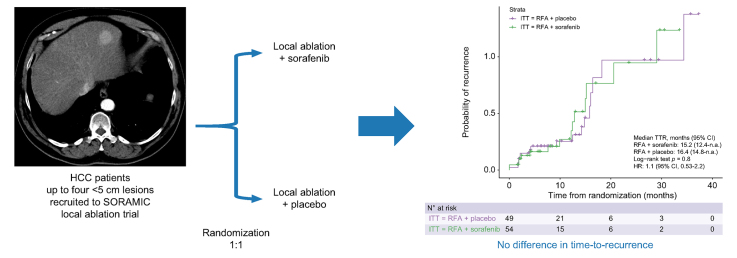
Abstract
Background & Aims
The aim of the study was to evaluate the efficacy and safety of adjuvant sorafenib treatment compared with placebo in patients with hepatocellular carcinoma who underwent local ablation.
Methods
The SORAMIC trial is a randomised controlled trial with diagnostic, local ablation, and palliative sub-study arms. After initial imaging within the diagnostic study, patients were assigned to local ablation or palliative arms. In the local ablation cohort, patients were randomised 1:1 to local ablation + sorafenib vs. local ablation + placebo. The primary endpoint was time-to-recurrence (TTR). Secondary endpoints were local control rate and safety in terms of adverse events and quality-of-life.
Results
The recruitment was terminated prematurely after 104 patients owing to slow recruitment. One patient was excluded because of a technical failure. Fifty-four patients were randomised to local ablation + sorafenib and 49 to local ablation + placebo. Eighty-eight patients who underwent standardised follow-up imaging comprised the per-protocol population. The median TTR was 15.2 months in the sorafenib arm and 16.4 months in the placebo arm (hazard ratio 1.1; 95% CI 0.53–2.2; p = 0.82). Out of 136 lesions ablated within the trial, there was no difference in local recurrence rate between sorafenib (6/69, 8.6%) and placebo groups (5/67, 5.9%; p = 0.792).
Overall (92.5% vs. 71.4%, p = 0.008) and drug-related (81.4% vs. 55.1%, p = 0.003) adverse events were more common in the sorafenib arm compared with the placebo arm. Dose reduction because of adverse events were common in the sorafenib arm (79.6% vs. 30.6%, p <0.001).
Conclusions
Adjuvant sorafenib did not improve in TTR or local control rate after local ablation in patients with hepatocellular carcinoma within the limitations of an early terminated trial.
Impact and implications
Local ablation is the standard of care treatment in patients with early stages of hepatocellular carcinoma, along with surgical therapies. However, there is a risk of disease recurrence during follow-up. Sorafenib, an oral medication, is a routinely used treatment for patients with advanced hepatocellular carcinoma. This study found that sorafenib treatment after local ablation in people with early hepatocellular carcinoma did not significantly improve the disease-free period compared with placebo.
Clinical trial number
EudraCT 2009-012576-27, NCT01126645.
Keywords: Hepatocellular carcinoma, Sorafenib, Local ablation, Adjuvant, Time-to-recurrence
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CONSORT, Consolidated Standards of Reporting Trials; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HCC, hepatocellular carcinoma; HR, hazard ratio; ITT, intention-to-treat; MRI, magnetic resonance imaging; MWA, microwave ablation; PP, per protocol; RFA, radiofrequency ablation; RFS, relapse-free survival; SIRT, selective internal radiation therapy; SORAMIC, SORAfenib in combination with local MICro-therapy guided by gadolinium-EOB-DTPA-enhanced MRI; TTR, time-to-recurrence
Graphical abstract
Highlights
-
•
Adjuvant sorafenib after local ablation for HCC does not extend time-to-recurrence.
-
•
Similarly, sorafenib treatment has no effect on local recurrence rate.
-
•
Adjuvant sorafenib has a significantly higher adverse event rate compared with placebo.
-
•
Sorafenib is associated with early decrease (at 2 and 3 months) in quality-of-life compared with placebo.
Introduction
Hepatocellular carcinoma (HCC) patients with very early Barcelona Clinic Liver Cancer (BCLC-0) and early (BCLC-A) stages are potential candidates for curative therapies.1 Surgical resection, liver transplantation, or local ablation are the standard of care in these patients. The primary ablation method is thermal ablation inducing coagulative tumour necrosis with heat up to 60–100 °C by either radiofrequency ablation (RFA) or microwave ablation (MWA).
However, resection and local ablation are associated with high recurrence rates by up to 80% within 5 years.2,3 Tumour recurrence is seen either as a result of progression of concomitant micrometastases or development of de novo primary lesions in underlying cirrhosis or chronic liver disease. Currently, no benefit of an adjuvant therapy has been shown in patients with HCC after resection or local ablation. The trials that evaluated adjuvant therapy with interferon, vitamin K2, and retinoids have failed to meet their endpoints.[4], [5], [6], [7]
Sorafenib, a multikinase inhibitor, improved the overall survival in patients with unresectable HCC,8,9 and was the only systemic therapy option for more than a decade. Its mechanism of action by inhibiting tumour cell proliferation and angiogenesis offers a potential rationale for adjuvant therapy after curative therapies.
SORAfenib in combination with local MICro-therapy guided by gadolinium-EOB-DTPA-enhanced MRI (SORAMIC; EudraCT 2009-012576-27, NCT01126645) is a prospective study that comprised three sub-studies: (i) comparison of gadoxetic acid-enhanced magnetic resonance imaging (MRI) vs. contrast-enhanced multislice computed tomography (CT) for the stratification of patients to a local ablation (curative treatment) or palliative treatment group; (ii) comparison of local ablation plus sorafenib vs. control (local ablation plus matching placebo) on time-to-recurrence; and (iii) comparison of selective internal radiation therapy (SIRT) with 90Y resin microspheres combined with sorafenib compared with control (sorafenib alone) on overall survival.
The local ablation study arm of the SORAMIC trial (part ii) was designed to determine the efficacy and safety of adjuvant sorafenib in patients with HCC who underwent local ablation.
Patients and methods
Study design and participants
SORAMIC is a randomised, double-blind, placebo-controlled, multicentre phase II trial conducted at 38 sites in 12 countries in Europe and Turkey. The study was approved by the institutional review boards of all participating centres as well as the competent authorities. All patients gave written informed consent before entering the study.
