Abstract
Immunotherapy, along with chemotherapy, targeted delivery, radiation and surgery has become one of the most common cancer treatments. The aim of cancer immunology is to use the bodys immune system to combat tumors and develop a robust antitumor immune response. In the last few years, immune checkpoint inhibitors and chimeric antigen receptor-modified T cells have made substantial advancements in cancer immunotherapy. By boosting cell type-specific delivery and immunological responses, nanocarriers like liposomes have the ability to enhance greater immune responses. The efficacy of anti-tumor therapeutics is being significantly improved as liposomes can assist in resolving a number of issues that can arise from a variety of cancer immunotherapies. Since, liposomes can be loaded with both hydrophilic and hydrophobic drugs and protect the immunotherapeutic agents loaded inside the core, they offer significant advantages over other nano delivery systems. The use of liposomes for accurate and timely delivery of immunotherapies to particular targeted neoplasms, with little or no injury to healthy cells, maximizes immunotherapy efficacy. Liposomes are also suitable vehicles for delivering medications simultaneously with other therapies such as chemotherapy, radiation, and phototherapy. Liposomal nanoparticles will be introduced and used as an objective immunotherapy delivery system for great precision, making them a viable cancer treatment approach.With an emphasis on dendritic cells, T cells, tumor and natural killer cells, and macrophages; outline of many forms of immune-therapies in oncology and cutting-edge advances in liposomal nanovesicles for cancer immunotherapy are covered in this review.
Keywords: Liposomes, Nanovesicles, Immunotherapy, Cancer, Combinational therapy
1. Introduction
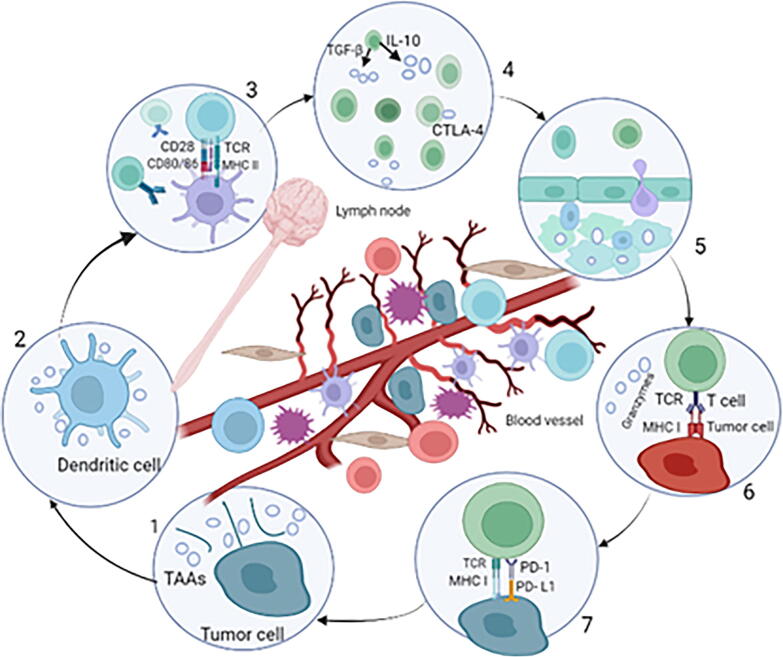
Cancer is defined as the uncontrolled growth of cells in a specific area of the body. The prevalence of newly diagnosed cancers is steadily increasing year after year, and there are over 100 different types of cancer that affect the human body (Rahamathulla et al., 2021a, Rahamathulla et al., 2021b, Banchereau and Steinman. 1998). Often, immunotherapy is considered a fairly recent medical breakthrough, having only been developed a few decades ago. In truth, immunotherapy can be traced back to China's Qin dynasty, which reigned from 3rd century BCE (Yu, 2020, Farkona et al., 2016, Yin, 2020). The hypothesis of cancer immune the idea that the body’s immune system has the potential to eradicate cancerous cells during the early phases of transformation (Farkona et al., 2016, Sharma et al., 2011). The therapeutic effectiveness of traditional treatments for cancer can be aided by the adaptive and innate immune systems. Evidence from pre-clinical trials suggests that immunotherapies are critical to cancer therapy's long-term success, establishing immunotherapy as the fourth pillar of treatment for cancer (Yu, H.J and De Geest, 2020, Javed et al., 2021). Generally, clinically effective antitumor responses require the efficient execution of numerous immunological mechanisms (Khan et al., 2022). An illustration An illustration of a typical immune response against cancerous cells in tumors is shown in Fig. 1. (1) Antigens of cancer, either tumor-associated (TAA) or tumor-specific antigens, are produced during tumor growth (2) Dendritic cells are antigen-presenting cells that phagocytose, process, and present cancer antigens (3) The major histocompatibility complex class I and class II (MHC I/II)receptor, which includes the epitope peptide from tumor-associated antigens, interacts with the corresponding T cell receptor. The majority of the time, T cells are primed in lymphoid tissue. (4) Immunological positive/negative factors that prevent/promote complete activation of T cells via cytokines e.g., transforming growth factor (TGF) and costimulatory receptors. Costimulatory receptors are vulnerable to T cells during priming (5) After being successfully stimulated, effector T cells expand, release inflammatory cytokines, gain cytolytic capabilities, and migrate to tumor sites. (6) Cytotoxic T cells recognize tumor cells and attach to the corresponding antigens of cancer on the major histocompatibility complex I surface of cancer cells, causing death mediated by T-cells. (7) T cell function can potentially be increased or suppressed in the tumor. Negative costimulatory signals e.g., programmed cell death ligand 1 (PD-L1) suppress T cells, resulting in anergy and fatigue (Dobosz and Dzieciątkowski, 2019).
Fig. 1.
An illustration of a typical immune response against cancerous cells in tumors (Graphpad prism software).
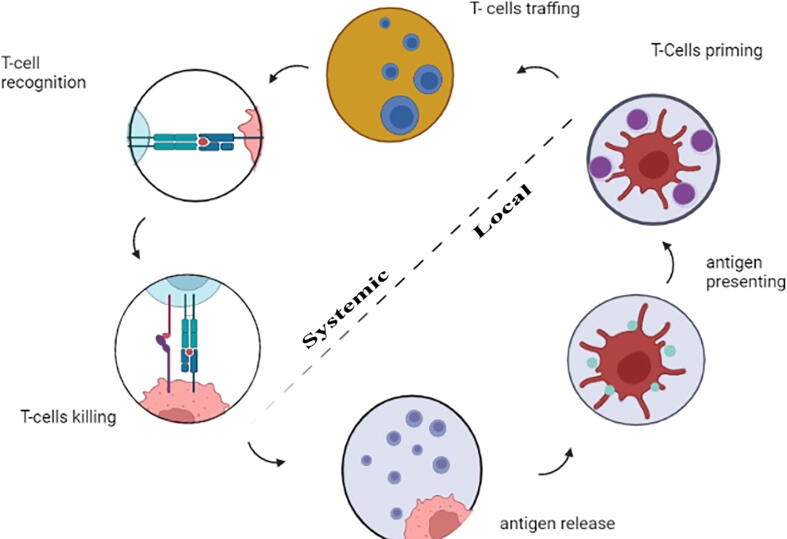
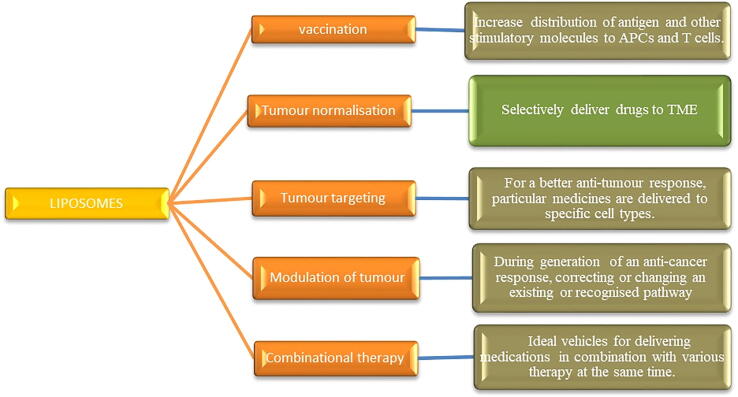
The cancer–immunity cycle, which is the cyclical evolution of a succession of incremental processes, is required for cancer immunity. Tumor-derived antigens produced by necrotic or apoptotic tumor cells are released. Dendritic cells (DCs) collect these antigens and deliver them extracellularly on molecules of the MHCI and MHCII. Activated dendritic cells stimulate and activate immature T cells to effector T cells in lymph nodes. TCR and MHC interactions allow effector T cells to travel to tumor sites and detect tumor cells selectively. When effector T cells recognize their target cancer cells, they initiate apoptosis. The cancer-immunity cycle is frequently disrupted, resulting in a reduced anti-tumor immune response and, in some cases, immunological escape. Cancer immunity cycle - the goal of cancer immunotherapy is to either start or restart the self-sustaining cancer immunity as shown in Fig. 2. (Chen and Mellman, 2013, Song et al., 2017, Motz and Coukos, 2013). The various strategies incorporated in cancer immunotherapy are shown in Table.1. As a result of the growing number of therapeutic delivery mechanisms, the number of immunotherapy strategies being explored has increased. Immunomodulatory medications such as recombinant cytokines (Interleukin-2 (IL-2), TGF-, Interferons), monoclonal antibodies, and small molecule adjuvants (e.g., CpG, Pam3CSK4, monophosphoryl lipid A). have progressed in terms of molecular understanding and availability. Antibodies against programmed cell death protein 1 (PD1), IL-10, and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) have contributed to the development of immunotherapy techniques (Smyth et al., 2015, Stewart and Keselowsky, 2017). In recent years, chimeric antigen receptor T cells (CAR-T) and immune checkpoint inhibitors have made substantial advances in cancer immunotherapy (since checkpoint inhibitors can activate immune cells back to life) (Xia et al., 2019, Wang et al., 2018, Yaddanapudi et al., 2013).
Fig. 2.
Cancer immunity cycle - the goal of cancer immunotherapy is to either start or restart the self-sustaining cancer immunity (Graphpad prism software).
Table 1.
The major immunotherapy spectrum.
| Approach | Action Mechanism | Reference |
|---|---|---|
| 1. To stimulate effector immune values | ||
| Vaccines | Stimulates the immune system of the host | (Farkona et al., 2016) |
| Cytokines | Either directly exerting an antitumor effect or indirect improving the antitumor immune response | (Disis, 2014) |
| Adoptive cellular therapy | By inducing a set of high avidity effector T cells and overcoming tumor antigen tolerance | (Farkona et al., 2016, Mellman et al., 2011, Disis, 2014) |
| Oncolytic virus therapy | To treat cancer, existing biological agents are being used. Despite the lack of initial virulence, genetically engineered viruses can infiltrate and lyse cancer cells. | ((Dobosz and Dzieciątkowski, 2019, Disis, 2014). |
| 2. To inhibit the immunosuppression mechanism | ||
| Immune checkpoint blockade | ||
| Monoclonal antibodies against CTLA-4 | Reactivates pre-existing antitumor T cell responses, which might be useful in cancer therapy and might initiate new | (Farkona et al., 2016) |
| Antibodies against PD1 and PD-L1 | New Sufficient clinical reactions are triggered, which are frequently long-lasting | (Farkona et al., 2016) |
CTLA-4 = Cytotoxic T-lymphocyte–associated antigen 4.
PD-L1 = Programmed cell death ligand 1.
