Abstract
This study investigated motor preparation and action-consequence prediction using the lateralized readiness potential (LRP). Motor impairments are common in autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD), which commonly co-occur. Alterations in predictive processes may impact motor planning. Whether motor planning deficits are characteristic of ASD broadly or magnified in the context of co-morbid ADHD is unclear. ASD children with (ASD + ADHD; n = 12) and without (ASD − ADHD; n = 9) comorbid ADHD and typical controls (n = 29) performed voluntary motor actions that either did or did not result in auditory consequences. ASD − ADHD children demonstrated LRP enhancement when their action produced an effect while ASD + ADHD children had attenuated responses regardless of action-effect pairings. Findings suggest influence of ADHD comorbidity on motor preparation and prediction in ASD.
Keywords: Lateralized readiness potential (LRP), Autism spectrum disorder (ASD), Attention-deficit/hyperactivity disorder (ADHD), Motor preparation, Prediction, EEG
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, characterized by differences in social interaction and communication, as well as restricted interests and repetitive behaviors. ASD is also associated with impairments in movement and motor skills (Reiersen & Todd, 2008). Common motor deficits include coordination issues (Fournier et al., 2010), impairments in fine and gross motor skills (MacDonald et al., 2013, 2014), and delays in achieving developmental motor milestones (Jasmin et al., 2009; Ornitz et al., 1977). Importantly, motor differences often emerge earlier than social communicative symptoms (LeBarton & Landa, 2019; Sacrey et al., 2018). In later childhood, both motor symptoms (Hirata et al., 2016; Kaur et al., 2018), and function and connectivity within motor systems in the brain (Lidstone et al. 2021; Wymbs et al., 2020) associate with symptom severity in core ASD domains. Indeed, it has recently been argued that deficits in motor behavior and cognition can lead to subsequent errors in the performance of external social behaviors, linking motor alterations to the core ASD phenotype (Moseley & Pulvermuller, 2018). While many studies have examined broad motor output in ASD, few have examined preparatory motor activity to understand aberrant neural mechanisms of motor processing.
The premotor cortices are instrumental in preparing sequences of motor actions (Dowell et al., 2009). Event-related potentials (ERPs), electroencephalogram (EEG) changes locked to the motor response, can serve as an index of this preparatory activity. In particular, the lateralized readiness potential (LRP) (Luck et al., 2009) reflects an increase in neural activity prior to the execution of a motor action. The deflection is typically negative in polarity, and its amplitude has been associated with motor preparation (Luck, 2014). The LRP is measured bilaterally over the motor cortices and the waveform is isolated by subtracting the baseline activity of the ipsilateral hemisphere of the brain, which corresponds to the side not executing the motor movement, from the neural activity of the contralateral hemisphere, which corresponds to the side executing the motor movement (Luck et al., 2009). Children with ASD produce larger LRP amplitudes compared to a control group (Sokhadze et al., 2016) when required to execute a motor action in response to a stimulus.
The LRP can be modulated by the causes and consequences of a movement, and this modulation may be relevant to ASD. Preparatory neural activity locked to stimulus-driven responses is inherently mixed with activity associated with stimulus processing, suggesting that motor preparation for self-generated events, i.e. when motor activity precedes an event outcome, may involve different cognitive processes, namely prediction-associated processes. In a study of healthy controls, a larger LRP amplitude was observed from self-generated responses with consequences (i.e. the presentation of a black and white checkerboard pattern) versus those that resulted in no event outcome (Hughes & Waszak, 2011). This difference in neural activity was thought to reflect the outcome of prediction processes related to action outcomes.
It has been hypothesized that individuals with ASD generate atypical predictions (for a review, see (Sinha et al., 2014)). In particular, individuals with ASD may place too much emphasis on minor deviations from their prior expectations, thereby relying heavily on bottom-up sensory input (and little on prior beliefs) in making perceptual inferences. Such a neural system would not be optimally configured for processing regularities in the environment: instead, what may typically be filtered out as “noise” is considered consequential, sensory input is poorly filtered, and learning and generalization of patterns are impacted. Indeed, the superior pitch discrimination abilities in ASD has been attributed to developing overly specific categories of sounds (Bonnel et al., 2010). These prediction atypicalities may impact motor planning and movement execution via disturbances in the expected consequences of self-initiated actions. Examining the neural correlates of self-initiated motor preparation in ASD may both contribute to comprehensive, canonical theories of ASD symptomatology as well as shed light on the mechanisms underlying motor abnormalities.
