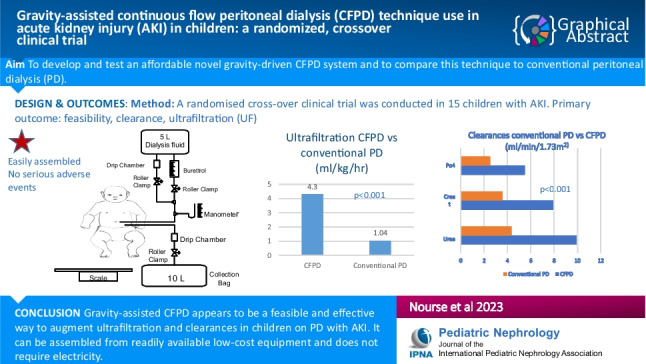
Abstract
Background
Our previously demonstrated continuous flow peritoneal dialysis (CFPD) technique in children with acute kidney injury (AKI), although effective, was manpower heavy and expensive due to the high-volume pumps required. The aim of this study was to develop and test a novel gravity-driven CFPD technique in children using readily available, inexpensive equipment and to compare this technique to conventional PD.
Methods
After development and initial in vitro testing, a randomised crossover clinical trial was conducted in 15 children with AKI requiring dialysis. Patients received both conventional PD and CFPD sequentially, in random order. Primary outcomes were measures of feasibility, clearance and ultrafiltration (UF). Secondary outcomes were complications and mass transfer coefficients (MTC). Paired t-tests were used to compare PD and CFPD outcomes.
Results
Median (range) age and weight of participants were 6.0 (0.2–14) months and 5.8 (2.3–14.0) kg, respectively. The CFPD system was easily and rapidly assembled. There were no serious adverse events attributed to CFPD. Mean ± SD UF was significantly higher on CFPD compared to conventional PD (4.3 ± 3.15 ml/kg/h vs. 1.04 ± 1.72 ml/kg/h; p < 0.001). Clearances for urea, creatinine and phosphate for children on CFPD were 9.9 ± 3.10 ml/min/1.73 m2, 7.9 ± 3.3 ml/min/1.73 m2 and 5.5 ± 1.5 ml/min/1.73 m2 compared to conventional PD with values of 4.3 ± 1.68 ml/min/1.73 m2, 3.57 ± 1.3 ml/min/1.73 m2 and 2.53 ± 0.85 ml/min/1.73 m2, respectively (all p < 0.001).
Conclusion
Gravity-assisted CFPD appears to be a feasible and effective way to augment ultrafiltration and clearances in children with AKI. It can be assembled from readily available non-expensive equipment.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-022-05852-3.
Keywords: Gravity-assisted continuous flow peritoneal dialysis, Acute kidney injury, Peritoneal dialysis
Introduction
Peritoneal dialysis (PD) has long been a mainstay of acute kidney injury (AKI) management in children of all ages [1–4], and it remains the most commonly used modality in low-income regions, where continuous kidney replacement therapy (CKRT) is not widely available [4, 5]. Compared to extracorporeal therapies, PD has lower clearance as well as lower and less precise fluid removal. We previously demonstrated increased ultrafiltration and clearances using continuous flow peritoneal dialysis (CFPD) in children with AKI, but the technique described required expensive high-volume CKRT pumps to circulate fluid, as well as high-level technical expertise, limiting its utility in resource-constrained settings [6, 7].
The objectives of this study were to test the feasibility of implementing a novel CFPD method using low-cost, readily available materials and which uses gravity as the driving force to circulate the fluid through the abdomen and to compare this CFPD system to conventional PD in children with AKI requiring dialysis.
Methods
Trial design
This study was a randomised crossover clinical trial of consecutive eligible patients admitted to a multidisciplinary paediatric intensive care unit (PICU). Participants were allocated 1:1 to one of two treatment sequences: CFPD/conventional PD or conventional PD/CFPD. The crossover design was chosen owing to the heterogenous population, with varying ages, sizes, severity of illness and underlying diagnoses. Within-patient variation is less than between-patient variation; therefore, fewer patients would be required to demonstrate a treatment effect in a crossover design than in a parallel group-randomised controlled trial. The main outcomes of clearance and ultrafiltration are not affected by the previous dialysis, and thus, there is no carry-over effect. The order in which patients received the interventions was randomised using an online random number generator (http://appstore.com/TheRandomNumberGenerator). One investigator (PN) generated the random allocation sequence and assigned patients to the sequence of interventions after obtaining written informed consent from the child’s legal guardian. It was not possible to blind the therapy to the researchers; however, patients were sedated and ventilated and therefore effectively blinded to the therapy.
Participants, setting and location
Inclusion criteria
Patients up to a maximum weight of 15 kg admitted to the PICU of Red Cross War Memorial Children’s Hospital, Cape Town, with AKI, defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [8] were eligible for inclusion in the study, once a decision to start acute dialysis was made by the attending paediatric nephrologists and intensivists. The Neonatal KDIGO definition of AKI was used for infants under 120 days old [9].
Exclusion criteria
Any patients whose clinical condition contraindicated PD catheter placement (e.g. abdominal wall defects, abdominal wall surgery, severe diaphragmatic defects, burns or septic skin lesions covering the entire abdominal wall) were excluded. Patients developing a pleural effusion secondary to PD were withdrawn from the study.
