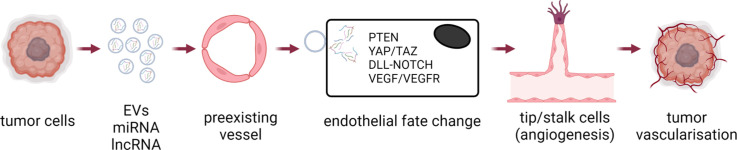
Highlights
-
•
Vascularization continues to be a challenge for medicine and tissue engineering.
-
•
Tumor cell-derived exosomes stimulate angiogenesis through PTEN.
-
•
PTEN that differentially controls proliferation to give vessel stability. In non-endothelial cells PTEN blocks the cell cycle but, in VEGF-activated endothelial cells, PTEN releases these from their hyper-mitogenic arrest.
-
•
PTEN and Hippo pathway components converge in the regulation of endothelial patterning during angiogenesis.
-
•
miRNAs and lncRNAs control the "dose" of activation of the PTEN and Hippo pathways, determining the physiological to pathological transition.
Keywords: Vascularization, Angiogenesis, Endothelial cells, miRNAs, lncRNAs, Exosomes, Cancer, Cell communication
Abbreviations: EC, endothelial cell; ECM, extracellular matrix; EVs, Extracellular vesicles; HIF-1α, Hypoxia-inducible factor 1 alpha; lncRNA, long noncoding RNAs; miRNA, microRNA; ncRNAs, Non-coding RNAs; PC, pericytes; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10; VEGFA, vascular endothelial growth factor 2; vSMC, vascular smooth muscle cells; TAZ, transcription activator with PDZ binding motif; YAP, Yes-associated protein
Abstract
Vascularization is a multifactorial and spatiotemporally regulated process, essential for cell and tissue survival. Vascular alterations have repercussions on the development and progression of diseases such as cancer, cardiovascular diseases, and diabetes, which are the leading causes of death worldwide. Additionally, vascularization continues to be a challenge for tissue engineering and regenerative medicine. Hence, vascularization is the center of interest for physiology, pathophysiology, and therapeutic processes. Within vascularization, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and Hippo signaling have pivotal roles in the development and homeostasis of the vascular system. Their suppression is related to several pathologies, including developmental defects and cancer. Non-coding RNAs (ncRNAs) are among the regulators of PTEN and/or Hippo pathways during development and disease. The purpose of this paper is to review and discuss the mechanisms by which exosome-derived ncRNAs modulate endothelial cell plasticity during physiological and pathological angiogenesis, through the regulation of PTEN and Hippo pathways, aiming to establish new perspectives on cellular communication during tumoral and regenerative vascularization.
Graphical abstract
Introduction
The vasculature is a complex, heterogeneous, and plastic system, which adapts to the tissue requirements to cope with microenvironmental challenges. Blood vessels are prone to respond to variations in mechanical stimuli such as cyclic strain and fluid shear stress that may keep endothelial cells (ECs) in quiescence or induce their activation. Such activation is followed by phenotypic adaptations of ECs and blood vessels which safeguard critical processes such as nutrient supply, waste product disposal, inflammation, and other processes that require cell-to-cell communication. Important vascular responses, namely vascular adaptation include (a) angiogenesis, i.e. the formation of new capillaries from existing vessels; (b) changes in vascular reactivity, where the vessel diameter adjusts to specific tissue needs; (c) changes in the architecture of the vascular wall due to reorganization of ECs, pericytes, vascular smooth muscle cells (vSMCs), and fibroblasts as well as the extracellular matrix (ECM) [1].
Cancer is a pathology that drives vascular adaptation, where tumor cells develop strategies to become irrigated in response to the local hypoxic microenvironment. According to these strategies tumors can be classified as pro-angiogenic or non-angiogenic. Angiogenic tumors grow vessels from the pre-existing vasculature toward the tumor site. Non-angiogenic tumors use alternative strategies such as vascular mimicry to meet their metabolic demands to sustain proliferation and metastasis [2]. These alternative strategies vary depending on the tissue of origin, the development status of the tumor, or the anti-tumoral therapy used [3].
The tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is frequently inactivated in tumor cells [4]. PTEN loss causes constitutive activation of the class I phosphoinositide 3-kinase (PI3K) pathway and is associated with increased endothelial Hypoxia-Inducible Factor 1α (HIF-1α) expression and vascularization stimulation through the PI3K/AKT/VEGF-ET-1 signaling pathway [5]. Furthermore, tumor angiogenesis is enhanced in PTEN-deficient models [6,7]. In contrast, PI3K signaling inactivation by PTEN impairs vascular sprouting by attenuating EC migration and proliferation. PTEN is also linked to vascular homeostasis through PI3K- independent mechanisms.
Tumor development and dissemination depend on cell communication to regulate pivotal processes such as vascularization. Besides the classical signaling through soluble (growth) factors, tumors exploit exosomes to influence their microenvironment. Exosomes are membrane-shelled extracellular vesicles (EVs) that mediate intercellular molecular communication, contributing to a wide range of biological processes in health and disease. Environmental alterations stimulate signaling pathways that influence exosome production rate and content. Tumor cells release around ten-fold more exosomes compared to non-tumoral cells [8], emphasizing the importance of these vesicles for tumor communication. Otherwise, non-coding RNAs (ncRNAs) which include microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), directly affect gene expression profile, and signaling pathway activity and therefore change the cell fate and plasticity.
Exosomal ncRNAs from tumors and ECs regulate the expression of surface receptors, growth factors, ECM components, and signaling molecules involved in vascular adaptation [9]. Some of these ncRNAs influence PTEN signaling during development and pathology, inducing migration, proliferation, and survival in EC and stromal cells. These microenvironmental changes favor the increase in vascular density [10,11]. Moreover, specific exosomal molecule enrichment is associated with the development, progression, and/or outcome of several diseases such as cancer [12]. In this review, we summarize the current knowledge on the mechanisms by which tumor cell-derived exosomal ncRNAs regulate the endothelial phenotype and tumor vascular adaptations via PTEN signaling pathway.
PTEN: a multi-tool protein phosphatase
With an estimated 200,000 different phosphorylation sites in a cellular proteome, the human genome encodes about 612 kinases and 170 phosphatases [13,14]. Interestingly, this small number of phosphatases revert the actions of the dominating sets of kinases, balancing their reduced gene numbers by high protein abundance; suggesting that individual phosphatases have multiple roles and are critical for homeostasis [14].
Many types of allelic losses and sequence alterations in chromosomal region 10q23 in several human cancers led to the search for a tumor suppressor gene in this region, receiving various names including PTEN [6], gene mutated in multiple advanced cancers 1 (MMAC1) [15] and transforming growth factor-β-regulated and epithelial-cell enriched phosphatase 1 (TEP1) [7]. PTEN encodes a ubiquitously expressed lipid and protein phosphatase of 403 amino acids and about 47.1 KDa in humans. The N-terminal domain contains the protein tyrosine phosphatase motif and the C-terminal has a C2 domain which has a membrane phospholipids affinity essential for the lipid phosphatase function [16]. PTEN has different subcellular localizations: membrane, cytoplasm, nucleus, and mitochondria, where its distribution is kept under strict control [17]. PTEN is an evolutionarily conserved and functionally non-redundant protein with crucial roles in vascular development, angiogenesis, motility, proliferation, and metabolism [18]. PTEN is also described as a haploinsufficient tumor suppressor, i.e., one functional allele is not sufficient to sustain a wild-type condition, whereby subtle variations in PTEN dose predispose to tumorigenesis in a tissue-specific manner. Hence, PTEN is on the list of ‘quasi-insufficient’ tumor suppressor genes [19,20]. The homozygous deletion of PTEN is embryonic lethal due to aberrant angiogenesis which underpins its relevance in endothelial biology.
PTEN regulation
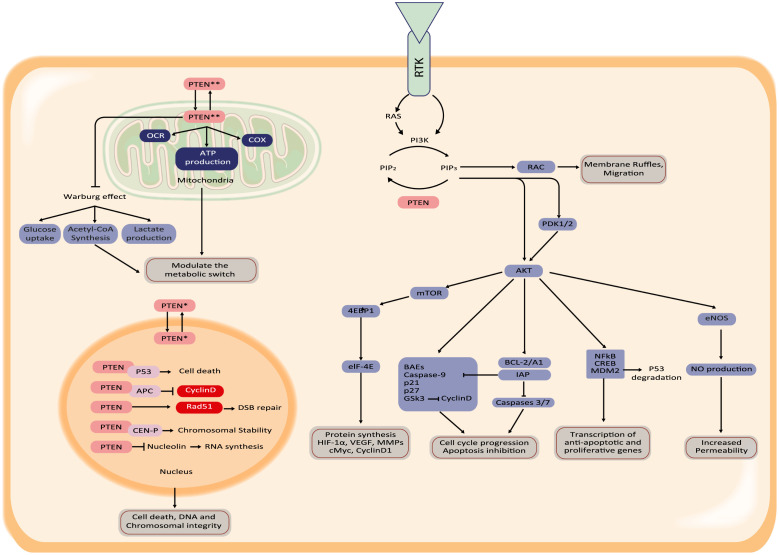
PTEN expression is regulated through transcriptional factors, epigenetic silencing (transcriptional); miRNAs silencing (post-transcriptional), and phosphorylation, SUMOylation, ubiquitination, redox regulation, acetylation, S-nitrosylation (post-translational). Its transcription is positively regulated by early growth-regulated transcription factor 1 (EGR1), peroxisome proliferator-activated receptor γ (PPARγ), and P53 among others, having effects on proliferation, cytoskeletal organization, apoptosis evasion, and metabolic adaptations. Additionally, PTEN transcription is negatively regulated by the Nuclear factor kappa-light-chain-enhancer of activated β cells (NFκβ) and Transforming growth factor-beta (TGFβ), as well as by methylation, and miRNAs that target PTEN mRNA. The most widely described role of PTEN is to antagonize the PI3K action through its lipid phosphatase activity at the plasma membrane [15]. PTEN dephosphorylates the lipid substrate phosphatidylinositol [3], [4], [5]-triphosphate (PIP3), converting it back into phosphatidylinositol [4,5]-biphosphate (PIP2), leading to reduced PIP3 levels and signaling. An overview of this process is shown in Fig. 1.
Fig. 1.
Schematic overview of general PTEN signaling. PTEN is a phosphatase that antagonizes the PI3K/AKT axis, while PTEN also acts as a transcriptional co-factor in the nucleus. In mitochondria, PTEN influences energy metabolism.
PI3K is a part of the PI3K/AKT/mTOR pathway. Therefore, a negative regulation of PI3K signaling by PTEN inhibits both protein synthesis through mTOR activity and phosphorylation of proteins involved in translational control. PTEN also acts as a negative regulatory signal for the PI3K mitogenic signaling pathway. For in-depth details, the reader is directed to Ref. [21]. During the last decade, other interesting functions for PTEN have been described. Nuclear translocation of PTEN has a role in chromosome stability through the interaction with CEN-P in primary mouse embryonic fibroblasts [22] and is also required for homologous recombination repair of DNA double-strand breaks induced by irradiation in cancer cells (e.g. glioblastoma, colorectal, and breast) [23]. PTEN phosphorylation by ataxia telangiectasia mutated (ATM) kinase in response to DNA damage in cancer cells, is associated with PTEN nuclear translocation and autophagy induction through p-JUN-SESN2/AMPK pathway [24]. Otherwise, PTEN also influences the metabolic switch through the expression of an alternative translation product, PTENα. PTENα stimulates mitochondrial cyclooxygenase (COX) activity through the maintenance of COX hypo-phosphorylation, increases ATP production, and helps to recruit PTEN to the mitochondria [25]. Consistent with the previous, mitochondrial PTEN is linked to increased oxygen-dependent metabolism in normal and tumor hepatic cells. Overexpression of PTEN not only prevents proliferation and migration but also reverts the Warburg effect from glycolysis to oxidative phosphorylation, increasing the oxygen consumption rate [26] (Fig. 1).
The identity of other protein substrates for PTEN is still under study and is also linked to vascular homeostasis through mechanisms independent of PI3K signaling. Growing literature demonstrates that PTEN is versatile beyond its canonical function in the AKT signaling pathway, but PTEN also requires both lipid and protein phosphatase activity for tumor-suppressing functions [27].
Vascularization during development and pathology
Vascular development is fundamental for multicellular organisms. During tumor growth, tumoral cells expand rapidly and lose access to the bloodstream, generating a vascular homeostasis disruption, leading the tumor to adopt different strategies to increase the capillary density. Three distinct vascularization processes are recognized: (1) angiogenesis, (2) vasculogenesis and (3) arteriogenesis. Tumors primarily depend on angiogenesis which is the hypoxia-driven sprouting of novel vascular branches from pre-existing vessels. This process is often called sprouting or sprouting angiogenesis. In contrast, vasculogenesis is a primarily growth factor-driven process, done by the new formation of a vascular network from aggregating precursor ECs or even adult ECs. Finally, arteriogenesis is the fluid shear stress-induced maturation of pre-existing collateral arteries to functional conduits [28,29].
