Abstract
Intradialytic hypotension (IDH) is the most frequent complication of hemodialysis (HD) treatments with a frequency of 10% to 12% for patients with chronic kidney disease attending for outpatient treatments and is associated with both temporary ischemic stress to vital organs, including the heart and brain, and increased patient mortality. Although there have been many different definitions of IDH over the years, an absolute nadir systolic blood pressure (SBP) has the strongest association with patient outcomes. The unifying pathophysiology is one of reduced effective blood volume, resulting in lower plasma tonicity, and if this cannot be adequately compensated for by activation of neurohumeral systems, then arteriolar tone and blood pressure fall. The risk factors for developing IDH are numerous, ranging from patient-related factors, including age and comorbidity with reduced cardiac reserve, to patient compliance with dietary and lifestyle advice, to reactions with the extracorporeal circuit and medications, choice of dialysate composition and temperature, setting of postdialysis target weight, ultrafiltration rate, and profiling. Advances in dialysis machine technology by providing real time estimates of the effective circulating volume and adjusting dialysate composition to maintain vascular tonicity are being developed, but currently require more sophisticated biofeedback loops to be clinically effective in preventing IDH. While awaiting advances in artificial intelligence, the clinician continues to rely on patient education to limit interdialytic weight gains, frequent assessment of the postdialysis target weight, adjusting dialysate composition and temperature, introducing convective therapies to increase thermal losses, and altering dialysis session duration and frequency to reduce ultrafiltration rate requirements.
Keywords: autonomic nervous system, bioimpedance, hemodialysis, hypotension, target weight
Hypotension is the most commonly reported complication of routine outpatient HD treatments.1 The incidence of IDH has been reported to range from 0.5% to 40% of all treatments, although more recent studies have suggested a prevalence of approximately 11%.2 In part, this variation is because of the numerous definitions of IDH that have been used, ranging from symptomatic hypotension requiring active management to symptomatic or asymptomatic absolute or percentage fall in SBP, or mean arterial blood pressure, or an absolute nadir SBP. In 2005 the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines in 2005 defined IDH as either a decrease in SBP ≥20 mm Hg or mean arterial blood pressure ≥10 mm Hg in conjunction with symptoms of hypotension.3 This was followed by the European Best Practice Guidelines, which defined IDH as a decrease in SBP ≥20 mm Hg in combination with associated clinical and nursing interventions.4 Other national and clinical guideline groups essentially adopted similar definitions based on the Kidney Foundation Kidney Disease Outcomes Quality Initiative or European Best Practice Guidelines definitions, with some variations including a fall in SBP of ≥30 mm Hg.5,6 Studies investigating the association between IDH and mortality reported that a nadir SBP had a stronger association with mortality, with a nadir of <100 mm Hg for patients starting dialysis with a SBP ≥160 mm Hg, and <90 mm Hg for those with a predialysis SBP of <160 mm Hg.6
Physiological Response to Hypovolemia
Venous Return
Most HD patients gain weight between dialysis sessions, and as such fluid removal and returning patients to a postdialysis target weight is a key objective of the dialysis treatment. Because veins can distend more than the arteries, approximately 70% of the blood volume is normally distributed in the venous system. Increased resistance to the vessels supplying a compliant vascular bed reduces inflow and distending pressure, resulting in a passive recoil of the venous bed and translocation of blood pooled in the venous bed back into the central circulation, thereby increasing right atrial filling pressure (DeJager-Krogh effect).7
Increased sympathetic drive and elevated plasma catecholamines predominantly increase cardiac venous return by reducing venous capacitance in the splanchnic and cutaneous circulations. Although the capacity of other vascular beds, such as muscle and kidney are also reduced, these only have a minor effect in supporting the central circulation. Studies in HD patients have demonstrated initial pooling of radio-labeled red blood cells in the splanchnic circulation, and when ultrafiltration was applied, these transferred to the systemic circulation, and bioimpedance measurements demonstrated a preferential initial movement of extracellular fluid to the splanchnic circulation.8,9 Similarly, intravital microscopy and doppler studies have demonstrated a reduction in skin and mucosal blood flow during HD in response to ultrafiltration.10, 11, 12, 13
Cardiovascular Response
The cardiovascular response to hypovolemia includes changes in heart rate, contractility, and peripheral vascular resistance. Heart rate initially increases, however, it reduces toward baseline as other compensatory mechanisms get activated. However, in severe cases of refractory hypovolemia the heart rate may slow because of the Bezold-Jarisch reflex. Although heart rate is thought to have only a relatively minor effect on the response to hypovolemia,13 many HD patients have diastolic dysfunction, estimated between 50% to 75%, and are therefore more vulnerable to a reduction in cardiac venous return if they cannot increase their heart rate response.14,15 Similarly, increased contractility by increasing cardiac output could potentially provide some degree of compensation for hypovolemia. However, magnetic resonance imaging studies conducted during HD have noted a reduction in myocardial blood flow by approximately 13% during the first 30 minutes when minimal fluid has been removed.16 Other magnetic resonance imaging studies have reported a 25% reduction in myocardial blood flow at the end of a dialysis session following 2.5 liter fluid removal, with both a reduction in ventricular volumes and left ventricular muscle mass, because of removal of intracardiac muscle water.16,17 Echocardiography studies have observed that these changes in myocardial blood flow can induce stress related segmental left ventricular dysfunction.18 Echocardiographic studies have suggested that approximately 6% of incident HD patients may have an ejection fraction of <25%.15 However, correcting volume overload often leads to an improvement in left ventricular function;19 even so, many dialysis patients have left ventricular hypertrophy20 because of chronic volume overload and hypertension.20, 21, 22
Thus, the response to hypovolemia for HD patients with normal cardiac function or diastolic dysfunction is predominantly dependent on sustaining venous return. However, patients with left ventricular dysfunction will be at greater risk of decompensation if cardiac return cannot be maintained.
Autonomic Response
Hypovolemia triggers cardiopulmonary receptors in atria and main pulmonary veins, and the baroreceptors in the aortic arch and carotid sinuses, resulting in neurohumeral activation of the sympathetic nervous system, followed by nonosmotic vasopressin and renin release. This leads to reduced blood flow to the skin and skeletal muscles, redistribution of blood from venous capacitance vessels with increased peripheral vascular resistance, and increased heart rate and contractility, designed to preserve blood flow for vital organs. Circulating catecholamines and aldosterone then increase with progressive hypovolemia.
There has been debate as to whether uremia per se causes autonomic dysfunction, because autonomic dysfunction increases with age, and comorbidities including diabetes, hypertension, and heart failure. Studies in HD patients have reported variable findings with some observing deterioration in autonomic function over time, whereas others noted improvement.23 Many HD patients have some degree of autonomic impairment, and greater autonomic dysfunction is associated with increased mortality.23 Sympathetic nervous system activity is increased in patients with chronic kidney disease,24 and this had generated a hypothesis that chronic overstimulation results in a down regulation of the sympathetic nervous system response.25 Although reports vary, majority have noted an increase in plasma catecholamines in keeping with chronic overactivation.23,26
Renin seems to have little effect in the acute response to hypovolemia in HD patients. Similarly, although vasopressin levels are increased, consequent on the increased plasma osmolality of HD patients, vasopressin levels do not rise during HD in response to hypotension27 because vasopressin is cleared.28
Hypotension During HD Sessions
Ultrafiltration and IDH
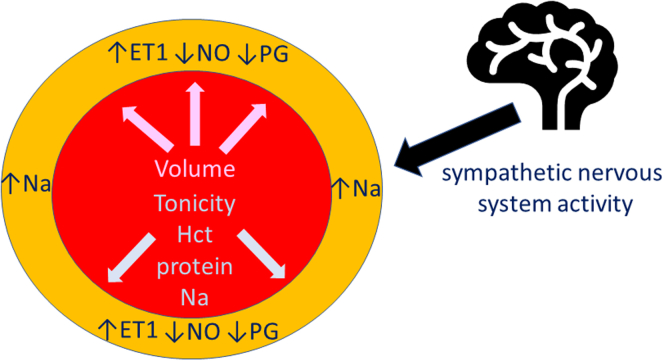
Most HD patients gain weight between dialysis sessions, and the fluid gained needs to be removed during HD to return the patient to their post-HD target weight, because persistent volume overload increases left ventricular hypertrophy and reduces survival.29 Fluid is removed during passage through the dialyzer by applying a transmembrane pressure to generate an ultrafiltrate. Most fluid gained between dialysis sessions is intracellular and extracellular, with only a modest increase in plasma volume.30 If the rate of fluid removal from plasma volume exceeds the rate at which fluid can be mobilized from the intracellular compartment and extracellular extravascular volume, termed plasma refill rate, plasma volume will be reduced and the patient becomes hypotensive unless compensated for by neurohumeral responses. Blood pressure is a measure of vascular tone. Tonicity in the vessel wall depends upon the internal pressure from the blood volume and viscosity, and the tone in the media of the arterial wall (Figure 1). Hematocrit has the greatest effect on blood rheology, followed by protein and then sodium concentration. This effect of anemia on vascular changes has been shown by studies demonstrating increasing orthostatic hypotension with greater severity of anemia in patients with chronic kidney disease.31 Similarly, observational reports have highlighted that hypoalbuminemia is a risk factor for IDH.32 Studies in HD patients have shown that vasopressin levels, a potent constrictor of the splanchnic circulation, do not increase in response to ultrafiltration during HD.33,34 Similarly, catecholamines, dopamine, norepinephrine, or epinephrine do not increase during HD.33,35 Over time, more older patients and those with diabetes and other comorbidities are now treated by HD, thereby increasing the number of patients with potentially impaired autonomic responses to hypotension (Table 1).
