Abstract
Background:
Although insufficient sleep has been shown to contribute to obesity-related elevated blood pressure, the circadian timing of sleep (CTS) has emerged as a novel risk factor. We hypothesized that deviations in sleep midpoint, a measure of CTS, modify the association between visceral adiposity and elevated blood pressure in adolescents.
Methods:
We studied 303 subjects from the Penn State Child Cohort (16.2±2.2y; 47.5% female; 21.5% racial/ethnic minority). Actigraphy (ACT)-measured sleep duration, midpoint, variability, and regularity were calculated across a 7-night period. Visceral adipose tissue (VAT) was measured with dual-energy X-ray absorptiometry. Systolic (SBP) and diastolic (DBP) blood pressure levels were measured in the seated position. Multivariable linear regression models tested sleep midpoint and its regularity as effect modifiers of VAT on SBP/DBP levels, while adjusting for demographic and sleep covariables. These associations were also examined as a function of being in-school or on-break.
Results:
Significant interactions were found between VAT and sleep irregularity, but not sleep midpoint, on SBP (p-interaction=0.007) and DBP (p-interaction=0.022). Additionally, significant interactions were found between VAT and schooldays sleep midpoint on SBP (p-interaction=0.026) and DBP (p-interaction=0.043), whereas significant interactions were found between VAT and on-break weekdays sleep irregularity on SBP (p-interaction=0.034).
Conclusion:
A delayed and an irregular sleep midpoint during school and during free-days, respectively, increase the impact of VAT on elevated BP in adolescents. These data suggest that deviations in the CTS contribute to the increased cardiovascular sequelae associated with obesity and that its distinct metrics require measurement under different entrainment conditions in adolescents.
Keywords: circadian misalignment, adolescents, blood pressure, visceral adiposity, entrainment
Graphical Abstract

INTRODUCTION
Obesity is a health condition of epidemic proportions,1 and it is particularly detrimental during adolescence as it increases the risk of developing chronic diseases later in life.2,3 Obesity is typically defined through global metrics such as body mass index (BMI), and although BMI can identify individuals with obesity that have greater risk of morbidity and mortality,4 its adipose-distribution profiles may vary greatly.5 Developmentally, there is also a shift in adipose tissue distribution during adolescence when fat is increasingly accumulated in the visceral depot.6 Visceral adipose tissue (VAT) has emerged as the phenotype of choice to identify metabolically unhealthy individuals, and it is a key risk factor for the adverse cardiovascular health outcomes associated with weight gain.7 VAT is a hormonally active fat tissue that has the biochemical characteristics capable of activating pro-inflammatory cytokines, causing a cascade of metabolic dysfunction.8 Research has shown that increased VAT is strongly associated with elevated blood pressure (eBP), cardiovascular disease (CVD) and type 2 diabetes (T2D), and it is impactful to the long-term health consequences.3,9 Adolescence is, thus, a critical period for the onset of such cardiovascular and metabolic disorders related to obesity.10 Preventive strategies have aimed to target risk factors that may contribute to the development of obesity and consequent chronic diseases, however, obesity is a highly complex condition due to its multifactorial etiology.11 To reduce the growing prevalence of obesity and associated cardiovascular consequences, research has focused on novel factors, such as disturbed, insufficient or misaligned sleep, that may contribute to obesity and its associated cardiovascular risk.12,13
Evidence supports that insufficient sleep duration is associated with obesity and eBP in adolescents.14–17 More recent evidence suggests that the intra-individual standard deviation (StDev) in sleep duration, depicted in Figure 1, may be more relevant dimensions of sleep health than metrics averaged across nights. Recent data indicate that such intra-individual StDev in sleep duration (i.e., sleep variability), indicative of nights of sufficient vs. insufficient vs. prolonged sleep, is more strongly associated with obesity and cardiovascular outcomes than average sleep duration alone.12,18–20 Furthermore, beyond disturbed and insufficient sleep,18,21–23 a circadian misalignment in the timing of the sleep-wake cycle has also been shown to further increase the pro-inflammatory response characteristic of increased VAT.24
Figure 1. Exemplary depiction of sleep variability and irregularity as sleep health metrics beyond average values.
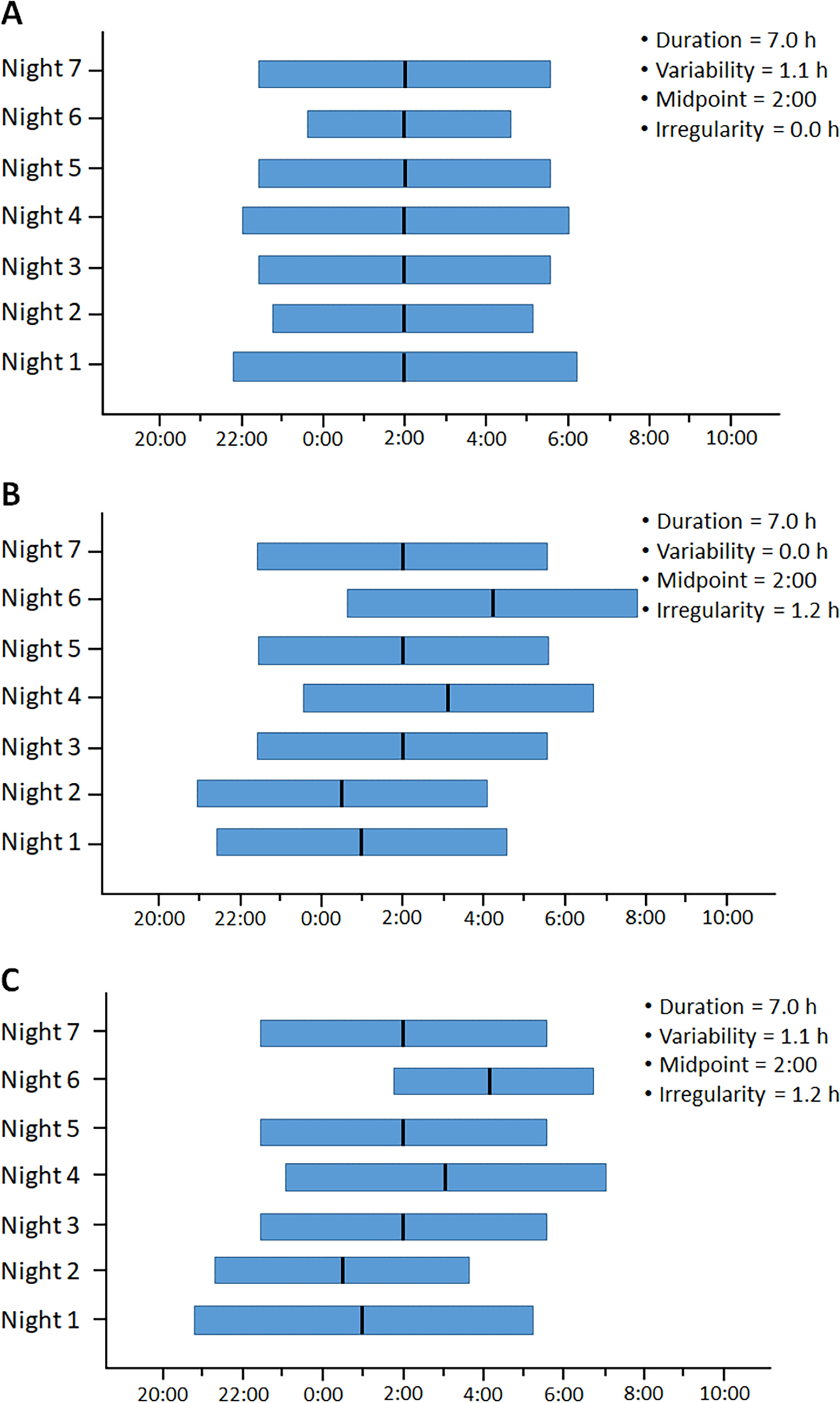
Deviations in average sleep duration (e.g., insufficient sleep of 6 h) and average sleep midpoint (e.g., delayed sleep phase at 4:00) have shown to be associated with adverse health outcomes; however, A) the intra-individual standard deviation of sleep duration across multiple nights helps identify individuals who have high variability in their sleep duration (1.1 h), despite obtaining a normative average sleep duration (7.0 h) with normative average timing/midpoint (2:00) and no irregularity (0.0 h). In addition, B) the intra-individual standard deviation of the sleep midpoint across multiple nights helps identify individuals who have high irregularity in their sleep midpoint (1.2 h), despite obtaining a normative average sleep duration (7.0 h) with no variability (0.0 h) and with normative average timing/midpoint (2:00). Of course, C) these metrics can overlap in some individuals, identifying those with high variability in their sleep duration (1.1 h) and irregularity in their sleep midpoint (1.2 h), despite obtaining a normative average sleep duration (7.0 h) with normative average timing/midpoint (2:00).