In the local ablation study arm of the SORAMIC trial, the primary objective was to determine if the sorafenib in combination with tumour ablation prolongs the time-to-recurrence (TTR) compared with local ablation and placebo. Secondary objectives were to assess the safety of the combination of local ablation and sorafenib in comparison with local ablation and placebo, and the local control rate. Recruitment in this arm was stopped owing to lack of recruitment when the endpoints of part i (diagnostic arm) and part iii (palliation arm) reached their endpoints. The study herein summarises the results of 104 patients randomised, when the recruitment goal had initially been 290 patients.
After study inclusion, patients with a diagnosis of HCC underwent gadoxetic acid-enhanced MRI and contrast-enhanced CT according to the imaging protocol of the SORAMIC study.10 Patients aged between 18 and 85 years with a diagnosis of treatment-naive HCC, Child-Pugh scores A or B up to 7 points, an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2 were eligible. The disease stage was evaluated by the local investigator using all available clinical information, including CT and MR imaging. Patients were allocated to either local ablation (part of the following analysis) or palliative treatment (previously reported11) by the treating physicians at the study sites based on the disease stage. Patients with up to four lesions of a maximum diameter of 5 cm each were recruited to the local ablation study arm. Main exclusion criteria in this study arm were: invasion of the portal vein (any extent), extrahepatic metastases, and patients in whom the surgical resection was the most appropriate treatment. Additionally, patients with significant cardiovascular disease (including myocardial infarction within 6 months of inclusion), uncontrolled hypertension, thrombotic or embolic events (including transient ischemic attacks) within the past 6 months, and history of organ transplantation were excluded (full list is in the Supplementary material).
Local ablation
Percutaneous or laparoscopic RFA or MWA were allowed for local tumour ablation. Local ablation was performed according to the manufacturer’s instructions, and routine procedures of the participating hospital were followed as far as possible. Applicators were selected according to the size, location, and configurations of the lesions to be treated. The imaging guidance was at the discretion of the operator. A maximum of two ablation sessions (maximum of 2 weeks apart) were permitted per patient, with a maximum of two liver lesions treated in each session. If ablation was deemed incomplete for any reason, one reintervention of a maximum of two lesions was allowed within 2 weeks of the last session. Patients who do not complete local therapy as planned were excluded from the study (technical failures).
Randomisation and masking
Following a pre-defined randomisation plan, patients eligible for the study were randomised 1:1 to the local ablation + sorafenib or local ablation + placebo after completion of local ablation sessions. Randomisation was performed using an interactive voice response system. The randomisation was done by centres and separately for patients with one, two, or three ablation sessions.
Sorafenib treatment
Patients received continuous treatment with sorafenib or matching placebo until disease recurrence. Treatment was started at a reduced dose of 200 mg b.i.d. at Day 3 after completion of local ablation and was increased to the full dose of 400 mg b.i.d. at Day 10. In case of toxicity, the sorafenib dose was modified according to pre-defined dosing guidelines. In brief, the lowest accepted dose was 200 mg b.i.d. on alternate days. Following the resolution of toxicities, maintaining the highest tolerable dose level was attempted with a stepwise dose re-escalation (Fig. S1). Treatment was continued until tumour progression or the emergence of a drug-related adverse event requiring discontinuation. Patients in whom sorafenib treatment had to be permanently discontinued because of adverse events within the first 28 days of treatment were excluded.
Follow-up
Patients were followed at 2 months intervals until recurrence or death. At each follow-up visit, gadoxetic acid-enhanced MRI and contrast-enhanced CT were repeated to assess disease recurrence. During the trial, the recurrence was diagnosed by the local investigator and confirmed by one external radiologist from the core centre with experience in liver imaging.
Image assessment
After completion of the study, all follow-up CT and MRI images were collected centrally and independently read according to a pre-specified imaging charter by two radiologists blinded to all clinical information. A third reader served as an adjudicator and evaluated only the image sets in which the two readers have reached different conclusions with respect to recurrence or time point of recurrence.
A newly detected hepatic lesion on either CT or MRI was classified as HCC (evidence of recurrence) when the longest diameter was at least 10 mm and it showed the typical vascular pattern of HCC (wash-in and wash-out); or in case of absent typical vascular pattern, by evidence of at least 1 cm interval growth in subsequent scans. Recurrence time was recorded at first visualisation of that lesion without matching the criteria. Local recurrence was diagnosed if the centre of the new lesion was located within 5 mm of the thermal scar.
Statistical analysis
A one-sided log-rank test with an overall sample size of 290 subjects (of which 145 are in the local ablation + sorafenib group and 145 are in the local ablation + placebo group) achieves at least 80% power at a one-sided 0.05 significance level to detect a difference of 0.11160 between 0.52738 and 0.63898-the proportions with no recurrence in the local ablation + sorafenib group and the local ablation + placebo group after 12 months assuming median times with no recurrence of 13 and 18.57 months, respectively. The hazard ratio (HR) was assumed to be 0.70. An accrual period of approximately 52 months had been planned, and 50% of the enrolment had been expected to be complete when 50% of the accrual time had passed. A follow-up period of 24 months of the last patient enrolled adds up to a total study period of 76 months. A 10% loss from the local ablation + sorafenib group and a 10% loss from the local ablation + placebo group per year of follow-up were considered for the sample size calculation. However, because of difficulties in enrolling the targeted number of participants after reaching endpoints in the other arms of the trial and the publication of another randomised study showing no benefit of sorafenib treatment in the adjuvant setting, recruitment was stopped prematurely on 19 April 2016 after enrolment of 104 patients.
TTR as the primary endpoint was evaluated by the Kaplan–Meier method (product-limit method) to compute non-parametric estimates of the survivor functions. Patients who were lost to follow-up or withdrawn from the study were censored at the date of the last visit. Similarly, patients who had transplantation were censored for time-to-recurrence analysis at the time of the last available follow-up images, unless they had already recurrence. However, those patients were followed until death or the end of the trial for overall survival analysis. Subjects alive who did not have any follow-up imaging were censored 1 day after the date of randomisation for TTR evaluation. Superiority of local ablation + sorafenib could be concluded when the one-sided log-rank test was significant. All subgroup analyses and other study testing were considered exploratory with α = 5%. All safety analyses were performed by study group and treatment arm.
Subgroup analyses
As pre-defined, separate analyses were performed (a) for patients complying with the Milan criteria, (b) patients outside the Milan criteria, (c) patients with complete local ablation after the first (one or two) local ablation session(s), and (d) patients requiring an additional local ablation procedure because of incomplete ablation. In addition, analyses according to age (<65 years, ≥65 years), sex (male), Child-Pugh class (A, B), hepatitis B (yes, no), hepatitis C (yes, no), alcoholic aetiology (yes, no), and lesion number (single, multiple) were done.
