Abstract
Background: Due to the interrupted blood supply in cerebral ischemic stroke (CIS), ischemic and hypoxia results in neuronal depolarization, insufficient NAD+, excessive levels of ROS, mitochondrial damages, and energy metabolism disorders, which triggers the ischemic cascades. Currently, improvement of mitochondrial functions and energy metabolism is as a vital therapeutic target and clinical strategy. Hence, it is greatly crucial to look for neuroprotective natural agents with mitochondria protection actions and explore the mediated targets for treating CIS. In the previous study, notoginseng leaf triterpenes (PNGL) from Panax notoginseng stems and leaves was demonstrated to have neuroprotective effects against cerebral ischemia/reperfusion injury. However, the potential mechanisms have been not completely elaborate. Methods: The model of middle cerebral artery occlusion and reperfusion (MCAO/R) was adopted to verify the neuroprotective effects and potential pharmacology mechanisms of PNGL in vivo. Antioxidant markers were evaluated by kit detection. Mitochondrial function was evaluated by ATP content measurement, ATPase, NAD and NADH kits. And the transmission electron microscopy (TEM) and pathological staining (H&E and Nissl) were used to detect cerebral morphological changes and mitochondrial structural damages. Western blotting, ELISA and immunofluorescence assay were utilized to explore the mitochondrial protection effects and its related mechanisms in vivo. Results: In vivo, treatment with PNGL markedly reduced excessive oxidative stress, inhibited mitochondrial injury, alleviated energy metabolism dysfunction, decreased neuronal loss and apoptosis, and thus notedly raised neuronal survival under ischemia and hypoxia. Meanwhile, PNGL significantly increased the expression of nicotinamide phosphoribosyltransferase (NAMPT) in the ischemic regions, and regulated its related downstream SIRT1/2/3-MnSOD/PGC-1α pathways. Conclusion: The study finds that the mitochondrial protective effects of PNGL are associated with the NAMPT-SIRT1/2/3-MnSOD/PGC-1α signal pathways. PNGL, as a novel candidate drug, has great application prospects for preventing and treating ischemic stroke.
Keywords: NAMPT, Ischemia stroke, Mitochondria, Energy metabolism, Notoginseng leaf triterpenes (PNGL)
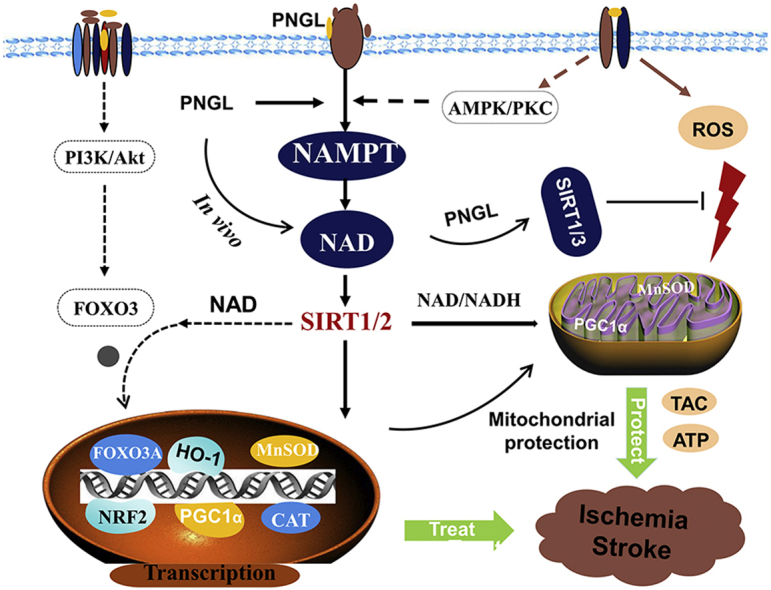
Graphical abstract
Highlights
-
•
PNGL obviously exerts neuroprotective effects against ischemic stroke.
-
•
PNGL significantly alleviates neuronal mitochondria injury caused by ischemic and improves energy metabolism.
-
•
Mitochondria protective mechanisms may be closely associated with the NAMPT-SIRT1/2/3 pathway.
Abbreviations
- ATP
adenosine triphosphate
- CIS
cerebral ischemic stroke
- NAD
nicotinamide adenine dinucleotide;
- ETC
electron transport chain
- H&E
hematoxylins and eosin
- I/R
ischemia and reperfusion
- MCAO/R
middle cerebral artery occlusion and reperfusion
- MDA
malondialdehyde
- NO
nitric oxide;
- MOJ
mitochondrial oxidative injury
- NAMPT
nicotinamide phosphoribosyltransferase
- PNGL
notoginseng leaf triterpenes of Panax notoginseng stem and leaves
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SIRT
sirtuin
- TEM
transmission electron microscopy
- DMEM
dulbecco’s modified eagle’s medium
- MnSOD
manganese superoxide dismutase
- PGC-1α
PPARγ coactivator-1α
- TAC
tricarboxylic acid cycle
1. Introduction
Stroke, especially cerebral ischemic stroke (CIS), is one of the leading causes of death worldwide; it has the characteristics of high morbidity, lethality, disability, and recurrence rate [[1], [2], [3]]. Although ischemic stroke pathogenesis is incompletely clear, the related researches and reports have proven that CIR is involved in energy metabolism disorders [4], oxidative stress [5], Ca2+ overload, excitatory neurotransmitters, apoptosis and necrosis [5]. Currently, tissue plasminogen activator (TPA) has been deveolped and regarded as the effective pharmacological therapy and drug for ischemic stroke, and some neuroprotective drugs have been developed for several aspects of ischemia-reperfusion injury [6,7], but it still remains limited and relatively difficult to meet needs for clinical treatment of stroke [3,5,7]. Therefore, novel therapeutic strategies and agents are urgently needed to develop for preventing and treating CIS.
The current reports have revealed that Nicotinamide adenine dinucleotide (NAD+) plays a crucial role in regulating metabolism and cell stress responses [8,9],.In the early stage of ischemia, the cerebral blood flow severely declines, which results in the severe oxygen and glucose deprivation [10,11] and insufficient NAD+, decreases the ratio of NAD+/NADH [[12], [13], [14], [15]], directly inhibits impaired H+ transmission in the oxidative respiratory chain and insufficient intracellular ATP synthesis, and further causes mitochondrial damages and energy metabolism disorders [10]. These mitochondrial disorders may further aggravate the multiple pathological progresses of cerebral ischemia and reperfusion (I/R) injury (CIRI), including excitotoxicity, free radical release, and inflammation, leading to cellular death and neuronal loss after stroke [[3], [4], [5],16]. Thus, mitochondrial metabolic of energy is regarded as one of key strategies against neuronal injury caused by I/R.
Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme for biosynthesizing NAD in mammals. Currently, much evidence supports NAMPT and the NAMPT-NAD + pathway as a therapeutic target against ischemic stroke [9,17,18]. NAMPT could increase neuronal ischemic tolerance, inhibit neuronal apoptosis and necrosis, and improve energy metabolism under ischemia [[19], [20], [21]]. Therefore, it is one of the hot tasks to find natural active substances, which effectively inhibits mitochondrial damages, improves energy metabolism via NAMPT.
