Abstract
Lifestyle changes should be the main basis for any treatment for metabolic dysfunction–associated fatty liver disease (MAFLD), aiming to increase energy expenditure, reduce energy intake and improve the quality of nutrients consumed. As it is a multifactorial disease, approaches such as physical exercise, a better dietary pattern, and possible pharmacological intervention are shown to be more efficient when used simultaneously to the detriment of their applications. The main treatment for MAFLD is a lifestyle change consisting of diet, activity, exercise, and weight loss. The variables for training prescription such as type of physical exercise (aerobic or strength training), the weekly frequency, and the intensity most indicated for the treatment of MAFLD remain uncertain, that is, the recommendations must be adapted to the clinical conditions comorbidities, and preferences of each subject in a way individual. This review addresses recent management options for MAFLD including diet, nutrients, gut microbiota, and physical exercise.
Keywords: dietary pattern, lifestyle changes, metabolic dysfunction–associated fatty liver disease (MAFLD), nonalcoholic fatty liver disease (NAFLD), physical exercise
Introduction
Fatty liver is a clinicopathological condition characterized by the accumulation of lipids within hepatocytes and encompasses a wide spectrum of diseases. It is one of the most common forms of liver disease primarily related to the progressive increase in obesity in the world.1 Initially, it was considered a benign liver disease; however, it is currently known that it is a multifactorial disease that involves environmental and genetic factors and can progress to more severe forms, such as cirrhosis and hepatocellular carcinoma (HCC) in patients without a history of alcohol consumption.2 Recently, Eslam et al.3 proposed to redefine the diagnostic criteria to cover fatty liver associated with metabolic dysfunction, including changing the nomenclature to metabolic dysfunction–associated fatty liver disease (MAFLD). Obesity, overweight, type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS), and genetic predisposition are the main risk factors.4 The prevalence of MAFLD has increased in all populations in parallel with the increase in obesity rates worldwide, mainly in the western world. However, these values can change significantly according to the diagnostic methods used and the population studied.5
A recent meta-analysis support previous studies and reports that the prevalence of MAFLD has been affecting more than a third of the global population,6 while Liu et al.7 estimated a global MAFLD prevalence as 50.7% among overweight/obese adults.
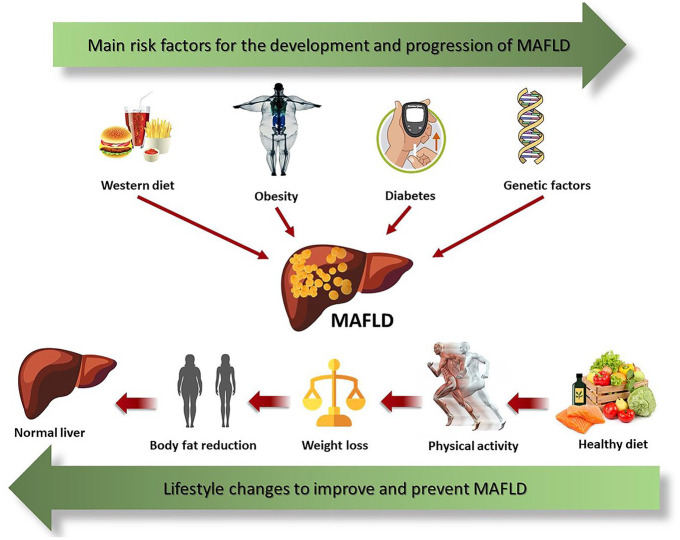
The perfect non-pharmacological strategy for the management of MAFLD is not yet well defined. However, there is a consensus that excessive consumption of carbohydrates and lipids is harmful.8 The high intake of carbohydrates (mainly soft drinks and products with added fructose syrup) and a high-fat diet have been identified as responsible for the development of MetS9 and the accumulation of fatty acids (FAs) in the liver,10 important predictors of MAFLD11 (Figure 1). To determine the contribution of the distribution of dietary macronutrients in the development and treatment of MAFLD, several studies have been conducted, but the results presented are still conflicting.12,13
Figure 1.
Genetic predisposition and environmental factors such as a diet rich in fats and simple sugars, mainly fructose, lead to weight gain, and the development of T2DM, important risk factors for MAFLD. Lifestyle changes including healthy eating and physical activity decrease weight and body fat mass which consequently improves MAFLD.
It has been shown that a sedentary lifestyle is an important factor in the predisposition of MetS, obesity, and T2DM.14 Studies show that people who have a physically active lifestyle develop less insulin resistance (IR) and T2DM than sedentary individuals.15,16 In addition, there is already a consensus that changes in lifestyle, including physical activity and weight loss, reduce risk factors for MAFLD.17 There is evidence that physical activity, induces beneficial metabolic effects, such as lowering blood glucose levels independent of weight loss.18 The effects of physical activity, strength training (weight training), and aerobics in the treatment of MAFLD have been evaluated in clinical trials and observational studies.19,20
Considering that so far data related to diet and lifestyle in this pathology using the current nomenclature are still scarce, in this review, we will use the term MAFLD/metabolic associated steatohepatitis (MASH) as a synonym for NAFLD/nonalcoholic steatohepatitis (NASH). In this review, we will present recent scientific evidence on the non-pharmacological treatment and management of MAFLD, including chapters on dietary patterns, foods, nutrients, and lifestyle.
