Abstract
Day-to-day choices often involve social information and can be influenced by prior social experience. When making a decision in a social context, a subject might need to: 1) recognize the other individual or individuals, 2) infer their intentions and emotions, and 3) weigh the values of all outcomes, social and non-social, prior to selecting an action. These elements of social information processing all rely, to some extent, on the medial prefrontal cortex (mPFC). Patients with neuropsychiatric disorders often have disruptions in prefrontal cortical function, likely contributing to deficits in social reasoning and decision making. To better understand these deficits, researchers have turned to rodents, which have revealed prefrontal cortical mechanisms for contending with the complex information processing demands inherent to making decisions in social contexts. Here, we first review literature regarding social decision making, and the information processing underlying it, in humans and patient populations. We then turn to research in rodents, discussing current procedures for studying social decision making, and underlying neural correlates.
Keywords: prelimbic, infralimbic, anterior cingulate, vmPFC, dmPFC, operant, social decision-making, sociability, choice
INTRODUCTION
Social experiences color our choices. From simple day-to-day choices such as what to have for dinner, to incredibly complex choices such as with whom to partner, social experiences affect how we behave and navigate a changing environment. This phenomenon extends to all mammals, as a degree of “social competence” is necessary for survival1. Thus, it is critically important that mammalian brains can contend with the complexities inherent to decision making, which involves the perception and integration of multiple sensory cues with historical knowledge and information about current internal states, ultimately guiding choices and behavior. In social contexts, this already complex process must also combine internal decision-making strategies with the perceived motivations and emotions of a dynamic group of agents. Many psychiatric illnesses are either co-morbid with, or defined by, disruptions in social behavior, including in decision making related to social experiences. Thus, it is imperative to investigate and uncover the neural processes inherent to this complicated and integrative process to further understand and develop new therapeutic avenues.
Across mammalian species, the prefrontal cortex, specifically the medial prefrontal cortex (mPFC), has emerged as a key regulator of the information processing demands necessary for decision making in social contexts. Historically, investigations of the mPFC focused on its function in adaptive control of behavior: utilizing motivationally salient information to guide decisions, and then altering decisions based on feedback2. This model applies to social situations; generally speaking, the mPFC functions within a “cognitive social brain” network3, exerting “top-down” cognitive control over decisions that involve social information in humans4, non-human primates5, and rodents6. These functions can be contrasted with the more innate social behaviors, such as aggressive or sexual responses, which are predominantly controlled by subcortical structures like the hypothalamus7, 8, and will largely not be discussed in this review.
Here, we review literature that deconstructs the complex information processing involved in social decision making in humans. We discuss three essential elements: social/emotional recognition, empathic processes, and social incentive/action selection. These three components of social information processing are controlled by overlapping but partially distinct circuits in the brain. As almost every neuropsychiatric disorder involves some degree of impairment in social decision making, better understanding the origins of impairment through investigations of social/emotional recognition, empathic processes, and social incentive/action selection is of utmost therapeutic importance. Finally, we discuss the tractable rodent models for understanding the precise functions of the mPFC in the information processing required for decisions in social contexts and discuss areas where future rodent studies could be translatable and beneficial.
SOCIAL DECISION MAKING IN HUMANS
Decision making in social contexts: deconstructing a complex process
Social interaction involves a very complex set of information-processing demands. To better understand how a social decision might be dissected, we turn to an example (modified from9): going to a bake sale fundraiser for your child’s school. As you walk by a stand, a fellow parent calls you over, attempting to persuade you to purchase their cookies. The first component of the interaction is social and emotional perception: do you recognize this person? Have you interacted with this parent before? Do they seem disappointed that nobody is at their stand? After you walk over to their stand, you infer the intentions of another person based on your perception of them, using empathic processes. Do you feel sorry for the parent who may not be selling as many cookies as they had hoped? Are you wondering whether they are trying to swindle you into purchasing overpriced cookies? At the same time, you are computing the value of the potential decisions you have in front of you. Importantly, this valuation must integrate not only the cost of the cookies, but the current “cost” of the relationship with the parent, and any other agent who might be paying attention to this interaction. Further, historical social associations with the food, such as enjoying these cookies with your child last year, might encourage you to buy them. As such, our brains are computing the social incentive of the interaction, alongside any non-social valuation. Lastly, you must select an action. You weigh the potential costs and benefits of purchasing the cookies from the parent, and you balance the social and non-social valuations of the interaction and reach a decision threshold as to whether you will purchase the cookies. In this relatively simple example of buying cookies at a bake sale, we are able to distinguish three elements of information processing when making a decision in a social context: social/emotional recognition, empathic processes, and social incentive/action selection9, 10.
Social decision making as an endophenotype for neuropsychiatric disease
“Endophenotype” refers to neurophysiological, biochemical, endocrinological, neuroanatomical, cognitive, or neuropsychological processes that are stable and have known or likely genetic causes; they are measurable processes that are associated with disease symptomatology, and understanding their mechanisms may provide insight into the etiology of a disease syndrome itself11. Deficits in the capacity for social decision making are prominent in many, if not all, neuropsychiatric conditions and are predictors of overall mortality, due to effects of diminished interpersonal relationships on overall health12, 13. This notion has led many clinicians to posit that investigations into social decision making may reveal potential endophenotypes more predictive of disease progression or treatment course than current diagnostic criteria. This is reflected in the National Institute for Mental Health’s strategic plan, which has transitioned from emphasizing traditional disease diagnostic criteria, as reported in the Diagnostic and Statistical Manual of Mental Disorders (DSM), to the neurobiological construct-based Research Domain Criteria (RDoC), which includes “social processes” as one of its key Domains14. For instance, neuroimaging studies are already being conducted using social interaction as a putative endophenotype for neuropsychiatric illness15. However, the ubiquity of social behavioral difficulties among clinical populations poses a significant challenge to these studies. Given that decisions in social contexts impose demands on a large number of interconnected brain structures, dissecting this process into more discrete component parts may be necessary to inform endophenotypes16. This next section will take a more in-depth look at the brain structures underlying social/emotional recognition, empathic processes, and social incentive/action selection, and emphasize how functional impairments can manifest in neuropsychiatric illnesses such as autism spectrum disorders (ASDs) and schizophrenia spectrum disorders (SSDs).
Anatomical and functional subdivisions of the mPFC in humans/primates
Understanding the neural correlates of social decision making requires a brief orientation to the subdivisions of the mPFC in humans (fig.1), which is involved in social decisions. Parcellation of the prefrontal cortex has proven to be a divisive endeavor; complexity in structure and function is the rule, not the exception17. The mPFC of humans classically consists of Brodmann areas (BAs) 9 and 10, 24, 25, and 32, with 11 and 14 constituting the medial orbital cortex4, 18. Major afferents to the mPFC come from the dorsolateral prefrontal cortex, temporal pole, anterior superior temporal gyrus, parietotemporal cortex, and posterior cingulate cortex (PCC). Connectivity of the mPFC broadly follows two axes: a dorso-ventral axis and a rostro-caudal axis19. The rhinal cortex, which acts as a gateway to hippocampal input and output, is more densely connected with the ventral mPFC (vmPFC) than the dorsal mPFC (dmPFC), which instead shares connections with the lateral premotor cortex, the supplementary motor area, and the cingulate motor area20. Further, the vmPFC, but not dmPFC, sends monosynaptic projections to the ventral striatum. Meanwhile, the amygdala has strong inputs to the more caudal anterior cingulate regions, also known as the anterior cingulate cortex (ACC; BAs 24, 25, and 32)21, but much weaker inputs to the frontopolar regions. Dorsocaudal regions (BAs 24 and 32) have robust connections with the premotor cortex22.
Figure 1. Comparative structural and functional anatomy of the “Social Brain” in humans and rodents.
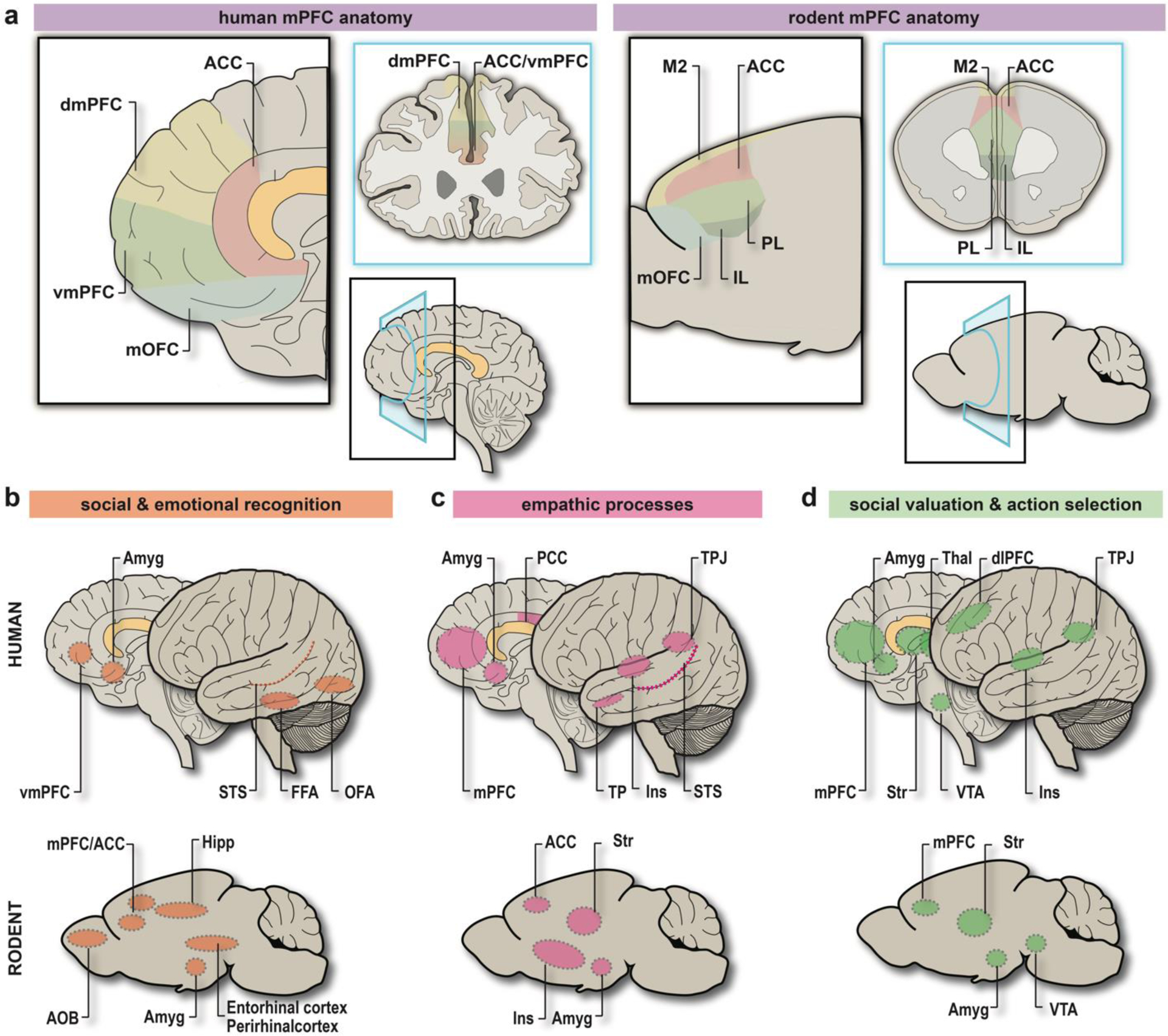
a. (left) Diagram detailing human mPFC anatomy from the sagittal (black) and coronal (blue) perspective. Corresponding BAs: ACC = BA24, BA25, BA32, dmPFC = BA9, BA10, vmPFC = BA10, BA32, mOFC = BA11, BA14. (right) Diagram detailing rodent mPFC anatomy from the sagittal (black) and coronal (blue) perspective. Putative homologies to BAs: M2 = BA6 or BA8, ACC = parts of BA24a-b, 25, 32; PL = BA32; IL = BA25; mOFC = BA11, BA14. b. Brain areas involved in social and emotional recognition in humans (top) and rodents (bottom). c. Brain areas involved in empathic processes in humans (top) and rodents (bottom). d. Brain areas involved in the incentive valuation of social interaction, and subsequent action selection in humans (top) and rodents (bottom). Abbreviations: anterior cingulate cortex (ACC), anterior olfactory bulb (AOB), amygdaloid complex (Amyg), Brodmann area (BA), dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), fusiform face area (FFA), hippocampus (Hipp), infralimbic subregion of mPFC (IL), insular cortex (Ins), medial orbitofrontal cortex (mOFC), medial prefrontal cortex (mPFC), occipital face area (OFA), posterior cingulate cortex (PCC), prelimbic subregion of mPFC (PL), thalamus (Thal), temporal pole (TP), temporo-parietal junction (TPJ), striatum (Str), superior temporal sulcus (STS), secondary motor cortex (M2), vmPFC = ventromedial prefrontal cortex (vmPFC), ventral tegmental area (VTA). Image created by author HK using Adobe Illustrator.
Canonical experiments focused on human and non-human primate PFC functions investigated non-social roles in working memory23, 24 and attention25. In social neuroscience, a functional distinction is often made between the ventral (BA10, 24, 25, parts of 32) and dorsal (BA9, parts of 32) regions of the mPFC along various spectra: such as emotional vs. cognitive26, or self-reliant vs. other-relevant social cognition27, 28, respectively; illustrating the lack of clear agreement as to their functions (fig.1; to see a more exhaustive review of functional axes in the prefrontal cortex, see29). Broadly, the dmPFC is preferentially connected to high-order association cortical areas of the lateral frontal, temporal, and parietal lobe, while the vmPFC is preferentially connected to limbic and reward-related medial brain areas30.
Social and emotional recognition in humans
Neural correlates.
Humans display a remarkable capacity for facial recognition, instantaneously discriminating between the relative similarity of human features while interpreting the vast complexity of information that can be transmitted through faces. This capacity appears to be intact from a very young age, as newborns will preferably look at photographs and cartoons of faces rather than objects or inverted faces31. The ability to identify a social context, including recognition of agents and recall of past interactions with them, is associated with activity in the medial temporal lobes and the fusiform gyrus (fig.1). In fMRI studies, several areas demonstrate a selective response to faces, including the fusiform face area (FFA), the lateral occipital lobe, the posterior superior temporal sulcus (pSTS), and the occipital face area32–34 (fig.1; reviewed in35).
