Abstract
Background and Aims
Patient-centric management of inflammatory bowel disease [IBD] is important, with consensus considering patient-reported outcomes alongside clinical and endoscopic assessment by healthcare providers. However, evidence regarding patients’ treatment priorities is still limited. This study aimed to elicit benefit–risk trade-offs that patients with IBD are willing to make, to help inform discussions about patient-centric treatment targets.
Methods
This was a cross-sectional online survey of adults with self-confirmed Crohn’s disease [CD] or ulcerative colitis [UC] receiving IBD treatment. The impact of efficacy, administration and safety on treatment preferences was elicited using a discrete choice experiment. Relative attribute importance [RAI] and maximum acceptable risk of mild-to-moderate side effects [SEs] were estimated from a mixed logit model.
Results
In total, 400 patients [CD: 54%; UC: 46%; female: 38.0%; age range: 18–78 years] were recruited. Efficacy, administration and safety affected treatment preferences to varying degrees, with abdominal pain being most important [RAI 33%] followed by risks of mild-to-moderate SEs [RAI 27%] and serious infections [RAI 16%]. To reduce abdominal pain from severe to moderate/mild, patients accepted an additional 18.8% or 30.6% risk of mild-to-moderate SEs, respectively. While average preferences between patients with CD and UC were similar, patients with CD placed greater importance on abdominal pain [p < 0.05], and patients with UC on bowel urgency [p < 0.05]. However, preferences varied notably.
Conclusions
While avoiding abdominal pain, SEs and serious infections had on average the highest treatment priority, preferences varied between patients. Treatment strategies should consider the trade-offs individuals are willing to make.
Keywords: Quality of life, socio-economical, psychological endpoints
1. Introduction
There is increasing evidence that a treat-to-target approach may improve outcomes for patients with inflammatory bowel disease [IBD],1 with the STRIDE-II recommendations identifying clinical remission, endoscopic healing, restoration of quality of life [QoL] and absence of disability as important and achievable long-term treatment targets.2 Integration of additional patient-reported outcomes [PROs] into recommendations highlights an increasing need to better understand what goals patients themselves deem most important and how these might be achieved with current therapies.
The treatment landscape for IBD is evolving, and new treatments have significantly contributed to reduced clinical morbidity, improvements in QoL, avoidance of work absence and/or loss of productivity, as each treatment offers a unique attribute profile in terms of safety, efficacy and administration.3 With the diversification of the treatment landscape as novel treatments are developed, the focus lies increasingly on optimal treatment selection as well as the definition of individual treatment targets and trade-offs patients make to optimize their treatment based on their needs. However, it is crucial to note that physicians may perceive the risk of side effects and other attributes differently to patients.4
Treat-to-target strategies through personalized care5 have been advocated and individualizing patient care in IBD may lead to improved outcomes.1 However, quantitative evidence of patients’ treatment priorities is still limited. Patient preference data can be used to help define patient-centric treatment targets by assessing the relative importance of different treatment aspects as well as acceptable benefit–risk profiles. Previously conducted preference studies in IBD may not be suitable for informing patient-centric treat-to-target approaches, because they have either focused on clinical endpoints that may not reflect all treatment options, or they only cover a narrow population of patients with IBD.5–9
This study used a discrete choice experiment [DCE] and a thresholding exercise [TE] to quantify the effect of different treatment attributes on patient preferences and to elicit the trade-offs that patients are willing to make between treatment risks, treatment administration and disease outcomes. The findings provide insights into patients’ treatment priorities and can form a basis for patient-centric treatment practice.
2. Materials and Methods
2.1 Study design and patients
The P-POWER IBD study was a cross-sectional online survey of adults with Crohn’s disease [CD] or ulcerative colitis [UC]. Patients were recruited through partnership with the European Federation of Crohn’s and Ulcerative Colitis Associations [EFCCA] and from nationally representative online access panels between October 2020 and December 2020. Eligible patients met the following self-reported criteria: ≥18 years; resident of Ireland, UK, Italy, Luxembourg, Belgium, Switzerland, France, Germany, Austria, Spain, the Netherlands or Portugal; diagnosed with CD or UC; and currently receiving treatment for IBD. In total, 181 patients with a self-reported diagnosis of irritable bowel syndrome [IBS] were excluded.
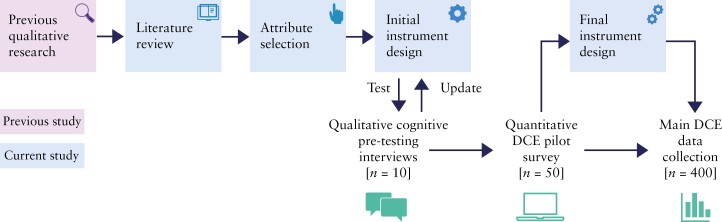
The overall study flow is presented in Figure 1. To ensure clinical relevance and patient-centricity, clinicians experienced in treating IBD [E.L., C.A.S., S.G.] and EFCCA representatives contributed to the protocol development as well as data interpretation. Patient input was considered throughout the instrument development phase.
Figure 1.
P-POWER mixed methodology study flow. DCE, discrete choice experiment.
The study was conducted in compliance with local laws and regulations and informed consent was collected from all participants. The Institutional Review Board [IRB] at the Ethical and Independent Review Services [E&I] reviewed and approved the study protocols, informed consent and other study documents [study number 20003 – 01C].
