Abstract
Background:
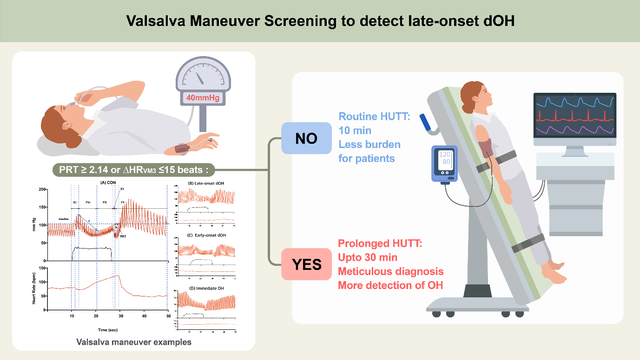
Standard autonomic testing includes a 10-min head-up tilt table test (HUTT) to detect orthostatic hypotension (OH). Although this test can detect delayed OH (dOH) between 3 and 10 min of standing, it cannot detect late-onset dOH after 10 min of standing.
Methods:
To determine whether Valsalva maneuver (VM) responses can identify patients who would require prolonged HUTT to diagnose late-onset dOH, patients with immediate OH (iOH: onset < 3 min; n = 176), early-onset dOH (edOH: onset between 3–10 min; n = 68), and late-onset dOH (ldOH: onset >10 min; n = 32) were retrospectively compared to controls (CON, n = 114) with normal HUTT and CASS (composite autonomic scoring scale) score of 0.
Results:
Changes in baseline systolic blood pressure at late phase 2 (ΔSBPVM2), heart rate at phase 3 (ΔHRVM3), and Valsalva ratio were lower, and pressure recovery time (PRT) at phase 4 was longer in late-onset dOH patients than in CON. Differences in PRT and ΔHRVM3 remained significant after correcting for age. A PRT ≥ 2.14 s and ΔHRVM3 ≤ 15 bpm distinguished late-onset dOH from age- and sex-matched controls. Patients with longer PRT (relative risk ratio (RR) = 2.189 [1.579–3.036]) and lower ΔHRVM3 (RR = 0.897 [0.847–0.951]) were more likely to have late-onset dOH. Patients with longer PRT (RR = 1.075 [1.012–1.133]) were more likely to have early-onset dOH than late-onset dOH.
Conclusions:
Long PRT and short ΔHRVM3 can help to identify patients who require prolonged HUTT to diagnose late-onset dOH.
Keywords: Late-onset delayed orthostatic hypotension, Valsalva maneuver, Autonomic function test, Prolonged head-up tilt table test
Graphical Abstract
INTRODUCTION
Delayed orthostatic hypotension (dOH) is defined as a decrease in blood pressure that meets the OH criteria (i.e., systolic blood pressure (SBP) of at least 20 mmHg or diastolic blood pressure (DBP) of 10 mmHg) after 3 min of standing or at least 60° in a head-up tilt table test (HUTT).1,2 The difference in the definition of orthostatic hypotension (OH) and dOH is only the timing of the first drop in orthostatic blood pressure (BP).
The prevalence of dOH in the general population remains unclear. However, dOH makes up for approximately 20–45% of all individuals with OH.3–10 dOH can be seen in patients with milder forms of OH, neurodegenerative autonomic disease, or incomplete autonomic dysfunction.4,11,12 The presence of dOH has been associated with both parasympathetic and sympathetic adrenergic impairments, although its severity is generally milder than that of classical OH.12 Although an exaggerated orthostatic norepinephrine response is observed in patients with dOH, it is rarely observed in classic OH, suggesting that dOH is a milder form of autonomic dysfunction than immediate OH.9 A long-term follow-up study suggested that 54% of individuals with dOH progressed to classic OH, and 31% developed a clinically diagnosed alpha-synucleinopathy, including Parkinson’s disease (PD), multiple systemic atrophy (MSA), and dementia with Lewy bodies (DLB).5
The diagnosis of patients with dOH can be missed by routine autonomic testing, which may delay treatment. In clinical practice, orthostatic vitals, if performed at all, are often measured 1 and 3 min after standing; hence, the definition of dOH is based on a drop in blood pressure beyond 3 min. Although standard autonomic function tests (AFTs) that include 10 min of HUTT can detect “early”-onset dOH (onset between 3–10 min), they may miss patients with “late”-onset dOH (occurring ≥10 min of standing).13,14 Diagnosing late-onset dOH requires a prolonged HUTT, which is not practical for all patients. Therefore, it would be beneficial for both patients and clinicians to have a clinical tool to select patients who are candidates for prolonged HUTT. Autonomic responses to the VM have been shown to provide valuable information about autonomic function based on their metrics, reflecting both sympathetic and parasympathetic functions with a lesser burden than HUTT.15–20 We hypothesized that the VM results may provide useful information for clinicians to detect which patients should undergo prolonged HUTT. To test this hypothesis, we performed a retrospective comprehensive analysis and detailed assessment of the VM parameters and HUTT results using a relatively large dataset of patients and controls.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Classification of the Patient Group
This study included 2,498 patients who completed AFTs in a university-affiliated hospital between March 2016 and May 2022. The collected data included age, sex, body mass index (BMI), current chronic disease status (hypertension (HTN), diabetes mellitus (DM), PD, MSA, and DLB), and medication at the time of AFTs.
Patients older than 19 years who were diagnosed with OH based on the consensus criteria1 were included. OH was subcategorized as immediate OH (onset < 3 min), early-onset dOH (onset between 3–10 min), and late-onset dOH (onset after 10 min). Patients taking antihypotensive medications (e.g., midodrine) before testing were excluded. Patients with a composite autonomic scoring scale (CASS) score of 0 and a normal autonomic function test were included in the control group (CON). Patients with normal HUTT results but CASS scores > 0 were excluded from the analysis. This study was approved by the Institutional Review Board (IRB) of the Korea University Anam Hospital, Seoul, Korea (IRB no.2021AN0591). The requirement for informed consent was waived.
Valsalva Maneuver and Definition of Parameters
VM was performed at a consistent room temperature in a quiet and dimly lit environment by following the guidelines from the consensus statement of the European Federation of Autonomic Societies, endorsed by the American Autonomic Society and the European Academy of Neurology.21–25 Patients were instructed to fast for at least 2 h before the test and avoid caffeine and smoking on the test day. Continuous BP monitoring was performed using a finger cuff recording system (Finometer®; FMS, Amsterdam, Netherlands). Patients were required to blow and maintain an exhale pressure of at least 40 mmHg for 15 s, and at least three repeated reproducible measurements were recorded after a practice run. Baseline BP and heart rate (HR) were recorded for at least 5 min before VM initiation. After forced exhalation, patients were asked to remain calm in a supine position until their BP returned to the baseline value. The VM results were divided into four phases (Figure 1), as described previously.17,21,22,24,26,27 In brief, phase 1 is the start of the VM (onset of strain), and the duration reflects the increase in intrathoracic pressure. Early phase 2 reflects the decreased venous return to the heart, which causes an eventual drop in BP and the activation of baroreceptors in the aortic arch and carotid sinus. BP and HR start to increase and BP returns to baseline during late phase 2 via vagal withdrawal and increased sympathetic nervous activity. An abrupt and transient decrease in BP is observed in phase 3 due to strain release. Lastly, phase 4 is the overshoot period, in which BP and HR return to baseline and climb even higher due to continuous sympathetic nervous activity. This, in turn, activates the baroreflex, and finally BP and HR return to baseline.
