Summary
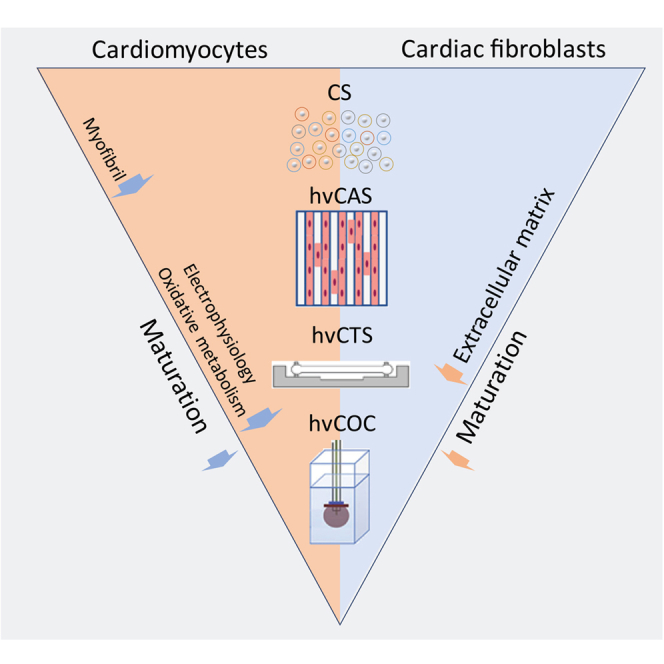
Cardiac in vitro models have become increasingly obtainable and affordable with the optimization of human pluripotent stem cell-derived cardiomyocyte (hPSC-CM) differentiation. However, these CMs are immature compared to their in vivo counterparts. Here we study the cellular phenotype of hPSC-CMs by comparing their single-cell gene expression and functional profiles in three engineered cardiac tissue configurations: human ventricular (hv) cardiac anisotropic sheet, cardiac tissue strip, and cardiac organoid chamber (hvCOC), with spontaneously aggregated 3D cardiac spheroids (CS) as control. The CM maturity was found to increase with increasing levels of complexity of the engineered tissues from CS to hvCOC. The contractile components are the first function to mature, followed by electrophysiology and oxidative metabolism. Notably, the 2D tissue constructs show a higher cellular organization whereas metabolic maturity preferentially increases in the 3D constructs. We conclude that the tissue engineering models resembling configurations of native tissues may be reliable for drug screening or disease modeling.
Subject areas: Cell biology, Stem cells research, Tissue Engineering
Graphical abstract
Highlights
-
•
scRNA-seq allows accurate evaluation of maturation of engineered cardiac tissues
-
•
Unique microenvironment in engineered cardiac tissues promote maturation
-
•
CM maturity increases with increasing levels of complexity of the engineered tissues
Cell biology; Stem cells research; Tissue Engineering
Introduction
In the emerging fields of cardiac regenerative medicine and in vitro disease modeling, research has been increasingly focused on developing more physiological human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs).1,2,3,4 While differentiation of hPSCs from the pluripotent state to beating CMs is achievable by a variety of protocols,5,6,7,8,9 the challenge of achieving mature cardiomyocytes (CMs) in vitro comparable to their counterpart in the native heart remains. Considering that CMs differentiated from hPSCs have yet to achieve maturation at the level observed in vivo,10 cardiac tissue engineering can provide a unique cellular niche environment to promote in vitro maturation of differentiated CMs.11,12,13,14,15,16,17,18 Developing accurate human cardiac models would also prove useful for drug screening, as currently the most common reason for withdrawal of a new drug is cardiotoxicity, including increased risk for life-threatening arrythmias that can go undetected in small animal models during the preclinical stage of drug development.19,20
Adult CMs are characterized by distinctive features including rod-like shape, polyploidy and organized myofibrils, mitochondrial networks, sarcoplasmic reticulum, and T-tubules.10,21 While there is some consensus on the physiological and structural indicators of CM maturation, the exact molecular mechanisms that drive maturation remain incompletely understood. Some hallmarks of maturation associated with cardiac function include a shift in the myofibril protein isoforms and increased expression of ventricular ion channels and proteins involved in calcium handling. Changes in cardiac energy metabolism have also been found to drive CM maturation, including a transition from glycolysis to oxidative phosphorylation and an increase in the utilization of fatty acid as the main source of energy substrates.22 Cell cycle silencing with the expression of cell adhesion genes was also shown to be a sign of mature CM.23
Recently, RNA sequencing has advanced to enable analysis of the transcriptome of single cells during differentiation and maturation processes.24 So far single-cell RNA sequencing (scRNA-seq) has mostly been used to study cardiac differentiation.24,25,26,27 In this study, we use scRNA-seq to compare the transcriptomes of hPSC-CMs isolated from four different engineered cardiac tissue models: 1) 3D cardiac spheroids (CSs), 2) human ventricular Cardiac Anisotropic Sheet (hvCAS),28,29,30,31 a 2D monolayer reproducing the anisotropic cell alignment found in the human heart for the assessment of electrophysiological functions and visualization of re-entrant arrhythmic events, 3) human ventricular Cardiac Tissue Strip (hvCTS),1,15,16,32 a 3D cardiac tissue strip composed of CMs embedded in a collagen matrix stretched between two polydimethylsiloxane (PDMS) posts for assessing contractile function, and 4) human ventricular Cardiac Organoid Chamber (hvCOC),33,34 a complex 3D fluid-ejecting cardiac tissue composed of embedded CMs in a collagen matrix that models complex physiological cardiac pump behavior and enables clinically familiar measurements such as the pressure-volume loop, cardiac output, stroke volume, and ejection fraction.
We first order the engineered tissue models based on the gene expression profiles of single CMs and characterize the phenotypes of different models. Then we describe genes involved in the cardiac contractile function, electrophysiology, and metabolism that define differential CM maturation between the 2D and 3D models and distinguish the more mature 3D tissue strip and mini-heart models from those tissue configurations with lesser biocomplexity. In addition to CMs, we also examine other differentiated cardiac cell types, including fibroblasts, to determine whether their expression profile shows similar maturation patterns across the engineered tissue configurations. The results are verified not only by replicates but also using both human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) sources.
Results
Analysis of human embryonic stem cell-derived cardiac tissues by single-cell RNA sequencing identified cardiac and non-cardiac cell populations
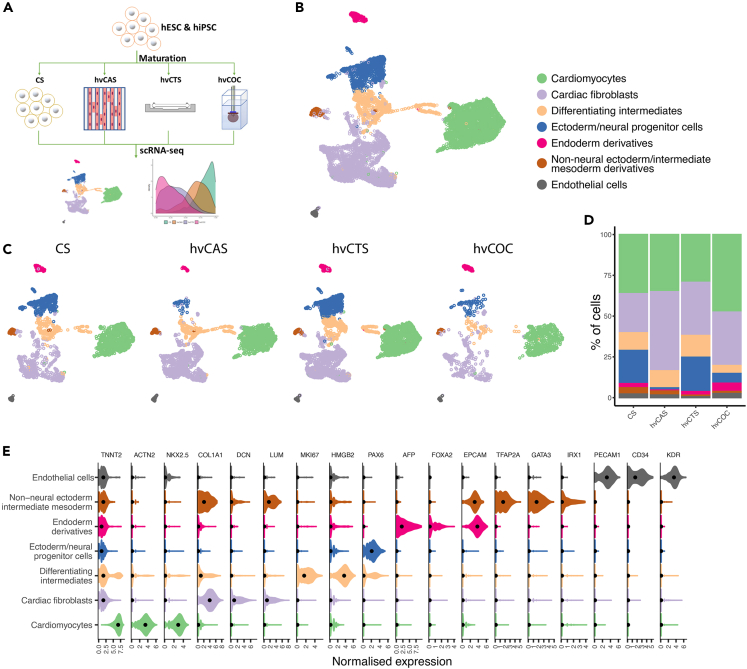
We first performed single-cell transcription profiling of derivatives of hESCs from our directed cardiac differentiation protocol fabricated into four tissue constructs—CS, hvCAS, hvCTS, and hvCOC—from CMs on day 15 post differentiation.8 The fabricated tissue constructs were then cultured until 29–30 days post-differentiation in standard incubator conditions with no electrical conditioning or specialized media treatments. We then extracted cells from each of the four engineered tissue configurations and performed scRNA-seq using the 10X Chromium platform (Figure 1A). After pre-processing, 10,482 cells were retained for subsequent analysis (Table S1). Clustering analysis resulted in seven distinct cell populations that were confirmed by known cell type-specific markers (Figures 1B–1D, Table S2). The identified cell populations included non-neural ectoderm/intermediate mesoderm derivatives, endoderm derivatives, ectoderm/neural progenitor cells, differentiating intermediates, and cardiac cells, including endothelial cells, cardiac fibroblasts and CMs (Figure 1E). Cell cycle analysis showed that most of the cells in the differentiating intermediates and the ectoderm/neural progenitor cell populations were still proliferating (Figures S1A and S1B).
