Abstract
Objective:
Little is known about the effects of over-the-counter fish oil (FO) supplements on circulating omega-3 polyunsaturated fatty acid (n-3 PUFA)-derived specialized pro-resolving mediators (SPMs), nor about whether having a chronic inflammatory disease such as rheumatoid arthritis (RA) influences SPM levels. We investigated associations between over-the-counter n-3 PUFA FO supplementation and circulating SPMs among patients with vs. without RA.
Methods:
We studied 104 participants: 26 with RA taking FO matched by age and sex to 26 with RA not taking FO, 26 without RA taking FO, and 26 without RA not taking FO. Targeted-liquid chromatography-tandem mass spectroscopy was performed on patient plasma to identify and quantify 27 lipid mediators (including eicosanoids and SPMs). We performed t-tests and then multivariable linear regression analyses to assess whether having RA or taking FO supplements was associated with circulating lipid mediator concentrations, adjusting for age, race, sex, smoking, body mass index, and current medication use (statins; prednisone and immunomodulators among RA cases only). We tested for interactions between FO supplementation and RA status. We also conducted Spearman’s correlations between EPA, DHA, and ARA and their downstream metabolites.
Results:
Among patients who were taking FO compared to those who were not, in multivariable- adjusted analyses, SPM substrates EPA and DHA were both elevated as were several of their pro-resolving bioactive products, including 15- and 18-HEPE from EPA, and 14- and 17-HDHA from DHA, which are substrates for specific SPMs. While E-series and D-series resolvins were present and identified, we did not find statistical elevations of other SPMs. Results were similar among patients with RA and patients without RA, taking vs. not taking FO supplementation (no formal statistical interaction observed). There was a strong positive correlation between EPA and DHA and their immediate downstream SPM precursors (18-HEPE and 15-HEPE from EPA; 17-HDHA and 14-HDHA from DHA) among all patients.
Conclusion:
Patients taking FO supplements, regardless of RA status, not only had higher blood levels of EPA and DHA, but also of their enzymatic products 18-HEPE (E-series resolvin precursors), 15-HEPE and 17-HDHA (D-series resolvin and protectin precursors). Patients with RA, an inflammatory autoimmune disease, may be able to augment some SPM precursor reserves, similarly to matched controls without RA, by taking oral FO supplements.
Keywords: rheumatoid arthritis, specialized pro-resolving mediator, omega-3 fatty acid, DHA, EPA, fish oil
1. INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune inflammatory polyarthritis of incompletely understood etiology that attacks and destroys synovial joints, causing systemic complications and early mortality. RA strikes 1–3% of the population, a majority women, and has a peak incidence in mid-life.[1–3] Although we have an expanding array of medications to treat RA, none are curative and the costs of therapy, disability and lost productivity are still very high.[4] A hallmark of the long-term period leading to RA onset includes an upregulation of inflammatory biomarkers.[5,6]
Fish oil (FO) supplements contain omega-3 polyunsaturated fatty acids (n-3 PUFA), a higher intake of which has been associated with a lower risk of RA and conversely a lower intake has been associated with a higher risk of RA,[7,8] and derived from these n-3 PUFAs are lipid mediators known as specialized pro-resolving mediators (SPMs).[9,10] (Figure 1) There is increasing evidence that the n-3 PUFA-derived SPMs, including resolvins, protectins, and maresins, have important pro-resolving capacities that are perhaps dysregulated or overwhelmed in the pathogenesis of RA.[11] These bioactive lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the main n-3 PUFAs, have now been extensively biochemically characterized.[10–13] Reports of the pro-resolving actions of SPMs continue to accumulate, including dampening production of inflammatory mediators such as PGE2, LTB4, IL-1β and IL-6, promoting phagocytosis and efferocytosis, and increasing polarization of pro-resolving type 2 macrophages (M2), and thus SPMs have been proposed to have potential as therapy for human rheumatic inflammatory disease.[10–20] However, the associations between long-term n-3 PUFA intake and plasma SPMs and their precursors in humans with and without RA still need to be thoroughly investigated..
FIGURE 1.
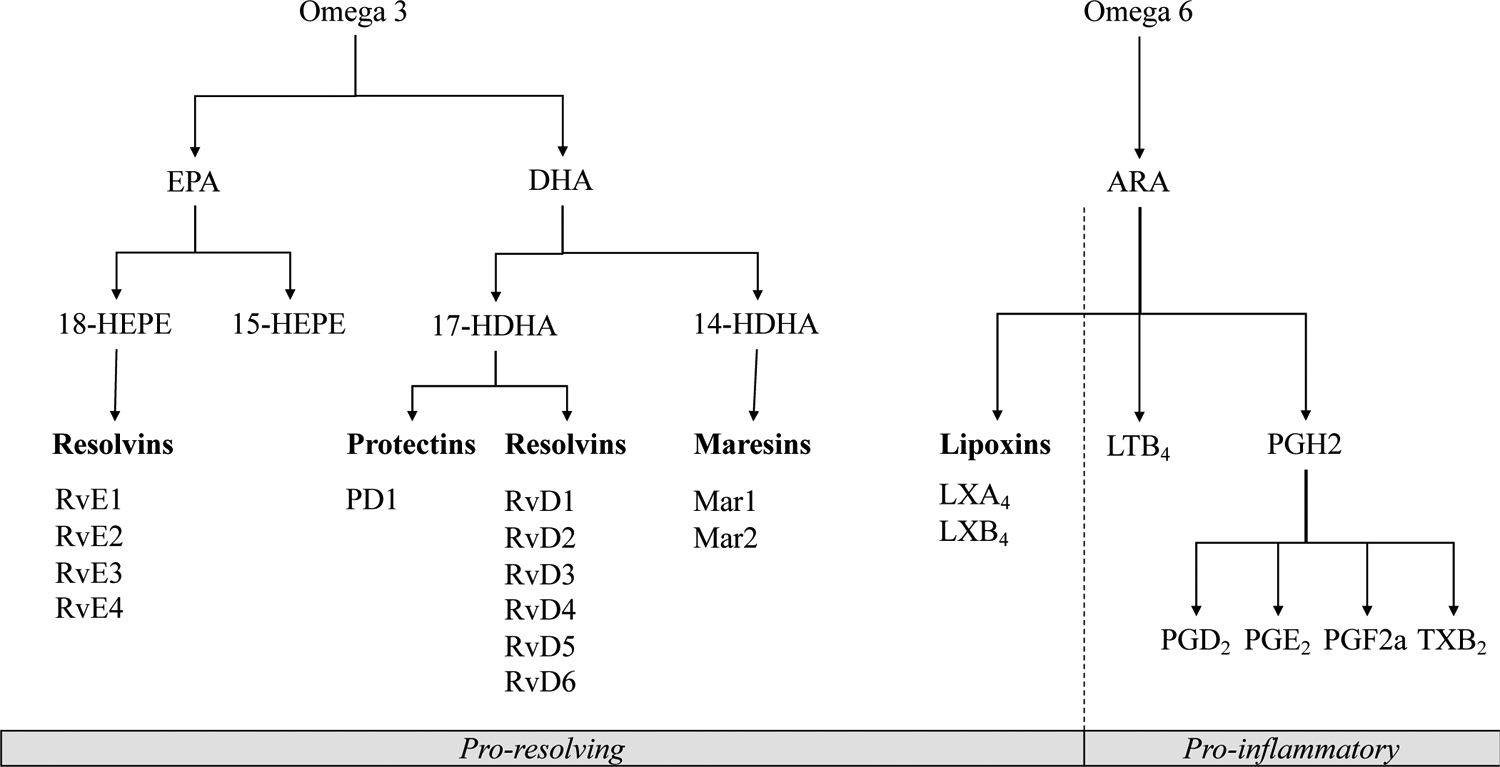
Specialized Pro-resolving Mediators derived from Omega-3 and Eicosanoids from Omega-6 fatty acids [9,10,41]
Specialized pro-resolving mediators are marked in bold font.
Abbreviations: ARA, Arachidonic acid, EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LTB4, Leukotriene B4; LXA4, Lipoxin A4; LXB4, Lipoxin B4; MaR1–2, Maresin 1–2; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PGF2a, Prostaglandin F2 alpha; TXB2, Thromboxane B2; PD1, Protectin D1; RA, rheumatoid arthritis; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6, D-series resolvins; RvE1, RvE2, RvE3, and RvE4, E-series resolvins; 14-HDHA, 14-hydroxy-docosahexaenoic acid; 15-HEPE, 15-hydroxyeicosapentaenoic acid; 17-HDHA, 17-hydroxy-docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid.
