Abstract
Introduction
The efficacy of taurolidine containing lock solutions for the prevention of central line-associated bloodstream infections (CLABSI) in paediatric oncology patients is still unknown. If the taurolidine-citrate-heparin lock appears to decrease the incidence of CLABSIs, we hope to increase the quality of life of children with cancer by subsequently reducing the central venous access device (CVAD)-removal rates, dispense of antibiotics, hospital admissions and incidence of severe sepsis resulting in intensive care unit admission.
Methods and analysis
This assessor-blinded randomised controlled trial including 462 patients was designed to compare the taurolidine-citrate-heparin lock to the heparin-only lock for the prevention of CLABSIs in paediatric oncology patients. Patients receiving their first CVAD at the Princess Máxima Centre for Paediatric Oncology, Utrecht, the Netherlands, are eligible for inclusion. The primary outcome of this study is the incidence of first CLABSIs from CVAD insertion until the end of the study, maximum follow-up of 90 days. An intention-to-treat and a per-protocol analysis will be performed. An interim analysis will be performed after the inclusion of 50% of the patients. The results of the interim analysis and overall conduct of the trial will be discussed by a data safety monitoring board.
Ethics and dissemination
The medical ethics committee NedMec, Utrecht, the Netherlands, has approved this research (number 20/370). Written informed consent for participation in this trial and publication of the trial data is obtained from all patients and/or their parents/guardians. The results of this trial will be published in a peer-reviewed journal and the data will be made available on reasonable request after publication of the main results manuscript.
Trial registration numbers
NTR6688; NCT05740150.
Keywords: Paediatric oncology, Infection control, PREVENTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Designed as an assessor-blinded randomised controlled trial.
Stratification for central venous access device type and diagnosis will be performed.
Large paediatric oncology patient cohort (N=462).
Inclusion and randomisation should take place as soon as possible after insertion of the central venous access device, which is not always possible due to clinical and psychological circumstances.
Locks are instilled once a week during the study since the maximum number of taurolidine-citrate-heparin locks that can be given during a certain time period is currently unknown; more frequent instillations of the lock might result in a higher efficacy.
Introduction
Central venous access devices (CVADs) are fundamental in paediatric oncology since they provide long-term venous access. The most commonly used CVADs in paediatric oncology patients are the totally implantable venous access ports (TIVAP) and external tunnelled CVADs. In this patient group, the incidence of central line-associated bloodstream infections (CLABSI) is high.1 CLABSI incidence rates (IRs) of 0.1–2.3 per 1000 CVAD days have previously been reported, mostly depending on the patient population, CVAD type and infection definitions used.2 In our hospital, the Princess Máxima Centre for paediatric oncology, a CLABSI IR of 1.51 per 1000 CVAD days has been reported; at least one CLABSI was observed in 30% of the children receiving a CVAD.3 CLABSI episodes often result in hospital admission, postponement of anticancer treatment, early CVAD removal (15% of all CVADs inserted) and can lead to severe sepsis requiring intensive care unit admission (5% of all patients receiving a CVAD).3 CLABSIs, therefore, have a great impact on the quality of life of children diagnosed with cancer and result in high healthcare costs.1 4
Taurolidine-citrate(-heparin) lock solutions (TCHL) are suggested as a promising and safe method for the prevention of CLABSIs.5 6 Taurolidine and citrate have anticoagulant, antimicrobial and antibiofilm properties. No antimicrobial resistance to taurolidine has been reported, which makes taurolidine a more attractable option compared with other antimicrobial lock solutions.7 Taurolidine causes a chemical reaction with the bacterial cell wall, endotoxins and exotoxins, resulting in irreversible damage to the bacteria, inhibition of bacterial pathogenicity and inhibition of surface adhesion of bacteria.5 7–11 The current standard of care in the Netherlands for paediatric oncology patients is to lock CVADs with a heparin-only lock (HL) solution for the prevention of malfunctions. The HL, however, does not have antimicrobial activity and its use is barely supported by literature.5 Our meta-analysis including all randomised controlled trials (RCTs) comparing the efficacy of taurolidine containing lock solutions to heparin-only, saline-only and citrate-only locks in haemodialysis, total parenteral nutrition and oncology patients showed a pooled IR ratio (IRR) of 0.30 (95% CI 0.19 to 0.46) in favour of the taurolidine containing lock solutions. Adverse events were all rare and mild.6 However, these studies were associated with a serious risk of bias and indirectness of evidence.6 More specifically, in paediatric oncology patients, only two open-labelled RCTs) (N≤112) and four non-RCTs) have been performed.12–17 To summarise, these studies did show promising results of the TCHL, but this was not enough evidence to implement the TCHL in paediatric oncology patients.12–17
Therefore, this assessor-blinded RCTs including a large patient cohort was designed to compare the TCHL to the HL for the prevention of CLABSIs in paediatric oncology patients. If the TCHL appears to be safe and decreases the incidence of CLABSI, we hope to increase the quality of life for children with cancer by subsequently reducing the CVAD-removal rate, dispense of antibiotics, days of hospital and incidence of severe sepsis resulting in intensive care unit admission.
