Abstract
Background
Chronic inflammation in IBD is postulated to drive NAFLD progression from steatosis to fibrosis.
Aims
To study the histopathological spectrum of NAFLD in Crohn disease (CD) and Ulcerative colitis (UC).
Methods
Patients with biopsy proven NAFLD at a quaternary center from 2008 to 2018 were included in this retrospective analysis. Inflammatory bowel disease (IBD) diagnosed either clinically and/or endoscopically at the time of liver biopsy. Multivariable regression and propensity score (PS) weighted analysis were conducted. Statistical analysis were performed using SAS statistical software.
Results
Among 1009 patients with NAFLD a diagnosis of IBD was identified in 50 cases (34 CD and 16 UC). On multivariable analysis; CD was independently associated with significantly higher odds of advanced fibrosis (AF) on liver biopsy (adjusted OR = 4.09, 95% CI = 1.40–11.94) compared to NAFLD patients without IBD. Similar results were obtained with both the overlap PS weighted model (OR = 3.17, 95% CI = 1.55–6.49) and the PS matched model (OR = 3.49, 95% CI = 1.50–8.13).
Conclusion
In a large cohort of patients with histologically well characterized NAFLD, AF was more common in CD patients than NAFLD patients without IBD. These findings must be confirmed in a larger cohort, but suggest CD patients with NAFLD could be at greater risk for liver fibrosis.
Keywords: NAFLD, Inflammatory bowel disease, Fibrosis, NASH
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a clinico-pathological spectrum ranging from simple steatosis (SS) with a relatively benign course, to Non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis. NASH is projected to be the leading indication for liver transplantation in the United States. The global prevalence of NAFLD is as high as 25% [1] however only a subset of these develop advanced liver disease characterized by hepatic fibrosis, cirrhosis or HCC. Chronic inflammation, oxidative stress and more recently alteration in the ‘gut-liver’ axis have been recognized as factors associated with progression from SS to NASH and cirrhosis [2]. Within NAFLD, the presence of advanced fibrosis (AF) is the most important parameter associated with outcomes such as decompensation of liver disease, mortality and need for liver transplantation [3]. Thus identification and treatment of AF is critical to improving outcomes in NAFLD. Despite advances in modalities to diagnose and follow disease activity non-invasively, a liver biopsy remains the gold standard to characterize and stage NAFLD [4].
Inflammatory bowel disease (IBD) is a group of chronic inflammatory diseases broadly divided into Crohn disease (CD) and ulcerative colitis (UC). IBD is characterized by an exaggerated systemic immune response to environmental factors based on a genetic predisposition resulting in chronic relapsing remitting inflammation of the gastrointestinal tract [5]. The inflammatory process is likely central to the pathophysiology of diseases associated with IBD, such as cardiovascular, neurological and gastrointestinal [4]. Even though NAFLD is known to have a higher prevalence in the IBD population [6, 7], it is unclear whether an increased prevalence is associated with higher rates of liver fibrosis which is the major risk factor for liver related outcomes. Most studies have used ultrasound as a surrogate marker of hepatic steatosis to define NAFLD in IBD but none has till now described the histopathological spectrum of NAFLD comparing patients with and without IBD.
Given a potential pathophysiological link between IBD and progression of NAFLD, we hypothesized that NAFLD patients with IBD to have a higher prevalence of advanced stages of NAFLD as well as histologically defined AF in comparison to NAFLD patients without IBD. In addition, we aimed to delineate risk factors related to AF in NAFLD patients with IBD and compare the performance of commonly used non-invasive tests (NITs) for liver fibrosis in NAFLD patients with and without IBD.
Methods
After IRB approval, records were reviewed for all patients aged over 18 with histologically diagnosed NAFLD at our institution from 2008 to 2019. In this retrospective analysis of data collected prospectively within the EMR, manual chart review was performed to identify patients with and without a diagnosis of IBD at the time of liver biopsy (Fig. 1). The presence of a diagnosis of IBD was confirmed based on clinical notes from an experienced gastroenterologist based on a combination of clinical, radiological and endoscopic features [8, 9].