Figure 1.
Cell types present in the hPSC-derived tissue configurations
(A) Workflow of hPSC-derived cell derivation and construction of four tissue configurations. Four tissue constructs—CS, hvCAS, hvCTS, and hvCOC—were fabricated from hPSC-CMs on day 15 post-differentiation and cultured until day 29-30 post-differentiation, followed by cell dissociation for scRNA-seq.
(B and C) UMAP plots identified seven cell types separated into distinct clusters showing cells isolated from all tissue constructs (B) and cells in each tissue construct (C).
(D) Bar plot showing the fraction of each cell type present in the four constructs.
(E) Violin plots showing expression of marker genes used to identify the seven cell types.
Progressive maturation from cardiac spheroids to human ventricular cardiac organoid chamber in the order of their cardiac physiological complexity
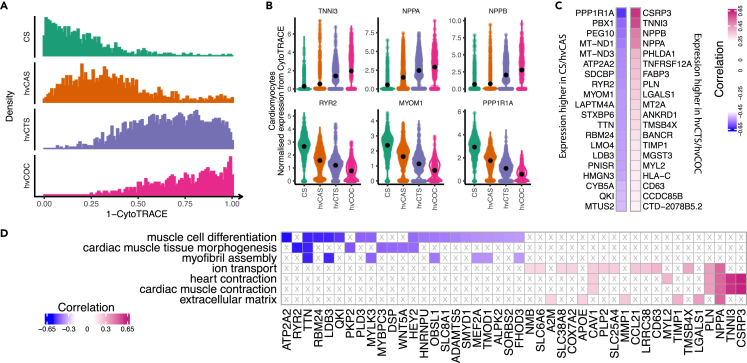
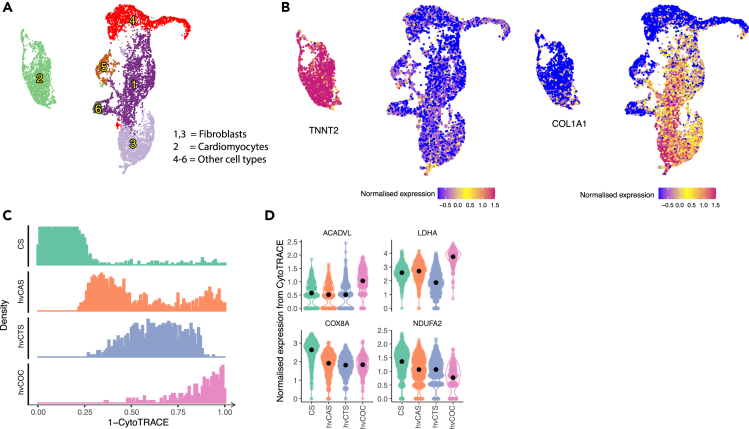
To investigate the relative maturation state of CMs within the 2D and 3D tissues, we performed trajectory analysis by using CytoTRACE,35 which allows the prediction of maturation states among different tissue constructs without prior knowledge of developmental direction. Our results showed that CMs from CS, hvCAS, hvCTS, and hvCOC were ordered along a trajectory with increasing state of maturation (Figure 2A). Analysis of genes governing the trajectory identified genes that were more abundantly expressed in CS and hvCAS compared to hvCOC and hvCTS, which also negatively correlated with the CM developmental state (Figures 2B and 2C, Table S3). These genes were overrepresented in Gene Ontology (GO) terms of cardiac muscle tissue morphogenesis, myofibril assembly and muscle cell differentiation, with notable enriched genes including MYOM1, RYR2, and TTN (Figures 2C and 2D, Table S4). The top genes that exhibited higher expression and positive correlation with a mature state in the hvCTS and hvCOC included cardiac genes encoding TNNI3, NPPA, and CSRP3 (Figures 2B and 2C). These genes were enriched in GO terms ion transport, heart contraction, cardiac muscle contraction, and extracellular matrix (Figure 2D, Table S4).
Figure 2.
Trajectory analysis of CMs in the hESC-derived tissues
(A) Maturation states of four tissue configurations characterised by CytoTRACE. X axis shows “1 - CytoTRACE” score that ranges from 0 to 1 indicating low to high maturation levels.
(B) Violin plots for normalized expression levels of genes with high positive and negative correlation with the maturation state. Black dots indicate the mean values.
(C) Top twenty positively and negatively correlated genes in hvCTS/hvCOC and CS/hvCAS.
(D) Enriched GO terms for genes with top negative and positive correlation with the maturation state.
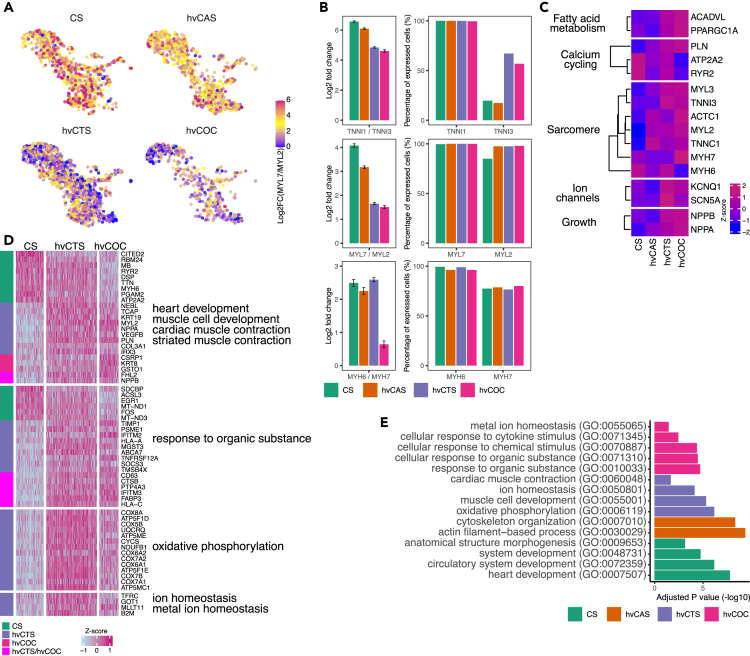
We then performed further analysis of the CM population and found that the ratio of TNNI1/TNNI3 and MYL7/MYL2 expression progressively decreased from CS → hvCAS → hvCTS → hvCOC, a more mature ventricular phenotype in higher ordered tissue constructs (Figures 3A and 3B). Although there was no significant difference in the number of cells expressing MYH6 and MYH7 in all the tissue constructs, the ratio of MYH6/MYH7 expression was much lower in CMs extracted from the hvCOC (Figure 3B). Moreover, TNNI3, MYL2, and MYH7, which are myofibrillar isoforms in mature ventricular CMs, were more abundantly expressed in hvCOC and hvCTS (Figures 3B and 3C), indicating that CMs in hvCOC and hvCTS are more mature, consistent with our previously reported RNA-seq results. Key genes in the maturation of fatty acid metabolism, sarcomere, and ion channels were also upregulated in CMs from the hvCTS and hvCOC relative to those from the CS and hvCAS (Figure 3C).11,23,36
Figure 3.
Comparison of CM expression between CS and other tissue configurations hvCAS, hvCTS, and hvCOC
(A) UMAP of each tissue configuration with relative expression level of myosin light chain 7 to 2 (MYL7/MYL2) represented as log2 scale.
