investigational therapies targeting lymphocyte antigens for the treatment of non‐Hodgkin's lymphoma. Expert Opin Investig Drugs. 2015;24(7):897‐912. [DOI] [PubMed] [Google Scholar]
- 120. Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA. 2002;99(24):15620‐15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J Neurosci Res. 2001;64(6):582‐589. [DOI] [PubMed] [Google Scholar]
- 122. Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion attenuates cellular infiltration following spinal cord contusion injury in rat. J Neurosci Res. 2000;59(1):63‐73. [PubMed] [Google Scholar]
- 123. Okada S, Nakamura M, Mikami Y, et al. Blockade of interleukin‐6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76(2):265‐276. [DOI] [PubMed] [Google Scholar]
- 124. Gonzalez R, Hickey MJ, Espinosa JM, Nistor G, Lane TE, Keirstead HS. Therapeutic neutralization of CXCL10 decreases secondary degeneration and functional deficit after spinal cord injury in mice. Regen Med. 2007;2(5):771‐783. [DOI] [PubMed] [Google Scholar]
- 125. Kurt G, Ergün E, Cemil B, et al. Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol. 2009;71(3):332‐336. discussion 336. [DOI] [PubMed] [Google Scholar]
- 126. Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst. 2006;11(4):294‐303. [DOI] [PubMed] [Google Scholar]
- 127. Wang XJ, Kong KM, Qi WL, Ye WL, Song PS. Interleukin‐1 beta induction of neuron apoptosis depends on p38 mitogen‐activated protein kinase activity after spinal cord injury. Acta Pharmacol Sin. 2005;26(8):934‐942. [DOI] [PubMed] [Google Scholar]
- 128. Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep. 2017;7:40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Papa S, Caron I, Erba E, et al. Early modulation of pro‐inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13‐24. [DOI] [PubMed] [Google Scholar]
- 131. Francos‐Quijorna I, Santos‐Nogueira E, Gronert K, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. 2017;37(48):11731‐11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wu X, Yan Y, Zhang Q. Neuroinflammation and modulation role of natural products after spinal cord injury. J Inflamm Res. 2021;14:5713‐5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shao A, Tu S, Lu J, Zhang J. Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther. 2019;10(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yamazaki K, Kawabori M, Seki T, Houkin K. Clinical trials of stem cell treatment for spinal cord injury. Int J Mol Sci. 2020;21(11):3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gao S, Guo X, Zhao S, et al. Differentiation of human adipose‐derived stem cells into neuron/motoneuron‐like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019;10(8):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Novikova LN, Brohlin M, Kingham PJ, Novikov LN, Wiberg M. Neuroprotective and growth‐promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy. 2011;13(7):873‐887. [DOI] [PubMed] [Google Scholar]
- 137. Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet‐Gendebien S. Concise review: spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells. 2014;32(4):829‐843. [DOI] [PubMed] [Google Scholar]
- 138. Ceci M, Mariano V, Romano N. Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment. Rev Neurosci. 2018;30(1):45‐66. [DOI] [PubMed] [Google Scholar]
- 139. Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fracaro L, Zoehler B, Rebelatto CLK. Mesenchymal stromal cells as a choice for spinal cord injury treatment. Neuroimmunol Neuroinflamm. 2020;7(1):1‐12. [Google Scholar]
- 141. Neves J, Sousa‐Victor P, Jasper H. Rejuvenating strategies for stem cell‐based therapies in aging. Cell Stem Cell. 2017;20(2):161‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gensel JC, Donnelly DJ, Popovich PG. Spinal cord injury therapies in humans: an overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin Ther Targets. 2011;15(4):505‐518. [DOI] [PubMed] [Google Scholar]
- 143. Cheng Z, Zhu W, Cao K, et al. Anti‐inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 2016;17(9):1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Führmann T, Anandakumaran PN, Shoichet MS. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater. 2017;6(10):1601130. [DOI] [PubMed] [Google Scholar]
