Abstract
Background
Osteoarthritis is common in the aging dog and is associated with chronic pain and impaired mobility. The main objective of this study was to determine whether low‐level laser therapy (LLLT) would increase physical activity in dogs with osteoarthritis.
Methods
Twenty‐three dogs with osteoarthritis were instrumented with an accelerometer 48 h before the first LLLT session (baseline), to record daily activity. Each dog underwent six consecutive weekly laser treatments. The scores of the Canine Brief Pain Inventory and the Liverpool Osteoarthritis in Dogs’ were recorded for clinical purposes, as a tool to titrate the analgesic therapy of each individual dog, before LLLT (as baseline) and then weekly for 6 weeks.
Results
The number of daily activities increased during week 2 (161,674; SD, 103,666) and remained higher than baseline (93,481; SD, 107,878) until week 6 (179,309; SD, 126,044; p < 0.001). Daily step count increased from week 1 (4472; SD, 3427) compared to baseline (1109; SD, 1061) and remained higher than the baseline until the end of week 6 (8416; SD, 3166; p < 0.001). Average energy expenditure during the study period was 179 [range, 2–536] kcal/day; there were no statistically significant differences in this variable between weeks of treatment. Systemic analgesics therapy was decreased in 50% of the dogs during the study period.
Conclusions
Laser therapy may advance the management of osteoarthritis by increasing the level of activity of dogs, therefore improving their quality of life.
Keywords: accelerometry, analgesia, dogs, low‐level laser therapy, osteoarthritis
Osteoarthritis is a common condition that in the aging dog is associated with chronic pain and impaired mobility. Twenty‐three dogs with osteoarthritis were treated with low‐level laser therapy and instrumented with an accelerometer to monitor their daily activity. In the study dogs, mobility started to improve after the first laser treatment, as demonstrated by the increased number of daily activities and daily step count. Laser therapy may advance the management of osteoarthritis by increasing the level of activity of the dogs, therefore improving their quality of life.

1. INTRODUCTION
Osteoarthritis (OA) is a common clinical condition that causes chronic pain, decreased joint function and reduced level of activity and quality of life in the affected dogs. Osteoarthritis‐associated pain and impaired mobility are usually addressed with long‐term administration of systemic analgesics, of which non‐steroidal anti‐inflammatory drugs (NSAIDs) are traditionally used as first line therapy, whilst gabapentinoids and opioid analgesics are usually added to treat unresponsive pain (Pettitt & German, 2015; Sanderson et al., 2009). Cannabidiol has recently been proposed as a novel therapeutic option for dogs with OA; however, in some countries this agent is not commercially available for use in animals (Mejia et al., 2021; Verrico et al., 2020). Beside the inconvenience of often inadequate pain relief, one drawback of administering NSAIDs on the long term is the potential for gastrointestinal, hepatic and renal side effects. The use of opioids in non‐hospitalised client‐owned pets, on the other hand, carries the risk for human drug abuse and therefore raises ethical concerns.
During the last decade, the challenging treatment of canine OA‐associated pain resulted in a growing interest in non‐pharmacological analgesic techniques, such as acupuncture and electroanalgesia (Baker‐Meuten et al., 2020; Barale et al., 2020; Kapatkin et al., 2006). The most promising electroanalgesic techniques developed to treat joint pain in humans imply the use of laser. Whilst low‐level laser therapy (LLLT) is widely used in humans with no clinically relevant reported adverse effects, only a few studies reported the use of laser for analgesic purposes in dogs (Huang et al., 2015; Wyszyńska & Bal‐Bocheńska, 2018; Youssef et al., 2016). The results of a recent survey study limited to the veterinarians in the state of Missouri suggest that LLLT is widely used for the treatment of canine OA, although treatment protocols were reportedly unknown and rather chosen by predetermined settings on the LLLT unit (Barger et al., 2020). One clinical trial conducted in 12 dogs investigated the effects of LLLT on bone healing and pain after stifle surgery, and to the best of the authors’ knowledge only three published studies reported the use of laser to treat OA‐associated chronic pain in dogs with naturally occurring OA, with promising results in terms of improved analgesia and lack of side effects (Barale et al., 2020; De Oliveira Reusing et al., 2021; Kennedy et al., 2018; Looney et al., 2018).
