Abstract
Background: Cancer, also known as a malignant tumor, is caused by the activation of oncogenes, which leads to the uncontrolled proliferation of cells that results in swelling. According to the World Health Organization (WHO), cancer is one of the main causes of death worldwide. The main variables limiting the efficacy of anti-tumor treatments are side effects and drug resistance. The search for natural, safe, low toxicity, and efficient chemical compounds in tumor research is essential. Berberine is a pentacyclic isoquinoline quaternary ammonium alkaloid isolated from Berberis and Coptis that has long been used in clinical settings. Studies in recent years have reported the use of berberine in cancer treatment. In this study, we performed a bibliometric analysis of berberine- and tumor-related research.
Materials and methods: Relevant articles from January 1, 2002, to December 31, 2021, were identified from the Web of Science Core Collection (WOSCC) of Clarivate Analytics. Microsoft Excel, CiteSpace, VOSviewer, and an online platform were used for the literary metrology analysis.
Results: A total of 1368 publications had unique characteristics. Publications from China were the most common (783 articles), and Y. B. Feng (from China) was the most productive author, with the highest total citations. China Medical University (Taiwan) and Sun Yat-sen University (China) were the two organizations with the largest numbers of publications (36 each). Frontiers in Pharmacology was the most commonly occurring journal (29 articles). The present body of research is focused on the mechanism, molecular docking, and oxidative stress of berberine in tumors.
Conclusion: Research on berberine and tumors was thoroughly reviewed using knowledge map and bibliometric methods. The results of this study reveal the dynamic evolution of berberine and tumor research and provide a basis for strategic planning in cancer research.
Keywords: berberine, tumor, pharmacology, bibliometric analysis, Web of Science
1 Introduction
In every nation worldwide, cancer ranks as a primary cause of mortality and a significant roadblock to increasing life expectancy (Sung et al., 2021). The mortality and incidence rates of malignant tumors have increased globally (Mullard, 2020). The World Health Organization (WHO) estimates for 2019 suggest that cancer is the third or fourth leading cause of death before the age of 70 years in 23 countries (among 183 nations in total) and the first or second leading cause in 112 countries (Sung et al., 2021). The development of cancer treatments has faced significant obstacles. While both traditional and modern approaches (surgery, radiation, chemotherapy, targeted therapy, and immunotherapy) have good efficacy, these treatment modalities also have negative consequences on patient quality of life, including the development of simultaneous resistance to multiple drugs and dermatologic toxicities (Szakacs et al., 2006; Nastiuk and Krolewski, 2016; Lacouture and Sibaud, 2018). One of the main clinical therapies for cancer is chemotherapy; however, cancer is prone to relapse and drug resistance, and most chemotherapy treatments fail; therefore, efforts to develop anti-cancer drugs are needed. Additionally, toxic side effects caused by chemotherapy also affect the quality of life of patients with cancer. Therefore, identifying anti-tumor medicines with minimal toxicity and high efficacy is crucial for the treatment of tumors.
In recent decades, scientists have conducted numerous clinical and laboratory studies on traditional Chinese medicine. Natural compounds have many medicinal properties, including multiple action targets, low toxicity, low side effects, few adverse reactions, and high safety and effectiveness. These compounds have been gradually applied to cancer treatment due to their safety, availability, accessibility, and low cost. Natural compounds have a variety of anti-cancer effects, including suppression of tumor cell growth, induction of tumor cell death, prevention of tumor spread and angiogenesis, regulation of tumor autophagy, reversal of tumor drug resistance, regulation of body immunity, and influence on tumor metabolic reprogramming (Yang et al., 2021). In addition, natural therapy can prevent many issues, increase tumor cell sensitivity to conventional treatment, reduce side effects, enhance patient quality of life, and extend patient lives to cure cancer (Sun et al., 2022a; Sun et al., 2022b). Therefore, natural medicines are receiving increasing attention. Berberine (BBR) is an isoquinoline alkaloid obtained from the Chinese herb Coptis chinensis and other Berberis species (Song et al., 2020). It is the main component of Coptidis Rhizome (CR), known as Huanglian in Chinese. Because of its pharmacological characteristics, BBR has been used as a drug to treat diseases. As a secondary metabolite of plants, it has several pharmacological characteristics (Song et al., 2020), including treatment efficacy for cardiovascular and metabolic disease (Feng et al., 2019), polycystic ovary syndrome (Zhang et al., 2021b), and non-alcoholic fatty liver disease (Koperska et al., 2022), in addition to anti-inflammatory (Kuo et al., 2004), antioxidant (Zhuang et al., 2018), and antibacterial (Li et al., 2019) properties. In recent years, BBR has also been used in the research of cancers. For example, BBR binds RXRα to suppress β-catenin signaling, leading to the inhibition of colorectal cancer proliferation (Ruan et al., 2017); BBR also regulates the HMGB1–TLR4 axis to repress the metastasis of breast cancer in vitro and in vivo (Zheng et al., 2021). Moreover, BBR exerts therapeutic actions on gastric cancer by multi-step actions such as inhibiting cell proliferation, migration, and angiogenesis (Liu et al., 2022a); BBR also combines with cisplatin to induce necroptosis and apoptosis in ovarian cancer (Liu et al., 2019). In recent years, more studies on BBR have been conducted in vitro and in vivo, which have largely proved the reproducibility and transformation potential of BBR’s anti-tumor effect in in vitro cell and in vivo animal models.
Using measurement techniques from mathematics and statistics, bibliometrics analysis assesses and forecasts the current state of science and technology by utilizing the features of the literature system as its research subject (Chen et al., 2014; Yu et al., 2020). By examining and evaluating the quantity and quality of scientific literature associated with a specific topic, bibliometric evaluation can objectively assess the state and level of development of that field (Musbahi et al., 2022). Bibliometrics assists researchers in swiftly identifying the information context in the target field, including the annual publishing trends of the literature, catalog of publishing institutes or journals, and popular research (Yin et al., 2022). The knowledge structure can be understood more methodically and intuitively using this approach, and frontiers or hotspots in a particular research area can be identified (Musbahi et al., 2022). Therefore, the present bibliometrics study aimed to investigate the role of BBR in tumors and to offer a broad perspective and roadmap for future research on BBR in pan-carcinoma treatment.
2 Materials and methods
2.1 Data source and searching strategies
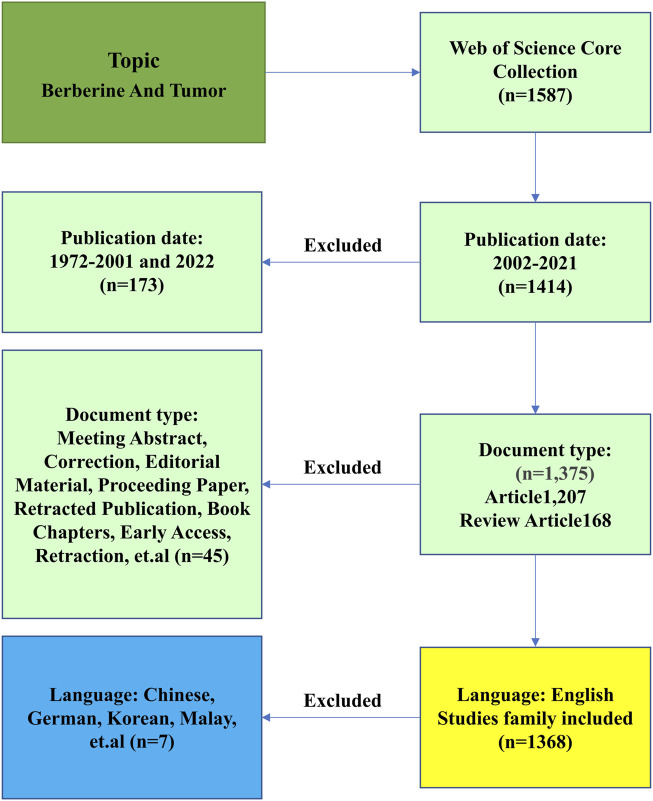
Published studies from January 1, 2002, to December 31, 2021, related to BBR and tumors were collected from the Science Citation Index Expanded (SCI-E) of the Web of Science (WoS) Core Collection (WOSCC) of Clarivate Analytics. To guarantee the reliability and accuracy of the findings, we performed pertinent pretests and improved the retrieval method. The retrieval method is illustrated in Figure 1. We used the WoS engine to search for terms related to BBR and cancer that were obtained from the Medical Subject Headings (MeSH) in PubMed. The search formula was TS = (Berberine or Umbellatine) and TS = (Tumor or Neoplasm or Tumors or Neoplasia or Neoplasias or Cancer or Cancers or “Malignant Neoplasm” or Malignancy or Malignancies or “Malignant Neoplasms” or “Neoplasm, Malignant” or “Neoplasms, Malignant” or “Benign Neoplasms” or “Benign Neoplasm” or “Neoplasms Benign” or “Neoplasm Benign”). Only publications written in English were included. The reasons for exclusion from the study were 1) no connection between BBR and tumors of any kind; 2) meeting abstract, correction, editorial material, proceeding paper, retracted publication, book chapters, early access, retraction, etc.; and 3) publications in a language other than English. A total of 1,368 papers were obtained.
FIGURE 1.
Flowchart of the screening process.
2.2 Data collection
All retrieved documents were used for the bibliometric analysis. We extracted information including titles, annual publications, countries and institutes, authors, references, keywords, and scientific cooperation analysis. Data were independently extracted from a set of articles by TY and YRZ. SP-H mediated the outcomes if a disagreement arose.
2.3 Data analysis and visualization
We used CiteSpace (version 6.3. R3), VOSviewer (version 1.6.18), Bibliometrix (version 3.1.4), R language (version 3.6.3), and Microsoft Excel 365 for data analysis and presentation.