Deficits in motor abilities are not, however, unique to ASD. The degree of impairment in motor preparation in ASD could be confounded by the high rates of Attention Deficit/Hyperactivity Disorder (ADHD) symptoms and comorbid diagnosis (Joshi et al., 2010; Mannion & Leader, 2013); indeed, over 50 percent of individuals with ASD also have an ADHD diagnosis (Antshel et al., 2016; Stevens et al., 2016). ADHD manifests early in development, presenting core symptoms of inattention, impulsivity, hyperactivity, and behavioral dysregulation (Winstanley et al., 2006). Individuals with ADHD share common motor anomalies with individuals with ASD, such as difficulties with fine and gross motor skill (Kaiser et al., 2015; Meyer & Sagvolden, 2006), as well as with motor skill preparation and execution (Klimkeit et al., 2005). Similar to individuals with ASD, individuals with ADHD display abnormal LRP amplitude when compared to controls. More specifically, children with ADHD present with both a smaller LRP amplitude than healthy controls, and a positive polarity LRP amplitude during a self-paced voluntary movement task (Jarczok et al., 2019; Szucs et al., 2009). Together, findings suggest that both individuals with ASD and ADHD exhibit atypical motor preparation as indexed by the LRP. However, the extent to which motor preparation issues identified in ASD may be driven by subsets of participants with comorbid ADHD remains unclear.
The present study aimed to investigate motor preparation associated with self-generated actions that did or did not result in an immediate consequence in participants with ASD in comparison with typically developing (TD) controls to explore how predictive processing influences motor preparation. We hypothesized (1) impaired prediction in ASD, manifesting as a lack of difference between LRP amplitude for actions resulting in an immediate consequence and actions not producing an outcome and (2) an attenuated LRP amplitude in children with ASD that is exacerbated in children with a comorbid ADHD diagnosis. Our hope was that, by examining neural markers of motor preparation and prediction in children with ASD with and without ASD, we would gain insight into shared or divergent motor disruptions in these subgroups that could eventually inform diagnosis and have implications for treatment.
Methods
Participants
Participants were recruited through flyers and advertisements posted both online and in the local community. In addition, some children were recruited through a database of previous research participants at the Seaver Autism Center for Research and Treatment at the Icahn School of Medicine at Mount Sinai. This study enrolled 25 participants with ASD and 32 TD controls between 8 and 17 years of age. All participants had intact cognitive skills (IQ > 70, measured by the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler, 2011), Wechsler Intelligence Scale for Children, Fifth Edition (WISC-5) (Weiss et al., 2010), or Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) (Wechsler, 2008)) with no history of neurological disorders and no history of hearing and vision impairments. In ASD participants, ASD was assessed using the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) (Lord et al., 2012) and Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). For ADHD, the Child Behavior Checklist (CBCL) (Achenbach & Edelbrock, 1979) was completed by participants’ caregivers to quantify symptoms and additional information was obtained via medical and educational record review and psychiatric interview with children and their caregivers. ASD and ADHD diagnoses were confirmed through a clinical assessment and consensus among a team of licensed psychologists and psychiatrists based on all available data and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria (Association, 2013). Additional inclusion criteria for TD controls included an absence of an ASD or ADHD diagnosis or other diagnosed psychiatric disorders, and no first-degree relatives with ASD. Participants with an ASD diagnosis were enrolled regardless of their ADHD severity. Legal guardians of participants provided signed informed consent and children gave assent prior to data collection. Procedures were approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai.
Procedure
Participants were seated in front of a 21.5-inch Dell computer monitor that displayed the visual cues. Stimulus timing was controlled using Presentation® software (Version 19.0, Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com). The auditory stimulus, a 50 ms, 1000 Hz pure tone at 77 dB, was presented using Etymotic Research ER-3A insert earphones bi-aurally. During the task, visual cues with the instructions “Press” and “Rest” were shown in white text on a gray background at the horizontal and vertical meridians of the screen. Participants used a Cedrus response box during the task, with the response key clearly marked in a blue color. Participants sat approximately 60 cm away from the monitor and a researcher was present in the room to cue participants on behavior, e.g. to slow down rate of button presses. All subjects were run in the same room and were given the same instructions.
Experimental Task
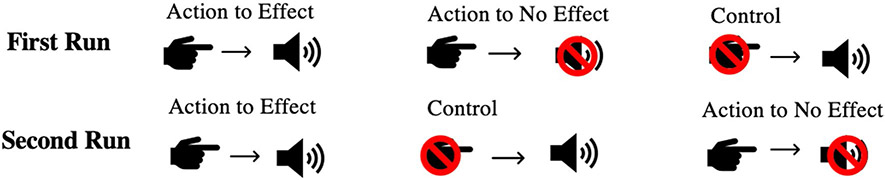
The task consisted of two runs, each consisting of three conditions: Action to Effect, Action to No Effect, and a Control condition. Each of the three conditions were presented in separate blocks. Figure 1 depicts the two run sequences; run order was counterbalanced across participants. The task was self-paced; however, prior to the task, participants were instructed to press a button every 2–3 s, with their right hand, and participants practiced with feedback from a research team member until they pressed reliably at the desired rate. During the experiment, a researcher sat near the participant in order to provide visually-cued feedback about button press rate, if needed.
Fig. 1.