Results
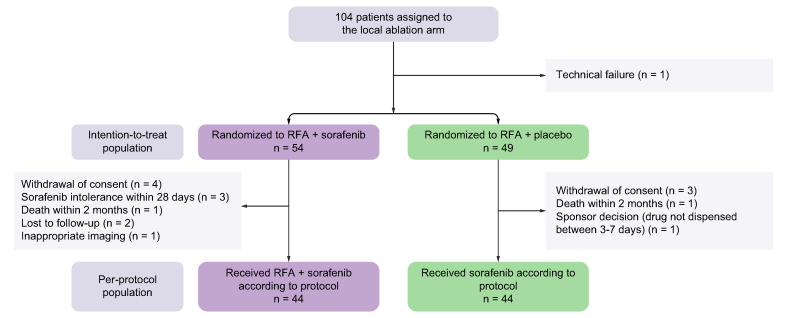
Patients were recruited into SORAMIC between 5 January 2011 and 19 April 2016, and 104 patients were allocated to the local ablation treatment arm. One patient was excluded from the study because of a technical failure (Fig. 1). In the intention-to-treat (ITT) population, 54 patients were randomised to local ablation + sorafenib and 49 to local ablation + placebo. The per-protocol (PP) population comprised 44 patients randomised to local ablation + sorafenib and 44 patients randomised to local ablation + placebo. Reasons for protocol violation were in the local ablation + sorafenib arm: withdrawal of consent, four; sorafenib intolerance within first 28 days, three; death before first follow-up, one; lost to follow-up, two; inappropriate follow-up imaging, one; and in the local ablation + placebo arm: withdrawal of consent, three; death before first follow-up, one; problem with drug dispensation at 3–7 days, one. Eighty-one patients underwent a single session of local ablation, 21 patients two sessions, and one patient three sessions.
Fig. 1.
CONSORT diagram.
CONSORT, consolidated standards of reporting trials; RFA, radiofrequency ablation.
Baseline characteristics of the ITT and PP population are summarised in Table 1. Most patients were male. Alcoholic liver disease was the main cause of HCC in both groups. Most patients had good liver function corresponding to Child-Pugh class A.
Table 1.
Baseline characteristics.
| ITT population |
PP population |
|||
|---|---|---|---|---|
| RFA + sorafenib (n = 54) | RFA + placebo (n = 49) | RFA + sorafenib (n = 44) | RFA + placebo (n = 44) | |
| Age, years: median (IQR) | 65.5 (10.8) | 68 (12) | 66 (11) | 68 (13.5) |
| Age, ≥65 years | 28 (52) | 32 (65) | 23 (52) | 29 (66) |
| Sex, male | 45 (83.3) | 45 (91.8) | 37 (84.1) | 40 (90.1) |
| Cirrhosis | 51 (94.4) | 42 (85.7) | 43 (97.7) | 37 (84.1) |
| ECOG | ||||
| 0 | 49 (90.7) | 45 (91.8) | 40 (90.1) | 40 (90.1) |
| 1 | 5 (9.3) | 4 (8.2) | 4 (9.9) | 4 (9.9) |
| Alcoholic aetiology | 32 (59.2) | 22 (44.8) | 29 (65.9) | 18 (40.9) |
| Hepatitis | ||||
| B | 3 (5.5) | 4 (8.1) | 2 (4.5) | 3 (6.8) |
| C | 10 (18.5) | 12 (24.4) | 6 (13.6) | 11 (25) |
| Child-Pugh | ||||
| A | 47 (87.1) | 44 (89.7) | 38 (86.3) | 39 (88.6) |
| B | 7 (12.9) | 5 (10.3) | 6 (13.7) | 5 (11.4) |
| Number of lesions | ||||
| 1 | 33 (61.1) | 34 (69.4) | 27 (61.4) | 30 (68.1) |
| 2 | 15 (27.8) | 6 (12.2) | 12 (27.2) | 6 (13.6) |
| 3 | 4 (7.4) | 6 (12.2) | 3 (6.8) | 5 (11.4) |
| 4 | 2 (3.7) | 3 (6.1) | 2 (4.5) | 3 (6.8) |
| Number of local ablation sessions | ||||
| 1 | 42 (77.7) | 39 (79.6) | 32 (72.7) | 34 (77.3) |
| 2 | 11 (20.3) | 10 (20.4) | 11 (25) | 10 (22.7) |
| 3 | 1 (1.9) | 1 (2.3) | ||
| Maximum tumour diameter | 24.5 (12-46) | 25 (10-50) | 24.5 (12-46) | 25 (12-50) |
| Albumin (g/dl)∗ | 38.3 (6.6) | 39.7 (5.4) | 38.5 (6.8) | 39.7 (5.3) |
| Total bilirubin (μmol/L)∗ | 19.2 (11.5) | 18.4 (10.5) | 19.2 (11.8) | 18.6 (10.6) |
| Alpha-foetoprotein (ng/ml) | 7 (0.01-6,499) | 8 (1.2-4,450) | 8.1 (0.01-6,499) | 7.6 (1.2-4,450) |
The data are represented as numbers (percentages).
ECOG, Eastern Cooperative Oncology Group; ITT, intention to treat; PP, per protocol; RFA, radiofrequency ablation.
Mean (SD).
Follow-up and events
The median follow-up duration for TTR was 8.0 months (IQR 3.6–10.8 months) in the ITT population and 9.3 months (IQR 4.5–12.6 months) in the PP population. Blinded follow-up read identified 32 recurrences (15 [34.1%] in the sorafenib group and 17 [38.6%] in the placebo group) in the PP population (n = 88). At the end of the study, 38 deaths (16 in the sorafenib group and 22 in the placebo group) in the ITT population and 34 deaths (14 in the sorafenib group and 20 in the placebo group) in the PP population had occurred.