Figure 1. The representative finger blood pressure and heart rate during the Valsalva maneuver.
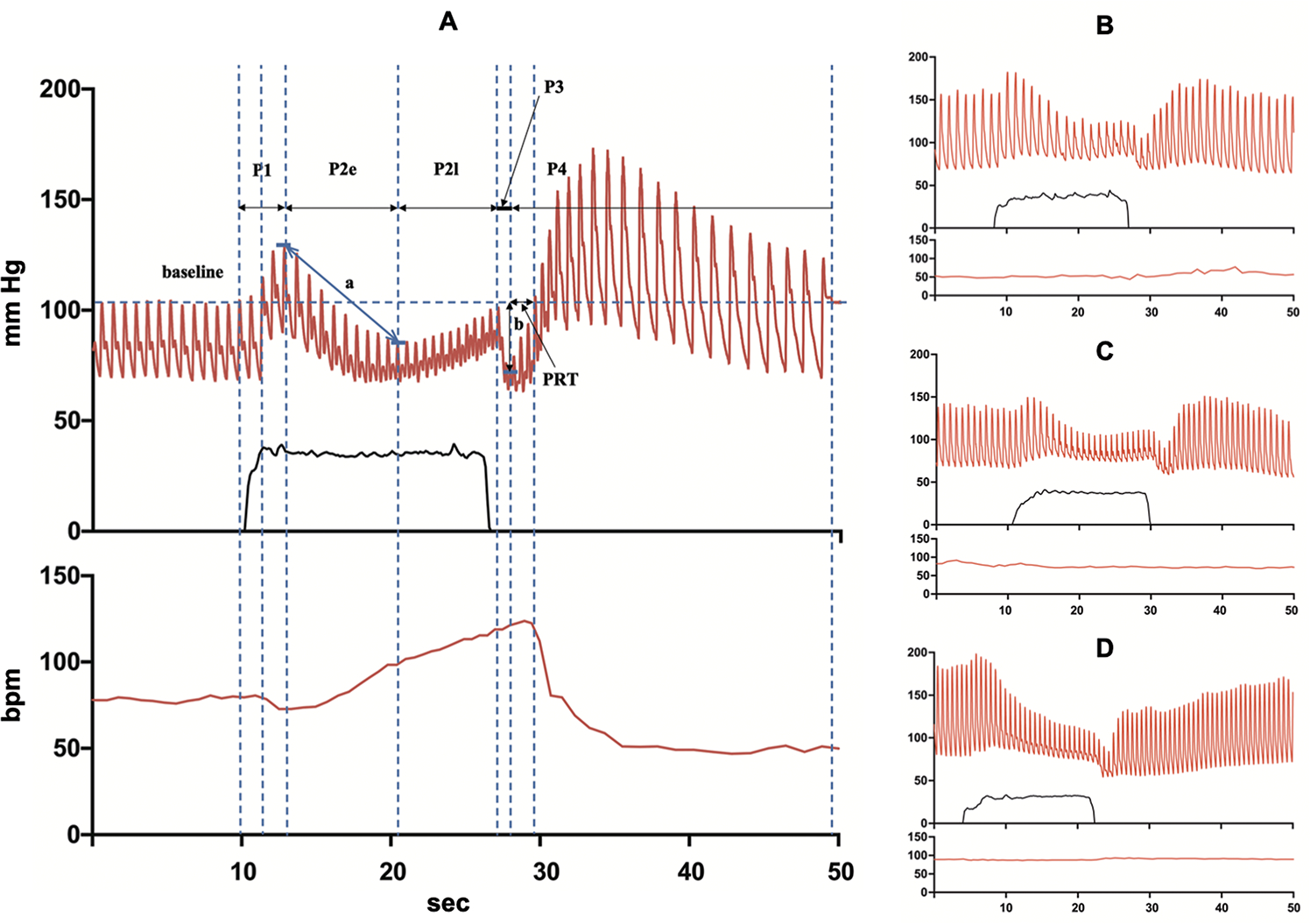
(A) Controls (CON), (B) late-onset delayed orthostatic hypotension (dOH, onset >10 min), (C) early-onset dOH (onset 3–10 min), and (D) immediate orthostatic hypotension (OH) (onset <3 min). Abbreviations: a, BRSv; b, difference between baseline and nadir systolic blood pressure of phase 3; PRT, pressure recovery time; P1, phase 1; P2e, early phase 2; P2l, late phase 2; P3, phase 3; P4, phase 4.
Valsalva metrics include parameters that assess adrenergic function, such as pressure recovery time (PRT, s), adrenergic baroreflex sensitivity (BRSa, mmHg/s), changes from baseline SBP, DBP, and mean BP (MBP) at late phase 2 (ΔSBPVM2, ΔDBPVM2, and ΔMBPVM2), and parameters that assess cardiovascular functions, such as vagal baroreflex sensitivity (BRSv, ms/mmHg), Valsalva ratio (VR), and ΔHRVM3.
PRT is the time required for the SBP to recover from the nadir SBP of phase 3 and reach baseline levels during phase 4, and is an indicator of the severity of sympathetic impairment.21 BRSa was defined as the decrease in SBP between the baseline and the nadir SBP of phase 3 divided by the PRT. ΔBPVM2 is the BP at the end of phase 2 − baseline BP. BRSv was defined as the regression curve slope between the RR interval expressed in milliseconds and the SBP values during early phase 2 and was automatically calculated using the Testwork 3 program (WR Medical Electronics Company, Stillwater, MN, USA). VR was defined as the maximum HR in phase 2 divided by the lowest HR in phase 4. ΔHRVM3 is the HR difference between baseline and phase 3, reflecting the capacity to mount a compensatory HR increase in response to the decrease in BP triggered in phase 2.
Head-up Tilt Table Test
The environmental setting was identical to that in VM.13,23,24,28 A 70° HUTT was performed using a Finometer recording system. A finger cuff was placed on the middle finger and a sphygmomanometer cuff was placed over the brachial artery. BP and HR were continuously measured using the finger cuff and automatically at 1-min intervals using the sphygmomanometer cuff. Patients were asked to rest calmly for at least 5 min in the supine position before tilting, and baseline BP and HR were recorded during this period. The tilt rate was approximately 5°/s and the tilt duration was at least 10 min. The test was stopped early if the patient could not tolerate symptoms of orthostatic intolerance. The tilt was extended for up to 30 min if the patient complained of definite orthostatic symptoms before HUTT, without evidence of an orthostatic BP fall within 10 min of HUTT. The resting-state BP was measured 10 min after returning the table to the supine position. Baseline SBP, DPB, nadir SBP, DBP, time of diagnosis of dOH or OH (the time when 20 mmHg of systolic or 10 mmHg of diastolic BP drop was first observed during the HUTT), and the degrees of maximal SBP and DBP drop obtained from the HUTT were included in the final dataset.