2.2 Development of the discrete choice experiment
The six treatment attributes [with three to four levels each] that were included in the DCE [Table 1] were informed by foundational qualitative research consisting of a targeted literature review, a focus group and two rounds of online voting with patients with IBD, which have been published previously.10 The targeted literature review of published patient preference studies in IBD was conducted to supplement the qualitative study findings and help refine the included treatment attributes [Supplementary Material: Appendix 1].
Table 1.
Final attributes and levels identified for inclusion in the discrete choice experiment
| Attribute | Level |
|---|---|
| Administration | (1)Oral at home, every day |
| (2)Injection at home, every few weeks | |
| (3)Injection at home, every few months | |
| (4)Infusion at hospital, every few monthsa | |
| Abdominal painb | (1)No pain |
| (2)Mild pain: aware but tolerable | |
| (3)Moderate pain: interferes with usual activities | |
| (4)Severe pain: intolerablea | |
| Bowel urgency | (1)You can usually postpone as long as necessary without fear of having an accident |
| (2)You usually can postpone for a short while without fear of an accident | |
| (3)You usually have to rush to the toilet, but cannot avoid having an occasional accidenta | |
| Fatigue | (1)No fatigue |
| (2)Mild fatigue: little effect on usual activities | |
| (3)Moderate fatigue: some effect on usual activities | |
| (4)Severe fatigue: considerable effect on usual activitiesa | |
| Risk of mild or moderate side effectsc,d | (1) 100 out of 1000 [10%] |
| (2) 200 out of 1000 [20%] | |
| (3)400 out of 1000 [40%]a | |
| Risk of serious infectionsc | (1) 0 out of 1000 [0%] |
| (2) 50 out of 1000 [5%] | |
| (3)100 out of 1000 [10%]a |
aReference level for attribute.
bSeverity was presented on a 0–100 visual analogue scale: 0–<25 = low; 25–<50 = mild; 50–<75 = moderate; 75–100 = severe.
cRisk was presented using coloured icon arrays.
dIncluded adverse events such as nausea, vomiting, headache, non-serious infections, skin reactions, laboratory abnormalities and infusion reactions.
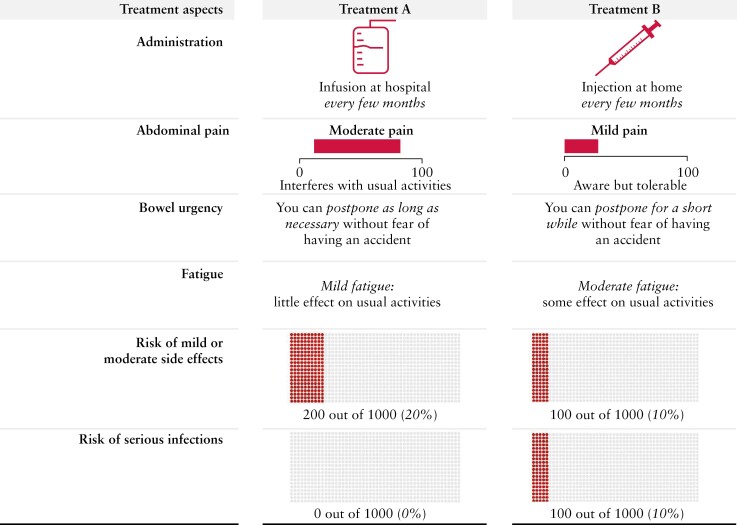
Within the DCE, patients with IBD were asked to complete a series of choices between two hypothetical treatment alternatives [Figure 2]. The performance of the two alternatives on the six attributes was varied systematically according to a D-efficient design with 36 choice tasks. To minimize the burden for each respondent, the design was split into three blocks of 12 choice tasks and every patient completed one randomly allocated block. The order of the experimental choice tasks was randomized to mitigate learning and fatigue effects. Task numbers 5 and 6 were fixed to construct a subsequent TE. In addition, the presentation order of symptoms and risks in the choice tasks was randomized between patients to avoid ordering effects.11 Finally, three non-experimental choice tasks were included: (1) the first non-experimental task was an interactive demonstration and practice choice task to familiarize patients with the format of the DCE; (2) the second was a repeated choice task to test the stability of patients’ preferences; and (3) the final task was a dominance test that included one superior and one inferior treatment alternative. Overall, patients completed 15 choice tasks.
Figure 2.
Example choice task between two hypothetical treatment alternativesa. aWhile reflecting typical features of IBD medications, the hypothetical treatment alternatives were not intended to replicate specific clinical treatments. IBD, inflammatory bowel disease.
The DCE was tested during ten iterative think-aloud pre-testing interviews that assessed the clarity of the choice tasks, the risk communication approach [e.g. graphical representations], the completeness of attributes and patients’ ability to trade off between attributes.12 In addition, the possibility of including an opt-out alternative was explored during the qualitative pilot, but taking no treatment was perceived as unrealistic by patients. Subsequently, a quantitative pilot was conducted with 50 patients from the UK. Details on the literature review, the think-aloud interviews and the quantitative pilot are included in Supplementary Material: Appendix 1.