Notoginseng leaf triterpenes (PNGL) is total saponins isolated and purified from stems and leaves of Panax notoginseng (Burk) F. H. Chen ex C. H. Our previous study has shown that PNGL exerts neuroprotective effects via attenuation of neuronal apoptosis caused by ischemia. And the chemical fingerprinting assay was used to identify and analyze eleven batches of PNGL samples [22]. It finds that PNGL mainly contains mainly 20(s)-protopanaxadiol saponins, such as ginsenoside Rb1, Rb2, Rb3, and Rc [23,24]. But the neuroprotective mechanisms of PNGL are not completely elaborated. In additions, our team previously has found that Panax notoginseng saponins (PNS) and its monomeric saponin components could reduce mitochondrial damages, such as notoginsenoside R1, R2, and ginsenoside Rg1 [25,26]. Thus, we speculated that PNGL might have protective effects on mitochondria. But it is essential to further verify whether PNGL exerts mitochondria-protective effects against CIS. And the relevant mechanisms are not fully aware that how PNGL may alleviate mitochondrial dysfunction, improve energy metabolism and thus suppress cerebral ischemic damages, which needs to further explore.
This present research was designed to further confirm the effects of PNGL against ischemic MCAO/R-induced brain injury, explore the effects and mechanisms of PNGL on inhibiting mitochondrial injury and alleviating metabolic disorder of energy via MCAO/R model rats, and conduct further researches on the regulation of PNGL on the NAMPT pathway.
2. Methods
2.1. Animals
Adult male Sprague-Dawley rats (220∼240g) were obtained from Beijing Vital Lihua Experimental Animals Co., Ltd. Rats were housed with standard conditions [22,27]. All operations and treatments were obliged to conform to the Declaration of Helsinki and the “3R” principles. And the protocol was approved by the Laboratory Animal Ethics Committee (Permit Number: SYXK 2017-0020).
2.2. Focal cerebral ischemia model
The ischemia model was produced by middle cerebral artery occlusion and reperfusion (MCAO/R) as previously described [22,27]. Briefly, after anaesthetization with Zoletil 50 (ip,10-15 mg·kg-1, Virbac S.A, Carros, France, Supplemental Material-S1), rats were exposed to the MCAO/R operations according to the standard operating specifications [22,27]. The occlusion last for 2h and reperfusion for 24h. Rats in sham-operated group underwent the same procedures except occluding the MCA. Stuporous animals were kept under the conditions of 32 ± 0.1 °C until woke up. In addition, the experimental design procedure was showed in Supplemental Material-S2.
2.3. Drug administrations for animal
Based on our previous experiments [22], rats were divided into 6 groups (n = 10 for each group): the Sham group, the MCAO/R group, the PNGL(73 mg/kg, L) + MCAO/R group, the PNGL(146 mg/kg, M) + MCAO/R group, the PNGL(192 mg/kg, H) + MCAO/R group, and the NBP group (butylphthalide, 60 mg/kg). PNGL samples were provided by the Jilin Academy of Chinese Medicine (Lot No. 2018-05-08, Changchun, China), and NBP was purchased from the Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical Co. Ltd (Lot No. 118180810). The solvent media was normal saline. Rats were by intragastric administration for two weeks, and rats were exposed to the same volume of normal saline in the Sham and MCAO/R group.
2.4. Histological examinations
After drug administration and MCAO/R operation, rats were anaesthetized, the intact brains were prepared, and the pathological paraffin sections were made. Sections were stained using H&E and Nissl staining according to the described standard protocol [22,27,28]. The staining images were acquired using a pathological scanner and analysis system (Aperio CS2, Leica, Wetzlar, Germany).
2.5. Detection of MDA, NO contents, SOD and GSH-Px activities
Following the MCAO/R operation and treatment, the blood serum was prepared for further measurement [22,28]. The malondialdehyde (MDA), nitric oxide (NO) contents, superoxide dismutase (SOD) and GSH-Px activities in blood serum were measured using colorimetric assay kits according to the manufacturer protocols [27]. The MDA level and NO level were measured using the kits from Beyotime (Beyotime, Shanghai, China). SOD and GSH-Px activities were detected by the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the kit operation specifications. And the results were obtained by the Tecan Infinite microplate reader (M1000, Tecan Infinite, Switzerland).
2.6. Transmission electron microscopy
The mitochondrial ultrastructural changes in neurons were observed using transmission electron microscopy (TEM). Brain sections were fixed with 4% glutaraldehyde for 1 h and then with 1% OsO4 for 2 h in 0.1 mol/L cacodylate buffer (pH 7.4). Then, they were stained with 1% aqueous uranyl acetate for overnight, dehydrated in an ascending series of ethanol and dry acetone, and embedded in the Hard-Plus Resin-812 (SPI). Ultrathin sections (0.1 μm) were cut, stained with 3% lead citrate, and examined with TEM (HT7700, HITACHI, Tokyo, Japan).
2.7. ELISA assay
After the MCAO/R operation and treatment, the brain samples in each group were prepared (as showed in Supplemental Material-S1), homogenized, and centrifuged according to the operation specifications [22,28]. The collected supernatants were used to assess the cytokines and protein levels. A BCA protein assay kit was used to determine protein concentrations of the supernatants (CWBIO, Beijing, China). The total antioxidant capacity (T-AOC) in blood serum was analysed using an ELISA kit (Hiton., Beijing, China). The sirtuins, superoxide dismutase 2 (SOD2), and nicotinamide adenine dinucleotide phosphate (NADPH) concentrations from the ischemic brains were detected by using ELISA kit (Hiton., Beijing, China) according to the operation instructions. The ATP, ATPase, NAD and NADH levels in the ischemic brains were detected by using an ELISA kit (Hiton., Beijing, China) according to the operation instructions.
2.8. Immunofluorescence
Immunofluorescence staining was performed as previously described [27,28]. Briefly, the micro-slides were prepared, and then co-incubated with anti-NAMPT (ab236873, Abcam), and anti-beta III Tubulin with Alexa Fluor® 488 (ab195879, Abcam) overnight at 4 °C. Sections were then incubated with a TRITC-conjugated goat anti-rabbit IgG (CW0160, CWBIO, Beijing, China) at room temperature for 1 hr. Finally, after DAPI for nuclear counterstaining, the sections were coverslipped with cover glass and examined under a fluorescence microscope (Leica, Germany Q9).
2.9. Protein extraction
After MCAO operation and treatment, the cerebral ischemic hippocampus and cortex region tissue were suspended in ice-cold lysis buffer and homogenized as described previously [22,28,29]. Then, the protein concentrations in the supernatant were determined by the BCA protein assay kit according to the protocol.
2.10. Immunoblotting
Western blotting was performed as previously reported [22,28,29]. Based on the standard operating specifications, protein samples were loaded, separated and transferred onto NC membranes (Millipore, Bedford, MA, USA). After blocking for 2h at 20 °C, the transferred membranes were incubated overnight at 4 °C with the special antibodies: NAMPT (ab236873, 1:1000), SIRT1 (ab189494, 1:1000), SIRT2 (ab211033, 1:2000), SIRT3 (cst5490, 1:1000), MnSOD (ab137037, 1:5000), PGC-1α (ab188102, 1:5000), and β-actin (EXP0041F, 1:3000). Protein expression was examined by using an enhanced chemiluminescence method and ChemiDoc XRS (Bio-Rad, Hercules, CA, USA). To eliminate variations in protein expression, three independent experiments were conducted.
