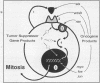
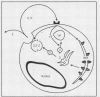
Abstract
The ability of malignant cells to escape the constraint that normally regulate cell growth and differentiation has been a primary focus of attention for investigators of cancer cell biology. An outcome of this attention has been the discovery that the protein products of oncogenes play a role in the activation of growth signal pathways. A second outcome, possibly related to abnormal oncogene expression, has been the discovery that malignant cells frequently show an ability to regulate their own growth by the release of autocrine growth modulatory substances. Most important, the growth of certain malignant cell types has been shown to depend on autocrine growth circuits. A malignant tumor whose continued growth depends on the release of an autocrine growth factor may be vulnerable to treatment with specific receptor antagonists or immunoneutralizing antibodies designed to break the autocrine circuit. Information is rapidly emerging concerning autocrine growth factors in selected human solid tissue malignancy.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Merlo G., Callahan R., Lidereau R. The amplification unit on chromosome 11q13 in aggressive primary human breast tumors entails the bcl-1, int-2 and hst loci. Oncogene. 1989 Jan;4(1):89–92. [PubMed] [Google Scholar]
- Anzano M. A., Rieman D., Prichett W., Bowen-Pope D. F., Greig R. Growth factor production by human colon carcinoma cell lines. Cancer Res. 1989 Jun 1;49(11):2898–2904. [PubMed] [Google Scholar]
- Arteaga C. L., Coronado E., Osborne C. K. Blockade of the epidermal growth factor receptor inhibits transforming growth factor alpha-induced but not estrogen-induced growth of hormone-dependent human breast cancer. Mol Endocrinol. 1988 Nov;2(11):1064–1069. doi: 10.1210/mend-2-11-1064. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Tandon A. K., Von Hoff D. D., Osborne C. K. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988 Jul 15;48(14):3898–3904. [PubMed] [Google Scholar]
- Augereau P., Garcia M., Mattei M. G., Cavailles V., Depadova F., Derocq D., Capony F., Ferrara P., Rochefort H. Cloning and sequencing of the 52K cathepsin D complementary deoxyribonucleic acid of MCF7 breast cancer cells and mapping on chromosome 11. Mol Endocrinol. 1988 Feb;2(2):186–192. doi: 10.1210/mend-2-2-186. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Davidson N. E., Valverius E. M., Freter C. E., Dickson R. B., Tam J. P., Kudlow J. E., Lippman M. E., Salomon D. S. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988 Jun;2(6):543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Valverius E. M., Ennis B. W., Bronzert D. A., Sheridan J. P., Stampfer M. R., Mendelsohn J., Lippman M. E., Dickson R. B. Expression of the transforming growth factor-alpha/epidermal growth factor receptor pathway in normal human breast epithelial cells. Endocrinology. 1990 Jan;126(1):596–607. doi: 10.1210/endo-126-1-596. [DOI] [PubMed] [Google Scholar]
- Battaglia F., Scambia G., Rossi S., Panici P. B., Bellantone R., Polizzi G., Querzoli P., Negrini R., Iacobelli S., Crucitti F. Epidermal growth factor receptor in human breast cancer: correlation with steroid hormone receptors and axillary lymph node involvement. Eur J Cancer Clin Oncol. 1988 Nov;24(11):1685–1690. doi: 10.1016/0277-5379(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Bennett C., Paterson I. M., Corbishley C. M., Luqmani Y. A. Expression of growth factor and epidermal growth factor receptor encoded transcripts in human gastric tissues. Cancer Res. 1989 Apr 15;49(8):2104–2111. [PubMed] [Google Scholar]
- Berthois Y., Dong X. F., Martin P. M. Regulation of epidermal growth factor-receptor by estrogen and antiestrogen in the human breast cancer cell line MCF-7. Biochem Biophys Res Commun. 1989 Feb 28;159(1):126–131. doi: 10.1016/0006-291x(89)92413-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Blatt J., White C., Dienes S., Friedman H., Foley T. P., Jr Production of an insulin-like growth factor by osteosarcoma. Biochem Biophys Res Commun. 1984 Aug 30;123(1):373–376. doi: 10.1016/0006-291x(84)90423-6. [DOI] [PubMed] [Google Scholar]
- Browder T. M., Dunbar C. E., Nienhuis A. W. Private and public autocrine loops in neoplastic cells. Cancer Cells. 1989 Sep;1(1):9–17. [PubMed] [Google Scholar]
- Capony F., Garcia M., Capdevielle J., Rougeot C., Ferrara P., Rochefort H. Purification and first characterization of the secreted and cellular 52-kDa proteins regulated by estrogens in human-breast cancer cells. Eur J Biochem. 1986 Dec 1;161(2):505–512. doi: 10.1111/j.1432-1033.1986.tb10471.x. [DOI] [PubMed] [Google Scholar]