2.3 Threshold exercise
To extend the scope of the DCE beyond the included attributes, a TE elicited patients’ average maximum acceptable risk [MAR] of cancer and maximum acceptable number of weeks on steroids per year. For this, patients were presented with choice tasks 5 and 6 after completion of the DCE, with their previously denoted preference being highlighted as a reminder. The risk of cancer or the number of weeks on steroids per year were added as additional attributes to one of each of these choice tasks. While the risk of the previously non-chosen treatment option was fixed at 4:1000 for the risk of cancer and 0 for the number of weeks on steroids per year, the corresponding levels of the previously chosen alternative were systematically increased until preference reversal was observed. The point at which this reversal was observed was used as a measure of the maximum acceptable level of each of the two attributes. More details are included in Supplementary Material: Appendix 2.
2.4 Questionnaire and data collection
The DCE and TE were integrated into a comprehensive questionnaire that consisted of the following sections: (1) sociodemographic data; (2) self-reported clinical data; and (3) health literacy assessment. The full survey questionnaire is included in Supplementary Material: Appendix 3. All patients answered direct questions about the type of treatment they would prefer and completed the DCE and TE sections. Furthermore, all patients completed the IBD disc13 to obtain a visual representation of IBD-related disability and also the Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F] scale14 to assess their level of fatigue during usual daily activities over the past week.
The questionnaire also included indication-specific instruments. Specifically, patients with UC completed the Patient Simple Clinical Colitis Activity Index [P-SCCAI] to quantify UC disease activity;15 an adapted partial Mayo score was determined for patients with UC based on patients’ responses to two questions about stool frequency and one question about rectal bleeding. Patients with CD completed two PRO measures [PRO2] [frequency of liquid or very soft stools and abdominal pain] to assess disease activity.
2.5 Statistical analysis
All statistical analyses were conducted in R version 4.0.5 [Supplementary Material: Appendix 4]. Frequency statistics were reported for the sociodemographic data, clinical data, ratings, internal validity tests and survey completion time. Data from the five PROs were analysed following published scoring methods.2,14–16
The collected DCE data were analysed within a random utility maximization [RUM] framework.17 A correlated mixed multinomial logit [MXL] model was used to estimate the effects of deviations from the reference level of each attribute [Table 1] on treatment preferences.18 Correlated MXL models account for variations in preferences between patients, as well as random variations in their choice consistency.18,19
Two behavioural output measures were derived from the MXL models: (1) relative attribute importance [RAI] scores were defined as the maximum percentage contribution of each attribute to a treatment’s desirability [i.e. utility]—in addition to the RAI scores for the overall sample, subgroup estimates were obtained for each IBD type [i.e. CD vs UC] using interaction effects in the main model; (2) to express attribute valuation as trade-offs with a common denominator, MAR was obtained as the ratio between each estimated parameter and the linear marginal disutility from the risk of mild-to-moderate side effects.
Subgroup analyses were also conducted based on gender, disease activity [based on P-SCCAI for UC and PRO2 for CD], time since diagnosis and advanced therapy experience using interaction effects in the model.
The data from the TE were analysed using a double-inflated interval regression model that estimated the average MAR of cancer and the average maximum acceptable number of weeks on steroids per year.20 Similar to zero inflation in the Poisson model,21 the mechanism of double inflation was introduced in the interval regression to account for the clustering of answers at the extremes, which is often observed in TE due to the bounds of the evaluated risk attributes. The analysis was conducted for the overall sample and the UC and CD subsamples separately.
3. Results
3.1 Patient characteristics
A total of 774 patients clicked on the link to participate in the study, with 181 excluded due to IBS diagnosis or due to the recruitment quota being filled. Of the remaining 593 patients, 191 had incomplete survey data and two entries were excluded due to duplicate IP addresses. A total of 400 patients (CD: 54%; UC: 46%; female: 38.0%; mean age: 41 years [range: 18–78]) had final complete data.
Patients with CD or UC had comparable demographics and disease characteristics [Table 2]. While many patients self-reported their IBD as mild or moderate, only 17% [n = 62] considered their disease to be in remission, and almost half of those not in remission reported experiencing an active flare at the time of the survey [42%, n = 141]. In contrast to the direct self-reported disease activity, PRO2 and P-SCCAI scores calculated from patient responses to clinical questions suggested that 28% [n = 61] of patients with CD and 55% [n = 101] of patients with UC were in remission.
Table 2.
