Abstract
The mechanism by which an enhancer activates transcription over large distances has been investigated. Activation of the glnAp2 promoter by the NtrC-dependent enhancer in Escherichia coli was analyzed using a purified system supporting multiple-round transcription in vitro. Our results suggest that the enhancer–promoter interaction and the initiation complex must be formed de novo during every round of transcription. No protein remained bound to the promoter after RNA polymerase escaped into elongation. Furthermore, the rate of initiation during the first and subsequent rounds of transcription were very similar, suggesting that there was no functional ‘memory’ facilitating multiple rounds of transcription. These studies exclude the hypothesis that enhancer action during multiple-round transcription involves the memory of the initial activation event.
INTRODUCTION
Transcriptional enhancers are relatively short (30–200 bp) DNA sequences usually composed of several binding sites for activator protein(s). The landmark of enhancers is their ability to activate genes over a considerable distance (up to 60 kb in Eukaryota and up to 15 kb in Prokaryota) (reviewed in 1,2). Gene regulation over such large distances is widespread in higher Eukaryota. In Prokaryota, enhancer-dependent gene regulation is less common; only a small number of promoters used by σ54-containing RNA polymerase are known to be regulated by enhancers (3–5).
The mechanism of enhancer action over a large distance is unknown. The ‘recruitment’ mechanism proposed for gene activation involves the recruitment of a promoter-binding protein by an enhancer-bound protein through protein–protein interaction (reviewed in 6). However, it explains the action of activators satisfactorily only over enhancer–promoter distances up to 1 kb. Therefore, it is unclear how efficient communication between enhancer and promoter can occur over distances >2 kb (reviewed in 7). Several models have been proposed to explain the mechanism of enhancer action. One class of models suggests that initial communication of an enhancer with a promoter leads to formation of a stable DNA–protein complex in the vicinity of the promoter. This stable complex may facilitate subsequent rounds of transcription serving as a ‘memory’ of initial enhancer–promoter contact (reviewed in 1). Conceivably, ‘memory’ may also be due to alteration of the template. In either case, ‘memory’ is expected to result in a vast difference in the rates of the first round of transcription (which by definition occurs in the absence of memory) and subsequent rounds of transcription.
Alternatively, the average distance between promoter and enhancer could be considerably decreased if intervening DNA is supercoiled or bent (reviewed in 7). In this case, formation of a stable DNA–protein complex at the promoter (or any other type of memory) may not be required because DNA supercoiling or bending bring the enhancer into close proximity to the promoter and thus allow more efficient recruitment of the transcriptional machinery. In this case, the rate of initiation of the first round of transcription and subsequent rounds of transcription from the promoter should be similar.
The NtrC (NRI)-dependent, σ54-dependent transcriptional enhancer participates in the regulation of genes involved in metabolism of nitrogen in Escherichia coli (see Fig. 5) (reviewed in 8). The mechanism of action of the NtrC-dependent enhancer has been intensely studied using the glnAp2 promoter as a model. The enhancer is localized ∼110 bp upstream of the glnAp2 promoter but strongly activates transcription when positioned up to at least 15 kb away in vivo (9) and up to at least 0.9 kb in vitro (10); it functions both upstream and downstream from the promoter. NtrC is an activator that binds to the enhancer, and, when phosphorylated by NtrB (NRII) protein kinase, forms a higher order homooligomer and is capable of activating the transcription of the glnAp2 gene (11–14). Phosphorylation of NtrC also activates its ATPase activity, which is required to stimulate conversion of the closed promoter–polymerase complex (RPc) to the transcriptionally active open complex (RPo), in which the strands of the template are melted near the site of initiation (11–14). Active enhancer-bound NtrC interacts with the Eσ54 RNA polymerase holoenzyme bound as the closed initiation complex (RPc) at the promoter (15–17). During enhancer–promoter interaction, intervening DNA is looped out (18,19). Interaction of the NRI with the σ54 subunit of the holoenzyme drives the transition from RPc into RPo (20–22).
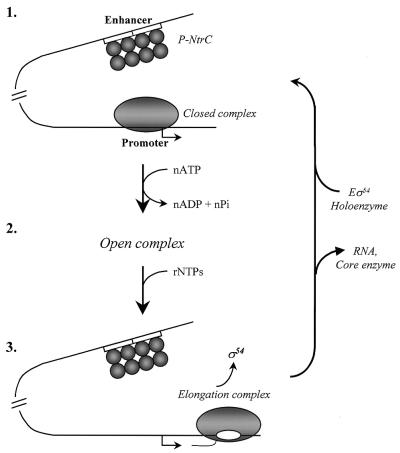
Figure 5.
