To the Editor:
Dr Sandkühler raises several relevant and interesting points regarding our review on activity-dependent central sensitization and synaptic plasticity, and we are happy to clarify our approach to, and understanding of, synaptic plasticity in the dorsal horn, particularly the relationship between central sensitization and long-term potentiation (LTP). The definition of LTP needs to be addressed, we believe, in the context of the type of preparation (in vivo or in vitro), nature of the neurons (cerebellum, neocortex, hippocampus, dorsal horn), the synaptic changes that occur (homo- or heterosynaptic, pre- or postsynaptic), duration of changes (short term or persistent), molecular mechanisms (signal transduction pathways engaged, transcription independent or dependent), and the biological function of the circuits involved (learning, memory, or pain). For our review,17 we focused on the relationship between the different forms of activity-dependent synaptic plasticity reported in the dorsal horn of the spinal cord, including those that have been called LTP, and central sensitization. We thought it was particularly relevant to address the phenomenon of central sensitization as a defense mechanism specific to nociception, one that contributes to maintaining the integrity of the organism by producing pain hypersensitivity following a conditioning nociceptive afferent barrage, and, in this way, protecting an organism from further damage. This is an evolutionary conserved process, with prominent central sensitization-like behavior in Aplysia and other invertebrates;2,30 and while it shares general mechanistic aspects with several forms of synaptic plasticity in other systems, there are also, we argue, some important differences that make central sensitization unique. At a conceptual level, for example, we believe that there is a major difference between the input-specific synaptic potentiation that constitutes classic LTP in cortical neurons4,20,21 and the more generalized heightened excitability in nociceptive pathways that underlies central sensitization.26,29 Memory, which is widely considered dependent on hippocampal LTP,4,19,20,21,23 is a convergent neural operation where the storage of defined information is the consequence of coincident temporal and spatial association between specific stimuli. In contrast, central sensitization represents a divergent neural operation, one where a conditioning nociceptive input triggers diffuse changes such that other unstimulated synaptic inputs can now generate pain. For example, nociceptive stimulation of deep tissue, like muscle, results in an enlargement and reduction in threshold of the cutaneous receptive field of dorsal horn neurons, to an extent and duration that is even greater than when a nociceptive input is generated from the skin.28 If central sensitization were entirely maintained by LTP, it would not have this fundamental feature, which characterizes it.
Several different lines of evidence support the conjecture that the enlargement of cutaneous receptive fields and recruitment of Aβ fiber inputs of nociceptive neurons in the spinal cord after a C-fiber conditioning input are caused by heterosynaptic facilitation, as defined in our review:
After a brief conditioning C-fiber input originating from L4 dorsal root fibers, subsequent stimulation of L5 Aβ fibers elicit an enhancement of evoked EPSPs (Fig 1).26 The test and conditioning inputs are different and act on different synapses, something distinct from LTP.
Secondary pinprick hyperalgesia in human volunteers can be mediated by fibers whose activity is not increased by the conditioning input, injection of capsaicin15 demonstrating an “input nonspecific” change quite unlike LTP.
The imaging of nociceptive activity in the spinal cord using an autofluorescent flavoprotein has recently allowed direct visualization of the increase of receptive fields to tactile stimulation after capsaicin injection, physically showing the spatial extent of central sensitization-related plasticity in the dorsal horn,14 again quite different to the synaptically restricted nature of LTP.
Figure 1.
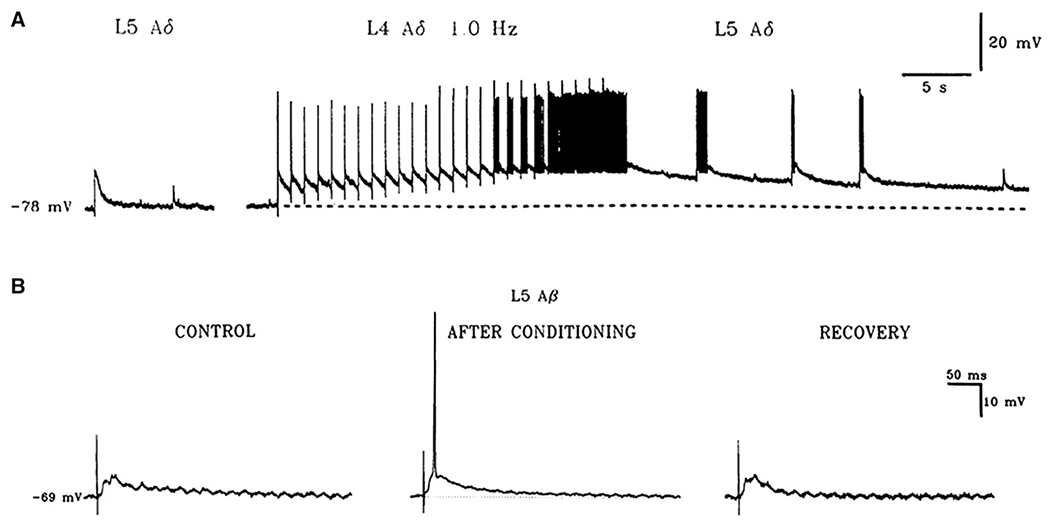
Evidence for the heterosynaptic nature of central sensitization caused by a nociceptive barrage. After a brief conditioning C-fiber input originating from L4 dorsal root fibers, subsequent stimulation of L5 Aδ or Aβ fibers elicits an enhancement of the EPSPs evoked by this input, which was not active during the conditioning input. Note how the subthreshold Aβ input becomes suprathreshold. Adapted from Thompson et al.26
The direct unequivocal and formal demonstration of the presence of either homosynaptic facilitation requires visualization of changes in a single synapse, as has been done for the activation of CaMKII in a single bouton during LTP in the hippocampus.18 This has not been done in the dorsal horn for LTP because of many technical problems, not least that there are no dendritic boutons on dorsal horn neurons, which seem not to be specialized for this kind of synaptic plasticity. We prefer simple operational definitions; if a conditioning nociceptive input enhances the synaptic strength of a nonstimulated input, this represents for us heterosynaptic facilitation. Furthermore, if a test and conditioning input activate the same synapse, and produce persistent facilitation of this synapse lasting several days, this would for us constitute LTP. We think that it is important to emphasize the relative short-term nature and reversibility of activity-dependent central sensitization, seen both at a synaptic and behavioral level, as opposed to the long-term, possibly irreversible effects of the hippocampal LTP that underlies formation of memory.4,19,20,21,23 Once triggered, activity-dependent central sensitization does not last more than a few hours after the conditioning stimulation ends, in contrast to hippocampal LTP that can last for at least several days.7,9,16 Pain-conditioning inputs that generate LTP-like phenomena in the dorsal horn do not produce irreversible pain hypersensitivity,27 and it does seem to us that the LTP-like changes in the spinal cord are sufficiently different from that in the hippocampus that they really should be called different names, to avoid the confusion that already abounds. Most recordings of LTP in the dorsal horn are for 30 minutes6,11,12,25 and have never demonstrated the sustained synaptic changes found in the hippocampus. If the changes are not long term, how can they be termed LTP? We agree that central sensitization and early LTP share many common factors including the trafficking of AMPAR and the activation of similar kinases.10,12,13,17 Once triggered, however, LTP in the hippocampus is systematically associated with a late, transcription-dependent phase that allows a synaptic potentiation for days.1,3,7–9,22 No such transcription-dependent component to LTP has been demonstrated in dorsal horn in response to those brief conditioning inputs that produce this synaptic enhancement.