Task schematic: Visual representation of the task conditions across two runs. In the Action to Effect condition a tone was initiated by the motor action of pressing a button. In the Action to No Effect condition, participants pressed the button, but no tone was presented. In the Control condition, tones were presented without participants having to press a button
During the Action to Effect condition, participants were cued, via a continuous display of the word “Press” on the computer screen, to press the button on the response box. Almost concurrently upon pressing the button (6 ms delay) the tone was delivered bi-aurally to the earphones. Participants were told that their action of pressing the button caused the tone to be played. The condition ended after 75 button-presses (trials), after which the screen changed to read “Rest” for 5 s before the following condition began.
During the Action to No Effect condition, participants performed the same simple motor activity as described above, but no tone played after each button press. The word “Press” was still displayed in the monitor. This condition also ended after the participants completed 75 button-presses and was followed by a 5 s “Rest” screen.
The Control condition always followed the Action to Effect condition. During this condition, participants performed no motor activity. Participants were instructed to simply listen, while the monitor displayed the word “Listen”. The temporal sequence of tone presentation preserved from the recently completed Action to Effect condition was reproduced during this condition, resulting in 75 trials with identical inter-trial intervals to those in the Action to Effect condition. This condition was not of interest in the current study examining motor activity.
ERP Acquisition and Processing
EEG recordings were acquired at 1000 Hz from 128 electrode sites using the EGI Geodesic system. Analyses focused on the Action to Effect and Action to No Effect conditions. Trials or button-presses that were less than 500 ms apart were discarded to avoid including any residual neural activity from the previous trial. EEG data processing was conducted using Net Station version 5.4.2 (r29917) software (EGI, Inc., Eugene, OR, USA). Continuous EEG data were band pass filtered at 0.1–30 Hz and then data were segmented into 1600 ms epochs time-locked to the button-press response from 800 ms before the button-press to 600 ms after (Luck, 2014; Luck et al., 2009). Next, artifacts (i.e., eye blinks, eye saccades, and any change in voltage larger than 100 uV) were detected using automated NetStation algorithms, and replaced via interpolation and remaining data were averaged and baseline corrected from – 800 to – 600 ms before the button-press response. The LRP was measured as the voltage difference between contralateral and ipsilateral sites each encompassing eight lateral channels surrounding electrodes C4 and C3 Fig. 2. Amplitude measurements, taken from each participant’s LRP waveform, reflected the largest peak value within the time interval prior to the button press (− 600 ms to 0 ms). In computing the peak amplitude, voltage maximum and absolute value of the minimum were compared to determine the polarity of the LRP. Finally, group averages of LRP amplitudes were calculated.
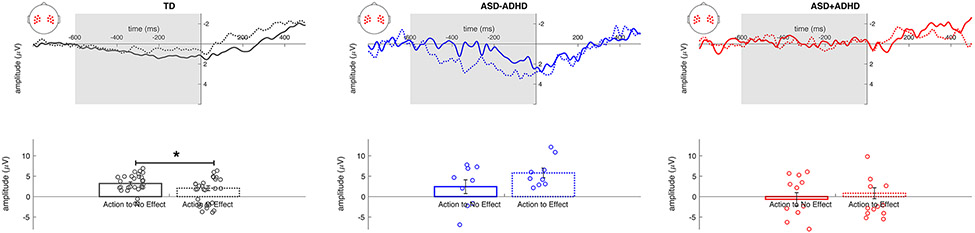
Fig. 2.
(Top) Response-locked group average LRP waveforms of the Action to No Effect (solid) and Action to Effect (dashed) conditions from TD (grey), ASD − ADHD (blue), and ASD + ADHD (red) groups. The button press occurred at time zero and the area highlighted in grey denotes the time window over which the LRP was calculated, – 600 to 0 ms. (Bottom) Group average peak amplitude of the LRP with individual data points represented as open circles. Error bars represent ± 1 SE. * p < 0.05
Data Analysis
We tested whether individuals with ASD had abnormal motor preparation, indexed by LRP amplitude, and whether this putative abnormality in children with ASD was different in those children with and without a comorbid ADHD diagnosis. Participants’ data were excluded if the averaged LRP amplitude exceeded ± 2 SDs of the averaged means for each group. Based on this criterion, five participants (ASD, n = 3; TD, n = 2) were excluded. Additionally, before calculating means, data were visually inspected for noise after filtering and artifact rejection. Those participants with more than 30% of unusable trials due to excessive noise were eliminated, resulting in the exclusion of three participants (ASD: n = 1, TD: n = 2). The final sample consisted of 50 participants (ASD − ADHD: n = 9; ASD + ADHD: n = 12; TD: n = 29).
The main variables of interest were diagnostic group (TD, ASD − ADHD, ASD + ADHD) and the consequence of a motor action (Action to Effect; Action to No Effect). To test the hypothesis concerning the effects of consequences on motor preparation, we submitted LRP peak amplitudes to a 2 × 3 mixed model analysis of variance (ANOVA), including condition (Action to Effect, Action to No Effect) as a within-subjects factor, and group as a between-subjects factor. One-way ANOVA was used to follow up on between-group effects, and paired-sample t-tests were used for within-group follow ups. In all tests, a criterion of alpha = 0.05 was applied with Bonferroni correction for post-hoc tests.