Figure 1.
Blood pressure is a measure of arterial tone, which reflects internal outward pressure on vessel wall by the circulating volume, plasma tonicity (Hct, total protein-protein and sodium) and vascular smooth muscle tone (sodium content vascular smooth muscle, balance of local vasoconstrictors (ET1) and vasodilators NO, PDG) and sympathetic nerve activity. ET1, endothelin; Hct, hematorcrit; Na, sodium; NO, nitric oxide; PDG, prostanoids.
Table 1.
Patient factors which potentially increase the risk of intradialytic hypotension
| Risk factors | Pathology | At risk groups |
|---|---|---|
| Normal plasma volume | Pulmonary hypertension | Chronic lung disease |
| High flow A-V shunt | ||
| Pericardial effusion | Cardiac tamponade | |
| Right ventricular dysfunction | Inferior myocardial infarction | |
| Heart failure with preserved ejection fraction | Diastolic dysfunction | |
| Cardiac conduction defect | Complete heart block | |
| Infiltrative cardiomyopathy | Amyloid | |
| Reduced plasma volume | Hemorrhage | Acute blood loss |
| Diarrhea | Gastroenteritis | |
| Vomiting | Gastric outflow obstruction | |
| Sodium losing nephropathy | Posterior urethral valves | |
| Reduced effective plasma volume | Systemic sepsis | Bacterial infection |
| Liver failure | Acute on chronic liver failure | |
| Anemia | Chronic kidney disease | |
| Hypoalbuminemia | Malnutrition & sepsis | |
| Cardiac afterload | Heart failure with reduced ejection fraction | Ischemic heat disease |
| Cardiomyopathy | ||
| Valvular heart disease | Aortic stenosis | |
| Autonomic dysfunction | Age | Elderly |
| Endocrine/metabolic | Diabetes | |
| Thyroid disease | ||
| Porphyria | ||
| Autoimmune | Systemic lupus erythematosus | |
| Sjogrens syndrome | ||
| Coeliac disease | ||
| Infiltrative | Amyloid | |
| Neurologic | Parkinson’s disease | |
| Life style | Alcohol | |
| Malignancy | Paraneoplastic | |
| Bortezomib | ||
| Doxorubicin | ||
| Sympathetic denervation | Cardiac transplant | |
| Artificial heart | ||
| Medications | Atenolol/metoprolol/propranolol/timolol | |
| Methyl dopa | ||
| Alpha blockers |
A-V, arterio-venous.
Predominantly because of reduced cardiac reserve to repond to a reduction in cardiac filling pressures and autonomic dysfunction AV shunt.
Determining Target Weight and Ultrafiltration
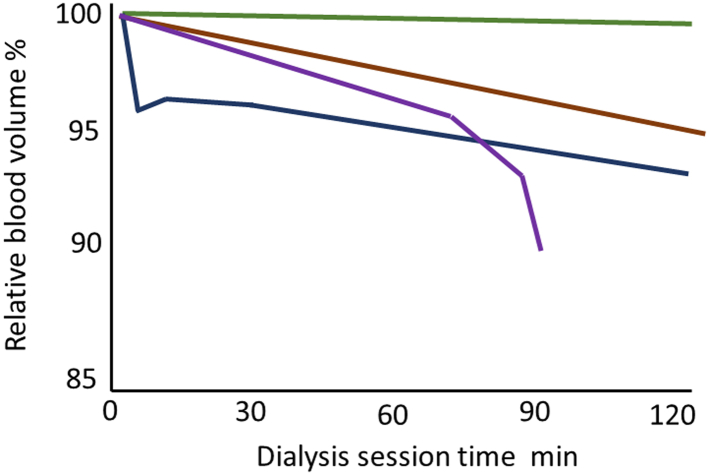
One of the differences between adult and pediatric practice is the number of children with sodium-losing nephropathies. Therefore, many children may have minimal interdialytic weight gains, and IDH is much more common in pediatric HD units.36 Similarly, adult patients attending for dialysis with minimal extracellular water overload are more likely to suffer IDH,37 and report more intradialytic symptoms.38,39 Therefore, it is important to determine and regularly review target weight for patients. Most dialysis centers use clinical assessment aided by laboratory and other investigations (Table 2).40 Although serial measurements of brain natriuretic peptides, cancer antigen 125, inferior vena cava diameter, lung ultrasound comets, and extracellular water by bioimpedance provide important information as to whether volume overload is increasing or decreasing, they have not been shown to reduce the risk of IDH.41, 42, 43, 44, 45, 46 Advances in dialysis machine technology have led to the integration of relative blood volume (RBV) monitoring or adding measurements of venous oxygenation.47 RBV monitoring measures hematocrit or blood density of blood density entering the dialyzer, on the basis that contraction of the plasma volume leads to an increase in hematocrit and density. Although multicenter trials of RBV did not show that this technology reduced IDH,48 they did not have nurses continuously monitoring the trends. Several patterns of RBV monitoring have been described (Figure 1) as follows: no change in RBV when patients are very volume overloaded; a gradual fall with ultrafiltration, suggesting that plasma refill is compensating for ultrafiltration losses, an initial fall at the start of HD; and a steep decline suggesting that the ultrafiltration rate exceeds the plasma refill rate.49,50 As patients approach their target weight, pulses of ultrafiltration lead to increasingly steeper slopes, and longer recovery50 (Figure 2). However, there are several confounders to consider with RBV monitoring, including setting the starting point and the Fahraeus effect, because hematocrit varies in different organ circulations so that changes in RBV may lag behind real time changes in the circulating volume. Attempts to add biofeedback control to respond to changes in RBV monitoring have not yet been successful in preventing IDH.51 There is no absolute critical threshold that predicts IDH, and not only is there marked variation in patient responses to ultrafiltration, but also different responses within the same patient during different dialysis sessions.52 Central venous oxygen saturation can be measured in patients dialyzing with catheters,53 and as with RBV monitoring there is a heterogenous patient response to ultrafiltration, with an overall trend for a fall in oxygenation in patients with IDH.54 However, studies using RBV monitoring did show that different prescriptions of ultrafiltration were associated with different risks of IDH. A linear constant fluid removal rate reduced the risk of IDH compared with intermittent periods of ultrafiltration, and the pattern with the least risk of IDH was one starting a little higher than the linear pattern and then slowly decreasing over the course of the dialysis session.55
Table 2.
Postdialysis target weight needs to be regularly assessed because an inappropriately low target increases the risk of intradialytic hypotension
| Assessment | Volume overload | Volume depleted |
|---|---|---|
| History | ↑Dietary salt intake | ↓Appetite |
| ↑Dyspnea | Diarrhea/vomiting | |
| Examination | No postural hypotension | Postural hypotension |
| ↑Blood pressure | ↓Blood pressure | |
| ↑Weight trend | ↓Weight trend | |
| ↑Neck veins | ↓Neck veins | |
| ↑Peripheral edema | No edema | |
| Laboratory | Low albumin | ↑Albumin |
| ↑Natriuretic peptides | ↓Natriuretic peptides | |
| ↑Serum CA125 | Normal serum CA125 | |
| Imaging chest X ray | ↑CTR | Normal/↓CTR |
| Septal lines | ||
| Kerley B lines | ||
| Lung ultrasound | >10 B lines | <5 B lines |
| Abdominal ultrasound | <50% collapse IVC | >50% IVC collapse |
| Bioimpedance | ↑ECW/ICW | ↓ECW/ICW |
| Dialysis session RBV | Flat line | Rapid decline |
| Dialysis session VO2 | Stable O2 saturation | ↓O2 saturation |
CA125, cancer antigen 125; CTR, cardio-thoracic ratio; ECW, extracellular water, ICW, intracellular water; RBV, relative blood volume; VO2, venous oxygen saturation.
Figure 2.
Relative blood volume patterns Linear flat line in a patient who is substantially volume overloaded and no change with ultrafiltration (Green line). Patient with stable downward trend so plasma refill is being compensated during ultrafiltration (Brown line). Initial fall in relative blood volume because of reaction with dialyzer, and then stabilization (Black line). Patient decompensating as sudden steep fall in relative blood volume, as plasma refill does not keep pace with ultrafiltration (Purple line).
Multiple observational studies have reported an association between high ultrafiltration rates >10 to >13 ml/h/kg, mortality, and increased IDH.56,57 Therefore, ultrafiltration by removing excess fluid improves cardiac performance and venous oxygen saturation in HD patients,53,58 whereas excessive ultrafiltration rates risk myocardial and other organ ischemia.18 In addition, excessive fluid removal can lead to postdialysis thirst, with increased interdialytic weight gains, thereby setting up a vicious cycle. Such patients require appropriate dietary advice, and if possible, longer or more frequent of more frequent dialysis sessions to control volume overload. Because weight naturally varies, applying a “soft” target weight, so that the target weight does not have to be achieved with every session, particularly after the longer interdialytic interval allows the development of a protocol which is less likely to cause patients to have greater interdialytic weight gains.59
Hypotension Unrelated to Ultrafiltration
Anaphylactoid Reactions
The extracorporeal circuit consists of plastic tubing and the dialyzer. Plastics are formed from a basic polymer to which other compounds are added, and the capillary fibers are assembled in a dialyzer casing that contains additional organic compounds. As blood flows through the extracorporeal circuit, the circuit has to be sterilized and anticoagulants administered to prevent clotting. Therefore, patients may develop profound hypotension within the first few minutes of starting dialysis because of an anaphylactoid reaction to some of these compounds. In the 1980s, a number of serious anaphylactoid reactions were reported because of the use of ethylene oxide (EtO) as a sterilant for dialyzers. EtO formed a complex with albumin resulting in the formation of IgG antibodies to the EtO-albumin complex.60 Today, very few dialyzers are sterilized with EtO, but some blood lines may still be sterilized with EtO.
