Abstract
Acquired von Willebrand syndrome (aVWS) develops with various underlying diseases. We herein report an individual with aVWS associated with mucosa-associated lymphoid tissue lymphoma in the lungs complicated by hyperviscosity syndrome, Sjögren's syndrome, and hypothyroidism. This patient developed life-threatening hemorrhaging during a lung biopsy despite transfusion of concentrate of plasma-derived VWF/factor VIII. The use of rituximab caused remission of the lymphoma and hyperviscosity syndrome in parallel with the resolution of aVWS. Thus, lymphoma and hyperviscosity might result in aVWS. Invasive procedures with a risk of bleeding should be avoided in individuals with aVWS.
Keywords: acquired von Willebrand syndrome, von Willebrand factor, MALT lymphoma, Sjögren's syndrome, hyperviscosity syndrome
Introduction
von Willebrand factor (VWF) plays an important role in hemostasis and thrombosis by binding glycoprotein (GP) Ib/IX on platelets and stabilizing clotting factor VIII (FVIII) (1,2). VWF is produced and secreted from endothelial cells and megakaryocytes as ultra-large VWF (UL-VWF) and cleaved into multimers of various sizes by an enzyme called a disintegrin and metalloprotease with a thrombospondin type-1 motif (ADAMTS13) under shear stress conditions (3,4). The structure of high-molecular-weight VWF multimers (HMWM) is the most effective at mediating platelet adhesion to endothelial damage.
von Willebrand disease (VWD) is a congenital bleeding disorder caused by quantitative and/or qualitative abnormalities of VWF with an estimated incidence of 100 per million people (5). VWD is classified into three categories, type 1 to 3, according to its pathogenesis; plasma levels of VWF are decreased in type 1, the activity of VWF is impaired in type 2, and VWF is totally absent in type 3 (6). Type 2 VWD is further categorized into four subtypes: 2A, 2B, 2M, and 2N. Types 2A and 2B are characterized by a decrease in HMWM due to impaired secretion or enhanced cleavage or hyperconsumption of VWF, respectively. Type 2M is characterized by impaired binding affinity of VWF to GPIb on platelets. Type 2N is accompanied by a marked decrease in the levels of FVIII.
Acquired von Willebrand syndrome (aVWS) is also caused by deficiency and/or dysfunction of VWF; however, the cause of abnormality of VWF is independent of genetic alterations. aVWS develops in the presence of various underlying diseases, including lymphoproliferative disorders, myeloproliferative neoplasms, cardiovascular diseases, autoimmune disorders, and hypothyroidism (1,7,8). The etiology of aVWS remains to be fully elucidated; however, possible mechanisms include diminished VWF synthesis in hypothyroidism (9), production of antibody to VWF associated with lymphoproliferative diseases, immune thrombocytopenic purpura and antiphospholipid syndrome (10-12), adsorption of VWF by multiple myeloma cells (13), and enhanced clearance of HMWM under high shear stress conditions in cardiovascular diseases (14).
We herein report an individual with aVWS who had multiple comorbidities, including mucosa-associated lymphoid tissue (MALT) lymphoma in the lungs, Sjögren's syndrome (SjS), hypothyroidism, and hyperviscosity syndrome.
Case Report
A 38-year-old woman with blood type A who had neither a family history nor previous bleeding disorder was referred to our hospital for a detailed examination of recurrent epistaxis, headache, easy fatigability, and exertional breathlessness. She had developed left parotid gland swelling 13 years earlier and been diagnosed with SjS. She had dry eyes but had been observed without medication. She had undergone surgery 12 years earlier to remove a mediastinal tumor, which had been diagnosed as MALT lymphoma and monitored without chemotherapy. Several months ago, a slight increase in serum levels of immunoglobulin had been noted, and since then, she had been aware of recurrent epistaxis.
