Abstract
Objective
To determine the effectiveness of exercise rehabilitation in people with multimorbidity. Exercise capacity was the primary outcome. Secondary outcomes were: health-related quality of life, activities of daily living, cardiometabolic outcomes, mental health outcomes, symptom scores, resource utilization, health behaviours, economic outcomes, and adverse events.
Data sources
A search was conducted in MEDLINE, CINHAL, EMBASE, and Cochrane Central Register of Controlled Trials databases.
Study selection and extraction
Randomized and non-randomized controlled trials and cohort studies of exercise rehabilitation vs any comparison in people with multimorbidity.
Data synthesis
Forty-four reports (38 studies) were included. Rehabilitation ranged from 8 weeks to 4 years, with 1–7 sessions of rehabilitation weekly. Exercise included aerobic and resistance, limb training, aquatic exercises and tai chi. Compared with usual care, exercise rehabilitation improved 6-min walk distance (weighted mean difference (WMD) 64 m, 95% CI 45–82) and peak oxygen consumption (WMD 2.74 mL/kg/min, 95% CI –3.32 to 8.79). Effects on cardiometabolic outcomes and health-related quality of life also favoured rehabilitation; however; few data were available for other secondary outcomes.
Conclusion
In people with multimorbidity, exercise rehabilitation improved exercise capacity, health-related quality of life, and cardiometabolic outcomes.
LAY ABSTRACT
Chronic disease is a common health problem world-wide. It is increasingly common for people to have more than 1 chronic disease, which is called multimorbidity, and the interaction of their multiple health problems may worsen their health outcomes. Exercise rehabilitation is an effective and established treatment to improve health for people with different chronic diseases, such as heart and lung disease; however, the benefit of structured rehabilitation in people with multimorbidity has not been systematically reviewed. A literature search was performed to investigate the clinical outcomes following exercise rehabilitation in people with multimorbidity. Compared with usual medical care, the results showed that exercise rehabilitation improved exercise capacity, measured by walking distance in a formal test, health-related quality of life and the body’s ability to use oxygen, in people with multimorbidity. There were few data regarding the benefit of rehabilitation on other outcomes, and more well-designed robust trials are needed.
Key words: rehabilitation, exercise, multimorbidity, comorbidity, chronic disease
Multimorbidity, defined as the co-existence of 2 or more chronic conditions (1), is common in clinical practice (2) and is associated with many negative consequences, including increased risk of disability (3), frailty (3) and mortality (4, 5), poorer functional status (6), reduced health-related quality of life (HRQoL) (7) and high healthcare costs (8). The increasing prevalence of multimorbidity generates financial pressures on healthcare systems, as expenditure increases almost exponentially with the number of chronic diseases in an individual (9).
Rehabilitation is integral to chronic disease management. It is described as therapies including exercise (aerobic and resistance) training, education and behaviour change (10), with interventions designed to optimize function and reduce disability in individuals with health conditions (11). Evidence has shown that exercise training, inclusive of aerobic and resistance regimens and education, improves outcomes, including exercise capacity, upper and lower limb function and muscular strength, and quality of life, and mitigates the progression of many chronic diseases (12). This accounts for the inclusion of exercise rehabilitation in clinical practice guidelines for several single diseases (12, 13). Worldwide healthcare delivery tends to be organized around the treatment of single diseases (1, 14, 15). As a result, people with multimorbidity are often managed according to several single-disease guidelines. This is reflected in rehabilitation, which is frequently structured as single-disease programmes, such as cardiac and pulmonary rehabilitation. While meta-analyses of single-disease programmes have demonstrated improvements in exercise capacity, symptoms, and HRQoL (16–19), recent multimorbidity guidelines suggest that single-disease care may not be appropriate for people with multimorbidity (20). The low inclusion of people with multimorbidity in randomized controlled trials (RCT) reinforces the difficulty faced by healthcare professionals in creating appropriate clinical protocols (3) and guidelines. In a review of guidelines relevant to single-disease rehabilitation, 3 out of 7 did not mention coexisting conditions, and an additional 3 only briefly mentioned minor programme adaptations to accommodate multimorbidity (21). This highlights the need to investigate rehabilitation in people with multimorbidity.
A systematic review on the interventions for improving outcomes in patients with multimorbidity found mixed results about the effectiveness of interventions (2). The interventions were predominantly focused on organization of care, such as case management or multidisciplinary team-work, and educational or self-management support (2). It found no clear positive improvements in clinical outcomes, health service use, patient-related health behaviours or costs (2). The review suggests that interventions designed to target difficulties that people experience with daily functioning (e.g. physiotherapy) may be more effective (2). However, exercise rehabilitation was not delivered in the included studies and exercise capacity was not a reported outcome measure.
Exercise rehabilitation for people with multimorbidity may have a role to play in addressing common symptoms of multiple chronic diseases. This systematic review aimed to determine clinical outcomes following exercise rehabilitation in people with multimorbidity. This review was registered on PROSPERO on 29 August 2018 (CRD42018100512).
METHODS
This systematic review was reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (22).
Types of studies
Due to the emerging nature of the field of multimorbidity, RCTs, non-randomized control trials (NRCT) and cohort studies were eligible for inclusion. Studies published in a language other than English were excluded due to lack of access to translation services. Systematic reviews, cross-sectional and case studies were excluded.
Types of participants
Any participants with multimorbidity, defined as 2 or more chronic diseases, were included, and no age criteria were applied (1). This study used the World Health Organization (WHO) definition of chronic disease: health problems that require ongoing management over a period of years or decades (23). No criteria to confirm diagnosis of a specific chronic disease was applied. If multimorbidity was present in only a proportion of the participant population, studies were included if there were separate data for participants with multimorbidity.
Types of interventions
The study included rehabilitation programmes of at least 4 weeks’ duration that included exercise with or without any form of education or psychological support (19, 24, 25), delivered in any setting (home-based, primary, secondary or tertiary care). These criteria are consistent with systematic reviews reporting on rehabilitation in chronic obstructive pulmonary disease, heart failure, and coronary heart disease populations (19, 24, 25). There were no criteria specified for exercise type, frequency or intensity or follow-up period, in order to enable widespread search results. The study excluded programmes without exercise training or those aimed at a single joint (e.g. hip), which focused on regaining function in the single joint via targeting range of motion or strength.
Comparisons of interest
Usual medical care (UMC) or other interventions that excluded exercise training (e.g. education or psychological support only) were comparisons of interest. Usual medical care was defined as general inpatient or outpatient care, including medical, nursing or allied health intervention. Studies comparing rehabilitation with UMC were analysed separately from those comparing with other interventions.
Types of outcomes
The primary outcome was exercise capacity, as measured by 1 or more of: laboratory-based exercise testing (e.g. cardiopulmonary exercise test; CPET) and/or field walking tests (e.g. 6-min walk test).
The secondary outcomes were: HRQoL (any generic or disease-specific questionnaires); activities of daily living (ADL) (any questionnaires); cardiometabolic outcomes (e.g. blood pressure; BP), lipid profiles, body mass index (BMI)); mental health outcomes (e.g. depression and anxiety scores); symptom scores (e.g. dyspnoea, fatigue); resource utilization (e.g. hospital admissions, general practitioner visits); health behaviours (exercise or medication adherence, physical activity); economic outcomes (e.g. analysis measuring cost; effectiveness or impact); and adverse events.
The primary and secondary outcomes were selected as they are common measures within the field of rehabilitation research and in clinical practice. The studies included reported on at least 1 outcome of interest and did not have to include the primary outcome.
Search strategy
The search strategy used the following electronic databases in English only: up to 21 December 2021: MEDLINE, 1946 to present, In-process and other non-indexed citations, Ovid MEDLINE; Cumulative Index to Nursing and Allied Health Literature (CINAHL), 1981 to present, EBSCO CINAHL; EMBASE, 1947 to present, Ovid EMBASE; and Cochrane Central Register of Controlled Trials (CENTRAL), 1966 to present.
The search strategy for MEDLINE is shown in Table SI and was adapted for other databases. Reference lists of the identified articles were hand searched. The following trial registry was also searched: www.clinicaltrials.gov. Only studies with data published were included.
Selection of studies
Citations identified were collated via reference manager software (Endnote X7.8) and duplicates were removed. Two review authors (KB, ALL) screened titles and abstracts independently. Potential articles that met the inclusion criteria were identified and retrieved in full text for independent assessment by both reviewers. Any disagreements were resolved by consensus or a third reviewer (AH), where necessary.
Data extraction and management
Two review authors (KB, ALL) completed data extraction using an a priori data extraction template developed by the authors. The following data from included studies were extracted: (i) details of the intervention including: provider, delivery, location, dosage and tailoring (26); additional components (e.g. education or psychological support); (ii) participants: nature of multimorbidity and how it was defined; age; (iii) trial setting; (iv) study design; (v) comparators; (vi) outcome measures; and (vii) results. In the event that another report, referenced in the methods of an included study provided further detail of this data, this report was sourced and used to obtain the information required. WebPlotDigitizer (Pacifica, California, USA) (27) was used to extract data from studies that displayed results via figures and graphs only.
Assessment of risk of bias
The risk of bias of the RCT, NRCT and cohort studies were assessed independently using the Joanna Briggs Institute critical appraisal tools for specific study design (28). If necessary, authors were contacted to obtain further information. The risk of bias was assessed for the following domains: selection; performance; detection; attrition; reporting; and other (29). Two review authors (KB, ALL) independently extracted the data, and clarification was obtained via consensus discussion to confirm complete agreement.
Data analysis
For continuous variables (e.g. exercise capacity and HRQoL), the study recorded mean change from baseline or mean post-intervention values and standard deviation (SD). When 95% confidence intervals (95% CIs) and standard errors (SE) were reported, SDs were calculated. For dichotomous variables (e.g. health behaviours), risk ratios or odds ratios were calculated.
Meta-analysis
Meta-analysis was performed if trials were sufficiently clinically or statistically homogeneous, determined by factors including length of rehabilitation and outcome measure. Effect sizes (ES) were calculated using an online calculator (30) and Cohen’s definition of ES of 0.2 as small, 0.5 as moderate and 0.8 or greater as large (31) was used to define magnitude.
Assessment of heterogeneity
Included studies were assessed in terms of clinical and statistical heterogeneity. Statistical heterogeneity was assessed by the inspection of forest plots and the I2 statistics. The Cochrane guide to interpreting I2 as follows, 0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: considerable heterogeneity (29). The fixed-effects model was used in the absence of heterogeneity; otherwise a random-effects model was used.
Subgroup analyses
Subgroup analyses were performed based on: (i) the definition of multimorbidity (i.e. 2 diseases vs 3 or more diseases), as these have been shown to have differences in prevalence (32, 33) and mortality (3); and (ii) the length of rehabilitation (4–8 weeks vs > 8 weeks); in clinical practice, it is common for rehabilitation programmes to have durations of 4–8 weeks and research trials may have durations longer than 8 weeks.
Sensitivity analysis
Sensitivity analyses were performed to determine the potential effects of intervention components on outcomes, examining studies of exercise training only vs exercise training combined with education or psychological support.
RESULTS
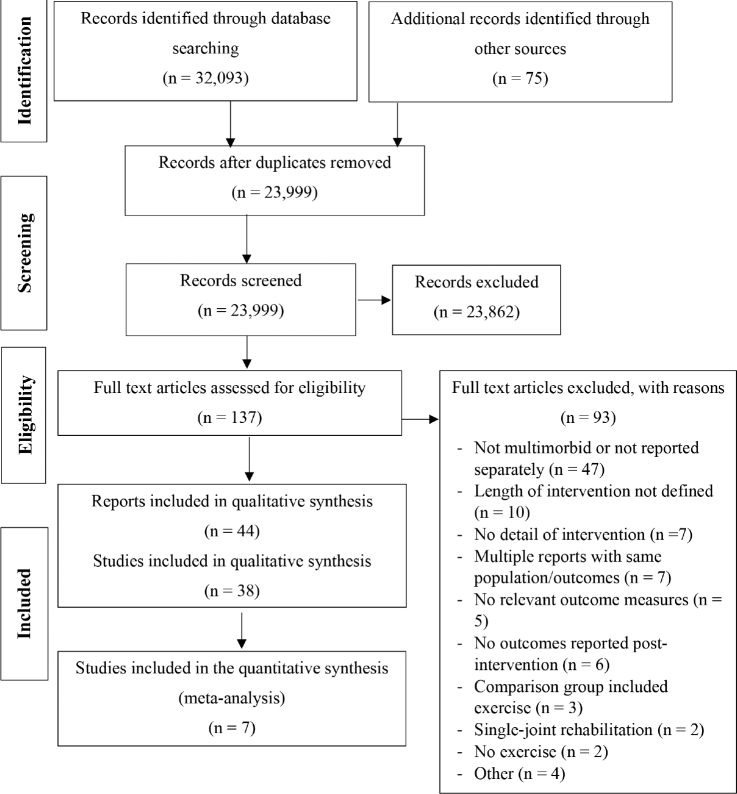
The searches identified 23,999 studies (excluding duplicates), of which 23,862 were excluded based on title and abstract. Of the 137 full-text studies screened, 93 were excluded. The reasons for exclusion are detailed in Fig. 1. The final search outcome was 44 reports, resulting from 38 studies. Four studies had multiple reports that focused on different outcomes and met the inclusion criteria. Nine studies (10 reports) were reported only as abstracts (34–43). There were 17 RCTs, 1 randomized crossover trial, 19 cohort studies, and 1 quasi-experimental study.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Study characteristics are shown in Table I. The most common sample in the studies was chronic obstructive pulmonary disease (COPD) and comorbidities [diagnosis not specified] (n = 6), followed by coronary heart disease (CHD) and diabetes (n = 4). Multimorbidity groups were defined as 2 and 3 or more (n = 6), 2–3 and 4 or more (n = 1), distinct clusters (e.g. respiratory conditions, musculoskeletal conditions or neurological conditions) (n = 4) or using a weighted comorbidity score (Charlson Comorbidity Index (CCI)) (n = 1).