Mechanism of action of NtrC-dependent enhancer during multiple-round transcription of the glnAp2 promoter. (1) Before transcription of NtrC-dependent genes is induced, the Eσ54 holoenzyme forms a closed complex (RPc) at the promoter (localized at the –24 to –12 DNA region) but cannot initiate transcription. NtrC is bound to the enhancer (two 17 bp NtrC-binding sites indicated by open boxes) but cannot communicate with the promoter. After induction and phosphorylation by NtrB, NtrC forms homooligomers and interacts with the holoenzyme causing looping out of the intervening DNA (not shown) and ATP-dependent formation of the open complex (RPo) at the promoter (2). After formation of RPo, enhancer–promoter interaction is broken and the DNA loop is opened. As the RNA polymerase leaves the promoter (3), the σ54 subunit dissociates into solution and the holoenzyme has to re-bind to the promoter and re-establish the interaction with the enhancer for the next round of transcription to occur.
In this work, a multiple-round in vitro transcription assay was employed to investigate the mechanism of NtrC-dependent enhancer action over a large distance. Previously, we have found that DNA supercoiling greatly facilitates prokaryotic enhancer action over a large distance (2.5 kb) but DNA supercoiling is not essential for action over a short distance (0.11 kb) (23). The experiments described here excluded the possibility that the initial activation event provided ‘memory’ at the promoter facilitating multiple rounds of transcription, and, in contrast, suggested that multiple rounds of transcription occur by the same activation mechanism as the initial round. Possible mechanisms of enhancer action are discussed.
MATERIALS AND METHODS
Purified proteins
All proteins and protein complexes were purified according to protocols previously described for core RNA polymerase (24), σ54 (24), NtrC (25) and NtrB (26). Isolated proteins have been analyzed in 10% SDS–PAGE and their purity was over 95% according to Coomassie and silver staining (see Fig. 3A).
Figure 3.
Escape of the RNA polymerase from the promoter is accompanied by dissociation of σ54 from DNA. (A) RNA polymerase subunit σ54 is depleted from early RPel. The experimental strategy for purification of RPc and RPo complexes is outlined at the top. The complexes were purified from DNA-free proteins on a Sephacryl S-400 column, separated in an SDS–PAGE and silver stained (lanes 6 and 7). Purified proteins used for preparation of the transcription complexes were of ∼95% purity and were loaded as additional markers (lanes 1–4). Total proteins present in the reaction mixture were stained with Coomassie (lane 5). In some experiments NtrC was under-represented in RPel as compared with RPo suggesting that NtrC was more stably bound to DNA in RPo. NtrC was not detectable in either of the complexes if column fractionation was conducted in the presence of excess competitor DNA containing strong NtrC binding site (data not shown). M, protein molecular mass markers. (B) Functional RPo and RPel complexes survive Sephacryl S-400 column. RPo and RPel complexes were analyzed using a single-round transcription assay before (–) or after (+) fractionation on a Sephacryl S-400 column. No DNA-free proteins were added to the reaction after the column.
DNA templates
For detailed description of all plasmid templates used in the experiments see Figure 1. Supercoiled DNA templates were purified using QIAGEN plasmid purification kit. Plasmids pTH8, pLR100 and pAN6 containing glnAp2 promoter were previously described by Ninfa et al. (10).
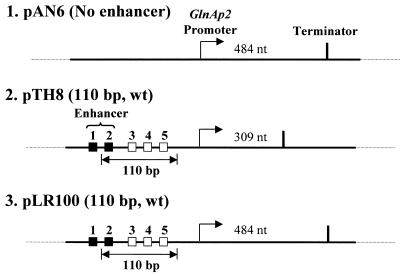
Figure 1.
Templates for analysis of the mechanism of glnAp2 promoter activation by NtrC-dependent enhancer. Plasmid templates having different enhancer–promoter spacing (constructs 2 and 3) and having no enhancer (construct 1). Strong and weak NtrC-binding sites are indicated by closed and open squares, respectively. Under our experimental conditions only the strong sites are occupied and contribute to the enhancer activity (10). The pTH8 and pLR100 plasmids (3.6 and 3.3 kb in size, respectively) have 110 bp wild-type (wt) enhancer–promoter spacing. The transcripts were terminated at the T7 terminator positioned over different distances downstream of the promoter. The lengths of the transcripts are indicated.