- 145. Enomoto M. Therapeutic effects of neurotrophic factors in experimental spinal cord injury models. J Neurorestoratol. 2016;4(1):15‐22. [Google Scholar]
- 146. Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27(44):11782‐11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Donovan J, Kirshblum S. Clinical trials in traumatic spinal cord injury. Neurotherapeutics. 2018;15(3):654‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36‐42. [DOI] [PubMed] [Google Scholar]
- 149. Liu Z, Yang Y, He L, et al. High‐dose methylprednisolone for acute traumatic spinal cord injury: a meta‐analysis. Neurology. 2019;93(9):e841‐e850. [DOI] [PubMed] [Google Scholar]
- 150. Al Mamun A, Monalisa I, Tul Kubra K, et al. Advances in immunotherapy for the treatment of spinal cord injury. Immunobiology. 2021;226(1):152033. [DOI] [PubMed] [Google Scholar]
- 151. Cheung V, Hoshide R, Bansal V, Kasper E, Chen CC. Methylprednisolone in the management of spinal cord injuries: lessons from randomized, controlled trials. Surg Neurol Int. 2015;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cabrera‐Aldana EE, Ruelas F, Aranda C, et al. Methylprednisolone administration following spinal cord injury reduces aquaporin 4 expression and exacerbates edema. Mediators Inflamm. 2017;2017:4792932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hadley MN, Walters BC, Grabb PA, et al. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50(3 Suppl):S63‐S72. [DOI] [PubMed] [Google Scholar]
- 154. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119‐125. [DOI] [PubMed] [Google Scholar]
- 155. Nutt SE, Chang EA, Suhr ST, et al. Caudalized human iPSC‐derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. 2013;248:491‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Oh J, Lee KI, Kim HT, et al. Human‐induced pluripotent stem cells generated from intervertebral disc cells improve neurologic functions in spinal cord injury. Stem Cell Res Ther. 2015;6(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Guzmán‐Lenis MS, Vallejo C, Navarro X, Casas C. Analysis of FK506‐mediated protection in an organotypic model of spinal cord damage: heat shock protein 70 levels are modulated in microglial cells. Neuroscience. 2008;155(1):104‐113. [DOI] [PubMed] [Google Scholar]
- 158. Sevc J, Goldberg D, van Gorp S, et al. Effective long‐term immunosuppression in rats by subcutaneously implanted sustained‐release tacrolimus pellet: effect on spinally grafted human neural precursor survival. Exp Neurol. 2013;248:85‐99. [DOI] [PubMed] [Google Scholar]
- 159. Itakura G, Kobayashi Y, Nishimura S, et al. Control of the survival and growth of human glioblastoma grafted into the spinal cord of mice by taking advantage of Immunorejection. Cell Transplant. 2015;24(7):1299‐1311. [DOI] [PubMed] [Google Scholar]
- 160. Torres‐Espín A, Redondo‐Castro E, Hernandez J, Navarro X. Immunosuppression of allogenic mesenchymal stem cells transplantation after spinal cord injury improves graft survival and beneficial outcomes. J Neurotrauma. 2015;32(6):367‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. López‐Vales R, García‐Alías G, Forés J, et al. FK 506 reduces tissue damage and prevents functional deficit after spinal cord injury in the rat. J Neurosci Res. 2005;81(6):827‐836. [DOI] [PubMed] [Google Scholar]
- 162. Hayashi Y, Shumsky JS, Connors T, et al. Immunosuppression with either cyclosporine a or FK506 supports survival of transplanted fibroblasts and promotes growth of host axons into the transplant after spinal cord injury. J Neurotrauma. 2005;22(11):1267‐1281. [DOI] [PubMed] [Google Scholar]
- 163. Pandit R, Chen L, Götz J. The blood‐brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020;165–166:1‐14. [DOI] [PubMed] [Google Scholar]
- 164. Gribkoff VK, Kaczmarek LK. The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. 2017;120:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo‐controlled trial. Lancet. 2004;364(9442):1321‐1328. [DOI] [PubMed] [Google Scholar]
- 166. Letko Khait N, Ho E, Shoichet MS. Wielding the double‐edged sword of inflammation: building biomaterial‐based strategies for immunomodulation in ischemic stroke treatment. Adv Funct Mater. 2021;31(44):2010674. [Google Scholar]