Statistical Analysis
All data are expressed as mean ± standard deviation unless otherwise indicated. Statistical significance was set at P < 0.05. Shapiro–Wilk and Durbin–Watson tests were performed to confirm the assumptions of normality and independence. Group comparisons were performed using analysis of variance (ANOVA) or the Kruskal–Wallis test, followed by Tukey’s or Bonferroni’s post hoc analysis. The comparison between the two groups (age- and sex-matched) was performed using a Student’s t-test or Mann–Whitney test. A chi-square analysis was performed for categorical variables. To compare the CON and late-onset dOH groups, patients were randomly matched in a 1:1 ratio using a propensity score matching strategy to minimize the effects of age and sex.29,30 The frequency distribution and cumulative frequency distribution of the time of diagnosis of late-onset dOH, early-onset dOH, and OH were calculated. Receiver operating characteristic (ROC) curves were calculated to differentiate the two groups based on the sensitivity and specificity of the Valsalva metrics. Subsequently, the following comparisons with age- and sex-matched control groups were performed: CON (reference) vs. late-onset dOH and late-onset dOH (reference) vs. early-onset dOH. The area under the curve (AUC), standard error (SE), 95% confidence interval (95% CI), and cutoff values were calculated. The optimum cutoff values from the ROC curves were obtained by applying the Youden index.31 Univariable and multivariable logistic regressions were performed to determine the association and RR between Valsalva metrics and analysis groups (CON, late-onset dOH, and early-onset dOH). The variables used for both ROC and logistic regression analyses were selected based on the results of ANOVA and age- and sex-matched comparisons.
Linear regression analysis was conducted to determine the association between the degree of drop in BP and Valsalva metrics (PRT, ΔSBPVM2, ΔDBPVM2, ΔMBPVM2, BRSa, BRSv, VR, and ΔHRVM3), and to determine the association between the time of diagnosis of dOH and OH with Valsalva metrics. Statistical analyses were performed using SAS statistical software package version 9.4. (SAS Institute, Cary, NC, United States).
RESULTS
Patient Characteristics
A total of 390 patients who met the inclusion criteria were included in the statistical analysis (n; CON = 114, late-onset dOH = 32, early-onset dOH = 68, and immediate OH = 176) (Figure S1). Demographic characteristics of the study groups are presented in Table 1. The mean ages of the late-onset dOH (61.4 ± 16.9), early-onset dOH (70.4 ± 11.6), and immediate OH groups (69.4 ± 11.4) were higher than that of the CON group (45.0 ± 17.9). Only the immediate OH group showed a predominance of women. There was no difference in BMI between the groups. The prevalence of HTN and DM was the highest in the immediate OH group (HTN, 42.6%; DM, 35.2%). Comorbid synucleinopathy (e.g., PD, MSA) was more likely to be present with early-onset dOH (20.6%) and OH (20.5%) compared to others.
Table 1.
Baseline Characteristics of Controls (CON), Patients with Delayed Orthostatic Hypotension (dOH), and Patients with Immediate Orthostatic Hypotension (OH)
| Baseline characteristics of participants | |||||
|---|---|---|---|---|---|
| CON (n = 114) | Late-onset dOH (onset > 10 min) (n = 32) | Early-onset dOH (onset 3–10 min) (n = 68) | Immediate OH (onset < 3 min) (n = 176) | P-value | |
| Age (years) | 45 ± 17.9 | 61.4 ± 16.9 | 70.4 ± 11.6 | 69.4 ± 11.4 | < 0.001 |
| Sex (M: F) | 62:52 | 18:14 | 41:27 | 52:124 | < 0.001 |
| BMI (kg/m2) | 23.5 ± 3.3 | 22.8 ± 2.5 | 24.5 ± 3.2 | 23.9 ± 3.2 | ns |
| HTN (n, %) | 17 (14.9) | 9 (28.1) | 28 (41.2) | 75 (42.6) | < 0.001 |
| CCBs (n, %) | 12 (10.5) | 3 (9.4) | 14 (20.6) | 39 (22.2) | 0.038 |
| ARBs (n, %) | 11 (9.6) | 5 (15.6) | 17 (25) | 44 (25) | 0.0026 |
| ACEis (n, %) | 1 (0.9) | 0 | 0 | 0 | ns |
| Alpha-blockers (n, %) | 1 (0.9) | 1 (3.1) | 1 (1.5) | 23 (13.1) | 0.0003 |
| Beta-blockers (n, %) | 2 (1.8) | 1 (3.1) | 6 (8.8) | 21 (11.9) | 0.0156 |
| Diuretics (n, %) | 1 (0.9) | 1 (3.1) | 3 (4.4) | 4 (2.3) | ns |
| DM (n, %) | 10 (8.8) | 4 (12.5) | 13 (19.1) | 62 (35.2) | < 0.001 |
| PD, MSA, and DLB (n, %)* | 12 (10.5) | 14 (3.1) | 1 (20.6) | 36 (20.5) | 0.0203 |
Statistical tests were performed using the Kruskal–Wallis test. Abbreviations: M, male; F, female; HTN, hypertension; DM, diabetes mellitus; PD, Parkinson’s disease; MSA, multiple systemic atrophy; DLB, dementia with Lewy bodies; CCBs, calcium channel blockers; ARBs, angiotensin receptor blockers; ACEis, angiotensin-converting enzyme inhibitors; ns, non-specific.
No patients were taking anti-parkinsonian medications at the time of testing.
Differences in Valsalva Metrics Among the Groups
Representative images of the VM in each patient group are shown in Figure 1. Statistically significant differences were found between the groups in terms of the Valsalva metrics (Figures 2 and S2). The Kruskal–Wallis test and Bonferroni’s post hoc analysis revealed that PRT, ΔSBPVM2, VR, and ΔHRVM3 were different between the early-onset dOH, late-onset dOH, and CON groups. PRT showed a gradual increase in the order of CON < late-onset dOH < early-onset dOH, while ΔSBPVM2, VR, and ΔHRVM3 decreased in the order of CON > late-onset dOH > early-onset OH. Although ΔDBPVM2, ΔMBPVM2, BRSa, and BRSv differed between the early-onset dOH and CON groups, they were not statistically different between the late-onset dOH and CON groups. Statistically significant differences between immediate OH and CON were found in all Valsalva metrics: 20.0 ± 15.4 vs. 1.7 ± 0.7 s (PRT); −36.6 ± 22.9 vs. 3.4 ± 12.2 mm Hg (ΔSBPVM2); 7.5 ± 8.0 vs. 16.0 ± 8.2 mm Hg/msec (BRSa); 3.1 ± 2.5 vs. 5.2 ± 2.3 msec/mm Hg (BRSv); 1.2 ± 0.2 vs. 1.5 ± 0.2 (VR); 11.2 ± 7.9 vs. 26.1 ± 8.3 bpm (ΔHRVM3). Statistically significant differences were also observed between the immediate OH and late-onset dOH groups in all Valsalva metrics: 20.0 ± 15.4 vs. 5.9 ± 9.4 s (PRT); −36.6 ± 22.9 vs. −9.0 ± 23.7 mm Hg (ΔSBPVM2); 7.5 ± 8.0 vs. 13.3 ± 8.9 mm Hg/msec (BRSa); 3.1 ± 2.5 vs. 4.8 ± 3.7 msec/mm Hg (BRSv); 1.2 ± 0.2 vs. 1.3 ± 0.2 (VR); 11.2 ± 7.9 vs. 16.5 ± 9.1 bpm (ΔHRVM3). However, no significant differences were found between the immediate and early-onset dOH groups (Table S1).