Methods and analysis
Design and setting
The CATERPILLAR-study is an investigator-initiated, assessor-blinded, randomised controlled superiority parallel trial comparing the incidence of CLABSI between the TCHL to the HL in paediatric oncology patients with a CVAD (ie, TIVAP and external tunnelled CVAD). The information in this manuscript aligns with the latest protocol, version number 4.0, 19 July 2022. In total 462 patients with a CVAD are expected to be recruited from the Princess Máxima Centre for paediatric oncology, Utrecht, the Netherlands over 29 months. The Princess Máxima Centre is the centralised hospital for paediatric oncology in the Netherlands (ie, all patients diagnosed with a paediatric oncological disease are treated here). Patients will be randomised (1:1) into the HL or TCHL study arm. Patients will be followed up from CVAD insertion until the first CLABSI episode (primary outcome), CVAD-removal, second CVAD insertion or death with a maximum study period of 90 days, whichever comes first. The maximum study period of 90 days was chosen since a great deal of the CLABSI episodes occurs within the first 90 days after insertion (median of 60 days after insertion).1–3
In the first months after diagnosis, patients will receive their oncological treatment at the Princess Máxima Centre. After 1–2 months, a minority of the patients will also be treated in one of the 15 shared care hospitals (see online supplemental file 1) close to their homes. These patients will return at least every 3 weeks to the Princess Máxima Centre. The randomised locks (HL or TCHL) will be given when the patient visits the Princess Máxima Centre. The locks are instilled after each treatment cycle, with a maximum of once weekly. When the CVAD is used in between these moments (ie, more frequent than once a week, in the home care setting or at one of the shared care hospitals), for both groups, the CVAD will be temporarily locked with a non-study related HL. This was done since the maximum lock frequency for this patient group is unknown and the administration of study locks in all shared care hospitals and the home care setting would logistically be too difficult and the costs would be too high. The effect of this method is deemed minimal since the vast majority of patients visits the Princess Máxima Centre once a week and will then receive their randomised lock as soon as possible. The total number of lock days per patient will be taken into account/corrected for during the analyses as described below. Shared care data of the included patients will be shared with the Princess Máxima Centre.
bmjopen-2022-069760supp001.pdf (58.6KB, pdf)
Subjects can leave the study at any time if they wish to do so without any consequences. The investigator can decide to withdraw a subject from the study for urgent medical reasons, if the patient is admitted in a hospital outside the Netherlands or non-participating shared care centre for more than 3 weeks, or if the patient experienced a hypersensitivity reaction after instillation of the TCHL solution.
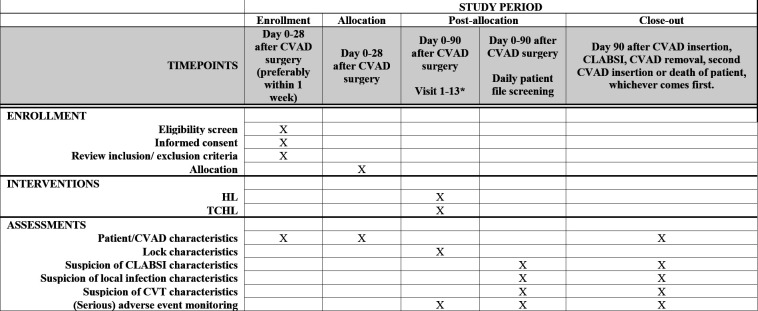
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) schedule for enrolment, interventions and assessments is described in figure 1, the SPIRIT checklist was completed (see online supplemental file 2). This trial is registered at ClinicalTrials.gov (registration under review). The items from the WHO Trial Registration Data Set can be found in table 1. All research staff working on this study is BROK-certified (https://nfu-ebrok.nl/), (see online supplemental file 3) for the roles and responsibilities of the study team).