- 167. Cerqueira SR, Ayad NG, Lee JK. Neuroinflammation treatment via targeted delivery of nanoparticles. Front Cell Neurosci. 2020;14:576037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of polymeric nanoparticles for blood‐brain barrier transfer‐strategies and challenges. Adv Sci. 2021;8(10):2003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Shen K, Sun G, Chan L, et al. Anti‐inflammatory Nanotherapeutics by targeting matrix metalloproteinases for immunotherapy of spinal cord injury. Small. 2021;17(41):e2102102. [DOI] [PubMed] [Google Scholar]
- 170. Zhu R, Zhu X, Zhu Y, et al. Immunomodulatory layered double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor‐beta receptor 2. ACS Nano. 2021;15(2):2812‐2830. [DOI] [PubMed] [Google Scholar]
- 171. Nazemi Z, Nourbakhsh MS, Kiani S, et al. Co‐delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145‐158. [DOI] [PubMed] [Google Scholar]
- 172. Wang Z, Nong J, Shultz RB, et al. Local delivery of minocycline from metal ion‐assisted self‐assembled complexes promotes neuroprotection and functional recovery after spinal cord injury. Biomaterials. 2017;112:62‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ghosh B, Nong J, Wang Z, et al. A hydrogel engineered to deliver minocycline locally to the injured cervical spinal cord protects respiratory neural circuitry and preserves diaphragm function. Neurobiol Dis. 2019;127:591‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yang L, Conley BM, Cerqueira SR, et al. Effective modulation of CNS inhibitory microenvironment using bioinspired hybrid‐Nanoscaffold‐based therapeutic interventions. Adv Mater. 2020;32(43):e2002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chvatal SA, Kim YT, Bratt‐Leal AM, Lee H, Bellamkonda RV. Spatial distribution and acute anti‐inflammatory effects of methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29(12):1967‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Papa S, Ferrari R, De Paola M, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15‐26. [DOI] [PubMed] [Google Scholar]
- 177. Han GH, Kim SJ, Ko WK, et al. Injectable hydrogel containing Tauroursodeoxycholic acid for anti‐neuroinflammatory therapy after spinal cord injury in rats. Mol Neurobiol. 2020;57(10):4007‐4017. [DOI] [PubMed] [Google Scholar]
- 178. Zheng XQ, Huang JF, Lin JL, et al. Controlled release of baricitinib from a thermos‐responsive hydrogel system inhibits inflammation by suppressing JAK2/STAT3 pathway in acute spinal cord injury. Colloids Surf B Biointerfaces. 2021;199:111532. [DOI] [PubMed] [Google Scholar]
- 179. Xiao Z, Yao Y, Wang Z, et al. Local delivery of Taxol from FGL‐functionalized self‐assembling peptide nanofiber scaffold promotes recovery after spinal cord injury. Front Cell Dev Biol. 2020;8:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu J, Li K, Zhou J, et al. Bisperoxovanadium induces M2‐type macrophages and promotes functional recovery after spinal cord injury. Mol Immunol. 2019;116:56‐62. [DOI] [PubMed] [Google Scholar]
- 181. Zhang K, Li J, Jin J, et al. Injectable, anti‐inflammatory and conductive hydrogels based on graphene oxide and diacerein‐terminated four‐armed polyethylene glycol for spinal cord injury repair. Mater Design. 2020;196:109092. [Google Scholar]
- 182. Luo J, Shi X, Li L, et al. An injectable and self‐healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact Mater. 2021;6(12):4816‐4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bighinati A, Focarete ML, Gualandi C, et al. Improved functional recovery in rat spinal cord injury induced by a drug combination administered with an implantable polymeric delivery system. J Neurotrauma. 2020;37(15):1708‐1719. [DOI] [PubMed] [Google Scholar]
- 184. Ko WK, Lee SJ, Kim SJ, et al. Direct injection of hydrogels embedding Gold nanoparticles for local therapy after spinal cord injury. Biomacromolecules. 2021;22(7):2887‐2901. [DOI] [PubMed] [Google Scholar]
- 185. Breen BA, Kraskiewicz H, Ronan R, et al. Therapeutic effect of neurotrophin‐3 treatment in an injectable collagen scaffold following rat spinal cord Hemisection injury. ACS Biomater Sci Eng. 2017;3(7):1287‐1295. [DOI] [PubMed] [Google Scholar]