Fig. 1.
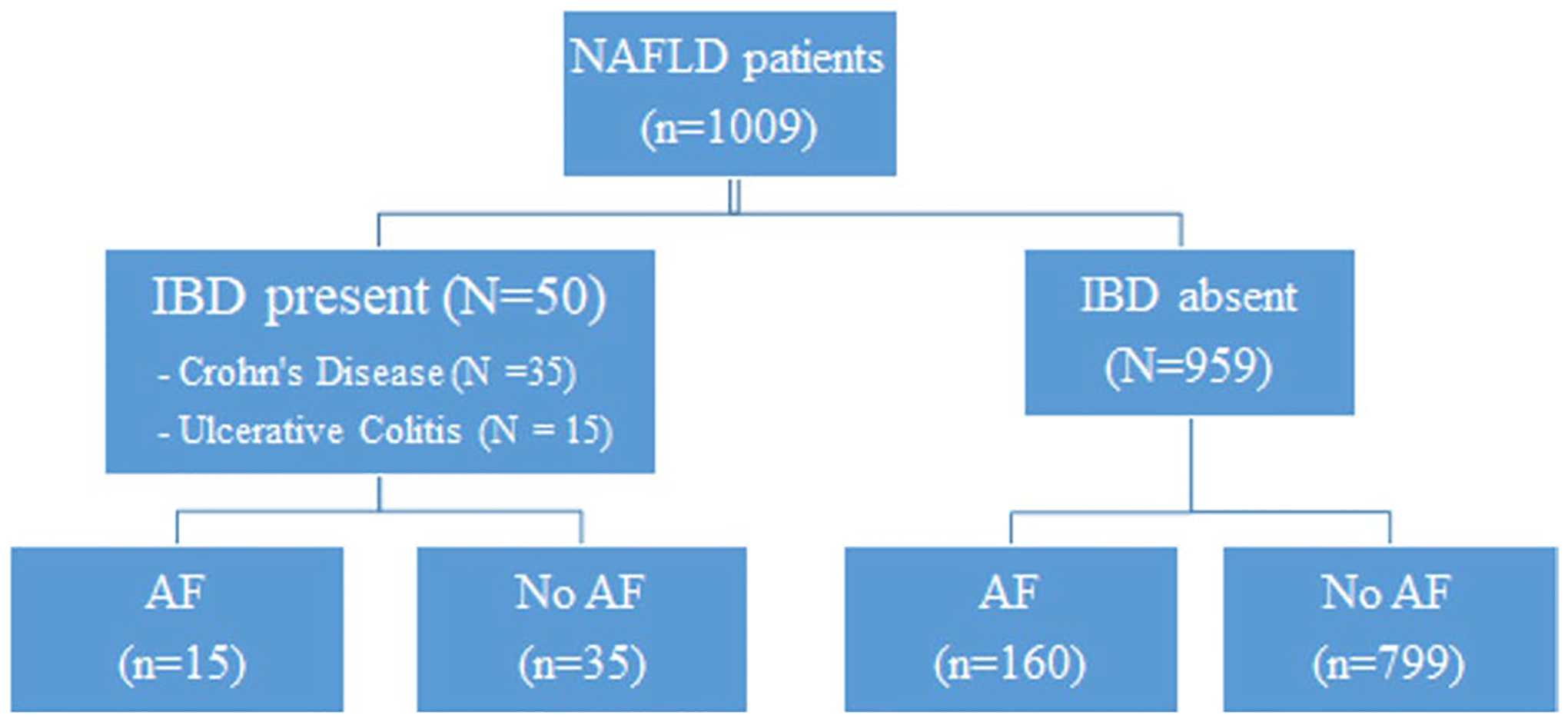
Flowchart of patients included in the study AF, Advanced fibrosis; IBD, Inflammatory bowel disease; NAFLD, Non-alcoholic fatty liver disease Advanced Fibrosis: Defined as Fibrosis stage 3–4
NAFLD Grading and Assessment of Fibrosis
NAFLD was diagnosed based on established histological criteria and graded per the NASH-Clinical Research Network (NASH-CRN) criteria by experienced pathologists [10]. Other etiologies of liver disease such as hemochromatosis, documented history of significant alcohol consumption (> 21 drinks per week in men and > 14 weeks per week in women), chronic viral hepatitis, hemochromatosis, autoimmune hepatitis, Wilson’s disease and or alpha-1 antitrypsin deficiency were excluded. Liver fibrosis was graded per the Kleiner staging system (grade I–IV) [10].
IBD Characteristics
Data regarding type of IBD (UC vs. CD); disease duration, location, phenotype and management was collected via manual chart review. We collected information regarding previous corticosteroid, immunomodulator (azathioprine, methotrexate) and biological agent use (antitumor necrosis factor-alpha [TNF-a], interleukin blockers, integrin inhibitors) prior to NAFLD diagnosis. History of previous intestinal surgery prior to NAFLD diagnosis was also collected and those with > 1 intestinal surgery were defined as having multiple surgeries.
Outcome of Interest
The primary outcome of interest was advanced liver fibrosis, defined as liver fibrosis stage III–IV. Secondary outcomes were presence of NASH, defined as evidence of steatohepatitis with NAFLD activity score (NAS) > 3 and separately, presence of any stage of liver fibrosis.
Comparison of Non-invasive Fibrosis Tests (NITs) for Diagnosis of AF
We calculated individual patient scores for three commonly used NITs (fibrosis-4[FIB-4] index, NAFLD fibrosis score [NFS] and AST/Platelet index[APRI]) [11] in all patients with data available for calculation using previously described formulae for each score.
Statistical Analysis
Descriptive statistics characterizing patient demographics, laboratory and histopathological features according to IBD status and IBD subgroup were summarized as either percentage or median with interquartile range. P values for group comparisons were obtained from chi-square, Fisher’s exact or Wilcoxon rank-sum tests as appropriate. Thirteen potential confounders were identified from the literature or expert opinion from the research team (MA, RG, FR and BLC). Focusing on the primary outcome of AF, first multivariable logistic regression models were built utilizing: (1) all variables (full model); (2) forward selection; and (3) backward elimination. The two variable selection techniques resulted in the same seven variable model. Adjusted odds ratios and 95% confidence intervals are reported. Additionally, a propensity score (PS) for IBD was estimated from a multivariable logistic regression model containing age, sex, race, body mass index (BMI), presence of hypertension and diabetes. The overlap propensity score weighting method was then applied, in which each patient’s weight is the probability of that patient belonging to the opposite IBD group. Logistic regression models adjusted for overlap PS weight investigated associations between IBD status and the probability of AF, NASH and liver fibrosis. Reported are weighted percentages, odds ratios and 95% confidence intervals. In a separate analysis, a PS matched cohort of CD and non-IBD subjects utilizing a caliper of ± 0.01 was created. Comparability of matched cohorts was assessed with absolute standardized differences (ASD), where a value greater than 0.20 indicates covariate imbalance. Simple logistic regressions were fit to the PS matched cohort assessing the association among CD status and AF, NASH and liver fibrosis. Reported are the odds ratios, 95% confidence intervals and P values. The diagnostic accuracy of NITs for AF was assessed by receiver operating characteristic (ROC) analysis. Areas under the ROC curves (AUROCs) were compared by the method of DeLong et al.[12] All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). P values were 2-sided with a significance threshold of 0.05.