Figure 1.
Schedule of enrolment, interventions and assessments. *Number of visits depending on the treatment schedule and unexpected admissions. Aim is to insert the lock after each visit with a maximum of once weekly. CLABSI, central line-associated bloodstream infections; CVT, central venous thrombosis; CVAD, central venous access device; HL, heparin-only lock; TCHL, taurolidine-citrate(-heparin) lock solutions.
Table 1.
Items from the WHO trial registration data set
| Data category | Information |
| Primary registry and trial identifying number | ClinicalTrials.gov, NCT05740150 |
| Date of registration in primary registry | 07-09-2017 |
| Secondary identifying numbers | NTR6688 Netherlands Trial Register 12617 Dutch Cancer Society |
| Source(s) of monetary or material support | Monetary: Dutch Cancer Society (KWF) Material: Cablon Medical and TauroPharm |
| Primary sponsor | Princess Máxima Centre for Paediatric Oncology |
| Secondary sponsor(s) | Not applicable |
| Contact for public queries | Ceder Hildegard van den Bosch C.H.vandenBosch-4@prinsesmaximacentrum.nl +31625395632 |
| Contact for scientific queries | Ceder Hildegard van den Bosch C.H.vandenBosch-4@prinsesmaximacentrum.nl +31625395632 |
| Public title | Central line-associated bloodstream infection prevention using TauroLock-Hep100 in paediatric oncology patients. |
| Scientific title | The efficacy of a lock solution containing taurolidine, citrate and heparin for the prevention of tunnelled central line-associated bloodstream infections in paediatric oncology patients, a randomised controlled, monocentre trial. |
| Countries of recruitment | The Netherlands |
| Health condition(s) or problem(s) studied | Central line-associated bloodstream infections |
| Intervention(s) | Experimental: TauroLock-Hep100 (taurolidine 1.35%, citrate 4%, heparin 100 IU/mL) Active Comparator: Heparin lock (heparin 100 IU/mL) |
| Key inclusion and exclusion criteria | Inclusion criteria:
Exclusion criteria:
|
| Study type | Interventional Allocation: Randomised in 2 arms 1:1 Masking: Assessor blinded Primary purpose: Prevention |
| Date of first enrolment | 27-10-2020 |
| Target sample size | 462 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Incidence of central line-associated bloodstream infections |
| Key secondary outcomes |
|
bmjopen-2022-069760supp002.pdf (5MB, pdf)
bmjopen-2022-069760supp003.pdf (50.3KB, pdf)
Patient and public involvement
The patient association Vereniging Kinderkanker Nederland (VKN; https://www.kinderkankernederland.nl/) was involved in the design of this study. The VKN reviewed the protocol and patient information forms, and they assessed the burden for patients to participate in the research. Currently, yearly meetings are held between the researcher and VKN to discuss the progress of the trial. The advice given by the VKN is strongly taken into account by the researchers. Furthermore, the VKN will be involved in the plan for the dissemination of the trial results after completion of the trial.
Participants
All consecutive paediatric oncology patients (haematologic, solid and neurological malignancies), treated at the Princess Máxima Centre for Paediatric Oncology, ranging from 0 to 19 years old, receiving a CVAD (tunnelled external CVAD or TIVAP) for the first time or if their previous CVAD has been removed >12 months ago, will be asked to participate in this study by a research physician or nurse. Further inclusion criteria are: a radiological, cytological or histological proven paediatric malignancy (hematologic, solid and neurological malignancies), planned need for central vascular access of >90 days, written consent signed according to local law and regulations, parents/guardians or patient are willing and able to comply with the trial procedure. Exclusion criteria are: a previous CVAD removed <12 months ago, expected treatment for a majority of the follow-up time in a different hospital than the Princess Maxima Centre for paediatric oncology in the first 90 days of inclusion resulting in difficulties/the inability to visit the Princess Maxima Centre at least once every 3 weeks, primary immunological disorder, contra indications, such as known hypersensitivity to taurolidine, citrate or heparin, and a history of heparin-induced thrombocytopaenia, documented bacteraemia in the period from 24 hours before catheter insertion until inclusion, insertion of the CVAD at the same site as a previously confirmed central venous thrombosis (CVT), pregnant, not willing to use adequate contraceptives or breastfeeding patients.
