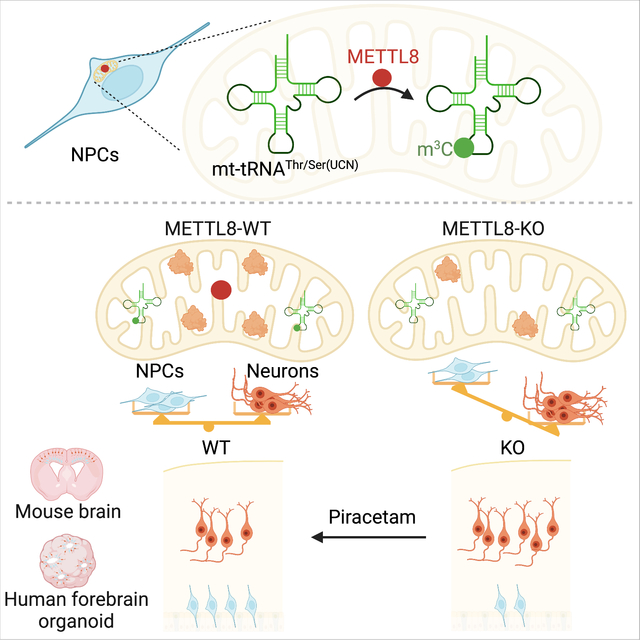
SUMMARY
Increasing evidence implicates critical roles of various epitranscriptomic RNA modifications in different biological processes. Methyltransferase METTL8 installs 3-methylcytosine (m3C) modification of mitochondrial tRNAs in vitro, however, its role in intact biological systems is unknown. Here we show that Mettl8 is localized in mitochondria and installs m3C specially on mitochondrial tRNAThr/Ser(UCN) in mouse embryonic cortical neural stem cells. At molecular and cellular levels, Mettl8 deletion in cortical neural stem cells leads to reduced mitochondrial protein translation and attenuated respiration activity. At the functional level, conditional Mettl8 deletion in mice results in impaired embryonic cortical neural stem cell maintenance in vivo, which can be rescued by pharmacologically enhancing mitochondrial functions. Similarly, METTL8 promotes mitochondrial protein expression and neural stem cell maintenance in human forebrain cortical organoids. Together, our study reveals a conserved epitranscriptomic mechanism of Mettl8 and mitochondrial tRNA m3C modification in maintaining embryonic cortical neural stem cells in mice and humans.
Graphical Abstract
ETOC BLURB
Zhang et al. identified an epitranscriptomic mechanism regulating cortical neural stem cells in the embryonic mouse brain and human forebrain organoids. Specifically, Mettl8 installs m3C modification on mitochondrial tRNAThr/Ser(UNC), which regulates mitochondrial protein translation and function, and loss of Mettl8 leads to accelerated embryonic cortical neural stem cell depletion
INTRODUCTION
The study of more than 170 chemically distinct types of RNA modifications, or epitranscriptomics, has attracted significant interest because of its critical involvement in tuning nearly every aspect of RNA biology, regulating various biological processes ranging from development to cancer, and profound successes in clinical applications of RNA modifications to improve efficacy and reduce immunogenicity of COVID19 mRNA vaccines1–4. Significant progress has been made in identifying writers, erasers and readers for different RNA modifications, which in turn provides entry points to investigate their biological functions and underlying mechanisms. Most epitranscriptomic studies have used immortal or cancer cell lines, while investigations in intact biological systems lag behind. In the mammalian nervous system, recent studies have identified diverse roles and mechanisms of epitranscriptomics, mostly focusing on mRNA m6A modification, yet many other RNA modifications remain to be explored5–7.
Besides modifications on mRNA, transfer RNA (tRNA) are also heavily modified (~ 13 modifications/molecule) to be fully active8–10. Modifications of mitochondrial tRNAs (mt-tRNAs, ~ 5 modifications/molecule11) are very important for utilizing only 22 mt-tRNAs to precisely recognize 64 codons specifying 20 amino acids and stop signals, and tuning mitochondria protein translation and function8,12. Several writers install m3C on tRNAs13–17. Mettl6 is a cytosolic tRNA (cyto-tRNA) m3C methyltransferase regulating stem cell pluripotency and tumor cell growth14, whereas Mettl8 is an mt-tRNA m3C methyltransferase regulating mitochondria protein translation and activity in immortal and cancer cell lines and promoting pancreatic cancer cell growth and regulating mouse embryonic stem cell differentiation in vitro15,16,18,15,19. However, distinct from normal cells, metabolic reprogramming is a hallmark of immortal cell lines and cancer cells, characterized by aerobic glycolysis and enhancement of mitochondrial biogenesis20, and the physiological role and mechanism of Mettl8 and mt-tRNA m3C modification in intact biological systems remain elusive. The genome-wide m3C modification targets of Mettl8 are also unknown.
During mammalian cortical neurogenesis, neural stem cells sequentially generate deep- and superficial-layer neurons and become gradually depleted21. The precise timing of neurogenesis and maintenance of neural stem cells are critical for producing proper numbers of neurons of different cortical layers22. m6A modification is known to regulate cortical neurogenesis tempo via promoting mRNA decay23,24 and nuclear export25. Mitochondria are also critically involved in regulating cortical neurogenesis through their dynamics, homeostasis, and generation of reactive oxygen species, ATP, and TCA cycle metabolites26–28. Here we investigated the physiological role and mechanism of Mettl8 and mt-tRNA m3C modification in cortical neurogenesis in mice and human induced pluripotent stem cell (iPSC)-derived forebrain organoids. We showed that Mettl8 deletion leads to decreased m3C modification specifically on mt-tRNAThr/Ser(UCN), reduced mitochondria protein translation, and deficient mitochondrial function in cortical neural stem cells. Functionally, Mettl8 deletion leads to accelerated depletion of cortical neural stem cells with increased neuronal differentiation, which were largely rescued by pharmacologically enhancing the respiration function of mitochondria.
RESULTS
Mettl8 installs m3C on mt-tRNAThr/Ser(UCN) in mouse embryonic cortical neural stem cells
To explore the physiological role of Mettl8 in the nervous system in vivo, we generated a Mettl8flox/flox (Mettl8f/f) mouse line by inserting two loxP sites flanking the 3rd exon (92 bp) of the Mettl8 gene, deletion of which results in a premature stop codon. We confirmed the exon 3 deletion in the Nestin-Cre::Mettl8f/f (cKO) mouse brain at embryonic day 13.5 (E13.5) and postnatal day 14 (P14) and in neural progenitor cells (NPCs) cultured from the E17.5 cerebral cortex of cKO mice (Figure S1A and Table S1). We found mainly one long isoform and one short splicing isoform of Mettl8 expressed in the wild-type (WT, Mettl8f/f) and cKO mouse brains (Figure S1B). We cloned both isoforms from WT and cKO brains into an expression vector with a HA tag and found that only the long isoform from WT cDNA can be translated into a detectable protein (Figure S1C).
Mettl8 cKO mice survive into adulthood. Analysis of the P1 mouse cortex showed similar cortical thickness (Figure S1D), but more Ctip2+ deep-layer neurons and fewer Cux1+ superficial-layer neurons in cKO compared to WT mice, indicating aberrant cortical neurogenesis (Figures S1E–G).
To explore the underlying molecular mechanism, we first examined the subcellular localization of Mettl8. To overcome a lack of functional antibodies that can detect the endogenous Mettl8 protein, probably due to its low expression levels, we overexpressed mouse full-length Mettl8 and found it was co-localized with a mitochondria marker mt-Co1 (Figure S1H) and mostly in the biochemically purified mitochondria fraction (Figure S1I) from mouse neuroblastoma N2a cell lines29, similar as in other cell lines15,16,18,30. Upon in utero electroporation, we found co-localization of overexpressed Mettl8 protein with mt-Co1 in Pax6+ neural stem cells in the ventricular zone (VZ) and in Dcx+ immature neurons in the intermediate zone (IZ) in the embryonic cerebral cortex (Figures S1J–M).
To identify targets of m3C modification by Mettl8, we performed Hydrazine-Aniline Cleavage sequencing (HAC-seq)13 using WT and cKO NPCs. Our unbiased analysis revealed Mettl8-dependent m3C modification for only one site with genome-wide significance at the 32 position of mt-tRNAThr (Figures 1A–B and Table S2). To confirm this result, we performed an independent qPCR assay based on the blockade of reverse transcription (RT) by m3C modification on RNA16. Since long and short fragments of cDNA are generated from tRNAs without and with m3C modification during RT, respectively, we designed primer sets to specifically target these long and short fragments for qPCR and used the ratio of long fragment (unmodified) versus short fragment (unmodified + modified) levels to evaluate the m3C modification levels of tRNAs (Figure S1N). Using WT and cKO NPCs, we examined all mt-tRNAs with Cytosine at the 32 position, with the exception of mt-tRNAGly due to a lack of effective primers, and 9 cyto-tRNAs previously identified to be m3C modified in the human breast cancer MCF7 cell line13. Among all tRNAs tested, mt-tRNAThr and mt-tRNASer(UCN) exhibited significant Mettl8-dependent m3C modification (Figure 1C). Careful inspection of HAC-seq data also revealed a trend of Mettl8-dependent m3C modification for mt-tRNASer(UCN) (Figure 1B). We further confirmed Mettl8-dependent m3C modification of mt-tRNAThr/Ser(UCN) in the mouse brain at E13.5 and P14 by qPCR (Figures 1D and S1O). Together, these results indicate that Mettl8 is localized in mitochondrial and regulates m3C modification levels specifically on mt-tRNAThr/Ser(UCN) in mouse cortical neural stem cells.
Figure 1. Mettl8 installs m3C modification of mt-tRNAThr/Ser(UCN) and regulates mitochondria protein translation in mouse NPCs.
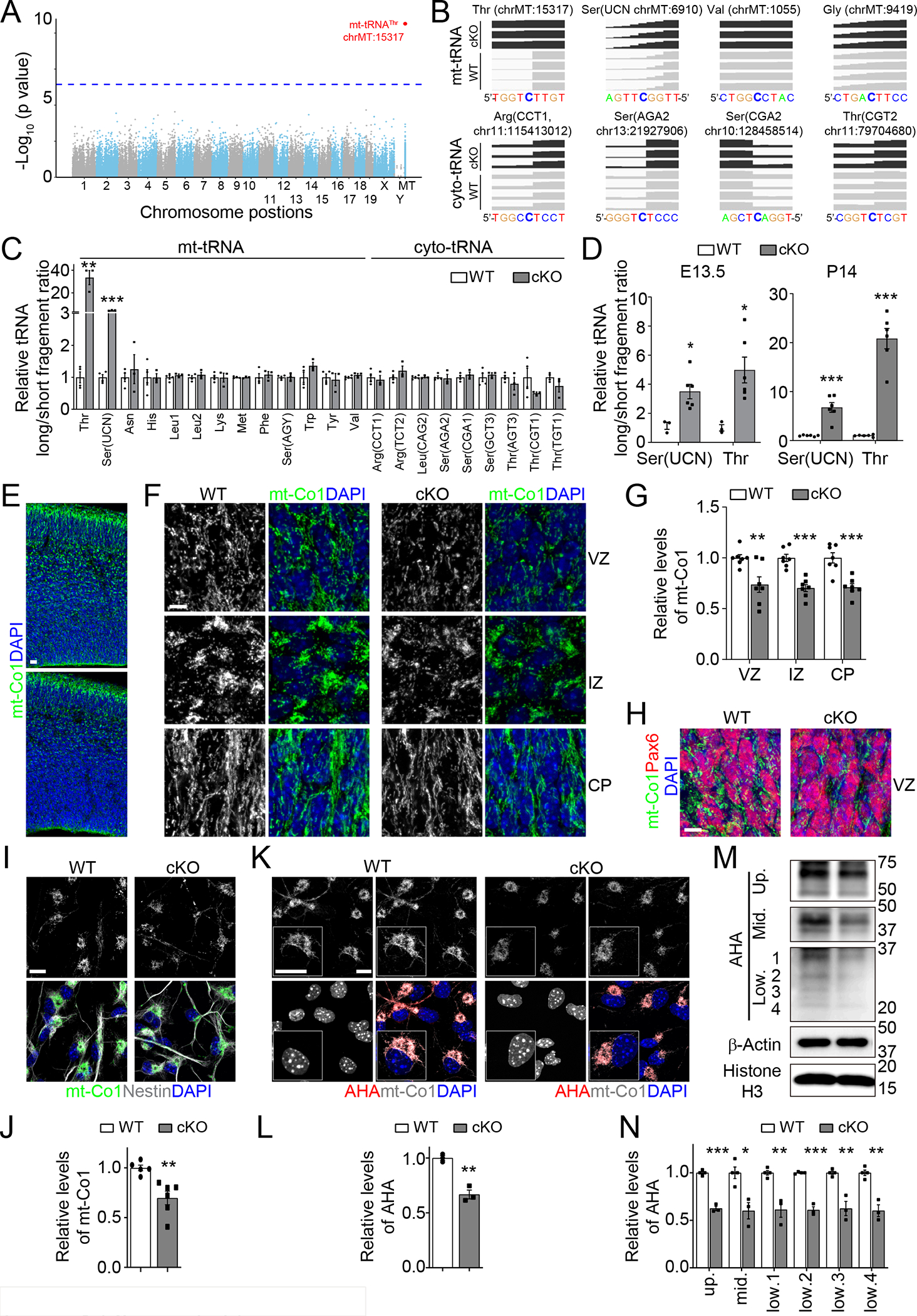
(A-B) HAC-seq analysis of NPCs derived from E17.5 WT and cKO cortex. Shown are a Manhattan plot of −Log10(P) of the differential cleavage ratio of analyzed Cytosine sites (n = 3 cKO and 4 WT) at their chromosomal positions with the blue dotted lines indicating the genome-wide significance threshold with the Bonferroni correction (p = 0.05/135582 = 3.69×10−7) (A) and alignment track of potential m3C modification sites of several mt-tRNAs and cyto-tRNAs.
(C) qPCR measurement of the long/short ratio of 13 mt-tRNAs with Cytosine at the 32 position and 9 cyto-tRNAs previously reported to contain m3C modifications13. Data were normalized to the value of WT NPCs. Values represent mean ± SEM (n = 4/WT; 3/cKO; **P < 0.01, ***P < 0.001; Student’s t-test).
