Abstract
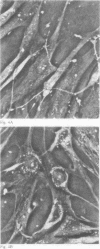
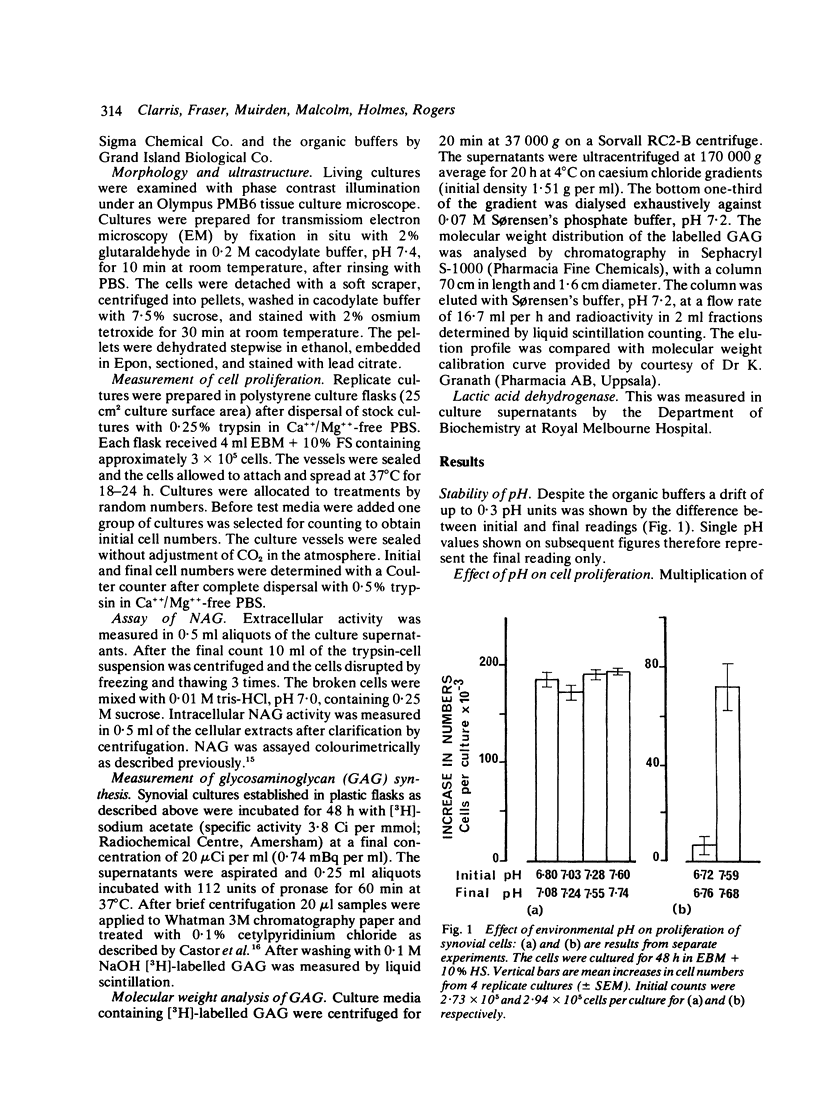
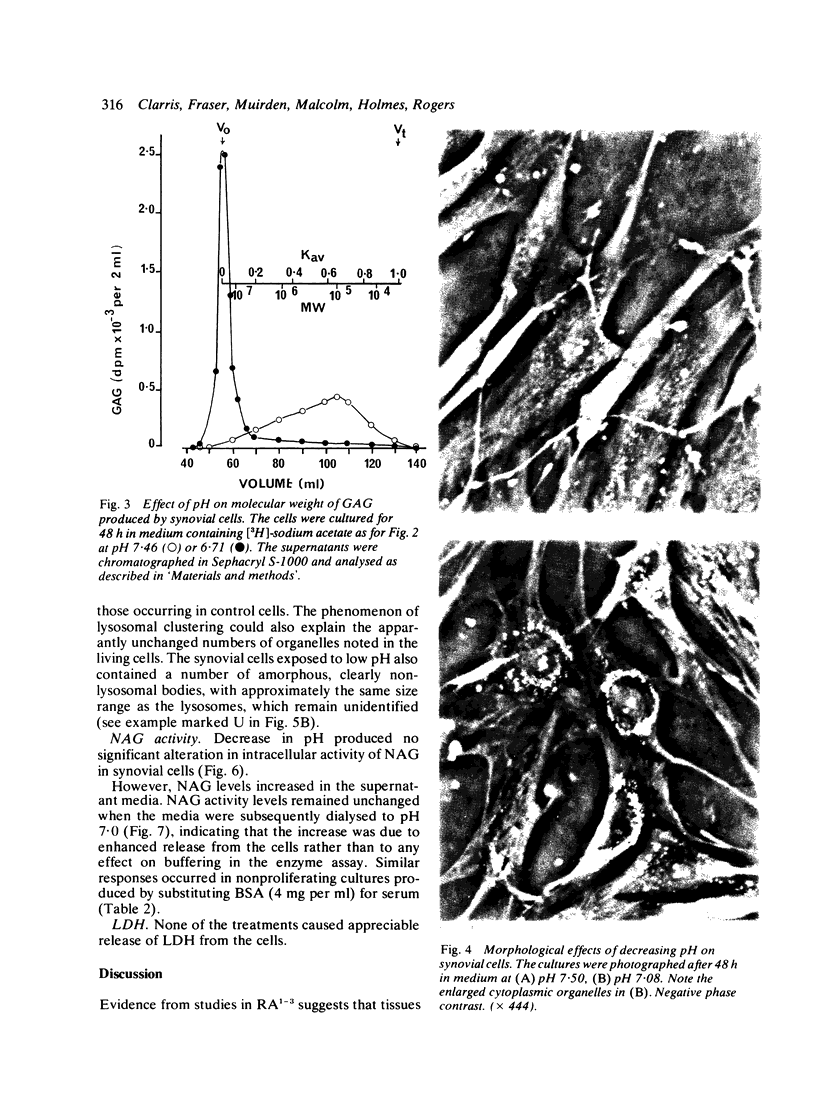
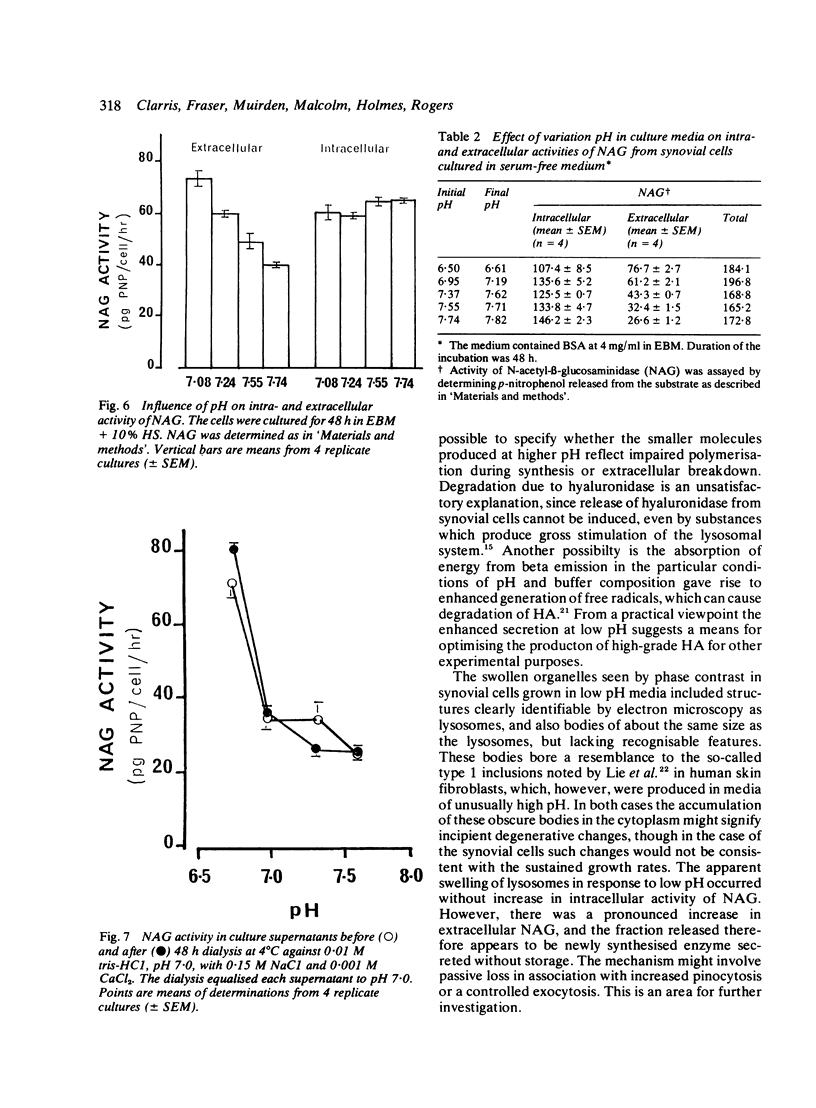
Glycosaminoglycan production, acid hydrolase activity, proliferation, and morphology were examined in human synovial cells subjected to low environmental pH. The amount and the molecular size of newly synthesised glycosaminoglycan (GAG) were increased without significant change in the rate of cell proliferation. Lowered pH produced an increase in the size of cytoplasmic organelles. Some of these possessed ultrastructural features of lysosomes, but others were clearly nonlysosomal and were of uncertain identity. Intracellular activity of the lysosomal acid hydrolase N-acetyl-beta-glucosaminidase (NAG) was not altered by low pH, but a marked increase occurred in extracellular NAG activity, indicating enhanced release.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter E., Fraser J. R., Harris G. S. Fractionation and recovery of secretions of synovial cells synthesized in culture with radioactive precursors. Ann Rheum Dis. 1973 Jan;32(1):35–40. doi: 10.1136/ard.32.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman G., Beckman L., Lemperg R. Acid phosphatase activity in the synovial fluid of patients with rheumatoid arthritis and other joint disorders. Acta Rheumatol Scand. 1971;17(1):47–56. doi: 10.3109/rhe1.1971.17.issue-1-4.07. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Bignall M. C., Hossler P. A., Roberts D. J. Connective tissue activation. XXI. Regulation of glycosaminoglycan metabolism by lymphocyte (CTAP-I) and platelet (CTAP-III) growth factors. In Vitro. 1981 Sep;17(9):777–785. doi: 10.1007/BF02618444. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Prince R. K., Hazelton M. J. Hyaluronic acid in human synovial effusions; a sensitive indicator of altered connective tissue cell function during inflammation. Arthritis Rheum. 1966 Dec;9(6):783–794. doi: 10.1002/art.1780090606. [DOI] [PubMed] [Google Scholar]