In vitro transcription
In vitro transcription was optimized for maximal utilization of promoter in initiation and elongation using supercoiled template pTH8 plasmid as a template. Purified proteins NtrC, NtrB, σ54 and core RNA polymerase used in this system were analyzed in a single-round transcription assay on supercoiled template. Exclusion of any of the protein components from the transcription assay abolished transcription from glnAp2 promoter. The buffer for the transcription assay contained 50 mM Tris-OAc (pH 8.0), 100 mM KOAc, 8 mM Mg(OAc)2, 27 mM NH4OAc, 0.7% PEG (8000) and 0.2 mM DTT. The transcription reactions were conducted in 50 µl aliquots and the template DNA was present at 2.8 nM. The final (saturating) concentrations of protein components were 500 nM core RNA polymerase, 1000 nM σ54, 120 nM NtrC and 400 nM NtrB. The reaction mix was incubated for 15 min at 37°C to form RPc. ATP was added to the reaction to 0.5 mM (single-round) or 2 mM (multiple-round) final concentration and reaction mixture was incubated at 37°C for 15 min (or for variable time when the time-course of single-round transcription was analyzed) to form RPo. Then, NTPs (final concentration 80 µM), 2.5 µCi [α-32P]UTP, RNase inhibitor (final concentration 0.2 U/µl) and heparin (final concentration 80 µg/ml, single-round transcription only) were added to the reaction to start transcription. The mixture was incubated at 37°C for 15 min (or for different times when time-course of multiple-round transcription was analyzed) and then, 50 µl of stop solution (200 µg/ml sheared DNA, 40 mM EDTA) was added to terminate the reaction. End-labeled 227 bp DNA fragments (1–2 µl) were added to the mixture as a loading control. The samples were extracted with 100 µl of phenol–chloroform (1:1), precipitated with ethanol, washed with 70% ethanol and dissolved in 100% formamide. The samples were separated in 8% denaturing urea-containing PAGE, dried and analyzed using a PhosphorImager.
DNase I and KMnO4 footprinting
The footprinting was conducted under conditions used in the transcription assays [see above and Sasse-Dwight and Gralla (27)]. After formation of RPc, ATP was added to the reaction (final concentration 0.5 mM) and samples were incubated at 37°C for 15 min to form RPo. GTP and CTP were added to the reaction (final concentration 0.05 mM) and incubation continued at 37°C for 10 min to form an elongation complex (RPel). Measurements of the length of the transcript formed in the –U reaction suggest that, consistent with the footprinting data (see Fig. 2), RPel terminates at position +32 (data not shown). The final volumes of the samples were 20 µl each. Before DNase I digestion, the enzyme preparation was calibrated before the experiment to achieve digestion of only 30–40% of protein-free DNA to guarantee primarily single-hit digestion of the samples. After formation of RPc, RPo and RPel, 2 µl of DNase I (0.15 U/µl; Sigma) was added and samples were incubated at 37°C for 30 s. The reaction was terminated with 2 µl of 0.5 M EDTA. KMnO4 footprinting was conducted by adding 2 µl of 80 mM KMnO4 and incubation at 37°C for 1 min. The reaction was terminated with 2 µl of 14.7 M 2-mercaptoethanol. After treatment with footprinting reagents, 20 µl of phenol–chloroform (1:1) was added. The samples were heated at 90°C for 4 min, cooled on ice and centrifuged at 14 000 g for 2 min. The upper aqueous layer was desalted on a MiniSpin G-50 column equilibrated with 10 mM Tris–HCl, 0.1 mM EDTA buffer. The modified templates were then subjected to primer extension using Klenow fragment (Gibco BRL) and the following 32P-labeled primers (28,29): 5′-CGTATGGGCTAAAGAATCCCCATTGACTTAGG (top DNA strand); 5′-TTCACATCGTGGTGCAGCCC (bottom DNA strand). The products of the reaction were resolved in 8% denaturing PAGE.
