Abstract
Euphrasia nankotaizanensis (Orobanchaceae) is a rare alpine herb that is endemic to Taiwan. Only four small populations remain in Xue, Nanhu, and Cilai Mountains of Taiwan. The distribution of alpine herbs is severely threatened by climate change, which influences genetic variation and population structure. In this study, we investigated the effects of the natural isolation of alpine habitats on the genetic diversity and geographic structure of populations of E. nankotaizanensis using chloroplast (cp) and nuclear DNA (nrDNA) markers. We found lower levels of genetic diversity in E. nankotaizanensis than in other alpine plants and little to no genetic variation within populations, which could be mainly attributed to the small population size and genetic drift. Only one nrDNA haplotype was present in each population. The lack of monophyly of the four populations in cpDNA probably resulted from lineage sorting or occasional long-distance seed dispersal. Phylogeographic analysis suggested that Nanhu Mountain was probably a refugium over the glacial maxima, agreeing with the potential refugia in central Taiwan. The STRUCTURE and AMOVA analyses revealed significant genetic differentiation in nrDNA among the mountains, which resulted from geographical isolation among these mountains. Estimates of the effective population size (Ne) and demography reflected lower Ne values and a recent population decline, probably implying a greater extinction risk for E. nankotaizanensis. We observed genetic depletion and considerable genetic differentiation among mountain populations, which should be considered in future conservation efforts for this species. In addition, this study provides important insights into the long-term potential of alpine herbs in Taiwan, which are useful for a better prediction of their responses to future climate change.
Keywords: Alpine Herbs, Climate change, Extinction risks, Euphrasia nankotaizanensis, Geographical isolation
1. Introduction
A central theme in conservation biology is understanding the level and apportioning patterns of genetic variation in endangered species across populations and geographical regions. Studying the level and distribution of genetic variations in rare plants enhances our understanding of their population dynamics, adaptation, and evolution. Many rare and endangered species possess reduced genetic variability and are differentiated into genetically unique populations adapted to local conditions [1]. The construction of management principles for rare flora necessitates the quantification of genetic variability to optimise the conservation of species diversity. Information on the genetic relationships between organisms and populations can help managers to focus their efforts on distinctive taxa or populations.
Population genetic studies have revealed that the populations of many organisms are structured into phylogenetic units that often correspond to geographical regions [2,3]. The patterns of population structure result either from physical barriers that restrict gene flow between geographical regions or from the localised extinction of haplotypes in widely distributed taxa with a limited capacity for dispersal [2]. The genetic structuring of populations depends on demographic, genetic, and historical factors [4]. These factors can vary so much that even closely related taxa in a common environment have different genetic structure patterns [5,6]. Habitat size, isolation, and effective population size are major factors affecting the demographic and genetic structures of locally fragmented populations [7].
Geographic isolation plays a critical role in determining the genetic structure of plant populations. Mountain ranges, rivers, and glaciers may act as barriers to prevent gene flow and cause the genetic differentiation of isolated natural populations [8]. Geographical isolation, combined with a reduction in habitat range and small population size, is expected to cause a decline in a population [9,10]. This can form a barrier to gene flow that can result in an increased impact of genetic drift, rising inbreeding rates, local adaptation and differentiation, and the probability of local extinction [10,11]. The alpine plant species Euphrasia nankotaizanensis Yamamoto was selected as a candidate to explore the effects of natural isolation of alpine habitats, genetic variability, and population structure because it is rare and grows in high mountain peaks of Taiwan which are isolated from each other by large geographical distances.
Euphrasia nankotaizanensis is a perennial herb that grows above 3000 m altitude on the peaks of Xue, Nanhu, and Cilai Mountains in Taiwan. This species is endemic and is listed as Near Threatened (NT) following the International Union for Conservation of Nature criteria [12]. Because the seeds of E. nankotaizanensis lack morphological adaptations for dispersal, their ability to disperse is limited. However, occasional long-distance seed dispersal occurs by birds or strong winds such as typhoons or monsoon [13]. Moreover, insect-mediated pollen dispersal of E. nankotaizanensis is also limited. Recent studies have shown an upward migration of plant distribution, leaving those plants surviving on mountain tops with no further dispersal [14,15]. The reduction of habitat poses the greatest threat to species and may cause species to become endangered. In Taiwan, some studies have indicated lower levels of genetic diversity in alpine plants, such as Juniperus morrisonicola [14], and Abies kawakamii [16]. Currently, the populations and habitats of alpine plants in Taiwan tend to reduce in size and distribution [14,17]. Wu et al. [18] assessed the extent of differentiation in the genetic structure of the E. transmorrisonensis complex in the alpine regions of Taiwan, indicating at least three origins and an increased level of genetic differentiation among populations. However, the detailed population structure and genetic diversity of E. nankotaizanensis have not yet been studied. Therefore, we aimed to analyse the population characteristics using chloroplast DNA (cpDNA) and nuclear DNA (nrDNA) and expected them to show low gene flow among populations, resulting in high population differentiation.
For rare or endemic species with limited and isolated geographical ranges and small/reducing populations, genetic variation and historical patterns of demography are important not only for determining the population structure but also for developing effective and sustainable management plans. In the present study, we analyzed genetic variations in E. nankotaizanensis at trnL-F intergenic spacer and rpL16 intron of cpDNA, and the internal transcribed spacer regions (ITS) of nrDNA. The aims were: 1) to examine the level of genetic diversity in E. nankotaizanensis; 2) to assess whether the strong isolation of mountains restricted gene flow and caused significant genetic differentiation among E. nankotaizanensis populations; and 3) to uncover the demographic histories and assess the effective population sizes of this rare species for future conservation management.