Hypocaloric diet and MAFLD
Several studies demonstrate the association between caloric intake and liver fat content.21,22 Evidence shows that patients with MAFLD ingest significantly more calories; however, the proportion of macronutrients shows a short difference compared to the general composition of the diet of healthy individuals.23,24
Hypocaloric diets have been used to promote faster body weight loss and consequently a significant reduction in adipose tissue.25 It is postulated that low-calorie intake can exert metabolic programming and promote a more effective expenditure of body energy reserves, decreasing oxidative damage to cells.26 Hypocaloric diets can have different macronutrient distributions from each other and present distinct results. A low-carb diet could be more effective in reducing body weight than a low-fat diet. However, the patients have difficulty maintaining their weight in the long term and this weight loss is not sustained.25 Some authors show that restricting carbohydrate intake (<20 g/day) and keeping a caloric deficit of 30% (500–750 kcal/day) improves NAS (Nonalcoholic fatty liver disease Activity Score), serum liver enzymes and liver lipids, i.e. a caloric deficit can be considered an initial therapeutic approach for MAFLD. The recommendation is a daily intake of 1.200 kcal/day for women and 1.500 kcal/day for men. However, these values can be adjusted according to the caloric expenditure from your physical activity (women: 1.500 kcal/day and men: 1.800 kcal/day).2,27
Fat intake and MAFLD
Some studies have shown that MASH patients eat more saturated fat and cholesterol and less polyunsaturated fatty acids (PUFAs) than healthy individuals.28,29 In addition, Eslam et al.30 reported that cholesterol intake was higher in patients with lean MAFLD (Asian body mass index (BMI) < 23 kg/m² or non-Asian BMI < 25 kg/m²) than in obesity patients and MAFLD. A high-fat diet (55% of the total energy) has been shown to increase fasting insulin levels and the number of intrahepatic triglycerides (TGs), regardless of body weight changes. Although the high consumption of saturated FA stimulates endoplasmic reticulum stress and hepatocytes damage. Previous studies have shown that severe restriction of these FAs may not be healthy for patients with MAFLD, since excessive FA restriction can also increase serum TG levels and reduce high-density lipoprotein (HDL).31,32
On the contrary, oleic acid (monounsaturated FA) present in avocado and olive oil brings benefits to MAFLD patients as they reduce TG levels and low-density lipoprotein (LDL) cholesterol. Diets that have between 20% and 40% of monounsaturated fats of the total caloric value have shown benefits for patients with MAFLD, decreasing lipogenesis [reducing activation of sterol regulatory element–binding proteins (SREBP)] and increasing the oxidation of other FAs (by activating receptors activated by α and γ peroxisome proliferators).33
Carbohydrate intake and MAFLD
In the last decades, the consumption of sweetened and fatty foods has increased gradually. Data show that increased consumption of high–glycemic index sugars, like fructose, is directly related to a higher risk of developing T2DM, MetS, cardiovascular disease (CVD), and MAFLD.34
Studies show that higher dairy carbohydrate intake can harm MAFLD patients demonstrating an association with inflammation and disease progression.8,33 Diets with low carbohydrate intake (⩽45%) show positive results in body weight loss, improvement of metabolic parameters, and reduction of intrahepatic TG content in obese individuals.35
Recently, special attention has been given to Fibroblast growth factor 21 (FGF21) gene polymorphisms, an endocrine hormone produced in the liver that regulates energy metabolism and stress responses, and also seems to be regulated by nutritional factors.36,37 Although some experimental studies demonstrate elevated FGF21 levels are reported in metabolic diseases,38–41 the role of FGF21 in metabolic diseases is still unclear. However, genetic studies have shown that higher serum levels of FGF21 are associated with the minor rs838133 allele and higher consumption of carbohydrates, particularly sugar, and alcohol and lower consumption of proteins.42,43 Recently, Bayoumi et al. described that patients with MAFLD have higher liver levels of FGF21 than healthy individuals. These findings have been related to the minor allele in rs838133, which leads to alterations in the synthesis of FGF21, inducing peripheral resistance to this hormone, leading to the increased FA supply to the liver and reducing FA utilization.37 Although the role of this variant in MAFLD is still unclear, the authors have associated this allele with increased liver inflammation in animal models.37 On the contrary, experimental studies in rodents, non-human primates, and preclinical and clinical trials have demonstrated that FGF21 analogs induce weight loss, regulate hepatic metabolic pathways, protect against hepatic lipotoxicity, improve steatosis, reduce inflammation, and attenuate fibrogenesis in MASH models.44,45 In addition, some studies demonstrated reduction FGF21 levels in response to lifestyle modification and bariatric surgery.46,47
Among the carbohydrate types, fructose is the sugar most associated with MAFLD progression.48,49 In the 1960s, high-fructose corn syrup was introduced into the food industry as a substitute for sugar, thus increasing its intake. Fructose affects lipid metabolism and increases plasma TG levels, leading to de novo lipogenesis, hyperferritinemia, hyperuricemia, and IR. These changes increase the risk of MAFLD. It has also been described that high sugar consumption positively regulates fructose transport through the GLUT5 transporter, leading to increased hepatic fructose kinase levels regardless of excess energy intake. It has been shown that high-fructose consumption is associated with the prevalence of MAFLD in obese children and adolescents.50 These data are supported by evidence that daily consumption of a high-fructose intake is associated with a higher stage of liver fibrosis in young and elderly patients.51
It is also known that a high-fructose diet promotes intestinal inflammation, increased epithelial dysfunction, endotoxin release, and reduced tight junction proteins, regardless of the fat and calorie diet. These data show the negative impact of excess fructose intake on intestinal barrier function.52 There is a hypothesis that fructose can cause dysbiosis, increasing intestinal permeability and favoring the passage of endotoxins into the blood.
High-protein diet and MAFLD
There is a limited number of studies that elucidate the relationship between a high-protein diet and MAFLD. It is known that the quality, quantity, and composition of dietary proteins in the development and treatment of MAFLD is still not clear, but it is known that malnutrition due to protein deficiency can cause steatosis.53 Evidence suggests that a high-protein diet leads to hepatic lipid oxidation since hepatic catabolism of ingested amino acids is a process of intense energy expenditure. This may be effective in the treatment of MAFLD.54 The short-term protein supplementation showed improvement in lipid profile and hepatic steatosis in obese sedentary women.55 In addition, a hypocaloric high-protein diet is associated with improved glucose homeostasis, lipid profile, and reduced liver evidence. This evidence is consistent with the positive results of caloric restriction in the treatment of MetS and improvement in liver histology. In addition, when comparing different diets for the treatment of MASH (hyperproteic, hypolipidemic and low in carbohydrates) the results are equivalent, showing a reduction in intrahepatic fat, the enzyme alanine aminotransferase (ALT), visceral adiposity, in total weight and improvement in insulin sensitivity.23,56
The ideal dietary pattern aims to ensure both adequate protein intake and moderate caloric restriction. According to European Society Guidelines (EASL), although more evidence is lacking, special attention should be paid to the protein intake needed to maintain muscle mass, due to the potential risk of exacerbating sarcopenia during restrictive interventions for weight loss.2
Fiber intake and MAFLD
The dietary fibers can be grouped into soluble fibers (pectins, gums, mucilages, and hemicelluloses) present in fruits, barley, oats, and legumes such as beans, lentils, chickpeas, and insoluble fibers (cellulose, lignin, and some hemicelluloses and mucilages) present in wheat, grains, and vegetables.57 It has been shown that MASH patients consume less-complex carbohydrates, more fat, and less fiber than healthy individuals.58 Intake of dietary fiber, mainly derived from whole grains, has shown benefits in reducing comorbidities associated with MetS and MAFLD, reducing liver fat and inflammation.59
Mediterranean diet and MAFLD
The traditional Mediterranean diet (MD) is the most studied dietary pattern in the management of MAFLD. It shows a beneficial role in the metabolic profile and reduces the risk of CVD and T2DM, comorbidities associated with MAFLD. These results can be obtained regardless of body weight loss.32,60