If dialyzers and tubing are not thoroughly rinsed before connection, a series of potentially toxic hydrocarbons and halocarbons can be released from the dialyzer and tubing set and be detected in the exhaled breath of HD patients.61 Hypotensive anaphylactoid reactions were reported from several countries when patients were dialyzed with polysulfone and polyethersulfone dialyzers from different manufacturers.62, 63, 64 Some patients demonstrated histamine release from mast cells, reacting to polyvinylpyrrolidone, and others to substances released on rinsing the dialyzers.62,64 Some of the most severe hypotensive anaphylactoid reactions, sometimes fatal, have been due to anticoagulants. These include heparin-induced thrombocytopenia with antibodies generated against heparin-platelet factor 4 complexes,65 and reactions to the serine protease inhibitor nafamostat maleate.66 Heparin contaminated with chondroitin sulfate, termed over-sulfated heparin, was also reported to cause severe hypotension.67,68 Other reactions causing early onset hypotension have been reported with the use of acetate containing bicarbonate dialysate during both HD and hemodiafiltration (HDF),69 and topical preparations of chlorhexidine used to cleanse the skin around central venous access catheters.70
Inflammatory Reactions to the Extracorporeal Circuit
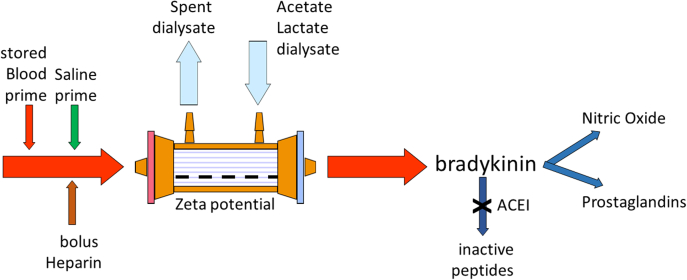
During the 1980s and 1990s, a variety of polymers were used in the manufacture of capillary dialyzers. One of the main differences in polymer composition was the surface charge, or zeta potential. Reports of severe hypotension soon after starting HD appeared in the pediatric literature. Because the volume of the extracorporeal circuit is relatively larger than that of an infant or small child, circuits were typically primed with whole blood. Blood is anticoagulated with approximately 40 mmol/l of citrate, which is quite negatively charged, and the amount of negative charge is increased with storage and cold temperature. The combination of negative charges from blood, saline, and heparin coupled with a negatively charged dialyzer membrane could potentially increase bradykinin and nitric oxide generation in the circuit, resulting in profound vasodilation and hypotension (Figure 3).71,72 This response was also dependent on patient factors in terms of activation of the acute inflammatory system, which is more common in patients with sepsis and liver failure.73,74 In addition, because nitric oxide can be scavenged by hemoglobin, anemic patients are at greater risk of IDH with these reactions.75
Figure 3.
Negative electrical charges in priming fluids (chloride in 0.9% saline, citate in stored blood) and heparin combine with negatively charged dialyzer membrane (negative zeta potential) combine to increase bradykinin and nitric oxide generation by inflammatory cells. angiotensin converting enzyme inhibitor reduces bradykinin breakdown to inactive peptides. ACEI, angiotensin converting enzyme inhibitor.
Even with the modern day dialyzer, leukocytes, monocytes, platelets, and complement are activated in the extracorporeal circuit, with initial leukocyte sequestration in the lung,76 and increased alveolar capillary permeability.77 As such, a substantial number of patients have an initial fall in RBV and blood pressure during the first 30 minutes of dialysis, at a time when there has been minimal ultrafiltration (Figure 1).78 Nitric oxide is generated by the activation of inflammatory cells, and although there is some clearance during dialysis, the rate of change supports ongoing production,33 and some studies have reported greater nitric oxide production in those patients with IDH.79
Dialysate Composition and Temperature
Historically, acetate was used for the buffer base for dialysate solutions, and acetate accumulation was linked to hypotension, particularly for patients with impaired cardiac function.80 Current bicarbonate dialysates contain a small amount of acetate (3−4 mmol/l), but even some studies have shown an early reduction in vascular tone, measured with aortic pulse wave velocity, in patients using a standard bicarbonate dialysate.81 Acetate-free biofiltration is a form of dialysis available in Europe, which uses no acetate, and observational studies have reported less IDH with acetate-free biofiltration compared with standard dialysis with a standard bicarbonate and low acetate dialysate.82,83 Acetate free dialysates are now becoming available for HD, replacing 3 mmol/l acetate with 1 mmol/l of citrate, and preliminary studies have reported a reduction in IDH with the acetate-free citrate dialysate.84
Sodium is important in maintaining plasma tonicity and vascular refilling. Single or dual center studies have demonstrated that using a high dialysate sodium leads to increased interdialytic weight gains and hypertension but less IDH, whereas a lower dialysate sodium reduces interdialytic weight gains, but increases IDH.85 The effects of dialysate sodium are also affected by patient factors, and meta-analysis particularly of multicenter studies failed to demonstrate an overall effect of different dialysate sodium concentrations.86,87 However, a number of studies have reported that the prescribed dialysate sodium and delivered sodium may differ, in which case this may have been a confounder in the results from the multicenter studies.88,89
During the first hour of HD, plasma urea concentrations and osmolality rapidly fall. To modify this fall in plasma tonicity, centers used a varying dialysate sodium, starting with a higher dialysate sodium and ending with a lower sodium,90 with reports that this practice reduced the incidence of IDH in the short term.91 Other studies have shown a marginal reduction in the fall of SBP during the first hour of dialysis when starting with a higher dialysate sodium, which was associated with a greater vasopressin response.33 However, as patients consume different amounts of sodium and have different interdialytic weight gains, this has led to the concept of an individual osmostat.92,93 Individualizing the dialysate sodium concentration according to the plasma sodium, so that delivering an iso-natric dialysate has been reported to reduce the incidence of IDH, but this technology requires further refinement and clinical testing.94
Plasma potassium declines rapidly during the early phase of dialysis, predominantly by diffusion and then plateaus. Choosing a lower dialysate potassium to maximize potassium removal potentially risks inducing hypokalemic intradialytic and postdialytic arrhythmias.95 Although the rapid change of intracellular and extracellular potassium concentrations may potentially alter cardiac conduction, this may be exacerbated when using a lower dialysate magnesium and high bicarbonate.47 However, there are no studies demonstrating an effect of dialysate potassium concentration and the prevalence of IDH.96
Following the introduction of active forms of Vitamin D3, the concentration of calcium in dialysates has been reduced from 1.75 mmol/l to much lower concentrations because of concerns over calciphylaxis. One major US dialysis provider reduced dialysate calcium to 1.0 and 1.125 mmol/L and observed an increased prevalence of IDH.97 Other studies reported a reduction in IDH when calcium dialysate was increased from 1.25 to 1.5 mmol/l.98 The differences in IDH with different calcium dialysate concentrations are typically reported during the latter phase of the dialysis session, when volume has been lost because of ultrafiltration. Though calcium can affect nerve transmission and muscle contraction, several short term interventional studies have reported that a reduction in dialysate calcium, from 1.75 to 1.5 mmol/l, or comparison between 1.75 to 1.25 mmol/l and 1.37 versus 1.12 mmol/l, led to a reduction in vascular stiffness, as measured by pulse wave velocity.99, 100, 101 However, longer term observational studies did not demonstrate a difference in the change in PWV over time.102 Most centers use a fixed dialysate calcium, but dialysate calcium profiling starting with a lower dialysate calcium of 1.25 mmol/l and then increasing to 1.75 mmol/l,was reported to cause less IDH than when dialyzing with 1.5 mmol/l.103 This is most likely because of calcium induced vasoconstriction.