Randomized controlled trials in the 1990s suggested that FO supplements were effective in decreasing pain and inflammation in patients with RA.[21,22] Oral FO supplements were also shown to decrease circulating leukotriene B4 (LTB4), a potent leukocyte chemoattractant and inflammatory mediator.[21] However, FO supplements are still not widely prescribed or taken in the current era of stronger biologic agents for RA. It is not known, although it is hypothesized, that much of the beneficial effect of marine n-3 PUFAs in RA is due to increased availability of SPMs for inflammation resolution, and taking FO supplements have been shown to increase SPM blood levels in clinical trials of healthy adults [23,24] and those with pre-existing conditions.[25,26] Moreover, it is not known whether taking an over-the-counter FO supplement, as many patients with RA do, is associated with higher chronic circulating concentrations of SPMs via an increase in the available substrate for their production, DHA and EPA.
We aimed to examine associations between over-the-counter FO supplementation and circulating SPMs in a clinical population of patients with RA enrolled in a large academic hospital biobank, and similar associations in age- and sex-matched individuals without RA. Our hypothesis was that the intake of FO supplements would be associated with higher circulating SPMs in both groups, but that we would detect less augmentation in circulating levels among those with RA due to their chronic inflammation.
2. MATERIALS AND METHODS
2.1. Study Population
We employed the Mass General Brigham (MGB) Biobank in Boston, MA to identify participants in our healthcare system who had provided stored plasma samples. We identified individuals with validated diagnoses of RA by American College of Rheumatology/European league against Rheumatism 2010 classification criteria and reviewed their medication lists at the time of sample donation to identify those who were listed as taking an over-the-counter FO/n-3 PUFA supplement;[27] supplement dosage was not routinely reported. These participants were then 1:1 matched by age and sex to a) participants with RA who were not taking a FO supplement, b) participants without RA who were taking a FO supplement and c) participants without RA who were not taking a FO supplement. Demographic and clinical features of all participants, including age, sex, race, body mass index (BMI in kg/m2), smoking status, and current prescribed medication use, were collected from detailed review of the electronic medical records, and from the Health Information Survey, completed by the participants at the time of enrollment in the MGB Biobank and blood collection and storage. Exact preparations/name brands and doses of FO supplements were not documented in all medical records. Of the 52 subjects, 38 had documented dose information (73%). Doses ranged from 100–4000 mg a day. The majority of patients reported a dose of 1000mg or less a day, which was true for 74% of the RA cases and 63% of matched non-RA controls. Medication data collected included prednisone, as well as immunosuppressants methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, cyclophosphamide, cyclosporine, mycophenolate mofetil, etanercept, adalimumab, rituximab, infliximab, belimumab, tofacitinib, tocilizumab). Data on current statin use (pravastatin, atorvastatin, lovastatin, rosuvastatin, simvastatin) were also collected for all participants. Additional data for the individuals with RA, including their serostatus (rheumatoid factor [RF], anti-cyclic citrullinated peptide [anti-CCP], and recent erythrocyte sedimentation rate [ESR] and/or C-reactive protein [CRP]), were abstracted from the linked electronic medical records. Informed consent was obtained from study participants, and the Mass General Brigham Institutional Review Board approved all aspects of this study.
2.2. Identification and Quantification of SPM and Lipid Mediators by Targeted LC-MS/MS
Blood samples were retrieved from the −80° C MGB Biobank storage facility and thawed. Liquid chromatography-tandem mass spectroscopy (LC-MS/MS) was performed on patient plasma to quantify 27 lipid mediators (eicosanoids and SPMs), which were pre-classified as pro-inflammatory and pro-resolving mediators, each identified by matching their retention time and MS-MS spectra to those obtained with authentic standards for each mediator in identical chromatographic conditions as well as with >6 diagnostic ions in MS-MS matching those of synthetic standards. Briefly, plasma samples (100 μL) were defrosted from −80°C on ice. For purpose of quantification and recovery of the lipid mediators, deuterium labeled internal standards including d8-5S-HETE, d5-RvD2, d5-LXA4, d4-LTB4, and d4-PGE2 (500 pg each, Cayman Chemical) in 1 mL of methanol were added to each sample thus covering the entire chromatographic profile by regions. Samples were then held in methanol at − 20 °C for 45 min to allow protein precipitation. After centrifugation at 1000 g for 10 minutes at 4° C, supernatants were collected and solid phase extracted using an automated lipid mediator extractor (Extrahera, Biotage) using optimized methods.[15] Briefly, samples were acidified to pH 3.5 and loaded onto Isolute SPE C18 columns (100 mg, 3 mL, Biotage). C18 columns were rapidly neutralized with double-distilled water and washed with hexane. Lipid mediators were eluted with methyl formate. These lipid mediators-containing fractions were taken to dryness under a gentle stream of nitrogen gas using the automated evaporation system (TurboVap LV, Biotage) and immediately resuspended in methanol-water mixture (50:50, v/v). Lipid mediators present in the methyl formate fraction from solid phase extraction of each sample were measured at the Hospital for Sick Children Analytical Facility for Bioactive Molecules (Toronto, ON) using liquid chromatography-tandem mass spectrometry. Chromatography was performed with a Kinetex C18 column (100 × 4.6 mm, 5 μ particle sizes) (Phenomenex, California, USA). The HPLC flow was maintained at 800 μL/minute with a gradient consisting of A= Water/Acetonitrile (90/10) + 0.02% acetic acid and B = Acetonitrile/Isopropanol with a total run time of 19 minutes. A QTRAP 6500plus triple-quadruple mass spectrometer (Sciex: Framingham, Massachusetts, USA) in negative electrospray ionization mode was used for multiple reaction monitoring data acquisition a SCIEX ExionLC HPLC (Framingham, Massachusetts, USA). Calibration curves were obtained for each mediator and used for quantitation of amounts present in pg/100 ul in each sample. For each lipid mediator (limits of detection ≈ 0.1 pg), the assayed levels were above the level of detection in the corresponding calibration curves.
2.3. Statistical Analyses
We examined the characteristics of the participants with RA and their matched controls without RA and compared them using Fisher’s exact tests for categorical variables and paired t-tests for continuous variables. Paired t-tests were initially performed to determine whether taking FO was associated with individual lipid mediator concentrations, overall and among the patients with RA and the matched controls without RA separately. The 27 lipid mediators, for which mean log-transformed concentrations were calculated to improve normality, were divided into two groups: pro-inflammatory and pro-resolving. Measured pro-inflammatory lipid mediators included: arachidonic acid (ARA), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2 alpha (PGF2a), thromboxane B2 (TXB2), and leukotriene B4 (LTB4), while pro-resolving SPMs and their precursors included: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), lipoxin A4 (LXA4), lipoxin B4 (LXB4), maresin 1–2 (MaR1–2), protectin D1 (PD1), D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6), E-series resolvins (RvE1, RvE2, RvE3, and RvE4), 14-hydroxy-docosahexaenoic acid (14-HDHA), 15-hydroxyeicosapentaenoic acid (15-HEPE), 17-hydroxy-docosahexaenoic acid (17-HDHA), 18-hydroxyeicosapentaenoic acid (18-HEPE) (Figure 1). For each lipid mediator, we compared log mean concentrations using t-tests among three groups: (1) all patients with RA to all matched controls without RA, and then (2) among patients with RA, those taking over-the-counter FO to those not taking FO, and (3) among matched controls without RA, those taking over-the-counter FO to those not taking FO.
We then performed multivariable linear regression analyses to assess whether RA status was associated with lipid mediator concentration, and whether FO supplementation was associated with lipid mediator concentration, adjusted for age and race, sex, smoking status, BMI, and statin medication use, among all participants. We tested for interactions between RA status and FO supplementation in an additional multivariable model, assessing whether the associations between taking FO differed among patients with or without RA, by including an RA * FO interaction term.
We repeated these multivariable linear regression analyses among RA cases only, further adjusting for ESR and CRP (either above normal range or not), assessing associations between FO intake and lipid mediator concentrations. To allow for correlation between biomarkers and to control for the proportion of incorrectly rejected null hypotheses (based upon a pre-specified p-value of 0.05), all analyses were corrected for multiple comparisons using the Benjamini and Yekutieli false discovery rate (FDR) method.[28] However, as these analyses were also hypothesis-generating, we have reported and interpreted the nominal results as well.