Table I.
Characteristics of included studies (n = 38)
| Study | Country N | Study type | Diseases | Participants | Intervention | Duration of rehabilitation | Outcomes |
|---|---|---|---|---|---|---|---|
| Abdelbasset (2019) (54) | Saudi Arabia 69 | Cohort | COPD HF |
Age = range 45–61 74% males |
Int 1 = low to moderate intensity aerobic exercise Int 2 = moderate intensity aerobic exercise Com = UC |
12 weeks | PHQ-9 |
| Abd El-Kader (2013) (44) | Saudi Arabia 80 |
RCT | Obesity | Age# (range) = 12–18 53% males# |
Int = aerobic exercise; diet; medical treatment | 8 weeks | BMI |
| Bronchial asthma | Com = usual medical care | ||||||
| Al-Jiffri (2013) (58) | Saudi Arabia 100 |
RCT | NAFLD | Age# (range) = 35–55 100% males# |
Int = aerobic exercise; diet | 3 months | BMI HOMA-IR |
| Diabetes | Com = diet only (no exercise) | ||||||
| Barnes (2009) (62) | Australia 12 |
Cohort | OSA | Age# = 42 (10.4) 25% males# |
Int = aerobic and resistance exercise; diet | 16 weeks | VO2 peak BMI BP Lipids Insulin and glucose SF-36 POMS BDI FOCQ SASQ CRP |
| Obesity | Com = N/A | ||||||
| Beaudoin (2017) (46) | Canada 17 |
RCT | Cystic fibrosis | Age* (mean, SEM) = 32 (24, 41) 38% males* Sedentary (< 100 min/wk structured exercise |
Int = aerobic and resistance exercise | 12 weeks | VO2 peak Cystic fibrosis questionnaire-revised Physical activity questionnaire Physical activity monitor (steps) CRP |
| Impaired glucose tolerance | Age* (mean, SEM) = 36 (22, 57) 50% males* Sedentary (< 100 min/wk structured exercise) |
Com = usual medical care | |||||
| Bernocchi (2018) (47) | Italy 112 |
RCT | COPD | Age* = 71 (9) 88% males* |
Int = aerobic and resistance exercises; education | 4 months | 6MWT MLHFQ CAT MRC PASE |
| Heart failure | Age* = 70 (9.5) 75% males* |
Com = usual medical care | |||||
| Byrkjeland (2015) (48) | Norway 137 |
RCT | T2DM | Age* = 65 (7.6) 87% males* |
Int = aerobic and resistance exercise | 12 months | VO2 peak HbA1c Glucose Insulin HOMA2-IR Adverse event (all medical events) |
| CAD | Age* = 63 (7.2) 81% males* |
Com = usual medical care | |||||
| Castro (2015) (35) | Portugal 19 |
Cohort | CKD | Age# = 72 (10) 33% males# |
Int = aerobic and resistance exercise; haemodialysis | 16 weeks | 6MWT Accident or complication |
| Diabetes | Com = N/A | ||||||
| Chiang (2020) (55) | Taiwan 50 |
RCT | Multimorbidity: 2 or more of HT, DM, HL, heart disease, metabolic syndrome, gout | Age = 60 (7.2) 72% males |
Int = aerobic exercise Com = UC |
12 weeks | IPAQ VO2 peak SF-36 |
| Collins (2010) (36) | USA 145 |
RCT | Diabetes | Age# = 67 (10.1) 69% males# |
Int = aerobic exercise; phone call | 6 months | Depressive symptoms |
| PAD | Com = phone call only (no exercise) | ||||||
| Crisafulli (2010) (63) | Italy 316 |
Cohort | COPD | Age# = 68 (7.6) 74% males# |
Int = peripheral limb training; education; psychological support | 21 sessions (9 weeks) |
6MWT SGRQ MRC |
| Comorbidities | Com = N/A | ||||||
| de Groot (2012) (64) | USA 50 |
Quasi-experimental | Diabetes | Age# = 57 (9.0) 32% males# |
Int = aerobic and resistance exercise; cognitive behavioural therapy | 12 weeks | VO2 peak Diabetes quality of life measure SF-36 HbA1c Lipids BMI BDI Chronic illness resource survey Minutes exercise/week Steps |
| Major depressive disorder | Com = N/A | ||||||
| Freitas (2018) (60) | Brazil 55 |
RCT | Asthma | Age* = 46 (7.7) 4% males* Performed < 60 min structured or planned PA per week |
Int = aerobic and resistance exercise; diet; education; psychological support | 3 months | BMI HADS |
| Obesity | Age* = 49 (9.6) 0% males* Performed < 60 min structured or planned PA per week |
Com = sham exercise (breathing and stretches); diet; education; psychological support | |||||
| Halvari (2017) (61) | Norway 137 |
RCT | CAD | Age# = 63 (7.9) 81% males# |
Int = aerobic and resistance exercise | 12 months | HbA1c |
| Diabetes | Physically active < 150 min/wk | Com = usual physical activity | |||||
| Hassan (2016) (65) | Egypt 55 |
Cohort | COPD | Age# = 60 (8.9) 93% males# |
Int = aerobic and resistance exercise; education | 8 weeks | 6MWT VO2 maximum mMRC SGRQ |
| Comorbidities | Com = N/A | ||||||
| Hsu (2021) (56) | Taiwan 66 |
RCT | Knee osteoarthritis Obesity |
Age = range 60–70 53% males |
Int 1 = Elastic band resistance exercise Int 2 = Elastic band resistance exercise, diet control Com = U/C |
12 weeks | BMI |
| Johnson (2014) (38) | Not stated 30 |
Randomized crossover trial | Diabetes | Age# = 68 (6.9) 53% males# |
Int = aquatic exercise (details not provided) | 12 weeks | 6MWT |
| Lower limb arthritis | Com = NS | ||||||
| Khadanga (2016) (66) | USA 898 |
Cohort | CHD | Age# = 64 (11.1) 73% males# |
Int = aerobic and resistance exercise; education | 3–4 months (maximum 36 sessions) | VO2 peak Peak METS BMI |
| Insulin resistance or diabetes | Com = N/A | ||||||
| Kurian (2010) (39) | USA 22 |
Cohort | Diabetes | Age# = elderly 68% males# |
Int = resistance exercise | 12 weeks | HbA1c Lipids |
| Peripheral neuropathy | Com = N/A | ||||||
| Listerman (2011) (67) | USA 749 |
Cohort | CHD | Age# = 62 (10.6) 71% males# |
Int = aerobic and resistance exercise; education; psychological support | 24–36 sessions | 6MWT BMI |
| Comorbidities | Com = N/A | ||||||
| Lo (2021) (57) | Taiwan 43 |
RCT | Multimorbidities: 2 of HT, HL, DM, stroke, cancer, heart disease, kidney disease, asthma, COPD, OP, degenerative arthritis, gout, depression, schizophrenia, bipolar disorder | Age range 40–≥ 65 49% males PA < 150 min moderate intensity or < 75 min vigorous intensity per week |
Int 1 = aerobic exercise and MI Com 1 = MI Com 2 = UC |
12 weeks | IPAQ SF-36 BMI VO2 max |
| Martin (2016) (40) | Canada 15,927 |
Cohort | CHD | Age and sex details not stated | Int = details not provided | 12 weeks | METs |
| PAD | Com = N/A | ||||||
| McNamara (2013) (50) | Australia 53 |
RCT | COPD | Age* = 73 (7) 50% males* |
Int 1 = aerobic and resistance exercise (land-based) | 8 weeks | 6MWT ESWT ISWT CRDQ HADS |
| Comorbidities | Age* = 72 (10) 28% males* |
Int 2 = aerobic and resistance exercise (water-based) | |||||
| Age* = 70 (9) 47% males* |
Com = no exercise | ||||||
| Mentz (2013) (51) | USA 2,331 |
RCT | Heart failure | Age# (median) = 59 72% males# |
Int = aerobic exercise; education | Up to 4 years | VO2 peak 6MWT KCCQ |
| COPD | Com = usual medical care; education | ||||||
| Mesquita (2015) (68) | Netherlands 213 |
Cohort | COPD | Age# = 64 (7) 59% males# |
Int = details not provided | 8 weeks (inpatient) Or 14 weeks (outpatient) |
6MWT CWRT SGRQ |
| Comorbidities | Com = N/A | ||||||
| Mundra (2013) (41) | USA 120 |
Cohort | CVD | Age details not stated 70% males# |
Int = details not provided | 8–12 weeks | METs BMI BP Lipids Glucose BDI |
| Obesity | Com = N/A | ||||||
| Naz (2019) (69) | Turkey 211 |
Cohort | COPD | Age# (median, IQ range) = 64 (58, 68) 89% males# |
Int = aerobic and resistance exercise | 8 weeks | 6MWT SGRQ mMRC HADS SF-36 |
| Comorbidities | Com = N/A | ||||||
| Nonoyama (2016) (70) | Canada 1,247 |
Cohort | IHD | Age¥ = 61 (8.3) 96% males¥ [no comorbidities] |
Int = aerobic and resistance exercise; education; psychological support | 6–12 months | VO2 peak BMI |
| Comorbidities | Age¥ = 67 (10.1) 78% males¥ [non-respiratory comorbidity] |
Com = N/A | |||||
| Age¥ = 61 (10.1) 89% males¥ [respiratory comorbidity] |
|||||||
| Servantes (2012) (52) | Brazil 50 |
RCT | Heart failure | Age* = 52 (9.83) 47% males* |
Int 1 = aerobic exercise; education | 3 months | VO2 peak MLHFQ |
| Sleep apnoea | Age* = 51 47% males* |
Int 2 = aerobic and resistance exercise; education | |||||
| Age* = 53 (8.19) 46% males* |
Com = no exercise | ||||||
| Soleimani (2009) (71) | Netherlands 284 |
Cohort | IHD | Age# = 57 (11.1) 72% males# |
Int = aerobic exercise; diet counselling; psychological support | 8 weeks | Resting HR Peak HR Post-exercise HR HR recovery |
| Diabetes | Com = N/A | ||||||
| Sridhar (2010) (53) | Malaysia 105 |
RCT | Diabetes | Age* = 62 (3.10) 55% males* |
Int = aerobic exercise | 12 months | BP HbA1c HR variability |
| Hypertension | Age* = 59 (2.75) 56% males* |
Com = no exercise | |||||
| Srinivasan (2014) (42) | USA 16 |
RCT | Major depressive disorder | Age# = 72 (5.24) Sex details not stated |
Int = Tai Chi; antidepressant treatment | 8 weeks | SIGHD |
| Arthritis pain disorder | Age# = 74 (7.07) Sex details not stated |
Com = mind-body education; antidepressant treatment | |||||
| Takaya (2014) (72) | Japan 528 |
Cohort | AMI | Age¥ = 62 (10) 81% males¥ [non-CKD] |
Int = aerobic exercise; education | 3 months | VO2 peak BMI HR recovery |
| CKD | Age¥ = 68 (9) 84% males¥ [CKD] |
Com = N/A | |||||
| Tunsupon (2017) (73) | USA 165 |
Cohort | COPD | Age# (mean) = 70 96% males# |
Int = aerobic and resistance exercise | 8 weeks | 6MWT MIET CWET CRQ |
| Comorbidities | Com = N/A | ||||||
| Verges (2004) (75) | France 95 |
Cohort | Acute coronary event | Age¥ = 57 (8.8) 86% males¥ [T2DM] |
Int = aerobic exercise; education | 2 months | VO2 peak |
| T2DM | Age¥ = 57 (11.3) 92% males¥ [Non-diabetic] |
Com = N/A | |||||
| Wang (2013) (76) | Taiwan 90 |
Cohort | Heart failure | Age¥ = 63 (2.10) 47% males¥ [HF and non-anaemic] |
Int = aerobic exercise | 12 weeks | VO2 peak |
| Anaemia | Age¥ = 64 (2.3) 40% males¥ [HF and anaemic] |
Com = N/A | |||||
| Age¥ = 62 (2.1) 47% males¥ [Normal control] |
|||||||
| Woodard (1994) (77) | USA 28 |
Cohort | CVD | Age¥ = 61 (1.7) Sex details not stated [Comorbidity] |
Int = aerobic exercise | 6 months | METs |
| Knee arthritis | Age¥ = 59 (2.0) Sex details not stated [CVD only] |
Com = N/A | |||||
| Zwerink (2010) (43) | Netherlands 6 |
Cohort | COPD | Age# = 70 (5) Sex details not stated |
Int = aerobic and resistance exercise; education | 10 weeks | 6MWT ISWT MLHFQ CRQ |
| Heart failure | Com = N/A |
Age is mean (standard deviation; SD) unless otherwise stated;
whole population;
intervention group;
disease group.
n: number; RCT: randomized control trial; Int: intervention; Com: comparison; BMI: body mass index; NAFLD: non-alcoholic fatty liver disease; HOMA-IR: homeostasis model assessment-insulin resistance-index; MI: motivational interviewing; N/A: not applicable; OSA: obstructive sleep apnoea; VO2: oxygen consumption; BP: blood pressure; SF-36: Short Form-36; POMS: Profile of Mood States; BDI: Beck Depression Index; FOCQ: Functional Outcomes of Sleep Questionnaire; SASQ: Sleep Apnoea Symptom Questionnaire; CRP: C-reactive protein; SEM: standard error of the mean; COPD: chronic obstructive pulmonary disease; 6MWT: 6-min walk test; MLHFQ: Minnesota Living with Heart Failure Questionnaire; CAT: COPD assessment test; MRC: dyspnoea by Medical Research Council; PASE: physical activity profile; T2DM: type 2 diabetes mellitus; CAD: coronary artery disease; HbA1c: haemoglobin A1c; HOMA2-IR: homeostasis model assessment 2-insulin resistance-index; CKD: chronic kidney disease; PAD: peripheral arterial disease; SGRQ: St George’s respiratory questionnaire; HADS: Hospital Anxiety Depression Scale; mMRC: modified dyspnoea by Medical Research Council; NS: not stated; CHD: coronary heart disease; METs: metabolic equivalents; ESWT: Endurance Shuttle Walk Test; ISWT; Incremental Shuttle Walk Test; CRDQ: Chronic Respiratory Disease Questionnaire; KCCQ: Kansas City Cardiomyopathy Questionnaire; CWRT: constant work rate cycling test; IHD: ischemic heart disease; HR: heart rate; SIGHD: structured interview for Hamilton depression scale; AMI: acute myocardial infarct; MIET: maximal symptom-limited incremental cycle ergometer test; CWET: constant workload cycle endurance time test; CRQ: Chronic Respiratory Questionnaire; CVD: cardiovascular disease; wk: weeks.