Traditionally, dialysates have had a low magnesium concentration to prevent magnesium accumulation in dialysis patients.104 Magnesium has an important role in generating cardiac myocyte action potentials and muscle contraction.105 Observational reports noted an association between the fall in intradialytic magnesium and an increased incidence of IDH.106 Prospective studies demonstrated that IDH was reduced following an increase in dialysate from 0.25 to 0.75 mmol/l when combined with a calcium dialysate of 1.35 mmol/l.107 Whereas another study investigating the effects of different dialysate magnesium and calcium concentrations reported that the combination with fewest episodes of IDH was one of magnesium 0.75 mmol/l and calcium 1.25 mmol/l.106 Other studies have observed lower postdialysis serum magnesium when using acetate compared with bicarbonate dialysates,107 and also with Citrasate (bicarbonate with citrate and acetate) (Health Tec Medical Ltd).108 However, studies using citrate as an anticoagulant have not shown any reduction in IHD.109
Despite warming the dialysate, there are thermal losses as blood flows through the extracorporeal circuit, which leads to a reduction in blood flow to the skin microvasculature and redistribution to the larger venous capacitance vessels.110,111 This leads to an increase in the central core temperature and coupled with additional thermal energy gain from the inflammatory reaction with the extracorporeal circuit. If the rise is too great, this will cause reflex vasodilatation and increased blood flow to the skin.110 Reports of the effects of thermal energy losses and IDH date back some 40 years.112,113 Since then, there have been many studies confirming these early reports, such that a meta-analysis of 26 randomized controlled trials, including 484 patients, reported that reducing the dialysate temperature significantly reduces the rate of IDH by 70% and increased intradialytic mean arterial blood pressure by 12 mm Hg.114 Although, another systematic review of 25 randomized controlled trials, including 712 patients, concluded that the prevention of IDH by cooled dialysate temperature was less certain, confounded by differences in study design and potential bias.115 A fixed reduction of dialysate temperature reduced IDH (rate ratio 0.52, 95% confidence interval 0.34–0.80), but potentially increased patient discomfort (rate ratio 8.31, 95% confidence interval 1.86–37.12) although the studies reporting were rated low as to certainty of evidence reported, and larger studies have not demonstrated an effect of dialysate temperature and self-reported symptoms.116,117 Thus, both international and national clinical guideline committees have recommended the use of cooled dialysate to prevent episodes of IDH.4,5 However, the practice of cooling dialysate varies between centers from simply reducing the dialysate temperature to a fixed lower temperature, or individualizing the temperature to 0.5 to 1.0 °C below the patient’s core temperature, or using dialysis machine technology to deliver isothermic (no change in temperature), or thermoneutral (no increase in thermal energy), or a prescribed negative thermal energy target.118 One study has demonstrated an advantage for isothermic compared with thermoneutral HD.119 However, cooled dialysate has a greater protective effect on IDH than increased dialysate sodium concentrations.120
HD Modes
IDH became an increasingly recognized problem as HD provision expanded. In the late 1970s, dialysis machines did not have the accurate volumetric pumps of today, and studies reported greater cardiovascular stability with isolated ultrafiltration compared with HD with ultrafiltration.121 The greater number of IDH with HD was variously ascribed to the use of acetate dialysate and fall in osmolality with changes in vessel tonicity. However, the advantage for ultrafiltration was primarily because of starting before HD, when patients were most volume overloaded and to experience greater peripheral vasoconstriction and venous tone to better support cardiac filling pressures, with a smaller rise in core temperature.122 Because ultrafiltration is a convective process, studies of hemofiltration similarly reported less IDH compared with comparable high-flux HD sessions.123 Initially, this was thought to be due to the removal of a cardio-depressant factor, or because of differences in sodium balance, if a higher dialysate sodium was used as the replacement fluid for convective volume losses.124 Similarly, HDF, in particular, high-volume postdilutional HDF was observed to cause fewer IDH episodes than high-flux HD.125 However, a series of interventional studies demonstrated that the reduction in IDH was related to the greater thermal losses with postdilutional HDF, and predilutional HDF, so that when HD treatments used cooled dialysate and thermal losses were comparable, blood pressure profiles were not different between modes.112,126,127 In-vitro studies demonstrated that cooled dialysates reduced endothelial nitric oxide production.128
Unusual Causes of Hypotension Unrelated to Ultrafiltration
Although ultrasound techniques have noted microembolic signals in the extracorporeal circuit that may derive from clots or gas embolies, it is very unusual for a sufficiently sized air embolus not to be detected and allowed to pass into the patient causing hypotension and cardiac arrest. Most fatal reports relate to central venous access catheters when tubing has not been adequately clamped, thereby allowing air entry and then faulty or silenced machine alarms, which have often been over-ridden.129 Clot emboli from venous catheters may occasionally be large enough to cause pleuritic pain and hypotension. Some years ago, dialyzers were tested for potential blood leaks in the capillary fibers with liquid perfluorocarbons. If these compounds were not fully removed during sterilization, they would transform to a gas at body temperature as warm blood flowed through the dialyzer, and result in a gas embolus.130
In extreme cases, exsanguination can occur during dialysis. The blood pump will automatically stop the dialysis machine alarm, if there is a disconnection with the arterial access. Whereas the dialysis blood pump will continue to pump blood, the machine would not alarm if there is disconnection or faulty venous needle or catheter connection, resulting in rapid exsanguination.131
Blood Pressure Targets and Medications
HD patients are at increased risk of stroke, and in the general population, stroke risk is associated with increased SBP.132 In an attempt to reduce stroke risk guideline targets for predialysis and postdialysis blood pressure control were introduced, but IDH rates were far greater for those dialysis centers with higher achievement of blood pressure targets.133 Further studies demonstrated that pre-HD and post-HD sessional measurements of blood pressure do not accurately reflect interdialytic blood pressures,134 and so peridialytic targets were discontinued.
There is a debate about antihypertensive medications for HD patients, with reports showing no advantage for angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and a possible cardioprotective effect with β-blockers.135 However, some drugs are cleared by HD, including water soluble β-blockers and some of the angiotensin converting enzyme inhibitors. Carvedilol is not cleared by dialysis,136 and carvedilol has been reported to improve outcomes for HD patients with heart failure.137 However, an observational study compared carvedilol with metoprolol, which is cleared during dialysis,136 and reported a greater number of IDH episodes with carvedilol.138 This would suggest that carvedilol potentially reduces any increase in heart rate to compensate for volume shifts. Although logical not to administer potent vasodilatory antihypertensive medications before a HD session, there are few reports and the PanThames review did not demonstrate a difference in IDH between those centers which advised patients not to take antihypertensive medications and those which did, although IDH was more frequent in those prescribed antihypertensives.133
Eating and Exercise During Dialysis Sessions
HD patients are at increased risk of sarcopenia,139 and unlike fat, there is no body protein store. Many patients spend time traveling to and from dialysis centers, and as such the “dialysis” day can be a long one, and postdialysis recovery times are variable, so patients may consume less food on the day of dialysis.140 There is a risk of peridialysis hypoglycemia, whether food is offered or not.141,142 However, providing patients with a large meal to eat during the HD session potentially risks IDH, because blood is diverted to the gut.143,144 Not all studies have demonstrated an increase in IDH,145 and many centers continue to provide patients with hot food and sandwiches, especially with the increasing number of diabetics. If however, patients develop IDH while eating during dialysis, then cold food could be provided after the session has been completed.146
Most reports on exercise during dialysis have used cycling or bands, and exercise that started during the early phase of dialysis before significant ultrafiltration has occurred, but only when patients have been cardiovascularly stable and for only a short duration. Coupled with patient selection, it is currently unclear as to whether exercise has a neutral or beneficial effect on IDH.147, 148, 149
Conclusion
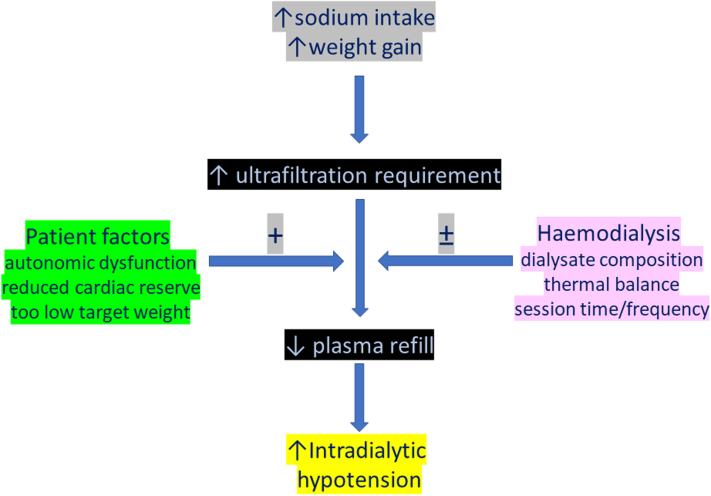
Despite numerous advances in dialysis practice, IDH remains the most common complication associated with dialysis sessions. IDH leads to episodes of transitory organ ischemia, and repetitive episodes lead to permanent organ damage. Although most episodes of IDH occur during the latter phase of the dialysis session with increasing ultrafiltration volumes (Figure 4), increased patient mortality is associated with IDH both during the early and later phases of the dialysis session.56,57,150
Figure 4.
Increased interdialytic weight gains driven by dietary sodium increase ultrafiltration requirements, and the risk of intradialytic hypotension is then potentially increased by patient comorbidities, and inappropriately low postdialysis target weight, but risk could be modified by adjusting dialysate composition and increasing thermal losses and altering dialysis session time and frequency of treatments.
Different clinical guideline groups and researchers have used different definitions of IDH and this has hampered research and advances in the prevention and treatment of IDH by the lack of a unifying definition, although the strongest association with mortality appears to be the nadir SBP.
The fundamental pathogenesis of IDH is a reduction in vascular tone, primarily because of a fall in the effective circulating volume without an effective compensatory neurohumeral response. The etiology is wide ranging from patient-related factors to the extracorporeal circuit and dialysis prescription.
Until artificial intelligence biofeedback systems can be developed to institute changes during dialysis, the clinician is reliant on patient compliance to limit interdialytic weight gains, reviewing postdialysis target weight, achieving isothermic or negative thermal energy losses, and adjusting dialysis session times and frequency to reduce ultrafiltration rate requirements.
Disclosure
The author decalred no competing interests.