2. Nanovesicles in cancer immunotherapy
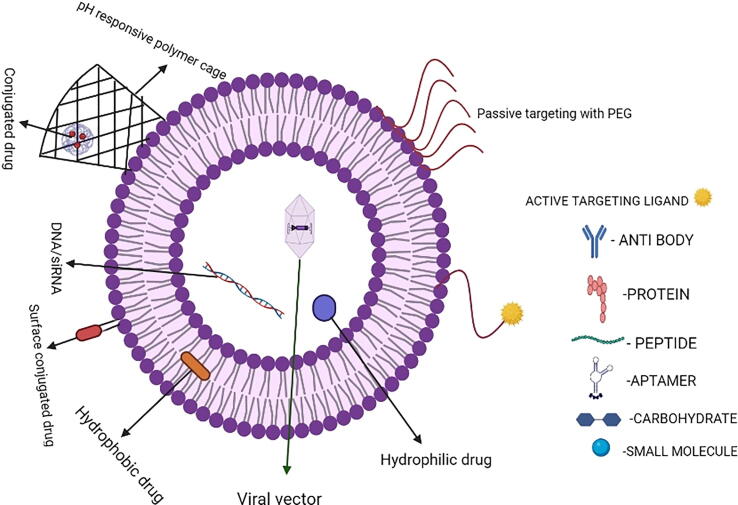
According to Maeda and Matsumura's pivotal research, they found out that nanoparticles accumulate passively in solid tumors and this paved the way for a new approach to treating these cancers a few decades ago (Kantoff et al., 2010, Chambers, et al., 2014, Allegra et al., 2021). The tumor’s immunosuppressive environment, poor T-cell response, and low immunogenicity, pose significant challenges to current cancer immunotherapy (Velcheti and schalper 2016). Advanced nano biomaterials, such as liposomes, polymer micelles, inorganic nanovesicles, drug conjugates, nanoparticles like virus and silica, are critical in promoting antitumor immune responses while minimizing toxic side effects (Alanazi et al., 2014, Alanazi et al., 2020a, Alanazi et al., 2020b). Nanoparticular delivery of immunotherapeutic agents can also beat biologicals in terms of complexity, manufacturing cost, shelf life, tissue penetration, and stability. However, on the other hand, nanoparticular delivery has a high risk of systemic distribution, which can lead to serious off-target consequences and unwanted side effects. Nanotechnology aids in the formulation of these therapeutic molecules, making them more successful in their delivery to certain immune cell subsets. (Yu, H.J and De Geest, 2020, Yu, 2020, Gu et al., 2020). Certain limitations are inevitably present as both hydrophilic and hydrophobic drugs/immunotherapeutic agents can be delivered. Only particular hydrophobic drugs/immunotherapeutic agents can be encapsulated in micelle monolayer structures. As a result of this, additional drugs/immunotherapeutic agents must be enclosed by a covalent link, limiting drug release significantly. Inorganic compounds that are used to induce covalent bonds can cause substantial tissue damage since they cannot be metabolized. Liposomes are utilized in this situation as a substitute in both circumstances. Since, they have a hydrophilic inner structure, they can deliver both hydrophilic and hydrophobic drugs/immunotherapeutic agents at the same time, considerably boosting drug loading efficiency. Liposomes are composed of lipids that act within the cell membrane's framework, making them less harmful to organs and more biocompatible than inorganic nanoparticles (Gao et al., 2019, Rahamathulla et al., 2020). In this review, we emphasize on recent breakthroughs in liposome-based delivery systems in immune-oncology. The structural considerations for liposomes as shown in Fig. 3. (Begum et al., 2022).
Fig. 3.
This figure depicts structural considerations for liposomes (Graphpad prism software).
3. Liposomes and their emergence as drug delivery vehicles in cancer immunotherapy
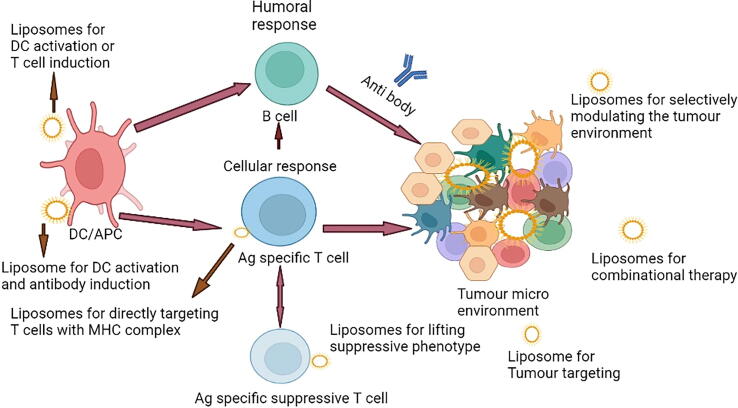
Because of their capacity to shield antibodies from degradation, high biocompatibility, and effective distribution, liposomes became the first therapeutic nanoparticle class to earn clinical approval for the treatment of cancer and have attracted researchers' interest. Liposomes have been found to be efficient drug carriers when it comes to delivering drugs to target cells (Shoaib et al., 2022). Liposomes are lipid-based nanovesicles that can include a wide range of cancer drugs, making cancer immunotherapies more effective. Polypeptide, nucleic acid, and antibody drugs are the most common types of immune agents. As a result, liposomes are anticipated to be effective carriers for cancer immunotherapy that activate either humoral or cellular immune responses in an immunotherapy treatment based on liposomes, as shown in Fig. 5. (Blume et al., 1993, Gao and Hu, 2019). Liposomes are particularly versatile, as they may be employed for a wide range of immunotherapeutic cancer treatments, such as checkpoint blockade and vaccination (Allen and Cullies 2013, Alanazi et al., 2022). Different delivery systems of drugs based on liposomes are illustrated in Fig. 4.
Fig. 5.
An immunotherapy treatment based on liposomes (Graphpad prism software).
Fig. 4.
Different drug delivery systems based on liposomes.
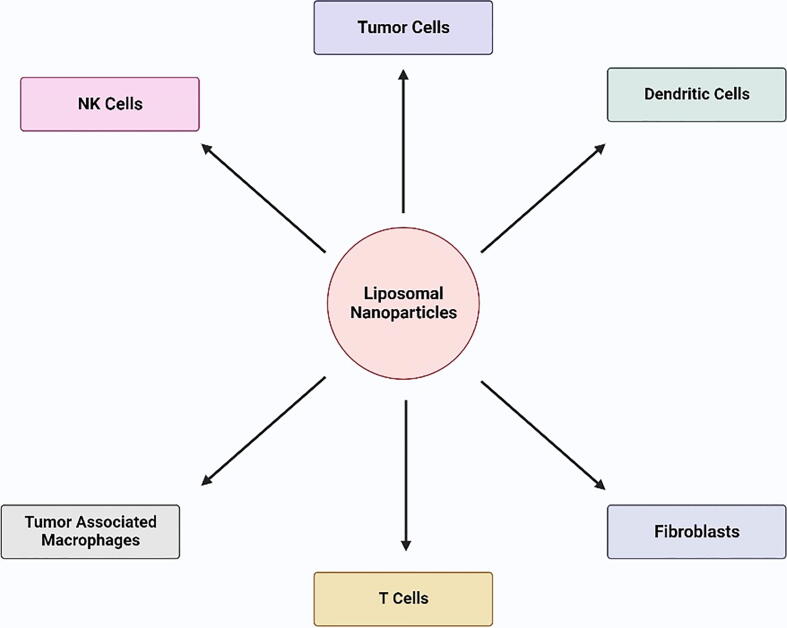
The liposomal formulation of vemurafenib was developed to deliver across the skin for treating subcutaneous melanoma by Weiping Ding et al. Vemurafenib was encapsulated in modified liposomes containing the peptide TD. In-vitro experiments concluded that vemurafenib has selective inhibition to A375 melanoma cells. Vemurafenib-TD liposomal administration through the skin was more effective than oral or intravenous administration because there was no damage to the liver, kidney, or lung. Additionally, the peptide TD alteration on the liposomes increased vemurafenib transdermal delivery considerably (Zou et al., 2018). The various immune related cells that liposomes target in immune-oncology to activate cellular or humoral immune response are represented in Fig. 6.
Fig. 6.
Liposomal nanoparticles are utilized in cancer immunotherapy to target immune-related cells.
4. Different delivery systems based on liposomes used in cancer immune-oncology
4.1. Various stimulatory molecules delivered through liposomes to induce immune responses
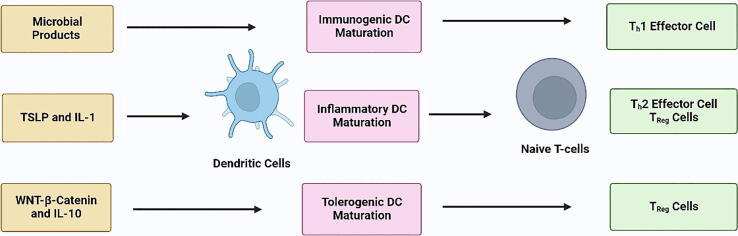
In cancer immunotherapy, attempts to activate or boost immune responses by altering regulatory pathways have been researched extensively for decades. These are some of the most effective cancer immunotherapy techniques available. Researchers recently investigated whether liposomes containing immune stimulatory compounds could boost the efficacy of cancer treatment (Gu et al., 2020, Allegra et al., 2021, Zou et al., 2018). Stealth (PEGylated) immunoliposomes with anti- CD137 and IL-12 attached surfaces demonstrated equivalent immunostimulatory effects to “free” medicines in a mouse melanoma model, with almost minimal systemic harm (Gu et al., 2020, Zhang et al., 2018a, Zhang et al., 2018b). Vaccination is a sort of immunotherapy in which an antigen is coupled with an adjuvant and administered to the body to activate therapeutic T cells. Dendritic cells (DCs) are sometimes referred to as “nature's adjuvants” because of their capacities, and they have evolved into natural antigen delivery agents. DCs are clearly at the heart of the immune system, as they control both immunological tolerance and immunity, according to four decades of studies (Palucka and Banchereau 2012). APCs such as DCs function as a connection between cellular and humoral immune responses. Antigens are collected by DCs, which digest them before exposing them to T cells (Palucka and Banchereau 2012). For adequate anti-tumour immune responses, effective dendritic cell activation and maturation are necessary, as shown in Fig. 7. Immunopotentiators (which stimulate immunological responses) and delivery systems are the most important components for achieving optimal efficacy. Liposomes can shield drugs from degradation while simultaneously delivering antigens and immunological adjuvants to trigger powerful immune responses (Sahdev et al., 2014, Riley et al., 2019). Guan and colleagues generated immunostimulatory spherical nucleic acids that specifically target TLRs 7/8. The antigen-loaded centre of the liposomes ensured more antigen-specific T-cell priming. TLR7/8 and TLR3 are effective in promoting DC maturation (Guan et al., 2018). Liposomes can trigger a strong immune response apart from acting as carriers. The use of archaeal lipids, or archaeosomes, as an example, has been proposed as a method for stimulating DCs and activating the immune system adjuvantly (Gao et al., 2019, Krishnan et al., 2001).
Fig. 7.
Maturation of the DC: Dendritic cell (DC) maturation is a straightforward notion that is complicated by the fact that not all mature or active. DCs are immunogenic in the same way (Graphpad prism software).