Circadian misalignment describes circumstances where there is a mismatch between the endogenous circadian system and 24-h environmental and behavioral cycles.10 The central biological pacemaker, located in the hypothalamus, regulates various behavioral and biological processes that ensure ideal physiological functioning by keeping organisms aligned to the dark-light cycle in 24-hour rhythms referred to as circadian rhythms, such as the sleep-wake cycle.25 However, changes in the circadian timing of sleep are common during adolescence; a developmental period marked by major physical, psychological and social changes. At the onset of puberty and throughout adolescence, youth’s sleep phase becomes more delayed as compared to their childhood years.26,27 Due to the potential mismatch between this maturational change in the circadian timing of sleep and the social/academic responsibilities during the schooldays (forced entrainment) and the weekend or on-break schedules (free days), adolescents may experience different forms of circadian misalignment, such as delayed sleep phase, sleep irregularity and/or social jetlag.26 Such deviations in the circadian timing of sleep have recently emerged as novel risk factors for adverse cardiovascular and metabolic outcomes, such as eBP, and may be the source of the observed insufficient sleep and sleep variability in adolescence; however, this evidence has been limited.10,28 Therefore, there is a potential for deviations in the circadian timing of sleep to play a role in the adverse cardiovascular sequelae of obesity particularly in adolescence, a developmental period vulnerable to circadian misalignment.
Our conceptual model posits a mechanistic role for alterations in the circadian timing of sleep during adolescence in the increased cardiovascular risk associated with obesity. From a conceptual standpoint, most studies reviewed above have focused on sleep variability and irregularity as sole predictors of either obesity (e.g., VAT) or cardiovascular health (e.g., BP).1–7 This study is based on a novel paradigm that tests the role of objectively-measured sleep variability and irregularity as effect modifiers (i.e., moderators) between traditional risk factors (i.e., obesity) and cardiovascular outcomes (i.e., BP). As shown in Figure 2, we propose that delays (sleep midpoint) and deviations (sleep regularity) of the sleep-wake cycle, defined as the mean and StDev of the midpoint of the sleep period respectively, act as effect modifiers of the association between VAT and eBP. This scientific premise is highly significant because it helps identify “under what circumstances”/“in whom” the impact of adolescent obesity on cardiovascular health is greatest. There is limited data on whether changes in the circadian timing of sleep that occur during adolescence increase the impact of obesity on adverse cardiovascular outcomes independent of insufficient sleep or underlying sleep disorders, and whether circadian misalignment may express differently if youth are studied during the academic year (in-school) or during free-days (on-break). Thus, this study addressed these questions by examining two metrics of circadian misalignment and their role in modifying the association of VAT with eBP in adolescents.
Figure 2. Conceptual model tested in this study.
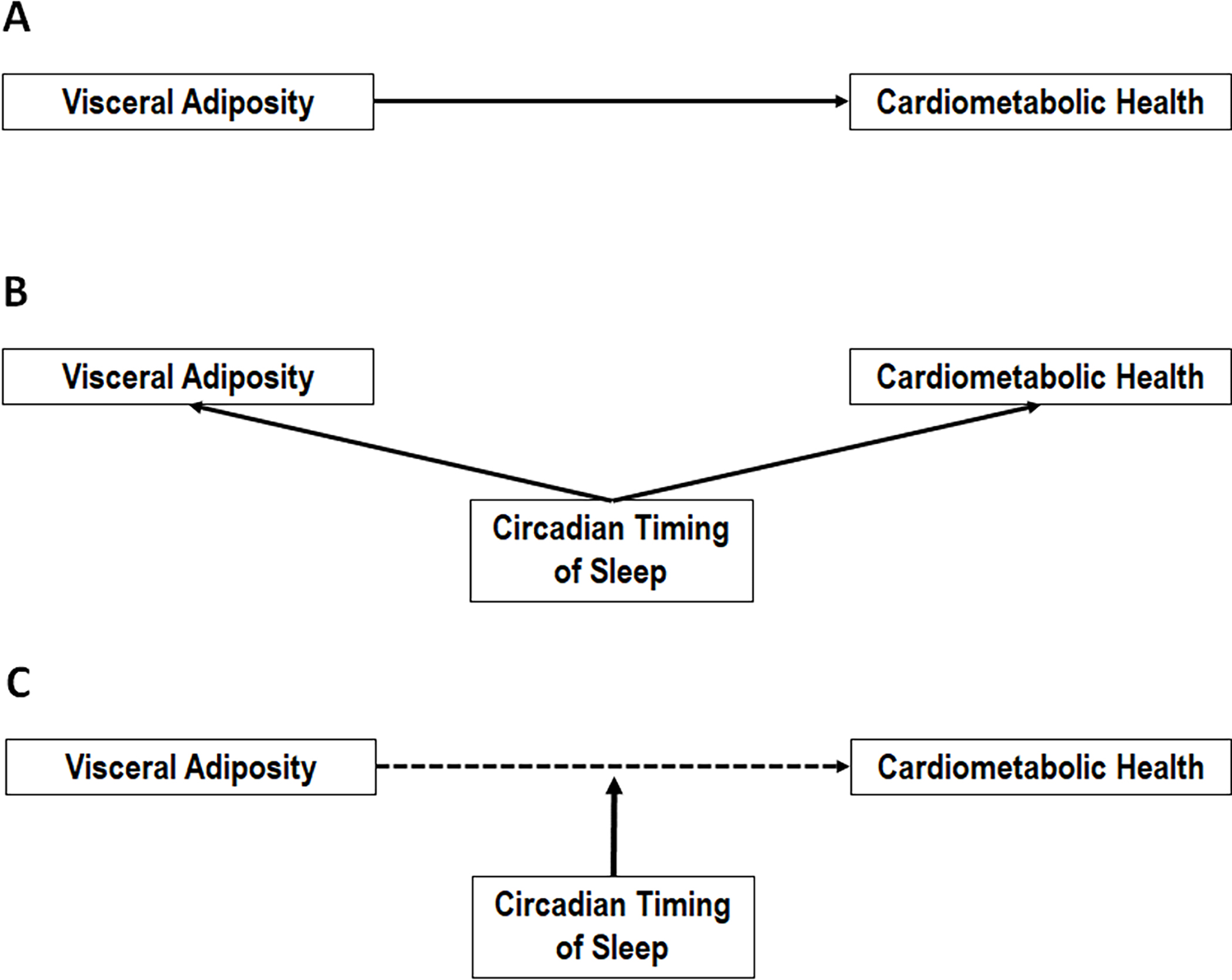
It is well-established that A) increased visceral adiposity is associated with adverse cardiometabolic outcomes (e.g., elevated blood pressure); however, most prior research that B) examined the role of the circadian timing of sleep has studied the independent association of metrics of circadian misalignment (e.g., sleep midpoint and its regularity) with either visceral adiposity or cardiometabolic health outcomes, without accounting for the known association between the latter two. Our conceptual model tested in this study posits that C) deviations in the circadian timing of sleep act as effect modifiers (moderators) of the known association between increased visceral adiposity and adverse cardiometabolic outcomes, helping identify “under what novel circumstances”/”in whom” visceral adiposity has the strongest impact on cardiometabolic health outcomes (e.g., highly irregular circadian timing of sleep) as well as “under what novel circumstances”/”in whom” there is resilience for such adverse health outcomes (e.g., a circadian timing of sleep aligned to the biological night).
