Keywords: mechanobiology, mechanosensitive ion channels, mechanotransduction, PIEZO1, PIEZO2
Abstract
PIEZO1 and PIEZO2 are mechanosensitive cation channels that are highly expressed in numerous tissues throughout the body and exhibit diverse, cell-specific functions in multiple organ systems. Within the musculoskeletal system, PIEZO1 functions to maintain muscle and bone mass, sense tendon stretch, and regulate senescence and apoptosis in response to mechanical stimuli within cartilage and the intervertebral disc. PIEZO2 is essential for transducing pain and touch sensations as well as proprioception in the nervous system, which can affect musculoskeletal health. PIEZO1 and PIEZO2 have been shown to act both independently as well as synergistically in different cell types. Conditions that alter PIEZO channel mechanosensitivity, such as inflammation or genetic mutations, can have drastic effects on these functions. For this reason, therapeutic approaches for PIEZO-related disease focus on altering PIEZO1 and/or PIEZO2 activity in a controlled manner, either through inhibition with small molecules, or through dietary control and supplementation to maintain a healthy cell membrane composition. Although many opportunities to better understand PIEZO1 and PIEZO2 remain, the studies summarized in this review highlight how crucial PIEZO channels are to musculoskeletal health and point to promising possible avenues for their modulation as a therapeutic target.
STRUCTURE AND FUNCTION OF PIEZO CHANNELS
PIEZO1 and PIEZO2 are multi-pass transmembrane proteins that serve as mechanosensitive cationic ion channels, directly activated by membrane stretch (1–3). Both channels are expressed in numerous mechanosensitive tissues, with PIEZO1 found more prominently in nonsensory tissues exposed to mechanical loading, fluid pressure, and shear, and PIEZO2 found more abundantly in sensory tissues involved in touch and pain (4). First identified in 2010 by Coste et al. (3) as homologs of the genes Fam38a and Fam38b, numerous studies have since demonstrated their expression in nearly all organ systems, including pulmonary, urinary, cardiovascular, musculoskeletal, and nervous systems, where they contribute toward tissue mechanosensation, development, and homeostasis, as well as cellular migration, differentiation, proliferation, apoptosis, and senescence.
PIEZO channels have a unique structure comprised of three subunits, each containing a curved blade with 26 transmembrane helices, which extend outward from a central pore region with 12 transmembrane helices (1, 2, 5). These blades are connected to the central pore by a beam domain (1, 2, 5). The combination of the three subunits forms the large (2,547 residues and 2,822 residues for PIEZO1 and PIEZO2, respectively), propeller-like structure of the PIEZO channels (1, 2, 5). PIEZO1 and PIEZO2 only share 42% homology, and this difference results in structural alterations between the two, with PIEZO1 containing a more dilated central pore when closed (4.5 Å vs. 0.9 Å), reduced transmembrane height (140 Å vs. 170 Å), and a contracted outer diameter (185 Å vs. 280 Å) with more tightly spiraling blades in the clockwise direction (5, 6). Despite these differences, PIEZO1 and PIEZO2 share a similar structure and are believed to gate in a similar manner. Studies have shown PIEZO1 responds directly to membrane stretch, while other studies have suggested that PIEZO2 may require interaction with intracellular and/or extracellular molecules to transduce membrane stretch (5–11).
Multiple studies have shown that PIEZO channels respond to various types of mechanical stimuli that induce membrane stretch, including cellular compression and tension, shear stress, and changes in substrate stiffness, as well as electrical stimuli in the form of voltage shifts (2, 4, 10, 12). Mechanistically, research suggests that membrane stretch deforms the blade regions, with the beam domains potentially acting as a lever arm to convert this deformation into minute motions within the pore to activate the channel and allow cation influx into the cell (1, 13). However, other studies have activated mutant PIEZO1 and PIEZO2 channels missing the proximal end of the beam domain, calling the proposed lever-like function of the beam domain into question (14). Evidence also suggests that the blades induce some warping of the surrounding membrane, which serves to magnify local strain surrounding the channel, increasing sensitivity to membrane stretch (1, 15).
Once activated, PIEZO1 is permeable to most monovalent and divalent cations with a hydrated diameter (diameter of ion plus bound water molecules) less than the open pore diameter of ∼8 Å. PIEZO1 displays the greatest permeability to K+, followed by Na+ and Cs+, with divalent cations Ca2+ and Mg2+ exhibiting lower permeability (16). However, PIEZO1 displays a slight selectivity preference for Ca2+ (3). The high affinity of Ca2+ for PIEZO1 causes Ca2+ to travel slowly through the pore and momentarily block conductance of monovalent ions such as K+ (16). The ion permeability and selectivity of PIEZO2 has not been characterized as clearly, but data suggest it nonselectively conducts cations (3, 17).
The triskelion structure of PIEZO channels allows for direct activation by membrane stretch, with PIEZO1 achieving half-maximal activation at pressures of −27 ± 3.4 mmHg (13). However, interactions with other cellular factors such as the actin cytoskeleton, extracellular matrix (ECM) proteins, integrins, and cell membrane lipid composition, as well as certain small molecules and peptides, can shift the threshold required for activation (13). The synthetic small molecule Yoda1, as well as the more recently discovered Jedi1 and Jedi2, reduce the half-maximal activation pressure of PIEZO1 (−15.1 ± 0.9 and −16.7 ± 2.2 mmHg for Yoda1 and Jedi1, respectively) (12, 13). Evidence suggests that these synthetic compounds alter how the beam domain transduces blade motion to the pore, encouraging activation at lower magnitudes of membrane stretch and energetically stabilizing the open channel configuration (13, 18, 19). Despite their similar effect on PIEZO1, Jedi1 and Jedi2 do not share any structural similarity with Yoda1, with Jedi1 and Jedi2 displaying rapid activation, decay, and reversibility, whereas Yoda1 results in slow activation with no decay and poor reversibility (13). A small inactive form of Yoda1, called Dooku1, may be used to antagonize the effects of Yoda1, but does not inhibit the activity of constitutive PIEZO1 channels (20).
Currently, there are no known small molecule agonists for PIEZO2, and the magnitude of stimuli required for PIEZO2 gating are still being determined (4). Several polycationic channel inhibitors, including ruthenium red, gadolinium chloride, and GsMTx4, effectively block PIEZO1 and PIEZO2 activation (3, 21). Ruthenium red inhibits PIEZO1 and PIEZO2 by blocking the open pore on the extracellular side in a voltage-dependent manner (22). Gadolinium chloride does not interact with ion channels directly, but rather binds to phospholipids and compacts the cellular membrane surrounding the ion channel to prevent opening (23). Similarly, GsMTx4, a peptide derived from spider venom, inserts into the outer layer of the surrounding cell membrane and interferes with its ability to transduce tension to the ion channel. Some models suggest this occurs primarily through increasing the surrounding phospholipids’ deformational capacity (24), whereas others propose the effect is simply due to GsMTx4 taking up space in the membrane, increasing its functional surface area to reduce tension (25).
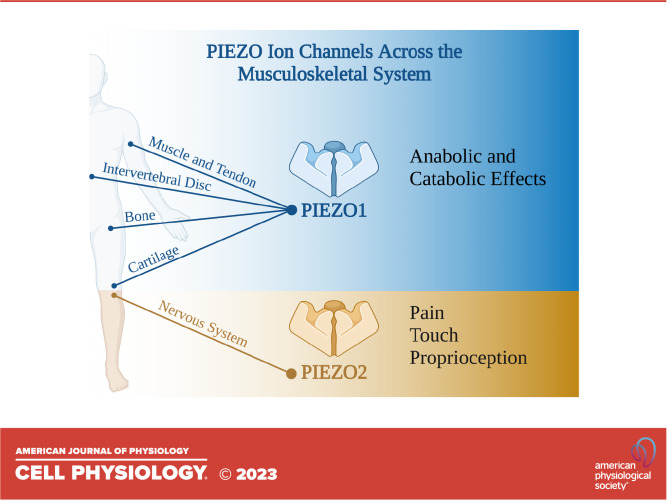
Various mutations in PIEZO1 and PIEZO2 channels can result in a range of phenotypic effects, ranging from minor adaptations to major disease phenotypes, primarily due to how the mutation affects PIEZO activation. Here, we review the contributions of PIEZO1 and PIEZO2 to the development and function of the neuro-musculoskeletal system, with examples of how dysregulation of these channels can have major consequences for tissue function. Finally, we will discuss the potential for modulating PIEZO activity as a therapeutic strategy to treat musculoskeletal diseases and enhance tissue regeneration (Fig. 1).
Figure 1.
PIEZO1 and PIEZO2 play important roles in development and function of multiple tissues in the musculoskeletal and nervous systems, and therapeutic strategies to control PIEZO1 and PIEZO2 activity include targeted inhibition of PIEZOs or other cation channels, and dietary supplementation.