CiteSpace, developed by Prof. Chao-mei Chen, is a bibliometric mapping analytical tool used worldwide, most predominantly in China (Qin et al., 2022). It is a free program for analyzing, identifying, and visualizing trends and patterns in scientific literature and was chosen as the analysis target (Pan et al., 2017). In our investigation, the specifics of the CiteSpace settings were as follows: time slices of 1 year each were taken from 2002 to 2021. The links (strength: cosine; scope: inside slices) and term sources (title, abstract, author keywords, and keywords plus) were set to the default values. Items with a g-index citation or occurrence were selected for this study. Links that were not necessary were removed using Pathfinder.
VOSviewer, developed by van Eck and Waltman, creates visual network maps of scientific information, including bibliometric network analysis (van Eck and Waltman, 2010). We used VOSviewer to create network, overlay, and density visualization maps. We chose the “full counting” method for our analysis. For keywords and co-cited journals, the thresholds for the minimum numbers were set at 100 and 5, respectively. Keywords or co-cited journals are displayed as nodes in the form of a network through a visualization diagram.
Visualization of the annual publication numbers; country, institute, author, and journal rankings; cited reference bursts; and most globally and locally cited Local Cited Documents, was performed using Microsoft Excel 365. Additionally, we performed a descriptive study of writer output over time and keyword evolution in Bibliometrix (Cheng et al., 2022). Bibliometrix (https://www.bibliometrix.org) is an open-source instrument for quantitative scient-metric research. Machine-learning software was used to evaluate the distribution of each component examined in the bibliometric investigation. The variables were annual scientific production, average citations per year, most impactful journals by H-index or total citations (TC), top journals’ production over time, most relevant authors, most globally and locally cited documents, most pertinent affiliations, country-level scientific output, international collaboration network, country of origin of the corresponding author, top producing nations over time, historical direct citation network, most widely cited publications, most pertinent keywords, and keyword cluster analysis. Impact factor (IF) and partition information for the journals referred to the “Journal Citation Report (JCR) 2022.” These analytical methods offer unbiased and varied viewpoints on the function of berberine in tumor formation. The variables are presented as numbers and percentages in the descriptive study. p-values were not reported because no comparisons were made.
3 Results
3.1 Global publication outputs
We assessed the historical development process, current research conditions, and forecasted future trends in development by statistically analyzing the general usage of BBR pharmacological functions in the number of publications over time.
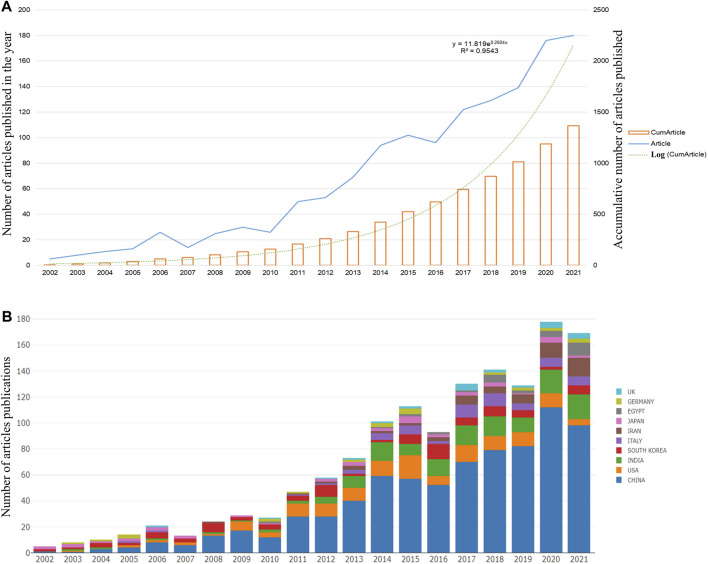
We counted the number of papers related to “berberine and tumors” from 1972 to 2021 and collected 1,587 articles for bibliographic records from the WOSCC. Ultimately, 1,368 articles were eligible for the next stage of analysis based on the exclusion criteria of publication time, document type, and language. Figure 2A shows the number of articles published each year and the cumulative number of articles published from 2002 to 2021. The overall trend has increased over the past 20 years. The greatest number of papers was published in 2021, with 180 research articles. The exponential curve equation for the rise in literature production was y = 11.819e0.2604x, which conforms to Price’s law. The simulation curve had a relatively strong coefficient of determination (R 2 = 0.9543), which fitted the annual literature growth trend well. Based on this curve, we predicted that the number of annual articles will steadily increase, indicating increased interest in research on BBR and tumors.
FIGURE 2.
Global trends of publications about berberine and tumor. (A) The number of articles published each year and the accumulative number of articles from 2002 to 2021. (B) The annual number of papers published by nations from 2002 to 2021.
3.2 Distributions of countries/regions
Statistics on the research countries reporting on publications related to “berberine and tumors” showed the stages of BBR pharmacology development in each country and made comparisons easier. According to WoS, many countries have participated in research in the last 20 years. Table 1 lists the top 10 productive countries. China had the most publications (783 articles), accounting for 57.24% of the total, followed by the United States and India (142 articles each, accounting for 10.38% each, respectively), South Korea (n = 97), Italy (n = 61), Iran (n = 60), Japan (n = 42), Egypt (n = 30), Germany (n = 26), and Poland (n = 24).
TABLE 1.
Top 10 countries that contributed publications on BBR and tumors.
| Rank | Countries | Article count | Percentage (n/1368) | H-index | Total citations | Average citation per article |
|---|---|---|---|---|---|---|
| 1 | China | 783 | 57.24% | 70 | 23,603 | 30.14 |
| 2 | United States | 142 | 10.38% | 48 | 6,886 | 48.49 |
| 3 | India | 142 | 10.38% | 35 | 4,137 | 29.13 |
| 4 | Republic of Korea | 97 | 7.09% | 34 | 3,198 | 32.97 |
| 5 | Italy | 61 | 4.46% | 25 | 2,257 | 37.00 |
| 6 | Iran | 60 | 4.39% | 22 | 1,800 | 30.00 |
| 7 | Japan | 42 | 3.07% | 22 | 1,403 | 33.40 |
| 8 | Egypt | 30 | 2.19% | 14 | 615 | 20.50 |
| 9 | Germany | 26 | 1.90% | 16 | 1,117 | 42.96 |
| 10 | Poland | 24 | 1.75% | 14 | 539 | 22.46 |
Publications from China ranked first in total number of citations, but the average number of citation per paper was very low, only 30.14%, compared to the top 10 countries. Although the number of articles published by Germany was limited compared to China, the average citation per article ranked second among the top 10 countries. This finding suggested that the publications were of very high quality and had certain reference values. The H-index is a mixed quantitative indicator that considers both the number of posts and the required number of citations and can be used to identify influential researchers (Hirsch, 2005). China’s researchers had the highest H-indexes, demonstrating the significant impact of their publications. After 2008, most global publications came from China. Figure 2B shows the total number of papers published worldwide from 2002 to 2021.
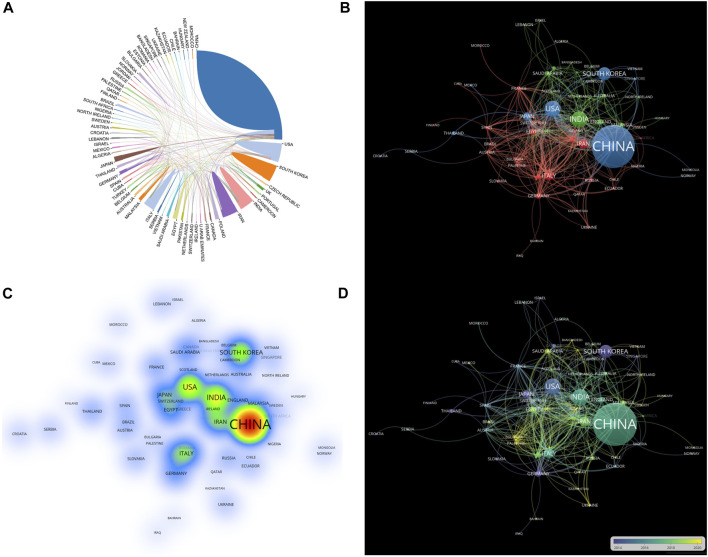
Using an online bibliometric analysis platform, we evaluated the significance of nations in the visualization of cooperative networks. Katz and Martin, two scientists, defined scientific cooperation as the study of academics collaborating to create new scientific knowledge, known as scientific collaboration (Katz and Martin, 1997). Figure 3A shows partnerships between countries, among which articles from China showed the most aggressive cooperation with other countries; most often between China and the United States. We conducted a visual analysis using VOSviewer (Figure 3B). The circles in the map represent countries and the lines indicate the connections between them. The larger the area of the circle, the larger the contribution of these countries to this field. We found that China made significant contributions to research on BBR and cancer. A density map can be used to determine the number of publications in each nation. The lighter the color, the greater the number of publications. The density map was used to determine the number of publications in each nation (Figure 3C ). As shown in Figure 3C, China had the highest number of publications. Figure 3D shows that American publications were generally concentrated before 2016, whereas Chinese, Indian, Italian, and Iranian articles were concentrated after 2016.
FIGURE 3.
Overview of national publications. (A) The scientific collaborations among worldwide countries. (B) Visual analysis of cooperation between countries based on VOSviewer. (C) A density visualization map of number of publications in each nation. (D) Median time chart of the number of articles issued by each country.