The pSTS belongs to another network as well, which recruits the amygdala and the vmPFC to process the emotional or affective value of observed stimuli (fig.1)36–39. Specifically, the amygdala is consistently recruited when emotional recognition provides motivational salience40, such as when the perceived emotion predicts danger41. In contrast, investigations of the vmPFC in emotional perception have been more equivocal: patients with vmPFC lesions have exhibited impairments in emotional recognition42, or exhibited no difference from controls43. More recent results suggest that the vmPFC coordinates visual attention to emotionally salient stimuli, particularly in the context of negative emotions, such as anger43. Thus, imaging studies suggest that typical facial recognition relies on the FFA and its accessory areas, while more salient stimuli, like an angry face, recruit the amygdala, and possibly, the vmPFC.
Alterations in ASDs and SSDs.
Both social recognition and emotional recognition are often impaired in neuropsychiatric and neurodevelopmental disorders and are necessary for typical decision making in social scenarios. Individuals with ASDs seem to lack early predispositions for social stimuli, and actually exhibit superior performance in the ability to match upside-down faces, consistent with the notion that these individuals may identify faces based on component parts as opposed to a gestalt44. A meta-analysis demonstrated that ASDs are consistently associated with facial and emotional recognition deficits, that the magnitude of these deficits increase with age, and that the deficits cannot be explained by levels of intelligence45. Indeed, face perecption in ASDs is accompanied by abnormal ventral temporal cortical activity, suggesting perhaps that patients with ASDs view faces similar to how neurotypical individuals view objects46.
Patients with SSDs cite interpersonal problems as among the most debilitating of the disorder47. Evidence suggests non-emotional facial processing might be impaired in schizophrenia48, but the neural bases underlying this deficit remain unclear, as FFA activation remains unchanged in patients with SSDs49. Comprehensive meta-analyses of facial affect research in SSDs confirmed that emotional recognition is a consistent and robust deficit, and that these deficits are predictive of deleterious outcomes in community functioning50. Deficits in emotional recognition are accompanied by decreased activation of the amygdala and the ACC and mPFC, with increases in areas outside those traditionally associated with emotional processing (such as the parietal lobule and cuneus), possibly representing compensatory processing51, 52. However, it is important to note that many studies of facial and emotional recognition in neuropsychiatric disorders suffer from inadequate validation in clinical settings (as discussed in53), pointing to fertile ground for new diagnostic and therapeutic approaches.
Empathic processes in humans
Neural correlates:
Empathy is considered by many to be a uniquely human trait. However, pro-social behaviors in non-human primates and rodents suggest that some empathic capacity may predate humans evolutionarily54. Frans de Waal posits a multi-level theory of empathy55, whereby the lowest common denominator of all empathic processes is when an individual is affected by another’s emotional state, a phenomenon called emotional contagion. Empathic processes then extend in a continuum, with the more complex levels involving concern about another’s state and attempts to ameliorate that state, and subsequently, at the most elaborate level – attributing another’s emotional state to oneself (i.e., putting yourself in someone else’s shoes).
Emotional contagion (also known as vicarious perception) is the notion that the observation of affective states in another induces a shared internal state in the observer56. Imaging studies have examined the brain regions that are activated when an observer watches someone taste the contents of a cup and look disgusted, and noted the same activation patterns when the observer themself tasted an unpleasant bitter liquid57. The functional circuits activated by both experiences have often been called the “emotional mirror neuron system” in the brain, relying on the activity of the ACC and the anterior insula56, 58. Most contend that emotional contagion, although likely fundamental to empathy, does not encompass the full empathic experience, as it does not involve a self-other distinction. In other words, a subject might become disgusted reflexively, without any real concern or understanding of another’s experience. As such, de Waal’s description of emotional contagion may constitute a “bottom-up” model of empathy, in which automatic responses are then sent to higher-order processing areas59. This model functions in tandem with the concept of “perspective taking,” or “top-down” executive control of cognition and emotion through selective attention and self-regulation, mediated by the prefrontal and cingulate cortices. These “top-down” processes are continuously updated by “bottom-up” signals and provide important feedback, adding flexibility and modulation to empathic processes56.
Prefrontal- and cingulate-mediated “top-down” executive control of empathy, or perspective taking, is often divided into two components: cognitive perspective taking (i.e., inferring what another thinks), and an affective perspective taking (i.e., inferring what another feels)56, 60. In this review, we will use the term “affective perspective taking” when tasks differentiate between the cognitive and affective components of “top-down” empathic processes. Otherwise, we will use “cognitive perspective taking,” which can also be called mental state attribution, or mentalizing. Both affective and cognitive perspective taking require intact Theory of Mind (ToM), or the ability to differentiate the beliefs of another from oneself. Investigations into the neural correlates of cognitive perspective taking have observed a characteristic network of activations when participants read stories about social interactions, including the temporo-parietal junction (TPJ), the STS, the temporal poles, the PCC, and the mPFC (fig.1)61–63. The dmPFC subnetwork, which is more strongly connected to these high-order association regions, is more heavily associated with cognitive perspective taking than the vmPFC64. In contrast, affective perspective taking preferentially activates the vmPFC65. Patients with vmPFC lesions demonstrate a functional dissociation in their perspective taking processes: they exhibit intact cognitive perspective taking but impaired affective perspective taking60. Thus, “top-down” regulation of cognitive and affective perspective taking may be dissociable functionally in the mPFC, requiring input from the dmPFC and vmPFC, respectively.
Alterations in ASDs and SSDs.
Empathic processes are altered in many neuropsychiatric disorders, most notably ASDs and SSDs66. In ASDs, there is no clear consensus as to what may underlie changes in empathic processes. Emotional contagion may be atypical in ASDs, but studies of vicarious pain have demonstrated reduced, similar, and increased brain responses to other’s pain in the ACC and AI58. A number of studies have demonstrated reduced cognitive perspective taking67, but not affective perspective taking68, 69, in patients with ASDs. In keeping with this perspective, patients with ASDs exhibit reduced covariance in networks centered on the dmPFC and TPJ, but not fronto-insular connectivity, suggestive of a dissociation between cognitive and affective networks. This perspective has come under question in recent years, however, with other studies finding intact cognitive perspective taking and even enhanced affective perspective taking70, 71.
In patients with SSDs, emotional contagion appears to remain intact, with comparable activation patterns to healthy control participants in the affective mirror neuron system during the observation and execution of complex facial emotional expressions72, despite lower self-reports of empathy. In contrast, there is robust evidence of changes in both cognitive and affective perspective taking73–75. When making judgments requiring either cognitive or affective perspective taking, patients with SSDs exhibit hypoactivation of the mPFC and the TPJ76, and decreased fronto-temporal connectivity77. Evidence suggests that inability to appreciate one’s own and others’ mental states is the single best predictor of poor social competence in SSDs78, which is consistently correlated with the functional disability of patients47. As such, determining the neurobiological etiology of empathetic impairments in SSDs, and using this knowledge as the basis for therapeutic interventions, may improve patient outcomes.
Social incentive and action selection in humans
Neural correlates.
Historically, non-social valuation research (i.e., responding for food or money) has focused on neural computations signaling rewarding properties of the choice options. This research has revealed a framework of brain structures known as the “brain valuation system.” Interestingly, many investigations into the neural correlates of the incentive value of social interactions have found overlap between the areas activated by social and non-social rewards. Meta-analyses comparing anticipation of monetary or social rewards found a consistent overlapping valuation network, including the ventral and dorsal striatum, the amygdala, the insula, and the supplemental motor cortex79 (fig.1). These studies have led many to contend that the brain utilizes a “common currency” to encode and utilize any reward information (social or non-social) to make decisions80, 81. The “common currency valuation” hypothesis suggests that a unified brain system that calculates value is utilized to determine the motivational salience of all stimuli, regardless of social nature (reviewed in82).
Another theory, “social-specific cognition,” asserts that social rewards and values are processed by a dedicated neural circuity that evolved specifically to deal with interactions with others. There are multiple derivations of how social-specific cognition may be encoded neurobiologically. Some contend that two types of neurons – those conveying social and non-social value information – might reside in different brain regions. For example, when subjects make decisions regarding a reward for themselves, the vmPFC is engaged, while making altruistic choices preferentially engages the TPJ83, 84. Social-specific cognition may involve the consistent differential activation of brain regions: although social and monetary rewards both activate the ventral striatum, social stimuli are more associated with amygdala activation whereas monetary rewards were more associated with thalamus activation85. Another possibility is that social-specific cognition may involve the same brain regions, but reside within distinct neuronal population codes82. Consistent with this notion, a recent multivariate pattern analysis identified distinct non-overlapping neuronal representations in the ACC following social rejection or physical pain86. Thus, there is still an open debate as to how social-specific value is encoded in the brain, and how that value guides decisions.
For a social decision to be performed, regardless of whether social or non-social circuitry is distinct or coalesced, putative values for each choice option must be computed, and an action must be selected. To operationalize decision making in a social context in humans, neuroscientists have adopted the tools of Game Theory, which is based on models aiming to identify the optimal choice for interacting agents. Typical examples of prosocial attitudes are investigated in tasks using two or more interacting players. One popular example is the Ultimatum Game, in which two players split a sum of money. The proposer offers a division, and the responder can accept or reject this. The Ultimatum Game thus relies on normative social principles of fairness as motivators of decision making, and involves the activation of the dlPFC and the anterior insula in normal contexts (fig.1), with heightened activity in the anterior insula when the responder rejects unfair offers87. In a modification of the Ultimatum Game, called the Dictator Game, the responder has no power to control the outcome of the bargain. The Dictator Game is thus used to evaluate the proposer only, demonstrating that even without responder input, a dictator will donate 20–30% of their earnings88. Another example, the Trust game, relies on interpersonal reciprocation89. In the Trust game, a first agent, the trustor, is given money and can choose what percentage of it goes to a second agent, the trustee. Before the transfer occurs, it is magnified by a certain factor (i.e., doubled or tripled). Lastly, the trustee can choose to either keep the whole amount or honor the initial sacrifice of the trustor and send a fraction of their earnings back. fMRI studies have exhibited higher mPFC activity when playing with a partner vs. a computer90, and that the TPJ, anterior insula, and ACC were activated by reciprocation of trust91.
Human choice behavior in the Ultimatum or Trust game can be understood using a reinforcement learning (RL) framework82, 92, 93. In RL, social and non-social factors have expected values. Expected values are then used to compute the reward value associated with the outcomes generated by the choice. Lastly, the expected value and the reward value are compared, resulting in a computed reward prediction error (RPE). For example, if the reward value far exceeds the expected value, a positive RPE is signaled in the ventral striatum and dopaminergic midbrain94. Subsequently, this RPE will reinforce the choice that led to it, guiding future decision making; this use of RPEs to guide decisions requires the vmPFC95. Imaging studies have combined variants of the Ultimatum Game with RL to analyze the social influences of decision making: the conversion of social value to a “social RPE” engaged the TPJ, the dlPFC, and the ACC96–99. This social RPE is then converted into a more generalized computational value in the vmPFC that guides decisions97, 98, 100. Thus, although social and non-social influences of decision making might engage different areas of the brain, these processes appear to converge onto the vmPFC before a decision is made. Perhaps unsurprisingly, patients with lesions in the vmPFC demonstrate deficits in outcome valuation-guided decision making in both non-social and social reward, suggesting a potential function in common currency valuation of decision making101.
Alterations in ASDs and SSDs.
In multiple clinical populations, there are specific reductions in the ability of individuals to find social stimuli incentivizing or motivating, typically termed social anhedonia, which is a distinct compared to general, or physical, anhedonia102. For example, the social motivation theory of ASDs posits that disruptions in social motivation constitute a primary alteration in ASDs, ultimately resulting in fewer experiences with social sources of information and decreased social learning103, 104. As discussed previously, very young children with ASDs exhibit decreased orienting to social stimuli105. This lack of social incentive over time decreases chances for social learning, decreasing social competence later in life106. Children with ASDs respond faster for monetary rewards than social ones107. Similarly, patients with SSDs rate genuine smiles as less rewarding than healthy controls, despite showing the same preference for monetary rewards108. Further, levels of social anhedonia in college undergraduates predict the development of later SSDs109, 110, suggesting that social amotivation may be a prodromal symptom of the disorder.
Performance in neuroeconomic games has also been informative in the analysis of patients with ASDs and SSDs89. In patients with ASDs, deficits emerge when subjects serve in the responder, but not proposer, role of the Ultimatum Game111, 112, and when serving in the trustee, but not trustor, role of the Trust Game113. In either game, there is a reduced responsivity to reciprocal cooperation or fairness, but the reasons for this are unclear. There is evidence that children with ASDs exhibit diminished middle cingulate cortex responses while playing the trustee in the Trust Game113, and reduced BOLD responses in regions associated with cognitive perspective taking (i.e., amygdala, insula, TPJ) during a variant of the Trust Game called the Prisoner’s Dilemma114. These findings have led researchers to speculate that alterations observed in neuroeconomic games may be attributable to reductions in cognitive perspective taking and ToM, resulting in the observed reductions in sensitivity to reciprocal fairness89, 112.
Patients with SSDs exhibit a similar deficit in perceived reciprocal fairness: accepting more unfair offers and rejecting more fair offers in the Ultimatum game115–117. In addition, patients with SSDs act less strategically as proposers – making higher offers in the Ultimatum Game – and exhibit less trust than control counterparts116, 118. Patients with SSDs also demonstrate a lack of flexibility or adaptive control in decision making in social contexts, failing to adapt to the availability of contextual information, or their partner’s emotions or performance115, 116. However, the reasons for these deficits are still unclear, and may emerge from a multifactorial combination of reduced social incentive, poor ToM, or poor integration of cognitive and affective information89. Notably, behavioral performance on these tasks correlates with symptom severity: patients with greater social deficits exhibit a higher degree of positive symptom severity119. Thus, a fine-tuned approach is needed to better understand how social decision making could function as an endophenotype for neuropsychiatric disorders.
CAN RODENTS REALLY BE USED TO STUDY SOCIAL DECISION MAKING?