References
- 1.Davenport A., Cox C., Thuraisingham R. Blood pressure control and symptomatic intradialytic hypotension in diabetic haemodialysis patients: a cross-sectional survey. Nephron Clin Pract. 2008;109:c65–c71. doi: 10.1159/000139991. [DOI] [PubMed] [Google Scholar]
- 2.Kuipers J., Verboom L.M., Ipema K.J.R., et al. The prevalence of intradialytic hypotension in patients on conventional hemodialysis: a systematic review with meta-analysis. Am J Nephrol. 2019;49:497–506. doi: 10.1159/000500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.K/DOQI Workgroup K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153. [PubMed] [Google Scholar]
- 4.Kooman J., Basci A., Pizzarelli F., et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22(suppl 2):ii22–ii44. doi: 10.1093/ndt/gfm019. [DOI] [PubMed] [Google Scholar]
- 5.Ashby D., Borman N., Burton J., et al. Renal Association clinical practice guideline on haemodialysis. BMC Nephrol. 2019;20:379. doi: 10.1186/s12882-019-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assimon M.M., Flythe J.E. Definitions of intradialytic hypotension. Semin Dial. 2017;30:464–472. doi: 10.1111/sdi.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugirdas J.T. Dialysis hypotension–a haemodynamic analysis. Kidney Int. 1991;39:233–246. doi: 10.1038/ki.1991.28. [DOI] [PubMed] [Google Scholar]
- 8.Daugirdas J.T. Intradialytic hypotension and splanchnic shifting: integrating an overlooked mechanism with the detection of ischemia-related signals during hemodialysis. Semin Dial. 2019;32:243–247. doi: 10.1111/sdi.12781. [DOI] [PubMed] [Google Scholar]
- 9.Haroon S., Tai B.C., Yeo X., Davenport A. Changes in total and segmental extracellular and intracellular volumes with hypotension during hemodialysis measured with bioimpedance spectroscopy. Artif Organs. 2022;46:666–676. doi: 10.1111/aor.14096. [DOI] [PubMed] [Google Scholar]
- 10.Bemelmans R.H., Boerma E.C., Barendregt J., Ince C., Rommes J.H., Spronk P.E. Changes in the volume status of haemodialysis patients are reflected in sublingual microvascular perfusion. Nephrol Dial Transplant. 2009;24:3487–3492. doi: 10.1093/ndt/gfp267. [DOI] [PubMed] [Google Scholar]
- 11.Meinders A.J., Nieuwenhuis L., Ince C., Bos W.J., Elbers P.W.G. Haemodialysis impairs the human microcirculation independent from macrohemodynamic parameters. Blood Purif. 2015;40:38–44. doi: 10.1159/000380902. [DOI] [PubMed] [Google Scholar]
- 12.Mistrík E., Dusilová Sulková S., Bláha V., et al. Evaluation of skin microcirculation during haemodialysis. Ren Fail. 2010;32:21–26. doi: 10.3109/08860220903375286. [DOI] [PubMed] [Google Scholar]
- 13.Daugirdas J.T. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis. 2001;38(4 suppl 4):S11–S17. doi: 10.1053/ajkd.2001.28090. [DOI] [PubMed] [Google Scholar]
- 14.Escoli R., Carvalho M.J., Cabrita A., Rodrigues A. Diastolic dysfunction, an underestimated new challenge in dialysis. Ther Apher Dial. 2019;23:108–117. doi: 10.1111/1744-9987.12756. [DOI] [PubMed] [Google Scholar]
- 15.Loutradis C., Sarafidis P.A., Papadopoulos C.E., Papagianni A., Zoccali C. The ebb and flow of echocardiographic cardiac function parameters in relationship to hemodialysis treatment in patients with ESRD. J Am Soc Nephrol. 2018;29:1372–1381. doi: 10.1681/ASN.2017101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasselaar J.J., Slart R.H.J.A., Knip M., et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–610. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha T., Martinez-Naharro A., Yoowannakul S., et al. Acute changes in cardiac structural and tissue characterisation parameters following hemodialysis measured using cardiovascular magnetic resonance. Sci Rep. 2019;9:1388. doi: 10.1038/s41598-018-37845-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton J.O., Jefferies H.J., Selby N.M., McIntyre C.W. Haemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirayama S., Ando Y., Sudo Y., Asano Y. Improvement of cardiac function by dry weight optimization based on interdialysis inferior vena caval diameter. ASAIO J. 2002;48:320–325. doi: 10.1097/00002480-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Chargui S., Allouche E., Dkhil W., et al. Left ventricular hypertrophy in hemodialysis patient: prevalence, electrocardiographic, echocardiographic study and associated risk factors. Nephrol Ther. 2022;18:247–254. doi: 10.1016/j.nephro.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Davies S.J., Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86:489–496. doi: 10.1038/ki.2014.207. [DOI] [PubMed] [Google Scholar]
- 22.Tangwonglert T., Davenport A. Changes in extracellular water and left ventricular mass in peritoneal dialysis patients. Kidney Res Clin Pract. 2021;40:135–142. doi: 10.23876/j.krcp.20.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson T.G., Carr S.J. Cardiovascular autonomic dysfunction in uraemia. Kidney Int. 2002;62:1921–1932. doi: 10.1046/j.1523-1755.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G., Fowler B., Scali B., et al. Sympathetic activation and heart rate thresholds for cardiovascular risk in chronic kidney disease. J Hypertens. 2022;40:1530–1536. doi: 10.1097/HJH.0000000000003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izzo J.L., Jr., Isso M.S., Sterns R.H., Freeman R.B. Sympathetic nervous system hyperactivity in maintenance hemodialysis patients. Trans Am Soc Artif Intern Organs. 1982;28:604–607. [PubMed] [Google Scholar]
- 26.Dziedzic M., Bednarek-Skublewska A., Solski J., Kapka-Skrzypczak L. Plasma and erythrocyte relationship of catecholamines in hemodialysis patients. Ann Agric Environ Med. 2014;21:562–566. doi: 10.5604/12321966.1120602. [DOI] [PubMed] [Google Scholar]
- 27.Ettema E.M., Zittema D., Kuipers J., et al. Dialysis hypotension: a role for inadequate increase in arginine vasopressin levels? A systematic literature review and meta-analysis. Am J Nephrol. 2014;39:100–109. doi: 10.1159/000358203. [DOI] [PubMed] [Google Scholar]
- 28.Kanbay M., Ertuglu L.A., Afsar B., et al. An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J. 2020;13:981–993. doi: 10.1093/ckj/sfaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beerenhout C.H., Noris M., Kooman J.P., et al. Nitric oxide synthetic capacity in relation to dialysate temperature. Blood Purif. 2004;22:203–209. doi: 10.1159/000076854. [DOI] [PubMed] [Google Scholar]
- 30.Zoccali C., Moissl U., Chazot C., et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–2497. doi: 10.1681/ASN.2016121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W., Wang L., Huang X., He W., Song Z., Yang J. Impaired orthostatic blood pressure stabilization and reduced hemoglobin in chronic kidney disease. J Clin Hypertens (Greenwich) 2019;21:1317–1324. doi: 10.1111/jch.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamoto H., Honda N., Mimura T., Suzuki H. Hypoalbuminaemia is an important risk factor of hypotension during hemodialysis. Hemodial Int. 2006;10(suppl 2):S10–S15. doi: 10.1111/j.1542-4758.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 33.Ettema E.M., Kuipers J., van Faassen M., et al. Effect of plasma sodium concentration on blood pressure regulators during haemodialysis: a randomized crossover study. BMC Nephrol. 2018;19:214. doi: 10.1186/s12882-018-0997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Movilli E., Cancarini G.C., Cassamali S., et al. Inter-dialytic variations in blood volume and total body water in uraemic patients treated by dialysis. Nephrol Dial Transplant. 2004;19:185–189. doi: 10.1093/ndt/gfg494. [DOI] [PubMed] [Google Scholar]
- 35.Frewin D.B., Bartholomeusz F.D., Cummings M.F., et al. Changes in plasma catecholamine levels during haemodialysis. Aust N Z J Med. 1984;14:31–34. doi: 10.1111/j.1445-5994.1984.tb03581.x. [DOI] [PubMed] [Google Scholar]
- 36.Thompson A.M., Oliver J.A. Endogenous and exogenous vasopressin during haemodialysis. Semin Dial. 2009;22:472–475. doi: 10.1111/j.1525-139X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 37.Hayes W., Hothi D.K. Intradialytic hypotension. Pediatr Nephrol. 2011;26:867–879. doi: 10.1007/s00467-010-1661-4. [DOI] [PubMed] [Google Scholar]
- 38.Yoowannakul S., Vongsanim S., Tangvoraphonkchai K., Mohammed A., Davenport A. Patient-reported symptoms during dialysis: the effect of pre-dialysis extracellular water and change in extracellular water post-dialysis. Ren Replace Ther. 2021;7:4. doi: 10.1186/s41100-021-00321-3. [DOI] [Google Scholar]
- 39.Yoowannakul S., Vongsanim S., Kotecha T., Fontana M., Davenport A. Hemodialysis patients with less extracellular water overload and smaller cardiac atrial chamber sizes are at greater risk of a fall in blood pressure during dialysis. Ther Apher Dial. 2021;25:16–23. doi: 10.1111/1744-9987.13490. [DOI] [PubMed] [Google Scholar]