3.3 Distribution of institutes
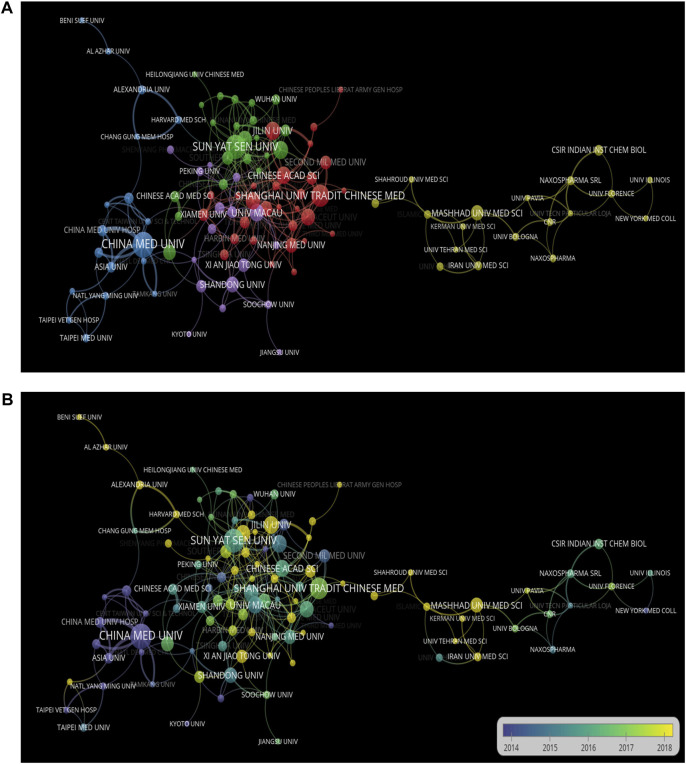
The top 10 productive institutes are listed in Table 2. China Medical University (Taiwan) and Sun Yat-Sen University (China) had the most publications, with 36 articles each. China Medical University (Taiwan) showed the highest total number of citations (1,417) and H-index (21), with an average number of citations of 39.26, demonstrating a high level of acclaim for its articles. We used VOSviewer to depict the cooperation between institutes in Figure 4A. Figure 4B shows each institute’s cooperation timeline from 2002 to 2021; the node color on this map corresponds to each institute’s cooperation respective average appearing year (AAY). Jilin University and Mashhad University of Medical Sciences had relatively fresh entries compared to the nations shown in purple, such as China Medical University (Taiwan), according to the color gradient in the lower right corner.
TABLE 2.
Top 10 institutes that contributed publications on BBR and tumors.
| Rank | Institute | Country | Article count | H-index | Total number of citations | Average number of citations per article |
|---|---|---|---|---|---|---|
| 1 | China Medical University (Taiwan) | China | 36 | 21 | 1,417 | 39.36 |
| 2 | Sun Yat-Sen University | China | 36 | 20 | 1,204 | 33.44 |
| 3 | Council of Scientific Industrial Research | India | 34 | 17 | 898 | 26.41 |
| 4 | Shanghai University of Traditional Chinese Medicine | China | 31 | 16 | 878 | 28.32 |
| 5 | Chinese Academy of Sciences | China | 29 | 18 | 755 | 26.03 |
| 6 | Egyptian Knowledge Bank | Egyptian | 28 | 14 | 606 | 21.64 |
| 7 | University of Hong Kong | China | 24 | 18 | 1,401 | 58.38 |
| 8 | Guangzhou University of Chinese Medicine | China | 24 | 14 | 587 | 24.46 |
| 9 | Jilin University | China | 24 | 14 | 562 | 23.42 |
| 10 | Jinan University | China | 24 | 14 | 484 | 20.17 |
FIGURE 4.
Cooperation networks between institutes based on VOSviewer. (A) Cooperative relationship of various institutions using network map. (B) Cooperative time of various institutions using network map.
3.4 Authors
The top 10 authors in terms of the number of articles in the past 20 years are listed in Table 3. Y. B. Feng of the University of Hong Kong, was the most prolific author (21 articles) and also received the highest number of total citations (1,281) and had an H-index of 17, demonstrating his significant contributions to the field. O Paolo from Naxospharma published 17 articles, while L Paolo and K Gopinatha Suresh (Indian Institution of Chemical Biology) both had H-index values of 12.
TABLE 3.
Top 10 authors that contributed publications on BBR and tumors.
| Rank | Author | Article count | H-index | Country | Total number of citations | Average number of citations per article | Institution |
|---|---|---|---|---|---|---|---|
| 1 | Feng, Y. B | 21 | 17 | China | 1,281 | 61.00 | University of Hong Kong |
| 2 | Paolo. L | 17 | 12 | Italy | 879 | 51.71 | Naxospharma |
| 3 | Gopinatha Suresh. K | 16 | 12 | Indian | 510 | 31.88 | Indian Institute of Chemical Biology |
| 4 | Tan, H.-Y | 13 | 9 | China | 408 | 31.38 | Hong Kong Baptist University |
| 5 | Wang, Y. T | 11 | 11 | China | 1,094 | 99.45 | University of Macau |
| 6 | Zheng, X | 11 | 9 | China | 275 | 25.00 | Jilin University |
| 7 | Bhupendra M. M | 10 | 8 | Republic of Korea | 129 | 12.90 | Konkuk University |
| 8 | Shao, D | 10 | 9 | China | 256 | 25.60 | Jilin University |
| 9 | Chen, L | 10 | 8 | China | 297 | 29.70 | Jilin University |
| 10 | Doo Hwan. K | 10 | 8 | Republic of Korea | 129 | 12.90 | Konkuk University |
3.5 Journals and co-cited journals
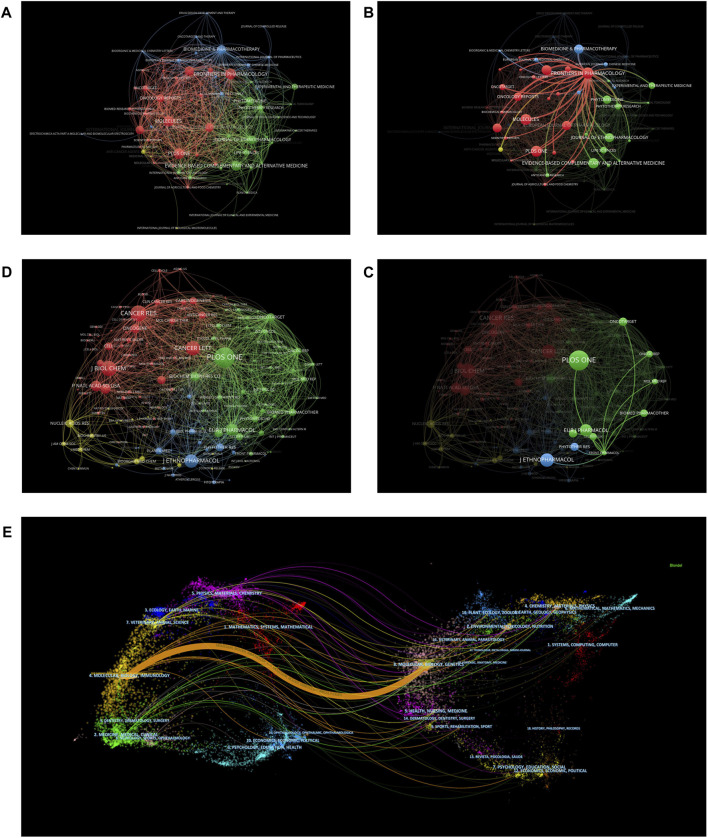
The top 10 journals according to the number of publications are shown in Table 4 and Figure 5. The top 10 journals published 252 papers, or 18.42% of all the included publications. Frontiers in Pharmacology (IF = 5.9879) was the most popular journal with 29 articles, followed by Molecules (IF = 4.9269) with 28 articles. PLOS One (IF = 3.7521) was the most frequently cited journal (1,388 citations). The distribution of journals across fields, evolution of citation trajectories, and drift of scientific research centers can be displayed using the dual-map overlay of journals (Chen and Leydesdorff, 2014; Zhang et al., 2021a). In our dual-map overlay analysis (Figure 5E), the left side shows the distribution of the journals where the citing documents were located, representing the main disciplines of science mapping, while the right side shows the distribution of the journals corresponding to the cited literature, representing which disciplines were mainly cited by science mapping. The results showed that research related to BBR and tumors was mainly associated with the areas of molecular/biology/immunology and was often cited by molecular/biology/genetics researchers.
TABLE 4.
Top 10 journals and co-cited journals that published articles on BBR and tumors.
| Rank | Journal | Count | Total number of citations | Average citation per article | IF and JCR division (2021) | Co-cited journal | Total number of co-citations |
|---|---|---|---|---|---|---|---|
| 1 | Frontiers in Pharmacology | 29 | 835 | 28.79 | 5.9879, Q1 | PLOS One | 1394 |
| 2 | Molecules | 28 | 690 | 24.64 | 4.9269, Q2 | Cancer Research | 1101 |
| 3 | PLOS One | 28 | 1,388 | 49.57 | 3.7521, Q2 | Journal of Biological Chemistry | 1037 |
| 4 | Evidence-Based Complementary and Alternative Medicine | 27 | 415 | 15.37 | 2.6498, Q3 | Cancer Letters | 973 |
| 5 | Molecular Medicine Reports | 25 | 484 | 19.36 | 3.4232, Q3 | Journal of Ethnopharmacology | 910 |
| 6 | Biomedicine and Pharmacotherapy | 24 | 954 | 39.75 | 7.4194, Q1 | European Journal of Pharmacology | 827 |
| 7 | European Journal of Pharmacology | 24 | 814 | 33.92 | 5.1948, Q2 | Proceedings of the National Academy of Sciences of the United States of America | 717 |
| 8 | International Journal of Molecular Sciences | 23 | 453 | 19.70 | 6.2082, Q1/Q2 | Nature | 687 |
| 9 | Journal of Ethnopharmacology | 23 | 1,234 | 53.65 | 5.1948, Q1/Q2 | Oncogene | 598 |
| 10 | Oncology Reports | 21 | 483 | 23.00 | 4.1361, Q3 | Biochemical and Biophysical Research Communications | 594 |
FIGURE 5.
The co-cited map of journals. (A,B) The map of journals that published papers about berberine and tumor. (C,D) The map of the cited journals based on Vosviewer. (E) The dual-map overlay of journals.