Figure 2. The comparison of Valsalva metrics in normal controls (CON) and the late-onset dOH (ldOH), early-onset dOH (edOH), and immediate OH (iOH) groups.
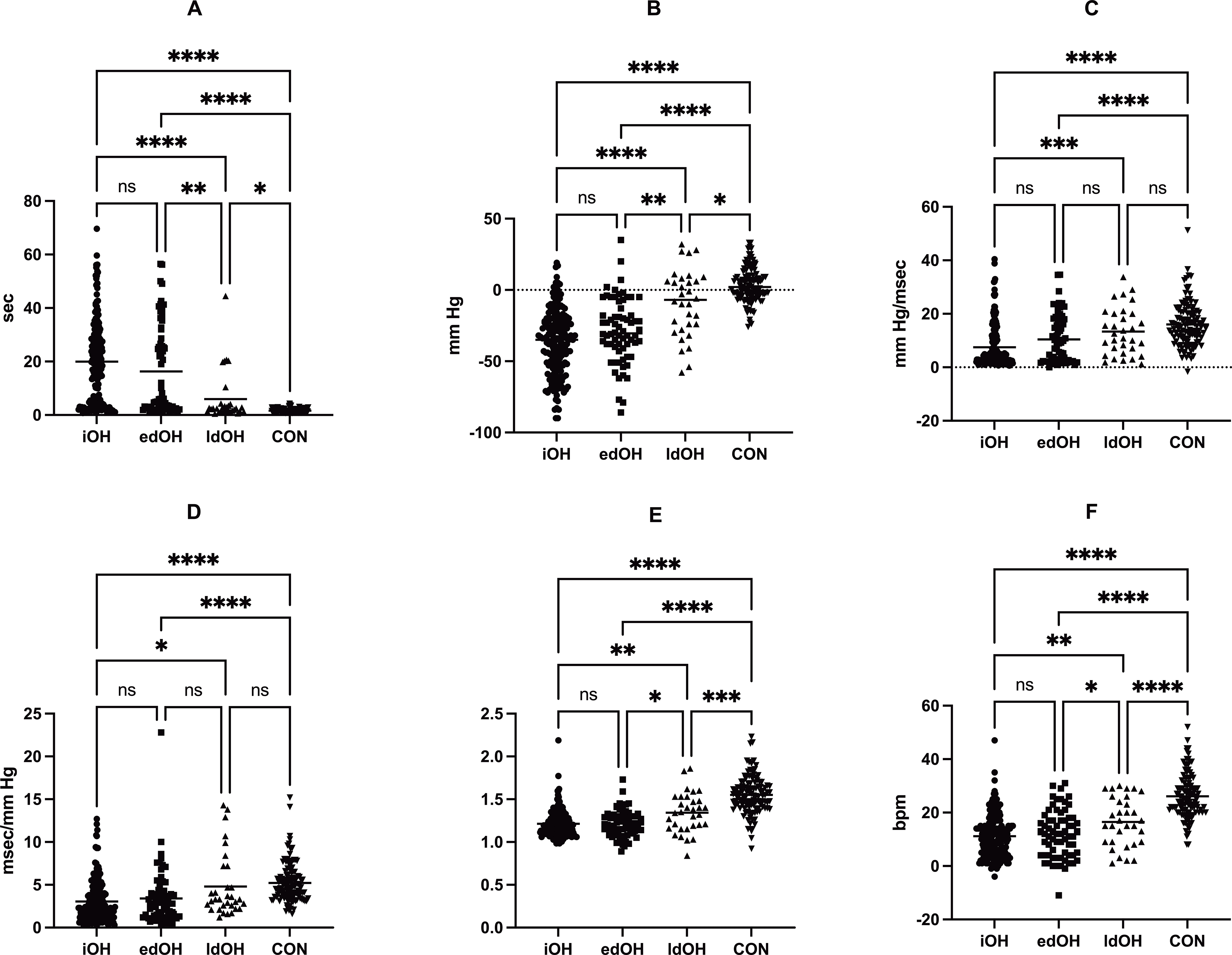
(A) PRT, (B) ΔSBPVM2, (C) BRSa, (D) BRSv, (E) Valsalva Ratio, (F) ΔHRVM3. ****, P ≤ 0.0001; ***, P ≤ 0.001; ns, P > 0.05. Statistical analyses were performed using the Kruskal–Wallis test with Bonferroni’s post hoc analysis. Abbreviations: bpm, beats per minute; ms, milliseconds. The dotted horizontal lines indicate the mean values.
Comparisons of Valsalva Metrics between the Late-Onset dOH Group and Controls (age- and sex-matched controls)
To avoid the possible confounding effects of age and sex,32–34 15 participants from the CON and late-onset dOH groups were randomly matched by age and sex and then compared. Only CON and patients with late-onset dOH were compared to determine differences in Valsalva metrics between these groups to help identify patients who need prolonged HUTT. The results showed significant differences between the CON and late-onset dOH groups in both PRT and ΔHRVM3; PRT was higher in the late-onset dOH group, whereas ΔHRVM3 was lower (Figures 3 and S3).
Figure 3. The age- and sex-matched comparison of the Valsalva metrics in normal controls (CON) and the late-onset dOH (ldOH) group.