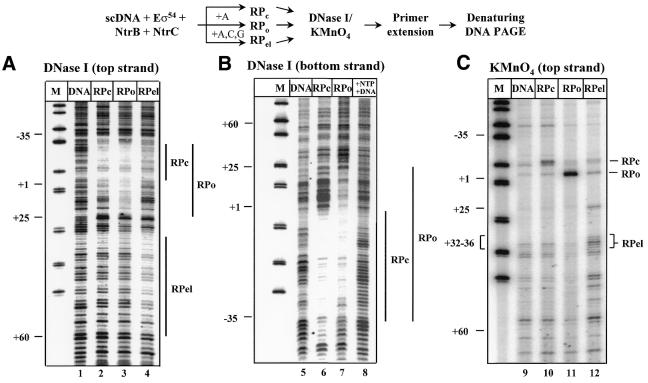
Figure 2.
Escape of the RNA polymerase from the glnAp2 promoter is accompanied by disappearance of KMnO4 and DNase I footprints characteristic for RPo complex. The experimental strategy for obtaining the RPc, RPo and RPel complexes is outlined at the top. In all experiments supercoiled pTH8 plasmid (110 bp enhancer–promoter spacing) was used. Reaction mixtures were incubated in the presence of ATP (+A; RPo) or partial mixture of nucleotides (+A, C, G; RPel), or in the absence of nucleotides (RPc). In lane 8, all NTPs were added to RPo in the presence of a large excess of competitor DNA (pLR90 plasmid, 100 nM). All experiments were performed as described by Tintut et al. (29). Protein-free DNA (lanes labeled DNA) was used as a negative control. After formation of the complexes and their incubation in the presence of DNase I (A and B) or KMnO4 (C) the samples were analyzed by single-round primer extension using 32P-labeled primers corresponding to the indicated DNA strand, and resolved in a denaturing PAGE. Vertical bars mark DNase I footprints characteristic for the corresponding complexes.
Analysis of protein composition of RPc, RPo and RPel complexes
RPc, RPo and RPel were formed as described above but the volumes of the reactions were increased 10 times. The DNA–protein complexes were loaded on 4 ml Sephacryl S-400 columns (Pharmacia). The column was washed with 1 ml of transcription buffer and then 100 µl fractions containing RPc, RPo or RPel complexes were collected. The DNA-bound proteins were eluted from the column in the void volume together with the plasmid DNA and thus separated from DNA-free proteins retained on the column. The purified complexes co-eluted with the DNA peak fractions, were resolved in SDS–PAGE and silver stained.
RESULTS
Initiation complexes are formed de novo on the glnAp2 promoter during every round of transcription: footprinting studies
To analyze the mechanism of enhancer action over a large distance, plasmids having 110 bp enhancer–promoter spacing or entirely lacking the enhancer (Fig. 1) were used in the experiments described below. Enhancer-dependent transcription of the glnAp2 promoter was initially characterized using a single-round transcription assay (10). As expected, transcription from the glnAp2 promoter requires the presence of all components described previously (core polymerase, promoter DNA, NtrC, NtrB and σ54) and occurs in an ATP- and enhancer-dependent manner (data not shown).
The goal of the initial experiments was to characterize DNA–protein interactions established during transcription initiation from the glnAp2 promoter. DNase I and KMnO4 footprinting methods (27) were utilized to analyze the structures of RPc, RPo and RPel complexes formed on supercoiled template containing the enhancer positioned 110 bp upstream the glnAp2 promoter (Fig. 2). KMnO4 preferentially modifies single-stranded DNA regions containing thimidines and is used for detection of melted DNA (27). As has been shown previously, the addition of NtrC, NtrB, σ54 and core RNA polymerase to the template in the absence of ATP results in the formation of RPc (30). In RPc, core RNA polymerase protects the –35 to +1 promoter DNA region from DNase I (Fig. 2, lanes 2 and 6) but promoter DNA remains double stranded and shows only weak sensitivity to KMnO4 (lane 10) as compared with free DNA (lane 9). Addition of ATP induces the formation of a footprint extending from –35 to +25 characteristic for RPo (lanes 3 and 7) and the appearance of a region of the promoter (at +1 position) highly reactive to KMnO4 (lane 11) indicating that RPo contains a melted DNA region at the start site (29).