- 186. He Z, Zang H, Zhu L, et al. An anti‐inflammatory peptide and brain‐derived neurotrophic factor‐modified hyaluronan‐methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int J Nanomedicine. 2019;14:721‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kwiecien JM, Zhang L, Yaron JR, et al. Local serpin treatment via chitosan‐collagen hydrogel after spinal cord injury reduces tissue damage and improves neurologic function. J Clin Med. 2020;9(4):1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Liu H, Xu X, Tu Y, et al. Engineering microenvironment for endogenous neural regeneration after spinal cord injury by reassembling extracellular matrix. ACS Appl Mater Interfaces. 2020;12(15):17207‐17219. [DOI] [PubMed] [Google Scholar]
- 189. Gong Z, Wang C, Ni L, et al. An injectable recombinant human milk fat globule‐epidermal growth factor 8‐loaded copolymer system for spinal cord injury reduces inflammation through NF‐kappaB and neuronal cell death. Cytotherapy. 2020;22(4):193‐203. [DOI] [PubMed] [Google Scholar]
- 190. Nguyen LH, Ong W, Wang K, Wang M, Nizetic D, Chew SY. Effects of miR‐219/miR‐338 on microglia and astrocyte behaviors and astrocyte‐oligodendrocyte precursor cell interactions. Neural Regen Res. 2020;15(4):739‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Park J, Decker JT, Margul DJ, et al. Local immunomodulation with anti‐inflammatory cytokine‐encoding lentivirus enhances functional recovery after spinal cord injury. Mol Ther. 2018;26(7):1756‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang C, Wang M, Xia K, et al. A bioactive injectable self‐healing anti‐inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact Mater. 2021;6(8):2523‐2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem‐cell‐derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20(6):4298‐4305. [DOI] [PubMed] [Google Scholar]
- 194. Xi K, Gu Y, Tang J, et al. Microenvironment‐responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat Commun. 2020;11(1):4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ye J, Jin S, Cai W, et al. Rationally designed, self‐assembling, multifunctional hydrogel depot repairs severe spinal cord injury. Adv Healthc Mater. 2021;10(13):e2100242. [DOI] [PubMed] [Google Scholar]
- 196. Matthews J, Surey S, Grover LM, Logan A, Ahmed Z. Thermosensitive collagen/fibrinogen gels loaded with decorin suppress lesion site cavitation and promote functional recovery after spinal cord injury. Sci Rep. 2021;11(1):18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Papa S, Rossi F, Ferrari R, et al. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano. 2013;7(11):9881‐9895. [DOI] [PubMed] [Google Scholar]
- 198. Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239‐240, 242, 244 passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yuan X, Yuan W, Ding L, et al. Cell‐adaptable dynamic hydrogel reinforced with stem cells improves the functional repair of spinal cord injury by alleviating neuroinflammation. Biomaterials. 2021;279:121190. [DOI] [PubMed] [Google Scholar]
- 200. Ropper AE, Thakor DK, Han I, et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix‐assisted hMSC implantation. Proc Natl Acad Sci USA. 2017;114(5):E820‐E829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Han IB, Thakor DK, Ropper AE, et al. Physical impacts of PLGA scaffolding on hMSCs: recovery neurobiology insight for implant design to treat spinal cord injury. Exp Neurol. 2019;320:112980. [DOI] [PubMed] [Google Scholar]
- 202. Shin JE, Jung K, Kim M, et al. Brain and spinal cord injury repair by implantation of human neural progenitor cells seeded onto polymer scaffolds. Exp Mol Med. 2018;50(4):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wang YH, Chen J, Zhou J, Nong F, Lv JH, Liu J. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13(1):203‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lin C, Ekblad‐Nordberg A, Michaelsson J, et al. In vitro study of human immune responses to hyaluronic acid hydrogels, recombinant spidroins and human neural progenitor cells of relevance to spinal cord injury repair. Cell. 2021;10(7):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Caron I, Rossi F, Papa S, et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135‐147. [DOI] [PubMed] [Google Scholar]
- 206. Fan L, Liu C, Chen X, et al. Directing induced pluripotent stem cell derived neural stem cell fate with a three‐dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater Interfaces. 2018;10(21):17742‐17755. [DOI] [PubMed] [Google Scholar]