METHODS
Sample
The data that support the findings of this study are available from the corresponding author upon reasonable request. The Penn State Child Cohort (PSCC) has been previously described in detail elsewhere.29 In brief, the PSCC was designed as a three-phase study in which 700 randomly selected participants ages 5–12 underwent an in-laboratory sleep study at baseline in 2002–2006 (70% response rate). The adolescent visit conducted from 2010 to 2013 consisted of 421 participants that returned 6 to 13 years later (median, 7.4 years) for a reexamination; no differences in baseline demographic characteristics were observed in the 279 participants who did not return for a follow-up.30 These 421 participants (60.1% response rate) were ages 12–23 (16.2 ± 2.2 years old; 47.5% female; 21.5% racial/ethnic minority). Participants were examined at the Clinical Research Center (CRC) at Penn State College of Medicine. The study was approved by The Penn State College of Medicine Institutional Review Board. Written informed consent was acquired from the parents or legal guardians and from participants 18 years or older, and assent from the participants younger than 18 as well.
Visceral adiposity
During their CRC visit, a certified technician conducted an in-lab whole body dual-energy X-ray absorptiometry (DXA) scan (Hologic QDR 4500W) on the participants to measure fat distribution and composition.30 This scan uses beams of low-energy x-ray that pass by the body tissue and are collected by detectors. Subjects are required to remove all metal, plastic, and rubber materials to avoid impact on x-ray beams. Daily quality control and calibrations are performed on the DXA scanning machine to assure that our standardized operation protocol is followed, assuring data validity. VAT and SAT were selected as the body adiposity regions of interest, with VAT in cm2 as the primary predictor of interest and measure of central obesity in this study.
Blood pressure
A clinical history and physical examination were conducted on the participants during their CRC visit, where SBP and DBP were measured using an automated system (Phillips SureSigns VM4; Koninklijke Philips NV). In the seated position, three consecutive assessments were taken after 5 minutes of rest, and a mean of the second 2 assessments was used in the analysis.30 Seated SBP and DBP levels were the primary outcomes in this study.
Actigraphy
During the week following the CRC visit, participants wore an accelerometer on their non-dominant wrist (ActiGraph GT3X) for 7 consecutive nights and completed sleep logs of their bedtime and rising times to increase the accuracy of the scoring of ACT-derived sleep estimates (Actlife 6 software, ActiGraph LLC, Pensacola, FL, USA). Sleep duration measured by the ACT was calculated as the intra-individual mean of the 7-night total sleep duration (total number of minutes slept since lights off until lights on), while sleep variability was calculated as the intra-individual standard deviation in total sleep duration across the 7 nights. Sleep midpoint was calculated as the ACT-measured mean midpoint of the sleep period such as in (sleep onset time – wakeup time)/2 across the 7 nights, while sleep regularity was calculated as the intra-individual standard deviation in sleep midpoint. Sleep duration, sleep variability, sleep midpoint and sleep regularity metrics were also calculated for weekdays (5-nights), while sleep duration and sleep midpoint were also calculated for weekends (2-nights) in order to capture whether there were any particular changes in the timing of sleep that varied depending on nights of the week.
The difference between mean sleep midpoint and sleep regularity on weekdays vs. weekends was also taken into consideration as a separate variable and calculated as, for example, Δsleep midpoint = weekends sleep midpoint – weekdays sleep midpoint (same for other sleep metrics). Sleep midpoint and sleep regularity were conceptualized and treated as moderators/effect modifiers in this study, while sleep duration and sleep variability were treated as covariates.
Finally, in order to consider the influence that entrainment may have on circadian misalignment as a function of free-days vs. days determined by environmental factors (i.e., schooling), we identified participants who were studied while “in-school” vs. while “on-break”.
Polysomnography
All participants visit were evaluated for one night in the sleep laboratory in a sound attenuated, light and temperature-controlled room using 16-channel PSG. Each subject was continuously monitored from 22:00 h until 07:00 h. The sleep recordings collected were scored based on standard criteria, and followed by an evaluation of the parameters of sleep continuity and sleep architecture. The focus for this paper was on sleep continuity factors such as sleep onset latency (SOL; the number of minutes to fall asleep since lights off), wake after sleep onset (WASO; the amount of time awake after the onset of sleep), and sleep duration (the total number of minutes slept since lights off until lights on). The apnea/hypopnea index (AHI) was determined based on the number of apneas and hypopneas per hour of sleep30 and was a key covariate in this study.
Other Key Measures
Demographic and some clinical information was collected via self-administered questionnaires. This included information on age (in years), race (black/white), ethnicity (Hispanic/non-Hispanic), and sex (assigned male/female at birth), Tanner staging31 and Morningness-Eveningness Questionnaire (MEQ).31 For the purpose of this study, age was treated as a continuous variable, while sex (male=0 and female=1) and race/ethnicity (non-Hispanic white=0 and racial/ethnic minority=1) were treated as binary variables. In our sample, there were 65 subjects (21.5%) who identified as a racial/ethnic minority and, given the inability to stratify with such sample size, we statistically controlled for the potential effect of underlying racial/ethnic disparities. Participants’ height (cm) and weight (kg) were measured during the physical examination and their body mass index (BMI) percentile was calculated.32
Statistical Analyses
Among the 421 participants, 391 had in-lab DXA data and 327 had at least 5 nights of ACT. Thus, the effective sample size for this study consisted of 303 participants with complete DXA and ACT data. There were no significant demographic differences (e.g., sex, race/ethnicity, BMI percentile, all p>0.340) between the 303 participants included in the analyses and the 118 excluded, except in terms of age with the former 0.8 years younger [16.2 (2.25)] than the latter [17.0 (2.19), p<0.001].