However, tumors also have specific non-angiogenic vascularization strategies which are not observed in normal tissues, like vessel co-option (the tumor grows exploiting pre-existing vessels), vascular mimicry (the cancer cells form structures, like channels, lined out by cancer cells), and cancer stem-like cells differentiation into ECs [2]. Some tumors are completely non-angiogenic. Also, non-angiogenic clusters of cells can transform into small angiogenic tumor islets, which progress to large vascularized tumors that metastasize, or even angiogenic aggressive tumors can switch to non-angiogenic growth in response to antitumoral therapy [2]. The strategy used by a tumor depends on the stage of development, and physiological and environmental conditions. Besides altering vascularization processes and vascular wall remodeling, tumors also induce changes in vascular reactivity to adapt to the tumor's needs [1,30].
Sprouting angiogenesis
Sprouting angiogenesis is the most extensively studied vascularization strategy. The angiogenic switch (quiescent vasculature is activated to sprout new capillaries) is triggered by changes in the relative balance of inducers and inhibitors of angiogenesis, while the main inducer is hypoxia [31]. In normoxic conditions, conserved proline residues in HIF-1α are continuously hydroxylated by prolyl hydroxylases, targeting HIF-1α for proteasomal degradation. However, hypoxia deactivates lysyl oxidases and HIF-1α is stabilized, translocated to the nucleus, and activates hypoxia-responsive gene transcription through binding to hypoxia-response elements (HRE) in their promoters. This signaling drives the transcription of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), endothelin-1 (ET-1), metalloproteinases 2 and 9 (MMP2 and MMP9). The VEGFA/VEGFR2 signaling stimulates PI3K signaling, which activates the serine/threonine kinase AKT. In turn, the AKT family member AKT1 activates eNOS expression to promote NO release. Meanwhile, ET-1 exerts sustained positive feedback on the HIF-1α signaling and its mitogenic and anti-apoptotic effects. The joint effect of these molecules is capillary sprouting through increased vessel permeability, EC proliferation, and migration [32].
In pathological conditions, local hypoxia, oxidative stress, and metabolic derangements drive tumor cells into a pro-angiogenic state, leading to the development of new tumor-penetrating vessels adapted to the tumor. The morphology of the resulting vessels is highly abnormal both structurally and functionally, disorganized, with irregular shapes and high tortuosity [1,33]. Also, due to the lack of pericyte support, tumor vessels are not able to maintain their shape and function, making those vessels leaky to support the exchange of gas, nutrients, and waste products but also to support metastasis. Paradoxically, tumors have a large number of microvessels, but these fail to perfuse properly because of diameter mismatches at vascular branch points [34]. This defective perfusion generates and maintains the metabolic switch, which includes fast energy production and increased carbohydrate fermentation. In consequence, the environmental pH lowers and exacerbates this defective angiogenesis in a positive feedback loop that reinforces the malignant environment [35] Also, disturbances in hemodynamic forces (fluid laminar shear stress, and cyclic strain) derived from under-perfused tissues facilitate the tumor progression through an increase in the cancer-associated fibroblasts [36], [37], [38].
Endothelial cell patterning during angiogenesis
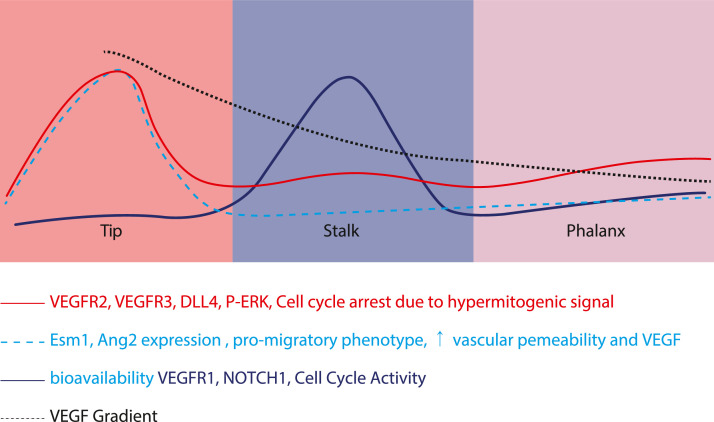
During angiogenesis, ECs form transient but phenotypically specialized EC populations. The tip cell EC phenotype is highly motile, non-proliferating, and polarized. This phenotype is associated with high levels of VEGFA/VEGFR2, which induces filopodia formation and extension through Cdc42 signaling (migratory phenotype), which guides to migration towards gradients of VEGFA [39], and helps with vasodilation due to an increase in eNOS expression [40]. The ECs with the stalk phenotype are located at the base of the sprout, proliferate, establish adherent/tight junctions, and form the vascular lumen. Phalanx cells are lumenized, non-proliferating, and immobile cells (Fig. 2).
Fig. 2.
Cellular patterning during angiogenesis. Tip cells are non-proliferative cells that form the leading edge of the protruding vascular tube. Immediately, juxtaposed to the tip cells are the proliferative stalk cells that essentially contribute to the lengthwise extension of the vascular tubes. After proliferation, stalk cells reach quiescence and become mature so-called phalanx cells.
Besides EC participation, correct and fully functional vessel development also implies mural cell recruitment. The platelet-derived growth factor B (PDGF-B) is secreted by ECs as a homodimer (PDGF-BB) and mediates the dimerization of its receptor in mural cells. This interaction of PDGF-BB/PDGFR is essential to recruit mural cells such as pericytes or vSMC, which regulate the proper vessel organization and capillary network maturation [41]. Tip cells also secrete angiopoietin-2 (Ang-2) and exert an autocrine proliferating signaling over ECs through the interaction of Ang-2 with the receptor Tie2. Recruited pericytes secrete angiopoietin-1 (Ang-1) that outcompetes Ang-2 from their joint receptor Tie-2. However, Ang-1 has no signaling activity, buffering the proliferation consistent with reduced VEGF-A secretion, and causing a complete arrest of EC proliferation. Therefore, a decrease in PDGFR signaling and Ang-1 expression concomitant with an increase in Ang-2/VEGF-A prevents the mural cell integration, supporting continued ECs proliferation and vessel destabilization during the tumor vascularization process [42].
Several molecules like VEGF, PDGF, and Ang-1 exert their action largely through a transitory PI3K/Akt and MAPK activation. A deranged activation of this signaling pathway triggers vascular development defects due to a disrupted specification and recruitment of vascular cells. Constitutively active PI3K/Akt expression in vivo, causes fetal vascular malformations and bleeding due to a failure in vascular development, and is associated with enhanced tumor angiogenesis, which suggests that the PI3K/Akt pathway is a key axis in vessel dynamics in ECs [43].
PTEN in vascularization process: canonical and non-canonical pathways
When considering the molecular mechanisms controlling vascularization through PTEN the most extensively investigated mechanism involves the canonical phosphatase activity of PTEN over PI3K/AKT signaling. PTEN deficiency in ECs causes vascular remodeling defects and contributes to both susceptibility to new tumorigenic mutations and accelerated tumor growth [43]. Ex vivo studies have shown that PTEN upregulation inhibits microvessel sprouting in arterial rings and pro-angiogenic processes in ECs including migration, tube formation, reduction in VEGF/ET-1 expression, and signaling. Conversely, PTEN silencing reverses that inhibition and triggers angiogenesis [44].
PTEN in endothelial patterning during angiogenesis
The specialization in ECs during angiogenesis correlates with increased Delta-like 4 (Dll4) expression in tip cells, which binds to and activates Notch 1 in adjacent stalk cells [45]. This specialization mechanism is transient and reversible, but indispensable for sufficient regulation of angiogenesis by preventing the stalk cell from becoming a new tip cell [46,47] (Fig. 2). In tip cells, the elevated VEGF/VEGFR2 signaling stimulates high Dll4 expression which blocks Notch signaling in these cells, increases filopodia protrusions, and arrests cell proliferation. Meanwhile, Notch signaling is increased in stalk cells, leading to a decrease in VEGF signaling, through reduced expression of VEGFR2/3 and enhanced expression of VEGFR1 and PTEN. Additionally, the number of filopodia is reduced while the cell cycle is increased both in vitro and in vivo [46], [47], [48]. This mechanism reinforces the tip cell position and suppresses the tip cell phenotype in stalk cells. During this transition, the lagging stalk cells adopt a phalanx cell phenotype which is a quiescent cell type and the prelude to newly formed quiescent blood vessels (Fig. 2).
It seems contradictory, that the expression of the antiproliferative PTEN is increased in stalk cells, because they are widely described to be proliferative, unlike tip cells. However, endothelial PTEN (in vitro and in vivo) is upregulated in stalk cells during vessel development through Notch signaling, by interaction with Dll4 on tip cells [48]. Why are the cells that have the greatest mitogenic stimulation not proliferating, and the ones that are proliferating express more PTEN? Tip cells express high VEGFR2 while producing high VEGF [49], generating a hyperactivation of VEGF/VEGFR2 signaling, which leads to high activation of the downstream MAPK pathway and ERK phosphorylation. This exacerbated mitogenic stimulation induces tip cells to exit the cell cycle, in a process known as hypermitogenic arrest, contributing to the induction of tip cell features, which include the already mentioned non-proliferation and pro-migratory phenotype [50].
Interestingly, overexpression of Notch signaling in stalk cells generates impaired proliferation and a gradual loss of ECs during development. If Notch signaling is lost, the cells do not “win” the vascular growth competition. In consequence, there is a transitory increase in the number and density of ECs without increasing the frequency of proliferating cells. Most likely by an increase in the cell cycle speed followed by a pronounced cell cycle arrest, which in total does not increase the proportion of the proliferating cells and impairs the appropriate vascular progression [50]. Data from this research show that most of the vessels are formed from cells with “normal” Notch levels [50]. Therefore, the physiological Notch activity in stalk cells is related to a decrease in hypermitogenic signaling (VEGF/VEGFR1 and MAPK/ERK phosphorylation). This allows the stalk cells both, to come out of the hypermitogenic arrest, and slow EC proliferation to prevent a premature expansion and exhaustion of cycling ECs in the angiogenic front [50].
Since the effect of a molecule depends on the context and the dose, PTEN expression in cells with a "normal" mitogenic signal decrease the proliferation. However, Dll4/Notch 1/PTEN signaling in cells under hypermitogenic arrest could help the cells to decrease the hypermitogenic signal and exit from the arrest to start cycling. As stalk cells proliferate, they escape from the influence of tip cells (Dll4 signal), Notch signaling wanes, and the balance is gradually tilted and PTEN helps transition from a proliferative to a quiescent phenotype, phalanx EC. If PTEN is lost, Notch signaling fails to limit stalk cell proliferation and results in defective sprout lengths and patterning [48]. The PTEN loss in ECs from zebrafish resulted in hypervascularization, ectopic vessel formation, and embryonic lethality [51]. Also, PTEN haploinsufficient zebrafish were prone to develop hemangiosarcoma at later life stages. All these vascular alterations were mediated by increased expression of VEGF-A after PTEN loss [51].
Most of the research focuses on tip and stalk cells, losing sight of the phalanx cells. Although PTEN itself is not required for Notch-dependent tip/stalk selection, phosphatase-dependent and -independent activities of PTEN are important to regulate stalk cell proliferation, vascular density, and vessel growth [48]. Also, most genetic studies are done using models of overexpression or underexpression and assign a pro or anti-angiogenic function to a given genetic product or signaling pathway, while in physiological conditions the dosage can be determinant for the cell plasticity and fate. Considering the above, PTEN could work as a hormetic rheostat helping the cells to reduce the hypermitogenic signal to make them able to cycle but later controlling the proliferation in the molecular transition between stalk and phalanx cells to give stability to the vessel.
Dual PTEN and Hippo communication in ECs: a strong feedback loop
Hippo signaling comprises the core kinases MST1/2, SAV1, MOB1, and LATS1/2. MST1/2 interaction with SAV1 activates MST1/2. MST1/2 phosphorylation leads to MOB1 phosphorylation, which in turn phosphorylates LATS1/2. The Hippo signaling is on when this cascade is phosphorylated and activated. Subsequently, Yes-associated protein (YAP)/transcription activator with PDZ binding motif (TAZ), effectors of the Hippo signaling, are phosphorylated, and retained in the cytoplasm or degraded. When the Hippo pathway is off, YAP/TAZ is not phosphorylated or actively dephosphorylated and shuttled to the nucleus, where it forms a complex with DNA-binding transcription factors including TEAD, Smads, and TBX5. The complex induces the expression of a wide range of genes (e.g., cell junctions, cytoskeletal regulators, kinases, and secreted proteins) which are involved in proliferation, survival, migration, and mechanical sensing [52]. In fact, YAP and TAZ have been identified as key mechanotransducers that detect mechanical stimuli and relay signals to control transcription of downstream genes involved in cellular mechanoresponses. The major upstream mechanosensors for YAP/TAZ signaling are integrins, G protein-coupled receptors, enzyme-linked receptors (e.g. receptor tyrosine kinases), and ion (calcium) channels (e.g. Piezo). These mechanosensors are modulated by disturbed blood flow, stretching or loss of cell contact, changes in cell geometry, or ECM stiffness, whose mechanotransduction elicits downstream transcriptional responses after YAP/TAZ activation or repression. Some of the effects on endothelial cells of disturbed mechanosensing are increased migration and proliferation and increased resistance to apoptosis (reviewed in Ref. [53])
An interesting association between PTEN and Hippo pathway is described in gastric cancer [54]. PTEN inactivation promotes gastric cancer cell proliferation and migration in vitro. In PTEN dominant-negative mutant cells, decrease the expression of SAV1, LATS1, LATS2, MOB1, and p-YAP, also decrease the MOB1–LATS1/2 interaction, and increase the expression of YAP/TAZ and YAP is redistributed to the nucleus [54]. A high expression and nuclear localization of YAP are associated with a more aggressive tumor phenotype and a shorter disease-free survival time for gastric cancer patients. In contrast, wild-type PTEN cells show the opposite effect over these Hippo signaling components [54], revealing a connection between those pathways.
