Abstract
Partially hydrolyzed guar gum dietary fiber is well recognized for a number of health benefits. In the present study, we aim to investigate the effects of partially hydrolyzed guar gum on constipation, intestinal microbiota as well as mental health in healthy subjects. In the randomized, parallel, double-blind, and placebo-controlled study the enrolled healthy men and women volunteers took either 3 g/day (T3) or 5 g/day (T5) of dietary fiber intakes for eight consecutive weeks compared to placebo (T0). The fecal characteristics, fecal microbiota, defecation characteristics, and quality of life (QOL) questionnaire were investigated. The results revealed a significant suppression in fecal potent harmful mucolytic bacteria in the T3 and T5 groups compared to the T0 group. The defecation frequency, excretory feeling, and scores of sleep and motivation questionnaire were also improved in the dietary fiber intake groups, showing a significant difference in the T5 group compared to the T0 group. In summary, the consumption of partially hydrolyzed guar gum dietary fiber is found effective in suppressing the potent harmful mucolytic bacteria that could be associated with the improvement of constipation-related symptoms including mental health in terms of sleep and motivation among the healthy subjects.
Keywords: partially hydrolyzed guar gum, constipation, mucolytic bacteria, sleep, motivation
Introduction
In recent years, dietary fiber intake has been declining due to the westernization of the diet.(1–3) Dietary fiber is essential for maintaining a healthy intestinal environment, as it can be utilized by beneficial microbes, which offer the host benefits through modulation of immune balance, production of useful metabolites such as short-chain fatty acids (SCFAs), and contribution to the maintenance of intestinal barrier integrity,(4,5) etc. Its deficiency deteriorates the balance of intestinal microbiota and causes defecation disorders such as constipation and diarrhea. Moreover, recent studies have shown that intestinal microbiota are involved not only in the digestive tract but also in the systemic health. It is becoming increasingly clear that intestinal microbiota affect a variety of diseases, including obesity and lifestyle-related diseases,(6–9) and that they have a significant impact not only on physical health but also on mental health.(10,11) The bi-directional relationship between the brain and the gut has been the focus of much attention and is referred to as the brain-gut axis.(12) Signals from the brain, e.g., stress, have been shown not only to alter gastrointestinal motility and cause gastrointestinal symptoms but also to induce abnormalities in the intestinal microbiota.(13) Conversely, gut bacteria act on the brain through their effects on metabolites, immunity, and gastrointestinal barrier function, and are involved in depression and anxiety.(14,15) Furthermore, intestinal microbiota have been shown to influence circadian rhythms and affect sleep.(16,17) Since intestinal microbiota play pivotal roles in the host’s physical and mental health as described above, probiotics and prebiotics, which have beneficial effects on the intestinal microbiota, are also attracting a great deal of attention. Various clinical trials are now being conducted to test the effects of probiotics and prebiotics on various diseases including immunity and brain function,(18–21) etc.
Partially Hydrolyzed Guar Gum (PHGG) is a prebiotic dietary fiber obtained from the endosperm of the guar bean (Cyanopsis tetragonolopus).(22) The PHGG is manufactured by treatment of guar bean seeds with a β-endogalactomannase derived from a strain of Aspergillus niger. It is galactomannans composed of galactose and mannose with a variety of prebiotic effects.(23,24) PHGG selectively increases the beneficial bacteria such as Bifidobacterium and butyrate-producing bacteria and promotes the production of SCFAs.(25–30) Not only does it improve constipation,(31,32) but it also improves diarrhea.(33–35) In addition, PHGG is reported to improve chronic kidney disease,(36) fatty liver,(37) and muscle atrophy via maintenance of intestinal barrier function and suppression of systemic inflammation in mice,(38,39) and to improve lifestyle-related diseases, such as abnormalities in glucose and lipid metabolism in humans.(40,41) Thus, PHGG may have various benefits on systemic health through improvement of the intestinal environment. In the present study, we evaluated and compared the effect of PHGG on gut and mental health in a placebo-controlled study using a randomized, double-blind design.
Materials and Methods
Study design
The present randomized, double-blind, placebo-controlled, parallel-group study was conducted at a clinical research organization [Healthcare Systems Co. Ltd. (HCS, Tokyo, Japan)]. Setsunan University (Osaka, Japan), Kyoto Prefectural University of Medicine (Kyoto, Japan), and Taiyo Kagaku Co., Ltd. (TKC, Mie, Japan) jointly prepared the study protocol, and TKC provided the test food. All study procedures were undertaken by HCS in the consignment from TKC. The study was conducted following the ethical principles based on the Declaration of Helsinki and the Ethical Guideline for Medical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare, 2021). The study protocol was approved by the institutional review board of HCS (approval number: 2122; Date of approval: October 26, 2021), and registered at the UMIN-CTR (Trial ID: UMIN000045324). The protocol was not modified from the time of final setup and during the study.
Study functional food material
The commercially available PHGG (Sunfiber®) used in this study was supplied by TKC. Most of the previous clinical trials have tested more than 5 g/day PHGG supplementation for defecation improvement.(32) In this study, we aim to investigate a smaller effective dose, therefore the PHGG dose was determined to be three-gram (3 g) and five-gram (5 g) per day (containing more than 80% of soluble dietary fiber). The soluble fiber content was measured by Association of Official Agricultural Chemists (AOAC) method. The placebo was maltodextrin (Dextrose equivalence 10.0–12.0; Matsutani Chemical Industry Co., Ltd., Hyogo, Japan).
Study participants
Sixty healthy Japanese men and women, aged 30–50 years were recruited as paid volunteers. Inclusion criteria were (1) subjects who have been fully informed of the purpose and content of the study, can consent, have volunteered of their own free will with full understanding, and have agreed in writing to participate in the study, (2) subjects with subjective awareness of constipation (defecation frequency of 2–5 times/week at pre-baseline screening), and (3) subjects with high average similarity of intestinal microbiota at pre-baseline screening. Exclusion criteria were (1) subjects who have a chronic disease and are receiving drug treatment or have a history of serious illness, (2) subjects who have less than one bowel movement per week, (3) subjects who are sensitive to a fiber products or other related foods, (4) subjects who usually consume high amounts of dietary fiber or oligosaccharide-containing supplements, (5) subjects who have a habit to ingest medicines or health-promoting foods that may affect the intestinal environment, (6) subjects who participated in other trials during the month before the start of the present study, or who plan to participate in other trials after consenting to this study, (7) subjects judged ineligible by the principal investigator and medical practitioner of the study, and (8) subjects who are or are possibly pregnant, or are lactating.
Sample size
Based on previous studies on the effect of PHGG supplementation on improving the intestinal environment, we performed a sample-size calculation for the 3 g/day PHGG dose. A tentative measurement predicted the requirement of approximately 55–60 participants for this study. Therefore, we decided to recruit 60 participants (20 in each intervention group), which is an adequate number of subjects that could be conveniently handled at the clinical research facility.