Intervention details are outlined in Table SII. Duration of interventions ranged from 8 weeks to up to 4 years, with a frequency of 1–7 sessions/week. The types of exercise included aerobic, aerobic and resistance, peripheral limb training, aquatic exercise and tai chi. The rehabilitation was performed in several different locations, including supervised setting (n = 1), centre-based (n = 2), community exercise facility (n = 1), medical centre (n = 2), community-based (n = 3), home-based (n = 11) and hospital-based (n = 14); with some studies at multiple locations. Comparisons included UMC (n = 7), no exercise (n = 3), diet control (n = 1), motivational interviewing (n = 1), diet and sham exercise (n = 1) (sham exercise consisting of yoga pranayama breathing exercises and upper and lower limb stretches), phone call only (n = 1), diet (n = 1), usual physical activity (n = 1) and mind-body education (n = 1). There were no studies that measured ADL, resource utilization or economic outcomes.
Full details of the quality assessment for all study types are shown in Tables SIII, SIV and SV. For the RCTs and randomized crossover trial, 17 out of 18 reported not having participant or therapist blinding. Only 6 studies reported assessor blinding, and the remaining 9 studies did not specify whether assessors were blinded. For the cohort studies, 14 out of 19 studies showed that the exposures were measured in a valid and reliable way, with the other studies being unclear.
Meta-analysis was limited, as studies were clinically and methodologically heterogeneous, as defined in the methods section. Meta-analysis was performed for 3 outcomes: 6-min walk distance (6MWD), peak oxygen consumption (VO2), and BMI.
Exercise rehabilitation vs usual medical care
Twelve studies (15 reports) compared exercise rehabilitation vs UMC (34, 38, 44–53, 54, 55, 57). The findings for studies are outlined in Table II.
Table II.
Outcomes of studies of exercise-rehabilitation vs usual medical care
| Study | Intervention (exercise type) | Outcome | Results (intervention) | Results (control) | Effect size | Notes |
|---|---|---|---|---|---|---|
|
Exercise capacity
| ||||||
| Ambrosy (2018) (45) | Aerobic | 6MWD (m) | HF+CKD: –7 (95% CI –13 to 0) | NR | NA |
p = 0.04* Mean change (within group p-value) |
| Bernocchi (2018) (47) | Aerobic and resistance | 6MWD (m) | 60 (95% CI 22.2–97.8) | –15 (95% CI –40.3 to 9.8) | d = 0.69 |
p = 0.004* Mean change (between group p-value) |
| Johnson (2014) (38) | Details not provided | 6MWD (m) | 17 | NR | – |
p = 0.046 mean change (SD not stated) |
| McNamara (2013) (50) | Aerobic and resistance | 6MWD (m) | land 43 (95% CI 22–63) water 48 (95% CI 22–70) |
–16 (95% CI –34 to 1) | land d = 1.76 water d = 1.86 |
land vs Con: p < 0.001* water vs Con: p < 0.0001* Mean change (between group p-values) |
| Mentz (2013) (51) | Aerobic | 6MWD (m) | 19 (IQR –9 to 69) | 1 (IQR –41 to 40) |
p = 0.16 Median (IQR) change |
|
| Beaudoin (2017) (46) | Aerobic and resistance | VO2 peak (mL/kg/min) | 24.53 (SD 4.01) | 25.35 (SD 6.79) | d = 0.15 | ns Post-intervention |
| Byrkjeland (2015) (48) | Aerobic and resistance | VO2 peak (mL/kg/min) | 25.4 (SD 5.4) | 25.2 (SD 6.7) | d = 0.03 |
p = 0.0777 Post-intervention |
| Chiang (2020) (55) | Aerobic | VO2 peak (mL/kg/min) | 27.3 (SD 7.1) | 24.1 (SD 7.3) | d = 0.44 |
p = 0.04 Post-intervention (between group p-value) |
| Lo (2021) (57) | Aerobic | VO2 peak (mL/kg/m2) | 25.9 (SD4.8) | MI: 24.2 (SD7.9) Con: 22.7 (SD 6.5) |
Int vs Con: d = 0.49 | Int vs Con: p = 0.002Post-intervention (between groups p-value) |
| Mentz (2013) (51) | Aerobic | VO2 peak (mL/kg/min) | 0.2 (IQR –0.6 to 1.5) | 0.1 (IQR –1.0 to 1.2) | – |
p = 0.82 Median (IQR) change |
| Servantes (2012) (52) | Aerobic and resistance | VO2 peak (mL/kg/min) | 20.9 (SD 4.2) | 12.8 (SD 3.2) | d = 2.17 |
p = 0.951 Post-intervention (between group p-value) |
| McNamara (2013) (50) | Aerobic and resistance | ESWD (m) | land 117 (95% CI –3 to 236) water 321 (95% CI 123–518) |
–50 (95% CI –240 to 140) | land d = 0.69 water d = 1.21 |
land vs Con: p = 0.456 water vs Con: p = 0.006* Mean change (between group p-values) |
| McNamara (2013) (50) | Aerobic and resistance | ISWD (m) | land 13 (95% CI –16 to 43) water 49 (95% CI 26–73) |
–1 (95% CI –24 to 22) | land d = 0.28 water d = 1.27 |
land vs Con: p = 0.542 water vs Con: p = 0.005* Mean change (between group p-values) |
|
Health-related quality of life
| ||||||
| Bernocchi (2018) (47) | Aerobic and resistance | MLHFQ | –10.5 (95% CI –14.2 to –6.8) | –0.44 (95% CI –4.0 to 4.0) | d = 0.73 |
p = 0.0007* Mean change (between group p-value) |
| Servantes (2012) (52) | Aerobic and resistance | MLHFQ | 25.1 (SD 16.5) | 51.0 (SD 16.8) | d = 1.56 |
p = 0.671 Post-intervention (between group p-value) |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: physical functioning (%) | 80.2 (SD 16.78) | 81.93 (SD 16.82) | d = 0.10 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: vitality (%) | 58.33 (SD 19.2) | 54.18 (SD 20.91) | d = 0.21 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: emotional state (%) | 81.66 (SD 12.73) | 83.33 (SD 15.06) | d = 0.12 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: eating disturbance (%) | 98.61 (SD 3.92) | 100 (SD 0) | d = unable to calc | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: treatment burden (%) | 65.29 (SD 28.14) | 68.52 (SD 21.59) | d = 0.13 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: health perception (%) | 58.34 (SD 23.59) | 74.1 (SD 15.17) | d = 0.79 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: social limitations (%) | 75.28 (SD 13.02) | 72.22 (SD 18.24) | d = 0.19 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: body image (%) | 84.74 (SD 8.26) | 81.5 (SD 18.13) | d = 0.23 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: role limitations (%) | 83.33 (SD 25.2) | 84.73 (SD 21.99) | d = 0.06 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: weight problems (%) | 87.5 (SD 24.81) | 83.33 (SD 40.82) | d = 0.12 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: respiratory symptoms (%) | 62.5 (SD 14.47) | 65.75 (SD 8.17) | d = 0.28 | ns Post-intervention |
| Beaudoin (2017) (46) | Aerobic and resistance | QCFQR: digestive symptoms (%) | 84.74 (SD 10.17) | 69.53 (SD 14.79) | d = 1.20 | ns Post-intervention |
| Bernocchi (2018) (47) | Aerobic and resistance | CAT | 5.3 (95% CI –6.9 to 3.7) | 1.6 (95% CI –0.4 to 3.5) | d = 1.17 |
p = 0.0001* Mean change (between group p-value) |
| McNamara (2013) (50) | Aerobic and resistance | CRDQ -dyspnoea | land vs Con: 1.6 (95% CI –0.8 to 4.0) water vs Con: 3.3 (95% CI 0.9–5.6) |
NA | – | land vs Con: p = 0.193 water vs Con: p = 0.007* Mean difference (between group p-values) |
| McNamara (2013) (50) | Aerobic and resistance | CRDQ - fatigue | land vs Con: 1.6 (95% CI –0.7 to 3.9) water vs Con: 4.7 (95% CI 2.4–7.0) |
NA | – | land vs Con: p = 0.163 water vs Con: p< 0.001* Mean difference (between group p-values) |
| McNamara (2013) (50) | Aerobic and resistance | CRDQ - emotion | land vs Con: 0.1 (95% CI –2.8 to 3.1) water vs Con: 3.1 (95% CI 0.1–6.1) |
NA | – | land vs Con: p = 0.921 water vs Con: p = 0.046* Mean difference (between group p-values) |
| McNamara (2013) (50) | Aerobic and resistance | CRDQ - mastery | land vs Con: 0.8 (95% CI –1.2 to 2.8) water vs Con: 1.9 (95% CI –0.2 to 4.0) |
NA | – | land vs Con: p = 0.414 water vs Con: p = 0.070 Mean difference (between group p-values) |
| Ambrosy (2018) (45) | Aerobic | KCCQ | HF+CKD: 3 months: –1 (95% CI –2 to 0) HF+CKD: 12 months: –3 (95% CI –4 to –1) |
NR | – | 3 months: p = 0.06 12 months: p< 0.01* Mean difference within groups |
| Mentz (2013) (51) | Aerobic | KCCQ | 2.1 (IQR –4.9 to 13.3) | 3.9 (IQR –5.2 to 13.5) | – |
p = 0.52 Median (IQR) change |
| Ambrosy (2018) (45) | Aerobic | EQ-5D | HF+CKD: 3 months: –1 (95% CI –3 to 0) HF+CKD: 12 months: –3 (95% CI –5 to –1) |
NR | – | 3 months: p = 0.09 12 months: p< 0.01* Mean difference within groups |
| Chiang (2020) (55) | Aerobic | SF-36 | PCS 52.8 (SD 6.3) MCS 48.6 (SD 7.7) |
PCS 49.4 (SD 7.4) MCS 51.4 (SD 6.6) |
d = 0.49 d = 0.39 |
p = 0.03 p = 0.0.03 Post-intervention (between groups p-value) |
|
Cardiometabolic
| ||||||
| Abd El-Kader (2013) (44) Hsu (2021) (56) Lo (2021) (57) |
Aerobic Resistance Aerobic |
BMI (kg/m2) BMI (kg/m2) BMI (kg/m2) |
27.15 (SD 2.38) Ex: 30.7 (SD 2.6) 66.5 (SD10.9) |
32.14 (SD 2.16) 29.5 (SD 2.6) MI: 70.3 (SD 19.2) Con: 68.8 (SD 6.6) |
d = 2.20 Ex vs Con: d = 0.46 d = 0.26 |
p< 0.05* Post-intervention (between group p-value) p< 0.001 Post-intervention (between group p-value) Int vs Con: p = 0.03 Int vs MI: p = NR Post-intervention (between group p-value) |
| Byrkjeland (2015) (48) | Aerobic and resistance | HOMA2-IR | 1.10 (IQR 0.80–1.70) | 1.25 (IQR 0.80–1.68) | NA |
p = 0.31 Post-intervention: median (IQR) |
| Byrkjeland (2015) (48) | Aerobic and resistance | HbA1c (%) | 7.2 (IQR 6.6–7.8) | 7.4 (IQR 6.5–8.2) | – |
p = 0.24 Post-intervention: median (IQR) |
| Sridhar (2010) (53) | Aerobic | HbA1c (%) | 7.44 (SD 0.44) | 9.84 (SD 0.53) | d = 4.93 |
p< 0.01* Post-intervention |
| Sridhar (2010) (53) | Aerobic | Systolic BP (mmHg) | 135.53 (SD 3.54) | 146.03 (SD 4.28) | d = 2.67 |
p< 0.05* Post-intervention |
| Sridhar (2010) (53) | Aerobic | Diastolic BP (mmHg) | 82.82 (SD 1.07) | 88.15 (SD 3.68) | d = 1.97 |
p< 0.05* Post-intervention |
| Byrkjeland (2015) (48) | Aerobic and resistance | Insulin (mmol/L) | 49 (IQR 32–78) | 48 (IQR 33–78) | NA |
p = 0.56 Post-intervention: median (IQR) |
| Byrkjeland (2015) (48) | Aerobic and resistance | Glucose (mmol/L) | 8.0 (IQR 6.7–9.3) | 7.8 (IQR 6.7–9.0) | – |
p = 0.63 Post-intervention: median (IQR) |
| Beaudoin (2017) (46) | Aerobic and resistance | CRP (mg/L) | 2.1 (SD 1.37) | 6.57 (SD 7.0) | d = 0.89 | ns Post-intervention |
| Sridhar (2010) (53) | Aerobic | HR variability (bpm) | 15.71 (SD 0.61) | 13.02 (SD 0.54) | d = 4.67 | ns Post-intervention |
| Hsu (2021) (56) | Resistance | Total cholesterol (mg/dL) | Ex: 165.8 (SD 26.9) Ex+Diet: 151.5 (SD 18.0) |
160.1 (SD 20.2) | Ex vs Con: d = 0.24 Ex+diet vs Con: d = 0.45 |
Ex vs Con: p < 0.001 Ex+Diet vs Con: p = NR Post-intervention (between group p-value) |
|
Mental health
| ||||||
| Abdelbassett (2019) (54) | Aerobic | PHQ-9 | LMIE 3.65 (SD 1.2) MICE 3.1 (SD 1.2) |
8.5 (SD 2.1) | LMIE vs Con: d = 2.84 MICE vs Con: d = 3.16 |
p< 0.001 Post-intervention (between groups p-value) |
| McNamara (2013) (50) | Aerobic and resistance | HADS - anxiety | land vs Con: 0 (95% CI –2 to 2) water vs Con: –1 (95% CI –4 to 1) |
NA | – | land vs Con: p = 0.990 water vs Con: p = 0.222 Mean difference (between group p-values) |
| McNamara (2013) (50) | Aerobic and resistance | HADS - depression | land vs Con: 0 (95% CI –2 to 1) water vs Con: –1 (95% CI –3 to 0) |
NA | – | land vs Con: p = 0.544 water vs Con: p = 0.068 Mean difference (between group p-values) |
|
Symptom score
| ||||||
| Bernocchi (2018) (47) | Aerobic and resistance | MRC | –0.17 (95% CI –0.3 to –0.02) | 0.07 (95% CI –0.1 to 0.3) | d = 0.37 |
p = 0.05* Mean change (between group p-value) |
|
Health behaviours
| ||||||
| Banks (2015) (34) | Aerobic | Exercise adherence (%) | HF+DM: 35.2% | NA | p = 0.02* | |
| Beaudoin (2017) (46) | Aerobic and resistance | Physical activity questionnaire (%) | 76.27 (SD 8.47) | 59.08 (SD 17.65) | d = 1.24 | ns Post-intervention |
| Chiang (2020) (55) Lo (2021) (57) |
Aerobic Aerobic |
Total Physical Activity (METS-min/wk) Total Physical Activity (Mets-min/wk) |
2898 (SD 2213) 1162 (SD 624) |
1411 (SD 773) MI: 1919 (SD 804) Con: 1068 (SD 781) |
d = 0.89 Int vs MI: d = 1.05 Int vs Con: d = 0.13 |
p = 0.003 Post-intervention (between group p-value) Int vs Con: p = 0.011 Int vs MI: p = NR Post-intervention (between groups p-value) |
| Beaudoin (2017) (46) | Aerobic and resistance | Steps (no./day) | 8644 (SD 1900) | 8848 (SD 2730) | d = 0.09 | ns Post-intervention |
|
Adverse events
| ||||||
| Byrkjeland (2015) (48) | Aerobic and resistance | No. adverse event (all medical events) | 45 | 31 | – | p = 0.032* |
Mean (SD) unless otherwise stated;
refers to whether study reported statistically significant improvement in this outcome; ns: not stated p-value; NR: no results; NA: not applicable; –: unable to calculate d.
