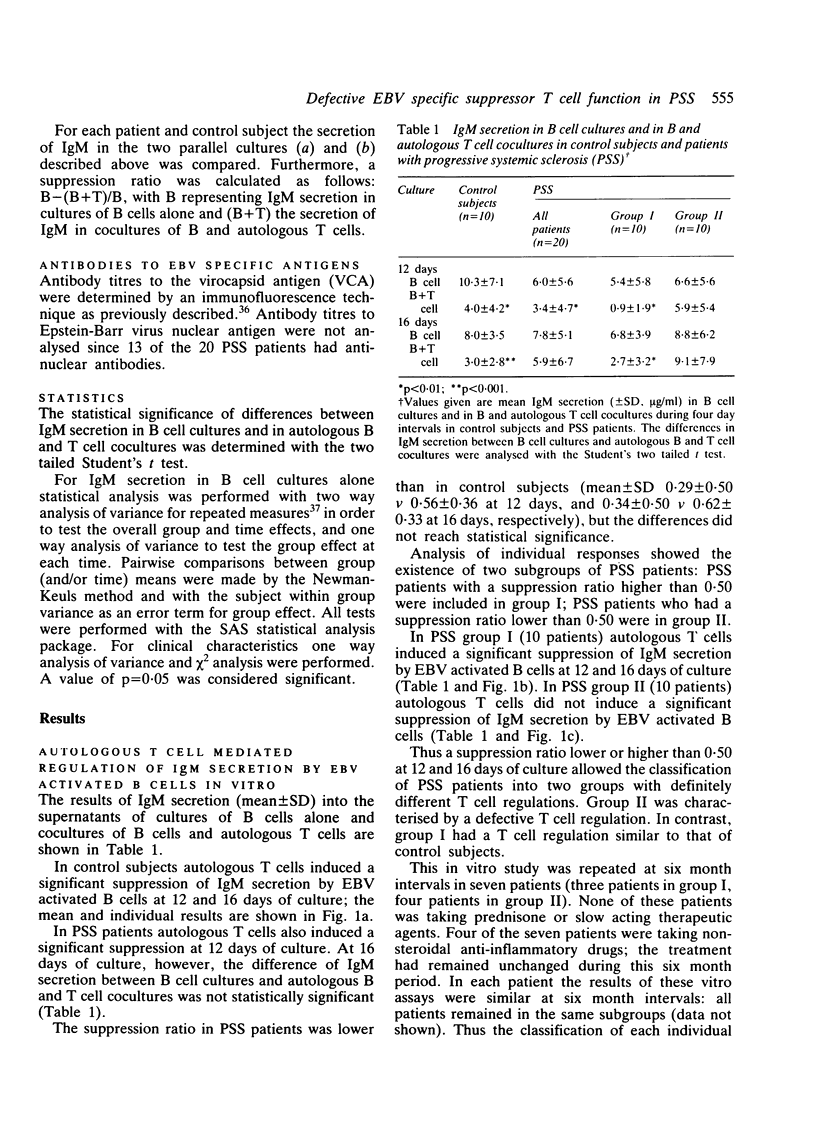
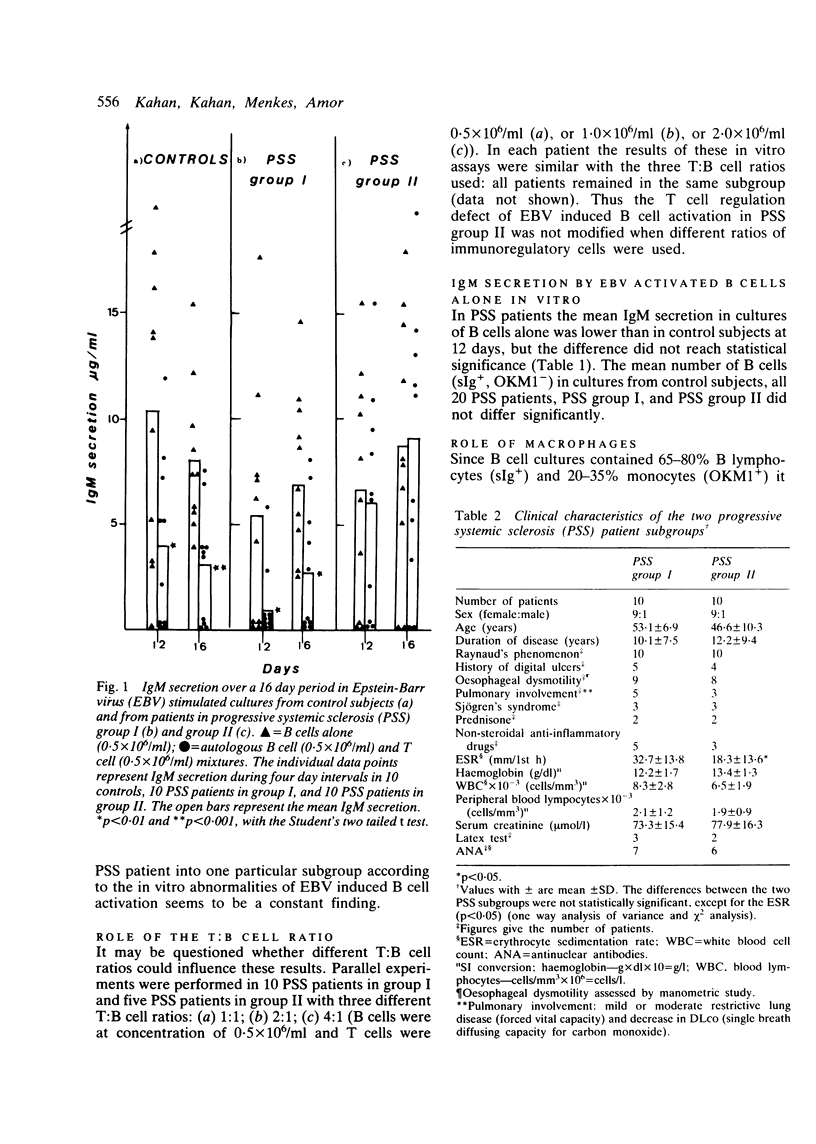
Abstract
Several immunoregulatory defects of Epstein-Barr virus (EBV) induced B cell activation have been described in patients with rheumatoid arthritis (RA), suggesting that EBV may have a role in the pathogenesis of RA. We assessed EBV specific T cell regulation in 20 patients with progressive systemic sclerosis (PSS) and immune to EBV and in 10 control subjects also immune to EBV by comparing the secretion of IgM into supernatants of 16 day cultures of B cells alone and cocultures of B and autologous T cells. In control subjects autologous T cells mediated a significant decrease in the secretion of IgM by B cells at 12 and 16 days of culture. Analysis of individual responses showed the existence of two subgroups of patients with PSS: group I (10 patients) had a suppressor T cell function similar to that of controls; group II (10 patients) had a defective T cell function. Differences in the duration or severity of the disease, the slow acting therapeutic agents, and anti-inflammatory drugs could not account for these subdivisions. These results suggest that several immunoregulatory defects of EBV induced B cell activation exist in different connective tissue diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Palacios R., Ibáez de Kasep G. Human postthymic precursor cells in health and disease. VII. Immunoregulatory circuits of the peripheral blood mononuclear cells from patients with progressive systemic sclerosis. J Clin Lab Immunol. 1981 May;5(3):143–148. [PubMed] [Google Scholar]
- Bardwick P. A., Bluestein H. G., Zvaifler N. J., Depper J. M., Seegmiller J. E. Altered regulation of Epstein-Barr virus induced lymphoblast proliferation in rheumatoid arthritis lymphoid cells. Arthritis Rheum. 1980 Jun;23(6):626–632. doi: 10.1002/art.1780230603. [DOI] [PubMed] [Google Scholar]
- Baron M., Keystone E. C., Gladman D. D., Lee P., Poplonski L. Lymphocyte subpopulations and reactivity to mitogens in patients with scleroderma. Clin Exp Immunol. 1981 Oct;46(1):70–76. [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Steigerwald J. C., Tan E. M. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982 Apr;48(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A new approach to the study of human B lymphocyte function using an indirect plaque assay and a direct B cell activator. Immunol Rev. 1979;45:41–67. doi: 10.1111/j.1600-065x.1979.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bluestein H. G., Hasler F. Epstein-Barr virus and rheumatoid arthritis. Surv Immunol Res. 1984;3(1):70–77. doi: 10.1007/BF02918600. [DOI] [PubMed] [Google Scholar]