2.11. Statistical analysis
GraphPad Prism 8.0 and statistical program SPSS 21.0. (SPSS, IBM, Chicago, IL, USA) were used for statistical analysis. Data expressed as means ± SD or standard error of the mean (SEM). When the Kruskal-Wallis H test showed a significant difference, the Mann-Whitney U test with the Bonferroni correction was in use. All other data were analyzed using one-way ANOVA followed by the least significant difference (LSD) or Bonferroni’s method, and p < 0.05 was considered statistically significant.
3. Results
3.1. PNGL inhibits neurological damages caused by ischemia in vivo
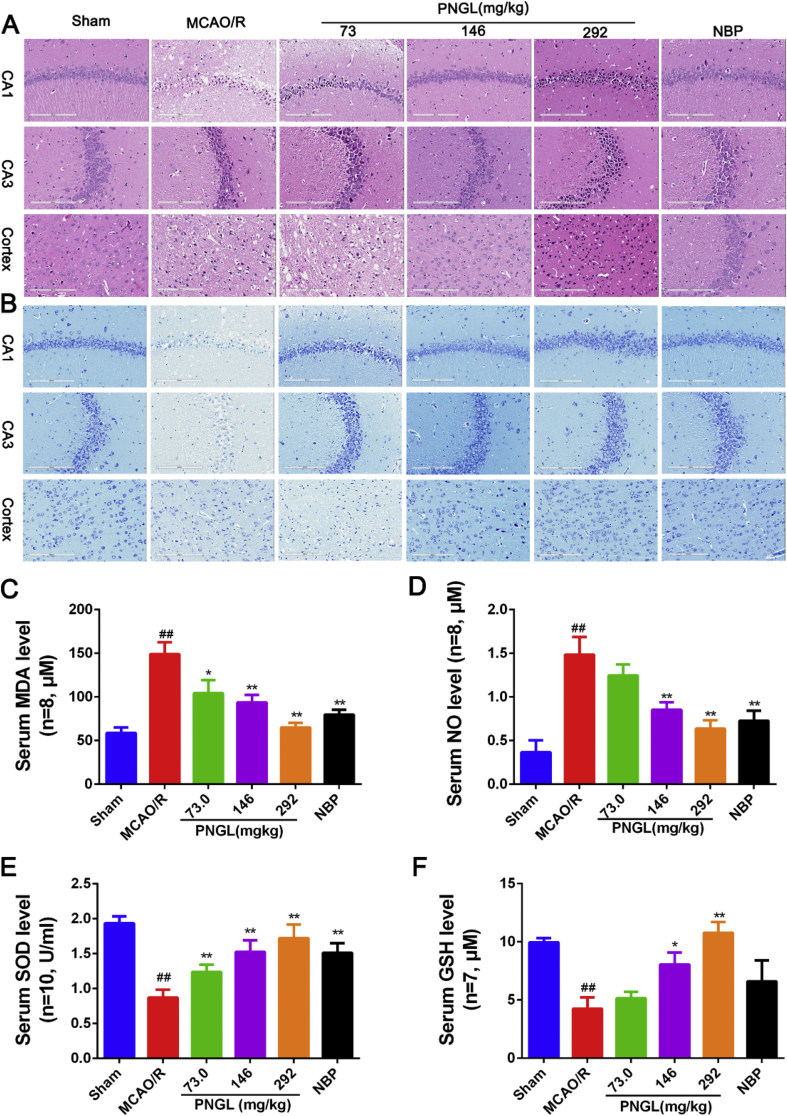
The morphological damages were detected by using Nissl and H&E staining. As showed in Fig. 1, the neuron cells possessed a clear and obvious outline of the nucleus without obvious tissue defects and edema, neurons and Nissl bodies arranged regularly in the hippocampus and cortex, and exhibited clear staining. After MCAO/R induction, most neurons and Nissl bodies exhibited weak staining, diffusely deteriorated, and neuron density and Nissl bodies decreased (Fig. 1A and B). Compared with the model group, PNGL (73, 146, 292 mg/kg) treatment dose-dependently exhibited obvious staining, possessed neurons arranged regularly in the hippocampus and cortex, and significantly increased neuron density and Nissl bodies (Fig. 1A and B). The PNGL group had less swollen cells, decreased issue space, reduced edema, and possessed more normal morphological cells, arranged more regularly with the nuclear shrinkage phenomenon (Fig. 1A and B).
Fig. 1.
Effects of PNGL on neuronal pathological changes, Nissl’s body loss and oxidative stress indicators in MCAO/R rats. (A) Representative images of H/E staining performed in hippocampus CA1, CA3, and cortex regions from ischaemic brains, obtained by the Digital Whole Slide Scanning System (Leica, Aperio CS2). (B) Representative images of Nissl staining performed in hippocampus CA1, CA3 regions, and cortex regions from ischaemic brains. (C) (D) (E) (F) the MDA, NO, SOD and GSH concentrations, determined by ELISA and specific assay kits (n = 6-10 in each group). Mean values ± SEM; ∗p < 0.05, ∗∗p < 0.01 versus MCAO/R group; #p < 0.05, ##p < 0.01, versus sham group. Scale bar, 200 μm.
In additions, PNGL (146 mg/kg) was equal to the NBP in the hippocampus regions. All of these results indicate that PNGL inhibits the neurological damages and decreases the neuronal density loss caused by CIRI.
In addition, we further investigated the MDA, NO, SOD, and GSH levels. In contrast with the sham group, the model group obviously increased the MDA and NO levels (Fig. 1C–D, p < 0.01 and p < 0.01), and significantly reduced the SOD activity and GSH levels (Fig. 1E–F, p < 0.01 and p < 0.01). Treatment of PNGL (73,146, 292 mg/kg) remarkably decreased the MDA and NO levels (Fig. 1C–D, p > 0.05, p < 0.01 and p < 0.01). And PNGL raised the SOD activity and GSH levels (Fig. 1E–F, p < 0.05 and p < 0.01) with a dose-dependent manner; PNGL (73 mg/kg) showed no significant differences on the NO and GSH concentrations with MCAO/R groups. Moreover, the NBP showed similar improvements with PNGL (146 mg/kg, p < 0.01), which suggested that PNGL could alleviate neuronal oxidative injury caused by CIRI.
3.2. PNGL alleviates mitochondrial injury and improves energetic metabolism in ischemic brains
Mitochondrial dysfunction is viewed as one of major causes of I/R-induced neuronal death [30,31]. To elucidate the effect of PNGL on mitochondrial function under the cerebral ischemia conditions, we assessed the mitochondrial structure and energy metabolism via TEM and ELISA assays.