(D) Mettl8-dependent m3C modification of mt-tRNAThr/Ser(UCN) measured by qPCR. Similar as in (C). Values represent mean ± SEM (E13.5 cortex: n = 3/WT, 6/cKO; P14 cortex: n = 6/WT, 6/cKO; *P < 0.05, ***P < 0.001; Student’s t-test).
(E-H) Sample confocal images of mt-Co1 immunostaining and DAPI of the E14.5 mouse cortex (E) and enlarged views for VZ, IZ and CP regions (F), and co-immunostaining of mt-Co1 and Pax6 (H). Scale bars, 20 μm (E) and 5 μm (F, H), and quantifications of the relative intensity of mt-Co1 signal of cKO compared to WT cortex (G). Values represent mean ± SEM (n = 7/WT, 7/cKO; **P < 0.01, ***P < 0.001; Student’s t-test).
(I-J) Sample immunostaining confocal images (I; Scale bar: 10 μm) and quantification of the relative intensity of mt-Co1 signal of cKO compared to WT NPCs (J). Values represent mean ± SEM (n = 5/WT, 6/cKO; **P < 0.01; Student’s t-test).
(K-N) Mettl8 deletion attenuates mitochondria protein translation in NPCs. WT and cKO NPCs were first cultured without Methionine for 1.5 hours, and then treated with cycloheximide (50 μg/mL) for 0.5 hour, followed by Methionine-depleted media containing cycloheximide and AHA (300 μM for K-L and 500 μM for M-N) for 3 hours. Shown are sample confocal images (K; Scale bar, 10 μm) and quantification of the relative intensity of AHA signal in cKO compared to WT NPCs (L). Values represent mean ± SEM (n = 3/WT, 3/cKO; **P < 0.01; Student’s t-test). Also shown are sample Western blot images (M) and quantification of the intensity of different AHA bands (1–4 bands indicating different mitochondria proteins) normalized first with that of β-Actin and then compared with WT samples (N). Values represent mean ± SEM (n = 4/WT, 3/cKO; *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t-test).
See also Figures S1, S2 and Tables S1, S2, S3
Mettl8 regulates mitochondrial protein translation in mouse cortical neural stem cells
To explore how Mettl8 deletion may impact neural stem cells, RNA-sequencing identified 190 down- and 198 up-regulated genes in cKO compared to WT NPCs (Figure S2A and Table S3). Gene Ontology enrichment analysis of down-regulated genes shows the majority of biological pathway terms related to ATP metabolic process, oxidative phosphorylation, cellular respiration, and electron transport chain, which are closely linked to mitochondria function, and most cellular component terms related to mitochondria (Figures S2B–D and Table S3). Protein-protein interaction network analysis of downregulated genes also revealed pathways related to electron transport chain and mitochondrial translation elongation process (Figure S2E and Table S3). Thus, unbiased transcriptome analysis suggests abnormal mitochondria function in cKO NPCs.
To investigate the mechanism underlying mitochondrial abnormalities in cKO NPCs, we examined mitochondrial protein translation. Immunostaining showed decreased expression of mitochondrial genome encoded mt-Co1 protein in different cortical layers of cKO compared to WT mice at E14.5, including Pax6+ neural stem cell-enriched VZ (Figures 1E–H), and in cultured cKO NPCs (Figures 1I–J). Western blot analysis showed decreased levels of mt-Co1 and mitochondrial genome-encoded mt-Co2 and mt-ATP6 proteins in the cKO E13.5 cortex and cultured NPCs compared to WT counterparts (Figures S2F–I). The mRNA levels of these genes were not different in E13.5 cortex and cultured NPCs between WT and cKO samples, with the exception of a mild reduction of mt-ATP6 mRNA levels in cKO NPCs (Figure S2J).
We specifically examined whether mitochondria protein translation is affected in NPCs using two independent assays. First, we treated cultured NPCs with chloramphenicol (CAP), a specific mitochondria protein translation inhibitor31, followed by time course analysis of recovery upon inhibition release. While CAP treatment drastically decreased mt-Co1 protein levels at 48 hours, expression levels were lower in cKO compared to WT NPCs at 1.5 and 5 hours upon release from CAP treatment (Figures S2K–L), indicating lower levels of de novo translation of mt-Co1 protein in cKO NPCs. Second, we directly monitored de novo mitochondria protein translation in NPCs with the incorporation of methionine analogue L-Azidohomoalanine (AHA)32. Upon inhibition of cytosolic protein translation by cycloheximide33, the AHA signal was exclusively concentrated in mt-Co1+ mitochondria (Figure 1K), indicating the specificity of this assay. Both immunostaining and Western blot analyses showed attenuated levels of mitochondria incorporation of AHA in cKO compared to WT NPCs under cycloheximide treatment (Figures 1K–N and S2M), whereas cytosolic protein translation was unaffected (Figures S2N–Q). Together, these results suggest that deficits of m3C modification of mt-tRNAs leads to impaired mitochondria protein translation in mouse cKO NPCs.
Mettl8−/− NPCs exhibit attenuated mitochondria activity and increased differentiation
We next examined the impact of Mettl8 deletion on mitochondria function of NPCs. Analysis using mitochondria membrane potential (MMP)-indicator MitoTracker Orange showed a modest but significant decrease of MMP in cKO compared to WT cultured NPCs, which became insignificant upon piracetam treatment, which enhances the respiration function of mitochondria34,35 (Figure 2A). We next performed the Seahorse assay to directly measure O2 consumption rates and mitochondria respiration activity36. Levels of basal respiration and ATP-linked respiration were reduced in cKO compared to WT NPCs, whereas piracetam treatment restored them to levels comparable with WT NPCs without treatment (Figures 2B–C). Together, these results indicate that mitochondria function in cKO NPCs is impaired, which can be restored by piracetam treatment.
Figure 2. Mettl8 deletion attenuates mitochondria respiration activity and NPC maintenance.
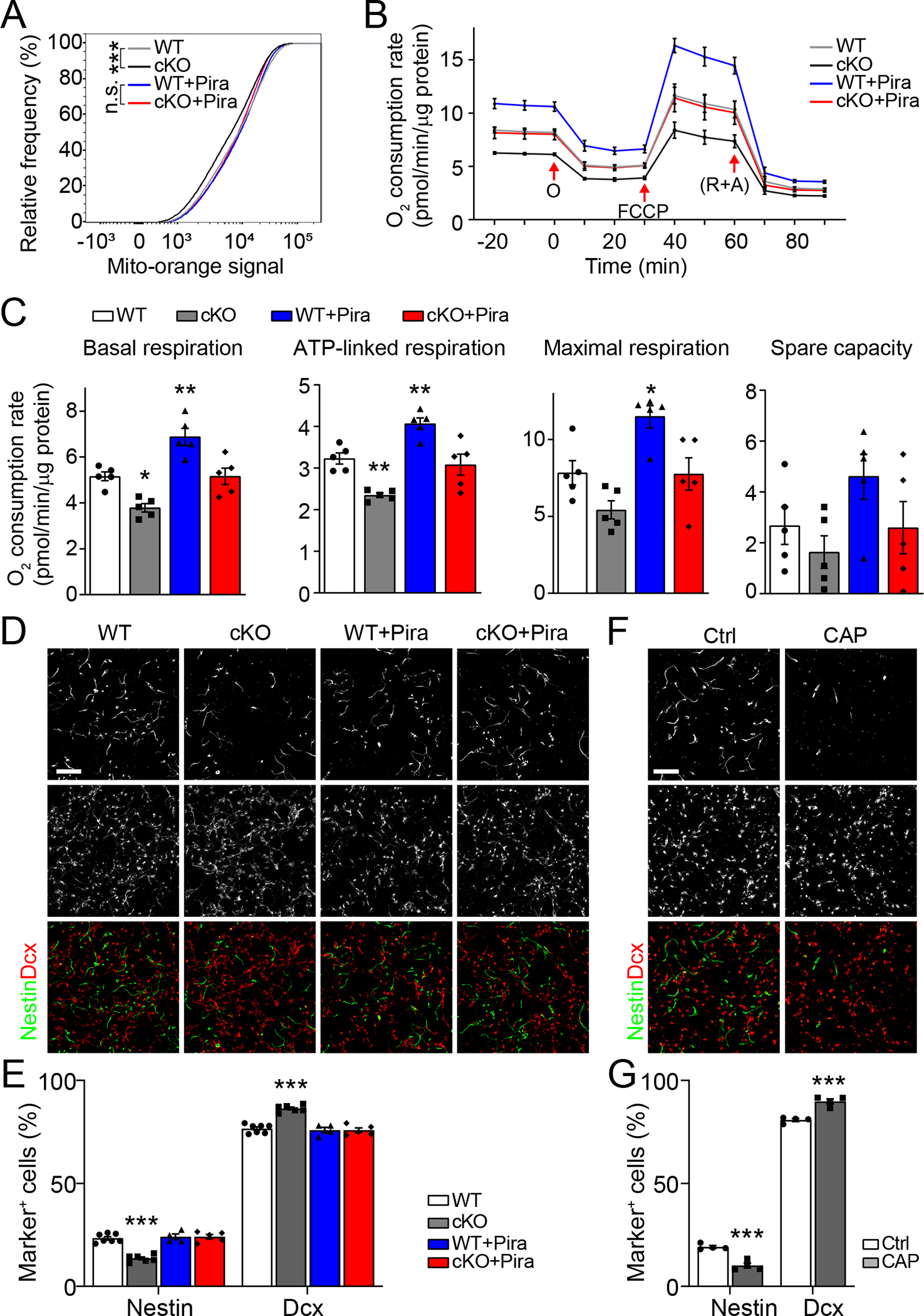
(A) Accumulative distributions of Mito-Orange signal levels. WT and cKO NPCs cultured with or without piracetam (Pira; 1 mM), were stained with 50 nM MMP-indicator Mito-Orange before flow cytometry analysis. The percent frequency distribution across every 400 bins of Mito-Orange signal intensity was used for quantification (number of cells analyzed: n = 48,546/WT, 49,548/KO, 50,688/WT+Pira, 53,196/KO+Pira; n.s.: P > 0.05, ***P < 0.001; Kolmogorov–Smirnov test).
(B-C) Summary of Seahorse analysis measuring O2 consumption of WT and cKO NPCs with or without piracetam (Pira) treatment (B) and quantification of levels of the basal respiration, ATP-linked respiration, maximal respiration and spare activity (C). Values represent mean ± SEM (n =5/WT, 5/cKO, 5/WT+Pira, 5/cKO+Pira; *P < 0.05; **P < 0.01; One-way ANOVA). O: oligomycin; (R + A): Rotenone + Antimycin A.
(D-E) Sample confocal images of immunostaining for Nestin and Dcx in WT and cKO primary NPCs cultured with or without piracetam (1 mM) for 48 hours (D; Scale bar: 100 μm) and quantification (E). Values represent mean ± SEM (n = 7/WT, 6/cKO, 4/WT+Pira, 5/cKO+Pira; ***P < 0.001; One-way ANOVA).
(F-G) Pharmacological inhibition of mitochondrial protein translation with CAP (10 μg/mL) for 48 hours leads to similar deficits in primary NPCs as Mettl8 deletion. Similar as in (D-E). Values represent mean ± SEM (n = 4/Ctrl, 4/CAP; ***P < 0.001; One-way ANOVA).
See also Figure S2
Next, we examined the impact of Mettl8 deletion and impaired mitochondrial function on NPC properties. Under the culture condition that supports primary NPCs to either maintain the neural stem cell state or differentiate37, we found the percentages of Nestin+ NPCs and Dcx+ neurons decreased and increased, respectively, in cKO compared to WT samples (Figures 2D–E). Importantly, piracetam treatment rescued these cell fate alterations in cKO samples (Figures 2D–E). The observed effect was not due to selective cell survival (Figures S2R–S).
To further support that reduced mitochondria protein translation in Mettl8 cKO NPCs is the underlying cause of impaired neural stem cell maintenance, we pharmacologically inhibited the mitochondria protein translation in primary WT NPCs by short-term CAP treatment (Figures S2T–U). Compared to the saline control, short-term CAP treatment led to a decreased percentage of Nestin+ NPCs and an increased percentage of Dcx+ neurons, phenocopying Mettl8 cKO samples in vitro (Figures 2F–G).
Mettl8−/− mice exhibit deficits in embryonic cortical neural stem cell maintenance
We next performed in vivo analysis. Pax6+ neural stem cells in the VZ become gradually depleted from E13.5 to E16.5 during embryonic mouse cortical development21 (Figures 3A–B). We found fewer Pax6+ neural stem cells, together with higher ratios of Tbr2+ intermediate neural progenitors (IPCs) over Pax6+ neural stem cells in the E13.5, E14.5 and E16.5 cerebral cortex of cKO compared to WT mice (Figures 3A–C). Analysis of cleaved-caspase3 expression and 2 hour EdU pulsing in cKO and WT E13.5 mice indicated no differences in cell survival or proliferation (Figures S3A–D). Together, these results suggest that Mettl8 deletion promotes depletion of the neural stem cell pool with increased neuronal differentiation during embryonic cortical development.
Figure 3. Mettl8 deletion leads to deficits in neural stem cell maintenance in vivo.