Results
Demographics and Lab Values
A total of 1023 patients with histologically assessed NAFLD were identified and 14 were excluded due to incomplete data. Among a total of 1009 histologically proven NAFLD patients, 50 patients had IBD (34 CD and 16 UC). The mean age at diagnosis of patients with NAFLD was similar between patients with and without IBD (50.4 vs. 50.7 years), however NAFLD patients with IBD had lower BMI (32.5 vs. 40.2 kg/m2, p < 0.0001). IBD patients were significantly less likely to have a history of hypertension (36% vs. 59%, p = 0.001) and a clinically notable but statistically non-significant lower frequency of history of T2DM (30% vs. 43%, p = 0.07). Their baseline demographic, clinical and laboratory features are depicted in Table 1.
Table 1.
Baseline characteristics of included patients
| Characteristic | Inflammatory bowel disease (IBD) | No inflammatory bowel disease | P valuea | Crohn’s Disease (CD) | Ulcerative Colitis (UC) | P valueb |
|---|---|---|---|---|---|---|
| Count | 50 | 959 | 34 | 16 | ||
| Age, years | 50.4 [39.7–56.8] | 50.7 [39.9–58.7] | 0.81 | 50.4 [37.0–56.7] | 51.6 [43.0–59.8] | 0.76 |
| Female | 62% | 62% | 0.97 | 59% | 69% | 0.50 |
| Caucasian race | 90% | 84% | 0.26 | 94% | 81% | 0.31 |
| BMI, Kg/m2 | 32.5 [27.6–39.7] | 40.2 [33.3–47.4] | <.0001 | 32.9 [27.7–37.1] | 30.8 [27.1–42.5] | 0.99 |
| Hypertension | 36% | 59% | 0.001 | 35% | 38% | 0.88 |
| Statin use | 32% | 48% | 0.02 | 29% | 38% | 0.57 |
| Diabetes Mellitus | 30% | 43% | 0.07 | 32% | 25% | 0.75 |
| Insulin use | 30% | 24% | 0.37 | 35% | 19% | 0.33 |
| Metformin use | 26% | 41% | 0.04 | 26% | 25% | 0.99 |
| Albumin, gm/dl | 4.2 [3.5–4.6] | 4.3 [4.0–4.5] | 0.52 | 4.2 [3.5–4.6] | 4.3 [3.7–4.7] | 0.95 |
| Alkaline phosphatase, IU L−1 | 89 [71–123] | 79 [64–95] | 0.004 | 92 [71–123] | 88 [71–118] | 0.72 |
| Alanine transaminase, IU L−1 | 47 [26–77] | 46 [27–82] | 0.82 | 46 [27–81] | 52 [23–75] | 0.76 |
| Aspartate aminotransferase, IU L−1 | 45 [28–56] | 36 [23–60] | 0.07 | 46 [28–68] | 44 [28–49] | 0.26 |
| Total Bilirubin, mg/dl | 0.5 [0.4–0.7] | 0.4 [0.3–0.6] | 0.002 | 0.6 [0.4–0.8] | 0.4 [0.3–0.6] | 0.04 |
| High-density lipoproteins, mg dl−1 | 50 [37–57] | 42 [36–51] | 0.03 | 47 [37–55] | 53 [35–61] | 0.49 |
| Iron, | 81 [49–100] | 76 [59–95] | 0.79 | 88 [58–107] | 70 [40–92] | 0.35 |
| Low-density lipoproteins, mg dl−1 | 94 [65–114] | 104 [83–129] | 0.03 | 81 [62–103] | 104 [85–124] | 0.14 |
| Total cholesterol, mg dl−1 | 176 [145–201] | 180 [157–209] | 0.37 | 167 [140–196] | 179 [168–224] | 0.26 |
| Triglycerides, mg dl−1 | 87 [71–117] | 79 [64–95] | 0.01 | 86 [71–117] | 88 [71–118] | 0.94 |
| Hematocrit, % | 40.6 [36.8–43.6] | 42.2 [39.3–44.6] | 0.009 | 40.4 [36.8–44.0] | 41.6 [37.2–42.8] | 0.98 |
| Hemoglobin, gm dl−1 | 13.5 [11.8–14.8] | 14.0 [12.9–15.0] | 0.02 | 13.6 [11.9–15.0] | 13.5 [11.6–14.3] | 0.51 |
| Mean platelet volume | 10.6 [10.1–11.2] | 10.6 [10.0–11.3] | 0.81 | 10.6 [9.8–11.2] | 10.6 [10.3–11.2] | 0.74 |
| Platelet count, k ul−1 | 254 [194–302] | 245 [203–289] | 0.92 | 238 [159–286] | 287 [202–334] | 0.15 |
| White blood cells count | 7.2 [5.5–8.3] | 7.4 [6.1–9.0] | 0.13 | 7.5 [5.9–9.1] | 6.7 [5.3–7.4] | 0.11 |
P values are obtained from chi-square/Fisher’s exact or Wilcoxon rank-sum tests
p values represent comparison between IBD and No IBD
p values represent comparison between CD and UC
Serum aminotransferase levels were not different but alkaline phosphatase (ALP) levels were higher in IBD patients (89 vs. 79 IU/L p = 0.004) compared to patients without IBD. Although IBD patients had a higher mean HDL (50 vs. 42 mg/dl, p = 0.03) and lower LDL levels (94 vs. 104 mg/dl, p = 0.03) supporting a favorable lipid profile, the mean triglyceride levels were significantly higher in IBD patients in comparison to patients without IBD (87 vs. 79 mg/dl, p = 0.01). IBD patients had a lower mean hemoglobin (13.5 vs. 14.0 gm/dl, p = 0.02) and the platelet count was similar between patients with and without IBD (245,000 vs. 254,000/mm3, p = 0.92).
Comparison Between NAFLD Patients with CD and UC
Demographics including age, BMI and gender were similar between CD and UC patients. A history of T2DM was seen more frequently in patients with CD as compared with UC (32% vs. 25%, p = 0.75). Metformin use was similar (26% vs. 25%, p = 0.99). However, there was a clinically notable, but statistically non-significant, higher insulin use in CD when compared to UC (35% vs. 19%, p = 0.33). Laboratory parameters were similar between patients with CD and UC except for total bilirubin which was slightly higher in CD patients versus UC (0.6 vs. 0.4 mg/dl, p = 0.04). The demographic, clinical and laboratory features between CD and UC patients are presented in Table 1.