2. Materials and methods
2.1. Plant materials
Four remnant populations of E. nankotaizanensis from Xue (one population), Nanhu (two populations), and Cilai (one population) Mountains in Taiwan, including 99 individuals, were sampled (Table 1; Fig. 1). Each population was collected about 25–35 individuals whenever samples were available. The NH2 population is very small in Nanhu Mountain, so only nine samples were collected. Young leaves were collected from the field and dried using silica gels. All samples were stored at −80 °C until they were processed.
Table 1.
Localities, symbols, and sample numbers of four sampled populations of Euphrasia nankotaizanensis in Taiwan.
| Locality | Symbol | Longitude | Latitude | Altitude | Sample size |
|---|---|---|---|---|---|
| Xue Mountain | XU | 121°13′ 54″ E | 24°23′ 09″ N | 3,780 m | 35 |
| Nanhu Mountain | NH1 | 121°26′ 35″ E | 24°21′ 45″ N | 3,530 m | 30 |
| NH2 | 121°26′ 29″ E | 24°21′ 40″ N | 3,640 m | 9 | |
| Cilai Mountain | CL | 121°20′ 01″ E | 24°07′ 03″ N | 3,490 m | 25 |
| Total | 99 |
Fig. 1.
Map showing the distributions and genetic compositions of Euphrasia nankotaizanensis populations. Pie diagrams indicate the frequencies of cpDNA and nrDNA haplotypes in each population. Abbreviations of populations are given in Table 1.
2.2. DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from powdered tissues following the CTAB procedure [19]. PCR amplification of the trnL-F intergenic spacer [20] and rpL16 intron of the cpDNA [21] and ITS region of nrDNA [22] were performed in a 50 μL reaction using 10 ng template DNA, 25 μL GoTaq ® Green Master Mix (Promega, Madison, WI, USA), and 5 pmol of each primer. PCRs was performed in a PCR cycler using the following profile: an initial 5 min denaturation at 94 °C, 30 cycles of 45 s denaturation at 94 °C, 1 min 15 s annealing at 50 °C for three markers, and 1 min 30 s extension at 72 °C, followed by a 10 min final extension at 72 °C. All PCR products were purified and then sequenced directly in both directions on an ABI 377XL automated sequencer (Applied Biosystems, Foster City, CA, USA). Direct sequencing of PCR products generates heterozygous base-calling fluorescence (double peak) chromatograms, which show paralogous genes within individuals [23]. In this study, the ITS sequences obtained from all individuals did not exhibit any double peaks for any of the sites in the chromatogram obtained by direct sequencing.
2.3. DNA sequence alignment and phylogenetic analyses
Nucleotide sequences were aligned using Clustal X2 [24]. The trnL-F intergenic spacer and rpL16 intron of the cpDNA were combined into a dataset for future analysis. Maximum-likelihood (ML) analyses were performed using PHYLIP v. 3.67 [25] for cp and nrDNA haplotypes, and bootstrap consensus values were calculated using 1000 replicates. Euphrasia stricta was included as an outgroup (GenBank accession numbers: FJ600681 for trnL-F spacer, EU259208 for rpL16 intron, and FJ790051 for ITS region). The program Network (http://www.fluxus-engineering.com/sharenet.htm) was used to construct a haplotype network using the median-joining method [26], indicating the genealogical relationships among all cp and nrDNA haplotypes.
2.4. Population genetic analyses
The levels of genetic diversity were quantified by pairwise estimates of nucleotide divergence (π) [27], haplotype number, and haplotype diversity using DnaSP 6 [28]. Genetic differentiation (Fst) was deduced based on pairwise comparisons between populations, and statistical tests of the neutrality of mutations were also conducted [29]. A hierarchical analysis of molecular variance (AMOVA) in ARLEQUIN v3.01 [30] was used to partition the genetic variability among populations and within populations for cp and nrDNA.
We then looked for evidence of genetic structure by analysing the data using the STRUCTURE program v. 2.3.4 [31]. This program applies a Bayesian method to infer the number of clusters (K) without using prior information on individual sampling locations. Initially, we converted the DNA sequences into the format of the STRUCTURE program using xmfa2struct (available from http://www.xavierdidelot.xtreemhost.com/clonalframe.htm). The program was run for K = 1 to K = 6 clusters, with 10 separate runs to estimate the stability of the results. Each run was pursued for 1,000,000 Markov chain Monte Carlo (MCMC) interactions, with an initial burn-in of 100,000, and an ancestry model that allowed for admixture [32]. The best fit number of grouping was assessed by ΔK [33] using STRUCTURE HARVESTER version 0.9.94 [34].
2.5. Molecular dating
In this study, the BEAST v2.6.4 program was used to estimate the divergence time of E. nankotaizanensis for both cp and nrDNAs [35]. Mutation rates of 2.61 × 10−9 and 9.86 × 10−9 per site per year for cp and nrDNA loci, respectively, were applied to estimate the divergence time [36]. We used the Hasegawa-Kishino-Yano (HKY) model of nucleotide substitution with estimated base frequencies, gamma shape distribution, proportion of invariant sites, and a relaxed molecular clock with an uncorrelated log-normal distribution of branch lengths. Posterior estimates of the mutation rate and age were examined using MCMC analysis, with samples drawn every 500 steps over a total of 1,000,000 steps. Adequate sampling and convergence to the stationary distribution were checked using TRACER v1.7 [37]. The ESS parameter was found to exceed 100, which suggested acceptable mixing and sufficient sampling. Posterior estimates of the parameters were all found to be distinctly unimodal, and all parameters appeared identifiable.