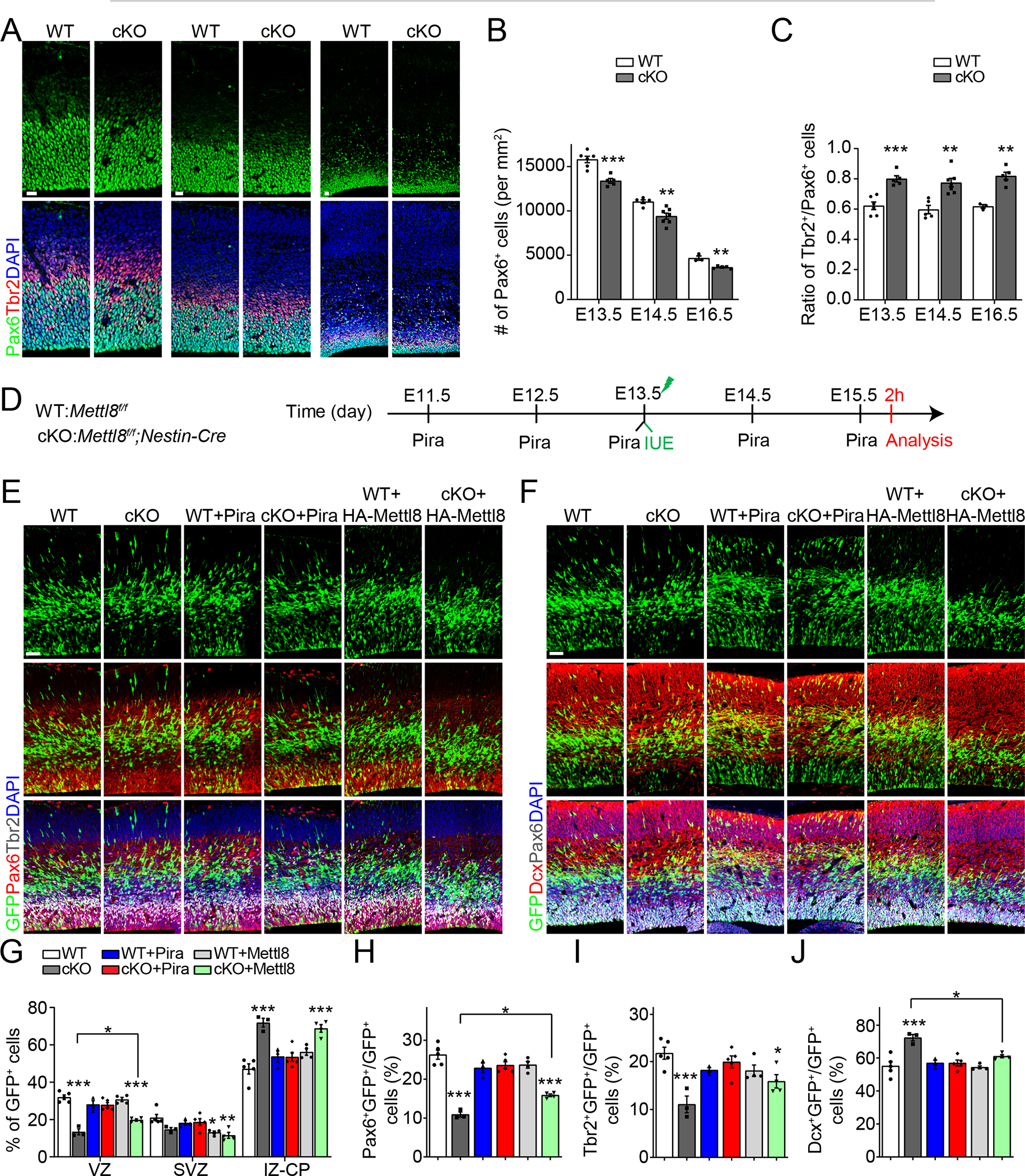
(A-C) Sample immunostaining confocal images (A; Scale bars: 20 μm) and quantification of the density of Pax6+ neural stem cells (B) and the ratio of Tbr2+ cells over Pax6+ cells (C) in WT and cKO cortex. Values represent mean ± SEM (E13.5: n = 6/WT, 5/cKO; E14.5: n = 5/WT, 7/cKO; E16.5: n = 3/WT, 5/cKO; *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t-test).
(D-J) Mettl8 cKO mice exhibit deficits in neural stem cell maintenance with increased neuronal differentiation, which were rescued by piracetam treatment (500 mg/kg body weight). Shown are schematic diagram of experimental design (D), sample immunostaining confocal images (E, F; Scale bars: 50 μm) and quantifications for percentages of GFP+ cells distributed across different layers of the cortex (G) and percentages of different cell types among all GFP+ cells (H-J) in WT and cKO mice. Values represent mean ± SEM (n = 5/WT, 3/cKO, 3/WT+Pira, 5/cKO+Pira, 4/WT+Mettl8, 4/cKO+Mettl8; *P < 0.05, ***P < 0.001; One-way ANOVA).
See also Figure S3
To further support this model, we injected EdU into mice at E13.5 and analysis of distribution of EdU+ cells across cortical layers 24 hours later showed a reduced percentage of EdU+ cells localized in the VZ and increased percentages of EdU+Ki67− cells and EdU+Ctip2+ cells among all EdU+ cells in cKO compared to WT cortex, indicating increased cell cycle exit and neuronal differentiation of dividing neural progenitors (Figures S3E–G). To specifically label neural stem cells lining the lateral ventricle surface, we performed in utero electroporation at E13.5 to express GFP and characterized the cell fate of GFP+ progenies in WT and cKO mice at E15.5 (Figure 3D). The percentage of GFP+ cells localized in the VZ was decreased, with an increase in the IZ and cortical plate (CP) in cKO compared to WT cortex (Figures 3E and 3G), consistent with the notion of decreased neural stem cells in the VZ and increased generation of neurons, which migrate to IZ and CP. Indeed, percentages of GFP+Pax6+ neural stem cells and GFP+Tbr2+ IPCs among GFP+ cells were reduced and the percentage of GFP+Dcx+ immature neurons among GFP+ cells was increased in cKO compared to WT cortex (Figures 3E–J). Electroporation of plasmids co-expressing GFP and full-length WT Mettl8 partially rescued cellular deficits in cKO mice and the partial rescue is likely due to the time delay before the construct was highly expressed (Figures 3E–J). Injection of piracetam, which can penetrate the placenta and blood brain barrier38, from E11.5 to E15.5, also largely rescued all cellular deficits in cKO mice (Figures 3E–J), indicating that impaired mitochondria function is the cause of deficits in neural stem cell maintenance with increased neuronal differentiation in vivo. Similar to Mettl8 overexpression, piracetam did not enhance neural stem cell maintenance in WT mice (Figures 3E–J), indicating a threshold effect for Mettl8 levels and mitochondrial activities on neural stem cell maintenance.
During embryonic cortical neurogenesis, cortical neurons are sequentially generated with deep-layer neurons born first and superficial-layer neurons born later21. To examine the impact of Mettl8 deletion-induced accelerated neural stem cell depletion on generation of cortical neurons of different layers, we injected EdU into WT and cKO mice at E11.5, E13.5 and E15.5 and examined the number of neurons born at these time points by analysis of EdU+ cells at P1. Numbers of EdU+Ctip2+ deep-layer neurons born at E11.5 and E13.5 were increased (Figures S3H–K), whereas numbers of EdU+Satb2+ and EdU+Cux1+ superficial-layer neurons born at E15.5 were decreased in cKO compared to WT mice (Figures S3L–O).
Together, these results show that Mettl8 deletion leads to maintenance deficits with increased neuronal differentiation of embryonic cortical neural stem cells, resulting in excess numbers of early-born deep-layer cortical neurons and reduced numbers of later-born superficial-layer cortical neurons in mice in vivo.
METTL8 regulates mitochondria protein expression and maintenance of cortical neural stem cells in human forebrain organoids
Finally, we examined the role of METTL8 in human cortical neurogenesis using a human iPSC-derived forebrain organoid model39 (Figure S4A). We generated isogenic METTL8 knockout human iPSCs with deletion of the third exon (92 bp) of the human METTL8 gene, which causes a reading frame shift and premature stop codon, and we used three different clones (KO1-3 iPSCs) for our analysis. We also transfected the same founder iPSC line with a gRNA targeting the human safe harbor-AAVS1 locus and selected four different clones as controls (WT1-4 iPSCs). We confirmed the deletion of Exon 3 of the METTL8 gene in KO organoids at day 33 (D33, Figure S4B). qPCR analysis of the long and short fragments of cDNAs generated from mt-tRNAThr and mt-tRNASer(UCN) during RT showed increased ratios in KO compared to WT organoids at D33, which were enriched with neural stem cells at this stage39 (Figures 4A–C). Thus, METTL8 also regulates m3C modification of mt-tRNAThr/Ser(UCN) in human cortical neural stem cells. Furthermore, immunostaining showed decreased levels of mt-CO1 in SOX2+ radial glia neural stem cells localized at the VZ region of KO compared to WT organoids (Figures 4C–D and S4C). Western blot analysis of D33 organoids also showed decreased expression levels of mt-CO2 and mt-ATP6 proteins (Figures S4D–E) with no change in mRNA levels (Figure S4F) in KO compared to WT organoids.
Figure 4. METTL8 deletion reduces mitochondria protein expression and impairs radial glia neuronal stem cell maintenance in human forebrain organoids.
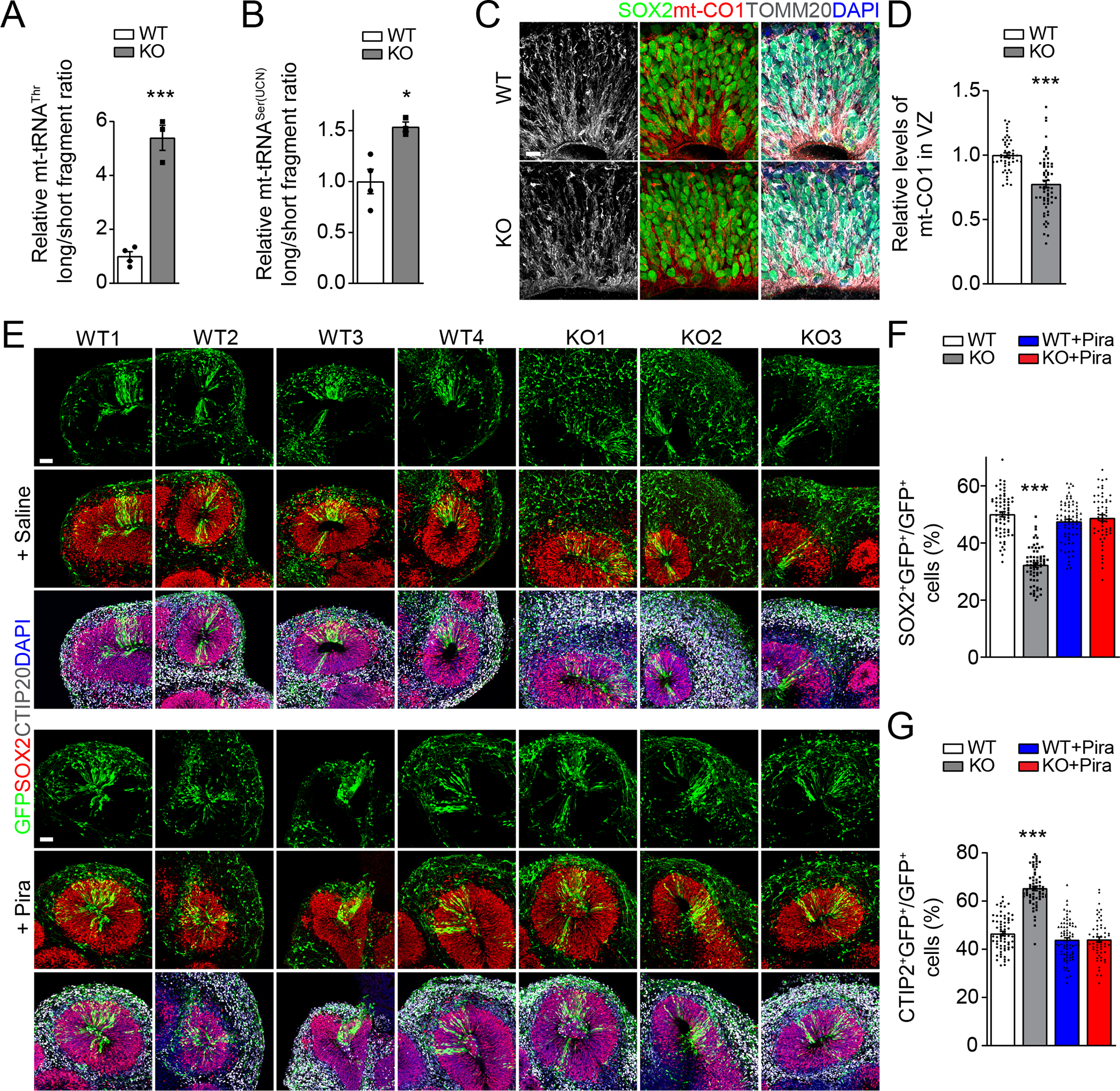
(A-B) Reduced m3C modification on mt-tRNAThr/Ser(UCN) in day 33 (D33) METTL8 KO organoids. Similar as in Figures 1C–D. Individual dots represent data from organoids derived from each iPSC line. Values represent mean ± SEM (n = 4/WT, 3/KO iPSC lines; *P < 0.05; ***P < 0.001; Student’s t-test).
(C-D) Sample immunostaining confocal images (C; Scale bar: 10 μm) and quantification of the relative intensity of mt-CO1 signal after normalization with the number of SOX2+ neural stem cells at the VZ regions of WT and KO D33 organoids (D). Individual dots represent data from each rosette of organoids derived from either WT or KO iPSC lines. Values represent mean ± SEM (n = 48 rosettes from 4 WT iPSC lines, 61 rosettes from 3 KO iPSC lines; ***P < 0.001; Student’s t-test).
(E-G) Sample immunostaining confocal images (E; Scale bars: 50 μm) for human organoids from each WT and KO iPSC line with or without treatment of piracetam (1 mM; from D34 to D56), injected with GFP-expressing retrovirus at D42 and analyzed 14 days later and quantification of percentages of SOX2+GFP+ neural stem cells (F) and CTIP2+GFP+ neurons (G) among all GFP+ cells in WT and KO organoids at D56. Values represent mean ± SEM (n = 68 sections from 4 WT iPSC lines, 64 sections from 3 KO iPSC lines, 76 sections from 4 WT iPSC lines with Pira treatment, 56 sections from 3 KO iPSC lines with Pira treatment; ***P < 0.001; One-way ANOVA).