- Caygill J. C., Pitkeathly D. A. A study of beta-acetylglucosaminase and acid phosphatase in pathological joint fluids. Ann Rheum Dis. 1966 Mar;25(2):137–144. doi: 10.1136/ard.25.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini C. Effect of pH on plating efficiency, serum reguirement, and incorporation of radioactive precursors into human cells. In Vitro. 1975 Mar-Apr;11(2):78–86. doi: 10.1007/BF02624079. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Fraser J. R. Relationship between chromosomal changes and alterations in the behaviour of a strain of human synovial cells during its life history in vitro. Ann Rheum Dis. 1968 Nov;27(6):597–603. doi: 10.1136/ard.27.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarris B. J., Fraser J. R. The effects of homologous and heterologous whole serum upon multiplication of recently-isolated human synovial cells in culture. Aust J Exp Biol Med Sci. 1967 Oct;45(5):549–560. doi: 10.1038/icb.1967.54. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Malcolm L. P. Effects of prostaglandins E1, E2, and F2 alpha on N-acetyl-beta-glucosaminidase activities of human synovial cells in culture. Ann Rheum Dis. 1983 Apr;42(2):187–191. doi: 10.1136/ard.42.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings N. A., Nordby G. L. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum. 1966 Feb;9(1):47–56. doi: 10.1002/art.1780090106. [DOI] [PubMed] [Google Scholar]
- Dingle J. T. The secretion of enzymes into the pericellular environment. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):315–324. doi: 10.1098/rstb.1975.0055. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., Clarris B. J., Baxter E. Patterns of induced variation in the morphology, hyaluronic acid secretion, and lysosomal enzyme activity of cultured human synovial cells. Ann Rheum Dis. 1979 Jun;38(3):287–294. doi: 10.1136/ard.38.