This study was approved by the University of Cape Town’s Human Research Ethics Committee (HREC 363/2017), and the protocol was registered on The Pan African Clinical Trials Registry (registry number: PACTR201801003005742 ). Informed consent was taken from the child’s parent or legal guardian.
Sample size
The sample size calculations were based on previous studies on CFPD using ultrafiltration (UF) and clearances of creatinine and urea [6, 7]. In a one-sided superiority trial, 18 children were estimated to be needed to show a 2.5% change in the primary outcome measure at a 95% significance level. Enrolment targets were not reached owing to COVID-19 research restrictions; however, after 15 patients had been enrolled, a post hoc power calculation confirmed that this sample size was sufficient and further recruitment was halted.
Baseline data
Baseline characteristics of the patients were recorded including age, sex, weight and height, primary admission diagnosis as well as comorbidities, indication for dialysis, medication and AKI score.
Interventions
Gravity-assisted CFPD intervention
In vitro modelling of the gravity-assisted CFPD system
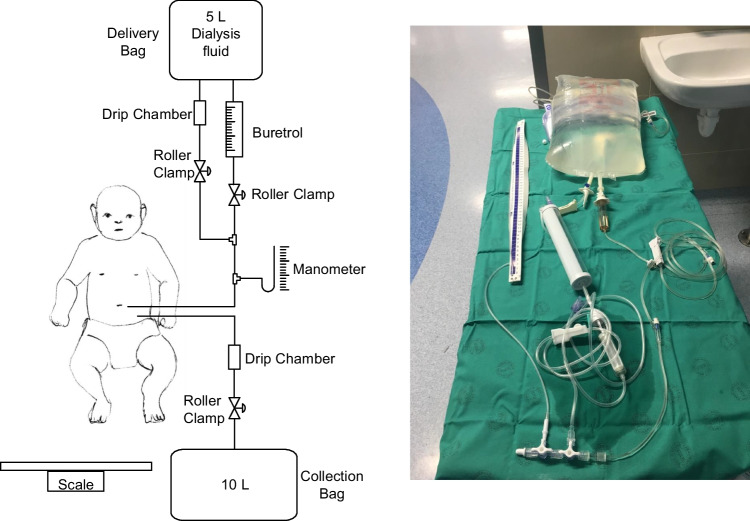
Initial in vitro measurements, described in Supplementary information A, were conducted to model the hydraulics of a gravity-assisted CFPD system (Fig. 1). The system was easily assembled using inexpensive and available equipment. Flow could be accurately measured by counting drops in the IVI, giving set drip chambers up to a maximum of 200 drops per minute. This flow rate was considered adequate for in vivo CFPD in children ≤15 kg body weight and could be achieved with a bag height of 15 cmH2O. Outflow and inflow could be adjusted by lifting or lowering the collection and dialysis bags, respectively, and both were fine-tuned with the roller clamps. Modelled intra-abdominal pressure (IAP) was not affected by flow rate when adjusting flow using the roller clamps while maintaining the same height as the inflow bag. The maximum modelled IAP with outflow fully constricted was equal to the height of the dialysate bag and occurred at a static system state when inlet flow had ceased. The IAP was much lower during flow than in the static system due to head losses in the system determined by the Poiseuille equation. Head losses over constrictions and the PD catheters increased with increased flow.
Fig. 1.
Set-up of in vitro experiments of gravity-assisted CFPD. Manometers inserted along the line at several points to measure pressure at different flow rates, the height of delivery and collection bags and during constriction of in and out flow. Drip chambers used to measure the flow rate
Technique used to deliver CFPD to patients
Equipment
Each participant required two PD catheters; a high sensitivity scale; fluid giving sets (20 or 10 droppers depending on the size of patient), 5 L bags of PD fluid; buretrol to measure intraperitoneal fill volume accurately; drainage bag; three 3-way taps; plus tubing to act as a manometer.
Catheter placement
Two PD catheters (8.5Fr Multipurpose Cook Catheters (MPCC); Cook Medical Inc, Bloomington, IN, USA) were placed at the bedside using the Seldinger technique as described in our previous study [7], except where a catheter had already been placed prior to study recruitment, in which case only one additional MPCC was inserted.
Dialysis fluid
The initial fill volume was aimed at 20 mL/kg; however, the fill volume was adjusted to maintain the initial IAP below 10 mmHg (15 cmH2O). Initial glucose concentration was generally 2.5%, but this was adjusted according to patient needs. The dialysis fluid was lactate-based unless the attending nephrologist preferred a bicarbonate-based fluid.
Circulation of fluid (Fig. 2): Once the initial fill volume had been infused via the buretrol, the continuous flow was started. The flow rate, both into and out of the peritoneum, was set by counting the number of drops that formed in the inlet and outlet drip chambers, respectively. The flow rate was adjusted by lowering or raising the collection bag or the inflow bag and then fine-tuned using the roller clamps. For the first three patients, the inflow was set at 25 mL/min/1.73 m2, and thereafter, the inflow rate was increased to 50 mL/min/1.73 m2, as interim analysis demonstrated that clearance at the lower rate was lower than expected. The outflow rate was set higher than the inflow rate by an increment of 2. 5 ml/min/1.73 m2 to compensate for ultrafiltration, as per our previous studies [6, 7] and derived from adult studies [10].