This prospective cohort clinical trial was designed to investigate whether a 6‐week LLLT would increase physical activity in a population of client‐owned dogs diagnosed with osteoarthritis, as determined with an accelerometry‐based monitor.
2. MATERIALS AND METHODS
2.1. Ethical approval
The study was conducted under ethical approval of the Clinical Research and Ethical Review Board of the Royal Veterinary College (reference number: URN 2019 1896‐3, date:14th of August 2019, validity: 3 years), and written, signed informed owner consent.
2.2. Animals’ recruitment and selection
This study was designed as a cohort observational study. The dogs with OA referred to the Clinica Veterinaria Europa that met the inclusion criteria, namely confirmed diagnosis of either knee or hip OA with both radiographic and orthopaedic exams, absence of painful conditions other than OA, absence of any systemic disease potentially affecting mentation and behaviour, access to open spaces for at least 1 h per day and willingness of their owners to standardise their physical activity, were included in the study. The dog owners were specifically instructed not to change the degree of exposure to physical activity of their dogs, in terms of duration of both walks on the lead and access to open spaces, during the study period. Dogs that were eligible for surgical treatment of OA, as determined by the orthopaedic surgeon, as well as dogs whose pharmacological therapy had been modified or adjusted during the 2 weeks before the beginning of the LLLT, were excluded from the study. The Canine OsteoArthritis Staging Tool (COAST; score: 0–4), assigned to each dog always by the same orthopaedic surgeon, was used as part of the clinical examination to stage the degree of OA (Cachon et al., 2018).
2.3. Accelerometry
A light‐weight accelerometer validated for use in dogs with OA (Model Z Actical Animal Device; Philips Electronics) was mounted within an armoured case on the neck collar of each dog 48 h prior to the first LLLT session and used to record the dogs’ daily activity over the whole study period (Figure 1) (Brown et al., 2010; Lascelles et al., 2015; Wernham et al., 2011). The Actical epoch length was set at 1 min. After study completion, data was downloaded using the Actical reader device (ActiReader; FCC version) and software (Actical Software; Global), and then extracted into a spreadsheet (Excel; Microsoft Corp.) to summarise activity data on a daily and weekly basis for all dogs, across a total collection period of 44 days (2 days baseline followed by 6 consecutive weeks). Data collected on the day on which the dogs were instrumented with Actical were discarded to allow for analysis of full days only. Accelerometry variables included number of total daily activities, average energy expenditure in kcal/day (not accounting for basal metabolic rate), number of steps per day and the amount of time spent daily in one of four categories, namely sedentary status and light, moderate and vigorous activity (Michel & Brown, 2011). The optimal setting for accelerometry data recording and analysis was determined based on previously published literature (Dow et al., 2009; Lascelles et al., 2015; Mejia et al., 2021).
FIGURE 1.
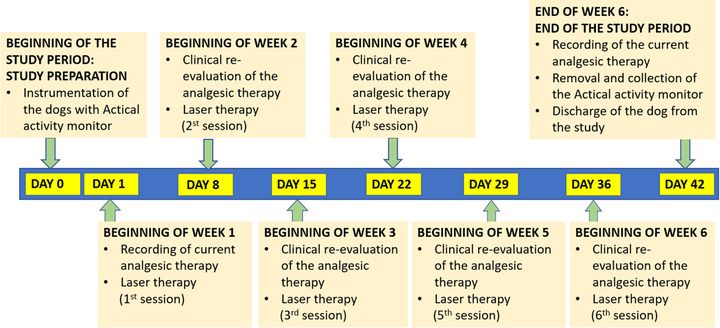
Timetable of the interventions and assessments performed in the study dogs over the 6‐week period
2.4. Low level laser therapy
A device designed for veterinary use and indicated for treatment of OA‐associated pain as specified by the manufacturer (LaserVet 1000; GlobusVet) was used for LLLT. Laser therapy was administered always by the same investigator (Loris Barale). The areas of the body treated with the laser were the affected joints and the associated muscles, namely the semitendinosus and semimembranosus for the stifle and the iliopsoas for the hip; the joints were always treated before the muscles. The laser probe was applied directly on the skin of the area to be treated. Each area was treated weekly, for a total of 6 weeks. By using a predetermined programme as per the manufacturer's instruction, the duration of laser exposure ranged from 50 s to 4 min, depending on coat pigmentation (light or dark), and body weight (1 to >25 kg). Other variables were set by the software as follows: 1000 mW potency, 1 W/cm2 density of potency, 808 nm laser beam wavelength, frequencies of 500–1000 (joints) and 3000–5000 Hz (muscles), energy of 5 (joints) and 4.2 (muscles) j/cm2, spot laser diameter varying from 3.5 to 11.5 mm and number of laser application points varying from 6 to 10 per site, depending on the surface area to be treated. Pulsate and continuous emittances were used for the joints and muscles, respectively, with cycles of the same duration, as per the manufacturer's instruction. The total energy (j) per treatment depended on the surface area to be treated (cm2), which ultimately depended on the size of the dog.