4.2. Liposomes in immune gene therapy
Nucleic acids are spontaneously immunogenic and they do not require the addition of an adjuvant. Liposome’s ability to concentrate in reticuloendothelial organs like the spleen and liver, which are rich in antigen-presenting cells, can be used to efficiently distribute antigen-coding RNA. When foreign RNA enters cells, pathogen recognition receptors recognise it, triggering the production of type I interferon and resulting in powerful immune responses (Gu et al., 2020, Barral et al., 2009). In Si RNA-based cancer therapy, cancer immunotherapy, gene therapy and other techniques, bioactive chemicals such as nucleic acids and proteins are used. The vast majority of these compounds are not able to cross biological membranes. As a result, proper carriers are needed to transport them into intracellular regions and to achieve their bioactivity efficiently once they are there. Liposomal membrane structure is useful for generating bio-related functions such as membrane destabilising and/or membrane fusing abilities, which are essential for moving membrane-impermeable substances across biological membranes, among other things. pH-sensitive polymer-lipid-incorporated liposomes produced by Kenji Kono et al. successfully conveyed their contents into the cytoplasm of DCs, most likely via destabilising and merging with endosomal and lysosomal membranes. These liposomes can also be utilised to transport antigens and promote antigen-specific immunity. Indeed, these polymer-lipid-incorporated liposomes' remarkable performance suggests that they could be employed to carry bioactive chemicals such as siRNA into the cytoplasm of target cells (Yuba et al., 2013, Cheng et al., 2003, Itaka and Kataoka, 2011). Colorectal cancer is being treated with immunogene therapy, which is a unique method. Because of its immune-stimulatory properties, the cytokine IL-15 has shown therapeutic anti-tumour promise. On the other hand, traditional cancer gene therapy research based on IL-15 has relied on plasmid DNA, which has drawbacks like poor transport efficiency and a backbone effect. In vitro transcript mRNA was employed by Sibei Lei, Xueyan Zhang, Ke Men, and colleagues to demonstrate an IL-15 immunogene therapy research for cancer in the colon. For in vivo mRNA transportation, a protamine/liposome system (CLPP) is being developed to enable effective delivery and condensation capacity (Lei et al., 2020). Liposomes improve immunological response to vaccination antigens by acting as an adjuvant and carrier of co-adjuvants viral glycolipids and glycoprotein (Daraee et al., 2014). Interleukin-12 (IL-12) gene therapy is predicted to be helpful against cancer because it primes the immune system to recognise cancer cells. In this therapy, inducing IL-12 gene expression in tumour tissue is crucial. Although sonoporation is a promising technology for producing non-invasive and non-viral gene delivery systems, the low efficacy of gene transport makes it ineffective for cancer gene therapy. Ultrasound and innovative ultrasound-sensitive liposomes were used to solve this problem (Suzuki et al., 2010). When siRNA-containing liposomes were administered to the skin alongside a vaccination, they were dry-coated onto microprojection arrays and maintained their particle integrity after dissolving. When employed in conjunction with the microprojection array, these liposomes diffused quickly after distribution and provided an optimal method for in vivo siRNA delivery to skin. The effectiveness of this method to influence immune responses when exposed to an antigen requires further investigation. The results of this work have wide significance for the delivery of siRNA to cause gene silencing in skin to treat illnesses or during exposure to immunogens, as well as specific ramifications for the siRNA-mediated gene silencing of CXCL1 in skin (Haigh et al., 2014). The approved immune checkpoint inhibitors in the cancer immune oncology example were tabulated in Table 2.
Table.2.
Examples of approved immune checkpoint inhibitors in cancer immune oncology.
| Target | Ligand/ receptor | Inducer | Mechanism of invasion | Therapeutics-year of approval | Cancer | Ref |
|---|---|---|---|---|---|---|
| CTLA-4 | CD80/CD86 | T-cells activation signal | PKC-M recruits SHP-2 by interacting with the cytoplasmic tail; ectodomain competition with the counter receptor | Ipilimumab [Yervoy].-March 2011 | Melanoma | Cheng et al., 2019, Velcheti et al., 2019, Sanaei et al., 2021. Vaddepally et al., 2020) |
| PD-1 | PD-L1, PD-L2 | T-cell receptor/ /B-cell receptor | The recruitment of SH2-domain protein tyrosine phosphatases to the ITSM cytoplasmic region of PD-1 suppresses the T-cell receptor's downstream signalling. | Pembrolizumab[keytruda].-September 2014 | Bladder cancer | Cheng et al., 2019, Velcheti et al., 2019, Sanaei et al., 2021. Vaddepally et al., 2020) |
| signalling | Nivolumab [Opdivo].-March 2015 | kidney cancer | ||||
| Cemiplimab [Libtayo].-September 2018 | Head and neck cancer | |||||
| Classical Hodgkin lymphoma | ||||||
| PD-L1, PD-L2 |
PD-1 |
FN-γ, cytokines, chemokine, STAT3 signaling |
The recruitment of SH2-domain-containing protein tyrosine phosphatases to the ITSM cytoplasmic region of PD-1 inhibits downstream signalling. |
Atezolizumab [Tecentriq].-May 2016 | Bladder cancer |
Cheng et al., 2019, Velcheti et al., 2019, Sanaei et al., 2021. Vaddepally et al., 2020) |
| Avelumab [Bavencio].-November 2015 | Merkel cell carcinoma | |||||
| Durvalumab [Imfinzi].-February 2016 |
4.3. Liposome- delivery of immunostimulatory molecules to T cells
In cell-mediated adaptive immunity, T cells play an important role. Since the 1980 s, when researcher's uncovered molecular evidence of T cell receptors (TCRs). TCR antigen recognition has been studied extensively, and the molecular mechanisms and regulations that govern this process have been established, setting the groundwork for cancer immunotherapy (Li et al., 2019). Helper T cells, are T cells that express the glycoprotein CD4 on their external surface. Helper T cells play an important role in immunologic processes such as cytotoxic T cell activation (Gao et al., 2019). T cell adoptive transfer was first studied in therapies of localized and disseminated lymphoma, and tumors in a syngeneic mouse model regressed after T cell infusion (Yuba et al., 2013); ever since, there has been research that has looked into therapeutic interventions of T cells and other immune cells to battle cancer and other diseases (Vaddepally et al., 2020). Tisa-genlecleucel and axicabtagene ciloleucel were recently approved by the FDA for the therapy of B-cell leukaemia and lymphomas, respectively (Khan et al., 2022, Pasternak, 2020). Both medications are CAR-T cell therapies as a basic need for therapeutic success, CAR-T cells must be delivered to tumour lesion. Once they've assembled in the location, they must quickly enter the tumour. One rationale for CAR-T cells' modest clinical success is that they must overcome hostile immunosuppressive components after migrating into the solid tumour lesion in order to induce targeted cytotoxicity. T-cell treatments are also being developed in significant quantities. Despite these significant advancements, many obstacles remain in the way of wider adoption of adoptively transferred T cells for cancer, which include insufficient T-cell expansion, inefficient T-cell trafficking into solid tumours, reduced activity of T cell due to an aggressive tumour immune microenvironment (TME), and failure of target antigen expression are all possible outcomes. since nanomaterials could be thoughtfully developed to boost T-cell proliferation, penetrate difficult physical barriers, and alter TMEs, they have the potential to revolutionise cancer treatment, they are uniquely suited to solve these issues. Although ACT has the potential to be an efficient cancer treatment, T cells produced by ex vivo may struggle to survive after infusion
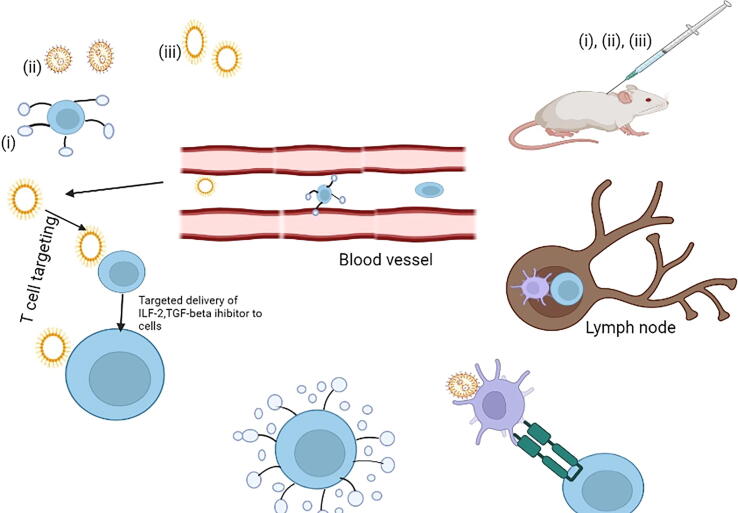
In order to overcome these constraints, nanovesicles are being developed to boost T-cell development in vivo through T-cell specific delivery, nanomaterial-based vaccines, and backpacking nanomaterials, as shown in Fig. 8 (Li et al., 2019, Gong, 2021, Zhang et al., 2016). When anti-CD 90 antibodies were added to a liposome to target T cells and distribute the TGF inhibitor chemical SB525334, tumor growth was drastically decreased in a melanoma of mouse model when compared to untargeted liposomes (Zheng et al., 2017). Researchers discovered that liposomes given IV successfully delivered to the outer layer of adoptively transferred T cells in a melanoma mouse model, provoking augmented T-cell proliferation in tumor-bearing mice using an IL-2–Fc fusion-protein-modified lipid nanovesicles (Zhang et al., 2013).
Fig. 8.
In vivo Nanomaterials for growth of T-cell (i) T-cell activation and growth can be induced in vivo using nanomaterials that are designed for T cells targeted delivery (ii) Backpacking nanoparticles adhere to the surface of T cells and release their load of stimulatory signals in response to external or administered stimuli, allowing for precise regulation of T cell growth in vivo (iii) in vivo, vaccine nanoparticles that target antigen-presenting cells can activate these cells and cause growth of T-cell (Graphpad prism software).
5. Improved cancer immunotherapy with liposome delivery-immune checkpoint blockade molecules
In cancer prevention and treatment, the immune system is critical. T-cell function has recently improved owing to the development of techniques that target negative regulatory components on T cells. T cells, also known as checkpoints, have helped patients with a variety of malignancies achieve extraordinary success rates. CTLA-4 was the first T-cell checkpoint to be clinically targeted. Based on the fact that patients with metastatic melanoma have a better overall survival rate, the FDA authorized ipilimumab, an anti-CTLA-4 antibody. A second immunologic checkpoint known as PD-1, as well as its major ligand, PD-L1, are exhibiting symptoms of activation. (Postow 2015).
It has a lot of potential as a therapeutic target. The FDA has approved nivolumab and pembrolizumab (anti-PD–1 blocking antibodies) for patients with metastatic melanoma. Other tumors that benefit from nivolumab and pembrolizumab, as well as other antibodies that target the PD-1 axis, include non-small cell lung cancer and other cancers, as well as Hodgkin lymphoma (Jacobson et al., 2022, Naidoo et al., 2015). Despite the fact that immune checkpoint blockade therapy (ICB) has had a lot of success in tumor immunotherapy, it has only helped a tiny fraction of patients. Patients who received ICB had a variety of side effects due to non-immune system up-regulation, according to a previous study, Immune-related adverse events (irAEs) can impact every organ system in variable patterns and intensity depending on which immune checkpoint is blocked. Nano-science has been viewed as an innovative technique for overcoming the issues of ICB systemic toxicity, and it has been intensively researched in cancer immunotherapy clinical trials. The ability to transport various payloads, deliver treatments sustainably, and adjust their pharmacodynamic effects are all advantages of nanoscience-based techniques (Postow, 2015, Hodi et al., 2010, Gammon et al., 2016).