See also Figure S4
To examine the impact of METTL8 deletion specifically on radial glia neural stem cells in forebrain organoids, we injected GFP-expressing retrovirus into the lumen of D42 organoids to specifically target dividing neural stem cells lining the lumen and permanently label their progeny for analysis of their cell fate 14 days later (Figure S4A). We found a decreased percentage of GFP+SOX2+ neural stem cells and an increased percentage of GFP+CTIP2+ neurons among all GFP+ cells in organoids from all KO iPSC lines compared to those of all WT iPSC lines (Figures 4E–G and S4G–H). Importantly, these deficits were largely rescued by long-term piracetam treatment starting from D34 (Figures 4E–G and S4A, S4G–H).
Together, these results indicate that METTL8 regulates m3C modification of mt-tRNAs and mitochondria protein expression, and METTL8 deletion leads to impaired maintenance of cortical neural stem cells with increased neuronal differentiation in human forebrain organoids, revealing a conserved function and mechanism of METTL8 in regulating both mouse and human brain development.
DISCUSSION
Our results support a model that Mettl8 installs m3C on mt-tRNAThr/Ser(UCN) in cortical neural stem cells to promote mitochondrial protein translation and function, which is required for maintaining neural stem cells during embryonic cortical development. Beyond previous findings of Mettl8-mediated m3C modifications on mt-tRNAs in immortal and cancer cells15,16,18,30, our study reveals its physiological role and mechanism in an intact biological system in vivo and showed its conservation in the human brain.
Role of Mettl8 and mt-tRNA m3C modification on mitochondrial properties and cortical neural stem cell maintenance
Using conditional brain-specific Mettl8 knockout mouse and isogenic METTL8 knockout human iPSC-derived forebrain organoid models, we showed that methyltransferase Mettl8 installs m3C modification specially on mt-tRNAThr/Ser(UCN) and plays a conserved role in regulating mitochondrial protein expression and promoting neural stem cell maintenance. Our results support a model that Mettl8 deletion shifts cell division of NPCs from the self-renewing mode to neurogenic stem cell depletion mode during embryonic cortical neurogenesis. Importantly, the Mettl8 deletion-induced cellular phenotypes are rescued by pharmacologically enhancing mitochondrial function, whereas short-term pharmacological reduction of mitochondrial protein translation is sufficient to mimic Mettl8 deletion-induced cellular phenotypes. Together, these results provide a causal link from Mettl8-mediated mt-tRNA m3C modification, mitochondrial protein translation and function, to cortical neural stem cell maintenance.
Our genome-wide HAC-seq and targeted qPCR results showed Mettl8-dependent m3C modification specifically at the position 32 of mt-tRNAThr and mt-tRNASer(UCN), but not on other mt-tRNAs, cyto-tRNAs or mRNA in NPCs. The m3C modification of mt-tRNA generates an extra positive charge16, potentially modulating the folded structure18, or the binding or dissociating capacity to ribosomes or proteins. About 90% of mt-tRNAThr is m3C modified based on HAC-seq, and the molecular amount and charged percentage of mt-tRNAThr are one of the lowest among mt-tRNAs40, raising the possibility that m3C modified mt-tRNAThr with one extra positive charge may serve as one of the rate-limiting and tunable factors for mitochondria protein elongation.
Although stem cells were previously thought to exhibit a highly glycolytic metabolism and little to no reliance on mitochondrial OXPHOS for ATP production, mitochondrial activity, beyond just ATP generation, has been accepted to play vital roles in regulating various aspects of neural stem cells, including affecting stem cell fate by intermediate metabolites from the TCA cycle and the NAD+:NADH ratio, promoting neural stem cell differentiation by physiological ROS, and regulating neural stem cell maintenance or differentiation by mitochondria dynamics26–28,41.
Diverse epitranscriptomic mechanisms that regulate cortical neurogenesis
The physiological and pathological functions of Mettl8 are not well understood. Notably, m3C has been implicated in human diseases42. For example, METTL8 upregulation is associated with aggressive pancreatic cancers and lung squamous cell carcinoma15,43,44, and frameshift mutations in METTL8 have been identified in colon cancers45. Our study reveals a critical epitranscriptomic mechanism involving mt-tRNA m3C modification in the nervous system, specifically in regulating cortical neurogenesis, via regulating mitochondrial protein translation. Our study expands a growing list of epitranscriptomic modifications regulating mammalian cortical neurogenesis. For example, deletion of m6A methyltransferase Mettl14 leads to prolonged cell cycle of radial glia neural stem cells in both mice and human forebrain organoids23. Mettl14 deletion in cortical neural stem cells leads to attenuated decay23,24, nuclear export deficits25, and alternative splicing46 of m6A-tagged mRNA. Loss-of-function mutations in m5C methyltransferase Nsun2 causes microcephaly in mouse and human models47. NSUN2-mediated m5C mRNA methylation promotes mRNA nuclear export48 and may cooperate with m6A to enhance translation of specific transcripts such as p2149. Mutations in PUS7, a tRNA and mRNA pseudouridine (Ψ) synthase, are associated with microcephaly, intellectual disability, speech delay, and aggressive behavior in multiple patients50,51, potentially due to dysregulated protein translation52. Hundreds of pre-mRNA sites are direct targets of Ψ synthases PUS1, PUS7, and RPUSD4, which regulate alternative pre-mRNA splicing53. Mutations of tRNA m1G9 methyltransferase TRMT10A lead to microcephaly in humans54. Mutations in METTL5, which adds m6A to 18S rRNA55, are associated with autosomal-recessive microcephaly and intellectual disability56. Together, these studies highlight diverse roles and potential mechanisms of various epitranscriptomic modifications in regulating mammalian cortical neurogenesis.
Limitations of the study
First, we presented evidence on the mitochondrial localization of Mettl8 based on overexpression, instead of the endogenous protein, due to a lack of sensitive antibodies. Our further results on deficits of specific mt-tRNA m3C modifications, mitochondria protein translation and properties in Mettl8 cKO NPCs support our conclusion. Future studies with better antibodies or with a knock-in tag may reveal the endogenous distribution of Mettl8 in neural stem cells. Second, our study focuses on the epitranscriptomic role of Mettl8 on neural stem cells, whereas Mettl8 also regulates m3C modification of mt-tRNAs and mitochondrial protein expression in neurons. Therefore, Mettl8 may regulate other aspects of the nervous system, which are interesting topics for future studies with tools generated in the current study.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Guo-li Ming (gming@pennmedicine.upenn.edu).
Material Availability
All unique/stable reagents and biological material generated in this study are available from the Lead Contact, Dr. Guo-li Ming (gming@pennmedicine.upenn.edu), with a completed Materials Transfer Agreement.
Data and Code Availability
The RNA-seq and HAC-seq data reported in this study are deposited in GEO (GSE214445).
The code for HAC-seq analysis can be found at https://doi.org/10.5281/zenodo.7535193, which is maintained by Dr. Daniel Y. Zhang.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All experimental procedures with mice used in this study were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of University of Pennsylvania. All mice were kept in cages with bedding materials and housed in a 14-hour light/10-hour dark cycle with regular food and water changes. C57BL/6 mice and pregnant CD1 mice at E17.5 were purchased from Charles River Laboratories. Mettl8f/f mice, with insertion of two loxp sites flanking the 3rd exon (92 bp) of the Mettl8 gene, were constructed through CRISPR/Cas9 mediated homologous recombination by injecting the mixture of Cas9 protein/gRNA/donor DNA into fertilized eggs. The Mettl8f/f mice used in this study were backcrossed for at least four generations and maintained on a C57BL/6 background. Nestin-Cre (B6.Cg-Tg(Nes-cre)1Kln/J) transgenic mice were purchased from the Jackson Laboratory57. Nestin-Cre::Mettl8f/f mice (cKO mice) were used as brain-specific conditional knockout mice with littermates Mettl8f/f (WT) mice as controls. For all of experiments involving mice at embryonic stages and P1, both male and female mice were used, and no obvious sex differences were observed in experiments performed in this study. The primers used for genotyping were listed in Table S1.
2D and 3D cell cultures
Neuroblastoma (N2a) cells, HEK293 and GP2-293 Packaging Cell Line (Cellosaurus) were cultured in DMEM (Corning) with 10% fetal bovine serum (FBS, Corning) and 1% penicillin/streptomycin (P/S, Gibco), and routinely passaged every three days after digestion by 0.05% (W/V) Trypsin-EDTA (Corning) for 3–5 mins. pCAGIG-GFP/HA-Mettl8 (full-length) plasmids were transfected into N2a cells or HEK293 cells with Lipo2000 (Invitrogen).
For primary NPC cultures, WT or Mettl8 cKO or CD1 mouse cerebral cortex at E17.5 were dissected and digested with Trypsin-EDTA at 37°C for 15 mins, and then dissociated into single cells. Primary NPCs were cultured (105/well) in plates pre-coated with 1% Matrigel and in the DMEM/F12 medium containing 2% B27 supplement, 1% N2 supplement, bFGF (20 ng/mL), EGF (20 ng/mL), 1% Glutamax, 1% P/S with digestion using Accutase (ThermoFisher) for passaging. For analysis of neural stem cell maintenance, primary NPCs were seeded (2×105/well) on plates pre-coated with 20 ng/mL poly-D-Lysine (pdL) and cultured first in DMEM/10% FBS/1% P/S for 2 hours and then in the DMEM/F12 medium containing 1% N2 supplement, bFGF (20 ng/mL), 1% Glutamax, 1% P/S for 48 hours37.
The founder human iPSC line (C1-2) used in the current study was previously generated from male neonatal foreskin fibroblasts from ATCC (CRL-2097) and fully characterized58–61. Generation of iPSC lines followed institutional IRB and ISCRO guidelines and was approved by Johns Hopkins University School of Medicine. All studies related with human iPSCs were performed in accordance with approved protocols of the University of Pennsylvania. Human iPSCs were cultured in the hESC-qualified Matrigel-coated plates in a feeder-free way and maintained in mTeSR plus medium (StemcellTech), with culture medium replaced every day. For passaging every week, iPSCs were incubated with ReLeSR reagent (StemcellTech) for 3–5 mins, and detached iPSC clumps were broken into smaller clusters by gentle trituration using 1 mL pipette tips and then were seeded onto freshly coated plates. C1-2 iPSC lines were confirmed to have a normal karyotype and tested negative for Mycoplasma, Acholeplasma, and Spiroplasma using EZ-PCR™ Mycoplasma Detection Kit (Biological Industries).
Generation of forebrain organoids was performed as described previously with minor modification39,62. Briefly, iPSCs were detached by incubation with ReLeSR reagent for 3–5 mins, transferred to an ultra-low attachment U-bottom 96-well plates (50K cells/well) and cultured in mTeSR plus media supplemented with 10 μM Y-27632 (StemcellTech) for 2 days for Embryoid Body (EB) aggregation. On Day 3–7, EBs were transferred to an ultra-low attachment 6-well plate (Corning Costar) and cultured in H1 neural induction medium containing DMEM/F12 supplemented with 20% KnockOut Serum Replacement, 1% P/S, 1% MEM-NEAAs, 1% GlutaMax, 0.1 mM 2-mercaptoethanol, 0.0002% Heparin, 5 μM SB-431542 (StemcellTech) and 1 μM LDN-193189 (StemcellTech). On Day 6, half of the medium was replaced with F2 forebrain induction medium containing DMEM/F12 supplemented with 1% N2 supplement, 1% P/S, 1% MEM-NEAAs, 1% GlutaMax, 0.1 mM 2-mercaptoethanol, 1 μM SB-431542, and 1 μM CHIR99021 (StemcellTech). On Day 7, organoids were embedded in Matrigel and cultured in F2 medium for 7 days. On Day 14, embedded organoids were dissociated from Matrigel by gentle pipetting, transferred to an ultra-low attachment 6-well plate placing on a CO2 resistant orbital shaker (ThermoFisher) and cultured in H3 differentiation medium containing DMEM/F12 supplemented with 1% B27 supplement, 2% N2 supplement, 1% P/S, 1% MEM-NEAAs, 1% GlutaMax, 0.1 mM 2-mercaptoethanol, and 3 mg/L human insulin (Sigma).
For mitochondria protein translation inhibition or piracetam rescue experiments, mouse NPCs or human forebrain organoids were treated with 10 μg/mL chloramphenicol (CAP, Sigma) dissolved in ethanol or 1 mM piracetam dissolved in PBS, respectively. For treatment of brain organoids with piracetam, H3 medium containing piracetam was changed every 48 hours.
METHOD DETAILS
Molecular cloning and constructs
Both the long and short isoforms of Mettl8 were amplified by PCR from complementary DNA cDNA) of WT and cKO mouse brains at P14 and cloned into the pCAGIG plasmids with a C-terminal HA tag63 (Addgene plasmid #11159). Paired gRNAs targeting the 2nd intron and 3rd intron of human METTL8, respectively, or gRNAs targeting human AAVS1 locus were designed by Benchling website (https://www.benchling.com/), and the corresponding DNA oligonucleotides were annealed and ligated with BbsI digested pSpCas9(BB)-2A-Puro plasmids (PX459, Addgene plasmid #62988), which express Cas9 protein, gRNA and are puromycin resistant64. The sequences of these plasmids were verified by Sanger sequencing. The sequences of primers used for Mettl8 cloning and DNA oligonucleotides of gRNA are listed in Table S1.
Tissue processing and immunostaining
NPCs or N2a cells were fixed with 4% paraformaldehyde (PFA) at room temperature for 30 mins, and immersed in PBS at 4°C. For tissue histology, the mice were first perfused with ice-cold PBS for 10 mins, followed by ice-cold 4% PFA for 5 mins. Dissected mouse brains were fixed in 4% PFA at 4°C overnight and then dehydrated in 30% sucrose in PBS at 4°C for 48 hours. Coronal brain serial sections at 30 μm thickness were sequentially attached on 5 slides using a cryostat (Leica, CM3050S) or a sliding microtome (Leica, SM2010R). Forebrain organoids were first fixed in 4% PFA at room temperature for 1 hour and dehydrated in 30% sucrose at 4°C overnight.