- Buckingham R. B., Prince R. K., Rodnan G. P., Taylor F. Increased collagen accumulation in dermal fibroblast cultures from patients with progressive systemic sclerosis (scleroderma). J Lab Clin Med. 1978 Jul;92(1):5–21. [PubMed] [Google Scholar]
- Buffone G. J., Lewis S. A. Manual immunochemical nephelometric assays for serum immunoglobulins IgG, IgA, and IgM. Clin Chem. 1979 Jun;25(6):1009–1012. [PubMed] [Google Scholar]
- Campbell P. M., LeRoy E. C. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin Arthritis Rheum. 1975 May;4(4):351–368. doi: 10.1016/0049-0172(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Carapeto F. J., Winkelmann R. K. Peripheral blood lymphocyte distribution in scleroderma. Dermatologica. 1975;151(4):228–235. doi: 10.1159/000251340. [DOI] [PubMed] [Google Scholar]
- Catoggio L. J., Bernstein R. M., Black C. M., Hughes G. R., Maddison P. J. Serological markers in progressive systemic sclerosis: clinical correlations. Ann Rheum Dis. 1983 Feb;42(1):23–27. doi: 10.1136/ard.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Harding B., Mirick G. R., Schneider J., Quismorio F. P., Friou G. J. Selective decrease in antibody-dependent cell-mediated cytotoxicity in systemic lupus erythematosus and progressive systemic sclerosis. Clin Exp Immunol. 1978 Nov;34(2):235–240. [PMC free article] [PubMed] [Google Scholar]
- D'Angelo W. A., Fries J. F., Masi A. T., Shulman L. E. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969 Mar;46(3):428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- Depper J. M., Zvaifler N. J. Epstein-Barr virus. Its relationship to the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1981 Jun;24(6):755–761. doi: 10.1002/art.1780240601. [DOI] [PubMed] [Google Scholar]
- French M. A., Harrison G., Penning C. A., Cunningham J., Hughes P., Rowell N. R. Serum immune complexes in systemic sclerosis: relationship with precipitating nuclear antibodies. Ann Rheum Dis. 1985 Feb;44(2):89–92. doi: 10.1136/ard.44.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Malaviya A. N., Rajagopalan P., Good R. A. Subpopulations of human T lymphocytes. IX. Imbalance of T cell subpopulations in patients with progressive systemic sclerosis. Clin Exp Immunol. 1979 Nov;38(2):342–347. [PMC free article] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983 Aug;131(2):768–772. [PubMed] [Google Scholar]