Selection, randomization, and blinding
The study was performed in a randomized, parallel, double-blind, and placebo-controlled manner. Sixty subjects were selected from 86 based on their relative abundance of 36 bacterial genera that are commonly found in Japanese,(42) excluding those who with significantly different intestinal microbiota composition. The selected participants were randomly allocated to 3 g/day PHGG (T3), 5 g/day PHGG (T5), or placebo (T0) supplementation groups using a block randomization design stratified by sex and defecation frequency at screening. The allocation was operated by an independent researcher of HCS who was not actively involved in the planning, conduct, and analysis of the study. The allocation was blinded from both the subjects as well as the investigators until the completion of the intervention and data tabulation of the study.
Intervention and outcomes
The study was carried out at the designated HCS facility from November to December 2021. The participants took either PHGG or placebo every day with water for eight consecutive weeks. During the study period, subjects were instructed to maintain their usual lifestyle and refrain from taking the functional foods. The day before the visit, subjects were asked to refrain from extreme exercise, to finish their meals until 9 pm, and to avoid eating, drinking, smoking, and staying up late. On the day of the visit, subjects were asked to avoid morning exercise and to keep fasting other than water until the test was completed. The subjects recorded their daily fecal defecation characteristics (frequency, quantity, shape, smell, and excretory feeling) in a diary during the entire study period. At the baseline (0 w), after 4 weeks of consumption (4 w), and after 8 weeks of consumption (8 w) of their respective treatments, the subjects visited the clinical center and submitted fecal samples. Also, the physical examination (height, weight, body mass index (BMI), and waist circumference), the physical condition questionnaire, and quality of life (QOL) questionnaire were investigated. The overall outcomes were a fecal defecation frequency and intestinal microbiota including the questionnaire on fecal defecation characteristics, fecal water content, fecal pH, fecal organic acids, fecal putrefactive metabolites, skin moisture content, weight, BMI, waist circumference, questionnaire on sleep, stress, and fatigue. Also, the safety of the study food was investigated.
Assessment of defecation
The fecal defecation frequency was assessed by recording defecation (times/day) from two weeks prior to the start of study at baseline, and until the end of the study duration (8 weeks). The fecal characteristics were assessed at each defecation according to the following criteria; the fecal amount: relative size of a commercial M-size egg as 1 unit; the fecal shape (Bristol stool scale): 1 (separate hard lumps, like nuts), 2 (“sausage-shaped” but lumpy), 3 (like a sausage but with cracks on its surface), 4 (like a sausage or snake, smooth and soft), 5 (soft blobs with clear-cut edges), 6 (fluffy pieces with ragged edges, a mushy stool), and 7 (watery, no solid pieces); the fecal smell: 1 (no smell), 2 (no too smelly), 3 (normal smell), 4 (slightly strong smell), 5 (strong smell); the fecal excretory feeling: 1 (completely clear), 2 (mostly clear but a little residual stool feeling), 3 (discomfort with residual stools). Scores were averaged per week before the start of the study at baseline (day −14∼day 0) and the entire intake period of 8 weeks; (day 0∼day 56) for within-group and between-group comparisons.
Analysis of intestinal microbiota by 16S rRNA metagenomics
Extraction of bacterial DNA from feces, library preparation, and deep sequencing (MiSeq, Illumina K.K., Tokyo, Japan) was carried out exactly as described by Morishima et al.(43) Sequence data analysis was carried out as depicted by Hashimoto et al.(44) with modification using QIIME 2.(45) At a taxonomy assignation step of the amplicon sequence variant, the SILVA database was adopted in this study.(46)
Estimation of fecal organic acids
Approximately 50 mg of fecal samples were suspended in 0.5 ml of 14% perchloric acid, centrifuged at 10,000 × g for 5 min at 4°C, and the supernatant solution was filtered through a 0.20 μm cellulose acetate membrane filter. The amount of organic acids (succinate, lactate, formate, acetate, propionate, iso-butyrate, butyrate, iso-valerate, and valerate) were measured by ion-exclusion high-performance liquid chromatography, as described elsewhere.(47)
Fecal water content and pH measurement
Approximately 0.5 g of feces were placed in a 2 ml of microcentrifuge tube and accurate weight was collected. Then, feces were freeze-dried and then weighted. Fecal water content was calculated based on fecal weight before and after drying. Feces was diluted 10-fold with distilled water and pH was measured by handy pencil-type pH meter (SPH70; AZ ONE, Tokyo, Japan).
Measurement of fecal putrefactive metabolites and ammonia content
The fecal putrefactive metabolites such as phenol, indole, skatole, para-cresol, and para-ethylphenol were measured by gas chromatography-mass spectrometry (GC-MS) as described elsewhere.(48) Fecal ammonia concentrations were measured using an ammonia test kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) according to the manufacturer’s protocol.
Skin moisture measurement
Skin surface stratum corneum moisture content was evaluated through high-frequency skin conductance using Skicon-200EX (I.B.S. Company, Ltd., Japan) at the inner arm site. Seven measurements were taken for each subject and five measurements with close approximation values were averaged.
Evaluation of QOL
QOL questionnaires on sleep, stress, and fatigue were assessed using a visual analog scale (VAS) at week 0, 4, and 8 of the study duration.(49) Since subjective evaluations such as mood and sensation are known to be strongly influenced by the placebo effect,(50) therefore the VAS was preferred to detect the subjective effect of the test food intake. The VAS measures the data of subjective evaluations as continuous variables and can detect even minute differences more sensitively than the stepwise subjective evaluations. The subjects were instructed to input the rate level of their condition on VAS from 0 (worst) to 10 (best). Among the VAS questionnaires, the sleep-related questionnaire was developed regarding the Ogri-Shirakawa-Azumi sleep inventory MA version (OSA-MA),(51) and the following items were evaluated: initiation of sleep, feeling of sufficient sleep, refreshment on waking up, fatigue on waking up, and daytime sleepiness. The stress-related questionnaire was developed regarding the SF-36 questionnaire,(52) and the stress-related activities and motivation toward work and study were evaluated. The fatigue-related questionnaire was conducted according to the method proposed by the Japan Society for Fatigue Science, and especially physical fatigue and mental fatigue were evaluated.
Statistical analysis
All data are presented as means ± SD. Data analysis were conducted using R (ver. 4.2.0), IBM SPSS Statistics (ver. 25), and JMP (ver. 13.2.0). P values of less than 0.05 were considered statistically significant (p<0.05). The male-female ratio was compared by the χ2 test. The Paired t test as a parametric test and the Wilcoxon’s signed-rank sum test as a non-parametric test was used for within-group comparison. Analysis of variance (ANOVA) and repeated-measures ANOVA was used to determine the effectiveness of factors in between-group and within-group comparison, respectively. The Dunnett’s test as a parametric test and the Steel’s test as a non-parametric test were used for multiple comparisons with the placebo group (T0). Tukey’s HSD post-hoc was used for multiple comparisons within all experimental groups to compare intestinal microbiota abundances. The nonparametric Spearman rank correlation coefficient was used to assess the relationship between the intestinal microbiota (genus level) and defecation characteristics.