3.6 Co-cited references
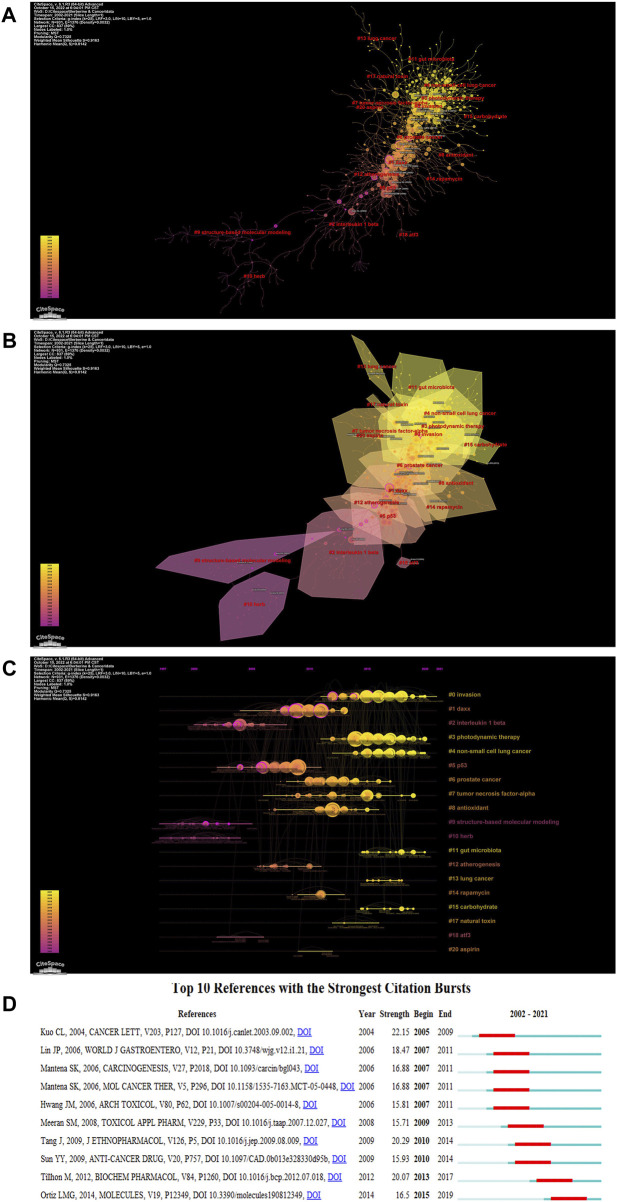
We quantified the knowledge and development of research on a particular topic by evaluating the significant nodes and clusters in the co-cited reference network (Yin et al., 2022). We used CiteSpace to create a timeline-visualized diagram network map of the co-cited articles, which was divided into 19 clusters (Table 5). The co-cited reference network is shown in Figure 6A, in which the node size is the proportion of the research frequency, color is the proportion of the time slice, and the connections are represented by lines. We further conducted cluster analysis on these co-cited articles based on the keywords in those articles (Figure 6B). By removing phrases from the titles of the cited publications, we identified the main research hotspots. Figure 6C shows a timeline of references in the field of BBR and tumors, as determined by CiteSpace. We observed a shift in the focus of research over time. The development of clusters 2 (interleukin 1 beta), 9 (structure-based molecular modeling), and 10 (herb) occurred the earliest, indicating that early research focused more on BBR structure and pharmacology. Clusters 1 (Daxx), 6 (prostate cancer), and 7 (tumor necrosis factor-alpha) occurred between 2007 and 2011, indicating a focus on the anti-cancer molecular mechanism of BBR. Clusters 15 (carbohydrate), 3 (photodynamic therapy), 4 (non-small cell lung cancer), and 11 (gut microbiota) are current research hotspots. Figure 6D shows the top 10 references with the strongest citation bursts, highlighting their significance in the fields of BBR and tumor-related research. In addition, we list the total citations of articles and local citations of articles in Tables 6 and Table 7, respectively.
TABLE 5.
Major clusters of co-cited references.
| Cluster ID | Size | Silhouette | Mean (year) | Top terms (LSI) | Top terms (LLR) | Terms (MI) |
|---|---|---|---|---|---|---|
| 0 | 96 | 0.842 | 2015 | Berberine; ERK1/2; and glioma | Invasion; migration; and glioma | Triptolide; RT-R breast cancer cells; and chemoprotective agents |
| 1 | 86 | 0.884 | 2008 | Breast cancer; signaling pathways; and leukemia | Daxx; side population; and invasiveness | Daxx; side population; and invasiveness |
| 2 | 85 | 0.935 | 2003 | Berberine; thermodynamics; and non-cooperative binding | Interleukin-1 beta; ARPE-19 cells; and interleukin-8 | Berberine; berberine interleukin-1 beta; and cooperative binding |
| 3 | 78 | 0.887 | 2016 | Berberine; carrier-free; and gram-scale | Photodynamic therapy; and epithelial–mesenchymal transition | Transforming growth factor; rat; and reverse pharmacophore mapping |
| 4 | 70 | 0.907 | 2017 | Berberine; doxorubicin; and neurotoxicity | Non-small-cell lung cancer; liquid crystalline nanoparticles; and neurotoxicity | Demethyleneberberine; modern science; and top2b |
| 5 | 69 | 0.95 | 2006 | Berberine; apoptosis; and ATF3 | P53; apoptosis; and Coscinium fenstratum | Human liver microsomes; anti-metastasis; and Tinospora cordifolia |
| 6 | 59 | 0.878 | 2012 | Berberine; IL-8; and adhesion | Prostate cancer; evodiamine; and epigenetics | Interleukin-8; traditional Chinese medicine; and gemcitabine |
| 7 | 51 | 0.914 | 2012 | Tumor necrosis factor-alpha; brain inflammation; and interleukin-1 beta | TNF-α; lipopolysaccharide; and NF-κB | Berberine intestinal mucosal barrier; interleukin-1; and interleukin-6 |
| 8 | 45 | 0.903 | 2011 | Cervical cancer; gastrointestinal disorders; and antitumor | Antioxidant; piperazine; and cervical cancer | RNA triplex; g-quadruplexes; and catenin |
| 9 | 45 | 0.971 | 2000 | Human topoisomerase; anti-cancer drugs; and structure-based molecular modeling | Structure-based molecular modeling; interleukin-12; p38 MAPK; and anti-cancer drugs | Berberine; apoptosis; and structure-based molecular modeling |
| 10 | 43 | 0.994 | 2000 | Anticancer activity; anticancer effects; and colorectal cancer | Herb; colon 26/clone 20 cells; and pancreatic cancer | Berberine; apoptosis; and colon 26/clone 20 cells |
| 11 | 30 | 0.97 | 2017 | Gut microbiota; pharmacokinetic study; and amino acid | Gut microbiota; atherosclerosis; and metabolomics | Metabolomics; fecal metabolites; and functional nano-vector |
| 12 | 22 | 0.942 | 2007 | Berberine; barrier function; plant alkaloid; and lipid mediators | Atherogenesis; anti-microbial; and acute intestinal symptoms | Berberine; atherogenesis; and anti-microbial |
| 13 | 16 | 0.997 | 2017 | Lung cancer; inhalable nanocomposites; and drug nanosuspension | Lung cancer; lactoferrin targeting; and drug nanosuspension | Berberine; apoptosis; and lactoferrin targeting |
| 14 | 13 | 0.957 | 2010 | Replication stress; H2AX phosphorylation; DNA damage; and ribosomal protein S6 phosphorylation | Rapamycin; replication stress; and salicylate | Berberine; salicylate; and ribosomal protein S6 phosphorylation |
| 15 | 10 | 0.995 | 2016 | Alkylated; HeLa cell; and carbohydrate | Carbohydrate; alkylated; and anticancer | Berberine; carbohydrate; and alkylated |
| 17 | 8 | 0.983 | 2014 | Kainic acid; status epilepticus; and oxidative stress | Natural toxin; chronic unpredictable mild stress; and temporal lobe epilepsy | Berberine; natural toxin; and chronic unpredictable mild stress |
| 18 | 7 | 0.998 | 2004 | Berberine; ATF3; and apoptosis | ATF3; nag-1; p53; and apoptosis | Berberine; apoptosis; cancer; and breast cancer |
| 20 | 4 | 0.99 | 2011 | AMPK; aspirin; and berberine | Aspirin; metformin; AMPK; and resveratrol | Berberine; apoptosis; and cancer |
FIGURE 6.
Analysis of co-cited references. (A) Visualized network diagram of co-cited documents. (B) Cluster Analysis of the co-cited articles. (C) Timeline view of the co-cited articles. (D) The top 10 references with the strongest citation bursts.
TABLE 6.
Total article citations.
| Rank | Title | DOI | Total number of citations | TC per year | Normalized TC |
|---|---|---|---|---|---|
| 1 | Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals | 10.1007/s10555-010-9235-2 | 570 | 43.85 | 7.15 |
| 2 | The anti-inflammatory potential of berberine in vitro and in vivo | 10.1016/j.canlet.2003.09.002 | 515 | 27.11 | 5.91 |
| 3 | Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine | 10.1002/ptr.2399 | 430 | 28.67 | 5.26 |
| 4 | Berberine and Coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations | 10.1016/j.jep.2009.08.009 | 394 | 28.14 | 5.20 |
| 5 | A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs | 10.1097/CAD.0b013e328330d95b | 309 | 22.07 | 4.08 |
| 6 | Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells | 10.1158/1535-7163.MCT-05-0448 | 285 | 16.76 | 3.59 |
| 7 | Berberine: New perspectives for old remedies | 10.1016/j.bcp.2012.07.018 | 284 | 25.82 | 6.05 |
| 8 | Anti-cancer natural products isolated from Chinese medicinal herbs | 10.1186/1749-8546-6-27 | 264 | 22.00 | 5.03 |
| 9 | Targeting apoptosis pathways in cancer by Chinese medicine | 10.1016/j.canlet.2010.07.015 | 220 | 22.00 | 5.41 |
| 10 | Cancer prevention and therapy through the modulation of the tumor microenvironment | 10.1016/j.semcancer.2015.02.007 | 212 | 26.50 | 6.11 |
TABLE 7.