- 40.Tangvoraphonkchai K., Davenport A. Changes in extracellular water with hemodialysis and fall in systolic blood pressure. Int J Artif Organs. 2022;45:140–145. doi: 10.1177/0391398821995503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasgupta I., Farrington K., Davies S.J., Davenport A., Mitra S. UK national survey of practice patterns of fluid volume management in haemodialysis patients: a need for evidence. Blood Purif. 2016;41:324–331. doi: 10.1159/000444246. [DOI] [PubMed] [Google Scholar]
- 42.Booth J., Pinney J., Davenport A. N-terminal proBNP--marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol. 2010;5:1036–1040. doi: 10.2215/CJN.09001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrigo M., Von Moos S., Gerritsen K., et al. Soluble CD146 and B-type natriuretic peptide dissect overhydration into functional components of prognostic relevance in haemodialysis patients. Nephrol Dial Transplant. 2018;33:2035–2042. doi: 10.1093/ndt/gfy113. [DOI] [PubMed] [Google Scholar]
- 44.Wijayaratne D., Muthuppalaniappan V.M., Davenport A. Serum CA125 a potential marker of volume status for peritoneal dialysis patients? Int J Artif Organs. 2021;44:1029–1033. doi: 10.1177/03913988211016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naruse M., Sakaguchi S., Nakayama Y., Nonoguchi H., Tomita K. A novel method for dry weight assessment in hemodialysis patients: utilization of inferior vena cava flat ratio to correct for individual variations in vessel diameter. Ther Apher Dial. 2007;11:42–48. doi: 10.1111/j.1744-9987.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 46.Zoccali C., Torino C., Mallamaci F., et al. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021;100:1325–1333. doi: 10.1016/j.kint.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Heguilen R.M., Sciurano C., Bellusci A.D., et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic haemodialysis patients. Nephrol Dial Transplant. 2005;20:591–597. doi: 10.1093/ndt/gfh661. [DOI] [PubMed] [Google Scholar]
- 48.Reddan D.N., Szczech L.A., Hasselblad V., et al. Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. J Am Soc Nephrol. 2005;16:2162–2169. doi: 10.1681/ASN.2004121053. [DOI] [PubMed] [Google Scholar]
- 49.Booth J., Pinney J., Davenport A. Do changes in relative blood volume monitoring correlate to hemodialysis-associated hypotension? Nephron Clin Pract. 2011;117:c179–c183. doi: 10.1159/000320196. [DOI] [PubMed] [Google Scholar]
- 50.Mitra S., Chamney P., Greenwood R., Farrington K. Linear decay of relative blood volume during ultrafiltration predicts hemodynamic instability. Am J Kidney Dis. 2002;40:556–565. doi: 10.1053/ajkd.2002.34914. [DOI] [PubMed] [Google Scholar]
- 51.Leung K.C.W., Quinn R.R., Ravani P., et al. Randomized crossover trial of blood volume monitoring-guided ultrafiltration biofeedback to reduce intradialytic hypotensive episodes with hemodialysis. Clin J Am Soc Nephrol. 2017;12:1831–1840. doi: 10.2215/CJN.01030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dasselaar J.J., van der Sande F.M., Franssen C.F. Critical evaluation of blood volume measurements during haemodialysis. Blood Purif. 2012;33:177–182. doi: 10.1159/000334142. [DOI] [PubMed] [Google Scholar]
- 53.Meyring-Wösten A., Luo Y., Zhang H., et al. Intradialytic hypertension is associated with low intradialytic arterial oxygen saturation. Nephrol Dial Transplant. 2018;33:1040–1045. doi: 10.1093/ndt/gfx309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Chan L., Meyring-Wösten A., et al. Association between intradialytic central venous oxygen saturation and ultrafiltration volume in chronic hemodialysis patients. Nephrol Dial Transplant. 2018;33:1636–1642. doi: 10.1093/ndt/gfx271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donauer J., Kölblin D., Bek M., Krause A., Böhler J. Ultrafiltration profiling and measurement of relative blood volume as strategies to reduce hemodialysis-related side effects. Am J Kidney Dis. 2000;36:115–123. doi: 10.1053/ajkd.2000.8280. [DOI] [PubMed] [Google Scholar]
- 56.Movilli E., Gaggia P., Zubani R., et al. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant. 2007;22:3547. doi: 10.1093/ndt/gfm466. 3552. [DOI] [PubMed] [Google Scholar]
- 57.Flythe J.E., Kimmel S.E., Brunelli S.M. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250. doi: 10.1038/ki.2010.383. 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchanan C., Mohammed A., Cox E., et al. Intradialytic Cardiac Magnetic Resonance Imaging to Assess Cardiovascular Responses in a Short-Term Trial of Hemodiafiltration and Hemodialysis. J Am Soc Nephrol. 2017;28:1269–1277. doi: 10.1681/ASN.2016060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashby D., Corbett R., Duncan N. Soft target weight: theory and simulation of a novel haemodialysis protocol which reduces excessive ultrafiltration. Nephron. 2022;146:160–166. doi: 10.1159/000519823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davenport A. New dialysis technology and biocompatible materials. Contrib Nephrol. 2017;189:130–136. doi: 10.1159/000450739. [DOI] [PubMed] [Google Scholar]
- 61.Lee H.J., Meinardi S., Pahl M.V., Vaziri N.D., Blake D.R. Exposure to potentially toxic hydrocarbons and halocarbons released from the dialyzer and tubing set during hemodialysis. Am J Kidney Dis. 2012;60:609–616. doi: 10.1053/j.ajkd.2012.02.327. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Sanz A., Sánchez-Villanueva R., Domínguez-Ortega J., et al. Mechanisms involved in hypersensitivity reactions to polysulfone hemodialysis membranes. Artif Organs. 2017;41:E285–E295. doi: 10.1111/aor.12954. [DOI] [PubMed] [Google Scholar]
- 63.Heegard K.D., Tilley M.A., Stewart I.J., et al. Anaphylactoid reaction during first hemofiltration with a PUREMA polysulfone membrane. Int J Artif Organs. 2013;36:363–366. doi: 10.5301/ijao.5000136. [DOI] [PubMed] [Google Scholar]
- 64.Konishi S., Fukunaga A., Yamashita H., Miyata M., Usami M. Eluted substances from hemodialysis membranes elicit positive skin prick tests in bioincompatible patients. Artif Organs. 2015;39:343–351. doi: 10.1111/aor.12392. [DOI] [PubMed] [Google Scholar]
- 65.Davenport A. Antibodies to heparin-platelet factor 4 complex: pathogenesis, epidemiology, and management of heparin-induced thrombocytopenia in haemodialysis. Am J Kidney Dis. 2009;54:361–374. doi: 10.1053/j.ajkd.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Kim J.H., Park J.Y., Jang S.H., et al. Fatal anaphylaxis due to nafamostat mesylate during hemodialysis. Allergy Asthma Immunol Res. 2021;13:517–519. doi: 10.4168/aair.2021.13.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warkentin T.E., Greinacher A. Heparin-induced anaphylactic and anaphylactoid reactions: two distinct but overlapping syndromes. Expert Opin Drug Saf. 2009;8:129–144. doi: 10.1517/14740330902778180. [DOI] [PubMed] [Google Scholar]
- 68.Blossom D.B., Kallen A.J., Patel P.R., et al. Outbreak of adverse reactions associated with contaminated heparin. N Engl J Med. 2008;359:2674–2684. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Misaki T., Suzuki Y., Naito Y., Shiooka T., Isozaki T. A case of anaphylactoid reaction to acetate in acetate-containing bicarbonate dialysate. CEN Case Rep. 2015;4:81–84. doi: 10.1007/s13730-014-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan J.N., Da Y., Sabrina Haroon S., Lau T. Chlorhexidine – a commonly used but often neglected culprit of dialysis associated anaphylactic reactions (case report) BMC Nephrol. 2022;23:18. doi: 10.1186/s12882-021-02646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coppo R., Amore A., Cirina P., et al. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney Int. 2000;58:881–888. doi: 10.1046/j.1523-1755.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 72.Ferraro B., Galli F., Frei B., et al. Peroxynitrite-induced oxidation of plasma lipids is enhanced in stable haemodialysis patients. Kidney Int. 2003;63:2207–2213. doi: 10.1046/j.1523-1755.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 73.Stoves J., Goode N.P., Visvanathan R., et al. The bradykinin response and early hypotension at the introduction of continuous renal replacement therapy in the intensive care unit. Artif Organs. 2001;25:1009–1013. doi: 10.1046/j.1525-1594.2001.06703.x. [DOI] [PubMed] [Google Scholar]
- 74.Davenport A., Kirby S.A. Intensive care management of patients with acute hepatic and renal failure. Contrib Nephrol. 1995;116:22–27. doi: 10.1159/000424608. [DOI] [PubMed] [Google Scholar]
- 75.Kang E.S., Miles D.E., Tevlin M.T., Cates T.B., Acchiardo S.R. Reversible sequestration of nitric oxide by hemoglobin during hemodialysis in end-stage renal disease. Am J Med Sci. 2001;321:113–123. doi: 10.1097/00000441-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Nyhlén K., Hultkvist-Bengtsson U., Nilsson M., Rippe B. Leukocyte sequestration in isolated guinea pig lungs during extracorporeal circulation: effects on microvascular function. Blood Purif. 2000;18:121–127. doi: 10.1159/000014435. [DOI] [PubMed] [Google Scholar]
- 77.Bell D., Nicoll J., Jackson M., Millar A., Winney R.J., Muir A.L. Altered lung vascular permeability during intermittent haemodialysis. Nephrol Dial Transplant. 1988;3:426–431. doi: 10.1093/oxfordjournals.ndt.a091692. [DOI] [PubMed] [Google Scholar]