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., McCall J. F. Culture of synovial cells in vitro. Notes on isolation and propagation. Ann Rheum Dis. 1965 Jul;24(4):351–359. doi: 10.1136/ard.24.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSFELD H., MEYER K., GODMAN G. Differentiation of fibroblasts in tissue culture, as determined by mucopolysaccharide production. Proc Soc Exp Biol Med. 1955 Jan;88(1):31–35. doi: 10.3181/00379727-88-21484. [DOI] [PubMed] [Google Scholar]
- JACOX R. F., FELDMAHN A. Variations of beta glucuronidase concentration in abnormal human synovial fluid. J Clin Invest. 1955 Feb;34(2):263–267. doi: 10.1172/JCI103079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittlick P. D., Neupert G. Experimentelle Beeinflussung der Synthese saurer Mucopolysaccharide (Glykosaminoglykane) in Fibroblastenkulturen. IV. Einfluss des pH-Wertes auf Zellproliferation und MPS-Muster. Exp Pathol (Jena) 1972;7(4):117–124. [PubMed] [Google Scholar]
- Lie S. O., Schofield B. H., Taylor H. A., Jr, Doty S. B. Structure and function of the lysosomes of human fibroblasts in culture: dependence on medium pH. Pediatr Res. 1973 Jan;7(1):13–19. doi: 10.1203/00006450-197301000-00003. [DOI] [PubMed] [Google Scholar]
- Marshall J. L., Fraser J. R., Muirden K. D. Lysosomal activation by neutral saccharides in cell cultures of synovium. Ann Rheum Dis. 1977 Apr;36(2):130–138. doi: 10.1136/ard.36.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Muirden K. D. Lysosomal enzymes in synovial membrane in rheumatoid arthritis. Relationship to joint damage. Ann Rheum Dis. 1972 Jul;31(4):265–271. doi: 10.1136/ard.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuhaft P. S., MCCarty D. J. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971 Jul-Aug;14(4):475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]
- Wegelius O., Klockars M., Vainio K. Acid phosphatase activity in rheumatoid synovia. Acta Med Scand. 1968 Jun;183(6):549–551. doi: 10.1111/j.0954-6820.1968.tb10521.x. [DOI] [PubMed] [Google Scholar]