Fig. 2.
Set-up of the system to deliver gravity-assisted CFPD to patients. Buretrol to measure the volume of initial fill volume. Drip chambers to measure flow rate. Flow adjusted by lifting and lowering delivery and collection bags. Roller clamps to fine-tune flow rate. Manometer inserted to measure intra-abdominal pressure during flow. Scale to weigh delivery and collection bags
Ultrafiltration volume
After 2 h and thereafter 4 hourly, the inflow was clamped, while the outflow continued draining for 20 min to allow the abdomen to empty completely. The ultrafiltration rate was calculated as follows: true UF = (mass of drainage bag at the end of session − mass of drainage bag at the beginning of session) – (mass of delivery bag at the beginning of session − mass of delivery bag at the end of session).
Monitoring
All children were in PICU and received continuous invasive and non-invasive monitoring as per standard practice. While the child was on CFPD, the intra-abdominal pressure was monitored hourly via a manometer directly from the PD catheter or via a bladder transducer. Tidal volume was also monitored for patients on pressure ventilation. If there was thought to be over-distension or the IAP was greater than or equal to 15 cmH2O, aliquots of 5 ml/kg were drained until the IAP was acceptable. Any dialysate removed from the abdomen because of over-distension was added to the outflow total volume for analysis.
Conventional peritoneal dialysis (control)
Conventional PD was performed as per international guidelines [11], with fill volume initially 20 ml/kg, glucose concentration 2.5%, dwell time 45–60 min, inflow 1–10 min and outflow 20 min, adjusted according to patient requirements. Dialysis was performed manually using the Fresenius PD Paeds system.
Deviation from the published protocol
We initially planned to treat each patient for 24 h with each dialysis technique, but after review of the first two patients and noting a considerable change in clinical condition over a two-day period, the total time on each intervention was limited to 6–8 h to account for the high acuity of illness. It was felt that shortening the duration of consecutive dialysis periods would therefore allow better comparison between the study periods and reduce the period effect.
Laboratory analysis
Blood
Blood was taken from the patients at baseline and then 6 hourly thereafter, coordinated with routine blood samples. The following analytes were measured using routine and standardised methodologies on the AU480 (Beckman Coulter, Brea, California, USA) platform: urea, creatinine, phosphate, sodium, potassium, albumin, bicarbonate and glucose. Briefly, urea was measured by kinetic enzymatic methodology using urease and glutamate dehydrogenase (coefficient of variation (CV) 1.1%). Creatinine was measured with coupled enzymatic reactions beginning with creatininase (CV 2.4%). Phosphate was measured colourimetrically using molybdate (CV 2.9%). Sodium and potassium were measured using ion-selective electrodes (CV 0.8% and 1.3%, respectively). Albumin was measured colourimetrically using bromocresol green (CV 1.0%). Bicarbonate was measured in a coupled enzymatic assay using phosphoenolpyruvate carboxylase and malate dehydrogenase (CV 2.3%). Glucose was measured using the enzymatic hexokinase method (CV 1.5%). Quality control checks were assessed and noted to be acceptable.
Dialysate
After measuring the volume of dialysate after the first 2-h session of CFPD or the first cycle of conventional PD, a sample of the dialysate was collected and sent to the laboratory for measurement of urea and creatinine. All the spent dialysate (inclusive) after the first session was then pooled. This fluid was then sent to the laboratory, and the following analytes were measured: urea, creatinine, phosphate, sodium and glucose. The analysis of these analytes was validated for routine measurement in fluid samples using the methodologies described above.
The values for phosphate measured in the dialysate fluid were expected to be lower than the reported sensitivity of the routine assay (0.48 mmol/L). Therefore, a modified, manually performed assay was developed utilising the same methodology, but with a significantly lower sensitivity (0.045 mmol/L). This modified assay was validated for use in this study. The measuring range was optimised to be linear at low concentrations of phosphate (0.045–2.890 mmol/L) with a CV of 3.9%, which is within allowable limits of imprecision according to biological variation [12].
Outcomes
Primary outcomes
Feasibility of technique
Ultrafiltration
Clearances
Secondary outcomes
-
Adverse events/complications
Complications systematically looked for were as follows: raised IAP with resultant deleterious effects on ventilation, leaking catheters, blocked catheters, peritonitis, hyperglycaemia and electrolyte disturbances. Any other unsuspected adverse events were documented.
Mass transfer coefficients (MTC)
Calculations
Ultrafiltration and clearance rates were calculated over the full duration of each modality, including filling and drainage times. For both interventions, UF was measured by the difference between the volume of infused fluid and the amount drained, expressed as mL/kg/h.
Clearances, expressed as ml/min/1.73 m2, for urea, creatinine and phosphate for both methods were calculated by dividing dialysate concentration (D) by average plasma concentration (P) multiplied by the total dialysate volume (V) collected after fully draining the abdomen at the end of the study period (clearance = D/P × V).
For patients on conventional dialysis, the MTC was calculated at the end of the first cycle according to the formulas of Garred, as modified by Krediet [13] and expressed per body surface area: MTC (mL/min/1.73 m2) = Vd/t × ln [Vi × P/(Vd × (P−Dt)]) × 1.73/BSA, where Vd is volume drained, t is dwell time, Vi is volume instilled, P is mean plasma concentration, Dt is dialysate concentration at the end of dwell, and BSA is body surface area.