Non-human primates exhibit a robust repertoire of social behaviors with undeniable translational potential120, but a smaller genetic toolbox relative to other experimental systems prohibits in-depth investigation of mechanistic factors in behavior – for example, functional analysis of projections between specific interconnected cortical, striatal, and limbic brain regions likely controlling social/emotional recognition, empathic processes, and social incentive/action selection. Rodents serve as a valuable complementary tool to non-human primates but are often dismissed as viable model organisms for studying complex social behaviors. This notion persists despite an emerging body of literature describing complex social behaviors in rodents, including sophisticated reciprocal interactions with conspecifics121.
Neural processes dedicated to social decision making appear to be remarkably conserved over the course of evolution, from rodents to humans122, 123. Still, how social information is processed in the rodent brain is a subject of debate. An obstacle in understanding the anatomical correlates underlying decision making in the rodent is that several parallel processes occur at once. To make a decision in a social context, a rodent must perceive and prioritize the sensory cues emitted by a conspecific, render those cues as relevant, use those cues to motivate behavior or encode reward, and generate a behavioral response. In this section, we will review how researchers have deconstructed social decision making in the rodent.
Anatomical and functional subdivision of the mPFC in rodents
Anatomical homologies between human and rodent mPFC have historically been a point of contention between researchers (for lively discussion, see18, 124, 125). Studies of functional homologies have been similarly fraught, but certain non-social functions of the human PFC, such as working memory126, behavioral flexibility127, 128, attention129, and impulse control130 have been localized to the rodent PFC. Nevertheless, we will briefly orient the reader to generally agreed upon subdivisions of the rodent mPFC before delving into their putative roles in social behavior. Historically, the rodent mPFC has been subdivided into five subregions along a dorsal-to-ventral axis: the supplementary motor area (M2; putatively homologous to BA6 or BA8, as this region is plagued by confusing nomenclature, for review, see131), the ACC (putatively homologous to BA24), the prelimbic cortex (PL; putatively homologous to BA32), the infralimbic cortex (IL; putatively homologous to BA25), and the medial orbitofrontal cortex (MO; putatively homologous to BA11/BA14) (fig.1)125, 132, 133. All subregions receive projections from adjacent regions of the mPFC, insular and entorhinal cortices, mediodorsal thalamus, amygdala, dorsal raphe, ventral tegmental area (VTA), and locus coeruleus (LC). Major inputs to the dorsal mPFC – M2 and caudal ACC – of rodents include cortical and thalamic sources, while the ventral mPFC – rostral ACC, PL, IL, MO – is more highly innervated by limbic sources and the midline thalamus (for extensive review, see133–135). Recent studies have highlighted antagonistic roles in cognition between the PL and IL in fear conditioning, with PL promoting fear expression while IL promotes fear extinction136. A similar dichotomy has emerged in the context of cortico-striatal control of action selection strategies: activation of PL supports flexible decision making, whereas activation of the IL supports inflexible (or habitual) behavior128, 137, 138.
Historically, studies examining the ACC in rodents have relied on distinguishing the region along a dorso-ventral axis, into the Cg1/Cg2 subregions, where Cg1 encompasses human BA24b and 24b’ while Cg2 encompasses BA24a and 24a’139. This has been observed to not be structurally or functionally homologous to the ACC/midcingulate cortex (MCC) distinction used in humans, which has led to a shift in rodent nomenclature such that the ACC/MCC distinction is made rostro-caudally, with rostral ACC encompassing putatively homologous parts of human BA24a-b, 25, and 32, whereas the caudal MCC is comparatively uniform and as such does not contain subdivision139. The rostral ACC shares reciprocal connections with the BLA, and dopaminergic centers, which may underlie functions in working memory and decision making140.
Social and emotional recognition in rodents
Tasks to test social recognition.
Perhaps the largest limitation in studying rodent social perception, from a translational perspective, is that the most salient sensory cues for the rodent differ significantly from that of humans141. In contrast to visual cues leading to neurobiological demands such as facial processing by the FFA, rodents rely much more heavily on the olfactory signatures and chemosensory cues emerging from social stimuli142 143. This is not to say that visual cues play no role in social recognition in rodents, as recent evidence suggests that integration of multiple sensory modalities is necessary for proper social recognition144, 145.
Many tasks have been developed to better understand social recognition in rodents (table 1). The first of which, the habituation/dishabituation task, was introduced in 1982 in rats146 (and subsequently adapted for use in mice147). In this task, the experimental subject is repeatedly exposed to an unfamiliar stimulus animal in a series of habituation sessions, interleaved by intervals in which the experimental animal is left in its home cage. Direct sniffing of, and interaction with, the stimulus animal is recorded, and a typical subject will decrease social investigative responses with repeated sessions. In the last phase of the task, termed “dishabituation,” a novel unfamiliar animal is introduced, and a typical subject will reinstate high levels of social investigation. This test is robust, but it does not control for any preference for novelty that may guide behavior in the last phase. Further, it has a limited ability to differentiate between short-term and long-term memory148.
Table 1.
Tasks used to investigate components of social information processing in the rodent.
| Behavioral Task | Species | Experimental Outcomes | Citation |
|---|---|---|---|
| Social recognition | |||
| Habituation/dishabituation task | Long-Evans rats, CD1, C57BL, DBA/2 mice |
|
146, 147 |
| Social recognition test/social novelty test | Wistar rats, CD1, C57BL, DBA/2 mice |
|
148, 149 |
| Odor block test | Mixed C57/129SvJ, C57BL/6J mice |
|
151 |
| Partner preference test | Prairie voles |
|
153 |
| Social hierarchies | A/alb, 03H, DBA/8 mice |
|
157 |
| Emotional recognition | |||
| Social affective preference test (SAP) Affective state discrimination task (ADT) | Sprague-Dawley Rats C57BL/6J mice |
|
187
186 |
| Empathic like processes - emotional contagion | |||
| Emotional reactions to the pain of others | Sprague-Dawley Rats |
|
176 |
| Social modulation of learning | Wistar rats |
|
247 |
| Vicarious freezing | C57BL/6J mice |
|
179, 192 |
| Fear conditioning-by-proxy (FCbP) | Sprague-Dawley rats |
|
183 |
| Social harm aversion | Sprague-Dawley rats |
|
184 |
| Empathic-like processes - more advanced | |||
| Rescue behavior | Sprague-Dawley rats |
|
188 |
| Consolation-like behavior | Prairie voles |
|
189 |
| Social valuation and action selection - approach behaviors | |||
| Social approach/social proximity | Hooded rats, BTBR T+ tf/J, Swiss albino, mice |
|
150, 200, 248, 249 |
| Three-chamber test | C57BL/6J, DBA/2J, FVB/NJ, and B6129PR2/J mice |
|
201 |
| Social preference/avoidance test | C57BL/6J mice |
|
250 |
| T-maze learning | Lister hooded rats |
|
202 |
| Social conditioned place preference (sCPP) | N:NIH rats |
|
206 |
| Transmission of food safety preference | Blue Spruce rats |
|
212 |
| Incentive value of social interaction and action selection - operant behaviors | |||
| Operant conditioning for social play | Wistar rats |
|
215 |
| Operant social preference (OSP) | Syrian hamsters |
|
219 |
| Volitional Social Interaction | Sprague-Dawley rats |
|
220 |
| Social incentivization of future choice (SIFC) | C57BL/6J mice |
|
221 |
| Social Decision Making (SDM) | C57BL/6J mice |
|
222 |
Each row describes a task. Multiple citations are included if validated in different model organisms. Tasks designed to examine social stress response, aggressive behaviors, or mating behaviors are not discussed in this review.
These limitations led to the development of the social discrimination task, perhaps the most widely used test of social recognition in rats and mice148, 149. This procedure consists of two sessions. In the first, a novel stimulus animal is introduced to the cage of the experimental animal, allowing for the acquisition of its olfactory signature. In the second, separated by any desired time interval, two stimulus animals, one familiar and one unfamiliar, are introduced at the same time to the experimental subject’s home cage. A typical experimental animal will spend more time with the unfamiliar stimulus animal than the familiar stimulus animal. This procedure is typically preferred over the habituation/dishabituation task as it can be used to test short-term memory or long-term memory, given that the period between the two sessions can be short or long.
Since the introduction of the social discrimination task, variants have been introduced in which the stimulus animals are confined in wire cups, or a three-chamber apparatus is used, called the social novelty test or social choice test149, 150. These variants limit the ecological validity of the test because the stimulus animals are constrained, but they reduce the complexity of the social interactions and are considered decent substitutes for more naturalistic approaches if the experimental subject can fully smell and interact with the olfactory signatures of the familiar and unfamiliar animals. The olfactory information emitted by a conspecific does appear critical for social recognition and approach in mice and rats, as tested in the odor block test. In this variant, an experimental animal is presented two wooden blocks: one scented with its own bedding or that of a conspecific. A typical experimental animal will prefer to engage with the block scented with the odor of the conspecific (table 1;151).
Other notable tests of social recognition include the partner preference test, which has been used in monogamous prairie voles152, 153. These monogamous voles, once they have formed a pair bond, will spend significantly more time with their partners than with strangers154. Also notable is the spontaneous formation of social hierarchies within colonies of mice155 and (although less aggressive and territorial) rats156, thought to maintain order and improve mating chances of community members with higher rank. Social hierarchies are typically determined using the tube test, whereby two mice are placed in opposing directions at either end of a narrow tube; the mouse that successfully pushes the other mouse out of its way is considered the successor and more dominant within a hierarchy157, 158. These dominant-subordinate relationships can be transmitted through olfactory signatures and appear to be regulated by prefrontal cortical circuits. Several studies have also investigated social recognition through the measurement of ultrasonic vocalization (reviewed in159).
Neural correlates.
For the purposes of this review, we will focus on one stream of sensory information – olfactory – as it reaches higher order processing areas responsible for social recognition (for a review of multisensory integration in social decisions, see145). The neural circuitry underlying olfactory social recognition in rodents begins in the accessory olfactory bulb (AOB; fig.1). The AOB houses a glomerular map for both the main olfactory epithelium (MOE), which detects olfactory signals, and the vomeronasal organ (VNO), which detects chemosensory signals. The MOE and VNO are both crucial for a wide variety of social behaviors in rodents160. For instance, ablation of the MOE or VNO decreases sexual behaviors in both sexes of mice161, 162.
From the AOB, projections reach the cortex or hypothalamus, while VNO outputs converge on the medial amygdala, which acts as a major site for the integration of olfactory output163. The medial amygdala subsequently activates following exposure to chemosensory stimuli, and blocking medial amygdala activity impairs social recognition memory in mice164. The entorhinal cortex, which receives inputs from the perirhinal cortex, the amygdala, the thalamus, and the hypothalamus, acts as a gateway to the hippocampus, as well as its primary output. Lesions of the entorhinal cortex cause deficits in short-term odor memory165. In contrast, the neighboring perirhinal cortex, which receives sensory information from the olfactory cortices, is involved in the social recognition of hamsters, and appears to be required for the long-term storage of olfactory signals166.
Surprisingly, initial studies in which lesions were placed in the hippocampus did not document impairments in social recognition memory167, 168. Subsequently, inactivation of the hippocampal CA2 subregion resulted in a pronounced loss of social memory with no effect on general sociability169, 170. Investigations of the CA1 region of the hippocampus, and its projections to the nucleus accumbens (NAc), documented higher neuronal activity in response to a familiar mouse relative to an unfamiliar mouse. In a so-called “social memory inception” protocol, activity-dependent labeling was used to “trap” CA1 neuronal ensembles during a novel experience with a stimulus mouse. Experimenters then optogenetically re-activated these CA1 ensembles previously activated by this stimulus mouse during a foot shock or cocaine exposure. Such experiences created “fake social memories” in the experimental mouse, causing avoidance or approach of the stimulus mouse in a subsequent social discrimination task, despite not having interacted with the stimulus mouse since that original contact171. Thus, the hippocampus appears to store manipulable ensembles encoding distinct social memories, or “engrams,” specific to certain individual social experiences (fig.1).
The consolidation of memory with social content increases protein synthesis and immediate early gene (IEG) activity in the basolateral amygdala (BLA), the mPFC and ACC, and the hippocampus, revealing the involvement of amygdalo-cortical structures in social recognition172. Chemogenetic excitation or inhibition of ventral hippocampal projections to the mPFC impairs social recognition173, and silencing of PL efferents to either the BLA or NAc can also reduce social recognition, highlighting an important role of the mPFC in this process, although more studies are needed to clarify its contributions174, 175.
Empathic-like processes in rodents
Tasks to test empathic-like processes in rodents.
Some of the first evidence that rodents were capable of emotional contagion came from an experiment demonstrating that rats, trained in a lever-press task to receive food, inhibited this behavior when viewing a shocked rat176 (table 1; for a review on rodent models of empathy, see177). The authors speculated that this phenomenon reflected emotional state matching between individual rodents. The most studied type of emotional contagion in rodents is called vicarious freezing, which is represented by a freezing response in a rat witnessing a conspecific being shocked and displaying fear responses178, 179. Mice also exhibit vicarious freezing responses180, and recent elegant studies demonstrated that emotional contagion in mice extends beyond fear, to feelings of pain and analgesia181.
Several other procedures provide evidence that rodents can use emotions, fear in particular, to socially transmit information182. In vicarious learning procedures, such as fear conditioning by proxy, a rat exposed to a novel tone in the presence of a cage-mate previously conditioned to that same tone will freeze183. In another variation of this type of procedure, termed social harm aversion184, rats will inhibit a behavior if this behavior is associated with harm to others. This phenomenon is bidirectional, in that the frequency of a behavior will increase if the behavior is associated with rewards to others185.
Two tasks have been recently developed specifically to test the ability of rodents to recognize the emotional states of conspecifics, and thereby guide behavior (table 1). The first of these tests is the affective state discrimination task (ADT). ADT allows for discrimination based on positive affective state, as an experimental mouse will prefer to investigate a “relieved” demonstrator – a conspecific that just received water after 24 hours of water deprivation – over a control mouse. ADT can also test detection of a negative affective state, as an experimental mouse will prefer to spend time investigating a “stressed” control mouse over a neutral demonstrator186. In an alternative to ADT, the social affective preference (SAP) test, adult rats will approach a stressed juvenile, but avoid a stressed conspecific adult187. Thus, the ADT and SAP provide avenues by which to investigate the neural correlates of emotional recognition and discrimination in rodents.