Informed consent procedure
Informed consent is obtained within 1 week after CVAD insertion, however, if this is not possible due to clinical circumstances, patients may be included within 4 weeks after CVAD insertion. Patients, parents and/or legal guardian are given verbal information and information in writing by the research physician or nurse. A dated and signed informed consent form will be obtained from each patient, parent and/or legal guardian depending on the age of the patients (see online supplemental file 4). The research physician or nurse will then also sign the consent form. A copy will be given to the patient and/or parents. The inclusion and exclusion criteria are thereafter checked by the researcher.
bmjopen-2022-069760supp004.pdf (17.3MB, pdf)
Randomisation and blinding
Patients will be randomised by the research physician or nurse with a method of minimisation into the HL or TCHL study arm (1:1) with the use of an online randomization service by internet called ALEA (https://www.aleaclinical.eu/). Stratification will be done according to two factors: CVAD type (TIVAP or external tunnelled CVAD) and diagnosis (haematological or solid, lymphoma and neurological malignancies). The expert panel, evaluating all possible CLABSI episodes, will be blinded for the allocated treatment. The allocated treatment will not be revealed to the expert panel or described in the parts of the electronic patient files which the expert panel will use to evaluate the possible CLABSI episodes. The patients, parents and/or legal guardians, and the rest of the research and clinical teams, will not be blinded. Complete blinding was logistically too difficult to execute and much more expensive since the design of the HL and TCHL ampoules is not similar.
Intervention
Patients will receive a lock solution of 0.8–1.5 mL, depending on the CVAD type as described in table 2, containing taurolidine 1.35%, citrate 4.0% and heparin 100 IU/mL (TauroLock-Hep100, Cablon Medical, Leusden, the Netherlands and TauroPharm, Waldbüttelbrunn, Germany) or heparin 100 IU/mL at the Princess Máxima Centre after each treatment cycle with a maximum of once a week. The locks will remain in situ until the CVAD is used again. Before the CVAD is used again, the previously instilled study locks (TCHL and HL) will be removed from all lumina. If a blood culture is obtained while the lock is still in situ, at least 2 mL of blood is aspirated and discarded for the prevention of false negative blood culture results. A dedicated research nurse will train the hospital staff, patients and parents/guardians and will monitoring adherence to the intervention study protocol as described above. All cointerventions that are needed during the trial can be used as in usual clinical practice.
Table 2.
Lock volumes
| CVAD | Type | Diameter (Fr) | Maximal catheter volume (mL) | Lock volume (mL) |
| TIVAP | Babyport | 4.5 | 0.80 | 1.0 |
| Low-profile | 6.5 | 1.04 | 1.5 | |
| Standard | 6.5 | 1.28 | 1.5 | |
| External tunnelled CVAD | Single lumen | 6.6 | 0.74 | 1.0 |
| Double lumen | 6.0 or 7.0 | 0.70/0.70 or 0.90/0.80 | 1.0/1.0 | |
| Triple lumen | 6.0 | 0.75/0.62/0.62 | 1.0/0.8/0.8 |
CVAD, central venous access device; TIVAP, totally implantable venous access port.
Outcomes
The primary outcome of this study is the incidence of first CLABSIs from CVAD insertion until the end of follow-up. A blinded expert panel of one paediatric infectiologist and two medical microbiologists will judge each positive blood culture episode during the study period as a CLABSI or non-CLABSI bacteraemia following the Centers for Disease Control and Prevention CLABSI criteria. The CLABSI criteria were chosen since they are the most applicable criteria for paediatric oncology patients, since no peripheral blood cultures are obtained in this patient group, which are needed for other existing diagnostic criteria.18 Judgement of the episodes will be performed based on the patient files and by contacting the treating physician if necessary, the randomisation group will not be described in the parts of the patient files that the experts will access for their assessment. All non-unanimous judgements will be discussed between the experts until they all agree. If the experts still disagree, the final judgement is based on the judgement of the majority. Additionally, all experts will be asked to answer if their result following the CLABSI criteria aligns with their clinical judgement.