When antigen retrieval was necessary for some antibodies, brain or organoid sections were incubated in 1x Target Retrieval Solution (Agilent Dako) at 95°C for 10 mins, followed by cooling to room temperature. For immunostaining, sections or fixed cells were first washed in the blocking buffer (5% BSA, 10% FBS, 0.3% Triton X-100, 0.01% NaN3 dissolved in PBS) at room temperature for 1 hour, and then incubated in the primary antibody diluted in the blocking buffer at 4°C overnight. After washes in PBS for 3×10 mins, the sections or cells were incubated in the Alexa Fluor 488, 568, 647 (Jackson ImmunoResearch or Invitrogen)-conjugated secondary antibodies (1:500) and DAPI (Roche, 1:500) diluted in the blocking buffer at room temperature for 1–2 hours. After a second round of washing with PBS, the sections or cells were mounted with Aqua-Mount Mounting Medium (EMSCO/FISHER). EdU staining was performed by following the manufacturer’s instructions of Click-iT EdU Alexa Fluor 647 Imaging Kit (EMSCO/FISHER) after secondary antibody staining. The antibodies used for immunostaining were listed in the Key Resource Table. The confocal images with multiple z stacks were acquired by Zeiss LSM810 confocal microscopy with 20X, 40X or 63X objectives.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-GFP | Rockland | Cat#600101215; RRID: AB 11181883 |
| Rabbit polyclonal anti-Cleaved caspase3 | Cell Signaling Technology | Cat#9661; RRID: AB_2341188 |
| Rat polyclonal anti-Ctip2 | Abcam | Cat#ab18465; RRID: AB_2064130 |
| Rabbit polyclonal anti-Cux1 | Santa Cruz | Cat#sc-13024; RRID: AB_2261231 |
| Guinea pig polyclonal anti-DCX | EMD Millipore | Cat#AB2253; RRID: AB_1586992 |
| Rabbit monoclonal anti-GAPDH | Cell Signaling Technology | Cat#2118; RRID: AB_561053 |
| Rat polyclonal anti-HA | Roche | Cat#11867423001; RRID: AB_390918 |
| Rabbit polyclonal anti-Histone H3 | Abcam | Cat#ab1791; RRID: AB_302613 |
| Mouse monoclonal anti-Ki67 | BD Biosciences | Cat#550609; RRID: AB_393778 |
| Rabbit polyclonal anti-Mettl8 | Abcam | Cat#ab122273; RRID: AB_11127706 |
| Rabbit polyclonal anti-mt-ATP6 | Sigma-Aldrich | Cat#SAB5700851; |
| Rabbit polyclonal anti-mt-ATP6 | Proteintech | Cat#55313-1-AP; RRID: AB_2881305 |
| Mouse monoclonal anti-mt-Co1 (Immunostaining) | Abcam | Cat#ab14705; RRID: AB_2084810 |
| Mouse monoclonal anti-mt-Co1 (WB) | Thermo Fisher Scientific | Cat#459600; RRID: AB_1501840 |
| Rabbit polyclonal anti-mt-Co2 | Proteintech | Cat#55070-1-AP; RRID: AB_10859832 |
| Chicken polyclonal anti-Nestin | Aves labs | Cat#NES; RRID: AB_2314882 |
| Mouse monoclonal anti-Pax6 | BD Biosciences | Cat#561462; RRID: AB_10715442 |
| Rabbit polyclonal anti-Pax6 | BioLegend | Cat#901301; RRID: AB_2565003 |
| Mouse monoclonal anti-Satb2 | Abcam | Cat#ab51502; RRID: AB_882455 |
| Goat polyclonal anti-Sox2 | R&D system | Cat#AF2018; RRID: AB_355110 |
| Rabbit monoclonal anti-TBR2 | Abcam | Cat#ab183991; RRID: AB_2721040 |
| Rabbit polyclonal anti-Tomm20 | THOMAS SCIENTIFIC LLC | Cat#11802-1-AP; RRID: AB_2207530 |
| Mouse monoclonal anti-α-tubulin | Cell Signaling Technology | Cat#3873S; RRID: AB_1904178 |
| Mouse monoclonal anti-β-actin | Thermo Fisher Scientific | Cat#AM4302; RRID: AB_2536382 |
| Donkey polyclonal anti-Goat IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-11055; RRID: AB_2534102 |
| Donkey polyclonal anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat#A-21447; RRID: AB_2535864 |
| Donkey polyclonal anti-Guinea Pig IgG (H+L) AffiniPure Secondary Antibody, Alexa Fluor 488 | Jackson ImmunoResearch | Cat#706-545-148; RRID: AB_2340472 |
| Donkey polyclonal anti-Guinea Pig IgG (H+L) AffiniPure Secondary Antibody, Alexa Fluor 647 | Jackson ImmunoResearch | Cat#706-605-148; RRID: AB_2340476 |
| Donkey polyclonal anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-21202; RRID: AB_141607 |
| Donkey polyclonal anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat#A-10037; RRID: AB_2534013 |
| Donkey polyclonal anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-21206; RRID: AB_2535792 |
| Donkey polyclonal anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat#A-10042; RRID: AB_2534017 |
| Donkey polyclonal anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat#A-31573; RRID: AB_2536183 |
| Donkey polyclonal anti-Rat IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-21208; RRID: AB_2535794 |
| Donkey polyclonal anti-Chicken IgY (IgG, H+L) AffiniPure Secondary Antibody, Alexa Fluor 488 | Jackson ImmunoResearch | Cat#703-545-155; RRID: AB_2340375 |
| Anti-mouse IgG, HRP-linked Antibody | Cell Signaling Technology | Cat#7076, RRID: AB_330924 |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signaling Technology | Cat#7074, RRID:AB_2099233 |
| Bacterial and virus strains | ||
| Retro-pSUBGW-U6-Ub-GFP | (Zhang et al., 2016) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 0.05% (W/V) Trypsin-EDTA | Corning | Cat#MT25052CI |
| 10X RT buffer | Thermo Fisher Scientific | Cat#53032LT |
| 2-Mercaptoethanol | Thermo Fisher Scientific | Cat#21985023 |
| Advantage UltraPure PCR deoxynucleotide mix (10mM each dNTP) | Takara Bio | Cat#639125 |
| AMPure XP beads | Beckman Coulter | Cat#A63880 |
| AMV Reverse Transcriptase | Takara Bio | Cat#2630A |
| Aniline | THOMAS SCIENTIFIC LLC | Cat#242284 |
| Antarctic Phosphatase | New England Biolabs | Cat#M0289S |
| Aqua-Mount Mounting Medium | EMSCO/FISHER | Cat#NC9428056 |
| B-27® Supplement | Gibco | Cat#17504044 |
| Blue Protein Loading sample buffer | New England Biolabs | Cat#B7703S |
| CHIR-99021 | StemCell Technologies | Cat#72054 |
| Chloramphenicol | Sigma-Aldrich | Cat#C0378 |
| Chloroform | Sigma-Aldrich | Cat#C2432 |
| cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail | Sigma-Aldrich | Cat#11836170001 |
| Cy5 conjugated Streptavidin | Jackson ImmunoResearch | Cat#016-170-084 |
| DAPI | BD Biosciences | Cat#564907 |
| DBCO-PEG4-biotin | Sigma-Aldrich | Cat#760749 |
| DirectPCR reagent | Fisher Scientific | Cat#NC9724951 |
| DL-Dithiothreitol solution | Millipore Sigma | Cat#43816 |
| Dulbecco’s Modification of Eagle’s Medium (DMEM) | Corning | Cat#10-013 |
| Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) | Gibco | Cat#11330057 |
| Dulbecco’s Phosphate-Buffered Saline (DPBS) | Corning | Cat#14190250 |
| EcoRI-HF restriction enzyme | New England Biolabs Inc | Cat#R3101S |
| EdU | Thermo Fisher Scientific | Cat#E10187 |
| EZ-Tn5 Transposase | Lucigen | Cat#TNP92110 |
| Fast Green | VWR Chemicals | Cat#E772-25G |
| Fast SYBR Green Master Mix | Thermo Fisher Scientific | Cat#4385612 |
| Fetal Bovine Serum (FBS) | Corning | Cat#35-010-CV |
| Formaldehyde, 16%, methanol free, Ultra Pure | Polysciences | Cat#18814-10 |
| Gibco DMEM, high glucose, no glutamine, no methionine, no cystine | EMSCO/FISHER | Cat#21013024 |
| GlutaMAX supplement | GIBCO | Cat#35050061 |
| Heparin solution | StemCell tech | Cat#7980 |
| Hibernate A Low Fluorescence buffer | Brain Bits | Cat#HALF100 |
| Human EGF | Peprotech | Cat#AF-100-15 |
| Human insulin solution | Sigma-Aldrich | Cat#I9278 |
| Hydrazine | THOMAS SCIENTIFIC LLC | Cat#215155 |
| KAPA HiFi Hotstart Readymix | Emsco/Fisher | Cat#KK2601 |
| KnockOut Serum Replacement | Thermo Fisher Scientific | Cat#10828028 |
| L-Azidohomoalanine (AHA) | EMSCO/FISHER | Cat#C10102 |
| LDN-193189 | StemCell Technologies | Cat#72147 |
| Lipofectamine 2000 | Invitrogen | Cat#11668019 |
| Lipofectamine Stem Reagent | Fisher Scientific | Cat#STEM00003 |
| Matrigel (hESC-qualified) | Corning | Cat#8774552 |
| MEM Non-Essential Amino Acids Solution | Gibco | Cat#11140050 |
| MitoTracker™ Orange CMTMRos | Thermofisher scientific | Cat#M7510 |
| mTeSR Plus | StemCell Technologies | Cat#5825 |
| N-2 Supplement (100X) | Thermo Fisher Scientific | Cat#17502048 |
| Neurobasal medium | Thermo Fisher Scientific | Cat#21103049 |
| Not1 restriction enzyme | New England Biolabs Inc | Cat#R0189S |
| Nuclease-Free Water (not DEPC-Treated) | Thermo Fisher Scientific | Cat#AM9937 |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat#15140163 |
| PhosSTOP | Sigma-Aldrich | Cat#4906845001 |
| Pierce™ ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat#PI32109 |
| Piracetam | Sigma-Aldrich | Cat#P5295 |
| Polybrene | Santa Cruz | Cat#sc-134220 |
| Poly-D-Lysine | Sigma-Aldrich | Cat#P6407 |
| Polyethylene glycol (PEG) solution, 40% | Sigma-Aldrich | Cat# P1458 |
| Protease Inhibitor Cocktail | Sigma-Aldrich | Cat#P8340 |
| Proteinase K | QIAGEN | Cat#19133 |
| Puromycin, Dihydrochloride | EMD MILLIPORE | Cat#540411 |
| Recombinant Human FGF-basic | Peprotech | Cat#100-18B |
| ReLeSR reagent | StemcellTech | Cat#5872 |
| RIPA lysis buffer | Thermofisher | Cat#89900 |
| RNase Inhibitor, Murine | New England Biolabs | Cat#M0314S |
| SB-431542 | StemCell Technologies | Cat#72234 |
| SDS (10% w/v) | Fisher Scientific | Cat#50-751-7490 |
| Seahorse XF Media & Calibrant | Agilent | Cat#103680-100 |
| Seahorse XFe96 FluxPak mini | Agilent | Cat#102601 |
| SMARTScribe Reverse Transcriptase | Takara Bio | Cat#639537 |
| StemCell freezing media | ATCC | Cat#ACS-3020 |
| StemPro Accutase Cell Dissociation Reagent | Thermo Fisher Scientific | Cat#A1110501 |
| Streptavidin-HRP Conjugate | PerkinElmer | Cat#NEL750001EA |
| Sucrose | VWR Chemicals | Cat#97061-430 |
| T4 Polynucleotide Kinase | New England Biolabs | Cat#M0289S |
| Target Retrieval Solution | Agilent Dako | Cat#S1699 |
| Triton X-100 | Sigma-Aldrich | Cat#T8787 |
| TRIzol reagent | Thermo Fisher Scientific | Cat#15596026 |
| TrypLE | Fisher scientific | Cat#12604013 |
| TWEEN 20 | Sigma-Aldrich | Cat#P1379 |
| XF DMEM medium pH 7.4 | Agilent | Cat#103575 |
| Y-27632 | StemCell Technologies | Cat#72304 |
| Critical commercial assays | ||
| Advantage 2 PCR Kit | Takara Bio | Cat#639206 |
| Cell Fractionation Kit | Abcam | Cat#ab109719 |
| Click-iT Plus EdU Alexa Fluor 647 Imaging Kit | Thermo Fisher Scientific | Cat#C10640 |
| EZ-PCR Mycoplasma test kit | Biological Industries | Cat#2070020 |
| KAPA Library Quantification Kit for Illumina NGS | Kapa Biosystems | Cat#KK4835 |
| NEBNext Magnesium RNA Fragmentation Modules | New England Biolabs | Cat#E6150S |
| NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (Set 1) | New England Biolabs | Cat#E7300S |
| NEBNext® rRNA Depletion Kit v2 (Human/Mouse/Rat) | New England Biolabs | Cat#E7405S |
| NextSeq High Output v2 75 Cycles | Illumina | Cat#20024906 |
| Pierce™ Rapid Gold BCA Protein Assay Kit | Thermo Fisher Scientific | Cat#A53226 |
| Qubit dsDNA HS Assay Kit | Thermo Fisher Scientific | Cat#Q33231 |
| RNA Clean & Concentrator 5 | Zymo Research | Cat#R1013 |
| Seahorse XF Cell Mito Stress Test Kit | Agilent | Cat#103015 |
| SuperScript III First-Strand Synthesis System | Thermo Fisher Scientific | Cat#18080051 |
| Deposited data | ||
| Raw and analyzed RNA-seq and m3C-HAC-seq data | This paper | GEO: GSE214445 |
| Code for HAC-seq analysis | This paper | https://doi.org/10.5281/zenodo.7535193 |
| Experimental models: Cell lines | ||
| Mouse: Neuro-2a Neuroblastoma (N2a) cell line | ATCC | Cat#CCL-131 |
| Human: C1-2 iPSC line | (Wen et al., 2014) | https://doi.org/10.1038/nature13716 |
| Human: iPSC METTL8−/− (3 clones) | This study | N/A |
| Human: iPSC AAVS1 WT (4 clones) | This study | N/A |
| Human: HEK293 cell line | ATCC | Cat#CRL-1573 |
| Human: GP2-293 cell line | Cellosaurus | Cat#CVCL_WI48, RRID: CVCL_WI48 |
| Experimental models: Organisms/strains | ||