If we apply de Waal’s multi-level theory of empathy, all of the behaviors described so far in this section can be explained through variants of emotional contagion, the “lowest common denominator” of empathic-like processes55. To anthropomorphize, subjects could simply be performing a behavior to reduce the stress they feel by having to watch another in stress. According to de Waal, the next evolutionary step occurs when emotional contagion is combined with an understanding of the other’s situation and attempts to understand the cause of the other’s emotions. Two elegant procedures recently demonstrated evidence of this more advanced level of empathy in rodents. This first describes rescue behavior, in which a free-roaming rats exhibit motivation to liberate a caged conspecific188. When given the choice between chocolate and a caged conspecific, the subject will choose to liberate at the same latency as retrieving a food reward, and will subsequently share the food reward with the recently freed conspecific188.
Another study in prairie voles demonstrated consolation-like behavior189. In this protocol, two prairie voles were given the opportunity to form a pair-bond prior to being separated. One of the two (the demonstrator) would receive foot shocks, while the other (the observer) would remain in the home cage. Following their reunion, the observer would greatly increase allogrooming behaviors towards the demonstrator, in comparison to a variant in which the demonstrator did not receive any shocks. No increase in grooming behaviors was observed when the demonstrator was an unfamiliar vole. Strikingly, the observer would continue this behavior, alleviating the plasma corticosterone levels of the demonstrator, apparently even at the cost of raising their own stress, as observers had significantly elevated plasma corticosterone following the interaction. These studies problematize the notion that rodents are not capable of more nuanced empathic-like behaviors and demonstrate the importance of generating tasks aimed at better understanding them (table 1).
Neural correlates.
Rodent studies have consistently connected empathic-like behaviors to ACC and prefrontal cortical function (table 2). The ACC is a hub of “emotional mirror neurons” in rodents, which are activated both by the self-experience of pain and witnessing pain in another190, 191. Inactivation of the ACC reduces emotional contagion, causing observers to freeze less while witnessing a demonstrator receiving foot shocks192, and vicarious freezing, causing observers to freeze less in the environment in which they had witnessed the demonstrator receiving foot shocks190. Meanwhile, consolation behavior in prairie voles requires oxytocinergic tone in the ACC189. Further, emotional discrimination in mice requires somatostatin-expressing interneurons, but not parvalbumin-expressing interneurons, in the mPFC186.
Table 2.
Prefrontal cortical control of social information processing in rodents.
| Species, Strain | Sex | Region | Stereotactic Coordinates | Manipulation or Measure | Findings | Cit. | ||
|---|---|---|---|---|---|---|---|---|
| A/P (+) | M/L (±) | D/V (−) | ||||||
| Social recognition and memory | ||||||||
| Lister hooded rats | M | ACC, OFC | 2.3–0.9 4.0–3.2 |
0.5 2.6–0.8 |
0.2–2.0 4.4–3.4 |
lesion lesion |
↓ social memory = social memory |
251 |
| C57BL/6J mice | M | mPFC, ACC | 2.10–1.98 0.8–1.0 |
-- | -- | IEG analysis | ↑ c-Fos and Arc | 172 |
| C57BL/6J mice | M | PFC | 2.25 | 0.45 | 1.4 | ↑ OXTR+ neurons, PL→BLA OXTR+ neurons |
↓ social recognition | 174 |
| C57BL/6J mice | M | IL | 1.97 | 0.3 | 3.0 | In vivo recording | ↑social olfactory
cues OR ↑non-social olfactory cues with experience-dependent sharpening of representations |
229 |
| C57BL/6J mice | M | mPFC | 1.45 | 0.5 | 1.45 | ↑↓vHC→mPFC | ↓↓ social recognition | 173 |
| C57BL/6J mice | M | PL | 1.9 | 0.3 | 2.3 | ↓vHC→mPFC (PV interneurons) | ↓ social recognition | 252 |
| C57BL/6J mice | M | PL | 1.9 | 0.25 | 2.0 | ↓ PL→NAc | ↓ social recognition | 175 |
| C57BL/6NHs d mice | M | IL | 2.4 (10°) | 1.0 (10°) | 2.3 | IEG analysis of IL→NAc ↓ IL→NAc |
↑ social recognition ↓ social recognition |
253 |
| Emotional recognition | ||||||||
| C57BL/6J mice | M, F | mPFC | 1.9 | 0.3 | 2.4 | In vivo recording ↓ of SST+ interneurons | ↑ in PL activity during
ADT ↓ ADT |
186 |
| Empathic-like processes | ||||||||
| Long-Evans rats | M | PL/IL | 2.7–3.2 | -- | -- | IEG analysis | ↑ c-Fos in PL/IL in subjects paired with shocked demonstrators | 254 |
| Sprague-Dawley rats | M | ACC | 1.8 (18°) | 1.6 (18 °) | 1.6 | IEG analysis ↓ACC | ↑ FCbP ↓ FCbP |
255 |
| Long Evans rats | M | ACC | 1.7 (20°) | 1.6 (20°) | 1.8 | in vivo recording ↓ACC | ↑ unit activity when experiencing pain
or observing another in pain ↓ OFL to observation of pain experience or associated CS+ |
190 |
| Sprague-Dawley rats | M | ACC | 1.35 | 0.6 | 2.4 | fMRI scanning
↓ACC ↓↑ACC→MDL |
↑ ACC activity ↓ OFL ↓↓ OFL |
256 |
| Sprague-Dawley rats | M,F | ACC | 1.17 (20°) | 1.16 (20°) | 1.8 | ↓ACC | ↓ social harm aversion | 184 |
| C57BL/6J mice | M | ACC | 1.0 | 0.2 | 1.2 | ↓ACC | ↓ OFL | 192 |
| C57BL/6J mice | M | ACC | 1.0 | 0.3 | 1.2 | ↓↑rACC ↓↑IACC |
↓↑ OFL == OFL |
257 |
| C57BL/6J mice | M | ACC | 1.0 1.0 |
0.3 0.25 |
2.1 2.1 |
In vivo recording ↓ACC→BLA | ↑ activity ACC→BLA during
OFL ↓ acquisition, but not expression, of cue-induced OFL |
193 |
| C57BL/6J mice | M | ACC | 1.0 | 0.3 | 1.5 | ↓↑ SST+
interneurons ↓ PV+ interneurons |
↑↓ OFL = OFL |
258 |
| C57BL/6J mice | M, F | ACC | 0.98 | 0.278 | 0.78 | IEG analysis ↓ACC, ACC neurons
”trapped” during social
interaction ↓↑ACC→NAc ↓ACC ; ↓ACC→NAc ↓ACC→BLA ↓ACC→BLA |
↑ACC→NAc by social transfer of
pain ↓ social transfer of pain ↓↑ social transfer of pain ↓ social transfer of analgesia = social transfer of pain ↓ retrieval of context-induced OFL |
181 |
| C57BL/6J mice | M | mPFC | 0.86–1.78 | 0.3 | 2.0 | In vivo recording from single neurons | ↑ self representation ↑ other representation |
259 |
| Prairie Voles | M,F | ACC PL |
1.4 2.4 |
0.8 0.8 |
1.3 2.2 |
IEG analysis OT Ø in ACC IEG analysis OT Ø in PL |
↑ during consolation ↓ during consolation = consolation = consolation |
189 |
| Sociability, social approach, incentive value of social interaction | ||||||||
| Lister hooded rats | M | ACC, OFC | 2.3–0.9 4.0–3.2 |
0.5 2.6–0.8 |
0.2–2.0 4.4–3.4 |
lesion (excitotoxic) lesion (excitotoxic) |
↓ social interaction = social interaction |
251 |
| Sprague-Dawley rats | M | PL/IL | 2.9 | 0.5 | 2.9 | inactivation (muscimol) | = social interaction | 260 |
| Sprague-Dawley rats | M | mPFC | 3.0 | 0.8 | 2.5 | In vivo recording | ↑social interaction
(32%) ↓social interaction (8%) =social interaction (60%) |
261 |
| Sprague-Dawley rats/Long-Evans rats | M,F | IL | 2.7 | 0.5 | 5.0 | § ↑excitability | ↓ social interaction | 262 |
| C57BL6/J mice | M | PL, IL | 1.8 | 0.35 | 2.85 | §↑↓ excitability | ↓= social interaction | 223 |
| C57BL/6J mice | M | PL | 1.9–2.8. | 0.3–0.4 | 2.5–1.5 | lesion IEG analysis |
↑ social interaction ↑ c-Fos during social interaction |
263 |
| C57BL/6J mice | M | mPFC | 1.7 | 0.3 | 1.8–2.5 | In vivo recording | ↑ firing during social approach behavior | 180 |
| C57BL/6J mice | M | mPFC | 1.7 | 0.3 0.9 (10 °) |
1.9 2.1 (10°) |
↑↓ BLA→PL | ↓↑ social interaction | 230 |
| C57BL/6J mice | M,F | PL | 1.8 | 0.5 | 2.5 | ↑↓ PL→NAc | ↓= social interaction | 224 |
| C57BL/6J mice | M | PL | 1.9 | 0.3 | 1.7 |
In vivo
recording In vivo recording |
↑or↓ during social approach (ON
and OFF ensembles) ↑to novel stimulus (ON ensembles sensitive to novelty) |
264 |
| C57BL/6J mice | M | ACC | 1.0 | 0.25 | 2.1 | ↓↑
ACC→BLA ↓ BLA→ACC |
↓= social interaction = social interaction |
193 |
| C57BL/6J mice | M | OFC | 2.6 | 1.26 | 2.8 |
In vivo
recording ↑”trapped” social ensembles ↑”trapped” feeding ensembles |
↑social behavior ↑feeding behavior ↓feeding behavior ↑feeding behavior |
239 |
| C57BL/6J mice | M | IL | 1.97 | 0.3 | 3.0 | In vivo recording | ↑activity to social odors | 229 |
| C57BL/6J mice | M | ACC | 1.0 | 0.35 | 2.0 | ↑ACC | ↑social interaction | 265 |
| C57BL/6J mice | M | dmPFC/PL | 2.0 | 0.3 | 1.8 | In vivo calcium imaging | Interbrain activity correlations of interacting mice | 228 |
| C57BL/6J mice | M,F | mPFC | 1.9 | 0.3 | 2.4 | ↓ PV+ interneurons | ↓ social interaction | 186 |
| C57BL6/J mice | M, F | dmPFC | 2.0 | 0.3 | 1.8 | In vivo calcium imaging | Single neurons selectively responsive to conspecific sex | 266 |
| C57BL6/J mice | F | PL IL |
2.2 2.2 |
0.4 0.4 |
2.1 2.8 |
↑↓PL→BLA ↑↓IL→BLA ↑”trapped” PL→BLA during footshock |
↓=social
interaction =↓social interaction ↓ social interaction |
267 |
| C57BL6/J mice | M | mPFC | 1.95 | 0.3 | 2.1 | In vivo calcium imaging of mPFC→VTA neurons | ↑social interaction | 225 |
| C57BL6/J mice | M, F | PL | 1.9 | 0.4 | 2.0–2.2 |
In vivo calcium imaging of
PL→ZIm neurons ↓PL→ZIm |
↑social
interaction ↓social interaction |
226 |
| C57BL6/J mice | M | mPFC | 1.3–2.3 | 0.4 | 1.0–1.7 | ↑↓mPFC→PVT | ↑↓social interaction | 227 |
| C57BL6/J mice | F | PL OFC |
1.7 2.6 |
0.17 1.2 |
2.5 2.8 |
↓BLA→PL, ↓PL→NAc ↓PL ↓BLA→PL, ↓PL→NAc ↓OFC |
=social
interaction ↓SIFC ↓SIFC = SIFC |
221 |
| C57BL6/J mice | M, F | PL | 2.0 | 0.25 | 2.4 | ↓BLA→PL ↓PL→BLA |
↓”altruistic”
choices ↓”selfish” choices |
222 |
“Region” is indicated as per author nomenclature, given the lack of coherence in prefrontal cortical nomenclature in the field125. Stereotactic coordinates are expressed in mm from lambda, ranges are provided for larger lesions or multiple infusions. For the Manipulation column, in vivo recording indicates calcium imaging or electrophysiological recording, ↑ indicates optogenetic or chemogenetic activation, ↓ indicates optogenetic or chemogenetic inhibition, Ø indicates the use of a pharmacological antagonist, § indicates the use of an SSFO to manipulate excitability of local cells223. For Findings column, ↑ indicates increase in measure, ↓ indicates decrease in measure, = indicates no change in measure. Tasks examining social stress response, aggressive behaviors, or mating behaviors are not discussed in this review. Specific protein knockouts, developmental insults, and models for disease are not included. Abbreviations: affective discrimination test (ADT), anterior cingulate cortex (ACC), basolateral amygdala (BLA), dorsomedial prefrontal cortex (dmPFC), fear conditioning-by-proxy (FCbP), immediate early gene (IEG), immunohistochemistry (IHC), infralimbic subregion of medial prefrontal cortex (IL), left anterior cingulate cortex (lACC), medial prefrontal cortex (mPFC), medial zona incerta (ZIm), observational fear learning (OFL), orbitofrontal cortex (OFC), oxytocin (OT), nucleus accumbens (NAc), paraventricular thalamus (PVT), parvalbumin (PV), prelimbic subregion of medial prefrontal cortex (PL), right anterior cingulate cortex (rACC), somatostatin (SST), ventral tegmental area (VTA).
An understanding of the neural network responsible for empathic-like behaviors in rodents is starting to emerge (fig.1). Projections from the ACC to the BLA are necessary for observational fear conditioning181, 193, while projections from the ACC to the NAc are necessary for the social transmission of pain181. The insular cortex, a site of multisensory integration with outputs to limbic, reward, and executive systems (reviewed in145), is implicated in emotional contagion. Inactivation of the insular cortex reduces the pain response induced by cohabitating a subject with a conspecific experiencing chronic pain194. Also, pharmacological or chemogenetic inactivation of the anterior insula leads to diminished helping behavior in a rat model of targeted helping195. Further, parts of the amygdaloid complex are involved in emotional contagion: inactivation of the lateral and medial amygdala reduces emotional contagion196. Lesions of the BLA reduce a rat’s preference for actions that reward themselves and another rat, over those that just reward themselves197.
Social incentive and action selection in rodents
Tasks to evaluate social incentive and action selection.