The secondary outcomes of this study are (measured from CVAD insertion until the end of follow-up): the time to first CLABSI, CLABSI incidence per 1000 CVAD days, the incidence of symptomatic CVT (ie, if the patient has (1) peripheral veins that have a non-compressible segment or (2) there is an echogenic intraluminal thrombus or an absence of flow in the central venous system (76)), bacteraemia episodes (ie, every non-CLABSI related positive blood culture), local infections (ie, positive exit-site culture, erythema, purulent drainage or tenderness within 2 cm of the CVAD track and exit-site), CVAD-removal (including reasons why CVAD was removed), cultured micro-organisms causing CLABSI, days of hospital admission due to CLABSIs/CVTs, the dispense of thrombolysis and systemic antibiotic treatment due to CLABSIs/CVTs, and safety of the locks in terms of (serious) adverse events, and intensive care unit admission or mortality due to CLABSIs/CVTs.
Data collection and management
Data are entered pseudonymised from paper case report forms and electronic patient files in Castor EDC (Castor EDC V.2021.1, CATERPILLAR-study V.6.21, password-protected access) by trained local data managers in the Princess Máxima Centre. In Castor EDC, range checks for data values are incorporated. All study information will be stored in locked cabinets in areas with limited access. Records with personal identifiers will be stored separately from records identified by a code number. Study information of the patients will not be released outside of the study without written permission of the patients. All data (including shared care hospital data) should be entered within 90 days after the end of study date of each patient. Regular quality checks are performed by a central data manager and independent monitor three times a year. The database will be locked after all data have been cleaned and all necessary changes have been made. The principal investigator and research physician will have access to the final trial dataset after completion of the trial. The data will be stored for at least 15 years. After the main results, manuscript is published, the data will become available on reasonable request.
The following data will be collected: patient characteristics (age, gender, diagnosis, treatment protocol, administration of prophylactic systemic antibiotics (ie, trimethoprim/sulfamethoxazole, ciprofloxacin or antimycotics)), CVAD characteristics (surgery date, type, introduction method, lumen amount/diameter, access vein and side, complications during procedure, removal date and reason), lock characteristics (date instillation and removal, type, method of removal, (serious) adverse events during lock instillation and removal (following common terminology criteria for adverse events (CTCAE) version 5.0, 27 November 2017)), treatment for possible malfunction (ie, impossibility to aspirate or flush the CVAD)), suspicion of CLABSI characteristics (start date episode, symptoms, neutropenia (including duration and lowest neutrophil count during episode: very severe <100, severe 500–1000, moderate 500–1000, mild 1000–1500×106/L)), blood culture results, treatment method of CLABSI, hospital/intensive care unit admission days, death, judgement of episode by expert panel (ie, CLABSI, mucosal barrier injury laboratory-confirmed bloodstream infection) or bacteraemia due to other reasons), reasons for non-CLABSI-related bacteraemia (ie, not enough blood cultures obtained, contamination/colonisation, CVAD in situ for <48 hours, infection at a different site)), suspicion of local infection characteristics (start date episode, symptoms, culture results, treatment, hospital/intensive care unit admission days, death), suspicion of a CVT characteristics (start date episode, symptoms, radiological imaging, location, treatment, hospital/intensive care unit admission days, death) and end of the study reasons. Data of patients that prematurely drop-out of the study will be collected until the day they dropped out.
Safety considerations
(Serious) adverse events with a possible or definite relationship to the locks are registered during the study (CTCAE version 5.0, 27 November 2017). Registration of all (serious) adverse events would lead to the registration of too many adverse events in these oncological patient groups. Adverse events of special interest, due to their known relationship to the HL or TCHL are: oral dysesthesias, neck/chest wall pain, dysgeusia, nausea, vomiting, allergic reactions and heparin induced thrombocytopaenia. Patients will be followed up for the occurrence of (serious) adverse events until 30 days after the last study lock was given. The Princess Máxima Centre will report serious adverse events within the appropriate time frame (ie, within 7 days of first knowledge in case of life-threatening situations or death and within 15 days in all other cases) to the accredited ethics committee that approved the protocol. The sponsor has a liability and subject insurance.