2.6. Demographic fluctuation
The effective population size (Ne) parameter (θ = 4Neμ, where Ne is the effective population size and μ is the mutation rate) and the Bayesian skyline plot (BSP) were estimated using the MIGRATE-n Program [38]. We calculated these estimates based on 20 short chains (10,000 trees) and three long chains (1,000,000 trees), with 10,000 trees discarded as the initial “burn-in”. In this study, the mutation rates of 2.61 × 10−9 and 9.86 × 10−9 per site/per year for cp and nrDNA loci were applied in Arabidopsis species, respectively [36]. We used the geometric mean of the mutation rate (μ = 5.43 × 10−6 per locus/per year) for the combination of cp and nrDNA loci to calculate the effective population size.
3. Results
3.1. Genetic variability of cpDNA and nrDNA
The levels of cp and nrDNA genetic diversity in E. nankotaizanensis are shown in Table 2. The cp and nrDNA consensus sequences were 1690 and 677 bp in length and contained 8 and 19 polymorphic sites, respectively. In total, five haplotypes (CP1–CP5) of cpDNA were identified (GenBank accession numbers: OU509538-OU509540 for trnL-F spacer; OU509541-OU509544 for rpL16 intron), and four nrDNA haplotypes (NR1–NR4) were identified (GenBank accession numbers: OU509545-OU509548).
Table 2.
Haplotype diversity (H), Haplotype diversity (Hd), and nucleotide diversity (π) of Euphrasia nankotaizanensis are indicated. for cp and nrDNA sequences.
| Population | Haplotype number |
Haplotype |
Haplotype diversity (Hd) |
Nucleotide diversity (π) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| cpDNA | nrDNA | cpDNA | nrDNA | cpDNA | nrDNA | cpDNA | nrDNA | ||
| Xue Mt. | XU | 3 | 1 | CP2(28), CP3(6), CP4(1) | NR2(35) | 0.292 | 0.000 | 0.00052 | 0.00000 |
| Nanhu Mt. | NH | 2 | 2 | CP1(30), CP5(9) | NR1(30), NR4(9) | 0364 | 0.364 | 0.00022 | 0.00054 |
| NH1 | 1 | 1 | CP1(30) | NR1(30) | 0.000 | 0.000 | 0.00000 | 0.00000 | |
| NH2 | 1 | 1 | CP5(9) | NR4(9) | 0.000 | 0.000 | 0.00000 | 0.00000 | |
| Cilai Mt. | CL | 1 | 1 | CP1(25) | NR3(25) | 0.000 | 0.000 | 0.00000 | 0.00000 |
| Total | 5 | 4 | – | – | 0.305 | 0.718 | 0.00033 | 0.01167 | |
Numbers in parentheses are sample sizes.
In total, the nucleotide diversity (π) and haplotype diversity (Hd) of E. nankotaizanensis were 0.00033 and 0.305 in cpDNA and 0.01167 and 0.718 in nrDNA, respectively. The nucleotide diversity of cp and nrDNA ranged from 0.00000 to 0.00052 and 0.00000 to 0.00054, respectively. The haplotype diversity (Hd) of cp and nrDNA ranged from 0.000 to 0.364 and 0.000 to 0.364, respectively. Higher levels of nucleotide and haplotype diversity were detected in nrDNA than in cpDNA (Table 2). All four sampled populations were fixed separately at a single haplotype for the nrDNA marker. Among all the populations examined, the Xue Mountain population possessed the highest nucleotide diversity in cpDNA, and the Nanhu Mountain population had the highest in nrDNA. Two subpopulations on Nanhu Mountain possessed no genetic diversity within the populations for either marker (Table 2).
3.2. Gene genealogies analyses
A maximum-likelihood (ML) tree and network were constructed based on the haplotypes of cp and nrDNA (Fig. 2A, B, C, D). Two major lineages (I and II) were identified in cpDNA, and the reciprocal monophyly of the geographical regions was not supported. Five haplotypes were identified in the ML tree and network corresponding to lineage I-II. Lineage I was found on the Xue and Nanhu (NH2) Mountains and lineage II on Nanhu (NH1) and Cilai Mountains (Fig. 2A, C). Two lineages (α and β) were identified for nrDNA. NR1, NR2, and NR4 were within lineage α, and NR3 was in lineage β (Fig. 2B). To further investigate the relationships between haplotypes, we constructed a network of cp and nrDNAs. For cpDNA, the CP1 haplotype in the Nanhu and Cilai Mountains was in an interior position, while the other haplotypes were exterior nodes in the network. This pattern suggests that the CP1 haplotype probably represents an ancient haplotype (Fig. 2C). For nrDNA, haplotypes NR1 and NR4 on Nanhu Mountain were nested in the network as interior nodes, indicating the Nanhu Mountain. The endemic haplotypes NR2 (Xue Mountain) and NR3 (Cilai Mountain) were linked to NR1 by seven and 12 mutation steps, respectively (Fig. 2D). According to cpDNA and nrDNA, the Nanhu Mountain were most likely ancient population.
Fig. 2.
Maximum likelihood trees and networks of Euphrasia nankotaizanensis rooted at outgroup sequences. The bootstrap values and divergent time (million years ago, MYA) are indicated at nodes. A) cpDNA tree. B) nrDNA tree. C) cpDNA network. D) nrDNA network.
Bayesian estimates of the mutation rates and divergence times of E. nankotaizanensis were obtained using the BEAST program (Fig. 2A and B). For cpDNA, all haplotypes of E. nankotaizanensis coalesced approximately 0.592 million years ago (MYA), while all nrDNA haplotypes could be traced back to a common ancestor about 1.670 MYA. For cpDNA, the haplotypes diverged about 0.385 in lineage I, and in the nrDNA tree, lineages α and β split approximately 1.670 MYA.
