Abstract
Background
Potent P2Y12 inhibitors such as ticagrelor and prasugrel are superior to clopidogrel in acute coronary syndrome (ACS) patients treated with percutaneous coronary intervention (PCI). Whether this benefit extends to a patient population with chronic coronary syndromes (CCS) is unclear.
Aims
We sought to compare the safety and efficacy of prasugrel and ticagrelor versus clopidogrel in patients undergoing PCI for CCS.
Methods
Consecutive patients undergoing PCI for CCS at a tertiary centre between 2014 and 2019 who were discharged on prasugrel or ticagrelor were compared with those on clopidogrel. The primary endpoint was the composite of death and myocardial infarction (MI), with secondary outcomes including rates of bleeding, stroke, and target vessel revascularisation at 1 year.
Results
Overall, 11,508 patients were included in the study (ticagrelor/prasugrel n=2,860 [24.9%], clopidogrel n=8,648 [75.1%]) with an increasing frequency of potent P2Y12 inhibitor use over the study period (ptrend<0.001). Clopidogrel was used more frequently in patients with multimorbid risk factors, whereas anatomical or procedural complexity was associated with ticagrelor/prasugrel use (left main PCI, bifurcation PCI, number of lesions, rotational atherectomy). No difference in the incidence of death or MI was noted across the groups (ticagrelor/prasugrel vs clopidogrel: 2.7% vs 3.1%, adjusted hazard ratio [adjHR] 0.86, 95% confidence interval [CI]: 0.62-1.17; p=0.33) or secondary outcomes including bleeding (adjHR 0.75, 95% CI: 0.46-1.21; p=0.23) on propensity score stratification analysis. Additionally, no difference in the primary outcome was observed across subgroups, including those undergoing complex PCI.
Conclusions
Ticagrelor and prasugrel are increasingly used in patients with CCS undergoing PCI with similar 1-year efficacy and safety when compared to clopidogrel. Whether use of these agents can be beneficial in patients undergoing PCI for CCS with a high thrombotic and low bleeding risk warrants further study.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the standard of care in patients undergoing percutaneous coronary intervention (PCI)1. In acute coronary syndromes (ACS), guidelines recommend the use of the newer, more potent P2Y12 inhibitors such as ticagrelor and prasugrel due to their rapid onset of action, potency and reduced interindividual variability, with a resultant reduction in major adverse cardiovascular events (MACE)1,2,3. However, in patients undergoing PCI for chronic coronary syndromes (CCS), clopidogrel is the preferred agent with a class I level of evidence supporting its use2. Despite this, recent administrative data indicate that ticagrelor and prasugrel are increasingly prescribed off-label in patients undergoing PCI for non-ACS indications4. Factors affecting the real-world, off-label use of these agents, as well as their comparative efficacy and safety when compared to clopidogrel, remain unclear. While the incidence of MACE is lower in patients undergoing PCI for CCS, the use of these more potent agents has the potential to reduce the risk of periprocedural and long-term cardiovascular events, particularly in high-risk patients. This is important to ascertain given the increased hazard of bleeding, the lower rates of adherence and the higher out-of-pocket costs in patients prescribed the newer P2Y12 agents5,6. Although randomised trials have been performed, they are limited by differences in study inclusion criteria and the patient populations assessed, as well as by a short duration of follow-up7,8,9,10. As such, we sought to compare the efficacy and safety of clopidogrel versus ticagrelor or prasugrel in a real-world population undergoing PCI for CCS.
Methods
Study population and design
A retrospective cohort study of consecutive patients undergoing PCI at a large tertiary hospital (Mount Sinai Hospital, New York, NY, USA) between 2014 and 2019 were enrolled in the institutional catheterisation laboratory registry and assessed for inclusion in this study. Patients undergoing PCI for CCS who were prescribed aspirin in addition to clopidogrel or a newer P2Y12 agent (either prasugrel or ticagrelor) were included. Patients presenting with ACS, cardiogenic shock and those on oral anticoagulants prior to PCI were excluded. PCI was performed according to standard techniques, and stent choice was per operator preference. Likewise, the DAPT duration and regimen was based on aspirin and a P2Y12 inhibitor according to the treating physician’s preference. The study was approved by the institutional review board.
Data collection
Informed consent was given for anonymised data collection and systematic follow-up by all patients. Clinical, laboratory and angiographic data were systematically obtained from electronic medical records by using standardised forms at the time of index hospitalisation for PCI. All patients were followed up by clinic visits or telephone interviews by experienced research personnel with a medical record review of recurrent hospitalisations up to 1 year post-PCI.
Antiplatelet regimen
Patients were divided into 2 groups based on the type of P2Y12 inhibitor (clopidogrel vs ticagrelor/or prasugrel) prescribed at discharge from hospital. All patients presenting to the catheterisation laboratory routinely received 325 mg aspirin >90 minutes prior to angiography with a maintenance dose of 81 mg daily. Therapy with ticagrelor was started at a loading dose of 180 mg and continued at a maintenance dose of 90 mg twice daily. Prasugrel therapy was commenced with a loading dose of 60 mg followed by a maintenance dose of 10 mg daily in patients with a body weight ≥100 kg or if 3 stents were deployed in the same vessel, as per our institutional protocol. The remainder of the patients received a dose of 5 mg daily. Clopidogrel was initiated at a loading dose of 600 mg with a maintenance dose of 75 mg daily. The timing of P2Y12 inhibitor initiation at our centre is typically following angiography and prior to undertaking PCI, and is continued for a period of 12 months in the absence of any contraindications. Routine escalation or de-escalation of antiplatelet therapy is not undertaken at our centre.
Study definitions and endpoints
The primary endpoint of the study was the composite of all-cause death or myocardial infarction (MI; Type 1) at 1 year following PCI. Secondary endpoints included rates of target lesion revascularisation (TLR), stent thrombosis, bleeding, stroke, and the incidence of the individual components of the primary endpoint. MI was defined according to the third universal definition of MI11. TLR was defined as repeat revascularisation within the 5 mm margin proximal and distal to the stent. Bleeding was defined as per the National Cardiovascular Data Registry CathPCI Registry (version 4.4), which included any bleeding occurring during hospitalisation for the index PCI associated with a haemoglobin decrease of >3 g/dL, blood transfusion, or requiring procedural intervention or surgery at the bleeding site, or a bleeding event requiring either hospitalisation or a blood transfusion. PCI was considered complex if fulfilling any of the following criteria: total stent length ≥60 mm, total number of stents implanted ≥3, total number of lesions ≥3, total number of target vessels ≥3, bifurcation lesion with ≥2 stents, or chronic total occlusion (CTO)12.