The dogs were observed after each treatment for the occurrence of side effects, namely itch, redness, swelling, changes in skin/coat pigmentation and any kind of discomfort perceived by either the dog owners or the clinician.
2.5. Titration of the pharmacological analgesic therapy to effect
Clinical Metrology was used for clinical purposes unrelated to the study, as a tool to titrate the analgesic therapy of each specific dog to effect. The quality of life, as perceived by the animal owners, was assessed based on the scores of two validated questionnaires for dog owners’ use: The Canine Brief Pain Inventory (CBPI; score: 0–100) and the Liverpool Osteoarthritis in Dogs’ (LOAD; score: 0–52) (Brown et al., 2008; Walton et al., 2013). Every dog owner was asked to compile both questionnaires before the beginning of laser therapy (baseline), and then weekly until the end of the study period. Previously compiled questionnaires were not made accessible to the dog owners. Following a decrease in either CBIP or LOAD scores by ≥30% of the values recorded on previous consultation, the systemic analgesic therapy was reduced stepwise as follows: For dogs on full dose of NSAIDs, the daily dose was halved; for dogs on either fragmental NSAIDs dose regimen or NSAIDs plus a second class of analgesics, the NSAIDs was withheld; for dogs receiving either gabapentin or pregabalin only, that analgesic was withheld in case of fragmental dose; otherwise, its daily dose was halved. In the event of an increase in LOAD and/or CBIP scores, or of any increase in pain or discomfort as perceived by either the dog owners (regardless of the questionnaires’ results) or one of the clinicians, the analgesic therapy was adjusted at the clinician's discretion based on the individual needs of the dog, which was excluded from the study.
2.6. Statistical analysis
The sample size calculation was performed, with a validated on‐line calculator (https://www.stat.ubc.ca), using as endpoint variable the total number of daily activities measured with accelerometry in dogs with OA before (mean: 100,000, with SD of 100%) and after (mean: 200,000, with SD of 100%) laser treatment. This resulted in a minimum sample of 22 subjects. Mean values were estimated based on unpublished pilot data from the authors. Other variables were set as follows: α value: 0.05; power: 0.9; type II error: 10%; and type of test: two‐sided test.
The Kolmogorov–Smirnov test was performed to analyse data distribution and descriptive statistics applied to demographic variables. Accelerometry variables were analysed with a two‐way mixed analysis of variance (ANOVA), with time as within‐subjects factor and each individual dog as between‐subjects factor. The Kruskal–Wallis ANOVA on ranks, followed by Dunn's method for multiple comparisons, was used to compare, between time points, the proportion of daily time, after arcsine transformation of the variable, spent by the dogs in each of the four activity categories (Feder et al., 2020).
Commercially available statistical software (SigmaPlot 10 and SigmaStat 3.5; Systat Software; SPSS statistics version 25; IBM Corp.) were used. p‐Values <0.05 were considered statistically significant. Data are represented as either means and SD or medians and interquartile (25%–75%) ranges, depending on data distribution.