3.3. Population structure and population demography
The genetic structure of the E. nankotaizanensis populations was evaluated using pairwise FST values and AMOVA. For cpDNA, a high level of genetic differentiation was detected between NH2 and CL populations (FST = 1.0000, Table 3). Levels of genetic differentiation among XU, NH, and CL were moderate (FST = 0.1428–0.2222). In contrast to cpDNA, higher levels of genetic differentiation in nrDNA (FST = 1.0000; Table 3) were detected among the four populations. The AMOVA revealed that 74.89% of the total variation was found among populations, while the rest (25.11%) was within populations for cpDNA. However, AMOVA of nrDNA indicated that 100.00% of the total genetic variation was contributed among populations and no variation existed within populations, which was consistent with the high genetic differentiation observed (Table 4).
Table 3.
Pairwise FST between Euphrasia nankotaizanensis populations using cp (below diagonal) and nrDNA (above diagonal) sequences.
| cpDNA\nrDNA |
|||||
|---|---|---|---|---|---|
| XU |
NH |
CL |
|||
| NH1 | NH2 | ||||
| XU | 0.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| NH | 0.1667 | 0.0000 | – | – | 1.0000 |
| NH1 | 0.1471 | – | 0.00000 | 1.0000 | 1.0000 |
| NH2 | 0.7103 | – | 1.00000 | 0.0000 | 1.0000 |
| CL | 0.1428 | 0.2222 | 0.00000 | 1.0000 | 0.0000 |
Table 4.
Hierarchical analysis of molecular variance for four populations of Euphrasia nankotaizanensis based on cp- and nrDNA sequences.
| Source of variation | d.f. | Sum of squares | Variance components | Percentage of variation (%) |
|---|---|---|---|---|
| cpDNA | ||||
| Among populations | 3 | 61.250 | 0.8577 | 74.89 |
| Within populations | 95 | 27.326 | 0.2876 | 25.11 |
| Total | 98 | 88.576 | 1.1454 | 100.00 |
| Fixation Index FST = 0.7489a | ||||
| nrDNA | ||||
| Among populations | 3 | 387.273 | 5.5007 | 100.00 |
| Within populations | 95 | 0.000 | 0.0000 | 0.00 |
| Total | 98 | 387.273 | 5.5007 | 100.00 |
| Fixation Index FST = 1.0000a | ||||
p < 0.001.
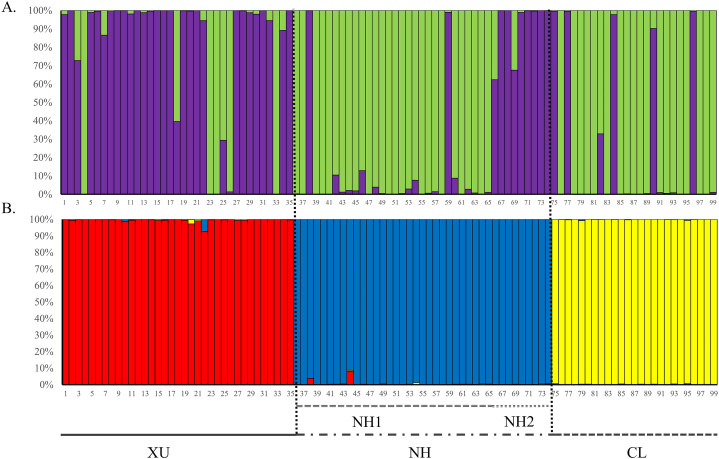
The population genetic structure of E. nankotaizanensis was revealed using STRUCTURE analyses. The optimum number of groups (K) was determined according to the procedure described by Evanno et al. (2005). The results indicated that the most likely number of genetic clusters for the four populations was two and three groups for cp and nrDNA, respectively (cpDNA: ΔK = 3.0003; nrDNA: ΔK = 631.2026, Fig. 3). At K = 2, E. nankotaizanensis was divided into two differentiated groups (green and purple segments), corresponding to phylogenetic tree in cpDNA (Fig. 3A). However, the XU population was assigned to Group I (red segment), the NH1 and NH2 populations to Group II (blue segment), and the remaining CL population to Group III (yellow segment) in nrDNA (Fig. 3B). The genetic composition of the populations based on STRUCTURE analysis was consistent with the geographical subdivisions of this species in nrDNA. However, individuals with intermixing compositions of the four populations were found in the cpDNA. These results were consistent with those of the genetic differentiation analysis (Table 3, Table 4). All the above analyses indicated significant genetic differentiation among populations, especially nrDNA.
Fig. 3.
Bar plots of individual Bayesian assignment probabilities of cpNDA and nrDNA for Euphrasia nankotaizanensis using the program STRUCTURE. A) cpDNA, K = 2. B) nrDNA, K = 3.
Bayesian skyline plots were able to uncover the demographic histories of E. nankotaizanensis. Based on the pattern of variation in cp and nrDNA, a long history of demographic expansion in the XU, NH, and CL populations, followed by a recent population decline, was uncovered (Fig. 4A, B, C). However, all populations displayed a pattern of rapid population shrinkage 1000 years before present (Fig. 4D). Accordingly, constant demographic shrinkage of E. nankotaizanensis was detected using the Bayesian skyline plot. Estimates of effective population sizes (θ = 4Neμ, where Ne = effective population size and μ = mutation rate, μ = 5.43 × 10−6 per locus/per year) were also calculated using the MIGRATE program. The results revealed a lower θ for each XU (θ = 0.0008, 95% CI = 0.00003–0.00213), NH (θ = 0.0009, 95% CI = 0.00003–0.00220), and CL (θ = 0.0008, 95% CI = 0.00003–0.00200) population, and θ = 0.0021 (95% CI = 0.00183–0.00380) for E. nankotaizanensis. In addition, the estimated current Ne values for each XU, NH, and CL population were approximately 37 (95% CI = 1.38–98.07), 41 (95% CI = 1.38–101.29), and 37 (95% CI = = 1.38–92.81) individuals, respectively, and 97 (95% CI = 84.25–174.95) individuals for E. nankotaizanensis.