For CFPD, the MTCs were calculated according to formulas by Gotch (ml/min) [10] adjusted for BSA: MTC = [Kt (Qp + Qf (1−0.33 × S) − 0.67Qf × S × (Qp + Qf)]/(Qp – Kt + Qf)) × 1.73/BSA, where Kt is clearance, Qp is flux into the patient in ml per minute, Qf is flux by UF in ml per minute, and S is sieving coefficient (which is approximately 1 for small solutes). The actual inflow and ultrafiltration rates were used rather than the initial target rates.
Statistical analysis
Data were entered manually into an Excel database (Microsoft, Washington, USA) and exported to SPSS version 7 (IBM, New York, USA) for statistical analysis. Descriptive statistics (proportions, means and/or medians) and central tendency were calculated for all outcomes and covariates at baseline and when participants underwent the comparative treatment (the two respective study arms). Primary outcome variables were found to have a normal distribution and were therefore analysed using parametric measures. Paired t-tests were performed to compare outcomes for the two dialysis techniques. A p-value of less than 0.05 was considered significant. A Bonferroni correction was applied to account for the effect of multiple testing.
Results
Participants
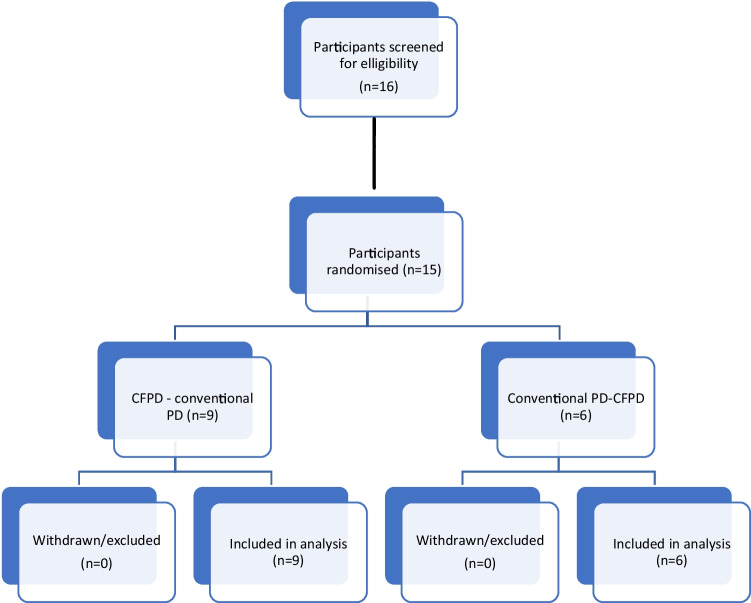
Between 2018 and 2021, consent was obtained from 16 eligible participants. Following consent but prior to randomisation, it was found that one patient had developed a pleural effusion while receiving out-of-study PD and this patient was therefore excluded. Fifteen patients were randomised, nine to first receive CFPD and six to first receive conventional PD (Fig. 3). All randomised patients were included in the final analysis (Table 1).
Fig. 3.
Participant flow
Table 1.
Baseline characteristics of patients
| Patient | Sequence | Age (months) | Wt (kg) | Underlying diagnosis | Percentage fluid overload (FO) since admission to PICU | AKI stage before dialysis |
|---|---|---|---|---|---|---|
| 1 | 1 | 0.2 | 2.3 | Diaphragmatic hernia | 4% | Urine stage 2; creat stage 2 |
| 2 | 0 | 0.33 | 2.5 | TGA post-cardiac surgery | Clinically FO++ 3% from the previous day, ++ fluid in OT | Urine stage 3; creat stage 2 |
| 3 | 1 | 15 | 9 | Shocked gastroenteritis paracetamol poisoning, liver failure | 9% | Urine stage 3; creat stage 2 |
| 4 | 0 | 7 | 5.8 | AVSD repair; sepsis; arrhythmia | 26% | Urine stage 2; creat stage 3 |
| 5 | 1 | 11 | 8.6 | Gastroenteritis, sepsis | 15% | Urine stage 3; creat stage 3 |
| 6 | 1 | 5.4 | 6.5 | Shocked gastroenteritis, sepsis, gangrenous foot | 8% | Urine stage 3; creat stage 3 |
| 7 | 0 | 3 | 3 | TOF post-central shunt | 9% | Urine stage 0; creat stage 3 |
| 8 | 1 | 0.26 | 3.3 | TGA post-surgery | 22% | Urine stage 3; creat stage 2 |
| 9 | 1 | 11.5 | 6.71 | TOF post-surgery repair | 9% | Urine stage 0; creat stage 2 |
| 10 | 0 | 0.5 | 2.7 | TGA post-surgery repair | 24% | urine stage 1; creat stage 0 |
| 11 | 0 | 10 | 10 | Septic shock | Clinically FO ++ (transferred from another hospital for dialysis) | Urine stage 2; creat stage 3 |
| 12 | 1 | 1.5 | 3.5 | Hemi truncus arteriosus; congenital syphilis | 6% FO | Neonatal creat stage 3 urine stage 0 |
| 13 | 1 | 35 | 14 | HUS diarrhoea associated | Clinically FO ++ (transferred from another hospital for dialysis) | Urine stage 2; creat stage 3 |
| 14 | 0 | 6 | 5 | TAPVD post-septostomy | 5% | Urine stage 0; creat stage 3 |
| 15 | 1 | 14 | 12 | Myocarditis | 2% | Urine stage 2; creat stage 2 |
| Median(range) | 6 (0.2–14) | 5.8(2.3–14) |
AKI, acute kidney injury; AVSD, atrioventricular septal defect; HUS, haemolytic uraemic syndrome; PICU, paediatric intensive care unit; TAPVD, total anomalous pulmonary venous drainage; TOF, tetralogy of fallot
Sequence assignment: 0 = conventional PD first, 1 = CFPD first
The mean (standard deviation) (SD) time of conventional PD was 8.1 h (5.02) compared to 8.7 (6.57) h on CFPD (p = 0.71). In thirteen patients, the percentage of glucose used in both modalities was identical, while in two patients, the percentage of glucose in the dialysis fluid used in conventional PD was higher than in CFPD (4.25% vs. 2.5%).