Results
Final groups did not differ in age (F(2,47) = 0.72, p = 0.49, ηp2 = 0.03), sex (χ2(2) = 0.11, p = 0.95), or IQ (F(2,44) = 1.87, p = 0.17, ηp2 = 0.08) (See Table 1). In addition, hand preference did not differ between ASD + ADHD (right hand preference: n = 10 (91%); missing response: n = 1), ASD − ADHD (right hand preference: n = 6 (75%); missing response: n = 1) and TD (right hand preference: n = 23 (85%); missing response: n = 2) groups (χ2(2) = 0.92, p = 0.63). Of the 84% of participants who provided race information, there were 23.81% that identified as African American, 2.4% as Asian, 35.71% Caucasian, and 38.10% as more than one race. Within the 94% of participants that provided ethnicity data, 23% identified as Hispanic or Latino.
Table 1.
Participant characteristics
| Group | Age mean (SD) | IQa mean (SD) | CBCL Attention Deficits/ Hyperactivity Problems T-score |
Sex |
|---|---|---|---|---|
| TD | 12.44 (2.73) | 106 (21.14) | 51.86 (3.84) | 18M, 11F |
| ASD − ADHD | 11.44 (2.01) | 100.67 (23.93) | 57.89 (8.04) | 6M, 3F |
| ASD + ADHD | 11.59 (2.89) | 93.27 (9.82) | 68.4 (6.48) | 8M, 4F |
Three participants (ASD = 1, TD = 2) did not have an IQ assessment available
CBCL attention deficits/hyperactivity problems T-scores differed among the groups F(2,45) = 35.37, p < 0.001, ηp2 = 0.61. The ASD + ADHD group (68.40 ± 6.48; mean +/− SD) scored higher than both ASD − ADHD (57.89 ± 8.04, p < 0.001) and TD (51.86 ± 3.84, p < 0.001) groups. The TD group also scored significantly lower than the ASD − ADHD participants (p = 0.016).
An analysis of behavioral performance using the rate at which participants made their button response revealed that performance did not differ between groups, F(2,46) = 1.60, p = 0.21, ηp2 = 0.065 (ASD + ADHD: 2.88 ± 11.87 s, ASD − ADHD: 2.15 ± 10.30 s, TD: 3.26 ± 19.07 s).
Average LRP peak amplitude, however, significantly differed among groups (F(2,47) = 10.41, p < 0.001, ηp2 = 0.31) (See Fig. 2). The ASD + ADHD group (− 0.74 ± 0.72 μV; mean ± SE) had a smaller amplitude regardless of condition compared to the ASD − ADHD (4.11 ± 0.84 μV, p < 0.001) and TD (2.21 ± 0.47 μV, p = 0.004) groups. However, there was no main effect of condition (F(1,47) = 0.19, p = 0.66, ηp2 = 0.004), indicating that, collapsed across groups, LRP peak amplitude was similar between Action to No Effect (1.67 ± 0.61 μV) (See Fig. 2) and Action to Effect (2.06 ± 0.58 μV) (See Fig. 2) conditions. A significant group by condition interaction (F(2,47) = 3.31, p = 0.045, ηp2 = 0.12) was parsed further with separate one-way ANOVAs for each condition and paired sample t-tests for each group, as follows.
There were significant LRP amplitude differences among groups for both the Action to No Effect (F(2,47) = 4.32, p = 0.019, ηp2 = 0.16) and Action to Effect (F(2,47) = 9.00, p < 0.001, ηp2 = 0.28) conditions. In the Action to No Effect condition, ASD + ADHD participants had a smaller LRP amplitude compared with the TD group (p = 0.016) but showed no difference in amplitude compared to the ASD − ADHD group (p = 0.23). The ASD − ADHD group did not differ in LRP amplitude from the TD group during this condition (p > 0.99). In the Action to Effect condition, the ASD − ADHD group had a significantly larger LRP amplitude than both ASD + ADHD (p < 0.001) and TD (p = 0.005) groups, whereas the TD and ASD + ADHD groups did not differ in amplitude (p = 0.32).
Within the TD group, LRP amplitude was significantly larger in the Action to No Effect (3.22 ± 0.41 μV) than the Action to Effect condition (1.20 ± 0.58 μV, t(28) = 3.45, p = 0.002, Cohen’s d = 0.64). In the ASD − ADHD group, the pattern trended in the opposite direction, with larger amplitude in the Action to Effect (5.80 ± 1.18 μV) than the Action to No Effect (2.43 ± 1.69 μV) condition; however, this result was not statistically significant, despite a medium effect size (t(8) = − 1.64, p = 0.14, d = 0.55). Finally, in the ASD + ADHD group, amplitude did not differ between conditions (Action to No Effect: − 0.65 ± 1.63 μV; Action to Effect: − 0.83 ± 1.34 μV; t(11) = 0.074, p = 0.94, d = 0.02).