Fig. 4.
Bayesian skyline plots for fluctuations in the effective population size through time. Y-axis, population size × generation time; X-axis, time, years before present (years ago). A) Xue Mountain. B) Nanhu Mountain. C) Cilai Mountain. D) all populations.
4. Discussion
4.1. The lower genetic diversity, and effective population size
Alpine plants are particularly vulnerable to climate change because of their restricted ranges, location on mountain summits, and increased habitat destruction. It is crucial to assess the genetic diversity and population structure of alpine plants to evaluate their long-term adaptive potential [14,39]. Studies addressing these issues are scarce in Taiwan, particularly for alpine herbs. Species with geographically restricted ranges are often considered more vulnerable to extinction because they possess reduced genetic diversity compared with more widespread species [40]. As expected, genetic variation in E. nankotaizanensis was lower in the cpDNA marker (Hd = 0.305, π = 0.00033) than in its relative E. transmorrisonensis (Hd = 0.612, π = 0.00090) (Wu et al., 2005) in Taiwan. However, the level of diversity found for E. nankotaizanensis in the nrDNA marker (Hd = 0.718, π = 0.01167) was similar to that of E. transmorrisonensis (Hd = 0.913, π = 0.0112), which is more widespread in Taiwan. Compared with other alpine plants, the genetic diversity of cpDNA was lower than that of Trochodendron aralioides (Hd = 0.658, π = 0.00088) [41], Rhododendron pseudochrysanthum (Hd = 0.879, π = 0.01092) [42], and R. hyperythrum (Hd = 0.879, π = 0.00510) [42], but similar to that of Juniperus morrisonicola (Hd = 0.857, π = 0.00030) [14] with recent demographic contraction. The nucleotide diversity of nrDNA was lower than that of R. hyperythrum (Hd = 0.879, π = 0.01549) [42], and similar to that of R. pseudochrysanthum (Hd = 0.881, π = 0.01135) [42]. The E. nankotaizanensis possessed lower level of genetic diversity than other alpine plants in Taiwan.
Numerous studies on rare and endangered plant species have demonstrated that species with spatially and environmentally isolated populations show lower levels of genetic variation, especially in high-mountain regions [1,43]. Significantly lower levels of genetic diversity have been detected within populations from the alpine zone than in other zones [43]. A small or declining population is another factor that causes a loss of genetic diversity. These populations show strong genetic drift, inbreeding, erosion of genetic diversity, and an increased extinction risk [44,45]. We found that lower levels of genetic diversity are associated with a decline in effective population size. The Ne value is important for assessing the genetic health of the population and extinction risk [46] and is usually smaller than the census population size. It is significantly correlated with the rate at which genetic diversity declines in a population [47,48]. When Ne is ≤ 50, inbreeding and demographic stochasticity can increase the extinction risk. For the long-term adaptive potential of a species, a Ne ≥ 500 is necessary [49]. In this study, the effective population sizes were small, approximately 37–41 individuals for each XU, NH, and CL population and less than 100 individuals for E. nankotaizanensis. In addition, the demographic history of E. nankotaizanensis showed rapid population shrinkage (Fig. 4D). Euphrasia nankotaizanensis, which grows on mountain peaks in Taiwan, is a good example to improve our understanding of the effect of climate change on alpine species. Its genetic information documented a greater extinction risk to E. nankotaizanensis owing to the loss of habitats under climate change. A critical aspect of E. nankotaizanensis conservation that has not been previously investigated is the genetic health of the population. Our study addresses this important topic and provides insights into the conservation of alpine herbs under climate change in Taiwan.
4.2. The phylogeographic pattern and population structure of Euphrasia nankotaizanensis
Taiwan is thought to have been connected to the Asian mainland, and most of its flora is thought to have originated during the Pleistocene glacial cycles [50,51]. Meanwhile, ancestral populations of many plants and animals may have migrated to Taiwan via land bridges [51]. Gussarova et al. [52] suggested that Euphrasia species are of Eurasian origin. Therefore, E. nankotaizanensis migrated from Asia in the early Pleistocene (0.592–1.670 MYA, Fig. 2A and B) into refugia for southward migration provided by the mountains in Taiwan, which is consistent with the history of land bridges between Asia and Taiwan [41,51,53]. In this study, the phylogeographic pattern of E. nankotaizanensis was assessed using the nrDNA dataset. Haplotypes NR1 and NR4 are nested as interior nodes in the network. According to the coalescent theory, these haplotypes as interior nodes linked to the outgroup of E. stricta may represent ancestral genotypes [54]. In contrast to nrDNA, a lack of monophyly in the four populations at cpDNA was found. The inconsistency between cpDNA and nrDNA may stem from the effects of lineage sorting caused by the relative ages of alleles at each locus [55]. For example, the haplotype of the CL population clustered with NH1 based on cpDNA but might be derived from NH2 based on nrDNA. Our phylogeographic analysis showed Nanhu Mountain to be a potential refugium during the last glaciations in central Taiwan. Accordingly, the populations of Nanhu Mountain maintained a higher genetic diversity than the other populations in terms of nrDNA. Many studies have also identified the north-central mountainous area of Taiwan as a possible refugia, coupled with higher genetic diversity. These species included Michelia formosana [56], Trochodendron aralioides [41], Cunninghamia konishii [57], Castanopsis carlesii [58], Machilus thunbergii [59], Abies kawakamii [16], Quercus variabilis [60], Musa basjoo var. formosana [61], and Chamaecyparis formosensis [62].