3. RESULTS
3.1. Demographic and general data
Data were collected from September 2019 until December 2021. Of the 25 dogs initially included in the study, two were excluded during week 3, one owing to the development of cranial cruciate rupture and another one because the accelerometer got lost. Changes of the analgesic therapy at the clinician's discretion without following the stepwise procedure as previously described were not deemed necessary in any of the study dogs. Twenty‐three dogs weighing 27 kg (SD, 10 kg) and aged 10 years (SD, 3 years), of which 12 were neutered females and 11 males (of which four were castrated), completed the study. The represented breeds were mixed breed (n = 11), German Shepherd (3), Beagle (2), Border Collie (1), Labrador (1), Rottweiler (1), Poodle (1), Australian Shepherd (1), Corso (1) and Setter Gordon (1). The affected joints were 25 hips and 10 stifles; in 12 dogs, more than one joint was affected and treated. The COAST score was 3 (Mejia et al., 2021; Pettitt & German, 2015; Verrico et al., 2020).
3.2. Low‐level laser therapy
The laser treatment was well tolerated by all the study dogs, and undesired effects following LLLT were not observed.
3.3. Accelerometry
Both the activity count and the number of daily steps increased compared to baseline during the 6‐week study period (p < 0.001; Figure 2). The study dogs spent the greatest proportion of their daily time as sedentary during the whole study period; however, the proportion of time spent on light activity increased from week 3 (25%) compared to baseline (18%; p < 0.001) and remained higher than baseline until the end of week 6 (28% [range, 27%–28%]). The proportion of daily time spent on moderate activity did not vary over time and was 4% [range, 2%–4%], whereas the proportion of daily time spent on vigorous activity was less than 1% for all weeks (Figure 3). Average energy expenditure during the study period was 179 [range, 2–536] kcal/day; there were no statistically significant differences in this variable between weeks of treatment (Figure 3).
FIGURE 2.
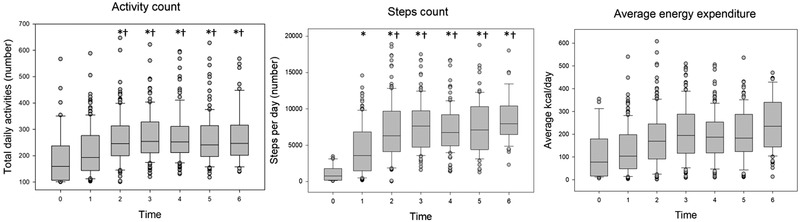
Number of total daily activities (a; within 60 s epoch; 100–700 are thousands of daily activities), average number of daily steps (b), and average daily energy expenditure (c), recorded by Actical accelerometer over a 6‐week laser therapy in 23 dogs with osteoarthritis. The boxes represent the second and third quartiles, with the horizontal lines between them representing the medians. The lower and upper quartiles (25% and 75%, respectively) are represented by the horizontal lines outside the boxes. The dots represent the outliers. 0: baseline (48 h prior to the first laser treatment); 1, 2, 3, 4, 5 and 6 are the weeks of treatment. The stars and the daggers indicate statistical significance for comparisons with baseline and with week 1, respectively (p < 0.001).
FIGURE 3.
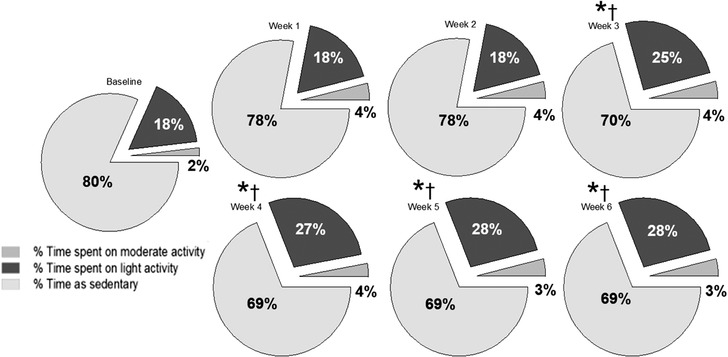
Proportion of daily (24 h) time spent by 23 dogs with osteoarthritis, before (baseline) and during a 6‐week laser treatment, in one of four categories, namely sedentary status and light, moderate and vigorous activity. The stars and the daggers indicate statistical significance for comparisons with baseline and with week 1, respectively (p < 0.001).