MUSCLE RELIES ON PIEZO1 FOR GROWTH, REGENERATION, AND MAINTENANCE
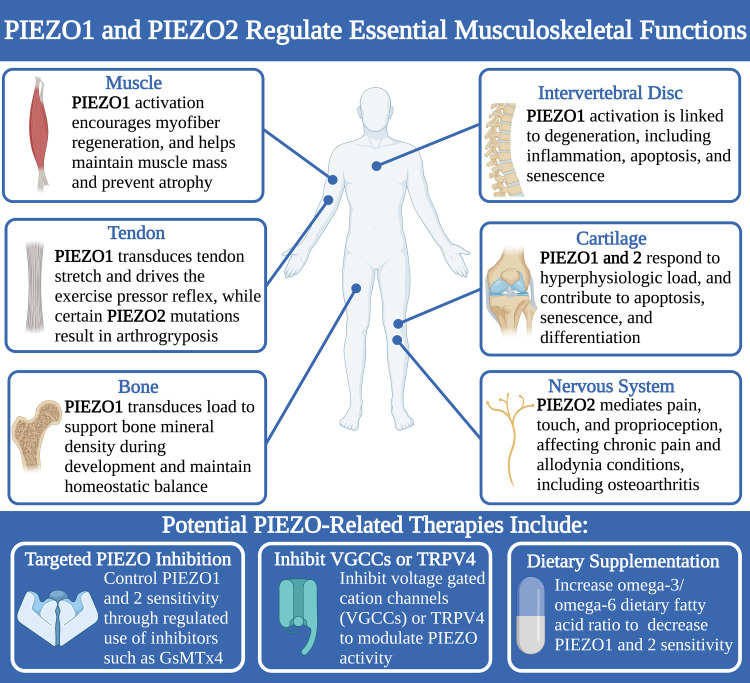
Skeletal muscle is a hierarchically organized tissue that provides stability and movement for the musculoskeletal system (26). Each muscle is composed of bundles of fascicles, which are in turn composed of multiple myofibers (26). Myofibers are long, multinucleated cells formed by the fusion of myoblasts during development (26). Skeletal muscle is well-vascularized and innervated, allowing for coordinated motor activity (26). However, when muscle tissue is damaged or degenerated, skeletal muscle must rely on skeletal muscle stem cells (MuSCs, also referred to as “satellite cells”), a population of quiescent adult stem cells within muscle fibers, for regeneration (27, 28). Activated MuSCs proliferate and subsequently differentiate into myoblasts, which fuse to existing myofibers or generate new ones to repair damaged muscle tissue (28). Multiple studies suggest that PIEZO1 contributes to the mechanosensation required to regulate MuSC activation and proliferation and to maintain muscle mass and prevent muscle atrophy.
Loss-of-function (LOF) studies performed using the MuSC marker Paired Box 7 (Pax7) to drive constitutive Cre recombinase action (a constitutive Pax7Cre;Piezo1f/f mouse line) to knock out Piezo1 in MuSCs suggest that MuSC mechanosensation modulates the dynamics of activation and differentiation via mechanosensitive ion channel PIEZO1, with drastic effects on muscle regeneration outcomes (28, 29). Piezo1 knockout resulted in an 80% reduction in the number of MuSCs and dysregulation of MuSC activation in the remaining population. Piezo1-deficient mice also displayed reduced MuSC activation and proliferation, with most MuSCs remaining in a quiescent state (29). Meanwhile, Yoda1 treatment prevented this shift and increased muscle regeneration. These data indicate that PIEZO1 plays a vital role in regulating and maintaining the MuSC population (28, 29).
Tamoxifen-inducible Pax7CreERT2;Piezo1f/f mice have been used to isolate the effects of PIEZO1 on MuSCs in adult muscle tissue from the effects of Piezo1 depletion during development. This model demonstrated that Piezo1 knockout reduces myogenic differentiation of MuSCs as well as MuSC proliferation following muscular injury (28). Furthermore, injured mice displayed delayed formation of myofibers and reduced myofiber diameter. Most notably, repetitive injury completely ablated the quiescent Pax7+ MuSC population in Pax7CreERT2;Piezo1f/f mice (28). PIEZO1 inhibition also drastically reduced myoblast fusion, a later step in myogenesis following MuSC activation (30). Conversely, treatment with Yoda1 significantly increased myoblast fusion. Piezo1 knockdown also reduced F-actin accumulation, indicating that PIEZO1 may play a role in cytoskeletal remodeling during myoblast differentiation (30).
Evidence suggests that PIEZO1 helps maintain muscle mass and prevent muscle atrophy. Mouse limb immobilization has been correlated with a downregulation of Piezo1, while intramuscular injection with Yoda1 has been shown to downregulate atrophy-associated genes (31). Similarly, tamoxifen-inducible Cre models have been used to achieve skeletal muscle-specific knockdown of Piezo1, resulting in decreased muscle mass, decreased intracellular Ca2+, and increased expression of atrophy-associated genes Klf15 and Il6 upon tamoxifen induction (31). These data align well with clinical findings, such as decreased PIEZO1 expression and increased expression of interleukin-6 (IL6) and other atrophy-associated genes in the skeletal muscle of patients following cast immobilization due to bone fractures (31). Taken together, these data demonstrate that PIEZO1 plays a vital role in the maintenance and regeneration of healthy skeletal muscle and suggest that PIEZO1 may be a key therapeutic target for muscle regeneration and treatment of muscle atrophy.
PIEZO1 TRANSDUCES STRETCH IN TENDON TO CONTROL ADAPTATION TO LOAD AND EXERCISE PRESSOR REFLEX
Tendons are dense, load-bearing connective tissues that transmit muscle forces to bone. Tendons contain a hierarchical structure of type I collagen positioned in a load-bearing direction, producing a highly anisotropic tissue that is ideally suited for transmission of uniaxial tensile strain (32). Tendons are home to a diverse and heterogeneous cell population, including specialized fibroblastic populations known as tenocytes (33, 34). Tenocytes produce ECM to maintain homeostasis in response to changing biomechanical stimuli (32). Single-cell RNA sequencing studies have demonstrated that PIEZO1 is highly expressed in cells positive for tendon-specific markers Scleraxis (Scx) and Mohawk Homeobox (Mkx), and both inhibition and knockout studies suggest it plays key roles in transducing the loading environment of the tendon to the tenocyte, with both local and systemic effects (35–38). Meanwhile, genotyping data have demonstrated that various gain-of-function (GOF) mutations in PIEZO1 range in effect from mild athletic advantages to severe pathologies (36, 38, 39).
Stretch induces a Ca2+ response via PIEZO1 activation in a strain-dependent manner in tendon explants from rat tails (36). Shear stress alone activated a Ca2+ influx in rat tenocytes, indicating that shear stress from collagen fibers rubbing against one another may be the primary form of stimulus experienced by tenocytes during tendon loading. Tendon-specific, tamoxifen-inducible Piezo1 knockout mice using Scx as a Cre recombinase driver (ScxCreERT2;Piezo1f/f mouse line) displayed a 40% reduction in PIEZO1 following tamoxifen injection and resulted in decreased stretch-induced Ca2+ influx, as well as a 10% reduction in fascicle stiffness despite no changes in fascicle diameter (36). The incomplete knockdown of PIEZO1 may have been due to tendon heterogeneity, as other studies have reported multiple tenocyte populations, some of which do not express Scx (33). However, stimulating wild-type fascicles with Yoda1 for 16 days in vitro resulted in a 4.1% increase in stiffness, which correlates well with physiological increases in tendon stiffness observed during high-strain exercise regimens of similar duration, indicating that PIEZO1 activation likely plays a key role in driving anabolic tendon stiffening (36).
PIEZO1 also modulates the exercise pressor reflex, which elevates heart rate and blood pressure via sympathetic nerve activity during skeletal muscle and tendon stretch (40). Achilles’ tendon stretch in rats elicited a strong exercise pressor reflex, with intra-arterial injection of GsMTx4 significantly reducing that response (37). Intermittent muscle contraction via sciatic nerve stimulation likewise induced a strong exercise pressor reflex, which was reduced by intra-arterial injection of GsMTx4 (37). PIEZO1 inhibition by intra-arterial injection of GsMTx4 has also been reported to reduce the exaggerated exercise pressor reflex observed in rats with induced type 1 diabetes mellitus (35). Since GsMTx4 is a nonspecific PIEZO1 inhibitor, a transient receptor potential cation (TRPC) channel-specific blocker (SKF 96365) was also used, but SKF 96365 injection did not replicate the reduced exercise pressor reflex observed following GsMTx4 injection (35). These studies indicate that PIEZO1 may transduce muscle and tendon stretch to drive the exercise pressor reflex, and that targeted blockade of PIEZO1 in muscle and tendon may have therapeutic potential in instances of exercise pressor reflex dysregulation.
The GOF mutations of Piezo1 that affect tendon do so primarily through increasing PIEZO1 activation. The R2482H GOF Piezo1 mutation has been shown to result in significantly greater jumping distance and maximum running speed, compared with wild-type controls (38). Tenocytes from both constitutive and tamoxifen-inducible Piezo1R2482H mice displayed higher Ca2+ influx and upregulation of tendon-specific marker genes compared with wild-type tenocytes, when treated with Yoda1 (38). Piezo1R2482H mice had wider Achilles tendons with greatly increased elastic limit, which resulted in increased compliance, extension, and energy storage during loading (38). In humans, the E756del mutation in PIEZO1 results in a similar, although milder, GOF to the R2482H mutation used in the mouse model. The frequency of E756del variants in professional sprinting, jumping, and throwing human athletes is significantly higher than in nonathletic controls (38). Human carriers of the E756del GOF mutation also performed better at one-legged countermovement jumps compared with individuals with typical PIEZO1 (36). This suggests that PIEZO1 GOF mutations in tendon may increase athletic performance for activities requiring sudden bursts of power and may be a target for improving tendon performance.
However, certain PIEZO mutations affecting muscle and tendon have also shown significant pathologic effects. Mutations of PIEZO2 have been clinically associated with arthrogryposis, a phenotype of over 300 different known disorders, characterized by multiple joint contractures (41). Specifically, studies have implicated PIEZO2 mutations to several subtypes of distal arthrogryposis (DA), a subgroup referring to patients who specifically demonstrate distal joint contractures (42–45). Dominant GOF mutations of PIEZO2 have been shown to result in DA type 3 (DA3/Gordon Syndrome), DA type 5 (DA5), and Marden–Walker syndrome (39, 46, 47). Patients with DA5 suffer from distal contractures, which are accompanied by additional symptoms such as limited ocular motility and ptosis, and in some cases by pulmonary disease (39). Certain mutations are shared between syndromes, suggesting that each of these syndromes could be a unique manifestation of the same underlying condition (39). This further underscores the need to conduct more robust genome-wide association studies, as well as more mechanistic studies to understand how PIEZO mutations cause malfunction and how these defects lead to disease progression.