The local citations of articles.
| Rank | Document | DOI | Year | Local Citations | Global Citations | LC/GC Ratio (%) | Normalized Local Citations | Normalized Global Citations |
|---|---|---|---|---|---|---|---|---|
| 1 | The anti-inflammatory potential of berberine in vitro and in vivo | 10.1016/j.canlet.2003.09.002 | 2004 | 155 | 515 | 30.10 | 6.25 | 5.91 |
| 2 | Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells | 10.1158/1535-7163.MCT-05-0448 | 2006 | 124 | 285 | 43.51 | 4.61 | 3.59 |
| 3 | A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs | 10.1097/CAD.0b013e328330d95b | 2009 | 121 | 309 | 39.16 | 4.63 | 4.08 |
| 4 | Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations | 10.1016/j.jep.2009.08.009 | 2009 | 115 | 394 | 29.19 | 4.40 | 5.20 |
| 5 | Berberine: new perspectives for old remedies | 10.1016/j.bcp.2012.07.018 | 2012 | 103 | 284 | 36.27 | 7.57 | 6.05 |
| 6 | Berberine induces apoptosis through a mitochondria/caspase’s pathway in human hepatoma cells | 10.1007/s00204-005-0014-8 | 2006 | 86 | 170 | 50.59 | 3.20 | 2.14 |
| 7 | Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism | 10.1002/jcb.22869 | 2010 | 85 | 200 | 42.50 | 5.44 | 2.51 |
| 8 | Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP | 10.1093/carcin/bgl043 | 2006 | 83 | 161 | 51.55 | 3.09 | 2.03 |
| 9 | Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine | 10.1002/ptr.2399 | 2008 | 83 | 430 | 19.30 | 3.31 | 5.26 |
| 10 | Berberine, an epiphany against cancer | 10.3390/molecules190812349 | 2014 | 79 | 165 | 47.88 | 10.53 | 4.19 |
3.7 Analysis of keywords
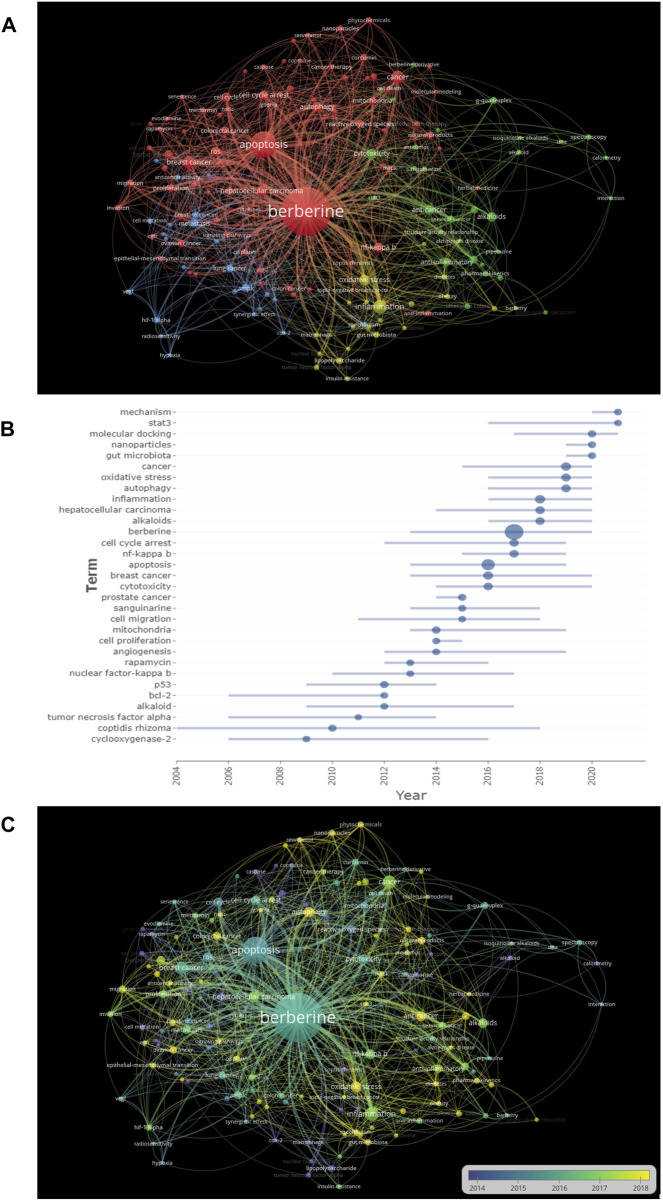
Keywords are generally regarded as a significant index to reflect research frontiers and hotspots in a certain topic (Zhong And Song, 2008; Wu et al., 2021). We produced a map showing the co-occurrence of keywords using VOSviewer. The cooperation network (Figure 7A) clearly shows keywords changing from 2002 to 2021. At present, the keywords are main focused on “berberine”, “apoptosis” and “cancer”, and so on. Figure 7B shows the evolution of keywords in a typical publishing year. Initially,research on BBR mainly focused on “alkaloid”, and “tumor necrosis factor-alpha”; then, the research keywords shifted towards “prostate cancer”, “rapamycin” and “p53”; finally, the most recent keywords have become more diverse, with topics including “mechanism”, “cancer” and “gut microbiota”, and so on. Figure 7C shows the frequency of keywords in different time periods, the larger the size of the node, the higher the search frequency. In conclusion, “apoptosis”, “non-small cell lung cancer”, and “gut microbiota” were the most frequently searched terms.
FIGURE 7.
Analysis of keywords (A) the changing trend of research keywords from 2002 to 2021. (B) The evolution of keyword frequency. (C) The network map of keywords. Minimum number of occurrences of keywords ≥5.
4 Discussion
Chemotherapy and radiation therapy are standard treatments for patients with cancer. However, the resistance of malignant tumors to these treatments and the occurrence of major organ damage severely restrict the clinical outcomes of this disease (35). Moreover, current cancer treatments are inefficient, and the surgical prognosis is poor because tumor cells can invade and spread after treatment with a variety of chemical medications (Colagiuri et al., 2013). Natural herbal nutraceuticals have recently gained increased attention among popular clinical medications owing to their safety, adaptability to overcome therapeutic resistance, potential to reduce the adverse side effects of cancer treatments, low cost, and wide availability (Choudhari et al., 2019). Natural remedies can not only prevent drug resistance (38) but also have anti-tumor effects through various signaling pathways (Kumar and Adki, 2018; Al-Bari et al., 2021; Hashem et al., 2022). The prognosis of patients with malignant tumors can be improved by combining Chinese herbal therapies with other therapies (Mignani et al., 2018; Rejhová et al., 2018). BBR is a multi-target Chinese medicine monomer compound that not only regulates the growth of cancer cells but also acts as a combination chemotherapy drug for the treatment of tumors.
In this study, we conducted a bibliometric analysis to evaluate the hotspots and development trends of research on disciplines connected to BBR and tumors. We analyzed a total of 1,368 studies published from January 1, 2001, to December 31, 2021, and found that research on BBR and tumors has become increasingly frequent, suggesting that BBR may be a natural medication for treating tumors. China had the most relevant publications in the last 20 years, but the average number of citations per article was only 30.14%, significantly lower than that of other nations in terms of publications, suggesting that articles from China still have room for improvement. Among the top 10 countries with the greatest number of articles, China engages the most in international collaborations, primarily with the United States. China Medical University (Taiwan) published the most studies related to BBR and tumors worldwide. However, the University of Hong Kong ranked first in terms of average citations per article. Y. B. Feng, an author based in China, was the most productive and had the highest total citations. The journal that published the most articles was Frontiers in Pharmacology (29 articles), followed by Molecules (28 articles). We also analyzed the reference with the strongest citation burst; the latest literature of the co-cited reference was “Berberine, an epiphany against cancer,” which was published in Molecules in 2014. This article summarizes the molecular targets of BBR, several mechanisms by which BBR inhibits cancer, and the potential discovery of BBR derivatives for anti-cancer drugs (Ortiz et al., 2014). Our analysis of cooperation between countries, institutes, and institutes, showed that academic cooperation can promote the development of clinical and academic research.
4.1 Mechanism of berberine on cancers
As shown in Figure 6B, BBR has been used in the treatment of lung cancer (#13), prostate cancer (#6), and non-small-cell lung cancer, and has been a research hotspot in recent years (Figure 6C). The mechanisms of action of BBR in cancer have also been studied and mainly consist of inhibiting migration and invasion, inducing apoptosis, arresting the cell cycle, inhibiting proliferation, and promoting autophagy in tumors.
4.1.1 Berberine can inhibit the migration and invasion of tumors
In clinical practice, most patients experience the invasion and migration of cancer cells from a single focus to other distant tissues. In 2015, research on migration and invasion became a hot topic (Figure 7B). A wide variety of migratory and invasion mechanisms are involved in cancer (Figure 6C). In triple-negative breast cancer cells, BBR reduces interleukin-8 (IL-8) expression by blocking the EGFR/MEK/ERK signaling pathway to prevent cell invasion and metastasis (Kim et al., 2018). BBR also affected cell migration and invasion in the human hepatoma HepG2 cell line by inhibiting the transforming growth factor (TGF-beta/Smad) signaling pathway (Du et al., 2021). Understanding cellular and molecular changes in these various migration/invasion programs will enable us to develop novel therapeutic approaches and provide insights into the spread of cancer cells.
4.1.2 Berberine can promote tumor cell apoptosis
Apoptosis was the main keyword in BBR-related research (Figure 7A) and the median time of the research boom occurred around 2016 (Figure 7C). Apoptosis is an evolutionarily conserved cell death system responsible for eliminating cells during embryonic development and maintaining organismal homeostasis (Singh et al., 2019). The pathways of apoptosis are divided into the exogenous pathway, endogenous pathway, caspase cascade reaction, and caspase effects. Many biochemical processes, such as a loss of mitochondrial membrane potential, release of cytochrome C into the cytoplasm, expression of Bcl-2 family and caspase family proteins, and cleavage of poly ADP ribose polymerase, induced apoptosis in the skin squamous cell carcinoma A431 cell line after treatment with BBR (Wartenberg et al., 2003). Moreover, BBR induced apoptosis involved in the caspase-related mitochondrial pathway compared to normal liver HL-7702 cells, which was mediated by adenosine monophosphate-activated protein kinase (AMPK) and reduced the survival of human hepatoma HepG2, SMMC-7721, and Bel-7402 cells (Mitani et al., 2001). To reach the goal of treatment, researchers are currently modeling small molecules with apoptosis-promoting protein activity, which renders cells susceptible to mitochondrial apoptosis and triggers apoptosis.