Like PTEN, Hippo signaling needs to be off in tip ECs and on in stalk ECs, regulating together ECs proliferation and patterning (Fig. 3) controlling the sprouting angiogenesis and vessel stabilization. Upon stimulation with VEGF, the VEGF/VEGFR2 interaction in brain ECs induce actin cytoskeleton changes and inhibits LATS1/2 and MTS1/2, allowing YAP/TAZ translocation into the nucleus [55]. This link between VEGF and Hippo pathway involves YAP/TAZ DNA binding complexes, controlling the expression of genes related to PI3K/AKT signaling, cell adhesion/ECM-receptor interaction (i.e., NINJ1, MFAP5), actin cytoskeleton remodeling (i.e., MACF1, FLNB, MICAL3, CTGF), VEGF/VEGFR2 signaling (i.e., NCK2, SHC2) and others (Fig. 2) [55]. Deletion of YAP/TAZ in ECs abrogates VEGF signaling; genes that are otherwise upregulated upon VEGF stimulation are decreased after YAP/TAZ depletion [55]. Also, ECs migration, filopodia/lamellipodia formation, and cdc42 regulation are impaired, inhibiting the normal sprouting process. For example, YAP/TAZ-depleted mice have defective sprouting and lumen formation, with extensive multifocal brain hemorrhages and visible leaking areas [55].
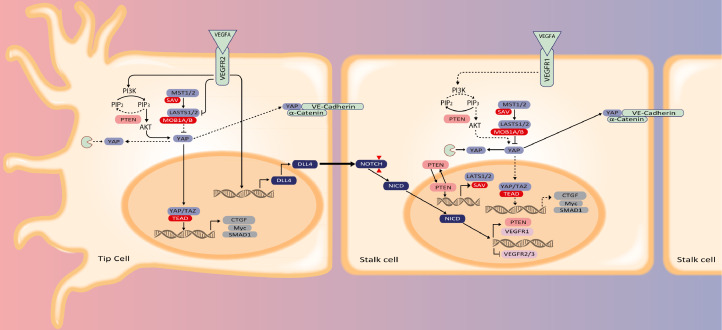
Fig. 3.
Molecular signaling in angiogenesis. In tip cells, VEGF/VEGFR2 signaling abrogates PTEN and activates AKT which causes nuclear localization of the YAP transcription factor. Nuclear YAP suppresses pro-proliferative signaling. In addition, VEGF signaling suppresses NOTCH expression while upregulating the NOTCH ligand Delta-like 4 (Dll4). The Dll4 activates NOTCH at the surface of stalk cells after which the NICD activates the expression of VEGFR1 while inhibiting VEGFR2 & 3. The decoy receptor VEGFR1 scavenges VEGF which releases PTEN activity and promotes YAP degradation, but part of the YAP is incorporated in adherens junctions and complexed with VE-Cadherin and α-catenin. These actions render stalk cells proliferative.
With an important role in tumor metastasis and drug resistance, tumor cells can acquire blood vessel-like structures through vascular mimicry. Like physiological angiogenesis, vascular mimicry is regulated by VEGFR. VEGFR-positive breast cancer cells form tubes when treated with VEGF, whereas knockdown of YAP/TAZ inhibits VEGF-induced tube formation. Therefore, the Hippo pathway contributes to VEGF-induced sprouting angiogenesis in ECs, and to vascular mimicry [56].
The exposure of ECs to VEGF inhibits the LATS1/2 kinase activity through stimulation of the VEGFR2-Src kinase complex. During the initiation phase of VEGF-induced endothelial sprouting Hippo kinases are off, in other words, expression of LATS1/2 and AMOT is reduced as well as their kinase activity. This causes nuclear translocation of YAP/TAZ and coincides with F-actin polymerization, filopodia extension, and stress fiber formation as the consequence. Thereafter, Hippo kinases turn on and YAP/TAZ activities turn off. Phosphorylated YAP binds to the 14-3-3 protein and is sequestered by binding to VE-cadherin-binding α-catenin at adherens junctions, followed by enhanced intercellular junction connectivity with the contractile actomyosin network (Fig. 3). Overall, Hippo-YAP/TAZ reads angiogenic signals to promote vascular sprouting and junction maturation [57,58].
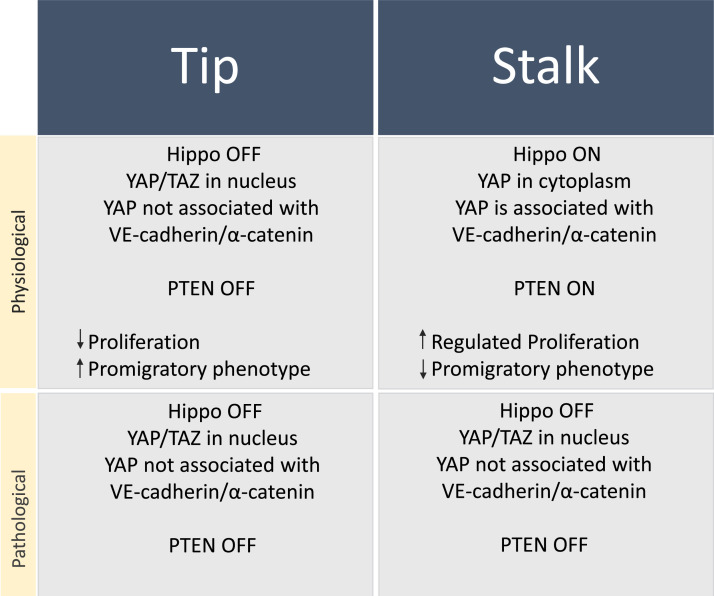
Based on the above, the on/off switch in tip and stalk cells works as follows. In a hypermitogenic arrest context, PTEN is absent (off), and PI3K/AKT will be active (on), switching off the Hippo signaling pathway, and consequently stimulating pro-migratory phenotype in tip cells. Conversely, if PTEN is actively expressed (on), PI3K/AKT will be inhibited (off), switching on the Hippo signaling pathway, and consequently helping the cells to come out of the hypermitogenic arrest, stimulating proliferation and inhibiting the pro-migratory phenotype in stalk cells (Box 1, Table 1, Fig. 3).
Box 1.
Cytoplasmic and nuclear functions of PTEN and Hippo signaling in EC patterning. Tip and stalk specification can be impaired by persistent silencing by ncRNA, affecting the stability and functionality of the new blood vessel.
Table 1.
miRNAs targeting PTEN (Ensemble: ENST00000371953) from miRWalk3.0.
| miR | Mechanism of action | Cell Type Described |
Inside EVs | Hippo Signaling | Ref. |
|---|---|---|---|---|---|
| miR-10a/b | Activates pro-metastatic genes, promoting tumor cell invasion and metastasis. Migration. | Ovarian cancer, bladder cancer, breast cancer, esophageal cancer, and gastric cancer cells. ECs. |  |
+ | [164,165,166,167,168,169,170,171] |
| miR-17-5p*/3p | Survival, proliferation, migration, regeneration. EC morphogenesis and angiogenesis. Target TIMP1 Tsp1, PTEN. |
ECs, tumor cells |  |
+ | ([78,88,89,172] |
| miR-19a/b-1*/-2⁎⁎ | Help to suppress the sensitivity to anticancer drugs, and anti-apoptotic. EC morphogenesis and tumor angiogenesis. Regeneration Target TIMP1 Tsp1, PTEN. |
Gastric cancer cell line, ECs, leukemia cells (K562). |  |
+ | [78,88,89,140,143,172,173] |
| miR-20a*/b-5p/3p⁎⁎ | EC morphogenesis and tumor angiogenesis. Regeneration Target TIMP1 Tsp1, PTEN. |
Breast cancer, myeloma cells, ECs. |  |
? | ([139,140] |
| miR-21 | Proliferation, EMT, angiogenesis. Repair spinal cord microvasculature. Maintaining of vSMC phenotype. | In vitro in vivo models (several tumors, vascular repair, healthy). |  |
+ | [83,86,97,134] |
| miR-23a/b-3p | Proliferation, tumor progression, metastasis. | Prostate cancer. | [90] | ||
| miR-25-3p*** | Up-regulation of miR-25 was correlated migration and invasion. | Esophageal squamous cell carcinoma. |
[174] |
||
| miR-26a-1-5p/3p | c-Myc increases miR-26a expression. miR-26a activates Akt signaling through direct PTEN targeting and increases VEGF, MMP2 expression. Increases proliferation and invasiveness. | High-grade glioma, glioblastoma multiforme, lung cancer cells |  |
+ | [90,91,175,176] |
| miR-26b | Enhance growth, survival, and tube formation through PTEN targeting. Inhibit the proliferation, migration, and EMT in epithelial cells through prostanoid pathway inhibition. |
EC, epithelial cells |  |
[177,178,179] |
|
| miR-29a-5p | Pro-angiogenic YAP induces miR-29 to inhibit PTEN translation which activates PI3K–mTOR signaling to regulate proliferation and cell size. |
Lung cancer cells, ECs, epithelial cells, mice. |  |
+ |
([138],[180] |
|
miR-92a-1-5p*/a-2⁎⁎ miR-92b-3p |
EC morphogenesis and tumor angiogenesis. Regeneration Target TIMP1 Tsp1, PTEN. |
ECs, leukemia cells (K562). |  |
+ |
[78,89,90,181,182,183] |
| miR-93-5p⁎⁎⁎ | High proliferation, anti-apoptotic, and influence tumor size. Cisplatin resistance, suppressing PTEN and over activating AKT pathway. Angiogenesis | Osteosarcoma cells, ovarian cancer, ECs, mouse hearts | + |
([135,184,185] |
|
| miR-103a-3p | Downregulation of angiogenesis inhibitors expression, like TIMP3. Promotes cell transformation, and disrupt the endothelium integrity, increasing the vascular permeability. | Endometrial and hepatocarcinoma cells, ECs. |  |
+ | [127,128,129,130,131,186] |
| miR-105-5p | Vascular permeability disruption. Downregulates ZO-1 expression, altering the vascular junctions. | In vitro, ex vivo and in vivo assay in rodents. |  |
[133] | |
| miR-106a-5p/3p⁎⁎/ miR-106b-5p/3p⁎⁎⁎ | |||||
| miR-130a/b | Proliferation, migration, and anti-apoptotic | Glioma cell lines, squamous cell carcinoma. | [187,188] | ||
| miR-181b/c-5p | |||||
| miR-193/a-3p | Cell proliferation, migration, 5-FU chemoresistance, and in vivo tumorigenicity through PTEN targeting. Contradictory, functions like tumor suppressor miRNA, inhibiting migration, invasion, and EMT in vitro and metastasis in vivo in lung cancer. |
Gastric cancer cell lines |
([189,190] |
||
| miR-200c-5p/3p | Alters wound-healing capacity and colony formation. MT1-MMP (Membrane type-1 matrix metalloproteinase) and PTEN expression are regulated. | Pancreatic cancer cell. | |||
| miR-205-5p | Directly targets SMAD4, CYR61, CTFG, PTEN. Promotes proliferation, anti-apoptotic, migration, chemoresistance, and tumorigenesis in vivo. Regulated Erb/HER, PTEN/AKT axis. Biomarker for cervical cancer patients. |
Ovarian cancers (OC) with poor diagnostic, cervical cancer cells, breast, lung, and NSCLC cancers. |  |
[93,94,95,191,192,193,194] |
|
| miR-221-3p | Downregulates PTEN and activates PI3K/Akt signaling. Induce cell survival, proliferation, carmustine, and cisplatin resistance. | Osteosarcoma cells, Epithelial ovarian cancer (EOC) cells | [195],[196] | ||
| miR-223 | Downregulates PTEN and activates PI3K/Akt signaling. | Ovarian cancer cells |  |
[124] | |
| miR-301a/b | M2 macrophage polarization through PTEN targeting. Promote migration, invasion, EMT, and facilitate the lung metastasis of pericytes. | Pancreatic cancer cells |  |
+ | [126] |
| miR-382-5p | Target PTEN and consequent activation of AKT/mTOR signaling. Increases proliferation, migration, and apoptosis avoidance. Increases vascular EC proliferation, migration, and tube formation. | Hemangioma-derived endothelial cell, hypoxia in human gastric cancer cells | [92,197] |
||
| miR-425 | MALAT1 interacts with miR-425 preventing PTEN silencing. Induce apoptosis | Plasma and plasma exosomes |  |
[141] |
|
| miR-494-5p/3p | Target PTEN and subsequent activation of AKT/eNOS pathway. Enhances proliferation, migration, invasion, and promotes angiogenesis. | Hypoxic lung cancer cells, glioblastoma cells, ECs. | [198,199] | ||
| miR-638 | Dual PTEN and p53-targeting. Cell migration, invasion, proliferation, and anchorage-independent growth. | Exogenous expression model in prostate cancer cells | [163] | ||
| miR-939 | ? | ([132] |
miR-17/92 cluster: located in chromosome 13, encodes miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92a-1, lso known as oncomiR-1.
miR-106a/363 cluster: located on chromosome X, encodes miR-106a, miR-18b, miR-19b-2, miR-20b, miR-92a-2, and miR-363.
miR-106b/25 cluster: located in chromosome 7, encodes miR-106b, miR-93, and miR-25.