3.4. Reduction in pharmacological analgesic therapy
At the time of their inclusion in the study, all dogs were on pharmacological therapy since at least 3 weeks. Prior to laser treatment, the analgesic therapy consisted of meloxicam for 15 dogs, grapiprant for five dogs, firocoxib for one dog and monoclonal antibodies for one dog; for all dogs, the drug dosages were based on the manufacturer's recommendations. At the beginning of week 2, the dose of NSAID was halved in seven out of 22 dogs, and two dogs required discontinuation of the NSAID administration owing to the development of gastrointestinal symptoms. At the beginning of week 2, the dose of the NSAID was halved in four dogs previously on full clinical dose and withdrawn in three dogs that were on half dose during week 2. During week 4, four dogs previously on half dose of NSAID had it withdrawn, and in one dog on half meloxicam dose the latter was replaced with grapiprant owing to mild gastrointestinal symptoms. In one dog, meloxicam, which had been withheld a week earlier, was reintroduced at full dose, owing to a fall from the stairs which caused discomfort to the dog. No further changes in therapy occurred during weeks 5 and 6.
4. DISCUSSION
The main finding of this study was that a weekly treatment with LLLT for 6 consecutive weeks effectively increased the level of activity of a population of dogs with OA. Most accelerometry variables consistently improved after 2 weekly sessions of laser therapy, which suggests increased ability and willingness of the study dogs to exercise.
The level of activity has been recognised as a reliable measure to quantify pain in dogs with OA; therefore, it is reasonable to assume that the increased activity following laser therapy was associated with a decrease in the intensity of pain as perceived by the dogs and, as a result, to an improvement of their quality of life (Rialland et al., 2012; Wernham et al., 2011).
Actical accelerometry has been validated as an objective and quantitative measure of activity and distance moved in dogs and was specifically evaluated to detect response to canine OA treatment (Hansen et al., 2007; Lascelles et al., 2015; Mejia et al., 2021). The piezoelectric sensor of Actical is capable of generating a voltage in response to even small, omnidirectional changes in acceleration, a feature which makes it suitable to detect changes of the activity pattern even in dogs with mild‐to‐moderate level of activity. Despite the objective nature of accelerometry variables, patient selection still plays a pivotal role in the obtainment of useful data. Whilst access to open spaces facilitates appreciation of changes in activity patterns associated with improved joint function, at the same time avoiding variations in the dogs’ opportunity to perform physical activity during the recording period is essential to avoid peaks in activity which could be totally unrelated to the willingness of the dogs to exercise. Moreover, another potential bias is that some dog owners may potentially be more willing to take their dogs for longer walks than usual knowing that an analgesic treatment has been administered. For these reasons, in this study routine physical activity limited to walk on the lead and unwillingness of the dog owners to be consistent with the duration of the free physical activity of their pets were both considered as exclusion criteria.
Accelerometry variables, including epochs of measurement and frequency interval for consecutive assessments, were determined based on previously published literature. Ideally, measuring the baseline values over periods longer than 48 hours and, potentially, extending the use of accelerometry a few weeks after discontinuation of the treatment, would have provided a better overall evaluation of the effects of LLLT, especially on the longer term. However, this would have been unpractical. Although the memory of Actical Z may extend to 200 days, data recording is battery limited, and relying on the dog owners for replacing the batteries was no suitable option. Moreover, extending the duration of data collection could have decreased the dog owners’ willingness to participate in the study, while increasing the risk of losing the accelerometers. For the accelerometry variables, which were measured more than once per time point, the choice of using a mixed model instead of repeated measures ANOVA was a statistical strategy to account for the lack of a complete balanced array of data, resulting from the fewer recordings at baseline (48 h) compared to the following time points (at seven day‐interval) (Krueger & Tian, 2004). For a thorough evaluation of locomotion, gait analysis would have been a valuable adjunction to accelerometry to assess weight bearing and activity patterns during the study period; however, this option was not available in the veterinary practice where the study was conducted (Moreau et al., 2014).