A Spearman’s correlation analysis was also run between all 27 lipid mediators to determine any relationship between EPA, DHA, ARA and their down-stream metabolites including their specialized pro-resolving lipid mediators.
All analyses were conducted in SAS v 9.4.
3. RESULTS
One hundred and four participants were studied: 26 participants with RA taking FO were matched three ways to 26 participants with RA not taking FO, 26 participants without RA taking FO, and 26 participants without RA or FO supplementation. Their demographic and clinical characteristics are shown in Table 1. Patients with RA and matched controls without RA had a similar mean age (approximately 49–50 years, 85% female, and 90–92% White) as expected; however, those with RA had a slightly higher BMI and more were past smokers. Half of the patients with RA were positive for RF and 29% were positive for anti-CCP antibodies. Among patients with RA, 50% were RF positive and biomarkers of inflammation (ESR and/or CRP) were elevated in 24, normal in 25, and missing in 3.
TABLE 1.
Characteristics of participants with rheumatoid arthritis and matched controls without rheumatoid arthritis from the Mass General Brigham Biobank
| Parameter | Rheumatoid Arthritis Cases (n=52) | Non-Rheumatoid Arthritis Matched Controls (n=52) | P value* |
|---|---|---|---|
| Matching Factors | |||
| Age at blood draw (mean, SD) | 50.0 (7.37) | 49.5 (7.35) | 0.73 |
| Female, n (%) | 44 (84.6) | 44 (84.6) | 1.00 |
| Race | 0.27 | ||
| -White, n (%) | 47 (90.4) | 48 (92.3) | |
| -Non-White, n (%) | 5 (9.6) | 2 (3.9) | |
| Smoking Status | 0.35 | ||
| -Never, n (%) | 33 (63.5) | 34 (65.4) | |
| -Past, n (%) | 17 (32.7) | 12 (23.1) | |
| -Current, n (%) | 2 (3.9) | 4 (7.7) | |
| Other Covariates | |||
| Body mass index kg/m2 (mean, SD) | 29.8 (10.42) | 26.0 (4.54) | 0.02 |
| Taking a statin medication, n (%) | 6 (11.5) | 4 (7.7) | 0.74 |
| Characteristics of Rheumatoid Arthritis cases | |||
| Rheumatoid factor positive, n (%) | 26 (50.0) | ||
| Anti-CCP positive, n (%) | 15 (28.9) | ||
| Elevated ESR or CRP, n (%) | 24 (46.2) | ||
| Taking steroid, n (%) | 8 (15.4) | ||
| Taking immunosuppressant, n (%) | 43 (82.7) | ||
Chi squared test for categorical values and t-test for continuous variables. N: number; SD: standard deviation; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; anti-CCP: anti-cyclic citrullinated peptide; Race data missing in 2 matched controls without RA. Smoking status missing in 2 matched controls without RA ESR or CCRP missing in 3 patients with RA
Figure 2 displays comparisons of the mean log-transformed lipid mediator levels by participant group. Among all patients with RA compared to all matched-controls without RA, TXB2 (pro-inflammatory) and LXA4 (pro-resolving) concentrations were higher. Among patients with RA, those taking FO had higher levels of EPA metabolites 15-HEPE and 18-HEPE, and DHA metabolite 17-HDHA; this was also true among participants without RA. However, the participants without RA taking FO compared to those not taking FO also had higher levels of both EPA and DHA, and the DHA metabolite, 14-HDHA, as well as lower amounts of resolvin RvE3. Differences in EPA, 18-HEPE, and 17-HDHA were significant after adjustment for multiple comparisons among the non-RA cases, but the other findings were no longer significant. (All results also shown in Supplementary Appendix Table 1.)
FIGURE 2.
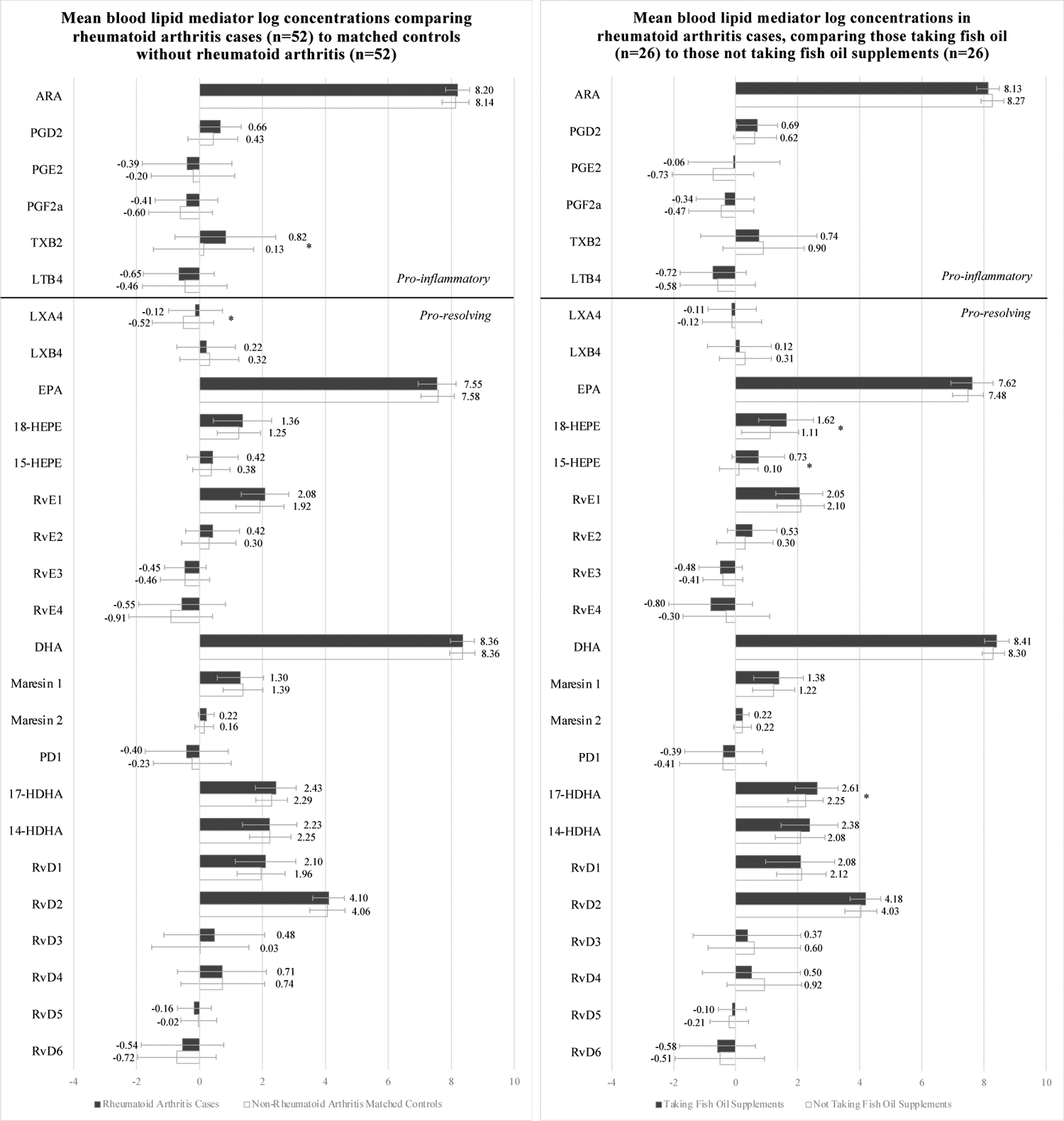
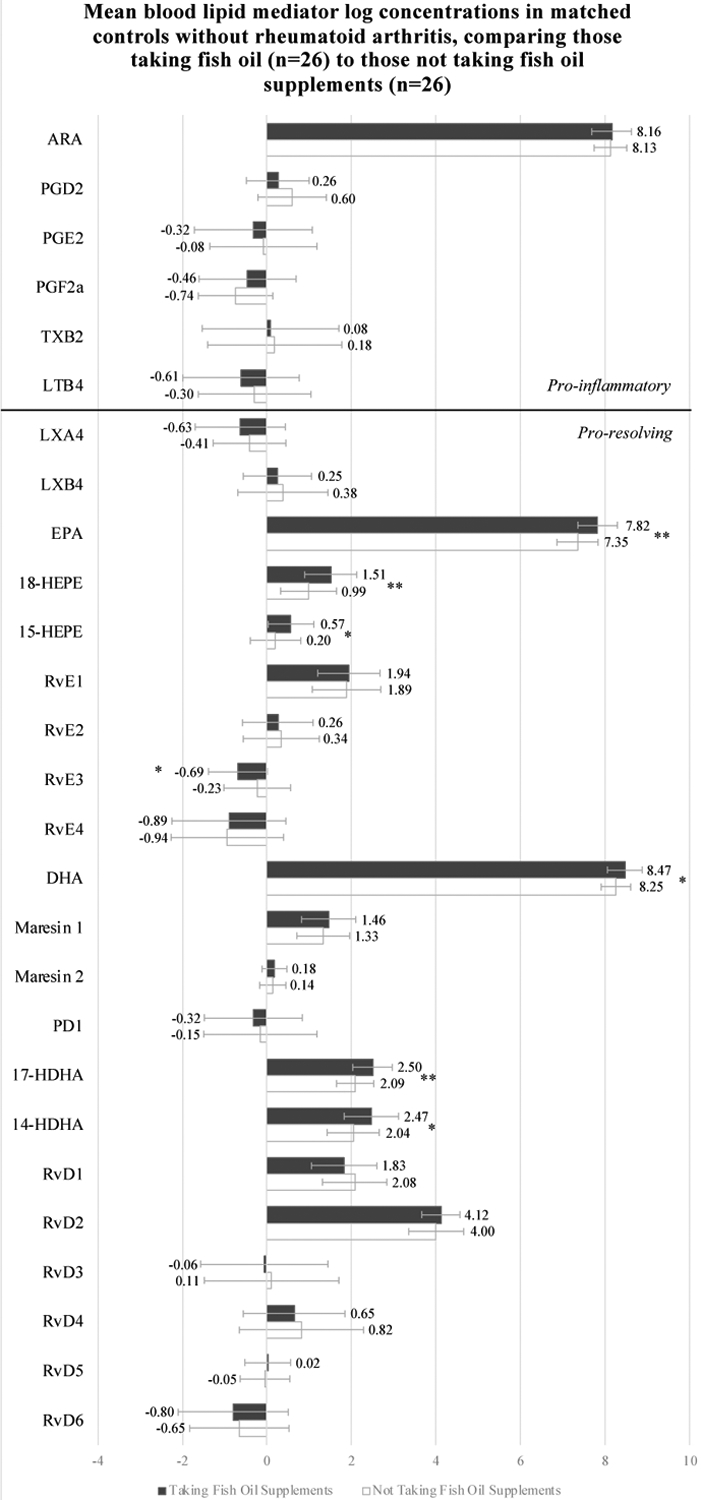
Mean blood lipid mediator log concentrations (pg/ul) among participants from the Mass General Brigham Biobank with rheumatoid arthritis cases and their matched controls without rheumatoid arthritis, taking and not taking fish oil supplements
Unadjusted analyses. Abbreviations: ARA, Arachidonic acid, EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LTB4, Leukotriene B4; LXA4, Lipoxin A4; LXB4, Lipoxin B4; MaR1–2, Maresin 1–2; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PGF2a, Prostaglandin F2 alpha; TXB2, Thromboxane B2; PD1, Protectin D1; RA, rheumatoid arthritis; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6, D-series resolvins; RvE1, RvE2, RvE3, and RvE4, E-series resolvins; 14-HDHA, 14-hydroxy-docosahexaenoic acid; 15-HEPE, 15-hydroxyeicosapentaenoic acid; 17-HDHA, 17-hydroxy-docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid.