CTLA-4 and CD28 are members of the Immunoglobulin-related receptor family, which regulates various aspects of T cell immunity (Rowshanravan et al., 2018). CTLA-4 is produced following T cell activation and competes with CD28 for the ability to bind to CD86 and CD80 on dendritic cells, reducing T cell survival and proliferation (Blume et al., 1993). The use of CD28/CTLA-4 pathway-targeting antibodies and fusion proteins in the therapy of cancer and autoimmune illnesses is of great interest. Because CTLA-4 restricts immune responses to self-tissues, increasing it may be a therapy option for autoimmunity, whereas decreasing it may result in anti-tumor responses. anti-CTLA-4 antibodies were the first to recognize the possibility of immunological rejection of cancer by harnessing immune responses, resulting in a surge of activity in this field (Gammon et al., 2016). Monoclonal antibody-based cancer immunotherapies CTLA-4 blocking monoclonal antibodies, for example, have a number of drawbacks, including low tumor penetration and organ damage. To overcome these obstacles, non– PEGylated and PEGylated liposomes containing CTLA-4 were created, characterized, and the anti-tumor treatment responses on mice with C26 colon cancer tumors were investigated PEGylated liposomes had longer blood half-lives and managed to accumulate much more in the tumor area than free CTLA-4 antibody and non-PEGylated liposomes, according to a biodistribution study (Nikpoor et al., 2017). Other inhibitory and costimulatory receptors, such as PD-1,inducible costimulatory (ICOS] on exterior of T cells, have joined the B7/CD28 superfamily, providing unique secondary signals that modify the immune response. PD-1 appears to play a role in peripheral tolerance, according to mounting evidence. When peripheral T and B cells, as well as monocytes, are activated, PD-1 expression is activated (Keir et al., 2006). Several successful liposome-based techniques have been developed to improve anti-PD-1 therapy. When compared to free DOX and IgG-Liposome-DOX, PD-1-Liposome-DOX demonstrated higher anticancer activity as measured by BLI imaging and tumor volume measurement, as well as a longer life, according to one study (Du et al., 2017). Several monoclonal antibodies (mAbs) targeting different immunological checkpoints (IC), or molecules involved in the immune system's negative modulation, have been developed and approved in cancer therapy in recent years. Some of the axes that can be expressed on activated cytotoxic T cells in this manner down regulate the immune response to tumors. In this way, PD-L1, which is commonly overexpressed on the surface of cells, inhibited tumor cells as well as its receptor, PD-1 (Merino et al., 2019). Despite significant progress, the efficacy of ICBs in the treatment of melanoma remains limited due to inflammatory side effects and auto immune disorders from PD-L1. Non-specific T cell-mediated reactions, in which reactivity is directed against normal cells, are thought to cause side effects. The low selectivity of anti-PD-L1 is linked to these reactions. As a result, anti-PD-L1 delivery must be enhanced in order to allow for targeted dispersion at the tumor site, boosting immunotherapy efficacy while lowering patient adverse effects. Overcoming hypoxia and reshaping the tumor microenvironment may boost PD-L1 inhibitor sensitivity, improve intra-tumor T cell activity, and aid tumor regression. Catalase (CAT) (Sigma Aldrich, St. Louis, USA) is an antioxidant enzyme that can degrade hydrogen peroxide (H2O2) in tumor cells into H2O and O2, lowering hypoxia-inducible factor (HIF)-117 and increasing immunotherapy efficacy. Combining antiPD-L1 and CAT in a liposome delivery method, pertaining to one study, may be an efficient method for enhancing antiPD-L1 effects and decreasing tumor growth (Hei et al., 2020, Noman et al., 2015, Naidoo et al., 2015, Postow, 2015). A study discovered that a pH-sensitive platform for co-loading docetaxel and PD-L1 antibody has showed increased clinical response and provided a good design for the conjunction of chemotherapeutics with different immunotherapies for further investigation (Gu et al., 2018, Al-Joufi et al., 2022).
6. Small molecule delivery using liposomes to modulate the tumor microenvironment selectively
Aside from cell contact, which contributes to a suppressive environment, soluble mediators like indoleamine 2,3-ioxygenase (IDO), adenosine, transforming growth factor, and interleukin-10 (IL-10) can also suppress anti-tumor. immunity (Gu et al, 2020). IDO is an intracellular enzyme that suppresses the immune system by lowering tryptophan levels, allowing tumors to go undetected (Moon et al, 2015). IDO inhibitors show promise for checkpoint blockage because they can reactivate the immune suppressive tumor microenvironment and also stimulate the host immune system, combining photo dynamic therapy (PDT) and IDO blockage to boost the therapeutic effect on both local and distant tumor inhibition, may be a better therapy method. A study showed a liposomal dual functional combination of NLG919 and PpIX for synthetic PDT and IDO inhibition in primary and metastatic tumors. PpIX-NLG@Lipo with synergistic IDO and PDT blockage showed promise for improved cancer therapy. Moreover, by suppressing both distant metastatic and primary tumors at the same time, a therapeutic approach that combines PDT with IDO inhibition has the ability to improve cancer therapy (Huang et al, 2019). Adenosine is an immunosuppressive chemical that hinders the surface activity of CD4 + and CD8 + T lymphocytes by binding to and initiating the A2a adenosine receptor (A2aR). The A2aR-specific small molecule antagonist SCH-58261 (SCH) can disrupt this suppression mechanism, but its applications are limited due to difficulties in delivering this medication to immune cells within the TME. To get around this limitation, we used CAR-engineered T cells as active chaperones (Shull et al., 1992, Tinoco-Veras et al., 2017, Zheng et al., 2017). TGF-1 (TGF-beta 1) is a multifunctional growth factor that regulates a variety of developmental and physiological processes, whose liposomes can increase anti-tumor activity by interfering with the TGF- pathway (Shull et al, 1992). TGF-inhibitors have a fairly low response rate and a higher rate of risk of autoimmune disorders caused by systemic inhibition, which greatly restricts their use. One method to strengthen their efficacy while lessening NEGATIVE effects is to target these therapies to immune effector cells or the TME. PEGylated liposomes effectively contained both TGF inhibitor and IL-2., according to Park et al. Tumor growth was inhibited as a result of enhanced NK cell activity and activation of CD8 cells infiltrating the tumor. T cells following intratumorally or systemic injection in a B16/B6 melanoma mouse model (Gu et al., 2020, Tinoco-Veras et al., 2017, Zheng et al., 2017). The recognized cancer immunotherapies were tabulated in Table 3.
Table 3.
Other approved cancer immunotherapies.
| Therapy& Drug | Approved Cancer | Type | Year of Approval |
|---|---|---|---|
| CAR -T cell therapy | |||
| Tisagenlecleucel | Large B cell lymphoma | CD19-specific CAR T cells | Pasternak, 2020 |
| Axicabtagene ciloleucel | Non-Hodgkin lymphoma | CD19-specific CAR T cells | Jacobson et al., 2022 |
| Antibodies | |||
| Blinatumomab | lymphocytic leukaemiaa | CD19 and CD3 bispecific antibody | Franquiz and Short, 2020 |
| Oncolytic viruses | |||
| Talimogene laherparepvec | Melanoma | HSV type 1 which has been genetically modified to replicate within tumours and produce GM -CSF | Lu et al., 2016 |
| Vaccines | |||
| Bacillus Calmette–Guérin | Bladder cancer | Mycobacterium tuberculosis variant bovis strain | Yaddanapudi et al., 2013 |
| Sipuleucel-T | Prostate cancer | Activation of autologous PBMCs with recombinant human PAP–GM-CSF | Kantoff et al., 2010 |
7. Combinational therapy delivery using liposomes for improving cancer immunotherapy
Even though these immunotherapies led to total regression in some patient populations, immunotherapy alone was only effective in 20–30 % of those who were treated (Gu et al., 2020, Le et al., 2018). A single immunotherapy has a moderate therapeutic impact given the low response rate. Chemoimmunotherapy has emerged as a new trend in cancer treatment due to its double role in cancer cell killing and immune activation, opening a new avenue for the reuse of conventional chemotherapeutic drugs (Salvati et al., 2013). Due to its low clinical efficacy, cancer immunotherapy poses a big obstacle. When cancer vaccines are coupled with chemotherapy in individuals with various forms of cancer, a number of clinical studies have lately revealed the coincidental discovery of high rates of objective clinical response (Ramakrishna et al., 2010). Targeted cancer medicines that can attack tumors early in their development are predicted to emerge as a result of a clever mix of nanomedicine, cancer immunotherapy, and chemotherapeutic engineering. Nanotechnology and combination therapy offers a novel cancer treatment strategy. The nanoparticle system increases drug therapy pharmacokinetics, enhancing therapeutic benefits while lessening the negative consequences associated with high dosage. Liposomes are regularly used as drug delivery vehicles, as well as several liposomal nanomedicines have received FDA approval (Rahamathulla et al., 2021a, Rahamathulla et al., 2021b, Mir et al., 2022). Since different medicines have different mechanisms and target areas, the selection of treatment drugs and the timing of these combinations are critical. Combination therapy has the potential to deliver powerful anti-tumor effects while simultaneously increasing the risk of systemic damage. A suitable liposome-based delivery system must: (1) Co-load several compounds in adequate amounts, (2) Employ cost-effective, safe and efficient, preparation processes, (3) Have synergistic or cumulative effects; deliver the drug at the desired place and time, (4) Target a specific tumor or cell type, (5) Release the drug at the desired site and time; and (6) Overcome biologic obstacles without compromising bioactivity (Gu et al., 2020). IDO1 expression within the solid tumor can increase the activation of immunosuppressive regulatory T cells while simultaneously inhibiting the proliferation of invading T cells in the G1 phase by catalyzing the necessary tryptophan to kynurenine. As a result, IDO1 has been identified as a potential new target for immunotherapeutic development. Due to the poor water solubility and bioavailability of currently available IDO inhibitors, it is vital to design appropriate delivery vehicles to enable efficient tumor-targeted delivery of such inhibitors Since oxaliplatin can trigger immunogenic cell death (ICD) in a variety of cancer cells, coupling it with IDO1 inhibitors like NLG919 prodrugs has been demonstrated to considerably boost treatment effectiveness (Shen et al., 2020, Mehmood, 2014, Dragovich et al., 2006). TLRs are important in innate immunity. While TLR7/8 agonists like Resiquimod (R848) can be given intradermally, direct delivery of TLR agonists to tumors results in situ vaccination, which enhances effectiveness by revealing activated immune cells to a cancer antigen. Thermosensitive liposomes (TSLs) were used as a delivery method in a recent study to create an intravenously injectable version of R848. In more than 100 days, an intravenously injectable formulation of R848 using thermosensitive liposomes (TSLs) as a delivery device demonstrated greater efficacy (Zhang et al., 2021, Barton and Medzhitov, 2002, Frank et al., 2018). Myeloid-derived suppressor cells (MDSCs), play a key role in tumor growth by inducing angiogenesis, metastasis, and treatment resistance. As a result, using techniques to diminish MDSCs in the tumor microenvironment could be a unique cancer immunotherapy strategy. According to various research, doxorubicin exhibits a tumoricidal effect as well as enhances the anti-tumor immune response. PEGylated liposome-encapsulated doxorubicin (Doxil®) has reduced cardiotoxicity than free doxorubicin. Mahmoud Reza Jaafari et al. did a study in which they used a liposomal formulation of P5 peptide and PEGylated liposomal doxorubicin (Doxil®) to treat mice with HER2 + tumor models (combination of immunotherapy and chemotherapy) (Muheem et al., 2017). The result indicated that using Doxil® in conjunction with liposomal P5 immunotherapy could enhance immunotherapeutic efficacy, allowing it to be used in treating HER2 positive breast cancer (Navashenaq et al., 2020, Alizadeh et al., 2014, Barenholz, 2012, Zamani et al., 2020). Reactive oxygen species (ROS) are oxidants that play an important role in cancer therapy by bringing about apoptosis of cells or acting as substrates in a variety of catalytic events. The elevated redox level in the tumor microenvironment limits oxidative therapy and the immune response. Minjie Sun and colleagues created two liposomal delivery systems for MA (maleimide) and ABTS&HRP (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) in vivo applications. Maleimide liposomes (ML) and ABTS&HRP liposomes were inserted separately into the hydrophilic cavities of liposomes (AHL).