PIEZO1 DRIVES ANABOLIC BONE REMODELING DURING DEVELOPMENT AND HOMEOSTASIS
Bone is a mineralized connective tissue in the musculoskeletal system that provides structure to the body and permits movement. In addition, bone supports and protects internal organs, houses bone marrow, functions as a calcium and phosphate mineral reservoir, and acts as an endocrine organ to regulate energy metabolism (48). Appropriate levels of mechanical loading are required for bone homeostasis, as well as for skeletal development, growth, and fracture repair (49–51). Bone adaptation to mechanical stimuli is primarily accomplished through mechanotransduction by osteocytes, which respond to mechanical loading by producing molecules that regulate bone remodeling (52, 53). Ion channel activation and subsequent calcium signaling represent an early response to mechanical activation in bone-resorbing osteoclasts and bone-forming osteoblasts (54–56). As such, evidence suggests that PIEZO1 plays a key role in the development, homeostasis, and healing of bone, modulating interactions between osteoblast and osteoclast populations to encourage anabolic bone remodeling in response to increased loading.
Several animal studies have associated Piezo1 deficiency during development with decreased bone formation rate, decreased bone size, and increased fracture risk. Cre recombinase models utilizing paired-related homeobox 1 (Prrx1), bone gamma carboxyglutamate protein/osteocalcin (Bglap/Ocn), or Runx family transcription factor 2 (Runx2) as osteoblast-specific Cre drivers have been used in multiple studies to constitutively knock out Piezo1 (Prrx1Cre;Piezo1f/f, OcnCre;Piezo1f/f, and Runx2Cre;Piezo1f/f mouse lines, respectively) (57–60). These studies all report decreased bone mass, bone shortening, and skeletal defects (57–60). Spontaneous fractures in load-bearing distal bones were observed in mice within days of birth, indicating that PIEZO1 plays a key role in the anabolic response of osteoblasts in load-bearing bones (57, 59, 60). Piezo1 knockout increased differentiation of bone marrow monocytes into osteoclasts, likely driven by decreased osteoblastic expression of several collagens, including Col1a1 reported in OcnCre;Piezo1f/f mice, as well as Col2a1, Col9a2, and Col10a1 reported in Prrx1Cre;Piezo1f/f mice (57, 58). Osteoclast markers cathepsin K (Ctsk) and tartrate-resistant acid phosphatase (Acp5/Trap) were increased in Prrx1Cre;Piezo1f/f mice, while osteoblast marker Sp7 transcription factor (Sp7) was decreased (59). Meanwhile, a model using Ctsk as an osteoclast-specific Cre driver to constitutively knock out Piezo1 (CtskCre;Piezo1f/f) did not show any reduction in bone mass or changes in bone resorption (57). This suggests that PIEZO1 primarily transduces load in osteoblasts, with an indirect effect on osteoclasts via interaction between osteoblast and osteoclast populations (57). RNA-sequencing on the developing humerus and femur tissues revealed that PIEZO1 activation in osteoblasts drives expression of protein phosphatase 3 catalytic subunit alpha (Ppp3ca), which induces expression of nuclear factor of activated T-cells (Nfat), Yes-1 associated transcription factor (Yap), and catenin beta 1 (Ctnnb1), which regulates expression of Col2a1 and Col9a2 to modulate osteoclastogenesis (59). These models suggest that Piezo1 deficiency inhibits this pathway, shifting bone marrow monocytes toward osteoclast production and increasing bone catabolism, while also decreasing bone mass and size. These changes ultimately result in a high number of spontaneous fractures.
PIEZO1 has also been shown to regulate adult bone physiology, with inducible knockout models demonstrating decreased bone mass and increased fracture risk, without affecting bone size. Tamoxifen-inducible Cre recombinase models utilizing collagen type I (Col1) or Ocn as osteoblast-specific Cre drivers (Col1CreERT2;Piezo1f/f or OcnCreERT2;Piezo1f/f) were used to induce Piezo1 knockout in adult mice. Col1CreERT2;Piezo1f/f mice displayed significantly reduced trabecular bone mass and cortical bone thickness, along with an increased osteoclast population and reduced collagen expression following Piezo1 knock out at 8 wk of age (57). Hindlimb suspension models applied to wild-type mice for 7–28 days resulted in hindlimb bone loss and increased osteoclast number (57, 58). No further bone loss beyond that caused by the Piezo1 knockout alone was observed when the hindlimb suspension model was applied to Col1CreERT2;Piezo1f/f mice or OcnCreERT2;Piezo1f/f mice, indicating that PIEZO1 mediates bone resorption in the absence of loading (57, 58). This result has also been found using botulinum toxin A injection to induce hindlimb paralysis in adult wild-type and constitutive Piezo1 knockout mice utilizing Sp7 as an osteoblast-specific Cre driver (Sp7CreERT2;Piezo1f/f) (59). Clinically, PIEZO1 is also significantly reduced at the mRNA and protein level in patients with osteoporosis (58), and several single-nucleotide polymorphisms (SNPs) within PIEZO1 are closely associated with variations in bone mineral density (61).
These studies demonstrate that PIEZO1 plays a vital role in the development and continued homeostatic maintenance of adult bone, with disruption of PIEZO1 being closely tied with disrupted bone development in immature load-bearing bones and bone loss in adult tissues. Since PIEZO1 is a critical regulator of bone mass, it may be a potential therapeutic target for osteoporosis patients and patients with bone fractures.
PIEZO1 IS ASSOCIATED WITH INTERVERTEBRAL DISC DEGENERATION AND NUCLEUS PULPOSUS CELL SENESCENCE
The intervertebral disks (IVDs) connect the vertebrae and are composed of the outer annulus fibrosus (AF), an inner gelatinous nucleus pulposus (NP), and cartilaginous endplates (62). With degeneration, NP cells favor a catabolic state resulting in ECM destruction and apoptosis (63, 64). PIEZO1 is expressed at the mRNA and protein level in NP cells and is overexpressed in degenerated tissue or in response to mechanical stress (65–70). PIEZO1 contributes to inflammation, apoptosis, and transduction of changes in substrate properties that lead to senescence and understanding the role of PIEZO1 in these processes is critical to identify potential targets for treating IVD degeneration.
The activation of PIEZO1 has been shown to regulate mechanical cues to inflammatory signals in NP cells (66, 67, 70). Inflammation is implicated in the pathogenesis of IVD degeneration, exhibited by upregulation of proinflammatory cytokines (71). PIEZO1 signaling has been shown to activate inflammasome NLR family pyrin domain containing 3 (NLRP3), leading to the production of interleukin-1β (IL-1β) (66, 67, 71). Suppressing PIEZO1 using siRNA reduced proinflammatory effects and dysfunctional metabolism, emphasizing the role of PIEZO1 in contributing to the degenerative IVD environment and its suppression as a protective tool (67).
Along with inflammation, excessive mechanical loading during IVD degeneration can induce cell apoptosis (72). Although the exact mechanisms are unknown, studies have shown apoptosis can be promoted through altering mitochondrial membrane potential triggered by PIEZO1 activation (65, 70). The application of mechanical stretch to NP cells in monolayer led to changes in mitochondrial potential and an increased apoptotic rate (65). Knocking down PIEZO1 via short hairpin RNA (shRNA) decreased changes in mitochondrial membrane potential and cell apoptosis (65). Similarly, mitochondrial dysfunction and increased apoptosis has been observed after compression of human IVDs, which was partly rescued by treatment with GsMTx4 (70).
The ECM mechanical environment is a critical regulator of NP differentiation and phenotype (73, 74). Degenerated NP tissue obtained from surgical samples display increased ECM stiffness, concurrent with overexpression of PIEZO1 (68–70). Specifically, PIEZO1-mediated sensation of increased substrate stiffness has been linked to the accumulation of reactive oxygen species (ROS), leading to apoptosis, autophagy, and senescence (70). Knocking down PIEZO1 in NP cells plated on stiff substrates reduced senescence, oxidative stress, and endoplasmic reticulum stress to soft substrate levels (68). PIEZO1 activation was found to trigger senescence in stiff, degenerated NP tissue by increasing the production of periostin (POSTN) (69). POSTN has been identified as a key player of IVD degeneration, increasing senescence and activating the nuclear factor kappa B (NF-κB) signaling pathway, which in turn leads to heightened Postn expression (69). This degenerative positive feedback loop was triggered by Yoda1 and blocked using GsMTx4 (69).
Taken together, PIEZO1 signaling in NP cells has been linked to the activation of intracellular pathways including inflammation, apoptosis, autophagy, and senescence—processes that drive IVD degeneration. Therefore, PIEZO1 may be an appealing therapeutic target for disk degeneration induced by inflammation and senescence. Further investigating the role of PIEZO2 in pain sensation in IVD could be another interesting path to develop effective treatments for low back pain.
Finally, although the role of PIEZO in NP mechanotransduction has been characterized, studies have yet to determine the ion channel’s role in the AF and cartilaginous endplates. Chondrocytes of the cartilaginous endplates have been shown to be mechanoresponsive (75–77). As injurious mechanical loading signals are transduced to chondrocytes in articular cartilage via PIEZO1, it would be of interest to determine whether chondrocytes in cartilaginous endplates are similarly regulated. Similarly, cells in the AF exhibit a response to mechanical forces dependent on the magnitude, frequency, and duration of loading (78–80). To gain a full understanding of the role of PIEZO channels in the IVD and fully harness it as a therapeutic target for low back pain, future studies may focus on the expression of PIEZO in the AF and cartilaginous end plates along with its role in regulating cells in these tissues in response to mechanical forces.
PIEZO CHANNELS TRANSDUCE HIGH-MAGNITUDE STRAINS TO MODULATE INFLAMMATION, APOPTOSIS, AND SENESCENCE IN CHONDROCYTES
Articular cartilage is an avascular and aneural tissue that lines the ends of long bones in diarthrodial joints and facilitates near-frictionless joint motion. Cartilage withstands various types of loading, including hydrostatic pressure, compression, tension, and shear, often at magnitudes much higher than body weight. Chondrocytes, the primary native cell population in cartilage, maintain dense ECM protein concentrations in dynamic equilibrium by responding to mechanical cues, specifically mechanical compression, that are propagated through the ECM (81). Chondrocytes sense mechanical loads through mechanically activated ion channels, including PIEZO1, PIEZO2, and transient receptor potential vanilloid 4 (TRPV4). PIEZO channels specifically have been implicated in pathogenic remodeling of cartilage in response to hyperphysiologic loading and changes in environmental cues, as well as driving senescence and apoptosis (82).