Histopathological Features
IBD patients were more likely to have AF on liver biopsy (30% vs. 17%, p = 0.02) although the prevalence of fibrosis and NASH was similar. On subgroup analysis, CD patients were more likely to have hepatocyte ballooning compared with non IBD patients (85% vs. 64%, p = 0.03). CD patients were also significantly more likely to have AF (41% vs. 17%, p = 0.0002), NASH (76% vs. 56%, p = 0.02) and liver fibrosis (88% vs. 63%, p = 0.003). Conversely UC patients had significantly lower rates of lobular inflammation (62% vs. 85%, p = 0.04) and hepatocyte ballooning (31% vs. 64%, p = 0.03). UC patients had a lower rate of AF (6% vs. 17%), however this was not statistically significant (p = 0.49). UC patients also had lower rates of NASH (31% vs. 56%, p = 0.04) and liver fibrosis (31% vs. 63%, p = 0.008). A detailed description of histological features on the liver biopsy is depicted in Table 2.
Table 2.
Histopathological features on liver biopsy among patients with and without inflammatory bowel disease (IBD) and stratified by Crohn disease (CD) and ulcerative colitis (UC)
| Biopsy outcomes | Inflammatory bowel disease | No inflammatory bowel disease | P value | Crohn’s Disease | P valuea (CD vs. No IBD) | Ulcerative Colitis | P valueb (UC vs. No IBD) |
|---|---|---|---|---|---|---|---|
| Count | 50 | 959 | 34 | 16 | |||
| Steatosis | 0.80 | 0.31 | 0.39 | ||||
| <5% | 2% | 2% | 0% | 6% | |||
| 5–33% | 42% | 48% | 35% | 56% | |||
| 34–66% | 40% | 33% | 47% | 25% | |||
| >66% | 16% | 17% | 18% | 13% | |||
| Lobular Inflammation | 0.41 | 0.91 | 0.04 | ||||
| No foci | 20% | 13% | 12% | 38% | |||
| <2 foci per 200×field | 48% | 50% | 47% | 50% | |||
| 2–4 foci per 200×field | 32% | 35% | 41% | 13% | |||
| >4 foci per 200×field | 0% | 2% | 0% | 0% | |||
| Hepatocyte Ballooning | 0.68 | 0.03 | 0.03 | ||||
| None | 32% | 36% | 15% | 69% | |||
| Few Balloon Cells | 46% | 46% | 59% | 19% | |||
| Many Cells/Prominent Ballooning | 22% | 18% | 26% | 12% | |||
| Fibrosis Stage | 0.06 | 0.001 | 0.09 | ||||
| 0 | 30% | 37% | 12% | 69% | |||
| 1 | 24% | 30% | 29% | 12% | |||
| 2 | 16% | 16% | 18% | 12% | |||
| 3 | 16% | 12% | 23% | 0% | |||
| 4 | 14% | 5% | 18% | 6% | |||
| Advanced Fibrosis | 30% | 17% | 0.02 | 41% | 0.0002 | 6% | 0.49 |
| NASH | 62% | 56% | 0.40 | 76% | 0.02 | 31% | 0.048 |
| Fibrosis | 70% | 63% | 0.34 | 88% | 0.003 | 31% | 0.008 |
Descriptive statistics are reported as either percentage or median [Q1–Q3]
P values are obtained from chi-square, Fisher’s exact or Wilcoxon rank-sum tests
Tests comparing Crohn’s Disease versus no Inflammatory Bowel Disease groups
Tests comparing Ulcerative Colitis versus no Inflammatory Bowel Disease groups
Advanced Fibrosis: Fibrosis stage 3–4
Fibrosis: Presence of any stage fibrosis
NASH: Nonalcoholic steatohepatitis (NASH) activity score > 3 and evidence of steatohepatitis
Multivariable Regression and Propensity Score Analyses
On multivariable analysis controlling for demographic features and comorbidities (Table 3); CD was independently associated with higher odds of AF on liver biopsy (adjusted OR = 4.09, 95% CI = 1.40–11.94) compared to NAFLD patients without IBD. This increased risk was observed with the fully adjusted model as well as a reduced model that utilized either forward selection or backward elimination for variable selection. Similar results were obtained with both the overlap PS weighted model (Fig. 2; weighted OR = 3.17, 95% CI = 1.55–6.49) and the PS matched model (Supplementary Table 1 & 2; OR = 3.49, 95% CI = 1.50–8.13).
Table 3.
Associations among Patient Characteristics and Advanced Fibrosis in non-alcoholic fatty liver disease (NAFLD) using Multivariable Logistic Regression
| Characteristic | Full model | Forward selection & backward elimination | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| IBD Status (reference=non-IBD) | 0.02 | 0.005 | ||||
| Crohn Disease | 4.09 | 1.40–11.94 | 3.23 | 1.47–7.12 | ||
| Ulcerative Colitis | 0.29 | 0.03–3.12 | 0.21 | 0.02–1.86 | ||
| Age | 1.03 | 1.01–1.05 | 0.003 | 1.04 | 1.02–1.05 | <.0001 |
| Female | 0.87 | 0.55–1.35 | 0.53 | |||
| White race | 1.88 | 1.01–3.51 | 0.048 | 1.74 | 0.99–3.04 | 0.05 |
| BMI | 0.98 | 0.95–0.99 | 0.03 | 0.98 | 0.96–1.00 | 0.09 |
| Hypertension | 1.03 | 0.63–1.69 | 0.89 | 0.90 | 0.61–1.34 | 0.60 |
| Statin | 1.00 | 0.64–1.56 | 0.99 | |||
| Diabetes Mellitus | 1.30 | 0.68–2.50 | 0.43 | |||
| Insulin | 1.31 | 0.81–2.11 | 0.28 | |||
| Metformin | 2.05 | 1.12–3.77 | 0.02 | 2.49 | 1.72–3.60 | <.0001 |
| High-density lipoproteins (HDL) | 0.99 | 0.98–1.01 | 0.49 | |||
| Low-density lipoproteins (LDL) | 0.99 | 0.99–1.01 | 0.86 | |||
| Total cholesterol | 0.99 | 0.99–1.01 | 0.89 | |||
| Triglycerides (Δ=10 units) | 1.10 | 1.04–1.16 | 0.0008 | 1.08 | 1.04–1.14 | 0.0004 |
Fig. 2.