(B) The left panel shows Log2 ratio (mean+/−SE) of expression of troponin I isoform 1 to 3 (TNNI1/TNNI3), MYL7/MLY2, and myosin heavy chain 6 to 7 (MYH6/MYH7) for each tissue type. The right panel shows the percentage of cells expressing the myofibril isoforms for each tissue types.
(C) Heatmap showing mean scaled expression (mean Z score) of the selected of CM-associated genes in the key categories for the four tissue types. All genes in the table are DE between at least two tissue configurations.
(D) Heatmap showing the scaled expression values of the DEGs between CS and hvCTS/hvCOC belonging to enriched GO categories. Vertical bar indicates the higher expressed tissue construct.
(E) Top enriched GO terms for the DEGs between CS and other tissue configurations hvCAS, hvCTS, and hvCOC.
Myofibrillar maturation precedes the maturation of electrophysiology and oxidative metabolism while cellular response pathways are the last to mature
To better understand the progression of functional maturation of CMs in the engineered tissue constructs, we analyzed the differentially expressed genes (DEGs) between hvCOC/hvCTS and CS (Figure 3D, Table S5). Genes upregulated in CS pertain to cardiac development and are overrepresented in GO terms, such as heart development and circulatory system development, with top enriched genes including ERBB2 and ADAMTS6 (Figure 3E, Table S6). Gene ontology analysis of the genes upregulated in hvCTS compared to CS not only revealed top enriched GO terms to be heart development, muscle cell development, and cardiac muscle contraction but also oxidative phosphorylation and ion homeostasis, with top enriched genes including NPPA, TNNI3, and CSRP3 (Figures 3D and 3E, Table S6). Comparing DEGs between hvCOC and CS revealed top enriched GO terms in hvCOC to be a cellular response to organic substance and metal ion homeostasis, highlighting the maturation of cellular response pathways and ion channels occurring later in the maturation process (Figure 3E, Table S6). In consideration of the maturity order of the tissue types from CS to hvCOC as analyzed by CytoTRACE, the functional maturation begins with cardiac morphogenesis, followed by electrophysiological and contractile maturation, and then terminates with acquired responsiveness to stimuli.
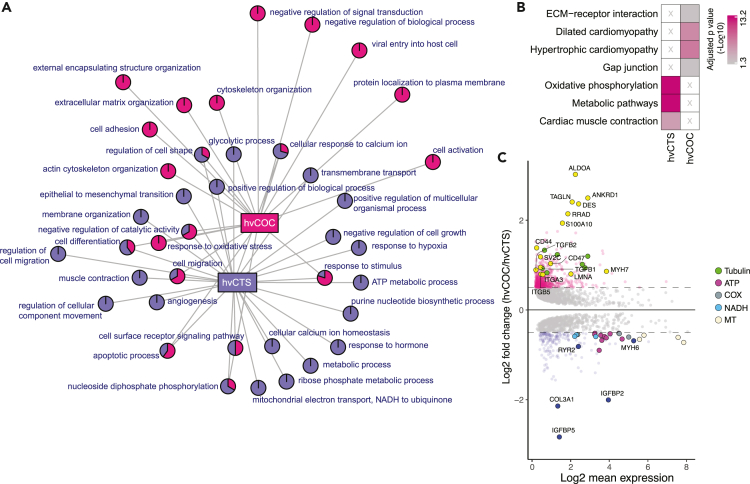
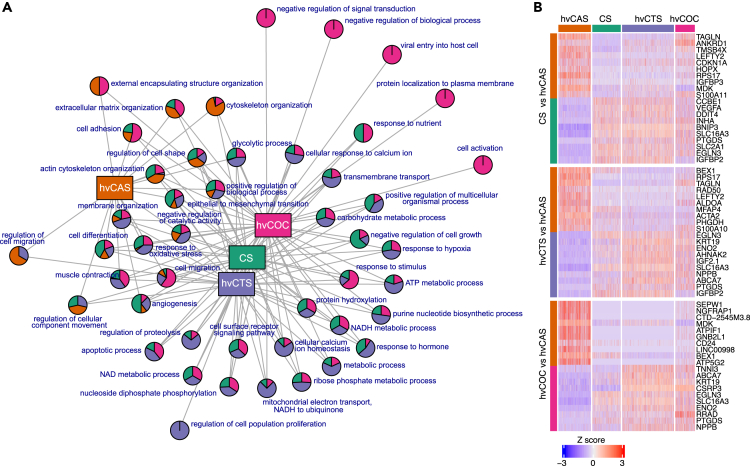
Next, we compared the DEGs between the two most mature engineered tissues hvCTS and hvCOC (Table S5). The network of parental GO terms showed that most (30 of 39) terms are either hvCTS- or hvCOC-specific, suggesting notable functional differences between the constructs (Figure 4A, Table S7). The top GO terms enriched in hvCTS are the generation of precursor metabolites and energy, oxidative phosphorylation, and ATP metabolic process. The hvCOC-specific genes were associated with extracellular matrix organization and response to the organic substance, corroborating the observation that extracellular organization and cellular response pathways are mature at a later time. Similar to the findings from GO analysis, the KEGG pathways enriched in hvCTS-specific genes are oxidative phosphorylation, metabolic pathways, and cardiac muscle contractions while the KEGG pathways enriched in hvCOC-specific genes are ECM-receptor interaction, GAP junction, and hypertrophic or dilated cardiomyopathy (Figures 4B and 4C, Table S6).
Figure 4.
Pathway and GO enrichment comparison between hvCTS and hvCOC
(A) Network graph shows parental GO terms enriched in pairwise comparisons between hvCTS and hvCOC. Pie charts represent parental GO terms and show the proportion of the GO terms enriched in each tissue configuration.
(B) Adjusted p values of KEGG pathways enriched in hvCTS and hvCOC.
(C) MA plot for the DEGs between hvCOC and hvCTS. Gene expression with Log2(FC) > 0.5 and Log2(FC) < −0.5 that are higher in hvCOC and hvCTS, respectively, are shown. Genes with largest fold change are named. Highlighted genes that belong to the enriched KEGG pathways shown in (B) are TUBULIN (Tubulin), ATP (ATP synthase subunit), COX (Cytochrome c oxidase subunits), NADH (NADH dehydrogenase subunits), and MT (Mitochondrial genes).
Cellular component organization and regulation of metabolism distinguishes 2D from 3D tissue configurations
We next compared the transcriptome between the CMs in the 2D hvCAS construct and the 3D constructs - CS, hvCTS, and hvCOC (Figures 5A and 5B, Table S5). The network of parental GO terms comparing any two different tissue constructs showed that most parental GO terms were shared between the 3D CS, hvCTS, and hvCOC, highlighting a fundamental difference between 2D and 3D tissue configurations (Figure 5A, Table S7). Gene ontology analysis revealed that cellular component organization was enriched in hvCAS (Figures 3E and 5A, Table S6), suggesting that the organization of cellular components is the biggest difference between CMs cultured in 2D vs 3D. GO terms pertaining to cell metabolic process, ATP metabolic process, ATP synthesis coupled proton transport, and response to oxygen levels were among the top enriched GO terms when hvCOC and hvCTS were compared to hvCAS (Table S6). Similarly, these GO terms were also enriched in CS when compared to hvCAS, suggesting that the 3D environment can alter the metabolism of CMs in the engineered tissues.
Figure 5.
Gene expression comparison between 2D and 3D tissue configurations
(A) Network graph showing parental GO terms enriched in pairwise comparisons between 2D hvCAS and the 3D tissue configurations CS, hvCTS and hvCOC. Pie charts represent parental GO terms and show the proportion of the GO terms enriched in each configuration.
(B) Heatmap showing scaled expression values for top ten DEGs between the 2D hvCAS and the other 3D tissue configurations individually.
