Abstract
Despite viral suppression with antiretroviral therapy (ART), people with human immunodeficiency virus (HIV) continue to experience central nervous system (CNS) complications, primarily in the form of mild cognitive impairment and mental health disorders (eg, depression, anxiety, other neuropsychiatric problems). The multifactorial pathogenesis and heterogeneity of mechanisms likely underlying CNS complications must be addressed in the development of preventive interventions and effective treatments. The biotyping approach has previously been useful to define phenotypes of other CNS diseases based on underlying mechanisms and could be translated to the field of neuroHIV. The purpose of the Biotype Workshop series, and the Virology, Immunology and Neuropathology Working Group in particular, is to capitalize on current and new technologies and guide future research efforts using the wealth of available immunological, virologic, and neuropathological data collected from people with HIV on and off ART.
Keywords: HIV-associated cognitive disorder, HAND, neuroHIV, biomarkers, inflammation, biotype
Human immunodeficiency virus type 1 (HIV) can be detected in the central nervous system (CNS) days after infection [1] and is associated with cognitive, neurological, and mental health complications [2]. Antiretroviral therapy (ART) has dramatically reduced CNS morbidity; however, many people with HIV (PWH) continue to experience CNS complications, primarily in the form of mild cognitive impairment and mental health disorders (eg, depression, anxiety, other neuropsychiatric problems) [3–5]. Understanding the complex relationship between HIV infection and neuropathogenesis remains a research priority even though ART that suppresses HIV replication reduces how HIV directly and indirectly affects the CNS.
Decades-long research efforts have not generated a complete understanding of the mechanisms by which HIV infection generates and maintains CNS complications during ART, and no effective therapies exist to reverse these complications. Inflammation has long been viewed as one of the primary drivers of CNS complications. While an early-stage study aimed at reducing monocyte activation in the CNS showed promise for improving cognition in PWH [6], several clinical trials aimed at reducing neuroinflammation in ART-treated PWH generated minimal or no improvement in cognition [7, 8]. Such findings may be explained by the following, not mutually exclusive, possibilities: (1) neuroinflammation is not the exclusive driver of adverse CNS outcomes; (2) clinical trials included interventions that were unable to sufficiently inhibit the type of inflammation responsible for CNS complications; (3) participants had accumulated irreversible CNS damage prior to the intervention (so-called legacy effect); (4) these trials only examined differences in specific subgroups, which might not be representative of the entire populations of PWH; and/or (5) most studies focused on global cognition and it is possible that certain drugs influence some, but not all, domains. These findings highlight the need for new approaches for studying neuroHIV.
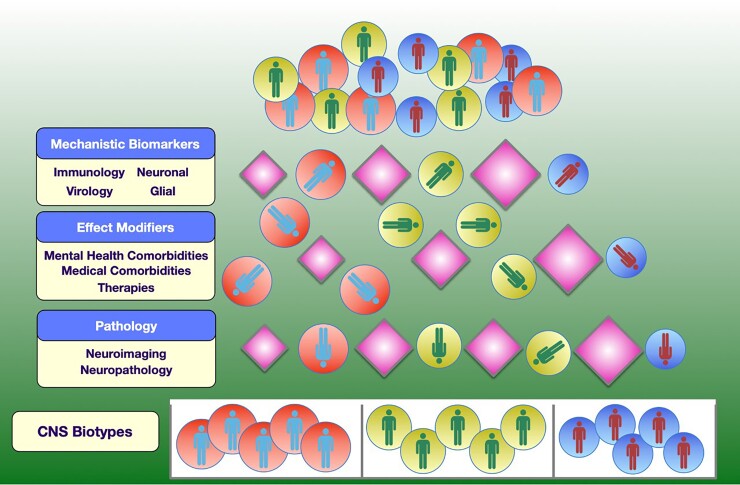
There is growing interest in using a biotyping approach to better understand CNS complications in the setting of treated HIV infection (Figure 1). Biotypes are defined as groups of individuals who are similar based on a combination of biomarkers and linked to specific adverse CNS outcomes and/or underlying mechanisms. This approach has long been used in the field of psychiatry to define biologically distinct disease phenotypes based on underlying mechanisms, and to capture such heterogeneity [9–13]. For example, one of the first large-scale National Institute of Mental Health–funded studies was the Bipolar Schizophrenia Network on Intermediate Phenotypes Consortium Study, which drew on neurobiological heterogeneity rather than clinical diagnoses to delineate subgroups independent of their clinical phenomenology [14]. Similar methods can be applied to neurodegenerative disease, for example, Alzheimer disease, in which biological changes such as amyloid deposition may occur decades before cognitive symptoms manifest [15]. Biotyping has led to remarkable changes in the neurodegenerative field. In June 2021, for the first time in 18 years, a new therapy, aducanumab, was approved for Alzheimer disease, and several more are in the pipeline for approval from the United States Food and Drug Administration. Other fields where biotyping approach have been successfully implemented outside HIV include cancer-related cognitive impairment [16], autism spectrum disorder [17], and depression-predicted response to treatment [18].
Figure 1.
It is likely that different neuropathologies in people infected with human immunodeficiency virus may be generated by different mechanisms and require different treatment strategies. Identifying groups of individuals with similar mechanistic biomarkers, effect modifiers, and neuropathologies may identify people whose pathologies are driven by similar mechanisms that require similar treatment strategies. Abbreviation: CNS, central nervous system.
Such an approach could include relevant virologic, inflammatory, immune activation, and tissue damage biomarkers, as well as pathologic findings, with the goal of identifying PWH with common mechanisms underlying their neuropathogenesis that might benefit from a specific adjunctive therapy in addition to suppressive ART. Here, we explore immunologic, virologic, and neuropathologic data that could be incorporated into biotype analyses, to guide future research efforts in this setting.