Loading magnitude and changes in environmental cues can modulate PIEZO activity in chondrocytes. Chondrocytes have been shown to require an average of 256 nm cell indentation to induce a Ca2+ influx, and this response was significantly reduced by knocking down either Piezo1 or Trpv4 (83). Cyclic tensile strain ranging from 3% to 18% deformation induced an increase in TRPV4, PIEZO1, and PIEZO2 protein levels in primary murine chondrocytes, and inhibiting these channels decreased Ca2+ influx at each loading magnitude (84). Increasing substrate stiffness decreased PIEZO1 and PIEZO2 protein expression (85). Mechanical compression of whole chondrocytes to >50% deformation via atomic force microscopy induced high levels of Ca2+ influx in primary porcine chondrocytes, mediated synergistically by PIEZO1 and PIEZO2 (86). Finally, applying one hour of cyclic loading to human chondrocytes encapsulated in polyethylene glycol (PEG) hydrogels for up to 14 days has been demonstrated to increase PIEZO1 protein expression (87).
Inflammatory damage and degeneration often associated with osteoarthritis (OA) can also affect PIEZO1 expression. PIEZO1 upregulation has been shown in primary porcine chondrocytes treated with interleukin-1α (IL-1α), which mimics similar increases in expression observed in osteoarthritic cartilage (88). Heightened PIEZO1 expression elevated Ca2+ transients and loosened the F-actin network, which decreased cellular Young’s modulus (88). Upon inhibition of PIEZO1 via GsMTx4 or depletion of PIEZO1 with PIEZO1-targeting small interfering RNA (siRNA), actin loosening was no longer observed (88). These data suggest that PIEZO1 contributes to a pathologic feedforward mechanism by elevating calcium transients, loosening the actin cytoskeleton, and contributing to a pathogenetic remodeling of cartilage, contributing to the development of OA (88).
Evidence suggests that expression and activation of PIEZO1 may be linked to senescence in chondrocytes. PIEZO1 mRNA and protein levels were significantly increased in human chondrocytes induced to senescence, and hyperphysiologic strain elevated senescence pathologies such as DNA damage, enlarged cell nuclei, and increased expression of Il6, cyclin-dependent kinase inhibitor 1A (Cdkn1a/P21) and cyclin-dependent kinase inhibitor 2A (Cdkn2a/P16) in murine and human chondrocytes (89, 90). Application of hyperphysiologic compressive loads to murine knee joints also increased the number of Cdkn1a/P21- and Cdkn2a/P16-positive cells and decreased proteoglycan production (89). PIEZO1 knockdown reversed senescence-related phenotypes, lowering senescence-related gene expression, ROS levels, and intracellular Ca2+ concentration (90). Yoda1 treatment increased senescence phenotypes, whereas intracellular Ca2+ chelation inhibited the effects of Yoda1, indicating the importance of Ca2+ signaling to the development of senescence (90).
Various studies have shown that intracellular Ca2+ concentration, modulated by PIEZO1 and PIEZO2, plays a critical role in cell death and apoptosis as well. Hyperphysiologic compression of bovine cartilage explants induced cell death and glycosaminoglycan release (91). Meanwhile, knocking down PIEZO1 in human chondrocytes was shown to reduce cell death induced by corticotropin-releasing factor receptor 1 antagonist (92). Urocortin-1 (UCN), a member of the corticotropin-releasing factor family of genes, mitigated cell death and Ca2+ overload experienced when PIEZO1 was activated either by Yoda1 or injurious mechanical loads in porcine cartilage explants (92, 93). Chondrocytes from patients with OA displayed reduced mRNA and protein levels of G protein coupled estrogen receptor (GPER), a key antiapoptotic protein. PIEZO1 knockdown increased GPER expression at mRNA and protein levels in response to 20% tensile loading (94). PIEZO1 inhibition via GsMTx4 has also been shown to protect against apoptosis induced by hyperphysiologic mechanical stress (95). Recent work has also shown that PIEZO1 activation drives apoptosis and cartilage degeneration by inducing lysosome dysfunction in C57BL/6 murine chondrocytes (96). However, these pathologies were completely abolished by knocking down PIEZO1 with siRNA or inhibiting with GsMTx4 (96). Mice with induced OA via destabilization of the medial meniscus were also treated with GsMTx4, and displayed suppressed OA progression, cartilage degeneration, and apoptosis within the joint (96).
The involvement of PIEZO1 in multiple chondrocyte processes such as inflammation, senescence, and apoptosis suggests that PIEZO1 activation likely modulates gene expression. Although the mechanism by which PIEZO1 activation regulates gene expression and which genes it regulates are still being explored, it is known that PIEZO1 activation can trigger several transient cell responses such as cytoskeletal remodeling and activation of the YAP-Tafazzin (TAZ) pathway (59, 88). Furthermore, ongoing transcriptomics work has suggested that several genes may be uniquely responsive to PIEZO1, including a gene regulatory network governed by hub gene hypoxia inducible factor 1 subunit alpha (HIF1A) (97).
Taken together, these studies highlight the important role of PIEZO channels in articular cartilage and suggest that targeting PIEZO channels to reduce inflammation, senescence, and apoptosis in response to mechanical loading has therapeutic potential. However, more studies are required to investigate the down-stream effects of modulating the activity of these channels before development of potential therapeutics.
PIEZO2 TRANSDUCES PAIN AND TOUCH SENSATION, AND REGULATES PROPRIOCEPTION
The nervous system contains multiple mechanoresponsive cell types sensitive to a wide array of signals. Noxious stimuli activate sensory neurons known as nociceptors, which are involved in a range of chronic pain conditions including peripheral neuropathies and even OA. Touch-related information is transduced by Merkel cells in the epidermis, while limb coordination is regulated by proprioceptive neurons. All these cells rely on mechanosensation via PIEZO2, implicating PIEZO2 in the processes of pain, touch, and proprioception.
PIEZO2 LOF studies have implicated PIEZO channels in mechanical pain and touch sensation, both of which affect musculoskeletal health. Neural cell Piezo2 deletion in mice was shown to impair touch but sensitize mice to mechanical pain (98, 99). Humans with PIEZO2 LOF report sensitivity to noxious stimuli but insensitivity to gentle touch (99). Mice lacking Piezo2 in both sensory neurons and Merkel cells had a profound loss of touch sensation but had unaffected pain phenotypes, indicating distinct roles in touch and pain sensation (100). Piezo2 knockout abrogated nerve growth factor (NGF)-induced sensitization of afferent neurons in murine bone (101). PIEZO2 has been shown to signal through Rap guanine nucleotide exchange factor 3 (RAPGEF3/EPAC1), a cyclic AMP sensor, to induce mechanical allodynia in neuropathic and bone cancer pain (102–104). In addition, Piezo2 LOF in mice attenuated mechanically stimulated pain in peripheral neuropathy (105). Intriguingly, PIEZO1 rescued defective touch due to Piezo2 deletion in mice, indicating that PIEZO1 may also play a role in touch (98).
PIEZO2 may also play a key role in vascular components of the synovial membrane and infrapatellar fat pad, which is known to transduce mechanical stimuli and mediate chronic pain in OA (106). Patients with OA had colocalized PIEZO1 and PIEZO2 expression in blood vessels of the synovial membrane and infrapatellar fat pad, compared with healthy controls, suggesting vascular remodeling may be a facet of OA progression (107). Nociceptor-specific, Piezo2 knockout mice are protected from NGF-induced joint pain in OA, further supporting that PIEZO2 is involved in OA joint pain (108).
In addition to the basic sensations of pain and touch, PIEZO2 is a major mechanotransducer of mammalian proprioceptors where a lack of PIEZO2 results in severely uncoordinated body movements and limb positions in mice and impaired performance on behavioral tests (109, 110). Humans with LOF variants of PIEZO2 had decreased joint proprioception but were still able to walk, talk, and write (111). Piezo2 deletion in proprioceptive neurons in mice resulted in hip dysplasia and spinal malalignment, which are conditions also seen in humans with PIEZO2 mutations (111, 112). However, loss of Piezo2 in osteogenic or chondrogenic lineages does not reproduce these skeletal phenotypes in mice (112). This distinction suggests that PIEZO2 may be an indirect regulator of skeletal morphogenesis through the proprioceptive system, potentially by disturbing normal muscle activity during development.
These studies together portray PIEZO2 as an integral mechanosensor in the nervous system, transducing pain, touch, and proprioceptive signals. Further research may further resolve the role of PIEZO2 in chronic pain and allodynia-associated conditions and reveal new areas of therapeutic potential.
POTENTIAL THERAPEUTIC APPLICATIONS OF PIEZO MODULATORS
The broad expression and activation of PIEZO channels in neuro-musculoskeletal systems make them an attractive therapeutic target to control pain and enhance regeneration following injury (113). Although the exact downstream cellular signaling cascades of PIEZO1 and PIEZO2 activation are still being characterized, PIEZO channels can clearly be activated by inflammation and play a key role in initiating apoptosis following high strain mechanical stress (86). Cell death and inflammation are key processes involved in tissue regeneration after injury, and the ability to modulate these processes via PIEZO ion channels holds great therapeutic promise. However, given the broad expression of PIEZO channels, their modulation could have detrimental off-target effects and preclinical therapeutic development should consider tissue-specific delivery mechanisms.
GsMTx4 blocks the activation of cationic stretch-activated ion channels, including PIEZO1 and PIEZO2, by changing membrane properties. Studies have shown that GsMTx4 can decrease chondrocyte cell death following injurious loading, suggesting a potential therapeutic use for post-traumatic OA (86). Moreover, studies have shown GsMTx4 to be effective in slowing the progression of Duchenne muscle dystrophy, increasing the functionality of the skeletal muscles, and elevating the neurite growth (114, 115). GsMTx4 treatment may be a potential therapy for PIEZO-related diseases; however, its side effects and toxicity to other tissues should be further evaluated.