* p value < 0.05
** FDR < 0.05
The results of multivariable adjusted linear regression analyses including all 104 participants examining associations between taking FO or not, and having RA or not, and log circulating lipid mediator concentrations are shown in Table 2. After adjustment for age, sex, race, BMI, and statin use, taking vs. not taking FO was significantly associated with higher levels of EPA, and its pro-resolving metabolites 15-HEPE and 18-HEPE, and DHA, and its pro-resolving metabolites, 14-HDHA and 17-HDHA. All but the result for DHA were significant after adjustment for multiple comparisons. RA status itself was associated with higher LXA4 concentrations in these multivariable models (although significance did not persist after adjustment for multiple comparisons). Moreover, we did not detect any significant interactions by the RA status of the participants, meaning that having RA did not significantly modify the association between taking FO and circulating lipid mediators.
TABLE 2.
Multivariable linear regression analyses of the associations between fish oil supplementation and circulating lipid mediators, including specialized pro-resolving mediators, among participants from the Mass General Brigham Biobank (n=104)
| Taking Fish Oil vs. Not Taking Fish Oil | Rheumatoid Arthritis vs. Non-Rheumatoid Arthritis | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| Lipid Mediator | Beta (95%CI) | P value | FDR | Beta (95%CI) | P value | FDR | P value | |
| Pro-Inflammatory | ARA | −0.049 (−0.206, 0.108) | 0.54 | 0.69 | 0.021 (−0.143, 0.185) | 0.80 | 0.93 | 0.36 |
| PGD2 | −0.156 (−0.436, 0.125) | 0.27 | 0.63 | 0.253 (−0.041, 0.546) | 0.09 | 0.66 | 0.17 | |
| PGE2 | 0.211 (−0.331, 0.754) | 0.44 | 0.63 | −0.210 (−0.777, 0.356) | 0.46 | 0.83 | 0.08 | |
| PGF2a | 0.202 (−0.188, 0.593) | 0.31 | 0.63 | 0.202 (−0.206, 0.609) | 0.33 | 0.74 | 0.82 | |
| TXB2 | −0.137 (−0.758, 0.485) | 0.66 | 0.78 | 0.552 (−0.097, 1.200) | 0.09 | 0.66 | 0.86 | |
| LTB4 | −0.194 (−0.661, 0.273) | 0.41 | 0.63 | −0.296 (−0.784, 0.191) | 0.23 | 0.66 | 0.70 | |
| Pro-Resolving | LXA4 | −0.113 (−0.475, 0.248) | 0.54 | 0.69 | 0.450 (0.072, 0.827) | 0.02 | 0.54 | 0.47 |
| LXB4 | −0.152 (−0.526, 0.222) | 0.42 | 0.63 | −0.130 (−0.520, 0.260) | 0.51 | 0.83 | 0.91 | |
| EPA | 0.309 (0.093, 0.524) | <0.01 | 0.04 | −0.049 (−0.274, 0.176) | 0.67 | 0.84 | 0.21 | |
| 18-HEPE | 0.515 (0.210, 0.819) | <0.01 | 0.01 | 0.099 (−0.218, 0.417) | 0.54 | 0.83 | 0.98 | |
| 15-HEPE | 0.496 (0.229, 0.763) | <0.01 | 0.01 | 0.027 (−0.252, 0.305) | 0.85 | 0.93 | 0.27 | |
| RvE1 | 0.027 (−0.267, 0.321) | 0.86 | 0.86 | 0.183 (−0.124, 0.490) | 0.24 | 0.66 | 0.96 | |
| RvE2 | 0.093 (−0.239, 0.425) | 0.58 | 0.71 | 0.213 (−0.134, 0.560) | 0.23 | 0.66 | 0.40 | |
| RvE3 | −0.256 (−0.539, 0.026) | 0.07 | 0.29 | 0.016 (−0.279, 0.310) | 0.92 | 0.93 | 0.18 | |
| RvE4 | −0.240 (−0.767, 0.287) | 0.37 | 0.63 | 0.252 (−0.299, 0.802) | 0.37 | 0.76 | 0.29 | |
| DHA | 0.170 (0.020, 0.320) | 0.03 | 0.12 | −0.007 (−0.164, 0.150) | 0.93 | 0.93 | 0.53 | |
| Mar1 | 0.161 (−0.108, 0.429) | 0.24 | 0.63 | −0.057 (−0.338, 0.223) | 0.69 | 0.84 | 0.91 | |
| Mar2 | 0.015 (−0.091, 0.122) | 0.78 | 0.84 | 0.041 (−0.070, 0.152) | 0.47 | 0.83 | 0.72 | |
| PD1 | −0.058 (−0.565, 0.450) | 0.82 | 0.85 | −0.147 (−0.677, 0.383) | 0.58 | 0.83 | 0.75 | |
| 17-HDHA | 0.395 (0.176, 0.614) | <0.01 | 0.01 | 0.141 (−0.087, 0.370) | 0.22 | 0.66 | 0.92 | |
| 14-HDHA | 0.366 (0.069, 0.663) | 0.02 | 0.09 | −0.088 (−0.398, 0.222) | 0.57 | 0.83 | 0.69 | |
| RvD1 | −0.139 (−0.477, 0.199) | 0.41 | 0.63 | 0.208 (−0.145, 0.560) | 0.25 | 0.66 | 0.40 | |
| RvD2 | 0.146 (−0.053, 0.345) | 0.15 | 0.50 | 0.104 (−0.104, 0.311) | 0.32 | 0.74 | 0.85 | |
| RvD3 | −0.249 (−0.869, 0.370) | 0.43 | 0.63 | 0.491 (−0.155, 1.137) | 0.13 | 0.66 | 0.84 | |
| RvD4 | −0.298 (−0.826, 0.229) | 0.26 | 0.63 | −0.139 (−0.691, 0.412) | 0.62 | 0.83 | 0.57 | |
| RvD5 | 0.099 (−0.118, 0.316) | 0.37 | 0.63 | −0.164 (−0.390, 0.063) | 0.16 | 0.66 | 0.63 | |
| RvD6 | −0.083 (−0.576, 0.410) | 0.74 | 0.83 | 0.028 (−0.487, 0.542) | 0.92 | 0.93 | 0.80 | |
All analyses are one model, adjusted for age, sex, race, smoking status, body mass index, and statin use.