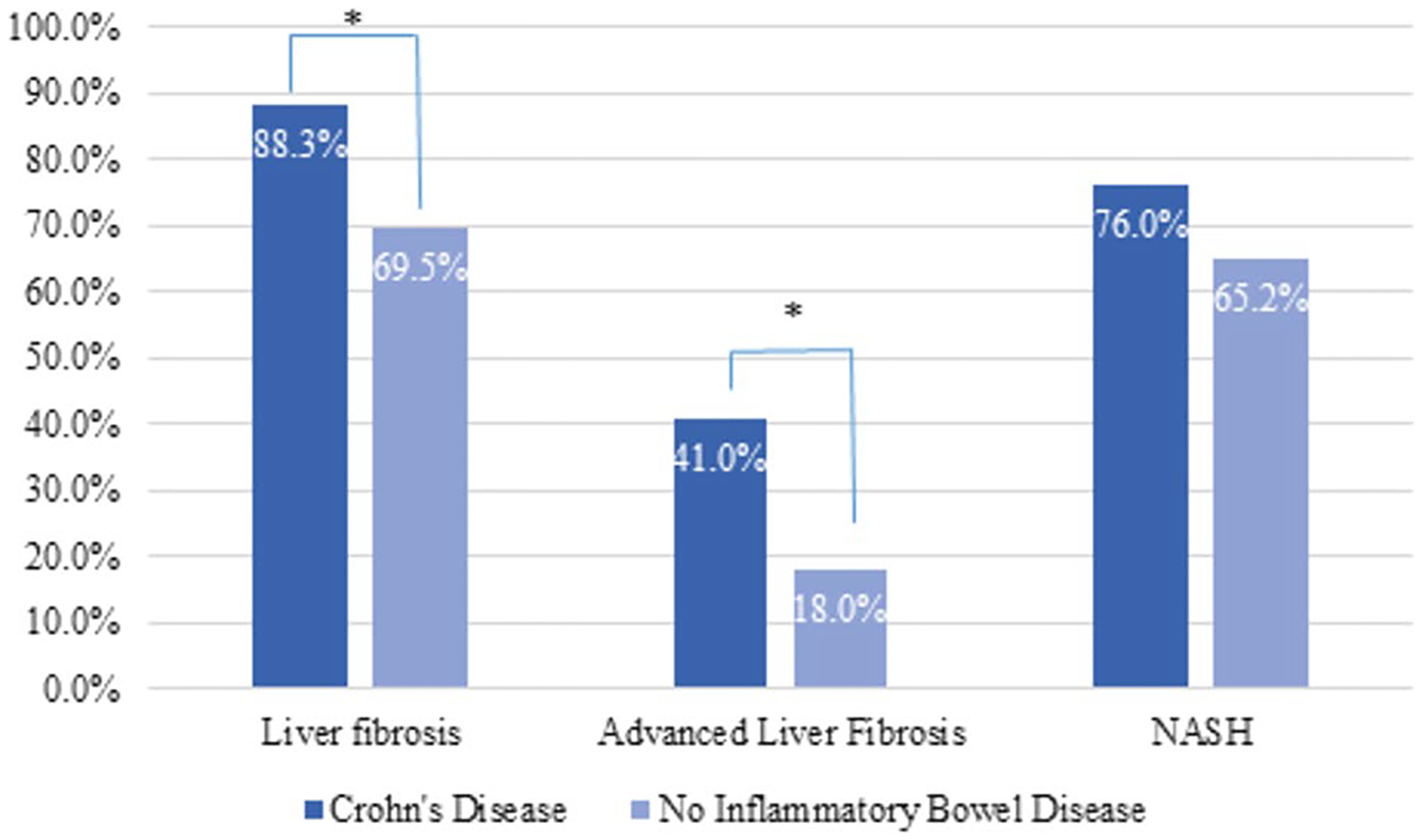
Rates of non-alcoholic fatty liver disease (NAFLD) histopathological features between NAFLD patients with Crohn’s disease (CD) and without inflammatory bowel disease (IBD) after overlap propensity score weighting
Both PS analyses were applied to the secondary outcomes with similar results. The risk of liver fibrosis was higher in CD patients (PS weighted OR = 3.30, 95% CI = 1.15–9.52; PS matched OR = 3.30, 95% CI = 1.09–10.02); however, the risk of NASH did not significantly differ among the CD patients and non-IBD controls (PS weighted OR = 1.69, 95% CI = 0.75–3.80; PS matched OR = 1.63, 95% CI = 0.68–3.92). UC patients, on the other hand, had odds of AF that did not significantly differ from those of NAFLD patients without IBD (OR = 0.29, 95% CI = 0.03–3.12).
Comparison Between CD Patients With and Without AF
On comparing characteristics of CD patients with and without AF (Table 4), CD patients with AF were older (median age 54y vs. 48y, p = 0.03) and were significantly more likely to have DM (57% vs. 15% p = 0.02). History of multiple (> 1) intestinal surgeries was the only IBD related charac teristic associated with AF (57% vs. 20% p = 0.04). Presence of fistulizing disease (p = 0.36) perianal disease (p = 0.72) or extra-intestinal manifestations (p = 0.25) were similar between CD patients with and without AF. There were no differences in the rates of corticosteroid use (p = 0.63), azathioprine (0.38) or biological use (0.49) between the groups.