- Capony F., Morisset M., Barrett A. J., Capony J. P., Broquet P., Vignon F., Chambon M., Louisot P., Rochefort H. Phosphorylation, glycosylation, and proteolytic activity of the 52-kD estrogen-induced protein secreted by MCF7 cells. J Cell Biol. 1987 Feb;104(2):253–262. doi: 10.1083/jcb.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Cartlidge S. A., Elder J. B. Transforming growth factor alpha and epidermal growth factor levels in normal human gastrointestinal mucosa. Br J Cancer. 1989 Nov;60(5):657–660. doi: 10.1038/bjc.1989.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles V., Garcia M., Rochefort H. Regulation of cathepsin-D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol Endocrinol. 1989 Mar;3(3):552–558. doi: 10.1210/mend-3-3-552. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S., Tobon A., Varani J., Brattain M. G. Induction of carcinoembryonic antigen secretion and modulation of protein secretion/expression and fibronectin/laminin expression in human colon carcinoma cells by transforming growth factor-beta. Cancer Res. 1988 Jul 15;48(14):4059–4064. [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Chicone L., Narayan S., Townsend C. M., Jr, Singh P. The presence of a 33-40 KDa gastrin binding protein on human and mouse colon cancer. Biochem Biophys Res Commun. 1989 Oct 16;164(1):512–519. doi: 10.1016/0006-291x(89)91749-x. [DOI] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katz D., Glanz P., Dickson R. B., Lippman M. E., Kern F. G. The effects of a constitutive expression of transforming growth factor-alpha on the growth of MCF-7 human breast cancer cells in vitro and in vivo. Mol Endocrinol. 1989 Feb;3(2):372–380. doi: 10.1210/mend-3-2-372. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Goustin A. S., Soderquist A. M., Shipley G. D., Wolfshohl J., Carpenter G., Moses H. L. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Shipley G. D., Moses H. L. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986 Mar;46(3):1164–1169. [PubMed] [Google Scholar]
- Cormier E. M., Jordan V. C. Contrasting ability of antiestrogens to inhibit MCF-7 growth stimulated by estradiol or epidermal growth factor. Eur J Cancer Clin Oncol. 1989 Jan;25(1):57–63. doi: 10.1016/0277-5379(89)90051-5. [DOI] [PubMed] [Google Scholar]
- Cormier E. M., Wolf M. F., Jordan V. C. Decrease in estradiol-stimulated progesterone receptor production in MCF-7 cells by epidermal growth factor and possible clinical implication for paracrine-regulated breast cancer growth. Cancer Res. 1989 Feb 1;49(3):576–580. [PubMed] [Google Scholar]
- Cozzolino F., Rubartelli A., Aldinucci D., Sitia R., Torcia M., Shaw A., Di Guglielmo R. Interleukin 1 as an autocrine growth factor for acute myeloid leukemia cells. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2369–2373. doi: 10.1073/pnas.86.7.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culouscou J. M., Remacle-Bonnet M., Garrouste F., Marvaldi J., Pommier G. Simultaneous production of IGF-I and EGF competing growth factors by HT-29 human colon cancer line. Int J Cancer. 1987 Nov 15;40(5):646–652. doi: 10.1002/ijc.2910400513. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Fedorko J., Gu J. A., Lebacq-Verheyden A. M., Linnoila R. I., Battey J. F. Gastrin-releasing peptide gene-associated peptides are expressed in normal human fetal lung and small cell lung cancer: a novel peptide family found in man. J Clin Endocrinol Metab. 1988 Sep;67(3):576–583. doi: 10.1210/jcem-67-3-576. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Emanuele M. A., Brooks M. H., Barbato A. L., Kapadia M., Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988 Dec 1;319(22):1434–1440. doi: 10.1056/NEJM198812013192202. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Tadaro G. J. A human fibrosarcoma cell line producing multiplication stimulating activity (MSA)-related peptides. Nature. 1978 Mar 23;272(5651):356–358. doi: 10.1038/272356a0. [DOI] [PubMed] [Google Scholar]
- De Leon D. D., Bakker B., Wilson D. M., Hintz R. L., Rosenfeld R. G. Demonstration of insulin-like growth factor (IGF-I and -II) receptors and binding protein in human breast cancer cell lines. Biochem Biophys Res Commun. 1988 Apr 15;152(1):398–405. doi: 10.1016/s0006-291x(88)80727-7. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Bates S. E., McManaway M. E., Lippman M. E. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 1986 Apr;46(4 Pt 1):1707–1713. [PubMed] [Google Scholar]