SOLUBLE AND CELLULAR BIOMARKERS (INFLAMMATION, IMMUNE ACTIVATION, AND NEURONAL INJURY)
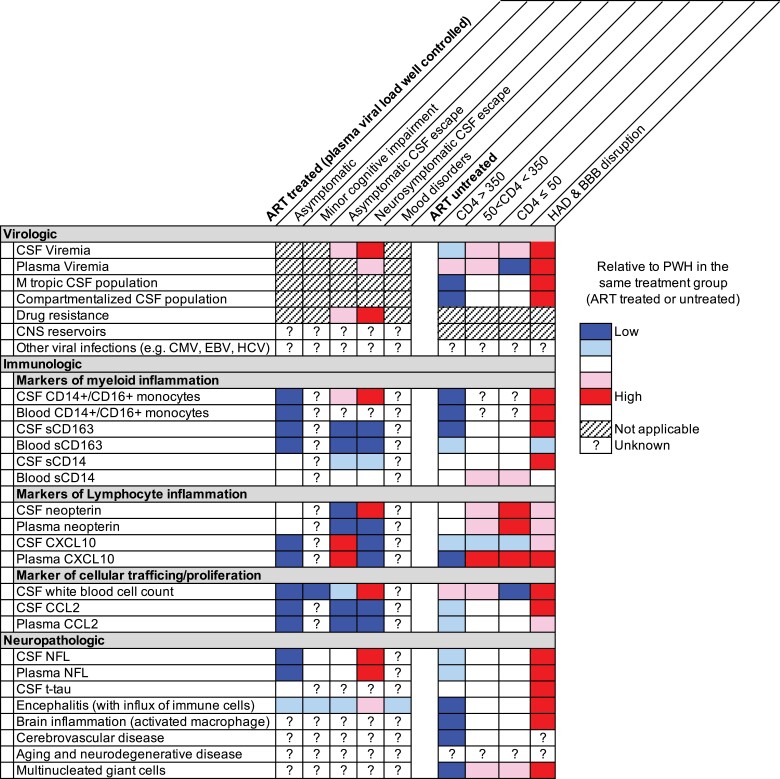
The need to identify immune biomarkers associated with lasting CNS damage is urgent. Ongoing viral replication in the CNS generates elevated levels of soluble and cellular biomarkers, which decline after ART initiation [19, 20]. Such biomarkers typically do not return to normal despite ART initiation [21–23] but can reach near-normal levels in PWH treated during the earliest phases of HIV infection [24]. Whether this is because they experience less damage related to HIV replication, retain better immune function [24–26], have a smaller CNS reservoir [25, 26], or/and some other factor is unknown. While the specific molecular pathways controlling biomarkers in the CNS are unresolved, biomarkers are often generated by specific cell types and can provide hints about the mechanisms that influence their expression. In this section, we describe soluble and cellular biomarkers that may be used in biotype analyses (see also Figure 2).
Figure 2.
Virologic, immunologic and neuropathologic biomarkers that may be informative for biotype analyses. This subset includes biomarkers that vary greatly across pathologic states associated with untreated and/or treated infection. The utility of these biomakers is difficult to assess due to a lack of relevant data and/or nonstandardized data collection.
Soluble biomarkers in plasma and cerebrospinal fluid (CSF) have many features that make them attractive for incorporation in biotype analyses including lower costs than other assays (eg, imaging and viral sequencing) and accessibility in clinical settings. Most importantly, the data linking soluble biomarkers with neuropathogenesis are substantial. For example, CSF neopterin, a biomarker of myeloid activation, is elevated during untreated HIV infection and reaches the highest levels during HIV-associated dementia (HAD) [27]. Similarly, ART-treated, virologically suppressed PWH with mild cognitive impairment and without significant comorbidities had higher levels of both neopterin and neurofilament light (NFL) in CSF than PWH without cognitive impairment [28]. Extensive data also show that elevation of 2 other plasma biomarkers of myeloid activation, soluble (s)CD163 and sCD14, in plasma are associated with cognitive impairment [29]. The association between sCD163 levels and cognitive impairment remains strong after ART initiation, with sCD163 levels declining in people who remain cognitively unimpaired but remaining elevated in impaired people [30]. Importantly, sCD163 and sCD14 are small components of a larger set of relevant biomarkers (eg, MMP9 [matrix metalloproteinase 9], CCL2, MCP-1 [monocyte chemoattractant protein 1], TNFα [tumor necrosis factor–α], CXCL10, sCD14, sCD163, and sCAMs [soluble vascular cell adhesion molecule]) that have been proposed to be associated with biological mechanisms that influence CNS complications in PWH [20], and might define certain CNS biotypes.
The use of biomarkers of lymphoid and myeloid activation is complicated by the fact that they do not always change in tandem across stages of untreated HIV infection and after ART initiation, likely reflecting the divergent biology of myeloid and lymphoid cells and raising the possibility that these cells contribute to CNS pathogenesis in different ways [20]. For example, biomarkers of lymphoid activation correlate closely with HIV RNA levels in CSF, whereas markers of myeloid activation are better correlated with neuronal injury (eg, NFL levels in CSF) [20]. A growing body of evidence from human studies suggests a link between monocytes and poor neurological outcomes in humans [31–33]. Of particular interest is the intermediate (activated) CD14+CD16+ monocyte subset that develops from classical monocytes [34] and represents <10% of total monocytes [35]. Consistent with them having a role in inflammation and T-cell activation, intermediate monocytes have been shown to express higher levels of antigen presentation molecules than other monocyte subsets [36, 37] and produce high levels of inflammatory cytokines when stimulated [35]. A study examining 2 cohorts of virally suppressed women with HIV found that higher proportions of intermediate monocytes in the blood were associated with worse global cognitive function, executive function, and processing speed, and also predicted neurocognitive impairment 12 months later [33]. Interestingly, expression of CCR2 was significantly increased on peripheral intermediate monocytes in individuals with HIV-associated cognitive disorder (HAND) compared to PWH with normal cognition, irrespective of ART status, viral load, and current or nadir CD4+ T-cell count [38, 39]. A single-arm, 24-week, open-label clinical trial of cenicriviroc, a dual CCR2 and CCR5 antagonist, was associated with improved neurocognitive performance and decreased plasma markers of myeloid activation in virally suppressed PWH [6]. These data potentially link changes in myeloid activation to cognitive performance, although the study was small and not randomized.