Outcome and estimations
Initial analysis showed that the order of sequence assignment did not affect the change in any of the primary outcome measures (see Supplementary information B). The interventions were therefore treated as independent samples in the final analysis.
Primary outcomes
-
Feasibility of gravity-assisted CFPD technique
The equipment was easily assembled in a sterile fashion, and treatment could be instituted within approximately 20 min of catheter insertion. Adequate flow rates were achieved with the investigational system, and it was possible to measure IAP directly from the peritoneal cavity during CFPD using a manometer. The target flow rate was 8.8 ml/min, and the average achieved flow rate was 8.2 ml/min. The mean actual flow rate of CFPD was 44 ml/min/1.73 m2 (range: 28–78) (Table 2). The mean ± SD fluid used in ml/kg/h on conventional PD was 13.3 ± 2.4 ml/kg/h compared to CFPD which was 73 ± 33 ml/kg/h. There were no major adverse events. An analysis of ventilatory settings, heart rate, blood pressure and blood saturation was performed in an attempt to demonstrate safety. These parameters were compared with each other using paired t-tests for each modality for the full time period that each patient was on the modality. There were no significant differences noted (see Supplementary information C for analysis).
Table 2.
Flow rates on CFPD in ml/min/1.73 m2
| Patient no. | Actual flow rate (ml/min/1.73 m2) | Target flow rate (ml/min/1.73 m2) |
|---|---|---|
| 1 | 30 | 25 |
| 2 | 30 | 25 |
| 3 | 34 | 25 |
| 4 | 78 | 50 |
| 5 | 28 | 50 |
| 6 | 56 | 50 |
| 7 | 61 | 50 |
| 8 | 46 | 50 |
| 9 | 29 | 50 |
| 10 | 56 | 50 |
| 11 | 48 | 50 |
| 12 | 50 | 50 |
| 13 | 33 | 50 |
| 14 | 52 | 50 |
| 15 | 31 | 50 |
| Mean flow rate | 44 (range: 28–78) |
-
2.
Ultrafiltration (Table 3)
Mean ± SD UF was significantly higher on CFPD (4.3 ± 3.15 ml/kg/h) compared to conventional PD (1.04 ± 1.72 ml/kg/h) (p < 0.001). Only one patient (no. 10) had a lower UF on CFPD.
Table 3.
Treatment effect
| Variable | Conventional PD, mean (SD) | CFPD, mean (SD) | Mean difference (CFPD-CONV) (95%CI) | Cohen’s D effect size (95%CI) | P* |
|---|---|---|---|---|---|
| UF (ml/kg/h) | 1.05 (1.73) | 4.10 (3.15) | 3.05 (1.62–4.49) | 1.18 (0.50–1.83) | <0.001 |
| Clearance urea (ml/min/1.73 m2) | 4.33 (1.69) | 9.91 (3.10) | 5.58(3.83–7.33) | 1.77(0.94–2.58) | <0.001 |
| Clearance creatinine (ml/min/1.73 m2) | 3.58 (1.31) | 7.91 (3.33) | 4.33 (2.75–5.90) | 1.52 (0.76–2.26) | <0.001 |
| Clearance phosphate (ml/min/1.73 m2) | 2.53 (0.85) | 5.48 (1.60) | 3.10(2.09–4.11) | 2.05 (0.98–3.12) | <0.001 |
| MTC urea (ml/min/1.73 m2) | 6.25 (5.13) | 12.80 (7.11) | 6.55 (2.48–10.61) | 0.89 (0.28–1.48) | 0.004 |
| MTC creatinine (ml/min/1.73 m2) | 4.17 (2.32) | 8.96 (5.31) | 4.79 (2.17–7.40) | 1.01 (0.37–1.63) | 0.002 |
CFPD continuous flow peritoneal dialysis, CI confidence interval, MTC mass transfer coefficient, PD peritoneal dialysis, UF ultrafiltration
*All variables remained statistically significant after the Bonferroni correction
-
3.