4.1.3 Berberine can block the cell cycle of tumor cells
Research on the effect of BBR on cell cycle arrest started around 2012 but was mainly concentrated in 2017 (Figure 7B). To some degree, changes in the cell cycle can either slow or accelerate cancer development. Previous studies demonstrated that BBR inhibits the growth of certain tumor cells by regulating their cell cycle. A study on the mechanism of BBR in breast cancer showed that the alkaloid BBR prevented breast cancer cells from entering the S phase and increased cancer cell sensitivity to treatment (Kim et al., 2010). In a human chondrosarcoma cell HBT-94 model, BBR activated the PI3K/Akt and p38 signaling pathways, increased the protein levels of p53 and p21, and triggered G2/M phase arrest, thus demonstrating anti-cancer effects (Eo et al., 2014). Gene stability is maintained by cell cycle arrest and cancer formation is significantly influenced by the incidence of cell cycle-regulated gene alterations. If DNA is damaged during a healthy cell cycle, the cell cycle stalls at a relevant checkpoint. Cell cycle arrest gives cells more time to repair damage, which lowers the likelihood of mutations and prevents tumor development. Therefore, targeting cell cycle checkpoints may significantly enhance cancer treatment.
4.1.4 Berberine can inhibit tumor proliferation
Numerous studies have reported that BBR regulates cell signal transduction by inhibiting tumor cell proliferation. As shown in Figure 7B, the keyword “tumor proliferation” mainly appeared in 2014. BBR inhibited the growth of human colon cancer cell lines Caco-2 and Lovo by reducing citrate synthase activity (Mantena et al., 2006). Moreover, BBR inhibited melanoma by increasing miRNA-582-5p and miRNA-188-5p levels and decreasing the expression of cell cycle-related proteins in melanoma A375 cells (Lin et al., 2005). Research on the mechanism associated with tumor proliferation mainly focuses on material metabolism; therefore, a deeper understanding of the molecular connections between cellular metabolism and growth regulation may ultimately result in more effective cancer treatments.
4.1.5 Berberine can induce autophagy of tumor cells
The cooperation network shown in Figure 7A shows that the keyword “autophagy” is closely connected with BBR. Autophagy, a type of programmed cell death, is crucial for preserving intracellular homeostasis. BBR induces autophagy by inhibiting the ERK1/2 signaling pathway in glioblastoma and further reduces temozolomide resistance (Qu et al., 2020). By inducing cytostatic autophagy and regulating the MAPK/mTOR/p70S6K and Akt signaling pathways, BBR inhibits the development of human gastric cancer cells both in vitro and in vivo (Zhang et al., 2020a). Despite substantial research on the regulation of autophagy by BBR in recent years, the precise mechanism remains unclear.
4.2 Applications of berberine
Figure 6 and Figure 7 list several conditions that can be regulated by BBR, including non-small-cell lung cancer, breast cancer, colorectal cancer, gut microbiota, and photodynamic therapy. Among these, the application of BBR in cancer has been the focus of research in recent years. The results are summarized in Table 8.
TABLE 8.
BBR applications.
| Application | Cell line | Effect | Reference |
|---|---|---|---|
| Non-small-cell lung cancer | A549, PC9, H1650, and H1299 | Activation of the p38α MAPK signaling pathway | Wu et al. (2021) |
| Induction of the protein expression of p53 and FOXO3a | |||
| Inhibit proliferation and induce apoptosis | |||
| A549, H157, H358, H460, H1299, H1975, and Lewis cells | Enhance tumor-infiltrating T-cell immunity and attenuate the activation of MDSCs and Tregs | Yang et al. (2021) | |
| Trigger PD-L1 degradation through the ubiquitin (Ub)/proteasome-dependent pathway | |||
| A549, NCI-H1299, and BEAS-2B | Suppression of epithelial–mesenchymal transition | Yin et al. (2022) | |
| Trigger cell cycle arrest | |||
| Suppression of HIF-1α expression | |||
| HCC827/AR, HCC827/AR0.5, HCC827/AR2, HCC827/ER, PC-9/AR, and PC-9/GR/AR | Act as a naturally existing MET inhibitor | Yu et al. (2020) | |
| Enhance induction of apoptosis through Bim elevation and Mcl-1 reduction | |||
| Breast cancer | HEK293, SMCC-7721, and ZR-75-30 | Decrease the expression of ephrin-B2 and its PDZ-binding proteins | Zhang et al. (2021a) |
| Downregulate the phosphorylation of VEGFR2 and downstream signaling members (AKT and Erk1/2) | |||
| Downregulate the expression of MMP-2 and MMP-9 | |||
| MCF-7 and MCF-7/MDR | Enhance sensitivity in drug-resistance breast cancer cells | Zhang et al. (2020a), Zhang et al. (2021b) | |
| Induce apoptosis | |||
| MDA-MB-231 and BT549 | Induce DSB | Zhang et al. (2020b) | |
| Increase the release of cytochrome c | |||
| Trigger the caspase-9-dependent apoptosis | |||
| Colorectal cancer | HT-29, SW480, and NIH3T3-Light2 cell lines | Suppression of the paracrine sonic hedgehog (SHH) signaling | Zhang et al. (2020c) |
| Inhibition of the secretion and expression of SHH protein | |||
| HCT116 and SW480 | Inhibit proliferation and induce G0/G1 phase arrest | Zhao et al. (2017) | |
| Knockdown of IGF2BP3 could suppress the PI3K/AKT pathway to inhibit cell proliferation and cycle transition | |||
| HCT-8, HCT-116, and HT-29 | Suppress lipogenesis via promotion of PLZF-mediated SCAP ubiquitination | Zhao et al. (2022) | |
| HCT116, SW48, RKO, Caco-2, SW480, and HT-29 | Suppression of DNA replication | Zheng et al. (2014) | |
| Gut microbiota | Brain neuron cells | Accelerate the production of L-DOPA by intestinal bacteria | Zheng et al. (2021) |
| P. mirabilis, S. boydii, and B. fragilis (intestinal bacterial strains) | Reduce the biosynthesis of TMAO by interacting with the enzyme/coenzyme containing (CutC) and (FMO) | Zhong and Song (2008) | |
| β-cells | Inhibit the biotransformation of DCA by Ruminococcus bromii | Zhuang et al. (2018) | |
| Photodynamic therapy | ACHN, 786-O, and HK-2 | Increase reactive oxygen species | [71] |
| Increase autophagy levels and apoptosis by caspase 3 activity | |||
| A375, M8, SK-Mel-19, and the cisplatin-resistant cell lines A375/DDP, M8/DDP, and SK-Mel-19/DDP | Activate the P38 MAPK signaling pathway | [72] |
4.2.1 Non-small-cell lung cancer
Figure 6C shows a research hotspot focused on non-small cell lung cancer. Several mechanisms by which BBR inhibits non-small cell lung cancer have been reported. For example, Zheng et al. (2014) reported that BBR induces apoptosis of NSCLC cells to prevent growth and is involved in activating the p38α MAPK signaling pathway and subsequent increased protein expression of p53 and FOXO3a. BBR plays also an anti-tumor role from the perspective of immunity, as it can specifically bind to glutamic acid 76 of constitutive photomorphogenic-9 signalosome 5 (CSN5) and inhibit the PD-1/PD-L1 axis through its deubiquitylation activity, leading to the PD-L1 ubiquitination and destruction (Liu et al., 2020). In addition, BBR and its derivatives are considered potential drugs for the treatment of NSCLC. Liu et al. (2021) demonstrated that the derivative of BBR, demethyleneberberine (DMB), exerts an anti-tumor effect leading to cell arrest and cellular senescence in NSCLC. In addition, BBR can be combined with other drugs such as osimertinib (Chen et al., 2022). These findings demonstrate that BBR exerts anti-tumor effects through various mechanisms.
4.2.2 Breast cancer
According to the keyword analysis (Figure 7A), “breast cancer” is also an aspect of BBR research. Ma et al. (2017) reported that BBR prevents the proliferation and migration of breast cancer ZR-75-30 cells by regulating ephrin-B2. BBR also increases chemosensitivity, reverses hypoxia-induced chemoresistance, and further induces apoptosis in breast cancer (Pan et al., 2017b; Pan et al., 2017c). Moreover, Zhao et al. (2017) reported that BBR inhibits triple-negative breast cancer. BBR induces caspase-9/cytochrome c-mediated apoptosis both in vitro and in vivo to inhibit the proliferation of TNBC cells. Thus, BBR has been used to treat breast cancer.
4.2.3 Colorectal cancer
BBR inhibits colorectal tumor development A previous study showed that BBR reduces paracrine sonic hedgehog (SHH) signaling, which in turn reduces colon cancer growth in vitro and in vivo (Shen et al., 2021). This revealed a novel molecular mechanism for the anti-cancer effects of BBR. BBR inhibits proliferation through cell cycle arrest-related pathways. BBR has been reported to induce G0/G1 phase arrest in colorectal cancer cells by downregulating the targeted gene IGF2BP3 (Zhang et al., 2020c). Moreover, BBR showed potential anti-migration and anti-invasion properties in cell lines including HCT-8, HCT-116, and HT-29. BBR reduces lipogenesis and the spread of colon cancer cells by promoting PLZF-mediated SCAP ubiquitination (Liu et al., 2022b). Meanwhile, several studies have reported on BBR combined with other drugs, for example, the combination with Andrographis to treat colorectal cancer (Zhao et al., 2022). BBR is well known as a potential drug for the treatment of colon cancer.