Patient demographics and disease characteristics
| Overall [N = 400] | CD [n = 215, 54%] | UC [n = 185, 46%] | |
|---|---|---|---|
| Age, years | |||
| Mean [SD] [range] | 41.0 [12.8] [18.0–78.0] | 39.9 [12.6] [18.0–74.0] | 42.3 [13.0] [19.0–78.0] |
| Gender, n [%] | |||
| Female | 152 [38.0] | 88 [40.9] | 64 [34.6] |
| Male | 247 [61.8] | 126 [58.6] | 121 [65.4] |
| Other | 1 [0.2] | 1 [0.5] | 0 [0.0] |
| Self-reported disease activity, n [%] | |||
| In remission | 67 [16.8] | 40 [18.6] | 27 [14.6] |
| Mild | 125 [31.2] | 65 [30.2] | 60 [32.4] |
| Moderate | 195 [48.8] | 103 [47.9] | 92 [49.7] |
| Severe | 13 [3.2] | 7 [3.3] | 6 [3.2] |
| Active IBD flare if not in remissiona, n [%] | |||
| Yes | 141 [42.3] | 77 [44.0] | 64 [40.5] |
| No | 192 [57.7] | 98 [56.0] | 94 [59.5] |
| Time since IBD diagnosis, n [%] | |||
| <1 year | 35 [8.8] | 21 [9.8] | 14 [7.6] |
| 1–2 years | 79 [19.8] | 42 [19.5] | 37 [20.0] |
| 2–5 years | 130 [32.5] | 58 [27.0] | 72 [38.9] |
| >5 years | 156 [39.0] | 94 [43.7] | 62 [33.5] |
| Current CD or UC medications, n [%] | |||
| Steroids | 260 [65.0] | 140 [65.1] | 120 [64.9] |
| 5-Aminosalicylates | 259 [64.8] | 116 [54.0] | 143 [77.3] |
| Immunomodulators | 179 [44.8] | 109 [50.7] | 70 [37.8] |
| Biologics | 157 [39.2] | 107 [49.8] | 50 [27.0] |
| Janus kinase inhibitors | 27 [6.8] | 16 [7.4] | 11 [5.9] |
| Advanced therapyb experience | |||
| Current or previous experience | 223 [55.8] | 145 [67.4] | 78 [42.2] |
| Surgery to treat IBD, n [%] | |||
| Yes | 107 [26.8] | 80 [37.2] | 27 [14.6] |
| No | 293 [73.2] | 135 [62.8] | 158 [85.4] |
CD, Crohn’s disease; IBD, inflammatory bowel disease; SD, standard deviation; UC, ulcerative colitis.
aThe questionnaire did not define either ‘remission’ or ‘active IBD flare’, meaning patients may have considered themselves to be simultaneously neither in remission nor experiencing an active IBD flare.
bBiologics or Janus kinase inhibitors.
At the time of the survey, 65% [n = 260] of patients were taking steroids [via any administration route], 65% [n = 259] were taking 5-aminosalicylates [5-ASAs] and 45% [n = 179] were taking immunomodulators. Almost half of the patients [46%, n = 184] were taking an advanced therapy [biologics 39%, n = 157; Janus kinase inhibitors 7%, n = 27]. There were some descriptive differences in current treatment use between the CD and UC populations, with more patients with UC taking 5-ASAs (UC: 77% [n = 143], CD: 54% [n = 116]) and more patients with CD having experience with advanced therapies (UC: 42% [n = 78], CD: 67% [n = 145]).
Patient self-reported symptoms and impacts are shown in Supplementary Material: Appendix 5. A complete list of patient characteristics is included in Supplementary Material: Appendix 6.
3.2 Discrete choice analyses
The DCE’s internal validity measures were in line with those observed in the literature.22 Most patients had a high level of self-reported health literacy [70%, n = 279] and adequate numeracy [84%, n = 334]. Also, most patients passed the repeated choice question [74%, n = 297] and dominance test [85%, n = 339]. These results were similar to those observed in previously reported studies.22 In addition, almost all patients varied their choices between alternatives [99%, n = 396], and most patients [88%, n = 352] spent an appropriate amount of time on the DCE questions, indicating that they were engaged throughout the survey.
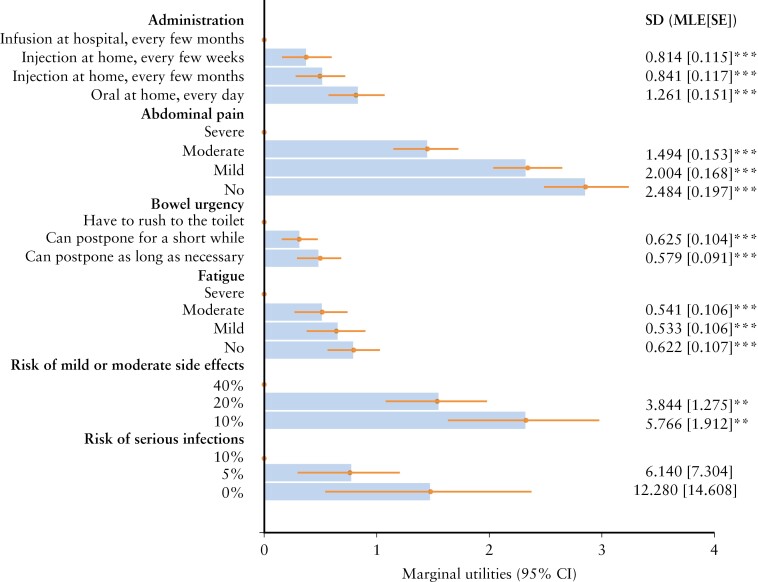
The main estimates are presented in Figure 3. The model had a good data fit with an adjusted McFadden R2 of 0.436 [0.2–0.4 is considered an excellent data fit]. All attributes mattered to patients and had a significant effect on preferences. On average, patients with IBD valued reductions in pain, avoiding having to rush to the toilet, avoiding severe levels of fatigue as well as lower risks of adverse events. In addition, patients significantly preferred injections over infusions and preferred daily oral pills at home over injections, even if injections were as infrequent as once every few months [all p < 0.05]. However, the significant standard deviations suggest that the effect of changes in attributes on treatment preferences varied between patients, except for the risk of serious infections. The effect of disease type on these estimates is included in Supplementary Material: Appendix 7.