| Mouse: Mettl8flox/flox | This Study | N/A |
| Mouse: B6.Cg-Tg(Nes-cre)1Kln/J | The Jackson Laboratory | JAX: 003771 |
| Mouse: Crl:CD1(ICR) | Charles River Laboratory | RRID: IMSR_CRL:22 |
| Mouse: C57BL/6 | Charles River Laboratory | RRID: IMSR_CRL:27 |
| Oligonucleotides | ||
| The primers and DNA oligos used for qPCR, cloning and genotyping | See Table S1 | N/A |
| Recombinant DNA | ||
| pCAGIG | Addgene (Matsuda and Cepko, 2004) | RRID:Addgene_11159 |
| pCAGIG/Mettl8-WT-Long | This study | N/A |
| pCAGIG/Mettl8-WT-Short | This study | N/A |
| pCAGIG/Mettl8-cKO-Long | This study | N/A |
| pCAGIG/Mettl8-cKO-Short | This study | N/A |
| pSpCas9(BB)-2A-Puro (PX459) V2.0 | Addgene (Ran et al., 2013) | RRID:Addgene_62988 |
| pSpCas9/METTL8-Upstream | This study | N/A |
| pSpCas9/METTL8-Downstream | This study | N/A |
| pSpCas9/AAVS1 | This study | N/A |
| Retro-X™ Universal Packaging System with pVSV-G | Clontech | Cat#631530 |
| pSUBGW vector | (Zhang et al., 2016) | N/A |
| Software and algorithms | ||
| Adobe Illustrator CC | Adobe | https://www.adobe.com/products/illustrator.html; RRID: SCR_010279 |
| Adobe Photoshop CC | Adobe | https://www.adobe.com/products/photoshop.html; RRID: SCR_014199 |
| Bcl2fastq v2.17.1.14 | Illumina | https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html; RRID: SCR_015058 |
| DESeq2 v1.36.0 | (Love et al., 2014) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html; RRID:SCR_015687 |
| FlowJo | FlowJo | https://www.flowjo.com/solutions/flowjo; RRID: SCR_008520 |
| GraphPad Prism | GraphPad Software | https://www.graphpad.com/scientific-software/prism/; RRID: SCR_002798 |
| ImageJ | NIH | https://imagej.nih.gov/ij/; RRID: SCR_003070 |
| Imaris | Bitplane | https://imaris.oxinst.com/packages;RRID:SCR_007370 |
| Metascape | (Zhou et al., 2019) | http://metascape.org/gp/index.html#/main/step1; RRID: SCR_016620 |
| PANTHER v15.0 | (Mi et al., 2019) | http://www.pantherdb.org/; RRID: SCR_004869 |
| R Project v3.6.0 | Open source | https://www.r-project.org/; RRID: SCR_001905 |
| RStudio v1.2.1335 | Open source | https://rstudio.com/; RRID: SCR_000432 |
| STAR v2.5.2a | (Dobin et al., 2013) | https://github.com/alexdobin/STAR; RRID: SCR_015899 |
| Trimmomatic v0.32 | (Bolger et al., 2014) | http://www.usadellab.org/cms/index.php?page=trimmomatic; RRID:SCR_011848 |
| Other | ||
| 4-20% Mini-Gel 15-well, | BIO-RAD | Cat#4561096 |
| 6-well ultra-low attachment culture plate | Fisher Scientific | Cat#7200601 |
| Amersham Imager 600 | GE healthcare | N/A |
| Charged microscope slides | Fisher Scientific | Cat#22-035813 |
| Corning 96-Well Clear Ultra Low Attachment Microplates | Corning/NETA | Cat#7201680 |
| Forma Steri-Cult CO2 Incubator | Thermo Fisher Scientific | 3310 |
| LSR II Flow Cytometer | BD Biosciences | N/A |
| MaxQ CO2 Plus Shaker | Thermo Fisher Scientific | 88881102 |
| NextSeq550 | Illumina | SY-415-1002 |
| PCR tube strips | Emsco/Fisher | Cat#AB0490 |
| Qubit 3 Fluorimeter | Thermo Fisher Scientific | Cat#Q33216 |
| Seahorse XFe96 Analyzer | Agilent | N/A |
| StepOnePlus Real-Time PCR | Applied Biosystems | 4376592 |
| TB Syringe (26 G × 3/8 in, 1 ml) | BD Biosciences | Cat#309625 |
| Trans-Blot Turbo Mini 0.2 um PVDF Transfer Packs | BIO-RAD | Cat#1704156 |
| Scanning Confocal microscope | Carl Zeiss | Zeiss LSM 780 |
Western blotting analysis
Different fractions, including whole cell lysates (W), nucleus (N), mitochondria (M) and cytosol (C), of N2a cells were separated with Cell Fractionation Kit (Abcam) following the manufacturer’s instructions. Briefly, N2a cells were collected and suspended in 1X Buffer A to 6.6 × 106 cells/ml. Then equal volume of Buffer B was added, incubated for 7 mins on a rotator at room temperature and subjected to centrifugation at 5,000 × g for 1 min at 4°C. The supernatant would be the cytosol fractions (C), and the resulting pellet was suspended with 1X Buffer A. The resuspended sample was added with equal volume of Buffer C, incubated for 10 minutes on a rotator at room temperature and subjected to centrifugation at 5,000 × g for 1 min at 4°C. The supernatant would be the mitochondria fractions (M), and the resulting pellet was suspended with 1X Buffer A, which would be the nuclear fractions (N).
Protein lysates of whole cells, cells fractions, mouse brain, or human forebrain organoids were prepared in the cell lysis buffer including RIPA lysis buffer (Thermofisher), Phosphatase Inhibitor Mixture (Sigma), and Protease Inhibitor Mixture (Sigma). After the protein concentration was determined by Pierce™ Rapid Gold BCA Protein Assay Kit, protein lysates were denatured in 1X Sample buffer (NEB) and boiled at 95°C for 10 mins. Then ~25 μg of protein was loaded on a 4–20% Mini-Gel (BIO-RAD), separated through SDS/PAGE and transferred into the Trans-Blot Turbo 0.2 μm PVDF membrane (BIO-RAD). After soaking PVDF membranes in 5% milk dissolved in TBS (20 mM TrisHCl pH 8.0, 50 mM NaCl) at room temperature for 2 hours, the membranes were incubated in primary antibodies diluted in the blocking buffer (TBS, 0.1% Tween-20, 5% BSA, 0.01% NaN3) at 4°C overnight and subsequently in the secondary antibodies conjugated with horseradish peroxidase (HRP) diluted in the blocking buffer at room temperature for 1–2 hours. Finally, Pierce™ ECL Western Blotting Substrate (EMSCO/FISHER) was used for visualization of the corresponding protein bands on membranes. The antibodies used for WB were listed in the Key Resource Table.
In utero electroporation and drug injection of mice
A stock solution of 10 mg/mL EdU (ThermoFisher) was prepared in PBS and incubated on a shaker at 37°C until fully dissolved. For the EdU labeling experiment, WT and Mettl8 cKO mice at E11.5, E13.5 or E15.5 were intraperitoneally injected with EdU (16.7 mg/kg) once and dissected 24 hours later or at P1.
In utero electroporation was performed as described previously37. Briefly, 2 μg/μL pCAGIG-GFP or pCAGIG-HA-Mettl8 (expressing long isoform of WT Mettl8) plasmids mixed with FastGreen (Sigma-Aldrich, F7252) were injected into the lateral ventricle of WT and cKO brains at E13.5 with a calibrated glass micropipette powered by an air pump. These brains were then given an electrical stimulation (50 V for 50 ms with a 950 ms interval) for 5 times and collected at E15.5. For piracetam rescue experiment, piracetam (Sigma-Aldrich, P5295) was dissolved in PBS (100 mg/mL) and injected intraperitoneally into WT and cKO mice (500 mg/Kg) once a day from E11.5 to E15.5.
RNA extraction, m3C modification analyses, and qPCR
RNA extraction and qPCR analysis was performed as described previously65. Briefly, the brain tissue, cell culture or human organoids were homogenized and dissolved in 1 mL TRIZOL (Invitrogen) for 15 mins on ice and 200 μL chloroform was subsequently added. After centrifugation at 12000 g for 15 mins at 4°C, about 400 μL supernatant was carefully taken out and mixed with the same volume of ethanol, and the mixture was transferred to Zymo-Spin™ IC Column in a Collection Tube from the kit of Zymo RNA Clean & Concentrator™-5. After centrifugation, 400 μL RNA Prep Buffer, 700 μL RNA Wash Buffer and 400 μL RNA Wash Buffer was sequentially added to the column with each procedure followed by centrifugation. Finally, about 15 μL RNase/DNase-free water was added to elute the column to collect the RNA sample. Subsequently, about 0.5–1 μg RNA was used for reverse transcription (RT) to synthesize the cDNA with poly-dT primers following the manufacturer’s instructions of SuperScript III First-Strand Synthesis System (Invitrogen).
m3C-HAC-seq was performed as described previously with minor modifications, based on the finding that the hydrazine treatment, followed by subsequent aniline treatment, could specifically induce the cleavage of RNA chain at the m3C modification sites13. 8 μg total RNA was fragmented by incubating in 20 μl of RNA fragmentation reagents (NEB, E6150S) at 95°C for 4 mins. The fragmentation reaction was stopped by adding 2 μl 10X RNA Fragmentation Stop Solution. Then 0.1 volume NaAc (3M, pH 5.2) and 3 volumes of cold ethanol was added for ethanol precipitation of RNA. The collected RNA fragments were dephosphorylated with Antarctic phosphatase (NEB) and re-phosphorylated with T4 PNK (NEB) following the manufacturer’s protocols for end repair, and then RNA was purified with the Zymo RNA-Clean&Concentrator-5 kit. Then RNA samples were incubated with 50 μl of ice-cold hydrazine buffer (10% hydrazine, 3M NaCl) on ice in the dark for 4 hours, which was stopped by ethanol precipitation. Next, RNA pellet was resuspended in 200 μl cleavage buffer (H2O:glacial acid:aniline = 7:3:1) and incubated at room temperature in the dark for 2 hours. RNA was then ethanol precipitated and dissolved in nuclease-free water, followed by rRNA depletion with NEBNext rRNA Depletion Kit (NEB, E7400). Lastly, 100 ng of ribominus RNA fragments were used for cDNA library preparation with the NEBNext Small RNA Library Prep Set (NEB, E7300) following the manufacturer’s protocol. Different libraries from 4 WT and 3 cKO NPCs were quantified using KAPA library Quantification kit and pooled together at equal molar amounts. The average fragment size of the final library fragment was determined as ~222 bp by using bioanalyzer (Agilent). About 2.7 pmol DNA was loaded on NextSeq High Output kit (75 cycles, Illumina) and subjected to a NextSeq 550 sequencer (Illumina) and 1×75 bp single-end sequencing was performed to an average depth of 40 million reads per sample.
For detection of m3C modification of mt-tRNAs and cyto-tRNAs by q-PCR, tRNA RT primers that have the reverse complement sequence of 3’ fragment of tRNA plus one 72-bp fragment of GFP were used to capture tRNA during RT reaction, respectively. For RT, about 0.5–1 μg RNA, 0.5 μL of mt-tRNA RT primer (2 mM) and 0.5 μL dNTP mixture (10 mM each) were mixed in total 5 μL of reaction system and incubated at 65°C for 5 mins. Then 2 μL of MgCl2 (25 mM), 1 μL of DTT (1 mM), 1 μL of 10X RT buffer (Invitrogen, 53032LT), 0.5 μL of RNase Inhibitor (40U/μL, NEB) and 0.5 μL of AMV Reverse Transcriptase (TAKARA) were mixed, and incubated at 50°C for 50 mins and 85°C for 5 mins. For qPCR reactions, 6 μL of H2O, 1 μL of forward primer (10 mM), 1 μL of reverse primer (10 mM),10 μL of Fast SYBR Green qPCR Master Mix (ThermoFisher) and 2 μL of 1:5 diluted cDNA generated by poly-dT or tRNA RT primers were mixed. Then qPCR reactions were performed on the StepOnePlus Real-Time PCR System (Applied Biosystems) with thermocycling conditions as follows: 95°C for 20 s, 44 cycles of 95°C for 3 s and 60°C for 30 s. The difference value between the Ct values (ΔCt) of the genes of interest and internal control genes was calculated and 2(−ΔΔCt) was used to calculate the fold-change in expression of the genes between two samples. For qPCR measuring the level of m3C modification of mt-/cyto-tRNAs, short primer sets can detect both long and short fragments, and long primer sets can only detect long fragment of mt-/cyto-tRNA cDNA generated from mt-/cyto-tRNAs without m3C modification in RT. The long/short ratio, indicated by the value of 2(the difference of CT values from qPCR when using short and long primer sets), reflects the m3C modification level. The higher the long/short value, the lower the m3C modification level. The primers used in qPCR and RT are listed in Table S1.