First, we evaluated the cross-sectional association of VAT, sleep midpoint and sleep regularity with seated SBD/DBP using multivariable linear regression. These models tested the main effects of VAT, sleep midpoint or sleep regularity on SBP/DBP levels; subsequently, the interaction between VAT and sleep midpoint or sleep regularity was further included in these models. (Table S1). Second, if the interaction term was significant (p<0.05), stratified analyses were conducted based on the quartile distribution of sleep midpoint and sleep regularity, as appropriate and rounded to clinically meaningful numbers (e.g., 2:00 AM or 45 minutes, respectively). A sleep midpoint occurring at 2:00 AM or later is considered delayed for a typical schooled adolescent,33 which coincided with the median sleep midpoint during schooldays in our sample. Similarly, 45 minutes was the median sleep irregularity in our sample. Covariates in these models included sex, age, race/ethnicity, AHI, sleep duration, and sleep variability, except when examining weekend-data only that variability could not be estimated for 2 nights, so week-long sleep variability was used. Sensitivity analyses added BMI percentile as a covariate to assure that results were not driven by the effect of global obesity vs. VAT (Table S2). Second, these models were stratified by “in-school” vs. “on-break” to test sleep midpoint and sleep regularity during weekdays or weekends as effect modifiers of VAT on SBP/DBP levels as a function of what entrainment conditions participants were evaluated under.
All analyses were performed using SPSS, version 27 (IBM Corp). All P values from two-sided tests and results were deemed statistically significant at P < 0.05.
RESULTS
Characteristics of the Sample
As shown in Table 1, among the 303 participants, who were 12 to 23 years old (median, 16.2 years), 159 were male (52.5%), 65 identified as a racial/ethnic minority (21.5%) and 51 were classified as having obesity as per BMI percentile ≥95 (16.8%). A total of 108 subjects were studied while on-break from school (35.6%). These latter participants were not significantly different in terms of sex (p=0.314), race/ethnicity (p=0.445), Tanner stage (p=0.155), BMI percentile (p=0.143), VAT (p=0.236) or SAT (p=0.224) as compared to those studied while in-school. Participants studied while on-break were on average 8.4 months older than those studied while in-school (p=0.009). Moreover, participants studied while on-break had an average sleep midpoint an hour and 10 minutes later (p<0.001), slept on average 24 minutes longer on weekdays (p<0.001) and with 15.8 minutes greater variability (p=0.001), but slept on average 36.5 minutes shorter on weekends (p<0.001) compared to those studied while in-school. Commensurate, participants studied while on-break had a lower difference from weekdays to weekends in sleep midpoint (p<0.001) and sleep duration (p<0.001) than those studied while in-school. There were no significant differences in overall sleep irregularity (p=0.492) or sleep regularity during weekdays (p=0.116) between the two sets of participants. Upon in-lab PSG, participants studied while on-break slept in the lab on average 22.3 minutes shorter (p=0.001) that those studied while in-school, consistent with their at-home sleep duration.
Table 1.
Characteristics of the sample
| All (N=303) |
In school (n=194) |
On break (n=109) |
P-value | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Female sex, % | 47.5% | 45.4% | 51.4% | 0.314 |
| Racial/ethnic minority, % | 21.5% | 20.1% | 23.9% | 0.445 |
| Age, years | 16.2 (2.2) | 16.0 (2.1) | 16.7 (2.4) | 0.009 |
| Tanner stage, score | 4.1 (0.8) | 4.1 (0.8) | 4.2 (0.8) | 0.155 |
| Anthropometrics | ||||
| BMI, percentile | 66.0 (28.0) | 64.3 (28.7) | 69.2 (26.4) | 0.143 |
| VAT, cm2 | 59.9 (39.4) | 57.9 (39.5) | 63.5 (39.2) | 0.236 |
| SAT, cm2 | 223.5 (159.0) | 215.1 (155.0) | 238.3 (165.5) | 0.224 |
| Blood pressure | ||||
| SBP, mmHg | 113.2 (11.7) | 112.5 (11.6) | 114.5 (11.6) | 0.141 |
| DBP, mmHg | 66.8 (8.6) | 66.1 (8.4) | 68.1 (8.4) | 0.053 |
| Actigraphy | ||||
| Overall | ||||
| Sleep midpoint, hh:mm | 3:55 (1:31) | 3:30 (1:34) | 4:40 (1:06) | <0.001 |
| Sleep irregularity, hh:mm | 0:56 (0:32) | 0:57 (0:34) | 0:54 (0:28) | 0.492 |
| Sleep duration, min | 420.1 (50.0) | 417.3 (47.9) | 425.2 (53.5) | 0.187 |
| Sleep variability, min | 71.5 (36.7) | 68.6 (34.5) | 76.5 (40.0) | 0.073 |
| Weekdays | ||||
| Bedtime, hh:mm | 23:23 (1:29) | 22:59 (1:24) | 24:08 (1:20) | <0.001 |
| Rising time, hh:mm | 7:52 (1:50) | 7:14 (1:46) | 8:59 (1:21) | <0.001 |
| Sleep midpoint, hh:mm | 3:04 (1:24) | 2:36 (1:16) | 3:55 (1:13) | <0.001 |
| Sleep irregularity, hh:mm | 0:50 (0:48) | 0:47 (0:54) | 0:56 (0:33) | 0.116 |
| Sleep duration, min | 418.6 (53.2) | 409.9 (48.7) | 434.1 (57.4) | <0.001 |
| Sleep variability, min | 64.0 (40.6) | 58.3 (37.6) | 74.1 (43.8) | 0.001 |
| Weekends | ||||
| Bedtime, hh:mm | 23:51 (1:30) | 23:34 (1:27) | 24:20 (1:28) | <0.001 |
| Rising time, hh:mm | 8:43 (1:26) | 8:34 (1:32) | 8:58 (1:14) | 0.024 |
| Sleep midpoint, hh:mm | 3:41 (1:20) | 3:30 (1:21) | 4:02 (1:13) | 0.001 |
| Sleep irregularity, hh:mm | n/a | n/a | n/a | n/a |
| Sleep duration, min | 446.0 (82.2) | 459.0 (80.5) | 422.5 (80.3) | <0.001 |
| Sleep variability, min | n/a | n/a | n/a | n/a |
| Weekends – Weekdays | ||||
| Bedtime, hh:mm | 0:27 (1:10) | 0:35 (1:13) | 0:12 (1:04) | 0.007 |
| Rising time, hh:mm | 0:50 (1:44) | 1:20 (1:42) | −0:02 (1:26) | <0.001 |
| Sleep midpoint, hh:mm | 0:37 (1:23) | 0:53 (1:27) | 0:07 (1:06) | <0.001 |
| Sleep irregularity, hh:mm | n/a | n/a | n/a | n/a |
| Sleep duration, min | 27.2 (84.6) | 49.1 (76.4) | −12.3 (84.7) | <0.001 |
| Sleep variability, min | n/a | n/a | n/a | n/a |
| Morningness-Eveningness | 26.0 (5.0) | 26.2 (5.0) | 25.6 (5.1) | 0.357 |
| Morning-type, % | 33.3% | 32.5% | 34.9% | 0.144 |
| Intermediate-type, % | 34.3% | 38.1% | 27.5% | |
| Evening-type, % | 32.3% | 29.4% | 37.6% | |
| Polysomnography | ||||
| Sleep duration, min | 446.6 (58.3) | 454.6 (52.1) | 432.3 (65.7) | 0.001 |
| AHI, events / hour | 2.6 (6.0) | 2.2 (2.8) | 3.2 (9.3) | 0.192 |
Data are mean (SD) or % for continuous and categorical variables, respectively. AHI = apnea/hypopnea index; BMI = body mass index; DBP = diastolic blood pressure; n/a = not applicable; SAT = Subcutaneous adipose tissue; SBP = systolic blood pressure; VAT = Visceral adipose tissue. Blood pressure levels between groups adjusted for age. Bold p-values are statistically significant at < 0.05
Sleep Midpoint and Regularity as Effect Modifiers
As shown in Table 2, no significant interactions were found between VAT and sleep midpoint on SBP (p-interaction=0.195) or DBP (p-interaction=0.936).