Discussion
In this study, we investigated motor preparation in children with ASD with and without comorbid ADHD by using a simple motor task in which participants made a self-initiated keypress at regular intervals, that either resulted in a consequence (i.e., onset of tone) or not. Our aim was to quantify the LRP as a measure of motor preparation and prediction in ASD, as well as to determine the impact of comorbid ADHD on this signal. As hypothesized, atypical LRP amplitude was observed in both ASD with and without ADHD, as compared to the TD control sample.
In the control group, we observed a smaller LRP amplitude when the action predicted a consequence as compared to when it did not. This finding suggests that, in controls, the neural signal associated with motor preparation was affected by learned associations between the action and the tone. The Prediction of Response Outcome (PRO) theory (Luck & Kappenman, 2011) suggests that some ERPs contain predictive information, based on past experiences, regarding the outcomes of an action. In essence, these ERPs may reflect the comparison of predicted response outcomes relative to the outcomes that actually occur. In light of this theory, our results suggest that more motor preparation is necessary in controls for those motor actions without subsequent consequence, perhaps reflecting that TD children expect that their motor actions usually elicit an event. This result observed in our control group contrasts with previous research reporting a larger (in previous cases, more negative) LRP amplitude in adults when the action led to an effect compared to when the action did not result in an immediate consequence (Ford et al., 2014; Hughes & Waszak, 2011). The difference in LRP—from negative to positive in polarity, and from a larger response in the effect-inducing condition to larger response in the no-effect condition—between previous adult samples and our current TD child sample may reflect a developmental shift both in LRP polarity and in prediction regarding the consequences of motor actions.
In individuals with ASD without comorbid ADHD, we found that, when actions did not result in a consequence, LRP amplitude was comparable to that of the control group. However, when the action resulted in an external event, the ASD without ADHD group did not follow the pattern observed in the control group (i.e., decreased amplitude). Instead, there was actually a significantly larger (i.e., more positive) LRP amplitude relative to both their own response in the action without effect condition and to the TD response in the action with effect condition. Previous findings have reported larger LRP amplitudes in ASD children as compared to TD children in stimulus-driven motor tasks (Sokhadze et al., 2016). Our results extend those findings by suggesting that ASD may also affect motor preparation during self-initiated action, specifically by inducing larger LRPs preceding self-initiated actions resulting in an external stimulus.
It has been hypothesized that individuals with ASD place disproportionate weight on sensory input (Sinha et al., 2014; Van de Cruys et al., 2017), relatively over-emphasizing minor sensory differences. Sensory input (bottom-up processes) and prior knowledge (top-down processes) influence perceptual experiences. Discrepancies between top-down and bottom-up signals yield a prediction error, which reflects a difference between what is expected based on the priors and what is experienced from the sensory evidence. A high-precision system, as has been predicted in ASD, would therefore bias perception towards bottom-up signals. In the paradigm used in this study, the action-stimulus relationship captures the motor process associated with an expected, upcoming auditory event. As such, the larger LRP we observe in ASD for the action-to-effect condition may reflect an over-anticipation of the outcome, preparing for any minor alteration that deviates from the expected. This interpretation aligns with an aberrant predictive coding mechanism in ASD. Moreover, it could explain some of the motor impairments commonly observed in ASD. For example, a common trait found in ASD is clumsiness (Ghaziuddin & Butler, 1998). Clumsiness could be the result of incorrectly predicting, for instance, where a foot might land, or over-anticipating any deviations in how forceful a kick will be when meeting a soccer ball before moving one’s leg, creating awkward movements.
In children with both an ASD and ADHD diagnosis, we found no discrimination of LRP amplitude as a function of whether an action produced an effect or not, suggesting broad impairments in motor preparation and prediction. Indeed, children with comorbid ASD and ADHD diagnoses produced a smaller LRP in comparison to children with only an ASD diagnosis and to TD controls, regardless of condition. This finding suggests that comorbid ASD and ADHD may be associated with atypical, reduced motor preparation, regardless of the anticipated outcome. Previous research has shown motor circuit differences in ASD with and without ADHD (Mahajan et al., 2016), and our findings are in line with this notion. Behaviorally, greater difficulties in motor response inhibition are observed in those with both ASD and ADHD compared to those with ASD alone (Mahone et al., 2006; Reiersen & Todd, 2008). Poor response inhibition could result from an inability to accurately prepare for a motor action, which could lead to poorly timed, impulsive, or absent responses, as has been observed in this population using a Go/No Go task (Sinzig et al., 2008). As a second example, poor preparation for applying vocal pressure when speaking could result in poorly modulated volume or odd intonation, as is also observed in ASD (Fusaroli et al., 2017; Sharda et al., 2010).