Results
Characteristics of participants
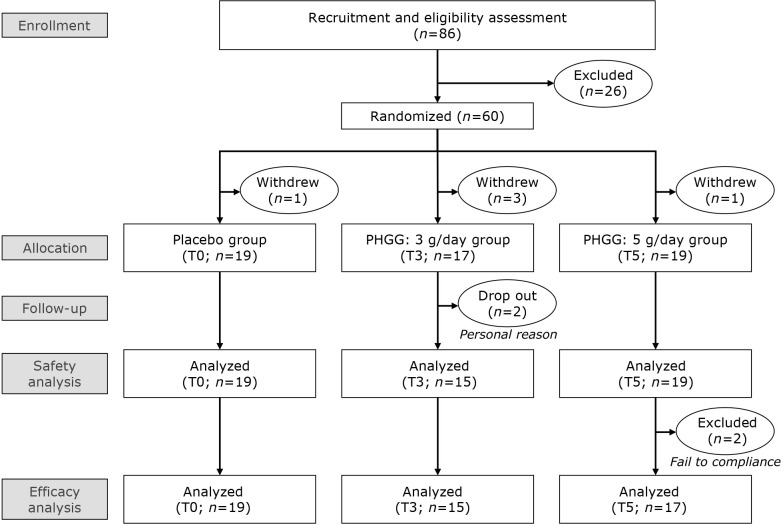
The study flow diagram is displayed in Fig. 1. Sixty subjects were selected from 86 subjects based on their common intestinal microbiota regimen and randomly allocated to T0, T3, and T5 groups. However, after screening, five subjects [T0 (n = 1), T3 (n = 3), and T5 (n = 1)] withdrew from the study, while during the study, two subjects [T3 (n = 2)] dropped out for personal reasons. Thus, 53 subjects completed the study. Two subjects [T5 (n = 2)] were excluded from the analysis due to noncompliance with the study protocol. As a result, 53 subjects were included in the safety analysis, and 51 subjects were included in the validity analysis. One subject [T3 (n = 1)] missed the clinical visit at week 4, therefore only the results of the defecation assessment were used and other data were excluded. Table 1 lists the baseline characteristics of 51 subjects. There was no significant difference in any of the parameters between the groups. Also, there was no significant inter-group difference in any parameters throughout the examination period (data not shown). Regarding skin moisture measurement, several subjects had very high values. It is possible that they were sweating and therefore could not be measured accurately.
Fig. 1.
Flow chart of study subjects.
Table 1.
Background of the subjects at baseline
| T0 (n = 19) | T3 (n = 15) | T5 (n = 17) | ||
|---|---|---|---|---|
| Gender | (male/female) | 10/9 | 8/7 | 8/9 |
| Age | (years) | 38.9 ± 5.7 | 39.3 ± 6.1 | 40.4 ± 5.0 |
| Hight | (cm) | 168.3 ± 9.3 | 164.4 ± 6.4 | 168.2 ± 9.0 |
| Weight | (kg) | 64.0 ± 9.5 | 65.3 ± 13.8 | 67.4 ± 16.5 |
| Body mass index | (kg/m2) | 22.5 ± 2.6 | 24.0 ± 3.8 | 23.6 ± 4.6 |
| Waist circumference | (cm) | 81.1 ± 7.2 | 82.8 ± 11.9 | 83.2 ± 14.8 |
| Skin moisture content | (μs) | 57.7 ± 33.3 | 62.9 ± 37.1 | 53.1 ± 21.9 |
Data are represented as Mean ± SD. T0, Placebo; T3, PHGG 3 g/day; T5, PHGG 5 g/day.
Fecal defecation characteristics
Table 2 reveals the fecal defecation characteristics from baseline to end of study (i.e., 8 weeks). Compared to the baseline, the PHGG intake tends to improve the excretory feeling, defecation episodes (days/week), and defecation frequency (times/week). However, the within-group significance could be reached only for the 5 g/day PHGG intake (T5) group. Also significant changes between T5 and T0 (placebo) group were noticed for the excretory feeling (p = 0.048). Additionally, the changes from the baseline of defecation frequency (times/week) at 8 weeks were significantly higher in the T5 group compared to the T0 group, while it should be noted that the baseline values were higher in the T0 group compared to the T5 group without significance.
Table 2.
The fecal defecation characteristics
| Category | Group | Baseline (B) | Intake period (I) | Δ (I) − (B) |
|---|---|---|---|---|
| Defecation frequency (times/week) | T0 (n = 19) | 6.82 ± 3.94 | 6.66 ± 3.28 | −0.16 ± 2.11 |
| T3 (n = 15) | 6.07 ± 3.40 | 6.62 ± 3.09 | 0.55 ± 1.26 | |
| T5 (n = 17) | 5.35 ± 2.12 | 6.50 ± 2.70## | 1.15 ± 1.32* | |
| Defecation episode (days/week) | T0 (n = 19) | 4.76 ± 1.65 | 5.08 ± 1.52 | 0.32 ± 1.07 |
| T3 (n = 15) | 5.00 ± 1.66 | 5.43 ± 1.24 | 0.43 ± 0.93 | |
| T5 (n = 17) | 4.79 ± 1.44 | 5.38 ± 1.41# | 0.58 ± 0.88 | |
| Amount | T0 (n = 19) | 2.03 ± 0.63 | 2.13 ± 0.65 | 0.10 ± 0.57 |
| T3 (n = 15) | 2.32 ± 0.95 | 2.23 ± 0.84 | −0.09 ± 0.49 | |
| T5 (n = 17) | 2.37 ± 1.08 | 2.55 ± 1.15 | 0.18 ± 0.44 | |
| Shape | T0 (n = 19) | 3.27 ± 1.08 | 3.34 ± 0.74 | 0.07 ± 0.67 |
| T3 (n = 15) | 3.75 ± 0.91 | 3.59 ± 0.76 | −0.16 ± 0.74 | |
| T5 (n = 17) | 3.63 ± 0.92 | 3.86 ± 0.58 | 0.23 ± 0.66 | |
| Smell | T0 (n = 19) | 3.15 ± 0.65 | 3.03 ± 0.61 | −0.12 ± 0.44 |
| T3 (n = 15) | 3.05 ± 0.57 | 2.80 ± 0.63# | −0.25 ± 0.32 | |
| T5 (n = 17) | 3.19 ± 0.54 | 2.98 ± 0.49 | −0.21 ± 0.51 | |
| Excretory feeling | T0 (n = 19) | 1.90 ± 0.60 | 1.74 ± 0.51 | −0.16 ± 0.40 |
| T3 (n = 15) | 1.77 ± 0.49 | 1.67 ± 0.33 | −0.10 ± 0.37 | |
| T5 (n = 17) | 1.76 ± 0.59 | 1.38 ± 0.35*,# | −0.39 ± 0.63 |
Data are represented as Mean ± SD. T0, Placebo; T3, PHGG 3 g/day; T5, PHGG 5 g/day. Between-group comparison with placebo: *p<0.05. Within-group comparison with baseline: ##p<0.01, #p<0.05. B, at baseline (day −14 to day 0). I, Intake period (day 0 to day 56).