Fibroblasts in more physiological tissue constructs contribute to extracellular structure and matrix organization
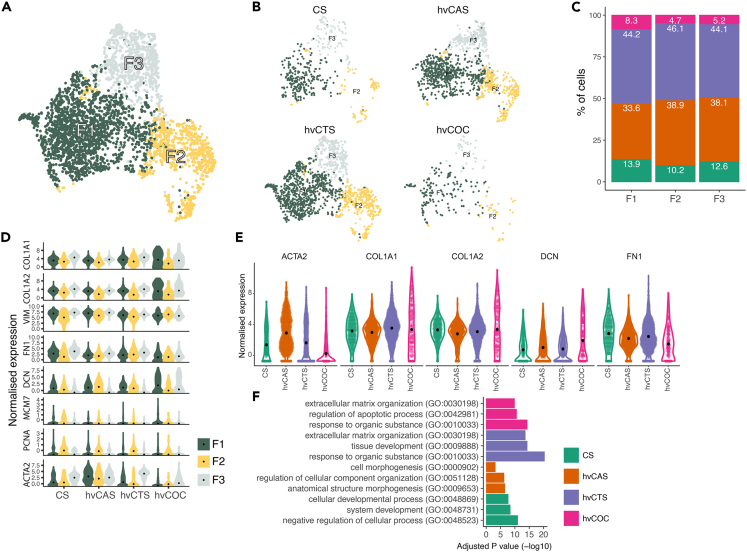
Besides CMs, fibroblast-like cells contributed to the largest population of cells that were extracted and analyzed from hESC-derived engineered tissue constructs. Further analysis of the fibroblast-like population identified three subpopulations that exhibited comparable expression of fibroblast marker, VIM, but with varying expression of collagen COL1A1, FN1, and ACTA2 (Figures 6A and 6D). The subpopulations were present in all four tissue types (Figure 6B). Of note, fewer fibroblast-like cells were extracted from the hvCOC than the other tissue constructs (Figures 6B and 6C). This observation in conjunction with a low expression of PCNA in the hvCOC suggests that fibroblasts in a 3D and more physiological cardiac tissue construct are less proliferative (Figure 6D). The ACTA2 expression was the lowest in the fibroblasts present in the hvCOC and highest in their counterpart in the hvCAS, indicating the hvCOC fibroblasts are quiescent in a homeostatic state (Figure 6E). The expression of extracellular matrix proteins also differed among fibroblasts extracted from the four tissue configurations. DCN expression was the highest in the fibroblasts of hvCOC (Figure 6E). The fibroblasts from the hvCOC exhibited higher expressions of COL1A1 and COL1A2 than FN1 (Figure 6E). We then analyzed the DEGs in the fibroblasts between hvCOC/hvCTS and CS (Figure S2, Table S8). Genes upregulated in fibroblasts from the CS were overrepresented in negative regulation of the cellular process, system development, and cellular development processes. Gene ontology analysis of the genes upregulated in hvCTS and hvCOC compared to CS revealed top enriched GO terms extracellular matrix organization, tissue development, and response to the organic substance. The hvCOC-specific GO terms also included regulation of the apoptotic process (Figure 6F, Table S9). Comparing DEGs between fibroblasts of hvCAS and CS, the GO terms cell morphogenesis, regulation of cellular component organization, and anatomical structure morphogenesis were overrepresented in hvCAS (Figures 6F and S2, Tables S8 and S9).
Figure 6.
Subclusters of cardiac fibroblasts and functional screening
(A) UMAP of cardiac fibroblasts showing three clusters, F1, F2, and F3.
(B) Individual UMAPs showing the fibroblasts from each tissue configuration.
(C) Bar graph on the fraction of each tissue configuration type contributing to the clusters.
(D) Violin plots showing normalised expression of highly expressed fibroblast genes. Black dots indicate the mean values.
(E) Violin plots showing normalised expression levels of ACTA2, COL1A1, COL1A2, DCN, and FN1 of tissue types in fibroblasts. Black dots indicate the mean values.
(F) Bar plot showing adjusted p value (-log10) of the enriched GO terms calculated for the top DEGs between CS and other configurations hvCAS, hvCTS, and hvCOC.
Human pluripotent stem cell-derived tissue constructs show similar phenotype progression as human embryonic stem cell-derived tissues
To similarly assess the effects of tissue types on hiPSC-CMs, cells extracted from hiPSC-derived CS, hvCAS, hvCTS, and hvCOC constructs were analyzed by scRNA-seq. A total of six clusters can be distinctively separated (Figures 7A, S3A and S3B, Table S10), with cluster 2 identified as CMs and clusters 1 and 3 as fibroblast-like cells by the abundance of TNNT2 and COL1A1 expression, respectively (Figure 7B). Interestingly, the hiPSC-CM cluster, in the UMAP plot of extracted cells from all tissue types, was well separated from all other clusters like the hESC-CM cluster (Figures 1B and 7A). CytoTRACE analysis scored the hiPSC-CMs in the four tissue constructs in the same increasing order of maturation from CS, hvCAS, hvCTS, to hvCOC as the hESC-derived tissue constructs (Figure 7C). Analysis of genes governing the trajectory identified COX8A and NDUFA2 as genes that were more highly expressed in CS and hvCAS compared to hvCOC and hvCTS, and negatively correlated with the trajectory, and ACADVL and LDHA as genes that were more highly expressed in hvCOC than in CS and hvCAS, and positively correlated with the trajectory (Figure 7D, Table S11).
Figure 7.
Identification of cell types and trajectory analysis of CMs in the hiPSC-derived tissues
(A) UMAP showing clustering of hiPSC-derived cells.
(B) UMAP highlighting cluster 2 with high expression of TNNT2 as CMs (left) and clusters 1 and 3 with high expression of COL1A1 as fibroblasts (right).
(C) Maturation stage of four tissue configurations characterised by CytoTRACE. X axis represents the maturation scores of “1 – CytoTRACE” ranging from 0 to 1 indicates maturation from low to high levels, respectively.
(D) Violin plots showing normalised expression level of genes with high positive or negative correlation with the maturation stage for the four tissue configurations. Black dots indicate the mean values.
Discussion
In this study, we used scRNA-seq to compare the transcriptome of hPSC-CMs isolated from different engineered tissue models by characterizing the phenotype and defining the molecular processes and marker genes that best distinguish differences in hPSC-CMs between the different models. The results showed that the CM maturity increased with increasing levels of tissue engineering biocomplexity, myofibril structure being the first to mature, followed by electrophysiological function and oxidative metabolism. A noticeable difference was found between the 2D model of hvCAS and 3D models of CS, hvCTS, and hvCOC: cellular organization was higher in the patterned 2D format hvCAS while increased metabolism was observed in the 3D models. These results suggest that even though all the CMs were analyzed at the same timepoint and were differentiated using the same protocol, the spatial structure and extracellular environment imposed by the engineered tissue configurations had a strong impact on their maturation and gene expression.
In vivo cardiac developmental process can be divided into three processes -specification, morphogenesis, and maturation,23 where maturation is the final process in which the cells undergo cell and tissue level changes to optimize the function of the heart for strong contraction and efficient pumping to enable systemic circulation. The maturation of CMs is loosely defined. However, a number of major hallmarks have been regarded as a general consensus of the field, including myofibril maturation, electrophysiology, calcium handling, metabolic maturation, hypertrophy, and integration of physiology.10,37,38
Despite advances in the development of directed cardiac differentiation from pluripotent stem cells during the past decade, these protocols can only produce relatively immature CMs when compared to their native adult counterparts.39 A number of strategies have been proposed for promoting the maturation of CMs in vitro, with microenvironmental conditions believed to be the key.40,41 These strategies include biophysical cues, biochemical cues as well as transcriptional regulation. In the present study, we investigated the effect of biophysical cues on cardiac maturation and constructed tissues to recreate 2D and 3D cardiac physiological microenvironments. Assessment of CM phenotype from these tissues is challenging due to the nature of differentiation that inevitably yields a heterogeneous population of cells including non-CMs. Moreover, the construction of tissues, specifically engineered 3D mimics, often necessitates the addition of stromal cells to facilitate proper formation of these tissue constructs.32,33
Earlier functional studies have shown that more complex tissue configurations display more robust cardiac function,34 but objective comparison of functional properties is difficult as the configurations of different engineered tissues do not allow for measurements of the same functional parameters. To characterize differences in maturation phenotype, we used scRNA-seq that enables analysis of the CMs in a heterogeneous population of cell types and CM subtypes. Our study highlights the advantage of scRNA-seq as an unbiased strategy to analyze the different tissue-engineered constructs of multi-cell types and tease out differences in their maturation state.