- 207. Tatic N, Rose F, des Rieux A, White LJ. Stem cells from the dental apical papilla in extracellular matrix hydrogels mitigate inflammation of microglial cells. Sci Rep. 2019;9(1):14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ma YH, Shi HJ, Wei QS, et al. Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix‐based neural repair of spinal cord injury. Biomaterials. 2021;279:121192. [DOI] [PubMed] [Google Scholar]
- 209. Peng Z, Gao W, Yue B, et al. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve‐guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med. 2018;12(3):e1725‐e1736. [DOI] [PubMed] [Google Scholar]
- 210. Yu Q, Liao M, Sun C, et al. LBO‐EMSC hydrogel serves a dual function in spinal cord injury restoration via the PI3K‐Akt‐mTOR pathway. ACS Appl Mater Interfaces. 2021;13(41):48365‐48377. [DOI] [PubMed] [Google Scholar]
- 211. Albashari A, He Y, Zhang Y, et al. Thermosensitive bFGF‐modified hydrogel with dental pulp stem cells on Neuroinflammation of spinal cord injury. ACS Omega. 2020;5(26):16064‐16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Papa S, Vismara I, Mariani A, et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J Control Release. 2018;278:49‐56. [DOI] [PubMed] [Google Scholar]
- 213. Li LM, Huang LL, Jiang XC, Chen JC, OuYang HW, Gao JQ. Transplantation of BDNF gene recombinant mesenchymal stem cells and adhesive peptide‐modified hydrogel scaffold for spinal cord repair. Curr Gene Ther. 2018;18(1):29‐39. [DOI] [PubMed] [Google Scholar]
- 214. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Leung BK, Biran R, Underwood CJ, Tresco PA. Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials. 2008;29(23):3289‐3297. [DOI] [PubMed] [Google Scholar]
- 216. Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Adv Mater. 2014;26(38):6530‐6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. von Boxberg Y, Soares S, Giraudon C, et al. Macrophage polarization in vitro and in vivo modified by contact with fragmented chitosan hydrogel. J Biomed Mater Res A. 2021;110(4):773‐787. [DOI] [PubMed] [Google Scholar]
- 218. Chedly J, Soares S, Montembault A, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91‐107. [DOI] [PubMed] [Google Scholar]
- 219. Hong JY, Seo Y, Davaa G, Kim HW, Kim SH, Hyun JK. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020;101:357‐371. [DOI] [PubMed] [Google Scholar]
- 220. Cornelison RC, Gonzalez‐Rothi EJ, Porvasnik SL, et al. Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed Mater. 2018;13(3):034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8(4):046033. [DOI] [PubMed] [Google Scholar]
- 222. Liu D, Chen J, Jiang T, et al. Biodegradable spheres protect traumatically injured spinal cord by alleviating the glutamate‐induced Excitotoxicity. Adv Mater. 2018;30(14):e1706032. [DOI] [PubMed] [Google Scholar]
- 223. Hong LTA, Kim YM, Park HH, et al. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8(1):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kushchayev SV, Giers MB, Hom Eng D, et al. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J Neurosurg Spine. 2016;25(1):114‐124. [DOI] [PubMed] [Google Scholar]
- 225. Austin JW, Kang CE, Baumann MD, et al. The effects of intrathecal injection of a hyaluronan‐based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials. 2012;33(18):4555‐4564. [DOI] [PubMed] [Google Scholar]
- 226. Lopez‐Dolado E, Gonzalez‐Mayorga A, Gutierrez MC, Serrano MC. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials. 2016;99:72‐81. [DOI] [PubMed] [Google Scholar]
- 227. Zhao T, Xu K, Wu Q, et al. Duraplasty of PHBV/PLA/col membranes promotes axonal regeneration by inhibiting NLRP3 complex and M1 macrophage polarization in rats with spinal cord injury. FASEB J. 2020;34(9):12147‐12162. [DOI] [PubMed] [Google Scholar]
- 228. Li X, Zhang C, Haggerty AE, et al. The effect of a nanofiber‐hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials. 2020;245:119978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Dumont CM, Carlson MA, Munsell MK, et al. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019;86:312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Haggerty AE, Maldonado‐Lasuncion I, Oudega M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed Mater. 2018;13(4):044105. [DOI] [PubMed] [Google Scholar]