6MWD: 6-min walk distance; HF: heart failure; CKD: chronic kidney disease; 95% CI: 95% confidence interval; Con: control; IQR: interquartile range; VO2 peak: oxygen consumption; mL/kg/min: millilitres/kilogram/min; SD: standard deviation; ESWD: Endurance Shuttle Walk Distance; IWSD: Incremental Shuttle Walk Distance; MLHFQ: Minnesota Living with Heart Failure Questionnaire; QCFQR: Quality Of Life Cystic Fibrosis Questionnaire-revised; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CRDQ: Chronic Respiratory Disease Questionnaire; KCCQ: Kansas City cardiomyopathy questionnaire; BMI: body mass index; HOMA2-IR: Homeostasis Model Assessment2-Insulin Resistance index; HbA1c: haemoglobin A1c; BP: blood pressure; mmHg: millimetres of mercury; CRP: C-reactive protein; HR: heart rate; bpm: beats per min; HADS: Hospital Anxiety and Depression Scale; MRC: Medical Research Council dyspnoea scale; no.: number.
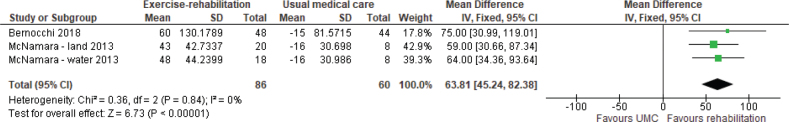
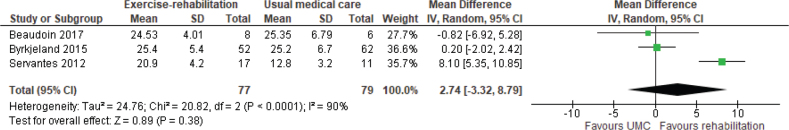
Exercise capacity. The 6MWD was reported in 4 studies (6 reports) (38, 45, 47, 49–51), of which 2 (47, 50) were included in meta-analysis. Meta-analysis showed a weighted mean difference (WMD) of 64 m (95% CI 45–82) in favour of exercise rehabilitation (Fig. 2). A randomized crossover trial of a 12-week aquatic exercise programme showed an increased 6MWD from 395 m (SD 143.9) to 412 m (SD 147.9), p = 0.046 (38). However, no details of the comparison group were provided; the assumption was that the comparison group was UMC. The VO2 peak (34, 45, 46, 48, 49, 51, 52, 55, 57) was reported in 6 studies (9 reports), of which 3 (46, 48, 52) were included in meta-analysis. Meta-analysis showed a WMD of 2.74 mL/kg/min (95% CI –3.32 to 8.79) in favour of exercise rehabilitation (Fig. 3) with significant heterogeneity, I2 = 90%. One study (50) reported on endurance shuttle walk distance (ESWD) and incremental shuttle walk distance (ISWD). Significant improvement in both outcomes was found in the water-based rehabilitation group compared with UMC, with large ESs of 1.21 and 1.27, respectively. There was a moderate ES for ESWD and a small ES for ISWD in favour of land-based rehabilitation; however, these results were not statistically significant.
Fig. 2.
Effect of exercise rehabilitation vs usual medical care (UMC) on 6-min walk distance (6MWD); reported in m. SD: standard deviation; IV: inverse variance; CI: confidence interval; df: degrees of freedom.
Fig. 3.
Effect of exercise rehabilitation vs usual medical care (UMC) on peak oxygen consumption (VO2) peak (mL/kg/min). SD: standard deviation; IV: inverse variance; CI: confidence interval; df: degrees of freedom.
Subgroup analysis
Duration. One study (50) with a rehabilitation duration of 4–8 weeks had an ES for 6MWD of 1.76 (land-based) and 1.86 (water-based), whereas 1 study (47) with a duration of greater than 8 weeks (16 weeks) had an ES of 0.69. All 4 studies (7 reports) reporting VO2 peak (34, 45, 46, 48, 49, 51, 52) had durations of greater than 8 weeks, and thus subgroup analysis for duration was not possible.
Number of coexisting conditions. Insufficient data were available to determine whether effects on exercise capacity varied according to the number of coexisting conditions.
Sensitivity analysis. The effect of adding education, psychological support or motivational interviewing to an exercise programme varied across studies and outcomes. Studies of exercise only had ESs of 1.76–1.86 for 6MWD (50) and 0.03–0.44 for VO2 peak (46, 47, 55). Studies of exercise plus education, psychological support or motivational interviewing has ESs of 0.69 for 6MWD (47) and 0.26–2.17 for VO2 peak (52, 57).
Health-related quality of life. The Minnesota Living with Heart Failure Questionnaire (MLHFQ) was reported in 2 studies (47, 52); both showed large ESs of 0.73 and 1.56, favouring exercise rehabilitation. One study (2 reports) (45, 51) showed a significant improvement in the Kansas City cardiomyopathy questionnaire following exercise rehabilitation (45). One study (46) applied the quality of life (QOL) cystic fibrosis questionnaire-revised, with no significant change in any of the 12 domains between exercise rehabilitation and UMC. One study (47) demonstrated significant improvement in the COPD assessment test in favour of exercise rehabilitation (ES of 1.17). One study (50) applied the chronic respiratory disease questionnaire (CRDQ); the water-based rehabilitation group compared with UMC showed significant difference in change for 3 out of 4 domains (dyspnoea, fatigue and emotion) in favour of exercise rehabilitation; however, it was not possible to calculate ESs. One study (55) applied the Short Form-26, with exercise training improving both the physical and mental composite score, with ESs of 0.49 and 0.39, respectively.
Subgroup analysis
Duration. Insufficient data were available to determine whether duration of rehabilitation impacted on QOL for the MLHFQ.
Number of coexisting conditions. Insufficient data were available to determine whether effects on QOL varied according to the number of coexisting conditions.
Sensitivity analysis. Insufficient data were available to determine whether the components of rehabilitation impacted on QOL for the MLHFQ.
Cardiometabolic. BMI was significantly reduced with exercise rehabilitation (ES ranging from 0.26 to 2.20) (44, 57). Two studies (48, 53) reported improvement in haemoglobin A1c (HbA1c), with 1 (53) study showing a large ES (4.93) in favour of exercise rehabilitation. The other study (48) showed a non-significant reduction in HbA1c following exercise rehabilitation, and ES could not be calculated. One study (53) reported improvement in systolic blood pressure (BP) and diastolic BP, with ESs of 2.67 and 1.97, respectively, favouring exercise rehabilitation. One study (46) reported C-reactive protein (CRP); there was no significant difference in the change in CRP level between groups, despite a large ES of 0.89 favouring exercise rehabilitation. One study (48) reported homeostasis model assessment-insulin resistance-index (HOMA-IR), insulin and glucose, with no significant differences in change between groups for these outcomes (ESs could not be calculated). One study (53) reported heart rate (HR) variability, with a large ES (4.67) favouring exercise rehabilitation; however, this was not statistically significant.
Subgroup analysis
Duration: Insufficient data were available to determine whether duration of rehabilitation impacted on HbA1c.
Number of coexisting conditions. Insufficient data were available to determine whether effects on cardiometabolic outcomes varied according to the number of coexisting conditions.
Sensitivity analysis. Insufficient data were available to determine whether the components of rehabilitation impacted on HbA1c.
Mental health. The Hospital Anxiety and Depression Scale (HADS) was reported in 1 study (50), with no significant differences between groups for either symptom. Exercise training at either a low to moderate intensity or moderate intensity improved depression according to the Patient Health Questionnaire (54).
Symptoms. Dyspnoea, measured by the Medical Research Council dyspnoea scale (MRC), was significantly reduced in 1 study (47) following exercise rehabilitation, with a moderate ES of 0.37.
Health behaviours. One study (46) reported steps and physical activity, with no significant differences found for either outcome. The step count showed a small ES of 0.09, while the physical activity questionnaire had a large ES of 1.24, favouring exercise rehabilitation. Two studies (55, 57) reported improvements in total physical activity with exercise training compared with control, with ESs of 0.13–0.89.
Adverse events. Only 1 of the 9 included studies reported adverse events (48); however, the study was limited by a lack of detail reporting the types and relative severity of these events. Adverse events were defined as all medical events (including cardiovascular events (worsening stable angina/heart failure, unstable angina, acute myocardial infarction, stroke, cardiac arrest), hypoglycaemia and musculoskeletal events (skin ulcers, lower back pain, tendinitis, joint pain and fractures)). The incidence of serious events (primarily cardiovascular events (type not specified)) was equally distributed between the rehabilitation and control groups (11 vs 12), and no cardiovascular events occurred in close relation to the exercise sessions or CPET (48). The rehabilitation group did have a higher reported incidence of all medical events (45 vs 31, p = 0.03), which appeared to be musculoskeletal in nature (21 vs 11, p = 0.077), although the type and severity were not reported.
Exercise rehabilitation vs other intervention
Seven studies (9 reports) reported exercise rehabilitation vs other interventions (36, 37, 42, 56–61), including diet and sham exercise (59, 60), phone call only (36, 37), diet (56, 58), usual physical activity (61), motivational interviewing (57) and mind-body education (42) interventions. The findings for studies are outlined in Table III.
Table III.