As an alternative, several studies have suggested that the inhibition of voltage-gated ion channels (VGCCs) can reduce mechanosensitivity. Activation of mechanosensitive ion channels such as PIEZO channels has been proposed to increase intracellular cation concentration, which increases cell membrane potential and triggers activation of VGCCs (116). Multiple studies suggest that L-type VGCCs inhibitors reduce mechanosensitivity by decreasing the response to direct mechanical compression, stretch, and shear stress, which has been shown to protect the cartilage from degradation and OA progression (86, 116–119). This not only demonstrates the mechanosensory nature of the VGCCs (117), but also indicates a potential link between the functionality of the voltage- and mechano-gated ion channels. Since VGCC inhibitors verapamil and nifedipine are FDA-approved for chest pain and high blood pressure, developing alternative musculoskeletal applications may yield novel strategies for managing PIEZO-associated diseases (119).
In addition to VGCCs, various studies have investigated the link between PIEZO channels and other mechanosensitive ion channels, including TRPV4. Activation of PIEZO channels by shear stress activated TRPV4 in murine pancreatic acinar cells (120). Moreover, blocking the TRPV4 channel mitigated pancreatitis pathologies that occur by PIEZO channel activation (120–122). Therefore, blocking TRPV4 instead of PIEZO channels is an alternative strategy to mitigate some PIEZO-associated pathologies. However, this also indicates that the therapeutic use of ion channel agonists or antagonists should be further evaluated in terms of their down-stream effects and modulation of other ion channels.
Instead of directly blocking PIEZO channels, specific dietary fatty acid species may be incorporated into the cell membrane to influence fluidity, stiffness, and cell signaling. Membrane lipid composition and cholesterol organization have been shown to influence PIEZO activity (123, 124). For example, depletion of phosphoinositides from the plasma membrane inhibits PIEZO channels (125, 126). Increased cholesterol in the plasma membrane increases stiffness and sensitizes PIEZO mechanical activation (127). Moreover, polyunsaturated fatty acids can also alter PIEZO channel activation to influence cellular mechanosensitivity (128). Eicosatetraenoic acid, docosahexaenoic acid, and margaric acid mediate the PIEZO channel sensitivity to mechanical indentation, whereas palmitic acid and stearic acid elevate autophagy, alter the activation of NF-κB pathways, and influence differentiation and proliferation in human chondrocytes through altered PIEZO signaling (70, 129–133). However, treating chondrocytes with lauric acid had the opposite effect and inhibited autophagy activation (70, 130–133). As such, dietary supplementation of specific fatty acid species may be a strategy to fine-tune cell membrane composition, thereby modulating PIEZO activity to restore optimal cellular mechanosensation. One clinical population that may especially benefit from PIEZO-modulating therapeutics are obese individuals. Obesogenic diets, especially those with high omega-6 content, increase OA severity in rodents and further exacerbate joint damage following injury (128, 134–138). However, mice fed an omega-3 enriched diet and those with a transgene to convert omega-6 to omega-3 fatty acids show less severe OA pathology (128, 139–141). Therefore, supplementation of specific fatty acids may be a preventative strategy to maintain cartilage integrity in obese individuals.
Obesity and type II diabetes are also risk factors for delayed wound healing and fracture repair (142). Both genetically induced (143) and diet-induced (144–146) obese mice display delayed fracture repair in part due to increased systemic inflammation and decreased vascular formation at the fracture site (143). PIEZO1 is a key mechanosensitive ion channel in the epithelial cells of the bone vasculature, and tissue-specific deletion of the Piezo1 gene in this cell population leads to impaired fracture healing (147). Furthermore, Piezo1 is expressed in periosteal stem cells and activation of PIEZO1 with Yoda1 improves fracture healing in a tail suspension unloading mouse model (148). Therefore, individuals with obesity or type II diabetes who are at a higher risk for impaired fracture repair may be a clinical population that would especially benefit from PIEZO modulation of mechanically gated ion channels. However, since post-traumatic injuries are an independent risk factor for OA, additional research is necessary to determine the ideal timing of PIEZO modulation following injury for optimal fracture repair and OA prevention.
CONCLUSIONS
The broad expression and activation of PIEZO channels in the neuro-musculoskeletal system highlights them as an attractive therapeutic target to control pain and enhance regeneration following injury (116). Although PIEZO inhibitors hold therapeutic promise for musculoskeletal aging, injury, and disease, PIEZO expression in other tissues such as the vasculature and sensory neurons regulating lung inflation should be considered and carefully monitored in preclinical studies (149–151). In addition, sexually dimorphic responses have been observed in the modulation of other mechanosensitive ion channels such as TRPV4, so it is imperative for preclinical studies investigating PIEZO modulation to include both sexes to maximize translational impact (152). As studies continue to reveal new functions and diseases associated with these ion channels, the potential for PIEZO1- and PIEZO2-based therapies may expand further.
GRANTS
This work was supported by the Shriners Hospitals for Children and the National Institutes of Health Grants AG46927, AG15768, AR080902, AR072999, AR073752, AR074992, AR078949, AR079045, and AR079260.
DISCLOSURES
F. Guilak is an employee and shareholder of Cytex Therapeutics Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.S., D.P., E.V.E., K.H.C., J.M.G.-C., Z.H., Y.S.K., A.O., F.Q., N.R., and F.G. conceived and designed research; A.S. and D.P. prepared figures; A.S., E.V.E., K.H.C., J.M.G.-C., Z.H., Y.S.K., A.O., F.Q., N.R., and D.P. drafted manuscript; A.S., D.P., E.V.E., K.H.C., J.M.G.-C., Z.H., Y.S.K., A.O., F.Q., N.R., and F.G. edited and revised manuscript; A.S., D.P., E.V.E., K.H.C., J.M.G.-C., Z.H., Y.S.K., A.O., F.Q., N.R., and F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Sara Oswald for technical editing. Figure 1 and Graphical Abstract created with BioRender and published with permission.
Footnotes
*A. Savadipour and D. Palmer contributed equally as first authors on this manuscript.
REFERENCES
- 1. Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature 554: 481–486, 2018. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin Y-C, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature 573: 230–234, 2019. doi: 10.1038/s41586-019-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu J, Lewis AH, Grandl J. Touch, tension, and transduction—the function and regulation of Piezo ion channels. Trends Biochem Sci 42: 57–71, 2017. doi: 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, Li Y, Lei J, Li X, Xiao B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 573: 225–229, 2019. doi: 10.1038/s41586-019-1505-8. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong M-Q, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature 554: 487–492, 2018. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]
- 7. Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng C-A, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7: 10366, 2016. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 channels are inherently mechanosensitive. Cell Rep 17: 1739–1746, 2016. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4: e12088, 2015. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moroni M, Servin-Vences MR, Fleischer R, Sánchez-Carranza O, Lewin GR. Voltage gating of mechanosensitive PIEZO channels. Nat Commun 9: 1096, 2018. doi: 10.1038/s41467-018-03502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin KC, Park HJ, Kim JG, Lee IH, Cho H, Park C, Sung TS, Koh SD, Park SW, Bae YM. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci Rep 9: 6446, 2019. doi: 10.1038/s41598-019-42492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M, Patapoutian A. Chemical activation of the mechanotransduction channel Piezo1. eLife 4: e07369, 2015. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q, Rao Y, Zu L, He W, Xiao B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun 9: 1300, 2018. doi: 10.1038/s41467-018-03570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taberner FJ, Prato V, Schaefer I, Schrenk-Siemens K, Heppenstall PA, Lechner SG. Structure-guided examination of the mechanogating mechanism of PIEZO2. Proc Natl Acad Sci USA 116: 14260–14269, 2019. doi: 10.1073/pnas.1905985116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haselwandter CA, MacKinnon R. Piezo’s membrane footprint and its contribution to mechanosensitivity. eLife 7: e41968, 2018. doi: 10.7554/eLife.41968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gnanasambandam R, Bae C, Gottlieb PA, Sachs F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One 10: e0125503, 2015. doi: 10.1371/journal.pone.0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G, Beyder A. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 595: 79–91, 2017. doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wijerathne TD, Ozkan AD, Lacroix JJ. Yoda1's energetic footprint on Piezo1 channels and its modulation by voltage and temperature. Proc Natl Acad Sci USA 119: e2202269119, 2022. doi: 10.1073/pnas.2202269119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin Y-C, Pham CN, Lacroix JJ, Luo Y. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun 10: 4503, 2019. doi: 10.1038/s41467-019-12501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, Hall SJ, Gaunt HJ, Ludlow MJ, Foster R, Beech DJ. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol 175: 1744–1759, 2018. doi: 10.1111/bph.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50: 6295–6300, 2011. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483: 176–181, 2012. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys J 98: 1018–1027, 2010. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430: 235–240, 2004. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 25. Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI, Suchyna TM. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J 112: 31–45, 2017. doi: 10.1016/j.bpj.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukund K, Subramaniam S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med 12: e1462, 2020. doi: 10.1002/wsbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernareggi A, Bosutti A, Massaria G, Giniatullin R, Malm T, Sciancalepore M, Lorenzon P. The state of the art of Piezo1 channels in skeletal muscle regeneration. Int J Mol Sci 23: 6616, 2022. doi: 10.3390/ijms23126616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng Y, Du J, Günther S, Guo X, Wang S, Schneider A, Zhu L, Braun T. Mechano-signaling via Piezo1 prevents activation and p53-mediated senescence of muscle stem cells. Redox Biol 52: 102309, 2022. doi: 10.1016/j.redox.2022.102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma N, Chen D, Lee J-H, Kuri P, Hernandez EB, Kocan J, Mahmood H, Tichy ED, Rompolas P, Mourkioti F. Piezo1 regulates the regenerative capacity of skeletal muscles via orchestration of stem cell morphological states. Sci Adv 8: eabn0485, 2022. doi: 10.1126/sciadv.abn0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ortuste Quiroga HP, Ganassi M, Yokoyama S, Nakamura K, Yamashita T, Raimbach D, Hagiwara A, Harrington O, Breach-Teji J, Asakura A, Suzuki Y, Tominaga M, Zammit PS, Goto K. Fine-tuning of Piezo1 expression and activity ensures efficient myoblast fusion during skeletal myogenesis. Cells 11: 393, 2022. doi: 10.3390/cells11030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirata Y, Nomura K, Kato D, Tachibana Y, Niikura T, Uchiyama K, Hosooka T, Fukui T, Oe K, Kuroda R, Hara Y, Adachi T, Shibasaki K, Wake H, Ogawa W. A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy. J Clin Invest 132: 1–13, 2022. doi: 10.1172/JCI154611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorpe CT, Screen HR. Tendon structure and composition. Adv Exp Med Biol 920: 3–10, 2016. doi: 10.1007/978-3-319-33943-6_1. [DOI] [PubMed] [Google Scholar]
- 33. Kendal AR, Layton T, Al-Mossawi H, Appleton L, Dakin S, Brown R, Loizou C, Rogers M, Sharp R, Carr A. Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. Sci Rep 10: 13939, 2020. doi: 10.1038/s41598-020-70786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buschmann J, Bürgisser GM. Biomechanics of Tendons and Ligaments: Tissue Reconstruction and Regeneration. Duxford, UK: Elsevier/Woodhead Publishing, 2017, p. 340. [Google Scholar]
- 35. Grotle A-K, Huo Y, Harrison ML, Ybarbo KM, Stone AJ. GsMTx-4 normalizes the exercise pressor reflex evoked by intermittent muscle contraction in early stage type 1 diabetic rats. Am J Physiol Heart Circ Physiol 320: H1738–H1748, 2021. doi: 10.1152/ajpheart.00794.2020. [DOI] [PubMed] [Google Scholar]
- 36. Passini FS, Jaeger PK, Saab AS, Hanlon S, Chittim NA, Arlt MJ, Ferrari KD, Haenni D, Caprara S, Bollhalder M, Niederöst B, Horvath AN, Götschi T, Ma S, Passini-Tall B, Fucentese SF, Blache U, Silván U, Weber B, Silbernagel KG, Snedeker JG. Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans. Nat Biomed Eng 5: 1457–1471, 2021. doi: 10.1038/s41551-021-00716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 310: H1233–H1241, 2016. doi: 10.1152/ajpheart.00974.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamichi R, Ma S, Nonoyama T, Chiba T, Kurimoto R, Ohzono H, Olmer M, Shukunami C, Fuku N, Wang G, Morrison E, Pitsiladis YP, Ozaki T, D'Lima D, Lotz M, Patapoutian A, Asahara H. The mechanosensitive ion channel PIEZO1 is expressed in tendons and regulates physical performance. Sci Transl Med 14: eabj5557, 2022. doi: 10.1126/scitranslmed.abj5557. [DOI] [PubMed] [Google Scholar]
- 39. McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HIS, et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet 94: 734–744, 2014. doi: 10.1016/j.ajhg.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchell JH, Smith SA. Unravelling the mysteries of the exercise pressor reflex at the cellular level. J Physiol 586: 3025–3026, 2008. doi: 10.1113/jphysiol.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hall JG. Genetic aspects of arthrogryposis. Clin Orthop Relat Res 194: 44–53, 1985. [PubMed] [Google Scholar]
- 42. Hall JG, Reed SD, Greene G. The distal arthrogryposes: delineation of new entities—review and nosologic discussion. Am J Med Genet 11: 185–239, 1982. doi: 10.1002/ajmg.1320110208. [DOI] [PubMed] [Google Scholar]
- 43. Bamshad M, Jorde LB, Carey JC. A revised and extended classification of the distal arthrogryposes. Am J Med Genet 65: 277–281, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 44. Masingue M, Fauré J, Solé G, Stojkovic T, Léonard-Louis S. A novel nonsense PIEZO2 mutation in a family with scoliosis and proprioceptive defect. Neuromuscul Disord 29: 75–79, 2019. doi: 10.1016/j.nmd.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 45. Ma S, Dubin AE, Romero LO, Loud M, Salazar A, Chu S, Klier N, Masri S, Zhang Y, Wang Y, Chesler AT, Wilkinson KA, Vásquez V, Marshall KL, Patapoutian A. Excessive mechanotransduction in sensory neurons causes joint contractures in a mouse model of arthrogryposis. Science 379: 201–206, 2023. doi: 10.1126/science.add3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haliloglu G, Becker K, Temucin C, Talim B, Küçükşahin N, Pergande M, Motameny S, Nürnberg P, Aydingoz U, Topaloglu H, Cirak S. Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J Hum Genet 62: 497–501, 2017. doi: 10.1038/jhg.2016.153. [DOI] [PubMed] [Google Scholar]
- 47. Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A, Magnusson O, Cirak S, Girisha KM, O'Driscoll M, Loeys B, Wirth B. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am J Hum Genet 99: 1206–1216, 2016. doi: 10.1016/j.ajhg.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du Y, Zhang L, Wang Z, Zhao X, Zou J. Endocrine regulation of extra-skeletal organs by bone-derived secreted protein and the effect of mechanical stimulation. Front Cell Dev Biol 9: 778015, 2021. doi: 10.3389/fcell.2021.778015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Watanabe-Takano H, Ochi H, Chiba A, Matsuo A, Kanai Y, Fukuhara S, Ito N, Sako K, Miyazaki T, Tainaka K, Harada I, Sato S, Sawada Y, Minamino N, Takeda S, Ueda HR, Yasoda A, Mochizuki N. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep 36: 109380, 2021. doi: 10.1016/j.celrep.2021.109380. [DOI] [PubMed] [Google Scholar]
- 50. Rolfe RA, Nowlan NC, Kenny EM, Cormican P, Morris DW, Prendergast PJ, Kelly D, Murphy P. Identification of mechanosensitive genes during skeletal development: alteration of genes associated with cytoskeletal rearrangement and cell signalling pathways. BMC Genomics 15: 48, 2014. doi: 10.1186/1471-2164-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morgan EF, Gleason RE, Hayward LNM, Leong PL, Palomares KTS. Mechanotransduction and fracture repair. J Bone Joint Surg Am 90, Suppl 1: 25–30, 2008. doi: 10.2106/JBJS.G.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bonewald LF. The amazing osteocyte. J Bone Miner Res 26: 229–238, 2011. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Florencio-Silva R, Sasso GRDS, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015: 421746, 2015. 2015. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mikuni-Takagaki Y, Naruse K, Azuma Y, Miyauchi A. The role of calcium channels in osteocyte function. J Musculoskelet Neuronal Interact 2: 252–255, 2002. [PubMed] [Google Scholar]
- 55. Yu K, Sellman DP, Bahraini A, Hagan ML, Elsherbini A, Vanpelt KT, Marshall PL, Hamrick MW, McNeil A, McNeil PL, McGee-Lawrence ME. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J Orthop Res 36: 653–662, 2018. doi: 10.1002/jor.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone 19: 609–614, 1996. doi: 10.1016/S8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 57. Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun 11: 282, 2020. doi: 10.1038/s41467-019-14146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, Jiang F, Li J, Liu C, Zhong G, Cao D, Jin X, Zhao D, Gao X, Liu Z, Xiao B, Li Y. The mechanosensitive Piezo1 channel is required for bone formation. eLlife 8: e47454, 2019. doi: 10.7554/eLife.47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, Zhang X, Zhou Y, Yadav PS, Lin J, Wu N, Zhao L, Huang D, Zhou S, Su P, Yang Y. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. eLife 9: e52779, 2020. doi: 10.7554/eLife.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hendrickx G, Fischer V, Liedert A, von Kroge S, Haffner-Luntzer M, Brylka L, Pawlus E, Schweizer M, Yorgan T, Baranowsky A, Rolvien T, Neven M, Schumacher U, Beech DJ, Amling M, Ignatius A, Schinke T. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J Bone Miner Res 36: 369–384, 2021. doi: 10.1002/jbmr.4198. [DOI] [PubMed] [Google Scholar]
- 61. Bai WY, Wang L, Ying ZM, Hu B, Xu L, Zhang GQ, Cong PK, Zhu X, Zou W, Zheng HF. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone 133: 115247, 2020. doi: 10.1016/j.bone.2020.115247. [DOI] [PubMed] [Google Scholar]
- 62. Walter BA, Torre OM, Laudier D, Naidich TP, Hecht AC, Iatridis JC. Form and function of the intervertebral disc in health and disease: a morphological and stain comparison study. J Anat 227: 707–716, 2015. doi: 10.1111/joa.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila PA 1976) 20: 1307–1314, 1995. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 64. Dou Y, Sun X, Ma X, Zhao X, Yang Q. Intervertebral disk degeneration: the microenvironment and tissue engineering strategies. Front Bioeng Biotechnol 9: 592118, 2021. doi: 10.3389/fbioe.2021.592118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Q, Zhou Y, Wang J, Fu W, Li X. Study on the mechanism of excessive apoptosis of nucleus pulposus cells induced by shRNA-Piezo1 under abnormal mechanical stretch stress. J Cell Biochem 120: 3989–3997, 2019. doi: 10.1002/jcb.27683. [DOI] [PubMed] [Google Scholar]