The MD is composed of a main higher intake of vegetables, fruits, whole grains, olive oil, nuts, and white meat (fish and poultry) and low consumption of dairy products, red meat, processed foods, sugary foods, and moderate alcohol intake.61 Another characteristic of MD is the low intake of carbohydrates (a maximum of 40% of the total caloric value), especially simple and refined sugars (fructose and sucrose). Reduced intake of beverages artificially sweetened with fructose improved MetS in obese subjects, disregarding dietary fruit consumption.62,63 The recommendation of fruit consumption in MD should be safely sustained due to the diversity of healthy nutrients present in fruits, such as antioxidants, phytosterols, and fiber. Phytosterols found in vegetables and derivatives (dark grapes, oranges, passion fruit, fresh plums, sunflower seeds, soybeans, olive oil, almonds, wheat germ, wheat bran, and cauliflower) compete with the cholesterol consumed in the diet and decrease the intestinal absorption of LDL.64
Studies showed that MD adherence with higher monounsaturated fatty acid (MUFA) and PUFA intake reduces hepatic TG, increases insulin sensitivity, and improves MASH and CVD risk-related benefits.65,66 Consumption of nuts and extra virgin olive oil present in MD significantly reduces the risk of cardiovascular events in people over 55 years old, hypertensive, smokers, and T2DM patients.66
Adherence to the Mediterranean pattern leads to a significant improvement in liver fat in MAFLD overweight patients with or without T2DM and was included as a therapeutic recommendation in European and Latin American guidelines for the treatment of MAFLD.32,60
Other nutritional strategies for MAFLD
Coffee and dark chocolate
Some studies showed that coffee consumption may be associated with liver enzymes reduction including ALT, aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT), as well as lower severity and lower rates of progression of liver disease.67,68 In addition, coffee consumption has been inversely related to nonalcoholic and alcoholic liver cirrhosis.69 Consuming two to three cups of coffee daily appears to have hepatoprotective benefits;70 however, there is still scientific disagreement about coffee consumption and the prevention or treatment of MAFLD. Different ways of preparing coffee can have different effects on MAFLD. Evidence attributes a hepatoprotective role to filtered coffee consumption, while unfiltered coffee has a harmful effect. Perhaps this difference is due to the presence of coffee oils (kahweol and cafestol), which are released from ground coffee beans but removed by paper filters.71,72 On the contrary, studies show that these substances, as well as caffeine, have anti-carcinogenic effects, through the downregulation of the antioxidant-responsive element signaling pathway. In addition, kahweol and cafestol also induce the activation of gamma-glutamylcysteine synthetase (GCS) and glutathione-S-transferase (GST), leading to protection against mutagenesis and inhibiting N-acetyltransferase (NAT).73,74
Like coffee, chocolate consumption, especially dark chocolate may have a therapeutic role in MAFLD.75 The epicatechin and other polyphenolic compounds present in dark chocolate are responsible for the antioxidant effect and reducing oxidative stress.76 Daily consumption of 40 g of dark chocolate reduced nicotinamide adenine dinucleotide phosphate oxidase (NOX) in MASH patients. NOX is considered the main cellular source of reactive oxygen species in humans, and its activation is associated with liver damage.77
Furthermore, the polyphenols found in cocoa improve endothelial function via NOX downregulation in MASH patients.75 Besides the antioxidant effect, dark chocolate can act as a prebiotic. Supplementing with 10 g of dark chocolate per day increases the bacterial diversity in the gut. This modulation in Gut microbiota (GM) may have a potential therapeutic effect for MAFLD in the future.78
Vegetarian diet
The consumption of fruits and vegetables present in the vegetarian diet has been shown to have a protective effect on metabolic diseases such as T2DM, CVD, and MAFLD. The majority of these foods their low caloric density and are rich in antioxidant vitamins and fiber that promotes satiety quickly, helping to lose weight.79,80
Fruits contain nutrients such as vitamin A, C, and E, antioxidants and carotenoids, and vegetables provide vitamins A and E, fiber, and phytochemicals (folate, tocopherols, and carotenoids) that have shown antitumor properties in different diseases64,81 and shows a protective effect on MAFLD.82 Antioxidants and phytochemicals are anti-inflammatory compounds that can keep blood glucose, insulin, and free FA within normal limits, besides preventing the development of hepatic steatosis. The consumption of non-starchy vegetables was associated with less fat deposition in the liver, and the consumption of orange and/or yellow or dark green vegetables with less visceral fat and better insulin sensitivity.83,84 Polyphenols found in these foods exert anti-inflammatory and anti-fibrotic effects, promote beneficial effects on metabolic homeostasis, inhibit de novo lipogenesis and stimulate β-oxidation in MAFLD.85
Ômega 3 (ω-3) supplementation
The administration of ω-3 PUFA improves the plasma lipid profile showing a protective effect in the development and progression of MAFLD and may be useful in its treatment; however, many clinical trials show heterogeneity, including formulation, supplementation time, and dose for the MAFLD treatment.86,87 The use of ω-3 PUFA showed improvement in steatosis and liver damage in patients with MAFLD. These data corroborate the findings obtained in an experimental study with fish oil, which demonstrated a reduction in steatosis, inflammation, and hepatic fibrogenesis.88 A randomized controlled trial evaluating ω-3 PUFA supplementation demonstrated a significant impact on the lipid profile in patients with MASH; however, no significant improvement in NAS was found.86
Supplementation of 4000 mg/day of a synthetic blend of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) compared to placebo showed a significant reduction in liver fat after 15–18 months.89 Similarly, another study supplementing 3000 mg/day of a fish oil–derived blend of EPA and DHA versus placebo soybean oil for 12 months demonstrated to improvement in liver fat on magnetic resonance imaging (MRI) scans.87 In addition, EPA supplementation (2700 mg/day) showed a decrease in steatosis after 12 months.90 Although the studies have demonstrated consistent improvement in liver fat content, recent evidence has not shown any significant benefit in liver histology or fibrosis.
Considering the difficulty for both science and clinical practice to understand the interactions between nutrients, foods and diets, recently, the use of the Geometric Structure for Nutrition (GFN) has been proposed to develop better analyzes between the relationship of nutrient intake, types of diets and health. The authors propose the use of GFN as a primary tool in precision medicine to apply population, clinical and preclinical data, first to visualize nutritional relationships and then generate testable predictions that can be used to develop nutritional interventions for prevention and treatment of diseases, including liver disease.91
Physical activity, exercise and MAFLD
The practice of physical exercise has been recommended as a non-drug strategy for MAFLD treatment. Regular physical exercise improves the liver profile, and reduces inflammation and oxidative stress, besides reducing some liver damage markers such as ALT and AST.15 In addition, physical exercise has the characteristic of increasing daily energy expenditure, favoring a negative energy balance, consequently, it is, together with food, one of the main tools for weight reduction, more specifically, reduction of body fat.24
Physical exercise acts directly or indirectly in the prevention of MAFLD. Some studies have demonstrated the importance of lifestyle changes in the development and progression of MAFLD.17,92,93 A systematic review found a positive correlation between a sedentary lifestyle and the prevalence of MAFLD.94
It has recently been shown that lifestyle changes including exercise and a healthy diet induce weight loss and are capable of reversing hepatic steatosis and even MASH and fibrosis. On the contrary, physical exercise is effective even in the absence of diet or body weight loss in patients with MAFLD.95
The latest guidelines from the American Gastroenterological Association (AGA), EASL, National Institute for Excellence in Health and Care (NICE), Italian Association for the Study of the Liver (AISF), and American Association for the Study of Liver Diseases (AASLD) recognize and encourages physical exercise as a fundamental part of treatment.5,96 In addition, the Asian Pacific Association for the Study of the Liver (APASL) published the first guidelines for MAFLD, including its epidemiology, diagnosis, screening, evaluation, and treatment.97
Aerobic exercise and MAFLD
Recent studies have demonstrated that aerobic exercise is beneficial for patients with MAFLD reducing ALT, BMI, and intrahepatic lipids.98 Although there is strong evidence of the beneficial effects of aerobic exercise for this population, a review study published by Machado95 suggests that aerobic or resistance exercise have similar effects on the management of MAFLD.