Addition of interaction term between RA status and fish oil intake in each model
Abbreviations: ARA, Arachidonic acid, EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LTB4, Leukotriene B4; LXA4, Lipoxin A4; LXB4, Lipoxin B4; MaR1–2, Maresin 1–2; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PGF2a, Prostaglandin F2 alpha; TXB2, Thromboxane B2; PD1, Protectin D1; RA, rheumatoid arthritis; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6, D-series resolvins; RvE1, RvE2, RvE3, and RvE4, E-series resolvins; 14-HDHA, 14-hydroxy-docosahexaenoic acid; 15-HEPE, 15-hydroxyeicosapentaenoic acid; 17-HDHA, 17-hydroxy-docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid.
In RA case-only analyses, taking FO was associated with significant elevation of 15-HEPE only (Table 3). This was the case in analyses adjusting for age, sex, race, BMI and smoking status, as well as after additional adjustment for steroid and immunosuppressant use and elevated inflammatory markers. This difference was not significant after adjustment for multiple comparisons.
TABLE 3.
Multivariable linear regression analyses of the association between fish oil supplementation and circulating lipid mediators, investigating the effect of adjusting for systemic inflammation, among patients with rheumatoid arthritis (n=49) from the Mass General Brigham Biobank
| Lipid Mediator | Beta (95%CI)* | P value* | FDR* | Beta (95%CI)** | P value** | FDR** | |
|---|---|---|---|---|---|---|---|
| Adjusted for age, sex, race, BMI, and smoking | Fully adjusted model for systemic inflammation | ||||||
| Pro-Inflammatory | ARA | −0.145 (−0.362, 0.073) | 0.19 | 0.750 | −0.114 (−0.345, 0.118) | 0.33 | 0.87 |
| PGD2 | 0.029 (−0.363, 0.421) | 0.88 | 0.946 | 0.113 (−0.310, 0.537) | 0.59 | 0.90 | |
| PGE2 | 0.690 (−0.132, 1.513) | 0.10 | 0.660 | 0.568 (−0.324, 1.460) | 0.21 | 0.87 | |
| PGF2a | 0.072 (−0.450, 0.593) | 0.78 | 0.946 | 0.128 (−0.438, 0.695) | 0.65 | 0.90 | |
| TXB2 | −0.149 (−1.095, 0.796) | 0.75 | 0.946 | −0.200 (−1.237, 0.837) | 0.70 | 0.90 | |
| LTB4 | −0.117 (−0.734, 0.500) | 0.70 | 0.946 | −0.148 (−0.856, 0.560) | 0.67 | 0.90 | |
| Pro-Resolving | LXA4 | −0.038 (−0.523, 0.447) | 0.87 | 0.946 | −0.075 (−0.591, 0.441) | 0.77 | 0.90 |
| LXB4 | −0.181 (−0.724, 0.362) | 0.51 | 0.853 | −0.091 (−0.671, 0.489) | 0.75 | 0.90 | |
| EPA | 0.148 (−0.210, 0.505) | 0.41 | 0.750 | 0.165 (−0.214, 0.543) | 0.38 | 0.87 | |
| 18-HEPE | 0.498 (−0.024, 1.019) | 0.06 | 0.547 | 0.500 (−0.027, 1.026) | 0.06 | 0.56 | |
| 15-HEPE | 0.637 (0.193, 1.082) | <.01 | 0.161 | 0.626 (0.179, 1.074) | <0.01 | 0.20 | |
| RvE1 | 0.026 (−0.406, 0.459) | 0.90 | 0.946 | −0.059 (−0.520, 0.402) | 0.80 | 0.90 | |
| RvE2 | 0.255 (−0.242, 0.751) | 0.31 | 0.750 | 0.206 (−0.341, 0.753) | 0.45 | 0.90 | |
| RvE3 | −0.027 (−0.413, 0.358) | 0.89 | 0.946 | −0.130 (−0.535, 0.275) | 0.52 | 0.90 | |
| RvE4 | −0.507 (−1.300, 0.286) | 0.20 | 0.750 | −0.511 (−1.396, 0.374) | 0.25 | 0.87 | |
| DHA | 0.097 (−0.126, 0.321) | 0.38 | 0.750 | 0.126 (−0.111, 0.363) | 0.29 | 0.87 | |
| Mar1 | 0.209 (−0.220, 0.638) | 0.33 | 0.750 | 0.140 (−0.343, 0.623) | 0.56 | 0.90 | |
| Mar2 | −0.005 (−0.158, 0.147) | 0.95 | 0.946 | 0.005 (−0.165, 0.174) | 0.96 | 0.96 | |
| PD1 | 0.028 (−0.739, 0.795) | 0.94 | 0.946 | 0.157 (−0.653, 0.967) | 0.70 | 0.90 | |
| 17-HDHA | 0.361 (−0.007, 0.729) | 0.05 | 0.547 | 0.388 (−0.016, 0.791) | 0.06 | 0.56 | |
| 14-HDHA | 0.289 (−0.211, 0.788) | 0.25 | 0.750 | 0.248 (−0.246, 0.743) | 0.32 | 0.87 | |
| RvD1 | −0.046 (−0.596, 0.503) | 0.87 | 0.946 | 0.194 (−0.347, 0.736) | 0.47 | 0.90 | |
| RvD2 | 0.196 (−0.085, 0.476) | 0.17 | 0.750 | 0.240 (−0.059, 0.538) | 0.11 | 0.76 | |
| RvD3 | −0.382 (−1.320, 0.556) | 0.42 | 0.750 | 0.031 (−0.889, 0.951) | 0.95 | 0.96 | |
| RvD4 | −0.376 (−1.191, 0.438) | 0.36 | 0.750 | −0.371 (−1.215, 0.472) | 0.38 | 0.87 | |
| RvD5 | 0.146 (−0.164, 0.456) | 0.35 | 0.750 | 0.171 (−0.171, 0.513) | 0.32 | 0.87 | |
| RvD6 | 0.129 (−0.612, 0.871) | 0.73 | 0.946 | 0.029 (−0.741, 0.799) | 0.94 | 0.96 | |
Adjusted for age, sex, race, body mass index, and smoking status.
Additionally adjusted for statin, steroid and immunosuppressant use, and ESR/CRP (elevated or not).
Abbreviations: ARA, Arachidonic acid, EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LTB4, Leukotriene B4; LXA4, Lipoxin A4; LXB4, Lipoxin B4; MaR1–2, Maresin 1–2; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PGF2a, Prostaglandin F2 alpha; TXB2, Thromboxane B2; PD1, Protectin D1; RA, rheumatoid arthritis; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6, D-series resolvins; RvE1, RvE2, RvE3, and RvE4, E-series resolvins; 14-HDHA, 14-hydroxy-docosahexaenoic acid; 15-HEPE, 15-hydroxyeicosapentaenoic acid; 17-HDHA, 17-hydroxy-docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid.