Statistical analysis
Continuous variables are reported as mean±standard deviation or median (interquartile range) and were compared using the Student’s t-test, the Mann-Whitney U test or the Wilcoxon test on the basis of normality. Categorical variables were compared using the chi-square test or Fisher’s exact test as appropriate. Trends in the use of antiplatelets over the study duration were estimated using the Cochran-Armitage test. Predictors of antiplatelet prescription were analysed by stepwise logistic regression with backward selection (p<0.10 for inclusion in the multivariate model andp>0.20 for exclusion) with risk estimates presented as odds ratios (OR) with 95% confidence intervals (CI). Clinical follow-up was censored at the date of death or latest available follow-up. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test.
To account for baseline differences between patients prescribed the different antiplatelet regimens, propensity score stratification analysis was performed. Propensity scores were calculated using a multivariable logistic regression model with the dependent outcome as treatment with clopidogrel (vs prasugrel or ticagrelor). The propensity model was generated in an iterative fashion using the method of Rosenbaum et al13. Propensity score stratification was then used to analyse outcomes using cause-specific Cox proportional hazards regression models to account for the time-to-event nature of the data and stratified by the propensity to receive clopidogrel versus prasugrel or ticagrelor. The following variables were used for multivariate adjustment for propensity score stratification: age, sex, body mass index (BMI), race, smoker, hypertension, hyperlipidaemia, cerebrovascular disease, prior MI, prior coronary artery bypass grafts (CABG), left ventricular ejection fraction, calcification, chronic total occlusion (CTO), and complex PCI as per Giustino’s criteria. Additionally, high bleeding risk (HBR) characteristics as per the Academic Research Consortium (ARC) criteria were also included in the propensity score stratification14. Patients were defined to be at HBR if they fulfilled at least 1 major or 2 minor criteria as recommended in the ARC-HBR definition. All other patients, including those presenting with only 1 minor criterion, were classified as not at HBR. The distribution of propensity scores for the entire cohort and each treatment group were visually examined. Mutually exclusive strata were then generated based on the propensity scores for the entire cohort, a process that was blinded to any outcome data in order to avoid bias in selection. The number of strata and their respective cut-off points were based on fulfilling previously established criteria and adequate balance in baseline covariates. All reported p-values are 2-tailed with p<0.05 considered significant. Statistical analysis was performed using Stata version 15.1 (StataCorp).
Results
During the study period, 30,675 PCI were performed. After the exclusion of patients who did not meet the study inclusion criteria (n=13,090), those undergoing repeat procedures (n=4,151), and those lost to follow-up (n=1,107), a total of 11,508 patients were included in the final analysis (Supplementary Figure 1). The mean age was 66±11 years, and 26.8% were female. Clopidogrel was used in the majority of patients (8,648 [75.1%]), followed by ticagrelor (1,717 [14.9%]) and prasugrel (1,143 [10.0%]). Baseline and procedural characteristics of the cohort are summarised in Table 1 and Table 2. Femoral access was utilised in the majority of patients (80.9%), and 35.7% of interventions met the criteria for complex PCI.
Table 1. Baseline characteristics, stratified by P2Y12 inhibitor prescribed at discharge.
| Ticagrelor or prasugrel (n=2,860) | Clopidogrel (n=8,648) | p-value | |
|---|---|---|---|
| Age, years | 62.3±10.2 | 67.2±10.8 | <0.001 |
| Female sex | 613 (21.4%) | 2,470 (28.6%) | <0.001 |
| Race | <0.001 | ||
| White | 1,170 (43.8%) | 3,677 (44.5%) | |
| African American | 222 (8.3%) | 883 (10.7%) | |
| Asian | 616 (23.0%) | 1,573 (19.0%) | |
| Hispanic | 490 (18.3%) | 1,728 (20.9%) | |
| Other | 175 (6.5%) | 404 (4.9%) | |
| BMI, kg/m2 | 29.1±5.4 | 28.6±5.5 | <0.001 |
| Clinical history | |||
| Hypertension | 2,674 (93.5%) | 8,121 (93.9%) | 0.468 |
| Hyperlipidaemia | 2,692 (94.1%) | 8,111 (93.8%) | 0.517 |
| Family history of coronary artery disease | 768 (26.9%) | 1,941 (22.4%) | <0.001 |
| Diabetes mellitus | 1,472 (51.5%) | 4,196 (48.5%) | 0.006 |
| Insulin-dependent diabetes | 517 (35.1%) | 1,399 (33.3%) | 0.214 |
| Prior MI | 850 (29.7%) | 1,894 (21.9%) | <0.001 |
| Prior PCI | 1,707 (59.7%) | 3,962 (45.8%) | <0.001 |
| Prior CABG | 425 (14.9%) | 1,445 (16.7%) | 0.02 |
| Peripheral artery disease (PAD) | 200 (7.0%) | 869 (10.0%) | <0.001 |
| Prior cerebrovascular disease (CVA) | 151 (5.3%) | 994 (11.5%) | <0.001 |
| Chronic kidney disease (CKD) | 560 (19.6%) | 2,562 (29.6%) | <0.001 |
| Dialysis | 54 (1.9%) | 346 (4.0%) | <0.001 |
| Anaemia | 1,030 (37.0%) | 3,383 (40.1%) | 0.004 |
| Neoplastic disease | 143 (5.0%) | 695 (8.0%) | <0.001 |
| Acute coronary syndrome (ACS) | 0 (0.0%) | 0 (0.0%) | N/A |
| LVEF, % | 55.6±9.6 | 55.7±9.8 | 0.816 |
| Laboratory values | |||
| Total cholesterol, mmol/L | 135.5±42.5 | 138.5±40.8 | 0.002 |
| LDL, mmol/L | 76.8±31.0 | 78.0±29.8 | 0.067 |
| HDL, mmol/L | 38.9±10.9 | 41.1±12.2 | <0.001 |
| Haemoglobin, baseline, g/dL | 13.2±1.6 | 12.9±1.7 | <0.001 |
| Platelet, baseline, 109/L | 202.0 [171.0-242.0] | 196.0 [161.0-234.0] | <0.001 |
| Socioeconomic parameters (insurance) | |||
| Medicare | 720 (26.5%) | 2,964 (36.1%) | <0.001 |
| Medicaid | 285 (10.5%) | 844 (10.3%) | 0.762 |
| Private | 1,614 (59.4%) | 4,250 (51.8%) | <0.001 |
| Military | 4 (0.1%) | 2 (0.0%) | 0.037 |
| None | 150 (5.5%) | 347 (4.2%) | 0.005 |
| Values are n (%), mean±SD or median [interquartile range]. BMI: body mass index; CABG: coronary artery bypass graft; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; MI myocardial infarction; N/A: not applicable; PCI: percutaneous coronary intervention; SD: standard deviation | |||
Table 2. Procedural characteristics by P2Y12 inhibitor use at discharge.