The mechanistic link between activated monocytes and HAND remains unclear. Studies of humans have observed that HAND is associated both with the proportion of activated monocytes in the blood [32] and with the proportion of those cells that are HIV infected [31]. Similarly, studies of nonhuman primates (NHPs) suggest that the effect of intermediate monocytes on cognition may be due to them facilitating viral seeding of the brain [40–43], but more recent studies in NHPs [44] and humans [45, 46] suggest that HIV is primarily trafficked into the CNS by infected CD4+ T cells. The importance of CD4+ T cells in seeding the brain and generating neuropathogenesis is further supported by a study observing that 12 weeks after initial simian human immunodeficiency virus (SHIV) infection, SHIV was detected in CD4+ T cells but not in other cell types in the brain [47].
We now appreciate that T cells likely contribute to the neuropathogenesis of HIV in many ways. This paradigm shift is related to the realization that, although T-cell entry into the CNS is generally restricted [48, 49], high numbers of T cells can be observed in untreated PWH during acute infection [50, 51], in the setting of CD8+ T-cell encephalitis [52] and in people with HAD [53], but also in treated PWH with suppressed HIV RNA in plasma but with symptomatic [54, 55] or asymptomatic CSF escape [56]. Furthermore, the concentration of HIV-infected cells [57] in the CSF of ART-treated PWH is associated with neurocognitive impairment. The mechanisms by which T cells contribute to neuropathogenesis are likely multiple. During CSF escape, CD4+ T cells are often the primary source of HIV in the CSF [56, 58]. In contrast, during CD8+ T-cell encephalitis, CD8+ T cells in the brain create a highly inflammatory environment [52]. These results indicate that T cells can influence neuropathogenesis both by producing HIV and contributing to inflammation.
Based on published data, 3 non–mutually exclusive “immune vector models” have been proposed to contribute to the natural history of HIV infection [20]. The lymphocyte vector model develops during primary HIV infection and increases as blood CD4+ T-cell count declines, reaching a peak in the midrange of blood CD4+ T-cell count, then falling as the counts decline further [20]. This pattern mirrors leukocyte and HIV RNA dynamics in the CSF. The macrophage vector model involves a gradual increase in sCD14, MCP-1, and neopterin concentrations with falling CD4+ T-cell count [20]. This vector appears to be associated with a noninflammatory type of CNS injury. The mixed vector model involves disruption of the blood-brain barrier (BBB), resulting in increased concentration of many inflammatory biomarkers. While the cause of disruption is unclear, this model is associated with an increase in all sets of biomarkers and with more severe CNS injury.
Biomarkers of neuronal damage are also of interest. For example, elevated NFL, reflecting injury to axons, is detected in the CSF during primary HIV infection and in untreated PWH with low CD4+ T-cell counts, but rarely during treated HIV infection with higher CD4+ T-cell counts [22, 59–61]. Very high levels of NFL are found in PWH with HAD [22, 62], and elevated levels can be found years before manifestation of dementia symptoms and often correlate with markers of immune activation [63]. Although NFL decreases after ART initiation, levels remain slightly elevated compared to controls without HIV [60, 64, 65]. Levels of NFL in CSF are very sensitive to ongoing neuronal injury, both in untreated PWH with HAD and those on ART with symptomatic CSF escape; NFL levels measured in plasma correlate with those in the CSF in the absence of ART, but more studies are needed to determine if they also decline in response to ART [66–68]. Both CSF and plasma NFL measures might be useful in biotype analysis.
In conclusion, neurocognitive impairment in untreated people likely involves systemic and local immune activation and neuronal damage, but we do not know yet which biomarkers (or vectors) might play the most important role in pathogenesis and at what stage of disease. Markers of myeloid activation seem to be particularly relevant in the context of CNS dysfunction. Importantly, these associations seem to persist regardless of HIV RNA and CD4+ T-cell count, but no consistent pathogenic association has yet been established between neuroinflammation and neurocognitive impairment in ART-suppressed PWH. Because sCD163 and sCD14 in plasma, as well as neopterin and MCP-1 in CSF, have been linked to neurocognitive impairment across several studies, they may be valuable targets for mitigating poor outcomes in neuroHIV, but they are not specific to the CNS. While these markers are useful for correlating with different diseases, their utility for biotyping remains unclear in the setting of HIV. Finally, emerging single-cell and spatial omics technologies and epigenetic tools for characterizing the immune state of the CNS can be applied to blood, CSF, and brain tissue, and discoveries from these methods should shed further light on neuroimmune pathogenesis and neuronal circuitry in PWH that will further inform biotypes in PWH [69, 70]. Going forward, researchers should identify biomarkers to facilitate a precision medicine approach that stratifies individuals into subgroups based on their unique immune and injury signatures, which might inform underlying mechanisms and therapeutic targets.
VIRAL NEUROPATHOGENESIS
Detectable HIV RNA in the CSF is a ubiquitous feature of untreated HIV and a defining feature of several pathogenic states affecting PWH on ART [71, 72]. These include neurosymptomatic CSF viral escape [54, 55], secondary CSF escape [73], and HIV encephalitis [74]. A mechanistic link between HIV viremia and neuropathogenesis is implied by the fact that these pathogenic states often resolve when ART is initiated that suppresses both systemic and CSF replication [54, 55, 75]. Recent studies [45, 57] suggest that even when PWH are ART suppressed, the presence of HIV-infected cells in the CSF is associated with impaired cognition and/or neuroinflammation [57]. However, ART intensification studies have failed to reduce intrathecal immune activation and residual low-level viral load in CSF in ART-suppressed PWH, suggesting that this is not due to ongoing HIV replication in the CNS [76, 77]. This lack of viral replication is further supported by the fact that drug-resistant variants rarely evolve in the CNS during ART. In the absence of ART, severe cognitive impairment is associated with extensive replication in the CNS [53], but the connection between mild impairment and viral replication has not been established. HIV replication in the CNS is influenced by several parameters including CSF white blood cells, tropism of HIV variants, drug concentration in the CNS, drug resistance mutations, and the presence of CNS coinfections (Figure 2). These variables are attractive candidates for incorporation into biotype analyses aimed at better characterizing the relationship between HIV replication and neuropathogenesis.