Time-to-recurrence
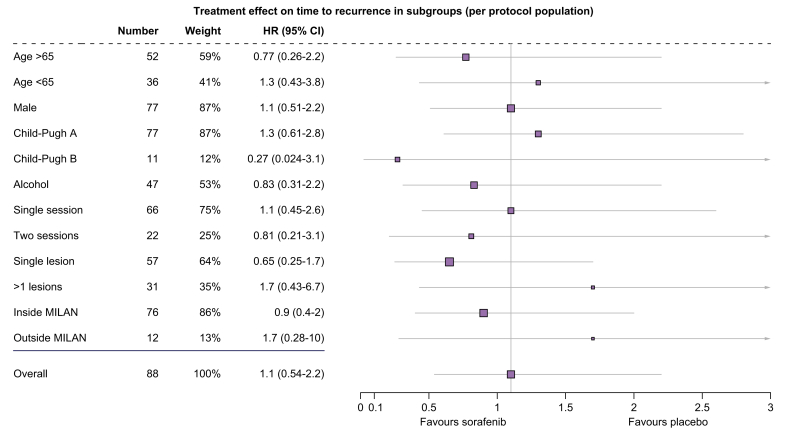
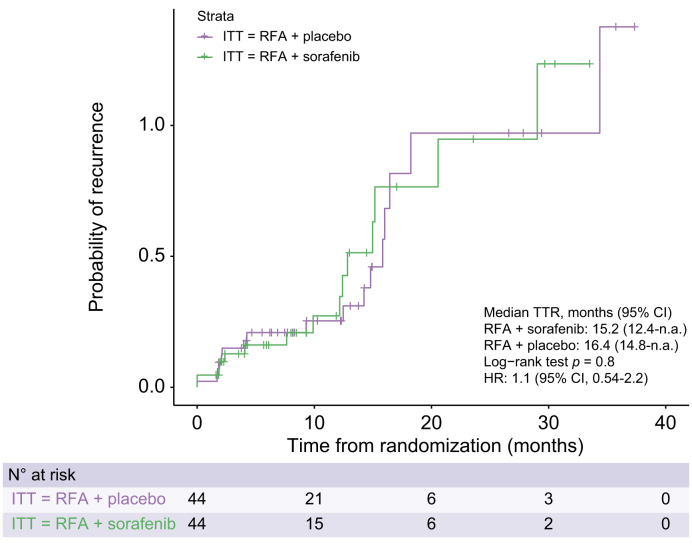
There was no significant treatment effect of sorafenib on TTR in the ITT (HR 1.1; 95% CI 0.53–2.2; p = 0.82) and the PP population (HR 1.1; 95% CI 0.54–2.2; p = 0.8). Median TTR was 15.2 months (95% CI 12.4–not applicable [n.a.]) in the sorafenib group and 16.4 months (95% CI, 14.8–n.a.) in the placebo group. (HR 1.1; 95% CI 0.53–2.2; p = 0.82; shown in Fig. S2). The 6-, 12-, and 24-month recurrence rates calculated via Kaplan–Meier curves were 18.8%, 22.4%, and 62.1% for the placebo arm and 14.9%, 23.9%, and 61.2% for the sorafenib group, respectively. Analysis of TTR according to blinded read, in subgroups defined by baseline stratification factor (number of local ablation sessions) as well as age, sex, Child-Pugh class, underlying aetiology (hepatitis B, hepatitis C, and alcoholic liver disease), lesion number (singe vs. multiple), Milan criteria (inside vs. outside), showed no significant benefit from sorafenib treatment compared with placebo across subgroups (Fig. 2). Similar to blinded read, assessment by investigators revealed the median TTR was 20.5 (95% CI, 15.1–29.8) months in the sorafenib group and 18.4 (95% CI, 13.8–29.1) months in the placebo group of the PP population, and the difference was not significant (p = 0.91; Fig. 3).
Fig. 2.
Forest plot of factors associated with time-to-recurrence in patients treated with RFA + sorafenib and RFA + placebo.
Subgroup analysis of time-to-recurrence by Cox regression based on independent assessment. The group effect was calculated with a Cox proportional-hazards model with hazard ratio (HR) and 95% confidence intervals. There was no significant difference in time-to-recurrence in any subgroup analyses. RFA, radiofrequency ablation.
Fig. 3.
Time-to-recurrence in PP population based on independent assessment.
Kaplan–Meier curves comparing time-to-recurrence between two treatment arms. No significant difference (p = 0.8, log-rank test). HR, hazard ratio; ITT, intention to treat; PP, per protocol; RFA, radiofrequency ablation; TTR, time-to-recurrence.
In the PP population, local recurrence was diagnosed in 11 of 136 lesions were treated (i.e. 91.9% local control rate). There was no difference in local recurrence rate between the sorafenib group (6/69, 8.6%) and the placebo group (5/67, 5.9%; p = 0.792).
Overall survival
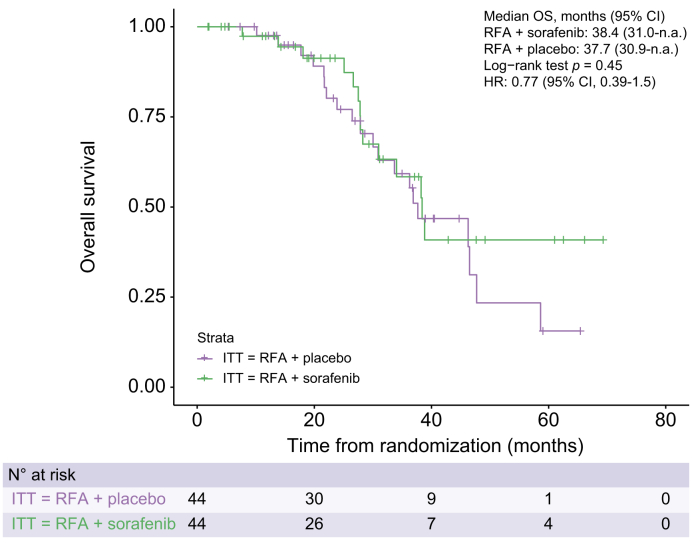
In the ITT population, the median overall survival was 38.4 (95% CI, 28.3–n.a.) months in the sorafenib arm and 36.9 (95% CI, 30.0–58.6) months in the placebo arm. There was no significant effect of sorafenib on overall survival (HR 0.81; 95% CI 0.42–1.5; p = 0.51; Fig. S3). Similarly, in the PP population, there was no significant difference (HR 0.77; 95% CI 0.39–1.5; p = 0.45; Fig. 4) in the median overall survival of the sorafenib arm (38.4 months, [95% CI, 31.0–n.a.]) and the placebo arm (37.7 months, [95% CI, 30.9–n.a.]).
Fig. 4.
Overall survival in PP population.
Kaplan–Meier curves comparing overall survival between two treatment arms. No significant difference (p = 0.45, log-rank test). HR, hazard ratio; ITT, intention to treat; n.a., not applicable; OS, overall survival; PP, per protocol; RFA, radiofrequency ablation.
Safety
Patients had received sorafenib for a mean number of 240.6 ± 233.8 days (range: 3 days to 33 months) and placebo for a mean number of 411.3 ± 299.3 days (range: 24 days to 37 months).