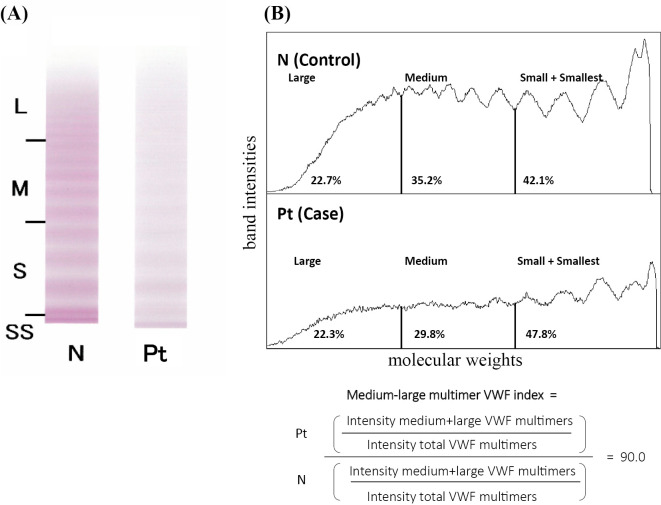
A physical examination found bilateral parotid gland swelling. No heart murmur was heard. Neither petechial hemorrhaging nor purpura was noted. Laboratory data revealed bicytopenia in peripheral blood (white blood cell count 2.1×103/μL, hemoglobin 10.7 g/dL, and platelet count 15.2×104/μL) and monoclonal IgA and polyclonal IgG gammopathy (IgA 3,789 mg/dL and IgG 3,801 mg/dL) as determined by immunofixation. In addition, prolongation of activated partial thromboplastin time (APTT) at 52.1 was noted (Table). We measured the bleeding time twice. The first result was 3.5 minutes, and the second one was longer than 10 minutes. Further tests found a decrease in FVIII coagulant activity (FVIII:C, 24%) and VWF antigen (VWF:Ag, 53%). Of note, VWF ristocetin cofactor activity (VWF:RCo) was profoundly decreased to 7%. The ratio of VWF:RCo to VWF:Ag (VWF:RCo/VWF:Ag) was 0.13. According to the clinical practice guideline of VWD, a VWF:RCo/VWF:Ag of <0.7 indicates a high probability of type 2 VWD (7,15,16). Furthermore, immunoelectrophoretic assays of the plasma followed by a densitometric analysis showed that the density of the VWF bands of all sizes, from low to high molecular weight, was decreased in this patient compared to healthy subjects (Fig. 1A B). These observations suggested quantitative abnormalities of VWF, a feature of type 1 VWD. Taken together, these findings prompted us to consider a mixture of type 1 and 2, such as aVWS, in this case.
Table.
Laboratory Findings.
| Complete Blood Count | Biocemistry | |||||||
| WBC | 2.1×103 | /µL | TP | 10.9 | g/dL | Fe | 49 | µg/dL |
| Blast | 0 | % | ALB | 2.7 | g/dL | Ft | 100 | mg/dL |
| Neu | 38 | % | TB | 0.4 | mg/dL | ß2MG | 3.55 | mg/dL |
| Lym | 46 | % | DB | <0.1 | mg/dL | CRP | 0.2 | mg/dL |
| Mono | 6 | % | AST | 25 | U/L | IgG | 3,801 | mg/dL |
| Eosin | 8 | % | ALT | 20 | U/L | IgA | 3,789 | mg/dL |
| Baso | 1 | % | LDH | 118 | U/L | IgM | 44 | mg/dL |
| Aty-Lym | 1 | % | ALP | 88 | U/L | κ | 35.8 | mg/dL |
| RBC | 3.07×106 | /µL | ChE | 143 | U/L | λ | 69.2 | mg/dL |
| Hb | 10.7 | g/dL | BUN | 13 | mg/dL | |||
| Hct | 27.7 | % | Crea | 0.71 | mg/dL | Immunology | ||
| PLT | 15.2×104 | /µL | UA | 6.9 | mg/dL | ANA | ×640 | |
| Na | 129 | mmol/L | Anti ds-DNA | 0.8 | IU/mL | |||
| Coagulation | K | 3.9 | mmol/L | U1-RNP | <0.5 | U/mL | ||
| PT-INR | 1.29 | Cl | 102 | mmol/L | Anti SS-A | >240 | U/mL | |
| APTT | 52.1 | s | Ca | 8.2 | mg/dL | Anti SS-B | >320 | U/mL |
| FBG | 146 | mg/dL | HbA1c | 6.1 | % | KL-6 | 765 | U/mL |
| D-dimer | <2.5 | µg/mL | s-IL2R | 308 | U/mL | TSH | 9 | µIU/mL |
| FDP | <0.5 | µg/mL | FT4 | 0.76 | ng/dL | |||
Figure 1.