- Dickson R. B., Huff K. K., Spencer E. M., Lippman M. E. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986 Jan;118(1):138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., McManaway M. E., Lippman M. E. Estrogen-induced factors of breast cancer cells partially replace estrogen to promote tumor growth. Science. 1986 Jun 20;232(4757):1540–1543. doi: 10.1126/science.3715461. [DOI] [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Duprez V., Lenoir G., Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by interleukin 2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badry O. M., Romanus J. A., Helman L. J., Cooper M. J., Rechler M. M., Israel M. A. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest. 1989 Sep;84(3):829–839. doi: 10.1172/JCI114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger U. New aspects in the molecular growth regulation of mammary tumors. Recent Results Cancer Res. 1989;113:1–3. doi: 10.1007/978-3-642-83638-1_1. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin P. R., Jr, Kaminski M. S., Cody R. L., Wicha M. S. Production of mammastatin, a tissue-specific growth inhibitor, by normal human mammary cells. Science. 1989 Jun 30;244(4912):1585–1587. doi: 10.1126/science.2662405. [DOI] [PubMed] [Google Scholar]
- Fagin J. A., Pixley S., Slanina S., Ong J., Melmed S. Insulin-like growth factor I gene expression in GH3 rat pituitary cells: messenger ribonucleic acid content, immunocytochemistry, and secretion. Endocrinology. 1987 May;120(5):2037–2043. doi: 10.1210/endo-120-5-2037. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Secretion and crinophagy in prolactin cells. Adv Exp Med Biol. 1977;80:37–94. doi: 10.1007/978-1-4615-6675-5_3. [DOI] [PubMed] [Google Scholar]
- Fekete M., Wittliff J. L., Schally A. V. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J Clin Lab Anal. 1989;3(3):137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., DiCarlo J. N. Somatomedin-C receptors and growth effects in human breast cells maintained in long-term tissue culture. Cancer Res. 1984 May;44(5):2122–2128. [PubMed] [Google Scholar]
- Furukawa Y., Ohta M., Miura Y., Saito M. Interleukin-1 derived from human monocytic leukemia cell line JOSK-I acts as an autocrine growth factor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):39–46. doi: 10.1016/s0006-291x(87)80084-0. [DOI] [PubMed] [Google Scholar]
- Garcia M., Lacombe M. J., Duplay H., Cavailles V., Derocq D., Delarue J. C., Krebs B., Contesso G., Sancho-Garnier H., Richer G. Immunohistochemical distribution of the 52-kDa protein in mammary tumors: a marker associated with cell proliferation rather than with hormone responsiveness. J Steroid Biochem. 1987;27(1-3):439–445. doi: 10.1016/0022-4731(87)90338-4. [DOI] [PubMed] [Google Scholar]
- Gazit A., Igarashi H., Chiu I. M., Srinivasan A., Yaniv A., Tronick S. R., Robbins K. C., Aaronson S. A. Expression of the normal human sis/PDGF-2 coding sequence induces cellular transformation. Cell. 1984 Nov;39(1):89–97. doi: 10.1016/0092-8674(84)90194-6. [DOI] [PubMed] [Google Scholar]
- Giss B. J., Walker A. M. Mammotroph autoregulation: intracellular fate of internalized prolactin. Mol Cell Endocrinol. 1985 Oct;42(3):259–267. doi: 10.1016/0303-7207(85)90057-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Greenan J. R., Balden E., Ho T. W., Walker A. M. Biosynthesis of the secreted 24 K isoforms of prolactin. Endocrinology. 1989 Oct;125(4):2041–2048. doi: 10.1210/endo-125-4-2041. [DOI] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Willshire I. R., Young J. A., Anderson H., Baildam A., Howell A. Epidermal and transforming growth factor alpha in patients with breast tumours. Br J Cancer. 1989 Apr;59(4):605–609. doi: 10.1038/bjc.1989.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Young J. A., Willshire I. R., Garner A. The contribution of the C-terminal undecapeptide sequence of urogastrone-epidermal growth factor to its biological action. Regul Pept. 1988 Aug;22(3):217–226. doi: 10.1016/0167-0115(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Hanauske A. R., Buchok J., Scheithauer W., Von Hoff D. D. Human colon cancer cell lines secrete alpha TGF-like activity. Br J Cancer. 1987 Jan;55(1):57–59. doi: 10.1038/bjc.1987.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Tumor suppressors: recessive mutations that lead to cancer. Cell. 1988 Apr 22;53(2):173–174. doi: 10.1016/0092-8674(88)90376-5. [DOI] [PubMed] [Google Scholar]