| Ticagrelor or prasugrel (n=2,860) | Clopidogrel (n=8,648) | p-value | |
|---|---|---|---|
| Radial access | 604 (21.1%) | 1,441 (16.7%) | <0.001 |
| Femoral access | 2,226 (77.8%) | 7,086 (81.9%) | <0.001 |
| Other access | 3 (0.1%) | 14 (0.2%) | 0.779 |
| LM | 201 (7.0%) | 374 (4.3%) | <0.001 |
| LAD | 1,687 (59.0%) | 4,494 (52.0%) | <0.001 |
| LCx | 1,191 (41.6%) | 3,230 (37.4%) | <0.001 |
| RCA | 1,057 (37.0%) | 3,131 (36.2%) | 0.473 |
| LIMA | 14 (0.5%) | 43 (0.5%) | 0.959 |
| SVG | 70 (2.4%) | 244 (2.8%) | 0.287 |
| Multivessel | 1,878 (65.7%) | 5,518 (63.8%) | 0.072 |
| B2C lesion | 2,107 (73.7%) | 5,993 (69.3%) | <0.001 |
| Bifurcation | 684 (23.9%) | 1,553 (18.0%) | <0.001 |
| Calcification, moderate or severe | 625 (21.9%) | 1,962 (22.8%) | 0.345 |
| Chronic total occlusion (CTO) | 264 (9.2%) | 754 (8.7%) | 0.403 |
| In-stent restenosis (ISR) | 614 (21.5%) | 1,179 (13.6%) | <0.001 |
| Stent implanted | 2,602 (91.0%) | 7,994 (92.4%) | 0.012 |
| First generation DES | 0 (0.0%) | 0 (0.0%) | N/A |
| Second generation DES | 2,588 (90.5%) | 7,795 (90.1%) | 0.582 |
| Bare metal stent | 17 (0.6%) | 208 (2.4%) | <0.001 |
| Total stent length, mm | 51.6±44.9 | 39.8±32.0 | <0.001 |
| Minimum stent diameter, mm | 2.9±0.5 | 2.9±0.5 | 0.731 |
| IVUS or OCT | 363 (12.7%) | 1,000 (11.6%) | 0.105 |
| Rotational atherectomy | 477 (16.7%) | 1,297 (15.0%) | 0.031 |
| Intra-aortic balloon pump (IABP) | 27 (0.9%) | 55 (0.6%) | 0.09 |
| Impella | 37 (1.3%) | 80 (0.9%) | 0.089 |
| Complex PCI | 1,198 (44.9%) | 2,669 (32.7%) | <0.001 |
| Values are n (%) or mean±SD. DES: drug-eluting stent; IVUS: intravascular ultrasound; LAD: left anterior descending; LCx: left circumflex; LIMA: left internal mammary artery; LM: left main; OCT : optical coherence tomography; PCI: percutaneous coronary intervention; RCA: right coronary artery; SD: standard deviation; SVG: saphenous vein grafts | |||
Trends in P2Y12 inhibitor prescription
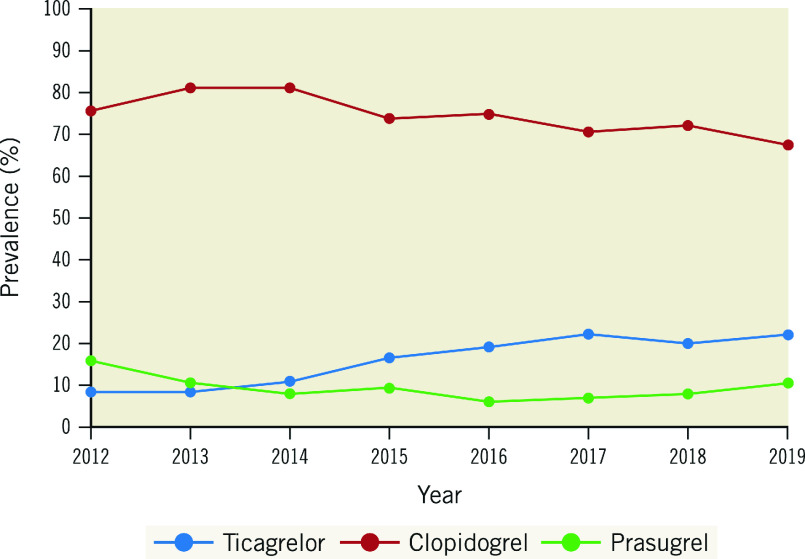
Trends in the rates of P2Y12 inhibitor use are shown in Figure 1. Prescription rates for clopidogrel reduced significantly from 76.0% in 2012 to 67.7% in 2019, with ticagrelor increasing from 8.2% to 21.9% (both ptrend<0.001), while the rates of prasugrel prescription were stable (~10%).
Figure 1. Trends in prescription of P2Y12 agents on discharge between 2012-2019.