Figure 3.
MXL model output [overall IBD population, n = 400]. Estimates quantify how deviating from the reference level [dot] in each attribute affects preferences. The larger the estimate, the larger the effect on preferences. The effects are ordinal. SDs next to the bar quantify the degree of heterogeneity in preferences. LL [pooled MXL] = –1770.7; adjusted McFadden R2 = 0.4362; BIC = 4431.3; alternative specific constant = 0.036 [SE = 0.051; p = NS]. BIC, Bayesian information criterion; CI, confidence interval; IBD, inflammatory bowel disease; LL, log-likelihood at convergence; MLE, mixed likelihood estimate; MXL, mixed multinomial logit; NS, non-significant; SD, standard deviation; SE, standard error. **p < 0.01; ***p < 0.001.
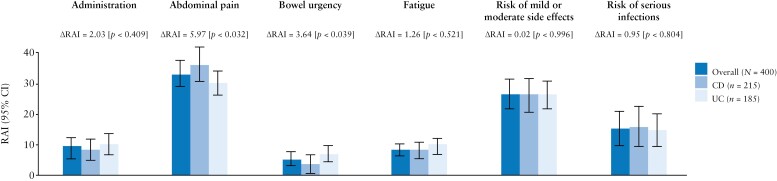
Although all attributes mattered to patients, their relative importance differed [Figure 4 and Supplementary Material: Appendix 8]. Avoiding abdominal pain was most important to patients [RAI: 33.4%, 95% confidence interval (CI): 29.2%, 37.5%] followed by the risk of mild or moderate side effects [RAI: 26.8%, 95% CI: 21.8%, 31.7%], risk of serious infections [RAI: 15.7%, 95% CI: 10.1%, 21.3%], treatment administration [RAI: 9.7%, 95% CI: 6.8%, 12.6%], fatigue [RAI: 8.8%, 95% CI: 6.9%, 10.7%] and bowel urgency [RAI: 5.7%, 95% CI: 3.4%, 7.9%]. Overall, all treatment benefits together [RAI: 50.9%] were approximately as important as all risks together [RAI: 49.1%]. While the preferences of patients with CD or UC were mostly aligned, patients with CD placed greater importance on abdominal pain [RAI: 36.4% vs 30.5%, respectively; p = 0.032] than patients with UC, and patients with UC placed greater importance on bowel urgency than patients with CD [RAI: 7.4% vs 3.7%, respectively; p = 0.039].
Figure 4.
RAI scores for the overall IBD, CD and UC populations. LL [interacted MXL] = –1751.3; adjusted McFadden R2 = 0.4379; BIC = 4511.3. ΔRAI, difference in RAI scores between CD and UC samples. RAI scores sum to 100% and measure how much variation in preferences can be attributed to changes in each attribute. BIC, Bayesian information criterion; CD, Crohn’s disease; CI, confidence interval; IBD, inflammatory bowel disease; LL, log-likelihood at convergence; MXL, mixed multinomial logit; RAI, relative attribute importance; UC, ulcerative colitis.
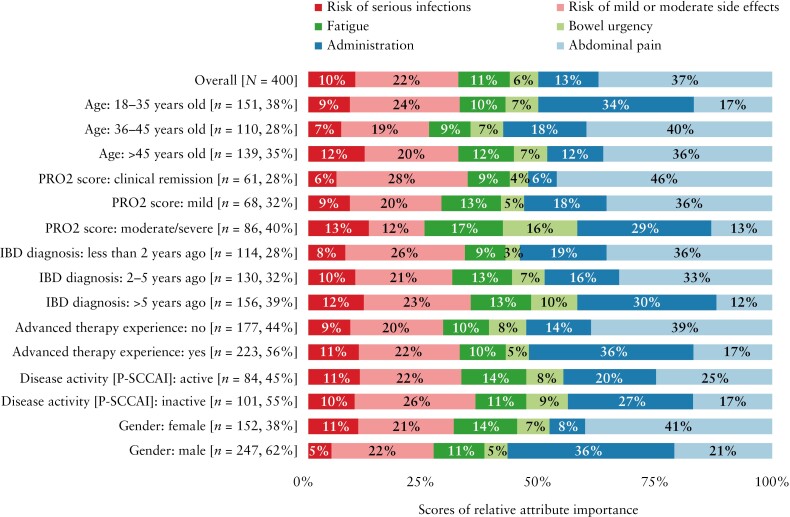
Pre-specified subgroup analyses indicated that females placed a lower relative importance on administration [p < 0.001] and more importance on abdominal pain [p < 0.001] than males and that patients aged ≤35 years old placed more importance on administration and less importance on abdominal pain than older patients [administration: 36–45 years p < 0.001, >45 years, p < 0.001; abdominal pain: ≥36 years p < 0.001; Figure 5 and Supplementary Material: Appendix 9]. Furthermore, patients diagnosed more than 5 years ago placed more importance on administration than those diagnosed less than 2 years ago or 2–5 years ago [both p < 0.001], more importance on bowel urgency than those diagnosed less than 2 years ago [p = 0.005], but less importance on abdominal pain than patients diagnosed more recently [p < 0.001]. Patients with active UC [defined as P-SCCAI ≥ 5] placed a higher importance on abdominal pain than those with inactive UC [P-SCCAI < 5; p = 0.044]. Patients with CD in clinical remission [defined as PRO2 score 0–7] or with mild CD [PRO2 score 8–13] placed a higher importance on abdominal pain vs patients with moderate-to-severe CD (PRO2 score 14–33 [moderate], ≥34 [severe]; p = 0.004). Patients with CD in clinical remission also placed a lower importance on administration [p = 0.009] and a higher importance on mild-to-moderate side effects [p = 0.033] vs patients with moderate-to-severe CD. Patients with advanced therapy experience placed a higher importance on administration [p = 0.001] and a lower importance on avoiding abdominal pain [p = 0.001].