- Haynes D. C., Gershwin M. E. The immunopathology of progressive systemic sclerosis (PSS). Semin Arthritis Rheum. 1982 Feb;11(3):331–351. doi: 10.1016/0049-0172(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R., Allonby I. D., Janis K., Dodd J. K. The relationship of defective cell-mediated immunity to visceral disease in systemic sclerosis. Clin Exp Immunol. 1977 May;28(2):233–240. [PMC free article] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R., Dodd J. Thymus-dependent (T) lymphocyte deficiency in progressive systemic sclerosis. Br J Dermatol. 1976 Nov;95(5):469–473. doi: 10.1111/j.1365-2133.1976.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Husson J. M., Le Go A., Engler R., Ollier M. P., Fiessinger J. N., Housset E., Hartmann L. Immunochemical study on serum proteins in systemic sclerosis. Biomedicine. 1977 May;26(3):182–187. [PubMed] [Google Scholar]
- Irving W. L., Walker P. R., Lydyard P. M. Abnormal responses of rheumatoid arthritis lymphocytes to Epstein-Barr virus infection in vitro: evidence for multiple defects. Ann Rheum Dis. 1985 Jul;44(7):462–468. doi: 10.1136/ard.44.7.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving W. L., Walker P. R., Lydyard P. M., Roitt I. M. Defective rheumatoid lymphocyte function assessed by Epstein-Barr virus infection in vitro. Lancet. 1982 Dec 11;2(8311):1337–1338. doi: 10.1016/s0140-6736(82)91536-7. [DOI] [PubMed] [Google Scholar]
- Jiménez S. A. Cellular immune dysfunction and the pathogenesis of scleroderma. Semin Arthritis Rheum. 1983 Aug;13(1 Suppl 1):104–113. doi: 10.1016/0049-0172(83)90029-x. [DOI] [PubMed] [Google Scholar]
- Kahan A., Kahan A., Amor B., Menkes C. J. Different defects of T cell regulation of Epstein-Barr virus-induced B cell activation in rheumatoid arthritis. Arthritis Rheum. 1985 Sep;28(9):961–970. doi: 10.1002/art.1780280902. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Gladman D. D., Baron M., Cane D., Poplonski L. Antigen-specific suppressor cell activity in patients with scleroderma. J Rheumatol. 1981 Sep-Oct;8(5):747–751. [PubMed] [Google Scholar]
- Keystone E. C., Lau C., Gladman D. D., Wilkinson S., Lee P., Shore A. Immunoregulatory T cell subpopulations in patients with scleroderma using monoclonal antibodies. Clin Exp Immunol. 1982 May;48(2):443–448. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Tosato G., Blaese R. M., Broder S., Magrath I. T. Polyclonal immunoglobulin secretion by human B lymphocytes exposed to Epstein-Barr virus in vitro. J Immunol. 1979 Apr;122(4):1310–1313. [PubMed] [Google Scholar]