Table 2.
Association of visceral adiposity and circadian timing of sleep with blood pressure
| Overall (N=303) |
In School (n=194) |
On Break (n=109) |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| SBP | DBP | SBP | DBP | SBP | DBP | |
|
|
||||||
| Sleep Midpoint | ||||||
| Visceral Adiposity | 3.52 (0.67) <0.001* | 1.82 (0.51) <0.001* | 4.44 (0.87) <0.001* | 1.99 (0.68) 0.004* | 2.58 (1.09) 0.020* | 2.23 (0.82) 0.008* |
| Sleep Midpoint | 1.11 (0.68) 0.101 | −0.13 (0.52) 0.806 | 1.63 (0.90) 0.071T | −0.19 (0.70) 0.788 | −2.52 (1.41) 0.077T | −2.04 (1.06) 0.056T |
| Visceral Adiposity * Sleep Midpoint | −0.84 (0.64) 0.195 | 0.04 (0.49) 0.936 | 1.27 (0.94) 0.179 | 1.29 (0.73) 0.078T | −1.95 (1.19) 0.104 | −1.28 (0.89) 0.153 |
| Sleep Regularity | ||||||
| Visceral Adiposity | 3.48 (0.67) <0.001* | 1.80 (0.51) <0.001* | 4.26 (0.88) <0.001* | 1.95 (0.67) 0.004* | 2.48 (1.11) 0.027* | 2.15 (0.83) 0.011* |
| Sleep Irregularity | 0.03 (0.65) 0.965 | −0.45 (0.50) 0.371 | 0.03 (0.79) 0.966 | −0.76 (0.60) 0.211 | 0.05 (1.19) 0.966 | −0.11 (0.89) 0.899 |
| Visceral Adiposity * Sleep Irregularity | 1.45 (0.53) 0.007* | 0.94 (0.41) 0.022* | 1.67 (0.59) 0.005* | 1.36 (0.45) 0.003* | 1.17 (1.22) 0.340 | −0.50 (0.92) 0.591 |
Data are unstandardized regression coefficients (standard error) p-value for each standard deviation increase (z-scores) associated with each independent variable (main effects without interaction in the model) and their interaction (with main effects in the model).
Adjusted for sex, age, race/ethnicity, polysomnography-measured apnea/hypopnea index, actigraphy-measured sleep duration, and actigraphy-measured sleep variability.
p-value<0.05
0.05<p-value<0.10
In contrast, significant interactions were found between VAT and sleep irregularity on SBP (p-interaction=0.007) and DBP (p-interaction=0.022). Among adolescents with high sleep irregularity (≥ 45 minutes; n=179) each standard deviation increase in VAT was associated with 5.67 (0.93) and 3.39 (0.71) mmHg higher SBP and DBP, respectively (both p<0.001), whereas among adolescents with low sleep irregularity (< 45 minutes; n=124) VAT was not significantly associated with significantly higher SBP [0.80 (1.00), p=0.424] or DBP [0.14 (0.77), p=0.854].
Sleep Midpoint and Regularity while In-School vs. On-Break
In participants studied while in-school, no significant interactions were found between VAT and sleep midpoint on SBP (p-interaction=0.179) and DBP (p-interaction=0.078), as shown in Table 2.
In contrast, significant interactions were found between VAT and sleep irregularity on SBP (p-interaction=0.005) and DBP (p-interaction=0.003) in participants studied while in-school. Among adolescents with high sleep irregularity while in-school (≥ 45 min; n=115) each standard deviation increase in VAT was associated with a 6.57 (1.11) and 3.33 (0.89) mmHg higher SBP and DBP levels, respectively (both p<0.001), whereas among adolescents with low sleep irregularity while in-school (< 45 min; n=79) VAT was not significantly associated with SBP [0.76 (1.32), p=0.569] or DBP [0.09 (1.01), p=0.932].
In participants studied while “on break”, no significant interactions were found between VAT and sleep midpoint or sleep irregularity on SBP (p-interaction=0.104 and p-interaction=0.340, respectively) or DBP (p-interaction=0.153 and p-interaction=0.591, respectively).
Weekdays and Weekends Sleep Midpoint and Regularity while In-School vs. On-Break
As shown in Table 3, significant interactions were found between VAT and weekdays sleep midpoint on SBP (p-interaction=0.026) and DBP (p-interaction= 0.043) in participants studied while in-school. Commensurate, among adolescents with a delayed sleep midpoint during school-(week)days (≥ 2:00 AM; n=133), each standard deviation increase in VAT was associated with a 4.93 (1.07) and 2.32 (0.80) mmHg increase in SBP (p<0.001) and DBP (p=0.004), respectively, whereas among adolescents with an earlier sleep midpoint during school-(week)days (< 2:00 AM; n=61) VAT was not significantly associated with SBP [2.49 (1.58), p=0.120] or DBP [0.93 (1.28), p=0.469].
Table 3.