The Spearman correlations between EPA, DHA, ARA and their metabolites were similar for patients with RA and their matched controls without RA (Table 4). There was a strong positive correlation between EPA and DHA and their immediate downstream SPM precursors, 18-HEPE and 15-HEPE, and 17-HDHA and 14-HDHA, respectively, but they were only weakly correlated with further downstream SPMs. ARA was not strongly correlated with its downstream metabolites.
TABLE 4.
Spearman correlations between plasma levels of omega-3 and omega-6 fatty acids and their down-stream metabolites including specialized pro-resolving lipid mediators among participants from the Mass General Brigham Biobank with rheumatoid arthritis and matched controls without rheumatoid arthritis
| ARA | EPA | DHA | |||||
|---|---|---|---|---|---|---|---|
| Lipid mediator | Rheumatoid Arthritis Cases ρ (p-value) | Matched Controls ρ (p-value) | Rheumatoid Arthritis Cases ρ (p-value) | Matched Controls ρ (p-value) | Rheumatoid Arthritis Cases ρ (p-value) | Matched Controls ρ (p-value) | |
| Pro-Inflammatory | PGD2 | −0.368 (0.01) | −0.323 (0.02) | ||||
| PGE2 | −0.061 (0.67) | −0.089 (0.53) | |||||
| PGF2a | −0.076 (0.59) | −0.154 (0.28) | |||||
| TXB2 | 0.138 (0.33) | 0.214 (0.13) | |||||
| LTB4 | −0.135 (0.34) | −0.011 (0.94) | |||||
| Pro-Resolving | LXA4 | −0.104 (0.46) | −0.290 (0.04) | ||||
| LXB4 | 0.116 (0.41) | 0.265 (0.06) | |||||
| 18-HEPE | 0.728 (<.001) | 0.800 (<.001) | |||||
| 15-HEPE | 0.660 (<.001) | 0.750 (<.001) | |||||
| RvE1 | −0.015 (0.91) | 0.031 (0.83) | |||||
| RvE2 | 0.056 (0.69) | 0.039 (0.78) | |||||
| RvE3 | −0.173 (0.22) | −0.041 (0.77) | |||||
| RvE4 | −0.186 (0.19) | 0.007 (0.96) | |||||
| Mar1 | −0.016 (0.91) | 0.051 (0.72) | |||||
| Mar2 | 0.136 (0.34) | 0.012 (0.93) | |||||
| PD1 | 0.180 (0.20) | 0.030 (0.83) | |||||
| 17-HDHA | 0.774 (<.001) | 0.766 (<.001) | |||||
| 14-HDHA | 0.519 (<.001) | 0.497 (<.001) | |||||
| RvD1 | 0.338 (0.01) | 0.325 (0.02) | |||||
| RvD2 | 0.056 (0.70) | −0.070 (0.62) | |||||
| RvD3 | −0.043 (0.77) | 0.015 (0.92) | |||||
| RvD4 | −0.059 (0.68) | −0.269 (0.05) | |||||
| RvD5 | −0.042 (0.77) | 0.149 (0.29) | |||||
| RvD6 | 0.063 (0.66) | ‒0.157 (0.27) | |||||
Abbreviations: ARA, Arachidonic acid, EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LTB4, Leukotriene B4; LXA4, Lipoxin A4; LXB4, Lipoxin B4; MaR1–2, Maresin 1–2; PGD2, Prostaglandin D2; PGE2, Prostaglandin E2; PGF2a, Prostaglandin F2 alpha; TXB2, Thromboxane B2; PD1, Protectin D1; RA, rheumatoid arthritis; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6, D-series resolvins; RvE1, RvE2, RvE3, and RvE4, E-series resolvins; 14-HDHA, 14-hydroxy-docosahexaenoic acid; 15-HEPE, 15-hydroxyeicosapentaenoic acid; 17-HDHA, 17-hydroxy-docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid.
4. DISCUSSION
Chronic inflammation in RA been posited to be due to a potential failure of endogenous pro-resolution mechanisms, which may be overwhelmed by extensive pro-inflammatory signals.[29,30] In this cross-sectional study, taking advantage of stored blood samples from patients with RA and those of well-matched controls, we examined both the association between RA itself and circulating lipid mediators and the association between taking over-the-counter FO supplements and these lipid mediator levels, among patients with and without RA. We found that patients with RA, compared to their matched controls without RA, had higher levels of TXB2 and LXA4 (neither association persisted after adjustment for multiple comparisons). It is easier to understand the elevation in pro-inflammatory TXB2 than perhaps it is to understand the elevated LXA4, although it may point to upregulated inflammation resolution responses ongoing in vivo or medication use. Taking oral FO was significantly associated with an increase in circulating levels of the omega-3 polyunsaturated fatty acids DHA and EPA, as well as SPM precursors, 14- and 17-HDHA (from DHA) and 15- and 18-HEPE (from EPA). Similar increases were observed in those with and without RA and there was no formal effect modification by RA status (although power was limited to detect differences in the subsets).
FO supplementation has been proven to reduce joint pain and swelling in RA, but the mechanisms have been incompletely understood. In double-blinded randomized controlled trials in the 1990s, Kremer et al demonstrated that oral n-3 PUFA supplementation had multiple beneficial effects in RA.[21,22] They reported significant reductions in tender joint counts, morning stiffness, physician and patient global assessments of disease activity, as well as a reduction in the use of non-steroidal anti-inflammatory drugs and a decrease in circulating IL-1β.[22] The mechanisms of these benefits were not completely understood, although it was hypothesized that the n-3 PUFAs led to a shift away from ARA inflammatory lipid mediators because DHA and EPA, the main n-3 PUFA components, suppress ARA-derived eicosanoids. In the current study, we did not find that individuals who took FO supplements had significantly lower circulating ARA-derived inflammatory eicosanoids, including PGD2, PGE2, PGF2a, TXB2 or LTB4. This contrasts to what has been shown in past animal model and human in vitro studies, and may reflect variation in FO doses, adherence, and timing of intake, as well as variation in individual inflammatory eicosanoid levels.[10–15] We did observe numerically lower levels of several of these inflammatory eicosanoids among those taking FO, but sample size was limited for the detection of small differences. It may also be that the beneficial effects of FO are through induction of SPM production, rather than suppression of inflammatory eicosanoids, thus further research is warranted.