A recent systematic review published by Farzanegi et al.15 highlighted some beneficial effects of exercise training on MAFLD such as reduced levels of ALT, AST, and alkaline phosphatase (ALP) enzymes, hepatic adipose components, IR, body weight, hepatic fat, obesity, hepatic steatosis, hepatic apoptotic cells, in addition to increased insulin sensitivity.
The main benefit of aerobic physical exercise is the improvement of cardiorespiratory capacity, consequently reducing cardiovascular risk.99 Some studies demonstrate the benefits of using moderate-high-intensity protocols with a volume of at least 150 minutes per week (40–45 minutes sessions, thrice per week) for 12 weeks.100,101 A limitation to these moderate-intensity continuous aerobic exercise protocols is time. Some studies show some benefits of using high-intensity interval training (HIIT) and verifying up to 27% reduction in steatosis, in addition to being a prescription alternative.100 Another limitation of this type of exercise is the cardiovascular condition of the patients, who are often impaired. It is up to professionals to verify the possibilities with each individual.
In MAFLD, the main physiological mechanisms consequent of aerobic physical exercise includes activation of adenosine monophosphate–activated protein kinase (AMPK) and reduction of malonyl coenzyme-A (malonyl Coa), allowing the increase in the action of carnitine acyl transferase 1 (CAT1), increasing the efficiency of transport and oxidation of FA in mitochondria. The mechanisms underlying the reduction in intrahepatic lipids resulting from aerobic physical exercise also appear to reflect improvements in insulin sensitivity. High levels of circulating insulin increase the expression of SREBP transcription factors, mainly SREBP-1c in the liver, increasing intrahepatic lipids and stimulating de novo lipogenesis.102–104
The lipogenesis is constantly elevated in MAFLD patients, contributing to the accumulation of intrahepatic lipids and a high concentration of circulating TG, which exacerbate MAFLD by creating a vicious cycle in which the high concentration of intrahepatic lipid prevents the action of hepatic insulin, raising the concentration of portal insulin which in turn increases intrahepatic lipids.105
Evidence suggests that the introduction of physical exercise (aerobic or resistance) breaks this vicious cycle improving lipid oxidation and blood glucose control due to the increase in the glucose transporter GLUT-4 and insulin receptors in the skeletal muscle, the expression and activity of the glycogen synthase enzyme, and muscle and liver glycogen stores.106 Aerobic exercise suppresses the general and specific pro-inflammatory state associated with IR, improves cardiovascular risk, and induces weight loss.107–109
Resistance exercise and MAFLD
A systematic review published by Hashida et al.20 showed that resistance to physical exercise can improve alterations caused by dyslipidemia, arterial hypertension, and IR. In addition, it may be more effective for some MAFLD patients, especially overweight patients and those who present low cardiorespiratory fitness or who cannot, for some reason, practice aerobic physical exercises, proving to be a useful and effective tool in the treatment. Resistance to physical exercise has been shown to increase body fat oxidizing capacity and decrease up to 13% of liver fat in MAFLD.100,110
Resistance to physical exercise can also contribute to weight management, both for the direct caloric cost while practicing the exercisan by the residual elevation of VO² post-exercise, besides by the greater fat oxidation post-exercise, due to the excessive oxygen consumption effect.110
Some authors demonstrated that resistance physical exercise was also able to reduce ALT and AST liver injury markers. Several studies demonstrated a reduction in steatosis independent of weight loss.62,100 In another study, participants trained for 8 weeks at a frequency of three non-consecutive days per week. After this period, the resistance physical exercise group also showed improvement in intra and intergroup comparisons in body composition, a significant decrease in body weight, and in serum levels of AST and ALT. In addition, liver fat levels assessed by ultrasound also decreased significantly compared to the pre-training period while the control group increased significantly.111
Therefore, it appears that resistance to physical exercise contributes to the improvement of MAFLD by increasing the uptake of circulating glucose and FA, reducing the impact of insulin-stimulated hepatic lipogenesis.112
During exercise, specifically during muscle fiber contraction, the hormone irisin is released, leading to increased energy consumption related to thermogenesis through mainly brown adipose tissue. Furthermore, in obese individuals, it appears at lower concentrations, and it appears that irisin can influence lipid metabolism in liver cells.113
Lifestyle and weight loss
Several studies reinforce that lifestyle modification, including physical activity and dietary habits should be the first line for the management of MAFLD.114–116 Recent studies emphasize that lifestyle change with weight loss is the most established therapy with a clear dose-response association.117,118 About 9% of body weight loss has been found to significantly improve MAFLD. A 10% of body weight loss resulted in a 45% reduction in liver fat content. Besides lifestyle change through exercise and caloric controlled ingestion, psychological behavior modification with guidance from health experts leads to improvements in MAFLD.2 The combination of an adequate diet and exercise practice improves fibrosis and the fat liver by an average of 40%. The intensity of the lifestyle modification can determine the degree of hepatic fat reduction, this way it is necessary a more active lifestyle for a 5–10% decrease in body weight. Randomized controlled trials showed that lifestyle modification in MAFLD patients leads to weight loss; improves MAFLD activity score (a composite of steatosis, hepatocyte balloon, and inflammation), and decreases hepatic TG content as determined by MRI scans.19,24,119 The majority of studies also elucidate that lifestyle change is accompanied by simultaneous improvement in the CVD risk factors such as IR and serum lipid levels.120,121
Study showed an interface between adipokines and neuroendocrine regulation of energy balance and MASH.122 Campos et al.123 demonstrated the importance of the study of interdisciplinary treatment in MAFLD obese adolescents, which observed in this study that at the beginning of therapy important clinical parameters such as visceral fat, BMI, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Quantitative Insulin sensitivity Check Index (QUICKI), TG, VLDL-cholesterol and liver enzymes were more altered in MASH patients, who improve after treatment.
