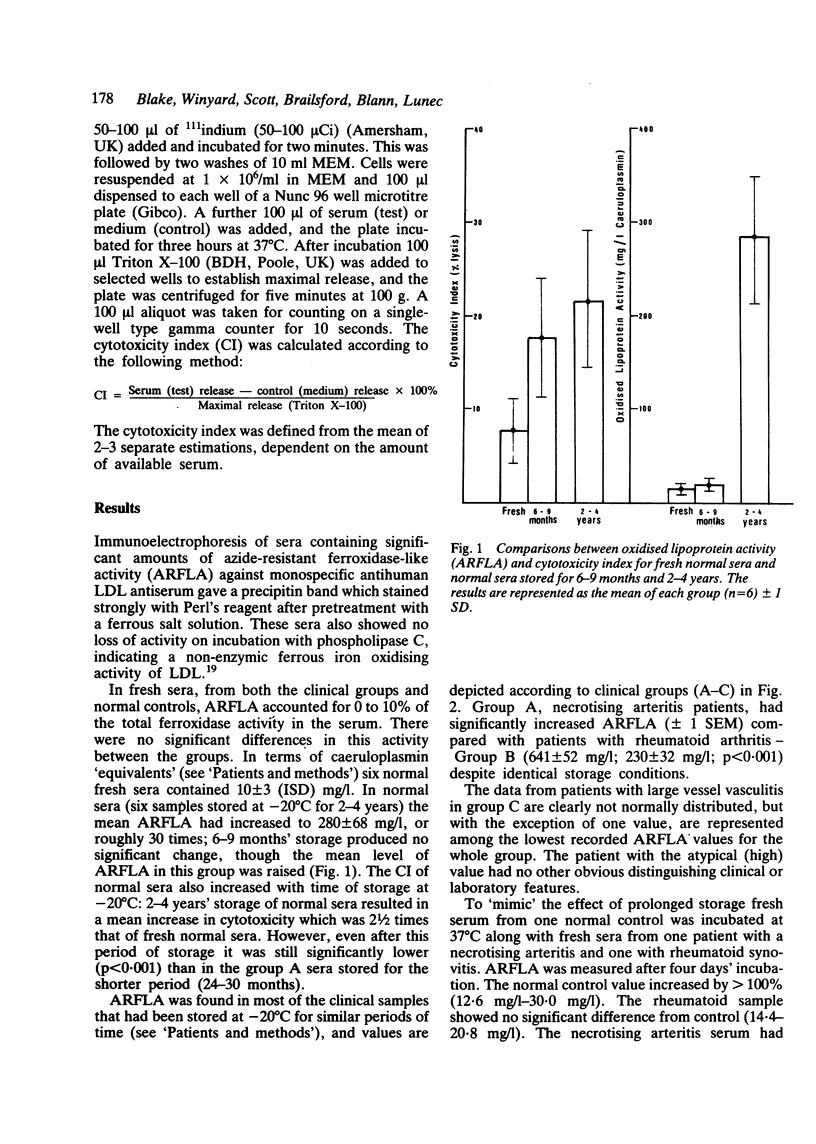
Abstract
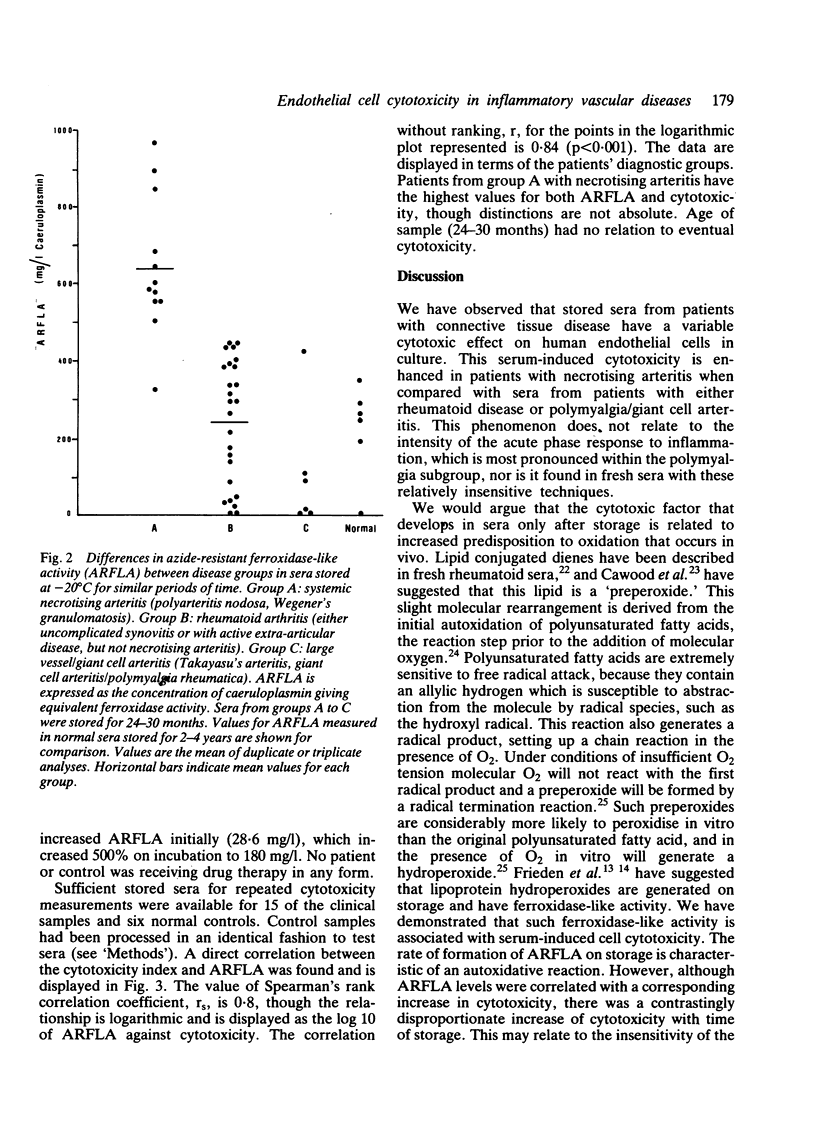
One of the proposed mechanisms of vascular damage in connective tissue disease is the direct action of a cytotoxic serum factor inducing endothelial cell damage. The nature of this serum factor is unclear, but has been suggested to be a lipoprotein. Sera from patients with (1) systemic necrotising arteritis (polyarteritis nodosa, Wegener's granulomatosis, and necrotising arteritis associated with rheumatoid synovitis), (2) systemic or joint restricted rheumatoid disease, and (3) large vessel/giant cell arteritis have been examined for cytotoxicity to human cultured endothelial cells and azide-resistant ferroxidase-like activity (indicative of the oxidised lipoprotein content). Stored sera from patients with necrotising arteritis showed a significantly enhanced tendency to develop oxidised lipoprotein, which correlated closely with human endothelial cell cytotoxicity. Fresh sera also contained this factor, but to a lesser extent. It is argued that the cytotoxic factor detected in previous clinical studies is in part an in-vitro artefact, although its accelerated development in certain patient groups might suggest an excess of pro-oxidants that have developed in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocanegra T. S., Germain B. F., Saba H. I., Bridgeford P. H., Saba S. R., Lowenstein M. B., Vasey F. B., Espinoza L. R. In vitro cytotoxicity of human endothelial cells in polymyalgia rheumatica and giant cell arteritis. Rheumatol Int. 1982;2(3):133–136. doi: 10.1007/BF00541166. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Johnson A. R., Hurd E. Cytotoxicity of sera from patients with scleroderma. Effects on human endothelial cells and fibroblasts in culture. Arthritis Rheum. 1983 Feb;26(2):170–178. doi: 10.1002/art.1780260208. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. An approach to free radicals. Lancet. 1983 Oct 29;2(8357):1010–1014. doi: 10.1016/s0140-6736(83)90989-3. [DOI] [PubMed] [Google Scholar]
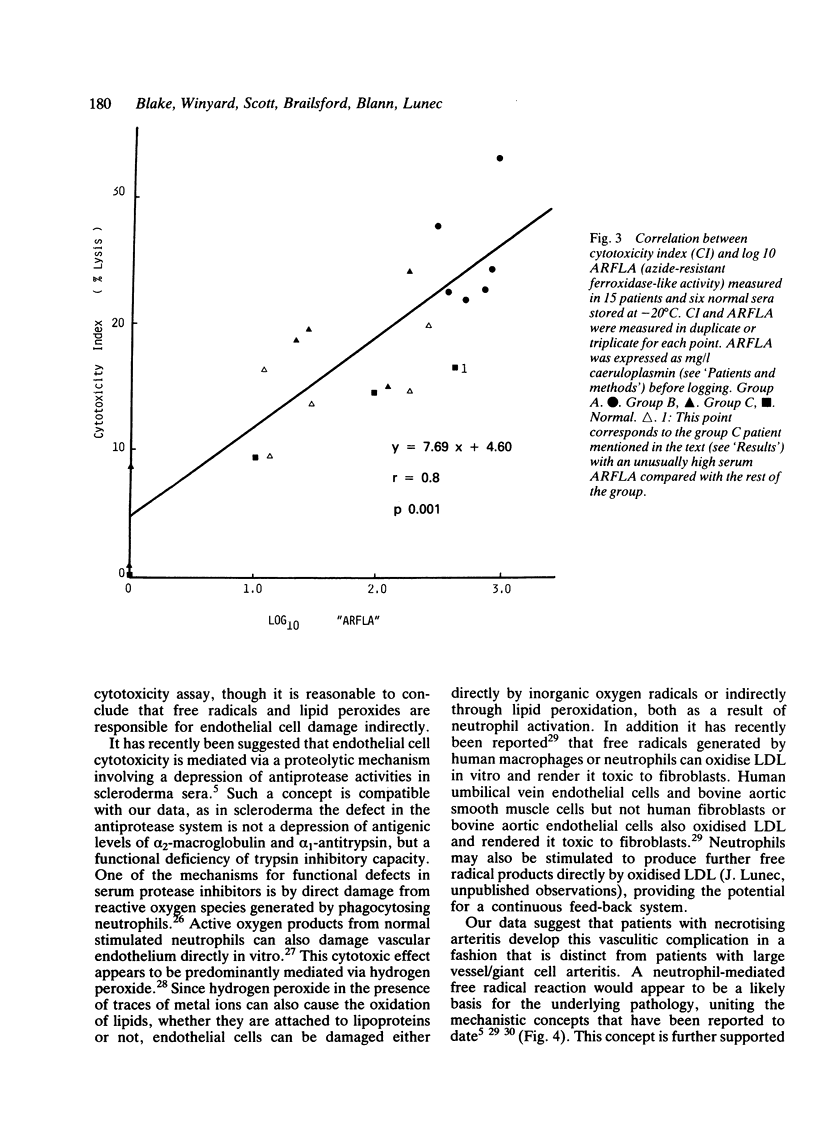
- Evensen S. A., Nilsen E., Galdal K. S. Injury to cultured human fibroblasts induced by low density lipoproteins: potentiating and protective factors. Scand J Clin Lab Invest. 1982 May;42(3):285–290. [PubMed] [Google Scholar]
- Frieden E., Hsieh H. S. Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol Relat Areas Mol Biol. 1976;44:187–236. doi: 10.1002/9780470122891.ch6. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Hawker R. J., Hawker L. M. A microcytotoxicity assay using 111indium oxine release from platelets. J Immunol Methods. 1980;33(1):45–53. doi: 10.1016/0022-1759(80)90081-2. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Osaki S., Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem. 1967 Feb;13(2):142–150. [PubMed] [Google Scholar]