- Hermansson M., Nistér M., Betsholtz C., Heldin C. H., Westermark B., Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T. W., Greenan J. R., Walker A. M. Mammotroph autoregulation: the differential roles of the 24K isoforms of prolactin. Endocrinology. 1989 Mar;124(3):1507–1514. doi: 10.1210/endo-124-3-1507. [DOI] [PubMed] [Google Scholar]
- Hollstein M. C., Smits A. M., Galiana C., Yamasaki H., Bos J. L., Mandard A., Partensky C., Montesano R. Amplification of epidermal growth factor receptor gene but no evidence of ras mutations in primary human esophageal cancers. Cancer Res. 1988 Sep 15;48(18):5119–5123. [PubMed] [Google Scholar]
- Hoosein N. M., Brattain D. E., McKnight M. K., Levine A. E., Brattain M. G. Characterization of the inhibitory effects of transforming growth factor-beta on a human colon carcinoma cell line. Cancer Res. 1987 Jun 1;47(11):2950–2954. [PubMed] [Google Scholar]
- Hoosein N. M., Kiener P. A., Curry R. C., Brattain M. G. Evidence for autocrine growth stimulation of cultured colon tumor cells by a gastrin/cholecystokinin-like peptide. Exp Cell Res. 1990 Jan;186(1):15–21. doi: 10.1016/0014-4827(90)90204-n. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Kiener P. A., Curry R. C., Rovati L. C., McGilbra D. K., Brattain M. G. Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human colon carcinoma cell lines. Cancer Res. 1988 Dec 15;48(24 Pt 1):7179–7183. [PubMed] [Google Scholar]
- Hoosein N. M., McKnight M. K., Levine A. E., Mulder K. M., Childress K. E., Brattain D. E., Brattain M. G. Differential sensitivity of subclasses of human colon carcinoma cell lines to the growth inhibitory effects of transforming growth factor-beta 1. Exp Cell Res. 1989 Apr;181(2):442–453. doi: 10.1016/0014-4827(89)90101-8. [DOI] [PubMed] [Google Scholar]
- Huff K. K., Kaufman D., Gabbay K. H., Spencer E. M., Lippman M. E., Dickson R. B. Secretion of an insulin-like growth factor-I-related protein by human breast cancer cells. Cancer Res. 1986 Sep;46(9):4613–4619. [PubMed] [Google Scholar]
- Huff K. K., Knabbe C., Lindsey R., Kaufman D., Bronzert D., Lippman M. E., Dickson R. B. Multihormonal regulation of insulin-like growth factor-I-related protein in MCF-7 human breast cancer cells. Mol Endocrinol. 1988 Mar;2(3):200–208. doi: 10.1210/mend-2-3-200. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., van Tol H., Van den Brande J. L., Sussenbach J. S. Nucleotide sequences of cDNAs encoding precursors of human insulin-like growth factor II (IGF-II) and an IGF-II variant. FEBS Lett. 1985 Jan 7;179(2):243–246. doi: 10.1016/0014-5793(85)80527-5. [DOI] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Johnson L. R. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977 Apr;72(4 PT2):788–792. [PubMed] [Google Scholar]
- Kadowaki J., Ku N., Oetting W. S., Walker A. M. Mammotroph autoregulation: uptake of secreted prolactin and inhibition of secretion. Endocrinology. 1984 Jun;114(6):2060–2067. doi: 10.1210/endo-114-6-2060. [DOI] [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988 Jul 15;48(14):4083–4092. [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Korman L. Y., Carney D. N., Citron M. L., Moody T. W. Secretin/vasoactive intestinal peptide-stimulated secretion of bombesin/gastrin releasing peptide from human small cell carcinoma of the lung. Cancer Res. 1986 Mar;46(3):1214–1218. [PubMed] [Google Scholar]
- Kudlow J. E., Cheung C. Y., Bjorge J. D. Epidermal growth factor stimulates the synthesis of its own receptor in a human breast cancer cell line. J Biol Chem. 1986 Mar 25;261(9):4134–4138. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Su Y. F., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Monoclonal antibodies to receptors for insulin and somatomedin-C. J Biol Chem. 1983 May 25;258(10):6561–6566. [PubMed] [Google Scholar]
- Lauweryns J. M., Peuskens J. C. Neuro-epithelial bodies (neuroreceptor or secretory organs?) in human infant bronchial and bronchiolar epithelium. Anat Rec. 1972 Mar;172(3):471–481. doi: 10.1002/ar.1091720301. [DOI] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Bertness V., Kirsch I., Hollis G. F., McBride O. W., Battey J. Human gastrin-releasing peptide gene maps to chromosome band 18q21. Somat Cell Mol Genet. 1987 Jan;13(1):81–86. doi: 10.1007/BF02422302. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Gelmann E. P., Rosen N., Knabbe C., Bates S., Bronzert D., Huff K., Kasid A. Growth regulation of human breast carcinoma occurs through regulated growth factor secretion. J Cell Biochem. 1987 Sep;35(1):1–16. doi: 10.1002/jcb.240350102. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Gelmann E. P., Rosen N., Knabbe C., Bates S., Bronzert D., Huff K., Kasid A. Growth regulatory peptide production by human breast carcinoma cells. J Steroid Biochem. 1988;30(1-6):53–61. doi: 10.1016/0022-4731(88)90076-3. [DOI] [PubMed] [Google Scholar]