The von Willebrand factor multimer assay. (A) Immune electrophoresis. Plasma samples from healthy volunteers and patients were electrophoresed and stained with an anti-VWF antibody. This assay was performed at SRL (Tokyo, Japan). The loss of large multimers and a marked decrease in medium-size multimers are shown. (B) A densitometric analysis. The density of VWF bands of all molecular weights in the patient was paler than that of the control sample. The medium-large VWF multimer index was 0.9. HMWM: high-molecular-weight VWF multimers, L: large size, M: medium size, N: normal pattern, Pt: patient pattern, S: small size, SS: smallest size, VWF: von Willebrand factor
Peripheral blood film showed marked rouleaux formation, indicating hyperviscosity. Platelet aggregation tests, which is required for making a diagnosis of type 2B VWD, could not be performed due to hyperviscosity. Furthermore, as shown in Table, a decrease in levels of free thyroxin hormone (FT4) and an increase in levels of thyroid-stimulating hormone (TSH) were noted, which led to a diagnosis of hypothyroidism, although neither anti-thyroglobulin nor anti-thyroid peroxidase was detected in her serum. Hormone replacement therapy with levothyroxine sodium hydrate (25 μg/day) was commenced, which promptly normalized the levels of both FT4 and TSH (data not shown).
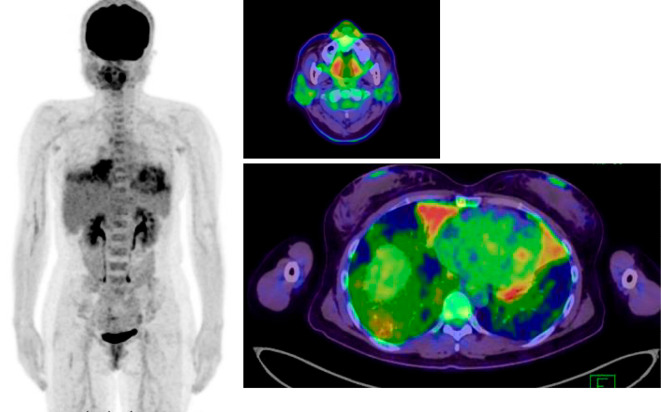
Positron emission tomography/computed tomography (PET-CT) showed the abnormal accumulation of fluorodeoxyglucose in the bilateral swollen parotid glands and the middle right and bilateral lower lungs (Fig. 2). These findings suggested interstitial pneumonia associated with SjS or recurrence of MALT lymphoma. A fine-needle aspiration biopsy of a swollen right parotid gland was performed without bleeding, although a pathological diagnosis was not made. Therefore, a pulmonary tissue biopsy was performed via bronchoscopy. Despite the replacement of plasma-derived (pd) VWF/FVIII (40 U/kg/day) before the biopsy, blood oozing from the bronchial tract occurred, which required management in the intensive care unit. The pathological findings were consistent with recurrence of MALT lymphoma.
Figure 2.