SPMs have been less studied in RA since they are relatively newly discovered, and their functions in resolving inflammation have only recently been elucidated.[9–13] When considering SPM precursors, which have been received research attention in the past, in observational studies, high fish and n-3 PUFA intakes were inversely associated with RA risk.[7,8,31] Low n-3 PUFA blood levels have been associated with increased risk of RA antibodies in healthy relatives of patients with RA, and higher risk of developing RA among asymptomatic individuals with anti-CCP antibodies.[32,33] In the VITamin D and omegA-3 Trial (VITAL), a 5-year double-blind randomized controlled trial of 25,871 community-dwelling adults, 1 gm/day n-3 PUFAs (460 mg EPA and 380 mg DHA) reduced the risk of all incident autoimmune disease by 15% and of RA by 44% (p 0.07).[34]
The discovery and complete stereochemical characterization of the SPMs have opened the possibility of new therapeutic avenues for inflammatory rheumatic diseases, and may provide the biochemical mechanisms for the benefits of n-3 PUFA supplementation in RA.[10] Murine models have implicated the SPM metabolites of n-3 PUFAs, in particular the resolvins, in resolution of inflammation in RA. Arnardottir and colleagues studied the temporality of SPM production in a murine model of self-resolving inflammatory arthritis, focusing in particular on the resolvins.[30] They found the D-series resolvins (from DHA) were temporally produced in self-resolving arthritis, and that reduced RvD3 levels were associated with delayed resolution of inflammation and the arthritis in mice. Norling and colleagues studied synovial joint fluid from the murine K/BxN serum transfer arthritis model, with and without oral n-3 PUFA supplementation, and found elevated levels of intraarticular SPMs, in particular RvD1, in the n-3 PUFA supplemented mice.[29]
However, only a few past studies have examined EPA and DHA-derived metabolites and SPMs in humans with RA, a disease marked by failure of inflammation resolution on a chronic basis. Arnardottir and colleagues reported that serum levels RvD3 were reduced in three patients with RA compared to three healthy controls.[30] Norling and colleagues profiled synovial fluid from four patients with RA and reported physiological levels of RvD1.[29] Jeffrey et al observed that among 22 patients with established RA newly starting etanercept, an anti-tumor necrosis factor (TNF) drug, high baseline blood EPA and EPA/ARA ratio were associated with more favorable European League against Rheumatism (EULAR) response to anti-TNF at 3 months.[35]
Thus, the current study of 52 patients with RA compared to 52 matched controls without RA, investigating the effects of taking oral over-the-counter FO supplementation contributes meaningfully to the literature. We have found that taking FO supplements increases DHA and EPA as expected and the pool of important SPM precursors in both patients with RA and their matched controls without RA, with no significant effect modification by RA status, although the results among RA cases did not appear to be as robust numerically. Taking over-the-counter FO supplements was associated with increased 18-HEPE (from EPA) in those with and without RA, which is particularly interesting given that 18-HEPE has been shown to have potent inhibitory effects on macrophage-mediated proinflammatory activation of cardiac fibroblasts in mouse models.[36] Further, both 18-HEPE and 17-HDHA are pro-resolving mediators that have proven to be potent mediators in addition to being substrate for resolvins.[37] Other studies of FO supplementation and SPM levels in humans have found increased plasma levels of SPMs following FO supplementation in healthy adults, those with peripheral artery disease, and in obese adults where increased plasma levels of resolvin E1 and maresin 1 were observed in one study.[23–26,38] Some studies have not found similar results, however, these were of short duration and contained a small number of participants (n=12)[39] or included only participants with mild hypertriacylglycerolemia at baseline.[40]
Limitations of this study include self-reporting which may be incomplete, lack of detailed data about the dose, preparation, and adherence to the FO supplements, and given the lack of standardization of these over-the-counter dietary supplements, there may have been variability. Moreover, while we did have access to complete medical records, we did not have simultaneous detailed physician-completed and standardized assessments of RA disease activity, or response to therapy, nor did we have measures of systemic inflammation or serostatus for the patients without RA. We assayed a panel of 27 central lipid mediators, including ARA, EPA, DHA and their metabolites, but were unable to study yet more potentially important lipid mediators and all their potentially interesting downstream metabolites due to financial constraints. This study focused on circulating blood concentrations and did not assess levels in specific tissues nor the time course of their production and metabolism, both subjects of research interest as well. Power was limited for stratified analyses and detection of interactions.
This is, however, the first study to examine the association between oral administration of FO and circulating levels of ARA, DHA and EPA and their important pro-inflammatory and pro-resolving mediators in the blood of humans with and without RA. These results are important and encouraging that patients with RA taking FO supplements generally have a similar increase in the pool of SPM precursors which may be essential for the local and timely production of SPMs at the site of inflammation. Future studies with more controlled administration of FO supplements should be undertaken among patients with RA and matched controls without RA to delineate the exact time course of their effects, to characterize circulating and local effects upon SPM production, as well as to decipher SPM actions on the cellular infiltrate, cytokine and chemokine production, and the resident components of synovial joints.
In conclusion, oral over-the-counter FO supplementation was associated with higher blood levels of pro-resolving 14- and 17-HDHA (from DHA) and 15- and 18-HEPE (from EPA), precursors of both protectins and resolvins. This was found in patients with and without RA, indicating that patients with RA do not appear to have a diminished ability to synthesize SPM precursors from fatty acid reserves, which may be important in resolving RA inflammation. Moreover, the powerful pro-resolution effects of the SPMs may offer a future non-immunosuppressive RA therapy. These novel findings deserve further confirmation and may point to how these pro-resolving mechanisms could be effectively targeted in RA.
Supplementary Material
HIGHLIGHTS.
Fish oil supplements contain omega-3 fatty acids from which specialized pro-resolving mediators (SPM) are derived, including resolvins, protectins, and maresins. These have potential as therapy for human rheumatic inflammatory disease, however, little is known about the effect of fish oil supplementation on SPM blood levels in rheumatoid arthritis patients. But, previous research has shown that fish oil supplementation is associated with a lower risk of RA. Thus, we investigated associations between over-the-counter omega-3 fatty acid FO supplementation and circulating SPMs among patients with and without RA.
Among all patients, both with and without RA, oral over-the-counter fish oil supplementation was associated with higher blood levels of pro-resolving lipid mediators including 17-HDHA (from DHA) and 15- and 18-HEPE (from EPA), precursors of both protectins and resolvins.
RA patients have the ability to augment their SPM precursor reserves, which may be important in resolving RA inflammation.
ACKNOWLEDGEMENTS
We would like to thank Mass General Brigham Biobank study participants for their contributions. The authors wish to acknowledge The Analytical Facility for Bioactive Molecules, the Hospital for Sick Children, Toronto, Canada for assistance with quantification of SPM and LM by targeted LC-MS/MS. We would also like to thank Denis Reynaud, Ph.D. and Charles N. Serhan, Ph.D., DSc for their expertise and contributions.
Funding Statement
This work was supported by The National Institutes of Health [grants numbers NIAMS R01AR059086 and K24AR066109 (Costenbader); NHBLI R01HL141826 and R01HL123915 (Lasky-Su); NHLBI R01HL134811, R01HL160799 (Mora)].
Role of the funding source
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, who were not involved in the study design, data collection, analysis or interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Abbreviations
- ARA
Arachidonic acid
- EPA
Eicosapentaenoic acid
- DHA
Docosahexaenoic acid
- LTB4
Leukotriene B4
- LXA4
Lipoxin A4
- LXB4
Lipoxin B4
- Mar1 and Mar2
Maresin 1 and Maresin 2
- PGD2
Prostaglandin D2
- PGE2
Prostaglandin E2
- PGF2a
Prostaglandin F2 alpha
- TXB2
Thromboxane B2
- PD1
Protectin D1
- RA
Rheumatoid Arthritis
- RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6
D-series resolvins
- RvE1, RvE2, RvE3, and RvE4
E-series resolvins
- 14-HDHA
14-hydroxy-docosahexaenoic acid
- 15-HEPE
15-hydroxyeicosapentaenoic acid
- 17-HDHA
17-hydroxy-docosahexaenoic acid
- 18-HEPE
18-hydroxyeicosapentaenoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
NM receives consulting fees from Pritikin Longevity Center. JL-S is on the scientific advisory board for Precion, Inc. All other authors report no conflict of interest.
Author Statement
Nathalie E. Marchand: Writing – Original Draft, Writing – Review & Editing, Visualization, May Y. Choi: Conceptualization, Writing –Review & Editing, Emily G. Oakes: Validation, Writing –Review & Editing, Nancy R. Cook: Writing –Review & Editing, Emma Stevens: Writing –Review & Editing, Natalya Gomelskaya: Validation, Writing –Review & Editing, Gregory Kotler: Software, Data curation, JoAnn E. Manson: Writing –Review & Editing, Jessica Lasky-Su: Writing –Review & Editing, Funding acquisition, Samia Mora: Writing –Review & Editing, I-Min Lee: Writing –Review & Editing, Raju Tatituri: Investigation, Karen H. Costenbader: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Supervision, Project Supervision, Funding acquisition
Data Availability
Data can be requested from the Mass General Brigham biobank at: https://rc.partners.org/research-apps-services/identify-subjects-request-data.