Although the fecal amount, shape and smell were not significantly different between the placebo (T0) and PHGG intake groups (T3 and T5), however, the smell was somewhat improved within T3 and T5 groups during the PHGG intake period. Wherein a significance (p = 0.023) could be noticed in the T3 group when compared to baseline.
Intestinal microbiota
As the alpha diversity indices of the intestinal microbiota, the Shannon index (species evenness) was significantly lower in the T3 group compared to the T0 group (T0: 5.78 ± 0.43%, T3: 5.19 ± 0.59%, p = 0.008) at week 8. There was no significant difference between groups during the study period for Chao-1 and beta diversity index. At the phylum level, the relative abundance of Desulfobacterota was significantly higher in the T5 group compared to the T0 group (T: 0.03 ± 0.06%, T5: 0.27 ± 0.06%, p = 0.028) at week 4. Table 3 shows the list of bacteria whose relative abundance differs within or between groups at each time point at the genus level. The relative abundance of Dorea and [Ruminococcus] torques_group which are potent mucin degraders increased in the T0 group,(53,54) whereas the relative abundance of Dorea was significantly lower in the T3 and T5 group, and the relative abundance of [Ruminococcus] torques_group was also significantly lower in the T5 group. Also, the relative abundance of Incertae_Sedis belonging to family Ruminococcaceae in the T0 group increased and was higher compared to the T3 and T5 group at week 4, and Monoglobus was significantly higher in the T0 compared to the T5 group at week 8. On the other hand, the relative abundance of Paraprevotella and Desulfovibrio was significantly higher in the T5 group compared to the T0 group at week 4, the relative abundance of Sellimonas was significantly higher in the T3 group compared to the T0 group at week 8. Although there was no significant difference between the groups, the relative abundance of Bacteroides increased during the study in the T5 group, and that at week 8 was significantly higher than at baseline. There was no significant difference between groups regarding the changes in the relative abundance of these microbes from baseline at 4 or 8 weeks (Supplemental Table 1*). The relative abundance of intestinal microbiota at the phyla and genus level are shown in Supplemental Fig. 1 and 2*.
Table 3.
Bacterial genera whose relative abundance differs within or between-groups
| Taxonomy | Group | 0 week | 4 weeks | 8 weeks | ||
|---|---|---|---|---|---|---|
| Order | Family | Genus | ||||
| Bacteroidales | Bacteroidaceae | Bacteroides | T0 (n = 19) | 6.686 ± 6.481 | 6.117 ± 5.371 | 11.47 ± 9.871 |
| T3 (n = 14) | 10.31 ± 9.623 | 10.17 ± 12.04 | 8.955 ± 8.699 | |||
| T5 (n = 17) | 8.029 ± 8.086 | 9.888 ± 8.142 | 16.04 ± 9.876# | |||
| Bacteroidales | Prevotellaceae | Paraprevotella | T0 (n = 19) | 0.193 ± 0.732 | 0.032 ± 0.099 | 0.222 ± 0.718 |
| T3 (n = 14) | 0.081 ± 0.166 | 0.028 ± 0.063 | 0.054 ± 0.113 | |||
| T5 (n = 17) | 0.369 ± 0.652 | 0.246 ± 0.431* | 0.195 ± 0.289 | |||
| Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | T0 (n = 19) | 0.046 ± 0.147 | 0.007 ± 0.014 | 0.125 ± 0.404 |
| T3 (n = 14) | 0.092 ± 0.345 | 0.026 ± 0.098 | 0.036 ± 0.094 | |||
| T5 (n = 17) | 0.141 ± 0.294 | 0.213 ± 0.385* | 0.317 ± 0.769 | |||
| Lachnospirales | Lachnospiraceae | Dorea | T0 (n = 19) | 2.139 ± 1.881 | 2.787 ± 2.656 | 2.252 ± 2.355 |
| T3 (n = 14) | 0.802 ± 1.164 | 0.949 ± 1.231* | 0.895 ± 1.114 | |||
| T5 (n = 17) | 1.190 ± 1.825 | 1.184 ± 1.329* | 0.821 ± 1.094 | |||
| Lachnospirales | Lachnospiraceae | [Ruminococcus]_torques_group | T0 (n = 19) | 2.454 ± 3.239 | 2.686 ± 3.407 | 2.809 ± 4.831 |
| T3 (n = 14) | 1.430 ± 1.479 | 1.482 ± 1.518 | 2.126 ± 2.436 | |||
| T5 (n = 17) | 1.034 ± 1.618 | 0.531 ± 0.668* | 0.445 ± 0.604 | |||
| Oscillospirales | Ruminococcaceae | Incertae_Sedis | T0 (n = 19) | 2.269 ± 5.175 | 2.821 ± 3.333 | 1.344 ± 1.488 |
| T3 (n = 14) | 1.090 ± 0.940 | 0.703 ± 0.629* | 2.014 ± 1.959 | |||
| T5 (n = 17) | 1.256 ± 1.586 | 0.967 ± 1.240* | 0.806 ± 0.640 | |||
| Oscillospirales | Unclassified | Unclassified | T0 (n = 19) | 0.001 ± 0.002 | 0.001 ± 0.002 | 0.005 ± 0.009# |
| T3 (n = 14) | 0.002 ± 0.005 | 0.002 ± 0.006 | 0.005 ± 0.016 | |||
| T5 (n = 17) | 0.001 ± 0.002 | 0.006 ± 0.017 | 0.001 ± 0.003 | |||
| Lachnospirales | Lachnospiraceae | Sellimonas | T0 (n = 19) | 0.137 ± 0.311 | 0.111 ± 0.272 | 0.087 ± 0.241 |
| T3 (n = 14) | 0.432 ± 0.652 | 0.501 ± 0.838 | 0.624 ± 0.951* | |||
| T5 (n = 17) | 0.441 ± 0.784 | 0.196 ± 0.355 | 0.176 ± 0.222 | |||
| Monoglobales | Monoglobaceae | Monoglobus | T0 (n = 19) | 1.126 ± 1.037 | 1.215 ± 0.981 | 0.870 ± 0.598 |
| T3 (n = 14) | 0.761 ± 0.959 | 0.716 ± 0.891 | 0.533 ± 0.535 | |||
| T5 (n = 17) | 0.450 ± 0.489 | 0.592 ± 0.725 | 0.411 ± 0.462* | |||
Data are represented as Mean ± SD. T0, Placebo; T3, PHGG 3 g/day; T5, PHGG 5 g/day. Between-group comparison with placebo: *p<0.05. Within-group comparison with baseline: #p<0.05.