The number of treatment days was significantly higher under placebo than sorafenib (p = 0.002). Additionally, the daily dose was higher in the placebo group (mean 409.2 ± 258.1 mg with sorafenib vs. 701.0 ± 195.5 mg with placebo; p <0.001). Dose reduction as a result of adverse events were more common in the sorafenib arm (79.6% vs. 30.6%, p <0.001).
Fifty (92.5%) of 54 patients who received sorafenib and 35 (71.4%) of 49 patients who received placebo had an adverse event of any grade. Drug-related adverse events were more common in the sorafenib arm compared with the placebo arm (81.4% vs. 55.1%, p = 0.003). The most common adverse events in the sorafenib arm were hand-foot reaction, diarrhoea, and hypertension (Table 2). Drug-related adverse events of grade 3–4 were reported more commonly in sorafenib arm (61.1% vs. 12.2%, p <0.001). Drug-related serious adverse events were seen in 22 (40.7%) patients in the sorafenib arm and 12 (24.4%) patients in the placebo arm. Two patients died because of grade 5 adverse events. One patient randomised to the sorafenib arm died as a result of haemorrhagic complications related to intraoperative local ablation. The other patient who was randomised to the placebo arm died after pulmonary embolism following surgery for a non-tumour related vertebra fracture 2 months after randomisation.
Table 2.
Adverse events and drug-related adverse events occurring in ≥5% of the patients.
| RFA + placebo (n = 49) |
RFA + sorafenib (n = 54) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug-related |
All |
Drug-related |
All |
|||||||||
| All | 3 | 4 | All | 3 | 4 | All | 3 | 4 | All | 3 | 4 | |
| Total | 27 (55.1) | 6 (12.2) | — | 35 (71.4) | 19 (38.7) | 1 (2) | 44 (81.4) | 31 (57.4) | 2 (3.7) | 50 (92.5) | 39 (72.2) | 3 (5.5) |
| Blood and lymphatic | ||||||||||||
| Anaemia | — | — | — | — | — | — | 3 (5.5) | — | — | 5 (9.2) | 1 (1.8) | 1 (1.8) |
| Constitutional symptoms | ||||||||||||
| Asthenia | 1 (2) | — | — | 2 (4) | — | — | 3 (5.5) | 1 (1.8) | — | 3 (5.5) | 1 (1.8) | — |
| Fatigue | 6 (12.2) | 1 (2) | — | 7 | 1 (2) | — | 7 (12.9) | 1 (1.8) | — | 8 (14.8) | 1 (1.8) | — |
| Dermatological events | ||||||||||||
| Alopecia | — | — | — | — | — | — | 7 (12.9) | — | — | 7 (12.9) | — | |
| Hand-foot skin reaction | 5 (10.2) | 1 (2) | — | 5 (10.2) | 1 (2) | — | 14 (25.9) | 11 (20.3) | — | 14 (25.9) | 11 (20.3) | — |
| Gastrointestinal symptoms | ||||||||||||
| Abdominal pain | 1 (0.2) | 1 (2) | — | 3 | 1 (2) | — | 3 (5.5) | — | — | 4 (7.4) | — | |
| Ascites | 3 | — | — | 4 (8.1) | — | — | 1 (1.8) | — | — | 1 (1.8) | — | |
| Diarrhoea | 8 | 5 (10.2) | — | 9 | 5 (10.2) | — | 17 (31.4) | 4 (7.4) | — | 19 (35.1) | 5 (9.2) | — |
| Nausea | 1 (2) | — | — | 1 (2) | — | — | 4 (7.4) | 1 (1.8) | — | 4 (7.4) | 1 (1.8) | — |
| General disorders | ||||||||||||
| General deterioration | — | — | — | — | — | — | 4 (7.4) | 3 (5.5) | — | 5 (9.2) | 4 (7.4) | |
| Laboratory investigations | ||||||||||||
| Decreased platelets | 4 (8.1) | 2 (4) | — | 4 (8.1) | 2 (4) | — | 1 (1.8) | — | — | 4 (7.4) | 1 (1.8) | — |
| Respiratory | ||||||||||||
| Cold | — | — | — | 2 (4) | — | — | — | — | — | 3 (5.5) | — | |
| Dyspnoea | 1 (2) | — | — | 1 (2) | — | — | 1 (1.8) | — | — | 4 (7.4) | 1 (1.8) | — |
| Pleural effusion | — | — | — | 2 (4) | — | — | — | — | — | 4 (7.4) | 1 (1.8) | — |
| Vascular disorders | ||||||||||||
| Hypertension | 1 (2) | 1 (2) | — | 1 (2) | 1 (2) | — | 9 | 6 (11.1) | — | 12 | 6 (11.1) | — |
The data are represented as numbers (percentages).
Quality of life
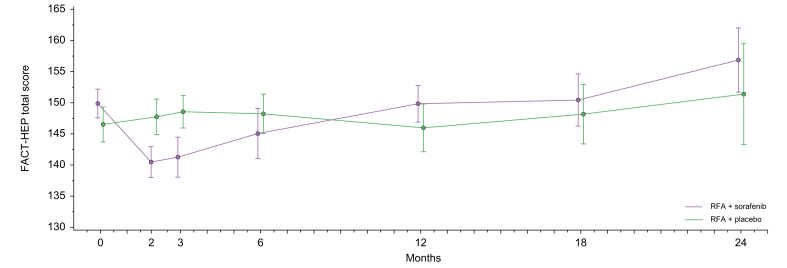
In the second month, scores of physical well-being (p = 0.004) and additional concerns (p = 0.012) were significantly lower in the sorafenib group than the placebo group in the ITT population (Table 3). Furthermore, there was a similar but marginally non-significant difference between the two groups in functional well-being score (p = 0.059) and FACT-HEP total score (p = 0.061) in the second month (Fig. 5). Similarly, there was a marginally non-significant decline in Functional Assessment of Cancer Therapy - General (FACT-G) total score in the sorafenib arm at the third month (Fig. S4). Also, the physical well-being score (p = 0.007) was lower in the sorafenib arm in the third month. After the third month, no clinically meaningful differences were noted between the sorafenib and placebo groups.
Table 3.