Graph illustrates a significant reduction in the use of clopidogrel and increase in the use of the potent P2Y12 inhibitors over the study period (ptrend<0.001).
Factors affecting P2Y12 therapy choice
Table 3 summarises the multivariate predictors affecting P2Y12 therapy choice. Patients prescribed ticagrelor or prasugrel were more likely male and had a higher prevalence of diabetes mellitus (DM) with prior PCI. Those discharged on clopidogrel tended to be older, have a greater prevalence of prior cerebrovascular disease, coronary artery bypass grafts, chronic kidney disease, and a history of cancer (all p<0.05). However, markers of anatomic complexity (left main PCI: OR 1.80, 95% CI: 1.48-2.19; p<0.001), lesion-specific complexity (bifurcation: OR 1.31, 95% CI: 1.17-1.46; p<0.001; lesion length: OR 1.01, 95% CI: 1.00-1.01; p<0.001) and procedural complexity (number of lesions treated: OR 1.22, 95% CI: 1.12-1.33; p<0.001; rotational atherectomy: OR 1.14, 95% CI: 1.00-1.30; p=0.04) were all significantly higher in patients prescribed ticagrelor or prasugrel (Table 3).
Table 3. Factors associated with being discharged on ticagrelor or prasugrel over clopidogrel.
| Variable | Multivariable adjusted odds ratio (95% CI) | p-value |
|---|---|---|
| Age, years | 0.96 (0.95-0.96) | <0.001 |
| Female sex | 0.84 (0.76-0.94) | 0.002 |
| Diabetes mellitus | 1.16 (1.06-1.27) | 0.002 |
| Prior cerebrovascular disease | 0.48 (0.40-0.58) | <0.001 |
| Neoplastic disease | 0.79 (0.65-0.96) | 0.018 |
| Dialysis | 0.36 (0.27-0.49) | <0.001 |
| Prior PCI | 1.89 (1.72-2.07) | <0.001 |
| Prior CABG | 0.86 (0.75-0.98) | 0.019 |
| Bifurcation | 1.31 (1.17-1.46) | <0.001 |
| PCI vessel – left main | 1.80 (1.48-2.19) | <0.001 |
| Number of lesions treated | 1.22 (1.12-1.33) | <0.001 |
| Total lesion length, mm | 1.01 (1.00-1.01) | <0.001 |
| Chronic total occlusion | 0.71 (0.60-0.84) | <0.001 |
| Rotational atherectomy | 1.14 (1.00-1.30) | 0.043 |
| CABG: coronary artery bypass graft; CI: confidence interval; PCI: percutaneous coronary intervention | ||
Periprocedural outcomes
No differences in the rates of periprocedural complications including side branch closure, slow-flow/no reflow and abrupt vessel closure were noted when comparing the 2 groups (p=ns) (Supplementary Table 1). Despite vascular access being predominantly femoral, the rates of access complications were low with no significant difference when stratified by the type of P2Y12 inhibitor used.
Clinical outcomes
Clinical outcomes at 1 year are reported in Table 4. The distribution of propensity scores for the entire cohort and treatment group were visually examined, demonstrating good overlap between the groups (Supplementary Figure 2). The primary endpoint of death or MI occurred in 68 (2.7%) and 223 (3.1%) of patients discharged with ticagrelor or prasugrel and clopidogrel, respectively (adjusted hazard ratio [adjHR] 1.19, 95% CI: 0.84-1.66; p=0.325) (Central illustration). When evaluating key secondary endpoints, no difference in stroke or stent thrombosis was noted (Table 4). Clinically indicated target vessel revascularisation occurred in 159 (6.4%) and 359 (5.1%) patients treated with ticagrelor/prasugrel and clopidogrel, respectively (adjHR 0.99, 95% CI: 0.74-1.31; p=0.92). Unadjusted (unadj) rates of bleeding were higher in patients on clopidogrel, although this was not statistically significant after propensity score stratification (adjHR 0.92, 95% CI: 0.54-1.58; p=0.76) (Central illustration). Sensitivity analyses were performed comparing clopidogrel to ticagrelor and prasugrel individually (Table 5, Table 6). A trend towards a lower rate of the primary outcome was noted when comparing prasugrel and clopidogrel (2.1% vs 3.1%, unadjHR 0.67, 95% CI: 0.43-1.05; p=0.08), although this was attenuated after propensity adjustment (adjHR 0.66, 95% CI: 0.38-1.13; p=0.13). A comparison of patients who received 10 mg and 5 mg of prasugrel versus clopidogrel also demonstrated similar results (Supplementary Table 2). Further, subgroup analysis was performed when stratified by ARC-HBR risk. While the incidence of ischaemic and bleeding outcomes was numerically higher in the HBR versus non-HBR patients, no significant difference was noted on propensity score stratification analysis across subgroups without evidence of interaction (Supplementary Table 3).
Table 4. Kaplan-Meier estimates, unadjusted & propensity score-stratified hazard ratios, stratified by P2Y12 inhibitor use.