Association of visceral adiposity and circadian timing of sleep during weekdays and weekends with blood pressure as a function of being in school vs. on break
| In School | On Break | |||
|---|---|---|---|---|
|
|
||||
| SBP | DBP | SBP | DBP | |
|
|
||||
| Weekdays | ||||
| Visceral Adiposity | 4.43 (0.87) <0.001* | 1.99 (0.67) 0.003* | 2.18 (1.08) 0.046* | 1.88 (0.82) 0.023* |
| Sleep Midpoint | 2.01 (1.13) 0.076 T | 0.58 (0.87) 0.504 | −2.54 (1.29) 0.052 T | −1.51 (0.98) 0.124 |
| Visceral Adiposity * Sleep Midpoint | 1.82 (0.81) 0.026* | 1.27 (0.62) 0.043* | −0.54 (1.15) 0.642 | −0.24 (0.87) 0.787 |
| Visceral Adiposity | 4.36 (0.87) <0.001* | 1.94 (0.67) 0.004* | 2.25 (1.10) 0.044* | 1.91 (0.83) 0.023* |
| Sleep Irregularity | 1.02 (0.72) 0.160 | −0.23 (0.55) 0.684 | −0.98 (1.75) 0.578 | −0.86 (1.31) 0.512 |
| Visceral Adiposity * Sleep Irregularity | 0.44 (1.03) 0.667 | 0.55 (0.79) 0.490 | 2.92 (1.36) 0.034* | 0.95 (1.04) 0.363 |
| Weekends | ||||
| Visceral Adiposity | 4.36 (0.87) <0.001* | 2.03 (0.67) 0.003* | 2.54 (1.12) 0.025* | 2.21 (0.84) 0.010* |
| Sleep Midpoint | −0.20 (0.80) 0.800 | −0.24 (0.62) 0.697 | −1.40 (1.28) 0.278 | −0.27 (0.96) 0.776 |
| Visceral Adiposity * Sleep Midpoint | −0.27 (0.76) 0.724 | 0.57 (0.59) 0.328 | −2.43 (1.36) 0.077 T | −0.60 (1.04) 0.566 |
| Weekends – Weekdays | ||||
| Visceral Adiposity | 4.20 (0.87) <0.001* | 1.99 (0.67) 0.004* | 2.41 (1.11) 0.033* | 2.08 (0.83) 0.014* |
| ΔSleep Midpoint | −1.47 (0.75) 0.051T | −0.51 (0.58) 0.374 | 1.31 (1.27) 0.305 | 1.35 (0.95) 0.159 |
| Visceral Adiposity * ΔSleep Midpoint | −1.96 (0.68) 0.004* | −0.69 (0.54) 0.201 | −1.00 (1.37) 0.467 | 0.14 (1.03) 0.889 |
Data are unstandardized regression coefficients (standard error) p-value for each standard deviation increase (z-scores) associated with each independent variable (main effects without interaction in the model) and their interaction (with main effects in the model). Adjusted for sex, age, race/ethnicity, polysomnography-measured apnea/hypopnea index, actigraphy-measured sleep duration, and actigraphy-measured sleep variability.
p-value<0.05
0.05<p-value<0.10
Also, significant interactions were found between VAT and weekdays sleep irregularity on SBP (p-interaction= 0.034) in participants studied while on-break, as shown in Table 3. Among adolescents with high sleep irregularity on weekdays while on-break (≥ 45 min, n=57), each standard deviation increase in VAT was marginally associated with greater SBP [3.25 (1.92), p=0.097], whereas among adolescents with low sleep irregularity on weekdays while on-break (<45 minutes; n=52) VAT was not significantly associated with greater SBP [1.01 (1.44), p=0.489].
Finally, there was a significant interaction between VAT and Δsleep midpoint from weekdays to weekends on SBP (p-interaction=0.004) in participants studied while in-school. Thus, among adolescents with a greater difference in sleep midpoint from weekdays to weekends while in-school (≥ 30 minutes later on weekends than weekdays; n=126), VAT was marginally associated with SBP [1.90 (1.09), p=0.085], while among adolescents with a lower difference in sleep midpoint from weekdays to weekends while in-school (< 30 minutes; n=68), each standard deviation increase in VAT was associated with a 7.94 (1.60) mmHg increase in SBP (p =0.001).
Self-reported Circadian Preference as an Effect Modifier
We also found significant interactions between VAT and MEQ scores on SBP (p-interaction=0.045) and DBP (p-interaction=0.014), as shown in Table S3. Among evening-type adolescents (n=98) each standard deviation increase in VAT was associated with 3.32 (0.88) mmHg higher DBP (p<0.001), whereas among morning-type adolescents (n=100) VAT was not significantly associated with higher DBP [0.62 (0.99) mmHg, p=0.531].
DISCUSSION
This study demonstrates that deviations in the circadian timing of sleep identify those adolescents with greater BP levels associated with visceral adiposity. Specifically, our novel findings showed that a delayed sleep phase during schooldays and an irregular sleep phase during free days (i.e., school breaks) identified those adolescents in whom visceral adiposity had a greater impact on BP levels. Importantly, these associations were independent of relevant demographic factors, such as age, sex and race/ethnicity, or clinical factors, such as sleep apnea, duration and variability. Our population-based findings, summarized in Table S4, indicate that different expressions of circadian misalignment require evaluation under different real-world entrainment scenarios, while both metrics help identify that a misaligned circadian timing of sleep contributes to increased cardiovascular risk associated with obesity in youth.
Given that obesity is a risk factor for chronic conditions, such as hypertension, atherosclerosis or T2D,3,34 but is a complex multifactorial condition,11 identifying novel factors, such as sleep and its circadian rhythmicity, that may influence its impact on adverse health outcomes is essential for the development of preventive measures and application of early interventions. A number of studies have examined the role that sleep plays in obesity and cardiovascular health,35 however, most of these studies predominantly focus on sleep duration20,36,37 rather than the circadian timing of sleep, which is known to shift with puberty.26 Furthermore, the studies that do evaluate deviations in the circadian timing of the sleep-wake cycle in association with obesity or cardiovascular health have focused mostly on adults.38–40 As a result, the influence of circadian misalignment on obesity-related eBP at the critical developmental period of adolescence has remained elusive.
Based on our novel findings, it appears that the risk of developing adverse cardiovascular outcomes in adolescence is not only contingent upon the level of visceral adiposity, and that an irregular or delayed sleep-wake cycle will increase the impact that this type of pro-inflammatory adiposity has on BP as early as adolescence. In the overall sample, we found that among those who had an irregular timing of sleep increased VAT had a stronger impact on eBP levels. Our findings are supported by previous studies showing that, beyond insufficient sleep or sleep apnea, deviations in the timing or irregularity of the sleep-wake cycle are related to increased hypertension risk,41 as previously observed in older populations and shift workers.39,40 Furthermore, our study builds upon previous findings by establishing that the effect of misaligned sleep may surface during adolescent years rather than adulthood,10,42 informing targeted early prevention. Indeed, those adolescents with a more regular timing of sleep showed no significant associations between their increased VAT and eBP levels, suggesting that a regular sleep-wake cycle may act as a protective factor against eBP and future development of clinical hypertension.
Our findings also indicate that the sleep midpoint and its regularity are meaningful moderators based on the entrainment conditions that adolescents are evaluated under; specifically, whether they are studied while in-school vs. on-break. Therefore, assessing these circadian measures under different entrainment scenarios (in-school vs. on-break) can better capture their role in increasing cardiovascular risk in youth. Adolescents evaluated while in-school who experienced a delay in their sleep midpoint were more likely to experience increased eBP levels in relation to higher visceral adiposity, therefore, sleep midpoint is a key marker of circadian misalignment under these entrainment conditions. Additionally, adolescents evaluated while in-school who have a smaller midpoint difference between weekdays and weekends were more likely to experience increased eBP levels in relation to higher visceral adiposity. In our adolescent sample, subjects were going to sleep late enough on school days that their weekday sleep midpoint matched their weekend midpoint, very likely driven by having to face increasing after-school academic/sports/social responsibilities coupled with early high-school start-times, which have been shown to be detrimental to health.12 Prior research has shown that a delayed sleep-wake phase is not only associated with negative educational and emotional outcomes in adolescents,43,44 but also with eBP levels41 and higher HOMA-IR and BMI in adults.45
Our novel stratified analyses showed that overall adolescents evaluated during the academic school year who had greater sleep irregularity experienced significant increases in eBP levels as a function of higher VAT. Although studies have not yet examined the role of entrainment and how irregular and delayed sleep impacts BP levels in adolescents, there is evidence suggesting that youth with obesity had a significantly later sleep midpoint and greater sleep irregularity from school nights to weekend nights,46 and worse cardiovascular outcomes.47 Our results are capturing the entire week for most subjects in-school, where it’s accounting for both weekdays and weekends and the social jetlag that occurs as a result of a delayed sleep midpoint on school days and highly irregular across the week. Therefore, the adverse impact of a delayed and irregular timing of sleep persisted in adolescents in-school, as did the protective nature of a regular sleep-wake cycle. Another important finding in our study is that adolescents evaluated while on-break who experienced greater sleep irregularity on weekdays showed a stronger impact between VAT and eBP levels, whereas among those with less irregularity in their sleep-wake cycle the association between visceral adiposity and BP levels was greatly diminished and not significant. While on-break, adolescents further delayed their sleep midpoint as they were free of forced entrainment to the school schedule, hence sleep irregularity becomes a key marker of circadian misalignment when adolescents are evaluated under these entrainment conditions. Previous studies and our own novel findings emphasize the importance of ascertaining the degree of circadian misalignment of the sleep-wake cycle under different real-world entrainment conditions in adolescents. Indeed, our data showed that adolescents who keep well aligned with their school and free day demands and do not experience delays in their sleep midpoint or irregularity in their sleep-wake schedule, have a more favorable, non-significant association between visceral adiposity and BP levels. These data further suggest that an aligned circadian timing of sleep may protect these adolescents from adverse cardiovascular outcomes.