Other functions of PTEN in vascular cells
Vascular homeostasis implies not only the ECs but also pericytes and vSMC phenotype and function. Other important downstream targets of the canonical PTEN pathway, are also linked with pathological vascular adaptations. PTEN depletion in mice induces a sustained PI3-kinase-Akt-mTOR signaling, leading to a decrease in vSMCs markers expression including αSMA and calponin. This PTEN loss concomitant with PI3-kinase-Akt enhancing is also linked to NFκβ activation and production of chemoattractant and profibrotic factors like MCP-1, IL-6, and KC/CXCL1, promoting injury-induced vascular adaptation. Interestingly, the PTEN loss was also associated with proliferation and neointima formation [59], promoting a general upregulation of proinflammatory and profibrotic genes [60]. The chemokine stromal cell-derived factor-1α (SDF-1α) is a PTEN downstream mediator. PTEN loss in vSMCs induces SDF-1α expression, and this helps to develop an inflammatory phenotype characterized by the recruitment of bone marrow-derived progenitor cells. The SDF-1α expression also induces vSMC hyperplasia by an autocrine growth loop through the interaction between SDF-1α and its receptor CXCR4. Interestingly, PTEN loss in vSMCs is directly associated with an increase in both HIF-1α expression and nuclear localization in an Akt-dependent manner irrespective of hypoxia [61,62]. Increased expression of PTEN confers protection against damage induced by angiotensin II, decreasing profibrotic and proinflammatory markers [63]. Derived from this, it has recently been identified that the use of 5-azacytidine, an inhibitor of DNMT1 (DNA methyltransferase-1), restores the expression of PTEN, promoting the maintenance of SMC differentiation and reducing pathological vascular remodeling [64]. All the above highlights PTEN signaling to play a central role in the vascular adaptation processes during development and tumor progression, which involve not only the ECs but all the cells that shape the architecture of the vascular wall.
Non-coding RNAs
The miRNAs and lncRNAs are in the large group of non-coding RNAs [65]. The miRNAs are small RNAs between 18 and 25 nucleotides in length and represent the most widely studied group of small ncRNAs [66]. miRNAs are predicted to target over 60% of all 3′ UTR mRNAs of human protein-coding genes [66]. They mediate their repressive effect through mRNA degradation or translational repression. Also, miRNAs may function as ligands directly binding to receptors, triggering downstream signaling pathways, regulating a plethora of physiological cell processes like differentiation, proliferation, metabolism, angiogenesis, apoptosis, and immune response. For in-depth details on miRNA biogenesis and mechanisms, the reader is directed to Ref. [67]. Besides, lncRNAs are usually larger than 200 nucleotides, which would originate from <1% of the human genome [65], and can also act as post-transcriptional regulators, suppressing the effect of the miRNAs. For in-depth details in lncRNA biogenesis, the reader is directed to Ref. [68].
Like virtually all mRNAs, PTEN mRNA is a target of this miRNAs/lncRNAs expression regulation. Since tumor development demands signaling, metabolic and microenvironmental shifts [69], a dysregulation of homeostatic ncRNA expression is involved in the pathophysiology of cancer [70,71]. The differential ncRNAs expression profiles in healthy and tumor tissues allow ncRNAs to be used as diagnostic and prognostic biomarkers, and for disease therapy design [71,72].
Angiogenesis and EC phenotype regulation by miRNA via PTEN
One of the most widely investigated onco-miRNAs (miRNAs associated with cancer) is miR-21, a multi-pathway regulation miRNA that contributes strongly to cancer pathology [73]. Different oncogenes like Ras trigger the expression of miR-21, stimulating proliferation and neoplastic transformation through the silencing of different tumor suppressor genes [74]. Moreover, the downregulation of tumor suppressor miRNAs may also stimulate tumorigenic processes due to the lack of inhibition of several oncogenes. The miRNA Let-7 is frequently downregulated during the onset and progression of cancer [75]. Let-7 inhibits the expression of Ras and c-Myc, thus, downregulation of miR-Let7 causes overexpression of Ras and c-Myc oncogenes [76,77]. This overexpression of Ras induces overexpression of miR-21, and c-Myc also stimulates a pro-tumor phenotype characterized by increased proliferation, migration, invasion, apoptosis inhibition, and immune escape [78]. These pathological positive feedback loops orchestrate a stimulatory symphony for tumor development such as vascular adaptation.
PTEN targeting by miR-21 in human hepatic, lung, and cervical cancer cells promotes epithelial-to-mesenchymal transition (EMT), angiogenesis and a pro-tumor phenotype [79], [80], [81], [82]. PTEN/PI3K/AKT, TGFβ1, and ERK/Bcl2 signaling are the main signaling pathways through which miR-21 operates in different tumor and vascular cells [83], [84], [85]. In addition, miR-21 also supports angiogenesis by targeting the angiogenesis inhibitor tissue inhibitor of metalloproteinase-3 (TIMP3) and stimulation of MMP2/9 expression and secretion in ECs. This pro-angiogenic role of miR-21 is a determining factor for the repair of the spinal cord microvasculature after injury [86]. Even, the deletion of miR-21 impairs vascularization and promotes apoptosis after ischemia, since it cannot suppress the activation of the PTEN/PI3K/AKT axis [87].
Besides, c-Myc overexpression induces the onco-miRNA miR-17/92 cluster [78]. Most of the members of the phylogenetically conserved cluster miR-17/92 and its two paralogues miR-106a/363 and miR-106b/25, target PTEN. Overexpression of miR-17/92 cluster in cervical and colorectal cancers increases PI3K/AKT/mTOR signaling targeting PTEN expression consequently, inducing pro-tumor phenotype [88]. Expression of the miR-17/92 cluster augments the angiogenic switch mediated by growth factors in a positive feedback loop [89]. VEGF-induced angiogenesis increases the expression of the miR-17/92 cluster, promoting an angiogenic phenotype through PTEN downregulation [89], but also directly repressing the anti-angiogenic factors thrombospondin-1 (TSP-1) and connective tissue growth factor (CTGF), stimulating angiogenesis [78]. Also, miR-23b and miR-26a help to impair PTEN/PI3/AKT signaling, increasing prostate and lung cancer cell proliferation, tumor progression, and metastatic potential [90,91].
Under hypoxia, stabilization of HIF-1α upregulates miR-382 expression. miR-382 targets PTEN leading to PI3K/AKT/mTOR signaling activation and VEGF secretion, promoting angiogenesis in ECs and chorioallantoic membrane [92]. PTEN is also targeted by miR-205, which increases PI3K/AKT/mTOR signaling pathway promoting migration, metastasis, and chemoresistance in lung and cervical tumor cells [93,94]. miR-205 directly targets PTEN, promoting endothelial progenitor cell (EPC) angiogenesis through an increase in MMP‐2 expression via AKT/autophagy pathway, helping to deep vein thrombosis recanalization process [95].
Otherwise, nuclear functions of PTEN are necessary to maintain the identity of other cells in the vascular unit like vSMCs [96]. Nuclear localization and interaction of PTEN with the transcription factor Serum Response Factor (SRF) is essential for SRF binding to vSMC promoters to maintain the vSMC differentiation program. SRF negatively regulates the expression of the transcription factor Fos-related antigen 1 (FRA-1) through an increase in the expression of miR-143, which targets FRA-1. FRA-1 in turn mediates the negative regulation of miR-21 promoter activity and this repression of miR-21 promoter activity avoids the translational repression of PTEN [97]. Silencing of SRF also inhibits PTEN expression through a miRNA-dependent mechanism. Loss of the SRF/PTEN axis promotes the vSMCs reprogramming, decreasing the expression of contractile genes and mediating a proliferative and inflammatory vSMC phenotype [96,97]. All the above makes PTEN an essential regulator to maintain the vSMC contractile gene expression. The loss of PTEN in intimal vSMCs is associated with the stabilization of atherosclerotic lesions and restenosis [60]. Thus, hinting at vSMC PTEN participation in the stabilization of a neo-vasculature in ischemic tissue injury and tumor progression. In this regard, the PTEN targeting by several miRNAs impacts vascular cells and EC phenotype during both development and malignant phenotype promotion. Given all the above, what is the PTEN right dose for proper vascularization? Not too much, nor too little. This will depend on the context: development, therapeutic or pathological.
Exosomal biogenesis and biological role in cell-to-cell communication
First described by Harding and co-workers [98] and Pan and Johnstone [99], exosomes are EVs produced through the secretory pathway and released by exocytosis by many cells. Exosomes comprise membranous nanoparticles that range between 30 and 150 nm in diameter. These can carry a repertoire of bioactive molecules such as proteins, lipids, carbohydrates, and nucleic acids i.e., coding- and non-coding RNAs [100].
Inside the cells, early endosomes fuse with endocytic vesicles to incorporate their content and be sorted for recycling, degradation, or exocytosis. During maturation, the transition from early to late endosomes, these vesicles undergo membrane invagination and fission events, generating multivesicular bodies (MVBs) [100]. These MVBs comprise numerous membrane-limited intraluminal vesicles (ILVs). Late endosomes are targeted to either fuse with lysosomes for cargo degradation or fuse with the plasma membrane to release the ILVs to the extracellular environment, after release ILVs are called exosomes [101]. The components of the endosomal sorting complex required for transport (ESCRT) and several Rab proteins (Rab3, −4, −5, −11, and Rab27) are required for MVB and ILV biogenesis. ESCRTs are assembled into four complexes (resp. ESCRT-0, -I, -II, and -III) with associated proteins (VPS4, VTA1, ALIX), which recognize and sequester proteins to the endosomal membrane [102], participate in membrane deformation into buds with sequestered cargo (I, II), and drive vesicle scission, which requires TSG101 and ALIX [103]. CD63 and ALIX have a pivotal role in vesicle sorting [104]. Finally, the dissociation and recycling of the ESCRT machinery requires the AAA-ATPase Vps4 [105]. The biogenesis processes of the extracellular vesicles are reviewed in detail elsewhere [106].
The exosome composition is also influenced by specific biogenesis pathways stimulation, which differs between normal cells and their tumorigenic derivatives [102]. The cells can have ESCRT-dependent and -independent exocytosis. Rab27a is linked to both ESCRT-dependent exocytosis and highly tumorigenic and metastatic exosome production [107]. The miRNAs and proteins inside these exosomes help to coordinate the metastatic cascade [108,109]. Therefore, exosomes facilitate the crosstalk between cells, mediating the reprogramming, transformation, and/or recruitment of several cells such as adipose-derived stem cells to tumor sites [110,111], changing the tumor microenvironment to promote angiogenesis [112], and metastasis [113].
The tumor microenvironment also influences the molecular exosome profile. Stressors like hypoxia, pro-inflammatory and metabolic challenges, activate transcription factors that change the cell expression profile and in consequence the exosomal content [109]. Exosomes from glioblastoma multiforme (GBM) patients and hypoxic GBM cells accumulate high concentrations of pro-angiogenic mediators like MMP9, pentraxin 3, IL8, PDGF-AB/AA, CD26 (aka dipeptidyl-peptidase-4), and plasminogen activator inhibitor 1 (PAI-1), stimulating proliferation, migration, and angiogenesis in ECs, mouse aorta rings and human GBM xenografts [114]. Interestingly, tumor cells exert an effect on surrounding ECs, while ECs also influence tumor cells through EV communication. Hence, constant crosstalk among cells persists. Cadherins mediate isotypic cell homophilic interactions, ECs express VE-cadherin, and epithelial breast cancer cells can be positive or negative for E-cadherin depending on the malignity. However, VE-cadherin expression is induced in breast cancer cells when they are co-cultivated with ECs [115]. As a “trojan horse”, the tumor cells can move through the endothelium having an EV-inducible homophilic interaction, maintaining the integrity of the endothelial monolayer, helping to both process tumor invasion and vascular mimicry [115].
Like other molecules, PTEN can also be regulated through exosomal communication [113]. Mouse embryonic fibroblasts secrete PTEN in exosomes into the extracellular environment conserving their phosphatase activity in the recipient cells having effects on the AKT pathway and decreasing cell proliferation [116]. All of the above reinforces the exosomes as potent mediators of intercellular communication, actively participatory in the preparation of the extracellular microenvironment supporting pivotal processes during cell specification, development, repair, and disease.