Table 4.
Comparison of Clinical features between Crohn diseases (CD) patients with and without advanced liver fibrosis
| Characteristic | Advanced fibrosis | No advanced fibrosis | P value |
|---|---|---|---|
| Count | 20 | 14 | |
| Age at NAFLD diagnosis, years | 54 [49–62] | 48 [31–55] | 0.03 |
| Duration of Crohn’s Disease (months) | 264 [48–357] | 161 [68–248] | 0.31 |
| Female gender | 43% | 70% | 0.11 |
| White race | 100% | 90% | 0.50 |
| Body Mass Index (BMI), Kg/m2 | 32 [29–35] | 34 [27–41] | 0.41 |
| Hypertension | 36% | 35% | 0.99 |
| Statin use | 43% | 20% | 0.25 |
| Diabetes Mellitus | 57% | 15% | 0.02 |
| Crohn’s disease characteristics | 0.16 | ||
| Crohn’s Disease Location | |||
| L1: Ileal | 14% | 20% | |
| L2: Colonic | 14% | 40% | |
| L3: Ileocolonic | 71% | 40% | |
| Fistulizing disease | 7% | 25% | 0.36 |
| Perianal disease | 29% | 40% | 0.72 |
| Extraintestinal Manifestations | 0% | 15% | 0.25 |
| Prior Intestinal Surgery | 71% | 55% | 0.33 |
| Multiple Prior Surgeries (> 1) | 57% | 20% | 0.04 |
| Aminosalicylic Acid (ASA) | 86% | 65% | 0.25 |
| Corticosteroid use | 79% | 90% | 0.63 |
| Azathioprine use | 50% | 65% | 0.38 |
| Methotrexate use | 0% | 5% | 0.99 |
| Biological use | 43% | 55% | 0.49 |
Descriptive statistics are reported as column percentages
NAFLD, Non-alcoholic fatty liver disease
P values are obtained from chi-square or Fisher’s exact tests
Comparison of Performance of NITs to Detect Advanced Fibrosis
On ROC analysis, NITs (FIB-4 and APRI) had similar performance in patients with and without IBD to detect AF except NAFLD fibrosis score [NFS] that had better performance in patients with IBD (AUROC = 0.87 vs. 0.65, p = 0.0001). Among patients with IBD, all three tests had similar performance to detect AF. The performance characteristics are depicted in supplementary table 3 and supplementary Fig. 1).
Discussion
This study described the association of IBD with histologic features of NAFLD in a large cohort of well phenotyped patients with IBD in a tertiary center. We found CD was associated with 4 times the odds of AF in comparison with NAFLD patients without IBD. UC was not associated with higher rates of AF. We compared the prevalence of liver fibrosis using the gold-standard histopathology in NAFLD patients with and without IBD and were able to control for several known risk factors for NAFLD progression. Based on our results, we suggest that CD patients with NAFLD may represent a higher risk subset for AF. Among patients with IBD and NAFLD, older age and history of DM were also associated with AF. Commonly used non-invasive test for diagnosis of AF (FIB-4, NFS and APRI) had similar performance in IBD patients suggesting that these tests can be used for stratification of NAFLD in patients with IBD.
Over the last decade, NAFLD has been shown to be more prevalent in patients with IBD with an overall prevalence ranging from 27.5 to 32% in different meta-analysis [6, 7]. Yet only a few studies have evaluated NASH or fibrosis in IBD patients and no study to date has compared the histological features between NAFLD patients with and without IBD. AF is the most important predictor of liver related morbidity and mortality in NAFLD [3] and thus it is critical to assess whether the higher prevalence of NAFLD in IBD patients is also associated with a higher prevalence of AF. In our study, NAFLD patients with CD had higher odds of AF in comparison with those without IBD. Recognizing that this study is observational, addressing potential confounding is important and we controlled for several factors known to be associated with NAFLD progression in our study [13]. CD patients had four times higher odds of having AF after controlling for age, gender and features of metabolic syndrome. IBD is characterized by chronic inflammation, oxidative stress and alteration of gut-liver axis, factors known to be associated with progression in NAFLD from steatosis to fibrosis [2]. Progression in NAFLD from liver steatosis to NASH and then liver fibrosis is complex and multifactorial [14]. In the initial stages of steatosis, lipid overload leads to lipotoxicity which contributes to inflammation and oxidative stress [15]. This ultimately leads to NASH and a milieu for progressive liver fibrosis. Chronic inflammation in NASH leads to hepatocyte death and a pro-fibrogenic response [16]. Multiple mediators such as transforming growth factor beta (TGF-β), platelet derived growth factor (PDGF) are upregulated which induce liver fibrosis [17]. Recently the influence of gut and gut microbiota on liver and vice-e-versa, termed the gut-liver axis is increasingly recognized to play a role in pathogenesis of NAFLD [18]. Breakdown of the intestinal barrier, a hallmark in IBD, results in exposure of liver to gut bacteria, bacterial products and immune mediators which can result in hepatic inflammation [19, 20]. Gut is essential for regulating nutrient intake and metabolism via gut derived hormones such as GLP-1 which can be decreased in IBD and predispose patients to hepatic inflammation and fibrosis [21]. Thus, there are multiple pathogenic pathways linking IBD and liver fibrosis explaining the observed increase in liver fibrosis (Fig. 3). To our surprise in contrast to CD, patients with UC did not have higher odds of having fibrosis or AF in our study. Although they are both types of IBD, CD and UC are known to have different pathobiology. Prior studies have shown that CD poses a greater risk for NAFLD than UC however the exact mechanism remains unknown [7]. Decreased expression of ileal farnesoid X receptor (FXR) gene and lower levels of fibroblast growth factor 19 (FGF 19) are postulated mechanisms behind increased rates of NAFLD seen in CD [2]. Activation of FXR in the small intestine by bile acids is crucial for regulation of carbohydrate and lipid metabolism as well as decreasing the production of pro-inflammatory and fibrogenic mediators [22]. Consequently FXR agonism has emerged as a therapeutic option for treatment of NAFLD. FXR is predominantly found in the small intestine which may partly contribute to increased rates of fibrosis seen in CD as compared with UC. In addition, CD is associated with higher degree of gut microbiome alteration in comparison with UC which is linked to progression of NAFLD [23]. For instance, F. Prausnitzii, the most abundant bacterium in healthy human gut with immunosuppressive effects [24], was shown to be depleted in CD whereas upregulated in UC patients [25]. Such alterations in the gut microbiome may be associated with a pro-inflammatory liver milieu and contribute to inflammation in CD patients resulting in liver fibrosis.