Visual inspection revealed a virtually flat LRP waveform across conditions for children with comorbid ASD and ADHD, which could either indicate that the execution of the motor movement, typically restricted to the ipsilateral hemisphere, was activated bilaterally, or that activation was lower overall. Review of the contra- and ipsi-lateral waveforms corroborated the latter. This observation suggests that, when executing a motor response, for children with ASD and ADHD in particular, neural response in premotor cortex may be less robust and/or less synchronized, resulting in a smaller LRP amplitude. In support of this notion, previous studies have shown that premotor cortex is differentially impacted in both ASD and ADHD (Dirlikov et al., 2015; Perkins et al., 2015; Puzzo et al., 2010; Suskauer et al., 2008). Moreover, previous studies exploring motor preparation in adult ADHD also have observed attenuated LRP signals, demonstrating altered response preparation (Gorman Bozorgpour et al., 2013). Our findings suggest that similar patterns also apply to childhood ADHD in the context of ASD. Furthermore, attention problems in the context of ASD may result in a unique profile that presents as a relative absence of the motor preparation signal. Our findings suggest that while ASD without ADHD may alter predictive motor outcome processes more than motor preparation alone, ASD with ADHD may confer broad impact to motor preparation itself, regardless of motor outcome or its prediction. If LRP findings were replicated in a bigger sample and observed in younger children with ASD prior to the age where ADHD diagnosis becomes more apparent, our results suggest attenuated LRP amplitude could be useful for predicting comorbid ADHD and for considering related interventions to address these symptoms early.
While past literature has reported that self-generated stimuli in both auditory and visual domains were associated with negative-going LRP amplitudes in healthy adults (Ford et al., 2014; Hughes & Waszak, 2011), our analysis yielded positive LRP amplitude values across groups. Our positive-going waveforms were unexpected, as LRPs tend to have a negative going deflection (Luck, 2014; Smulders et al., 2012). However, some studies in children have yielded a positive LRP in both stimulus-driven response (Szucs et al., 2009) and self-generated event (Jarczok et al., 2019) tasks. Thus, it may be that the morphology of the LRP changes with development and brain maturation, shifting from positive-going in childhood to negative-going by adulthood. Across childhood and adolescence, inter- and intra-hemispheric connections are still undergoing synaptic pruning impacting surface-potentials (Szucs et al., 2009), where axodendritic synapses, which produce positive surface potentials, are replaced by axosomatic synapses, which produce negative surface potentials, as the brain develops (Otto & Reiter, 1984). Therefore, the polarity inversion we and others have observed may be related to an immaturity of the sensory-motor system in children. Longitudinal studies exploring the LRP in typical development may be helpful in understanding the change in LRP polarity during maturation.
Our results should be considered in light of several study limitations. Replication with substantially larger sample sizes of individuals with ASD with versus without comorbid ADHD will be essential to confirm the value of our observations. Additionally, a cross-sectional study exploring polarity shifts in LRP across typical development and into adulthood would confirm developmental effects in the maturation of these processes, giving more clarity to our findings of positive LRP for self-generated events in children. Notably, three of the children with ASD and comorbid ADHD (n = 3) were receiving pharmacological treatment for ADHD symptoms, raising the possibility that our effects may have been confounded by presence of medication during testing and/or medication history. However, post-hoc analyses revealed that all significant findings remained significant or trending towards significance when removing those three participants from the sample. While our main interest was to explore ADHD as a comorbidity in ASD, to further understand motor problems that are specific to ASD, an additional ADHD-only comparison group would add clarification. Finally, future research should explore whether alterations in LRP amplitude extend to a sample of younger children with ASD. This direction may be useful for predicting an individual’s risk of developing comorbid ADHD, which is often diagnosed after school entry (Jang et al., 2013), years after the ASD diagnosis is first made.
In summary, whereas previous studies have utilized LRP measures to analyze motor preparation in both ASD and ADHD separately, neural correlates of motor preparation and prediction have never been examined in the context of comorbid ADHD and ASD. This study identified dissociable alterations in motor preparation and outcome prediction in ASD with and without ADHD. We found intriguing evidence that motor preparation appears abnormal in ASD without ADHD for voluntary, self-generated actions causing an effect. These findings suggest that prediction-related processes may be atypical in ASD, wherein there is an over-anticipation of a highly specific sensory outcome. Further, we observed that participants with ASD and comorbid ADHD displayed significantly attenuated motor preparation signals, regardless of action outcome, relative to both controls and to participants with ASD without comorbid ADHD. Together, these finding suggests that ASD and ADHD may affect distinct components of motor preparation.
Acknowledgments
The authors would like to acknowledge the clinical faculty and trainees at the Seaver Autism Center for their help with participant diagnostic characterization and the student research assistants who helped with data collection, entry, and cleaning. The authors would also like to thank the participants and their families who devoted their time and effort to make this project possible.
Funding
This research was supported in part by the National Center for Advancing Translational Sciences TL1TR001434 (SBG), the National Institute of Mental Health Grant R21MH115297 (JHF, KNT), the Seaver Foundation (JHF), and New York University’s Dean’s Undergraduate Research Fund (MM).
Footnotes
Conflicts of interest All authors declare that they have no conflict of interest.
Informed Consent Legal guardian of participants provided signed informed consent and children gave assent prior to data collection. Procedures were approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai.
Research Involving Human Participants and/or Animals The present research involved human subjects.