In the current study, it was decided not to standardise the analgesic pharmacological therapy but to titrate it to the individual needs of each dog instead. This approach was deemed necessary owing to the involvement of client‐owned dogs, with the resulting ethical implications. Dogs with OA often undergo long‐term treatment with drugs that have potential side effects, such as the NSAIDs (Lomas & Grauer, 2015; Monteiro‐Steagall et al., 2013). In this perspective, aiming at titrating the dose to effect—which could include decreasing it or discontinuing the administration when applicable—is common clinical practice. Titration of the analgesic therapy was done as part of the clinical assessment based on unobjective indicators, namely the overall clinical evaluation of both the pain therapist and the orthopaedic surgeon and the feedback of the animal owners as determined by the questionnaires’ scores. For these reasons, evaluating a decrease in the requirement of systemic analgesics was not a primary outcome of this study. Nevertheless, it is interesting to observe that, despite the analgesic therapy was decreased in half of the dogs during the study period, their level of activity continued to increase, which further corroborates the findings of this study.
This study was designed so that each dog acted as its own control over time, to evaluate the effect of treatment over the 6‐week study period. As a result, its biggest limitation is that the information provided can only be restricted to variables that can be measured objectively and recorded without the intervention of an observer, as it is the case for accelerometry data. For a more comprehensive and conclusive evaluation of the analgesic effects of LLLT, the use of complementary tools such as mechanical thresholds and gait analysis, as well as a double‐blind study design with the addition of a control group would be desirable (Briley et al., 2014; Knazovicky et al., 2016; Krueger & Tian, 2004; Machin et al., 2020).
5. CONCLUSIONS
LLLT increased physical activity in dogs with OA and may be used as an adjuvant therapy to treat chronic joint pain and reduce pharmacological therapy. More prospective clinical trials are needed to allow refinement of case‐specific protocols, and to investigate the risk for side effects of LLLT on a larger sample population.
AUTHOR CONTRIBUTIONS
Conceptualisation, investigation, and writing—original draft: Loris Barale. Conceptualisation, resources, and writing—review and editing: Paolo Monticelli. Conceptualisation, data curation, formal analysis, methodology, resources, software, supervision, writing—original draft, and writing—review and editing: Chiara Adami.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING INFORMATION
No third‐party funding or support was received in connection with this study or the publication of the manuscript.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
PEER REVIEW
I would not like my name to appear with my report on Publons https://publons.com/publon/10.1002/vms3.997.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Massimo Raviola (Clinica Veterinaria Europa, Turin, Italy) for performing the orthopaedic assessments in the study dogs.
Barale, L. , Monticelli, P. , & Adami, C. (2023). Effects of low‐level laser therapy on impaired mobility in dogs with naturally occurring osteoarthritis. Veterinary Medicine and Science, 9, 653–659. 10.1002/vms3.997
Loris Barale and Chiara Adami contributed equally to this work and share the first authorship.
DATA AVAILABILITY STATEMENT
All the data relevant to this study are presented in the result paragraph. Raw data may be made available by the corresponding author upon reasonable request.