Fig. 3.
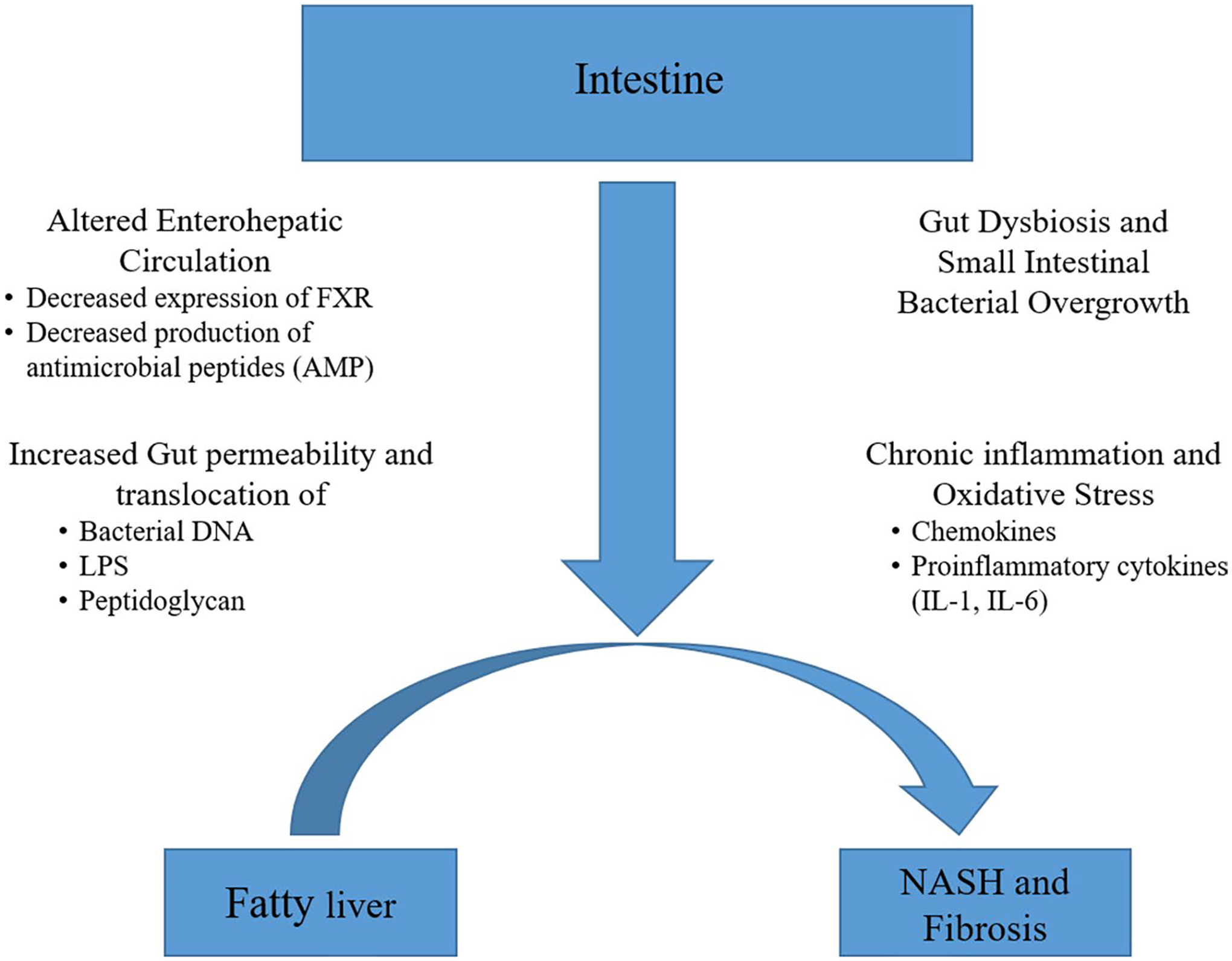
Proposed pathogenesis of non-alcoholic fatty liver disease progression in patients with inflammatory bowel disease
Among CD patients, older age and history of DM were significantly associated with AF. No other IBD related factors such as disease duration, extent of involvement or treatment were associated with higher rates of AF in this study. Advancing age and DM are well established as risk factors for progression of NAFLD from SS to fibrosis [26]. As the survival of patients with CD continues to improve, the burden of AF and consequently NASH cirrhosis is likely to increase. Factors such as duration of IBD, history of bowel resection, exposure to corticosteroids, methotrexate and biological agents have been postulated to be related to a higher risk of developing NAFLD but data is conflicting [6, 7]. However no previous study till date has evaluated these factors in relation to liver fibrosis. Although we did not identify any IBD specific factors to be related to survival, this may have been due to a small sample size of IBD patients and lack of statistical power. Larger studies with IBD patients in future should evaluate the impact of disease activity and control on NAFLD progression in this sub-group of patients.