Rodents are incredibly social creatures, with maternal contact, social play behavior, and sexual behavior being the most intensively researched forms of pro-social interactions in rats and mice (reviewed in198, 199). Some of the most common assays of rodent social behavior are derivations of the social approach test, which was first developed as an assay of anxiety-like behavior in 1978200 (table 1). Modern variants detect an experimental subject’s preference for investigating a novel conspecific compared to a novel object, typically in a three-chamber apparatus similar to the one used for the social discrimination task described above201. This task defines “sociability” as the propensity to spend time with another animal, as opposed to time spent in an identical but empty chamber. In a variation of this social approach task, a T-maze is used, and subjects will choose to spend time with a social stimulus over a non-social stimulus. Mice and rats will retrieve pups or find a mate in a T-maze198, and adolescent rats will prefer one end of the “T” over another to participate in social play202.
One limitation of social approach tasks is that “sociability” is a relatively abstract concept, as it is unclear if an individual actually values the social experience, or simply finds it more stimulating than an empty chamber. Dissecting the “value” of social interaction (or, in an RL framework, the expected value of a social experience used to compute a generalized common effective state to guide decisions) is inherently trickier. Historically, a way to infer the “rewarding” properties of social interaction in rodents was borrowed from the drug addiction literature: conditioned place preference (CPP)203. CPP uses Pavlovian conditioning principles whereby neutral environmental cues gain salience after being repeatedly paired with a rewarding stimulus. In this procedure, the rewarding event serves as the unconditioned stimulus (US), while the neutral environment functions as the conditioned stimulus (CS). Thus, a rodent will choose to spend more time in the previously neutral environment following CS-US pairings. Postpartum rat dams deprived of their pups, but not virgin females, exhibit social CPP (sCPP) when one chamber is paired with reunion with pups204, and this effect is dependent on the olfactory signature of the pups205. sCPP has also been demonstrated in adolescent rats when one chamber is paired with social play206, an age-matched conspecific207, or aggression-related reward behavior208. sCPP has been adapted for use in mice209, but with varying degrees of success, as the effect has been found to be more spurious210.
Along the lines of sCPP, researchers have attempted to compute the value of a social interaction by showing how it influences a decision and/or elicits a behavior. Most times, that social interaction is either an affiliative or aggressive interaction8. But other times, rodents must take information from a social experience and use it to guide a non-social decision. For example, rodents use information from others to learn where, what, how, and even when to eat211. In the social transmission of food preference task, the olfactory cues from the breath of conspecific mice and rats can induce food preferences that even overrule innate preferences212 (table 1). In this task, the olfactory cues from social investigation provide information regarding safety and palatability and thus will alter the decision to consume a typically avoided novel food.
Another form of ascertaining the reinforcing properties of social interaction on behavior in rodents is to see if they will perform an action to obtain a social interaction, which can be assayed through operant conditioning. Rats will lever-press to retrieve pups213, 214, and male rats will lever-press for the opportunity to play with a juvenile conspecific215, to gain access to a sexually receptive female216, or to act aggressively217, 218.
Another operant-based task, deemed Operant social preference (OSP), has been validated in adult male Syrian hamsters219. The apparatus in OSP consists of a main chamber with two small adjacent chambers, separated from the main chamber by a one-way vertical-swing door. The vertical swing door could be attached to weights, increasing the amount of effort needed to enter one of the side chambers. When tested, animals entered the chamber containing a conspecific significantly more than an empty chamber, and the motivational effort to obtain social interaction and food were similar219. A limitation of OSP is that hamsters are not asked to choose between different rewards; they are instead either entering a chamber with a novel stimulus hamster or food, as compared to an empty chamber. This limitation was addressed by Venniro and colleagues (2018), who developed a procedure in which rats could lever press for access to a novel conspecific, and strikingly, demonstrated that rats chronically exposed to cocaine or methamphetamine would still prefer to lever press for social interaction over drug exposure220. This report was particularly notable for introducing discrete choice at the time of tests: rats could choose social interaction or drug exposure and consistently chose social interaction over cocaine or methamphetamine220. This study demonstrates the intensely reinforcing nature of social interaction in rats.
In contrast to investigations in rats, the current repertoire of tasks validated to investigate social influences on non-social behaviors and action selection strategies in the mouse is limited (table 1). To add to this repertoire, our group devised a new task, social incentivization of future choice (SIFC)221. In SIFC, a female mouse is trained to nose poke on two distinct nose poke apertures for two distinct food rewards, either grain or chocolate. Once trained, the mice undergo social conditioning, whereby one of those two pellets is paired with a social experience with a novel conspecific, while the other is paired with a novel object. This social experience is sufficient to incentivize responding on the nose poke aperture predictive of the “social” pellet221. Said another way, the value of the social experience may be transferred to an external reward, biasing responses towards that pellet. SIFC is pervasive, expressed after varying social experiences, even presumably aversive ones, such as a social experience with a shocked conspecific or a sexually experienced unknown male. Our interpretation is that the opportunity to gain novel social information, writ large, is adaptively valuable, and that SIFC reflects that value, rather than strictly the affiliative properties of the social interaction itself. We find that SIFC cannot be attributable to olfactory or warmth cues alone and does not rely on any single behavior (e.g., anogenital sniffing) exhibited during social conditioning. As such, SIFC provides a new tool to examine the influence of social behavior on instrumental action in mice221. We imagine that it could have utility in rats, as well, given that rats can readily perform all aspects of this task. Further, this SIFC task was directly inspired by the classical operant conditioning assay termed Pavlovian-to-instrumental transfer (PIT), which has historically been tested in rats221.
Scheggia and colleagues also recently developed a mouse version of the Ultimatum/Dictator’s Game, deemed Social Decision Making (SDM), to analyze social influences of operant choices. Using a modified instrumental conditioning chamber with a connected chamber and magazine for a cagemate conspecific, mice could either act to gain a food reward for themselves (e.g., act in a selfish manner), or act to also provide a food reward for their neighbor (e.g., act in an altruistic manner)222. Interestingly, male mice were more likely to act altruistically than female mice, and altruism-like behavior varied based on the hunger state, familiarity, and hierarchical status of the neighbor222. Further, mice particularly susceptible to observational fear learning were more likely to act in an altruistic manner, suggesting a connection between emotional contagion and “altruism.” Thus, prosocial and “altruistic” behaviors are being identified and elucidated in species previously believed too primitive to perform such actions, mainly due to nuanced behavioral paradigms that lend themselves to larger scale rodent investigations. Future studies using these paradigms should aim to better understand the mechanisms underlying and evolution of altruism and prosociality in rodents.
Neural correlates.
How the incentive value of social interaction is encoded in the brain of rodents has only recently come more into focus (table 2; fig.1). In 2011, Yizhar and colleagues used a fine-tuned set of stable step-function opsins (SSFOs) to activate or inhibit excitatory or inhibitory neurons in the PL and IL, thereby disrupting the excitatory/inhibitory (E/I) balance. Elevation of the E/I balance in the mPFC through excitatory activation disrupted social interaction, an effect that could be partially ameliorated by increased inhibitory activation223. It was subsequently demonstrated that a subset of mPFC neurons elevates in discharge rates when an experimental subject approaches a stranger mouse but not an empty cup, suggestive of a role for the mPFC in social approach180. Recent real-time investigations have established that projections to the NAc224, to the VTA225, to the medial zona incerta (ZIm)226, and to the paraventricular thalamus (PVT)227 are among those active during social interaction. In a remarkable study, microendoscopic calcium imaging was used to monitor activity from hundreds of dmPFC neurons of two interacting mice in an open arena228. Kingsbury and colleagues computed aggregate signals of dmPFC activity to compare the “interbrain synchrony” between these two mice. Strikingly, much higher levels of interbrain synchrony were observed during social behaviors compared to non-social behaviors. Further, interbrain synchrony depended on cells responsive to specific social behaviors in the subject, as well as cells responsive to specific social behaviors in their partner228.
Rodent studies examining sociability identify a functional network of connectivity surrounding the PFC. Firstly, many studies have established a circuitry including amygdalo-cortical projections, and cortico-striatal projections in typical social interactions. Excitation and inhibition of BLA→PFC projections can decrease or increase social interaction, respectively, similar to the bidirectional control of these projections in anxiety-like behavior229, 230. Remarkably, PL→NAc projections can encode socio-spatial associations in a modified three-chamber task, meaning that a large proportion of PL→NAc neuronal ensembles preferentially fire in response to a social target in one spatial location (either the right or left side of the chamber)224. Further, Murugan and colleagues (2017) showed in a modified sCPP task that inhibiting PL→NAc ensembles can inhibit the development of sCPP, and, when activated, can enhance sCPP. The involvement of the NAc in sociability is perhaps unsurprising, as oxytocinergic tone in the NAc is required for intact sociability231 and inactivation of the NAc in rats abolishes approach toward stressed juveniles, but not avoidance of stressed adults, in the SAP task232. In prairie voles, in vivo miniscope imaging has revealed that distinct ensembles in the core of the NAc increase in firing during approach towards partners, but not novel conspecifics233.
New tasks investigating social influences of action have centered on the PL and its connections with subcortical structures. In SIFC, the PL is necessary for rodents to motivate instrumental responding based on previous social experiences, but not the neighboring orbitofrontal cortex (OFC)221. In contrast, classical non-social PIT requires the OFC but not the PL234 235, suggestive of unique recruitment of PL circuits in decision-making behavior involving social content. Further, BLA→PL→NAc circuitry is required for social experiences to incentivize instrumental responding in SIFC, and immediate-early gene levels in the BLA predict the degree of SIFC expressed221. In SDM, BLA→PL connections mediate “altruistic” behavior in mice, while projections from the PL→BLA mediate “selfish” choices, suggesting dissociable circuitry underlying the decision to help a conspecific222. As BLA→PL projections appear to be necessary for social memories to guide future choices, and for mice to act “altruistically,” future studies should aim to understand the time course of BLA→PL ensembles in social decision making, and what molecular mediators within the BLA may underly its ability to signal the social salience necessary to guide actions.
Regions outside amygdalo-cortical-striatal circuits also of course exert control over rodent sociability. Social interactions in the rodent activate dopaminergic cells in the ventral tegmental area (VTA), which densely project onto the NAc236. Further, optogenetic activation of VTA-NAc cells facilitates social approach, whereas inhibition decreases it236. The insular cortex is similarly emerging as an area necessary for rodent social behavior (reviewed in145). In the SAP procedure, chemogenetic stimulation of insular cortical projections to the NAc increased approach towards stressed juveniles while inhibition reduced this behavior187. The volitional social interaction model shown to be protective against addictive drug seeking described above relies on activation of central amygdala inhibitory neurons and inhibition of anterior insular cortex activity220. Importantly, the mPFC, NAc, VTA, and amygdala have all been implicated in non-social reward systems as well237.
Some evidence suggests that amygdalo-cortical circuits might encode social and asocial behaviors in an antagonistic manner. For example, an inhibitory GABAergic neuronal ensemble in the MeA promotes aggression and mating behaviors, while a glutamatergic population promotes repetitive self-grooming. Moreover, this glutamatergic subpopulation inhibits social interactions independently of its ability to promote self-grooming, while the GABAergic subpopulation inhibits self-grooming238. In the OFC, distinct neuronal ensembles fire in response to food or social stimuli. While activity of food-sensitive neuronal ensembles facilitated feeding behavior, the activity of socially-selective neuronal ensembles exerted inhibitory control over feeding behavior239. In both cases, activity in neuronal ensembles required for a social behavior inhibited a non-social behavior, suggesting that social and non-social behaviors might be regulated to some extent in an antagonistic manner in some regions of the brain. However, future studies will be needed to better understand the divergent and convergent processes the rodent brain uses to evaluate and act upon social vs. non-social information.
Next steps: translational studies aimed at understanding social decision making
In both humans and rodents, the dissection of social decisions into their component parts – social/emotional recognition, empathic processes, social incentive/action selection – has allowed researchers to investigate the underlying brain regions, circuits, and neurochemical events necessary for incredibly complex social behaviors to occur. Human subjects-focused work can capture the nuanced interpersonal landscape contained within the human social world and can translate experiments to clinical populations such as individuals with ASDs and SSDs, for which animal models are limited. Meanwhile, rodent-focused work can function at sub-second timescales, using novel genetic approaches to better understand circuit function at a resolution currently untenable in human studies. Further, hypotheses concerning specific genetic and/or biochemical mechanisms can be empirically tested. The concomitant use of non-human primates in social neuroscience research (which has not been discussed in the current review, but see5) may also be imperative to better understanding complex social cognitive processes, given the ever-growing toolbox allowing for genetic access to specific cell types and circuits in these sophisticated organisms240.
Future investigations into social decision making would do well to integrate cross-species research strategies to improve mechanistic and translatable knowledge, hopefully to ultimately improve treatment for illness. In one recent report, humans and mice with less bioavailable Brain-derived Neurotrophic Factor (BDNF) appeared to detect negative social information in experiences that control organisms deemed neutral. In mice, social information processing could be normalized by replacing BDNF during development in the MO, a cortical region argued to have a high degree of functional homology between organisms241. This cross-species investigation suggests that bioavailable BDNF during postnatal development is a fundamental building block for social information processing. The val66met BDNF gene variant is associated with worse long-term psychiatric outcomes for individuals who suffer early-life adversity.242 Thus, understanding BDNF control of social information processing may shed light onto disease mechanisms.
In this review, we attempted to identify human-rodent homologies in the mechanistic factors necessary for decisions to be made in social contexts. Connections between the vmPFC and amygdala routinely appear important for detecting salience in social contexts, with subsequent incorporation of information from reward systems (e.g., VTA) prior to engagement of downstream motor systems (e.g., striatal structures) as social information guides actions. The PL and ACC also recur as key social information processing hubs in rodents, corresponding with the aspects of the mPFC and ACC in humans, respectively. Future studies will be needed to better understand how social information is processed and valued in humans and rodents – are the neurobiological processes (from circuits to intracellular mechanisms and beyond) occurring within these structures conserved or divergent between species?