- MacDonald R. G., Pfeffer S. R., Coussens L., Tepper M. A., Brocklebank C. M., Mole J. E., Anderson J. K., Chen E., Czech M. P., Ullrich A. A single receptor binds both insulin-like growth factor II and mannose-6-phosphate. Science. 1988 Mar 4;239(4844):1134–1137. doi: 10.1126/science.2964083. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Burgess A. W. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989 Mar 15;43(3):380–384. doi: 10.1002/ijc.2910430305. [DOI] [PubMed] [Google Scholar]
- Markowitz S. D., Molkentin K., Gerbic C., Jackson J., Stellato T., Willson J. K. Growth stimulation by coexpression of transforming growth factor-alpha and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J Clin Invest. 1990 Jul;86(1):356–362. doi: 10.1172/JCI114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Halter S. A., Holt J. T., Hogan B. L., Coffey R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990 Jun 15;61(6):1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- McGregor D. B., Morriss L. L., Manalo P. B., Bomberger R. A., Pardini R. S. Pentagastrin stimulation of human colon carcinoma. Arch Surg. 1989 Apr;124(4):470–472. doi: 10.1001/archsurg.1989.01410040080019. [DOI] [PubMed] [Google Scholar]
- Melmed S., Carlson H. E., Briggs J., Hershman J. M. Autofeedback of prolactin in cultured prolactin-secreting pituitary cells. Horm Res. 1980;12(6):340–344. doi: 10.1159/000179140. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Trowbridge I., Castagnola J. Inhibition of human lymphocyte proliferation by monoclonal antibody to transferrin receptor. Blood. 1983 Oct;62(4):821–826. [PubMed] [Google Scholar]
- Minuto F., Del Monte P., Barreca A., Alama A., Cariola G., Giordano G. Evidence for autocrine mitogenic stimulation by somatomedin-C/insulin-like growth factor I on an established human lung cancer cell line. Cancer Res. 1988 Jul 1;48(13):3716–3719. [PubMed] [Google Scholar]
- Minuto F., Del Monte P., Barreca A., Fortini P., Cariola G., Catrambone G., Giordano G. Evidence for an increased somatomedin-C/insulin-like growth factor I content in primary human lung tumors. Cancer Res. 1986 Feb;46(2):985–988. [PubMed] [Google Scholar]
- Moody T. W., Bertness V., Carney D. N. Bombesin-like peptides and receptors in human tumor cell lines. Peptides. 1983 Sep-Oct;4(5):683–686. doi: 10.1016/0196-9781(83)90018-9. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Carney D. N., Cuttitta F., Quattrocchi K., Minna J. D. High affinity receptors for bombesin/GRP-like peptides on human small cell lung cancer. Life Sci. 1985 Jul 15;37(2):105–113. doi: 10.1016/0024-3205(85)90413-8. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Russell E. K., O'Donohue T. L., Linden C. D., Gazdar A. F. Bombesin-like peptides in small cell lung cancer: biochemical characterization and secretion from a cell line. Life Sci. 1983 Jan 31;32(5):487–493. doi: 10.1016/0024-3205(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Mori K., Ibaragi S., Kurobe M., Furukawa S., Hayashi K. Production of an hEGF-like immunoreactive factor by human gastric cancer cells depends on differentiational state of the cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1019–1025. doi: 10.1016/0006-291x(87)91537-3. [DOI] [PubMed] [Google Scholar]
- Mulder K. M., Childress-Fields K. E. Characterization of a serum-free culture system comparing growth factor requirements of transformed and untransformed cells. Exp Cell Res. 1990 Jun;188(2):254–261. doi: 10.1016/0014-4827(90)90167-9. [DOI] [PubMed] [Google Scholar]
- Mulshine J. L., Treston A. M., Natale R. B., Kasprzyk P. G., Avis I., Nakanishi Y., Cuttitta F. Autocrine growth factors as therapeutic targets in lung cancer. Chest. 1989 Jul;96(1 Suppl):31S–34S. doi: 10.1378/chest.96.1_supplement.31s. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Kurata S., Mishim Y. Regulation of human estrogen receptor gene, epidermal growth factor receptor gene, and oncogenes by estrogen and antiestrogen in MCF-7 breast cancer cells. Cancer Detect Prev. 1988;13(2):103–107. [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Regulation of transforming growth factor alpha and transforming growth factor beta messenger ribonucleic acid abundance in T-47D, human breast cancer cells. Mol Endocrinol. 1989 Apr;3(4):611–617. doi: 10.1210/mend-3-4-611. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Cuttitta F., Kasprzyk P. G., Avis I., Steinberg S. M., Gazdar A. F., Mulshine J. L. Growth factor effects on small cell lung cancer cells using a colorimetric assay: can a transferrin-like factor mediate autocrine growth? Exp Cell Biol. 1988;56(1-2):74–85. doi: 10.1159/000163465. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Mulshine J. L., Kasprzyk P. G., Natale R. B., Maneckjee R., Avis I., Treston A. M., Gazdar A. F., Minna J. D., Cuttitta F. Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J Clin Invest. 1988 Jul;82(1):354–359. doi: 10.1172/JCI113594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S., Halcrow P., Sainsbury J. R., Angus B., Chambers P., Farndon J. R., Harris A. L. Epidermal growth factor receptor (EGFr) status associated with failure of primary endocrine therapy in elderly postmenopausal patients with breast cancer. Br J Cancer. 1988 Dec;58(6):810–814. doi: 10.1038/bjc.1988.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S., Sainsbury J. R., Halcrow P., Chambers P., Farndon J. R., Harris A. L. Expression of epidermal growth factor receptors associated with lack of response to endocrine therapy in recurrent breast cancer. Lancet. 1989 Jan 28;1(8631):182–185. doi: 10.1016/s0140-6736(89)91202-6. [DOI] [PubMed] [Google Scholar]
- Nyberg F., Roos P., Wide L. Human pituitary prolactin: isolation and characterization of three isohormones with different bioassay and radioimmunoassay activities. Biochim Biophys Acta. 1980 Oct 21;625(2):255–265. doi: 10.1016/0005-2795(80)90289-5. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Takanashi A., Takekura N., Yoshida K., Miyamori S., Harada T., Tahara E. Effect of human epidermal growth factor on cell growth and its receptor in human gastric carcinoma cell lines. Jpn J Clin Oncol. 1988 Mar;18(1):15–25. doi: 10.1093/jjco/18.1.15. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Ho T. W., Greenan J. R., Walker A. M. Production and secretion of the 21-23.5 kDa prolactin-like molecules. Mol Cell Endocrinol. 1989 Feb;61(2):189–199. doi: 10.1016/0303-7207(89)90130-5. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Tuazon P. T., Traugh J. A., Walker A. M. Phosphorylation of prolactin. J Biol Chem. 1986 Feb 5;261(4):1649–1652. [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Shimizu N. Epidermal growth factor receptors in cancer tissues of esophagus, lung, pancreas, colorectum, breast and stomach. Jpn J Cancer Res. 1988 Nov;79(11):1201–1207. doi: 10.1111/j.1349-7006.1988.tb01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen F., Partanen S., Mäkinen T., Rutanen E. M. Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res. 1988 Mar 1;48(5):1343–1347. [PubMed] [Google Scholar]
- Peres R., Betsholtz C., Westermark B., Heldin C. H. Frequent expression of growth factors for mesenchymal cells in human mammary carcinoma cell lines. Cancer Res. 1987 Jul 1;47(13):3425–3429. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Beuscart R., Djiane J., Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer Res. 1988 Nov 15;48(22):6429–6433. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Dusanter-Fourt I., Leroy-Martin B., Djiane J., Demaille A. Characterization of insulin-like growth factor 1 receptors (IGF1-R) in human breast cancer cell lines. Bull Cancer. 1989;76(3):311–319. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Laurent J. C., Louchez M. M., Amrani S., Leroy-Martin B., Vilain M. O., Delobelle A., Demaille A. Presence and characterization of insulin-like growth factor 1 receptors in human benign breast disease. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1425–1431. doi: 10.1016/0277-5379(88)90332-x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Rothbauer E., Wiebecke B., Pratschke E., Krämling H. J., Mann K. Increased epidermal growth factor receptors in gastric carcinomas. Gastroenterology. 1990 Apr;98(4):961–967. doi: 10.1016/0016-5085(90)90020-2. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Reeve J. R., Jr, Cuttitta F., Vigna S. R., Heubner V., Lee T. D., Shively J. E., Ho F. J., Fedorko J., Minna J. D., Walsh J. H. Multiple gastrin-releasing peptide gene-associated peptides are produced by a human small cell lung cancer line. J Biol Chem. 1989 Feb 5;264(4):1928–1932. [PubMed] [Google Scholar]
- Ro J., Bresser J., Ro J. Y., Brasfield F., Hortobagyi G., Blick M. SIS/PDGF-B expression in benign and malignant human breast lesions. Oncogene. 1989 Mar;4(3):351–354. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U., Herlyn M., Herlyn D., Molthoff C., Atkinson B., Varello M., Steplewski Z., Koprowski H. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects. Cancer Res. 1987 Jul 15;47(14):3692–3696. [PubMed] [Google Scholar]
- Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci U S A. 1986 Jan;83(1):77–81. doi: 10.1073/pnas.83.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D. S., Zwiebel J. A., Bano M., Losonczy I., Fehnel P., Kidwell W. R. Presence of transforming growth factors in human breast cancer cells. Cancer Res. 1984 Sep;44(9):4069–4077. [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Lebacq-Verheyden A. M., Spindel E. R., Cuttitta F., Gazdar A. F., Battey J. F. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986 Feb 15;261(5):2451–2457. [PubMed] [Google Scholar]
- Scammell J. G., Burrage T. G., Dannies P. S. Hormonal induction of secretory granules in a pituitary tumor cell line. Endocrinology. 1986 Oct;119(4):1543–1548. doi: 10.1210/endo-119-4-1543. [DOI] [PubMed] [Google Scholar]
- Siegfried J. M. Detection of human lung epithelial cell growth factors produced by a lung carcinoma cell line: use in culture of primary solid lung tumors. Cancer Res. 1987 Jun 1;47(11):2903–2910. [PubMed] [Google Scholar]
- Singh P., Rae-Venter B., Townsend C. M., Jr, Khalil T., Thompson J. C. Gastrin receptors in normal and malignant gastrointestinal mucosa: age-associated changes. Am J Physiol. 1985 Dec;249(6 Pt 1):G761–G769. doi: 10.1152/ajpgi.1985.249.6.G761. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Smith J. J., Derynck R., Korc M. Production of transforming growth factor alpha in human pancreatic cancer cells: evidence for a superagonist autocrine cycle. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7567–7570. doi: 10.1073/pnas.84.21.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. P., Solomon T. E. Effects of gastrin, proglumide, and somatostatin on growth of human colon cancer. Gastroenterology. 1988 Dec;95(6):1541–1548. doi: 10.1016/s0016-5085(88)80075-1. [DOI] [PubMed] [Google Scholar]
- Smith J. P., Wood J. G., Solomon T. E. Elevated gastrin levels in patients with colon cancer or adenomatous polyps. Dig Dis Sci. 1989 Feb;34(2):171–174. doi: 10.1007/BF01536047. [DOI] [PubMed] [Google Scholar]
- Sorenson G. D., Bloom S. R., Ghatei M. A., Del Prete S. A., Cate C. C., Pettengill O. S. Bombesin production by human small cell carcinoma of the lung. Regul Pept. 1982 Jul;4(2):59–66. doi: 10.1016/0167-0115(82)90095-7. [DOI] [PubMed] [Google Scholar]
- Sorenson G. D., Pettengill O. S., Brinck-Johnsen T., Cate C. C., Maurer L. H. Hormone production by cultures of small-cell carcinoma of the lung. Cancer. 1981 Mar 15;47(6):1289–1296. doi: 10.1002/1097-0142(19810315)47:6<1289::aid-cncr2820470610>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine, paracrine and endocrine mechanisms of growth control. Cancer Surv. 1985;4(4):627–632. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Tahara E., Sumiyoshi H., Hata J., Yasui W., Taniyama K., Hayashi T., Nagae S., Sakamoto S. Human epidermal growth factor in gastric carcinoma as a biologic marker of high malignancy. Jpn J Cancer Res. 1986 Feb;77(2):145–152. [PubMed] [Google Scholar]
- Takahashi K., Suzuki K., Kawahara S., Ono T. Growth stimulation of human breast epithelial cells by basic fibroblast growth factor in serum-free medium. Int J Cancer. 1989 May 15;43(5):870–874. doi: 10.1002/ijc.2910430522. [DOI] [PubMed] [Google Scholar]
- Tally M., Enberg G., Li C. H., Hall K. The specificity of the human IGF-2 receptor. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1206–1212. doi: 10.1016/s0006-291x(87)80198-5. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Theillet C., Le Roy X., De Lapeyrière O., Grosgeorges J., Adnane J., Raynaud S. D., Simony-Lafontaine J., Goldfarb M., Escot C., Birnbaum D. Amplification of FGF-related genes in human tumors: possible involvement of HST in breast carcinomas. Oncogene. 1989 Jul;4(7):915–922. [PubMed] [Google Scholar]
- Toi M., Hamada Y., Nakamura T., Mukaida H., Suehiro S., Wada T., Toge T., Niimoto M., Hattori T. Immunocytochemical and biochemical analysis of epidermal growth factor receptor expression in human breast cancer tissues: relationship to estrogen receptor and lymphatic invasion. Int J Cancer. 1989 Feb 15;43(2):220–225. doi: 10.1002/ijc.2910430208. [DOI] [PubMed] [Google Scholar]