REFERENCES
- Baker‐Meuten, A. , Wendland, T. , Shamir, S. K. , Hess, A. M. , & Duerr, F. M. (2020). Evaluation of acupuncture for the treatment of pain associated with naturally‐occurring osteoarthritis in dogs: A prospective, randomized, placebo‐controlled, blinded clinical trial. BMC Veterinary Reseach, 16, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barale, L. , Monticelli, P. , Raviola, M. , & Adami, C. (2020). Preliminary clinical experience of low‐level laser therapy for the treatment of canine osteoarthritis‐associated pain: A retrospective investigation on 17 dogs. Open Veterinary Journal, 10, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger, B. K. , Bisges, A. M. , Fox, D. B. , & Torres, B. (2020). Low‐level laser therapy for osteoarthritis treatment in dogs at Missouri Veterinary Practice. Journal of the American Animal Hospital Association, 56, 139–145. [DOI] [PubMed] [Google Scholar]
- Briley, J. D. , Williams, M. D. , Freire, M. , Griffith, E. H. , & Lascelles, B. D. (2014). Feasibility and repeatability of cold and mechanical quantitative sensory testing in normal dogs. Veterinary Journal, 199, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. C. , Boston, R. C. , Coyne, J. C. , & Farrar, J. T. (2008). Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. Journal of the American Veterinary Medical Association, 233, 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. C. , Boston, R. C. , & Farrar, J. T. (2010). Use of an activity monitor to detect response to treatment in dogs with osteoarthritis. Journal of the American Veterinary Medical Association, 237, 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachon, T. , Frykman, O. , Innes, J. F. , Lascelles, B. D. X. , Okumura, M. , Sousa, P. , Staffieri, F. , Steagall, P. V. , & Van Ryssen, B. (2018). Face validity of a proposed tool for staging canine osteoarthritis: Canine OsteoArthritis Staging Tool (COAST). Veterinary Journal, 235, 1–8. [DOI] [PubMed] [Google Scholar]
- De Oliveira Reusing, M. S. , do Amaral, C. H. , Zanettin, K. , Weber, S. , & Villanova, J. (2021). Effects of hydrotherapy and low‐level laser therapy in canine hip dysplasia: A randomized, prospective, blinded clinical study. Revue Vétérinaire Clinique, 56, 4, 10.1016/j.anicom.2021.08.001 [DOI] [Google Scholar]
- Dow, C. , Michel, K. E. , Love, M. , & Brown, D. C. (2009). Evaluation of optimal sampling interval for activity monitoring in companion dogs. American Journal of Veterinary Research, 70, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder, P. I. , Aume, L. L. , Triplett, C. A. , Simmons, J. E. , & Narotsky, M. G. (2020). Analysis of proportional data in reproductive and developmental toxicity studies: Comparison of sensitivities of logit transformation, arcsine square root transformation, and nonparametric analysis. Birth Defects Research, 112, 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, B. D. , Lascelles, D. X. , Keene, B. W. , Adams, A. K. , & Thomson, A. E. (2007). Evaluation of an accelerometer for at‐home monitoring of spontaneous activity in dogs. American Journal of Veterinary Research, 68, 5468–5475. [DOI] [PubMed] [Google Scholar]
- Huang, Z. , Chen, J. , Ma, J. , Shen, B. , Pei, F. , & Kraus, V. B. (2015). Effectiveness of low‐level laser therapy in patients with knee osteoarthritis: A systematic review and meta‐analysis. Osteoarthritis and Cartilage, 23, 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapatkin, A. S. , Tomasic, M. , Beech, J. , Meadows, C. , Boston, R. C. , Mayhew, P. D. , Powers, M. Y. , & Smith, G. K. (2006). Effects of electrostimulated acupuncture on ground reaction forces and pain scores in dogs with chronic elbow joint arthritis. Journal of the American Veterinary Medical Association, 228, 1350–1354. [DOI] [PubMed] [Google Scholar]
- Kennedy, K. C. , Martinez, S. A. , Martinez, S. E. , Tucker, R. L. , & Davies, N. M. (2018). Effects of low‐level laser therapy on bone healing and signs of pain in dogs following tibial plateau levelling osteotomy. American Journal of Veterinary Research, 79, 893–904. [DOI] [PubMed] [Google Scholar]
- Knazovicky, D. , Helgeson, E. S. , Case, B. , Gruen, M. E. , Maixner, W. , & Lascelles, B. D. X. (2016). Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain, 157, 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, C. , & Tian, L. (2004). A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biological Research for Nursing, 6, 151–157. [DOI] [PubMed] [Google Scholar]