Figure 5.
RAIs by subgroup [overall IBD population, n = 400]. Bold values denote a significant difference from the overall average [p < 0.05]. Proportions do not total 100% for all subgroups due to rounding of individual scores. IBD, inflammatory bowel disease; PRO, patient-reported outcome; P-SCCAI, Patient Simple Clinical Colitis Activity Index; RAI, relative attribute importance.
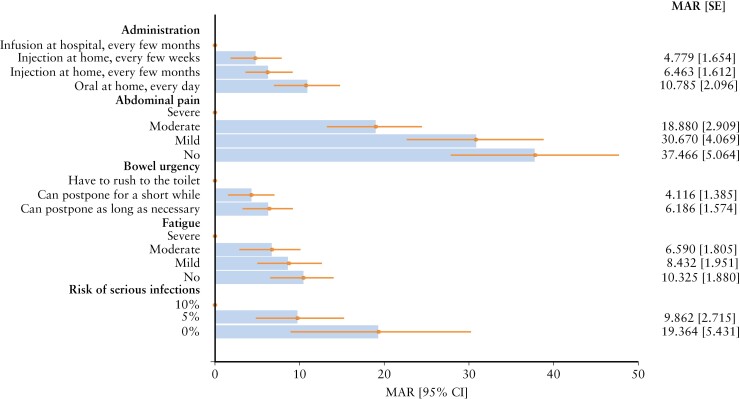
Using marginal rates of substitution, as implied by patients’ choices in the DCE, MARs of mild or moderate side effects were estimated as the ratio between the parameter of an attribute under evaluation, and the negative of the linear coded risk parameter. MAR estimates provided insights into the benefit–risk trade-offs patients were willing to make [Figure 6 and Supplementary Material: Appendix 10]. Most notably, to reduce their abdominal pain from severe to moderate, patients were willing to accept an additional 18.8% [95% CI: 13.1%, 24.5%] risk of mild or moderate side effects, 30.6% [95% CI: 22.6%, 38.6%] if the pain was reduced to mild and 37.4% [95% CI: 27.5%, 47.3%] to avoid abdominal pain entirely. To reduce the risk of serious infections from 10% to 5%, patients were willing to accept an additional 9.6% [95% CI: 4.3%, 15.0%] risk of mild or moderate side effects and an additional 19.3% [95% CI: 8.7%, 30.0%] to reduce the risk to 0%. Patients were also willing to accept an additional 6.5% risk of mild or moderate side effects to reduce their level of fatigue from severe to moderate [95% CI, 3.0%, 10.1%], 8.4% greater risk to reduce bowel urgency so that they can postpone as long as necessary [95% CI, 4.6%, 12.2%], and 10.3% added risk [95% CI, 6.6%, 14.0%] to replace an infusion at hospital with a daily oral treatment or an injection at home.
Figure 6.
Maximum acceptable risk of mild or moderate side effects to improve other attributes [overall IBD population, n = 400]. MAR estimates express the value that patients place on each attribute in risk equivalences. They quantify how much risk of mild or moderate side effects has the same value as a deviation from the reference level [dot]. MAR levels can be compared across attributes. CI, confidence interval; IBD, inflammatory bowel disease; MAR, maximum acceptable risk; SE, standard error.
3.3 Thresholding analysis
The results of the thresholding analysis are summarized in Supplementary Material: Appendix 11. The average MAR of cancer in the overall sample was 4.8%. This measure of MAR differed significantly between the type of disease [UC = 4.0% vs CD = 5.6%, p = 0.023]. Furthermore, patients were on average willing to accept a maximum of 10 weeks on steroids per year in the overall sample. This tolerance did not differ significantly between the two diseases [UC = 11.0 weeks vs CD = 9.2 weeks, p = 0.112]. Full model estimates are included in Supplementary Material: Appendix 12.
4. Discussion
This study demonstrated that patients with IBD were willing to make trade-offs between symptom improvement, administration convenience and treatment risk, which can form the basis for setting patient-centric treatment targets. The overall IBD population valued all six attributes included in the DCE, although their treatment preferences were mostly affected by the potential for amelioration of abdominal pain followed by the risk of mild or moderate side effects, and serious infections. Patients also significantly preferred a daily oral treatment regimen over other modes of administration. From a symptom perspective, patients valued avoiding severe fatigue but did not distinguish between improvements to mild or moderate fatigue. Furthermore, patients’ valuations of avoiding bowel urgency were largely driven by concerns about having to rush to the toilet to avoid a potential accident. Concerning tolerability and safety, patients with IBD generally preferred treatment with a lower risk of serious infection. Looking at the specific disease types, the effect of the different attributes on patients’ treatment preferences were similar for both patients with CD and UC, although bowel urgency was a significantly larger driver of treatment preferences in patients with UC, whereas patients with CD were driven more by abdominal pain. The fact that all attributes mattered to patients confirmed that these were relevant.