Sustained replication in the CNS (CSF or brain cells) can be identified based on the presence of viral lineages in the CNS that are genetically distinct (compartmentalized) from lineages in the blood [78]. Sequence analyses of virus in the CNS of untreated people with HAD [53] frequently observe viral lineages that are genetically distinct from those in the blood; such lineages are less frequent in early infection but emerge in some PWH within the first year after HIV transmission [51]. Studies of compartmentalization are typically performed by sequencing viral RNA or DNA from the blood and CNS (CSF or brain tissue) of untreated people [79]. Similarly, viral RNA can be sequenced from people on ART and experiencing CSF escape. Such analyses have observed that viral drug resistance is often found in the setting of neurosymptomatic CSF escape [55, 80] and has also been observed in asymptomatic CSF escape [56]. Drug concentrations can be lower in the CNS, with some drugs being particularly poor at crossing the BBB [81, 82]. This creates a situation in which partially drug-resistant variants may replicate in the CNS despite being well suppressed in the periphery (ie, CSF escape). More recently, sequence analyses have examined viral DNA in CSF cells and brain tissue collected from individuals on suppressive ART [83].
An additional potential consequence of sustained replication in the CNS is a shift in tropism from T cells to macrophages. The blood and lymphoid organs typically contain a high frequency of CD4+ T cells, and viruses isolated from the blood have the nearly universal ability to efficiently infected CD4+ T cells and inefficiently infect myeloid lineage cells (ie, T-cell tropism [84–87]). In contrast, in the CNS where CD4+ T cells are typically rare and myeloid lineage cells are common, some HIV variants have been observed to be well-adapted to infecting myeloid lineage cells (ie, macrophage-tropic) [53, 88–93]. Most of these macrophage-tropic lineages have been observed in untreated people late in disease with high viral loads and severe neurologic involvement [53, 88, 90, 94–96]. In contrast, variants in the CSF early in infection typically have a poor to moderate ability to infect macrophages [51] and do not reach the level of true macrophage-tropic variants from the CNS of people with HAD. The evolution of macrophage tropism has been shown to also generate variants with an increased ability to infect cells expressing a low density of CD4 [53, 89, 92, 93], making it possible to assess macrophage tropism using a cell line with inducible levels of CD4 [97]. Astrocytes are another cell type within the brain that has been reported to be infected by HIV, typically via cell-to-cell contact with HIV-infected T cells (reviewed in [98, 99]), and observed to produce virus after cytokine stimulation [100–103]. However, there is also evidence that HIV-1 detected within astrocytes may be due to phagocytosis rather than infection [104].
In ART-suppressed PWH, HIV-1 may continue to contribute to neuropathogenesis by producing HIV proteins. Currently available ART regimens can prevent the formation of infectious viral particles but cannot prevent formation of viral transcripts and proteins. Surprisingly, in a subset of PWH, the intranuclear protein Tat can be detected in the CSF even when virus cannot be detected in blood or CSF [105]. Furthermore, HIV trans-activating region sequences and Tat protein have been detected in exosomes derived from the CSF of patients on long-term ART [106]. The detection of Tat in CSF has also been associated with cognitive impairment [106].
One of the most poorly understood factors thought to contribute to HIV neuropathogenesis is the presence of other infectious agents in periphery or the CNS and the accompanying lymphocytes. Herpes zoster infection in the periphery has been associated with neuropathogenesis and elevated CSF white blood cells in untreated people [107] and in people on ART with CSF escape [73]. Similarly, measures of anti-cytomegalovirus immunoglobulin G in blood, prior systemic syphilis infection, and pulmonary tuberculosis have all been associated with cognitive dysfunction and/or depression in the setting of HIV [108–111]. It is uncertain whether neuropathogenesis in these conditions relates to heightened systemic immune activation with secondary CNS effects, or presence of various pathogens in the CNS. The use of next-generation sequencing may present new opportunities for addressing this issue and incorporating the resulting data in biotype analyses.
Variables relating HIV replication and/or persistence with neuropathogenesis (Figure 2) present several challenges for incorporation into biotype analyses. First, all require CSF or brain tissue. Second, sequencing analyses of compartmentalization and drug resistance are limited by the quality of sequences available. Thus, sequencing data incorporated into biotype analyses will require metadata characterizing how the data were generated and extensive quality assurance. Third, given that the genetic determinants of macrophage tropism have not been identified (reviewed by [112]), assessment requires endpoint dilution polymerase chain reaction, cloning of full length viral envs or genomes, and assessment of viral entry into macrophage or a surrogate low CD4+ T-cell line—a set of steps unlikely to be performed on a grand scale. Finally, detection of HIV proteins and transcripts is still a research tool not available in clinical laboratories and is not widely performed. These issues will need to be considered to incorporate some types of virologic data into biotype analyses. In contrast, other virologic variables such as the concentration of viral RNA and HIV-infected cells in the blood and CSF and the presence of other viral infections may be assessed on a wide range of participants using highly standardized methods.
INCORPORATING PATHOLOGY IN HIV BIOTYPING
Modern clinical practice iteratively integrates laboratory-based observations with clinical phenotypes to define appropriate diagnostic categories. Similarly, an iterative process involving neuropathologic study has informed scientific knowledge of HIV in the brain since the inception of the pandemic. For example, neuropathology demonstrated that only half of PWH who died with dementia had HIV encephalitis, suggesting that more research was needed to connect clinical phenotypes with underlying neuropathologic findings [113]. Subsequently, analysis of CD68+ macrophage accumulation in brain tissue found that cognitive performance was correlated with the quantity of infiltrating brain macrophages in the pretherapeutic era [114]. However, the mechanistic connection between infected macrophages and neurodegeneration remained elusive. Further studies showed that HIV neuropathogenesis is much more complex including the release of cell-derived neurotoxic molecules, the breakdown of the BBB, glial cell activation, release of cytokines and chemokines, and neurodegeneration [2, 115].