This template contains a U-less cassette corresponding to the first 18 nt in the transcript. In the presence of ATP, CTP and GTP (–UTP reaction) the polymerase was expected to stall at the position where it needs to incorporate UTP (nucleotide 18). In fact, the majority of the elongation complexes were stalled at the position before the next U in the RNA sequence (position +36; Fig. 2, lane 12 and data not shown), presumably due to read-through of the first U. Under these conditions a strong protection of the downstream region of the top strand (extending from +30 to +60) from DNase I was accompanied by disappearance of the RPo-specific footprint at the upstream promoter region; a weak RPc footprint was still detectable at the promoter (Fig. 2, lane 4). This was accompanied by the disappearance of the KMnO4-sensitive RPo-specific region at the promoter and the appearance of a new sensitive region at position +32 to +36 (lane 12) confirming that RPels were stalled at position +36. These results indicate that the RNA polymerase quantitatively escapes from the promoter and that the promoter is then occupied by another molecule of RNA polymerase that cannot form RPo, probably because the downstream promoter DNA is blocked by RPel. Thus, the second molecule of RNA polymerase remains arrested in RPc. When transcription was conducted in the presence of all NTPs and an excess of competitor DNA, no footprint was detected at the promoter (lane 8). This indicates that another molecule of RNA polymerase cannot bind to the promoter under these conditions, in agreement with previously published data (16).
In summary, the footprinting studies suggest that when RNA polymerase escapes from the glnAp2 promoter into RPel, both DNase I and KMnO4 footprints disappear from the promoter unless experimental conditions allow binding of another molecule of RNA polymerase to the promoter.
The σ54 subunit is displaced into solution during escape of the RNA polymerase from the glnAp2 promoter
To determine the protein composition of RPc, RPo and RPel, the complexes were formed and then purified from DNA-free proteins by gel-filtration on a Sephacryl S-400 (31) allowing purification of functionally active transcription complexes (Fig. 3B). Fractions containing transcriptionally active complexes had the same elution profile as the plasmid DNA template and came out in the void volume of the column. Pooled fractions were analyzed by SDS–PAGE (Fig. 3A). RPc did not survive the gel-filtration: only NtrC was detected in the gel indicating that the affinity of RPc to DNA is not very high (data not shown). NtrB protein was quantitatively depleted during the chromatography which provided an internal control for quality of purification from DNA-free proteins. Purified RPo (Fig. 3A, lane 6) consisted of three protein complexes stably bound to DNA: core RNA polymerase, σ54 and NtrC. RPel had the same protein composition but did not contain σ54 (lane 7); thus, the σ54 subunit is stably associated with DNA only in RPo.
In summary, both the data on the protein composition of RPo and RPel (Fig. 3A) and the footprinting data (Fig. 2) are consistent and indicate that in RPo, Eσ54 and NtrC are stably bound to the promoter and the promoter DNA is partially melted around the +1 region. After RNA polymerase escapes from the promoter and forms RPel, the σ54 subunit is displaced from the complex and the promoter DNA is not melted before the next molecule of RNA polymerase binds and RPo is formed again. Thus, the RPo complex must form de novo during every round of transcription initiation from glnAp2 promoter. No evidence for the presence of a DNA–protein complex surviving multiple rounds of transcription was obtained by any of the techniques described above.
Lack of functional ‘memory’ during enhancer action
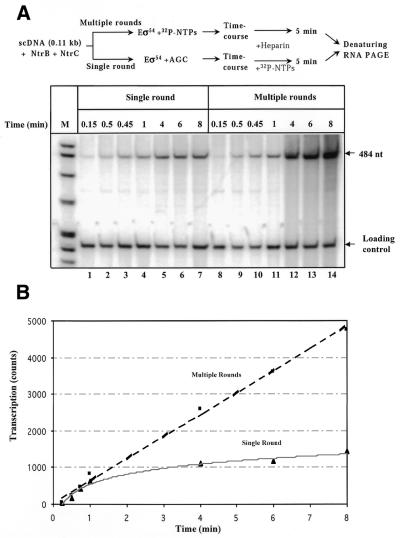
The structural data described above do not rule out the possibility that there is a functional ‘memory’ facilitating multiple rounds of transcription. For example, some DNA–protein complexes may remain weakly bound to promoter in RPel, but are not detectable by the methods described in Figures 2 and 3. Or, functional memory could result from alteration of the template DNA during the first round of transcription. If a functional ‘memory’ exists, the first round of transcription should occur more slowly than the subsequent rounds. This possibility was investigated by comparison of the rates of single- and multiple-round transcription on supercoiled pLR100 template (containing the natural 110 bp enhancer–promoter spacing; Fig. 4). The half-time for transcription initiation in the first round was ∼1 min and during multiple-round transcription one transcript was synthesized every 2 min (Fig. 4B). Thus, transcription initiation during the first and subsequent rounds occurs with similar rates suggesting that all rounds of transcription are functionally equivalent and that there is no ‘memory’ facilitating multiple-round transcription.