- 231. Dumont CM, Margul DJ, Shea LD. Tissue engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs. 2016;202(1–2):52‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Rowley AT, Nagalla RR, Wang SW, Liu WF. Extracellular matrix‐based strategies for immunomodulatory biomaterials engineering. Adv Healthc Mater. 2019;8(8):e1801578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1(7):481‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Austin JW, Gilchrist C, Fehlings MG. High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J Neurochem. 2012;122(2):344‐355. [DOI] [PubMed] [Google Scholar]
- 235. Sadtler K, Estrellas K, Allen BW, et al. Developing a pro‐regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Brown BN, Valentin JE, Stewart‐Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Abaricia JO, Farzad N, Heath TJ, Simmons J, Morandini L, Olivares‐Navarrete R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021;133:58‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Hume PS, Anseth KS. Inducing local T cell apoptosis with anti‐Fas‐functionalized polymeric coatings fabricated via surface‐initiated photopolymerizations. Biomaterials. 2010;31(12):3166‐3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Min SK, Kim SH, Kim CR, Paik SM, Jung SM, Shin HS. Effect of topography of an electrospun nanofiber on modulation of activity of primary rat astrocytes. Neurosci Lett. 2013;534:80‐84. [DOI] [PubMed] [Google Scholar]
- 240. Hezi‐Yamit A, Sullivan C, Wong J, et al. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A. 2009;90(1):133‐141. [DOI] [PubMed] [Google Scholar]
- 241. Jones JA, Chang DT, Meyerson H, et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface‐adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83(3):585‐596. [DOI] [PubMed] [Google Scholar]
- 242. Woeppel KM, Cui XT. Nanoparticle and biomolecule surface modification synergistically increases neural electrode recording yield and minimizes inflammatory host response. Adv Healthc Mater. 2021;10(16):e2002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)‐based hydrogels. J Biomed Mater Res A. 2012;100(6):1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Abaricia JO, Shah AH, Olivares‐Navarrete R. Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials. 2021;271:120715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Erpenbeck L, Gruhn AL, Kudryasheva G, et al. Effect of adhesion and substrate elasticity on neutrophil extracellular trap formation. Front Immunol. 2019;10:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Chakraborty M, Chu K, Shrestha A, et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 2021;34(2):108609. [DOI] [PubMed] [Google Scholar]
- 247. O'Connor RS, Hao X, Shen K, et al. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189(3):1330‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Saitakis M, Dogniaux S, Goudot C, et al. Different TCR‐induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. Elife. 2017;6:e23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wan Z, Zhang S, Fan Y, et al. B cell activation is regulated by the stiffness properties of the substrate presenting the antigens. J Immunol. 2013;190(9):4661‐4675. [DOI] [PubMed] [Google Scholar]
- 250. Zeng Y, Yi J, Wan Z, et al. Substrate stiffness regulates B‐cell activation, proliferation, class switch, and T‐cell‐independent antibody responses in vivo. Eur J Immunol. 2015;45(6):1621‐1634. [DOI] [PubMed] [Google Scholar]
- 251. Moshayedi P, Ng G, Kwok JC, et al. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials. 2014;35(13):3919‐3925. [DOI] [PubMed] [Google Scholar]
- 252. Blaschke SJ, Demir S, Konig A, et al. Substrate elasticity exerts functional effects on primary microglia. Front Cell Neurosci. 2020;14:590500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wilson CL, Hayward SL, Kidambi S. Astrogliosis in a dish: substrate stiffness induces astrogliosis in primary rat astrocytes. RSC Adv. 2016;6(41):34447‐34457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hu Y, Huang G, Tian J, et al. Matrix stiffness changes affect astrocyte phenotype in an in vitro injury model. NPG Asia Mater. 2021;13(1):35. [Google Scholar]