- 66. Sun Y, Leng P, Song M, Li D, Guo P, Xu X, Gao H, Li Z, Li C, Zhang H. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca(2+)/NF-kappaB pathway. Int Immunopharmacol 85: 106681, 2020. doi: 10.1016/j.intimp.2020.106681. [DOI] [PubMed] [Google Scholar]
- 67. Sun Z, Zheng X, Li S, Zeng B, Yang J, Ling Z, Liu X, Wei F. Single impact injury of vertebral endplates without structural disruption, initiates disc degeneration through Piezo1 mediated inflammation and metabolism dysfunction. Spine (Phila PA 1976) 47: E203–E213, 2022. doi: 10.1097/BRS.0000000000004203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang B, Ke W, Wang K, Li G, Ma L, Lu S, Xiang Q, Liao Z, Luo R, Song Y, Hua W, Wu X, Zhang Y, Zeng X, Yang C. Mechanosensitive ion channel piezo1 activated by matrix stiffness regulates oxidative stress-induced senescence and apoptosis in human intervertebral disc degeneration. Oxid Med Cell Longev 2021: 8884922, 2021. doi: 10.1155/2021/8884922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu J, Chen Y, Liao Z, Liu H, Zhang S, Zhong D, Qiu X, Chen T, Su D, Ke X, Wan Y, Zhou T, Su P. Self-amplifying loop of NF-kappaB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol Ther 30: 3241–3256, 2022. doi: 10.1016/j.ymthe.2022.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi S, Kang X-J, Zhou Z, He Z-M, Zheng S, He S-S. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther 24: 119, 2022. doi: 10.1186/s13075-022-02804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface 12: 20150429, 2015. doi: 10.1098/rsif.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vergroesen P-PA, Kingma I, Emanuel KS, Hoogendoorn RJW, Welting TJ, van Royen BJ, van Dieën JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage 23: 1057–1070, 2015. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 73. Gilchrist CL, Darling EM, Chen J, Setton LA. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell-cell interactions. PLoS One 6: e27170, 2011. doi: 10.1371/journal.pone.0027170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Navaro Y, Bleich-Kimelman N, Hazanov L, Mironi-Harpaz I, Shachaf Y, Garty S, Smith Y, Pelled G, Gazit D, Seliktar D, Gazit Z. Matrix stiffness determines the fate of nucleus pulposus-derived stem cells. Biomaterials 49: 68–76, 2015. doi: 10.1016/j.biomaterials.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 75. Xu HG, Zhang XH, Wang H, Liu P, Wang LT, Zuo CJ, Tong WX, Zhang XL. Intermittent cyclic mechanical tension-induced calcification and downregulation of ankh gene expression of end plate chondrocyte. Spine (Phila Pa 1976) 37: 1192–1197, 2012. doi: 10.1097/BRS.0b013e318244d989. [DOI] [PubMed] [Google Scholar]
- 76. Xu HG, Zheng Q, Song JX, Li J, Wang H, Liu P, Wang J, Wang CD, Zhang XL. Intermittent cyclic mechanical tension promotes endplate cartilage degeneration via canonical Wnt signaling pathway and E-cadherin/beta-catenin complex cross-talk. Osteoarthritis Cartilage 24: 158–168, 2016. doi: 10.1016/j.joca.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 77. Ariga K, Yonenobu K, Nakase T, Hosono N, Okuda S, Meng W, Tamura Y, Yoshikawa H. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine (Phila Pa 1976) 28: 1528–1533, 2003. [PubMed] [Google Scholar]
- 78. Abbott RD, Howe AK, Langevin HM, Iatridis JC. Live free or die: stretch-induced apoptosis occurs when adaptive reorientation of annulus fibrosus cells is restricted. Biochem Biophys Res Commun 421: 361–366, 2012. doi: 10.1016/j.bbrc.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tisherman R, Coelho P, Phillibert D, Wang D, Dong Q, Vo N, Kang J, Sowa G. NF-kappaB signaling pathway in controlling intervertebral disk cell response to inflammatory and mechanical stressors. Phys Ther 96: 704–711, 2016. doi: 10.2522/ptj.20150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gilbert HT, Hoyland JA, Freemont AJ, Millward-Sadler SJ. The involvement of interleukin-1 and interleukin-4 in the response of human annulus fibrosus cells to cyclic tensile strain: an altered mechanotransduction pathway with degeneration. Arthritis Res Ther 13: R8, 2011. doi: 10.1186/ar3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health 1: 461–468, 2009. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hall AC. The role of chondrocyte morphology and volume in controlling phenotype-implications for osteoarthritis, cartilage repair, and cartilage engineering. Curr Rheumatol Rep 21: 38, 2019. doi: 10.1007/s11926-019-0837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. eLife 6: e21074, 2017. doi: 10.7554/eLife.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Du G, Li L, Zhang X, Liu J, Hao J, Zhu J, Wu H, Chen W, Zhang Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp Biol Med (Maywood) 245: 180–189, 2020. doi: 10.1177/1535370219892601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Du G, Chen W, Li L, Zhang Q. The potential role of mechanosensitive ion channels in substrate stiffness-regulated Ca(2+) response in chondrocytes. Connect Tissue Res 63: 453–462, 2022. doi: 10.1080/03008207.2021.2007902. [DOI] [PubMed] [Google Scholar]
- 86. Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Liedtke WB. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA 111: E5114–E5122, 2014. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xie M, Fritch MR, He Y, Fu H, Hong Y, Lin H. Dynamic loading enhances chondrogenesis of human chondrocytes within a biodegradable resilient hydrogel. Biomater Sci 9: 5011–5024, 2021. doi: 10.1039/d1bm00413a. [DOI] [PubMed] [Google Scholar]
- 88. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F, McNulty AL, Chen Y, Guilak F, Liedtke WB. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci USA 118: e2001611118, 2021. doi: 10.1073/pnas.2001611118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang H, Shao Y, Yao Z, Liu L, Zhang H, Yin J, Xie H, Li K, Lai P, Zeng H, Xiao G, Zeng C, Cai D, Bai X. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. Ann Rheum Dis 81: 676–686, 2022. doi: 10.1136/annrheumdis-2021-221513. [DOI] [PubMed] [Google Scholar]
- 90. Ren X, Li B, Xu C, Zhuang H, Lei T, Jiang F, Zhou P. High expression of Piezo1 induces senescence in chondrocytes through calcium ions accumulation. Biochem Biophys Res Commun 607: 138–145, 2022. doi: 10.1016/j.bbrc.2022.03.119. [DOI] [PubMed] [Google Scholar]
- 91. Imgenberg J, Rolauffs B, Grodzinsky AJ, Schünke M, Kurz B. Estrogen reduces mechanical injury-related cell death and proteoglycan degradation in mature articular cartilage independent of the presence of the superficial zone tissue. Osteoarthritis Cartilage 21: 1738–1745, 2013. doi: 10.1016/j.joca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 92. Lawrence KM, Jones RC, Jackson TR, Baylie RL, Abbott B, Bruhn-Olszewska B, Board TN, Locke IC, Richardson SM, Townsend PA. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep 7: 5147, 2017. doi: 10.1038/s41598-017-04367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jones RC, Lawrence KM, Higgins SM, Richardson SM, Townsend PA. Urocortin-1 is chondroprotective in response to acute cartilage injury via modulation of Piezo1. Int J Mol Sci 23: 5119, 2022. doi: 10.3390/ijms23095119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sun Y, Leng P, Guo P, Gao H, Liu Y, Li C, Li Z, Zhang H. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol Med 27: 96, 2021. doi: 10.1186/s10020-021-00360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li XF, Zhang Z, Li XD, Wang TB, Zhang HN. Mechanism of the Piezo1 protein-induced apoptosis of the chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi Xue Za Zhi 96: 2472–2477, 2016. doi: 10.3760/cma.j.issn.0376-2491.2016.31.007. [DOI] [PubMed] [Google Scholar]
- 96. He Z, Yan Z, Zhang Y, Xv Y, Chen K, Zhan J, Zhang Y. Excessive mechanical stress mediated Piezo1 activation regulates lysosomal membrane permeabilization-induced chondrocyte apoptosis in mouse osteoarthritis model (Preprint). Res Sq, 2022. doi: 10.21203/rs.3.rs-2237583/v1. [DOI] [Google Scholar]
- 97. Nims RJ, Palmer D, Kassab J, Zhang B, Liedtke W, Guilak F. Physiologic and pathologic chondrocyte mechanotransduction is mediated through TRPV4 or Piezo1 signaling and activation of distinct downstream transcriptional pathways. Orthopedic Research Society 2023 Annual Meeting. Dallas, TX, February 10–14, 2023, #305. [Google Scholar]
- 98. Zhang M, Wang Y, Geng J, Zhou S, Xiao B. Mechanically activated Piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep 26: 1419–1431.e4, 2019. doi: 10.1016/j.celrep.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 99. Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, Bharucha-Goebel D, Saade D, Necaise A, Donkervoort S, Foley AR, Gordon T, Case L, Bushnell MC, Bönnemann CG, Chesler AT. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med 10: eaat9892, 2018. doi: 10.1126/scitranslmed.aat9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516: 121–125, 2014. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nencini S, Morgan M, Thai J, Jobling AI, Mazzone SB, Ivanusic JJ. Piezo2 knockdown inhibits noxious mechanical stimulation and NGF-induced sensitization in a-delta bone afferent neurons. Front Physiol 12: 644929, 2021. doi: 10.3389/fphys.2021.644929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 4: 1682, 2013. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo Z, Liao X, Luo L, Fan Q, Zhang X, Guo Y, Wang F, Ye Z, Luo D. Extracellular ATP and cAMP signaling promote Piezo2-dependent mechanical allodynia after trigeminal nerve compression injury. J Neurochem 160: 376–391, 2022. doi: 10.1111/jnc.15537. [DOI] [PubMed] [Google Scholar]
- 104. Ni K, Zhang W, Ni Y, Mao Y-T, Wang Y, Gu X-P, Ma Z-L. Dorsal root ganglia NR2B-mediated Epac1-Piezo2 signaling pathway contributes to mechanical allodynia of bone cancer pain. Oncol Lett 21: 338, 2021. doi: 10.3892/ol.2021.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ferrari LF, Bogen O, Green P, Levine JD. Contribution of Piezo2 to endothelium-dependent pain. Mol Pain 11: 65, 2015. doi: 10.1186/s12990-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fontanella CG, Belluzzi E, Pozzuoli A, Favero M, Ruggieri P, Macchi V, Carniel EL. Mechanical behavior of infrapatellar fat pad of patients affected by osteoarthritis. J Biomech 131: 110931, 2022. doi: 10.1016/j.jbiomech.2021.110931. [DOI] [PubMed] [Google Scholar]