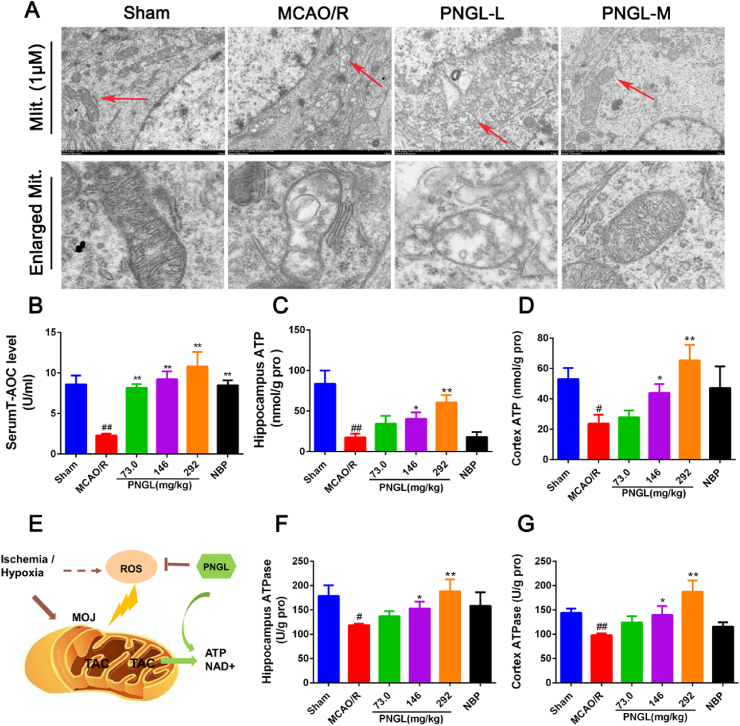
As showed in Fig. 2A, in the sham groups, mitochondrial cristae were clear, almost no mitochondrial vacuolization occurred, and a disruption of mitochondrial distribution was re regularly arranged and showed no polymorphism of the mitochondrial ultrastructure via TEM images (Fig. 2A). In the MCAO/R group, mitochondria showed swelling, disorder of sparse cristae and fracture, and obvious mitochondrial vacuoles. Contrast with MCAO/R. PNGL (73,146mg/kg) reduced the mitochondrial vacuolization, swelling, mitochondrial ultrastructure polymorphism, and disorder of sparse cristae and fracture. And the mitochondria were slightly swollen with less vacuolation and more visible mitochondrial cristae (Fig. 2A), indicating that PNGL may protect the ischemic brains against the mitochondrial injury caused by I/R.
Fig. 2.
Effects of PNGL on mitochondrial structure and energetic metabolism in MCAO/R rats. (A) Representative images of mitochondrial structure performed in cortex, measured by TEM (HITACHI, HT7700). (B) the total antioxidant capacity (T-AOC) in MCAO/R model rats, determined by a ELISA and specific assay kit (n = 6-10 in each group). (C) (F) (D) (G) the ATP concentrations and ATPase activities in hippocampus and cortex regions, determined by ELISA and specific assay kits (n = 7-10 in each group). (E) these results indicated PNGL may inhibit the ischemia-induced mitochondrial oxidative injury (MOJ) and thus improve the energy metabolism in vivo (TAC, Tricarboxylic acid cycle). Mean values ± SEM; ∗p < 0.05, ∗∗p < 0.01 versus MCAO/R group; #p < 0.05, ##p < 0.01, versus sham group.
Next, ATP production and energy metabolism were evaluated by ELISA kits in vivo. As showed in Fig. 2, the levels of ATP and ATPase decreased in brain hippocampus and cortex after I/R injury (Fig. 2C–D, p < 0.01, p < 0.05; Fig. 2F–G, p < 0.05, p < 0.01). Meanwhile, the antioxidant capability (T-AOC) significantly decreased (Fig. 2B, p < 0.01). In contrast, treatment with PNGL (73,146, 292 mg/kg) increased the ATP and ATPase levels in ischemia hippocampus and cortex (Fig. 2C–D, p > 0.05, p < 0.05, p < 0.01; Fig. 2F–G, p > 0.05, p < 0.05, p < 0.01), and raised the T-AOC, SOD, CAT, and GSH-Px levels (Fig. 2B, p < 0.01) with a dose-dependent manner. In additions, NBP treatment evidently improved the ATP and ATPase levels, which was similar to PNGL (146 mg/kg).
3.3. PNGL upregulates cerebral NAMPT levels and its mediated NAD + levels in vivo
To further elucidate the effect of PNGL on mitochondrial oxidative respiration and explore whether PNGL could regulate the target NAMPT under ischemia and hypoxia, we assessed the NAMPT level via immunofluorescence, the NAD+ and NADH levels by ELISA kits in the ischemic brains.
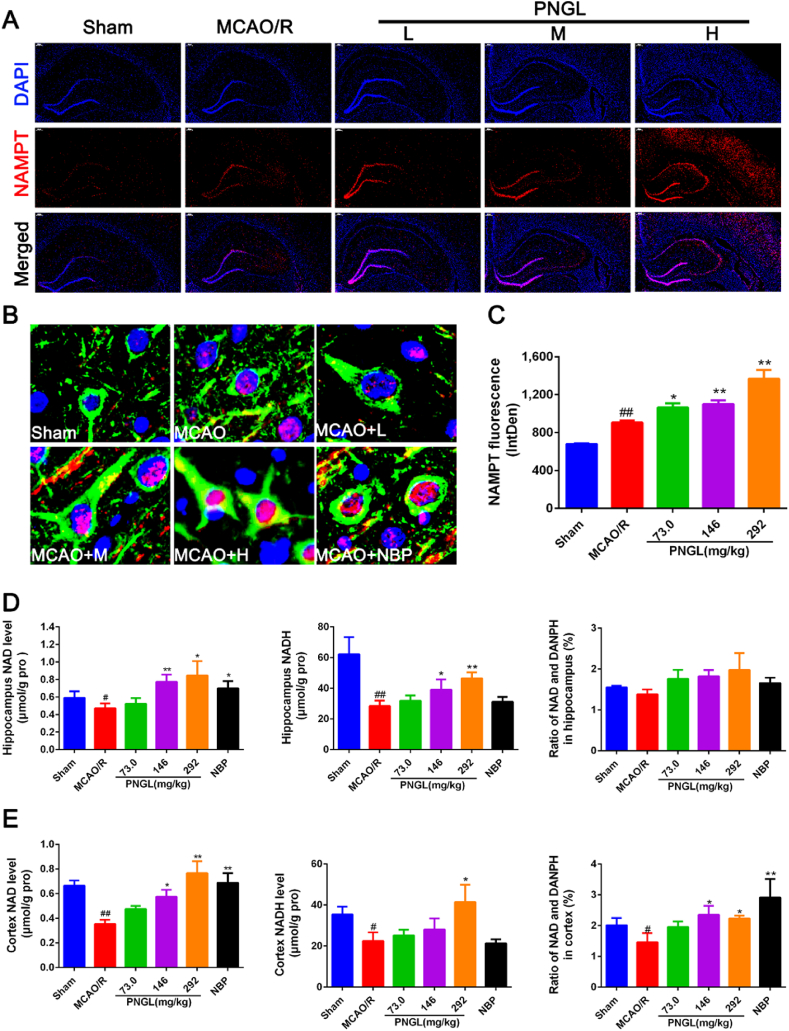
Immunofluorescence staining of the NAMPT, beta-III tubulin and DAPI revealed that NAMPT was mainly located in neurons and its nucleus, mainly expressed in ischemic cortex and hippocampus areas, and then other brain regions 24 hours after MCAO (Fig. 3A and B). And it demonstrated that NAMPT level was increased in the ischemic hemisphere (Fig. 3C, p < 0.01 vs. Sham). Meanwhile, PNGL (73, 146, 292mg/kg) administration further increased the number of NAMPT-positive neurons and its fluorescence value in the ischemic brain (Fig.3A–C, p < 0.05, p < 0.01, p < 0.01 vs. MCAO/R), The ELISA results suggested that the NAD+ and NADH levels decreased after MCAO/R (Fig. 3D–E, p < 0.05, p < 0.01); PNGL (73, 146, 292mg/kg) treatment increased the NAD+ and NADH levels with dose-dependence in ischemic cortex and hippocampus areas (Fig. 3D–E, p < 0.05, p < 0.01). And thus PNGL raised the ratio of NAD+ and NADH in cortex (Fig. 3D–E, p < 0.05) and in hippocampus, accompanied by the increase of NAMPT level; however, the trend did not reach statistical significance in hippocampus (Fig. 3D and E p > 0.05). Additionally, NBP (60 mg/kg) has similar increases in the NAD+ (Fig. 3D and E).