The ML, as a glutathione (GSH) depletion adjuvant, promotes immunogenic cell death activation and maturation of DCs for photothermal enhancement due to glutathione deficiency. As a result, ML's dual role in the treatment of primary tumors, breast cancer, metastatic cancer, and distant cancer worked well. Despite the fact that MLs have been found to have an excellent immune-enhancing impact, there are several limitations in this research that need to be investigated more in the future (Zhou et al., 2020, Yang et al., 2018, Fu et al., 2018). By binding to Signal regulatory protein (SIRP), CD47 (integrin-associated protein), which is overexpressed on tumor cells, activates a “don't eat me” signal, allowing immunological escape from the mononuclear phagocyte system (MPS). It is also difficult to deliver therapeutic medications to tumor areas due to its short blood residence time, poor targeting of tumor cells, and rapid clearance by MPS. Amidst this, the inability to reduce MPS absorption, poor tumor targeting, and short blood retention duration continue to be significant barriers to effective drug administration in nanovesicle. Exosomes containing the transmembrane protein CD47 on their exterior were shown to circumvent immune clearance by MPS and to prolong blood circulation time (Salvati et al., 2013, Cheng et al., 2021, Kooijmans et al., 2017). Immunogenic cell death (ICD) is a type of chemo-immunotherapy that seeks to promote antigen synthesis, recognition, procession, and initiation of immune cells in order to damage tumor cells while also stimulating the immune response. An efficient ICD, on the other hand, necessitates a high dose of ICD stimuli, which may be connected with dose-dependent toxicity. Yongjun Wang et al. designed a liposome remote-loaded with shikonin (SHK)., a potent ICD stimulus, a Chinese traditional medicine had shown antitumor activity through multi-mechanisms, via a copper ion gradient, which augmented the pharmacokinetics, stability, and tumor accumulation of SHK, with the ability to effectively enhance ICD at a large dose in vivo, but it showed hepatotoxicity (Li et al., 2021, Casares et al., 2005, Andújar et al., 2013). Immune checkpoint blockade therapy has a number of drawbacks, including toxicity, inefficiency, and tumor relapse. A cationic polymer–lipid nanocomposite vesicle-based delivery system (P/LNV) was proposed for peptide vaccine, to deliver tumor vaccines composed of anionic antigen epitopes, TLR9 agonist, CpG (AE/CpG) inhibitor, and indoleamine-2, 3-dioxygenase (IDO). inhibitor 1-methyl P/LNV to enhance immunogenicity of peptide antigens while blocking the immunological Liposomes effectively improved vaccination absorption by DCs (Su et al., 2021, Tornesello et al., 2020, Banchereau and Steinman, 1998). Because of their immunogenicity without central tolerance, tumor-specific neoantigens have emerged as promising targets for personalized cancer treatment. MHC binding prediction-based computational methods are heavily used in the development and selection of neoantigen vaccines. Shuqing Chen et al. created a DNA-based neoantigen vaccine platform that included whole-exome sequencing (WES) and RNA-seq-based identification of individual somatic mutations, bioinformatic prediction of neoepitopes, DC.-based effectiveness prevalidation of vaccine candidates, and optimization of DNA vaccine candidates, as well as their nanocarrier and adjuvant, but also the development of a liposome. The vaccination was well-absorbed by DCs and elicited a strong immune response against mouse melanoma cells, resulting in significant tumor shrinkage and reductions in lung metastasis (Yang et al., 2021, Sahin and Türeci, 2018, Garcia-Garijo et al., 2019). Inhibitors of the immune checkpoint PD-1/PD-L1 have demonstrated promising results in a variety of human cancers. Nonetheless, the low response rate remains the most significant impediment. dMMR or MSI-H, for example, are found in only 5 % of colorectal cancer patients. Immune checkpoint therapy, by altering the tumor immunological microenvironment, may improve response rates when used in conjunction with conventional therapy (e.g., chemotherapeutics and radiotherapeutics) (TIME). In one study, researchers developed a PD-L1-targeting immune liposome for co-delivery. Irinotecan (IRI), a first-line chemotherapy drug for colorectal cancer, and JQ1, which regulates the expression of critical oncogenes in a variety of cancers, can successfully elicit antitumor immunity in colorectal cancer by inducing immunogenic cell death. IRI facilitates adaptive immune responses against tumor growth by promoting intratumorally drug accumulation and DC maturation. JQ1 suppresses the immunosuppressive PD-1/PD-L1 pathway, whereas IRI promotes DC maturation and intratumorally drug accumulation (He et al., 2020, Goldstein et al., 2014, Floyd et al., 2013). Cancer vaccines can be used to elicit completely novel immune responses to antigens unique to tumors. Before being delivered locally, archetypal vaccines are normally constructed comprised of an antigen mixed with an adjuvant. Poor antigenicity has been a substantial drawback, especially when used in conjunction with intratumorally immune suppression tumor RNA encoding-related epitopes can be used as a potent immunogenic and rapid anti-tumor trigger. RNA activates innate immunity by activating toll-like receptors, resulting in the production of type I interferon. Interferon is a protein that helps to transmit powerful adaptive responses. Because RNA is inherently unstable, it is difficult to administer. In vivo delivery methods have been developed to protect it and deliver it to its intended targets (Sayour, 2018, Lorenzer et al., 2015, Chiang et al., 2011) MDSCs are immune suppressor cells that promote tumor progression. They can be distinguished from other immune cells by the expression of myeloid cell markers on their cell surface, such as Gr-1 (Ly6G and/or Ly6C) and CD11b in mice and CD33 and CD11b in men. Doxorubicin for example, which is commonly used in conventional chemotherapy (Dox) has been shown to reduce the number of MDSCs in tumor tissues, and it has been shown to reduce the number even more when used in conjunction with immunotherapy. However, Dox anticancer treatment is limited due to poor Dox dose distribution to tumor tissues following intravenous (IV) injection. According to Mahmoud Reza Jaafari et al., combining chemo- and immunotherapy with liposomal formulations of Dox and immunogenic peptide E75, provided a high tumor-related immunosuppressive effect against tumor cells. (Zamani et al., 2020, Wesolowski et al., 2013).
To encapsulate doxorubicin (DXR) (Liu et al., 2017) have developed liposomes comprised of malate dehydrogenase (MDH),1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- (amino (PEG)-2000) (DSPE-PEG2000), and cholesterol (CHOL) (MLP-DXR). Hexadecanedioic acid (HA) was coupled to the hypoxic radiosensitizer 2-methyl-5-nitroimidazole-1-ethanol (metronidazole) to create (16-(2-(2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethoxy)-16- oxohexadecanoic acid (MHA). To create ester-linked MDH, the MHA was next combined with 3-dimethylaminopropane-1, 2-diol (DA). To make liposomes containing a radiosensitizer for hypoxia, MDH lipid was employed. As a control, liposomes without MDH were created (DLP-DXR). The highest reduction of glioma growth was clearly seen with MLP-DXR with radiation therapy (RT) when it was tested in a xenograft glioma model created by intracranial injection of human glioblastoma U87 cells. Animals treated with MLP-DXR plus RT displayed tumor with 580 mm3 14 days after treatment, while those treated with RT alone and DLP-DXR plus RT showed tumor with 4000 and 1000 mm3, respectively (Liu et al., 2017). In a mouse model with an LL2 (murine Lewis lung carcinoma) tumour, Shi et al. 2012, have synthesized liposomes encapsulating CUR (l-CUR) and assessed its sensitization effects. Compared to mice treated with either l-CUR or RT alone, mice treated with l-CUR + RT (5 Gy) significantly inhibited tumour growth (5 Gy). Animals treated with l-CUR with RT displayed a tumour volume of less than 300 mm3 22 days after treatment, while those treated with radiation alone (6 Gy) presented a tumour volume of more than 600 mm3 (Gao et al., 2019). X-ray-triggered liposomes were created by Deng et al. In this study, gold nanoparticles and a photosensitizer (verteporfin) were inserted in a liposomal formulation. The photosensitizer induces 1O2 production in response to radiation. Singlet oxygen works by oxidising unsaturated lipids, which causes cargo release and liposomal membrane instability. The gold nanoparticles serve as a radiosensitizer when they interact with X-rays. These liposomes were used to encapsulate DXR, and the activity of DXR was assessed in a mouse xenograft model carrying the colorectal malignancy HCT 116. It was determined whether PBS, liposome alone, irradiation alone (4 Gy), liposome plus irradiation (4 Gy), or liposome plus irradiation (4 Gy) could inhibit tumour growth in the animals. The growth in tumour volume was confirmed in rats receiving PBS (threefold), liposomes alone (2.9fold), and irradiation alone two weeks after therapy (3.4-fold). However, compared to the PBS control group, treatment with liposome with irradiation resulted in a reduction (74 %) in tumour size (Deng et al., 2018). Few lipid-based formulations that have been approved in pre-clinical and clinical trials for cancer immune oncology were tabulated in Table 4.
Table 4.
Few lipid-based formulations, which are approved, in pre-clinical and clinical trials for cancer immune oncology.
| Nano Material | Pay Load | Strategy | Tumour | Stage | Reference |
|---|---|---|---|---|---|
| Liposomal nanohybrid cerasomes | PD-L1, Tubulin | Antibody and chemotherapy [Paclitaxel]. | Breast and colon | Pre-clinical | Sanaei et al., 2021, |
| Liposome | IL-2–Fc fusion protein | T-cell expansion in vivo | Melanoma in mouse | Pre-clinical | Gong, 2021, Goldstein et al., 2014, Chen and Mellman, 2013, Floyd et al., 2013, Zheng et al., 2013 |
| Liposomal-polymer core–shell | TGF-β and IL-2 inhibitors | Enhance NK population | Melanoma | ||
| Liposome | TGF-β inhibitor [SB525334]. | T-cell expansion in vivo | Mouse melanoma | Pre-clinical | Sayour, 2018, Zheng et al., 2013, |
| PEGylated -liposomes | anti-CD40 and CpG | DC activation | Melanoma | Approved | Kwong et al., 2011 |
| Liposome-coated polymeric gel | TGF inhibitor and mouse IL-2 [SB505124]. | T-cell expansion in vivo | Mouse melanoma | Pre-clinical | Park et al., 2012 |
| pDNA-loaded liposomal NPs | IL-2 and IL-12 | SCC VII | Squamous cell cancer of the head and neck in a mouse model | Pre-clinical | Gao and Hu, 2019, Xian et al., 2005 |
| Liposomes | gemcitabine | MSDC depletion | Melanoma | Sasso et al., 2016 | |
| Multilamellar liposomal vesicles | Inhibitor of the adenosine receptor A2a [SCH-58261]. | T-cell expansion in vivo | Mouse model of human ovarian cancer | Pre-clinical | Gong, 2021, Siriwon et al., 2018 |
| liposome-protamine-hyaluronic acid and lipid-calcium-phosphate | TGF- and tumour antigens/CpG are silenced by siRNA. | Higher infiltration CD8 + T cells after removal of immunosuppressive environment | Melanoma | Clinical | Lorenzer et al., 2015 |
| Liposome-protamine-hyaluronic acid Nano particles | TGF-β | B16-F10 | Mouse model of melanoma | Pre-clinical | Gao and Hu, 2019, Xu et al., 2014 |
| Liposomes | Hidrazinocurcumin | M2-M1 repolarization of TAM caused by SAT3 inhibition | Breast cancer | Clinical | Zhang et al., 2013 |
| Hyaluronic acid-modified cationic lipid nano particles | Anti-PD-1, CpG, and mitoxantrone- treated tumour cells | CT26 and B16-F10-OVA | Melanoma in mouse model and colon carcinoma | Pre-clinical | Fan et al., 2017 |
| Lipid nanomaterials | A T-cell stimulator [7DW8-5]. and a PI3K inhibitor [PI-3065]. | To overcome physical obstacles and tumour microenvironments that are unfriendly | Mouse breast cancer | Pre-clinical | Zhang et al., 2018a, Zhang et al., 2018b |
| Liposomal NPs | CpG and cisplatin | B16-F10 | Mouse model of melanoma | Pre-clinical | Lu et al., 2016 |
| Liposome | CD20 antibodies and Human epidermal growth factor receptor 2 [HER2]. | NBiTEs | Mouse breast cancer | Pre-clinical | Gong et al., 2021 |
| Lipid-dendrimer-calcium-phosphate | PD-L1 and an IL-2-encoding plasmid | Improve CD8 + T cell infiltration | Lung metastasis | Approved | Chiu et al., 2007 |
| Hyaluronic coated cationic liposome | CpG ODN, IR-7 dye | Agonist for PTT + TLR9 | CT-26 | Pre-clinical | Le et al., 2018 |
| Lipid-coated hollow mesoporous silica Nano particles | All-trans retinoic acid, interleukin-2, doxo | DC promotion + cytokine + chemotherapy | B16F10 | Pre-clinical | Kong et al., 2017; Le et al., |
| Lipid-based nanodisc | CpG ODN, neoantigen peptide | Vaccine + TLR9 agonist + CTLA-4/PD-1 antibody | B16F10 MC-38 | Pre-clinical | Kuai et al., 2016, Le et al., 2018 |
| Lipid vesicle coated mesoporous silica Nano particles | Indoximod, oxaliplatin | IDO inhibitor + Chemotherapy | KPC | Clinical | Lu et al., 2017, Chiang et al., 2011 |
| CpG and -CD40 are covalently linked with PEGylated liposomes. | CD40 and CpG conjugated to the surface | Dendritic cells | melanoma tumour mice | Pre-clinical | Kwong et al., 2011 |
| Lipoprotein Nano disks | CpG, anti-CTLA4 antigen, and soluble anti-PD-1 | DCs | melanoma mouse models and colon carcinoma | Pre-clinical | Kuai et al., 2016 |
| Hybrid lipid polymeric nano | SB505124, interleukin-12 | TGF-β inhibitor + cytokine | B16F10 | Pre-clinical | Le et al., 2018, Park et al., 2012 |
| Liposomes | PI103, Selumetinib | PI3K inhibitor combined with anti-PD-L1 antibody | B16F10 4 T1 | Pre-clinical | Kulkarni et al., 2016 |
| ipid/calcium/phosphate | MUC-1 mRNA | Vaccine plus anti-CTLA-4 antibody | 4 T1 | Approved | Li et al., 2018 |
| Lipid-coated calcium phosphate | CXCL-12, PD-L1 | Trapping protein-encoding plasmids | Colorectal liver metastasis | Clinical | Sansei et al., 2021 |
High manufacturing costs and low yield values are the primary drawbacks of liposomal nanovesicles, which discourages the growth of their industrial use in cancer immunology. Additionaly, on-shelf stability, the strict control over quality, purity, and sterility is required by pharmaceutical regulations, which can be a barrier to efficient technology transfer.