We used the computational framework CytoTRACE35 to perform an unsupervised prediction of relative differentiation states between the CMs in the four engineered constructs, namely, CS, hvCAS, hvCTS, and hvCOC. CytoTRACE has been used to predict the differentiation and developmental state of cells across tissues and species.35 It is based on the observation that less mature cells maintain looser chromatin to permit wider sampling of the transcriptome, whereas more differentiated cells generally restrict chromatin accessibility and transcriptional diversity as they specialize. Consequently, the number of expressed genes per cell can be considered a hallmark of the developmental potential of a cell. Similarly, a decrease in Shannon entropy, characterized by a decrease in the number of genes expressed in a cell, has also been shown to correlate with the maturation of pluripotent stem cell-derived CMs.42 This framework allows for the construction of a trajectory of the relative maturation state of the CMs by comparison of the tissue constructs. We adopt this unique approach where we compare CMs after being subjected to the various culture environments in the tissue models but of the same age post-differentiation. This approach allows us to determine the dependence of the CM phenotype on the complexities of engineered tissues, including a 2D vs. 3D structure as well as a unidirectional contraction hvCTS model vs multidirectional contraction hvCOC model.
CytoTRACE analysis showed that the CMs from CS were less mature compared to those from the higher order engineered tissues, hvCTS and hvCOC, which corroborates with findings from our previous study using bulk RNA-sequencing analysis.33 Identified key genes governing the trajectory confirm that these indeed define the maturation state of the CMs as the representative genes have been reported to be related to functional aspects of maturation of CMs. For example, top genes that have a positive correlation with the “1 – CytoTRACE”, pertain to cardiac muscle development, while a negative correlation with the “1 - CytoTRACE” pertains to other aspects of cardiac physiology, including electrophysiology and chamber specification.
With the establishment of a trajectory for maturation, a timeline for the different hallmarks of maturation can be determined by comparing the transcriptome of the CMs from different engineered constructs along the trajectory. In less physiological tissue structures, i.e., CS, the CMs are immature and continue to undergo developmental processes, as genes involved in development are upregulated, with the development of sarcomeric structure among the top enriched processes. In hvCAS, which is the next tissue construct along the maturation trajectory, biological processes including cellular component, cytoskeleton, and organelle organization are enriched, suggesting that structural and especially myofibril maturation begins after cardiac developmental processes. The more complex 3D hvCTS and hvCOC configurations, which are at the end of the maturation trajectory, show enrichment in metabolic processes, suggesting that metabolic processes mature at a later time after myofibril and sarcomeric development. Finally, comparing hvCTS and hvCOC, extracellular matrix organization and cellular response pathways continue to develop in hvCOC, further highlighting the fact that integrating physiology is a hallmark that is found in the most mature CMs along the trajectory. Results from other studies suggest that metabolic maturation and maturation on myofibril and sarcomeric development occur in parallel. Studies using metabolic conditioning to induce maturation in hPSC-CMs showed an increased expression in cardiac genes, increased contractility, and a mature ventricular-like electrophysiology.43 In another study, engineered heart tissue functions did not further increase after metabolic conditioning, suggesting that myofibril and sarcomeric development precedes or occurs in parallel with metabolic maturation.44 Consistent with our study, further maturation was observed in the extracellular matrix organization.
The mature phenotype of CMs in the more cardiomimetic tissue configurations, as revealed by the maturation trajectory, may be attributed to the 3D environment that can affect different cellular structures of CMs, with top differentially expressed genes and biological processes relating to the cellular organization. The observation that there are very few GO terms common between the 2D hvCAS and its 3D counterparts, hvCOC, hvCTS, and the CS, further supports this notion. Furthermore, GO terms that are commonly enriched between hvCTS and CS are related to oxidative metabolic processes, suggesting the maturation of oxidative metabolism in the CMs to counter an increase in energy consumption. CMs in hvCOC and hvCTS have to contract against an added mechanical load in the bioreactors housing these two tissues. Those cells in the hvCTS are contracting against posts with comparable physiological tissue stiffness that are anchoring the tissue ends. Similarly, CMs in the hvCOC are contracting against a fluid pre-load that inflates the chamber organoid. These mechanical loads impose an additional energy demand on the actively contracting CMs. This observation agrees with previous findings showing that mechanical loading could enhance mitochondrial biogenesis, hence promoting the maturation of oxidative metabolism.22,45
While microenvironment within the tissue construct may contribute to the maturation of hPSC-CMs within the engineered tissues, interaction between different cell types within the construct may also play a role in the promotion of maturation of hPSC-CMs. Cell compositions within different engineered constructs were different. Interestingly, the two constructs hvCTS and hvCOC contained added dermal fibroblasts when they were fabricated. Further studies would be needed in order to study the effect of dermal fibroblasts on the maturation of hPSC-CMs by comparing these cells with those that were fabricated without dermal fibroblasts.
Aside from the CMs present in the tissue constructs, hPSC-derived fibroblasts, a major non-CM population in the differentiation culture, also exhibit differential phenotypes among the models. Those in the 2D hvCAS exhibit an upregulated expression of ACTA2 compared to those 3D tissue models with physiological stiffness, suggesting that they have a myofibroblastic phenotype. Fibroblasts are known to undergo the transition to activated myofibroblasts at stiffness greater than 20 kPa.46 Indeed, fibroblasts in the hvCAS were in direct contact with a micropatterned PDMS substrate of supra-physiological stiffness. Therefore, the stiff substrate likely upregulated the ACTA2 in these cells. Of note, the maturity of the fibroblasts may also be modulated by the tissue models. These support cells in the hvCOC model express a lower level of PCNA, indicating that they are less proliferative in a 3D microenvironment of physiological stiffness. Quiescent fibroblasts are typically present in postnatal hearts entering a homeostatic state.47 Therefore, the low number of proliferative fibroblasts in the hvCOC may indicate cell maturity beyond the embryonic heart. In addition, COL1A1, COL1A2, and DCN, which play a role in collagen fibril assembly, are higher in those fibroblasts in the hvCOC compared to the other tissue models. Conversely, fibroblasts in the hvCOC have a lower expression of FN1 in comparison to the other tissue configurations. Cardiac fibroblasts in the mature, non-regenerative heart are known to produce more collagen than fibronectin.48 Hence, the extracellular matrix production profile of those fibroblasts in the hvCOC suggests that a 3D tissue of physiological stiffness may also be promoting maturation in fibroblasts.
In conclusion, our study shows that single-cell RNA-sequencing allows accurate evaluation of maturation in engineered cardiac tissues by analyzing the CMs and fibroblasts separately. It confirms that engineered cardiac tissues subjected to unique microenvironment and cell-cell interaction that more closely resembles the native cardiac tissue configurations allow CMs and cardiac fibroblasts within to display a higher maturity compared to less physiological cell population aggregated at random with little organization or alignment. Our advanced cardiomimetic engineered cardiac tissue models with improved cellular maturation may contribute to in vitro screening applications relevant to preclinical human studies. Future efforts should also focus on increasing the throughput and efficiency of such complex engineered cardiac tissue assays.
Limitations of the study
Analysis of cells from tissue constructs fabricated with hiPSC-CMs showed a similar trend as observed in hESC-CM tissue constructs. This is evident from the CytoTRACE analysis showing CS being least mature while hvCOC being most mature, although DEG analyses could not be performed due to the lack of replicates of samples. Nonetheless, the hiPSC-CM data corroborates our finding that increased maturation is observed in the higher order tissue configurations. This is universal to all hPSCs including hESCs and hiPSCs.