Fig. 3.
Effects of PNGL on Nampt expresssition and NAD + levels in the ischemic brains. (A) Representative images of Nampt (red) with DAPI (blue) staining in rat ischemic brains after MCAO/R injury, measured by the immunofluorescence assay; scale bar, 200μm. (B) The enlarged images of Nampt (Red) and the beta-III tublin(Green) with DAPI (Blue) staining in rat ischemic cortex regions, measured by the immunofluorescence assay, scale bar, 10μm. (C) The statistical data of Nampt fluorescence value, analyzed by using the Image J 2.44 softwae. (D) (E) The NAD and NADH concentrations in hippocampus and cortex in MCAO/R rat brains, determined by ELISA and specific assay kits. (n = 5-8 in each group). Mean values ± SEM; ∗p < 0.05, ∗∗p < 0.01 versus MCAO/R group; #p < 0.05, ##p < 0.01, versus sham group.
All of these results above indicated the effects of PNGL on improving the NAD+ and ATP synthesis might be tightly relevant to the activation and upregulation of the target NAMPT.
3.4. PNGL activates NAMPT-SIRT1/2/3 pathway in ischemic brains
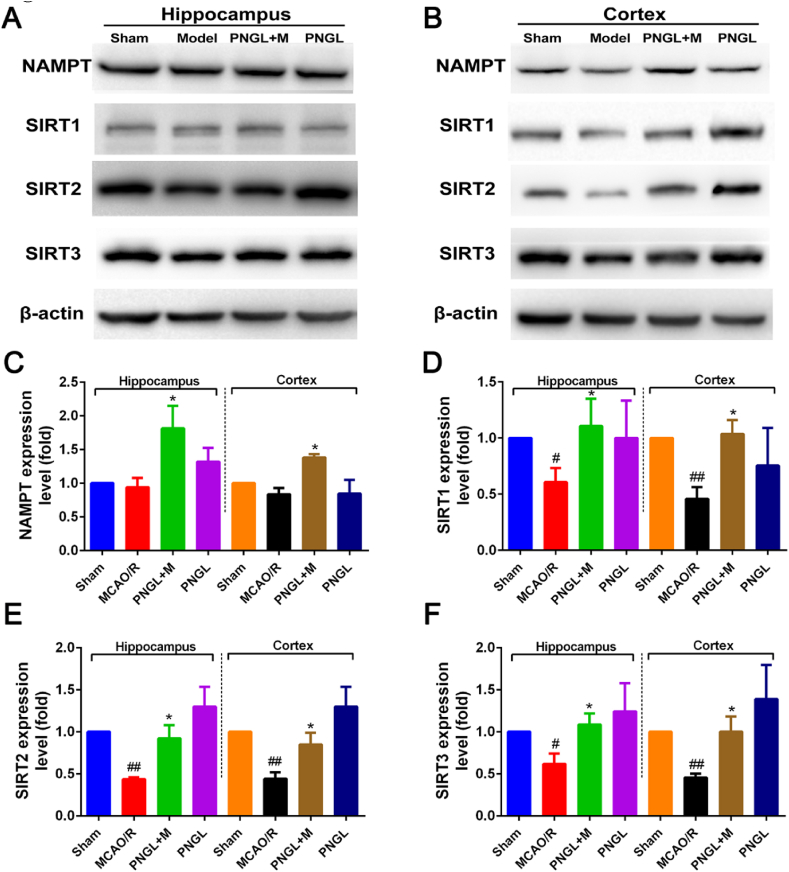
To further determine the molecular pathways underlying NAMPT-induced protection, we detected the protein levels of the NAMPT-NAD-SIRT1/2/3 signaling pathways by western blot.
As shown in Fig. 4, the results revealed that the NAMPT expression showed no significant improvement (Fig. 4A-C), and the expression levels of SIRT1/2/3 was remarkably decreased by the MCAO/R operation (Fig. 4D–F, p < 0.01, p < 0.01, p < 0.01). Compared to the MCAO/R, treatment of PNGL (146mg/kg) upregulated the NAMPT expression levels in cortex and hippocampus areas (Fig. 4C, p < 0.05, p < 0.05). In addition, the PNGL inhibited the decreases of SIRT1/2/3 expression in ischemic cortex and hippocampus areas (Fig. 4D–F, p < 0.05, p < 0.05, p < 0.05).
Fig. 4.
Effects of PNGL on the Nampt-NAD-SIRT1/2/3 signaling pathways in the ischemic brain. (A-B) The protein bands of Nampt and SIRT1/2/3 in the ischaemic brain sections examined by the western blot analysis. (C–F) the relative expression levels of Nampt, Sirt1, Sirt2 and Sirt3 proteins, respectively, quantified and analyzed by using Gel-Pro analyzer software. Mean values ± SEM (n = 3); p < 0.05, ∗∗p < 0.01, versus MCAO/R group; #p < 0.05, ##p < 0.01 versus sham-operated group.
These results demonstrate that PNGL exerts protective effects against mitochondrial injury and cerebral oxidative stress injury, which may be related to the regulation of NAMPT-NAD and SIRT1/2/3 pathways under ischemia conditions.
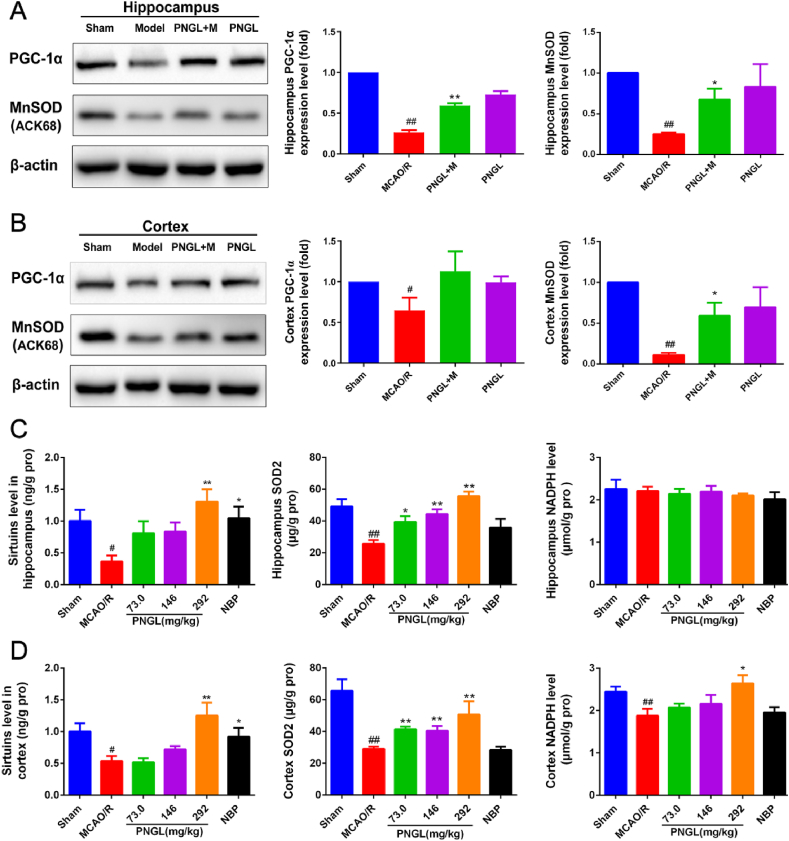
3.5. PNGL improves the SIRT1/2/3-MnSOD/PGC-1α pathway mediated by the NAMPT in vivo
Then our experiment focused on the PGC-1α [32], MnSOD and NADPH [[33], [34], [35]], the important downstreams of the NAMPT-SIRT1/2/3 pathway, which could regulate transcriptional activity of the targeted antioxidant and cell cycle genes, including the UCP2, PECPK, PGC-1α, MnSOD, and catalse genes [[33], [34], [35]].