Clearances (Table 3)
Mean ± SD clearances for urea, creatinine and phosphate for children on CFPD were 9.9 ± 3.10 ml/min/1.73 m2, 7.9 ± 3.3 ml/min/1.73 m2 and 5.5 ± 1.5 ml/min/1.73 m2, respectively, compared to 4.3 ± 1.68 ml/min/1.73 m2, 3.57 ± 1.3 ml/min/1.73 m2 and 2.53 ± 0.85 ml/min/1.73 m2, respectively, with conventional PD (all p < 0.001). Full data were available for urea and creatinine, but phosphate clearance data were only available for 11 (73.3%) patients. When expressed as ml/kg/h, the mean ± SD clearance for urea on CFPD was 19.5 ml ± 5.4 ml/kg/h.
Secondary outcomes
-
Complications (Table 4)
Lactate, in all but two patients, decreased or remained stable on dialysis, despite mostly using lactate-based fluids. Bicarbonate-based fluids were used for one patient (no. 3) with rising lactate and acute liver failure. For the other patient (no. 14), the persistent lactic acidosis was thought to be due to very poor cardiac output.
Table 4.
Complications
| Adverse event | Number of events | Action taken | |
|---|---|---|---|
| Raised intra-abdominal pressure whilst on dialysis | No adverse event under either conv PD or CFPD | 0 | |
| No adverse event under conv PD but adverse event under CFPD | 1 | 5 ml/kg fluid drained from the abdomen | |
| Adverse event observed under conv PD but not under CFPD | 0 | ||
| Adverse events observed under both conv PD and CFPD | 0 | ||
| Peritonitis | No adverse events under either conv PD or CFPD | 15 | |
| No adverse event under conv PD but adverse event under CFPD | 0 | ||
| Adverse event observed under conv PD but not under CFPD | 0 | ||
| Adverse events observed under both conv PD and CFPD | 0 | ||
| Catheter leak | No adverse events under either conv PD or CFPD | 0 | |
| No adverse event under conv PD but adverse event under CFPD | 1 | Small leak therefore treatment continued | |
| Adverse event observed under conv PD but not under CFPD | 0 | ||
| Adverse events observed under both conv PD and CFPD | 0 | ||
| Catheter block/poor drainage | No adverse event under either conv PD or CFPD | 0 | |
| No adverse event under conv PD but adverse event under CFPD | 3 | In and outflow catheters swapped in 2 patients. Treatment discontinued after block in one patient | |
| Adverse event observed under conv PD but not under CFPD | 1 | Treatment terminated after catheter block | |
| Adverse events observed under both conv PD and CFPD | 0 | ||
| Hyperglycaemia requiring insulin therapy | No adverse event under either conv PD or CFPD | 0 | |
| No adverse event under conv PD but adverse event under CFPD | 0 | ||
| Adverse event observed under conv PD but not under CFPD | 0 | ||
| Adverse events observed under both conv PD and CFPD | 1 | Insulin infusion started | |
| Hypokalaemia requiring replacement | No adverse event under either conv PD or CFPD | 0 | |
| No adverse event under conv PD but adverse event under CFPD | 4 | Potassium supplemented | |
| Adverse event observed under conv PD but not under CFPD | 0 | ||
| Adverse events observed under both conv PD and CFPD | 0 |
CFPD continuous flow peritoneal dialysis, PD peritoneal dialysis
-
2.
Mass transfer coefficients
Mean ± SD MTCs for urea and creatinine on CFPD were 12.7 ± 7.11 ml/min/1.73 m2 and 8.96 ± 5.3 ml/min/1.73 m2 compared to 6.2 ± 5.13 ml/min/1.73 m2 and 4.17 ± 2.3 ml/min/1.73 m2 on conventional PD (p = 0.004 and p = 0.002, respectively)
Discussion
In this paper, we demonstrated a simple, technologically light method of performing gravity-assisted CFPD in children with AKI using low-cost, readily available equipment. The method was easily and rapidly assembled at the bedside with readily available equipment. Counting drops formed in the drip chambers were an acceptable way of achieving the desired flow rates for CFPD.
UF and clearances were significantly higher in CFPD, similar to the results of our previous studies [6, 7], as well as experimental studies in adult patients with chronic kidney disease [14–16]. In this study, we used half the continuous flow rate compared to our previous studies, yet managed to achieve a similar increase in UF relative to conventional PD, although a slightly lower relative increase in clearance. This indicates that the faster rates may be unnecessary. In critically ill children and neonates, a relatively high fluid volume, often in excess of 150 ml/kg, is frequently necessary to deliver adequate nutrition and continuous drug infusions [17, 18]. In low- and middle-income countries where extracorporeal techniques are not available, the increased ultrafiltration and clearance attained by this technique may be useful when conventional PD is insufficient. The increased clearances may be an advantage in hypercatabolic AKI, where there are difficulties getting sufficient clearance of small solutes. Small solute clearances achieved of 9.91 ml/min/1.73 m2 (19.5 ml/kg/h) approximate the recommended prescription for extracorporeal CKRT [8]. It can also be postulated that this technique may be beneficial for the removal of ammonia in neonates with inborn errors of metabolism, but this has not been demonstrated.