4.2.4 Gut microbiota
Figure 6C shows that cluster 11 (gut microbiota) is a current research hotspot. The gut microbiota includes a large number and a wide range of species that are interdependent and interact with the host. The occurrence, development, and prognosis of many human diseases are closely related to intestinal flora. In 2021, Wang et al. (2021b) introduced oral BBR to accelerate the production of L-dopa by intestinal bacteria. The L-DOPA produced by intestinal bacteria enters the brain through circulation and is converted to dopamine, significantly increasing the brain dopamine levels of mice and improving PD expression. Ma et al. (2022) demonstrated that oral BBR reduced the biosynthesis of trimethylamine-N-oxide (TMAO), an atherogenic metabolite derived from the gut microbiota in the intestine, by interacting with the enzyme/coenzyme containing choline trimethylamine lyase (CutC) and lutein monooxygenase (FMO) in the intestinal microbiota, thus playing a role in the treatment of atherosclerosis. In addition, the human intestinal microbiome is a promising target for the treatment of type 2 diabetes. BBR reduces blood sugar levels by inhibiting the biotransformation of DCA by Ruminococcus bromii (Zhang et al., 2020b). Intestinal microorganisms affect the occurrence and development of metabolic diseases by regulating the metabolism of sugars, lipids, and amino acids and are inextricably linked with diseases of the neuropsychiatric, cardiovascular, urinary, and other systems. Therefore, it is of great significance to study the correlation between intestinal flora and diseases for the prevention and treatment of diseases and the maintenance of human health.
4.2.5 Photodynamic therapy
BBR is well known for its anti-inflammatory, antioxidant, anti-diabetes, anti-obesity, and anti-cancer properties; however, little is known about its photosensitive properties, and it might serve as a new kind of photodynamic therapeutic agent. Our analysis of co-cited references (Figure 6C) showed that photodynamic therapy is a hotspot of current research in BBR. For example, a study that assessed the effects of BBR on PDT in renal cancer cell lines reported increased reactive oxygen species (ROS) levels after treatment with BBR associated with PDT, which was accompanied by increased autophagy levels and apoptosis due to caspase 3 activity (Lopes et al., 2020). Wang et al. (2021a) reported that a combination of cisplatin and BBR-PDT played a role in cisplatin-resistant melanoma cells. The experimental findings showed that mitochondrial apoptotic pathways that depend on caspases were the mode of cell death and that BBR photodynamic therapy modulated apoptosis by activating the P38 MAPK signaling pathway. The number of articles on BBR photosensitivity therapy is currently small, and most have focused on the treatment and application of cancer, which may suggest that photosensitivity therapy could provide a new method for cancer treatment.
BBR is a natural compound with great biological activity that is effective against various diseases. The literature review showed that increasing numbers of BBR derivatives have been created and used in disease research. BBR can be used as a combination drug in the study of drug-resistant cell lines and has shown significant effects. The ability of BBR to disrupt intracellular pathways and its intrinsic features has been the subject of numerous investigations in recent years. More importantly, BBR has been investigated as a curative medication in both animal models and human cell lines. However, there remain many issues to resolve; for example, BBR alone has not been tested in humans and it has weak water solubility, reduced oral absorption, and low bioavailability. Therefore, future studies should focus on the clinical use of BBR.
5 Conclusion
This study analyzed publications, research topics, research hotspots, and development trends of research in the field of BBR and tumors through systematic bibliometric analysis. Chinese researchers have produced the most publications; however, articles published in the United States ranked first in terms of average citations per article. The most prolific universities are China Medical University (Taiwan) and Sun Yat-sen University. The terms “mechanism,” “molecular docking,” and “oxidative stress” are now popular study topics related to BBR in tumors.
Funding Statement
This study was supported by the Shenzhen Natural Science Foundation (KCXFZ20201221173600001).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
All authors contributed significantly to the work that was published, whether it was in ideation, study design, data collection, analysis, etc. All authors also participated in writing, revising, or critically evaluating the article; gave their final approval for the published version; decided on the journal to which the article would be submitted; and agreed to be responsible for all aspects of the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Al-Bari M. A. A., Ito Y., Ahmed S., Radwan N., Ahmed H. S., Eid N. (2021). Targeting autophagy with natural products as a potential therapeutic approach for cancer. Int. J. Mol. Sci. 22, 9807. 10.3390/ijms22189807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Dubin R., Kim M. C. (2014). Emerging trends and new developments in regenerative medicine: A scientometric update (2000 - 2014). Expert Opin. Biol. Ther. 14, 1295–1317. 10.1517/14712598.2014.920813 [DOI] [PubMed] [Google Scholar]
- Chen C., Leydesdorff L. (2014). Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J. Assoc. Inf. Sci. Technol. 65, 334–351. 10.1002/asi.22968 [DOI] [Google Scholar]
- Chen Z., Vallega K. A., Chen H., Zhou J., Ramalingam S. S., Sun S.-Y. (2022). The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 175, 105998. 10.1016/j.phrs.2021.105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K., Guo Q., Yang W., Wang Y., Sun Z., Wu H. (2022). Mapping knowledge landscapes and emerging trends of the links between bone metabolism and diabetes mellitus: A bibliometric analysis from 2000 to 2021. Front. Public Health 10, 918483. 10.3389/fpubh.2022.918483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhari A. S., Mandave P. C., Deshpande M., Ranjekar P., Prakash O. (2019). Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 10, 1614. 10.3389/fphar.2019.01614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiuri B., Dhillon H., Butow P. N., Jansen J., Cox K., Jacquet J. (2013). Does assessing patients' expectancies about chemotherapy side effects influence their occurrence? J. Pain Symptom Manag. 46, 275–281. 10.1016/j.jpainsymman.2012.07.013 [DOI] [PubMed] [Google Scholar]
- Dalian (2008). Peoples R China, 11529–11532. [Google Scholar]
- Du H., Gu J., Peng Q., Wang X., Liu L., Shu X., et al. (2021). Berberine suppresses EMT in liver and gastric carcinoma cells through combination with TGFR regulating TGF-/smad pathway. Oxidative Med. Cell. Longev. 2021, 2337818. 10.1155/2021/2337818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo S.-H., Kim J.-H., Kim S.-J. (2014). Induction of G₂/M arrest by berberine via activation of PI3K/Akt and p38 in human chondrosarcoma cell line. Oncol. Res. 22, 147–157. 10.3727/096504015X14298122915583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X. J., Sureda A., Jafari S., Memariani Z., Tewari D., Annunziata G., et al. (2019). Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 9, 1923–1951. 10.7150/thno.30787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem S., Ali T. A., Akhtar S., Nisar S., Sageena G., Ali S., et al. (2022). Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. = Biomedecine Pharmacother. 150, 113054. 10.1016/j.biopha.2022.113054 [DOI] [PubMed] [Google Scholar]
- Hirsch J. E. (2005). An index to quantify an individual's scientific research output. Proc. Natl. Acad. Sci. U. S. A. 102, 16569–16572. 10.1073/pnas.0507655102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. S., Martin B. R. (1997). What is research collaboration? Res. Policy 26, 1–18. 10.1016/s0048-7333(96)00917-1 [DOI] [Google Scholar]
- Kim J. B., Yu J. H., Ko E., Lee K. W., Song A. K., Park S. Y., et al. (2010). The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine Int. J. Phytotherapy Phytopharm. 17, 436–440. 10.1016/j.phymed.2009.08.012 [DOI] [PubMed] [Google Scholar]
- Kim S., You D., Jeong Y., Yu J., Kim S. W., Nam S. J., et al. (2018). Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomedicine Int. J. Phytotherapy Phytopharm. 50, 43–49. 10.1016/j.phymed.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Koperska A., Wesołek A., Moszak M., Szulińska M. (2022). Berberine in non-alcoholic fatty liver disease-A review. Nutrients 14, 3459. 10.3390/nu14173459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M. S., Adki K. M. (2018). Marine natural products for multi-targeted cancer treatment: A future insight. Biomed. Pharmacother. = Biomedecine Pharmacother. 105, 233–245. 10.1016/j.biopha.2018.05.142 [DOI] [PubMed] [Google Scholar]
- Kuo C.-L., Chi C.-W., Liu T.-Y. (2004). The anti-inflammatory potential of berberine in vitro and in vivo . Cancer Lett. 203, 127–137. 10.1016/j.canlet.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Lacouture M., Sibaud V. (2018). Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am. J. Clin. Dermatology 19, 31–39. 10.1007/s40257-018-0384-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wang P., Guo W., Huang X., Tian X., Wu G., et al. (2019). Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 13, 6770–6781. 10.1021/acsnano.9b01346 [DOI] [PubMed] [Google Scholar]
- Lin S. S., Chung J. G., Lin J. P., Chuang J. Y., Chang W. C., Wu J. Y., et al. (2005). Berberine inhibits arylamine N-acetyltransferase activity and gene expression in mouse leukemia L 1210 cells. Phytomedicine Int. J. Phytotherapy Phytopharm. 12, 351–358. 10.1016/j.phymed.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Liu J., Huang X., Liu D., Ji K., Tao C., Zhang R., et al. (2021). Demethyleneberberine induces cell cycle arrest and cellular senescence of NSCLC cells via c-Myc/HIF-1α pathway. Phytomedicine Int. J. Phytotherapy Phytopharm. 91, 153678. 10.1016/j.phymed.2021.153678 [DOI] [PubMed] [Google Scholar]
- Liu L., Fan J., Ai G., Liu J., Luo N., Li C., et al. (2019). Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 52, 37. 10.1186/s40659-019-0243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Tang J., Chen S., Hu S., Shen C., Xiang J., et al. (2022a). Berberine for gastric cancer prevention and treatment: Multi-step actions on the Correa's cascade underlie its therapeutic effects. Pharmacol. Res. 184, 106440. 10.1016/j.phrs.2022.106440 [DOI] [PubMed] [Google Scholar]