Correlation between intestinal microbiota and defecation characteristics
Supplemental Fig. 3* shows the correlation between the relative abundance of each intestinal microbiome (genus level) and defecation characteristics. Although there was no strong correlation between any of the items, for the defecation frequency and defecation episodes, Megasphaera, Megamonas, and [Ruminococcus]_gnavus_group showed positive correlations while Akkermansia, Subdoligranulum, Agathobacter, and UCG-002 belonging to family Oscillospiraceae showed negative correlations. For the excretory feeling (discomfort with residual stools), Monoglobus showed a positive correlation while Selimonas and Agathobacter showed negative correlations. For fecal amount, Holdemanella and Prevotella showed a positive correlation, while Incertae_Sedis and Monoglobus showed a negative correlation. For fecal shape (softness), Erysipelotrichaceae_UCG-003, Holdemanella, and Veilonella showed a positive correlation, while Incertae_Sedis and Ruminococcus showed a negative correlation.
Fecal characteristics
The fecal moisture and pH are shown in Supplemental Table 2*. The fecal pH was significantly higher in the T0 group after 8 weeks compared to the baseline, and was significantly (p = 0.037) lower in the T3 group (7.07 ± 0.67) compared to the T0 group (7.53 ± 0.44) at week 8. There were no significant inter-group differences in moisture, organic acids, and putrefactive metabolites in feces throughout the study examination period (data not shown).
QOL questionnaire
Table 4 lists the mean VAS scores for each QOL questionnaire item. The scores of most of the items significantly decreased at week 4 compared to baseline in all groups. However, there were significant improvement (increase) in values after eight weeks in both PHGG intake groups. Additionally, in the T5 group, the mean VAS scores of “refreshment on waking up” (T0: 3.77 ± 1.14, T5: 4.76 ± 1.21, p = 0.037) and “fatigue on waking up” (T0: 3.39 ± 1.16, T5: 4.54 ± 1.30, p = 0.009) in the sleep-related questionnaire, and “motivation toward work and study” (T0: 3.60 ± 1.10, T5: 4.46 ± 0.88, p = 0.030) in the stress-related questionnaire were significantly higher than that of the T0 group at week 8, indicating the improvement. There was no significant difference between groups for any questionnaire item regarding the changes from the baseline at 4 or 8 weeks (Supplemental Table 3*).
Table 4.
VAS scores for QOL questionnaire
| Category | Group | 0 week | 4 weeks | 8 weeks |
|---|---|---|---|---|
| Initiation of sleep | T0 (n = 19) | 5.52 ± 2.14 | 4.03 ± 1.11## | 4.31 ± 1.16# |
| T3 (n = 14) | 5.81 ± 2.32 | 4.11 ± 0.95# | 4.33 ± 1.50# | |
| T5 (n = 17) | 6.36 ± 2.14 | 4.27 ± 1.45## | 5.09 ± 1.17# | |
| Feeling of sufficient sleep | T0 (n = 19) | 4.77 ± 2.49 | 3.67 ± 1.22# | 3.90 ± 1.10 |
| T3 (n = 14) | 4.62 ± 2.71 | 3.95 ± 1.22 | 4.04 ± 1.55 | |
| T5 (n = 17) | 4.49 ± 2.54 | 3.54 ± 1.33 | 4.45 ± 1.36 | |
| Refreshment on waking up | T0 (n = 19) | 4.51 ± 2.38 | 3.73 ± 1.33 | 3.77 ± 1.14 |
| T3 (n = 14) | 5.30 ± 2.37 | 3.71 ± 1.36## | 4.01 ± 1.35# | |
| T5 (n = 17) | 4.95 ± 2.71 | 4.55 ± 1.25 | 4.76 ± 1.21* | |
| Fatigue on waking up | T0 (n = 19) | 3.86 ± 2.22 | 3.00 ± 1.29 | 3.39 ± 1.16 |
| T3 (n = 14) | 4.60 ± 2.03 | 3.41 ± 1.37# | 3.50 ± 0.94 | |
| T5 (n = 17) | 4.36 ± 2.55 | 3.86 ± 1.26 | 4.54 ± 1.30** | |
| Daytime sleepiness | T0 (n = 19) | 4.66 ± 2.39 | 3.51 ± 1.38## | 3.78 ± 1.68# |
| T3 (n = 14) | 4.74 ± 2.26 | 3.82 ± 1.33 | 3.64 ± 1.46# | |
| T5 (n = 17) | 4.42 ± 2.34 | 3.74 ± 1.42 | 4.27 ± 1.60 | |
| Stress related activities | T0 (n = 19) | 3.83 ± 1.85 | 2.71 ± 1.50## | 3.12 ± 1.29# |
| T3 (n = 14) | 3.55 ± 1.91 | 3.23 ± 1.39 | 3.44 ± 1.23 | |
| T5 (n = 17) | 4.45 ± 2.18 | 3.56 ± 1.30 | 3.94 ± 1.57 | |
| Motivation toward work and study | T0 (n = 19) | 4.88 ± 2.51 | 3.58 ± 1.27# | 3.60 ± 1.10 |
| T3 (n = 14) | 5.04 ± 1.72 | 3.91 ± 1.06# | 3.78 ± 1.11## | |
| T5 (n = 17) | 5.64 ± 1.88 | 4.31 ± 1.09## | 4.46 ± 0.88*,## | |
| Physical fatigue | T0 (n = 19) | 3.59 ± 2.10 | 3.20 ± 1.34 | 3.54 ± 1.50 |
| T3 (n = 14) | 4.31 ± 1.79 | 3.49 ± 1.26 | 3.26 ± 1.20# | |
| T5 (n = 17) | 5.02 ± 1.62* | 4.05 ± 1.23 | 4.46 ± 1.34 | |
| Mental fatigue | T0 (n = 19) | 3.77 ± 2.38 | 3.03 ± 1.51 | 3.24 ± 1.36 |
| T3 (n = 14) | 4.51 ± 2.25 | 3.61 ± 1.62# | 3.37 ± 1.46# | |
| T5 (n = 17) | 5.49 ± 2.31 | 3.88 ± 1.55## | 4.26 ± 1.57# |
Data are represented as Mean ± SD. T0, Placebo; T3, PHGG 3 g/day; T5, PHGG 5 g/day. Between-group comparison with placebo: **p<0.01, *p<0.05. Within-group comparison with baseline: ##p<0.01, #p<0.05.
Safety aspects
Although the results of this study showed that PHGG intake increased the Desulfovibrio abundance which is a sulfate-reducing bacterium that could causes infections in some species,(55) however, no serious adverse reports were observed during the study, indicating that this bacterium might not harmful. Desulfovibrio has also been shown not to be necessarily associated with adverse health in a recent large cohort study.(56) Therefore, it was concluded that there was no adverse effect caused by the intake of the PHGG dietary fiber as a test food in the present study.