Exosomal PTEN-targeting miRNAs in endothelial phenotype and tumor angiogenesis
The exchange of vesicle-loaded ncRNAs redirects the cell phenotype but also the neighboring cellular environment. Several cell types ranging from MSCs (therapeutic context) to tumor cells (pathological context) influence angiogenesis-PTEN-related mechanisms in paracrine ways. That paracrine intercellular communication is modulated through exosomes-derived ncRNAs, including miRNAs and lncRNAs, helping to redirect the target cell functions alike a cuckoo laying its eggs in the nest of a host bird. Table 1 lists the main miRNA whose target is PTEN in several tissues. The regulation by miRNAs is highly versatile, not only do specific mRNAs harbor binding sites for multiple miRNAs but a single miRNA generally binds to multiple target mRNAs transcribed from genes that often reside in the same signaling pathway. Thus, one miRNA might hit an entire pathway.
miR-21, miR-205-5p, miR-223 and miR-301
miRNAs play key roles in vascular and tumor development, targeting molecules like VEGF/VEGFR family members and PTEN signaling. In prostate tumor cells, miR-21-5p targets PTEN which activates HIF-1α. This upregulates VEGF expression and secretion which promotes tumor angiogenesis [83]. The exosomal miR-21 leads to STAT3 activation, which increases VEGF levels in recipient cells, leading to angiogenesis and malignant transformation of human bronchial epithelial cells [117]. Exosomal miR-21 from hepatocellular carcinoma cells targets PTEN mRNA in proximal hepatic stellate cells (HSC), activating and transforming these HSCs into cancer-associated fibroblasts (CAFs). CAFs secrete pro-angiogenic factors such as VEGF promoting tumor vascularization and remodeling the ECM to generate a pro-tumorigenic microenvironment [118]. miR-21 secreted on exosomes from colorectal cancer cells also promotes angiogenesis and vascular permeability [119]. miR-205-5p influences development and cancer by regulating cell proliferation, migration, tissue polarity, and morphogenesis [120]. Like a double-edged sword, pathological loss of miR-205 creates discontinuities in the basal membrane of prostate glands by an expression reduction in both laminin-332 and the cellular matrix receptor integrin α6β4, which favors malignant transformation [121]. Also, a pathological increase of miR-205-5p in different cancer cells and tumor-derived exosomes inhibit the PTEN expression causing the upregulation of PI3K/AKT signaling, promoting proliferation, and angiogenesis [95].
In a multi-pathway communication mechanism, the local hypoxia, the metabolic switch induction, and in consequence the low pH, change the cellular miRNA expression profile in tumor cells and ECs alike [122]. This altered expression profile confers to the cells the ability to modify the microenvironment through different mechanisms including exosomal communication. Altered exosomal lipid/protein/miRNAs expression profiles influence both exosome release and uptake, increasing the exosome fusion with cell membranes [123]. Hypoxic epithelial ovarian cancer cells recruit macrophages and stimulate these to produce exosomes enriched in miR-223, which target the PTEN-PI3K/AKT pathway, helping the cancer cells to proliferate and evade chemotherapy [124]. M2-type macrophages are anti-inflammatory and immunosuppressive and secrete IL-10, TGFβ, and VEGF, leading to proliferation, tissue repair, and angiogenesis [125]. Exosomes from hypoxic pancreatic cancer cells harbor miR-301a-3p that induces M2 polarization through PTEN expression inhibition, and consequently activation of PI3K/AKT/mTOR signaling. This promotes migration, invasion, and EMT, facilitating lung metastases [126].
miR-103, miR-105, and miR-939
Several miRNAs described to target PTEN also target adherens and tight junction proteins in ECs, like VE-cadherin and zonula occludens 1 (ZO-1), respectively. The loss of these junction proteins constitutes a determinant step in vasculogenesis during development, but also in physiological and pathological sprouting angiogenesis. miR-103 targets PTEN, with a context-dependent outcome. miR-103 targets PTEN [127] and TIMP-3 [128] in endometrial cancer cells, stimulating growth, invasion, and apoptosis decrease. Upregulation of miR‐103 was observed in oxidized-LDL-treated human ECs, a model for atherosclerosis. Direct PTEN targeting by miR-103 allows MAPK signaling activation to promote the expression of inflammatory cytokines (MMP‐9, MMP‐1, IL‐6, IL‐1β) and endoplasmic reticulum stress markers (GRP78, CHOP, XBP‐1, and GRP94); and miR-103 inhibition attenuates these markers through PTEN expression recovery [129]. Additionally, EVs derived from hypoxic lung cancer cells decreased macrophage PTEN levels caused by EV-miR-103a transfer, increasing the activation of AKT and STAT3 as well as the expression of several immunosuppressive and pro-angiogenic genes related to M2 phenotype [130]. Interestingly, xenografts of miR-103 overexpressing-hepatocellular carcinoma cells (HCCs) in mice, help tumor metastasis and alter the EC phenotype via exosomal communication. The exosomal miR-103-HCC xenograft derived, destroys the ECs adherent junctions integrity by direct targeting of VE-cadherin, p120‐catenin, and ZO-1. This facilitates the transendothelial invasion of tumor cells, increasing the vascular permeability, the number of tumor cells in blood circulation, and the hepatic and pulmonary metastases, compared to control mice [131]. miR-939 is upregulated in human breast cancer cells, particularly in the most aggressive subtypes. Indeed, lymph node-positive tumors overexpressing miR-939 have an increased risk of relapse as compared with those with lower levels of miR-939 and lacking lymph node involvement. miR-939 is expressed and released in exosomes of triple-negative breast cancer cell lines and its uptake in HUVECs downregulates the junctional protein VE-cadherin [132]. Exosomal transference of miR-105 downregulates the tight junction protein ZO-1 in ECs [133]. Together, miR-939 and miR-105 increase vascular permeability and destroy EC integrity, leading to enhanced metastasis.
Exosomal YAP/TAZ-targeting miRNAs in endothelial phenotype and tumor angiogenesis
As discussed above, a strong feedback loop between PTEN and Hippo signaling pathway drives vascular adaptations in ECs during development and disease (Fig. 3). This signaling network may seem to act as an on/off switch, counteracting the balance of signaling in tip and stalk. This allows for angiogenic stimulation and warrants the proper maturation of the newly sprouted vessels to render these to function as microvessels. Any perturbance of this communication i.e., disturbance of the balance in tip and stalk cells, has effects on regeneration and recovery of tissues after damage. Moreover, it may cause the formation of non-functional vessels.
Interestingly multiple endogenous miRNAs redundantly converge on PTEN and Hippo signaling pathways to activate robust and sustained proliferation and EC patterning (Table 1). For example, the miR-17/92 cluster, which targets PTEN, also induces cardiomyocyte proliferation and regeneration through modulation of Hippo signaling through LATS2 targeting, thus increasing YAP nuclear translocation and transcriptional activity (Table 1). Also, miR-21 induce proliferation and angiogenesis through Hippo interference in cancer cells [134]. In ECs and ischemic hearts, overexpression of miR-93 was directly associated with a decrease in LATS2 expression and an increase in nuclear YAP, in other words, inactivation of the Hippo pathway. This protective response prevented ischemia/reperfusion damage to the endothelium by promoting angiogenesis (cardiac and in vitro), increasing microvascular density in the infarcted myocardium, and decreasing endothelial activation (ICAM-1 and VCAM-1 staining) [135].
Exosomal lncRNA in endothelial phenotype and tumor angiogenesis via PTEN
Like miRNAs, lncRNAs are post-transcriptional regulators. One particularly interesting way of lncRNA functioning is the endogenous sponge function, in which the lncRNA binds a target molecule e.g. a miRNA, and thereby prevents this molecule from binding to its original target [136]. In this way, the lncRNA abolishes the potentially inhibitory effect of its targeted miRNA on specific mRNAs. Such lncRNAs that regulate the expression of other transcripts by competing for miRNAs are referred to as competing endogenous RNAs (ceRNAs) [137]. In this last chapter, we focus on lncRNAs that regulate PTEN signaling.
Growth arrest-specific 5 (GAS5) is a lncRNA that functions as a ceRNA for the PTEN target miR-29-3p. Lung cancer-derived exosome profiles showed reduced expression of GAS5 compared to the exosomes of healthy lung cells. Low GAS5 levels promoted tumor angiogenesis, while high levels of GAS5 resulted in inhibited proliferation and tube formation, and increased apoptosis [138]. Interestingly, the tumor increases angiogenesis by suppressing the amount of GAS5 in its exosomes. This suggests that there is an important role for healthy lung cells in maintaining homeostasis in the lung through the exosomal delivery of GAS5 to suppress pro-carcinogenic processes. These results show that lung cancer cells regulate tumor angiogenesis via PTEN by controlling GAS5 levels in their exosomes (Table 2).
Table 2.
lncRNAs targeting PTEN.
| LncRNA | Endogenous Sponge | Main function | Cell Type | Role exosomes | Interaction PTEN | Effect on angiogenesis | Refs. |
|---|---|---|---|---|---|---|---|
| PTENP1 | Yes | Binding miR-20a and thereby promoting PTEN | Cancer cell | ? | Promoting | Suppressed malignant behaviors like metastasis | [139,141] |
| APC-1 | No | Disrupting mRNA Rab5b stability and thereby reducing exosome production/release | Cancer cell | APC-1 affects the production/release? | No direct effect, possibly indirect by affecting exosome production | Suppressing | [149] |
| GAS5 | Yes | Binding miR-20a-3p and thereby promoting PTEN | Endothelial cell | Transport GAS5 from the cancer cells into the ECs | Promoting | Suppressing | [138] |
| H19 | Yes | Promotes angiogenesis in the tumor microenvironments and related with vascular repair | Endothelial cell | Transport H19 from the cancer cells into the ECs | Downregulation | Promoting | [[142], [143], [144],200] |
| POU3F3 | No? | Promotes angiogenesis after the exosomal transfer from the glioma cell into the endothelial cell. Downregulate GAS5 | Endothelial cell | Transport POU3F3 from the cancer cells into the ECs | ? | Promoting | [147,201] |
| MALAT1 | Yes | Therapeutic angiogenesis and metastasis | Coronary vessels | Transport MALAT1 which sponges miR-92a | ? | Promoting | [145,146,202] |
PTEN pseudogene 1 (PTENP1) is a lncRNA, a member of the PTENP1/miR-20a/PTEN axis. This axis is involved in the progression of breast cancer by mediating cell proliferation, metastasis, and apoptosis [139]. MiR-20a is known as a suppressor of apoptosis and as a promoter of cell proliferation due to binding and thereby suppressing PTEN. PTENP1 is called a pseudogene because it is, just like PTEN, a target for miR-20a. This ceRNA function of PTENP1 promotes PTEN signaling by decreasing miR-20a activity. Consequently, low PTENP1 expression promoted the malignant behavior of breast cells, while overexpression of PTENP1 suppressed breast cancer progression [140]. Furthermore, PTENP1 is also reported as a member of the PTENP1/miR-17/PTEN axis. PTENP1 was demonstrated to be transported from normal cells to bladder cancer cells by exosomes, where it induced a tumor-suppressing effect. In contrast, low levels of PTENP1 showed a malignant effect in these cells [141]. Again, this seems a way in which an exosomal lncRNA is involved in an effort of the body to defend itself against cancer (Table 2).
H19 is a lncRNA that was upregulated in cancer stem cell CD90+ liver cancer cell-derived exosomes, but not in normal liver cancer cell-derived exosomes. These exosomes induced the promotion of angiogenesis in ECs, by increasing VEGF and VEGFR. Besides, H19 overexpression in ECs displayed the same effect [142]. Although H19-mediated interaction with PTEN-targeting miRNAs, such as miR-19a-3p [143] and miR-675 [144], has been documented in previous years, the precise pathways in which cancer cell-derived exosomal H19 induces angiogenesis remains to be elucidated. Another lncRNA, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), acts as an angiogenesis promoter after exosomal delivery to ECs, suppressing miR-92a. Being also a predictor of poor prognosis for epithelial ovarian cancer patients [145,146]. POU3F3 lncRNA also promotes angiogenesis after their transference to ECs by cancer-derived exosomes [147,148] (Table 2). Angiogenesis stimulation via downregulation of PTEN after cancer-derived exosomal delivery of H19 and MALAT1 has not yet been reported. However, available literature suggests that these lncRNAs interact in multiple ways with this process, which renders these interesting for future research.
LncRNA-APC1, named after its activator, is a lncRNA active in the APC/PPARα/lncRNA-APC1/Rab5b axis. In colorectal cancer cells, lncRNA-APC1 binds to Rab5b mRNA, leading to reduced stability of this mRNA and decreased RAB5B protein levels. This led to decreased exosome production, inhibition of the overreaction of the MAPK pathway, and suppression of angiogenesis. Low levels of lncRNA-APC1 are associated with metastasis and poor prognosis in colorectal cancer patients [149] (Table 2). Besides the lncRNAs transported in the exosomes, it is important to evaluate other effects of lncRNAs on exosome delivery. Effects of lncRNAs on exosome production, release, and uptake can also contribute to the effect that a parent cell establishes in a recipient cell due to the delivery of its exosomes.
LncRNAs are a relatively novel field in biomedicine that warrants more thorough exploration. Revealing how lncRNAs manipulate signaling pathways can provide a new important element in the understanding of cellular functioning. Especially the interaction with lncRNAs and miRNAs seems to add an extra layer to the interplay of molecules involved in post-transcriptional signaling. In addition to the oncogenic and tumor suppressor miRNAs, mapping of pro-angiogenic and anti-angiogenic lncRNAs can serve as important new information to get a more complete view of the functioning of both PTEN-dependent and PTEN-independent intracellular signaling pathways.
Although not the focus of this review, ncRNAs comprise a class of degradation-resistant RNA, so-called circular RNAs (circRNAs), which have an average half-life of approximately five times longer than mRNAs [150]. Indeed, EVs also carry circRNAs [151]. Whereby, it has been suggested to use circRNA as biomarkers of progression and severity in different diseases, including colorectal cancer [152,153]. In 2017 exoRBase was created, which is a repository of exosome-derived long RNAs (exLRs) derived from RNA-seq data analyses in different human body fluids. The exLRs contain mRNA, lncRNA, and circRNA [154]. Several circRNAs participate in the regulation of PTEN and Hippo signaling, such as circPTEN1 and circ-PPP1R12A, respectively [153,155]. However, the role of cirRNAs in vascular plasticity regulation, beyond tumor angiogenesis models [156], should be the subject of future study.