Another limitation of this study is the lack of objective comparison in more than one parameter related to maturation. Indeed, as discussed earlier, an objective comparison of parameters related to maturation is difficult due to the different measurable parameters in the various tissue constructs. For example, while hvCTS and hvCOC were both designed to provide quantifiable readouts for contractility, a direct comparison between the two tissues is not possible due to the two different sets of measurable functional parameters. Thus, transcriptomic analysis remains the most objective analysis that allows us to compare between different constructs.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Cardiac troponin T | Abcam | Abcam Cat# ab8295; RRID:AB_306445 |
| Chemicals, peptides, and recombinant proteins | ||
| BMP4 | Thermo Fisher | PHC9531 |
| Activin A | Thermo Fisher | PHC9564 |
| IWR-1 | STEMCELL Technologies | 72,564 |
| Papain | Sigma | 76,220 |
| EDTA | Roth | 804.2 |
| Deposited data | ||
| Raw and preprocessed data | This paper | GSE200277 |
| Raw and preprocessed data | Paper: PMC7763394 | GSE157157 |
| Human reference, GRCh38 (Ensembl 93) | 10X GENOMICS | https://support.10xgenomics.com/ |
| Experimental models: Cell lines | ||
| Human embryonic stem cell ES02 | Wicell, Madison, WI | ES02 |
| Skin fibroblast | ATCC | CRL-2522 |
| Software and algorithms | ||
| bcl2fastq | Illumina | https://emea.support.illumina.com/ |
| R version 4.1.1 | The R Project for Statistical Computing | https://www.r-project.org/ |
| CytoTRACE version 0.33 | CytoTRACE | https://cytotrace.stanford.edu/ |
| Seurat version 4.0.5 | SEURAT | https://satijalab.org/seurat/ |
| MetaNeighbor version 1.12.0 | Bioconductor | https://www.bioconductor.org/ |
| Linnorm version 2.16.0 | Bioconductor | https://www.bioconductor.org/ |
| Limma version 3.48.3 | Bioconductor | https://www.bioconductor.org/ |
| DESeq2 version 1.32.0 | Bioconductor | https://www.bioconductor.org/ |
| gprofiler2 version 0.2.1 | CRAN | https://cran.r-project.org/ |
| rrvgo version 1.4.4 | Bioconductor | https://www.bioconductor.org/ |
| LabVIEW | In house | PostTracking_HK2 |
| Brainvision Analyzer | Brain Products GmbH | BV_Ana x64 v.16.04.20 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr Wendy Keung, wendywkeung@connect.hku.hk.
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human stem cell culture and CM differentiation
Cells and tissues were generated from the female hESC line, hES2 (ES02, Wicell, Madison, WI). For the hiPSC counterparts, in-house reprogrammed male hiPSC line was used.1 All hPSCs were cultured in a 6-well tissue culture plate coated with Matrigel, using mTeSR1 medium (Stem Cell Technologies). The cell line was passaged with Accutase after reaching 80% confluency at 3 × 105 cells per well. All hPSCs were differentiated in a 6-well low-attachment culture plate using the embryoid body suspension method in two induction stages: stage 1 with the addition of BMP4 and Activin A for mesodermal induction and stage 2 with the addition of IWR-1 to inhibit Wnt signaling pathways for cardiac specification. Detailed differentiation protocol is described in our previous publication.8
Generation of hvCAS, hvCTS and hvCOC constructs
The cardiac spheroids were maintained in StemPro-34 medium until dissociation on day 14 with 0.5% Trypsin/EDTA (Life Technologies, Waltham, MA). The cells were triturated at 37°C for 20 min. The differentiated cultures were then labeled for cardiac troponin T to identify hPSC-CMs. Only those differentiated batches with purity of hPSC-CM greater than 60% were used for the experiments. The tissue fabrication of hvCAS, hvCTS and hvCOC follows our previously published reports without deviations.28,32,33 A total of 1.3 million hPSC-CM was used to construct each hvCTS and 10 million for each hvCOC, with an additional 10% of male dermal fetal fibroblasts. One hvCAS construct per batch was quality checked for calcium transients using Rohd2-AM dye (Thermofisher Scientific, Waltham, MA) and optical mapped for quality control. Contractility of hvCTS was measured via video recording of PDMS post deflections using a custom software as described previously.34 Only hvCTS with at least 50 μN of force was used for the experiment. Pressure-volume parameters in hvCOC were measured via video recording and a custom analysis software according to Li et al.33 to ensure viability and normal function before collection of tissue construct for scRNA-seq.
Method details
Dissociation of tissue constructs and sequencing
All tissue constructs were washed with PBS without Ca2+ and Mg2+ and incubated in a solution containing 10 unit/mL of papain (Sigma, 76,220) and 1 mM of EDTA (Roth, 8043.2) for 45 min at 37°C. The cells were then gently triturated for ∼1 min until a single-cell suspension was obtained. Medium with 10% fetal calf serum (FCS, Merck-Milipore, S 0615) was added to inhibit the digestion process. The cells were then washed in PBS and counted.
For hES2 cells, we used two replicate CSs and hvCAS, and three replicate hvCTS and hvCOC constructs generated from three separate differentiation batches (Table S1), while for hiPSC only one replicate was used. The dissociated cells from each sample were filtered through a 40-μm strainer (pluriStrainer) and processed with Single Cell 3′ Reagent Kit v2 (Chromium Single Cell 3′ Library & Gel Bead Kit v2, Chromium Single Cell A Chip Kit, 48 runs, 10x Genomics) and Chromium Controller (10x Genomics) according to the protocol provided. The constructed libraries for hES2 batches B1 and B2 were sequenced using Illumina NovaSeq 6000 Systems with read length of 151bp, while libraries for hES2 batch B3 and hiPSC were sequenced with Illumina NextSeq500 using read length of 96bp.
Pre-processing of scRNA-seq data
After sequencing, the reads from each sample were assigned by using blc2fastq from Illumina. Then, the unique molecular identifier (UMI) counts of each barcode were analysed by using Cell Ranger (version 3.0.2) following the pipeline with default parameters. Cells were filtered using total UMI and total feature counts as criteria. The low-quality cells with total UMI counts of less than 1,000 or more than 50,000 or with mitochondrial genes accounting for more than 15% out of the total UMI counts were excluded from the analysis. Furthermore, the cells expressing less than 1,000 or more than 7,500 features were also disregarded.
Identification of cell types in tissue constructs
All analyses were carried out with R (version 4.1.1). To identify the cell types in the hESC and hiPSC-derived tissue constructs, 2,000 most variable genes were selected by using R package Seurat (version 4.0.5),49 and the cells were projected into two-dimensional space using Uniform Manifold Approximation and Projection (UMAP) technique with the first 20 principal components (PCs).50 Next, the cells were clustered with the same 20 PCs using a graph-based clustering method in Seurat (Figure S4A). Finally, CMs were identified by choosing the cluster with the highest expressions of known cell-specific genes for CMs such as MYL2 and TNNI1 (Figure S4B).51
The fibroblast-like clusters, identified by expression of COL1A1 (Figure S4B), consisted of cardiac and dermal fibroblasts, as for the hvCTS and hvCOC, the dermal fibroblasts were supplemented, However, for the hESC-derived cells added dermal fibroblasts were male cells, and could therefore be distinguished from the female fibroblasts by the raw Y chromosome UMI counts. Those cells with a sum of the Y-chromosome UMI counts of greater than 1 were then identified as dermal fibroblast (Figures S4C–S4E) and removed from the final data set. Since the clusters representing cardiac fibroblasts in the remaining cells has no clear separation from the other clusters, cells were also clustered within each sample, and the fibroblast population was identified using expressions of COL1A1 and DCN which are known to be highly expressed in fibroblasts (Figure S5A).52,53 The identity of cardiac fibroblasts was then confirmed by using the intersection of cardiac fibroblasts identified within each sample (Figure S5A) and the pooled collective of all samples that exhibited the highest expressions of COL1A1 and DCN (Figures S5B).
Clustering and prediction of trajectories
Clustering of CMs and fibroblasts was performed separately for both cell types using Seurat with the first 20 PCs. The optimal number of clusters was then evaluated by area under the receiver operating characteristic (AUROC) using R package MetaNeighbor (version 1.12.0).54 The clusters with AUROC less than 0.8 were merged and identified as one cluster.
To study the differentiation and maturation state of CMs from the four tissue constructs, we estimated cell trajectory. Within each tissue configuration, expressions of the CMs were normalized using R package Linnorm (version 2.16.0),55 and batch effect was corrected with removeBatchEffect function from R package Limma (version3.48.3).56 The CMs were then ranked using Cellular Trajectory Reconstruction Analysis (R package CytoTRACE, version 0.3.3). CytoTRACE score was calculated from a set of 19,000 annotated genes to determine the differentiation status of cells. Then the cells were ordered by the numeric CytoTRACE vector which represents the differentiation status ranging from one for the least differentiated phenotype to zero for most differentiated phenotype.35 For clarity, we use “1 - CytoTRACE” to order the rank of the cells from the least to the most mature phenotype.