Importantly, the neuropathology of HIV is (likely) different in PWH with ART-mediated viral suppression. A large study of 589 brains collected in the National NeuroAIDS Tissue Consortium found that HAND was associated with a variety of nonspecific, noninfectious pathologies and minimal nondiagnostic changes, suggesting that histologic tools centered on virally induced, grossly inflammatory pathologies, so useful in earlier stages of the pandemic, did not have the same efficacy in elaborating the neuropathogenesis of HAND in the current era of ART [116].
Nevertheless, neuropathology is still important to the understanding of HAND neuropathogenesis, as it can reveal important relationships between HAND, HIV, and an increasingly prevalent spectrum of other brain pathologies and smaller-scale neuroimmune perturbations that cannot be directly visualized by clinical means. An example of this is seen in our understanding of the relationship between HIV infections and large-artery cerebrovascular disease, as arterial walls in the circle of Willis are not easily visualized by neuroradiology [117, 118]. For example, increased wall thickness predicted decreased global t-scores and decrements in several cognitive domains in PWH [119]. This implicates that arteriosclerosis is an important contributor to cognitive outcomes in the ART era and is associated with traditional risk factors such as hypertension and diabetes, as well as exposures to protease inhibitor–based ART [120, 121]. Another important pathological feature to consider is amyloid deposition, a phenomenon for which there are excellent premortem manners of investigation, including positron emission tomography (PET) ligand studies and well-developed biomarkers [122–124]. In one study, the earliest cortical deposition of amyloid was better predicted by duration of HIV than by age, suggesting that HIV-associated accelerated aging and brain biologic age are more important determinants of incipient Alzheimer-type neurodegeneration than simple chronological age [125]. The mechanisms by which HIV leads to increased amyloid deposition involved inhibition of its breakdown, increased production, and increased aggregation when complexed with HIV-Tat protein.
In summary, human tissue analysis offers highly sensitive and specific diagnostics, allows detection and more specific localization of diverse pathologies, and enables stronger querying of pathogenesis and mechanisms underlying various biotypes. This is important since blood and even CSF are imperfect windows into the brain and direct visualization of brain tissue is currently the only way to confirm our observations. While usually available only at the time of death, and thus subject to the typical biases of autopsy populations, it remains a crucial tool to refine existing biotypes, albeit in a retrospective manner. New developments in high-resolution mapping of brain cell composition and function using single-nucleus and single-cell omics approaches hold promise to reveal novel cell types, targets of infection, and perturbations in neuronal circuitry underlying neuroHIV, with large-scale efforts underway (https://scorch.igs.umaryland.edu/). Furthermore, neuroimaging using PET ligands for detection of viral reservoirs, glial cell activation, synaptic density, amyloid and tau, neurotransmitter receptors such as dopamine and serotonin, high-resolution magnetic resonance imaging techniques, and novel methods of CSF analyses provide hope that the neuropathology of the brain might be studied by corollary studies available in living humans.
CONCLUSIONS
As part of this workshop, the Virology, Immunology and Neuropathology Working Group has identified a list of immunologic, virologic, and neuropathological biomarkers that could be used to create biotypes in the field of neuroHIV (Figure 2). These efforts will require the development of new, highly quantitative methods, coordination of biospecimen collection and processing procedures, and standardized data generation to identify biotypes that are robust and reproducible and that distinguish varying neurobiology between diverse populations. These biotypes might map onto our existing understanding of CNS outcomes (eg, cognitive impairment, depression), and likely will be influenced by many factors, such as source of sampling, stage of infection, treatment status, race/ethnicity, age, sex/gender, comorbidities, and many more. These unique biotypes should be continuously refined as new knowledge becomes available.
Importantly, factors other than HIV need to be carefully considered and are difficult to exclude in many studies, unless appropriate controls are included (ie, participants who have similar risk and lifestyle factors). For example, a study including PWH with suppressed plasma HIV RNA and well-matched controls without HIV found similar levels of myeloid activation in blood, suggesting that factors other than HIV drive myeloid activation and inflammation and may thereby contribute to CNS complications [126]. Likewise, people without HIV on preexposure prophylaxis with high-risk sexual behavior often have increased levels of inflammatory biomarkers in blood and CSF [127], suggesting that lifestyle factors or ART exposure may explain some of the findings in treated PWH. Thus, the use of appropriate controls (with similar risk factors and demographic profiles) will be crucial.
Biotypes should be reproducible across cohorts and longitudinally within an individual and creating platforms for data harmonization and standardization across studies (which include source of samples, various assays, platforms, protocols) will be important. Data transparency is also important, and all data should be made publicly available. This is particularly important for multiomics data. Having reference laboratories would be a good practice to standardize assays and serve as repository of reagents. Finally, all neuroHIV-related biotype data should incorporate the guiding principles of findability, accessibility, interoperability, and reusability (FAIR) to allow optimum reuse of the data.
Although the biotyping approach may prove to enhance diagnosis and treatment decisions, some challenges need to be considered that might hinder its clinical translation. These include the clinical diversity of mental health disorders, the technical complexity, costs, and the need for specialized training for laboratory staff, in addition to ethical concerns such as protecting the privacy and security of participants’ data and maintaining health equity.
The primary challenge is determining whether these individuals represent distinct biotypes with distinct underlying mechanisms. Examination of individuals across a range of suppression levels reveals the range of possible phenotypes. Unsuppressed individuals also show a range of phenotypes that may result from distinct underlying mechanisms.
In conclusion, understanding the impact of HIV on cognition and mental health from a personalized, brain-based perspective could lead to the development and implementation of maximally effective intervention strategies. Additionally, better understanding the impact of HIV in this way could provide crucial insights on the ways in which it affects the trajectory of individuals’ mental health and cognitive functioning. More research and interdisciplinary discussions are needed to successfully implement these methods to the field of HIV.
Contributor Information
Sarah B Joseph, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Sara Gianella, Department of Medicine, Division of Infectious Disease and Global Public Health, University of California, San Diego, La Jolla, California, USA.