- Lascelles, B. D. X. , Knazovicky, D. , Case, B. , Freire, M. , Innes, J. F. , Drew, A. C. , & Gearing, D. P. (2015). A canine‐specific anti‐nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease‐associated pain. BMC Veterinary Research, 11, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas, A. L. , & Grauer, G. F. (2015). The renal effects of NSAIDs in dogs. Journal of the American Animal Hospital Association, 51, 197–203. [DOI] [PubMed] [Google Scholar]
- Looney, A. L. , Huntingford, J. L. , Blaeser, L. L. , & Mann, S. (2018). A randomized blind placebo‐controlled trial investigating the effects of photobiomodulation therapy (PBMT) on canine elbow osteoarthritis. Canadian Veterinary Journal, 59, 959–966. [PMC free article] [PubMed] [Google Scholar]
- Machin, H. , Taylor‐Brown, F. , & Adami, C. (2020). Use of acupuncture as adjuvant analgesic technique in dogs undergoing thoracolumbar hemilaminectomy. Veterinary Journal, 264, 105536. [DOI] [PubMed] [Google Scholar]
- Mejia, S. , Duerr, F. M. , Griffenhagen, G. , & McGrath, S. (2021). Evaluation of the effect of cannabidiol on naturally occurring osteoarthritis‐associated pain: A pilot study in dogs. Journal of the American Veterinary Medical Association, 57, 81–90. [DOI] [PubMed] [Google Scholar]
- Michel, K. E. , & Brown, D. C. (2011). Determination and application of cut points for accelerometer‐based activity counts of activities with differing intensity in pet dogs. American Journal of Veterinary Research, 72, 866–870. [DOI] [PubMed] [Google Scholar]
- Monteiro‐Steagall, B. P. , Steagall, P. V. , & Lascelles, B. D. (2013). Systematic review of nonsteroidal anti‐inflammatory drug‐induced adverse effects in dogs. Journal of Veterinary Internal Medicine, 27, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Moreau, M. , Lussier, B. , Ballaz, L. , & Troncy, T. (2014). Kinetic measurements of gait for osteoarthritis research in dogs and cats. Canadian Veterinary Journal, 55, 1057–1065. [PMC free article] [PubMed] [Google Scholar]
- Pettitt, R. A. , & German, A. J. (2015). Investigation and management of canine osteoarthritis. In Practice, 37, 1–8. [Google Scholar]
- Rialland, P. , Bichot, S. , Moreau, M. , Guillot, M. , Lussier, B. , Gauvin, D. , Martel‐Pelletier, J. , Pelletier, J. P. , & Troncy, E. (2012). Clinical validity of outcome pain measures in naturally occurring canine osteoarthritis. BMC Veterinary Research, 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, R. O. , Beata, C. , Flipo, R. M. , Genevois, J. P. , Macias, C. , Tacke, S. , Vezzoni, A. , & Innes, J. F. (2009). Systematic review of the management of canine osteoarthritis. The Veterinary Record, 164, 418–424. [DOI] [PubMed] [Google Scholar]
- Verrico, C. D. , Wesson, S. , Konduri, V. , Hofferek, C. J. , Vazquez‐Perez, J. , Blair, E. , Dunner, K. , Salimpour, P. , Decker, W. K. , & Halpert, M. M. (2020). A randomized, double‐blind, placebo‐controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain, 161, 2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, M. B. , Cowderoy, E. , Lascelles, D. , & Innes, J. F. (2013). Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS One, 8, e58125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernham, B. G. , Trumpatori, B. , Hash, J. , Lipsett, J. , Davidson, G. , Wackerow, P. , Thomson, A. , & Lascelles, B. D. (2011). Dose reduction of meloxicam in dogs with osteoarthritis‐associated pain and impaired mobility. Journal of Veterinary Internal Medicine, 25, 1298–1305. [DOI] [PubMed] [Google Scholar]
- Wyszyńska, J. , & Bal‐Bocheńska, M. (2018). Efficacy of high‐intensity laser therapy in treating knee osteoarthritis: A first systematic review. Photomedicine and Laser Surgery, 36, 343–353. [DOI] [PubMed] [Google Scholar]
- Youssef, E. F. , Muaidi, Q. I. , & Shanb, A. A. (2016). Effect of laser therapy on chronic osteoarthritis of the knee in older subjects. Journal of Lasers in Medical Sciences, 7, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this study are presented in the result paragraph. Raw data may be made available by the corresponding author upon reasonable request.