Concluding remarks and perspectives: vascular adaptation lessons from cancer cells
A strong feedback loop between PTEN and Hippo signaling pathway seems to rule vascular adaptations (angiogenesis, vascular reactivity, and remodeling of the vessel wall) during development and disease. PTEN has a pivotal role in vascular homeostasis, and its influence lies not only over PI3K signaling pathway. PTEN signaling regulation is a fertile area, as more than 2000 miRNAs are predicted to target PTEN [157]. Although several miRNAs targeting PTEN have been described, there is still a huge gap between the predicted and biologically validated ncRNAs, and a large percentage of validated ncRNAs has been described only in a single context. Some of the ncRNAs that have been validated in different biological models show a context-dependent outcome, which increases the complexity when drawing conclusions with these molecules (Tables 1 and 2).
As a further point of consideration, the miRNA signatures of 2D cultured human ECs massively differ from 3D cultured ECs [158]. miRNA profiling comparing freshly isolated and cultured ECs, revealed major alterations in miRNA signatures between tissue-derived, cultured and aging ECs, differing around 30–40% in miRNA expression in cultured ECs compared to the fresh ECs [158]. Also, the stiffness of the substrates where the cells are planted has an impact on the EC miRNA expression profile and cell fate [159], as mentioned in the part 4.2 of the present review. This research reveals the existence of a mechanosensitive miRNA-based program conformed by 122 microRNA families that target 73 mRNAs encoding cytoskeletal, contractile, adhesive, and ECM, being YAP/TAZ signaling one of the main regulatory targets [159]. Although PTEN is not widely mentioned in that research, this is of special relevance since many of the miRNAs described to regulate this mechanosensitive program can also target PTEN. This indicates that the miRNA profile is highly adaptable and influenceable by the culture conditions, and cultured cells may not always represent the best model system for studying miRNA function, proper in vivo validation will remain as the gold standard.
Some of the studies cited here, described endogenous downregulation of PTEN expression by miRNAs. However, we do not know if they also influence exogenous expression. This allows us to determine if the predicted miRNA response elements (MREs) in PTEN are bona fide MREs and if the observed miRNA effect on endogenous PTEN is not indirect. Similarly, abundant literature describes the biological phenomena in which a miRNA interferes, but there is a gap in the description of the molecular mechanisms through which they execute its action.
The role of exosomes in pathophysiology as well as in normal physiology is investigated comprehensively during the last decades, with exponential growth in the number of publications associated with exosomes and ncRNAs, especially miRNA. Is well-recognized that ncRNAs packaged in exosomes alter physiological and pathological vascularization, reprogram the recipient cells, and thus change the cellular microenvironment, being PTEN one of the targets. Conversely, the cells’ microenvironment continuously demands the adaptation of the biological cargo in exosomes. Nevertheless, exosomes not only transport miRNAs but a wide variety of molecules with biological activity as lncRNAs. The stoichiometry of exosomal-miRNA transference (relation between copies number of miRNA/exosome/cell) describes that there is no more than one copy of each miRNA per exosome, delineating functional boundaries of exosome-mediated communication, their mechanism, and physiologic relevance over gene expression in a biological context [160,161]. Therefore, trying to explain the effect of exosomes from a single molecule is a reductionistic act. In the future, it will be necessary to integrate expression profiles of the total content of the vesicle, to understand cellular communication in detail, and the entire message.
As Calabrese and Mattson say: “the hormesis is not only constrained by plasticity but also describes the key characteristics of biological plasticity” [162]. Understanding the importance of the dose-response, makes us think about the molecules not only as on/off switches but rather as a hormetic rheostat. Transformed cancer cells are characterized by many chromosomal alterations, which include copy number amplifications and loss of heterozygosity in several loci. These genetic alterations imply drastic increases or decreases in the genic product expression (mRNAs/ncRNAs) encoded in these areas [163], changing the proper gene dose expression. In angiogenic tumors, the angiogenic switch must be induced frequently. However, the vascularization of the tumor is highly disordered. Therefore, the analysis of angiogenic factors regulated by tumors is relevant in the mapping of the effector's hierarchy, as well as their implied pathways. Since it is currently easier to inhibit angiogenesis than to induce the formation of functional vessels, this information is important not only to learn how to suppress such angiogenic flux in growing tumors but also to make a bridge to define optimal therapeutic miRNA doses to improve regenerative processes.
Ethics approval, consent to participate, and consent for publication
Not applicable.
Funding
This project was supported by the University Medical Center Groningen, (UMCG), the University of Antioquia (Medellin. Colombia), and the Colombian Ministry of Science and Technology (Minciencias) Project 111577757581.
CRediT authorship contribution statement
Elizabeth Orozco-García: Conceptualization, Visualization, Data curation, Investigation, Formal analysis, Writing – original draft, Supervision. D.J. van Meurs: Investigation, Formal analysis, Writing – original draft, Visualization, Supervision. JC. Calderón: Writing – review & editing, Visualization, Supervision. Raul Narvaez-Sanchez: Conceptualization, Visualization, Supervision, Writing – original draft, Writing – review & editing. M.C. Harmsen: Conceptualization, Investigation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, interpretation of data, or in the writing of the manuscript.
Acknowledgments
We acknowledge Dr. Francisco Drusso Martinez for critically reading the manuscript.
References
- 1.Nagy J.A., Dvorak A.M., Dvorak H.F. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb. Perspect. Med. 2012;2(2) doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnem T., Reynolds A.R., Kuczynski E.A., Gatter K., Vermeulen P.B., Kerbel R.S., et al. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer. 2018;18(5):323–336. doi: 10.1038/nrc.2018.14. [DOI] [PubMed] [Google Scholar]
- 3.Pezzella F., Harris A.L., Tavassoli M., Gatter K.C. Blood vessels and cancer much more than just angiogenesis. Cell Death Discov. 2015;1:15064. doi: 10.1038/cddiscovery.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins P.T., Anderson K.E., Davidson K., Stephens L.R. Signalling through class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006;34(Pt 5):647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 5.Kaur B., Khwaja F.W., Severson E.A., Matheny S.L., Brat D.J., Van Meir E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis1. Neuro-oncology. 2005;7(2):134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S.I., et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 7.Li D.M., Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57(11):2124–2129. [PubMed] [Google Scholar]
- 8.Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Wang L., Chen C., Chu X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol. Cancer. 2018;17(1):22. doi: 10.1186/s12943-018-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S., Alharbi M., Kobayashi M., Lai A., Guanzon D., Zuniga F., et al. Proteomic analysis of exosomes reveals an association between cell invasiveness and exosomal bioactivity on endothelial and mesenchymal cell migration in vitro. Clin. Sci. (Lond.) 2018;132(18):2029–2044. doi: 10.1042/CS20180425. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z., Wei K., Yang F., Guo Z., Pan C., He Y., et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. 2021;12(9) doi: 10.1038/s41419-021-04037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai Y., Chen K., Hsieh J., Shen T. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109(8):2364–2374. doi: 10.1111/cas.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardito F., Giuliani M., Perrone D., Troiano G., Muzio L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Int. J. Mol. Med. 2017;40(2):271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smoly I., Shemesh N., Ziv-Ukelson M., Ben-Zvi A., Yeger-Lotem E. An asymmetrically balanced organization of kinases versus phosphatases across eukaryotes determines their distinct impacts. PLOS Comput. Biol. 2017;13(1) doi: 10.1371/journal.pcbi.1005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steck P.A., Pershouse M.A., Jasser S.A., Yung W.K., Lin H., Ligon A.H., et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.O., Yang H., Georgescu M.M., Di Cristofano A., Maehama T., Shi Y., et al. Crystal structure of the PTEN tumor suppressor. Cell. 1999;99(3):323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 17.Tamguney T., Stokoe D. New insights into PTEN. J. Cell Sci. 2007;120(Pt 23):4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 18.Keniry M., Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27(41):5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 19.Alimonti A., Carracedo A., Clohessy J.G., Trotman L.C., Nardella C., Egia A., et al. Subtle variations in PTEN dose determine cancer susceptibility. Nat. Genet. 2010;42(5):454–458. doi: 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carracedo A., Alimonti A., Pandolfi P.P. PTEN level in tumor suppression: how much is too little? Cancer Res. 2011;71(3):629–633. doi: 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C.Y., Chen J., He L., Stiles B.L. PTEN: tumor suppressor and metabolic regulator. Front. Endocrinol. (Lausanne) 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen W.H., Balajee A.S., Wang J., Wu H., Eng C., Pandolfi P.P., et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Bassi C., Ho J., Srikumar T., Dowling R.J., Gorrini C., Miller S.J., et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341(6144):395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J.H., Zhang P., Chen W.D., Li D.D., Wu X.Q., Deng R., et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy. 2015;11(2):239–252. doi: 10.1080/15548627.2015.1009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang H., He S., Yang J., Jia X., Wang P., Chen X., et al. PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab. 2014;19(5):836–848. doi: 10.1016/j.cmet.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C., Wang B., Liu E., Zhang Z. Loss of PTEN expression is associated with PI3K pathway-dependent metabolic reprogramming in hepatocellular carcinoma. Cell Commun. Signal. 2020;18(1):131. doi: 10.1186/s12964-020-00622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tibarewal P., Zilidis G., Spinelli L., Schurch N., Maccario H., Gray A., et al. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci. Signal. 2012;5(213):ra18. doi: 10.1126/scisignal.2002138. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 29.Krenning G., van Luyn M.J., Harmsen M.C. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol. Med. 2009;15(4):180–189. doi: 10.1016/j.molmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Ferrero E., Labalde M., Fernandez N., Monge L., Salcedo A., Narvaez-Sanchez R., et al. Response to endothelin-1 in arteries from human colorectal tumours: role of endothelin receptors. Exp. Biol. Med. (Maywood) 2008;233(12):1602–1607. doi: 10.3181/0802-RM-69. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 32.Davies E.M., Gurung R., Le K.Q., Mitchell C.A. Effective angiogenesis requires regulation of phosphoinositide signaling. Adv. Biol. Regul. 2019;71:69–78. doi: 10.1016/j.jbior.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak H.F., Nagy J.A., Dvorak J.T., Dvorak A.M. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am. J. Pathol. 1988;133(1):95–109. [PMC free article] [PubMed] [Google Scholar]
- 34.Pries A.R., Cornelissen A.J.M., Sloot A.A., Hinkeldey M., Dreher M.R., Höpfner M., et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLOS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bock K., Cauwenberghs S., Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr. Opin. Genet. Dev. 2011;21(1):73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Krenning G., Barauna V.G., Krieger J.E., Harmsen M.C., Moonen J.R. Endothelial plasticity: shifting phenotypes through force feedback. Stem Cells Int. 2016;2016 doi: 10.1155/2016/9762959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan R., Emery T., Zhang Y., Xia Y., Sun J., Wan J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 2016;6(1):27073. doi: 10.1038/srep27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisberg E.M., Potenta S., Xie L., Zeisberg M., Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 39.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4(4):241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobov I.B., Renard R.A., Papadopoulos N., Gale N.W., Thurston G., Yancopoulos G.D., et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellstrom M., et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17(15):1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darden J., Payne L.B., Zhao H., Chappell J.C. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis. 2019;22(1):167–183. doi: 10.1007/s10456-018-9648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada K., Sasaki T., Koni P.A., Natsui M., Kishimoto H., Sasaki J., et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19(17):2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo H.M., Lin C.Y., Lam H.C., Lin P.R., Chan H.H., Tseng J.C., et al. PTEN overexpression attenuates angiogenic processes of endothelial cells by blockade of endothelin-1/endothelin B receptor signaling. Atherosclerosis. 2012;221(2):341–349. doi: 10.1016/j.atherosclerosis.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 45.Hellstrom M., Phng L.K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P., et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 46.Herbert S.P., Huisken J., Kim T.N., Feldman M.E., Houseman B.T., Wang R.A., et al. Arterial/venous segregation by selective cell sprouting: an alternative mode of blood vessel formation*. Science. 2009;326(5950):294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco R., Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013;3(1) doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serra H., Chivite I., Angulo-Urarte A., Soler A., Sutherland J.D., Arruabarrena-Aristorena A., et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat. Commun. 2015;6:7935. doi: 10.1038/ncomms8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontes-Quero S., Fernández-Chacón M., Luo W., Lunella F.F., Casquero-Garcia V., Garcia-Gonzalez I., et al. High mitogenic stimulation arrests angiogenesis. Nat. Commun. 2019;10(1):2016. doi: 10.1038/s41467-019-09875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choorapoikayil S., Weijts B., Kers R., de Bruin A., den Hertog J. Loss of PTEN promotes angiogenesis and enhanced vegfaa expression in zebrafish. Dis. Model Mech. 2013;6(5):1159–1166. doi: 10.1242/dmm.012377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Totaro A., Panciera T., Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018;20(8):888–899. doi: 10.1038/s41556-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai X., Wang K.C., Meng Z. Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front Cell Dev. Biol. 2021;9:01–12. doi: 10.3389/fcell.2021.673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu W., Yang Z., Xie C., Zhu Y., Shu X., Zhang Z., et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J. Exp. Clin. Cancer Res. 2018;37(1):198–214. doi: 10.1186/s13046-018-0795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Freire Valls A., Schermann G., Shen Y., Moya I.M., Castro L., et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell. 2017;42(5):462–478. doi: 10.1016/j.devcel.2017.08.002. .e7. [DOI] [PubMed] [Google Scholar]