- 107. Emmi A, Stocco E, Boscolo-Berto R, Contran M, Belluzzi E, Favero M, Ramonda R, Porzionato A, Ruggieri P, De Caro R, Macchi V. Infrapatellar fat pad-synovial membrane anatomo-fuctional unit: microscopic basis for Piezo1/2 mechanosensors involvement in osteoarthritis pain. Front Cell Dev Biol 10: 886604, 2022. doi: 10.3389/fcell.2022.886604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Obeidat AM, Wood MJ, Ishihara S, Li J, Wang L, Ren D, Bennett DA, Miller RJ, Malfait A-M, Miller RE. Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis (Preprint). bioRxiv 2022. doi: 10.1101/2022.03.12.484097. [DOI] [PMC free article] [PubMed]
- 109. Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18: 1756–1762, 2015. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Florez-Paz D, Bali KK, Kuner R, Gomis A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci Rep 6: 25923, 2016. doi: 10.1038/srep25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, Innes AM, Mah JK, Grosmann CM, Bradley N, Nguyen D, Foley AR, Le Pichon CE, Bönnemann CG. The role of PIEZO2 in human mechanosensation. N Engl J Med 375: 1355–1364, 2016. doi: 10.1056/NEJMoa1602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Assaraf E, Blecher R, Heinemann-Yerushalmi L, Krief S, Carmel Vinestock R, Biton IE, Brumfeld V, Rotkopf R, Avisar E, Agar G, Zelzer E. Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat Commun 11: 3168, 2020. doi: 10.1038/s41467-020-16971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lee W, Guilak F, Liedtke W. Role of piezo channels in joint health and injury. Curr Top Membr 79: 263–273, 2017. doi: 10.1016/bs.ctm.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ward CW, Sachs F, Bush ED, Suchyna TM. GsMTx4-D provides protection to the D2.mdx mouse. Neuromuscul Disord 28: 868–877, 2018. doi: 10.1016/j.nmd.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci 26: 5656–5664, 2006. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hara M, Tabata K, Suzuki T, Do M-KQ, Mizunoya W, Nakamura M, Nishimura S, Tabata S, Ikeuchi Y, Sunagawa K, Anderson JE, Allen RE, Tatsumi R. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am J Physiol Cell Physiol 302: C1741–C1750, 2012. doi: 10.1152/ajpcell.00068.2012. [DOI] [PubMed] [Google Scholar]
- 117. Efremov AK, Yao M, Sun Y, Tee YH, Sheetz MP, Bershadsky AD, Martinac B, Yan J. Application of piconewton forces to individual filopodia reveals mechanosensory role of L-type Ca(2+) channels. Biomaterials 284: 121477, 2022. doi: 10.1016/j.biomaterials.2022.121477. [DOI] [PubMed] [Google Scholar]
- 118. Takahashi K, Hayashi S, Miyajima M, Omori M, Wang J, Kaihara K, Morimatsu M, Wang C, Chen J, Iribe G, Naruse K, Sokabe M. L-type calcium channel modulates mechanosensitivity of the cardiomyocyte cell line H9c2. Cell Calcium 79: 68–74, 2019. doi: 10.1016/j.ceca.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 119. Takamatsu A, Ohkawara B, Ito M, Masuda A, Sakai T, Ishiguro N, Ohno K. Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/beta-catenin signaling. PLoS One 9: e92699, 2014. doi: 10.1371/journal.pone.0092699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Swain SM, Romac JM-J, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, Liddle RA. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest 130: 2527–2541, 2020. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Romac JM-J, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun 9: 1715, 2018. doi: 10.1038/s41467-018-04194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Swain SM, Romac JM, Vigna SR, Liddle RA. Piezo1-mediated stellate cell activation causes pressure-induced pancreatic fibrosis in mice. JCI Insight 7: e158288, 2022. doi: 10.1172/jci.insight.158288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Xiao B. Levering mechanically activated Piezo channels for potential pharmacological intervention. Annu Rev Pharmacol Toxicol 60: 195–218, 2020. doi: 10.1146/annurev-pharmtox-010919-023703. [DOI] [PubMed] [Google Scholar]
- 124. Ridone P, Pandzic E, Vassalli M, Cox CD, Macmillan A, Gottlieb PA, Martinac B. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J Gen Physiol 152: e201912515, 2020. doi: 10.1085/jgp.201912515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 8: ra15, 2015. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Narayanan P, Hütte M, Kudryasheva G, Taberner FJ, Lechner SG, Rehfeldt F, Gomez-Varela D, Schmidt M. Myotubularin related protein-2 and its phospholipid substrate PIP2 control Piezo2-mediated mechanotransduction in peripheral sensory neurons. eLife 7: e32346, 2018. doi: 10.7554/eLife.32346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun 6: 8512, 2015. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wu C-L, Jain D, McNeill JN, Little D, Anderson JA, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Guilak F. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis 74: 2076–2083, 2015. doi: 10.1136/annrheumdis-2014-205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF, Vásquez V. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun 10: 1200, 2019. doi: 10.1038/s41467-019-09055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sekar S, Wu X, Friis T, Crawford R, Prasadam I, Xiao Y. Saturated fatty acids promote chondrocyte matrix remodeling through reprogramming of autophagy pathways. Nutrition 54: 144–152, 2018. doi: 10.1016/j.nut.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 131. Han Y, Liu C, Zhang D, Men H, Huo L, Geng Q, Wang S, Gao Y, Zhang W, Zhang Y, Jia Z. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int J Oncol 55: 629–644, 2019. doi: 10.3892/ijo.2019.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z, Zhao Z. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod 85: 87–94, 2015. doi: 10.2319/123113-955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhong M, Komarova Y, Rehman J, Malik AB. Mechanosensing Piezo channels in tissue homeostasis including their role in lungs. Pulm Circ 8: 2045894018767393, 2018. doi: 10.1177/2045894018767393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Cao L, Setton LA, Guilak F. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther 12: R130, 2010. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Collins KH, Hart DA, Reimer RA, Seerattan RA, Herzog W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J Orthop Res 34: 1010–1018, 2016. doi: 10.1002/jor.23103. [DOI] [PubMed] [Google Scholar]
- 136. Collins KH, Hart DA, Seerattan RA, Reimer RA, Herzog W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res 7: 274–281, 2018. doi: 10.1302/2046-3758.74.BJR-2017-0201.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wu C-L, Kimmerling KA, Little D, Guilak F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci Rep 7: 44315, 2017. doi: 10.1038/srep44315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Harasymowicz NS, Dicks A, Wu C-L, Guilak F. Physiologic and pathologic effects of dietary free fatty acids on cells of the joint. Ann N Y Acad Sci 1440: 36–53, 2019. doi: 10.1111/nyas.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xie Y, Zhou W, Zhong Z, Yu H, Zhang P, Shen H. Docosahexaenoic acid inhibits bone remodeling and vessel formation in the osteochondral unit in a rat model. Biomed Pharmacother 114: 108811, 2019. doi: 10.1016/j.biopha.2019.108811. [DOI] [PubMed] [Google Scholar]
- 140. Kimmerling KA, Oswald SJ, Huebner JL, Little D, Kraus VB, Kang JX, Wu C-L, Guilak F. Transgenic conversion of omega-6 to omega-3 polyunsaturated fatty acids via fat-1 reduces the severity of post-traumatic osteoarthritis. Arthritis Res Ther 22: 83, 2020. doi: 10.1186/s13075-020-02170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cordingley DM, Cornish SM. Omega-3 fatty acids for the management of osteoarthritis: a narrative review. Nutrients 14: 3362, 2022. doi: 10.3390/nu14163362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jiao H, Xiao E, Graves DT. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep 13: 327–335, 2015. doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gao F, Lv T-R, Zhou J-C, Qin X-D. Effects of obesity on the healing of bone fracture in mice. J Orthop Surg Res 13: 145, 2018. doi: 10.1186/s13018-018-0837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ, O'Keefe RJ, Xing L, Mooney RA, Zuscik MJ. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS One 9: e99656, 2014. doi: 10.1371/journal.pone.0099656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Khajuria DK, Soliman M, Elfar JC, Lewis GS, Abraham T, Kamal F, Elbarbary RA. Aberrant structure of fibrillar collagen and elevated levels of advanced glycation end products typify delayed fracture healing in the diet-induced obesity mouse model. Bone 137: 115436, 2020. doi: 10.1016/j.bone.2020.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Figeac F, Tencerova M, Ali D, Andersen TL, Appadoo DRC, Kerckhofs G, Ditzel N, Kowal JM, Rauch A, Kassem M. Impaired bone fracture healing in type 2 diabetes is caused by defective functions of skeletal progenitor cells. Stem Cells 40: 149–164, 2022. doi: 10.1093/stmcls/sxab011. [DOI] [PubMed] [Google Scholar]
- 147. Chen P, Zhang G, Jiang S, Ning Y, Deng B, Pan X, Liu S, He Y, Zhang L, Wan R, Wu Z, He Q, Yin J, Wang H, Li J. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium 97: 102431, 2021. doi: 10.1016/j.ceca.2021.102431. [DOI] [PubMed] [Google Scholar]
- 148. Liu Y, Tian H, Hu Y, Cao Y, Song H, Lan S, Dai Z, Chen W, Zhang Y, Shao Z, Liu Y, Tong W. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci 18: 3961–3980, 2022. doi: 10.7150/ijbs.71390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ranade SS, Qiu Z, Woo S-H, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li Y-SJ, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111: 10347–10352, 2014. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282, 2014. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nonomura K, Woo S-H, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541: 176–181, 2017. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McNulty AL, Leddy HA, Liedtke W, Guilak F. TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch Pharmacol 388: 437–450, 2015. doi: 10.1007/s00210-014-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]