FDG PET-CT findings before the lung biopsy. The abnormal accumulation of FDG in bilateral swollen parotid glands and the middle right and bilateral lower lungs are shown. FDG: fluorodeoxyglucose, PET-CT: positron emission tomography/computed tomography
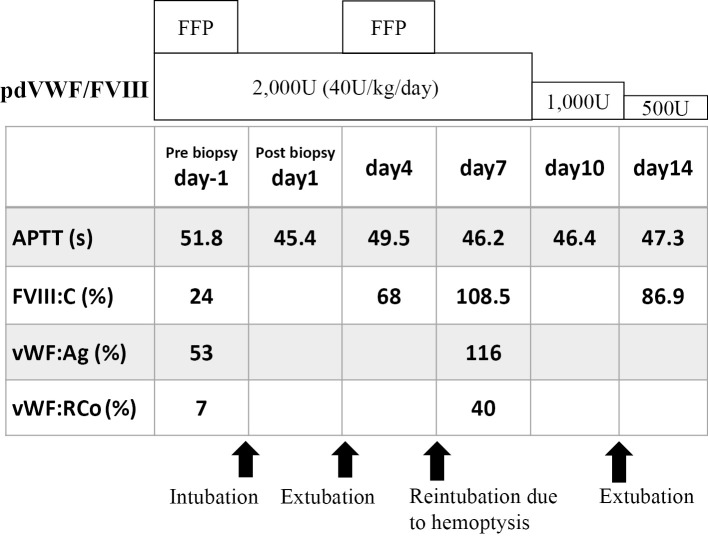
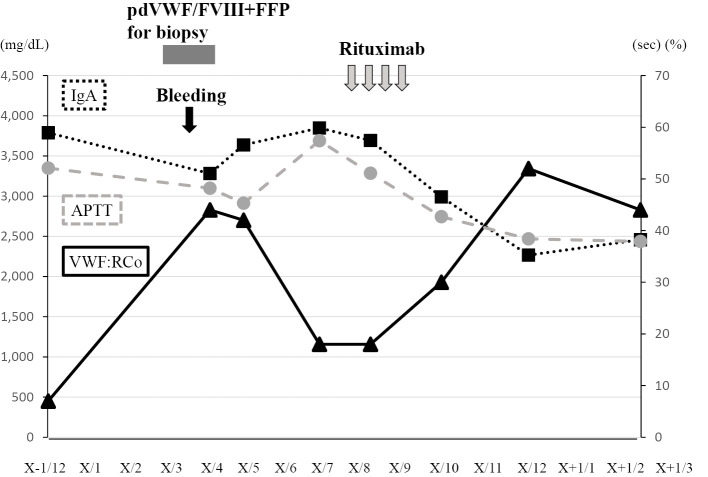
Immunohistochemistry showed that the lymphoma cells were positive for IgA-λ. Repeated transfusion of pdVWF/FVIII (40 U/kg/day) and fresh-frozen plasma (FFP) gradually increased the VWF:RCo, and pulmonary bleeding was stopped (Fig. 3). We did not closely monitor the VWF:Ag or VWF:RCo after injection of pdVWF/FVIII, so we were unable to calculate the recovery rate and half-life of this agent. Subsequently, the anti-CD20 monoclonal antibody rituximab was administered weekly for four times, which caused remission of the lymphoma in parallel with improvement of the hyperviscosity syndrome and normalization of the APTT and serum levels of IgA (Fig. 4).
Figure 3.
Clinical course and hemostatic parameters after the lung biopsy. Prior to the lung biopsy, hemostatic parameters showed prolonged APTT and decreased plasma levels of FVIII, vWF:Ag and VWF:RCo. Despite administration of FFP and pdVWF/FVIII before the procedure, the patient developed pulmonary hemorrhaging after the lung biopsy. Repeated administration of pdVWF/FVIII increased the levels of FVIII:c and vWF:Ag on day 7. However, the VWF:RCo increased only up to 40%. APTT: activated partial thromboplastin time, FFP: fresh-frozen plasma, FVIII:c: factor VIII activity, pdFWF/FVIII: plasma-derived von Willebrand factor/factor VIII, VWF:Ag: von Willebrand factor antigen, VWF:RCo: von Willebrand factor ristocetin cofactor activity
Figure 4.