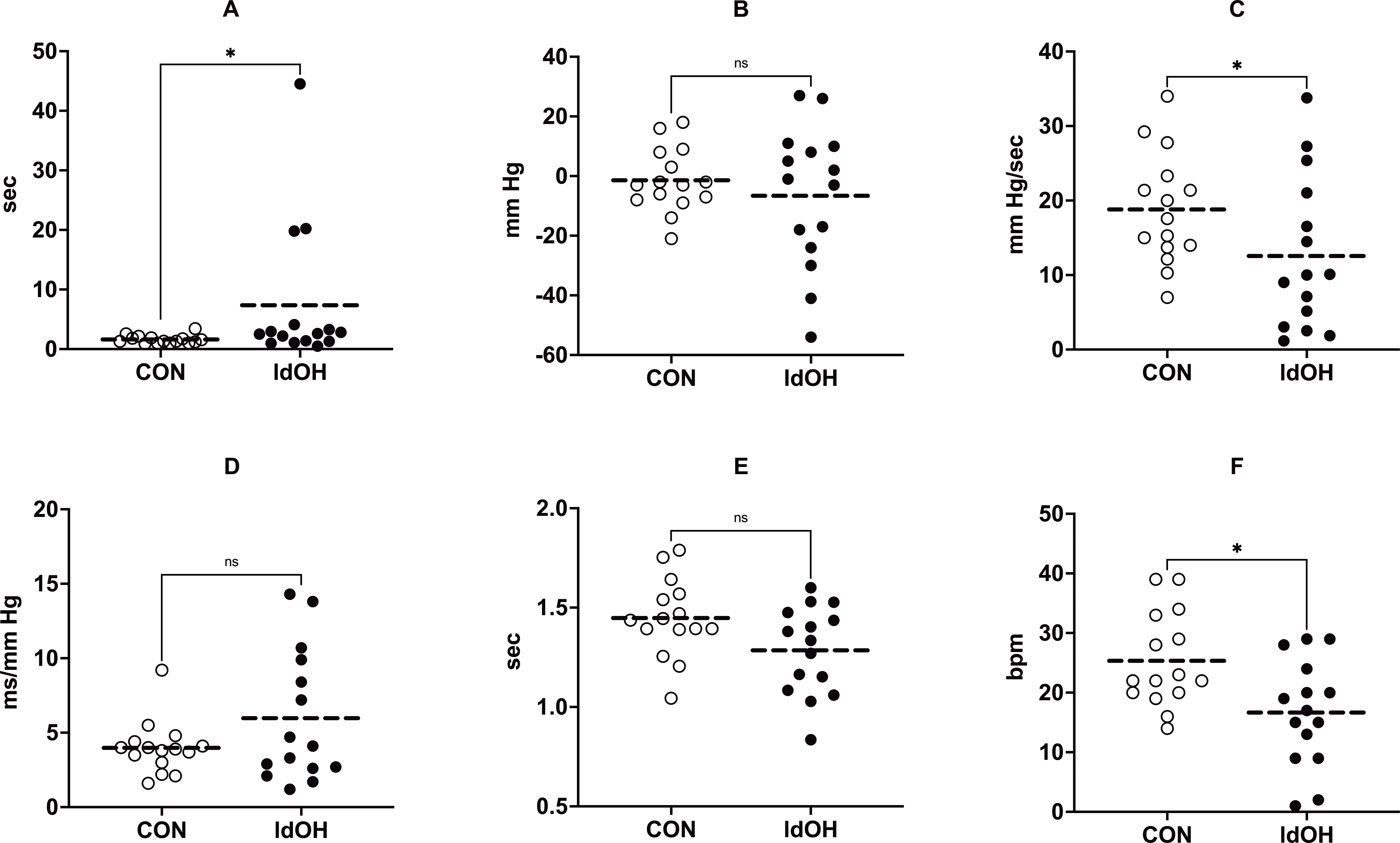
(A) PRT, (B) ΔSBPVM2, (C) BRSa, (D) BRSv, (E) Valsalva Ratio, (F) ΔHRVM3. ****, P ≤ 0.0001; ***, P ≤ 0.001; ns, P > 0.05. Statistical analyses were performed using the Mann–Whitney U test.
Time of Diagnosis of the Early-Onset and Late-Onset dOH Groups in HUTT
The average time of overall dOH diagnosis was 10.6 min (median, 9 min; min, 4 min; max, 30 min, 95 percentile: 26 min, 99 percentile: 30 min; Figure S4). In particular, the average diagnosis time was 6.9 min (median: 7 min, min: 4 min, max: 10 min, 95 percentile: 10 min, 99 percentile: 10 min) for the early-onset dOH group and 18.5 min (median: 18 min, min: 11 min, max: 30 min, 95 percentile: 30 min, 99 percentile: 30 min) for the late-onset dOH group.
Sensitivity and Specificity of Valsalva Metrics Using ROC Curves
Based on the results of the comparisons, PRT and ΔHRVM3 were selected as variables for ROC curve calculations and logistic regression. The ROC curves showed that Valsalva metrics, including PRT and ΔHRVM3, helped to predict the risk of late-onset dOH when compared to those of the CON group (reference) in all subjects (all Ps < 0.05, AUCs: 0.696 [PRT], 0.765 [ΔHRVM3]) and age- and sex-matched groups (all Ps < 0.05, AUCs: 0.713 [PRT] and 0.760 [ΔHRVM3]) (Table 2). This trend was also confirmed between the early-onset dOH and CON groups, with higher AUC, sensitivity, and specificity (Table 2).
Table 2.
The Sensitivity and Specificity Evaluation of Pressure Recovery time (PRT) and Change in Heart Rate at Phase 3 (ΔHRVM3) (results from ROC curves) for the Comparisons of the Normal Autonomic Function Test Group (CON, reference) vs. Late-Onset dOH, and Late-Onset dOH (reference) vs. Early-Onset dOH
| Valsalva metrics | AUC | SE | P-value | 95% CI | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| CON (reference) vs. late-onset dOH | |||||||
| All subjects | |||||||
| PRT | 0.696 | 0.06 | 0.0011 | 0.615–0.770 | 2.38 | 53.1 | 83.3 |
| ΔHR VM3 | 0.765 | 0.05 | < 0.0001 | 0.688–0.831 | 17 | 56.2 | 88.6 |
| Age- and sex-matched controls | |||||||
| PRT | 0.713 | 0.10 | 0.0381 | 0.520–0.863 | 2.14 | 66.7 | 86.7 |
| ΔHR VM3 | 0.760 | 0.09 | 0.0035 | 0.570–0.896 | 15 | 46.7 | 93.3 |
| Late-onset dOH (reference) vs. early-onset dOH | |||||||
| All subjects | |||||||
| PRT | 0.896 | 0.02 | < 0.0001 | 0.843–0.937 | 3.13 | 70.6 | 96.5 |
| ΔHR VM3 | 0.877 | 0.03 | < 0.0001 | 0.820–0.921 | 18 | 77.9 | 86.0 |
| Age- and sex-matched controls | |||||||
| PRT | 0.832 | 0.07 | < 0.0001 | 0.670–0.935 | 2 | 72.2 | 88.9 |
| ΔHR VM3 | 0.719 | 0.09 | 0.0115 | 0.545–0.856 | 17 | 55.56 | 83.3 |
Abbreviations: AUC, area under the curve; SE, standard error; CI, confidence interval.
Logistic Regression Between Groups and Valsalva Metrics
PRT and ΔHRVM3 remained statistically significant in both univariable and multivariable analyses (Table 3). A higher PRT and lower ΔHRVM3 increased RR in both the early- and late-onset dOH groups than in the CON (reference). The results of the multivariable logistic regression analysis revealed that patients with longer PRT (RR = 2.189 [1.579–3.036]) and lower ΔHRVM3 (RR = 0.897 [0.847–0.951]) were more likely to have late-onset dOH. The results were similar between the CON and early-onset dOH groups; however, RR was higher in PRT (RR = 2.355 [1.701–3.26]) and lower in ΔHRVM3 (RR = 0.869 [0.823–0.917]). Moreover, only PRT remained significant in distinguishing early-onset dOH from late-onset dOH (reference) in multivariable logistic regression (RR = 1.075 [1.012–1.133]).
Table 3.