The genetic structure of populations is usually influenced by multiple factors such as genetic drift, natural selection, gene flow, mating system, and demographic history [51]. Genetic drift is an important factor in shaping the genetic structure of a population, and an increase in homozygosity within populations is often observed in small and spatially isolated populations of rare and threatened species. Usually, geographic isolation can increase the chance of random genetic drift, increase inbreeding rates, reduce gene flow, and result in the eventual species extinction [63]. Euphrasia nankotaizanensis has a restricted distribution, with small population size, and only four remaining populations were found in Taiwan. It inhabits mountaintops isolated from the surrounding low-elevation habitats, forming small and fragmented populations. Seed- and insect-mediated pollen dispersal is limited in E. nankotaizanensis, and high genetic differentiation and low gene flow among populations are expected.
Nevertheless, the analysis of cpDNA variation revealed medium to high levels of genetic differentiation among mountain ranges (FST = 0.1428–0.2222). NH1 and NH2 populations within the same mountain range showed higher genetic differentiation (FST = 1.0000) than between populations from different mountain ranges (FST = 0.1428–0.2222) (Table 3). The short period of isolation since the last glacial period is seemingly not long enough to differentiate the populations of different mountain ranges and has resulted in the sharing of ancestral genetic polymorphisms. Occasional seed dispersal and random drift in small populations may be other factors influencing the genetic composition of E. nankotaizanensis. Compared to cpDNA, the lower capabilities of insect-mediated pollen dispersal in current environments may have contributed to the higher levels of genetic differentiation between populations in nrDNA (FST = 1.0000), and only one unique haplotype existed for each population (Fig. 1 and Table 2). The AMOVA indicated that 74.89% (cpDNA) and 100% (nrDNA) of the genetic variation was found among the populations (Table 4). A significant structure corresponded to the geographical division between the three mountains. The genetic composition based on STRUCTURE analysis revealed significant genetic structuring of Euphrasia in nrDNA (Fig. 3). Based on these analyses, a clear geographical isolation exists among the populations of these three mountains. Currently, E. nankotaizanensis populations on different mountain ranges represent isolated patches. Given such geographical barriers, seeds and pollen are less likely to be dispersed across mountain ranges. Random genetic drift caused the loss of genetic diversity within populations and enhanced genetic differentiation in small and isolated populations of E. nankotaizanensis.
5. Conclusion
High mountain ecosystems are among the most threatened ecosystems in the world. If the human disturbance continues, genetic variation in alpine plants will inevitably be lost due to habitat destruction, human pressure, and global warming. Rapid losses of genetic variation and effective population size can cause a decline in the adaptive ability and ultimately extirpation of this species. Preventing further habitat destruction, germplasm collection is critical for the long-term persistence of E. nankotaizanensis. In the present study, reciprocal monophyly in nrDNA, considerable genetic differentiation, and clear geographical isolation between mountains should be considered evolutionarily significant units for conservation. This information provides important insights into the long-term conservation management of alpine herbs in Taiwan.
Declarations
Author contribution statement
Syuan-Yu, Chen, Chi-Chun Huang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yu-Tzu Cheng: Performed the experiments; Analyzed and interpreted the data.
Chih-Chiang Wang, Chiuan-Yu Li, I-Ling Lai: Contributed reagents, materials, analysis tools or data.
Kuo-Hsiang Hung: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Kuo-Hsiang Hung was supported by Shei-Pa National Park and Taroko National Park in Taiwan [10704].
Data availability statement
Data associated with this study has been deposited at NCBI GenBank databases (https://www.ncbi.nlm.nih.gov/genbank/) under accession number OU509538-OU509540, OU509541-OU509544, and OU509545-OU509548.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Szczecinska M., Sramko G., Wolosz K., Sawicki J. Genetic diversity and population structure of the rare and endangered plant species Pulsatilla patens (L.) Mill in east central Europe. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avise J.C., Arnold J., Ball R.M., Bermingham E., Lamb T., Neigel J.E., Reeb C.A., Saunders N.C. Intrpspecific phylogeography: the Mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Evol. Syst. 1987;18:489–522. [Google Scholar]
- 3.Avise J.C. Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos. 1992;63:62–76. [Google Scholar]
- 4.Slatkin M. In: Ecological Genetics. Real L.A., editor. Princeton University Press; Princeton: 1994. Gene flow and population structure; pp. 19–34. [Google Scholar]
- 5.Patton J.L., Da Silva M.N., Malcolm J.R. Hierarchical genetic structure and gene flow in three sympatric species of Amazonian rodents. Mol. Ecol. 1996;5:229–238. doi: 10.1111/j.1365-294x.1996.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyer A., Knowles L.L., Verheyen E. Widespread geographical distribution of mitochondrial haplotypes in rock-dwelling cichlid fishes from Lake Tanganyika. Mol. Ecol. 1996;5:341–350. doi: 10.1111/j.1365-294x.1996.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 7.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003;34:487–515. [Google Scholar]