The last decade of rodent social neuroscience research has disabused the notion that rodents do not utilize complex social information to inform their choices, and/or that these processes cannot be systematically detected, quantified, and investigated. For instance, efforts aimed at translating social decision-making tasks across species have resulted in rodent versions of the Dictator’s game222. These efforts are reminiscent of other explorations of decision making outside of social contexts, including investigations concerning complex “human” phenomena such as paranoid beliefs. One theorized mechanism contributing to paranoia is the propensity of an individual to re-categorize held beliefs in the face of uncertainty. Specifically, in situations when one is presented with uncertainty, beliefs may be recategorized (e.g., my friend is actually my enemy). To investigate learning styles in humans with paranoid beliefs and rodents with altered expectancy updating (e.g., post-methamphetamine exposure), researchers can carefully quantify responses to uncertainty utilizing probabilistic reversal learning in parallel cross-species investigations243. In the human subjects research domain, interesting recent investigations have combined machine learning with virtual reality and eye tracking to better understand empathic processes244; with the recent advent of virtual reality protocols in rodents245, translational approaches could be similarly employed to dissect underlying neural circuits in rodents. This translation could be bidirectional, with the upscaling of certain rodent tasks (such as ADT or SIFC) for use with clinical populations. Cross-species investigations could help to determine whether: 1) comparable brain regions are involved or implicated in decisions made in social contexts, and/or 2) how current or novel pharmacotherapeutic and psychotherapeutics could be leveraged to ameliorate specific deficits in social decision making.
Conclusions
Social interactions, and how they affect our decisions, are difficult to dissect, even in controlled laboratory settings. This is due to a large dynamic environment of multiple agents, each with their own internal states, desires, and histories. It is perhaps unsurprising that deficits in social interactions, and social decision making, emerge in practically every neuropsychiatric disorder246, given how many underlying components must co-occur for social behaviors to be expressed typically. Even then, defining a “good” or “fair” (even “typical”) decision in a social context inherently includes some moralistic view of how individuals should act, which makes the study of social behavior all the more complicated. As genetic and physiological tools to manipulate specific neuronal ensembles and projections become more refined, so should the behavioral tasks that examine social behavior. Rodents provide a valuable tool to examine social decision making at a cellular and molecular level and can likely shed light on dysregulatory processes that characterize neuropsychiatric disorders.
Highlights.
Many illnesses are co-morbid with, or defined by, disruptions in social behavior.
Rodents are a viable tool for studying decision making related to social experiences.
This review breaks down “social decisions” into their component parts.
We discuss social/emotional recognition, empathy, and social incentive/value.
We also pay homage to investigations that paved the way for current knowledge.
Acknowledgements:
Preparation of this manuscript was supported by NIH F30 MH117878, P50 MH100023, and R01 MH117103. The Emory National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51 OD011132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement: The authors do not declare any competing interests.
REFERENCES
- 1.Taborsky B, Oliveira RF. Social competence: an evolutionary approach. Trends Ecol Evol. 2012;27(12):679–88. doi: 10.1016/j.tree.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 3.Prounis GS, Ophir AG. One cranium, two brains not yet introduced: Distinct but complementary views of the social brain. Neurosci Biobehav Rev. 2020;108:231–45. doi: 10.1016/j.neubiorev.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 5.Gangopadhyay P, Chawla M, Dal Monte O, Chang SWC. Prefrontal–amygdala circuits in social decision-making. Nature Neuroscience. 2021;24(1):5–18. doi: 10.1038/s41593-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko J Neuroanatomical Substrates of Rodent Social Behavior: The Medial Prefrontal Cortex and Its Projection Patterns. Frontiers in Neural Circuits. 2017;11(41). doi: 10.3389/fncir.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei D, Talwar V, Lin D. Neural circuits of social behaviors: innate yet flexible. Neuron. 2021. doi: 10.1016/j.neuron.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lischinsky JE, Lin D. Neural mechanisms of aggression across species. Nat Neurosci. 2020;23(11):1317–28. doi: 10.1038/s41593-020-00715-2. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay S, Sharika KM, Platt ML. Social Decision-Making and the Brain: A Comparative Perspective. Trends in cognitive sciences. 2017;21(4):265–76. doi: 10.1016/j.tics.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arioli M, Crespi C, Canessa N. Social Cognition through the Lens of Cognitive and Clinical Neuroscience. Biomed Res Int. 2018;2018:4283427-. doi: 10.1155/2018/4283427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American journal of psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald EM, Hayes RL, Baglioni AJ Jr. The quantity and quality of the social networks of young people with early psychosis compared with closely matched controls. Schizophr Res. 2000;46(1):25–30. doi: 10.1016/s0920-9964(00)00024-4. [DOI] [PubMed] [Google Scholar]
- 13.Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, Adler N. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health. 2013;103(11):2056–62. doi: 10.2105/ajph.2013.301261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbert BN. Research Domain Criteria (RDoC): Progress and Potential. Current Directions in Psychological Science. 2022;31(2):107–14. doi: 10.1177/09637214211051363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishida KT, Montague PR. Imaging models of valuation during social interaction in humans. Biol Psychiatry. 2012;72(2):93–100. doi: 10.1016/j.biopsych.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16(11):559–72. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chafee MV, Heilbronner SR. Prefrontal cortex. Current Biology. 2022;32(8):R346–R51. doi: 10.1016/j.cub.2022.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlén M What constitutes the prefrontal cortex? Science. 2017;358(6362):478–82. doi: 10.1126/science.aan8868. [DOI] [PubMed] [Google Scholar]
- 19.Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- 20.Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial Prefrontal Cortices Are Unified by Common Connections With Superior Temporal Cortices and Distinguished by Input From Memory-Related Areas in the Rhesus Monkey. Journal of Comparative Neurology. 1999;410(3):343–67. doi: . [DOI] [PubMed] [Google Scholar]
- 21.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology. 1984;230(4):465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 22.Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. Journal of Comparative Neurology. 1993;336(2):211–28. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS. Circuitry of Primate Prefrontal Cortex and Regulation of Behavior by Representational Memory. Comprehensive Physiology. p. 373–417. [Google Scholar]
- 24.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 25.Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121 (Pt 5):815–42. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 26.Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Social Neuroscience. 2006;1(3–4):149–66. doi: 10.1080/17470910600985589. [DOI] [PubMed] [Google Scholar]
- 27.Van Overwalle F Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell JP, Macrae CN, Banaji MR. Dissociable Medial Prefrontal Contributions to Judgments of Similar and Dissimilar Others. Neuron. 2006;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 29.O’Reilly RC. The What and How of prefrontal cortical organization. Trends in neurosciences. 2010;33(8):355–61. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Frontiers in human neuroscience. 2013;7:232-. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. Proc Natl Acad Sci U S A. 2005;102(47):17245–50. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furl N, Garrido L, Dolan RJ, Driver J, Duchaine B. Fusiform gyrus face selectivity relates to individual differences in facial recognition ability. J Cogn Neurosci. 2011;23(7):1723–40. doi: 10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein M, Erez Y, Blank I, Yovel G. An Integrated Neural Framework for Dynamic and Static Face Processing. Sci Rep. 2018;8(1):7036. doi: 10.1038/s41598-018-25405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrmann M, Scherf KS, Avidan G. Neural mechanisms of face perception, their emergence over development, and their breakdown. Wiley Interdiscip Rev Cogn Sci. 2016;7(4):247–63. doi: 10.1002/wcs.1388. [DOI] [PubMed] [Google Scholar]
- 36.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 37.Adolphs R Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 38.Adolphs R Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- 39.Ferretti V, Papaleo F. Understanding others: Emotion recognition in humans and other animals. Genes, Brain and Behavior. 2019;18(1):e12544. doi: 10.1111/gbb.12544. [DOI] [PubMed] [Google Scholar]
- 40.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 41.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 42.Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008;20(4):721–33. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- 43.Wolf RC, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain : a journal of neurology. 2014;137(Pt 6):1772–80. doi: 10.1093/brain/awu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobson RP, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988;79(4):441–53. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- 45.Lozier LM, Vanmeter JW, Marsh AA. Impairments in facial affect recognition associated with autism spectrum disorders: a meta-analysis. Dev Psychopathol. 2014;26(4 Pt 1):933–45. doi: 10.1017/s0954579414000479. [DOI] [PubMed] [Google Scholar]
- 46.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal Ventral Temporal Cortical Activity During Face Discrimination Among Individuals With Autism and Asperger Syndrome. Archives of General Psychiatry. 2000;57(4):331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 47.Fett A-KJ, Viechtbauer W, Dominguez M-d-G, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Darke H, Peterman J, Park S, Sundram S, Carter O. Are patients with schizophrenia impaired in processing non-emotional features of human faces? Frontiers in Psychology. 2013;4(529). doi: 10.3389/fpsyg.2013.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon JH, D’Esposito M, Carter CS. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Research: Neuroimaging. 2006;148(2):205–16. doi: 10.1016/j.pscychresns.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Irani F, Seligman S, Kamath V, Kohler C, Gur RC. A meta-analysis of emotion perception and functional outcomes in schizophrenia. Schizophrenia Research. 2012;137(1):203–11. doi: 10.1016/j.schres.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71(2):136–45. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36(5):1029–39. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grabowski K, Rynkiewicz A, Lassalle A, Baron-Cohen S, Schuller B, Cummins N, Baird A, Podgórska-Bednarz J, Pieniążek A, Łucka I. Emotional expression in psychiatric conditions: New technology for clinicians. Psychiatry Clin Neurosci. 2019;73(2):50–62. doi: 10.1111/pcn.12799. [DOI] [PubMed] [Google Scholar]
- 54.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, Knapska E. The roots of empathy: Through the lens of rodent models. Neurosci Biobehav Rev. 2017;76(Pt B):216–34. doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Waal FBM. Putting the Altruism Back into Altruism: The Evolution of Empathy. Annual review of psychology. 2007;59(1):279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 56.Singer T, Lamm C. The Social Neuroscience of Empathy. Annals of the New York Academy of Sciences. 2009;1156(1):81–96. doi: 10.1111/j.17496632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 57.Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PloS one. 2008;3(8):e2939–e. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lockwood PL. The anatomy of empathy: Vicarious experience and disorders of social cognition. Behav Brain Res. 2016;311:255–66. doi: 10.1016/j.bbr.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25(1):1–20; discussion −71. doi: . [DOI] [PubMed] [Google Scholar]
- 60.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(3):617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–28. doi: 10.1016/0010-0277(95)00692-R. [DOI] [PubMed] [Google Scholar]
- 62.Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. NeuroReport. 1995;6(13). [DOI] [PubMed] [Google Scholar]
- 63.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2010;22(7):1623–35. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- 65.Sebastian CL, Fontaine NMG, Bird G, Blakemore S-J, Brito SAD, McCrory EJP, Viding E. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Social cognitive and affective neuroscience. 2012;7(1):53–63. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Neerven T, Bos DJ, van Haren NE. Deficiencies in Theory of Mind in patients with schizophrenia, bipolar disorder, and major depressive disorder: A systematic review of secondary literature. Neurosci Biobehav Rev. 2021;120:249–61. doi: 10.1016/j.neubiorev.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Andreou M, Skrimpa V. Theory of Mind Deficits and Neurophysiological Operations in Autism Spectrum Disorders: A Review. Brain sciences. 2020;10(6):393. doi: 10.3390/brainsci10060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord. 2008;38(3):464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- 69.Schwenck C, Mergenthaler J, Keller K, Zech J, Salehi S, Taurines R, Romanos M, Schecklmann M, Schneider W, Warnke A, Freitag CM. Empathy in children with autism and conduct disorder: group-specific profiles and developmental aspects. J Child Psychol Psychiatry. 2012;53(6):651–9. doi: 10.1111/j.1469-7610.2011.02499.x. [DOI] [PubMed] [Google Scholar]
- 70.Gernsbacher MA, Yergeau M. Empirical Failures of the Claim That Autistic People Lack a Theory of Mind. Arch Sci Psychol. 2019;7(1):102–18. doi: 10.1037/arc0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith A Emotional empathy in autism spectrum conditions: weak, intact, or heightened? J Autism Dev Disord. 2009;39(12):1747–8; author reply 9–54. doi: 10.1007/s10803-009-0799-z. [DOI] [PubMed] [Google Scholar]
- 72.Horan WP, Iacoboni M, Cross KA, Korb A, Lee J, Nori P, Quintana J, Wynn JK, Green MF. Self-reported empathy and neural activity during action imitation and observation in schizophrenia. Neuroimage Clin. 2014;5:100–8. doi: 10.1016/j.nicl.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. American Psychological Association; 2007. p. 431–8. [DOI] [PubMed] [Google Scholar]
- 74.Bonfils KA, Lysaker PH, Minor KS, Salyers MP. Affective empathy in schizophrenia: a meta-analysis. Schizophr Res. 2016;175(1–3):109–17. doi: 10.1016/j.schres.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 75.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, Riccaboni R, Scotti G, Smeraldi E. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114(1–3):154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 77.Abram SV, Wisner KM, Fox JM, Barch DM, Wang L, Csernansky JG, MacDonald AW 3rd, Smith MJ. Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Hum Brain Mapp. 2017;38(3):1111–24. doi: 10.1002/hbm.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brüne M, Abdel-Hamid M, Lehmkämper C, Sonntag C. Mental state attribution, neurocognitive functioning, and psychopathology: what predicts poor social competence in schizophrenia best? Schizophr Res. 2007;92(1–3):151–9. doi: 10.1016/j.schres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Gu R, Huang W, Camilleri J, Xu P, Wei P, Eickhoff SB, Feng C. Love is analogous to money in human brain: Coordinate-based and functional connectivity meta-analyses of social and monetary reward anticipation. Neuroscience & Biobehavioral Reviews. 2019;100:108–28. doi: 10.1016/j.neubiorev.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Current opinion in neurobiology. 2012;22(6):1027–38. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64(3):431–9. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 82.Ruff CC, Fehr E. The neurobiology of rewards and values in social decision making. Nat Rev Neurosci. 2014;15(8):549–62. doi: 10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- 83.Morishima Y, Schunk D, Bruhin A, Ruff Christian C, Fehr E. Linking Brain Structure and Activation in Temporoparietal Junction to Explain the Neurobiology of Human Altruism. Neuron. 2012;75(1):73–9. doi: 10.1016/j.neuron.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Strombach T, Weber B, Hangebrauk Z, Kenning P, Karipidis II, Tobler PN, Kalenscher T. Social discounting involves modulation of neural value signals by temporoparietal junction. Proceedings of the National Academy of Sciences. 2015;112(5):1619–24. doi: doi: 10.1073/pnas.1414715112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 86.Woo C-W, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD. Separate neural representations for physical pain and social rejection. Nature Communications. 2014;5(1):5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 88.Guala F, Mittone L. Paradigmatic experiments: The Dictator Game. The Journal of Socio-Economics. 2010;39(5):578–84. doi: 10.1016/j.socec.2009.05.007. [DOI] [Google Scholar]