Differential gene expression and enrichment analyses
The differentially expressed genes were calculated using R package DESeq2 (version 1.32.0) with default parameters.57 Potential effect of batch was eliminated by including batch information into the model. The DEGs with an adjusted p value less than 0.05 and an absolute log2 fold change (log2FC) higher than 0.5 were regarded as statistically significant. The normalised expression values used in the figures were calculated by using variance stabilising transformation.
The Gene Ontology enrichment analyses were carried out for GO terms and pathway enrichment analyses for KEGG: Kyoto Encyclopedia of Genes and Genomes database by using g:Profiler (R package gprofiler2 version 0.2.1).58 The enrichment analyses were carried out for top 200 positively and negatively correlated genes in the CytoTRACE analysis, and top 200 DEGs ordered based on log2FC for the other comparisons. The enriched GO terms were summarised into non-redundant parental groups with REVIGO (R package rrvgo version 1.4.4 59,60) (Table S7). To evaluate the functional differences of the CMs from the different tissue constructs, GO networks were constructed (Figures 4A and 5A). The non-redundant parental GO groups which are enriched among the upregulated DEGs in each tissue configuration were designated as nodes in the GO networks. Then, for each non-redundant group, the proportion of the GO terms for each tissue configuration was calculated and illustrated as pie graph. Finally, the links were assigned between non-redundant groups and tissue configurations.
Quantification and statistical analysis
Data analysis is based on free software and R which are described in the STAR Methods and key resources table.
Acknowledgments
We thank the Genomics Core of the Centre for PanorOmic Sciences, the University of Hong Kong for scRNA-seq library preparation and sequencing. We thank Miller Leung and Renee Chou for their help in dissociating the tissues used for the scRNA-seq.
Author contributions
Conceptualization, R.A.L., W.K., K.D.C., and D.K.L.; methodology, S.C. and V.A.; software; formal analysis; visualization, S.C.; validation, D.B-L and S.C; investigation, D.B-L, S.C., V.A., D.K.L, and W.K; resources, D.B-L, A.O.T.W, H.Y.K, B.G., and W.K; writing – original draft writing D.B-L, W.K., D.K.L, S.C., and V.A.; writing – review & editing, all authors; supervision, W.K., V.A., D.K.L, and Z.Z.; project administration, D.B-L, V.A., and W.K.; funding acquisition, R.A.L.
Declaration of interests
K.D.C, and R.A.L. hold equities in Novoheart, whose value may potentially be affected by the publication of this article. S.C., D.B-L., V.A. A.O.T.W., H.Y.K, B.G., Z.Z., D.K.L, and W.K. have no competing interests.
Published: March 1, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106302.
Supplemental information
Data and code availability
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Lam Y.Y., Keung W., Chan C.H., Geng L., Wong N., Brenière-Letuffe D., Li R.A., Cheung Y.F. Single-cell transcriptomics of engineered cardiac tissues from patient-specific induced pluripotent stem cell-derived cardiomyocytes reveals abnormal developmental trajectory and intrinsic contractile defects in hypoplastic right heart syndrome. J. Am. Heart Assoc. 2020;9:e016528. doi: 10.1161/JAHA.120.016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannhardt I., Breckwoldt K., Letuffe-Brenière D., Schaaf S., Schulz H., Neuber C., Benzin A., Werner T., Eder A., Schulze T., et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Rep. 2016;7:29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye L., Chang Y.H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S., et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwi-Dantsis L., Huber I., Habib M., Winterstern A., Gepstein A., Arbel G., Gepstein L. Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients. Eur. Heart J. 2013;34:1575–1586. doi: 10.1093/eurheartj/ehs096. [DOI] [PubMed] [Google Scholar]
- 5.Breckwoldt K., Letuffe-Brenière D., Mannhardt I., Schulze T., Ulmer B., Werner T., Benzin A., Klampe B., Reinsch M.C., Laufer S., et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017;12:1177–1197. doi: 10.1038/nprot.2017.033. [DOI] [PubMed] [Google Scholar]
- 6.Burridge P.W., Thompson S., Millrod M.A., Weinberg S., Yuan X., Peters A., Mahairaki V., Koliatsos V.E., Tung L., Zambidis E.T. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng Z., Kong C.W., Ren L., Karakikes I., Geng L., He J., Chow M.Z.Y., Mok C.F., Chan H.Y.S., Webb S.E., et al. A simple, cost-effective but highly efficient system for deriving ventricular cardiomyocytes from human pluripotent stem cells. Stem Cells Dev. 2014;23:1704–1716. doi: 10.1089/scd.2013.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W.Z., Van Biber B., Laflamme M.A. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol. Biol. 2011;767:419–431. doi: 10.1007/978-1-61779-201-4_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Pabon L., Murry C.E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronaldson-Bouchard K., Ma S.P., Yeager K., Chen T., Song L., Sirabella D., Morikawa K., Teles D., Yazawa M., Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan J.L., Tulloch N.L., Razumova M.V., Saiget M., Muskheli V., Pabon L., Reinecke H., Regnier M., Murry C.E. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. 2016;134:1557–1567. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieu D.K., Fu J.D., Chiamvimonvat N., Tung K.C., McNerney G.P., Huser T., Keller G., Kong C.W., Li R.A. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythm. Electrophysiol. 2013;6:191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong A.O.T., Wong N., Geng L., Chow M.Z.Y., Lee E.K., Wu H., Khine M., Kong C.W., Costa K.D., Keung W., et al. Combinatorial treatment of human cardiac engineered tissues with biomimetic cues induces functional maturation as revealed by optical mapping of action potentials and calcium transients. Front. Physiol. 2020;11:165. doi: 10.3389/fphys.2020.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Kong C.W., Tong M.H., Chooi W.H., Huang N., Li R.A., Chan B.P. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: effects of niche cell supplementation and mechanical stimulation. Acta Biomater. 2017;49:204–217. doi: 10.1016/j.actbio.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 16.Keung W., Ren L., Sen L., Wong A.O.T., Chopra A., Kong C.W., Tomaselli G.F., Chen C.S., Li R.A. Non-cell autonomous cues for enhanced functionality of human embryonic stem cell-derived cardiomyocytes via maturation of sarcolemmal and mitochondrial KATP channels. Sci. Rep. 2016;6:34154. doi: 10.1038/srep34154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon E., Keung W., Liang Y., Ramalingam R., Yan B., Zhang S., Chopra A., Moore J., Herren A., Lieu D.K., et al. Proteomic analysis of human pluripotent stem cell-derived, fetal, and adult ventricular cardiomyocytes reveals pathways crucial for cardiac metabolism and maturation. Circ. Cardiovasc. Genet. 2015;8:427–436. doi: 10.1161/CIRCGENETICS.114.000918. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Lieu D.K., Siu C.W., Fu J.D., Tse H.F., Li R.A. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am. J. Physiol. Cell Physiol. 2009;297:C152–C159. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farraj A.K., Hazari M.S., Cascio W.E. The utility of the small rodent electrocardiogram in toxicology. Toxicol. Sci. 2011;121:11–30. doi: 10.1093/toxsci/kfr021. [DOI] [PubMed] [Google Scholar]
- 20.Kaese S., Verheule S. Cardiac electrophysiology in mice: a matter of size. Front. Physiol. 2012;3:345. doi: 10.3389/fphys.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdes A.M., Kellerman S.E., Moore J.A., Muffly K.E., Clark L.C., Reaves P.Y., Malec K.B., McKeown P.P., Schocken D.D. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. doi: 10.1161/01.cir.86.2.426. [DOI] [PubMed] [Google Scholar]