- Kondo H., Rabin B. S., Rodnan G. P. Cutaneous antigen-stimulating lymphokine production by lymphocytes of patients with progressive systemic sclerosis (scleroderma). J Clin Invest. 1976 Dec;58(6):1388–1394. doi: 10.1172/JCI108594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitt E. L., Holdstock G., Bland J. H., Chastenay B. F., Albertini R. J. Suppressor cell activity in progressive systemic sclerosis. J Rheumatol. 1982 Mar-Apr;9(2):263–267. [PubMed] [Google Scholar]
- Leroy E. C. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972 Jun 1;135(6):1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. L., Nardo J. M. Vascular disease in progressive systemic sclerosis (scleroderma). Ann Intern Med. 1970 Aug;73(2):317–324. doi: 10.7326/0003-4819-73-2-317. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Salem N. B., Morse J. H. Lymphocyte response to mitogens in progressive systemic sclerosis. Arthritis Rheum. 1976 Sep-Oct;19(5):875–882. doi: 10.1002/art.1780190507. [DOI] [PubMed] [Google Scholar]
- Segond P., Salliere D., Galanaud P., Desmottes R. M., Massias P., Fiessinger J. N. Impaired primary in-vitro antibody response in progressive systemic sclerosis patients: rôle of suppressor monocytes. Clin Exp Immunol. 1982 Jan;47(1):147–154. [PMC free article] [PubMed] [Google Scholar]
- Stierle H. E., Brown K. A., Perry J. D., Holborow E. J. Abnormal responsiveness of rheumatoid B cells to Epstein-Barr virus infection in vitro. Lancet. 1981 Dec 12;2(8259):1347–1347. doi: 10.1016/s0140-6736(81)91367-2. [DOI] [PubMed] [Google Scholar]
- Stierle H. E., Brown K. A., Perry J. D., Holborow E. J. Increased responsiveness of rheumatoid B lymphocytes to stimulation by Epstein-Barr virus. Rheumatol Int. 1983;3(1):7–11. doi: 10.1007/BF00541225. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Tsokos G. C., Magrath I. T., Balow J. E. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol. 1983 Oct;131(4):1797–1801. [PubMed] [Google Scholar]
- Vaughan J. H. Dunlop-Dottridge lecture. Rehumatoid arthritis, rheumatoid factor and the Epstein-Barr virus. J Rheumatol. 1979 Jul-Aug;6(4):381–388. [PubMed] [Google Scholar]
- Whiteside T. L., Kumagai Y., Roumm A. D., Almendinger R., Rodnan G. P. Suppressor cell function and T lymphocyte subpopulations in peripheral blood of patients with progressive systemic sclerosis. Arthritis Rheum. 1983 Jul;26(7):841–847. doi: 10.1002/art.1780260704. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Hughes P., Rowell N. R., Sneddon I. B. Antibody-dependent and phytohaemagglutinin-induced lymphocyte cytotoxicity in systemic sclerosis. Clin Exp Immunol. 1979 Apr;36(1):175–182. [PMC free article] [PubMed] [Google Scholar]
- de Jesus D. G., Clancy R. L. Circulating T and B lymphocytes in progressive systemic sclerosis. J Rheumatol. 1975 Sep;2(3):336–339. [PubMed] [Google Scholar]