- 89.Robson SE, Repetto L, Gountouna V-E, Nicodemus KK. A review of neuroeconomic gameplay in psychiatric disorders. Molecular psychiatry. 2020;25(1):67–81. doi: 10.1038/s41380-019-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci U S A. 2001;98(20):11832–5. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Soc Cogn Affect Neurosci. 2009;4(3):294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki S, Harasawa N, Ueno K, Gardner Justin L, Ichinohe N, Haruno M, Cheng K, Nakahara H. Learning to Simulate Others’ Decisions. Neuron. 2012;74(6):1125–37. doi: 10.1016/j.neuron.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 93.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. Science. 2004;304(5669):452. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 95.Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63(6):733–45. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith DV, Clithero JA, Boltuck SE, Huettel SA. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Social Cognitive and Affective Neuroscience. 2014;9(12):2017–25. doi: 10.1093/scan/nsu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fukuda H, Ma N, Suzuki S, Harasawa N, Ueno K, Gardner JL, Ichinohe N, Haruno M, Cheng K, Nakahara H. Computing Social Value Conversion in the Human Brain. The Journal of Neuroscience. 2019;39(26):5153. doi: 10.1523/JNEUROSCI.3117-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456(7219):245–9. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang L, Gläscher J. A brain network supporting social influences in human decision-making. Science Advances. 2020;6(34):eabb4159. doi: 10.1126/sciadv.abb4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci. 2012;7(3):274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moretti L, Dragone D, di Pellegrino G. Reward and social valuation deficits following ventromedial prefrontal damage. J Cogn Neurosci. 2009;21(1):128–40. doi: 10.1162/jocn.2009.21011. [DOI] [PubMed] [Google Scholar]
- 102.Barkus E, Badcock JC. A Transdiagnostic Perspective on Social Anhedonia. Frontiers in psychiatry. 2019;10:216-. doi: 10.3389/fpsyt.2019.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dichter G, Adolphs R. Reward processing in autism: a thematic series. J Neurodev Disord. 2012;4(1):20. doi: 10.1186/1866-1955-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–61. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 107.Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(11):1164–73. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- 108.Catalano LT, Heerey EA, Gold JM. The valuation of social rewards in schizophrenia. J Abnorm Psychol. 2018;127(6):602–11. doi: 10.1037/abn0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J Abnorm Psychol. 1998;107(4):558–65. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- 110.Robustelli BL, Newberry RE, Whisman MA, Mittal VA. Social relationships in young adults at ultra high risk for psychosis. Psychiatry Res. 2017;247:345–51. doi: 10.1016/j.psychres.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sally D, Hill E. The development of interpersonal strategy: Autism, theory-of-mind, cooperation and fairness. Journal of Economic Psychology. 2006;27(1):73–97. doi: 10.1016/j.joep.2005.06.015. [DOI] [Google Scholar]
- 112.Hartley C, Fisher S. Do Children with Autism Spectrum Disorder Share Fairly and Reciprocally? J Autism Dev Disord. 2018;48(8):2714–26. doi: 10.1007/s10803-018-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57(3):463–73. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edmiston EK, Merkle K, Corbett BA. Neural and cortisol responses during play with human and computer partners in children with autism. Soc Cogn Affect Neurosci. 2015;10(8):1074–83. doi: 10.1093/scan/nsu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, Li P, Mao H, Wang H, Shu C, Bliksted V, Zhou Y. Theory of mind deficits partly mediate impaired social decision-making in schizophrenia. BMC Psychiatry. 2017;17(1):168. doi: 10.1186/s12888-017-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agay N, Kron S, Carmel Z, Mendlovic S, Levkovitz Y. Ultimatum bargaining behavior of people affected by schizophrenia. Psychiatry Res. 2008;157(1–3):39–46. doi: 10.1016/j.psychres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 117.Horat SK, Favre G, Prévot A, Ventura J, Herrmann FR, Gothuey I, Merlo MCG, Missonnier P. Impaired social cognition in schizophrenia during the Ultimatum Game: An EEG study. Schizophr Res. 2018;192:308–16. doi: 10.1016/j.schres.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 118.Fett AK, Shergill SS, Joyce DW, Riedl A, Strobel M, Gromann PM, Krabbendam L. To trust or not to trust: the dynamics of social interaction in psychosis. Brain. 2012;135(Pt 3):976–84. doi: 10.1093/brain/awr359. [DOI] [PubMed] [Google Scholar]
- 119.Billeke P, Armijo A, Castillo D, López T, Zamorano F, Cosmelli D, Aboitiz F. Paradoxical Expectation: Oscillatory Brain Activity Reveals Social Interaction Impairment in Schizophrenia. Biological Psychiatry. 2015;78(6):421–31. doi: 10.1016/j.biopsych.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 120.Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Neuroethology of primate social behavior. Proc Natl Acad Sci U S A. 2013;110 Suppl 2(Suppl 2):10387–94. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. Elife. 2014;3:e01385–e. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085):1154–7. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- 123.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal Cortex and Social Cognition in Mouse and Man. Frontiers in Psychology. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 125.Laubach M, Amarante LM, Swanson K, White SR. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro. 2018;5(5):ENEURO.0315–18.2018. doi: 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Funahashi S Working Memory in the Prefrontal Cortex. Brain Sci. 2017;7(5). doi: 10.3390/brainsci7050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woon EP, Sequeira MK, Barbee BR, Gourley SL. Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: A brief review. Journal of Neuroscience Research. 2020;98(6):1020–30. doi: 10.1002/jnr.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balleine BW, O’Doherty JP. Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morè L, Lauterborn JC, Papaleo F, Brambilla R. Enhancing cognition through pharmacological and environmental interventions: Examples from preclinical models of neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews. 2020;110:28–45. doi: 10.1016/j.neubiorev.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Robbins TW. Cross-species studies of cognition relevant to drug discovery: a translational approach. Br J Pharmacol. 2017;174(19):3191–9. doi: 10.1111/bph.13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barthas F, Kwan AC. Secondary Motor Cortex: Where ‘Sensory’ Meets ‘Motor’ in the Rodent Frontal Cortex. Trends in neurosciences. 2017;40(3):181–93. doi: 10.1016/j.tins.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Van De Werd HJ, Rajkowska G, Evers P, Uylings HB. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214(4):339–53. doi: 10.1007/s00429-010-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–79. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212(2):149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 135.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends in Neurosciences. 2008;31(12):599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in Behavioral Neuroscience. 2015;9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barker JM, Glen WB, Linsenbardt DN, Lapish CC, Chandler LJ. Habitual Behavior Is Mediated by a Shift in Response-Outcome Encoding by Infralimbic Cortex. eNeuro. 2017;4(6). doi: 10.1523/eneuro.0337-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gourley SL, Taylor JR. Going and stopping: Dichotomies in behavioral control by the prefrontal cortex. Nature neuroscience. 2016;19(6):656–64. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, Vogt BA, Glennon JC, Havenith MN. Where is Cingulate Cortex? A Cross-Species View. Trends in Neurosciences. 2020;43(5):285–99. doi: 10.1016/j.tins.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 140.Fillinger C, Yalcin I, Barrot M, Veinante P. Efferents of anterior cingulate areas 24a and 24b and midcingulate areas 24a’ and 24b’ in the mouse. Brain Struct Funct. 2018;223(4):1747–78. doi: 10.1007/s00429-017-1585-x. [DOI] [PubMed] [Google Scholar]
- 141.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biological Psychiatry. 2002;51(1):18–26. doi: 10.1016/S0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 142.Noack J, Richter K, Laube G, Haghgoo HA, Veh RW, Engelmann M. Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol Learn Mem. 2010;94(4):568–75. doi: 10.1016/j.nlm.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 143.Natynczuk SE, Macdonald DW. Scent, sex, and the self-calibrating rat. J Chem Ecol. 1994;20(8):1843–57. doi: 10.1007/bf02066226. [DOI] [PubMed] [Google Scholar]
- 144.de la Zerda SH, Netser S, Magalnik H, Briller M, Marzan D, Glatt S, Abergel Y, Wagner S. Social recognition in laboratory mice requires integration of behaviorally-induced somatosensory, auditory and olfactory cues. Psychoneuroendocrinology. 2022;143:105859. doi: 10.1016/j.psyneuen.2022.105859. [DOI] [PubMed] [Google Scholar]
- 145.Rogers-Carter MM, Christianson JP. An insular view of the social decision-making network. Neurosci Biobehav Rev. 2019;103:119–32. doi: 10.1016/j.neubiorev.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thor DH, Holloway WR. Social memory of the male laboratory rat. American Psychological Association; 1982. p. 1000–6. [Google Scholar]
- 147.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8:Unit 8.24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58(2):315–21. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- 149.Winslow JT. Mouse social recognition and preference. Curr Protoc Neurosci. 2003;Chapter 8:Unit 8.16. doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- 150.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of Social Interaction Behaviors. Journal of Visualized Experiments : JoVE. 2011(48):2473. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behavioural Brain Research. 2006;172(1):97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 152.Carter CS, Getz LL. Monogamy and the Prairie Vole. Scientific American. 1993;268(6):100–6. [DOI] [PubMed] [Google Scholar]
- 153.Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26(3):339–49. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 154.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. US: American Psychological Association; 1995. p. 782–9. [DOI] [PubMed] [Google Scholar]
- 155.Wang F, Kessels HW, Hu H. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 2014;37(11):674–82. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 156.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–87. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lindzey G, Winston H, Manosevitz M. Social Dominance in Inbred Mouse Strains. Nature. 1961;191(4787):474–6. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- 158.Fan Z, Zhu H, Zhou T, Wang S, Wu Y, Hu H. Using the tube test to measure social hierarchy in mice. Nature Protocols. 2019;14(3):819–31. doi: 10.1038/s41596-018-0116-4. [DOI] [PubMed] [Google Scholar]
- 159.Wöhr M, Schwarting RK. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013;354(1):81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- 160.Brignall AC, Cloutier J-F. Neural map formation and sensory coding in the vomeronasal system. Cellular and Molecular Life Sciences. 2015;72(24):4697–709. doi: 10.1007/s00018-015-2029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006;31(4):315–23. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. European Journal of Neuroscience. 2006;23(2):521–30. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Camats Perna J, Engelmann M. Recognizing Others: Rodent’s Social Memories. Curr Top Behav Neurosci. 2017;30:25–45. doi: 10.1007/7854_2015_413. [DOI] [PubMed] [Google Scholar]
- 164.Noack J, Murau R, Engelmann M. Consequences of temporary inhibition of the medial amygdala on social recognition memory performance in mice. Front Neurosci. 2015;9:152. doi: 10.3389/fnins.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaut KP, Bunsey MD. The effects of lesions to the rat hippocampus or rhinal cortex on olfactory and spatial memory: retrograde and anterograde findings. Cogn Affect Behav Neurosci. 2001;1(3):270–86. doi: 10.3758/cabn.1.3.270. [DOI] [PubMed] [Google Scholar]
- 166.Feinberg LM, Allen TA, Ly D, Fortin NJ. Recognition memory for social and non-social odors: differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem. 2012;97(1):7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 167.Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res. 2001;138(1):100–9. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- 168.Squires AS, Peddle R, Milway SJ, Harley CW. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol Learn Mem. 2006;85(1):95–101. doi: 10.1016/j.nlm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 169.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508(7494):88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40(9):3294–301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353(6307):1536. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. The Journal of Neuroscience. 2017;37(15):4103. doi: 10.1523/JNEUROSCI.3451-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Phillips ML, Robinson HA, Pozzo-Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife. 2019;8:e44182. doi: 10.7554/eLife.44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen D-TM, Colon-Perez LM, Sahagian TJ, Thinschmidt JS, de Kloet AD, Febo M, Frazier CJ, Krause EG. Oxytocin Receptors Are Expressed by Glutamatergic Prefrontal Cortical Neurons That Selectively Modulate Social Recognition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(17):3249–63. doi: 10.1523/JNEUROSCI.2944-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xing B, Mack NR, Guo K-M, Zhang Y-X, Ramirez B, Yang S-S, Lin L, Wang DV, Li Y-C, Gao W-J. A Subpopulation of Prefrontal Cortical Neurons Is Required for Social Memory. Biological Psychiatry. 2020. doi: 10.1016/j.biopsych.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52(2):132–4. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 177.Cox SS, Reichel CM. Current rodent models for the study of empathic processes. Behav Pharmacol. 2021;32(2&3):96–111. doi: 10.1097/fbp.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, Keysers C. Experience modulates vicarious freezing in rats: a model for empathy. PloS one. 2011;6(7):e21855–e. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PloS one. 2009;4(2):e4387–e. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee E, Rhim I, Lee JW, Ghim J-W, Lee S, Kim E, Jung MW. Enhanced Neuronal Activity in the Medial Prefrontal Cortex during Social Approach Behavior. The Journal of Neuroscience. 2016;36(26):6926. doi: 10.1523/JNEUROSCI.0307-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Smith ML, Asada N, Malenka RC. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science. 2021;371(6525):153. doi: 10.1126/science.abe3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Debiec J, Olsson A. Social Fear Learning: from Animal Models to Human Function. Trends Cogn Sci. 2017;21(7):546–55. doi: 10.1016/j.tics.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bruchey AK, Jones CE, Monfils M-H. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behavioural brain research. 2010;214(1):80–4. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hernandez-Lallement J, Attah AT, Soyman E, Pinhal CM, Gazzola V, Keysers C. Harm to Others Acts as a Negative Reinforcer in Rats. Curr Biol. 2020;30(6):949–61.e7. doi: 10.1016/j.cub.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 185.Hernandez-Lallement J, van Wingerden M, Marx C, Srejic M, Kalenscher T. Rats prefer mutual rewards in a prosocial choice task. Frontiers in Neuroscience. 2015;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Scheggia D, Managò F, Maltese F, Bruni S, Nigro M, Dautan D, Latuske P, Contarini G, Gomez-Gonzalo M, Requie LM, Ferretti V, Castellani G, Mauro D, Bonavia A, Carmignoto G, Yizhar O, Papaleo F. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nature Neuroscience. 2020;23(1):47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- 187.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, Christianson JP. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nature neuroscience. 2018;21(3):404–14. doi: 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334(6061):1427–30. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, Keysers C. Emotional Mirror Neurons in the Rat’s Anterior Cingulate Cortex. Current Biology. 2019;29(8):1301–12.e6. doi: 10.1016/j.cub.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sakaguchi T, Iwasaki S, Okada M, Okamoto K, Ikegaya Y. Ethanol facilitates socially evoked memory recall in mice by recruiting pain-sensitive anterior cingulate cortical neurons. Nature Communications. 2018;9(1):3526. doi: 10.1038/s41467-018-05894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13(4):482–8. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang C-J, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, Ba D, Brown EN, Tye KM. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell. 2018;173(6):1329–42.e18. doi: 10.1016/j.cell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zaniboni CR, Pelarin V, Baptista-de-Souza D, Canto-de-Souza A. Empathy for Pain: Insula Inactivation and Systemic Treatment With Midazolam Reverses the Hyperalgesia Induced by Cohabitation With a Pair in Chronic Pain Condition. Frontiers in Behavioral Neuroscience. 2018;12(278). doi: 10.3389/fnbeh.2018.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cox SS, Kearns AM, Woods SK, Brown BJ, Brown SJ, Reichel CM. The role of the anterior insula during targeted helping behavior in male rats. Scientific Reports. 2022;12(1):3315. doi: 10.1038/s41598-022-07365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Twining RC, Vantrease JE, Love S, Padival M, Rosenkranz JA. An intra-amygdala circuit specifically regulates social fear learning. Nature Neuroscience. 2017;20(3):459–69. doi: 10.1038/nn.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hernandez-Lallement J, van Wingerden M, Schäble S, Kalenscher T. Basolateral amygdala lesions abolish mutual reward preferences in rats. Neurobiology of Learning and Memory. 2016;127:1–9. doi: 10.1016/j.nlm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 198.Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience. 2011;1(4):444–58. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176(1):40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 200.File SE, Hyde JRG. CAN SOCIAL INTERACTION BE USED TO MEASURE ANXIETY? British Journal of Pharmacology. 1978;62(1):19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 202.Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Animal Behaviour. 1981;29(1):259–70. doi: 10.1016/S0003-3472(81)80173-X. [DOI] [Google Scholar]
- 203.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 204.Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: Effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22(1):44–53. doi: 10.3758/BF03327079. [DOI] [Google Scholar]
- 205.Magnusson JE, Fleming AS. Rat pups are reinforcing to the maternal rat: Role of sensory cues. Psychobiology. 1995;23(1):69–75. doi: 10.3758/BF03327061. [DOI] [Google Scholar]
- 206.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51(4):667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 207.Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Frontiers in behavioral neuroscience. 2011;5:80-. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han M-H, Shapiro ML, Russo SJ. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534(7609):688–92. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, Dölen G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature. 2019;569(7754):116–20. doi: 10.1038/s41586-019-1075-9. [DOI] [PubMed] [Google Scholar]
- 210.Cann C, Venniro M, Hope BT, Ramsey LA. Parametric investigation of social place preference in adolescent mice. Behav Neurosci. 2020;134(5):435–43. doi: 10.1037/bne0000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Galef BG, Giraldeau LA. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav. 2001;61(1):3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- 212.Galef BG. Socially induced diet preference can partially reverse a LiCl-induced diet aversion. Animal Learning & Behavior. 1985;13(4):415–8. doi: 10.3758/BF03208018. [DOI] [Google Scholar]
- 213.Van Hemel SB. Pup retrieving as a reinforcer in nulliparous mice. J Exp Anal Behav. 1973;19(2):233–8. doi: 10.1901/jeab.1973.19-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100(1–2):15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 215.Achterberg EJ, van Kerkhof LW, Servadio M, van Swieten MM, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJ. Contrasting Roles of Dopamine and Noradrenaline in the Motivational Properties of Social Play Behavior in Rats. Neuropsychopharmacology. 2016;41(3):858–68. doi: 10.1038/npp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Everitt BJ, Fray P, Kostarczyk E, Taylor S, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): I. Control by brief visual stimuli paired with a receptive female. J Comp Psychol. 1987;101(4):395–406. [PubMed] [Google Scholar]
- 217.Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 2002;163(3):459–66. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- 218.Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, Shaham Y. Compulsive Addiction-like Aggressive Behavior in Mice. Biol Psychiatry. 2017;82(4):239–48. doi: 10.1016/j.biopsych.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Borland JM, Frantz KJ, Aiani LM, Grantham KN, Song Z, Albers HE. A novel operant task to assess social reward and motivation in rodents. J Neurosci Methods. 2017;287:80–8. doi: 10.1016/j.jneumeth.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y. Volitional social interaction prevents drug addiction in rat models. Nature neuroscience. 2018;21(11):1520–9. doi: 10.1038/s41593-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kietzman HW, Trinoskey-Rice G, Blumenthal SA, Guo JD, Gourley SL. Social incentivization of instrumental choice in mice requires amygdala-prelimbic cortex-nucleus accumbens connectivity. Nature Communications. 2022;13(1):4768. doi: 10.1038/s41467-022-32388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Scheggia D, La Greca F, Maltese F, Chiacchierini G, Italia M, Molent C, Bernardi F, Coccia G, Carrano N, Zianni E, Gardoni F, Di Luca M, Papaleo F. Reciprocal cortico-amygdala connections regulate prosocial and selfish choices in mice. Nat Neurosci. 2022;25(11):1505–18. doi: 10.1038/s41593-022-01179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, Parker NF, Bhave V, Hur H, Liang Y, Nectow AR, Pillow JW, Witten IB. Combined Social and Spatial Coding in a Descending Projection from the Prefrontal Cortex. Cell. 2017;171(7):1663–77.e16. doi: 10.1016/j.cell.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Solié C, Contestabile A, Espinosa P, Musardo S, Bariselli S, Huber C, Carleton A, Bellone C. Superior Colliculus to VTA pathway controls orienting response and influences social interaction in mice. Nature communications. 2022;13(1):817-. doi: 10.1038/s41467-022-28512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ahmadlou M, Houba JHW, van Vierbergen JFM, Giannouli M, Gimenez GA, van Weeghel C, Darbanfouladi M, Shirazi MY, Dziubek J, Kacem M, de Winter F, Heimel JA. A cell type-specific cortico-subcortical brain circuit for investigatory and novelty-seeking behavior. Science. 2021;372(6543). doi: 10.1126/science.abe9681. [DOI] [PubMed] [Google Scholar]
- 227.Yamamuro K, Bicks LK, Leventhal MB, Kato D, Im S, Flanigan ME, Garkun Y, Norman KJ, Caro K, Sadahiro M, Kullander K, Akbarian S, Russo SJ, Morishita H. A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat Neurosci. 2020;23(10):1240–52. doi: 10.1038/s41593-020-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, Hong W. Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell. 2019;178(2):429–46.e16. doi: 10.1016/j.cell.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Levy DR, Tamir T, Kaufman M, Parabucki A, Weissbrod A, Schneidman E, Yizhar O. Dynamics of social representation in the mouse prefrontal cortex. Nature Neuroscience. 2019;22(12):2013–22. doi: 10.1038/s41593-019-0531-z. [DOI] [PubMed] [Google Scholar]
- 230.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–84. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, Christianson JP. Insular Cortex Projections to Nucleus Accumbens Core Mediate Social Approach to Stressed Juvenile Rats. The Journal of Neuroscience. 2019;39(44):8717. doi: 10.1523/JNEUROSCI.0316-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, Donaldson ZR. A neuronal signature for monogamous reunion. Proceedings of the National Academy of Sciences. 2020;117(20):11076. doi: 10.1073/pnas.1917287117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1–2):145–57. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 235.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27(18):4819–25. doi: 10.1523/jneurosci.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gunaydin Lisa A, Grosenick L, Finkelstein Joel C, Kauvar Isaac V, Fenno Lief E, Adhikari A, Lammel S, Mirzabekov Julie J, Airan Raag D, Zalocusky Kelly A, Tye Kay M, Anikeeva P, Malenka Robert C, Deisseroth K. Natural Neural Projection Dynamics Underlying Social Behavior. Cell. 2014;157(7):1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79(3):351–7. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 238.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158(6):1348–61. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jennings JH, Kim CK, Marshel JH, Raffiee M, Ye L, Quirin S, Pak S, Ramakrishnan C, Deisseroth K. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature. 2019;565(7741):645–9. doi: 10.1038/s41586-018-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Raper J, Galvan A. Applications of chemogenetics in non-human primates. Curr Opin Pharmacol. 2022;64:102204. doi: 10.1016/j.coph.2022.102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li A, Jing D, Dellarco DV, Hall BS, Yang R, Heilberg RT, Huang C, Liston C, Casey BJ, Lee FS. Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. Mol Psychiatry. 2021;26(3):955–73. doi: 10.1038/s41380-019-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cruz-Fuentes CS, Benjet C, Martínez-Levy GA, Pérez-Molina A, Briones-Velasco M, Suárez-González J. BDNF Met66 modulates the cumulative effect of psychosocial childhood adversities on major depression in adolescents. Brain Behav. 2014;4(2):290–7. doi: 10.1002/brb3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Reed EJ, Uddenberg S, Suthaharan P, Mathys CD, Taylor JR, Groman SM, Corlett PR. Paranoia as a deficit in non-social belief updating. Elife. 2020;9. doi: 10.7554/eLife.56345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Parra Vargas E, García Delgado A, Torres SC, Carrasco-Ribelles LA, Marín-Morales J, Alcañiz Raya M. Virtual reality stimulation and organizational neuroscience for the assessment of empathy. Front Psychol. 2022;13:993162. doi: 10.3389/fpsyg.2022.993162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thurley K, Ayaz A. Virtual reality systems for rodents. Curr Zool. 2017;63(1):109–19. doi: 10.1093/cz/zow070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Derntl B, Habel U. Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci. 2011;261 Suppl 2:S145–9. doi: 10.1007/s00406-011-0244-0. [DOI] [PubMed] [Google Scholar]
- 247.Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A. 2006;103(10):3858–62. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463(1):35–53. doi: 10.1016/S0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 249.Defensor EB, Pearson BL, Pobbe RLH, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behavioural Brain Research. 2011;217(2):302–8. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science. 2006;311(5762):864. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 251.Rudebeck PH, Walton ME, Millette BHP, Shirley E, Rushworth MFS, Bannerman DM. Distinct contributions of frontal areas to emotion and social behaviour in the rat. European Journal of Neuroscience. 2007;26(8):2315–26. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sun Q, Li X, Li A, Zhang J, Ding Z, Gong H, Luo Q. Ventral Hippocampal-Prefrontal Interaction Affects Social Behavior via Parvalbumin Positive Neurons in the Medial Prefrontal Cortex. iScience. 2020;23(3). doi: 10.1016/j.isci.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Park G, Ryu C, Kim S, Jeong SJ, Koo JW, Lee Y-S, Kim SJ. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Reports. 2021;35(6). doi: 10.1016/j.celrep.2021.109104. [DOI] [PubMed] [Google Scholar]
- 254.Mikosz M, Nowak A, Werka T, Knapska E. Sex differences in social modulation of learning in rats. Scientific reports. 2015;5:18114-. doi: 10.1038/srep18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jones CE, Monfils M-H. Dominance status predicts social fear transmission in laboratory rats. Animal cognition. 2016;19(6):1051–69. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zheng C, Huang Y, Bo B, Wei L, Liang Z, Wang Z. Projection from the Anterior Cingulate Cortex to the Lateral Part of Mediodorsal Thalamus Modulates Vicarious Freezing Behavior. Neuroscience Bulletin. 2020;36(3):217–29. doi: 10.1007/s12264-019-00427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim S, Mátyás F, Lee S, Acsády L, Shin H-S. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proceedings of the National Academy of Sciences. 2012;109(38):15497. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Keum S, Kim A, Shin JJ, Kim J-H, Park J, Shin H-S. A Missense Variant at the Nrxn3 Locus Enhances Empathy Fear in the Mouse. Neuron. 2018;98(3):588–601.e5. doi: 10.1016/j.neuron.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 259.Lee DK, Li SW, Bounni F, Friedman G, Jamali M, Strahs L, Zeliger O, Gabrieli P, Stankovich MA, Demaree J, Williams ZM. Reduced sociability and social agency encoding in adult Shank3-mutant mice are restored through gene re-expression in real time. Nature neuroscience. 2021;24(9):1243–55. doi: 10.1038/s41593-021-00888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress (Amsterdam, Netherlands). 2009;12(5):445–50. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jodo E, Katayama T, Okamoto M, Suzuki Y, Hoshino K, Kayama Y. Differences in responsiveness of mediodorsal thalamic and medial prefrontal cortical neurons to social interaction and systemically administered phencyclidine in rats. Neuroscience. 2010;170(4):1153–64. doi: 10.1016/j.neuroscience.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 262.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351(6268):aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin J-C, Bourgeois J-P, Maskos U, Changeux J-P, Granon S. Prefrontal nicotinic receptors control novel social interaction between mice. The FASEB Journal. 2011;25(7):2145–55. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- 264.Liang B, Zhang L, Barbera G, Fang W, Zhang J, Chen X, Chen R, Li Y, Lin DT. Distinct and Dynamic ON and OFF Neural Ensembles in the Prefrontal Cortex Code Social Exploration. Neuron. 2018;100(3):700–14.e9. doi: 10.1016/j.neuron.2018.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, Yao H, Yang J, Liu H, Liu Y, Jia F, Qi C, Lynn-Jones T, Hu H, Fu Z, Feng G, Wang W, Wu S. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nature Neuroscience. 2019;22(8):1223–34. doi: 10.1038/s41593-019-0445-9. [DOI] [PubMed] [Google Scholar]
- 266.Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, Ye L, Hong W. Cortical Representations of Conspecific Sex Shape Social Behavior. Neuron. 2020;107(5):941–53.e7. doi: 10.1016/j.neuron.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Huang WC, Zucca A, Levy J, Page DT. Social Behavior Is Modulated by Valence-Encoding mPFC-Amygdala Sub-circuitry. Cell Rep. 2020;32(2):107899. doi: 10.1016/j.celrep.2020.107899. [DOI] [PMC free article] [PubMed] [Google Scholar]