Together, our data further support that circadian misalignment, independent of sleep apnea, insufficient sleep, and sleep variability, may be a key determinant of the cardiovascular sequelae associated with central obesity as early as adolescence, and it may express differently based on whether forced entrainment (to school schedules) is present or not (free days on break). Consequently, our study showed that the distinct sleep-related markers of circadian misalignment require measurement under different entrainment conditions in adolescents. In fact, we also found evidence for self-reported morningness-eveningness (MEQ) scores to modify the association between VAT and BP levels, indicating that the association of VAT with eBP was stronger among evening-type adolescents than among morning-type adolescents. While MEQ scores do not allow a fine-grained analysis of the circadian timing of the sleep-wake cycle under real-world conditions as at-home ACT does, these data are consistent with our ACT-measured findings and suggest that simple metrics such as the MEQ can be used as a first step in screening adolescents at greatest risk for circadian misalignment and cardiovascular morbidity. Randomized clinical trials should test whether aligning the circadian timing of sleep in overweight adolescents improves their cardiovascular health profile. Additionally, future research should investigate whether circadian misalignment in adolescents correlates with greater irregularity in lifestyle factors, such as nutrition and physical activity, as some studies have found that irregular bedtimes and rising times are associated with poor diet composition and skipping breakfast.48 The findings of our study should be interpreted in light of some potential limitations. First, we only had 7 nights of ACT data, which precluded calculating sleep regularity during weekends, as we only had 2 nights out of a week recording instead of 8 nights for a month recording. Second, these data also precluded using other metrics of sleep regularity, such as IS, CPD, and SRI.49 The accuracy of these different measures of deviations in habitual sleep patterns vary depending on the methodology of the study. Since our study had a smaller sample size and limited data, the most accurate metric would be the intra-individual standard deviation of the sleep midpoint, as used herein.49 Third, the design of the study was cross-sectional, which limits causal inference in the associations found. Future studies should examine these associations longitudinally as adolescents’ transition to young adulthood. Fourth, while we were able to study these associations in adolescents who were evaluated either in-school or on-break, we did not have repeated measures data allowing to examine within-subjects effects when adolescents shift from being in-school to on-break. Finally, eBP was not based on 24-hour average ambulatory BP, represented by 3 or more measures across different time points within a single day as well as across 3 different office visits, as performed in clinical settings.50
PERSPECTIVES
In summary, this cohort study found that circadian misalignment of the sleep-wake cycle may increase the impact of visceral adiposity on elevated blood pressure in adolescents. These data support that an irregular sleep-wake phase, independent of sleep apnea and insufficient sleep, may contribute to the development of cardiovascular sequelae associated with central obesity. Furthermore, when considering how entrainment plays a role, our findings suggest that a delayed sleep-wake phase during school days and an irregular sleep-wake phase during breaks best identify those adolescents with greater cardiovascular risk associated with central obesity. These data suggest that deviations in the circadian timing of sleep contribute to adverse cardiovascular outcomes and that its distinct misalignment metrics require measurement under different entrainment conditions in youth. Our findings are highly significant from a translational standpoint to public health prevention and clinical care, as they inform behavioral and pharmacological sleep and circadian interventions in youth. Furthermore, it addresses the need to conduct more research on how circadian misalignment may impact health domains beyond blood pressure, particularly from a longitudinal standpoint that examines the impact across the lifespan.
Supplementary Material
NOVELTY AND RELEVANCE.
What is new?
Our novel findings demonstrate that the association of visceral adiposity with eBP is strong in adolescents with high sleep irregularity, while non-significant in those with optimal sleep regularity.
A delayed sleep midpoint during school days and an irregular sleep midpoint during free days more reliably identifies those adolescents with eBP associated with visceral adiposity.
What is relevant?
Circadian misalignment in adolescence contributes to eBP independent of insufficient sleep, a risk factor for obesity-related cardiovascular morbidity.
What are the clinical implications?
Adolescents with obesity should be screened for circadian misalignment as they may carry an adverse cardiovascular prognosis.
Clinical evaluations of sleep-wake patterns should account for different entrainment scenarios in order to uncover distinct forms of circadian misalignment.
ACKNOWLEDGEMENTS
This work would not be possible without the support of funding agencies, the faculty, technologists, and trainees of the Sleep Research and Treatment Center at the Penn State University Milton S. Hershey Medical Center, as well as our collaborators from Public Health Science and the Heart Vascular Institute.
SOURCES OF FUNDING
Research reported in this publication was supported in part by the National Heart, Lung, and Blood Institute, National Institute of Mental Health, and the National Center for Advancing Translational Sciences of the National Institutes of Health under Awards Number R01HL136587 (Fernandez-Mendoza), R01MH118308 (Fernandez-Mendoza), and UL1TR000127 (Penn State University). Ms. Morales-Ghinaglia is funded by the American Heart Association through a predoctoral fellowship (Award Number 23PRE1011962). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- CTS
circadian timing of sleep
- ACT
actigraphy
- VAT
visceral adipose tissue
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- eBP
elevated blood pressure
- BMI
body mass index
- T2D
type 2 diabetes
- StDev
standard deviation
- PSCC
penn state child cohort
- CRC
clinical research center
- DXA
dual-energy x-ray absorptiometry
- SAT
subcutaneous adipose tissue
- PSG
polysomnography
- SOL
sleep onset latency
- WASO
wake after sleep onset
- AHI
apnea hypopnea index
- MEQ
morningness-eveningness questionnaire
- HOMA-IR
homeostasis model assessment of insulin resistance
- IS
interdaily stability
- CPD
composite phase deviation
- SRI
sleep regularity index
Footnotes
DISCLOSURES
Dr. Fernandez-Mendoza reported receiving grants from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Mental Health (NIMH) during the conduct of the study; and grants from Pfizer Inc, the American Heart Association, the National Institute on Drug Abuse, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Vgontzas reported receiving grants from the National Foundation for Research and Innovation EU/Greece outside the submitted work. No other disclosures were reported.