Lifestyle changes are also necessary for lean MAFLD treatment. As with overweight and obese patients, MD, physical activity including aerobics and resistance exercise is effective in reducing body weight, improving inflammation, liver enzymes and intrahepatic fat.124 Younossi et al.125 showed a lean MAFLD prevalence of 7%, mainly in females. Lean patients with MAFLD despite have less severe metabolic disturbance than overweight MAFLD, display altered body fat distribution and more IR.2 The weight loss for lean MAFLD patients of 3% to 5% shows similar histological benefits in steatosis and MASH as overweight or obese MAFLD patients.126
Gut microbiota and MAFLD
The GM is a dynamic and complex ecosystem, which assists in the proliferation, growth, and differentiation of epithelial cells to fight infections and improve immunity. Despite its important role in the synthesis of vitamin K, folate, short-chain fatty acids (SCFA) and peroxides, GM acts as a major environmental and etiological factor for the progression of many metabolic and liver diseases including MAFLD.127
Several pathogens, including viruses and intestinal microorganisms, use the mucous membranes as a gateway.128 Liver viruses violate intestinal permeability, leading to intestinal dysbiosis and releasing pro-inflammatory cytokines, which are key to the development of liver cirrhosis and HCC. It is also observed that the use of probiotics reduces the tolerogenic response and increases the mucosal defense against viral pathogens.129 In most liver diseases, especially cirrhosis, intestinal dysbiosis increases Proteobacteria, Enterobacteriaceae, and Veillonellaceae, while decreasing Bacteroidetes and Lachnospiraceae.130
Evidence shows that GM composition may be related to different stages of liver disease. A study by Boursier et al.131 demonstrated that the reduced amount of Bacteroides was independently associated with MASH and the prevalence of Ruminococcus was associated with moderate fibrosis stage. In addition, Zhang et al.132 showed that the composition and function of the intestinal microbiota in patients with a higher degree of liver fibrosis is significantly altered, mainly with an increase in Prevotella, Phascolarctobacterium succinatutens, Eubacterium biform, and Collinsella aerofaciens bacteria in MAFLD patients.
Gut Microbiota in Lean MAFLD
Endogenous factors such as GM may contribute to the development of MAFLD in both obese and lean patients.133 The GM alterated and its interaction with bile acids impair Farnesoid X receptor (FXR)-mediated signaling in both the liver and intestine and may be one of the factors responsible for the development of lean MAFLD.127 Bacteria of the phylum Firmicutes are the main intestinal commensal microorganisms that present a greater diversity of bacteria capable of metabolizing bile acids;134 however, when there is intestinal dysbiosis process, this metabolization is slowed down, which in turn can affect the energy balance.135,136
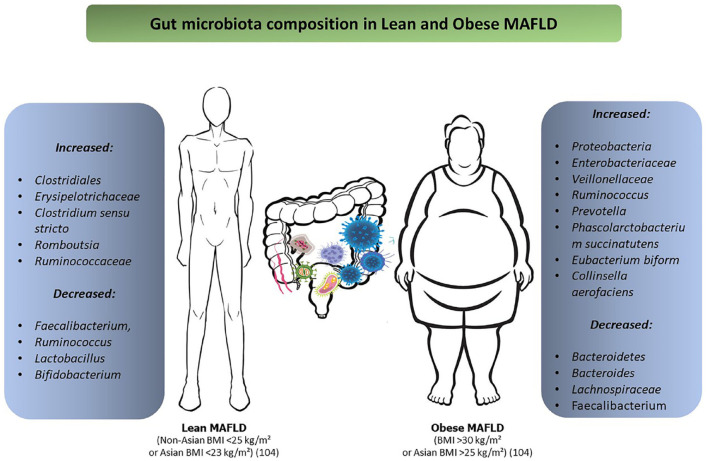
Recently, Chen et al.136 evaluated the intestinal microbial composition of lean and obese subjects with MAFLD and found no differencesin bacterial phyla but observed differences in the genera and order of bacteria such as Erysipelotrichaceae UCG-003, Clostridiales Ruminococcus, Clostridium sensu stricto, Romboutsia, and Ruminococcaceae that were more abundant in lean MAFLD patients than in obese MAFLD patients. This bacterial population is associated with lower metabolism of bile acids and greater stimulation of the inflammatory process.137 In addition, Duarte et al.138 found a significant difference in the abundance of Faecalibacterium, Ruminococcus, Lactobacillus, and Bifidobacterium in lean MASH patients when compared to obese controls. Another aspect of the lean MAFLD microbiota is the presence of Gram-negative bacteria that produce lipopolysaccharides (LPSs) which in the intestine activate nuclear factor kβ (NF-kβ) and tumor necrosis factor–alpha (TNF-α) production, increasing the exposure of the liver to endotoxins, an important role in the progression of steatosis to MASH.139,140 Figure 2 shows the differences in microbiota composition between lean and obese patients with MAFLD. In addition, the increase in intestinal permeability caused by dysbiosis leads to bacterial translocation and the endotoxins produced by these bacteria penetrate the portal vein and activate inflammatory cells of the Toll-like receptors (TLRs) type in hepatocytes141 and decreases FIAF secretion by increasing LPL activity and hepatic TGs accumulation.142,143
Figure 2.
The differences in microbiota composition between lean and obese patients with MAFLD.
Diet and nutritional supplementation may be an important factor for GM modulation in lean MAFLD patients.136 Consumption of probiotics is associated with reduced liver inflammation, decreased concentrations of LPS and levels of aminotransferases.137,144,145 Supplementation with Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 increases the expression of genes involved in hepatic oxidation of FAs146 and consumption of Bifidobacterium pseudocatenulatum improves glucose tolerance, the inflammatory state and hepatic steatosis.147 The clinical trial by Mofidi et al.148 demonstrated that patients with MAFLD supplemented with probiotics have a reduction in total cholesterol, liver enzymes, improvement in IR, and TNF-α. In addition, the use of probiotics has also been associated with a decrease in LDLs in animals and humans with hypercholesterolemia.149,150 The use of prebiotics increases the number of beneficial bacteria in the gut, in particular Bifidobacteria and Lactobacillus, and inhibits bacterial activities harmful to the health of the host;151 in addition, prebiotics can induce an increase in Eubacterium-type bacteria rectale, Roseburia, and Ruminicoccus bromii that are important producers of butyrate in the colon.152,153 This metabolite is related to the prevention of steatosis and improvement of oxidative stress indices and liver inflammation and improvement of intestinal integrity.
Conclusion
In conclusion, several evidence has shown that changes in lifestyle should be the main basis for the menagenement of MAFLD in lean and obese patients; however, further randomized studies involving diet, nutrient, nutritional supplementation, and lifestyle changes should be performed.
In the Table 1, we list the main therapeutic recommendations related to lifestyle changes and dietary treatment in MAFLD.
Table 1.
Therapeutic recommendations related to lifestyle changes and dietary treatment in MAFLD.
| 5–10% reduction in initial body weight for overweight/obese patients, increasing these targets when there are higher levels of MASH and fibrosis |
| 3–5% reduction in initial body weight for lean MAFLD patients (especially if their excess abdominal fat and is present has been recent weight gain) |
| Visceral fat reduction (abdominal circumference) |
| Visceral fat reduction (abdominal circumference) A healthy diet with calorie restriction (daily reduction of 0.5000–1.000 kcal) or total intake between 1.200−1.800 kcal/day adapted to patient preferences |
| Prioritize consumption of olive oil, nuts, and fish, reduction of saturated fat, and avoid trans fat intake |
| Avoid foods and drinks that contain added fructose There is no evidence of the harmful effect of fruits in their natural form if consumed in a reasonable amount (generally 1–3 servings/day) |
| The daily protein intake recommendation should be equal to or more than 1.2–1.5 g/kg/day |
| The Mediterranean diet is the better dietary strategy to improve steatosis and insulin sensitivity and their adherence should be advised. Partial adherence can be advantageous if it reduced saturated fat, and fructose intake, avoid added refined sugar, and prioritizes homemade food and minimally processed foods |
| Consumption of coffee in moderate amounts is recommended for patients with MAFLD |
| The ω-3 PUFAs may be considered to treat hypertriglyceridemia in patients with MAFLD, however, are not recommended for the specific treatment of MAFLD or MASH |
| Avoiding excessive alcohol consumption and smoking can be beneficial both in preventing the progression of MAFLD and in reducing the risk of hepatocellular carcinoma |
| Moderate-intensity aerobic exercise (150–200 min/week of 3–5
sessions) Resistance training (weight training) promotes musculoskeletal fitness and is also effective to improve effects on metabolic risk factors. Moderate-intensity exercise is effective to promote 7–10% of total weight loss and sustaining this loss over time |
| Stimulate fat loss and lean mass increase in lean MAFLD patients with diet and exercise |
MAFLD, metabolic dysfunction–associated fatty liver disease; MASH, metabolic associated steatohepatitis; PUFA, polyunsaturated fatty acids.