Clinical course and hemostatic parameters after rituximab treatment. Rituximab monotherapy decreased the serum levels of IgA, in parallel with an increase in plasma levels of VWF:RCo and normalization of APTT. IgA: immunoglobulin A, pdFWF/FVIII: plasma-derived von Willebrand factor/factor VIII, VWF/RCo: von Willebrand factor ristocetin cofactor activity
Neither an enzyme-linked immunosorbent assay (ELISA) nor a pathological examination detected antibody to VWF or adsorption of VWF on lymphoma cells, respectively (Supplementary materials 1 and 2). When APTT cross-mixing tests were performed by mixing the patient's and normal plasma in various ratios, both the immediate and delayed responses showed a downward convex pattern, and the presence of inhibitors to FVIII was ruled out (Supplementary material 3).
Discussion
aVWS should be suspected in patients with a current bleeding tendency, normal prothrombin time (PT), prolonged APTT, and no family history of bleeding. In such patients, FVIII activity, VWF:Ag, and VWF:RCo should be measured. Bleeding time is prolonged in patients with VWD, but the measurements vary widely among practitioners, and the diagnostic accuracy is not high. In fact, the first bleeding time measurement in this case was 3.5 minutes, while the second one was longer than 10 minutes. For this reason, in the United Kingdom, the guideline of VWD recommends against measuring the bleeding time to screen for a diagnosis (17). The level of VWF:Ag is physiologically influenced by the ABO blood type. Although VWF:Ag is often decreased in individuals with blood type O, the blood type of this patient was type A (18).
The etiology of aVWS depends on the underlying diseases. A decrease in the ratio of VWF:RCo/VWF:Ag indicates the presence of inhibitory antibodies or loss/decrease in HMWM (15). We therefore assumed that the antibody to VWF produced by lymphoma cells might contribute to a decrease in the ratio of VWF:RCo/VWF:Ag and the development of aVWS in this case. There are two types of antibodies to VWF: non-neutralizing antibodies, which enhance the clearance of VWF, and an inhibitor that impairs the binding of VWF to GPIb on platelets (10,19,20). Unexpectedly, neither type of antibody was detectable by an ELISA (Supplementary material 1). However, it should be noted that the sensitivity and specificity of ELISAs for detecting anti-VWF antibodies are low (19,21).
Another possible mechanism explaining the pathogenesis of aVWS in this case relates to hyperviscosity. In addition to marked rouleaux formation on blood film, blood agglutination in a 22-gauge needle was noted while collecting blood in this case (data not shown). Both symptoms disappeared and were associated with a decrease in serum levels of IgA and IgG (from 3,801 to 3,275 mg/dL) after treatment with rituximab. Hyperviscosity-related symptoms become prominent if the serum IgA levels exceed 6,000 mg/dL (22), which is much higher than in the present case (3,789 mg/dL). In this case, the serum IgG levels were also increased, probably because of SjS. This phenomenon may also have contributed to the hyperviscosity in this case. Hyperviscosity enhances shear stress, which allows HMWH to be cleaved by ADAMTS13. Plasma exchange might have been an option to improve hyperviscosity and reduce the risk of bleeding before the lung biopsy in this patient (23-25).
Enhanced-shear stress-related type 2 VWS is also noted in other medical conditions, including Hyde syndrome characterized by severe aortic valve stenosis (AS) and gastrointestinal bleeding (26,27) and heart failure requiring left ventricular assist devices (LVAD) or extracorporeal membrane oxygenation (ECMO) (14,28). In the present case, no murmur was heard on a physical examination. In addition, no left ventricular hypertrophy was observed by contrast-enhanced CT (figure not shown). Therefore, it is unlikely that she had severe valvular heart disease, such as AS, which can be a pathogenesis of VWS.