Outflow blockages and with continued inflow as well as unpredictable UF during CFPD could both precipitate a rise in IAP. Recent in vitro studies have attempted to predict UF in CFPD based on mass transfer equations and using the transporter status of patients [19]. In this current model of gravity-assisted CFPD, we believe that there is a degree of autoregulation: As the IAP increases (due to increased UF), back pressure on the inflow arm temporarily decreases inflow and increased pressure on the outflow arm increases outflow. This contrasts with when pumps are used, which regulate inflow and outflow more rigidly. In only one patient was fluid drained from the abdomen because of rising IAP while on CFPD. In cases where IAP is a concern, it is possible to use low fill volumes but still maintain UF. This was previously demonstrated in children by Sagy et al. [20], who successfully treated severe fluid overload in children using no fill volume and a continuous flow of fluid through the abdomen. Generally, we feel it is preferable to regulate the outflow to maintain an intraperitoneal volume. In this way, more of the peritoneal membrane is recruited with more surface area available for exchange. It is important, as shown in our experimental studies, to keep the inflow bag as low as possible but still maintain adequate flow. If outflow blocks, the static pressure in the abdomen (IPP) would equal the column of water above the abdomen. IAP during continuous flow will also be higher if the inflow bag is higher.
In four patients, the potassium level dropped and required replacement. As with conventional PD, we would recommend that potassium be added to the bags once the plasma potassium falls below 4 mmol/L [11]. When using lactate-based fluids, there is a risk of causing increased serum lactate (as with conventional PD) in cases where lactate handling by the liver is compromised. This was not found to be a problem with our patients.
MTCs in CFPD were increased compared to conventional PD, in keeping with previous studies [21] but lower than those calculated in chronic adult PD patients on CFPD [10]. Postulated reasons for this could be the lower fill volumes in paediatric patients leading to lower peritoneal membrane recruitment as well as the acute illness of the children which may affect perfusion of the peritoneal membrane. The flow rates in the adult study [10] were also double which could affect the mixing of the dialysate fluid.
CFPD, in this format, uses approximately six times as much fluid as conventional PD. Because of the small size of children with AKI, the fluid volumes are still relatively low compared to what it would be in adults, thus keeping the costs down.
Limitations of this study: This was a single-centre, small sample study of a highly heterogeneous population with different underlying conditions and severity of illness, and without blinding to intervention allocation. Despite these limitations, the results were consistent among most participants, supporting the study’s internal validity. However, the findings may not be generalisable to all patients treated in the PICU or to different socio-geographic settings. The short time each patient was on each modality may not be long enough to demonstrate feasibility and sustainability. Further studies would be necessary to demonstrate sustainability without increased risk of complications such as peritonitis. A further limitation may be that the initial patient number, according to the original power calculation, was not achieved. A post hoc power calculation however did confirm that the number of patients was sufficient.
In conclusion, gravity-assisted CFPD is an effective technique for increasing UF and clearances in children with acute PD. It can be rapidly assembled in low-resource settings with readily available, inexpensive equipment and without the need for electricity. This could be done by physicians accustomed to performing acute PD in children.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Darryl Brown and Dominic De Oliveira for their help in the design and construction of the in vitro testing rig and for carrying out the experimental programme described in the Supplementary information. The authors would also like to thank Kate Webb for her help with the statistical analysis.
Data availability
Individual participant data that underlie the results reported in this article will be made available after de-identification. Availability will follow publication, with no end date. The data will be made available to researchers whose use of the data has been approved by an independent review committee. The purpose of the data access will be to achieve the aims of the research proposal. Proposals should be directed to <peter.nourse@uct.ac.za>. To gain access to data, requesters will need to sign a data transfer agreement with the University of Cape Town.
Declarations
Conflict of interest
The authors have nothing to declare with respect to this research project. Funding for the study was from the primary author’s research fund only. All research equipment was bought from this fund.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swan H, Gordon HH. Peritoneal lavage in the treatment of anuria in children. Pediatrics. 1949;4:586–595. doi: 10.1542/peds.4.5.586. [DOI] [PubMed] [Google Scholar]
- 2.Guzzo I, de Galasso L, Mir S, Bulut IK, Jankauskiene A, Burokiene V, Cvetkovic M, Kostic M, Bayazit AK, Yildizdas D, Schmitt CP, Paglialonga F, Montini G, Yilmaz E, Oh J, Weber L, Taylan C, Hayes W, Shroff R, Vidal E, Murer L, Mencarelli F, Pasini A, Teixeira A, Afonso AC, Drozdz D, Schaefer F, Picca S. Acute dialysis in children: results of a European survey. J Nephrol. 2019;32:445–451. doi: 10.1007/s40620-019-00606-1. [DOI] [PubMed] [Google Scholar]