Data safety monitoring board
A data safety monitoring board (DSMB) is established to safeguard the interests of trial participants, assess the safety and efficacy of the interventions during the trial, and monitor the overall conduct of the clinical trial. Three DSMB meetings will be held: one start of the study session, a second closed session after the inclusion of 50% of the patients where the interim analysis will be presented, and a third session at the end of the study. The results of the interim analysis will only be presented to the principal and coordinating investigators, trial statistician and DSMB members. The DSMB will not be blinded and consists of a paediatric surgeon, infectious disease specialist and medical statistician. All three members are independent from the sponsor and have no competing interests. The DSMB will give an advice to the principal investigator, who will make the final decision to terminate or continue the trial (see online supplemental file 5 for the DSMB charter).
bmjopen-2022-069760supp005.pdf (4.3MB, pdf)
Statistical methods
Sample size calculation
Assuming a CLABSI rate of 12.8%, an estimated total number of 412 patients is needed to detect a difference between group proportion of 7.8%, with a two-sided α of 0.05 and power of 80% (two-sided Z-test with unpooled variance).19–24 The CLABSI rate of 12.8% was based on the data from the CVAD complication database of the Princess Máxima Centre, partially published by van den Bosch et al, 3 using the same inclusion and exclusion criteria and follow-up period as described for this study.3 The estimated reduction of 12.8%–5.0% was based on previously performed RCT, of which the vast majority showed a reduction of at least more than 60%; IRR of 0.30 (95% CI 0.19 to 0.46). For paediatric oncology specifically, two RCTs have been performed which showed reductions of 74% and 77%.6 For each patient that prematurely drops-out of the study an extra patient will be included, we estimated that an extra 50 patients would be needed to account for potential drop-outs. The drop-out inflated total sample size is therefore calculated as 462 patients, 231 per group.
Interim analysis
An interim analysis will be performed after the inclusion of 231 patients. A stopping rule was defined for a one sided test at an α level of 0.025 for the null hypothesis: experimental incidence ≥control incidence. The test is one sided because there is no need to prove superiority of the control treatment in case it is better than the experimental. The stopping rule allows stopping for acceptance of the alternative hypothesis (superiority) as well as stopping for acceptance of the null hypothesis (futility). The stopping boundaries are based on α-spending and β-spending functions. As α-spending function we have chosen the Jennison and Turnbull power family function with ρ=2.35 and as β-spending function we have chosen the Jennison and Turnbull power family function with ρ=3.2.
Statistical analysis
The primary data analyses will be performed with the intention-to-treat principle (ie, inclusion of all patients that were randomised). Additionally, a per-protocol analysis will be performed excluding patients who were not included within one week after CVAD insertion, patients who never received the intervention and patients who missed three or more of the minimal amount (once every three weeks) of locks during the follow-up period. Categorical data will be presented as contingency tables (frequencies and percentages). All patients will be analysed in the intervention group they were initially randomised in. For continuous data summary statistics of mean, SD, median, minimum and maximum will be presented. Differences between treatment groups with respect to baseline characteristics will be analysed by using a χ2 (or Fisher’s exact in the presence of small numbers) and two-tailed t-test for categorical or continuous variables, respectively. In case of violation of the normality assumption, a non-parametric test such as the Wilcoxon rank test will be applied.