8. Conclusions
In summary, along with the existing anticancer immunotherapies and incorporation of nanotechnology heralds the start of a new era in cancer treatment. With the amount of research that is available and ongoing on these therapeutics, some of which are discussed above, these therapeutic agents will bring more advanced and safer antitumor treatments. Tumor immunotherapy is classified into two types: humoral immunity and cellular immunity. However, immunotherapy response rates are still low, and additional research is certainly needed to improve these treatments' outcomes. A plethora of distinct nanocarriers endowed with extraordinary features have been described throughout the previous few decades. As a result, these nanocarriers can be designed to discharge medications at a targeted site by releasing drugs at right time, right place and right concentration. Cost and quality control are the major limitations in the development of liposomes. Quality assurance concerns both the manufacturing process and the stability of the formulation. Nano-delivery systems are influenced by scalability, consistency, and repeatability of the finished product, lack of equipment and/or in-house expertise, chemical instability or denaturation of the encapsulated compound in the manufacturing process, and challenges with long-term stability. When the liposomal delivery system's functionality is more complex/complicated, such as surface modification with coatings and/or ligands. Multiple chemical synthesis steps and formulation processes are required for the integration of multiple components into a single nanosized carrier, which inevitably causes issues for large-scale good manufacturing (cGMP) production, which ultimately elevating the cost of production, and making it more challenging to evaluate such products.
Liposomal nanovesicle’s targeting efficacy is still lower than ideal because of the presence of similar ligands on different cells. Improvement needed in some areas Liposomal nanovesicles are (1) modifying the liposomes in accordance with the function of the specific immunomodulator and its target and (2) enhancing the pharmacokinetics of liposomes to minimize biodistribution to obtain the least toxic effect. Future studies should concentrate on the specific ligands (neoantigens). of tumour cells in enhancing clinical outcomes. Liposomes have a number of advantages in cancer immunotherapy, including excellent safety, effective drug delivery, the ability to induce cell death, and self-activation of the immune system. Liposomal NPs, we believe, will be used in cancer immunotherapy for safe and high-performance clinical applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group (Large). Project number RGP. 2/251/43.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alanazi S.A., Alanazi F., Haq N., Shakeel F., Badran M.M., Harisa G.I. Lipoproteins-Nanocarriers as a Promising Approach for Targeting Liver Cancer: Present Status and Application Prospects. Curr. Drug Deliv. 2020;17:826–844. doi: 10.2174/1567201817666200206104338. [DOI] [PubMed] [Google Scholar]
- Alanazi, F.K., Lu, D.R., Shakeel, F., Haq, N., 2014. Density gradient separation of carborane-containing liposome from low density lipoprotein and detection by inductively coupled plasma spectrometry. 53–58. https://doi.org/10.3109/08982104.2013.833224. [DOI] [PubMed]
- Alanazi S.A., Harisa G.I., Badran M.M., Haq N., Radwan A.A., Kumar A., Shakeel F., Alanazi F.K. Cholesterol-Conjugate as a New Strategy to Improve the Cytotoxic Effect of 5-Fluorouracil on Liver Cancer: Impact of Liposomal Composition. Curr. Drug Deliv. 2020;17:898–910. doi: 10.2174/1567201817666200211095452. [DOI] [PubMed] [Google Scholar]
- Alizadeh D., Trad M., Hanke N.T., Larmonier C.B., Janikashvili N., Bonnotte B., Katsanis E., Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545/651271/AM/DOXORUBICIN-ELIMINATES-MYELOID-DERIVED-SUPPRESSOR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Joufi F.A., Salem-Bekhit M.M., Taha E.I., Ibrahim M.A., Muharram M.M., Alshehri S., Ghoneim M.M., Shakeel F. Enhancing Ocular Bioavailability of Ciprofloxacin Using Colloidal Lipid-Based Carrier for the Management of Post-Surgical Infection. Molecules. 2022;27:733–827. doi: 10.3390/MOLECULES27030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra A., Di Gioacchino M., Tonacci A., Petrarca C., Gangemi S. Nanomedicine for Immunotherapy Targeting Hematological Malignancies: Current Approaches and Perspective. Nanomater. 2021;2021(11):2792. doi: 10.3390/NANO11112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T.M., Cullis P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/J.ADDR.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Andújar I., Ríos J.L., Giner R.M., Recio M.C. Pharmacological Properties of Shikonin – A Review of Literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/S-0033-1350934. [DOI] [PubMed] [Google Scholar]
- Banchereau, J., Steinman, R.M., 1998. Dendritic cells and the control of immunity. Nat. 1998 3926673 392, 245–252. https://doi.org/10.1038/32588. [DOI] [PubMed]
- Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/J.JCONREL.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Barral P.M., Sarkar D., Su Zhong Z., Barber G.N., DeSalle R., Racaniello V.R., Fisher P.B. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: Key regulators of innate immunity. Pharmacol. Ther. 2009;124:219–234. doi: 10.1016/J.PHARMTHERA.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton G.M., Medzhitov R. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5/COVER. [DOI] [PubMed] [Google Scholar]
- Begum M.Y., Osmani R.A.M., Alqahtani A., Ghazwani M., Hani U., Ather H., Atiya A., Rahamathulla M., Siddiqua A. Development of stealth liposomal formulation of celecoxib: In vitro and in vivo evaluation. PLoS One. 2022;17:e0264518. doi: 10.1371/journal.pone.0264518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume G., Cevc G., Crommelin M.D.J.A., Bakker-Woudenberg I.A.J.M., Kluft C., Storm G. Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim. Biophys. Acta - Biomembr. 1993;1149:180–184. doi: 10.1016/0005-2736(93)90039-3. [DOI] [PubMed] [Google Scholar]
- Casares N., Pequignot M.O., Tesniere A., Ghiringhelli F., Roux S., Chaput N., Schmitt E., Hamai A., Hervas-Stubbs S., Obeid M., Coutant F., Métivier D., Pichard E., Aucouturier P., Pierron G., Garrido C., Zitvogel L., Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/JEM.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Cheng C.T., Castro G., Liu C.H., Lau P. Advanced nanotechnology: An arsenal to enhance immunotherapy in fighting cancer. Clin. Chim. Acta. 2019;492:12–19. doi: 10.1016/J.CCA.2019.01.027. [DOI] [PubMed] [Google Scholar]
- Cheng J.C., Moore T.B., Sakamoto K.M. RNA interference and human disease. Mol. Genet. Metab. 2003;80:121–128. doi: 10.1016/J.YMGME.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Cheng L., Zhang X., Tang J., Lv Q., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanoparticles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275 doi: 10.1016/J.BIOMATERIALS.2021.120964. [DOI] [PubMed] [Google Scholar]
- Chiang, C.L.L., Kandalaft, L.E., Coukos, G., 2011. Adjuvants for Enhancing the Immunogenicity of Whole Tumor Cell Vaccines. 150–182. https://doi.org/10.3109/08830185.2011.572210. [DOI] [PubMed]
- Chiu G.N.C., Edwards L.A., Kapanen A.I., Malinen M.M., Dragowska W.H., Warburton C., Chikh G.G., Fang K.Y.Y., Tan S., Sy J., Tucker C., Waterhouse D.N., Klasa R., Bally M.B. Modulation of cancer cell survival pathways using multivalent liposomal therapeutic antibody constructs. Mol. Cancer Ther. 2007;6:844–855. doi: 10.1158/1535-7163.MCT-06-0159/357274/P/MODULATION-OF-CANCER-CELL-SURVIVAL-PATHWAYS-USING. [DOI] [PubMed] [Google Scholar]
- Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. 2014;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- Deng W., Chen W., Clement S., Guller A., Zhao Z., Engel A., Goldys E.M. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat. Commun. 2018;91(9):1–11. doi: 10.1038/s41467-018-05118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.D., Zhang M., Wang Y.H., He Y., Wang H.R., Chen B.F., Tu B., Zhu S.Q., Huang Y.Z. Anti-PD-L1 mediating tumor-targeted codelivery of liposomal irinotecan/JQ1 for chemo-immunotherapy. Acta Pharmacol. Sin. 2020;429(42):1516–1523. doi: 10.1038/s41401-020-00570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis M.L. Mechanism of Action of Immunotherapy. Semin. Oncol. 2014;41:S3–S13. doi: 10.1053/J.SEMINONCOL.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Dobosz P., Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovich T., Mendelson D., Kurtin S., Richardson K., Von Hoff D., Hoos A. A Phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother. Pharmacol. 2006;586(58):759–764. doi: 10.1007/S00280-006-0235-4. [DOI] [PubMed] [Google Scholar]
- Du Y., Liang X., Li Y., Sun T., Jin Z., Xue H., Tian J. Nuclear and Fluorescent Labeled PD-1-Liposome-DOX-64Cu/IRDye800CW Allows Improved Breast Tumor Targeted Imaging and Therapy. Mol. Pharm. 2017;14:3978–3986. doi: 10.1021/ACS.MOLPHARMACEUT.7B00649/SUPPL_FILE/MP7B00649_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Fan Y., Kuai R., Xu Y., Ochyl L.J., Irvine D.J., Moon J.J. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017;17:7387–7393. doi: 10.1021/ACS.NANOLETT.7B03218/SUPPL_FILE/NL7B03218_SI_003.MOV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkona S., Diamandis E.P., Blasutig I.M. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd, S.R., Pacold, M.E., Huang, Q., Clarke, S.M., Lam, F.C., Cannell, I.G., Bryson, B.D., Rameseder, J., Lee, M.J., Blake, E.J., Fydrych, A., Ho, R., Greenberger, B.A., Chen, G.C., Maffa, A., Del Rosario, A.M., Root, D.E., Carpenter, A.E., Hahn, W.C., Sabatini, D.M., Chen, C.C., White, F.M., Bradner, J.E., Yaffe, M.B., 2013. The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nat. 2013 4987453 498, 246–250. https://doi.org/10.1038/nature12147. [DOI] [PMC free article] [PubMed]
- Frank M.J., Reagan P.M., Bartlett N.L., Gordon L.I., Friedberg J.W., Czerwinski D.K., Long S.R., Hoppe R.T., Janssen R., Candia A.F., Coffman R.L., Levy R. In situ vaccination with a tlr9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018;8:1258–1269. doi: 10.1158/2159-8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franquiz M.J., Short N.J. Blinatumomab for the Treatment of Adult B-Cell Acute Lymphoblastic Leukemia: Toward a New Era of Targeted Immunotherapy. Biologics: Targets and Therapy. 2020;14:23–34. doi: 10.2147/BTT.S202746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.H., Qi C., Lin J., Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018;47:6454–6472. doi: 10.1039/C7CS00891K. [DOI] [PubMed] [Google Scholar]