In terms of trade-offs between treatment attributes, patients with IBD were willing to accept an additional 19% risk of mild or moderate side effects to reduce their abdominal pain from severe to moderate. Accepting additional risk of side effects to ameliorate or control symptoms has been seen in previous studies exploring trade-offs between risks and remission.8,10,23 Patients with IBD were willing to accept an average of 28% chance of serious infection and 1.8% risk of lymphoma to avoid a disease relapse in the next 5–10 years.10 Patients with UC also considered symptom control to be 2.5 times as important as symptom improvement11 and, importantly, pain management ranks high among patients with IBD.10 This supports the results of our study that found avoiding abdominal pain was paramount [RAI 33%] for this patient population, accepting up to 30.6% additional risk of mild-to-moderate side effects to reduce abdominal pain from severe to moderate/mild. Abdominal pain was of greater importance for patients with active UC than those with inactive UC, and is of greater importance for patients with CD in clinical remission or mild CD compared with patients with moderate-to-severe CD.
For every attribute except serious infections, there was significant preference heterogeneity. Most of the preference heterogeneity was due to individual idiosyncrasies rather than observable aspects such as type of IBD, age or gender. This suggests that optimal treatment selection is likely to be specific to the individual. Thus, an individualized approach to treatment may be required to provide patients with the treatment they value most.
While previous studies have looked at patient preferences on treatment characteristics,5–9 only few ventured to cover both CD and UC, and many were not comprehensive in assessing a range of treatment attributes. The present analysis provides broad and novel insights in this area and highlights the need for personalized treatment discussions and decisions between patients and their healthcare providers. Identifying and characterizing the breadth of heterogeneity in patient preferences among those with CD and UC allows physicians to develop a highly personalized patient-centric treat-to-target strategy,24 which is recommended in management guidelines.1 Our study showed aggregate treatment benefit attributes were almost equally important to patients as treatment risks, thus highlighting a need for education on overall benefit–risk profiles of individual therapy options, particularly within the context of a treat-to-target approach.25 Furthermore, this study includes a novel methodological approach, with the development of a model for the analysis of thresholding data [double inflated interval regression] that aims to account for bi-modality in the data.
All findings from this study should be interpreted in the context of the following limitations: all findings are conditional on the selected attributes; the study was based on patient-reported data [e.g. there was no documented confirmation of the diagnosis or disease activity]; there may have been variability in patients’ interpretations of some questions, potentially influencing the results obtained; there is possible intra-patient variability in the data collected at a specific point-in-time in life or disease stage of the patients; because all respondents completed the questionnaire voluntarily, rather than being selected at random, selection bias is a potential limitation, meaning that the comparability of this sample to the overall IBD population is unknown; information bias due to measurement error in reporting cannot be entirely ruled out, although dedicated screening questions ensured that respondents fulfilled all inclusion criteria and only patients pre-profiled as having IBD were invited to participate; and recruitment was affected by the COVID-19 pandemic and it is unknown whether COVID-19 affected elicited preferences [e.g. for home- vs hospital-based treatment administration]. To minimize this risk, the DCE’s plausibility to participants was explicitly tested during the qualitative pre-testing.
In summary, patients with IBD are willing to make trade-offs between multiple potential treatment benefits and risks, as well as administration attributes. Patients emphasized the importance of improving a range of symptoms, with a focus on abdominal pain and the avoidance of side effects. The differences in the relative importance that patients placed on treatment attributes indicate that treatment selection should consider multiple features carefully and, due to the considerable preference heterogeneity observed, treatment selection may benefit from highly individualized approaches. Future research should explore how to best consider patient treatment preferences in clinical development and routine clinical practice.
Supplementary Material
Acknowledgments
AbbVie and the authors thank Kathleen O’Hara of AbbVie [North Chicago, IL], Katelyn Cutts of Evidera Inc. [Bethesda, MD], and Luisa Avedano and Bella Haaf of the European Federation of Crohn’s and Colitis Association [EFCCA] for contributing to the design and conduct of the study and would also like to thank the patients who participated in the trial. AbbVie was the study sponsor and contributed to study design, data collection, analysis, and interpretation, reviewing and approval of the manuscript. No honoraria or payments were made for authorship. Medical writing support was provided by Parastoo Momeni, PhD, of 2 the Nth [Cheshire, UK], and was funded by AbbVie.
Contributor Information
Edouard Louis, CHU de Liège et Université de Liège, Liège, Belgium.
Corey A Siegel, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA.
Barbara James, AbbVie Inc., North Chicago, IL, USA.
Sebastian Heidenreich, Evidera Ltd, London, UK.
Nicolas Krucien, Evidera Ltd, London, UK.
Subrata Ghosh, APC Microbiome Ireland, College of Medicine and Health, University College Cork, Cork, Ireland.
Funding
This work was supported by AbbVie.