REFERENCES
- 1.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and Adolescent Obesity in the United States: A Public Health Concern. Glob Pediatr Health. 2019. Dec 1;6:2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slyper AH, Rosenberg H, Kabra A, Weiss MJ, Blech B, Gensler S, Matsumura M. Early atherogenesis and visceral fat in obese adolescents. Int J Obes (Lond). 2014. Jul;38(7):954–8. [DOI] [PubMed] [Google Scholar]
- 3.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014. Dec 4;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010. Dec 2;363(23):2211–9. doi: 10.1056/NEJMoa1000367. Erratum in: N Engl J Med. 2011 Sep 1;365(9):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012. Sep 4;126(10):1301–13. [DOI] [PubMed] [Google Scholar]
- 6.Suliga E Visceral adipose tissue in children and adolescents: a review. Nutr Res Rev. 2009. Dec;22(2):137–47. [DOI] [PubMed] [Google Scholar]
- 7.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015. Oct 27;132(17):1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi W, Kim K, Im M, Ryang S, Kim EH, Kim M, Jeon YK, Kim SS, Kim BH, Pak K, Kim IJ, Kim SJ. Association between visceral adipose tissue volume, measured using computed tomography, and cardio-metabolic risk factors. Sci Rep. 2022. Jan 10;12(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean HJ, Sellers EA. Comorbidities and microvascular complications of type 2 diabetes in children and adolescents. Pediatr Diabetes. 2007. Dec;8 Suppl 9:35–41. [DOI] [PubMed] [Google Scholar]
- 10.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016. Mar 8;113(10):E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rippe JM, Crossley S, Ringer R. Obesity as a chronic disease: modern medical and lifestyle management. J Am Diet Assoc. 1998. Oct;98(10 Suppl 2):S9–15. [DOI] [PubMed] [Google Scholar]
- 12.Kjeldsen JS, Hjorth MF, Andersen R, Michaelsen KF, Tetens I, Astrup A, Chaput JP, Sjödin A. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond). 2014. Jan;38(1):32–9. [DOI] [PubMed] [Google Scholar]
- 13.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008. Sep 2;118(10):1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cespedes Feliciano EM, Quante M, Rifas-Shiman SL, Redline S, Oken E, Taveras EM. Objective Sleep Characteristics and Cardiometabolic Health in Young Adolescents. Pediatrics. 2018. Jul;142(1):e20174085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meininger JC, Gallagher MR, Eissa MA, Nguyen TQ, Chan W. Sleep duration and its association with ambulatory blood pressure in a school-based, diverse sample of adolescents. Am J Hypertens. 2014. Jul;27(7):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quist JS, Sjödin A, Chaput JP, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev. 2016. Oct;29:76–100. [DOI] [PubMed] [Google Scholar]
- 17.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012. Mar;59(3):747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He F, Bixler EO, Berg A, Imamura Kawasawa Y, Vgontzas AN, Fernandez-Mendoza J, Yanosky J, Liao D. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015. Jul;16(7):856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, Vgontzas AN, Liao D. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015. Dec;16(12):1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T, Mariani S, Redline S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2020. Mar 10;75(9):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Calhoun SL, He F, Liao D, Sawyer MD, Bixler EO. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016. Nov 1;311(5):E851–E858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017. Mar;61:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016. Jul 1;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JY, Choi WJ, Lee HS, Lee JW. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine (Baltimore). 2019. Mar;98(9):e14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012. Feb 5;349(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011. Jun;58(3):637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014. Jun;54(6):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, Malik BH, Gupta D, Rutkofsky I. The Role of Circadian Misalignment due to Insomnia, Lack of Sleep, and Shift Work in Increasing the Risk of Cardiac Diseases: A Systematic Review. Cureus. 2020. Jan 9;12(1):e6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Fedok F, Vlasic V, Graff G. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008. Nov;52(5):841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Mendoza J, He F, Calhoun SL, Vgontzas AN, Liao D, Bixler EO. Association of Pediatric Obstructive Sleep Apnea With Elevated Blood Pressure and Orthostatic Hypertension in Adolescence. JAMA Cardiol. 2021. Oct 1;6(10):1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993. Apr;16(3):258–62. [DOI] [PubMed] [Google Scholar]
- 32.Freedman DS. Interpreting Weight, Height, and Body Mass Index Percentiles in the US Centers for Disease Control and Prevention Growth Charts. JAMA Pediatr. 2022;176(4):424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pande B, Parveen N, Parganiha A et al. Shortening of sleep length and delayed mid-sleep on free days are the characteristic features of predominantly morning active population of Indian teenagers. Sleep Biol. Rhythms 2018;16, 431–439 [Google Scholar]
- 34.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001. Jul 9;161(13):1581–6. [DOI] [PubMed] [Google Scholar]
- 35.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007. Apr;5(2):93–102. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006. Aug;29(8):1009–14. [DOI] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011. Jun;32(12):1484–92. [DOI] [PubMed] [Google Scholar]
- 38.Makarem N, Zuraikat FM, Aggarwal B, Jelic S, St-Onge MP. Variability in Sleep Patterns: an Emerging Risk Factor for Hypertension. Curr Hypertens Rep. 2020. Feb 21;22(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kervezee L, Kosmadopoulos A, Boivin DB. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur J Neurosci. 2020. Jan;51(1):396–412. [DOI] [PubMed] [Google Scholar]
- 40.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009. Mar 17;106(11):4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, Nyenhuis SM, Ramos AR, Shah NA, Sotres-Alvarez D, et al. Sleep Timing, Stability, and BP in the Sueño Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Chest. 2019. Jan;155(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse Impact of Sleep Restriction and Circadian Misalignment on Autonomic Function in Healthy Young Adults. Hypertension. 2016. Jul;68(1):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asarnow LD, McGlinchey E, Harvey AG. The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. J Adolesc Health. 2014. Mar;54(3):350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merikanto I, Lahti T, Puusniekka R, Partonen T. Late bedtimes weaken school performance and predispose adolescents to health hazards. Sleep Med. 2013. Nov;14(11):1105–11. [DOI] [PubMed] [Google Scholar]
- 45.Taylor BJ, Matthews KA, Hasler BP, Roecklein KA, Kline CE, Buysse DJ, Kravitz HM, Tiani AG, Harlow SD, Hall MH. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep. 2016. Feb 1;39(2):457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skjåkødegård HF, Danielsen YS, Frisk B, Hystad SW, Roelants M, Pallesen S, Conlon RPK, Wilfley DE, Juliusson PB. Beyond sleep duration: Sleep timing as a risk factor for childhood obesity. Pediatr Obes. 2021. Jan;16(1):e12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins S, Stoner L, Lubransky A, Howe AS, Wong JE, Black K, Skidmore P. Social jetlag is associated with cardiorespiratory fitness in male but not female adolescents. Sleep Med. 2020. Nov;75:163–170. [DOI] [PubMed] [Google Scholar]
- 48.Mathew GM, Reichenberger DAgi, Master L, Buxton OM, Hale L, Chang AM. Worse sleep health predicts less frequent breakfast consumption among adolescents in a micro-longitudinal analysis. Int J Behav Nutr Phys Act. 2022. Jun 17;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer D, Klerman EB, Phillips AJK. Measuring sleep regularity: theoretical properties and practical usage of existing metrics. Sleep. 2021. Oct 11;44(10):zsab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020. Jun;75(6):1334–1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