Outcomes of exercise-rehabilitation vs other intervention
| Study | Intervention | Outcome | Results (intervention) | Results (control) | Effect size (between groups) | Notes |
|---|---|---|---|---|---|---|
| Exercise capacity | ||||||
| Freitas (2017) (59) | Int = aerobic and resistance exercise | VO2 peak (mL/kg/min) | 18.8 (IQR 16.8–21) | 15 (IQR 13–17.5) | – |
p< 0.001* Post-intervention: median (IQR) |
| Com = sham exercise (breathing and stretches) | ||||||
| Health-related quality of life | ||||||
| Freitas (2017) (59) | Int = aerobic and resistance exercise | AQQ | 4.7 (IQR 4.1–6.3) | 3.8 (IQR 3.1–4.9) | – |
p = 0.038* Post-intervention: median (IQR) |
| Com = sham exercise (breathing and stretches) | ||||||
| Cardiometabolic | ||||||
| Al-Jiffri (2013) (58) | Int = aerobic exercise | BMI (kg/m2) | 27.25 (SD 2.68) | 32.64 (SD 4.26) | d = 1.51 |
p = 0.0088* Post-intervention (between group p-value) |
| Com = diet only (no exercise) | ||||||
| Freitas (2018) (60) | Int = aerobic and resistance exercise | BMI (kg/m2) | –2.6 (SD 1.3) | –1.0 (SD 1.1) | d = 1.33 | ns Mean change |
| Com = sham exercise (breathing and stretches) | ||||||
| Freitas (2017) (58) | Int = aerobic and resistance exercise | BMI (kg/m2) | –2.7 (IQR –3.3 to –1.8) | –1.1 (IQR –1.8 to –0.4) | – | ns Post-intervention: median (IQR) |
| Com = sham exercise (breathing and stretches) | ||||||
| Hsu (2021) (56) | Int = resistance exercise Com: diet advice |
BMI (kg/m2) | 30.7 (SD 2.6) | 29.5 (SD 2.6) | d = 0.46 |
p< 0.001 Post-intervention (between group p-value |
| Lo (2021) (57) | Int = aerobic exercise Com = motivational interviewing |
BMI (kg/m2) | 25.9 (SD 4.8) | 24.2 (SD7.9) | d = 0.24 | p = NR |
| Al-Jiffri (2013) (58) | Int = aerobic exercise | HOMA-IR | 2.64 (SD 1.37) | 5.13 (SD 2.44) | d = 1.26 |
p = 0.0091* Post-intervention (between group p-value) |
| Com = diet only (no exercise) | ||||||
| Halvari (2017) (61) | Int = aerobic and resistance exercise | HbA1c (%) | 7.57 (SD 1.41) | 7.56 (SD 1.23) | d = 0.01 | ns Post-intervention |
| Com = usual physical activity | ||||||
| Collins (2011) (36) | Int = aerobic exercise | Total cholesterol (mmol/L) | –9.77 (SE 6.85) | NR | – | ns Change in intervention minus change in control |
| Com = phone call only (no exercise) | ||||||
| Collins (2011) (36) | Int = aerobic exercise | LDL (mmol/L) | –1.67 (SE 5.83) | NR | – | ns Change in intervention minus change in control |
| Com = phone call only (no exercise) | ||||||
| Collins (2011) (36) | Int = aerobic exercise | HDL (mmol/L) | –3.61 (SE 2.09) | NR | – | ns Change in intervention minus change in control |
| Com = phone call only (no exercise) | ||||||
| Collins (2011) (36) | Int = aerobic exercise | Triglycerides (mmol/L) | 10.9 (SE 34.53) | NR | – | ns Change in intervention minus change in control |
| Com = phone call only (no exercise) | ||||||
| Collins (2011) (36) | Int = aerobic exercise | CRP (mg/L) | 0.19 (SE 1.41) | NR | – |
p> 0.2 Change in intervention minus change in control |
| Com = phone call only (no exercise) | ||||||
| Mental health | ||||||
| Collins (2010) (36) | Int = aerobic exercise | Depressive symptoms | 3.2 (SD 1.5) | –2.4 (SD 1.5) | d = 3.73 | ns Mean change |
| Com = phone call only (no exercise) | ||||||
| Freitas (2018) (60) | Int = aerobic and resistance exercise | HADS | Anxiety: –4.0 (SD 4.6) Depression: –4.6 (SD 4.2) |
Anxiety: –1.0 (SD 3.7) Depression: –0.4 (SD 3.3) |
Anxiety: d = 0.72 Depression: d = 1.11 |
Anxiety: p = 0.63 Depression: p< 0.01* Mean change |
| Com = sham exercise (breathing and stretches) | ||||||
| Symptom score | ||||||
| Freitas (2017) (59) | Int = aerobic and resistance exercise | ACQ | 1.1 (IQR 0.4–1.6) | 1.7 (IQR 1.4–2.0) | – |
p = 0.003* Median (IQR) |
| Com = sham exercise (breathing and stretches) | ||||||
Mean (SD) unless otherwise stated;
refers to whether study reported statistically significant improvement in this outcome; ns: not stated p-value; NR: no results; –: unable to calculate d.
Int: intervention; Com: comparison; VO2 peak: oxygen consumption; IQR: interquartile range; AQQ: Asthma Quality of Life Questionnaire; BMI: body mass index; SD: standard deviation; HOMA-IR: Homeostasis Model Assessment-Insulin Resistance index; HbA1c: haemoglobin A1c; SE: standard error; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; HADS: Hospital Anxiety and Depression Scale; ACQ: Asthma Control Questionnaire.
Exercise capacity. One study reported VO2 peak (59) and demonstrated significant improvement post-intervention for aerobic and resistance exercise with diet compared with sham-exercise (breathing and stretches) with diet.
Health-related quality of life. The asthma QOL questionnaire was reported in 1 study (59), demonstrating better HRQoL post-intervention for aerobic and resistance exercise with diet compared with sham-exercise (breathing and stretches) with diet.
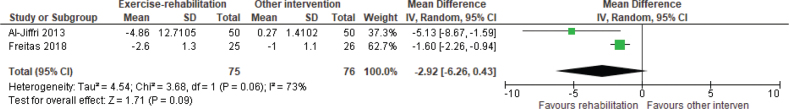
Cardiometabolic. BMI was reported in 4 studies (5 reports) (56–60). Meta-analysis showed a WMD of –2.92 kg/m2 (95% CI –6.26 to 0.43) in favour of exercise rehabilitation and diet compared with sham exercise (breathing and stretches) and diet or diet only, but heterogeneity was high (Fig. 4). Diet alone resulted in a greater reduction in BMI and total cholesterol levels compared with resistance training (56). There was no difference in the effects of motivational interviewing and aerobic training (57). One study (61) reported HbA1c with no significant differences and a small ES of 0.01, comparing aerobic and resistance exercise rehabilitation with usual physical activity. One study (37) found no significant differences in CRP between aerobic exercise rehabilitation with a weekly phone call or a weekly phone call only. One study (58) reported homeostasis model assessment 2-insulin resistance-index (HOMA2-IR) and showed a large ES (1.26) in favour of exercise rehabilitation with low calorie diet, compared with low calorie diet alone. Total cholesterol, triglycerides, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were reported in 1 study (37) and there were no significant differences found for any of these outcomes, comparing aerobic and resistance exercise rehabilitation with usual physical activity.
Fig. 4.
Effect of exercise rehabilitation vs other intervention on body mass index (BMI); reported in kg/m2. SD: standard deviation; IV: inverse variance; CI: confidence interval; df: degrees of freedom.
Subgroup analysis
Duration: Insufficient data were available to determine whether duration of rehabilitation impacted on BMI.
Number of coexisting conditions: Insufficient data were available to determine whether effects on cardiometabolic outcomes varied according to the number of coexisting conditions.
Sensitivity analysis. A study of exercise and diet had an ES of 1.51 for BMI, favouring exercise rehabilitation (58). A study of exercise and diet plus education or psychological support had an ES of 1.33 for BMI, favouring exercise rehabilitation and diet (60) compared with sham exercise (breathing and stretches) and diet.
Mental health. One study (60) reported significant reduction in depression measured on HADS, in favour of aerobic and resistance exercise rehabilitation with diet compared with sham-exercise (breathing and stretches) with diet. One study (36) reported depressive symptoms; however, the tool used was not stated and no significant difference was demonstrated, despite a large ES of 3.73 favouring exercise rehabilitation and weekly phone call compared with weekly phone call only. The structured interview for the Hamilton depression rating scale was reported in 1 study (42). No data were provided, but it stated there were statistically significant reductions in scores that demonstrated improvement for both the intervention (tai chi) and control (mind-body education) groups at the end of the intervention (42).
Symptoms. The asthma control questionnaire was reported in 1 study (59). There was improved asthma control in those who undertook aerobic and resistance exercise rehabilitation with diet compared with sham-exercise (breathing and stretches) with diet.
Exercise rehabilitation in cohort/quasi-experimental studies
Twenty studies (21 reports) reported the effects of exercise rehabilitation using cohort or quasi-experimental designs (35, 39–41, 43, 58–73) (Table IV).
Table IV.
Outcomes of exercise-rehabilitation in cohort/quasi-experimental studies
| Study | Intervention (exercise type) | Outcome | Subgroup | Results | Notes |
|---|---|---|---|---|---|
|
Exercise capacity
| |||||
| Castro+ (2015) (35) | Aerobic and resistance | 6MWD (m) | n/a | ↑50 | p< 0.01* |
| Crisafulli+ (2010) (63) | Peripheral limb training | 6MWD (m) | 1 comorbidity ≥ 2 comorbidities |
45% 44% |
ns no. of participants who achieved MCID |
| Hassan+ (2016) (65) | Aerobic and resistance | 6MWD (m) | 1 comorbidity > 1 comorbidity |
↑173 ↑149 |
ns |
| Listerman+ (2011) (67) | Aerobic and resistance | 6MWD (m) | 1–2 comorbidities > 2 comorbidities |
↑77¥ ↑61¥ |
ns Participants < 56 years |
| Listerman+ (2011) (67) | Aerobic and resistance | 6MWD (m) | 1–2 comorbidities > 2 comorbidities |
↑71¥ ↑74¥ |
ns Participants 56–65 years |
| Listerman+ (2011) (67) | Aerobic and resistance | 6MWD (m) | 1–2 comorbidities > 2 comorbidities |
↑56¥ ↑61¥ |
ns* Participants > 65 years |
| Mesquita+ (2015) (68) | Details not provided | 6MWD (m) | All participants | ↑30 | ns* |
| Naz+ (2019) (69) | Aerobic and resistance | 6MWD (m) | CCI 1 CCI 2 CCI ≥ 3 |
↑40 ↑40 ↑50 |
ns |
| Tunsupona (2017)+ (73) | Aerobic and resistance | 6MWD (m) | 1 comorbidity ≥ 2 comorbidities |
↑44 ↑28 |
ns |
| Tunsuponb (2017)+ (74) | Aerobic and resistance | 6MWD (m) | Obese Morbidly obese |
↑44 ↑42 |
ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | 6MWD (m) | n/a | ↑2 | ns |
| Barnes+ (2009) (62) | Aerobic and resistance | VO2 peak (mL/kg/min) | n/a | ↑3.8 | p = 0.003* |
| de Groot# (2012) (64) | Aerobic and resistance | VO2 peak (mL/kg/min) | n/a | ↑1.1 | p< 0.01* |
| Khadanga+ (2016) (66) | Aerobic and resistance | VO2 peak (mL/kg/min) | Diabetes Insulin resistance |
Insufficient data Insufficient data |
|
| Nonoyama+ (2016) (70) | Aerobic and resistance | VO2 peak (mL/kg/min) | Respiratory comorbidity Non-respiratory comorbidity |
↑1.2 ↑2.6 |
ns* |
| Takaya+ (2014) (72) | Aerobic | VO2 peak (mL/kg/min) | AMI+CKD | ↑2.3 | ns |
| Verges+ (2004) (75) | Aerobic | VO2 peak (mL/kg/min) | ACE+DM | ↑2.4 | ns* |
| Wang+ (2013) (76) | Aerobic | VO2 peak (mL/kg/min) | HF+A | ↑3.6 | ns* |
| Hassan+ (2016) (65) | Aerobic and resistance | VO2 max (mL/kg/min) | 1 comorbidity > 1 comorbidity |
↑22.72 ↑24.25 |
ns |
| Khadanga+ (2016) (66) | Aerobic and resistance | Peak METS | Diabetes Insulin resistance |
Insufficient data Insufficient data |
|
| Martin+ (2016) (40) | Details not provided | METs | IHD+PAD | ↑0.76 | ns |
| Woodard+ (1994) (77) | Aerobic | METs | CVD+arthritis | ↑0.92 | p = 0.005* |
| Zwerink+ (2010) (43) | Aerobic and resistance | ISWD (m) | n/a | ↑18 | ns |