| Outcome |
Ticagrelor or prasugrel (n=2,860) |
Clopidogrel (n=8,648) |
Unadjusted hazard ratio (95% CI) |
p-value |
PS-stratified hazard ratio* |
p-value |
|---|---|---|---|---|---|---|
| Primary composite | 68 (2.7%) | 223 (3.1%) | 0.89 (0.67-1.16) | 0.378 | 1.19 (0.84-1.66) | 0.325 |
| Death | 25 (1.0%) | 108 (1.5%) | 0.67 (0.43-1.03) | 0.07 | 1.01 (0.57-1.78) | 0.972 |
| Myocardial infarction | 39 (1.6%) | 104 (1.4%) | 1.09 (0.75-1.58) | 0.644 | 1.36 (0.86–2.15 | 0.189 |
| Stroke | 5 (0.2%) | 19 (0.3%) | 0.77 (0.29-2.06) | 0.599 | 1.17 (0.42–3.28) | 0.762 |
| Definite or probable stent thrombosis | 11 (0.4%) | 20 (0.3%) | 1.62 (0.78-3.39) | 0.197 | 2.17 (0.95–4.98) | 0.066 |
| Bleeding (follow-up) | 25 (1.0%) | 115 (1.5%) | 0.64 (0.41-0.98) | 0.04 | 0.92 (0.54-1.58) | 0.764 |
| Target lesion revascularisation | 159 (6.4%) | 359 (5.1%) | 1.29 (1.07-1.56) | 0.007 | 0.99 (0.74-1.31) | 0.929 |
| *Propensity score (PS) was generated using the following variables: age2, age, gender, BMI (kg/m2), current smoking status, hypertension, hyperlipidaemia, cerebrovascular disease, prior MI, prior CABG, LVEF, acute coronary syndrome, moderate/severe calcification, chronic total occlusion, and complex PCI as per Giustino’s criteria, anaemia, malignancy, CKD, diabetes mellitus, planned surgery, thrombocytopaenia, age greater than 75 years, prior CVA, and prior bleeding. Complex PCI is defined as having one of the following criteria: total stent length ≥60 mm, total number of stents ≥3, number of lesions ≥3, number of vessels ≥3, bifurcation with ≥2 stents, or chronic total occlusion. BMI: body mass index; CABG: coronary artery bypass graft; CI: confidence interval; CKD: chronic kidney disease; CVA: cerebrovascular disease; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention | ||||||
Central illustration. Efficacy and safety of ticagrelor/prasugrel versus clopidogrel.
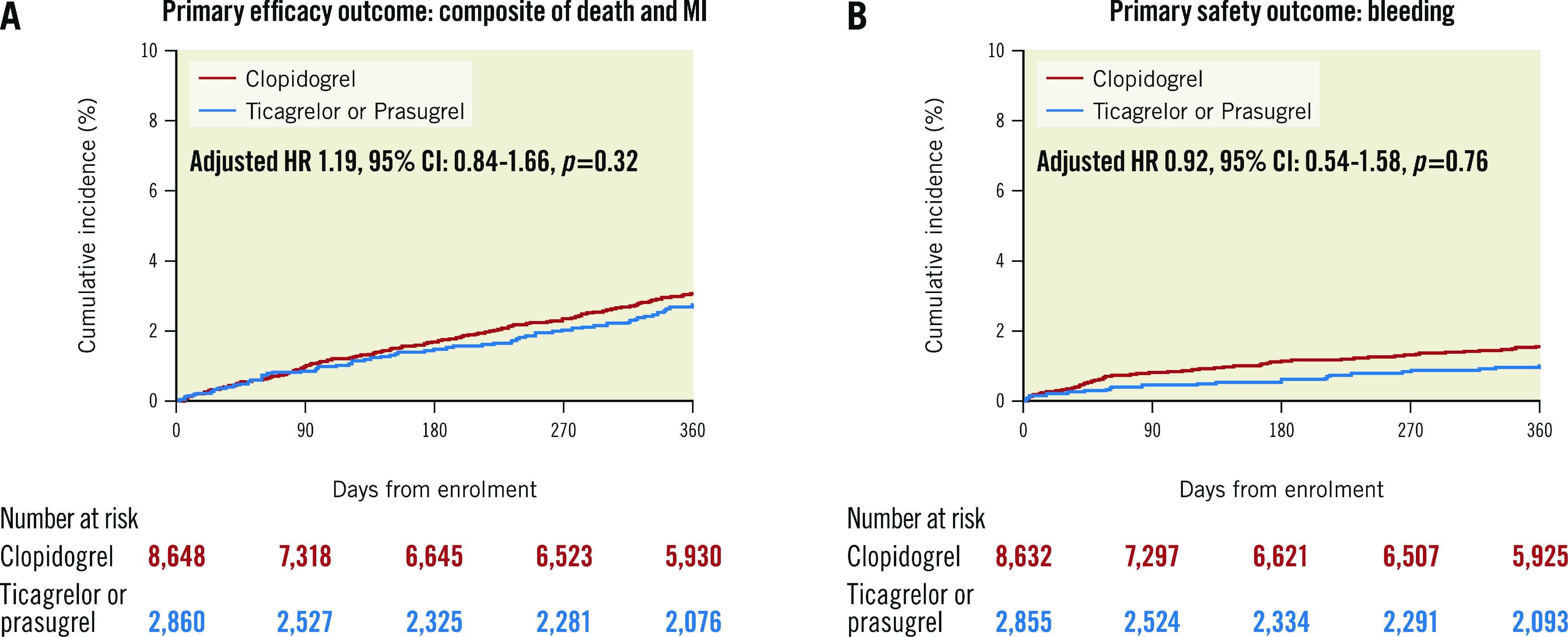
A) Primary efficacy outcome comparing the composite of death and myocardial infarction; B) Primary safety outcome: bleeding. CI: confidence interval; HR: hazard ratio; MI: myocardial infarction
Table 5. Kaplan-Meier estimates, unadjusted & PS-stratified hazard ratios, stratified by clopidogrel or ticagrelor prescribed at discharge.
| Outcome | Ticagrelor (n=1,717) | Clopidogrel (n=8,648) | Unadjusted hazard ratio (95% CI) | p-value | PS-stratified hazard ratio* | p-value |
|---|---|---|---|---|---|---|
| Death or MI | 47 (3.1%) | 223 (3.1%) | 1.03 (0.75-1.41) | 0.849 | 0.96 (0.68-1.38) | 0.844 |
| Death | 16 (1.1%) | 108 (1.5%) | 0.72 (0.43-1.22) | 0.221 | 0.60 (0.31-1.17) | 0.132 |
| Myocardial infarction | 27 (1.8%) | 104 (1.4%) | 1.27 (0.83-1.94) | 0.267 | 1.25 (0.78 - 1.98) | 0.354 |
| * Propensity score was generated using the following variables: age2, age, gender, BMI (kg/m2), current smoking status, hypertension, hyperlipidaemia, cerebrovascular disease, prior MI, prior CABG, LVEF, acute coronary syndrome, calcification-moderate/severe, chronic total occlusion, and complex PCI. MI: myocardial infarction; PS: propensity score | ||||||
Table 6. Kaplan-Meier estimates, unadjusted, & PS-stratified hazard ratios, stratified by clopidogrel or prasugrel use.