Discussion
Defecation disorders can greatly impair the QOL, not only physically but also mentally. In this study, we investigated whether PHGG intake contributes to improving constipation and gastrointestinal health, including mental health in healthy subjects with constipation symptoms. In addition to the 5 g/day PHGG intake group, the 3 g/day intake group was also examined to determine if a smaller dose of PHGG could be effective.
In the Japanese Clinical Guidelines, constipation is defined as a “state in which feces that should be defecated from the body cannot be defecated in sufficient quantity and comfortably”.(57) In this study, 5 g/day PHGG intake significantly improved the excretory feeling, or comfort of defecation, indicating the efficacy of PHGG dietary fiber on constipation. In addition, an increase in the frequency of defecation was also observed. These results are consistent with previously reported clinical studies.(31) A 5 g/day PHGG intake significantly improved the excretory feeling and the defecation frequency compared to a placebo intake. Whereas, 3 g/day PHGG intake did not significantly improve the excretory feeling, however, the rate of the defecation frequency was higher during the intake period than at baseline. The fecal smell during the intake period was also significantly improved compared to baseline. The overall results confirmed the effectiveness of PHGG dietary fiber for improved QOL.
Concerning the intestinal microbiota, Paraprevotella and Sellimonas which are well known to be involved in organic acid production were maintained at higher levels in either PHGG intake groups compared to the placebo.(58–60) Sellimonas negatively correlates with the excretory feeling (discomfort with residual stools) in the correlation analysis (Supplemental Fig. 3*) suggesting that it might be involved in constipation remediation. The relative abundance of Bacteroides which are involved in succinate and propionate production was also significantly increased with 5 g/day PHGG intake compared to the baseline.(61) These results indicate the prebiotic effect of PHGG dietary fiber, consistent with the previous clinical studies that showed PHGG intake was associated with increases in organic acid-producing bacteria such as Ruminococcus, Fusicatenibacter, Faecalibacterium, Bacteroides, and Bifidobacterium.(27,28,62) In addition, this study has newly confirmed that PHGG intake greatly suppresses the mucolytic bacteria, which could be potentially harmful in healthy subjects. The relative abundance of Dorea and [Ruminococcus] torques_group was maintained significantly lower in the PHGG dietary fiber intake groups compared to the placebo group. Ruminococcus_torques is a mucin-degrading bacterium known to cause inflammation by weakening the gut barrier function,(63–65) increase under conditions of dysbiosis in patients with irritable bowel disease and autism spectrum disorder.(54,66) Dorea produces ethanol, formate, and acetate from carbohydrates,(53) while it is also reported to involve in mucin degradation associated with inflammation,(67) irritable bowel syndrome,(68) fatty liver,(69) and multiple sclerosis.(70) Since the disruption of the mucosal barrier is closely associated with chronic systemic inflammation and various kinds of diseases,(71) suppressing the increase of these bacteria might contribute to the maintenance of systemic health. Ruminococcaceae Incertae_Sedis and Monoglobus, which were similarly kept in lower abundance in PHGG intake groups, are not been characterized in detail. However, Ruminococcaceae Incertae_Sedis negatively correlates with the fecal amount and fecal shape (softness), and Monoglobus positively correlates with excretory feeling (discomfort with residual stools) while negatively correlates with the fecal amount in the correlation analysis (Supplemental Fig. 3*), suggesting that they might be involved in constipation. The suppressing effect of PHGG on such potentially harmful bacteria in addition to the promoting effect on organic acid-producing bacteria might be involved in constipation improvement.
Furthermore, PHGG’s efficacy on intestinal microbiota may have contributed to mental health. In this study, we also newly confirmed the effectiveness of PHGG intake on sleep and motivation with the QOL questionnaire. In all questionnaire items related to sleep, stress, and fatigue, all groups had worse scores during the study period compared to baseline. The COVID-19 infection increased sharply during the study period in Japan, and restrictions on daily life, such as the recommendation to stay home, might have caused psychological stress.(72,73) Even under such circumstances, refreshment and fatigue on waking up and motivation toward work and study were significantly improved with 5 g/day PHGG intake. Thus, PHGG intake might alleviate mental stress and improve sleep and motivation. There are numerous studies on the relationship between gut bacteria, stress, and sleep.(16,17) And the involvement of mucolytic bacteria in insomnia is also shown.(74) Increases in Dorea and Ruminococcus torques were observed in shift workers whose circadian rhythms were disrupted,(75) and increases in Ruminococcus torques were observed in a mouse model of disrupted circadian rhythms,(76) which were just the same bacteria whose relative abundances were kept lower by PHGG intake in this study. Excessive increase of mucolytic bacteria disrupts the intestinal barrier function via degradation of the mucin layer, allowing entry of pathogens and their metabolites into the body and causing inflammation.(71) Systemic inflammation causes permeability of the blood-brain barrier, and neuro-inflammation due to the influx of inflammatory substances into the brain might cause insomnia and stress.(77) Based on the above mechanisms, PHGG might improve sleep and motivation through the suppression of mucin-degrading bacteria associated with gut barrier dysfunction, systemic inflammation, and neuroinflammation. In the previous clinical trial of PHGG in children with autism spectrum disorder, in addition to improving intestinal health, PHGG also reduced inflammatory cytokines in the blood and suppressed irritable behavior,(78) similarly suggesting that PHGG may reduce systemic inflammation and neuroinflammation by improving the intestinal environment. In addition, intestinal microbial metabolites altered by PHGG ingestion might affect brain function. It is reported that SCFAs produced by intestinal bacteria regulate circadian rhythms,(79) and that neurotransmitters produced via tryptophan metabolism in gut bacteria are supposed to affect brain function.(80) Since PHGG intake is shown to increase serotonin and dopamine in serum and the striatum and hippocampus, and improve depressive behavior in stressed mice,(81) it could be possible that PHGG intake improves mental health through the modulation of neurotransmitters also in humans. Comprehensive and detailed mechanism elucidation is desirable in the future. Regarding the metabolites of intestinal bacteria, previous clinical trials have shown that PHGG intake promotes SCFA production and reduces putrefactive metabolites,(26,62,82) whereas no significant differences were found between the groups in the present study. The limitation of this study is that we did not examine the usual diet of study participants outside of restricted probiotic and prebiotic intake to investigate the impact of PHGG intake in their daily lives. Since SCFAs are produced by carbohydrates reaching the digestive tract and putrefactive metabolites are produced by metabolizing amino acids derived from the diet, the base diet other than test food may have a great influence. In the future studies, it will be necessary to fully consider the influence of the base diet. In addition, since individual differences in intestinal microbiota may have a significant impact, further investigation is desirable through larger-scale clinical trials. Concerning the skin-moisture content analysis, we believe that data were not completed, therefore we decided to omit the disussion on the effect of PHGG on skin-moisture content. This will be a topic of a large-scale future clinical study with dietary PHGG fiber.