Other physicians have also reported cases of aVWS complicated by MALT and splenic marginal zone lymphoma (29-32). Two cases had concomitant autoimmune diseases SjS (30) and Hashimoto's thyroiditis (31). Intriguingly, all cases were categorized as the type 2 VWD pattern; however, the etiology varied among cases. For example, non-neutralizing autoantibodies to VWF were found in one case (31), and the aberrant expression of GPIb on lymphoma cells, which may cause a consumptive reduction in VWF, was found in another case (29). The etiology of enhanced VWF clearance remained unknown in the other two cases. In the present case, adsorption of VWF on lymphoma cells was not noted by a pathological examination (Supplementary material 2), although we did not examine the expression of GPIb by lymphoma cells in our patient (29). The resolution of aVWS was correlated with the remission of lymphoma. Three cases received chemotherapy for lymphoma (29,30,32). Two of these cases achieved remission of lymphoma after chemotherapy in association with improvement of aVWS (29,30), while the remaining case did not respond to rituximab monotherapy, and aVWS persisted (32). We recently reported a case of aVWS complicated by acute myeloid leukemia (AML). aVWS improved in parallel with the achievement of complete remission of AML after induction chemotherapy (33).
We performed a densitometric analysis following immunoelectrophoretic assays of VWFM in this case (34). This analysis showed that the density of VWF bands of all sizes, from low to high molecular weight, was decreased in our patient (Fig. 1B). In addition, the proportion of large VWF multimers (22.3%) was almost equal to that of control samples (22.7%), and the medium-large VWF multimer index (14), which is well associated with VWF activity, was slightly decreased (0.9) in this case (Fig. 1B). Boender et al. measured this index in a large cohort of VWD patients (type 1, n=328 and type 2, n=211) and found that the index was 1.23 (1.04-1.40) (median, interquartile range) in type 1 and 0.53 (0.29-0.89) in type 2 VWD (34). Thus, it is difficult to categorize this case as type 1 or 2 VWD. The present patient had multiple comorbidities, which might have made it difficult to evaluate the subtype of VWD. Our patient had hypothyroidism, which is a well-known underlying disease that causes type 1-like aVWS in association with a decrease in the synthesis of VWF; however, replacement of thyroid hormone did not improve APTT (data not shown), thus ruling out the involvement of hypothyroidism in the development of aVWS in this case. In addition, Pikta et al. reported that the proportion of HMWM was 61.2% (approximately twice that of this case) in aVWS that developed in association with autoimmune thyroiditis (35).
Conclusion
We observed a patient with aVWS with multiple comorbidities, including MALT lymphoma, IgA monoclonal gammopathy, SjS, hypothyroidism, and hyperviscosity syndrome. Treatment of each underlying disease is required in aVWS with multiple comorbidities. Hyperviscosity associated with IgA monoclonal gammopathy caused by MALT lymphoma may play a major role in the development of aVWS in this case, as the use of rituximab caused remission of lymphoma and hyperviscosity syndrome in parallel with improvement in aVWS. Replacement therapy with pdVWF/FVIII before a lung biopsy was unable to prevent pulmonary hemorrhaging. Invasive procedures should be avoided in cases with aVWS with a marked decrease or loss of HMWM.
The authors state that they have no Conflict of Interest (COI).