Acknowledgments
None.
Footnotes
ORCID iD: Claudia P. Oliveira  https://orcid.org/0000-0002-2848-417X
https://orcid.org/0000-0002-2848-417X
Contributor Information
José Tadeu Stefano, Laboratório de Gastroenterologia Clínica e Experimental LIM-07, Division of Clinical Gastroenterology and Hepatology, Hospital das Clínicas HCFMUSP, Department of Gastroenterology, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil.
Sebastião Mauro Bezerra Duarte, Laboratório de Gastroenterologia Clínica e Experimental LIM-07, Division of Clinical Gastroenterology and Hepatology, Hospital das Clínicas HCFMUSP, Department of Gastroenterology, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil.
Renato Gama Ribeiro Leite Altikes, Departament of Gastroenterology, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Claudia P. Oliveira, Laboratório de Gastroenterologia Clínica e Experimental LIM-07, Division of Clinical Gastroenterology and Hepatology, Hospital das Clínicas HCFMUSP, Department of Gastroenterology, Faculdade de Medicina, Universidade de Sao Paulo, Av. Dr. Enéas de Carvalho Aguiar no 255, Instituto Central, # 9159, Sao Paulo 05403-000, Brazil; Departament of Gastroenterology, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: José Tadeu Stefano: Writing – original draft; Writing – review & editing.
Sebastião Mauro Bezerra Duarte: Writing – original draft; Writing – review & editing.
Renato Gama Ribeiro Leite Altikes: Writing – review & editing.
Claudia P. Oliveira: Investigation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 2019; 92: 82–97. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 3. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73: 202–209. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Scorletti E, Mosca A, et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020; 111S: 154170. [DOI] [PubMed] [Google Scholar]
- 5. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 6. Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab 2022; 107: 2691–2700. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Ayada I, Zhang X, et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol 2022; 20: e573–e82. [DOI] [PubMed] [Google Scholar]
- 8. Duarte SMB, Stefano JT, Vanni DS, et al. Impact of current diet at the risk of Non-Alcoholic Fatty Liver Disease (NAFLD). Arq Gastroenterol 2019; 56: 431–439. [DOI] [PubMed] [Google Scholar]
- 9. Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr 2007; 86: 285–300. [DOI] [PubMed] [Google Scholar]
- 10. Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2006; 91: 4753–4761. [DOI] [PubMed] [Google Scholar]
- 11. Yasutake K, Kohjima M, Nakashima M, et al. Nutrition therapy for liver diseases based on the status of nutritional intake. Gastroenterol Res Pract 2012; 2012: 859697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashemi Kani A, Alavian SM, Haghighatdoost F, et al. Diet macronutrients composition in nonalcoholic Fatty liver disease: a review on the related documents. Hepat Mon 2014; 14: e10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan S. Implications of diet on nonalcoholic fatty liver disease. Curr Opin Gastroenterol 2010; 26: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryu S, Chang Y, Jung HS, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol 2015; 63: 1229–1237. [DOI] [PubMed] [Google Scholar]
- 15. Farzanegi P, Dana A, Ebrahimpoor Z, et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci 2019; 19: 994–1003. [DOI] [PubMed] [Google Scholar]
- 16. Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci 2016; 130: 93–104. [DOI] [PubMed] [Google Scholar]
- 17. Semmler G, Datz C, Reiberger T, et al. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int 2021; 41: 2249–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rezende RE, Duarte SM, Stefano JT, et al. Impact of aerobic exercise in postmenopausal women with nonalcoholic fatty liver disease: a 24 weeks randomized clinical trial. J Hepatol 2015; 62: S724. [DOI] [PubMed] [Google Scholar]
- 19. Zelber-Sagi S, Buch A, Yeshua H, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 2014; 20: 4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 2017; 66: 142–152. [DOI] [PubMed] [Google Scholar]
- 21. Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 2012; 56: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 22. Wehmeyer MH, Zyriax BC, Jagemann B, et al. Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine 2016; 95: e3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bezerra Duarte SM, Faintuch J, Stefano JT, et al. Hypocaloric high-protein diet improves clinical and biochemical markers in patients with nonalcoholic fatty liver disease (NAFLD). Nutr Hosp 2014; 29: 94–101. [DOI] [PubMed] [Google Scholar]
- 24. Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology 2016; 63: 2032–2043. [DOI] [PubMed] [Google Scholar]
- 25. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab 2010; 21: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003; 37: 1202–1219. [DOI] [PubMed] [Google Scholar]
- 28. Musso G, Gambino R, De Michieli F, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003; 37: 909–916. [DOI] [PubMed] [Google Scholar]
- 29. Toshimitsu K, Matsuura B, Ohkubo I, et al. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 2007; 23: 46–52. [DOI] [PubMed] [Google Scholar]
- 30. Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol 2022; 19: 638–651. [DOI] [PubMed] [Google Scholar]
- 31. Westerbacka J, Lammi K, Häkkinen AM, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab 2005; 90: 2804–2809. [DOI] [PubMed] [Google Scholar]
- 32. Anania C, Perla FM, Olivero F, et al. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol 2018; 24: 2083–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antonucci L, Porcu C, Iannucci G, et al. Non-alcoholic fatty liver disease and nutritional implications: special focus on copper. Nutrients 2017; 9: 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010; 121: 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 2012; 15: 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab 2019; 29: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bayoumi A, Elsayed A, Han S, et al. Mistranslation drives alterations in protein levels and the effects of a synonymous variant at the fibroblast growth factor 21 locus. Adv Sci 2021; 8: 2004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 39. Chavez AO, Molina-Carrion M, Abdul-Ghani MA, et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lehtonen JM, Forsström S, Bottani E, et al. FGF21 is a biomarker for mitochondrial translation and mtDNA maintenance disorders. Neurology 2016; 87: 2290–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shenoy VK, Beaver KM, Fisher FM, et al. Elevated serum fibroblast growth factor 21 in humans with acute pancreatitis. PLoS ONE 2016; 11: e0164351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frayling TM, Beaumont RN, Jones SE, et al. A common allele in FGF21 associated with sugar intake is associated with body shape, lower total body-fat percentage, and higher blood pressure. Cell Rep 2018; 23: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hill CM, Qualls-Creekmore E, Berthoud HR, et al. FGF21 and the physiological regulation of macronutrient preference. Endocrinology 2020; 161: bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keinicke H, Sun G, Mentzel CMJ, et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect 2020; 9: 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu C, Schönke M, Spoorenberg B, et al. FGF21 protects against hepatic lipotoxicity and macrophage activation to attenuate fibrogenesis in nonalcoholic steatohepatitis. bioRxiv preprint, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao Y, Zhang W, Zeng LQ, et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol 2020; 36: 101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji Y, Lee H, Kaura S, et al. Effect of bariatric surgery on metabolic diseases and underlying mechanisms. Biomolecules 2021; 11: 1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fedirko V, Lukanova A, Bamia C, et al. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol 2013; 24: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008; 48: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sullivan JS, Le MT, Pan Z, et al. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr Obes 2015; 10: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010; 51: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Volynets V, Louis S, Pretz D, et al. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a western-style diet or drinking water supplemented with fructose. J Nutr 2017; 147: 770–780. [DOI] [PubMed] [Google Scholar]