Tricia H Burdo, Department of Microbiology, Immunology and Inflammation, Center for NeuroVirology and Gene Editing, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania, USA.
Paola Cinque, Unit of Infectious Diseases, San Raffaele Scientific Institute, Milano, Italy.
Magnus Gisslen, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Scott Letendre, Department of Medicine, Division of Infectious Disease and Global Public Health, University of California, San Diego, La Jolla, California, USA.
Avindra Nath, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA.
Susan Morgello, Departments of Neurology, Neuroscience, and Pathology, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Lishomwa C Ndhlovu, Department of Medicine, Division of Infectious Diseases, Brain and Mind Research Institute, Weill Cornell Medicine, New York, New York, USA.
Serena Spudich, Department of Neurology, Yale School of Medicine, New Haven, Connecticut, USA.
Notes
Financial support. S. B. J. is supported by the National Institutes of Health (NIH) (grant numbers R01MH118990, UM1AI126619, UM1AI164567, R01AI47849, and R01DA051890). S. G. is supported by the NIH (grant numbers P30 AI036214, AI147821, DA051915, AI164559, and MH062512). M. G. is supported by the Swedish state, under an agreement between the Swedish government and the county councils (ALF agreement ALFGBG-965885). S. L. is supported by the NIH (grant numbers P30AI036214, P30MH062512, R01MH125720, R01DA050491, R24MH129166, R25MH108389, R01AG063659, T32AI007384, R01DA047879, and R21MH122243). A. N. is supported by funds from the Office of AIDS Research at the NIH. S. M. is supported by the NIH (grant number U24MH100931). L. C. N. was supported during the draft of the manuscript in part by the National Institute on Drug Abuse and the NIH (grant numbers R01 DA042027, R01AG063846, R01 NS117458, UM1AI164559, and U01DA53625). S. S. was supported during the writing of this manuscript by the NIH (grant numbers R01MH125737, R01MH125396, UM1DA051410, and R01MH106466).
Supplement sponsorship. This article appears as part of the supplement “State of the Science of Central Nervous System Complications in People With HIV,” sponsored by the National Institutes of Health, National Institute of Mental Health.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin LH, Maki PM. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep 2019; 16:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’Antoni ML, Paul RH, Mitchell BI, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV following dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr 2018; 79:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambrosius B, Gold R, Chan A, Faissner S. Antineuroinflammatory drugs in HIV-associated neurocognitive disorders as potential therapy. Neurol Neuroimmunol 2019; 6:e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bougea A, Spantideas N, Galanis P, Gkekas G, Thomaides T. Optimal treatment of HIV-associated neurocognitive disorders: myths and reality. A critical review. Ther Adv Infect Dis 2019; 6:2049936119838228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 2016; 173:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamminga CA, Clementz BA, Pearlson G, et al. Biotyping in psychosis: using multiple computational approaches with one data set. Neuropsychopharmacol 2021; 46:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-Linsenbarth I, Planchuelo-Gómez Á, Beño-Ruiz-de-la-Sierra RM, et al. Search for schizophrenia and bipolar biotypes using functional network properties. Brain Behav 2021; 11:e2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagerty SL, Williams LM. The impact of COVID-19 on mental health: the interactive roles of brain biotypes and human connection. BBI Health 2020; 5:100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson TP, Nath A. Biotypes of HIV-associated neurocognitive disorders based on viral and immune pathogenesis. Curr Opin Infect Dis 2022; 35:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull 2014; 40:S131–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira D, Nordberg A, Westman E. Biological subtypes of Alzheimer disease: a systematic review and meta-analysis. Neurology 2020; 94:436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kesler SR, Petersen ML, Rao V, Harrison RA, Palesh O. Functional connectome biotypes of chemotherapy-related cognitive impairment. J Cancer Surviv 2020; 14:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong S-J, Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multidimensional neuroanatomical subtyping of autism spectrum disorder. Cereb Cortex 2018; 28:3578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wager TD, Woo C-W. Imaging biomarkers and biotypes for depression. Nat Med 2017; 23:16–7. [DOI] [PubMed] [Google Scholar]
- 19. Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis 2008; 197:S294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gisslen M, Keating SM, Spudich S, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One 2021; 16:e0250987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulfhammer G, Edén A, Mellgren Å, et al. Persistent central nervous system immune activation following more than 10 years of effective HIV antiretroviral treatment. AIDS 2018; 32:2171–8. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz A, Blennow K, Hagberg L, et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn 2017; 17:761–70. [DOI] [PubMed] [Google Scholar]
- 23. Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hellmuth J, Slike BM, Sacdalan C, et al. Very early initiation of antiretroviral therapy during acute HIV infection is associated with normalized levels of immune activation markers in cerebrospinal fluid but not in plasma. J Infect Dis 2019; 220:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gisslén M, Hunt PW. Antiretroviral treatment of acute HIV infection normalizes levels of cerebrospinal fluid markers of central nervous system (CNS) inflammation: a consequence of a reduced CNS reservoir? J Infect Dis 2019; 220:1867–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burbelo PD, Price RW, Hagberg L, et al. Anti-human immunodeficiency virus antibodies in the cerebrospinal fluid: evidence of early treatment impact on central nervous system reservoir? J Infect Dis 2018; 217:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edén A, Marcotte TD, Heaton RK, et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Royal W III, Cherner M, Burdo TH, et al. Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PLoS One 2016; 11:e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valcour VG, Shiramizu BT, Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 2010; 87:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet 1997; 349:692–5. [DOI] [PubMed] [Google Scholar]
- 33. Veenhuis RT, Williams DW, Shirk EN, et al. Higher circulating intermediate monocytes are associated with cognitive function in women with HIV. JCI Insight 2021; 6:e146215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel AA, Zhang Y, Fullerton JN, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 2017; 214:1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 2017; 12:e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J, Tam H, Adler L, Ilstad-Minnihan A, Macaubas C, Mellins ED. The MHC class II antigen presentation pathway in human monocytes differs by subset and is regulated by cytokines. PLoS One 2017; 12:e0183594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong KL, Tai JJ-Y, Wong W-C, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011; 118:e16–31. [DOI] [PubMed] [Google Scholar]