As shown in Fig. 5A-B, the protein levels of PGC-1α and MnSOD were significantly reduced in the MCAO/R model rats (Fig. 5 A, p < 0.05 and p < 0.01, respectively, Fig. 5 B, p < 0.01 and p < 0.01, respectively). Compared to the MCAO/R group, treatment with PNGL increased the expression levels of PGC-1α and MnSOD in hippocampus (Fig. 5 A, p < 0.01 and p < 0.05), and upregulated the levels of PGC-1α and MnSOD in cortex (Fig. 5 B, p > 0.05 and p < 0.05). Moreover, PNGL showed no significant upregulation of the PGC-1α and MnSOD in the non-ischemia brains.
Fig. 5.
Effects of PNGL on the downstream SIRT1/2/3-MnSOD/PGC-1α signaling pathway mediated by the target Nampt in the ischemic brains. (A) The protein bands and relative expression levels of the PGC-1α and MnSOD in hippocampus, respectively, examined by western blot analysis and analyzed by using the Gel-Pro analyzer software. (B) The protein bands and relative expression levels of the PGC-1α and MnSOD in cortex. (C) the sirtuins, SOD2, and NADPH concentrations and levels in the ischemic hippocampus regions, detected by the ELISA assay kits. (D) the sirtuins, SOD2, and NADPH concentrations and levels in the ischemic cortex regions, detected by the ELISA assay kits Mean values ± SEM (n = 3); p < 0.05, ∗∗p < 0.01, versus MCAO/R group; #p < 0.05, ##p < 0.01 versus sham-operated group.
The ELISA results showed that the MCAO/R decreased the levels of the sirtuins, MnSOD, and NADPH in ischemic hippocampus (Fig. 5 B, p < 0.05, p<0.01 and p>0.05). In ischemic hippocampus, treatment of PNGL (73, 146, 292mg/kg) improved the levels of sirtuins and MnSOD with dose-dependence (Fig. 5 C, p < 0.05, p < 0.01), and the NADPH level did not showed significant increases. Similarly, in ischemic cortex regions, the decreases of sirtuins, MnSOD, and NADPH levels were abrogated by PNGL treatment in a dose-dependent manner (Fig. 5 C, p < 0.01, p < 0.01, p < 0.05); and PNGL (73, 146mg/kg) raised the levels of the sirtuins and NADPH proteins without significance. In additions, NBP group showed significant changes in the levels of sirtuins in hippocampus and cotex.
These findings suggest that PNGL may upregulate the PGC-1α and MnSOD expression, and improve the protein levels of sirtuins and SOD2, and regulate the NAD-NADPH process in the ischemic hippocampus and cortex.
4. Discussion
In the previous work, we found that PNGL attenuated the brain swelling, and reduced the infarct volume and BBB disruption in MCAO/R rats [22,36]. PNGL might exert the neuroprotective effects via suppression of apoptosis [22,36]. But the mechanisms of PNGL are not completely interpreted. Thus, in the present study, the mechanisms of PNGL against I/R injury were further investigated. The results demonstrate that PNGL significantly inhibits the neurological morphological damages, decreases neuronal density loss in rats subjected to MCAO/R (Fig. 1). Therefore, PNGL is a promising agent for preventing and treating ischemic stroke.
Once ischemic stroke occurs, the critical reduction of regional cerebral blood flow may lead to mitochondrial dysfunctions within minutes after ischemia [10,11]. Maintaining the mitochondrial function is critical in promoting neuron survival and neurological improvement [10,11]. The TEM images and detection data showed that treatment with PNGL (73, 146, 292 mg/kg) remarkably alleviated mitochondrial structure injury caused by CIRI (Fig. 2), and markedly increased ATP and ATPase levels in vivo (Fig. 2), suggesting that the neuroprotection of PNGL may be tightly associated with inhibiting mitochondrial injury and improving metabolism of energy.
NAD plays an important role in energy balance and cellular redox reactions in ischemic stroke [13,37,38]. Under the hypoxia-ischemia conditions, NADH gets oxidized in the cytoplasm through the reduction of pyruvate to lactate, which leads to the mitochondria dysfunction that the NAD + level is reduced [[12], [13], [14], [15]], ETC is obviously blocked, and the ATP synthesis was inhibited [10,18,39]. And thus the absence of mitochondrial NAD + pool further causes excessive production of ROS [11,37,38,40], which results in the aggravated mitochondria impairment, depletion of ATP production, depolarization of mitochondrial membrane potential, and its induced neuronal injury [11,41]. Our study found that the MCAO/R operation significantly decreased the NAD + levels (Fig. 3), inhibited oxidation resistance (Fig. 1, T-AOC, SOD, GSH-Px), and increased the MDA and NO levles (Fig. 1) in vivo, and induced the NAD + insufficiency, which was consistent with the previous reports. After further treatment of PNGL for the model rats, PNGL reversed these alterations of NAD+ and mitochondria impairment caused by IR. All of these indicate that PNGL may exert mitochondria protective effects via the maintenance of mitochondrial NAD + pool and the inhibition of oxidative injury.
Intracellular NAMPT is able to convert nicotinamide into to NAD [[42], [43], [44]], which exerts important roles in energy metabolism and cellular biological functions. NAMPT have a comparable effect on neuronal protection and suppression of apoptosis-inducing factor translocation [15]. The in vivo experiments demonstrated that the intracellular NAMPT level was induced by ischemia along with the NAD + decrease (Fig. 3A–C, Fig. 4A–C), which was in accordance with the related previous researches. In contrast, PNGL treatment markedly improved the intracellular level in the MCAO/R rats (Fig. 3A–C, Fig. 4A–C). These results show that PNGL may regulate the NAMPT-NAD + pathway against mitochondria dysfunctions and I/R injury.
Sirtuins are an evolutionarily conserved family of NAD + -dependent lysine deacylases and ADP ribosylases; the sirtuin family proteins consists of seven members (SIRT1-7) in mammals [17,38,45]. SIRT1, SIRT2, and SIRT3 show the highest deacetylase activity, and the SIRT1/2/3 mainly exerts great neuroprotective effects in cerebral ischemia [34,46,47]. As an NAD + -dependent deacetylase, SIRT1, SIRT2, and SIRT3 specifically promote mitochondrial functions [48], energy metabolism [[48], [49], [50]], and oxidizing reactions [35,48]. Our further researches suggested the MCAO/R model remarkably reduced the expression levels of SIRT1/2/3 (Fig. 4D-F), which was consistent with those reported results. Meanwhile, our further researches suggested PNGL upregulated these expression levels (Fig. 4D-F). Hence, SIRT1/2/3 may be involved in the regulation of PNGL.