- 8.Qiong L., Zhang W., Wang H., Zeng L., Birks H.J.B., Zhong Y. Testing the effect of the Himalayan mountains as a physical barrier to gene flow in Hippophae tibetana Schlect. (Elaeagnaceae) PLoS One. 2017;12 doi: 10.1371/journal.pone.0172948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterling K.A., Reed D.H., Noonan B.P., Warren M.L. Genetic effects of habitat fragmentation and population isolation on Etheostoma raneyi (Percidae) Conserv. Genet. 2012;13:859–872. [Google Scholar]
- 10.Toth E.G., Tremblay F., Housset J.M., Bergeron Y., Carcaillet C. Geographic isolation and climatic variability contribute to genetic differentiation in fragmented populations of the long-lived subalpine conifer Pinus cembra L. in the western Alps. BMC Evol. Biol. 2019;19:190. doi: 10.1186/s12862-019-1510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Futuyma D.J. Sinauer Associates; Sunderland, MA: 1998. Evolutionary Biology. [Google Scholar]
- 12.Editorial Committee of the Red List of Taiwan Plants, the Red List of Vascular Plants of Taiwan. Endemic Species Research Institute, Forestry Bureau, Council of Agriculture, Executive Yuan and Taiwan Society of Plant Systematics; Taiwan: 2017. 2017. [Google Scholar]
- 13.Wu M.J., Huang T.C., Huang S.F. Phylogenetic biogeography of Euphrasia section malesianae (orobanchaceae) in taiwan and malesia. Blumea. 2009;54:242–247. [Google Scholar]
- 14.Huang C.C., Hsu T.W., Wang H.V., Liu Z.H., Chen Y.Y., Chiu C.T., Huang C.L., Hung K.H., Chiang T.Y. Multilocus analyses reveal postglacial demographic shrinkage of Juniperus morrisonicola (Cupressaceae), a dominant alpine species in Taiwan. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jump A.S., Peñuelas J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 16.Shih F.L., Hwang S.Y., Cheng Y.P., Lee P.F., Lin T.P. Uniform genetic diversity, low differentiation, and neutral evolution characterize contemporary refuge populations of Taiwan Fir (Abies kawakamii, Pinaceae) Am. J. Bot. 2007;94:194–202. doi: 10.3732/ajb.94.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Jump A.S., Huang T.J., Chou C.H. Rapid altitudinal migration of mountain plants in Taiwan and its implications for high altitude biodiversity. Ecography. 2012;35:204–210. [Google Scholar]
- 18.Wu M.J., Huang S.F., Huang T.C., Lee P.F., Lin T.P. Evolution of the Euphrasia transmorrisonensis complex (Orobanchaceae) in alpine areas of Taiwan. J. Biogeogr. 2005;32:1921–1929. [Google Scholar]
- 19.Murray M.G., Thompson W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taberlet P., Gielly L., Pautou G., Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J., Lickey E.B., Beck J.T., Farmer S.B., Liu W., Miller J., Siripun K.C., Winder C.T., Schilling E.E., Small R.L. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- 22.White T.J., Bruns T., Lee S., Taylor J. In: Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. Academic Press; San Diego: 1990. pp. 315–322. (PCR Protocols). [Google Scholar]
- 23.Chang C.T., Tsai C.N., Tang C.Y., Chen C.H., Lian J.H., Hu C.Y., Tsai C.L., Chao A., Lai C.H., Wang T.H., Lee Y.S. Mixed sequence reader: a program for analyzing DNA sequences with heterozygous base calling. Sci. World J. 2012;2012 doi: 10.1100/2012/365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 25.Felsenstein J. University of Washington; Seattle: 1993. PHYLIP: Phylogeny Inference Package (Version 3.5c) [Google Scholar]
- 26.Bandelt H.J., Forster P., Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 27.Nei M. Columbia University Press; New York Chichester, West Sussex: 1987. Molecular Evolutionary Genetics. [Google Scholar]
- 28.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S.E., Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 29.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Excoffier L., Laval G., Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2007;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 31.Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubisz M.J., Falush D., Stephens M., Pritchard J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 34.Earl D., vonHoldt B. Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012;4:359–361. [Google Scholar]
- 35.Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.H., Xie D., Suchard M.A., Rambaut A., Drummond A.J. Beast 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C.C., Hung K.H., Wang W.K., Ho C.W., Huang C.L., Hsu T.W., Osada N., Hwang C.C., Chiang T.Y. Evolutionary rates of commonly used nuclear and organelle markers of Arabidopsis relatives (Brassicaceae) Gene. 2012;499:194–201. doi: 10.1016/j.gene.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beerli P., Felsenstein J. Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4563–4568. doi: 10.1073/pnas.081068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Till-Bottraud I., Gaudeul M. In: Mountain Biodiversity: A Global Assessment. Körner C., Spehn E.M., editors. Parthenon Publishing; London, UK: 2002. Intraspecific genetic diversity in alpine plants; pp. 23–34. [Google Scholar]
- 40.Tian H.Z., Han L.X., Zhang J.L., Li X.L., Kawahara T., Yukawa T., López-Pujol J., Kumar P., Chung M.G., Chung M.Y. Genetic diversity in the endangered terrestrial orchid Cypripedium japonicum in East Asia: insights into population history and implications for conservation. Sci. Rep. 2018;8:6467. doi: 10.1038/s41598-018-24912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S.F., Hwang S.Y., Wang J.C., Lin T.P. Phylogeography of Trochodendron aralioides (trochodendraceae) in taiwan and adjacent areas. J. Biogeogr. 2004;31:1251–1259. [Google Scholar]