| Outcome | Prasugrel (n=1,143) | Clopidogrel (n=8,648) | Unadjusted hazard ratio (95% CI) | p-value | PS-stratified hazard ratio* | p-value |
|---|---|---|---|---|---|---|
| Death or MI | 21 (2.1%) | 223 (3.1%) | 0.67 (0.43-1.05) | 0.082 | 0.66 (0.38-1.13) | 0.13 |
| Death | 9 (0.9%) | 108 (1.5%) | 0.59 (0.30-1.17) | 0.133 | 0.57 (0.23-1.44) | 0.236 |
| Myocardial infarction | 12 (1.2%) | 104 (1.4%) | 0.83 (0.45-1.50) | 0.533 | 0.85 (0.44-1.67) | 0.64 |
| * Propensity score was generated using the following variables: age2, age, gender, BMI (kg/m2), current smoking status, hypertension, hyperlipidaemia, cerebrovascular disease, prior MI, prior CABG, LVEF, acute coronary syndrome, calcification-moderate/severe, chronic total occlusion, and complex PCI. Complex PCI is defined as having one of the following criteria: total stent length ≥60 mm, total number of stents ≥3, number of lesions ≥3, number of vessels ≥3, bifurcation with ≥2 stents, or chronic total occlusion. BMI: body mass index; CABG: coronary artery bypass graft; CI: confidence interval; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; PS: propensity score | ||||||
Subgroup analysis
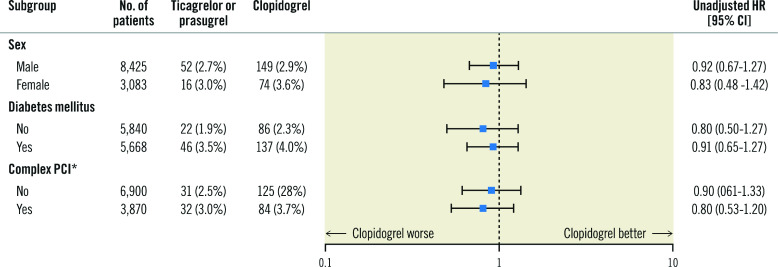
Additional subgroup analyses were performed comparing the primary outcome in patients by sex, DM and those undergoing complex PCI. Consistent with the overall study findings, no overall difference in the primary endpoint of death or MI was noted between patients treated with ticagrelor/prasugrel or clopidogrel (Figure 2).
Figure 2. Subgroup analysis comparing ticagrelor/prasugrel versus clopidogrel.
*Complex PCI is a composite of the following: multivessel PCI, ≥3 stents implanted, ≥3 lesions treated, or total stent length >60 mm. CI: confidence interval; HR: hazard ratio; PCI: percutaneous coronary intervention
Discussion
In this study of consecutive patients undergoing PCI for CCS, a number of findings merit attention. First, ticagrelor or prasugrel was the P2Y12 inhibitor of choice in 1 in 3 patients, with an increasing rate of use of these agents during the study period. Second, while clopidogrel was used more frequently in patients with multimorbid risk factors, the newer P2Y12 agents were prescribed more often in patients with a greater degree of procedural complexity. Third, no significant differences were observed in periprocedural events, or death or MI at 1 year. Lastly, the adjusted rates of bleeding were similar between groups. Taken together, our study highlights real-world factors affecting clinicians’ choice of clopidogrel versus ticagrelor/prasugrel as well as their comparative efficacy and safety in a large, contemporary patient population undergoing PCI for CCS.
Ticagrelor and prasugrel are both more potent P2Y12 agents with a higher level of platelet inhibition and faster onset of action than clopidogrel, with randomised controlled trials demonstrating their superiority in patients with ACS3,6,15,16,17. Subsequent studies have been performed to evaluate whether the use of these potent P2Y12 inhibitors can improve periprocedural safety and long-term efficacy in a typically lower-risk CCS population undergoing PCI. The ALPHEUS Study, which compared ticagrelor against clopidogrel in an elective PCI cohort with at least 1 high-risk characteristic, demonstrated no difference in efficacy, with a higher incidence of minor bleeding with ticagrelor10. Similarly, the SASSICAIA (Strategies of Loading With Prasugrel Versus Clopidogrel in PCI-Treated Biomarker Negative Angina) trial also delivered neutral results, although this study only compared the impact of a loading dose of prasugrel to clopidogrel8. The limitations of these studies included the use of periprocedural MI and myocardial injury as surrogates for hard clinical endpoints and a short 30-day follow-up. In contrast, the results of the PRASFIT-Elective (PRASugrel For Japanese patIenTs with coronary artery disease undergoing Elective PCI) study which evaluated the effect of an adjusted dose of prasugrel with up to a year of follow-up demonstrated a reduction in cardiovascular events with prasugrel when compared to clopidogrel7. The CHAMPION PHOENIX trial demonstrated significant reductions in ischaemic events with cangrelor compared to clopidogrel in patients with stable angina undergoing PCI18. The comparative efficacy and safety of ticagrelor in a predominantly CCS population was also demonstrated by our group in the TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention) trial19. Additionally, the potential for longer-term benefits of the newer P2Y12 agents in high-risk patient subgroups at a modified dose (for example, a 60 mg twice daily dose of ticagrelor used in THEMIS-PCI) has also been demonstrated in recent studies20,21. As such, some discrepant evidence remains when comparing the efficacy and safety of utilising more potent P2Y12 inhibition in the CCS population undergoing PCI.
The present study demonstrated no difference in MACE at 1 year when comparing clopidogrel versus ticagrelor or prasugrel in a large contemporary population of patients undergoing PCI for CCS. A trend towards a reduction in MACE was noted when comparing prasugrel to clopidogrel. While this analysis is likely underpowered, it raises the question whether this difference may have become evident with a broader use of prasugrel over ticagrelor in this study population. Periprocedural outcomes including rates of no reflow, side branch closure and stent thrombosis were also comparable across the groups. No differences were noted across subgroups, including among patients that underwent complex PCI. This indicates a potential lack of benefit with these potent agents compared to clopidogrel for the prevention of both periprocedural and longer-term MACE. Also, it is worth considering the risk of bleeding, which is typically higher with use of potent P2Y12 inhibitors. The lack of any significant difference in bleeding likely represents selection bias, whereby those patients who were felt to be at a higher risk of bleeding may have been more likely to have received clopidogrel. This is also noteworthy, as the majority of our patient population underwent PCI via femoral access. Notwithstanding, it is of interest to highlight that in an appropriately selected patient population, bleeding risk can be mitigated to a degree, irrespective of the choice of P2Y12 inhibitor.