References
- Achenbach TM, & Edelbrock CS (1979). The child behavior profile: II. Boys aged 12–16 and girls aged 6–11 and 12–16. Journal of Consulting and Clinical Psychology, 47(2), 223–233. 10.1037//0022-006x.47.2.223 [DOI] [PubMed] [Google Scholar]
- Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, & Faraone SV (2016). An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert Review of Neurotherapeutics, 16(3), 279–293. 10.1586/14737175.2016.1146591 [DOI] [PubMed] [Google Scholar]
- Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, et al. (2010). Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia, 48(9), 2465–2475. [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Shiels Rosch K, Crocetti D, Denckla MB, Mahone EM, & Mostofsky SH (2015). Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage Clin, 7, 222–229. 10.1016/j.nicl.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, & Mostofsky SH (2009). Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology, 23(5), 563–570. 10.1037/a0015640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, & Mathalon DH (2014). Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophrenia Bulletin, 40(4), 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- Fusaroli R, Lambrechts A, Bang D, Bowler DM, & Gaigg SB (2017). Is voice a marker for Autism spectrum disorder? A systematic review and meta-analysis. Autism Research, 10(3), 384–407. 10.1002/aur.1678 [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, & Butler E (1998). Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research, 42(Pt 1), 43–48. 10.1046/j.1365-2788.1998.00065.x [DOI] [PubMed] [Google Scholar]
- Gorman Bozorgpour EB, Klorman R, & Gift TE (2013). Effects of subtype of attention-deficit/hyperactivity disorder in adults on lateralized readiness potentials during a go/no-go choice reaction time task. Journal of Abnormal Psychology, 122(3), 868–878. 10.1037/a0033992 [DOI] [PubMed] [Google Scholar]
- Hirata I, Mohri I, Kato-Nishimura K, Tachibana M, Kuwada A, Kagitani-Shimono K, et al. (2016). Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Research in Developmental Disabilities, 49–50, 86–99. 10.1016/j.ridd.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Hughes G, & Waszak F (2011). ERP correlates of action effect prediction and visual sensory attenuation in voluntary action. Neuro-Image, 56(3), 1632–1640. 10.1016/j.neuroimage.2011.02.057 [DOI] [PubMed] [Google Scholar]
- Jang J, Matson JL, Williams LW, Tureck K, Goldin RL, & Cervantes PE (2013). Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Research in Developmental Disabilities, 34(8), 2369–2378. 10.1016/j.ridd.2013.04.021 [DOI] [PubMed] [Google Scholar]
- Jarczok TA, Haase R, Bluschke A, Thiemann U, & Bender S (2019). Bereitschaftspotential and lateralized readiness potential in children with attention deficit hyperactivity disorder: Altered motor system activation and effects of methylphenidate. European Neuropsychopharmacology, 29(8), 960–970. 10.1016/j.euroneuro.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, & Gisel E (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(2), 231–241. 10.1007/s10803-008-0617-z [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, et al. (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40(11), 1361–1370. 10.1007/s10803-010-0996-9 [DOI] [PubMed] [Google Scholar]
- Kaiser M-L, Schoemaker M, Albaret J-M, & Geuze R (2015). What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Research in Developmental Disabilities, 36, 338–357. [DOI] [PubMed] [Google Scholar]
- Kaur M, Srinivasan SM, & Bhat AN (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Research in Developmental Disabilities, 72, 79–95. 10.1016/j.ridd.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkeit EI, Mattingley JB, Sheppard DM, Lee P, & Bradshaw JL (2005). Motor preparation, motor execution, attention, and executive functions in attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology, 11(2), 153–173. 10.1080/092970490911298 [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Landa RJ (2019). Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behavior & Development, 54, 37–47. 10.1016/j.infbeh.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Lidstone DE, Rochowiak R, Mostofsky SH, & Nebel MB (2021). A data driven approach reveals that anomalous motor system connectivity is associated with the severity of core autism symptoms. Autism Research 10.1002/aur.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Western Psychological Corporation. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/bf02172145 [DOI] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique. MIT Press. [Google Scholar]
- Luck SJ, & Kappenman ES (2011). The Oxford handbook of event-related potential components. Oxford University Press. [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summer-felt A, & Gold JM (2009). Impaired response selection in schizophrenia: Evidence from the P3 wave and the lateralized readiness potential. Psychophysiology, 46(4), 776–786. 10.1111/j.1469-8986.2009.00817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich DA (2013). The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapted Physical Activity Quarterly, 30(3), 271–282. 10.1123/apaq.30.3.271 [DOI] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich DA (2014). Motor skills and calibrated autism severity in young children with autism spectrum disorder. Adapted Physical Activity Quarterly, 31(2), 95–105. 10.1123/apaq.2013-0068 [DOI] [PubMed] [Google Scholar]