- 22.Lopaschuk G.D., Jaswal J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y., Pu W.T. Cardiomyocyte maturation: new phase in development. Circ. Res. 2020;126:1086–1106. doi: 10.1161/CIRCRESAHA.119.315862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selewa A., Dohn R., Eckart H., Lozano S., Xie B., Gauchat E., Elorbany R., Rhodes K., Burnett J., Gilad Y., et al. Systematic comparison of high-throughput single-cell and single-nucleus transcriptomes during cardiomyocyte differentiation. Sci. Rep. 2020;10:1535. doi: 10.1038/s41598-020-58327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biendarra-Tiegs S.M., Li X., Ye D., Brandt E.B., Ackerman M.J., Nelson T.J. Single-cell RNA-sequencing and optical electrophysiology of human induced pluripotent stem cell-derived cardiomyocytes reveal discordance between cardiac subtype-associated gene expression patterns and electrophysiological phenotypes. Stem Cells Dev. 2019;28:659–673. doi: 10.1089/scd.2019.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman C.E., Nguyen Q., Lukowski S.W., Helfer A., Chiu H.S., Miklas J., Levy S., Suo S., Han J.D.J., Osteil P., et al. Single-cell transcriptomic analysis of cardiac differentiation from human PSCs reveals HOPX-dependent cardiomyocyte maturation. Cell Stem Cell. 2018;23:586–598.e8. doi: 10.1016/j.stem.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan H., Liao Y., Ren Z., Mao L., Yao F., Yu P., Ye Y., Zhang Z., Li S., Xu H., et al. Single-cell reconstruction of differentiation trajectory reveals a critical role of ETS1 in human cardiac lineage commitment. BMC Biol. 2019;17:89. doi: 10.1186/s12915-019-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shum A.M.Y., Che H., Wong A.O.T., Zhang C., Wu H., Chan C.W.Y., Costa K., Khine M., Kong C.W., Li R.A. A micropatterned human pluripotent stem cell-based ventricular cardiac anisotropic sheet for visualizing drug-induced arrhythmogenicity. Adv. Mater. 2017;29:1602448. doi: 10.1002/adma.201602448. [DOI] [PubMed] [Google Scholar]
- 29.Chen A., Lieu D.K., Freschauf L., Lew V., Sharma H., Wang J., Nguyen D., Karakikes I., Hajjar R.J., Gopinathan A., et al. Shrink-film configurable multiscale wrinkles for functional alignment of human embryonic stem cells and their cardiac derivatives. Adv. Mater. 2011;23:5785–5791. doi: 10.1002/adma.201103463. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Chen A., Lieu D.K., Karakikes I., Chen G., Keung W., Chan C.W., Hajjar R.J., Costa K.D., Khine M., Li R.A. Effect of engineered anisotropy on the susceptibility of human pluripotent stem cell-derived ventricular cardiomyocytes to arrhythmias. Biomaterials. 2013;34:8878–8886. doi: 10.1016/j.biomaterials.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Luna J.I., Ciriza J., Garcia-Ojeda M.E., Kong M., Herren A., Lieu D.K., Li R.A., Fowlkes C.C., Khine M., McCloskey K.E. Multiscale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng. Part C Methods. 2011;17:579–588. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 32.Turnbull I.C., Karakikes I., Serrao G.W., Backeris P., Lee J.J., Xie C., Senyei G., Gordon R.E., Li R.A., Akar F.G., et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28:644–654. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R.A., Keung W., Cashman T.J., Backeris P.C., Johnson B.V., Bardot E.S., Wong A.O.T., Chan P.K.W., Chan C.W.Y., Costa K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. doi: 10.1016/j.biomaterials.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keung W., Chan P.K.W., Backeris P.C., Lee E.K., Wong N., Wong A.O.T., Wong G.K.Y., Chan C.W.Y., Fermini B., Costa K.D., Li R.A. Human cardiac ventricular-like organoid chambers and tissue strips from pluripotent stem cells as a two-tiered assay for inotropic responses. Clin. Pharmacol. Ther. 2019;106:402–414. doi: 10.1002/cpt.1385. [DOI] [PubMed] [Google Scholar]
- 35.Gulati G.S., Sikandar S.S., Wesche D.J., Manjunath A., Bharadwaj A., Berger M.J., Ilagan F., Kuo A.H., Hsieh R.W., Cai S., et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science. 2020;367:405–411. doi: 10.1126/science.aax0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y., Jardin B.D., Zhou P., Sethi I., Akerberg B.N., Toepfer C.N., Ai Y., Li Y., Ma Q., Guatimosim S., et al. Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor. Nat. Commun. 2018;9:3837. doi: 10.1038/s41467-018-06347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keung W., Boheler K.R., Li R.A. Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2014;5:17. doi: 10.1186/scrt406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundy S.D., Zhu W.Z., Regnier M., Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machiraju P., Greenway S.C. Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J. Stem Cells. 2019;11:33–43. doi: 10.4252/wjsc.v11.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correia C., Koshkin A., Duarte P., Hu D., Carido M., Sebastião M.J., Gomes-Alves P., Elliott D.A., Domian I.J., Teixeira A.P., et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2018;115:630–644. doi: 10.1002/bit.26504. [DOI] [PubMed] [Google Scholar]
- 41.Zhu R., Blazeski A., Poon E., Costa K.D., Tung L., Boheler K.R. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2014;5:117. doi: 10.1186/scrt507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannan S., Farid M., Lin B.L., Miyamoto M., Kwon C. Transcriptomic entropy benchmarks stem cell-derived cardiomyocyte maturation against endogenous tissue at single cell level. PLoS Comput. Biol. 2021;17:e1009305. doi: 10.1371/journal.pcbi.1009305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feyen D.A.M., McKeithan W.L., Bruyneel A.A.N., Spiering S., Hörmann L., Ulmer B., Zhang H., Briganti F., Schweizer M., Hegyi B., et al. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020;32:107925. doi: 10.1016/j.celrep.2020.107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills R.J., Titmarsh D.M., Koenig X., Parker B.L., Ryall J.G., Quaife-Ryan G.A., Voges H.K., Hodson M.P., Ferguson C., Drowley L., et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA. 2017;114:E8372–E8381. doi: 10.1073/pnas.1707316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter G.A., Jr., Hom J., Hoffman D., Quintanilla R., de Mesy Bentley K., Sheu S.S. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011;31:75–81. doi: 10.1016/j.ppedcard.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwager S.C., Bordeleau F., Zhang J., Antonyak M.A., Cerione R.A., Reinhart-King C.A. Matrix stiffness regulates microvesicle-induced fibroblast activation. Am. J. Physiol. Cell Physiol. 2019;317:C82–C92. doi: 10.1152/ajpcell.00418.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivey M.J., Kuwabara J.T., Pai J.T., Moore R.E., Sun Z., Tallquist M.D. Resident fibroblast expansion during cardiac growth and remodeling. J. Mol. Cell. Cardiol. 2018;114:161–174. doi: 10.1016/j.yjmcc.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hortells L., Johansen A.K.Z., Yutzey K.E. Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J. Cardiovasc. Dev. Dis. 2019;6:29. doi: 10.3390/jcdd6030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McInnes L., Healy J., Melville J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv. 2018 doi: 10.48550/arXiv.1802.03426. Preprint at. [DOI] [Google Scholar]
- 51.Forough R., Scarcello C., Perkins M. Cardiac biomarkers: a focus on cardiac regeneration. J. Tehran Heart Cent. 2011;6:179–186. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Yang S., Lovisa S., Ambrose C.G., McAndrews K.M., Sugimoto H., Kalluri R. Type-I collagen produced by distinct fibroblast lineages reveals specific function during embryogenesis and Osteogenesis Imperfecta. Nat. Commun. 2021;12:7199. doi: 10.1038/s41467-021-27563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keene D.R., San Antonio J.D., Mayne R., McQuillan D.J., Sarris G., Santoro S.A., Iozzo R.V. Decorin binds near the C terminus of type I collagen. J. Biol. Chem. 2000;275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 54.Crow M., Paul A., Ballouz S., Huang Z.J., Gillis J. Characterizing the replicability of cell types defined by single cell RNA-sequencing data using MetaNeighbor. Nat. Commun. 2018;9:884. doi: 10.1038/s41467-018-03282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yip S.H., Wang P., Kocher J.P.A., Sham P.C., Wang J. Linnorm: improved statistical analysis for single cell RNA-seq expression data. Nucleic Acids Res. 2017;45:e179. doi: 10.1093/nar/gkx828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Supek F., Bošnjak M., Škunca N., Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sayols S. rrvgo: a Bioconductor package to reduce and visualize Gene Ontology terms. 2020. https://ssayols.github.io/rrvgo [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.