The 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) coronary revascularisation guidelines indicate a paucity of data supporting the use of ticagrelor and prasugrel in CCS patients undergoing PCI1. In contrast, the European Society of Cardiology guidelines provide a Class IIb recommendation for the use of these agents in elective patients at a high risk of stent thrombosis, complex left main or multivessel PCI (level of evidence C)2. Despite the inconsistencies in guideline recommendations, ticagrelor and prasugrel were prescribed in up to 1 in 3 patients undergoing PCI for CCS at our centre4. Increasing clinician familiarity with the newer P2Y12 agents and supportive pharmacodynamic data may account for the selective off-label use of these agents3,16,22. When comparing the predictors of use of these agents, it was evident that anatomic and procedural complexity led to preferential use of ticagrelor or prasugrel, whereas markers of clinical frailty such as advanced age, cancer and chronic kidney disease were predictors of clopidogrel use. While the increasing off-label use of ticagrelor and prasugrel for non-ACS indications has been highlighted previously, the present study, to our knowledge, is the first to report on the real-world factors affecting decision-making regarding the choice of these agents. While no significant differences in the rates of MACE were noted in this study, which was stratified by type of P2Y12 inhibitor, it is conceivable that the selective use of ticagrelor and prasugrel in patients with higher procedural complexity may have mitigated a potentially higher risk of ischaemic events. An assessment of trends demonstrated a significant increase in the use of the newer P2Y12 agents, particularly ticagrelor. This is of concern given previous data from the United States demonstrating that patients prescribed prasugrel or ticagrelor had lower rates of adherence and incurred higher out-of-pocket costs than those prescribed clopidogrel5. The absence of any benefit seen in this study, among others, indicates that these agents should be used judiciously in patients undergoing PCI for CCS. Notwithstanding, our findings raise the question whether an individualised decision-making strategy of using either potent or moderate intensity P2Y12 agents, whilst accounting for the competing risks of bleeding and thrombosis, may have neutralised a risk of cardiac events or bleeding in the study. Future studies evaluating a patient population enriched with a high degree of clinical risk and procedural complexity may offer clarity on this question.
Study limitations
The strengths of this study include the inclusion of a consecutive all-comer cohort undergoing PCI for CCS with systematic recording of procedural data and clinical events. However, certain limitations warrant mention. First, the observational, retrospective design precludes drawing any causal inferences. While we used propensity stratification methods to account for imbalances between treatment groups, the possibility of residual confounding cannot be excluded. Second, detailed data on medication adherence and phenotypic/genotypic assessment of platelet reactivity were not collected. Antiplatelet therapy guided by genotypic data may be beneficial in appropriately selecting CCS patients that may derive benefit from the use of more intensified platelet inhibition. Third, our institutional protocol for the dosing of prasugrel is different from in previously published studies. However, modified prasugrel dosing in certain populations has been shown to have similar efficacy with less bleeding, and analysis stratified by prasugrel dosing also did not demonstrate any significant differences in outcomes23,24. Fourth, as this study was based on data from a PCI registry, the comparison of outcomes in patients managed medically during the study time frame were not available. Fifth, routine measurement of biomarkers and assessment of periprocedural MI were not included in this study. Lastly, we utilised all-cause mortality not cardiovascular mortality in the assessment of the primary outcome. However, given the difficulties in establishing the cause of death, utilising all-cause mortality is less likely to introduce bias and is in keeping with recommendations from the Academic Research Consortium-2 consensus recommendations25.
Conclusions
Ticagrelor and prasugrel are increasingly used in patients with CCS undergoing PCI with similar 1-year efficacy and safety when compared to clopidogrel. Future studies are needed to evaluate whether the use of the more potent P2Y12 agents in selected patients with a high thrombotic and low bleeding risk offers potential benefits over clopidogrel in a CCS population undergoing PCI.
Impact on clinical practice
The role of potent P2Y12 inhibitors, such as prasugrel and ticagrelor, compared with clopidogrel in patients undergoing PCI for stable ischaemic heart disease is unclear. This real-world study highlights the lack of any added benefit for the use of prasugrel or ticagrelor in patients undergoing PCI for stable coronary artery disease.
Supplementary data
Periprocedural complications.
Propensity score stratification outcomes comparing prasugrel 5 mg and prasugrel 10 mg versus clopidogrel.
Adjusted associations between meds at discharge and adverse events at 1 year after index procedure, stratified by Academic Research Consortium high bleeding risk.
Study inclusion flowchart.
Distribution of propensity scores.
Acknowledgments
Conflict of interest statement
R. Mehran has received institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; has received personal fees from the American College of Cardiology, Boston Scientific, the California Institute for Regenerative Medicine, Cine-Med Research, Janssen, WebMD, and the Society for Cardiovascular Angiography and Interventions; has received consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, Cardiawave, CeloNova, Chiesi, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, and Philips; holds equity (<1%) in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); and is a scientific advisory board member for the American Medical Association and Biosensors (spouse). S. Khera is a consultant for Abbott, Medtronic, and Boston Scientific and receives speaker honoraria from Medtronic. S K. Sharma has received speakers’ bureau fees from Abbott Vascular, Boston Scientific, and Cardiovascular Systems. G. Dangas has received personal fees from Biosensors and Philips. The other authors have no conflicts of interest to declare.
Abbreviations
- ACC/AHA/SCAI
American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions
- ACS
acute coronary syndromes
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CCS
chronic coronary syndromes
- CTO
chronic total occlusion
- DAPT
dual antiplatelet therapy
- HR
hazard ratio
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- PSS
propensity score stratification
- TLR
target lesion revascularisation
Contributor Information
Anoop N. Koshy, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Cardiology, Austin Health, The University of Melbourne, Melbourne, Australia.