Supplementary Materials
Anti-VWF immunoglobulin (Ig) in the patient’s sample (black circles; depicted as an amount equivalent to undiluted volume) was detected by using anti-human Ig (common for IgG, IgM and IgA) as a detecting antibody and purified VWF (200 ng/well) coated on a 96-well plate in a sandwich-enzyme-liked immunosorbent assay (ELISA).
a. Pathological examination by immunostaining did not find the adsorption of VWF on lymphoma cells. Magnification, ×200 b. Immunohistochemistry of lymphoma cells of the lung tissue. The expressions of VWF were not detected in the lymphoma cells. Magnification, ×400 c. Endothelial cells of a bone marrow biopsy specimen, which was obtained from an unrelated patient with chronic myeloid leukemia in complete remission, serve as positive control. Magnification, ×400
The patient and normal plasma were mixed with various ratios. APTT was measured immediately after mixing or after 2 hours of reaction. Both lines showed the downward convex pattern, ruling out the presence of the inhibitors to factor VIII.
References
- 1. Mital A. Acquired von Willebrand syndrome. Adv Clin Med 25: 1337-1344, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Ng C, Motto DG, Di Paola J. Diagnostic approach to von Willebrand disease. Blood 125: 2029-2037, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gogia S, Neelamegham S. Role of fluid shear stress in regulating VWF structure, function and related blood disorders. Biorheology 52: 319-335, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor cleaving protease and its identification as a new member of the metalloprotease family. Blood 98: 1662-1666, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 14: 171-232, 2008. [DOI] [PubMed] [Google Scholar]
- 6. James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv 5: 280-300, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Federici AB, Rand JH, Bucciarelli P, et al. ; Subcommittee on von Willebrand Factor. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost 84: 345-349, 2000. [PubMed] [Google Scholar]
- 8. Shetty S, Kasatkar P, Ghosh K. Pathophysiology of acquired von Willebrand disease: a concise review. Eur J Haematol 87: 99-106, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Dalton RG, Dewar MS, Savidge GF, et al. Hypothyroidism as a cause of acquired von Willebrand's disease. Lancet 1: 1007-1009, 1987. [DOI] [PubMed] [Google Scholar]
- 10. Mannucci PM, Lombardi R, Bader R, et al. Studies of the pathophysiology of acquired von Willebrand's disease in seven patients with lymphoproliferative disorders or benign monoclonal gammopathies. Blood 64: 614-621, 1984. [PubMed] [Google Scholar]
- 11. Ihara A, Suzuki N, Matsushita T, Ichinose A. Acquired von Willebrand syndrome in a patient with immune thrombocytopenic purpura. Rinsho Ketsueki (Jpn J Clin Hematol) 56: 901-904, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi N, Ogawa Y, Yanagisawa K, et al. Acquired immune-mediated von Willebrand syndrome accompanied by antiphospholipid syndrome. Rinsho Ketsueki (Jpn J Clin Hematol) 58: 613-618, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Richard C, Cuadrado MA, Prieto M, et al. Acquired von Willebrand disease in multiple myeloma secondary to absorption of von Willebrand factor by plasma cells. Am J Hematol 35: 114-117, 1990. [DOI] [PubMed] [Google Scholar]
- 14. Tamura T, Horiuchi H, Imai M, et al. Unexpectedly high prevalence of acquired von Willebrand syndrome in patients with severe aortic stenosis as evaluated with a novel large multimer index. J Atherosclero Thromb 22: 1115-1123, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Tiede A, Priesack J, Werwitzke S, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J Thromb Haemost 6: 569-576, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med 373: 2067-2080, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Laffan MA, Lester W, O'Donnel JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophlia Center Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol 167: 453-465, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill JC, Endes-Brooks J, Bauer PJ, Marks Jr WJ, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 69: 1691-1695, 1987. [PubMed] [Google Scholar]
- 19. Mohri H, Motomura S, Kanamori H, et al. Clinical significance of inhibitors in acquired von Willebrand syndrome. Blood 91: 3623-3629, 1998. [PubMed] [Google Scholar]