- 38. Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14+ CD16+ monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol 2014; 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Veenstra M, Byrd DA, Inglese M, et al. CCR2 On peripheral blood CD14+ CD16+ monocytes correlates with neuronal damage, HIV-associated neurocognitive disorders, and peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol 2019; 14:120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim W-K, Alvarez X, Fisher J, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol 2006; 168:822–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 2003; 74:650–6. [DOI] [PubMed] [Google Scholar]
- 42. Williams KC, Corey S, Westmoreland SV, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 2001; 193:905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell JH, Ratai E-M, Autissier P, et al. Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog 2014; 10:e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma V, Creegan M, Tokarev A, et al. Cerebrospinal fluid CD4+ T cell infection in humans and macaques during acute HIV-1 and SHIV infection. PLoS Pathog 2021; 17:e1010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzuki K, Zaunders J, Gates TM, et al. Elevation of cell-associated HIV-1 RNA transcripts in CSF CD4+ T cells, despite suppressive antiretroviral therapy, is linked to in vivo brain injury. medRxiv [Preprint]. Posted online 27 December 2021. doi: 10.1101/2021.12.22.21268288 [DOI] [Google Scholar]
- 46. Massanella M, Bakeman W, Sithinamsuwan P, et al. Infrequent HIV infection of circulating monocytes during antiretroviral therapy. J Virol 2019; 94:e01174-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hsu DC, Sunyakumthorn P, Wegner M, et al. Central nervous system inflammation and infection during early, nonaccelerated simian-human immunodeficiency virus infection in rhesus macaques. J Virol 2018; 92:e00222-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015; 36:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 2017; 18:123–31. [DOI] [PubMed] [Google Scholar]
- 50. Kessing CF, Spudich S, Valcour V, et al. High number of activated CD8+ T cells targeting HIV antigens are present in cerebrospinal fluid in acute HIV infection. J Acquir Immune Defic Syndr 2017; 75:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog 2015; 11:e1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lucas SB, Wong KT, Nightingale S, Miller RF. HIV-associated CD8 encephalitis: a UK case series and review of histopathologically confirmed cases. Front Neurol 2021; 12:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog 2011; 7:e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Canestri A, Lescure F-X, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50:773–8. [DOI] [PubMed] [Google Scholar]
- 55. Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well-controlled plasma viral load. AIDS 2012; 26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joseph SB, Kincer LP, Bowman NM, et al. Human immunodeficiency virus type 1 RNA detected in the central nervous system (CNS) after years of suppressive antiretroviral therapy can originate from a replicating CNS reservoir or clonally expanded cells. Clin Infect Dis 2019; 69:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spudich S, Robertson KR, Bosch RJ, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Investig 2019; 129:3339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lustig G, Cele S, Karim F, et al. T cell derived HIV-1 is present in the CSF in the face of suppressive antiretroviral therapy. PLoS Pathog 2021; 17:e1009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peterson J, Gisslen M, Zetterberg H, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 2014; 9:e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 2014; 9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013; 207:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abdulle S, Mellgren Å, Brew BJ, et al. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J Neurol 2007; 254:1026–32. [DOI] [PubMed] [Google Scholar]
- 63. Gisslén M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 2007; 195:1774–8. [DOI] [PubMed] [Google Scholar]
- 64. Mellgren Å, Price R, Hagberg L, Rosengren L, Brew B, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology 2007; 69:1536–41. [DOI] [PubMed] [Google Scholar]
- 65. Van Zoest RA, Underwood J, De Francesco D, et al. Structural brain abnormalities in successfully treated HIV infection: associations with disease and cerebrospinal fluid biomarkers. J Infect Dis 2018; 217:69–81. [DOI] [PubMed] [Google Scholar]
- 66. Alagaratnam J, De Francesco D, Zetterberg H, et al. Correlation between cerebrospinal fluid and plasma neurofilament light protein in treated HIV infection: results from the COBRA study. J Neurovirol 2021; 28:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anderson AM, Easley KA, Kasher N, et al. Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol 2018; 24:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farhadian SF, Mehta SS, Zografou C, et al. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI Insight 2018; 3:e121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Corley MJ, Farhadian SF. Emerging single-cell approaches to understand HIV in the central nervous system. Curr HIV/AIDS Rep 2022; 19:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulfhammer G, Edén A, Antinori A, et al. Cerebrospinal fluid viral load across the spectrum of untreated HIV-1 infection: a cross-sectional multi-center study. Clin Infect Dis 2022; 75:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gelman BB, Lisinicchia JG, Morgello S, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr 2013; 62:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hagberg L, Price RW, Zetterberg H, Fuchs D, Gisslén M. Herpes zoster in HIV-1 infection: the role of CSF pleocytosis in secondary CSF escape and discordance. PLoS One 2020; 15:e0236162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol 1991; 1:163–75. [DOI] [PubMed] [Google Scholar]
- 75. Dravid AN, Gawali R, Betha TP, et al. Two treatment strategies for management of neurosymptomatic cerebrospinal fluid HIV escape in Pune, India. Medicine 2020; 99:e20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yilmaz A, Verhofstede C, D’Avolio A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 2010; 55:590–6. [DOI] [PubMed] [Google Scholar]
- 77. Dahl V, Lee E, Peterson J, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis 2011; 204:1936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gianella S, Kosakovsky Pond SL, Oliveira MF, et al. Compartmentalized HIV rebound in the central nervous system after interruption of antiretroviral therapy. Virus Evol 2016; 2:vew020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chaillon A, Gianella S, Dellicour S, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Investig 2020; 130:1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mukerji SS, Misra V, Lorenz DR, et al. Impact of antiretroviral regimens on cerebrospinal fluid viral escape in a prospective multicohort study of antiretroviral therapy–experienced human immunodeficiency virus-1–infected adults in the United States. Clin Infect Dis 2018; 67:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yilmaz A, Price RW, Gisslén M. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother 2012; 67:299–311. [DOI] [PubMed] [Google Scholar]