Non-invasive tests are routinely used to detect AF in patients with NAFLD however these have not been validated in patients with IBD. This study found that routinely used NITs (FIB-4, NFS, APRI) had similar (FIB-4 and APRI) or even better performance (NFS) to detect AF in IBD patients in comparison with non-IBD patients. Among IBD patients, the three tests had similar performance. Validation of NITs in IBD is key to stratify NAFLD in patients with IBD which can help guide referral to hepatologists for specialized care and further investigation. NITs can also facilitate larger scale studies to accurately determine the prevalence of liver fibrosis in larger IBD populations without a need for liver biopsy and provide a marker to assess progression of disease. These results should be validated in further larger IBD populations.
Our study has the following key strengths. We focused on the most important predictor of clinically relevant outcomes in liver fibrosis, which was defined per the validated NASH-CRN system [10]. Further, liver biopsy is gold standard for assessment and grading of fibrosis. All biopsies were systematically evaluated using the NASH-CRN criteria by expert pathologists. This is in contrast to previous studies which used surrogate marker for fibrosis assessment not yet validated in patients with IBD. We also included detailed clinical data regarding both NAFLD and IBD related features, and adjust for several key variables that confound the association between both disease phenotypes. We used robust statistical methods including multivariable and propensity score weighted and matched analysis. However, we acknowledge limitations of our study. As we started from a cohort of patients who underwent liver biopsy, this selection bias would have excluded IBD patients who did not get a liver biopsy, which could have contributed to the relatively small subset of IBD patients. As this is a single center study conducted at a referral center, there is a possibility of referral bias selecting for patients with more complications. However, the rates of NASH and AF in our study were similar to prior epidemiological studies from other academic centers. We focused on fibrosis and did not evaluate the prevalence of NAFLD as assessed by other noninvasive modalities, which has already been described in patients with IBD [7]. Although we collected several characteristics of IBD phenotype and management in our cohort, we were not able to define IBD disease activity using standardized disease activity score and determine its correlation with NAFLD.
In summary, this is the first study to provide detailed clinical and histopathological characteristics of NAFLD in IBD patients and compare it with NAFLD patients without IBD. We recommend that further prospective data be collected in CD patients with NAFLD to ascertain their true risk for liver fibrosis. Results of such studies can help in prognosticating CD patients with NAFLD, and support screening and prevention strategies in the same. Future studies should evaluate effects of aggressive control of IBD disease activity and treatment of metabolic risk factors on liver fibrosis in patients with CD and NAFLD.
Supplementary Material
Conflict of interest
Benjamin Click reports consulting fees for TAR-GET-RWE, speakers bureau for Takeda. Florian Rieder reports consulting and advisory boards for Adnovate, Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios, 89Bio and research funding from the NIH, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, AbbVie, Pfizer, Boehringer Ingelheim, Morphic, Kenneth Rainin Foundation. Benjamin Cohen receives financial support for advisory boards and consultant for Abbvie, CelgeneBristol Myers Squibb, Lilly, Pfizer, Sublimity Therapeutics, Takeda, TARGET RWE; CME Companies: Cornerstones, Vindico; Speaking: Abbvie. Other authors have no conflict of interest to declare.
Abbreviations
- AF
Advanced fibrosis
- CD
Crohn Disease
- IBD
Inflammatory bowel disease
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- PS
Propensity score
- TNF
Tumor necrosis factor
- UC
Ulcerative colitis
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10620-022-07562-0.
References
- 1.Younossi Z, Anstee QM, Marietti M et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Publ Gr. 2017;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholicsteatohepatitis: an overview. Hepatol Commun. 2020;4:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RS, Taylor RJ, Bayliss S et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–1625.e12. [DOI] [PubMed] [Google Scholar]
- 4.Argollo M, Gilardi D, Peyrin-Biroulet C, Chabot J-F, Peyrin-Biroulet L, Danese S. Comorbidities in inflammatory bowel disease: a call for action. LancetGastroenterol Hepatol. 2019;4:643–54. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, He C, Cong Y, Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal-Immunol. 2015;8:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou ZY, Shen B, Fan JG. Systematic review with meta-analysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm BowelDis. 2019;25:1764–1772. [DOI] [PubMed] [Google Scholar]
- 7.Lin A, Roth H, Anyane-Yeboa A, Rubin DT, Paul S. Prevalence of nonalcoholic fatty liver disease in patients with inflammatory bowel disease: a systematicreview and meta-analysis. Inflamm BowelDis. 2020;27:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s Disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 9.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020;69:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837. [PubMed] [Google Scholar]
- 13.Hartmann P, Schnabl B. Risk factors for progression of and treatment options for NAFLD in children. Clin Liver Dis. 2018;11:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-alcoholicsteatohepatitis: a review of its mechanism models and medical treatments. Front Pharmacol. 2020. 10.3389/fphar.2020.603926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akazawa Y, Nakao K. Lipotoxicity pathways intersect in hepatocytes: endoplasmic reticulum stress, c-Jun N-terminal kinase-1, and death receptors. Hepatol Res. 2016;46:977–984. [DOI] [PubMed] [Google Scholar]
- 16.Rolla S, Alchera E, Imarisio C et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci. 2016;130:193–203. [DOI] [PubMed] [Google Scholar]
- 17.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFβ1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi A, Debelius J, Brenner DA et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009;137:1716–1724.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa Y, Imajo K, Honda Y et al. Palmitate-induced lipotoxicity is crucial for the pathogenesis of nonalcoholic fatty liver disease in cooperation with gutderived endotoxin. Sci Rep. 2018;8:11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zietek T, Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol. 2016. 10.3389/fimmu.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parséus A, Sommer N, Sommer F et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017;66:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sankarasubramanian J, Ahmad R, Avuthu N, Singh AB, Guda C. Gut Microbiota and metabolic specificity in ulcerative colitis and Crohn’s disease. Front Med. 2020. 10.3389/fmed.2020.606298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokol H, Pigneur B, Watterlot L et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci. 2008;105:16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín R, Miquel S, Benevides L et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol. 2017. 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain N, Afendy A, Stepanova M et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–1229.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