To conclude, we believe that the most important notion we can gather from more than 25 years of experience by many labs is the formidable plasticity and complexity of nociceptive neurons in the spinal cord. Many experiments are still required to further characterize the precise nature of this plasticity. Since similar cascades can underlie different mechanisms,5 and possible new, unexpected physiological mechanisms remain to be discovered,24 we believe that while the synaptic facilitation observed in the spinal cord during central sensitization may well include features similar to the early phase of homosynaptic LTP in the hippocampus, overall the differences are even greater; its short duration, heterosynaptic nature, and divergent function. William Shakespeare asked, “What’s in a name?” His answer, “That which we call a rose by any other name would smell as sweet,” and while that may indicate in a literary setting that a name is only a convention, in science a name does have specific meaning; and we feel that LTP is perhaps not technically the most accurate name for the synaptic plasticity responsible for the major features of central sensitization.
References
- 1.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R: Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615–626, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Antonov I, Kandel ER, Hawkins RD: The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J Neurosci 19:10438–10450, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CH, Bartsch D, Kandel ER: Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A 93:13445–13452, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collingridge GL, Isaac JT, Wang YT: Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5:952–962, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Constantine-Paton M, Cline HT: LTP and activity-dependent synaptogenesis: The more alike they are, the more different they become. Curr Opin Neurobiol 8:139–148, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Drdla R, Gassner M, Gingl E, Sandkuhler J: Induction of synaptic long-term potentiation after opioid withdrawal. Science 325:207–210, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Frey S, Schollmeier F, Krug M: Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol 490(Pt 3):703–711, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey U, Huang YY, Kandel ER: Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260:1661–1664, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K: Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38:447–460, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD: LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 5:27–33, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J: Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299:1237–1240, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J: Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312:1659–1662, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Kohno T, Moore KA, Woolf CJ: Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci 26:696–705, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Jongen JL, Pederzani T, Koekkoek SK, Shapiro J, van der Burg J, De Zeeuw CI, Huygen FJ, Holstege JC: Autofluorescent flavoprotein imaging of spinal nociceptive activity. J Neurosci 30:4081–4087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koltzenburg M, Lundberg LE, Torebjork HE: Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51:207–219, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Krug M, Lossner B, Ott T: Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull 13:39–42, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Latremoliere A, Woolf CJ: Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain 10:895–926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R: Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458:299–304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison DV, Malenka RC, Nicoll RA: Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci 14:379–397, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Malenka RC, Bear MF: LTP and LTD: An embarrassment of riches. Neuron 44:5–21, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Malenka RC, Nicoll RA: Long-term potentiation–a decade of progress? Science 285:1870–1874, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM: Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104:675–686, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Neves G, Cooke SF, Bliss TV: Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci 9:65–75, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Pozo K, Goda Y: Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66:337–351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandkuhler J, Liu X: Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 10:2476–2480, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Thompson SW, Woolf CJ, Sivilotti LG: Small-caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal rat spinal cord in vitro. J Neurophysiol 69:2116–2128, 1993 [DOI] [PubMed] [Google Scholar]
- 27.van den Broeke EN, van Rijn CM: Biurrun Manresa JA, Andersen OK, Arendt-Nielsen L, Wilder-Smith OH: Neurophysiological Correlates of Nociceptive Heterosynaptic Long-Term Potentiation in Humans. J Neurophysiol 2010. Feb 17; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Wall PD, Woolf CJ: Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol 356:443–458, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolf CJ: Evidence for a central component of post-injury pain hypersensitivity. Nature 306:686–688, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ, Walters ET: Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia. Trends Neurosci 14:74–78, 1991 [DOI] [PubMed] [Google Scholar]