Univariable and Multivariable Regression Analysis for the Comparisons of Controls (CON) (reference) vs. Late-onset dOH, CON (reference) vs. Early-onset dOH, and Late-onset dOH (reference) vs. Early-onset dOH
| Univariable logistic regression | Multivariable logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Valsalva metrics | RR | Lower 95% CI | Upper 95% CI | P-value | RR | Lower 95% CI | Upper 95% CI | P-value |
| CON (reference) vs. late-onset dOH | ||||||||
| PRT | 2.497 | 1.806 | 3.454 | < 0.001 | 2.189 | 1.579 | 3.036 | < 0.001 |
| ΔHR VM3 | 0.878 | 0.833 | 0.925 | < 0.001 | 0.897 | 0.847 | 0.951 | < 0.001 |
| CON (reference) vs. early-onset dOH | ||||||||
| PRT | 2.716 | 1.966 | 3.752 | < 0.001 | 2.355 | 1.701 | 3.26 | < 0.001 |
| ΔHR VM3 | 0.819 | 0.78 | 0.861 | < 0.001 | 0.869 | 0.823 | 0.917 | < 0.001 |
| Late-onset dOH (reference) vs. early-onset dOH | ||||||||
| PRT | 1.088 | 1.035 | 1.143 | < 0.001 | 1.075 | 1.012 | 1.133 | 0.006 |
| ΔHR VM3 | 0.934 | 0.886 | 0.983 | 0.009 | 0.968 | 0.916 | 1.024 | ns |
Abbreviations: RR, relative risk ratio.
Valsalva Metrics and the Time of Diagnosis of OH in HUTT
We evaluated whether the time of diagnosis of late-onset dOH, early-onset dOH, and OH was correlated with Valsalva metrics (Figure S5). Although the correlations were extremely weak, a significant tendency for a decrease in PRT was observed as the time of diagnosis increased, while ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3 increased (all Ps < 0.05).
Valsalva Metrics and the Maximal Degree of BP Drop in HUTT
We further evaluated the association between the degree of maximal BP drop during the HUTT and Valsalva metrics in all patients and explored whether there were trends among the CON, late-onset dOH, early-onset dOH, and immediate OH groups. The analysis revealed that PRT, ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3 were significantly correlated with the maximal magnitude of both SBP and DBP drop during the HUTT (all Ps < 0.001; Figures S6 and S7). Specifically, PRT increased as the maximal degree of BP drop increased, while ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3 decreased.
DISCUSSION
Our results revealed significant differences in the Valsalva metrics between CON and patients with late-onset dOH, both in adrenergic and cardiovagal indices, particularly in PRT and ΔHRVM3 (Figure 2). These differences remained significant after correcting for age and sex (Figure 3) and expanding the analysis to ROC analysis (Table 2) and multivariable logistic regression (Table 3). Thus, these Valsalva metrics can help identify patients who require prolonged HUTT for dOH diagnosis; however, this novel approach has not been suggested previously.
The present results are consistent with our previous understanding of the pathophysiological nature of dOH, which suggests that dOH is a milder or perhaps prodromal form of autonomic failure. Furthermore, the subclassification of patients with dOH as early- and late-onset dOH indicates that these forms represent a spectrum of diseases, with a gradual increase in the severity of autonomic impairment from late- and early-onset dOH to OH.4,9–12
The results of VM are reproducible and reliable, and its usefulness has been validated in several studies,17,18,27,35 including some in dOH.9,12 Gibbons and Freeman reported that the VM characteristics of dOH were diagnosable after 10 min of HUTT.12 dOH was associated with a smaller fall in BP during phase 2 of the VM and had a greater phase 4 overshoot compared to OH.12 The extent of the BP change in phases 2 and 4 of the VM correlated with the time of the BP fall (i.e., time of diagnosis). Patients with earlier BP falls on the HUTT had more severe BP falls during phase 2 of the VM and a reduced phase 4 overshoot. In contrast, those with later falls in BP on HUTT were less likely to demonstrate sympathetic adrenergic abnormalities in the VM. These findings suggest that dOH may be a mild or early manifestation of sympathetic adrenergic failure, a finding reproduced in our study. Additionally, our data included the CON group for comparison and more Valsalva metrics such as PRT and ΔHRVM3, which were unique findings that are probably clinically useful.
We were able to distinguish between the CON, late-onset dOH, and early-onset dOH groups based on Valsalva metrics, specifically in terms of PRT, ΔSBPVM2, VR, and ΔHRVM3. The differences in PRT and ΔHRVM3 remained significant after correcting for age and sex. ROC curve analysis confirmed that PRT and ΔHRVM3 could distinguish between the late-onset dOH and CON groups (AUC: PRT = 0.696, ΔHRVM3 = 0.765; specificity: PRT = 83.3%, ΔHRVM3 = 88.6%). Specificity increased when the age-matched control groups were compared (AUC: PRT = 0.713, ΔHRVM3 = 0.760; specificity: PRT = 86.7%, ΔHRVM3 = 93.3%). Moreover, based on the ROC curve analysis, we were able to suggest the optimal cutoff values of PRT (2.14 s) and ΔHRVM3 (15 bpm), which may be beneficial for selecting candidates for prolonged HUTT from the VM results. The suggestion of cutoff values may be clinically helpful in selecting patients for prolonged HUTT to detect late-onset dOH. These values would also help interpret the HUTT results when the magnitude of BP drop is near the borderline to satisfy the definition of OH (i.e., SBP 20 mmHg and DPB 10 mmHg), and/or if the drop was incidentally observed with compensatory mechanisms. In such cases, prolonged PRT and short ΔHRVM3 can be helpful to clinicians in supporting a diagnosis.
Logistic regression analysis also confirmed that PRT and ΔHRVM3 are valuable parameters for screening for late-onset dOH. The RR for PRT was 2.497 in the univariate analysis and 2.189 in the multivariable analysis, while the values for ΔHRVM3 were 0.878 and 0.897, respectively. In other words, patients with late-onset dOH tended to have delayed recovery in BP during Valsalva release and a lower compensatory HR increase at the end of the VM strain compared to the CON group. Similar trends were confirmed by comparing the CON and early-onset dOH groups in both univariable and multivariable logistic regression analyses. PRT, and not ΔHRVM3, helped to distinguish between late-onset and early-onset dOH; however, our ability to differentiate between patients with late-onset dOH and CON is of greater clinical relevance (i.e., detecting candidates for prolonged HUTT).
We also evaluated whether there were associations between the Valsalva metrics and the HUTT results (e.g., time of OH diagnosis and magnitude of maximal BP drop during HUTT). As previously described,12 there were statistically significant associations between the time of OH diagnosis (Figure S5) and the magnitude of BP drop (Figures S6 and S7), as seen with the Valsalva metrics, including PRT, ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3. Relatively higher correlations between the Valsalva metrics were observed with the magnitude of the BP drop. As expected, the time of diagnosis of OH and the magnitude of BP drop during HUTT were positively correlated with ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3 and negatively correlated with PRT. In contrast, the magnitude of BP drop during HUTT was positively correlated with PRT and negatively correlated with ΔBPVM2, BRSa, BRSv, VR, and ΔHRVM3. These findings are consistent with the proposition that Valsalva responses reflect the clinical severity of autonomic manifestations. In other words, indicators of clinical severity of OH (e.g., earlier diagnosis of OH and a greater decrease in BP during HUTT) were correlated with the tendency for a decrease in both adrenergic and cardiovagal functions reflected in Valsalva metrics.