- 20. Kumar S, Pruthi RK, Nichols WL. Acquired von Willebrand disease. Mayo Clin Proc 77: 181-187, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Federici AB, Budde U, Castaman G, Rand JH, Tiede A. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: a 2013 update. Semin Tromb Hemost 39: 191-201, 2013. [DOI] [PubMed] [Google Scholar]
- 22. Metha J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 29: 467-471, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Federici AB, Stabile F, Castaman G, Canciani MT, Mannucci PM. Treatment of acquired von Willebrand syndrome in patients with monoclonal gammopathy of uncertain significance: comparison of three different therapeutic approaches. Blood 92: 2707-2711, 1998. [PubMed] [Google Scholar]
- 24. Kwaan HC. Hyperviscosity in plasma cell dyscrasias. Clin Hemorheol Microcirc 55: 75-83, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Abou-Ismail MY, Rodgers GM, Bray PF, Lim MY. Acquired von Willebrand syndrome in monoclonal gammopathy - a scoping review on hemostatic management. Res Pract Thromb Haemost 5: 356-365, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heyde EC. Gastrointestinal bleeding in aortic stenosis. N Engl J Med 259: 196, 1958. [Google Scholar]
- 27. Loscalzo J. From clinical observation to mechanism - Heyde's syndrome. N Engl J Med 367: 1954-1956, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Horiuchi H, Doman T, kokame K, Saiki Y, Matsumoto M. Acquired von Willebrand syndrome associated with cardiovascular diseases. J Atherosclero Thromb 26: 303-314, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tefferi A, Hanson CA, Kurtin PJ, Katzmann JA, Dalton RJ, William LN. Acquired von Willebrand's disease due to aberrant expression of platelet glycoprotein Ib by marginal zone lymphoma cell. Br J Haematol 96: 850-853, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Iwabuchi T, Kimura Y, Suzuki T, et al. Successful treatment with rituximab in a patient with primary thymic MALT lymphoma complicated with acquired von Willebrand syndrome and Sjögren syndrome. Rinsho Ketsueki (Jpn J Clin Hematol) 52: 210-215, 2011. [PubMed] [Google Scholar]
- 31. Koyama T, Fujimoto K, Shima M. Acquired von Willebrand syndrome associated with Hashimoto's thyroiditis and subcutaneous mucosa-associated lymphoid tissue lymphoma. Intern Med 52: 2661-2663, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Komeno Y, Shibuya N, Uryu H, et al. Splenic marginal zone lymphoma with acquired von Willebrand syndrome diagnosed via splenic bleeding. Intern Med 56: 557-562, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukatsu M, Ohkawara H, Takahashi H, et al. A case of acquired von Willebrand syndrome complicated by acute myelomonocytic leukemia. Case Rep Oncol 14: 1152-1158, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boender J, Atiq F, Cnossen MH, et al. Von Willebrand factor multimer densitometric analysis: validation of the clinical accuracy and clinical implications in von Willebrand disease. Hemasphere 17:5: e542, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pikta M, Banys V, Szanto T, et al. Von Willebrand factor multimeric assay in acquired von Willebrand disease diagnosis: a report of experience from North Estonia Medical Center. J Lab Physicians 13: 195-201, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-VWF immunoglobulin (Ig) in the patient’s sample (black circles; depicted as an amount equivalent to undiluted volume) was detected by using anti-human Ig (common for IgG, IgM and IgA) as a detecting antibody and purified VWF (200 ng/well) coated on a 96-well plate in a sandwich-enzyme-liked immunosorbent assay (ELISA).
a. Pathological examination by immunostaining did not find the adsorption of VWF on lymphoma cells. Magnification, ×200 b. Immunohistochemistry of lymphoma cells of the lung tissue. The expressions of VWF were not detected in the lymphoma cells. Magnification, ×400 c. Endothelial cells of a bone marrow biopsy specimen, which was obtained from an unrelated patient with chronic myeloid leukemia in complete remission, serve as positive control. Magnification, ×400
The patient and normal plasma were mixed with various ratios. APTT was measured immediately after mixing or after 2 hours of reaction. Both lines showed the downward convex pattern, ruling out the presence of the inhibitors to factor VIII.