REFERENCES
- [1].Soussi B, Cordtz R, Kristensen S, Bork C, Christensen J, Schmidt E, Torp-Pedersen C, Prieto-Alhambra D, Dreyer L, Incidence and prevalence of rheumatoid arthritis in Denmark from 1998 to 2018: a nationwide register-based study, Scandinavian Journal of Rheumatology. (2021) 1–9. 10.1080/03009742.2021.1957557. [DOI] [PubMed] [Google Scholar]
- [2].Myasoedova E, Davis J, Matteson EL, Crowson CS, Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014, Ann Rheum Dis. 79 (2020) 440–444. 10.1136/annrheumdis-2019-216694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bengtsson C, Malspeis S, Orellana C, Sparks JA, Costenbader KH, Karlson EW, Association Between Menopausal Factors and the Risk of Seronegative and Seropositive Rheumatoid Arthritis: Results From the Nurses’ Health Studies, Arthritis Care Res (Hoboken). 69 (2017) 1676–1684. 10.1002/acr.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dalal DS, Zhang T, Shireman TI, Medicare expenditures for conventional and biologic disease modifying agents commonly used for treatment of rheumatoid arthritis, Seminars in Arthritis and Rheumatism. 50 (2020) 822–826. 10.1016/j.semarthrit.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arkema EV, Lu B, Malspeis S, Karlson EW, Costenbader KH, Monocyte chemotactic protein-1 elevation prior to the onset of rheumatoid arthritis among women, Biomark Med. 9 (2015) 723–729. 10.2217/BMM.15.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karlson EW, Chibnik LB, Tworoger SS, Lee I-M, Buring JE, Shadick NA, Manson JE, Costenbader KH, Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies, Arthritis Rheum. 60 (2009) 641–652. 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosell M, Wesley A-M, Rydin K, Klareskog L, Alfredsson L, EIRA study group, Dietary fish and fish oil and the risk of rheumatoid arthritis, Epidemiology. 20 (2009) 896–901. 10.1097/EDE.0b013e3181b5f0ce. [DOI] [PubMed] [Google Scholar]
- [8].Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A, Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women, Ann Rheum Dis. 73 (2014) 1949–1953. 10.1136/annrheumdis-2013-203338. [DOI] [PubMed] [Google Scholar]
- [9].Dyall SC, Balas L, Bazan NG, Brenna JT, Chiang N, da Costa Souza F, Dalli J, Durand T, Galano J-M, Lein PJ, Serhan CN, Taha AY, Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions, Progress in Lipid Research. 86 (2022) 101165. 10.1016/j.plipres.2022.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature. 510 (2014) 92–101. 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Serhan CN, Chiang N, Dalli J, The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution, Semin Immunol. 27 (2015) 200–215. 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N, Novel Anti-Inflammatory-Pro-Resolving Mediators and Their Receptors, CTMC. 11 (2011) 629–647. 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K, Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing, Journal of Experimental Medicine. 192 (2000) 1197–1204. 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chiang N, Serhan CN, Specialized pro-resolving mediator network: an update on production and actions, Essays in Biochemistry. 64 (2020) 443–462. 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN, Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue, Am J Physiol Cell Physiol. 307 (2014) C39–54. 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG, Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression, Journal of Biological Chemistry. 278 (2003) 43807–43817. 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- [17].Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M, Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1, Am J Neurodegener Dis. 1 (2012) 60–74. [PMC free article] [PubMed] [Google Scholar]
- [18].Schwab JM, Chiang N, Arita M, Serhan CN, Resolvin E1 and protectin D1 activate inflammation-resolution programmes, Nature. 447 (2007) 869–874. 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dalli J, Serhan CN, Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators, Blood. 120 (2012) e60–e72. 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sobrino A, Walker ME, Colas RA, Dalli J, Protective activities of distinct omega-3 enriched oils are linked to their ability to upregulate specialized pro-resolving mediators, PLoS ONE. 15 (2020) e0242543. 10.1371/journal.pone.0242543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kremer JM, Lawrence DA, Jubiz W, Digiacomo R, Rynes R, Bartholomew LE, Sherman M, Dietary fish oil and olive oil supplementation in patients with Rheumatoid Arthritis clinical and immunologic effects, Arthritis & Rheumatism. 33 (1990) 810–820. 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- [22].Kremer JM, Lawrence DA, Petrillo GF, Litts LL, Mullaly PM, Rynes RI, Stocker RP, Parhami N, Greenstein NS, Fuchs BR, Mathur A, Robinson DR, Sperling RI, Bigaouette J, Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs clinical and immune correlates, Arthritis & Rheumatism. 38 (1995) 1107–1114. 10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- [23].Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ, Dalli J, Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study, Circ Res. 126 (2020) 75–90. 10.1161/CIRCRESAHA.119.315506. [DOI] [PubMed] [Google Scholar]
- [24].Mas E, Croft KD, Zahra P, Barden A, Mori TA, Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood following n-3 Fatty Acid Supplementation, Clinical Chemistry. 58 (2012) 1476–1484. 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- [25].Al-Shaer AE, Regan J, Buddenbaum N, Tharwani S, Drawdy C, Behee M, Sergin S, Fenton JI, Maddipati KR, Kane S, Butler E, Shaikh SR, Enriched Marine Oil Supplement Increases Specific Plasma Specialized Pro-Resolving Mediators in Adults with Obesity, The Journal of Nutrition. 152 (2022) 1783–1791. 10.1093/jn/nxac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ramirez JL, Gasper WJ, Khetani SA, Zahner GJ, Hills NK, Mitchell PT, Sansbury BE, Conte MS, Spite M, Grenon SM, Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial), Journal of Surgical Research. 238 (2019) 164–174. 10.1016/j.jss.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JMW, Hobbs K, Huizinga TWJ, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G, 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative, Arthritis Rheum. 62 (2010) 2569–2581. 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- [28].Benjamini Y, Yekutieli D, False Discovery Rate–Adjusted Multiple Confidence Intervals for Selected Parameters, Journal of the American Statistical Association. 100 (2005) 71–81. 10.1198/016214504000001907. [DOI] [Google Scholar]
- [29].Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M, Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis, JCI Insight. 1 (2016). 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M, Serhan CN, Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation, J.I 197 (2016) 2362–2368. 10.4049/jimmunol.1502268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sparks JA, O’Reilly ÉJ, Barbhaiya M, Tedeschi SK, Malspeis S, Lu B, Willett WC, Costenbader KH, Karlson EW, Association of fish intake and smoking with risk of rheumatoid arthritis and age of onset: a prospective cohort study, BMC Musculoskelet Disord. 20 (2019) 2. 10.1186/s12891-018-2381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gan RW, Demoruelle MK, Deane KD, Weisman MH, Buckner JH, Gregersen PK, Mikuls TR, O’Dell JR, Keating RM, Fingerlin TE, Zerbe GO, Clare-Salzler MJ, Holers VM, Norris JM, Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis, Ann Rheum Dis. 76 (2017) 147–152. 10.1136/annrheumdis-2016-209154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gan RW, Young KA, Zerbe GO, Demoruelle MK, Weisman MH, Buckner JH, Gregersen PK, Mikuls TR, O’Dell JR, Keating RM, Clare-Salzler MJ, Deane KD, Holers VM, Norris JM, Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: a nested case-control study, Rheumatology. 55 (2016) 367–376. 10.1093/rheumatology/kev266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, Kotler G, Lee I-M, Manson JE, Costenbader KH, Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial, BMJ. (2022) e066452. 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jeffery L, Fisk HL, Calder PC, Filer A, Raza K, Buckley CD, McInnes I, Taylor PC, Fisher BA, Plasma Levels of Eicosapentaenoic Acid Are Associated with Anti-TNF Responsiveness in Rheumatoid Arthritis and Inhibit the Etanercept-driven Rise in Th17 Cell Differentiation in Vitro, J Rheumatol. 44 (2017) 748–756. 10.3899/jrheum.161068. [DOI] [PubMed] [Google Scholar]
- [36].Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M, 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload–induced maladaptive cardiac remodeling, Journal of Experimental Medicine. 211 (2014) 1673–1687. 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Serhan CN, Rosa X, Jouvene C, Novel mediators and mechanisms in the resolution of infectious inflammation: evidence for vagus regulation, J Intern Med. 286 (2019) 240–258. 10.1111/joim.12871. [DOI] [PubMed] [Google Scholar]
- [38].Lamon-Fava S, So J, Mischoulon D, Ziegler TR, Dunlop BW, Kinkead B, Schettler PJ, Nierenberg AA, Felger JC, Maddipati KR, Fava M, Rapaport MH, Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation, Prostaglandins, Leukotrienes and Essential Fatty Acids. 164 (2021) 102219. 10.1016/j.plefa.2020.102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Skarke C, Alamuddin N, Lawson JA, Li X, Ferguson JF, Reilly MP, FitzGerald GA, Bioactive products formed in humans from fish oils, Journal of Lipid Research. 56 (2015) 1808–1820. 10.1194/jlr.M060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M, Grün M, Nicolaou A, Jahreis G, Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: Effects on circulating eicosanoids and cardiovascular risk factors, Clinical Nutrition. 32 (2013) 686–696. 10.1016/j.clnu.2012.12.010. [DOI] [PubMed] [Google Scholar]
- [41].Li C, Wu X, Liu S, Shen D, Zhu J, Liu K, Role of Resolvins in the Inflammatory Resolution of Neurological Diseases, Front. Pharmacol 11 (2020) 612. 10.3389/fphar.2020.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be requested from the Mass General Brigham biobank at: https://rc.partners.org/research-apps-services/identify-subjects-request-data.