- 78.Os I., Nordby G., Lyngdal P.T., Eide I. Plasma vasopressin, catecholamines and atrial natriuretic factor during hemodialysis and sequential ultrafiltration. Scand J Urol Nephrol. 1993;27:93–99. doi: 10.3109/00365599309180422. [DOI] [PubMed] [Google Scholar]
- 79.Yokokawa K., Mankus R., Saklayen M.G., et al. Increased nitric oxide production in patients with hypotension during hemodialysis. Ann Intern Med. 1995;123:35–37. doi: 10.7326/0003-4819-123-1-199507010-00005. [DOI] [PubMed] [Google Scholar]
- 80.Leunissen K.M., Hoorntje S.J., Fiers H.A., Dekkers W.T., Mulder A.W. Acetate versus bicarbonate hemodialysis in critically ill patients. Nephron. 1986;42:146–151. doi: 10.1159/000183654. [DOI] [PubMed] [Google Scholar]
- 81.Power A., Charitaki E., Davenport A. Changes in vascular tone occur early during hemodialysis treatments independently of volume reduction. Artif Organs. 2016;40:678–683. doi: 10.1111/aor.12610. [DOI] [PubMed] [Google Scholar]
- 82.Tessitore N., Santoro A., Panzetta G.O., et al. Acetate-free biofiltration reduces intradialytic hypotension: a European multicenter randomized controlled trial. Blood Purif. 2012;34:354–363. doi: 10.1159/000346293. [DOI] [PubMed] [Google Scholar]
- 83.Kosmadakis G., Correia E.D.C., Albaret J., Somda F., Aguilera D. Comparison of the hemodynamic tolerance and the biological parameters of four acetate-free hemodialysis methods. Nephrol Ther. 2017;13:532–536. doi: 10.1016/j.nephro.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 84.de Sequera P., Pérez-García R., Molina M., et al. Advantages of the use of citrate over acetate as a stabilizer in hemodialysis fluid: a randomized ABC-treat study. Nefrologia (Engl Ed) 2021 doi: 10.1016/j.nefro.2021.06.006. Published online August 11. [DOI] [PubMed] [Google Scholar]
- 85.Sandhu E., Crawford C., Davenport A. Weight gains and increased blood pressure in outpatient hemodialysis patients due to change in acid dialysate concentrate supplier. Int J Artif Organs. 2012;35:642–647. doi: 10.5301/ijao.5000114. [DOI] [PubMed] [Google Scholar]
- 86.Marshall M.R., Vandal A.C., de Zoysa J.R., et al. Effect of low-sodium versus conventional sodium dialysate on left ventricular mass in home and self-care satellite facility hemodialysis patients: a randomized clinical trial. J Am Soc Nephrol. 2020;31:1078–1091. doi: 10.1681/ASN.2019090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah A., Davenport A. Does a reduction in dialysate sodium improve blood pressure control in haemodialysis patients? Nephrology (Carlton) 2012;17:358–363. doi: 10.1111/j.1440-1797.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- 88.Ekbal N.J., Consalus A., Persaud J., Davenport A. Reliability of delivered dialysate sodium concentration. Hemodial Int. 2016;20(suppl 1):S2–S6. doi: 10.1111/hdi.12465. [DOI] [PubMed] [Google Scholar]
- 89.Shendi A.M., Davenport A. The difference between delivered and prescribed dialysate sodium in haemodialysis machines. Clin Kidney J. 2020;14:863–868. doi: 10.1093/ckj/sfaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tangvoraphonkchai K., Davenport A. Why does the choice of dialysate sodium concentration remain controversial? Hemodial Int. 2018;22:435–444. doi: 10.1111/hdi.12645. [DOI] [PubMed] [Google Scholar]
- 91.Ebrahimi H., Safavi M., Saeidi M.H., Emamian M.H. Effects of sodium concentration and dialysate temperature changes on blood pressure in hemodialysis patients: a randomized, triple-blind crossover clinical trial. Ther Apher Dial. 2017;21:117–125. doi: 10.1111/1744-9987.12506. [DOI] [PubMed] [Google Scholar]
- 92.Gkza A., Davenport A. Estimated dietary sodium intake in haemodialysis patients using food frequency questionnaires. Clin Kidney J. 2017;10:715–720. doi: 10.1093/ckj/sfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donati G., Ursino M., Spazzoli A., et al. Sodium prescription in the prevention of intradialytic hypotension: new insights into an old concept. Blood Purif. 2018;45:61–70. doi: 10.1159/000480221. [DOI] [PubMed] [Google Scholar]
- 94.Coli L., La Manna G., Comai G., et al. Automatic adaptive system dialysis for hemodialysis-associated hypotension and intolerance: a noncontrolled multicenter trial. Am J Kidney Dis. 2011;58:93–100. doi: 10.1053/j.ajkd.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 95.Buemi M., Aloisi E., Coppolino G., et al. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrol Dial Transplant. 2005;20:1148–1154. doi: 10.1093/ndt/gfh770. [DOI] [PubMed] [Google Scholar]
- 96.Pun P.H. Dialysate potassium concentration: should mass balance trump electrophysiology? Semin Dial. 2018;31:569–575. doi: 10.1111/sdi.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brunelli S.M., Sibbel S., Do T.P., Cooper K., Bradbury B.D. Facility dialysate calcium practices and clinical outcomes among patients receiving hemodialysis: a retrospective observational study. Am J Kidney Dis. 2015;66:655–665. doi: 10.1053/j.ajkd.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 98.Gabutti L., Bianchi G., Soldini D., Marone C., Burnier M. Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol Dial Transplant. 2009;24:973–981. doi: 10.1093/ndt/gfn541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J.K., Moon S.J., Park H.C., et al. Effects of lowering dialysate calcium concentrations on arterial stiffness in patients undergoing hemodialysis. Korean J Intern Med. 2011;26:320–327. doi: 10.3904/kjim.2011.26.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LeBoeuf A., Mac-Way F., Utescu M.S., et al. Impact of dialysate calcium concentration on the progression of aortic stiffness in patients on haemodialysis. Nephrol Dial Transplant. 2011;26:3695–3701. doi: 10.1093/ndt/gfr138. [DOI] [PubMed] [Google Scholar]
- 101.Kyriazis J., Katsipi I., Stylianou K., Jenakis N., Karida A., Daphnis E. Arterial stiffness alterations during haemodialysis: the role of dialysate calcium. Nephron Clin Pract. 2007;106:c34–c42. doi: 10.1159/000101482. [DOI] [PubMed] [Google Scholar]
- 102.Charitaki E., Davenport A. Do higher dialysate calcium concentrations increase vascular stiffness in hemodialysis patients as measured by aortic pulse wave velocity? BMC Nephrol. 2013;14:189. doi: 10.1186/1471-2369-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kyriazis J., Glotsos J., Bilirakis L., et al. Dialysate calcium profiling during hemodialysis: use and clinical implications. Kidney Int. 2002;61:276–287. doi: 10.1046/j.1523-1755.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 104.Tangvoraphonkchai K., Davenport A. Magnesium and cardiovascular disease. Adv Chronic Kidney Dis. 2018;25:251–260. doi: 10.1053/j.ackd.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 105.Pakfetrat M., Roozbeh Shahroodi J., Malekmakan L., Zare N., Hashemi Nasab M., Hossein Nikoo M. Is there an association between intradialytic hypotension and serum magnesium changes? Hemodial Int. 2010;14:492–497. doi: 10.1111/j.1542-4758.2010.00477.x. [DOI] [PubMed] [Google Scholar]
- 106.Kyriazis J., Kalogeropoulou K., Bilirakis L., et al. Dialysate magnesium level and blood pressure. Kidney Int. 2004;66:1221–1231. doi: 10.1111/j.1523-1755.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 107.Elsharkawy M.M., Youssef A.M., Zayoon M.Y. Intradialytic changes of serum magnesium and their relation to hypotensive episodes in hemodialysis patients on different dialysates. Hemodial Int. 2006;10(suppl 2):S16. doi: 10.1111/j.1542-4758.2006.00120.x. S23. [DOI] [PubMed] [Google Scholar]
- 108.Davenport A. What are the anticoagulation options for intermittent haemodialysis? Nat Rev Nephrol. 2011;7:499. doi: 10.1038/nrneph.2011.88. 450. [DOI] [PubMed] [Google Scholar]
- 109.Gritters M., Borgdorff P., Grooteman M.P., et al. Reduction in platelet activation by citrate anticoagulation does not prevent intradialytic hemodynamic instability. Nephron Clin Pract. 2007;106:c9–c16. doi: 10.1159/000100496. [DOI] [PubMed] [Google Scholar]
- 110.Ezquerro Rodríguez E., Montes García Y., Marín Fernández B. Physical methods used to control body temperature. Rev Enferm. 2012;35:32–39. [PubMed] [Google Scholar]
- 111.Yoowannakul S., Leung T.S., Davenport A. Pilot study to detect changes in blood flow in the external auditory meatus during haemodialysis. Ther Apher Dial. 2020;24:307–311. doi: 10.1111/1744-9987.13433. [DOI] [PubMed] [Google Scholar]
- 112.Maggiore Q., Pizzarelli F., Sisca S., et al. Blood temperature and vascular stability during hemodialysis and haemofiltration. Trans Am Soc Artif Intern Organs. 1982;28:523. 527. [PubMed] [Google Scholar]
- 113.Pizzarelli F., Sisca S., Zoccali C., et al. Blood temperature and cardiovascular stability in haemofiltration. Int J Artif Organs. 1983;6:37. 34. [PubMed] [Google Scholar]