Figure 4.
Lack of functional ‘memory’ during enhancer-dependent activation of the glnAp2 promoter. (A) Time-courses of single-round and multiple-round transcription of supercoiled pLR100 plasmid having 110 bp enhancer–promoter spacing. The experimental strategy for comparison of the rates of single- and multiple-round transcription is outlined at the top. Reaction mixtures were incubated in the presence of all nucleotides (multiple-round) or with ACG mixture only (single-round). Use of an ACG mixture instead of ATP prevents conversion of RPo back to RPc and thus allows comparison of single- and multiple-round transcription under similar conditions. The reaction was terminated by adding heparin with subsequent incubation for 5 min. Heparin prevents formation of new initiation complexes but allows completion of already initiated transcripts. Labeled transcripts were analyzed in a denaturing PAGE. The loading control (a 227 bp end-labeled DNA fragment) was added to the reaction mixtures immediately after terminating the reaction. (B) Initiation of transcription occurs at similar rates during the first and subsequent rounds of transcription on supercoiled DNA. Quantitative analysis of the data is shown in (A). The intensities of the bands containing 484 nt transcripts were analyzed using a PhosphorImager.
Previously, we have shown that the efficiencies of transcription of supercoiled templates having an enhancer–promoter spacing of 110 or 2500 bp were not very different (23). In fact, multiple-round transcription of a template with 2.5 kb enhancer–promoter spacing was only ∼2-fold less efficient as compared with the template having 0.11 kb spacing (23). Thus, in spite of the fact that under our experimental conditions neither functional nor structural memory were detected at the glnAp2 promoter during multiple rounds of transcription, the enhancer can work efficiently over a large distance. The data suggest that the ‘memory’ is not required for efficient enhancer action over a distance.
DISCUSSION
In summary, an experimental system supporting multiple (up to four) rounds of transcription from the glnAp2 promoter in vitro has been established (Fig. 4). Using this system, it has been shown that neither structural nor functional ‘memory’ is established during the first round of transcription: the first and subsequent rounds are kinetically and structurally identical (Figs 2–4). In our previous studies it has been shown that NtrC-dependent enhancer can efficiently work over a large distance (23). Taken together, the data suggest that the ‘memory’ is not required for enhancer action over a short or long distance.
It has been shown previously that after RNA polymerase escapes from the glnAp2 promoter and RPel is formed, a DNase I footprint and DNA melting are still detected at the promoter, suggesting that some protein (presumably the σ54 subunit) remains bound there (29). However, analysis of the protein content of the early RPel purified by gel-purification (16) or gel-filtration (31) revealed the absence of the σ54 in the complex. In agreement with the latter data, no σ54 subunit was detected in the RPel purified by gel-filtration (Fig. 3A). Moreover, DNase I and KMnO4 footprinting studies did not reveal the presence of any footprint at the glnAp2 promoter after escape of the polymerase from the promoter (Fig. 2). This apparent disagreement of our data and some earlier published results (29) could be explained by the use of different protein preparations. In any case, the lack of the ‘memory’ does not prevent very efficient enhancer action over different distances and activating of up to four rounds of transcription. Taken together, the data suggest that the structural ‘memory’ observed in some studies (16,29,31) is not essential for efficient enhancer action. In fact, the protein that remains bound at the promoter was inhibitory for subsequent rounds of transcription (29). In agreement with the structural studies, functional studies revealed no indication of ‘memory’ facilitating re-initiation after the first round of transcription (Fig. 4). Thus, on supercoiled DNA the first and the subsequent rounds of transcription are structurally and functionally identical (Figs 2–4).
Our previous data suggested that DNA supercoiling greatly facilitates action of the NtrC-dependent enhancer over a large distance probably bringing enhancer and promoter in close proximity (23). In combination with our present results on the lack of ‘memory’ during enhancer-dependent transcription, the data suggest that DNA supercoiling is a principal factor mediating the action of NtrC-dependent enhancer over a distance, and eliminates the possibility that supercoiling facilitates enhancer action by establishing ‘memory’ at the promoter (see Introduction).