- 56.Azad T., Janse Van Rensburg H.J., Lightbody E.D., Neveu B., Champagne A., Ghaffari A., et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park J.A., Kwon Y.G. Hippo-YAP/TAZ signaling in angiogenesis. BMB Rep. 2018;51(3):157–162. doi: 10.5483/BMBRep.2018.51.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakabe M., Fan J., Odaka Y., Liu N., Hassan A., Duan X., et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl. Acad. Sci. USA. 2017;114(41):10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furgeson S.B., Simpson P.A., Park I., Vanputten V., Horita H., Kontos C.D., et al. Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc. Res. 2010;86(2):274–282. doi: 10.1093/cvr/cvp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moulton K.S., Li M., Strand K., Burgett S., McClatchey P., Tucker R., et al. PTEN deficiency promotes pathological vascular remodeling of human coronary arteries. JCI Insight. 2018;3(4):e97228. doi: 10.1172/jci.insight.97228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemenoff R.A., Simpson P.A., Furgeson S.B., Kaplan-Albuquerque N., Crossno J., Garl P.J., et al. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ. Res. 2008;102(9):1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- 62.Nemenoff R.A., Horita H., Ostriker A.C., Furgeson S.B., Simpson P.A., VanPutten V., et al. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler. Thromb. Vasc. Biol. 2011;31(6):1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu S., Strand K.A., Mutryn M.F., Tucker R.M., Jolly A.J., Furgeson S.B., et al. PTEN (Phosphatase and Tensin Homolog) protects against Ang II (Angiotensin II)-induced pathological vascular fibrosis and remodeling-brief report. Arterioscler. Thromb. Vasc. Biol. 2020;40(2):394–403. doi: 10.1161/ATVBAHA.119.313757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strand K.A., Lu S., Mutryn M.F., Li L., Zhou Q., Enyart B.T., et al. High throughput screen identifies the DNMT1 (DNA Methyltransferase-1) inhibitor, 5-azacytidine, as a potent inducer of PTEN (Phosphatase and Tensin Homolog): central role for PTEN in 5-azacytidine protection Against pathological vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2020;40(8):1854–1869. doi: 10.1161/ATVBAHA.120.314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palazzo A.F., Lee E.S. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12(12):861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 67.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. (Lausanne). 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22(2):96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Tan S., Xia L., Yi P., Han Y., Tang L., Pan Q., et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 2020;39(1):67–82. doi: 10.1186/s13046-020-01570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z., Xu F., Wang H., Teschendorff A.E., Xie F., He Y. Pan-cancer characterization of long non-coding RNA and DNA methylation mediated transcriptional dysregulation. EBioMedicine. 2021;68 doi: 10.1016/j.ebiom.2021.103399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Condrat C.E., Thompson D.C., Barbu M.G., Bugnar O.L., Boboc A., Cretoiu D., et al. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9(2):276. doi: 10.3390/cells9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krichevsky A.M., Gabriely G. miR-21: a small multi-faceted RNA. J. Cell. Mol. Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frezzetti D., Menna M.D., Zoppoli P., Guerra C., Ferraro A., Bello A.M., et al. Upregulation of miR-21 by Ras in vivo and its role in tumor growth. Oncogene. 2011;30(3):275–286. doi: 10.1038/onc.2010.416. [DOI] [PubMed] [Google Scholar]
- 75.Xie Y., Zhang H., Guo X.J., Feng Y.C., He R.Z., Li X., et al. Let-7c inhibits cholangiocarcinoma growth but promotes tumor cell invasion and growth at extrahepatic sites. Cell Death Dis. 2018;9(2):01–14. doi: 10.1038/s41419-018-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X., Wu L., Yao J., Jiang H., Wang Q., Yang Z., et al. MicroRNA let-7c inhibits cell proliferation and induces cell cycle arrest by targeting CDC25A in human hepatocellular carcinoma. PLOS One. 2015;10(4) doi: 10.1371/journal.pone.0124266. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Sampson V.B., Rong N.H., Han J., Yang Q., Aris V., Soteropoulos P., et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67(20):9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 78.Dews M., Homayouni A., Yu D., Murphy D., Sevignani C., Wentzel E., et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006;38(9):1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng F., Henson R., Wehbe–Janek H., Ghoshal K., Jacob S.T., Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Mattos-Arruda L., Bottai G., Nuciforo P.G., Di Tommaso L., Giovannetti E., Peg V., et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 2015;6(35):37269–37280. doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao W., Shen H., Liu L., Xu J., Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J. Cancer Res. Clin. Oncol. 2011;137(4):557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 82.Peralta-Zaragoza O., Deas J., Meneses-Acosta A., De la O.G.F., Fernandez-Tilapa G., Gomez-Ceron C., et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells. BMC Cancer. 2016;16:215. doi: 10.1186/s12885-016-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu L.Z., Li C., Chen Q., Jing Y., Carpenter R., Jiang Y., et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLOS One. 2011;6(4):e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J.Y., Gao Y.B., Zhang N., Zou D.W., Wang P., Zhu Z.Y., et al. miR-21 overexpression enhances TGF-beta1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol. Cell. Endocrinol. 2014;392(1–2):163–172. doi: 10.1016/j.mce.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Xu L.F., Wu Z.P., Chen Y., Zhu Q.S., Hamidi S., Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLOS One. 2014;9(8) doi: 10.1371/journal.pone.0103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu J., Ni S., Cao Y., Zhang T., Wu T., Yin X., et al. The angiogenic effect of microRNA-21 targeting TIMP3 through the regulation of MMP2 and MMP9. PLOS One. 2016;11(2) doi: 10.1371/journal.pone.0149537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang W.T., Lin Y.W., Huang P.S., Lin Y.C., Tseng S.Y., Chao T.H., et al. Deletion of MicroRNA-21 impairs neovascularization following limb ischemia: from bedside to bench. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.826478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu S.Q., Jiang S., Li C., Zhang B., Li Q.J. miR-17-92 cluster targets phosphatase and tensin homology and Ikaros Family Zinc Finger 4 to promote TH17-mediated inflammation. J. Biol. Chem. 2014;289(18):12446–12456. doi: 10.1074/jbc.M114.550723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chamorro-Jorganes A., Lee M.Y., Araldi E., Landskroner-Eiger S., Fernandez-Fuertes M., Sahraei M., et al. VEGF-induced expression of miR-17-92 cluster in endothelial cells is mediated by ERK/ELK1 activation and regulates angiogenesis. Circ. Res. 2016;118(1):38–47. doi: 10.1161/CIRCRESAHA.115.307408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian L., Fang Y., Xue J., Chen J. Four MicroRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLOS One. 2013;8(9) doi: 10.1371/journal.pone.0075885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu B., Wu X., Wang C., Liu Y., Zhou Q., Xu K. MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim. Biophys. Acta. 2012;1822(11):1692–1704. doi: 10.1016/j.bbadis.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Seok J.K., Lee S.H., Kim M.J., Lee Y.M. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42(12):8062–8072. doi: 10.1093/nar/gku515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai J., Fang L., Huang Y., Li R., Yuan J., Yang Y., et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73(17):5402–5415. doi: 10.1158/0008-5472.CAN-13-0297. [DOI] [PubMed] [Google Scholar]
- 94.Xie H., Zhao Y., Caramuta S., Larsson C., Lui W.O. miR-205 expression promotes cell proliferation and migration of human cervical cancer cells. PLOS One. 2012;7(10):e46990. doi: 10.1371/journal.pone.0046990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun L.L., Xiao L., Du X.L., Hong L., Li C.L., Jiao J., et al. MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression. J. Cell. Mol. Med. 2019;23(12):8493–8504. doi: 10.1111/jcmm.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horita H., Wysoczynski C.L., Walker L.A., Moulton K.S., Li M., Ostriker A., et al. Nuclear PTEN functions as an essential regulator of SRF-dependent transcription to control smooth muscle differentiation. Nat. Commun. 2016;7:10830. doi: 10.1038/ncomms10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horita H.N., Simpson P.A., Ostriker A., Furgeson S., Van Putten V., Weiser-Evans M.C., et al. Serum response factor regulates expression of PTEN through a micro-RNA network in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2011;31(12):2909–2919. doi: 10.1161/ATVBAHA.111.233585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 100.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 101.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell. Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 103.Blanc L., Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9(1–2):95–106. doi: 10.1080/21541248.2016.1264352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jackson C.E., Scruggs B.S., Schaffer J.E., Hanson P.I. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys. J. 2017;113(6):1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 107.Bobrie A., Krumeich S., Reyal F., Recchi C., Moita L.F., Seabra M.C., et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 108.Ostenfeld M.S., Jeppesen D.K., Laurberg J.R., Boysen A.T., Bramsen J.B., Primdal-Bengtson B., et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 109.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 110.Abd Elmageed Z.Y., Yang Y., Thomas R., Ranjan M., Mondal D., Moroz K., et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–997. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park J.E., Tan H.S., Datta A., Lai R.C., Zhang H., Meng W., et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteomics. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L., Zhang S., Yao J., Lowery F.J., Zhang Q., Huang W.C., et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kucharzewska P., Christianson H.C., Welch J.E., Svensson K.J., Fredlund E., Ringnér M., et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rezaei M., Martins Cavaco A.C., Stehling M., Nottebaum A., Brockhaus K., Caliandro M.F., et al. Extracellular vesicle transfer from endothelial cells drives VE-cadherin expression in breast cancer cells, thereby causing heterotypic cell contacts. Cancers (Basel) 2020;12(8):2138. doi: 10.3390/cancers12082138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Putz U., Howitt J., Doan A., Goh C.P., Low L.H., Silke J., et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci. Signal. 2012;5(243):ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y., Ren H., Dai B., Li J., Shang L., Huang J., et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018;37(1):324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He Q., Ye A., Ye W., Liao X., Qin G., Xu Y., et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell. Death Dis. 2021;12(6):576–590. doi: 10.1038/s41419-021-03803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferrari E., Gandellini P. Unveiling the ups and downs of miR-205 in physiology and cancer: transcriptional and post-transcriptional mechanisms. Cell. Death Dis. 2020;11(11):980–1000. doi: 10.1038/s41419-020-03192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gandellini P., Profumo V., Casamichele A., Fenderico N., Borrelli S., Petrovich G., et al. miR-205 regulates basement membrane deposition in human prostate: implications for cancer development. Cell Death Differ. 2012;19(11):1750–1760. doi: 10.1038/cdd.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Jong O.G., Verhaar M.C., Chen Y., Vader P., Gremmels H., Posthuma G., et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles. 2012;1:18396–18408. doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu X., Shen H., Yin X., Yang M., Wei H., Chen Q., et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. 2019;38(1):81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79(1):541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 126.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 127.Guo C., Song W.Q., Sun P., Jin L., Dai H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015;22(1):100. doi: 10.1186/s12929-015-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu D., Zhou H., Xun Q., Xu X., Ling J., Hu Y. microRNA-103 regulates the growth and invasion of endometrial cancer cells through the downregulation of tissue inhibitor of metalloproteinase 3. Oncol. Lett. 2012;3(6):1221–1226. doi: 10.3892/ol.2012.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jiang L., Qiao Y., Wang Z., Ma X., Wang H., Li J. Inhibition of microRNA-103 attenuates inflammation and endoplasmic reticulum stress in atherosclerosis through disrupting the PTEN-mediated MAPK signaling. J. Cell. Physiol. 2020;235(1):380–393. doi: 10.1002/jcp.28979. [DOI] [PubMed] [Google Scholar]
- 130.Hsu Y.L., Hung J.Y., Chang W.A., Jian S.F., Lin Y.S., Pan Y.C., et al. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol. Therapy. 2018;26(2):568–581. doi: 10.1016/j.ymthe.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y., et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68(4):1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 132.Di Modica M., Regondi V., Sandri M., Iorio M.V., Zanetti A., Tagliabue E., et al. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017;384:94–100. doi: 10.1016/j.canlet.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 133.Zhou W., Fong M.Y., Min Y., Somlo G., Liu L., Palomares M.R., et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.An Y., Zhang Q., Li X., Wang Z., Li Y., Tang X. Upregulated microRNA miR-21 promotes the progression of lung adenocarcinoma through inhibition of KIBRA and the Hippo signaling pathway. Biomed. Pharmacother. 2018;108:1845–1855. doi: 10.1016/j.biopha.2018.09.125. [DOI] [PubMed] [Google Scholar]