Conflict of Interest
E.L. reports research grants from Janssen, Pfizer and Takeda; educational grants from AbbVie, Janssen and Takeda; speaker fees from AbbVie, Celgene, Falk, Ferring, Janssen, Pfizer, Roche and Takeda; consultancy fees from AbbVie, Arena, Celgene, Eli Lilly, Ferring, Galapagos, Gilead, Janssen and Takeda. C.A.S. reports consultancy fees from AbbVie, BMS, Janssen, Lilly, Pfizer, Prometheus and Takeda; speaker fees from Continuing Medical Education activities for AbbVie, BMS, Pfizer and Takeda; research grants from AbbVie, Janssen, Pfizer, and Takeda. B.J. is an employee of AbbVie and may own stock and/or options. S.H. and N.K. are employees of Evidera, a business unit of Pharmaceutical Product Development, LLC. Evidera received funding for conducting the work outlined in this paper. S.H. is a minority stockholder of Thermo Fisher Scientific as part of his employment. S.G. reports speaker fees from AbbVie, Janssen, Pfizer and Takeda; consultancy fees from Boehringer Ingelheim, Celgene, Eli Lilly, Gilead and Roche; is a steering committee member for AbbVie, Boehringer Ingelheim, Celgene and Janssen; and is an advisory committee member for AbbVie.
Author Contributions
B.J. and S.H. contributed to the conception and design of the study, acquisition of data, and analysis and interpretation of data as well as drafting the article or revising it critically for important intellectual content. E.L., C.A.S., S.G. and N.K. contributed to the analysis and interpretation of the data together with drafting the article or revising it critically for important intellectual content. All authors provided final approval of the manuscript to be submitted.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Colombel J-F, D’haens G, Lee W-J, Petersson J, Panaccione R.. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis 2020;14:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 3. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al Khoury A, Balram B, Bessissow T, et al. Patient perspectives and expectations in inflammatory bowel disease: a systematic review. Dig Dis Sci 2021;67:1956–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregor JC, Williamson M, Dajnowiec D, Sattin B, Sabot E, Salh B.. Inflammatory bowel disease patients prioritize mucosal healing, symptom control, and pain when choosing therapies: results of a prospective cross-sectional willingness-to-pay study. Patient Prefer Adherence 2018;12:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almario CV, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol 2018;113:58–71. [DOI] [PubMed] [Google Scholar]
- 7. Bewtra M, Reed SD, Johnson FR, et al. Variation among patients with Crohn’s disease in benefit vs risk preferences and remission time equivalents. Clin Gastroenterol Hepatol 2020;18:406–14.e7. [DOI] [PubMed] [Google Scholar]
- 8. Boeri M, Myers K, Ervin C, et al. Patient and physician preferences for ulcerative colitis treatments in the United States. Clin Exp Gastroenterol 2019;12:263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson FR, Hauber B, Özdemir S, Siegel CA, Hass S, Sands BE.. Are gastroenterologists less tolerant of treatment risks than patients? Benefit-risk preferences in Crohn’s disease management. J Manag Care Pharm 2010;16:616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louis E, Ramos-Goñi JM, Cuervo J, et al. A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient 2020;13:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heidenreich S, Phillips-Beyer A, Flamion B, Ross M, Seo J, Marsh K.. Benefit-risk or risk-benefit trade-offs? Another look at attribute ordering effects in a pilot choice experiment. Patient 2021;14:65–74. [DOI] [PubMed] [Google Scholar]
- 12. Ryan M, Watson V, Entwistle V.. Rationalising the ‘irrational’: a think aloud study of discrete choice experiment responses. Health Econ 2009;18:321–36. [DOI] [PubMed] [Google Scholar]
- 13. Ghosh S, Louis E, Beaugerie L, et al. Development of the IBD disk: a visual self-administered tool for assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis 2017;23:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FACIT. Facit fatigue scale (version 4).http://www.ser.es/wp-content/uploads/2015/03/facit-f_indice.pdf. Accessed July 30, 2021.
- 15. Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis 2013;7:890–900. [DOI] [PubMed] [Google Scholar]
- 16. Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther 2015;41:77–86. [DOI] [PubMed] [Google Scholar]
- 17. Manski CF. The structure of random utility models. Theory Decis 1977;8:229–54. [Google Scholar]
- 18. Hensher DA, Greene WH.. The mixed logit model: the state of practice. Transportation 2003;30:133–76. [Google Scholar]
- 19. Hess S, Train K.. Correlation and scale in mixed logit models. J Choice Model 2017;23:1–8. [Google Scholar]
- 20. Zhigang Z, Jianguo S.. Interval censoring. Stat Methods Med Res 2009;19:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambert D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 1992;34:1–14. [Google Scholar]
- 22. Johnson FR, Yang JC, Reed SD.. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health 2019;22:157–60. [DOI] [PubMed] [Google Scholar]
- 23. Bewtra M, Reed SD, Johnson FR, et al. Variation among patients with Crohn’s disease in benefit vs risk preferences and remission time equivalents. Clin Gastroenterol Hepatol 2020;18:406–14 e7. [DOI] [PubMed] [Google Scholar]
- 24. Hazlewood GS, Pokharel G, Deardon R, et al. Patient preferences for maintenance therapy in Crohn’s disease: a discrete-choice experiment. PLoS One 2020;15:e0227635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selinger C, Carbonell J, Kane J, Omer M, Ford AC.. Acceptability of a ‘treat to target’ approach in inflammatory bowel disease to patients in clinical remission. Frontline Gastroenterol 2021;12:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.