Results of quality-of-life in the ITT population.
| Local ablation | RFA + sorafenib |
RFA + placebo |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | p value | |
| Physical well-being | ||||||||||
| Baseline | 49 | 25.4 | 3.2 | 44 | 24.4 | 3.8 | 93 | 25.0 | 3.5 | 0.2440 |
| 2 months | 39 | 21.8 | 5.0 | 34 | 24.9 | 3.6 | 73 | 23.2 | 4.6 | 0.0049 |
| 3 months | 34 | 21.6 | 5.4 | 35 | 24.7 | 3.3 | 69 | 23.2 | 4.7 | 0.0074 |
| 6 months | 26 | 22.3 | 5.9 | 36 | 23.9 | 4.2 | 62 | 23.2 | 5.0 | 0.4817 |
| 12 months | 17 | 23.8 | 4.9 | 25 | 23.9 | 4.3 | 42 | 23.8 | 4.5 | 0.7667 |
| 18 months | 12 | 25.0 | 2.4 | 13 | 25.3 | 2.9 | 25 | 25.1 | 2.6 | 0.4925 |
| 24 months | 7 | 25.1 | 2.9 | 7 | 24.3 | 5.2 | 14 | 24.7 | 4.1 | 0.9476 |
| Social/family well-being | ||||||||||
| Baseline | 50 | 22.4 | 6.3 | 42 | 22.5 | 5.3 | 92 | 22.5 | 5.8 | 0.7586 |
| 2 months | 39 | 22.2 | 6.0 | 33 | 22.2 | 5.8 | 72 | 22.2 | 5.9 | 0.9278 |
| 3 months | 33 | 21.5 | 5.5 | 34 | 23.2 | 4.9 | 67 | 22.4 | 5.2 | 0.0825 |
| 6 months | 24 | 23.4 | 2.6 | 36 | 22.7 | 5.7 | 60 | 23.0 | 4.7 | 0.6716 |
| 12 months | 16 | 22.2 | 3.5 | 24 | 22.5 | 5.8 | 40 | 22.4 | 4.9 | 0.3973 |
| 18 months | 12 | 22.1 | 3.3 | 12 | 22.8 | 4.8 | 24 | 22.4 | 4.1 | 0.6927 |
| 24 months | 7 | 22.9 | 2.1 | 6 | 24.3 | 4.1 | 13 | 23.5 | 3.1 | 0.4335 |
| Emotional well-being | ||||||||||
| Baseline | 49 | 19.2 | 3.6 | 43 | 18.0 | 3.8 | 92 | 18.6 | 3.7 | 0.1169 |
| 2 months | 38 | 19.3 | 3.0 | 33 | 19.6 | 2.9 | 71 | 19.5 | 3.0 | 0.6846 |
| 3 months | 34 | 18.8 | 4.1 | 35 | 19.8 | 2.9 | 69 | 19.3 | 3.6 | 0.4769 |
| 6 months | 27 | 19.1 | 4.4 | 36 | 18.6 | 4.1 | 63 | 18.8 | 4.2 | 0.5529 |
| 12 months | 17 | 19.7 | 4.7 | 25 | 19.8 | 2.9 | 42 | 19.8 | 3.7 | 0.6145 |
| 18 months | 12 | 20.2 | 1.6 | 13 | 19.8 | 3.7 | 25 | 20.0 | 2.8 | 0.7254 |
| 24 months | 7 | 21.0 | 1.2 | 7 | 20.3 | 3.9 | 14 | 20.6 | 2.8 | 0.6597 |
| Functional well-being | ||||||||||
| Baseline | 50 | 19.2 | 5.8 | 43 | 18.6 | 5.0 | 93 | 18.9 | 5.4 | 0.3998 |
| 2 months | 40 | 17.3 | 5.2 | 33 | 19.6 | 4.0 | 73 | 18.3 | 4.8 | 0.0596 |
| 3 months | 34 | 18.2 | 5.6 | 35 | 19.5 | 4.3 | 69 | 18.9 | 5.0 | 0.2853 |
| 6 months | 27 | 18.2 | 5.1 | 36 | 20.2 | 4.6 | 63 | 19.3 | 4.9 | 0.1158 |
| 12 months | 16 | 20.1 | 3.4 | 25 | 18.5 | 5.0 | 41 | 19.1 | 4.5 | 0.2607 |
| 18 months | 12 | 20.7 | 4.5 | 13 | 19.3 | 4.9 | 25 | 20.0 | 4.7 | 0.4819 |
| 24 months | 7 | 21.4 | 4.6 | 7 | 20.1 | 5.6 | 14 | 20.8 | 5.0 | 0.7475 |
| FACT-G total score | ||||||||||
| Baseline | 48 | 85.8 | 12.0 | 41 | 83.0 | 13.9 | 89 | 84.5 | 12.9 | 0.3586 |
| 2 months | 37 | 81.0 | 12.5 | 32 | 85.9 | 12.4 | 69 | 83.3 | 12.6 | 0.1105 |
| 3 months | 33 | 81.2 | 13.0 | 34 | 87.0 | 11.7 | 67 | 84.2 | 12.6 | 0.0617 |
| 6 months | 24 | 84.0 | 13.9 | 36 | 85.3 | 14.2 | 60 | 84.8 | 14.0 | 0.6891 |
| 12 months | 16 | 87.8 | 7.8 | 24 | 84.2 | 14.0 | 40 | 85.7 | 11.9 | 0.3068 |
| 18 months | 12 | 87.9 | 10.0 | 12 | 86.1 | 11.9 | 24 | 87.0 | 10.8 | 0.7037 |
| 24 months | 7 | 90.4 | 9.5 | 6 | 87.3 | 16.0 | 13 | 89.0 | 12.4 | 0.6703 |
| Additional concerns-HCS subscale | ||||||||||
| Baseline | 50 | 63.8 | 5.9 | 44 | 63.6 | 5.9 | 94 | 63.7 | 5.9 | 0.8197 |
| 2 months | 39 | 59.0 | 5.3 | 34 | 62.3 | 5.7 | 73 | 60.5 | 5.7 | 0.0127 |
| 3 months | 32 | 59.1 | 8.6 | 35 | 61.7 | 5.7 | 67 | 60.5 | 7.3 | 0.2605 |
| 6 months | 26 | 60.1 | 8.3 | 36 | 62.9 | 6.3 | 62 | 61.7 | 7.3 | 0.1551 |
| 12 months | 16 | 62.0 | 5.3 | 25 | 62.1 | 7.1 | 41 | 62.1 | 6.4 | 0.6974 |
| 18 months | 12 | 62.6 | 5.9 | 13 | 62.4 | 6.5 | 25 | 62.5 | 6.1 | 0.9384 |
| 24 months | 7 | 66.4 | 4.4 | 7 | 64.6 | 4.9 | 14 | 65.5 | 4.6 | 0.4817 |
| FACT-HEP total score | ||||||||||
| Baseline | 49 | 149.9 | 16.4 | 41 | 146.5 | 18.3 | 90 | 148.4 | 17.3 | 0.3621 |
| 2 months | 37 | 140.5 | 15.4 | 32 | 147.7 | 16.2 | 69 | 143.9 | 16.1 | 0.0611 |
| 3 months | 31 | 141.3 | 18.2 | 34 | 148.6 | 15.5 | 65 | 145.1 | 17.1 | 0.1048 |
| 6 months | 24 | 145.1 | 19.9 | 36 | 148.2 | 19.1 | 60 | 147.0 | 19.3 | 0.5922 |
| 12 months | 16 | 149.8 | 11.9 | 24 | 146.0 | 18.9 | 40 | 147.5 | 16.4 | 0.5808 |
| 18 months | 12 | 150.4 | 14.7 | 12 | 148.2 | 16.7 | 24 | 149.3 | 15.4 | 0.7279 |
| 24 months | 7 | 156.8 | 13.7 | 6 | 151.4 | 19.9 | 13 | 154.3 | 16.3 | 0.5723 |
| TOI score | ||||||||||
| Baseline | 49 | 108.4 | 12.6 | 43 | 106.6 | 12.8 | 92 | 107.6 | 12.7 | 0.4620 |
| 2 months | 38 | 98.6 | 12.3 | 33 | 106.6 | 11.6 | 71 | 102.3 | 12.6 | 0.0026 |
| 3 months | 32 | 99.0 | 17.7 | 35 | 105.9 | 11.4 | 67 | 102.6 | 15.0 | 0.1226 |
| 6 months | 26 | 100.7 | 16.5 | 36 | 106.9 | 13.4 | 62 | 104.3 | 14.9 | 0.1708 |
| 12 months | 16 | 107.0 | 9.2 | 25 | 104.5 | 14.0 | 41 | 105.5 | 12.2 | 0.7586 |
| 18 months | 12 | 108.2 | 11.4 | 13 | 107.0 | 12.1 | 25 | 107.6 | 11.5 | 0.8026 |
| 24 months | 7 | 113.0 | 11.2 | 7 | 109.0 | 14.0 | 14 | 111.0 | 12.3 | 0.5686 |
The Student t test and Mann–Whitney U test were used to compare the quality-of-life of treatment arms appropriately after the normality of the data was determined using the Kolmogorov-Smirnov test. Values of p in bold denote statistical significance.