| Mesquita+ (2015) (68) | Details not provided | CWRT (seconds) | All participants | ↑202 | ns* |
| Soleimani+ (2009) (71) | Aerobic | Resting HR (bpm) | CAD+DM | ↓6 |
p = 0.852 Men |
| Soleimani+ (2009) (71) | Aerobic | Resting HR (bpm) | CAD+DM | ↓14 |
p = 0.699 Women < 50 years |
| Soleimani+ (2009) (71) | Aerobic | Resting HR (bpm) | CAD+DM | ↓10 |
p = 0.753 Women ≥ 50 years |
| Soleimani+ (2009) (71) | Aerobic | Peak HR (bpm) | CAD+DM | ↑24 |
p = 0.019* Men |
| Soleimani+ (2009) (71) | Aerobic | Peak HR (bpm) | CAD+DM | 0 |
p = 0.012* Women < 50 years |
| Soleimani+ (2009) (71) | Aerobic | Peak HR (bpm) | CAD+DM | ↑5 |
p = 0.529 Women ≥ 50 years |
| Soleimani+ (2009) (71) | Aerobic | Post-exercise HR (bpm) | CAD+DM | ↑5 |
p = 0.471 Men |
| Soleimani+ (2009) (71) | Aerobic | Post-exercise HR (bpm) | CAD+DM | ↑1 |
p = 0.606 Women < 50 years |
| Soleimani+ (2009) (71) | Aerobic | Post-exercise HR (bpm) | CAD+DM | ↓7 |
p = 0.902 Women ≥ 50 years |
| Soleimani+ (2009) (71) | Aerobic | HR recovery (bpm) | CAD+DM | ↑18 |
p = 0.029* Men |
| Soleimani+ (2009) (71) | Aerobic | HR recovery (bpm) | CAD+DM | ↑9 |
p = 0.019* Women < 50 years |
| Soleimani+ (2009) (71) | Aerobic | HR recovery (bpm) | CAD+DM | ↑14 |
p = 0.913 Women ≥ 50 years |
| Takaya+ (2014) (72) | Aerobic | HR recovery (bpm) | AMI+CKD | ↑3 | ns |
| Tunsupona (2017)+ (73) | Aerobic and resistance | MIET (W) | 1 comorbidity ≥ 2 comorbidities |
↑9.49 ↑15.01 |
ns* |
| Tunsuponb (2017)+ (74) | Aerobic and resistance | MIET (W) | Obese Morbidly obese |
↑10.47 ↑15.13 |
ns |
| Tunsupona (2017)+ (73) | Aerobic and resistance | CWET (seconds) | 1 comorbidity ≥ 2 comorbidities |
↑803.39 ↑870.59 |
ns |
| Tunsuponb (2017)+ (74) | Aerobic and resistance | CWET (seconds) | Obese Morbidly obese |
↑12.18 ↑14.31 |
ns |
|
Health-related quality of life
| |||||
| Barnes+ (2009) (62) | Aerobic and resistance | SF-36 | n/a | ↑18.2 | p = 0.03* |
| de Groot# (2012) (64) | Aerobic and resistance | SF-36 | n/a | ↑7.5 | p< 0.01* |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – physical function | CCI 1 CCI 2 CCI ≥ 3 |
↑10 ↑15 ↑10 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – social function | CCI 1 CCI 2 CCI ≥ 3 |
↑13 ↑6 ↑13 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – physical role | CCI 1 CCI 2 CCI ≥ 3 |
0 ↑25 0 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – emotional role | CCI 1 CCI 2 CCI ≥ 3 |
0 0 0 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – general | CCI 1 CCI 2 CCI ≥ 3 |
↑6 ↑13 ↑5 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – mental | CCI 1 CCI 2 CCI ≥ 3 |
↑4 ↑8 ↑4 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – pain | CCI 1 CCI 2 CCI ≥ 3 |
↑10 ↑19 0 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | SF-36 – vitality | CCI 1 CCI 2 CCI ≥ 3 |
↑10 ↑15 ↑10 |
ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | MLHFQ | n/a | ↑3.3 | ns |
| Crisafulli+ (2010) (63) | Peripheral limb training | SGRQ | 1 comorbidity ≥ 2 comorbidities |
66% 71% |
ns no. of participants who achieved MCID |
| Hassan+ (2016) (65) | Aerobic and resistance | SGRQ | 1 comorbidity > 1 comorbidity |
↓23.26 ↓25.75 |
ns |
| Mesquita+ (2015) (68) | Details not provided | SGRQ | All participants | ↓4.0 | ns |
| Naz+ (2019) (69) | Aerobic and resistance | SGRQ | CCI 1 CCI 2 CCI ≥ 3 |
↓8 ↓11 ↓5 |
ns |
| Barnes+ (2009) (62) | Aerobic and resistance | FOSQ | n/a | ↑0.5 | p = 0.07 |
| de Groot# (2012) (64) | Aerobic and resistance | Diabetes quality of life measure | n/a | ↑8.2 | p< 0.01* |
| Tunsupona (2017)+ (73) | Aerobic and resistance | CRQ – score | 1 comorbidity ≥ 2 comorbidities |
↑7.0 ↑14.31 |
ns |
| Tunsuponb (2017)+ (74) | Aerobic and resistance | CRQ – score | Obese Morbidly obese |
↑12.73 ↑8.13 |
ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | CRQ – dyspnoea | n/a | ↓1.1 | ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | CRQ – fatigue | n/a | ↑0.5 | ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | CRQ – emotional | n/a | ↑0.1 | ns |
| Zwerink+ (2010) (43) | Aerobic and resistance | CRQ - mastery | n/a | ↑0.4 | ns |
|
Cardiometabolic
| |||||
| Barnes+ (2009) (62) | Aerobic and resistance | BMI (kg/m2) | n/a | ↓6.0 | p< 0.001* |
| de Groot# (2012) (64) | Aerobic and resistance | BMI (kg/m2) | n/a | ↑0.6 | ns |
| Khadanga+ (2016) (66) | Aerobic and resistance | BMI (kg/m2) | Diabetes Insulin resistance |
Insufficient data Insufficient data |
|
| Listerman+ (2011) (67) | Aerobic and resistance | BMI (kg/m2) | 1–2 comorbidities > 2 comorbidities |
↓0.4 ↓0.1 |
ns Participants< 56 years |
| Listerman+ (2011) (67) | Aerobic and resistance | BMI (kg/m2) | 1–2 comorbidities > 2 comorbidities |
↓0.7 ↓0.3 |
p< 0.001* (1–2 comorbidities) Participants 56–65 years |
| Listerman+ (2011) (67) | Aerobic and resistance | BMI (kg/m2) | 1–2 comorbidities > 2 comorbidities |
↓0.5 ↓0.3 |
p< 0.001* (1–2 comorbidities) Participants> 65 years |
| Nonoyama+ (2016) (70) | Aerobic and resistance | BMI (kg/m2) | Respiratory comorbidity Non-respiratory comorbidity |
↑0.3 ↑0.2 |
ns |
| Takaya+ (2014) (72) | Aerobic | BMI (kg/m2) | AMI+CKD | ↓0.2 | ns |
| de Groot# (2012) (64) | Aerobic and resistance | HbA1c (%) | n/a | ↓0.4 | p< 0.05* |
| Barnes+ (2009) (62) | Aerobic and resistance | Total cholesterol (mmol/L) | n/a | ↓0.9 | p = 0.006* |
| de Groot# (2012) (64) | Aerobic and resistance | Total cholesterol (mg/dL) | n/a | ↓7.2 | ns |
| Barnes+ (2009) (62) | Aerobic and resistance | LDL (mmol/L) | n/a | ↓0.6 | p = 0.04* |
| de Groot# (2012) (64) | Aerobic and resistance | LDL (mg/dL) | n/a | ↓11.1 | p< 0.01* |
| de Groot# (2012) (64) | Aerobic and resistance | HDL (mg/dL) | n/a | ↑0.7 | ns |
| Barnes+ (2009) (62) | Aerobic and resistance | Triglycerides (mmol/L) | n/a | ↓0.5 | p = 0.003* |
| de Groot# (2012) (64) | Aerobic and resistance | Triglycerides (mg/dL) | n/a | ↑7.1 | ns |
| Barnes+ (2009) (62) | Aerobic and resistance | Systolic BP (mmHg) | n/a | ↓5.3 | p = 0.02* |
| Barnes+ (2009) (62) | Aerobic and resistance | Diastolic BP (mmHg) | n/a | ↓4.0 | p = 0.09 |
| Barnes+ (2009) (62) | Aerobic and resistance | Insulin (mIU/L) | n/a | ↓4.8 | p< 0.001* |
| Barnes+ (2009) (62) | Aerobic and resistance | Glucose (mg/L) | n/a | ↓0.3 | p = 0.37 |
| Barnes+ (2009) (62) | Aerobic and resistance | CRP (mg/L) | n/a | ↓2.9 | p = 0.01* |
|
Mental health
| |||||
| Barnes+ (2009) (56) | Aerobic and resistance | BDI | n/a | ↓7.9 | p< 0.001* |
| de Groot# (2012) (64) | Aerobic and resistance | BDI | n/a | ↓10.7 | p< 0.01* |
| Barnes+ (2009) (62) | Aerobic and resistance | Profile of mood states | n/a | ↑15.6 | p = 0.11 |
| Naz+ (2019) (69) | Aerobic and resistance | HADS - anxiety | CCI 1 CCI 2 CCI ≥ 3 |
↓2 ↓2 ↓2 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | HADS - depression | CCI 1 CCI 2 CCI ≥ 3 |
↓1 ↓2 0 |
ns |
|
Symptom score
| |||||
| Barnes+ (2009) (62) | Aerobic and resistance | SAAQ | n/a | ↓20.8 | p = 0.08 |
| Crisafulli+ (2010) (63) | Peripheral limb training | MRC | 1 comorbidity ≥ 2 comorbidities |
84% 70% |
ns no. of participants who achieved MCID |
| Hassan+ (2016) (65) | Aerobic and resistance | mMRC | 1 comorbidity > 1 comorbidity |
↓1.35 ↓1.55 |
ns |
| Naz+ (2019) (69) | Aerobic and resistance | mMRC | CCI 1 CCI 2 CCI ≥ 3 |
↓1 ↓1 ↓1 |
ns |
|
Health behaviours
| |||||
| de Groot# (2012) (64) | Aerobic and resistance | Chronic illness survey | n/a | ↑6.3 | p< 0.01* |
| de Groot# (2012) (64) | Aerobic and resistance | Steps (no./week) | n/a | ↓179 | ns |
| de Groot# (2012) (64) | Aerobic and resistance | Exercise (min/week) | n/a | ↑41.1 | Ns |
Mean (SD) unless otherwise stated;
cohort study;
quasi-experimental study;
refers to whether study reported statistically significant improvement in this outcome; ns: not stated p-value;
distance converted from feet to meters; NA: not applicable; –: unable to calculate d.
6MWD: 6-min walk distance; SD: standard deviation; no.: number; MCID: minimally clinical important difference; CCI: Charlson Comorbidity Index; CI: confidence interval; IQR: interquartile range; VO2: oxygen consumption; AMI: acute myocardial infarction; CKD: chronic kidney disease; ACE: acute coronary event; DM: diabetes mellitus; HF: heart failure; A: anaemia; SEM: standard error of the mean; METS: metabolic equivalents; IHD: ischaemic heart disease; PAD: peripheral arterial disease; CVD: cardiovascular disease; SE: standard error; IWSD: incremental shuttle walk distance; CWRT: constant work rate time; HR: heart rate; bpm: beats per min; CAD: coronary artery disease; MIET: maximal symptom limited incremental cycle ergometer time; W: Watts; CWET: constant workload endurance time; SF-36: Short Form-36; MLHFQ: Minnesota Living with Heart Failure Questionnaire; SGRQ: St George’s Respiratory Questionnaire; FOSQ: Functional Outcomes of Sleep Questionnaire; CRQ: Chronic Respiratory Questionnaire; BMI: body mass index; HbA1c: haemoglobin A1c; LDL: low-density lipoprotein; HDL: high-density lipoprotein; BP: blood pressure; mmHg: millimeters of mercury; mIU/L: milli-international units/litre; CRP: C-reactive protein; BDI: Beck Depression Index; HADS: Hospital Anxiety and Depression Scale; SAAQ: Sleep Apnoa Symptom Questionnaire; MRC: Medical Research Council dyspnea scale; mMRC: modified Medical Research Council dyspnea scale.
Exercise capacity. Nineteen studies (20 reports) (35, 40, 41, 43, 62–77) reported measures of exercise capacity with clinically significant improvements following exercise rehabilitation in 6MWD (35, 67, 68), VO2 peak (62, 64, 66, 70, 75, 76), metabolic equivalents (41, 66, 77), HR recovery (71), maximal symptom limited incremental cycle ergometer time (73) and peak HR (71).
Health-related quality of life. Eight studies (9 reports) (43, 62–65, 68, 69, 73, 74) reported measures of HRQoL with clinically significant improvements following exercise rehabilitation in Short Form-36 (62,64) and diabetes diabetes quality of life (QOL) measure questionnaire (64).
Cardiometabolic. Eight studies (39, 41, 62, 64, 66, 67, 70, 72) reported cardiometabolic measures with clinically significant improvements following exercise rehabilitation in BMI (62, 66, 67), HbA1c (39, 64), systolic BP (62), diastolic BP (41), CRP (62), insulin (62), glucose (41), total cholesterol (41,62), triglycerides (39, 41, 62), HDL (41, 62, 64) and LDL (41).
Mental health. Four studies (41, 62, 64, 69) reported measures of mental health with clinically significant improvements following exercise rehabilitation in the Beck Depression Index (41, 62, 64).
Symptoms. Three studies (62, 65, 69) reported symptom measures with no clinically significant improvements following exercise rehabilitation.
Health behaviours. One study (64) reported health behaviour measures with clinically significant improvements following exercise rehabilitation in the chronic illness survey.
Adverse events. One study (35) reported an adverse event outcome, defined as an accident or complication, but reported that this did not occur during the intervention.