- 42.Huang C.C., Hung K.H., Hwang C.C., Huang J.C., Lin H.D., Wang W.K., Wu P.Y., Hsu T.W., Chiang T.Y. Genetic population structure of the alpine species Rhododendron pseudochrysanthum sensu lato (Ericaceae) inferred from chloroplast and nuclear DNA. BMC Ecol. Evol. 2011;11:108. doi: 10.1186/1471-2148-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisch C., Rosbakh S. Patterns of genetic variation in European plant species depend on altitude. Divers. Distrib. 2021;27:157–163. [Google Scholar]
- 44.Frankham R. Genetics and conservation biology. C. R. Biol. 2003;326:22–29. doi: 10.1016/s1631-0691(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 45.Hatmaker E.A., Staton M.E., Dattilo A.J., Hadziabdic Ð., Rinehart T.A., Schilling E.E., Trigiano R.N., Wadl P.A. Population structure and genetic diversity within the endangered species Pityopsis ruthii (Asteraceae) Front. Plant Sci. 2018;9:943. doi: 10.3389/fpls.2018.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palstra F.P., Ruzzante D.E. Genetic estimates of contemporary effective population size: what can they tell us about the importance of genetic stochasticity for wild population persistence? Mol. Ecol. 2008;17:3428–3447. doi: 10.1111/j.1365-294x.2008.03842.x. [DOI] [PubMed] [Google Scholar]
- 47.Hare M.P., Nunney L., Schwartz M.K., Ruzzante D.E., Burford M., Waples R.S., Ruegg K., Palstra F. Understanding and estimating effective population size for practical application in marine species management. Conserv. Biol. 2011;25:438–449. doi: 10.1111/j.1523-1739.2010.01637.x. [DOI] [PubMed] [Google Scholar]
- 48.Frankham R. Genetics and extinction. Biol. Conserv. 2005;126:131–140. [Google Scholar]
- 49.Franklin I.R. In: Conservation Biology: an Evolutionary-Ecological Perspective. Soulé M.E., Wilcox B.M., editors. Sinauer Associates; Sunderland, MA: 1980. Evolutionary change in small populations; pp. 135–140. [Google Scholar]
- 50.Boggs S., Jr., Wang W.C., Lewis F.S., Chen C.J. Sediment properties and water characteristics of the Taiwan shelf and slope. Acta Oceanogr. Taiwan. 1979;10:10–49. [Google Scholar]
- 51.Chiang T.Y., Schaal B.A. Phylogeography of plants in taiwan and the ryukyu archipelago. Taxon. 2006;55:31–41. [Google Scholar]
- 52.Gussarova G., Popp M., Vitek E., Brochmann C. Molecular phylogeny and biogeography of the bipolar Euphrasia (Orobanchaceae): recent radiations in an old genus. Mol. Phylogenet. Evol. 2008;48:444–460. doi: 10.1016/j.ympev.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Huang S.S.F., Hwang S.Y., Lin T.P. Spatial pattern of chloroplast DNA variation of Cyclobalanopsis glauca in taiwan and east Asia. Mol. Ecol. 2002;11:2349–2358. doi: 10.1046/j.1365-294x.2002.01624.x. [DOI] [PubMed] [Google Scholar]
- 54.Crandall K.A., Templeton A.R. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics. 1993;134:959–969. doi: 10.1093/genetics/134.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang T.Y. Lineage sorting accounting for the disassociation between chloroplast and mitochondrial lineages in oaks of southern France. Genome. 2000;43:1090–1094. [PubMed] [Google Scholar]
- 56.Lin T.P. Allozyme variations in Michelia formosana (kanehira) masamune (magnoliaceae), and the inference of a glacial refugium in taiwan. Theor. Appl. Genet. 2001;102:450–457. [Google Scholar]
- 57.Chung J.D., Lin T.P., Tan Y.C., Lin M.Y., Hwang S.Y. Genetic diversity and biogeography of Cunninghamia konishii (Cupressaceae), an island species in Taiwan: a comparison with Cunninghamia lanceolata, a mainland species in China. Mol. Phylogenet. Evol. 2004;33:791–801. doi: 10.1016/j.ympev.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Y.P., Hwang S.Y., Lin T.P. Potential refugia in Taiwan revealed by the phylogeographical study of Castanopsis carlesii Hayata (Fagaceae) Mol. Ecol. 2005;14:2075–2085. doi: 10.1111/j.1365-294X.2005.02567.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu S.H., Hwang C.Y., Lin T.P., Chung J.D., Cheng Y.P., Hwang S.Y. Contrasting phylogeographical patterns of two closely related species, Machilus thunbergii and Machilus kusanoi (Lauraceae), in Taiwan. J. Biogeogr. 2006;33:936–947. [Google Scholar]
- 60.Chen D., Zhang X., Kang H., Sun X., Yin S., Du H., Yamanaka N., Gapare W., Wu H.X., Liu C. Phylogeography of Quercus variabilis based on chloroplast DNA sequence in East Asia: multiple glacial refugia and mainland-migrated island populations. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J.H., Huang C.L., Lai Y.L., Chang C.T., Liao P.C., Hwang S.Y., Sun C.W. Postglacial range expansion and the role of ecological factors in driving adaptive evolution of Musa basjoo var. formosana. Sci. Rep. 2017;7:5341. doi: 10.1038/s41598-017-05256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C.J., Chu F.H., Huang Y.S., Tu Y.C., Hung Y.M., Tseng Y.H., Pu C.E., Hsu C.T., Chao C.H., Chou Y.S., Liu S.C., You Y.T., Hsu S.Y., Hsieh H.C., Wang C.T., Chen C.T. SSR individual identification system construction and population genetics analysis for Chamaecyparis formosensis. Sci. Rep. 2022;12:4126. doi: 10.1038/s41598-022-07870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellstrand N., Elam D. Population genetic consequences of small population size: implications for plant conservation. Annu. Rev. Ecol. Evol. Syst. 2003;24:217–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at NCBI GenBank databases (https://www.ncbi.nlm.nih.gov/genbank/) under accession number OU509538-OU509540, OU509541-OU509544, and OU509545-OU509548.