The results of the present study demonstrated that intervention of PHGG was found effective in improving constipation, intestinal microbiota, as well as sleep, and motivation in the healthy subjects. However, further comprehensive studies are needed to design with large sample size and diversity of the population to evaluate the potential of PHGG.
Supplementary Materials
The following online documents are available: Supplemental Table 1*. The changes from the baseline of the relative abundance of each intestinal microbes; Supplemental Table 2*. The fecal moisture and pH; Supplemental Table 3*. The changes from the baseline of VAS scores for QOL questionnaire; Supplemental Fig. 1*. The relative abundance of intestinal microbiota at the phyla level; Supplemental Fig. 2*. The relative abundance of intestinal microbiota at the genus level; Supplemental Fig. 3*. The correlation analysis between intestinal microbiome and defecation characteristics.
Author Contributions
AA and MO conceived this experiment; AA, MO, RI, and YN designed the research; RI and TT conducted the experiments and analyzed data; AA, SM, and MPK wrote the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This research received funding from Taiyo Kagaku Co., Ltd. The authors appreciate the staff at Healthcare Systems Co., Ltd., and Akasaka Family Clinic who supported the present study.
Conflict of Interest
There were no particular conflicts of interest. However, referring to a potential conflict of interest, AA, SM, MPK, and MO were employed by Taiyo Kagaku Co., Ltd.; RI received a research support fund from Taiyo Kagaku Co., Ltd.; YN received a scholarship for research from Taiyo Kagaku Co., Ltd.
Supplementary Material
References
- 1.Nakaji S, Sugawara K, Saito D, et al. Trends in dietary fiber intake in Japan over the last century. Eur J Nutr 2002; 41: 222–227. [DOI] [PubMed] [Google Scholar]
- 2.Clemens R, Kranz S, Mobley AR, et al. Filling America’s fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J Nutr 2012; 142: 1390S–1401S. [DOI] [PubMed] [Google Scholar]
- 3.Stephen AM, Champ MM, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 2017; 30: 149–190. [DOI] [PubMed] [Google Scholar]
- 4.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013; 5: 1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017; 8: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 9.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019; 129: 4050–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci 2015; 16: 7493–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 2018; 6: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10: 735–742. [DOI] [PubMed] [Google Scholar]
- 14.Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 2015; 13: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor AM, Holscher HD. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr Neurosci 2020; 23: 237–250. [DOI] [PubMed] [Google Scholar]
- 16.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev 2020; 53: 101340. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Hao Y, Fan F, Zhang B. The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatry 2018; 9: 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017; 67: 1084–1103. [DOI] [PubMed] [Google Scholar]
- 19.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 2003; 16: 658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett 2012; 334: 1–15. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RS, Vargas F, Dorrestein PC, Chichlowski M, Berg BM, Fleshner M. Dietary prebiotics alter novel microbial dependent fecal metabolites that improve sleep. Sci Rep 2020; 10: 3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghani Soltani M, Meftahizadeh H, Barani M, et al. Guar (Cyamopsis tetragonoloba L.) plant gum: from biological applications to advanced nanomedicine. Int J Biol Macromol 2021; 193 (Pt B): 1972–1985. [DOI] [PubMed] [Google Scholar]
- 23.Rao TP, Quartarone G. Role of guar fiber in improving digestive health and function. Nutrition 2019; 59: 158–169. [DOI] [PubMed] [Google Scholar]
- 24.Slavin JL, Greenberg NA. Partially hydrolyzed guar gum: clinical nutrition uses. Nutrition 2003; 19: 549–552. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi Y, Sumitani K, Tokunaga M, Ishihara N, Okubo T, Fujisawa T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef Microbes 2015; 6: 451–455. [DOI] [PubMed] [Google Scholar]
- 26.Okubo T, Ishihara N, Takahashi H, et al. Effects of partially hydrolyzed guar gum intake on human intestinal microflora and its metabolism. Biosci Biotechnol Biochem 1994; 58: 1364–1369. [Google Scholar]
- 27.Yasukawa Z, Inoue R, Ozeki M, et al. Effect of repeated consumption of partially hydrolyzed guar gum on fecal characteristics and gut microbiota: a randomized, double-blind, placebo-controlled, and parallel-group clinical trial. Nutrients 2019; 11: 2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor MP, Koido M, Kawaguchi M, et al. Lifestyle related changes with partially hydrolyzed guar gum dietary fiber in healthy athlete individuals—A randomized, double-blind, crossover, placebo-controlled gut microbiome clinical study. J Funct Foods 2020; 72: 104067. [Google Scholar]
- 29.Pylkas AM, Juneja LR, Slavin JL. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J Med Food 2005; 8: 113–116. [DOI] [PubMed] [Google Scholar]
- 30.Velázquez M, Davies C, Marett R, Slavin JL, Feirtag JM. Effect of oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe 2000; 6: 87–92. [Google Scholar]
- 31.Kapoor MP, Sugita M, Fukuzawa Y, Okubo T. Impact of partially hydrolyzed guar gum (PHGG) on constipation prevention: a systematic review and meta-analysis. J Funct Foods 2017; 33: 52–66. [Google Scholar]
- 32.Polymeros D, Beintaris I, Gaglia A, et al. Partially hydrolyzed guar gum accelerates colonic transit time and improves symptoms in adults with chronic constipation. Dig Dis Sci 2014; 59: 2207–2214. [DOI] [PubMed] [Google Scholar]
- 33.Alam NH, Ashraf H, Kamruzzaman M, et al. Efficacy of partially hydrolyzed guar gum (PHGG) supplemented modified oral rehydration solution in the treatment of severely malnourished children with watery diarrhoea: a randomised double-blind controlled trial. J Health Popul Nutr 2015; 34: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rushdi TA, Pichard C, Khater YH. Control of diarrhea by fiber-enriched diet in ICU patients on enteral nutrition: a prospective randomized controlled trial. Clin Nutr 2004; 23: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 35.Homann HH, Kemen M, Fuessenich C, Senkal MZV. Reduction in diarrhea incidence by soluble fiber in patients receiving total or supplemental enteral nutrition. J Parenter Enter Nutr 1994; 18: 486–490. [DOI] [PubMed] [Google Scholar]
- 36.Hung TV, Suzuki T. Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J Nutr 2018; 148: 552–561. [DOI] [PubMed] [Google Scholar]