- 114.Mustafa R.A., Bdair F., Akl E.A., et al. Effect of lowering the dialysate temperature in chronic hemodialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016;11:442–457. doi: 10.2215/CJN.04580415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsujimoto Y., Tsujimoto H., Nakata Y., et al. Dialysate temperature reduction for intradialytic hypotension for people with chronic kidney disease requiring haemodialysis. Cochrane Database Syst Rev. 2019;7:CD012598. doi: 10.1002/14651858.CD012598.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoowannakul S., Vongsanim S., Tangvoraphonkchai K., Mohammed A., Davenport A. Falls in systolic blood pressure during dialysis which require no nursing intervention are associated with increased patient intra-dialytic symptom self-reporting and prolonged post-dialysis recovery times. Ren Replace Ther. 2020;6:2. doi: 10.1186/s41100-019-0249-0. [DOI] [Google Scholar]
- 117.Davenport A. Using dialysis machine technology to reduce intradialytic hypotension. Hemodial Int. 2011;15(suppl 1):S37–S42. doi: 10.1111/j.1542-4758.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 118.Larkin J.W., Reviriego-Mendoza M.M., Usvyat L.A., Kotanko P., Maddux F.W. To cool, or too cool: is reducing dialysate temperature the optimal approach to preventing intradialytic hypotension? Semin Dial. 2017;30:501–508. doi: 10.1111/sdi.12628. [DOI] [PubMed] [Google Scholar]
- 119.Maggiore Q., Pizzarelli F., Santoro A., et al. The effects of control of thermal balance on vascular stability in haemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis. 2002;40:280–290. doi: 10.1053/ajkd.2002.34506. [DOI] [PubMed] [Google Scholar]
- 120.Davenport A., Cox C., Thuraisingham R., PanThames Renal Audit Group The importance of dialysate sodium concentration in determining interdialytic weight gains in chronic hemodialysis patients: the PanThames Renal Audit. Int J Artif Organs. 2008;31:411–417. doi: 10.1177/039139880803100506. [DOI] [PubMed] [Google Scholar]
- 121.Ing T.S., Quon M.J., Chen W.T., Daugirdas J.T. Sequential isolated ultrafiltration and dialysis. Int J Artif Organs. 1979;2:265. [PubMed] [Google Scholar]
- 122.Van der Sande F.M., Gladziwa U., Kooman J.P., Böcker G., Leunissen K.M.L. Energy transfer is the single most important factor for the difference in vascular response between isolated ultrafiltration and hemodialysis. J Am Soc Nephrol. 2000;11:1512–1517. doi: 10.1681/ASN.V1181512. [DOI] [PubMed] [Google Scholar]
- 123.Altieri P., Sorba G.B., Bolasco P.G., et al. On-line predilution hemofiltration versus ultrapure high-flux hemodialysis: a multicenter prospective study in 23 patients. Sardinian collaborative study group of on-line hemofiltration. Blood Purif. 1997;15:169–181. doi: 10.1159/000170328. [DOI] [PubMed] [Google Scholar]
- 124.Davenport A. Can advances in hemodialysis machine technology prevent intradialytic hypotension? Semin Dial. 2009;22:231–236. doi: 10.1111/j.1525-139X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 125.Vareesangthip K., Davenport A. Reducing the risk of intradialytic hypotension by altering the composition of the dialysate. Hemodial Int. 2020;24:276. doi: 10.1111/hdi.12840. 228. [DOI] [PubMed] [Google Scholar]
- 126.Maduell F., Moreso F., Pons M., et al. High-efficiency post-dilution online hemodiafiltration reduces all-cause mortality in haemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pinney J.H., Oates T., Davenport A. Haemodiafiltration does not reduce the frequency of intradialytic hypotensive episodes when compared to cooled high-flux haemodialysis. Nephron Clin Pract. 2011;119:c138–c144. doi: 10.1159/000324428. [DOI] [PubMed] [Google Scholar]
- 128.Sande F.M.V., Kooman J.P., Konings C.J., Leunissen K.M.L. Thermal effects and blood pressure response during post-dilution haemodiafiltration and haemodialysis: the effect of amount of replacement fluid and dialysate temperature. J Am Soc Nephrol. 2001;12:1916–1920. doi: 10.1681/ASN.V1291916. [DOI] [PubMed] [Google Scholar]
- 129.Greenberg K.I., Choi M.J. Hemodialysis emergencies: core curriculum 2021. Am J Kidney Dis. 2021;77:796–809. doi: 10.1053/j.ajkd.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 130.Canaud B., Aljama P., Tielemans C., Gasparovic V., Gutierrez A., Locatelli F. Pathochemical toxicity of perfluorocarbon-5070, a liquid test performance fluid previously used in dialyzer manufacturing, confirmed in animal experiment. J Am Soc Nephrol. 2005;16:1819–1823. doi: 10.1681/ASN.2004050361. [DOI] [PubMed] [Google Scholar]
- 131.Davenport A. Intradialytic complications during haemodialysis. Hemodial Int. 2006;10:162–167. doi: 10.1111/j.1542-4758.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 132.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 133.Davenport A., Cox C., Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73:759–764. doi: 10.1038/sj.ki.5002745. [DOI] [PubMed] [Google Scholar]
- 134.Mitra S., Chandna S.M., Farrington K. What is hypertension in chronic haemodialysis? The role of interdialytic blood pressure monitoring. Nephrol Dial Transplant. 1999;14:2915–2921. doi: 10.1093/ndt/14.12.2915. [DOI] [PubMed] [Google Scholar]
- 135.Georgianos P.I., Agarwal R. Antihypertensive therapy in patients Re6eiving maintenance hemodialysis: a narrative review of the available clinical-trial evidence. Curr Vasc Pharmacol. 2021;19:12–20. doi: 10.2174/1570161118666200317151000. [DOI] [PubMed] [Google Scholar]
- 136.Tieu A., Velenosi T.J., Kucey A.S., Weir M.A., Urquhart B.L. β-blocker dialyzability in maintenance hemodialysis patients: a randomized clinical trial. Clin J Am Soc Nephrol. 2018;13:604–611. doi: 10.2215/CJN.07470717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou H., Sim J.J., Shi J., et al. β-blocker use and risk of mortality in heart failure patients initiating maintenance dialysis. Am J Kidney Dis. 2021;77:704–771. doi: 10.1053/j.ajkd.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 138.Assimon M.M., Brookhart M.A., Fine J.P., Heiss G., Layton J.B., Flythe J.E. A comparative study of carvedilol versus metoprolol initiation and 1-year mortality among individuals receiving maintenance hemodialysis. Am J Kidney Dis. 2018;72:337–348. doi: 10.1053/j.ajkd.2018.02.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davenport A. Application of the Clinical Frailty Score and body composition and upper arm strength in haemodialysis patients. Clin Kidney J. 2021;15:553–559. doi: 10.1093/ckj/sfab228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martins A.M., Dias Rodrigues J.C., de Oliveira Santin F.G., et al. Food intake assessment of elderly patients on haemodialysis. J Ren Nutr. 2015;25:321–326. doi: 10.1053/j.jrn.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 141.Watha K., Davenport A., Tangvoraphonkchai K. Changes in blood glucose and lactate concentrations with haemodialysis. Artif Organs. 2022;46:138–145. doi: 10.1111/aor.14097. [DOI] [PubMed] [Google Scholar]
- 142.Davenport A. Peri-dialytic hypoglycemia with hemodialysis and online post-dilutional hemodiafiltration. Ther Apher Dial. Published online March 8. 2022 doi: 10.1111/1744-9987.13835. [DOI] [PubMed] [Google Scholar]
- 143.Strong J., Burgett M., Buss M.L., Carver M., Kwankin S., Walker D. Effects of calorie and fluid intake on adverse events during hemodialysis. J Ren Nutr. 2001;11:97–100. doi: 10.1016/s1051-2276(01)51664-7. [DOI] [PubMed] [Google Scholar]
- 144.Zoccali C., Mallamaci F., Ciccarelli M., Maggiore Q. Postprandial alterations in arterial pressure control during haemodialysis in uremic patients. Clin Nephrol. 1989;31:323–326. [PubMed] [Google Scholar]
- 145.Benaroia M., Iliescu E.A. Oral intake during haemodialysis: is there an association with intradialytic hypotension? Hemodial Int. 2008;12:62–65. doi: 10.1111/j.1542-4758.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- 146.Kistler B.M., Benner D., Burrowes J.D., et al. Eating during haemodialysis treatment: a consensus statement. J Ren Nutr. 2018;28:4–12. doi: 10.1053/j.jrn.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 147.Greenwood S.A., Koufaki P., Macdonald J.H., et al. Randomized trial-PrEscription of intradialytic exercise to improve quAlity of life in patients receiving hemodialysis. Kidney Int Rep. 2021;6:2159–2170. doi: 10.1016/j.ekir.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Desai M., Mohamed A., Davenport A. A pilot study investigating the effect of pedalling exercise during dialysis on 6-min walking test and hand grip and pinch strength. Int J Artif Organs. 2019;42:161–166. doi: 10.1177/0391398818823761. [DOI] [PubMed] [Google Scholar]
- 149.Rhee S.Y., Song J.K., Hong S.C., et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J Intern Med. 2019;34:588–598. doi: 10.3904/kjim.2017.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keane D.F., Raimann J.G., Zhang H., Willetts J., Thijssen S., Kotanko P. The time of onset of intradialytic hypotension during a hemodialysis session associates with clinical parameters and mortality. Kidney Int. 2021;99:1408–1417. doi: 10.1016/j.kint.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]