The above data suggest the following mechanism of action of the NtrC-dependent enhancer (Fig. 5). Before activation, non-phosphorylated NtrC is bound at the enhancer and the holoenzyme forms RPc at the glnAp2 promoter both in vitro (Fig. 2) (10,16,17) and in vivo (15). Transcription activation starts when NtrC is phosphorylated by NtrB protein (32 and references therein) and NtrC forms higher order homooligomer complexes essential for activation of transcription (intermediate 1; Fig. 5) (11–14). Phosphorylation of the NtrC also activates its ATPase activity required to stimulate conversion from RPc to RPo (11–14). Phosphorylated NtrC interacts with the holoenzyme bound at the promoter such that the intervening DNA forms a loop (18,19). This step is greatly facilitated by DNA supercoiling when the enhancer is positioned far from the promoter (23). Once established, enhancer–promoter interaction greatly stimulates the RPc→RPo transition, the rate-limiting step in the absence of the enhancer (10,16,21,33). When formation of RPo is completed (intermediate 2), enhancer–promoter interaction is destabilized (19); RPo is stable and does not require the continued presence of the enhancer for completion of the first round of transcription initiation (16). In the presence of NTPs, the RPel leaves the promoter and the σ54 subunit is displaced into solution (intermediate 3), leaving no structural or functional ‘memory’ at the promoter. Finally, the next molecule of RNA polymerase arrives at the promoter and forms RPc starting a new transcription cycle (intermediate 1).
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Drs D. Clark, M. Kashlev, L. Lutter, R. Needleman and K. Mizuuchi for valuable discussion and comments on the manuscript. The work was supported in part by the NIH grant GM58650 to V.M.S.
REFERENCES
- 1.Blackwood E.M. and Kadonaga,J.T. (1998) Going the distance: a current view of enhancer action. Science, 281, 61–63. [DOI] [PubMed] [Google Scholar]
- 2.Buck M., Gallegos,M.T., Studholme,D.J., Guo,Y. and Gralla,J.D. (2000) The bacterial enhancer-dependent σ(54) (σ(N)) transcription factor. J. Bacteriol., 182, 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magasanik B. (1993) The regulation of nitrogen utilization in enteric bacteria. J. Cell. Biochem., 51, 34–40. [DOI] [PubMed] [Google Scholar]
- 4.Gralla J.D. (1996) Activation and repression of E. coli promoters. Curr. Opin. Genet. Dev., 6, 526–530. [DOI] [PubMed] [Google Scholar]
- 5.Studholme D.J. and Buck,M. (2000) The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences. FEMS Microbiol. Lett., 186, 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Ptashne M. and Gann,A. (1997) Transcriptional activation by recruitment. Nature, 386, 569–577. [DOI] [PubMed] [Google Scholar]
- 7.Rippe K., von Hippel,P.H. and Langowski,J. (1995) Action at a distance: DNA-looping and initiation of transcription. Trends Biochem. Sci., 20, 500–506. [DOI] [PubMed] [Google Scholar]
- 8.Magasanik B. (1989) Regulation of transcription of the glnALG operon of Escherichia coli by protein phosphorylation. Biochimie, 71, 1005–1012. [DOI] [PubMed] [Google Scholar]
- 9.Reitzer L.J. and Magasanik,B. (1986) Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell, 45, 785–792. [DOI] [PubMed] [Google Scholar]
- 10.Ninfa A.J., Reitzer,L.J. and Magasanik,B. (1987) Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell, 50, 1039–1046. [DOI] [PubMed] [Google Scholar]
- 11.Porter S.C., North,A.K., Wedel,A.B. and Kustu,S. (1993) Oligomerization of NTRC at the glnA enhancer is required for transcriptional activation. Genes Dev., 7, 2258–2273. [DOI] [PubMed] [Google Scholar]
- 12.Wedel A. and Kustu,S. (1995) The bacterial enhancer-binding protein NTRC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev., 9, 2042–2052. [DOI] [PubMed] [Google Scholar]
- 13.Wyman C., Rombel,I., North,A.K., Bustamante,C. and Kustu,S. (1997) Unusual oligomerization required for activity of NtrC, a bacterial enhancer-binding protein. Science, 275, 1658–1661. [DOI] [PubMed] [Google Scholar]