- 135.Ma C., Peng P., Zhou Y., Liu T., Wang L., Lu C. MicroRNA‑93 promotes angiogenesis and attenuates remodeling via inactivation of the Hippo/Yap pathway by targeting Lats2 after myocardial infarction. Mol. Med. Rep. 2020;22(1):483–493. doi: 10.3892/mmr.2020.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 2016;17(5):272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 137.Qi X., Zhang D.H., Wu N., Xiao J.H., Wang X., Ma W. ceRNA in cancer: possible functions and clinical implications. J. Med. Genet. 2015;52(10):710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 138.Cheng Y., Dai X., Yang T., Zhang N., Liu Z., Jiang Y. Low long noncoding RNA growth arrest-specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J. Oncol. 2019;2019:1–13. doi: 10.1155/2019/2476175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao X., Qin T., Mao J., Zhang J., Fan S., Lu Y., et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J. Exp. Clin. Cancer Res. CR. 2019;38(1):256. doi: 10.1186/s13046-019-1260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan J., Su Z., Gu W., Shen X., Zhao Q., Shi L., et al. MiR-19b and miR-20a suppress apoptosis, promote proliferation and induce tumorigenicity of multiple myeloma cells by targeting PTEN. Cancer Biomark. 2019;24(3):279–289. doi: 10.3233/CBM-182182. [DOI] [PubMed] [Google Scholar]
- 141.Zheng R., Du M., Wang X., Xu W., Liang J., Wang W., et al. Exosome–transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer. 2018;17(1):143–155. doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao N., Tang H., Gao L., Tu G.L., Luo H., Xia Y. LncRNA H19 aggravates cerebral ischemia/reperfusion injury by functioning as a ceRNA for miR-19a-3p to target PTEN. Neuroscience. 2020;437:117–129. doi: 10.1016/j.neuroscience.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 144.Lv J., Wang L., Zhang J., Lin R., Sun W., Wu H., et al. Long noncoding RNA H19-derived miR-675 aggravates restenosis by targeting PTEN. Biochem. Biophys. Res. Commun. 2018;497(4):1154–1161. doi: 10.1016/j.bbrc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 145.Shyu K.G., Wang B.W., Pan C.M., Fang W.J., Lin C.M. Hyperbaric oxygen boosts long noncoding RNA MALAT1 exosome secretion to suppress microRNA-92a expression in therapeutic angiogenesis. Int. J. Cardiol. 2019;274:271–278. doi: 10.1016/j.ijcard.2018.09.118. [DOI] [PubMed] [Google Scholar]
- 146.Qiu J.J., Lin X.J., Tang X.Y., Zheng T.T., Lin Y.Y., Hua K.Q. Exosomal metastasis‑associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 2018;14(14):1960–1973. doi: 10.7150/ijbs.28048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lang H.L., Hu G.W., Chen Y., Liu Y., Tu W., Lu Y.M., et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017;21(5):959–972. [PubMed] [Google Scholar]
- 148.Wu Q., Wu X., Ying X., Zhu Q., Wang X., Jiang L., et al. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int. 2017;17(1):62. doi: 10.1186/s12935-017-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang F.W., Cao C.H., Han K., Zhao Y.X., Cai M.Y., Xiang Z.C., et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J. Clin. Investig. 2019;129(2):727–743. doi: 10.1172/JCI122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32(5):453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hon K.W., Ab-Mutalib N.S., Abdullah N.M.A., Jamal R., Abu N. Extracellular vesicle-derived circular RNAs confers chemoresistance in colorectal cancer. Sci.. Rep. 2019;9(1):16497. doi: 10.1038/s41598-019-53063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang S., Cheng L., Wu H., Li G. Mechanisms and prospects of circular RNAs and their interacting signaling pathways in colorectal cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.949656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai H., Li Y., Zhang H., Hu J., Liao J., Su Y., et al. exoRBase 2.0: an atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022;50(D1):D118–DD28. doi: 10.1093/nar/gkab1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng L., Liang H., Zhang Q., Shen Z., Sun Y., Zhao X., et al. circPTEN1, a circular RNA generated from PTEN, suppresses cancer progression through inhibition of TGF-β/Smad signaling. Mol. Cancer. 2022;21(1):41. doi: 10.1186/s12943-022-01495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang S., Fu R., Shi J., Wu H., Mai J., Hua X., et al. CircRNA-mediated regulation of angiogenesis: a new chapter in cancer biology. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.553706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dweep H., Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat. Methods. 2015;12(8):697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 158.Kuosmanen S.M., Kansanen E., Sihvola V., Levonen A.L. MicroRNA profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Sci. Rep. 2017;7(1):10943. doi: 10.1038/s41598-017-11487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moro A., Driscoll T.P., Boraas L.C., Armero W., Kasper D.M., Baeyens N., et al. MicroRNA-dependent regulation of biomechanical genes establishes tissue stiffness homeostasis. Nat. Cell Biol. 2019;21(3):348–358. doi: 10.1038/s41556-019-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chevillet J.R., Kang Q., Ruf I.K., Briggs H.A., Vojtech L.N., Hughes S.M., et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. 2014;111(41):14888. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stevanato L., Thanabalasundaram L., Vysokov N., Sinden J.D. Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. PLOS One. 2016;11(1) doi: 10.1371/journal.pone.0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calabrese E.J., Mattson M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017;3(1):13–29. doi: 10.1038/s41514-017-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tay Y., Tan S.M., Karreth F.A., Lieberman J., Pandolfi P.P. Characterization of dual PTEN and p53-targeting microRNAs identifies microRNA-638/Dnm2 as a two-hit oncogenic locus. Cell. Rep. 2014;8(3):714–722. doi: 10.1016/j.celrep.2014.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakayama I., Shibazaki M., Yashima-Abo A., Miura F., Sugiyama T., Masuda T., et al. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int. J. Oncol. 2013;43(1):63–71. doi: 10.3892/ijo.2013.1935. [DOI] [PubMed] [Google Scholar]
- 165.Xiao H., Li H., Yu G., Xiao W., Hu J., Tang K., et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol. Rep. 2014;31(4):1832–1838. doi: 10.3892/or.2014.3048. [DOI] [PubMed] [Google Scholar]
- 166.Tian Y., Luo A., Cai Y., Su Q., Ding F., Chen H., et al. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J. Biol. Chem. 2010;285(11):7986–7994. doi: 10.1074/jbc.M109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Z., Zhu J., Cao H., Ren H., Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int. J. Oncol. 2012;40(5):1553–1560. doi: 10.3892/ijo.2012.1342. [DOI] [PubMed] [Google Scholar]
- 168.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 169.Zhu J., Du S., Zhang J., Huang G., Dong L., Ren E., et al. microRNA-10a-5p from gastric cancer cell-derived exosomes enhances viability and migration of human umbilical vein endothelial cells by targeting zinc finger MYND-type containing 11. Bioengineered. 2022;13(1):496–507. doi: 10.1080/21655979.2021.2009962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Wang C., Yin W., Liu H. MicroRNA-10a promotes epithelial-to-mesenchymal transition and stemness maintenance of pancreatic cancer stem cells via upregulating the Hippo signaling pathway through WWC2 inhibition. J. Cell. Biochem. 2020;121(11):4505–4521. doi: 10.1002/jcb.29716. [DOI] [PubMed] [Google Scholar]
- 171.Xie Y., Wang Q., Gao N., Wu F., Lan F., Zhang F., et al. MircroRNA-10b promotes human embryonic stem cell-derived cardiomyocyte proliferation via novel target gene LATS1. Mol. Ther. Nucleic Acids. 2020;19:437–445. doi: 10.1016/j.omtn.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gao F., Kataoka M., Liu N., Liang T., Huang Z.P., Gu F., et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019;10(1):1802–1817. doi: 10.1038/s41467-019-09530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang F., Li T., Zhang B., Li H., Wu Q., Yang L., et al. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem. Biophys. Res. Commun. 2013;434(3):688–694. doi: 10.1016/j.bbrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 174.Xu X., Chen Z., Zhao X., Wang J., Ding D., Wang Z., et al. MicroRNA-25 promotes cell migration and invasion in esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2012;421(4):640–645. doi: 10.1016/j.bbrc.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 175.Huse J.T., Brennan C., Hambardzumyan D., Wee B., Pena J., Rouhanifard S.H., et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23(11):1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guo P., Nie Q., Lan J., Ge J., Qiu Y., Mao Q. C-Myc negatively controls the tumor suppressor PTEN by upregulating miR-26a in glioblastoma multiforme cells. Biochem. Biophys. Res. Commun. 2013;441(1):186–190. doi: 10.1016/j.bbrc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 177.Martello A., Mellis D., Meloni M., Howarth A., Ebner D., Caporali A., et al. Phenotypic miRNA screen identifies miR-26b to promote the growth and survival of endothelial cells. Mol. Ther. Nucleic Acids. 2018;13:29–43. doi: 10.1016/j.omtn.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dong N., Xu B., Benya S.R., Tang X. MiRNA-26b inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Mol. Cell. Biochem. 2014;396(1–2):229–238. doi: 10.1007/s11010-014-2158-4. [DOI] [PubMed] [Google Scholar]
- 179.Li J., Kong X., Zhang J., Luo Q., Li X., Fang L. MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell. Int. 2013;13(1):7. doi: 10.1186/1475-2867-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tumaneng K., Schlegelmilch K., Russell R.C., Yimlamai D., Basnet H., Mahadevan N., et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 2012;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suarez Y., Fernandez-Hernando C., Yu J., Gerber S.A., Harrison K.D., Pober J.S., et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. 2008;105(37):14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Umezu T., Ohyashiki K., Kuroda M., Ohyashiki J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 183.Tian Y., Liu Y., Wang T., Zhou N., Kong J., Chen L., et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015;7(279):279ra38. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawano M., Tanaka K., Itonaga I., Ikeda S., Iwasaki T., Tsumura H. microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells. J. Exp. Clin. Cancer Res. 2015;34(1):76. doi: 10.1186/s13046-015-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fu X., Tian J., Zhang L., Chen Y., Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586(9):1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 186.Zhang M.L., Sun W.H., Wu H.Q., Liu Z.D., Wang P. Knockdown of microRNA-103a-3p inhibits the malignancy of thyroid cancer cells through Hippo signaling pathway by upregulating LATS1. Neoplasma. 2020;67(6):1266–1278. doi: 10.4149/neo_2020_191224N1331. [DOI] [PubMed] [Google Scholar]
- 187.Yu T., Cao R., Li S., Fu M., Ren L., Chen W., et al. MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer. 2015;15:29. doi: 10.1186/s12885-015-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gu J.J., Fan K.C., Zhang J.H., Chen H.J., Wang S.S. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int. J. Mol. Med. 2018;41(1):284–292. doi: 10.3892/ijmm.2017.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jian B., Li Z., Xiao D., He G., Bai L., Yang Q. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumour. Biol. 2016;37(7):8941–8949. doi: 10.1007/s13277-015-4727-x. [DOI] [PubMed] [Google Scholar]
- 190.Yu T., Li J., Yan M., Liu L., Lin H., Zhao F., et al. MicroRNA-193a-3p and -5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34(4):413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 191.De Cola A., Volpe S., Budani M.C., Ferracin M., Lattanzio R., Turdo A., et al. miR-205-5p-mediated downregulation of ErbB/HER receptors in breast cancer stem cells results in targeted therapy resistance. Cell Death Dis. 2015;6:e1823. doi: 10.1038/cddis.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lei L., Huang Y., Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol. Rep. 2013;30(6):2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 193.Li J., Hu K., Gong G., Zhu D., Wang Y., Liu H., et al. Upregulation of MiR-205 transcriptionally suppresses SMAD4 and PTEN and contributes to human ovarian cancer progression. Sci. Rep. 2017;7:41330–41339. doi: 10.1038/srep41330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ma Q., Wan G., Wang S., Yang W., Zhang J., Yao X. Serum microRNA-205 as a novel biomarker for cervical cancer patients. Cancer Cell Int. 2014;14:81. doi: 10.1186/s12935-014-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhao G., Cai C., Yang T., Qiu X., Liao B., Li W., et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLOS One. 2013;8(1):e53906. doi: 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie Q., Yan Y., Huang Z., Zhong X., Huang L. MicroRNA-221 targeting PI3-K/Akt signaling axis induces cell proliferation and BCNU resistance in human glioblastoma. Neuropathology. 2014;34(5):455–464. doi: 10.1111/neup.12129. [DOI] [PubMed] [Google Scholar]
- 197.Li D., Li P., Guo Z., Wang H., Pan W. Downregulation of miR-382 by propranolol inhibits the progression of infantile hemangioma via the PTEN-mediated AKT/mTOR pathway. Int. J. Mol. Med. 2017;39(3):757–763. doi: 10.3892/ijmm.2017.2863. [DOI] [PubMed] [Google Scholar]
- 198.Mao G., Liu Y., Fang X., Fang L., Lin L., Liu X., et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18(3):373–382. doi: 10.1007/s10456-015-9474-5. [DOI] [PubMed] [Google Scholar]
- 199.Li X.T., Wang H.Z., Wu Z.W., Yang T.Q., Zhao Z.H., Chen G.L., et al. miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell. Mol. Neurobiol. 2015;35(5):679–687. doi: 10.1007/s10571-015-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhuo D., Ning J.Z., Zhang J.W., Chen Y., Cheng L., Ba Z. [Role of the lncRNA H19/microRNA-203a/PTEN axis in ischemia-reperfusion injury of mouse GC-1 cells and its mechanism] Zhonghua Nan Ke Xue. 2020;26(6):499–504. [PubMed] [Google Scholar]
- 201.Zhang L., Wang C., Ma M. LncRNA POU3F3 Promotes cancer cell proliferation, migration, and invasion in renal cell carcinoma by downregulating LncRNA GAS5. Kidney Blood Press. Res. 2021;46(5):613–619. doi: 10.1159/000511174. [DOI] [PubMed] [Google Scholar]
- 202.Wang L., Liu J., Xie W., Li G., Yao L., Zhang R., et al. Overexpression of MALAT1 relates to lung injury through sponging miR-425 and promoting cell apoptosis during ARDS. Can. Respir. J. 2019;2019 doi: 10.1155/2019/1871394. [DOI] [PMC free article] [PubMed] [Google Scholar]