- 255. Moeendarbary E, Weber IP, Sheridan GK, et al. The soft mechanical signature of glial scars in the central nervous system. Nat Commun. 2017;8:14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Wang Z, Cui Y, Wang J, et al. The effect of thick fibers and large pores of electrospun poly(epsilon‐caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials. 2014;35(22):5700‐5710. [DOI] [PubMed] [Google Scholar]
- 257. Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42(7):1508‐1516. [DOI] [PubMed] [Google Scholar]
- 258. Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M. Impact of 3‐D printed PLA‐ and chitosan‐based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3‐D structures on inflammation. Acta Biomater. 2014;10(2):613‐622. [DOI] [PubMed] [Google Scholar]
- 259. Snider S, Cavalli A, Colombo F, et al. A novel composite type I collagen scaffold with micropatterned porosity regulates the entrance of phagocytes in a severe model of spinal cord injury. J Biomed Mater Res B Appl Biomater. 2017;105(5):1040‐1053. [DOI] [PubMed] [Google Scholar]
- 260. Yang CY, Huang WY, Chen LH, et al. Neural tissue engineering: the influence of scaffold surface topography and extracellular matrix microenvironment. J Mater Chem B. 2021;9(3):567‐584. [DOI] [PubMed] [Google Scholar]
- 261. McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA. 2013;110(43):17253‐17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Luu TU, Gott SC, Woo BW, Rao MP, Liu WF. Micro‐ and Nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl Mater Interfaces. 2015;7(51):28665‐28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Saino E, Focarete ML, Gualandi C, et al. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12(5):1900‐1911. [DOI] [PubMed] [Google Scholar]
- 264. Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93(3):1151‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Abaricia JO, Shah AH, Musselman RM, Olivares‐Navarrete R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater Sci. 2020;8(8):2289‐2299. [DOI] [PubMed] [Google Scholar]
- 266. Chang S, Popowich Y, Greco RS, Haimovich B. Neutrophil survival on biomaterials is determined by surface topography. J Vasc Surg. 2003;37(5):1082‐1090. [DOI] [PubMed] [Google Scholar]
- 267. Hotchkiss KM, Clark NM, Olivares‐Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T‐helper cell populations. Biomaterials. 2018;182:202‐215. [DOI] [PubMed] [Google Scholar]
- 268. Zuidema JM, Hyzinski‐Garcia MC, Van Vlasselaer K, et al. Enhanced GLT‐1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin‐coated electrospun poly‐L‐lactic acid fibers. Biomaterials. 2014;35(5):1439‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gottipati MK, D'Amato AR, Ziemba AM, Popovich PG, Gilbert RJ. TGFbeta3 is neuroprotective and alleviates the neurotoxic response induced by aligned poly‐l‐lactic acid fibers on naive and activated primary astrocytes. Acta Biomater. 2020;117:273‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Pires LR, Rocha DN, Ambrosio L, Pego AP. The role of the surface on microglia function: implications for central nervous system tissue engineering. J R Soc Interface. 2015;12(103):20141224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Song K‐H, Harvey BK, Borden MA. State‐of‐the‐art of microbubble‐assisted blood‐brain barrier disruption. Theranostics. 2018;8(16):4393‐4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. McMahon D, Poon C, Hynynen K. Evaluating the safety profile of focused ultrasound and microbubble‐mediated treatments to increase blood‐brain barrier permeability. Expert Opin Drug Deliv. 2019;16(2):129‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Tsui C, Koss K, Churchward MA, Todd KG. Biomaterials and glia: Progress on designs to modulate neuroinflammation. Acta Biomater. 2019;83:13‐28. [DOI] [PubMed] [Google Scholar]
- 274. Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O'Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18(6):313‐325. [Google Scholar]
- 275. Ma D, Zhao Y, Huang L, et al. A novel hydrogel‐based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020;237:119830. [DOI] [PubMed] [Google Scholar]