This study has several limitations. This was a retrospective analysis of existing data, with limitations inherent to this approach. We included only participants with a CASS score of 0 in the control group to ensure that they did not have autonomic or orthostatic symptoms. However, only 37% of the patients in the CON underwent HUTT for > 20 min. Therefore, it is possible that some would have developed dOH if exposed to a more prolonged tilt. However, patients with a CASS score of 0 who did not undergo prolonged HUTT were unlikely to have typical orthostatic symptoms on clinical evaluation. For safety reasons, antihypertensive medications were not discontinued before the AFTs. Individual plasma volume changes are not available in the database. Although these factors may have affected the AFT results, we believe that these are not important confounders that may have impacted our analysis. The maximum duration of HUTT in our study was 30 min, and we may have missed some cases of dOH. However, our results suggest that most cases are likely to occur within 30 min (Figure S4), consistent with the observation of Gibbons and Freeman’s study.12 Therefore, if there is no clear clinical evidence of orthostatic intolerance, HUTT for more than 30 min may not be necessary. As expected, there was an overlap in the individual data points for any given VM parameter. Adequate diagnosis of dOH requires that physicians be aware of the possibility of this problem in patients complaining of orthostatic intolerance, but with negative orthostatic vitals and AFTs. Nevertheless, the VM results could be helpful in the decision to prolong HUTT for over 30 min or until diagnosis.
Our results suggest that the cutoff values of PRT and ΔHRVM3 could distinguish the late-onset dOH group from the CON group. PRT was also able to distinguish early-onset dOH from late-onset dOH. Therefore, we believe that PRT and ΔHRVM3 can provide clinical guidance to determine whether patients with orthostatic intolerance should undergo prolonged HUTT for longer than 10 min. Based on the frequency distribution results of our data, we suggest that at least 30 min HUTT is required to diagnose 99% of late-onset dOH cases, and at least 24.6% of the patients may not be diagnosed by routine autonomic testing that includes a 10-min HUTT. Patients with a PRT longer than 2.14 s and an ΔHRVM3 less than 15 bpm may benefit from prolonged HUTT. In conclusion, we demonstrated the utility of VM in the diagnosis of dOH to assist in deciding which patients would benefit from prolonged HUTT.
PERSPECTIVE
At present, there is no objective guidance to enable clinicians to decide which patients should undergo prolonged HUTT. For the first time, using VM, we proposed the optimal cutoff values of Valsalva metrics, especially in terms of PRT and ΔHRVM3. We also found that patients with late-onset dOH had blunt compensatory tachycardia in response to the hypotensive phase of VM (lower ΔHRVM3) and delayed recovery of BP after release (longer PRT). The severity of impairment of these parameters was sequentially worse in late-onset dOH (> 10 min), early-onset dOH (3–10 min), and immediate OH (< 3 min). These results are consistent with the concept that dOH is the initial stage of autonomic neuropathy. These findings will help diagnose patients with dOH who will otherwise have normal orthostatic blood pressure measurements and negative 10 minutes of HUTT as part of autonomic testing.
Supplementary Material
NOVELTY AND RELEVANCE:
WHAT IS NEW?
We discovered VM responses that can help identify late-onset dOH patients.
WHAT IS RELEVANT?
Using VM, we found that late-onset dOH patients (>10 min of HUTT) had blunted compensatory tachycardia in response to the hypotensive phase of VM (lower ΔHRVM3) and delayed recovery of BP after release (longer PRT).
The severity of impairment of these parameters was sequentially worse in late-onset dOH (>10 min), early-onset dOH (3–10 min), and immediate OH (<3 min).
These results are consistent with the concept that dOH is the initial stage of autonomic neuropathy.
CLINICAL/PATHOPHYSIOLOGICAL IMPLICATIONS?
PRT and ΔHRVM3 can help diagnose patients with dOH and determine candidates for prolonged HUTT.
Sources of Funding:
This work was supported by National Institutes of Health (NIH) grants R01 HL144568 (LO) and RO1 HL161095 (LO, IB). AD was partly funded by the NIH grant R01 HL142583.
Nonstandard Abbreviations and Acronyms
- HUTT
head-up tilt table test
- OH
orthostatic hypotension
- dOH
delayed orthostatic hypotension
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MBP
mean blood pressure
- iOH
immediate orthostatic hypotension
- edOH
early onset delayed orthostatic hypotension
- ldOH
late onset delayed orthostatic hypotension
- ΔSBPVM2
changes from baseline systolic blood pressure at late phase 2
- ΔDBPVM2
changes from baseline diastolic blood pressure at late phase 2
- ΔMBPVM2
changes from baseline mean blood pressure at late phase 2
- HRVM3
heart rate at phase 3
- RR
relative risk ratio
- PRT
pressure recovery time
- PD
Parkinson’s disease
- MSA
multiple system atrophy
- DLB
dementia with Lewy bodies
- AFT
autonomic function test
- VM
Valsalva maneuver
- HTN
hypertension
- DM
diabetes mellitus
- CASS
composite autonomic scoring scale
- CON
controls
- HR
heart rate
- IRB
Institutional Review Board
- BRSa
adrenergic baroreflex sensitivity
- BRSv
vagal baroreflex sensitivity
- VR
Valsalva ratio
- ΔHRVM3
HR difference between baseline and phase 3
- ROC
Receiver operating characteristics
- HRVdb
Heart rate variability with deep breathing
Footnotes
Disclosures: None.
Patient consent for publication: Not applicable.
Contributor Information
Jin-Woo Park, Department of Neurology, Korea University Medicine, Seoul, Korea; Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Luis E Okamoto, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Sung-Hwan Kim, Department of Neurology, Korea University Medicine, Seoul, Korea.
Seol-Hee Baek, Department of Neurology, Korea University Medicine, Seoul, Korea.
Joo Hye Sung, Department of Neurology, Korea University Medicine, Seoul, Korea.
Namjoon Jeon, Department of Neurology, Korea University Medicine, Seoul, Korea.
Alfredo Gamboa, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Cyndya A Shibao, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
André Diedrich, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA; Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA.
Byung-Jo Kim, Department of Neurology, Korea University Medicine, Seoul, Korea; BK21 FOUR Program in Learning Health Systems, Korea University, Seoul, Korea.