FACT-G, functional assessment of cancer therapy - general; FACT-HEP, functional assessment of cancer therapy – hepatobiliary; HCS, hepatobiliary cancer subscale; RFA, radiofrequency ablation; TOI, trial outcome index.
Fig. 5.
Longitudinal analysis of FACT-HEP questionnaire for ITT population.
The blue line represents the RFA + sorafenib arm and red line represents the RFA + placebo arm. FACT-HEP, functional assessment of cancer therapy-hepatobiliary; ITT, intention-to-treat; RFA, radiofrequency ablation.
Discussion
In this placebo-controlled randomised trial, which terminated early because of the lack of recruitment after reaching endpoints in other arms of the trial, adjuvant sorafenib treatment after local ablation failed to meet the primary and secondary endpoints of TTR and the local control rate. Additionally, there was no significant improvement in overall survival after sorafenib treatment.
Currently, no adjuvant therapy is recommended in patients with HCC who received surgical resection or local ablation.1 There are currently ongoing randomised-controlled trials evaluating immune checkpoint inhibitors in adjuvant setting, after showing safety in single-arm studies.[12], [13], [14] The STORM trial, a placebo-controlled phase III trial comparing testing the efficiency of adjuvant sorafenib, did not show an improvement in relapse free-survival (RFS; 33.3 vs. 33.7 months, HR 0.94; 0.78–1.13) and overall survival (HR 0.99; 0.76–1.33).15 Similar to this trial, our results have demonstrated no additional benefit from adjuvant sorafenib treatment after local ablation. The STORM trial recruited patients who underwent either local ablation (RFA or ethanol injection, n = 214) or resection (n = 900) at 202 centres in 28 countries. Complete resection or ablation in each patient was confirmed 3–7 weeks after the procedure with imaging, and randomisation was done 6–12 weeks after the last treatment session with risk stratification based on images up to 4 months before treatment. Similar to the overall study population of the STORM trial, subanalysis of patients who underwent local ablation revealed no difference in RFS between sorafenib and placebo arms. In the STORM trial, 106 patients were randomised to the local ablation plus sorafenib arm and 108 patients to the local ablation plus placebo arm, and RFS was 19.6 and 22.8 months (p = 0.97), respectively. As relevant differences to STORM, in the SORAMIC trial, all patients underwent CT and MRI with gadoxetic-acid according to a standardised protocol, and local ablation was performed within 1 month after imaging. Complete ablation was performed with contrast-enhanced imaging at the end of the procedure, and sorafenib treatment was started on Day 3 after the last ablation session. Additionally, the local ablation arm of the SORAMIC trial was conducted in a lower number of centres (n = 17) from Europe with high experience in local ablation in HCC, which provides fewer variations in technical approach as compared with the STORM trial. Furthermore, while 52.4% of our patients had alcoholic liver disease, 75% of the patients had viral hepatitis in the STORM trial. Thus, our study delivers important novel information on the adjuvant use of sorafenib and important data of a real adjuvant setting starting immediately after ablation with drug treatment and reports for the first-time results on local control rate.
TTR in our study (20.5 and 18.4 months in sorafenib and placebo arms) was similar or shorter than previously reported cohorts, being 18.2 months in the placebo arm of a vitamin K trial, 6 and 38.5 months after sorafenib or 35.8 months after placebo in the STORM trial.15 In SORAMIC, patients with up to four lesions smaller than 5 cm were allocated to local ablation, which also included patients with BCLC-B, who inherently have a higher risk of disease progression. In contrast, only BCLC-A patients were recruited in the STORM trial.
Similar to TTR, sorafenib treatment did not improve local tumour control rate compared with placebo. Local control rate was 91.9% in the overall study population, which is consistent with previous cohorts of local ablation in HCC with 90.4%,16 82.4%,17 90.0%,18 and 88.0%19 in the RFA arms of previous prospective ablation trials. However, SORAMIC has reported that the adjuvant sorafenib treatment has no beneficial effect on local control rate for the first time in the literature.
Adverse events reported during the trial were consistent with the safety profile of sorafenib. However, the rate of grade ≥3 drug-related (61.1%) and overall (81.4%) adverse events were higher compared with the sorafenib group of the palliative study-arm in SORAMIC trial (26.4% and 53.3%), as well as other similar cohorts (37.7% and 74.7% in SIRveNIB, 63% and 94% in SARAH, 39% and 98% in SHARP). This is probably because of the longer duration of sorafenib treatment in this cohort of patients with early HCC (240 days) than in patients with advanced HCC (i.e. 135 days in palliative cohort or SORAMIC).