- 82. Srinivas N, Rosen EP, Gilliland WM Jr, et al. Antiretroviral concentrations and surrogate measures of efficacy in the brain tissue and CSF of preclinical species. Xenobiotica 2019; 49:1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oliveira MF, Chaillon A, Nakazawa M, et al. Early antiretroviral therapy is associated with lower HIV DNA molecular diversity and lower inflammation in cerebrospinal fluid but does not prevent the establishment of compartmentalized HIV DNA populations. PLoS Pathog 2017; 13:e1006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ping L-H, Joseph SB, Anderson JA, et al. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol 2013; 87:7218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Parrish NF, Wilen CB, Banks LB, et al. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog 2012; 8:e1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Isaacman-Beck J, Hermann EA, Yi Y, et al. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol 2009; 83:8208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ochsenbauer C, Edmonds TG, Ding H, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 2012; 86:2715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peters PJ, Bhattacharya J, Hibbitts S, et al. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol 2004; 78:6915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol 2001; 75:10073–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gorry PR, Taylor J, Holm GH, et al. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol 2002; 76:6277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li S, Juarez J, Alali M, et al. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol 1999; 73:9741–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Joseph SB, Arrildt KT, Swanstrom AE, et al. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol 2014; 88:1858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas ER, Dunfee RL, Stanton J, et al. Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology 2007; 360:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Duenas-Decamp MJ, Peters PJ, Burton D, Clapham PR. Determinants flanking the CD4 binding loop modulate macrophage tropism of human immunodeficiency virus type 1 R5 envelopes. J Virol 2009; 83:2575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dunfee RL, Thomas ER, Gorry PR, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A 2006; 103:15160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martín-García J, Cao W, Varela-Rohena A, Plassmeyer ML, González-Scarano F. HIV-1 tropism for the central nervous system: brain-derived envelope glycoproteins with lower CD4 dependence and reduced sensitivity to a fusion inhibitor. Virology 2006; 346:169–79. [DOI] [PubMed] [Google Scholar]
- 97. Johnston SH, Lobritz MA, Nguyen S, et al. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J Virol 2009; 83:11016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li G-H, Henderson L, Nath A. Astrocytes as an HIV reservoir: mechanism of HIV infection. Curr HIV Res 2016; 14:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Al-Harti L, Joseph J, Nath A. Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. J Neurovirol 2018; 24:665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor (s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol 1991; 65:6094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li G-H, Maric D, Major EO, Nath A. Productive HIV infection in astrocytes can be established via a non-classical mechanism. AIDS 2020; 34:963–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lutgen V, Narasipura SD, Barbian HJ, et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog 2020; 16:e1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo X, He JJ. Cell–cell contact viral transfer contributes to HIV infection and persistence in astrocytes. J Neurovirol 2015; 21:66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Russell RA, Chojnacki J, Jones DM, et al. Astrocytes resist HIV-1 fusion but engulf infected macrophage material. Cell Rep 2017; 18:1473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnson TP, Patel K, Johnson KR, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 2013; 110:13588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Henderson LJ, Johnson TP, Smith BR, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019; 33:S145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brown M, Scarborough M, Brink N, Manji H, Miller R. Varicella zoster virus–associated neurological disease in HIV-infected patients. Int J STD AIDS 2001; 12:79–83. [DOI] [PubMed] [Google Scholar]
- 108. Letendre S, Bharti A, Perez-Valero I, et al. Higher anti-CMV IgG concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin Infect Dis 2018; 67:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gianella S, Letendre S. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 2016; 214(Suppl 2):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Robertson KR, Oladeji B, Jiang H, et al. Human immunodeficiency virus type 1 and tuberculosis coinfection in multinational, resource-limited settings: increased neurological dysfunction. Clin Infect Dis 2019; 68:1739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Marra C, Deutsch R, Collier A, et al. Neurocognitive impairment in HIV-infected individuals with previous syphilis. Int J STD AIDS 2013; 24:351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Arrildt KT, Joseph SB, Swanstrom R. The HIV-1 env protein: a coat of many colors. Curr HIV/AIDS Rep 2012; 9:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 1993; 43:2230. [DOI] [PubMed] [Google Scholar]
- 114. Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 1995; 38:755–62. [DOI] [PubMed] [Google Scholar]
- 115. McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus–associated neurocognitive disorders: mind the gap. Ann Neurol 2010; 67:699–714. [DOI] [PubMed] [Google Scholar]
- 116. Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 2009; 15:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology 2015; 85:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gutierrez J, Menshawy K, Gonzalez M, et al. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS 2016; 30:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gutierrez J, Byrd D, Yin MT, Morgello S. Relationship between brain arterial pathology and neurocognitive performance among individuals with human immunodeficiency virus. Clin Infect Dis 2019; 68:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Morgello S, Murray J, Van Der Elst S, Byrd D. HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neurol Neuroimmunol Neuroinflamm 2014; 1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 2014; 28:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Krut JJ, Zetterberg H, Blennow K, et al. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol 2013; 260:620–6. [DOI] [PubMed] [Google Scholar]
- 123. Ances BM, Benzinger TL, Christensen JJ, et al. 11C-PiB imaging of human immunodeficiency virus–associated neurocognitive disorder. Arch Neurol 2012; 69:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cooley SA, Strain JF, Beaumont H, et al. Tau positron emission tomography binding is not elevated in HIV-infected individuals. J Infect Dis 2019; 220:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Morgello S, Cortes EP, Gensler G, et al. HIV disease duration, but not active brain infection, predicts cortical amyloid beta deposition. AIDS 2021; 35:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Booiman T, Wit FW, Maurer I, et al. High cellular monocyte activation in people living with human immunodeficiency virus on combination antiretroviral therapy and lifestyle-matched controls is associated with greater inflammation in cerebrospinal fluid. Open Forum Infect Dis 2017; 4:ofx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Robertson J, Edén A, Nyström K, et al. Increased immune activation and signs of neuronal injury in HIV-negative people on preexposure prophylaxis. AIDS 2021; 35:2129–36. [DOI] [PubMed] [Google Scholar]