- Gammon J.M., Dold N.M., Jewell C.M., Gammon J.M., Dold N.M., Jewell C.M. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget. 2016;7:15421–15443. doi: 10.18632/ONCOTARGET.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A., Hu X.L., Saeed M., Chen B.F., Li Y.P., Yu H.J. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019;409(40):1129–1137. doi: 10.1038/s41401-019-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garijo A., Fajardo C.A., Gros A. Determinants for neoantigen identification. Front. Immunol. 2019;10:1392. doi: 10.3389/FIMMU.2019.01392/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Tran B., Ensor J., Gibbs P., Wong H.L., Wong S.F., Vilar E., Tie J., Broaddus R., Kopetz S., Desai J., Overman M.J. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann. Oncol. 2014;25:1032–1038. doi: 10.1093/ANNONC/MDU100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N., Sheppard N.C., Billingsley M.M., June C.H., Mitchell M.J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 2021;161(16):25–36. doi: 10.1038/s41565-020-00822-y. [DOI] [PubMed] [Google Scholar]
- Gu Z., Wang Q., Shi Y., Huang Y., Zhang J., Zhang X., Lin G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control. Release. 2018;286:369–380. doi: 10.1016/J.JCONREL.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Gu Z., Da Silva C.G., van der Maaden K., Ossendorp F., Cruz L.J. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmceutics. 2020;12:1054. doi: 10.3390/PHARMACEUTICS12111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C., Chernyak N., Dominguez D., Cole L., Zhang B., Mirkin C.A. RNA-Based Immunostimulatory Liposomal Spherical Nucleic Acids as Potent TLR7/8 Modulators. Small. 2018;14:1803284. doi: 10.1002/SMLL.201803284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh O., Depelsenaire A.C.I., Meliga S.C., Yukiko S.R., McMillan N.A.J., Frazer I.H., Kendall M.A.F. CXCL1 gene silencing in skin using liposome-encapsulated siRNA delivered by microprojection array. J. Control. Release. 2014;194:148–156. doi: 10.1016/J.JCONREL.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Hei Y., Teng B., Zeng Z., Zhang S., Li Q., Pan J., Luo Z., Xiong C., Wei S. Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma. Int. J. Nanomedicine. 2020;15:1677. doi: 10.2147/IJN.S225807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., Akerley W., van den Eertwegh A.J.M., Lutzky J., Lorigan P., Vaubel J.M., Linette G.P., Hogg D., Ottensmeier C.H., Lebbé C., Peschel C., Quirt I., Clark J.I., Wolchok J.D., Weber J.S., Tian J., Yellin M.J., Nichol G.M., Hoos A., Urba W.J. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMOA1003466/SUPPL_FILE/NEJMOA1003466_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Wei G., Zeng Z., Huang Y., Huang L., Shen Y., Sun X., Xu C., Zhao C. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics. 2019;9:5542. doi: 10.7150/THNO.35343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaka K., Kataoka K. Progress and Prospects of Polyplex Nanomicelles for Plasmid DNA Delivery. Curr. Gene Ther. 2011;11:457–465. doi: 10.2174/156652311798192879. [DOI] [PubMed] [Google Scholar]
- Jacobson C.A., Chavez J.C., Sehgal A.R., William B.M., Munoz J., Salles G., Munshi P.N., Casulo C., Maloney D.G., de Vos S., Reshef R., Leslie L.A., Yakoub-Agha I., Oluwole O.O., Fung H.C.H., Rosenblatt J., Rossi J.M., Goyal L., Plaks V., Yang Y., Vezan R., Avanzi M.P., Neelapu S.S. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
- Javed S., Alshehri S., Shoaib A., Ahsan W., Sultan M.H., Alqahtani S.S., Kazi M., Shakeel F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics. 2021;13:368. doi: 10.3390/pharmaceutics13030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., Xu Y., Frohlich M.W., Schellhammer P.F. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMOA1001294/SUPPL_FILE/NEJMOA1001294_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A., Koulmanda M., Freeman G.J., Sayegh M.H., Sharpe A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/JEM.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Mansoor S., Rafi Z., Kumari B., Shoaib A., Saeed M., Alshehri S., Ghoneim M.M., Rahamathulla M., Hani U., Shakeel F. A review on nanotechnology: Properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 2022;348 doi: 10.1016/J.MOLLIQ.2021.118008. [DOI] [Google Scholar]
- Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S., Gao X., Zhang Z. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics. 2017;7:3276–3292. doi: 10.7150/THNO.19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans S.A.A., Vader P., Schiffelers R.M. Tumour-bound RNA-laden exosomes. Nat. Biomed. Eng. 2017;18(1):634–636. doi: 10.1038/s41551-017-0119-4. [DOI] [PubMed] [Google Scholar]
- Krishnan L., Sad S., Patel G.B., Sprott G.D. The Potent Adjuvant Activity of Archaeosomes Correlates to the Recruitment and Activation of Macrophages and Dendritic Cells In Vivo. J. Immunol. 2001;166:1885–1893. doi: 10.4049/JIMMUNOL.166.3.1885. [DOI] [PubMed] [Google Scholar]
- Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2016;164(16):489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A., Natarajan S.K., Chandrasekar V., Pandey P.R., Sengupta S. Combining Immune Checkpoint Inhibitors and Kinase-Inhibiting Supramolecular Therapeutics for Enhanced Anticancer Efficacy. ACS Nano. 2016;10:9227–9242. doi: 10.1021/ACSNANO.6B01600/SUPPL_FILE/NN6B01600_SI_005.AVI. [DOI] [PubMed] [Google Scholar]
- Kwong B., Liu H., Irvine D.J. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32:5134–5147. doi: 10.1016/J.BIOMATERIALS.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q.V., Choi J., Oh Y.K. Nano delivery systems and cancer immunotherapy. J. Pharm. Investig. 2018;485(48):527–539. doi: 10.1007/S40005-018-0399-Z. [DOI] [Google Scholar]
- Lei S., Zhang X., Men K., Gao Y., Yang X., Wu S., Duan X., Wei Y., Tong R. Efficient Colorectal Cancer Gene Therapy with IL-15 mRNA Nanoformulation. Mol. Pharm. 2020;17:3378–3391. doi: 10.1021/ACS.MOLPHARMACEUT.0C00451/ASSET/IMAGES/MEDIUM/MP0C00451_0013.GIF. [DOI] [PubMed] [Google Scholar]
- Li D., Li X., Zhou W.L., Huang Y., Liang X., Jiang L., Yang X., Sun J., Li Z., Han W.D., Wang W. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019;41(4):1–17. doi: 10.1038/s41392-019-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yang S., Song L., Zeng Y., He T., Wang N., Yu C., Yin T., Liu L., Wei X., Wu Q., Wei Y., Yang L., Gong C. An Endogenous Vaccine Based on Fluorophores and Multivalent Immunoadjuvants Regulates Tumor Micro-Environment for Synergistic Photothermal and Immunotherapy. Theranostics. 2018;8:860–873. doi: 10.7150/THNO.19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou S., Yu J., Cai W., Yang Y., Kuang X., Liu H., He Z., Wang Y. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J. Control. Release. 2021;335:306–319. doi: 10.1016/J.JCONREL.2021.05.040. [DOI] [PubMed] [Google Scholar]
- Liu H., Xie Y., Zhang Y., Cai Y., Li B., Mao H., Liu Y., Lu J., Zhang L., Yu R. Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma. Biomaterials. 2017;121:130–143. doi: 10.1016/J.BIOMATERIALS.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Lorenzer C., Dirin M., Winkler A.M., Baumann V., Winkler J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J. Control. Release. 2015;203:1–15. doi: 10.1016/J.JCONREL.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Lu J., Liu X., Liao Y.P., Salazar F., Sun B., Jiang W., Chang C.H., Jiang J., Wang X., Wu A.M., Meng H., Nel A.E. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 2017;8:1–14. doi: 10.1038/s41467-017-01651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang Y., Miao L., Haynes M., Xiang G., Huang L. Exploiting in situ antigen generation and immune modulation to enhance chemotherapy response in advanced melanoma: A combination nanomedicine approach. Cancer Lett. 2016;379:32–38. doi: 10.1016/J.CANLET.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Mehmood R.K. Review of cisplatin and oxaliplatin in current immunogenic and monoclonal antibodies perspective. Oncol. Rev. 2014;8:36–43. doi: 10.4081/oncol.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nat. 2011;4807378(480):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino M., Contreras A., Casares N., Troconiz I.F., ten Hagen T.L.M., Berraondo P., Zalba S., Garrido M.J. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomedicine Nanotechnology. Biol. Med. 2019;17:13–25. doi: 10.1016/J.NANO.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Mir S.A., Hamid L., Bader G.N., Shoaib A., Rahamathulla M., Alshahrani M.Y., Alam P., Shakeel F. Role of Nanotechnology in Overcoming the Multidrug Resistance in Cancer Therapy: A Review. Molecules. 2022;27:6608. doi: 10.3390/MOLECULES27196608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y.W., Hajjar J., Hwu P., Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer. 2015;3:1–10. doi: 10.1186/S40425-015-0094-9/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz G.T., Coukos G. Deciphering and Reversing Tumor Immune Suppression. Immunity. 2013;39:61–73. doi: 10.1016/J.IMMUNI.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheem A., Shakeel F., Warsi M.H., Jain G.K., Ahmad F.J. A Combinatorial Statistical Design Approach to Optimize the Nanostructured Cubosomal Carrier System for Oral Delivery of Ubidecarenone for Management of Doxorubicin-Induced Cardiotoxicity. In Vitro–In Vivo Investigations. J. Pharm. Sci. 2017;106:3050–3065. doi: 10.1016/j.xphs.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Naidoo J., Page D.B., Li B.T., Connell L.C., Schindler K., Lacouture M.E., Postow M.A., Wolchok J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/ANNONC/MDV383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navashenaq J.G., Zamani P., Nikpoor A.R., Tavakkol-Afshari J., Jaafari M.R. Doxil chemotherapy plus liposomal P5 immunotherapy decreased myeloid-derived suppressor cells in murine model of breast cancer. Nanomedicine Nanotechnology. Biol. Med. 2020;24 doi: 10.1016/J.NANO.2020.102150. [DOI] [PubMed] [Google Scholar]
- Nikpoor A.R., Tavakkol-Afshari J., Sadri K., Jalali S.A., Jaafari M.R. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: In vitro and in vivo studies. Nanomedicine Nanotechnology. Biol. Med. 2017;13:2671–2682. doi: 10.1016/J.NANO.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Noman M.Z., Hasmim M., Messai Y., Terry S., Kieda C., Janji B., Chouaib S. Hypoxia: A key player in antitumor immune response. A review in the theme: Cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015;309:C569–C579. doi: 10.1152/AJPCELL.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;124(12):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wrzesinski S.H., Stern E., Look M., Criscione J., Ragheb R., Jay S.M., Demento S.L., Agawu A., Licona Limon P., Ferrandino A.F., Gonzalez D., Habermann A., Flavell R.A., Fahmy T.M. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012;1110(11):895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A. Complete response to pixantrone as a salvage therapy in a relapsed/refractory diffuse large B-cell lymphoma. OncoReview. 2020;10:62–68. doi: 10.24292/01.OR.220300620.5. [DOI] [Google Scholar]
- Postow M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. B. 2015:76–83. doi: 10.14694/edbook_am.2015.35.76. [DOI] [PubMed] [Google Scholar]