Gennaro Giustino, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Samantha Sartori, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Amit Hooda, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Clayton Snyder, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Shabitri Dasgupta, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kartik R. Kumar, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Parasuram Krishnamoorthy-Melarcode, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Joseph Sweeny, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Sahil Khera, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Gregory W. Serrao, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Raman Sharma, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
George Dangas, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Annapoorna S. Kini, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Roxana Mehran, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Center for Interventional Cardiovascular Research and Clinical Trials, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Samin K. Sharma, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
References
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4–17. doi: 10.1161/CIR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- Hochholzer W, Amann M, Titov A, Younas I, Löffelhardt N, Riede F, Potocnik C, Stratz C, Hauschke D, Trenk D, Neumann FJ, Valina CM. Randomized Comparison of Different Thienopyridine Loading Strategies in Patients Undergoing Elective Coronary Intervention: The ExcelsiorLOAD Trial. JACC Cardiovasc Interv. 2016;9:219–27. doi: 10.1016/j.jcin.2015.10.036. [DOI] [PubMed] [Google Scholar]
- Dayoub EJ, Nathan AS, Khatana SAM, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Groeneveld PW, Giri J. Use of Prasugrel and Ticagrelor in Stable Ischemic Heart Disease After Percutaneous Coronary Intervention, 2009-2016. Circ Cardiovasc Interv. 2019;12:e007434. doi: 10.1161/CIRCINTERVENTIONS.118.007434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayoub EJ, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Giri J, Groeneveld PW. Trends in Platelet Adenosine Diphosphate P2Y12 Receptor Inhibitor Use and Adherence Among Antiplatelet-Naive Patients After Percutaneous Coronary Intervention, 2008-2016. JAMA Intern Med. 2018;178:943–50. doi: 10.1001/jamainternmed.2018.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Ikeda Y, Nakamura M, Saito S PRASFIT-Elective Investigators. Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J. 2014;78:2926–34. doi: 10.1253/circj.cj-14-0266. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Baquet M, Hochholzer W, Mayer K, Tesche C, Aradi D, Xu Y, Thienel M, Gschwendtner S, Zadrozny M, Jochheim D, Sibbing D, Schüpke S, Mansmann U, Hoffmann E, Kastrati A, Neumann FJ, Massberg S. Randomized Comparison of Intensified and Standard P2Y12-Receptor-Inhibition Before Elective Percutaneous Coronary Intervention: The SASSICAIA Trial. Circ Cardiovasc Interv. 2020;13:e008649. doi: 10.1161/CIRCINTERVENTIONS.119.008649. [DOI] [PubMed] [Google Scholar]
- Orme RC, Parker WAE, Thomas MR, Judge HM, Baster K, Sumaya W, Morgan KP, McMellon HC, Richardson JD, Grech ED, Wheeldon NM, Hall IR, Iqbal J, Barmby D, Gunn JP, Storey RF. Study of Two Dose Regimens of Ticagrelor Compared with Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention for Stable Coronary Artery Disease (STEEL-PCI). Circulation. 2018 Jun 21;138(13):1290–300. doi: 10.1161/CIRCULATIONAHA.118.034790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvain J, Lattuca B, Beygui F, Rangé G, Motovska Z, Dillinger JG, Boueri Z, Brunel P, Lhermusier T, Pouillot C, Larrieu-Ardilouze E, Boccara F, Labeque JN, Guedeney P, El Kasty, Laredo M, Dumaine R, Ducrocq G, Collet JP, Cayla G, Blanchart K, Kala P, Vicaut E, Montalescot G ALPHEUS investigators. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet. 2020;396:1737–44. doi: 10.1016/S0140-6736(20)32236-4. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. [Google Scholar]
- Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Gilard M, Morice MC, Sawaya F, Sardella G, Genereux P, Redfors B, Leon MB, Bhatt DL, Stone GW, Colombo A. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol. 2016;68:1851–64. doi: 10.1016/j.jacc.2016.07.760. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical Association. 1984;79:516–24. [Google Scholar]
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632–53. doi: 10.1093/eurheartj/ehz372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisset T, Cayla G, Quilici J, Pankert M, Deharo P, Bonnet G, Grosdidier C, Beguin S, Morange P, Bonnet JL, Alessi MC. Off-label use of prasugrel in stable coronary artery disease is associated with greater degree of platelet inhibition compared with use after acute coronary syndrome. Int J Cardiol. 2013;168:2988–9. doi: 10.1016/j.ijcard.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–42. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimsky P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–13. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381:2032–42. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, Held C, Andersson M, Himmelmann A, Ridderstråle W, Chen J, Song Y, Diaz R, Goto S, James SK, Ray KK, Parkhomenko AN, Kosiborod MN, McGuire DK, Harrington RA THEMIS Steering Committee and Investigators. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394:1169–80. doi: 10.1016/S0140-6736(19)31887-2. [DOI] [PubMed] [Google Scholar]
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- Hwang D, Lim YH, Park KW, Chun KJ, Han JK, Yang HM, Kang HJ, Koo BK, Kang J, Cho YK, Hong SJ, Kim S, Jo SH, Kim YH, Kim W, Lee SY, Kim YD, Oh SK, Lee JH, Kim HS HOST-RP-ACS investigators. Prasugrel Dose De-escalation Therapy After Complex Percutaneous Coronary Intervention in Patients With Acute Coronary Syndrome: A Post Hoc Analysis From the HOST-REDUCE-POLYTECH-ACS Trial. JAMA Cardiol. 2022;7:418–26. doi: 10.1001/jamacardio.2022.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Nakamura M. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–92. doi: 10.1253/circj.cj-13-1482. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur Heart J. 2018;39:2192–207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Periprocedural complications.
Propensity score stratification outcomes comparing prasugrel 5 mg and prasugrel 10 mg versus clopidogrel.
Adjusted associations between meds at discharge and adverse events at 1 year after index procedure, stratified by Academic Research Consortium high bleeding risk.
Study inclusion flowchart.
Distribution of propensity scores.