An additional important result of our study was deterioration in the quality of life at 2 and 3 months after treatment start in patients receiving sorafenib compared with patients receiving placebo. Similar to our cohort, time to deterioration in global health status was 2.6 months in the sorafenib arm of the SARAH trial.20 Health-related quality of life analysis is important in the assessment of clinical benefit and should be taken into consideration in clinical decision-making. Additionally, public health agencies also consider results of quality-of-life analyses before approval of therapies.
The major limitation of our study was the small number of patients because of the early discontinuation of the recruitment after reaching endpoints in the other two arms of the trial and the publication of a similar negative trial (STORM trial). Although this published data potentially reduces the overall novelty of our results, our paper reports for the first time a cohort with early start of adjuvant sorafenib in a cohort of patients who received local ablation only in highly experienced European centres. Geographical differences also reflect patient characteristics, such as the main aetiology being alcoholic liver disease in our cohort. Additionally, our paper reports the local control rate after adjuvant sorafenib treatment for the first time in a cohort allowing a maximum tumour diameter beyond established guidelines for local ablation, with up to 5 cm instead of up to 3 cm recruited.21 Up to 5 cm was allowed for inclusion to mirror current clinical practice at the time of protocol development. In light of guidelines and likely clinical practice today, inclusion of patients with up to four lesions with a diameter of ≤5 cm may suggest a limitation for a local ablation cohort. The addition of BCLC-B patients also explains the relatively short median overall survival in this trial with an adjuvant setting. However, our local control rate was favourable with >90% in both arms. The study endpoint had been time to tumour recurrence, and the risk of undertreatment at ablation served as an additional risk factor for recurrence.
In conclusion, adjuvant sorafenib treatment did not improve tumour control in terms of TTR or local tumour control rate, and overall survival after local ablation for HCC compared with placebo in this early terminated randomised trial.
Financial support
SORAMIC is an investigator-initiated trial sponsored by the University of Magdeburg. Financial support was granted by Sirtex Medical and Bayer Healthcare.
Authors’ contributions
Conception and design of the study: MS, OÖ, PM, JR. Generation, collection, assembly, analysis and/or interpretation of data: KS, TB, CL, HJK, OvD, MRÜ, NBK, ENdT, AA, AT, J-PB, BP, BS, MP. Drafting or revision of the manuscript: KS, TB, CL, HJK, OvD, MRÜ, NBK, ENdT, AA, AT, J-PB, BP, BS, MP. Approval of the final version of the manuscript: KS, TB, CL, HJK, OvD, MRÜ, NBK, ENdT, AA, AT, J-PB, BP, BS, MP.
Data availability statement
The data that support the findings of this study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of interest
MS: Personal fees: Bayer, Sirtex. OÖ: Honoraria: Bayer. PM: Grants: Bayer, Sirtex. TB: Grants/research support: Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Novartis, Sequana Medical. Honoraria or consultation fees: Abbvie, Alexion, Bayer, Gilead, Eisai, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi. ENdT: Consultant fees: AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, and Roche. Travel fees: Arqule, Astrazeneca, BMS, Bayer, Celsion, and Roche and lecture honoraria from BMS and Falk. Grants: Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. BS: Consultancy fees: Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, BTG, Eisai, Eli Lilly, H3 Biomedicine, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo. Speaker fees: Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo. Grants (to Institution): BMS and Sirtex Medical. MP: Grants: Sirtex, Bayer; Personal fees: Sirtex. JR: Grants: Sirtex, Bayer; Personal fees: Sirtex, Bayer.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100699.
Supplementary data
The following are the supplementary data to this article:
References
- 1.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Li L., Zhang J., Liu X., Li X., Jiao B., Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51–58. doi: 10.1111/j.1440-1746.2011.06947.x. [DOI] [PubMed] [Google Scholar]
- 3.Lim K.C., Chow P.K., Allen J.C., Siddiqui F.J., Chan E.S., Tan S.B. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V., Romito R., Schiavo M., Mariani L., Camerini T., Bhoori S., et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 5.Lo C.M., Liu C.L., Chan S.C., Lam C.M., Poon R.T., Ng I.O., et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H., Shiratori Y., Kudo M., Shiina S., Mizuta T., Kojiro M., et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532–540. doi: 10.1002/hep.24430. [DOI] [PubMed] [Google Scholar]
- 7.Okita K., Izumi N., Matsui O., Tanaka K., Kaneko S., Moriwaki H., et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191–202. doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Ricke J., Steffen I.G., Bargellini I., Berg T., Jaureguizar J.I.B., Gebauer B., et al. Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricke J., Klümpen H.J., Amthauer H., Bargellini I., Bartenstein P., de Toni E.N., et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Knox J., Cheng A., Cleary S., Galle P., Kokudo N., Lencioni R., et al. A phase 3 study of durvalumab with or without bevacizumab as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after curative hepatic resection or ablation: EMERALD-2. Ann Oncol. 2019;30:iv59–iv60. [Google Scholar]
- 13.Hack S.P., Spahn J., Chen M., Cheng A.-L., Kaseb A., Kudo M., et al. IMbrave 050: a phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975–989. doi: 10.2217/fon-2020-0162. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M., Ueshima K., Nakahira S., Nishida N., Ida H., Minami Y., et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): a phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. J Clin Oncol. 2021;39(Suppl):4070. [Google Scholar]
- 15.Bruix J., Takayama T., Mazzaferro V., Chau G.-Y., Yang J., Kudo M., et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 16.Feng K., Yan J., Li X., Xia F., Ma K., Wang S., et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen K., Chen G., Wang H., Li H., Xiao J., Duan X., et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol. 2014;61:1304–1311. doi: 10.1016/j.jhep.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Wang C., Wang H., Yang W., Hu K., Xie H., Hu K.Q., et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 19.Vietti Violi N., Duran R., Guiu B., Cercueil J.P., Aubé C., Digklia A., et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325. doi: 10.1016/S2468-1253(18)30029-3. [DOI] [PubMed] [Google Scholar]
- 20.Pereira H., Bouattour M., Dioguardi Burgio M., Assenat E., Grégory J., Bronowicki J.P., et al. Health-related quality of life in locally advanced hepatocellular carcinoma treated by either radioembolisation or sorafenib (SARAH trial) Eur J Cancer. 2021;154:46–56. doi: 10.1016/j.ejca.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available but are available from the corresponding author on reasonable request.