- Trepel J. B., Moyer J. D., Cuttitta F., Frucht H., Coy D. H., Natale R. B., Mulshine J. L., Jensen R. T., Sausville E. A. A novel bombesin receptor antagonist inhibits autocrine signals in a small cell lung carcinoma cell line. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1383–1389. doi: 10.1016/s0006-291x(88)80785-x. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Karakousis C. P., Herrera L., Petrelli N. J., Bell G. I., Shows T. B. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986 Dec;46(12 Pt 1):6169–6173. [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Scott J., Bell G. I., Shows T. B. Localization of insulin-like growth factor genes to human chromosomes 11 and 12. 1984 Aug 30-Sep 5Nature. 310(5980):784–786. doi: 10.1038/310784a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upp J. R., Jr, Saydjari R., Townsend C. M., Jr, Singh P., Barranco S. C., Thompson J. C. Polyamine levels and gastrin receptors in colon cancers. Ann Surg. 1988 Jun;207(6):662–669. doi: 10.1097/00000658-198806000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Vignon F., Capony F., Chambon M., Freiss G., Garcia M., Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986 Apr;118(4):1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- Vostrejs M., Moran P. L., Seligman P. A. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. doi: 10.1172/JCI113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling A. E., Newboult E., Peters S. W. Effects of antioestrogens on the proliferation of MCF-7 human breast cancer cells. J Mol Endocrinol. 1989 May;2(3):225–234. doi: 10.1677/jme.0.0020225. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Lazar E., Sporn M. B. Transformation of normal rat kidney (NRK) cells by an infectious retrovirus carrying a synthetic rat type alpha transforming growth factor gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1258–1262. doi: 10.1073/pnas.84.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L. F., Brattain M. G., Levine A. E. Modulation of a high molecular weight form of transforming growth factor-alpha in human colon carcinoma cell lines. Cancer Lett. 1988 May;40(1):59–70. doi: 10.1016/0304-3835(88)90262-5. [DOI] [PubMed] [Google Scholar]
- Watson S. A., Durrant L. G., Crosbie J. D., Morris D. L. The in vitro growth response of primary human colorectal and gastric cancer cells to gastrin. Int J Cancer. 1989 Apr 15;43(4):692–696. doi: 10.1002/ijc.2910430425. [DOI] [PubMed] [Google Scholar]
- Watson S., Durrant L., Morris D. Gastrin: growth enhancing effects on human gastric and colonic tumour cells. Br J Cancer. 1989 Apr;59(4):554–558. doi: 10.1038/bjc.1989.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Weinstock J., Baldwin G. S. Binding of gastrin(17) to human gastric carcinoma cell lines. Cancer Res. 1988 Feb 15;48(4):932–937. [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Wood S. M., Wood J. R., Ghatei M. A., Lee Y. C., O'Shaughnessy D., Bloom S. R. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. J Clin Endocrinol Metab. 1981 Dec;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Abe K., Kameya T., Adachi I., Taguchi S., Otsubo K., Yanaihara N. Production and molecular size heterogeneity of immunoreactive gastrin-releasing peptide in fetal and adult lungs and primary lung tumors. Cancer Res. 1983 Aug;43(8):3932–3939. [PubMed] [Google Scholar]
- Yasui W., Hata J., Yokozaki H., Nakatani H., Ochiai A., Ito H., Tahara E. Interaction between epidermal growth factor and its receptor in progression of human gastric carcinoma. Int J Cancer. 1988 Feb 15;41(2):211–217. doi: 10.1002/ijc.2910410209. [DOI] [PubMed] [Google Scholar]
- Yasui W., Sumiyoshi H., Hata J., Kameda T., Ochiai A., Ito H., Tahara E. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988 Jan 1;48(1):137–141. [PubMed] [Google Scholar]
- Yee D., Cullen K. J., Paik S., Perdue J. F., Hampton B., Schwartz A., Lippman M. E., Rosen N. Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 1988 Dec 1;48(23):6691–6696. [PubMed] [Google Scholar]
- Yoshida K., Kyo E., Tsuda T., Tsujino T., Ito M., Niimoto M., Tahara E. EGF and TGF-alpha, the ligands of hyperproduced EGFR in human esophageal carcinoma cells, act as autocrine growth factors. Int J Cancer. 1990 Jan 15;45(1):131–135. doi: 10.1002/ijc.2910450124. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kyo E., Tsujino T., Sano T., Niimoto M., Tahara E. Expression of epidermal growth factor, transforming growth factor-alpha and their receptor genes in human gastric carcinomas; implication for autocrine growth. Jpn J Cancer Res. 1990 Jan;81(1):43–51. doi: 10.1111/j.1349-7006.1990.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeillinger R., Kury F., Czerwenka K., Kubista E., Sliutz G., Knogler W., Huber J., Zielinski C., Reiner G., Jakesz R. HER-2 amplification, steroid receptors and epidermal growth factor receptor in primary breast cancer. Oncogene. 1989 Jan;4(1):109–114. [PubMed] [Google Scholar]