Italo Biaggioni, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
REFERENCES
- 1.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 2.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567–1582. doi: 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurevich T, Machmid H, Klepikov D, Ezra A, Giladi N, Peretz C. Head-up tilt testing for detecting orthostatic hypotension: how long do we need to wait? Neuroepidemiology. 2014;43:239–243. doi: 10.1159/000368699 [DOI] [PubMed] [Google Scholar]
- 4.Gibbons CH, Freeman R. Delayed orthostatic hypotension. Auton Neurosci. 2020;229:102724. doi: 10.1016/j.autneu.2020.102724 [DOI] [PubMed] [Google Scholar]
- 5.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: A 10-year follow-up study. Neurology. 2015;85:1362–1367. doi: 10.1212/wnl.0000000000002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedorowski A, van Wijnen VK, Wieling W. Delayed orthostatic hypotension and vasovagal syncope: a diagnostic dilemma. Clin Auton Res. 2017;27:289–291. doi: 10.1007/s10286-017-0424-8 [DOI] [PubMed] [Google Scholar]
- 7.Roy AG, Gopinath S, Kumar S, Kannoth S, Kumar A. Delayed Orthostatic Hypotension: A Pilot Study from India. Ann Indian Acad Neurol. 2017;20:248–251. doi: 10.4103/aian.AIAN_498_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byun JI, Moon J, Kim DY, Shin H, Sunwoo JS, Lim JA, Kim TJ, Lee WJ, Lee HS, Jun JS, et al. Delayed orthostatic hypotension: Severity of clinical symptoms and response to medical treatment. Auton Neurosci. 2018;213:81–85. doi: 10.1016/j.autneu.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 9.Torabi P, Ricci F, Hamrefors V, Sutton R, Fedorowski A. Classical and Delayed Orthostatic Hypotension in Patients With Unexplained Syncope and Severe Orthostatic Intolerance. Front Cardiovasc Med. 2020;7:21. doi: 10.3389/fcvm.2020.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzur I, Barchel D, Khateb Z, Swarka M, Izhakian S, Gorelik O. Delayed versus classic orthostatic hypotension: clinical and prognostic implications. Blood Press. 2020;29:209–219. doi: 10.1080/08037051.2020.1733389 [DOI] [PubMed] [Google Scholar]
- 11.Streeten DH, Anderson GH Jr. Delayed orthostatic intolerance. Arch Intern Med. 1992;152:1066–1072. doi: 10.1001/archinte.1992.00400170138025 [DOI] [PubMed] [Google Scholar]
- 12.Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67:28–32. doi: 10.1212/01.wnl.0000223828.28215.0b [DOI] [PubMed] [Google Scholar]
- 13.Cheshire WP Jr., Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res. 2019;29:215–230. doi: 10.1007/s10286-019-00598-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aponte-Becerra L, Novak P. Tilt Test: A Review. J Clin Neurophysiol. 2021;38:279–286. doi: 10.1097/wnp.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 15.Nathanielsz PW, Ross EJ. Abnormal response to Valsalva maneuver in diabetics: relation to autonomic neuropathy. Diabetes. 1967;16:462–465. doi: 10.2337/diab.16.7.462 [DOI] [PubMed] [Google Scholar]
- 16.Maser RE, Lenhard MJ. Obesity is not a confounding factor for performing autonomic function tests in individuals with diabetes mellitus. Diabetes Obes Metab. 2002;4:113–117. doi: 10.1046/j.1463-1326.2002.00188.x [DOI] [PubMed] [Google Scholar]
- 17.Mar PL, Nwazue V, Black BK, Biaggioni I, Diedrich A, Paranjape SY, Loyd JE, Hemnes AR, Robbins IM, Robertson D, et al. Valsalva Maneuver in Pulmonary Arterial Hypertension: Susceptibility to Syncope and Autonomic Dysfunction. Chest. 2016;149:1252–1260. doi: 10.1016/j.chest.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palamarchuk IS, Baker J, Kimpinski K. The utility of Valsalva maneuver in the diagnoses of orthostatic disorders. Am J Physiol Regul Integr Comp Physiol. 2016;310:R243–252. doi: 10.1152/ajpregu.00290.2015 [DOI] [PubMed] [Google Scholar]
- 19.Lamotte G, Takahashi M, Wu T, Sullivan P, Cherup J, Holmes C, Goldstein DS. Do indices of baroreflex failure and peripheral noradrenergic deficiency predict the magnitude of orthostatic hypotension in Lewy body diseases? Clin Auton Res. 2021;31:543–551. doi: 10.1007/s10286-021-00788-4 [DOI] [PubMed] [Google Scholar]
- 20.Low PA, Tomalia VA. Orthostatic Hypotension: Mechanisms, Causes, Management. J Clin Neurol. 2015;11:220–226. doi: 10.3988/jcn.2015.11.3.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65:1533–1537. doi: 10.1212/01.wnl.0000184504.13173.ef [DOI] [PubMed] [Google Scholar]
- 22.Novak P Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76:2010–2016. doi: 10.1212/WNL.0b013e31821e5563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seok HY, Kim YH, Kim H, Kim BJ. Patterns of Orthostatic Blood Pressure Changes in Patients with Orthostatic Hypotension. J Clin Neurol. 2018;14:283–290. doi: 10.3988/jcn.2018.14.3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low PA, Tomalia VA, Park KJ. Autonomic function tests: some clinical applications. J Clin Neurol. 2013;9:1–8. doi: 10.3988/jcn.2013.9.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JB, Kim H, Sung JH, Baek SH, Kim BJ. Heart-Rate-Based Machine-Learning Algorithms for Screening Orthostatic Hypotension. J Clin Neurol. 2020;16:448–454. doi: 10.3988/jcn.2020.16.3.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JW, Okamoto LE, Shibao CA, Biaggioni I. Pharmacologic treatment of orthostatic hypotension. Auton Neurosci. 2020;229:102721. doi: 10.1016/j.autneu.2020.102721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura RA, Tajik AJ. The Valsalva maneuver and response revisited. Mayo Clin Proc. 1986;61:211–217. doi: 10.1016/s0025-6196(12)61852-7 [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Paik SH, Phillips JV, Jeon NJ, B.J K, B.M K. Cerebral perfusion monitoring using near-infrared spectroscopy during head-up tilt table test in patients with orthostatic intolerance. Front Hum Neurosci. 2019;13:55. doi: 10.3389/fnhum.2019.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung TK, Rosenthal EL, Porterfield JR, Carroll WR, Richman J, Hawn MT. Examining national outcomes after thyroidectomy with nerve monitoring. J Am Coll Surg. 2014;219:765–770. doi: 10.1016/j.jamcollsurg.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wachter R, Halbach M, Bakris GL, Bisognano JD, Haller H, Beige J, Kroon AA, Nadim MK, Lovett EG, Schafer JE, et al. An exploratory propensity score matched comparison of second-generation and first-generation baroreflex activation therapy systems. J Am Soc Hypertens. 2017;11:81–91. doi: 10.1016/j.jash.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 31.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135 [DOI] [PubMed] [Google Scholar]
- 32.Méndez AS, Melgarejo JD, Mena LJ, Chávez CA, González AC, Boggia J, Terwilliger JD, Lee JH, Maestre GE. Risk Factors for Orthostatic Hypotension: Differences Between Elderly Men and Women. Am J Hypertens. 2018;31:797–803. doi: 10.1093/ajh/hpy050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juraschek SP, Simpson LM, Davis BR, Beach JL, Ishak A, Mukamal KJ. Effects of Antihypertensive Class on Falls, Syncope, and Orthostatic Hypotension in Older Adults: The ALLHAT Trial. Hypertension. 2019;74:1033–1040. doi: 10.1161/hypertensionaha.119.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arik F, Soysal P, Capar E, Kalan U, Smith L, Trott M, Isik AT. The association between fear of falling and orthostatic hypotension in older adults. Aging Clin Exp Res. 2021;33:3199–3204. doi: 10.1007/s40520-020-01584-2 [DOI] [PubMed] [Google Scholar]
- 35.Chuang YM, Hu HH, Pan PJ. Cerebral syncope: insights from Valsalva maneuver. Eur Neurol. 2005;54:98–102. doi: 10.1159/000088644 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