RNA-seq, m3C-HAC-seq and data analysis
RNA-seq libraries of WT and cKO NPCs were prepared based on the SMART-seq2 method as previously described with minor modifications66. In brief, for RT, 3.2 μL RNA (100 ng/μL), 0.25 μL RNase inhibitor (NEB) and 1 μL CDS primer (10 μM, 5′-AAGCAGTGGTATCAACGCAGAGTACT30VN-3′) were mixed in an 8-well PCR tube strip and incubated at 70°C for 2 mins. Then, 2 μL of 5X SMARTScribe RT buffer (Takara), 0.5 μL of DTT (100 mM), 0.3 μL of MgCl2 (200 mM), 1 μL of dNTPs (10 mM), 1 μL of TSO primer (10 μM, 5′-AAGCAGTGGTATCAACGCAGAGTACATrGrGrG-3′), 0.25 μL of RNase inhibitor (NEB), and 0.5 μL SMARTScribe reverse transcriptase (Takara) was added. RT was performed at 42°C for 90 mins, followed by 10 cycles of 50°C for 2 mins and 42°C for 2 mins, and then 70°C for 15 mins. To amplify the full-length cDNA, 2 μL of the RT reaction, 2.5 μL of 10X Advantage 2 buffer (Takara), 2.5 μL of dNTPs (2.5 mM), 0.25 μL of IS PCR primer (10 μM, 5′-AAGCAGTGGTATCAACGCAGAGT-3′), 0.5 μL Advantage DNA Polymerase (Takara) and 17.25 μL nuclease-free water were mixed, and thermocycling conditions of PCR were as follows: 94°C for 3 mins, 8 cycles of 94°C for 15 s, 65°C for 30 s, and 68°C for 6 mins, followed by 72°C for 10 mins, and 4°C indefinitely. PCR products were then purified with 0.8X AMPure XP beads (Beckman Coulter), eluted in nuclease-free water and quantified following the instructions of Qubit dsDNA HS assay kit (ThermoFisher). For tagmentation of cDNA, 2 μL cDNA (50 pg/μL), 2.5 μL 2X TD buffer (20 mM Tris/pH 8.0, 10 mM MgCl2, and 16% PEG 8000) and 0.5 μL adaptor-loaded Tn5 transposase (Lucigen) were mixed and incubated at 55°C for 12 mins. Then the reaction was terminated by adding 1.25 μL of 0.2% SDS (Fisher) and incubated at room temperature for 10 mins. PCR reaction was performed after addition of 16.75 μL H2O, 1 μL of Nextera i7 primer (10 mM), 1 μL of Nextera i5 primer (10 mM), and 25 μL KAPA HiFi hotstart readymix (EMSCO/FISHER), with the thermocycling conditions as follows: 72°C for 5 min, 95°C for 1 minute, 14 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by 72°C for 1 minute, and 4°C indefinitely. PCR products were then purified twice with 0.8X AMPure XP beads, eluted in nuclease-free water, and quantified following the instructions of Qubit dsDNA HS assay kit. Different libraries from 6 WT and 6 cKO NPCs were quantified using KAPA library Quantification kit and pooled together at equal molar amounts. The average fragment size of the final library fragment was determined as ~420 bp by using a bioanalyzer (Agilent), and the concentration of the library was determined by qPCR. About 2.7 pmol DNA was loaded on NextSeq High Output kit (75 cycles, Illumina) and subjected to a NextSeq 550 sequencer (Illumina) and 1×58 bp single-end sequencing was performed to an average depth of 10 million reads per sample.
Raw sequencing data from RNA-seq and m3C-HAC-seq were demultiplexed with bcl2fastq2 v2.17.1.14 (Illumina), and adaptors were trimmed using Trimmomatic v0.32 software with MINLEN setting as 18 and 26, respectively67. Alignments were made using STAR v2.5.2a to GENCODE mouse reference genome GRCm38.p668. For m3C-seq analysis, all cytosine sites were analyzed when their +1 bp downstream sites were a) within genomic regions with known features including genes and tRNAs, b) at the start end of more than one read in each WT sample, and c) with coverage in all samples. The cleavage ratio of sitei was calculated as the ratio of the number of reads starting at sitei+1 to the read depth of sitei+1. The cleavage ratio of all analyzed 135582 cytosine sites are listed in Table S2. The genome wide significance was determined with Bonferroni correction (p = 0.05/135582 = 3.69×10−7). For RNA-seq analysis, only uniquely mapped reads were quantified at the gene level and summarized to gene counts using STAR-quantMode (GeneCounts), with multimapping and chimeric alignments discarded. The other further analyses were performed in R (v3.6.0). Differential gene expression analysis between WT and cKO NPCs was performed using DESeq2 v1.36.0 with genes whose average counts were lower than the 20 discarded69, and identified upregulated and downregulated gene lists between WT and cKO NPCs were used for Gene Ontology (GO) enrichment analysis using PANTHER v15.070,71 (Table S3) Normalized gene counts of genes of selected GO terms calculated by DESeq2 were converted to row Z-scores per gene for visualization in heatmaps. A protein-protein interaction (PPI) network of downregulated genes and pathway enrichment analysis of identified Molecular Complex Detection (MCODE) components of PPI network was performed with Metascape Database72.
Mitochondria and cytosolic protein translation assay
Mitochondria protein translation assay was performed as previously described32. WT and cKO NPCs cultured on Matrigel-coated plates were incubated in NPC (-Met) medium containing methionine-free DMEM supplemented with 2% B27 supplement, 1% N2 supplement, bFGF (20 ng/mL), EGF (20 ng/mL), 1% Glutamax, 1% P/S for 1.5 hours, and then treated with 50 μg/mL cycloheximide, which specifically inhibits cytosolic translation, for 0.5 hour. Then medium was replaced with NPC (-Met) medium containing 50 μg/mL cycloheximide and 500 μM (for Western blot) or 300 μM (for immunostaining) AHA reagent (L-Azidohomoalanine, EMSCO/FISHER, C10102). After 3 hours, cells were washed with PBS for two times and then lysed with cell lysis buffer or fixed with 4% PFA. For Western blotting analysis, after measuring the protein concentration, WT and cKO cell lysates containing the same amount of proteins were reconstituted to the same volume and treated with 50 μM DBCO-PEG4-biotin (SIGMA ALDRICH INC) at 37°C for 30 mins. Then 1X sample buffer was added and the protein sample was boiled at 95°C for 10 min and subjected to Western blot analysis. The PVDF membrane was blotted with Streptavidin-conjugated HRP at room temperature for 1.5 hours and AHA was visualized by addition of ECL Western Blotting Substrate. For staining, the fixed cells were permeabilized with the blocking buffer at room temperature for 1 hour, and then incubated in PBS containing 50 μM DBCO-PEG4-biotin at room temperature for 30 mins. After washing with PBS, the cells were incubated in conjugated-Cy5 Streptavidin dissolved in blocking buffer, and then subjected to confocal imaging. Cytosolic protein translation assay was performed similarly, except that 10 μg/mL CAP was used to inhibit the mitochondria protein translation.
Analysis of mitochondria membrane potential
To measure the mitochondria membrane potential (MMP), WT and cKO NPCs with/without piracetam treatment were washed with DMEM/F12 twice and incubated with 50 nM MitoTracker™ Orange CMTMRos (ThermoFisher) dissolved in DMEM/F12 for 30 mins. After washing with DMEM/F12, the NPCs were incubated with Accutase for 10 mins and dissociated to single cells. Single cells were suspended by cold Hibernate A Low Fluorescence buffer (Brain Bits) containing 0.1% DAPI (BD, 564907) and then subjected to LSR II Flow Cytometer (BD) for flow cytometry analysis. Forward and side scatter gating was used to identify single cells and live cells were indicated by negative DAPI staining. The signal of Mito-tracker Orange of each cell was measured with the 585/42 channel, and quantification was analyzed with FlowJo software.
Oxygen consumption rate assay
Oxygen consumption rate (OCR) was performed following the manufacturer’s instructions for the Agilent Seahorse XF96 analyzer. Briefly, WT and cKO NPCs (105 cells/well) with/without 1mM piracetam treatment were seeded on Matrigel-coated 96-well Seahorse XF96 cell culture microplates. On Day 2, cells were equilibrated in XF DMEM medium (pH 7.4, Agilent) containing 10 mM glucose, 1 mM sodium pyruvate and 2 mM glutamine with/without 1mM piracetam in a non-CO2 incubator at 37°C for 1 hour. Then the sensory cartridge (Agilent, 102601), which was pre-immersed in Seahorse XF Calibrant overnight at 37°C, was loaded with appropriate amount of Oligomycin, FCCP and Rotenone/antimycin A (R/A, Agilent), put into the cell culture microplates and loaded on the Seahorse XFe96 analyzer. OCR at the baseline and after sequential injections of 1.5 μM Oligomycin, 1 μM FCCP and R/A (0.5 μM each) was monitored with 3 cycles for each step. OCR for each well was normalized to total protein amount as measured with Pierce™ Rapid Gold BCA Protein Assay Kit. The levels of basal, ATP-linked, maximal and spare respiration was calculated accordingly36.
Generation of METTL8 knockout human iPSCs
After washing with PBS, human C1-2 iPSCs were digested with TrypLE (Life Technologies) at 37°C for 5 mins and dissociated to single cells. The single cells were seeded on Matrigel-coated plates and cultured in mTeSR plus medium supplemented with 10 μM Y-27632. On Day 2, culture medium was replaced with 2 mL Opti-Medium containing 10 μM Y-27632, and double PX459 plasmids expressing gRNA targeting METTL8 gene (as KO) or AAVS1 locus (as WT) were transfected with LipoStem reagent (Thermo). After 4 hours, 2 mL mTeSR plus medium containing 10 μM Y-27632 was added. On Day 3, mTeSR plus medium containing 10 μM Y-27632 and 0.5 μg/mL puromycin (Millipore) was used to select for puromycin resistant cells. On Day 5 or 6, mTeSR plus medium was added until iPSC clones formed. Several WT and KO clones were picked and seeded on new plates for expansion. The genotype was determined by PCR and knockout of 3rd Exon of METTL8 was confirmed by qPCR. Only clones with the correct genotype and validated knockout of METTL8 were maintained and used for organoid generation.
Retrovirus preparation and injection into organoids
To prepare engineered self-inactivating GFP-expressing murine onco-retroviruses73, when GP2-293 cells cultured on the 15 cm dish reached ~80% confluence, pSUbGW vectors74 and pVSV-G Vectors from Retro-X Universal Packaging Vector Set (Clontech) were transfected into GP2-293 cells with Lipofectamine 2000. The culture medium was first changed 6 hours later, and then collected once every 24 hours for three times. The retrovirus in the collected medium was concentrated by ultracentrifugation at 29,000 g for 2 hours, with the supernatant discarded and pellet reconstituted with PBS. The titer of virus was measured by counting the number of infected cell clusters in a serial dilution experiment. Retrovirus GFP mixed with FastGreen and 10 μg/mL Polybrene (Santa Cruz) were microinjected into the lumen of D42 organoids to label dividing cells in the ventricular zone using a calibrated glass micropipette.
QUANTIFICATION AND STATISTICAL ANALYSIS
All the images were analyzed with Photoshop (Adobe), ImageJ (NIH) or Imaris 7.6 software (Bitplane) software. The “Spots” function of Imaris software or the “cell counter” plugin of ImageJ software was used to count the number of cells positive for single or multiple markers. Images were cropped and edited using ImageJ and Adobe Illustrator (Adobe) software.
For analysis of mouse brains, the average value of two sequential sections around Bregma - 0.10 mm or three sequential sections around Bregma −1.06 mm for each mouse brain at embryonic stage or at P1 was used for quantification, respectively. The density of markers+ cells was calculated by the number of markers+ cells in the cortex divided by the area of analyzed cortex. For quantification of mouse brains after in utero electroporation, the average value of three sequential sections from similar regions of WT and cKO brains was used. For quantification of human organoids, because one section may contain GFP+ cells originated from different nearby rosettes, all the GFP+ cells within the image of one section were counted. A range of 56–76 sections from 1113 different organoids from 3 KO iPSC lines and 4 WT iPSC lines were examined and quantified.
For quantification of immunostaining intensity, tissue sections were attached to the same slides or cells were put into the same well to make sure that the samples being compared were processed in parallel with the same primary/secondary antibodies or reagents; and the corresponding images were taken and processed using the same settings. The sum of intensity of each channel measured with “Measure” function of ImageJ software was used for quantification, and the relative intensity in the KO or drug-treated groups was normalized with that in WT or control groups from the same batch. In Figures 1I–J, 1K–L, S2K–L and S2P–Q, around 50, 100, 100 and 100 cells for each sample in these experiments were quantified, respectively.
For quantification analysis of protein bands in Western blot, the images were cropped with ImageJ software, and the image type was switched to 8-bit. After subtracting the background with setting rolling ball radius as 50.0 pixels, the white and black colors of the images were inverted. Then the intensity of each protein band was measured with “Measure” function of ImageJ, and the sum of intensity of each protein after normalization with that of loading control from the same sample was used for quantification. For quantification analysis of protein bands in mitochondria protein translation assay, the normalized intensity of one upper band between 50–75 kD, one middle band between 37–50kD and four lower bands between 15–37 kD were quantified.
No statistical methods were used to predetermine the sample size. For analyzing WT and Mettl8 cKO mice in each experiment, at least 3 mice and up to 7 mice were included for quantification, and the exact number can be found in the Figure legends. For quantification of human organoids labeled with retrovirus, WT and METTL8 KO organoids with/without piracetam treatment generated from 3 batches were used, and the number of analyzed sections with different rosettes were shown in Figure legends. The quantification of confocal images was performed blinded to experiment conditions. No data were excluded. For the statistical analysis, an estimate of variation within each group of data is evaluated with standard error of the mean (SEM). Unpaired Student’s t-test and One-way analysis of variance (ANOVA) was used through GraphPad Prism or Excel software to calculate significant differences as indicated: ns: not significant, P > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Shown is the list of the cleavage ratio of analyzed Cytosine sites with more than one read in each WT sample in m3C-HAC-seq starting at their +1 bp downstream sites.
HIGHLIGHTS.
Mettl8 installs m3C modification on mt-tRNAThr/Ser(UCN) in cortical neural stem cells
Mettl8 regulates mitochondrial protein translation and function in neural stem cells
Mettl8 deleted mice exhibit accelerated embryonic cortical neural stem cell depletion
METTL8 plays a conserved role in neurogenesis in human forebrain organoids as in mice
ACKNOWLEDGEMENTS
We thank members of Ming and Song laboratories for comments and suggestions, B. Temsamrit, E. LaNoce and A. Angelucci for laboratory support, and the Penn transgenic and chimeric mouse core for helping to generate the Mettl8f/f mice. This work was supported by grants from National Institutes of Health (R35NS097370 and RF1MH123979 to G-l.M., and R35NS116843 and RM1HG008935 to H.S.), and from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to G-l.M.).