- 3.Guzzo I, de Galasso L, Bayazit AK, Yildizdas D, Schmitt CP, Hayes W, Shroff R, Jankauskiene A, Virsilas E, Longo G, Vidal E, Mir S, Bulut IK, Tkaczyk M, Mencarelli F, Bertulli C, Cvetkovic M, Kostic M, Paglialonga F, Montini G, Yilmaz E, Teixeira A, Atmis B, Schaefer F. Acute pediatric kidney replacement therapies in Europe: demographic results from the EurAKId Registry. Nephrol Dial Transplant. 2022;37:770–780. doi: 10.1093/ndt/gfab280. [DOI] [PubMed] [Google Scholar]
- 4.Raina R, Chauvin AM, Bunchman T, Askenazi D, Deep A, Ensley MJ, Krishnappa V, Sethi SK. Treatment of AKI in developing and developed countries: an international survey of pediatric dialysis modalities. PloS One. 2017;12:e0178233. doi: 10.1371/journal.pone.0178233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olowu WA, Niang A, Osafo C, Ashuntantang G, Arogundade FA, Porter J, Naicker S, Luyckx VA. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4:e242–250. doi: 10.1016/S2214-109X(15)00322-8. [DOI] [PubMed] [Google Scholar]
- 6.Nourse P, Sinclair G, Gajjar P, du Plessis M, Argent AC. Continuous flow peritoneal dialysis (CFPD) improves ultrafiltration in children with acute kidney injury on conventional PD using a 4.25 % dextrose solution. Pediatr Nephrol. 2016;31:1137–1143. doi: 10.1007/s00467-016-3341-5. [DOI] [PubMed] [Google Scholar]
- 7.Raaijmakers R, Schroder CH, Gajjar P, Argent A, Nourse P. Continuous flow peritoneal dialysis: first experience in children with acute renal failure. Clin J Am Soc Nephrol. 2011;6:311–318. doi: 10.2215/CJN.00330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Diseases Improving Global Outcomes Working Group KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 9.Starr MC, Charlton JR, Guillet R, Reidy K, Tipple TE, Jetton JG, Kent AL, Abitbol CL, Ambalavanan N, Mhanna MJ, Askenazi DJ, Selewski DT, Harer MW. Advances in neonatal acute kidney injury. Pediatrics. 2021;148:e2021051220. doi: 10.1542/peds.2021-051220. [DOI] [PubMed] [Google Scholar]
- 10.Gotch FA. Kinetic modeling of continuous flow peritoneal dialysis. Semin Dial. 2001;14:378–383. doi: 10.1046/j.1525-139X.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 11.Nourse P, Cullis B, Finkelstein F, Numanoglu A, Warady B, Antwi S, McCulloch M. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (paediatrics) Perit Dial Int. 2021;41:139–157. doi: 10.1177/0896860820982120. [DOI] [PubMed] [Google Scholar]
- 12.Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, Minchinela J, Perich C, Simón M. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 13.Krediet RT, Boeschoten EW, Zuyderhoudt FM, Strackee J, Arisz L. Simple assessment of the efficacy of peritoneal transport in continuous ambulatory peritoneal dialysis patients. Blood Purif. 1986;4:194–203. doi: 10.1159/000169445. [DOI] [PubMed] [Google Scholar]
- 14.Ronco C, Dell'Aquila R, Bonello M, Gloukhoff A, Amerling R, Cruz C, Levin N. Continuous flow peritoneal dialysis: a new double lumen catheter. Int J Artif Organs. 2003;26:984–990. doi: 10.1177/039139880302601103. [DOI] [PubMed] [Google Scholar]
- 15.Freida P, Issad B. Continuous flow peritoneal dialysis: assessment of fluid and solute removal in a high-flow model of "fresh dialysate single pass". Perit Dial Int. 2003;23:348–355. doi: 10.1177/089686080302300407. [DOI] [PubMed] [Google Scholar]
- 16.Amerling R, DeSimone L, Inciong-Reyes R, Pangilinan A, Folden T, Ronco C, Gotch FA, Levin N. Clinical experience with continuous flow and flow-through peritoneal dialysis. Semin Dial. 2001;14:388–390. doi: 10.1046/j.1525-139X.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- 17.Langer T, D'Oria V, Spolidoro GCI, Chidini G, Scalia Catenacci S, Marchesi T, Guerrini M, Cislaghi A, Agostoni C, Pesenti A, Calderini E. Fluid therapy in mechanically ventilated critically ill children: the sodium, chloride and water burden of fluid creep. BMC Pediatr. 2020;20:424. doi: 10.1186/s12887-020-02322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Lawati ZH, Sur M, Kennedy CE, Akcan Arikan A. Profile of fluid exposure and recognition of fluid overload in critically ill children. Pediatr Crit Care Med. 2020;21:760–766. doi: 10.1097/PCC.0000000000002337. [DOI] [PubMed] [Google Scholar]
- 19.Öberg CM, Martuseviciene G. Computer simulations of continuous flow peritoneal dialysis using the 3-pore model – a first experience. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. Perit Dial Int. 2019;39:236–242. doi: 10.3747/pdi.2018.00225. [DOI] [PubMed] [Google Scholar]
- 20.Sagy M, Silver P. Continuous flow peritoneal dialysis as a method to treat severe anasarca in children with acute respiratory distress syndrome. Crit Care Med. 1999;27:2532–2536. doi: 10.1097/00003246-199911000-00034. [DOI] [PubMed] [Google Scholar]
- 21.Cruz C, Melendez A, Gotch FA, Folden T, Crawford TL, Diaz-Buxo JA. Single-pass continuous flow peritoneal dialysis using two catheters. Semin Dial. 2001;14:391–394. doi: 10.1046/j.1525-139X.2001.00098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article will be made available after de-identification. Availability will follow publication, with no end date. The data will be made available to researchers whose use of the data has been approved by an independent review committee. The purpose of the data access will be to achieve the aims of the research proposal. Proposals should be directed to <peter.nourse@uct.ac.za>. To gain access to data, requesters will need to sign a data transfer agreement with the University of Cape Town.