DISCUSSION
This is the first systematic review of studies of exercise rehabilitation in people with multimorbidity. Compared with UMC, improvement in exercise capacity (peak exercise and selected measures of functional exercise tolerance), HRQoL and a mix of cardiometabolic outcomes were evident for exercise rehabilitation. These findings were consistent with outcomes from single-disease rehabilitation programmes, which included individuals with multimorbidities (78, 79), with noted improvements in BP and other cardiometabolic parameters. This suggests that either disease-specific or multimorbidity exercise rehabilitation programmes may be suitable for people with multimorbidities, in order to target improvements in these outcomes. A range of ES were identified for the included studies (range 0.03–4.93) which may be attributable to the type of programme applied (e.g. water-based vs land-based) (50) or difference in exercise prescription across studies. With the small number of included studies, it is not possible to establish the contribution of rehabilitation duration, adjuncts to exercise or the role of the number of coexisting conditions for exercise rehabilitation compared with UMC.
The majority of studies did not report on adverse events during rehabilitation. While a single study reported a greater number of medical events in the rehabilitation group (48), there was no difference in serious cardiovascular events, none occurred in proximity to rehabilitation classes or testing, and most were classified as musculoskeletal, with no reporting on the frequency of severe events (i.e. fractures). It is plausible that people participating in an exercise programme may encounter musculoskeletal events (80, 81), and the likelihood of this may be increased in the multimorbidity population with a history of sedentary behaviour. The absence of cardiovascular events in those with multimorbidity during exercise rehabilitation is reassuring, particularly as this population is likely to have several cardiovascular risk factors. Recently, the concerns regarding providing a safe exercise programme for the multimorbidity population have been illustrated (82, 83). We recommend that future studies report on adverse events, and specify the type, severity and timing of these events and their temporal relationship to the intervention.
Compared with other interventions (ranging from dietary advice, usual physical activity, distant support and education), improvement in exercise capacity, HRQoL, selected cardiometabolic parameters of BMI and HOMA2-IR, depression and asthma symptom were evident for exercise rehabilitation; however, the number of studies was very small. With the known benefits of exercise on exercise capacity, cardiometabolic parameters and depression (84), these findings support what has been previously demonstrated when comparing exercise rehabilitation with other interventions (85–87). The lack of difference between groups for HbA1c (61) may be attributed to the inclusion of exercise as part of usual physical activity (< 150 min per week) in the comparative group. While there were no statistically significant differences found for CRP, cholesterol and triglyceride measures, ESs could not be calculated (37); therefore the results may have some clinical significance, with the magnitude unable to be determined.
A mix of approaches of varying intensities for exercise rehabilitation were tested in the included studies, with findings demonstrating that multiple approaches can achieve improvements in those with multimorbidity. Some studies used existing single-disease exercise rehabilitation programmes, such as cardiac and pulmonary rehabilitation, which implies that rehabilitation programmes including aerobic exercise with or without resistance training appear to be beneficial in this population. Other studies have established new exercise rehabilitation programmes. This heterogeneity may impact the conclusions for the outcomes reviewed. Further research into the feasibility of multimorbidity rehabilitation programmes, the varying models of exercises, their prescription with regards to frequency and intensity and the outcomes achieved in those with multimorbidity will enhance the ability to make guidelines and recommendations for best-practice models of care for this cohort. It was not possible to determine whether there is an optimal length of rehabilitation programmes for multimorbidity, or whether effects differed according to the number of chronic conditions. The addition of education or psychological support appeared to have minimal impact, suggesting that the benefits achieved may be attributed to effects of exercise, although few studies included a comprehensive assessment of psychological outcomes. The lack of impact on anxiety, depression or dyspnoea may be attributable to the baseline levels of these psychological symptoms or severity of breathlessness in the participants; if baseline levels demonstrate minimal impact on an individual, there is limited room for improvement.
Few data were available to understand the impact of exercise rehabilitation on outcomes such as mental health, ADL, health behaviours such as physical activity or medication adherence, or healthcare costs. There is also limited information regarding the impact on resistance training on specific measures of strength in this population, despite 20 of the 38 included studies incorporating resistance training as part of the intervention. This lack of defined strength assessment as an outcome measure is also reflected in a recent Delphi study identifying the core outcome set for multimorbidity research (88). While physical activity and function is included (and perhaps represented in measures of exercise capacity in the studies included in this review), strength measurement is absent. This is despite recent reviews highlighting that exercise prescription for people with various diseases should include resistance training (12, 89). Collectively, each of these outcomes are likely to be of critical importance for people with multimorbidity and should be addressed in future trials. People with multimorbidity define good health and wellbeing as enjoyment of life, maintenance of independence, having social relationships and participating in society (90), which reinforces the importance of optimizing these outcomes. It has been suggested that optimal care for people with multimorbidity should focus on maximizing the health goals of individual patients, rather than on improving disease-focused outcomes (15). Whilst exercise rehabilitation directly addresses goals related to physical function and wellbeing, it should be acknowledged that goals related to psychological, social and participatory outcomes may require a more complex intervention, of which exercise may be only 1 component.
This systematic review had a number of limitations. Because this is a relatively new field, we chose to include studies with a broad range of designs, including non-randomized trials, to ensure that studies with relevant data were not excluded. As a result, risk of bias also varied widely across the studies, and interpretation of data from non-randomized trials was difficult. Subgroup analysis was not performed according to the number of coexisting conditions, as data were not reported in sufficient detail for this to occur. Although the current study did not limit the age of participants in the inclusion criteria, only 1 study was conducted in a paediatric population; the current findings are largely confined to studies of adults with multimorbidities. The exclusion of studies aimed at rehabilitation of a single joint (e.g. hip) may have led to exclusion of some that included interventions aimed at improving exercise capacity. Reporting of dosage, frequency and intensity of exercise were often very limited, which made it difficult to account for some of the changes, or lack of, in the outcome measures of interest. These factors can affect the magnitude of change for outcomes such as exercise capacity. The use of English language only may have had an effect by not including studies and data published in other languages. There is also a risk of publication bias through the impact of negative studies potentially being less likely to be published. The lack of ability to blind participants and therapists in rehabilitation trials, due to the nature of the intervention, may affect the outcomes achieved. For the RCTs, assessor blinding was unclear for 64% of the studies. This also could have a significant effect on outcomes such as bias towards positive results, particularly if assessors were not blinded. Sample sizes ranged from 6 to 2,331. Therefore, the results of some studies may be more powerful than others and the results from smaller sized studies should be considered with discretion.
In conclusion, in people with multimorbidity, improvement in exercise capacity, HRQoL and cardiometabolic outcomes were evident with exercise rehabilitation. Outcomes were similar to those seen following exercise rehabilitation in people with single diseases, regardless of the intervention type. Therefore, exercise rehabilitation can be effectively delivered to people with multimorbidity, both within current single-disease rehabilitation programmes or in specialized multimorbidity exercise rehabilitation programmes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Kathryn Ritchie, from the Western Health Library services, for her assistance with the electronic database searches. The authors would like to acknowledge the Community Based Rehabilitation service at Western Health for their support of the review.
Footnotes
The authors have conflicts of interest to declare.
REFERENCES
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. DOI: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: CD006560. DOI: 10.1002/14651858.CD006560.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–138. DOI: 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GAM. Causes and consequences of comorbidity: a review. J Clin Epidemiol 2001; 54: 661–674. DOI: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 5.Caughey GE, Ramsay EN, Vitry AI, Gilbert AL, Luszcz MA, Ryan P, et al. Comorbid chronic diseases, discordant impact on mortality in older people: a 14-year longitudinal population study. J Epidemiol Comm Health 2010; 64: 1036–1342. DOI: 10.1136/jech.2009.088260 [DOI] [PubMed] [Google Scholar]
- 6.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Family Med 2003; 1: 15–21. DOI: 10.1370/afm.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortin M, Hudon C, Dubois M-F, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health Qual Life Outcomes 2005; 3: 1–7. DOI: 10.1186/1477-7525-3-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–439. DOI: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 9.Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012; 380: 7–9. DOI: 10.1016/S0140-6736(12)60482-6 [DOI] [PubMed] [Google Scholar]
- 10.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. DOI: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Rehabilitation in health systems. Geneva: World Health Organization; 2017. (accessed 5 May 2020) Available from: https://apps.who.int/iris/handle/10665/254506 [Google Scholar]
- 12.Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, et al. Exercise as a prescription for patients with various diseases. J Sports Health Sci 2019; 8: 422–441. DOI 10.1016/j.jshs.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhalwani NN, O’Donovan G, Zaccardi F, Hamer M, Yates T, Davies M, et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act 2016; 13: 8. DOI: 10.1186/s12966-016-0330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britt HC, Harrison CM, Miller GC, Knox SA. Prevalence and patterns of multimorbidity in Australia. Med J Aust 2008; 189: 72–77. DOI: 10.5694/j.1326-5377.2008.tb01919.x [DOI] [PubMed] [Google Scholar]
- 15.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition-multimorbidity. JAMA 2012; 307: 2493–2494. DOI: 10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacasse Y, Goldstein RS, Lasserson T, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease (Review). Cochrane Database Syst Rev 2006: 4: CD003793. DOI: 10.1002/14651858.CD003793.pub2 [DOI] [PubMed] [Google Scholar]
- 17.Oldridge N. Exercise-based cardiac rehabilitation in patients with coronary heart disease: meta-analysis outcomes revisited. Future Cardiol 2012; 8: 729–751. DOI: 10.2217/fca.12.34 [DOI] [PubMed] [Google Scholar]
- 18.Piepoli MF, Davos C, Francis DP, Coats AJS, ExTraMATCH Collaborative . Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328: 189. DOI: 10.1136/bmj.37938.645220.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies E, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise based rehabilitation for heart failure (Review). Cochrane Database Syst Rev 2010; 4: CD003331. DOI: 10.1002/14651858.CD003331.pub3 [DOI] [PubMed] [Google Scholar]
- 20.Multimorbidity: assessment, prioritisation and management of care for people with commonly occurring multimorbidity. National Institute for Health Care Excellence; 2016. (accessed 5 May 2020) Available from: https://www.ncbi.nlm.nih.gov/books/NBK385543/ [PubMed] [Google Scholar]
- 21.Holland AE, Harrison SL, Brooks D. Multimorbidity, frailty and chronic obstructive pulmonary disease: are the challenges for pulmonary rehabilitation in the name? Chron Respir Dis 2016; 13: 372–382. DOI: 10.1177/1479972316670104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG,PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009; 6: e1000097. DOI: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Innovative care for chronic conditions: building blocks for action: global report. Geneva: World Health Organization; 2002. (accessed 29 January 2019) Available from: https://apps.who.int/iris/handle/10665/42500 [Google Scholar]
- 24.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. DOI: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016; 1: CD001800. DOI: 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med 2016; 50: 1428–1437. DOI: 10.1136/bjsports-2016-096651 [DOI] [PubMed] [Google Scholar]
- 27.Rohatgi A. WebPlotDigitizer San Francisco, California, USA2019 [4.2:]. Pacifica, California, USA. Available from: https://automeris.io/WebPlotDigitizer [Google Scholar]
- 28.Joanna Briggs Institute Reviewer’s Manual: The Joanna Briggs Institute; 2017. (accessed 7 January 2019) Available from: https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 29.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration; 2011. [updated March 2011. (accessed 28 February 2019) Available from: http://handbook.cochrane.org [Google Scholar]