- Liu Y., Fang X., Li Y., Bing L., Li Y., Fang J., et al. (2022b). Berberine suppresses the migration and invasion of colon cancer cells by inhibition of lipogenesis through modulation of promyelocytic leukemia zinc finger-mediated sterol-regulatory element binding proteins cleavage-activating protein ubiquitination. J. Pharm. Pharmacol. 74, 1353–1363. 10.1093/jpp/rgac026 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu X., Zhang N., Yin M., Dong J., Zeng Q., et al. (2020). Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 10, 2299–2312. 10.1016/j.apsb.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes T. Z., De Moraes F. R., Tedesco A. C., Arni R. K., Rahal P., Calmon M. F. (2020). Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. = Biomedecine Pharmacother. 123, 109794. 10.1016/j.biopha.2019.109794 [DOI] [PubMed] [Google Scholar]
- Ma S.-R., Tong Q., Lin Y., Pan L.-B., Fu J., Peng R., et al. (2022). Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 7, 207. 10.1038/s41392-022-01027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Zhu M., Zhang D., Yang L., Yang T., Li X., et al. (2017). Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomedicine Int. J. Phytotherapy Phytopharm. 25, 45–51. 10.1016/j.phymed.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Mantena S. K., Sharma S. D., Katiyar S. K. (2006). Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 5, 296–308. 10.1158/1535-7163.MCT-05-0448 [DOI] [PubMed] [Google Scholar]
- Mignani S., Rodrigues J., Tomas H., Zablocka M., Shi X., Caminade A.-M., et al. (2018). Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 47, 514–532. 10.1039/c7cs00550d [DOI] [PubMed] [Google Scholar]
- Mitani N., Murakami K., Yamaura T., Ikeda T., Saiki I. (2001). Inhibitory effect of berberine on the mediastinal lymph node metastasis produced by orthotopic implantation of Lewis lung carcinoma. Cancer Lett. 165, 35–42. 10.1016/s0304-3835(00)00710-2 [DOI] [PubMed] [Google Scholar]
- Mullard A. (2020). Addressing cancer's grand challenges. Nat. Rev. Drug Discov. 19, 825–826. 10.1038/d41573-020-00202-0 [DOI] [PubMed] [Google Scholar]
- Musbahi A., Rao C. B., Immanuel A. (2022). A bibliometric analysis of robotic surgery from 2001 to 2021. World J. Surg. 46, 1314–1324. 10.1007/s00268-022-06492-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastiuk K. L., Krolewski J. J. (2016). Opportunities and challenges in combination gene cancer therapy. Adv. Drug Deliv. Rev. 98, 35–40. 10.1016/j.addr.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz L. M. G., Lombardi P., Tillhon M., Scovassi A. I. (2014). Berberine, an epiphany against cancer. Mol. (Basel, Switz. 19, 12349–12367. 10.3390/molecules190812349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. L., Cui M., Yu X. T., Hua W. N. (2017). “How is CiteSpace used and cited in the literature? An analysis of the articles published in English and Chinese core journals,” in 16th International Conference on Scientometrics and Informetrics (ISSI), Oct 16-20 2017a Wuhan Univ (Wuhan, PEOPLES R CHINA: ISSI; ), 158–165. [Google Scholar]
- Pan Y., Shao D., Zhao Y., Zhang F., Zheng X., Tan Y., et al. (2017b). Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK- HIF-1α. Int. J. Biol. Sci. 13, 794–803. 10.7150/ijbs.18969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang F., Zhao Y., Shao D., Zheng X., Chen Y., et al. (2017c). Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J. Cancer 8, 1679–1689. 10.7150/jca.19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.-F., Ren S.-H., Shao B., Qin H., Wang H.-D., Li G.-M., et al. (2022). The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front. Immunol. 13, 931783. 10.3389/fimmu.2022.931783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Song X., Song Z., Jiang X., Gao X., Bai L., et al. (2020). Berberine reduces temozolomide resistance by inducing autophagy via the ERK1/2 signaling pathway in glioblastoma. Cancer Cell Int. 20, 592. 10.1186/s12935-020-01693-y [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rejhová A., Opattová A., Čumová A., SlíVA D., Vodička P. (2018). Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 144, 582–594. 10.1016/j.ejmech.2017.12.039 [DOI] [PubMed] [Google Scholar]
- Ruan H., Zhan Y. Y., Hou J., Xu B., Chen B., Tian Y., et al. (2017). Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene 36, 6906–6918. 10.1038/onc.2017.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z.-Q., Wang J., Tan W.-F., Huang T.-M. (2021). Berberine inhibits colorectal tumor growth by suppressing SHH secretion. Acta Pharmacol. Sin. 42, 1190–1194. 10.1038/s41401-020-00514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Letai A., Sarosiek K. (2019). Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 20, 175–193. 10.1038/s41580-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Hao J., Fan D. (2020). Biological properties and clinical applications of berberine. Front. Med. 14, 564–582. 10.1007/s11684-019-0724-6 [DOI] [PubMed] [Google Scholar]
- Sun Q., Gong T., Liu M. L., Ren S., Yang H., Zeng S., et al. (2022a). Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine 94, 153805. 10.1016/j.phymed.2021.153805 [DOI] [PubMed] [Google Scholar]
- Sun Q., Yang H., Liu M. L., Ren S., Zhao H., Ming T. Q., et al. (2022b). Berberine suppresses colorectal cancer by regulation of Hedgehog signaling pathway activity and gut microbiota. Phytomedicine 103, 154227. 10.1016/j.phymed.2022.154227 [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J. Clin. 71, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Szakacs G., Paterson J. K., Ludwig J. A., Booth-Genthe C., Gottesman M. M. (2006). Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234. 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- Van Eck N. J., Waltman L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Gong Q., Song C., Fang J., Yang Y., Liang X., et al. (2021a). Berberine-photodynamic therapy sensitizes melanoma cells to cisplatin-induced apoptosis through ROS-mediated P38 MAPK pathways. Toxicol. Appl. Pharmacol. 418, 115484. 10.1016/j.taap.2021.115484 [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong Q., Ma S.-R., Zhao Z.-X., Pan L.-B., Cong L., et al. (2021b). Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota. Signal Transduct. Target. Ther. 6, 77. 10.1038/s41392-020-00456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenberg M., Budde P., De MareéS M., GrüNHECK F., Tsang S. Y., Huang Y., et al. (2003). Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional Chinese medicine. Laboratory Investigation; a J. Tech. Methods Pathology 83, 87–98. 10.1097/01.lab.0000049348.51663.2f [DOI] [PubMed] [Google Scholar]
- Wu H., Zhou Y., Wang Y., Tong L., Wang F., Song S., et al. (2021). Current state and future directions of intranasal delivery route for central nervous system disorders: A scientometric and visualization analysis. Front. Pharmacol. 12, 717192. 10.3389/fphar.2021.717192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-H., Zhang L.-J., Luo M.-J., Luo S., Gong Y.-Y., Chen T. (2021). Research progress in mechanism of berberine's antitumor action. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Materia Medica 46, 2449–2455. 10.19540/j.cnki.cjcmm.20210209.601 [DOI] [PubMed] [Google Scholar]
- Yin H., Zhang F., Yang X., Meng X., Miao Y., Hussain M. S. N., et al. (2022). Research trends of artificial intelligence in pancreatic cancer: A bibliometric analysis. Front. Oncol. 12, 973999. 10.3389/fonc.2022.973999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Li Y. J., Zhang Z. H., Gu Z. C., Zhong H., Zha Q. F., et al. (2020). A bibliometric analysis using VOSviewer of publications on COVID-19. Ann. Transl. Med. 8, 816. 10.21037/atm-20-4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Song L., Xu L., Fan Y., Wang T., Tian W., et al. (2021a). Knowledge domain and emerging trends in ferroptosis research: A bibliometric and knowledge-map analysis. Front. Oncol. 11, 686726. 10.3389/fonc.2021.686726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang X., Cao S., Sun Y., He X., Jiang B., et al. (2020a). Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. = Biomedecine Pharmacother. 128, 110245. 10.1016/j.biopha.2020.110245 [DOI] [PubMed] [Google Scholar]
- Zhang S.-W., Zhou J., Gober H.-J., Leung W. T., Wang L. (2021b). Effect and mechanism of berberine against polycystic ovary syndrome. Biomed. Pharmacother. = Biomedecine Pharmacother. 138, 111468. 10.1016/j.biopha.2021.111468 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gu Y., Ren H., Wang S., Zhong H., Zhao X., et al. (2020b). Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 11, 5015. 10.1038/s41467-020-18414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu X., Yu M., Xu M., Xiao Y., Ma W., et al. (2020c). Berberine inhibits proliferation and induces G0/G1 phase arrest in colorectal cancer cells by downregulating IGF2BP3. Life Sci. 260, 118413. 10.1016/j.lfs.2020.118413 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Jing Z., Lv J., Zhang Z., Lin J., Cao X., et al. (2017). Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo . Biomed. Pharmacother. = Biomedecine Pharmacother. 95, 18–24. 10.1016/j.biopha.2017.08.045 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Roy S., Wang C., Goel A. (2022)., 15. Pharmaceuticals (Basel, Switzerland), 262. 10.3390/ph15030262 A combined treatment with berberine and andrographis exhibits enhanced anti-cancer activity through suppression of DNA replication in colorectal cancer Pharmaceuticals [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Tang Q., Wu J., Zhao S., Liang Z., Li L., et al. (2014). p38α MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J. Exp. Clin. Cancer Res. CR 33, 36. 10.1186/1756-9966-33-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhao Y., Jia Y., Shao D., Zhang F., Sun M., et al. (2021). Biomimetic co-assembled nanodrug of doxorubicin and berberine suppresses chemotherapy-exacerbated breast cancer metastasis. Biomaterials 271, 120716. 10.1016/j.biomaterials.2021.120716 [DOI] [PubMed] [Google Scholar]
- Zhong Q., Song J. (2008). “The developing trend research of knowledge management overseas based on word frequency analysis,” in 4th International Conference on Wireless Communications, Networking and Mobile Computing (IEEE; ). [Google Scholar]
- Zhuang W., Li T., Wang C., Shi X., Li Y., Zhang S., et al. (2018). Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic. Biol. Med. 121, 127–135. 10.1016/j.freeradbiomed.2018.04.575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.