It is reported that both SIRT1 and SIRT2 are major regulators of cellular anti-oxidative and anti-apoptotic responses [49,51,52]; and similar to SIRT1/2, NRF2 plays an important role in promoting mitochondrial biogenesis and regulating mitochondrial function [44]. Then the transcriptional activity of the NRF2-HO1 pathway is improved, which in turn, induces the increase of antioxidant genes expression (SOD and catalase), the decrease of ROS production, and the upregulation of mitochondrial superoxide dismutase (MnSOD) expression [[52], [53], [54]]. Consistent with the current reports, this study showed that the SIRT1/2 downregulation increased ROS production, reduced the activities of antioxidant proteins and factors (SOD, CAT, MnSOD), and thus caused oxidative stress and mitochondria injury in the MCAO/R rats. However, PNGL significantly improved the expression of antioxidant proteins in vivo (Fig. 5). These results suggested that the protective effects of PNGL might be related to the NAMPT-NAD + pathway and its related downstream SIRT1/2-MnSOD pathways.
In additions, some related study demonstrates that SIRT1 is not the only mediator of NAMPT to maintain mitochondrial NAD + pool [33]. Sirt3 is the primary mitochondria-targeted deacetylase, and has been shown to bind to and deacetylate several metabolic and respiratory enzymes that regulate mROS generation and mitochondrial functions [34,46,55]. Mitochondrial Sirt3 induces forkhead box O3 (FoxO3a) translocation to the nucleus and augments FoxO3a-dependent antioxidant defense systems [[52], [53], [54]] through upregulation of PGC-1α [[33], [34], [35]] and SOD2 [33,48]. In addition, they suppress ROS production and protect cells from mROS-induced oxidative damages. Our data indicated that pretreatment with PNGL obviously increased the PGC-1α and SOD2 levels in the MCAO/R-operated rats (Fig. 4), resulting in the inhibition of mitochondrial oxidizing injury, improvement of energy metabolism, and maintenance of mitochondrial functions.
This study suggests that PNGL has greatly neuroprotective effects, exerts anti-oxidative and mitochondria-protective effects, improves the energy metabolism, and thus inhibits neuronal apoptosis and necrosis. The underlying mechanisms may be involved in the NAMPT-SIRT1/2/3-MnSOD/PGC-1α signaling pathways. All of these results provide the strong scientific basis and guidances for the current onging clinical tests of PNGL as a new agent for treating CIS [22], which may contribute to confirming its effect characteristics, intensity and different stages of CIS; and it also further shows the promising perspercives of targeting NAMPT and mitochondrial protection as a therapeutic strategy against stroke. And these results give us a hint that PNGL may play a vital role by protecting mitochondria and promoting neurogenesis in the post-ischemia injury and recovery. Nonetheless, the related mechanisms of PNGL have not been confirmed by in vitro ischemic models. Therefore, further investigations is needed to deeply elucidate the mechanisms.
Author contributions
W.X. and T.Z. designed the research; T.Z., P.Z, X.M., and W.X. performed the experimental work; W.X., T.D. and F.N. wrote the manuscript; W.X. and F.N. performed the statistical analysis; W.X., F.N and H.X. helped map the figures and revise the manuscript. All authors discussed, edited, and approved the final version.
Declaration of competing interest
The authors declare no conflicts of competing interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81773938), the National Key Research and Development Project (No. 2017YFC1702504), the Key Laboratory Construction Project of Chinese Academy of Medical Sciences (No. 2018PT35030), the National Natural Science Foundation of China (No. 81891012), and the Clinical Research Project of Chinese Medicine “Qiye Tongmai Capsule” (No. 2015ZX09101020). We express our sincere gratitude to the foundations. In additions, it was appreciated that PNGL samples were provided by Jilin Academy of Chinese Medicine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.11.004.
Contributor Information
Guibo Sun, Email: sunguibo@126.com.
Xiaobo Sun, Email: sun_xiaobo163@163.com, xbsun@implad.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Eckert B. Acute stroke therapy 1981–2009∗. Clinical Neuroradiology. 2009;19(1):8. doi: 10.1007/s00062-009-8033-0. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L., Truelsen T., et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. The Lancet. 2014;383(9913):245–255. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turley K.R., Toledo-Pereyra L.H., Kothari R.U. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg. 2005;18(4):207–218. doi: 10.1080/08941930591004449. [DOI] [PubMed] [Google Scholar]
- 4.Bolaños J.P., Moro M.A., Lizasoain I., Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: therapeutic implications. Advanced Drug Delivery Reviews. 2009;61(14):1299–1315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. The Lancet Neurology. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 6.Tuttolomondo A., Di S.R., Di R.D., Pedone C., La P.S., Pinto A., Licata G. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. International Journal of Cardiology. 2011;151(3):318–322. doi: 10.1016/j.ijcard.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Woodruff T.M., Thundyil J., Tang S.-C., Sobey C.G., Taylor S.M., Arumugam T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Molecular Neurodegeneration. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsyuba E., Romani M., Hofer D., Auwerx J. NAD+ homeostasis in health and disease. Nature Metabolism. 2020;2(1):9–31. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 9.Stein L.R., Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23(9):420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F., Lu J., Manaenko A., Tang J., Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9(5):924–937. doi: 10.14336/AD.2017.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters A.M., Porter G.A., Jr., Brookes P.S. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111(9):1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel C.S., McCullough L.D. NAD+ and nicotinamide: sex differences in cerebral ischemia. Neuroscience. 2013;237:223–231. doi: 10.1016/j.neuroscience.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T., Byun J., Zhai P., Ikeda Y., Oka S., Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D., Gharavi R., Pitta M., Gleichmann M., Mattson M.P. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11(1):28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Zhang Z., Zhang N., Li H., Zhang L., Baines C.P., Ding S. Subcellular NAMPT-mediated NAD(+) salvage pathways and their roles in bioenergetics and neuronal protection after ischemic injury. J Neurochem. 2019;151(6):732–748. doi: 10.1111/jnc.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crack P.J., Taylor J.M. Reactive oxygen species and the modulation of stroke. Free Radical Biology and Medicine. 2005;38(11):1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Imai S.I., Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P., Miao C.Y. NAMPT as a therapeutic target against stroke. Trends Pharmacol Sci. 2015;36(12):891–905. doi: 10.1016/j.tips.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Tan C.L., Chin T., Tan C.Y.R., Rovito H.A., Quek L.S., Oblong J.E., Bellanger S. Nicotinamide metabolism modulates the proliferation/differentiation balance and senescence of human primary keratinocytes. J Invest Dermatol. 2019;139(8):1638–1647 e3. doi: 10.1016/j.jid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Liu X.Z., Tian W.W., Guan Y.F., Wang P., Miao C.Y. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther. 2014;20(6):539–547. doi: 10.1111/cns.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou X.D., Guo S.Q., Hu Z.W., Li W.L. NAMPT protects against 6-hydroxydopamine-induced neurotoxicity in PC12 cells through modulating SIRT1 activity. Mol Med Rep. 2016;13(5):4058–4064. doi: 10.3892/mmr.2016.5034. [DOI] [PubMed] [Google Scholar]