- Kahaleh M. B., Leroy E. C. Endothelial injury in scleroderma. A protease mechanism. J Lab Clin Med. 1983 Apr;101(4):553–560. [PubMed] [Google Scholar]
- Kahaleh M. B., Sherer G. K., LeRoy E. C. Endothelial injury in scleroderma. J Exp Med. 1979 Jun 1;149(6):1326–1335. doi: 10.1084/jem.149.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Rabinovitz M., Chayen R., Schen R. J., Goldschmidt L. The oxidation of ferrous ions by serum proteins demonstrated by immunoelectrophoresis. Clin Chim Acta. 1966 Aug;14(2):270–273. doi: 10.1016/0009-8981(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan W. R., Jr, Korn J. H. Cytotoxic activity of sera from scleroderma and other connective tissue diseases. Lack of cellular and disease specificity. Arthritis Rheum. 1982 Dec;25(12):1391–1395. doi: 10.1002/art.1780251201. [DOI] [PubMed] [Google Scholar]
- Sung C. S., Topham R. W. Lipid components of human ferroxidase-II. Biochem Biophys Res Commun. 1973 Aug 6;53(3):824–829. doi: 10.1016/0006-291x(73)90167-8. [DOI] [PubMed] [Google Scholar]
- Tribe C. R., Scott D. G., Bacon P. A. Rectal biopsy in the diagnosis of systemic vasculitis. J Clin Pathol. 1981 Aug;34(8):843–850. doi: 10.1136/jcp.34.8.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Christensen D. D., Lee G. R., Cartwright G. E. Serum azide-resistant ferroxidase activity. Biochim Biophys Acta. 1974 May 20;350(1):129–134. doi: 10.1016/0005-2744(74)90210-1. [DOI] [PubMed] [Google Scholar]