- 37.Takayama S, Katada K, Takagi T, et al. Partially hydrolyzed guar gum attenuates non-alcoholic fatty liver disease in mice through the gut-liver axis. World J Gastroenterol 2021; 27: 2160–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakakida T, Ishikawa T, Doi T, et al. Water‐soluble dietary fiber alleviates cancer‐induced muscle wasting through changes in gut microenvironment in mice. Cancer Sci 2022; 113: 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura T, Hamaguchi M, Mori J, et al. Partially hydrolyzed guar gum suppresses the development of sarcopenic obesity. Nutrients 2022; 14: 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dall'Alba V, Silva FM, Antonio JP, et al. Improvement of the metabolic syndrome profile by soluble fibre - guar gum - in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 2013; 110: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 41.Kapoor MP, Ishihara N, Okubo T. Soluble dietary fibre partially hydrolysed guar gum markedly impacts on postprandial hyperglycaemia, hyperlipidaemia and incretins metabolic hormones over time in healthy and glucose intolerant subjects. J Funct Foods 2016; 24: 207–220. [Google Scholar]
- 42.Takagi T, Inoue R, Oshima A, et al. Typing of the gut microbiota community in Japanese subjects. Microorganisms 2022; 10: 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishima S, Oda N, Ikeda H, et al. Altered fecal microbiotas and organic acid concentrations indicate possible gut dysbiosis in university rugby players: an observational study. Microorganisms 2021; 9: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimoto Y, Hamaguchi M, Kaji A, et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J Diabetes Investig 2020; 11: 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019; 37: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glöckner FO, Yilmaz P, Quast C, et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol 2017; 261: 169–176. [DOI] [PubMed] [Google Scholar]
- 47.Ushida K, Sakata T. Effect of pH on oligosaccharide fermentation by porcine cecal digesta. Nihon Chikusan Gakkaiho 1998; 69: 100–107. [Google Scholar]
- 48.Morishima S, Aoi W, Kawamura A, et al. Intensive, prolonged exercise seemingly causes gut dysbiosis in female endurance runners. J Clin Biochem Nutr 2021; 68: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxwell C. Sensitivity and accuracy of the visual analogue scale: a psycho-physical classroom experiment. Br J Clin Pharmacol 1978; 6: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015; 16: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto Y, Tanaka H, Takase H, Shirakawa M. Standardization of revised version of OSA sleep inventory for middle age and aged. Brain Sci Ment Disord 1999; 10: 401–409. [Google Scholar]
- 52.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for Use in Japan. J Clin Epidemiol 1998; 51: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 53.Taras D, Simmering R, Collins MD, Lawson PA, Blaut M. Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2002; 52 (Pt 2): 423–428. [DOI] [PubMed] [Google Scholar]
- 54.Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 2010; 105: 2420–2428. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein EJ, Citron DM, Peraino VA, Cross SA. Desulfovibrio desulfuricans bacteremia and review of human Desulfovibrio infections. J Clin Microbiol 2003; 41: 2752–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YR, Jing QL, Chen FL, Zheng H, Chen LD, Yang ZC. Desulfovibrio is not always associated with adverse health effects in the Guangdong Gut Microbiome Project. PeerJ 2021; 9: e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Japan Society of Gastroenterology-affiliated Study Group, Chronic Constipation Diagnosis and Treatment Study Group. Clinical Guidelines for Chronic Constipation 2017. Tokyo: Nankodo, 2017. [Google Scholar]
- 58.Morotomi M, Nagai F, Sakon H, Tanaka R. Paraprevotella clara gen. nov., sp. nov. and Paraprevotella xylaniphila sp. nov., members of the family ‘Prevotellaceae’ isolated from human faeces. Int J Syst Evol Microbiol 2009; 59 (Pt 8): 1895–1900. [DOI] [PubMed] [Google Scholar]
- 59.Seo B, Yoo JE, Lee YM, Ko G. Sellimonas intestinalis gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2016; 66: 951–956. [DOI] [PubMed] [Google Scholar]
- 60.Muñoz M, Guerrero-Araya E, Cortés-Tapia C, Plaza-Garrido A, Lawley TD, Paredes-Sabja D. Comprehensive genome analyses of sellimonas intestinalis, a potential biomarker of homeostasis gut recovery. Microb Genomics 2020; 6: mgen000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017; 19: 29–41. [DOI] [PubMed] [Google Scholar]
- 62.Reider SJ, Moosmang S, Tragust J, et al. Prebiotic effects of partially hydrolyzed guar gum on the composition and function of the human microbiota-results from the PAGODA trial. Nutrients 2020; 12: 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson G, Falk P, Hoskins LC. Degradation of human intestinal glycosphingolipids by extracellular glycosidases from mucin-degrading bacteria of the human fecal flora. J Biol Chem 1988; 263: 10790–10798. [PubMed] [Google Scholar]
- 64.Hoskins LC, Boulding ET, Gerken TA, Harouny VR, Kriaris MS. Mucin glycoprotein degradation by mucin oligosaccharide-degrading strains of human faecal bacteria. Characterisation of saccharide cleavage products and their potential role in nutritional support of larger faecal bacterial populations. Microb Ecol Health Dis 1992; 5: 193–207. [Google Scholar]
- 65.Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018; 6: 1539595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism 2013; 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms 2020; 8: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajilić-Stojanović M, Biagi E, Heilig HGHJ, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 69.Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017; 65: 451–464. [DOI] [PubMed] [Google Scholar]
- 70.Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes 2017; 8: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selwal KK, Selwal MK, Yu Z. Mucolytic bacteria: prevalence in various pathological diseases. World J Microbiol Biotechnol 2021; 37: 176. [DOI] [PubMed] [Google Scholar]
- 72.Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord 2021; 281: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueda M, Stickley A, Sueki H, Matsubayashi T. Mental health status of the general population in Japan during the COVID-19 pandemic. Psychiatry Clin Neurosci 2020; 74: 505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Shao L, Mou Y, Zhang Y, Ping Y. Sleep, circadian rhythm and gut microbiota: alterations in Alzheimer’s disease and their potential links in the pathogenesis. Gut Microbes 2021; 13: 1957407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mortaş H, Bilici S, Karakan T. The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chronobiol Int 2020; 37: 1067–1081. [DOI] [PubMed] [Google Scholar]
- 76.Deaver JA, Eum SY, Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front Microbiol 2018; 9: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun 2017; 60: 1–12. [DOI] [PubMed] [Google Scholar]
- 78.Inoue R, Sakaue Y, Kawada Y, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr 2019; 64: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tahara Y, Yamazaki M, Sukigara H, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 2018; 8: 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015; 277: 32–48. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y, Wan M, Zhong Y, et al. Partially hydrolyzed guar gum modulates gut microbiota, regulates the levels of neurotransmitters, and prevents CUMS-induced depressive-like behavior in mice. Mol Nutr Food Res 2021; 65: e2100146. [DOI] [PubMed] [Google Scholar]
- 82.Miyoshi M, Kadoguchi H, Usami M, Hori Y. Synbiotics improved stool form via changes in the microbiota and short-chain fatty acids in hemodialysis patients. Kobe J Med Sci 2021; 67: E112–E118. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.