- 14.Hwang I., Thorgeirsson,T., Lee,J., Kustu,S. and Shin,Y.K. (1999) Physical evidence for a phosphorylation-dependent conformational change in the enhancer-binding protein NtrC. Proc. Natl Acad. Sci. USA, 96, 4880–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasse-Dwight S. and Gralla,J.D. (1988) Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc. Natl Acad. Sci. USA, 85, 8934–8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popham D.L., Szeto,D., Keener,J. and Kustu,S. (1989) Function of a bacterial activator protein that binds to transcriptional enhancers. Science, 243, 629–635. [DOI] [PubMed] [Google Scholar]
- 17.Buck M. and Cannon,W. (1992) Activator-independent formation of a closed complex between σ54-holoenzyme and nifH and nifU promoters of Klebsiella pneumoniae. Mol. Microbiol., 6, 1625–1630. [DOI] [PubMed] [Google Scholar]
- 18.Su W., Porter,S., Kustu,S. and Echols,H. (1990) DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc. Natl Acad. Sci. USA, 87, 5504–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rippe K., Guthold,M., von Hippel,P.H. and Bustamante,C. (1997) Transcriptional activation via DNA-looping: visualization of intermediates in the activation pathway of E.coli RNA polymerase ×σ54 holoenzyme by scanning force microscopy. J. Mol. Biol., 270, 125–138. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.T., Syed,A., Hsieh,M. and Gralla,J.D. (1995) Converting Escherichia coli RNA polymerase into an enhancer-responsive enzyme: role of an NH2-terminal leucine patch in σ54. Science, 270, 992–994. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y., Wang,L. and Gralla,J.D. (1999) A fork junction DNA–protein switch that controls promoter melting by the bacterial enhancer-dependent σ factor. EMBO J., 18, 3736–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon W.V., Gallegos,M.T. and Buck,M. (2000) Isomerization of a binary σ-promoter DNA complex by transcription activators. Nat. Struct. Biol., 7, 594–601. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y., Bondarenko,V., Ninfa,A.J. and Studitsky,V.M. (2001) DNA supercoiling allows enhancer action over a large distance. Proc. Natl Acad. Sci. USA, 98, 14883–14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt T.P. and Magasanik,B. (1985) Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG and glnL. Proc. Natl Acad. Sci. USA, 82, 8453–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitzer L.J. and Magasanik,B. (1985) Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc. Natl Acad. Sci. USA, 82, 1979–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninfa A.J., Ueno-Nishio,S., Hunt,T.P., Robustell,B. and Magasanik,B. (1986) Purification of nitrogen regulator II, the product of the glnL (ntrB) gene of Escherichia coli. J. Bacteriol., 168, 1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasse-Dwight S. and Gralla,J.D. (1991) Footprinting protein–DNA complexes in vivo. Methods Enzymol., 208, 146–168. [DOI] [PubMed] [Google Scholar]
- 28.Sasse-Dwight S. and Gralla,J.D. (1989) KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J. Biol. Chem., 264, 8074–8081. [PubMed] [Google Scholar]
- 29.Tintut Y., Wang,J.T. and Gralla,J.D. (1995) A novel bacterial transcription cycle involving σ54. Genes Dev., 9, 2305–2313. [DOI] [PubMed] [Google Scholar]
- 30.Popham D., Keener,J. and Kustu,S. (1991) Purification of the alternative σ factor, σ54, from Salmonella typhimurium and characterization of σ54-holoenzyme. J. Biol. Chem., 266, 19510–19518. [PubMed] [Google Scholar]
- 31.Ninfa A.J., Brodsky,E. and Magasanik,B. (1989) The role of NRI-phosphate in the activation of transcription from the nitrogen regulated promoter glnAp2 of Escherichia coli. In Gralla,J. (ed.), DNA–protein Interactions in Transcription. Alan R. Liss, Inc., New York, pp. 43–52.
- 32.Jiang P., Peliska,J.A. and Ninfa,A.J. (1998) Reconstitution of the signal-transduction bicyclic cascade responsible for the regulation of Ntr gene transcription in Escherichia coli. Biochemistry, 37, 12795–12801. [DOI] [PubMed] [Google Scholar]
- 33.Weiss D.S., Batut,J., Klose,K.E., Keener,J. and Kustu,S. (1991) The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell, 67, 155–167. [DOI] [PubMed] [Google Scholar]