- Mahajan R, Dirlikov B, Crocetti D, & Mostofsky SH (2016). Motor circuit anatomy in children with autism spectrum disorder with or without attention deficit hyperactivity disorder. Autism Research, 9(1), 67–81. 10.1002/aur.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Powell SK, Loftis CW, Goldberg MC, Denckla MB, & Mostofsky SH (2006). Motor persistence and inhibition in autism and ADHD. Journal of the International Neuropsychological Society, 12(5), 622–631. 10.1017/S1355617706060814 [DOI] [PubMed] [Google Scholar]
- Mannion A, & Leader G (2013). Comorbidity in autism spectrum disorder: A literature review. Research in Autism Spectrum Disorders, 7(12), 1595–1616. [Google Scholar]
- Meyer A, & Sagvolden T (2006). Fine motor skills in South African children with symptoms of ADHD: Influence of subtype, gender, age, and hand dominance. Behavioral and Brain Functions, 2, 33. 10.1186/1744-9081-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, & Pulvermuller F (2018). What can autism teach us about the role of sensorimotor systems in higher cognition? New clues from studies on language, action semantics, and abstract emotional concept processing. Cortex, 100, 149–190. 10.1016/j.cortex.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, & Farley AH (1977). The early development of autistic children. Journal of Autism and Childhood Schizophrenia, 7(3), 207–229. 10.1007/bf01538999 [DOI] [PubMed] [Google Scholar]
- Otto D, & Reiter L (1984). Developmental changes in slow cortical potentials of young children with elevated body lead burden. Neurophysiological considerations. Annals of the New York Academy of Sciences, 425, 377–383. 10.1111/j.1749-6632.1984.tb23559.x [DOI] [PubMed] [Google Scholar]
- Perkins TJ, Bittar RG, McGillivray JA, Cox II, & Stokes MA (2015). Increased premotor cortex activation in high functioning autism during action observation. Journal of Clinical Neuroscience, 22(4), 664–669. 10.1016/j.jocn.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Puzzo I, Cooper NR, Vetter P, & Russo R (2010). EEG activation differences in the pre-motor cortex and supplementary motor area between normal individuals with high and low traits of autism. Brain Research, 1342, 104–110. 10.1016/j.brainres.2010.04.060 [DOI] [PubMed] [Google Scholar]
- Reiersen AM, & Todd RD (2008). Co-occurrence of ADHD and autism spectrum disorders: Phenomenology and treatment. Expert Review of Neurotherapeutics, 8(4), 657–669. 10.1586/14737175.8.4.657 [DOI] [PubMed] [Google Scholar]
- Sacrey LR, Zwaigenbaum L, Bryson S, Brian J, & Smith IM (2018). The reach-to-grasp movement in infants later diagnosed with autism spectrum disorder: A high-risk sibling cohort study. Journal of Neurodevelopmental Disorders, 10(1), 41. 10.1186/s11689-018-9259-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharda M, Subhadra TP, Sahay S, Nagaraja C, Singh L, Mishra R, et al. (2010). Sounds of melody–pitch patterns of speech in autism. Neuroscience Letters, 478(1), 42–45. 10.1016/j.neulet.2010.04.066 [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, et al. (2014). Autism as a disorder of prediction. Proceedings of the National Academy of Sciences, 111(42), 15220–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, & Lehmkuhl G (2008). Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child and Adolescent Psychiatry and Mental Health, 2(1), 4. 10.1186/1753-2000-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders FT, Miller JO, & Luck S (2012). The lateralized readiness potential. The Oxford handbook of event-related potential components (pp. 209–229). [Google Scholar]
- Sokhadze EM, Tasman A, Sokhadze GE, El-Baz AS, & Casanova MF (2016). Behavioral, cognitive, and motor preparation deficits in a visual cued spatial attention task in autism spectrum disorder. Applied Psychophysiology and Biofeedback, 41(1), 81–92. 10.1007/s10484-015-9313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Peng L, & Barnard-Brak L (2016). The comorbidity of ADHD in children diagnosed with autism spectrum disorder. Research in Autism Spectrum Disorders, 31, 11–18. [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, & Mostofsky SH (2008). fMRI of intrasubject variability in ADHD: Anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry, 47(10), 1141–1150. 10.1097/CHI.0b013e3181825b1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D, Soltesz F, Bryce D, & Whitebread D (2009). Real-time tracking of motor response activation and response competition in a Stroop task in young children: A lateralized readiness potential study. Journal of Cognitive Neuroscience, 21(11), 2195–2206. 10.1162/jocn.2009.21220 [DOI] [PubMed] [Google Scholar]
- Van de Cruys S, Van der Hallen R, & Wagemans J (2017). Disentangling signal and noise in autism spectrum disorder. Brain and Cognition, 112, 78–83. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) (Vol. 22(498), pp. 816–827). NCS Pearson. [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- Weiss LG, Saklofske DH, Coalson D, & Raiford SE (2010). WAIS-IV clinical use and interpretation: Scientist-practitioner perspectives. Academic Press. [Google Scholar]
- Winstanley CA, Eagle DM, & Robbins TW (2006). Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review, 26(4), 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Nebel MB, Ewen JB, & Mostofsky SH (2020). Altered inferior parietal functional connectivity is correlated with praxis and social skill performance in children with autism spectrum disorder. Cerebral Cortex, 31(5), 2639–2652. 10.1093/cercor/bhaa380 [DOI] [PMC free article] [PubMed] [Google Scholar]