- 53. Moore MP, Cunningham RP, Dashek RJ, et al. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity 2020; 28: 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Wit NJ, Afman LA, Mensink M, et al. Phenotyping the effect of diet on non-alcoholic fatty liver disease. J Hepatol 2012; 57: 1370–1373. [DOI] [PubMed] [Google Scholar]
- 55. Bortolotti M, Maiolo E, Corazza M, et al. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin Nutr 2011; 30: 494–498. [DOI] [PubMed] [Google Scholar]
- 56. Xu C, Markova M, Seebeck N, et al. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and FGF21 levels. Liver Int 2020; 40: 2982–2997. [DOI] [PubMed] [Google Scholar]
- 57. Alfonzo González GC. [Effect of thermal treatment on total dietetic fiber, soluble and insoluble contents in legumes]. Arch Latinoam Nutr 2000; 50: 281–285. [PubMed] [Google Scholar]
- 58. Cortez-Pinto H, Jesus L, Barros H, et al. How different is the dietary pattern in non-alcoholic steatohepatitis patients. Clin Nutr 2006; 25: 816–823. [DOI] [PubMed] [Google Scholar]
- 59. Arslanow A, Teutsch M, Walle H, et al. Short-term hypocaloric high-fiber and high-protein diet improves hepatic steatosis assessed by controlled attenuation parameter. Clin Transl Gastroenterol 2016; 7: e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plaz Torres MC, Aghemo A, Lleo A, et al. Mediterranean diet and NAFLD: what we know and questions that still need to be answered. Nutrients 2019; 11: 2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis C, Bryan J, Hodgson J, et al. Definition of the Mediterranean diet; a literature review. Nutrients 2015; 7: 9139–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017; 67: 829–846. [DOI] [PubMed] [Google Scholar]
- 63. Madero M, Arriaga JC, Jalal D, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011; 60: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 64. Salomone F, Godos J, Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int 2016; 36: 5–20. [DOI] [PubMed] [Google Scholar]
- 65. Grosso G, Mistretta A, Frigiola A, et al. Mediterranean diet and cardiovascular risk factors: a systematic review. Crit Rev Food Sci Nutr 2014; 54: 593–610. [DOI] [PubMed] [Google Scholar]
- 66. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 67. Hiramatsu T, Tajima O, Uezono K, et al. Coffee consumption, serum γ-glutamyltransferase, and glucose tolerance status in middle-aged Japanese men. Clin Chem Lab Med 2013; 51: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 68. Yesil A, Yilmaz Y. Review article: coffee consumption, the metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2013; 38: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 69. Bravi F, Tavani A, Bosetti C, et al. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev 2017; 26: 368–377. [DOI] [PubMed] [Google Scholar]
- 70. Torres DM, Harrison SA. Is it time to write a prescription for coffee? Coffee and liver disease. Gastroenterology 2013; 144: 670–672. [DOI] [PubMed] [Google Scholar]
- 71. Lee KJ, Choi JH, Jeong HG. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol 2007; 45: 2118–2125. [DOI] [PubMed] [Google Scholar]
- 72. Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2005; 128: 24–32. [DOI] [PubMed] [Google Scholar]
- 73. Molloy JW, Calcagno CJ, Williams CD, et al. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012; 55: 429–436. [DOI] [PubMed] [Google Scholar]
- 74. Miranda AM, Steluti J, Fisberg RM, et al. Association between coffee consumption and its polyphenols with cardiovascular risk factors: a population-based study. Nutrients 2017; 9: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loffredo L, Baratta F, Ludovica P, et al. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr Metab Cardiovasc Dis 2017; 28: 143–149. [DOI] [PubMed] [Google Scholar]
- 76. Malhi H, Loomba R. Editorial: dark chocolate may improve NAFLD and metabolic syndrome by reducing oxidative stress. Aliment Pharmacol Ther 2016; 44: 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Loffredo L, Del Ben M, Perri L, et al. Effects of dark chocolate on NOX-2-generated oxidative stress in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2016; 44: 279–286. [DOI] [PubMed] [Google Scholar]
- 78. Wiese M, Bashmakov Y, Chalyk N, et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. Biomed Res Int 2019; 2019: 4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cook LT, O’Reilly GA, Goran MI, et al. Vegetable consumption is linked to decreased visceral and liver fat and improved insulin resistance in overweight Latino youth. J Acad Nutr Diet 2014; 114: 1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang PY, Fang JC, Gao ZH, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Investig 2016; 7: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology 2014; 147: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 82. Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc 2010; 69: 333–340. [DOI] [PubMed] [Google Scholar]
- 83. Han JM, Jo AN, Lee SM, et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 2014; 29: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 84. Mirabelli M, Chiefari E, Arcidiacono B, et al. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients 2020; 12: 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li H, Wang X, Ye M, et al. Does a high intake of green leafy vegetables protect from NAFLD? Evidence from a large population study. Nutr Metab Cardiovasc Dis 2021; 31: 1691–1701. [DOI] [PubMed] [Google Scholar]
- 86. Nogueira MA, Oliveira CP, Ferreira Alves VA, et al. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016; 35: 578–586. [DOI] [PubMed] [Google Scholar]
- 87. Argo CK, Patrie JT, Lackner C, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol 2015; 62: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yuan F, Wang H, Tian Y, et al. Fish oil alleviated high-fat diet-induced non-alcoholic fatty liver disease via regulating hepatic lipids metabolism and metaflammation: a transcriptomic study. Lipids Health Dis 2016; 15: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scorletti E, Bhatia L, McCormick KG, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology 2014; 60: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 90. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014; 147: 377–384. [DOI] [PubMed] [Google Scholar]
- 91. Simpson SJ, Raubenheimer D, Cogger VC, et al. The nutritional geometry of liver disease including non-alcoholic fatty liver disease. J Hepatol 2018; 68: 316–325. [DOI] [PubMed] [Google Scholar]
- 92. Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol 2016; 13: 196–205. [DOI] [PubMed] [Google Scholar]
- 93. Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005; 43: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 94. Stevanović J, Beleza J, Coxito P, et al. Physical exercise and liver ‘fitness’: role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol Metab 2020; 32: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Machado MV. Aerobic exercise in the management of metabolic dysfunction associated fatty liver disease. Diabetes Metab Syndr Obes 2021; 14: 3627–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Leoni S, Tovoli F, Napoli L, et al. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol 2018; 24: 3361–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020; 14: 889–919. [DOI] [PubMed] [Google Scholar]