Footnotes
DECLARE OF INTEREST
G-l.M. is on the advisory board of Cell Stem Cell. The authors declare no other competing interests.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Helm M, and Motorin Y (2017). Detecting RNA modifications in the epitranscriptome: predict and validate. Nat Rev Genet 18, 275–291. 10.1038/nrg.2016.169. [DOI] [PubMed] [Google Scholar]
- 2.Zhao BS, Roundtree IA, and He C (2017). Post-transcriptional gene regulation by mRNA modifications. Nature reviews. Molecular cell biology 18, 31–42. 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morais P, Adachi H, and Yu YT (2021). The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Frontiers in cell and developmental biology 9, 789427. 10.3389/fcell.2021.789427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener D, and Schwartz S (2021). The epitranscriptome beyond m(6)A. Nat Rev Genet 22, 119–131. 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 5.Vissers C, Sinha A, Ming GL, and Song H (2020). The epitranscriptome in stem cell biology and neural development. Neurobiology of disease 146, 105139. 10.1016/j.nbd.2020.105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafik AM, Allen EG, and Jin P (2020). Dynamic N6-methyladenosine RNA methylation in brain and diseases. Epigenomics 12, 371–380. 10.2217/epi-2019-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livneh I, Moshitch-Moshkovitz S, Amariglio N, Rechavi G, and Dominissini D (2020). The m(6)A epitranscriptome: transcriptome plasticity in brain development and function. Nat Rev Neurosci 21, 36–51. 10.1038/s41583-019-0244-z. [DOI] [PubMed] [Google Scholar]
- 8.Kirchner S, and Ignatova Z (2015). Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 16, 98–112. 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 9.Roundtree IA, Evans ME, Pan T, and He C (2017). Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellner K, and Morl M (2019). Post-Transcriptional Regulation of tRNA Pools To Govern the Central Dogma: A Perspective. Biochemistry 58, 299–304. 10.1021/acs.biochem.8b00862. [DOI] [PubMed] [Google Scholar]
- 11.Pan T (2018). Modifications and functional genomics of human transfer RNA. Cell Res 28, 395–404. 10.1038/s41422-018-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Yashiro Y, Kikuchi I, Ishigami Y, Saito H, Matsuzawa I, Okada S, Mito M, Iwasaki S, Ma D, et al. (2020). Complete chemical structures of human mitochondrial tRNAs. Nat Commun 11, 4269. 10.1038/s41467-020-18068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui J, Liu Q, Sendinc E, Shi Y, and Gregory RI (2021). Nucleotide resolution profiling of m3C RNA modification by HAC-seq. Nucleic Acids Res 49, e27. 10.1093/nar/gkaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatova VV, Kaiser S, Ho JSY, Bing X, Stolz P, Tan YX, Lee CL, Gay FPH, Lastres PR, Gerlini R, et al. (2020). METTL6 is a tRNA m(3)C methyltransferase that regulates pluripotency and tumor cell growth. Science advances 6, eaaz4551. 10.1126/sciadv.aaz4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholler E, Marks J, Marchand V, Bruckmann A, Powell CA, Reichold M, Mutti CD, Dettmer K, Feederle R, Huttelmaier S, et al. (2021). Balancing of mitochondrial translation through METTL8-mediated m(3)C modification of mitochondrial tRNAs. Mol Cell 81, 4810–4825 e4812. 10.1016/j.molcel.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiber N, Lemus-Diaz N, Stiller C, Heinrichs M, Mai MM, Hackert P, Richter-Dennerlein R, Hobartner C, Bohnsack KE, and Bohnsack MT (2022). The RNA methyltransferase METTL8 installs m(3)C32 in mitochondrial tRNAs(Thr/Ser(UCN)) to optimise tRNA structure and mitochondrial translation. Nat Commun 13, 209. 10.1038/s41467-021-27905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Liu X, Sheng N, Oo KS, Liang J, Chionh YH, Xu J, Ye F, Gao YG, Dedon PC, and Fu XY (2017). Three distinct 3-methylcytidine (m(3)C) methyltransferases modify tRNA and mRNA in mice and humans. J Biol Chem 292, 14695–14703. 10.1074/jbc.M117.798298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lentini JM, Bargabos R, Chen C, and Fu D (2022). Methyltransferase METTL8 is required for 3-methylcytosine modification in human mitochondrial tRNAs. J Biol Chem 298, 101788. 10.1016/j.jbc.2022.101788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu H, Do DV, Liu X, Xu L, Su Y, Nah JM, Wong Y, Li Y, Sheng N, Tilaye GA, et al. (2018). The STAT3 Target Mettl8 Regulates Mouse ESC Differentiation via Inhibiting the JNK Pathway. Stem Cell Reports 10, 1807–1820. 10.1016/j.stemcr.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faubert B, Solmonson A, and DeBerardinis RJ (2020). Metabolic reprogramming and cancer progression. Science 368. 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotz M, and Huttner WB (2005). The cell biology of neurogenesis. Nature reviews. Molecular cell biology 6, 777–788. [DOI] [PubMed] [Google Scholar]
- 22.Koo B, Lee KH, Ming GL, Yoon KJ, and Song H (2022). Setting the clock of neural progenitor cells during mammalian corticogenesis. Semin Cell Dev Biol. 10.1016/j.semcdb.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. (2017). Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 171, 877–889 e817. 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ, Zhang Z, Ogawa Y, Kellis M, Duester G, and Zhao JC (2018). N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci 21, 195–206. 10.1038/s41593-017-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edens BM, Vissers C, Su J, Arumugam S, Xu Z, Shi H, Miller N, Rojas Ringeling F, Ming GL, He C, et al. (2019). FMRP Modulates Neural Differentiation through m(6)A-Dependent mRNA Nuclear Export. Cell Rep 28, 845–854 e845. 10.1016/j.celrep.2019.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katajisto P, Dohla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, and Sabatini DM (2015). Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 348, 340–343. 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khacho M, Harris R, and Slack RS (2019). Mitochondria as central regulators of neural stem cell fate and cognitive function. Nature reviews. Neuroscience 20, 34–48. 10.1038/s41583-018-0091-3. [DOI] [PubMed] [Google Scholar]
- 28.Chakrabarty RP, and Chandel NS (2021). Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell Stem Cell 28, 394–408. 10.1016/j.stem.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olmsted JB, Carlson K, Klebe R, Ruddle F, and Rosenbaum J (1970). Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A 65, 129–136. 10.1073/pnas.65.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang MH, Peng GX, Mao XL, Wang JT, Zhou JB, Zhang JH, Chen M, Wang ED, and Zhou XL (2022). Molecular basis for human mitochondrial tRNA m3C modification by alternatively spliced METTL8. Nucleic Acids Res 50, 4012–4028. 10.1093/nar/gkac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran A, Moellering DR, Ceaser E, Shiva S, Xu J, and Darley-Usmar V (2002). Inhibition of mitochondrial protein synthesis results in increased endothelial cell susceptibility to nitric oxide-induced apoptosis. Proc Natl Acad Sci U S A 99, 6643–6648. 10.1073/pnas.102019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LS, Xiong QP, Pena Perez S, Liu C, Wei J, Le C, Zhang L, Harada BT, Dai Q, Feng X, et al. (2021). ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat Cell Biol 23, 684–691. 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousefi R, Fornasiero EF, Cyganek L, Montoya J, Jakobs S, Rizzoli SO, Rehling P, and Pacheu-Grau D (2021). Monitoring mitochondrial translation in living cells. EMBO Rep 22, e51635. 10.15252/embr.202051635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockburger C, Kurz C, Koch KA, Eckert SH, Leuner K, and Muller WE (2013). Improvement of mitochondrial function and dynamics by the metabolic enhancer piracetam. Biochemical Society transactions 41, 1331–1334. 10.1042/BST20130054. [DOI] [PubMed] [Google Scholar]
- 35.Beckervordersandforth R, Ebert B, Schaffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, et al. (2017). Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 93, 560–573 e566. 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu X, Ma Y, Liu Y, and Wan Q (2021). Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR protocols 2, 100245. 10.1016/j.xpro.2020.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Xu D, Yuan L, Sun Y, and Xu Z (2014). Epigenetic regulation of Atrophin1 by lysine-specific demethylase 1 is required for cortical progenitor maintenance. Nat Commun 5, 5815. 10.1038/ncomms6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winblad B (2005). Piracetam: a review of pharmacological properties and clinical uses. CNS drug reviews 11, 169–182. 10.1111/j.1527-3458.2005.tb00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans ME, Clark WC, Zheng G, and Pan T (2017). Determination of tRNA aminoacylation levels by high-throughput sequencing. Nucleic Acids Res 45, e133. 10.1093/nar/gkx514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, et al. (2016). Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 19, 232–247. 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Bohnsack KE, Kleiber N, Lemus-Diaz N, and Bohnsack MT (2022). Roles and dynamics of 3-methylcytidine in cellular RNAs. Trends in biochemical sciences 47, 596–608. 10.1016/j.tibs.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Begik O, Lucas MC, Liu H, Ramirez JM, Mattick JS, and Novoa EM (2020). Integrative analyses of the RNA modification machinery reveal tissue- and cancer-specific signatures. Genome biology 21, 97. 10.1186/s13059-020-02009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang M, Li Y, Luo X, Xiao J, Wang J, Zeng X, Hu Q, Chen X, Tan SJ, and Hu J (2021). Identification of Biomarkers Related to CD8(+) T Cell Infiltration With Gene Co-expression Network in Lung Squamous Cell Carcinoma. Frontiers in cell and developmental biology 9, 606106. 10.3389/fcell.2021.606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeon SY, Jo YS, Choi EJ, Kim MS, Yoo NJ, and Lee SH (2018). Frameshift Mutations in Repeat Sequences of ANK3, HACD4, TCP10L, TP53BP1, MFN1, LCMT2, RNMT, TRMT6, METTL8 and METTL16 Genes in Colon Cancers. Pathology oncology research : POR 24, 617–622. 10.1007/s12253-017-0287-2. [DOI] [PubMed] [Google Scholar]
- 46.Ringeling FR, Chakraborty S, Vissers C, Reiman D, Patel AM, Lee KH, Hong A, Park CW, Reska T, Gagneur J, et al. (2022). Partitioning RNAs by length improves transcriptome reconstruction from short-read RNA-seq data. Nat Biotechnol 40, 741–750. 10.1038/s41587-021-01136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flores JV, Cordero-Espinoza L, Oeztuerk-Winder F, Andersson-Rolf A, Selmi T, Blanco S, Tailor J, Dietmann S, and Frye M (2017). Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Reports 8, 112–124. 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, Li A, Wang X, Bhattarai DP, Xiao W, et al. (2017). 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res 27, 606–625. 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Li X, Tang H, Jiang B, Dou Y, Gorospe M, and Wang W (2017). NSUN2-Mediated m5C Methylation and METTL3/METTL14-Mediated m6A Methylation Cooperatively Enhance p21 Translation. J Cell Biochem 118, 2587–2598. 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Brouwer APM, Abou Jamra R, Kortel N, Soyris C, Polla DL, Safra M, Zisso A, Powell CA, Rebelo-Guiomar P, Dinges N, et al. (2018). Variants in PUS7 Cause Intellectual Disability with Speech Delay, Microcephaly, Short Stature, and Aggressive Behavior. Am J Hum Genet 103, 1045–1052. 10.1016/j.ajhg.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaheen R, Tasak M, Maddirevula S, Abdel-Salam GMH, Sayed ISM, Alazami AM, Al-Sheddi T, Alobeid E, Phizicky EM, and Alkuraya FS (2019). PUS7 mutations impair pseudouridylation in humans and cause intellectual disability and microcephaly. Hum Genet 138, 231–239. 10.1007/s00439-019-01980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han ST, Kim AC, Garcia K, Schimmenti LA, Macnamara E, Network UD, Gahl WA, Malicdan MC, and Tifft CJ (2022). PUS7 deficiency in human patients causes profound neurodevelopmental phenotype by dysregulating protein translation. Mol Genet Metab 135, 221–229. 10.1016/j.ymgme.2022.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez NM, Su A, Burns MC, Nussbacher JK, Schaening C, Sathe S, Yeo GW, and Gilbert WV (2022). Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol Cell 82, 645–659 e649. 10.1016/j.molcel.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igoillo-Esteve M, Genin A, Lambert N, Desir J, Pirson I, Abdulkarim B, Simonis N, Drielsma A, Marselli L, Marchetti P, et al. (2013). tRNA methyltransferase homolog gene TRMT10A mutation in young onset diabetes and primary microcephaly in humans. PLoS Genet 9, e1003888. 10.1371/journal.pgen.1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR, Graille M, and Lafontaine DLJ (2019). The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res 47, 7719–7733. 10.1093/nar/gkz619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richard EM, Polla DL, Assir MZ, Contreras M, Shahzad M, Khan AA, Razzaq A, Akram J, Tarar MN, Blanpied TA, et al. (2019). Bi-allelic Variants in METTL5 Cause Autosomal-Recessive Intellectual Disability and Microcephaly. Am J Hum Genet 105, 869–878. 10.1016/j.ajhg.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, and Schutz G (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23, 99–103. 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 58.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al. (2014). Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418. 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, Makri G, Nauen D, Shin JH, Park Y, et al. (2014). Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 15, 79–91. 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiang CH, Su Y, Wen Z, Yoritomo N, Ross CA, Margolis RL, Song H, and Ming GL (2011). Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol Psychiatry 16, 358–360. 10.1038/mp.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, et al. (2020). Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 26, 766–781 e769. 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang WK, Wong SZH, Pather SR, Nguyen PTT, Zhang F, Zhang DY, Zhang Z, Lu L, Fang W, Chen L, et al. (2021). Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28, 1657–1670 e1610. 10.1016/j.stem.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda T, and Cepko CL (2004). Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A 101, 16–22. 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308. 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmittgen TD, and Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 66.Jacob F, Pather SR, Huang WK, Zhang F, Wong SZH, Zhou H, Cubitt B, Fan W, Chen CZ, Xu M, et al. (2020). Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 27, 937–950 e939. 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. (2000). Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29. 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mi H, Muruganujan A, Ebert D, Huang X, and Thomas PD (2019). PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47, D419–D426. 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, and Chanda SK (2019). Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10, 1523. 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, and Song H (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593. 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Kang E, Wang Y, Yang C, Yu H, Wang Q, Chen Z, Zhang C, Christian KM, Song H, et al. (2016). Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nature communications 7. 10.1038/ncomms11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown is the list of the cleavage ratio of analyzed Cytosine sites with more than one read in each WT sample in m3C-HAC-seq starting at their +1 bp downstream sites.
Data Availability Statement
The RNA-seq and HAC-seq data reported in this study are deposited in GEO (GSE214445).
The code for HAC-seq analysis can be found at https://doi.org/10.5281/zenodo.7535193, which is maintained by Dr. Daniel Y. Zhang.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