- 22.Xie W., Zhu T., Dong X., Nan F., Meng X., Zhou P., Sun G., Sun X. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules. 2019;9(10):512. doi: 10.3390/biom9100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui H.M., Zhang C.G., Lin H., Lu W.L., Cheng H.P., Wang J. [Determination of effective components in different positions of Panax notoginseng by HPLC] Zhong Yao Cai. 2009;32(12):1810–1813. [PubMed] [Google Scholar]
- 24.Jiang B., Wang C., Han Y., Hu X., Zheng L., Zhao Y. [Isolation and identification of minor bioactive saponins from the leaves of Panax notoginseng] Zhong Yao Cai. 2004;27(7):489–491. [PubMed] [Google Scholar]
- 25.Yu Y., Sun G., Luo Y., Wang M., Chen R., Zhang J., Ai Q., Xing N., Sun X. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. 2016;6:21730. doi: 10.1038/srep21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma B., Meng X., Wang J., Sun J., Ren X., Qin M., Sun J., Sun G., Sun X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int Immunopharmacol. 2014;22(1):151–159. doi: 10.1016/j.intimp.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Meng X., Xie W., Xu Q., Liang T., Xu X., Sun G., Sun X. Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules. 2018;23(9) doi: 10.3390/molecules23092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie W., Meng X., Zhai Y., Ye T., Zhou P., Nan F., Sun G., Sun X. Antidepressant-like effects of the Guanxin Danshen formula via mediation of the CaMK II-CREB-BDNF signalling pathway in chronic unpredictable mild stress-induced depressive rats. Annals of Translational Medicine. 2019 doi: 10.21037/atm.2019.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou P., Xie W., Meng X., Zhai Y., Dong X., Zhang X., Sun G., Sun X. Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells. 2019;8(3) doi: 10.3390/cells8030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guaragnella N., Antonacci L., Passarella S., Marra E., Giannattasio S. Achievements and perspectives in yeast acetic acid-induced programmed cell death pathways. Biochem Soc Trans. 2011;39(5):1538–1543. doi: 10.1042/BST0391538. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal B., Stowe D.F., Dash R.K., Bosnjak Z.J., Camara A.K. Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol. 2014;5:341. doi: 10.3389/fphys.2014.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S.D., Yang D.I., Lin T.K., Shaw F.Z., Liou C.W., Chuang Y.C. Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12(10):7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu B., Zhao J., Peng W., Wu H., Zhang Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1alpha and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem Biophys Res Commun. 2017;486(1):198–204. doi: 10.1016/j.bbrc.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Sidorova-Darmos E., Sommer R., Eubanks J.H. The role of SIRT3 in the brain under physiological and pathological conditions. Front Cell Neurosci. 2018;12:196. doi: 10.3389/fncel.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Li L., Li C.Y., Pei Z., Zhou M., Li N. SIRT3 protects cells from hypoxia via PGC-1α- and MnSOD-dependent pathways. Neuroscience. 2015;286:109–121. doi: 10.1016/j.neuroscience.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 36.Xie W., Zhou P., Sun Y., Meng X., Dai Z., Sun G., Sun X. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells. 2018;7(12) doi: 10.3390/cells7120270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouble A.N., Storey K.B. Characterization of the SIRT family of NAD+-dependent protein deacetylases in the context of a mammalian model of hibernation, the thirteen-lined ground squirrel. Cryobiology. 2015;71(2):334–343. doi: 10.1016/j.cryobiol.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Khoury N., Koronowski K.B., Young J.I., Perez-Pinzon M.A. The NAD+-Dependent family of sirtuins in cerebral ischemia and preconditioning. Antioxidants & Redox Signaling. 2018;28(8):691–710. doi: 10.1089/ars.2017.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P., Xu T.Y., Guan Y.F., Tian W.W., Viollet B., Rui Y.C., Zhai Q.W., Su D.F., Miao C.Y. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69(2):360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- 40.Zhao D., Sun X., Lv S., Sun M., Guo H., Zhai Y., Wang Z., Dai P., Zheng L., Ye M., et al. Salidroside attenuates oxidized lowdensity lipoproteininduced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019;43(6):2279–2290. doi: 10.3892/ijmm.2019.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Liu M., Sun R., Zeng Y., Chen S., Zhang P. Protective approaches against myocardial ischemia reperfusion injury. Exp Ther Med. 2016;12(6):3823–3829. doi: 10.3892/etm.2016.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garten A., Grohmann T., Kluckova K., Lavery G.G., Kiess W., Penke M. Sorafenib-induced apoptosis in hepatocellular carcinoma is reversed by SIRT1. Int J Mol Sci. 2019;20(16) doi: 10.3390/ijms20164048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S.N., Miao C.Y. Targeting NAMPT as a therapeutic strategy against stroke. Stroke Vasc Neurol. 2019;4(2):83–89. doi: 10.1136/svn-2018-000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu A., Zhou R., Xia B., Dang W., Yang Z., Chen X. NAMPT maintains mitochondria content via NRF2-PPARα/AMPKα pathway to promote cell survival under oxidative stress. Cellular Signalling. 2019;66:109496. doi: 10.1016/j.cellsig.2019.109496. [DOI] [PubMed] [Google Scholar]
- 45.Lin J.B., Kubota S., Ban N., Yoshida M., Santeford A., Sene A., Nakamura R., Zapata N., Kubota M., Tsubota K., et al. NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Rep. 2016;17(1):69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma R., Ritzel R.M., Crapser J., Friedler B.D., McCullough L.D. Evaluation of the neuroprotective effect of Sirt3 in experimental stroke. Transl Stroke Res. 2019;10(1):57–66. doi: 10.1007/s12975-017-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Libert S., Cohen D., Guarente L. Neurogenesis directed by Sirt1. Nat Cell Biol. 2008;10(4):373–374. doi: 10.1038/ncb0408-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Chen C.-L., Kang P.T., Jin Z., Chen Y.-R. Differential protein acetylation assists import of excess SOD2 into mitochondria and mediates SOD2 aggregation associated with cardiac hypertrophy in the murine SOD2-tg heart. Free Radical Biology and Medicine. 2017;108:595–609. doi: 10.1016/j.freeradbiomed.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potenza M.A., Sgarra L., Nacci C., Leo V., De Salvia M.A., Montagnani M. Activation of AMPK/SIRT1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia-reperfusion (I/R) injury in rats. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song S.B., Jang S.Y., Kang H.T., Wei B., Jeoun U.W., Yoon G.S., Hwang E.S. Modulation of mitochondrial membrane potential and ROS generation by nicotinamide in a manner independent of SIRT1 and mitophagy. Mol Cells. 2017;40(7):503–514. doi: 10.14348/molcells.2017.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., Sun J., Chen G., Niu C., Wang Y., Zhao C., Sun J., Huang H., Huang S., Liang Y., et al. Resveratrol promotes diabetic wound healing via SIRT1-FOXO1-c-myc signaling pathway-mediated angiogenesis. Front Pharmacol. 2019;10:421. doi: 10.3389/fphar.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olmos Y., Sanchez-Gomez F.J., Wild B., Garcia-Quintans N., Cabezudo S., Lamas S., Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 2013;19(13):1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H., Zhang J.J., Wang Z., Chen-Yang D.U., Zheng H., Shen Z.Y. Effects of FOXO3a on regulating mitophagy in hepatic ischemia reperfusion injury. Tianjin Medical Journal. 2017 [Google Scholar]
- 54.She D.T., Wong L.J., Baik S.-H., Arumugam T.V. SIRT2 inhibition confers neuroprotection by downregulation of FOXO3a and MAPK signaling pathways in ischemic stroke. Molecular Neurobiology. 2018;55(12):9188–9203. doi: 10.1007/s12035-018-1058-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W., Zhang L., Chen R., Lu H., Sui M., Zhu Y., Zeng L. SIRT3 protects against acute kidney injury via AMPK/mTOR-Regulated autophagy. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.