- 98. Słomko J, Zalewska M, Niemiro W, et al. Evidence-based aerobic exercise training in metabolic-associated fatty liver disease: systematic review with meta-analysis. J Clin Med 2021; 10: 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011; 60: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rezende RE, Duarte SM, Stefano JT, et al. Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause 2016; 23: 876–883. [DOI] [PubMed] [Google Scholar]
- 102. Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 2011; 301: E1033–E1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci 2006; 63: 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008; 51: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 105. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 2018; 75: 3313–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013; 58: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 107. Duarte SM, Rezende RE, Stefano JT, et al. Impaired aerobic capacity and cardiac autonomic control in sedentary postmenopausal women with Nonalcoholic Fatty Liver Disease (NAFLD). J Hepat 2015; 62: S733. [Google Scholar]
- 108. Garcia CB, Perandini LA, Seguro LP, et al. Impaired aerobic exercise capacity and cardiac autonomic control in primary antiphospholipid syndrome. Lupus 2013; 22: 928–931. [DOI] [PubMed] [Google Scholar]
- 109. Wycherley TP, Buckley JD, Noakes M, et al. Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. Eur J Nutr 2013; 52: 317–325. [DOI] [PubMed] [Google Scholar]
- 110. Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep 2019; 1: 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shamsoddini A, Sobhani V, Ghamar Chehreh ME, et al. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat Mon 2015; 15: e31434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dubé JJ, Amati F, Stefanovic-Racic M, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 2008; 294: E882–E888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen N, Li Q, Liu J, et al. Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev 2016; 32: 51–59. [DOI] [PubMed] [Google Scholar]
- 114. Bellentani S, Dalle Grave R, Suppini A, et al. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 2008; 47: 746–754. [DOI] [PubMed] [Google Scholar]
- 115. Bradford V, Dillon J, Miller M. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease. Hepat Med 2014; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Finelli C, Tarantino G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J Gastrointestin Liver Dis 2012; 21: 293–302. [PubMed] [Google Scholar]
- 117. Raza S, Rajak S, Upadhyay A, et al. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci 2021; 26: 206–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mundi MS, Velapati S, Patel J, et al. Evolution of NAFLD and its management. Nutr Clin Pract 2020; 35: 72–84. [DOI] [PubMed] [Google Scholar]
- 119. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012; 57: 157–166. [DOI] [PubMed] [Google Scholar]
- 120. van Oort S, Beulens JWJ, van Ballegooijen AJ, et al. Association of cardiovascular risk factors and lifestyle behaviors with hypertension: a mendelian randomization study. Hypertension 2020; 76: 1971–1979. [DOI] [PubMed] [Google Scholar]
- 121. Mikusova V, Mikus J, Grilusova K, et al. Insulin resistance and need for a lifestyle change to eliminate it. Bratisl Lek Listy 2021; 122: 567–571. [DOI] [PubMed] [Google Scholar]
- 122. Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis 2001; 21: 27–41. [DOI] [PubMed] [Google Scholar]
- 123. Campos RM, de Piano A, da Silva PL, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: effects of long-term interdisciplinary therapy. Endocrine 2012; 42: 146–156. [DOI] [PubMed] [Google Scholar]
- 124. Maier S, Wieland A, Cree-Green M, et al. Lean NAFLD: an underrecognized and challenging disorder in medicine. Rev Endocr Metab Disord 2021; 22: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 2012; 91: 319–327. [DOI] [PubMed] [Google Scholar]
- 126. Alam S, Jahid Hasan M, Khan MAS, et al. Effect of weight reduction on histological activity and fibrosis of lean nonalcoholic steatohepatitis patient. J Transl Int Med 2019; 7: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Martín-Mateos R, Albillos A. The role of the gut-liver axis in metabolic dysfunction-associated fatty liver disease. Front Immunol 2021; 12: 660179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol 2016; 14: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rigo-Adrover MDM, van Limpt K, Knipping K, et al. Preventive effect of a synbiotic combination of galacto- and fructooligosaccharides mixture with. Front Immunol 2018; 9: 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sanduzzi Zamparelli M, Rocco A, Compare D, et al. The gut microbiota: a new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterol J 2017; 5: 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016; 63: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang Y, Yan S, Sheng S, et al. Comparison of gut microbiota in male MAFLD patients with varying liver stiffness. Front Cell Infect Microbiol 2022; 12: 873048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Madrid AM, Poniachik J, Quera R, et al. Small intestinal clustered contractions and bacterial overgrowth: a frequent finding in obese patients. Dig Dis Sci 2011; 56: 155–160. [DOI] [PubMed] [Google Scholar]
- 134. Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol 2015; 11: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci 2020; 21: 5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 2020; 71: 1213–1227. [DOI] [PubMed] [Google Scholar]
- 137. Wong VW, Tse CH, Lam TT, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis – a longitudinal study. PLoS ONE 2013; 8: e62885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Duarte SMB, Stefano JT, Miele L, et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: a prospective pilot study. Nutr Metab Cardiovasc Dis 2018; 28: 369–384. [DOI] [PubMed] [Google Scholar]
- 139. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2013; 19: 6911–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Beaumont M, Neyrinck AM, Olivares M, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J 2018; 32: fj201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, et al. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int 2010; 4: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bäckhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 2007; 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr 2017; 8: 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Everard A, Matamoros S, Geurts L, et al. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio 2014; 5: e01011–e01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Park DY, Ahn YT, Park SH, et al. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013; 8: e59470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhao Z, Chen L, Zhao Y, et al. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol 2020; 104: 5273–5282. [DOI] [PubMed] [Google Scholar]
- 148. Mofidi F, Poustchi H, Yari Z, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr 2017; 117: 662–668. [DOI] [PubMed] [Google Scholar]
- 149. Mofidi F, Yari Z, Poustchi H, et al. Effects of synbiotics supplementation in lean patients with nonalcoholic fatty liver disease: study protocol of a pilot randomized double-blind clinical trial. Arch Iran Med 2016; 19: 282–284. [PubMed] [Google Scholar]
- 150. Usman Hosono A. Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. J Dairy Sci 2000; 83: 1705–1711. [DOI] [PubMed] [Google Scholar]
- 151. Younes R, Bugianesi E. NASH in lean individuals. Semin Liver Dis 2019; 39: 86–95. [DOI] [PubMed] [Google Scholar]
- 152. Louis P, Young P, Holtrop G, et al. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 2010; 12: 304–314. [DOI] [PubMed] [Google Scholar]
- 153. Endo H, Niioka M, Kobayashi N, et al. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS ONE 2013; 8: e63388. [DOI] [PMC free article] [PubMed] [Google Scholar]