- Rahamathulla M., Alam N., Hani U., Ibrahim Q., Alhamhoom Y. Development and in vitro evaluation of effervescent floating matrix tablet of neritinib: An anticancer drug. Pak. J. Pharm Sci. 2021;34:1297–1303. doi: 10.36721/PJPS.2021.34.4.REG.1297-1303.1. [DOI] [PubMed] [Google Scholar]
- Rahamathulla M., Alsahhrani S.M., Al Saqr A., Alshetaili A., Shakeel F. Effervescent floating matrix tablets of a novel anti-cancer drug neratinib for breast cancer treatment. J. Drug Deliv. Sci. Technol. 2021;66 doi: 10.1016/J.JDDST.2021.102788. [DOI] [Google Scholar]
- Rahamathulla M., Veerapu G., Hani U., Alhamhoom Y., Alqahtani A., Moin A. Characterization, Optimization, In Vitro and In Vivo Evaluation of Simvastatin Proliposomes, as a Drug Delivery. AAPS PharmSciTech. 2020;214(21):1–15. doi: 10.1208/S12249-020-01666-4. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R., Assudani D., Nagaraj S., Hunter T., Cho H.I., Antonia S., Altiok S., Celis E., Gabrilovich D.I. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;183(18):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowshanravan B., Halliday N., Sansom D.M. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/BLOOD-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahdev P., Ochyl L.J., Moon J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014;31:2563–2582. doi: 10.1007/S11095-014-1419-Y/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science (80-.) 2018;359:1355–1360. doi: 10.1126/SCIENCE.AAR7112. [DOI] [PubMed] [Google Scholar]
- Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R., Kelly P.M., Åberg C., Mahon E., Dawson K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;82(8):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- Sanaei M.J., Pourbagheri-Sigaroodi A., Kaveh V., Sheikholeslami S.A., Salari S., Bashash D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit. Rev. Oncol. Hematol. 2021;157 doi: 10.1016/J.CRITREVONC.2020.103160. [DOI] [PubMed] [Google Scholar]
- Sasso M.S., Lollo G., Pitorre M., Solito S., Pinton L., Valpione S., Bastiat G., Mandruzzato S., Bronte V., Marigo I., Benoit J.P. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials. 2016;96:47–62. doi: 10.1016/J.BIOMATERIALS.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Sayour E.J., Mendez-Gomez H.R., Mitchell D.A. Cancer Vaccine Immunotherapy with RNA-Loaded Liposomes. Int. J. Mol. Sci. 2018;19:2890. doi: 10.3390/IJMS19102890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Wagner K., Wolchok J.D., Allison J.P. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat. Rev. Cancer. 2011;1111(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Feng L., Zhu Y., Tao D., Xu J., Peng R., Liu Z. Oxaliplatin-/NLG919 prodrugs-constructed liposomes for effective chemo-immunotherapy of colorectal cancer. Biomaterials. 2020;255 doi: 10.1016/J.BIOMATERIALS.2020.120190. [DOI] [PubMed] [Google Scholar]
- Shi H.S., Gao X., Li D., Zhang Q.W., Wang Y.S., Zheng Y., Cai L.L., Zhong R.M., Rui A., Li Z.Y., Zheng H. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomedicine. 2012;7:2601–2611. doi: 10.2147/IJN.S31439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib A., Azmi L., Pal S., Alqahtani S.S., Rahamathulla M., Hani U., Alshehri S., Ghoneim M.M., Shakeel F. Integrating nanotechnology with naturally occurring phytochemicals in neuropathy induced by diabetes. J. Mol. Liq. 2022;350 doi: 10.1016/j.molliq.2021.118189. [DOI] [Google Scholar]
- Shull M.M., Ormsby I., Kier A.B., Pawlowski S., Diebold R.J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., Annunziata N., Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nat. 1992;3596397(359):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwon N., Kim Y.J., Siegler E., Chen X., Rohrs J.A., Liu Y., Wang P. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol. Res. 2018;6:812–824. doi: 10.1158/2326-6066.CIR-17-0502/470613/AM/CAR-T-CELLS-SURFACE-ENGINEERED-WITH-DRUG. [DOI] [PubMed] [Google Scholar]
- Smyth M.J., Ngiow S.F., Ribas A., Teng M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2015;133(13):143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- Song W., Musetti S.N., Huang L. Nanomaterials for cancer immunotherapy. Biomaterials. 2017;148:16–30. doi: 10.1016/J.BIOMATERIALS.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Keselowsky B.G. Combinatorial drug delivery approaches for immunomodulation. Adv. Drug Deliv. Rev. 2017;114:161–174. doi: 10.1016/J.ADDR.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q., Wang C., Song H., Zhang C., Liu J., Huang P., Zhang Y., Zhang J., Wang W. Co-delivery of anionic epitope/CpG vaccine and IDO inhibitor by self-assembled cationic liposomes for combination melanoma immunotherapy. J. Mater. Chem. B. 2021;9:3892–3899. doi: 10.1039/D1TB00256B. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Namai E., Oda Y., Nishiie N., Otake S., Koshima R., Hirata K., Taira Y., Utoguchi N., Negishi Y., Nakagawa S., Maruyama K. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J. Control. Release. 2010;142:245–250. doi: 10.1016/J.JCONREL.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Tinoco-Veras C.M., Santos A.A.Q.A., Stipursky J., Meloni M., Araujo A.P.B., Foschetti D.A., López-Ureña D., Quesada-Gómez C., Leitão R.F.C., Gomes F.C.A., de Brito G.A., C., Transforming growth factor β1/SMAD signaling pathway activation protects the intestinal epithelium from Clostridium difficile toxin A-induced damage. Infect. Immun. 2017;85 doi: 10.1128/IAI.00430-17/SUPPL_FILE/ZII999092181S1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornesello, A.L., Tagliamonte, M., Tornesello, M.L., Buonaguro, F.M., Buonaguro, L., 2020. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines (Basel) 12, 1049. https://doi: 10.3390/cancers12041049. [DOI] [PMC free article] [PubMed]
- Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers. 2020;12:738. doi: 10.3390/CANCERS12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcheti V., Schalper K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. B. 2016;298–308 doi: 10.1200/edbk_156572. [DOI] [PubMed] [Google Scholar]
- Wang N., Wang Z., Xu Z., Chen X., Zhu G. A Cisplatin-Loaded Immunochemotherapeutic Nanohybrid Bearing Immune Checkpoint Inhibitors for Enhanced Cervical Cancer Therapy. Angew. Chemie Int. Ed. 2018;57:3426–3430. doi: 10.1002/ANIE.201800422. [DOI] [PubMed] [Google Scholar]
- Wesolowski R., Markowitz J., Carson W.E. Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;1:1–11. doi: 10.1186/2051-1426-1-10/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia A.L., Xu Y., Lu X.J. Cancer immunotherapy: challenges and clinical applications. J. Med. Genet. 2019;56:1–3. doi: 10.1136/JMEDGENET-2018-105852. [DOI] [PubMed] [Google Scholar]
- Xian J., Yang H., Lin Y., Liu S. Combination Nonviral Murine Interleukin 2 and Interleukin 12 Gene Therapy and Radiotherapy for Head and Neck Squamous Cell Carcinoma. Arch. Otolaryngol. Neck Surg. 2005;131:1079–1085. doi: 10.1001/ARCHOTOL.131.12.1079. [DOI] [PubMed] [Google Scholar]
- Xu Z., Wang Y., Zhang L., Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636–3645. doi: 10.1021/NN500216Y/ASSET/IMAGES/NN-2014-00216Y_M002.GIF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaddanapudi, K., Mitchell, R.A., Eaton, J.W., 2013. Cancer vaccines. 2. https://doi.org/10.4161/ONCI.23403.
- Yang X., Fan J., Wu Y., Ma Z., Huang J., Zhang Y., Zhou Z., Mo F., Liu X., Yuan H., Xu Y., Pan L., Chen S. Synthetic multiepitope neoantigen DNA vaccine for personalized cancer immunotherapy. Nanomedicine Nanotechnology. Biol. Med. 2021;37 doi: 10.1016/J.NANO.2021.102443. [DOI] [PubMed] [Google Scholar]
- Yang W., Shi X., Shi Y., Yao D., Chen S., Zhou X., Zhang B. Beyond the Roles in Biomimetic Chemistry: An Insight into the Intrinsic Catalytic Activity of an Enzyme for Tumor-Selective Phototheranostics. ACS Nano. 2018;12:12169–12180. doi: 10.1021/ACSNANO.8B05797/SUPPL_FILE/NN8B05797_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Yin W.M., Li Y.W., Gu Y.Q., Luo M. Nanoengineered targeting strategy for cancer immunotherapy. Acta Pharmacol. Sin. 2020;417(41):902–910. doi: 10.1038/s41401-020-0417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.J., De Geest B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol. Sin. 2020;417(41):879–880. doi: 10.1038/s41401-020-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuba E., Kono Y., Harada A., Yokoyama S., Arai M., Kubo K., Kono K. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials. 2013;34:5711–5721. doi: 10.1016/J.BIOMATERIALS.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Zamani P., Navashenaq J.G., Teymouri M., Karimi M., Mashreghi M., Jaafari M.R. Combination therapy with liposomal doxorubicin and liposomal vaccine containing E75, an HER-2/neu-derived peptide, reduces myeloid-derived suppressor cells and improved tumor therapy. Life Sci. 2020;252 doi: 10.1016/J.LFS.2020.117646. [DOI] [PubMed] [Google Scholar]
- Zhang B.L., Qin D.Y., Mo Z.M., Li Y., Wei W., Wang Y.S., Wang W., Wei Y.Q. Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci. China Life Sci. 2016;594(59):340–348. doi: 10.1007/S11427-016-5027-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li N., Suh H., Irvine D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018;9:6. doi: 10.1038/s41467-017-02251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Stephan S.B., Ene C.I., Smith T.T., Holland E.C., Stephan M.T. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective t-cell therapy in solid malignancies. Cancer Res. 2018;78:3718–3730. doi: 10.1158/0008-5472.CAN-18-0306/653226/AM/NANOPARTICLES-THAT-RESHAPE-THE-TUMOR-MILIEU-CREATE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tang W.L., Kheirolomoom A., Fite B.Z., Wu B., Lau K., Baikoghli M., Raie M.N., Tumbale S.K., Foiret J., Ingham E.S., Mahakian L.M., Tam S.M., Cheng R.H., Borowsky A.D., Ferrara K.W. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release. 2021;330:1080–1094. doi: 10.1016/J.JCONREL.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tian W., Cai X., Wang X., Dang W., Tang H., Cao H., Wang L., Chen T. Hydrazinocurcumin Encapsuled Nanoparticles “Re-Educate” Tumor-Associated Macrophages and Exhibit Anti-Tumor Effects on Breast Cancer Following STAT3 Suppression. PLoS One. 2013;8:e65896. doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Stephan M.T., Gai S.A., Abraham W., Shearer A., Irvine D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release. 2013;172:426–435. doi: 10.1016/J.JCONREL.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Tang L., Mabardi L., Kumari S., Irvine D.J. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS Nano. 2017;11:3089–3100. doi: 10.1021/ACSNANO.7B00078/SUPPL_FILE/NN7B00078_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wu H., Yang R., Xu A., Zhang Q., Dong J., Qian C., Sun M. GSH depletion liposome adjuvant for augmenting the photothermal immunotherapy of breast cancer. Sci. Adv. 2020;6 doi: 10.1126/SCIADV.ABC4373/SUPPL_FILE/ABC4373_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ding W., Zhang Y., Cheng S., Li F., Ruan R., Wei P., Qiu B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials. 2018;182:1–12. doi: 10.1016/J.BIOMATERIALS.2018.08.013. [DOI] [PubMed] [Google Scholar]