- 30.Social Science Statistics [Available from: https://www.socscistatistics.com/effectsize/default3.aspx (accessed 4 March 2019)
- 31.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care 1989; 27: S178–S189. DOI: 10.1097/00005650-198903001-00015 [DOI] [PubMed] [Google Scholar]
- 32.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10: 142–151. DOI: 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison C, Britt H, Miller G, Henderson J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 2014; 4: e004694. DOI: 10.1136/bmjopen-2013-004694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks AZ, Mentz R, Stebbins A, Mikus C, Schulte P, Fleg J, et al. Response to exercise training and outcomes in heart failure patients with diabetes mellitus: Insights from HF-action. J Am Coll Cardiol 2015; 65: A1034 DOI: 10.1016/j.cardfail.2015.12.007 [DOI] [Google Scholar]
- 35.Castro C, Gonzalez N, Oliveira M. Changing diabetic dialysis patients physical functioning with exercise training. Nephrol Dial Transplant 2015; 3: iii527–iii528. [Google Scholar]
- 36.Collins T, Lunos S. Home-based walking therapy improves walking ability and quality of life in persons with diabetes mellitus and peripheral arterial disease. Vasc Med 2010; 15: 155. [DOI] [PubMed] [Google Scholar]
- 37.Collins T, Lunos S, Hodges J. Effects of a walking intervention on systemic inflammation in persons with diabetes mellitus and peripheral arterial disease. J Gen Internal Med 2011; 1: S174–S175. [Google Scholar]
- 38.Johnson ST, Mundt C, Boule N, Bell G, Vallance J, Taylor L, et al. Improved functional status following the aquatic physical exercise for arthritis and diabetes (APEXD) study. Can J Diabet 2014; 5: S63. [Google Scholar]
- 39.Kurian R, Gobejishvili L, Barve S, Mokshagundam SP. Effect of resistance training on cytokine responses in elderly subjects with peripheral neuropathy-effect of gender. Proceedings of the 70th Scientific Sessions of the American Diabetes Association. 2010 June 25–29; Orlando, USA. [Google Scholar]
- 40.Martin B, Hauer T, Austford LD, Arena R, Stone JA, Aggarwal S. Cardiac rehabilitation in subjects with peripheral arterial disease: a higher risk patient population who benefit from attendance. Can J Cardiol 2016; 134: A18122. [Google Scholar]
- 41.Mundra V, Henquinet S, Moudry G. Outcomes of cardiac rehabilitation in obese patients. Cardiol 2013; 2: 482. [Google Scholar]
- 42.Srinivasan S, Reagan LP, Hardin JW, Matthews M, Leaphart E, Grillo CA, et al. Adjunctive tai chi in geriatric depression with comorbid arthritis: a randomized, controlled trial. Am J Geriatr Psych 2014; 1: S135–S136. DOI: 10.1016/j.jagp.2013.12.158 [DOI] [Google Scholar]
- 43.Zwerink M, Van Der Meer S, Van Der Valk P, Van Der Palen J. Safety, feasibility and effectiveness of a community-based physiotherapy exercise program for patients with both copd and chronic heart failure a pilot study. Am J Respir Crit Care Med 2010; 181: A3452. DOI 10.1164/ajrccm-conference.2010.181.1. [DOI] [Google Scholar]
- 44.Abd El-Kader MS, Al-Jiffri O, Ashmawy EM. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. Afr Health Sci 2013; 13: 682–688. DOI: 10.4314/ahs.v13i3.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambrosy AP, Mulder H, Coles A, Krauss WE, Lam CSP, McCullough PA, et al. Renal function and exercise training in ambulatory heart failure patients with a reduced ejection fraction. Am J Cardiol 2018; 122: 999–1007. DOI: 10.1016/j.amjcard.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 46.Beaudoin N, Bouvet GF, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Combined exercise training improves glycemic control in adult with cystic fibrosis. Med Sci Sports Exerc 2017; 49: 231–237. DOI: 10.1249/MSS.0000000000001104 [DOI] [PubMed] [Google Scholar]
- 47.Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing 2018; 47: 82–88. DOI: 10.1093/ageing/afx146 [DOI] [PubMed] [Google Scholar]
- 48.Byrkjeland R, Njerve IU, Anderssen S, Arnesen H, Seljeflot I, Solheim S. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: a randomised clinical trial. Diab Vasc Dis Res 2015; 12: 325–333. DOI: 10.1177/1479164115590552 [DOI] [PubMed] [Google Scholar]
- 49.Jones WS, Clare R, Ellis SJ, Mills JS, Fischman DL, Kraus WE, et al. Effect of peripheral arterial disease on functional and clinical outcomes in patients with heart failure (from HF-ACTION). Am J Cardiol 2011; 108: 380–384. DOI: 10.1016/j.amjcard.2011.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise in COPD with physical comorbidities: a randomised controlled trial. Eur Respir J 2013; 41: 1284–1291. DOI: 10.1183/09031936.00034312 [DOI] [PubMed] [Google Scholar]
- 51.Mentz RJ, Schulte PJ, Fleg JL, Fiuzat M, Kraus WE, Pina IL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF–ACTION). Am Heart J 2013; 165: 193-199. DOI: 10.1016/j.ahj.2012.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Servantes DM, Pelcerman A, Salvetti XM, Salles AF, de Albuquerque PF, de Salles FC, et al. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil 2012; 26: 45–57. DOI: 10.1177/0269215511403941 [DOI] [PubMed] [Google Scholar]
- 53.Sridhar B, Haleagrahara N, Bhat R, Kulur AB, Avabratha S, Adhikary P. Increase in the heart rate variability with deep breathing in diabetic patients after 12-month exercise training. Tohoku J Exp Med 2010; 220: 107–113. DOI: 10.1620/tjem.220.107 [DOI] [PubMed] [Google Scholar]
- 54.Abdelbasset WK, Alqahtani BA, Alrawaili SM, Ahmed AS, Elnegamy TE, Ibrahim AA, Soliman GS. Similar effects of low to moderate-intensity exercise program vs moderate-intensity continuous exercise program on depressive disorder in heart failure patients. Medicine 2019; 98: e16820. DOI: 10.1097/MD.0000000000016820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang S-L, Shen C-L, Chen L-C, Lo Y-P, Lin C-H, Lin C-H. Effectiveness of a home-based telehealth exercise training program for patients with cardiometabolic multimorbidity. J Cardiovasc Nurs 2020; 35: 491–501. DOI: 10.1097/JCN.0000000000000693 [DOI] [PubMed] [Google Scholar]
- 56.Hsu Y-I, Chen Y-C, Lee C-L, Chang N-J. Effects of diet control and telemedicine-based resistance exercise intervention on patients with obesity and knee osteoarthritis: a randomised control trial. Int J Environ Res Public Health 2021; 18: 7744. DOI: 10.3390/ijerph18157744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo Y-P, Chiang S-L, Lin C-H, Liu H-C, Chiang L-C. Effects of individualised aerobic exercise training on physical activity and health-related physical fitness among middle-aged and older adults with multimorbidity: a randomised controlled trial. Int J Environ Res Public Health 2021; 18: 101. DOI: 10.3390/ijerph18010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, Ashmawy EM. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci 2013; 13: 667–672. DOI: 10.4314/ahs.v13i3.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 32–42. DOI: 10.1164/rccm.201603-0446OC [DOI] [PubMed] [Google Scholar]
- 60.Freitas PD, Silva AG, Ferreira PG, Carvalho CRF, Da Silva A, Salge JM, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Med Sci Sports Exerc 2018; 50: 1367–1376. DOI: 10.1249/MSS.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 61.Halvari H, Healey J, Olafsen AH, Byrkjeland R, Deci EL, Williams GC. Physical activity and motivational predictors of changes in health behavior and health among DM2 and CAD patients. Scand J Med Sci Sports 2017; 27: 1454–1469. DOI: 10.1111/sms.12757 [DOI] [PubMed] [Google Scholar]
- 62.Barnes M, Goldsworthy UR, Cary BA, Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea – a feasibility study. J Clin Sleep Med 2009; 5: 409–415. [PMC free article] [PubMed] [Google Scholar]
- 63.Crisafulli E, Gorgone P, Vagaggini B, Pagani M, Rossi G, Costa F, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J 2010; 36: 1042–1048. DOI: 10.1183/09031936.00203809 [DOI] [PubMed] [Google Scholar]
- 64.de Groot M, Doyle T, Kushnick M, Shubrook J, Merrill J, Rabideau E, et al. Can lifestyle interventions do more than reduce diabetes risk? Treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Cur Diab Rep 2012; 12: 157–166. DOI: 10.1007/s11892-012-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassan M, Mourad S, Abdel Wahab NH, Daabis R, Younis G. Effect of comorbidities on response to pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Egyp J Chest Dis Tuberc 2016; 65: 63–69. DOI: 10.1016/j.ejcdt.2015.11.006 [DOI] [Google Scholar]
- 66.Khadanga S, Savage PD, Ades PA. Insulin resistance and diabetes mellitus in contemporary cardiac rehabilitation. J Cardiopul Rehabil Prev 2016; 36: 331–338. DOI: 10.1097/HCR.0000000000000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Listerman J, Bittner V, Sanderson BK, Brown TM. Cardiac rehabilitation outcomes: impact of comorbidities and age. J Cardiopul Rehabil Prev 2011; 31: 342–348. DOI: 10.1097/HCR.0b013e31822f189c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesquita R, Vanfleteren LE, Franssen FM, Sarv J, Taib Z, Groenen MT, et al. Objectively identified comorbidities in COPD: impact on pulmonary rehabilitation outcomes. Eur Respir J 2015; 46: 545–548. DOI: 10.1183/09031936.00026215 [DOI] [PubMed] [Google Scholar]
- 69.Naz I, Sahin H, Varol Y, Komurcuoglu B. The effect of comorbidity severity on pulmonary rehabilitation outcomes in chronic obstructive pulmonary disease patients. Chron Respir Dis 2019; 16: 1479972318809472. DOI: 10.1177/1479972318809472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nonoyama ML, Marzolini S, Brooks D, Oh P. Comparison of cardiac rehabilitation outcomes in individuals with respiratory, cardiac or no comorbidities: a retrospective review. Can J Respir Ther 2016; 52: 43–49. [PMC free article] [PubMed] [Google Scholar]
- 71.Soleimani A, Nejatian M, Hajizaynali MA, Abbasi SH, Alidoosti M, Sheikhfathollahi M, et al. Effect of gender and type 2 diabetes mellitus on heart rate recovery in patients with coronary artery disease after cardiac rehabilitation. Endokrynol Pol 2009; 60: 430–436. [PubMed] [Google Scholar]
- 72.Takaya Y, Kumasaka R, Arakawa T, Ohara T, Nakanishi M, Noguchi T, et al. Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction. Circ J 2014; 78: 377–384. DOI: 10.1253/circj.cj-13-0779 [DOI] [PubMed] [Google Scholar]
- 73.Tunsupon P, Lal A, Khamis MA, Mador MJ. Comorbidities in patients with chronic obstructive pulmonary disease and pulmonary rehabilitation outcomes. J Cardiopul Rehabil Prev 2017; 37: 283–289. DOI: 10.1097/HCR.0000000000000236 [DOI] [PubMed] [Google Scholar]
- 74.Tunsupon P, Mador MJ. The influence of body composition on pulmonary rehabilitation outcomes in chronic obstructive pulmonary disease patients. Lung 2017; 195: 729–738. DOI: 10.1007/s00408-017-0053-y [DOI] [PubMed] [Google Scholar]
- 75.Verges B, Patois-Verges B, Cohen M, Lucas B, Galland-Jos C, Casillas JM. Effects of cardiac rehabilitation on exercise capacity in type 2 diabetic patients with coronary artery disease. Diabet Med 2004; 21: 889–895. DOI: 10.1111/j.1464-5491.2004.01262.x [DOI] [PubMed] [Google Scholar]
- 76.Wang JS, Fu TC, Lien HY, Wang CH, Hsu CC, Wu WC, et al. Effect of aerobic interval training on erythrocyte rheological and hemodynamic functions in heart failure patients with anemia. Int J Cardiol 2013; 168: 1243–1250. DOI: 10.1016/j.ijcard.2012.11.053 [DOI] [PubMed] [Google Scholar]
- 77.Woodard CM, Berry MJ, Rejeski WJ, Ribisl PM, Miller HS. Exercise training in patients with cardiovascular disease and coexistent knee arthritis. J Cardiopul Rehabil 1994; 14: 255–261. [Google Scholar]
- 78.Butler SJ, Li LSK, Ellerton L, Gershon AS, Goldstein RS, Brooks D. Prevalence of comorbidities and impact on pulmonary rehabilitation outcomes. ERJ Open Res 2019; 5: 4. DOI: 10.1183/23120541.00264-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maniar S, Sanderson BK, Bittner V. Comparison of baseline characteristics and outcomes in younger and older patients completing cardiac rehabilitation. J Cardiopul Rehabil Prev 2009; 29: 220–229. DOI: 10.1097/HCR.0b013e3181ac7870 [DOI] [PubMed] [Google Scholar]
- 80.Campbell KL, Foster-Schubert K, Xiao L, Cadmus Bertram LA, Duggan C, Irwin M, et al. Injuries in sedentary individuals enrolled in a 12 month, randomized, controlled, exercise trial. J Phys Act Health 2012; 9: 198–207. DOI: 10.1123/jpah.9.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Little RM, Paterson DH, Humphreys DA, Stathokostas L. A 12-month incidence of exercise-related injuries in previously sedentary community-dwelling older adults following an exercise intervention. BMJ Open 2013; 3:6. DOI: 10.1136/bmjopen-2013-002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dekker J, Buurman BM, van der Leeden M. Exercise in people with comorbidity or multimorbidity. Health Psychol 2019; 38: 822–830. DOI: 10.1037/hea0000750 [DOI] [PubMed] [Google Scholar]
- 83.van der Leeden M, Stuiver MM, Huijsmans R, Geleijn E, de Rooij M, Dekker J. Structured clinical reasoning for exercise prescription in patients with comorbidity. Disabil Rehabil 2020; 42: 1474–1479. DOI: 10.1080/09638288.2018.1527953 [DOI] [PubMed] [Google Scholar]
- 84.Turner-Warwick MEH, Pentecost BL, Jones JH, Bannister SR, Chambers TL, Clayton RN, et al. Medical aspects of exercise. Benefits and risks. Summary of a report of the Royal College of Physicians. J R Coll Physicians Lond 1991; 25: 193–196. [PMC free article] [PubMed] [Google Scholar]
- 85.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16: 3–63. DOI: 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- 86.Kujala UM. Evidence for exercise therapy in the treatment of chronic disease based on at least three randomized controlled trials – summary of published systematic reviews. Scand J Med Sci Sports 2004; 14: 339–345. DOI: 10.1111/j.1600-0838.2004.413.x [DOI] [PubMed] [Google Scholar]
- 87.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009; 10: 313–323. DOI: 10.1111/j.1467-789X.2008.00547.x [DOI] [PubMed] [Google Scholar]
- 88.Smith SM, Wallace E, Salisbury C, Sasseville M, Bayliss E, Fortin M. A core outcome set for multimorbidity research (COSmm). Ann Fam Med 2018; 16: 132–138. DOI: 10.1370/afm.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlesso LC, Skou ST, Tang LH, Simony C, Brooks D. Multimorbidity: making the case for an end to disease-specific rehabilitation. Physiother Can 2020; 72: 1–3. DOI: 10.3138/ptc-72-1-gee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leijten FRM, Hoedemakers M, Struckmann V, Kraus M, Cheraghi-Sohi S, Zemplenyi A, et al. Defining good health and care from the perspective of persons with multimorbidity: results from a qualitative study of focus groups in eight European countries. BMJ Open 2018; 8: e021072. DOI: 10.1136/bmjopen-2017-021072 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.