- 276. Zhang N, Lin J, Lin VPH, et al. A 3D fiber‐hydrogel based non‐viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv Sci. 2021;8(15):e2100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Gaojian T, Dingfei Q, Linwei L, et al. Parthenolide promotes the repair of spinal cord injury by modulating M1/M2 polarization via the NF‐kappaB and STAT 1/3 signaling pathway. Cell Death Dis. 2020;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Rong Y, Liu W, Lv C, et al. Neural stem cell small extracellular vesicle‐based delivery of 14‐3‐3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin‐1. Aging (Albany, NY). 2019;11(18):7723‐7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wang M, Li Z, Zuo Q. miR‐194‐5p inhibits LPS‐induced astrocytes activation by directly targeting neurexophilin 1. Mol Cell Biochem. 2020;471(1–2):203‐213. [DOI] [PubMed] [Google Scholar]
- 280. Khankan RR, Griffis KG, Haggerty‐Skeans JR, et al. Olfactory Ensheathing cell transplantation after a complete spinal cord transection mediates neuroprotective and immunomodulatory mechanisms to facilitate regeneration. J Neurosci. 2016;36(23):6269‐6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ishii H, Jin X, Ueno M, et al. Adoptive transfer of Th1‐conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 2012;3:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Dombrowski Y, O'Hagan T, Dittmer M, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR‐124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314(14):2618‐2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR‐124 antagonizes the anti‐neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21(7):744‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA‐124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP‐alpha‐PU.1 pathway. Nat Med. 2011;17(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zou D, Chen Y, Han Y, Lv C, Tu G. Overexpression of microRNA‐124 promotes the neuronal differentiation of bone marrow‐derived mesenchymal stem cells. Neural Regen Res. 2014;9(12):1241‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Taj SH, Kho W, Aswendt M, et al. Dynamic modulation of microglia/macrophage polarization by miR‐124 after focal cerebral ischemia. J Neuroimmune Pharmacol. 2016;11(4):733‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ong W, Pinese C, Chew SY. Scaffold‐mediated sequential drug/gene delivery to promote nerve regeneration and remyelination following traumatic nerve injuries. Adv Drug Deliv Rev. 2019;149–150:19‐48. [DOI] [PubMed] [Google Scholar]
- 289. Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: evidence and future perspectives. Exp Neurol. 2021;341:113704. [DOI] [PubMed] [Google Scholar]
- 290. Zuidema JM, Gilbert RJ, Gottipati MK. Biomaterial approaches to modulate reactive Astroglial response. Cells Tissues Organs. 2018;205(5–6):372‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Nakkala JR, Li Z, Ahmad W, Wang K, Gao C. Immunomodulatory biomaterials and their application in therapies for chronic inflammation‐related diseases. Acta Biomater. 2021;123:1‐30. [DOI] [PubMed] [Google Scholar]
- 294. Zhang B, Su Y, Zhou J, Zheng Y, Zhu D. Toward a better regeneration through implant‐mediated immunomodulation: harnessing the immune responses. Adv Sci. 2021;8(16):e2100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Dellacherie MO, Seo BR, Mooney DJ. Macroscale biomaterials strategies for local immunomodulation. Nat Rev Mater. 2019;4(6):379‐397. [Google Scholar]
- 296. Lasola JJM, Kamdem H, McDaniel MW, Pearson RM. Biomaterial‐driven immunomodulation: cell biology‐based strategies to mitigate severe inflammation and sepsis. Front Immunol. 2020;11:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Lin J, Anopas D, Milbreta U, et al. Regenerative rehabilitation: exploring the synergistic effects of rehabilitation and implantation of a bio‐functional scaffold in enhancing nerve regeneration. Biomater Sci. 2019;7(12):5150‐5160. [DOI] [PubMed] [Google Scholar]
- 299. Anopas D, Junquan L, Milbreta U, et al. Exploring new treatment for spinalized rats by synergising robotic rehabilitation system and regenerative medicine. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:4205‐4208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