For the primary outcome, the percentages and IRs of first CLABSIs per 1000 CVAD days will be reported for both study groups and compared by computing an IRR. The exact confidence limits for the IRRs will be based on the polynomial algorithm for person time data.25 26 The nominal alpha level for the primary outcome in the final analysis will be equal to 0.045 due to the interim analysis.19–24
The cumulative incidence of CLABSI from CVAD insertion will be estimated by using a competing risk model27 with CVAD removal due to non-CLABSI-related reasons or death as competing events. To assess the difference between the cumulative incidence for the intervention (TCHL) and control (HL) group, the Gray’s test will be used.28
To estimate the effect of risk factors on the occurrence of CLABSI, a Cox-specific proportional hazard regression model from CVAD insertion will be estimated. Well-known time fixed risk factors for a CLABSI to be incorporated into the model are diagnosis (haematological disease vs other diagnoses), CVAD type (TIVAP vs tunnelled external CVADs). Furthermore, total parenteral nutrition (TPN) administration will be used in the model as time-dependent covariate).27
A landmark analysis at 28 days after CVAD insertion will be performed. The same risk factors as discussed above will be incorporated in the Cox-specific hazard regression model with additional covariate number of lock days. The landmark point of 28 days was chosen based on clinical reasons, the first lock should have been given within the first 4 weeks after CVAD insertion.29
For the secondary outcomes, the percentages and IRs per 1000 CVAD days will be reported and compared by computing IRRs. Furthermore, the above-described analyses will be repeated for subgroups based on diagnosis and CVAD type.
All analyses concerning the competing risk model will be performed in RStudio V.1.3.1093 (USA) environment by using the cmprisk library. IBM SPSS Statistics for Windows V.26.0 (USA) will be used to perform all other statistical analyses.
Study timeline
Inclusion of the study began on 27 October 2020. We expect that the planned number of patients can be recruited in 29 months from the defined source population. The planned study timeline is described in table 3.
Table 3.
Planned study schedule
| Months after start inclusion | What? | Description |
| 0 | Start inclusion | Planned start of the study |
| 14.5 | Interim database lock and interim analysis | After the inclusion of 50% of the patients |
| 29 | Stop inclusion | After the inclusion of 462 patients |
| 32 | Stop follow-up | After a period of 3 months after the inclusion of the last patient |
| 32 – 36 |
Database lock, statistical analysis, writing the clinical study reports and drafting of the manuscript based on the clinical study reports. | From the stop of follow-up until manuscript submission |
| 36 | Manuscript submission | Four months after the study has stopped |
Ethics and dissemination
The medical ethics committee NedMec, Utrecht, the Netherlands, has approved this research registered under number 20/370 (https://www.metcutrecht.nl/); a copy of the trial protocol submitted to the ethics committee can be in online supplemental file 6. Modifications to the protocol that impact the conduct of the study will require a formal amendment, which will be agreed on by the medical ethics committee. Written informed consent is obtained from all patients and/or their parents/guardians for participation in the trial and for the publication of their data. The results of this trial will be published in an open access, peer-reviewed journal, presented at international congresses and subsequently the data (stored for at least 15 years) will be made available on reasonable request after publication of the main results manuscript. The VKN will be involved in the plan for the dissemination of the trial results to the participants and the public after completion of the trial. All eventually listed authors of the publication of the main results manuscript will have made a substantial, direct, intellectual contribution to the work.
bmjopen-2022-069760supp006.pdf (45.2MB, pdf)
Supplementary Material
Acknowledgments
We would like to thank all patients and their families for participating in this study. We thank the Vereniging Kinderkanker Nederland and especially W. Plieger for her/their advice during the development and execution of this trial. We thank the research nurses, research assistants, data managers and trial managers of the trial and data centre of the Princess Máxima Centre for paediatric oncology for their tremendous efforts and dedication during the design and execution of this study. We thank all shared care hospitals and Bureau Zorgbemiddeling Utrecht for their collaboration and all their efforts during the follow-up of the patients of this study. We thank the pharmacy of the Princess Máxima Centre for paediatric oncology for the distribution of the locks. We thank Cablon Medical (https://cablon.nl/nl/) and TauroPharm (https://www.taurolock.com/en/about/tauropharm-gmbh) for the supply of the TCHLs for this study. We thank the DSMB members M. Witvliet, B. Rijnders and H. Putter for their part during this study.
Footnotes
Contributors: All authors have contributed to the manuscript in significant ways and meet the journal’s authorship guidelines. They have reviewed and agreed on the contents and have no conflicts of interest. CHvdB, YL, AFWvdS, J-TTvdB, FNJF, MF, CPvdV, MDvdW and MHWAW designed methodology of the study. MF and CHvdB wrote the statistical plan. CHvdB, AFWvdS, MF and MDvdW wrote the original draft of the manuscript. CHvdB, YL, AFWvdS, J-TTvdB, FNJF, MF, CPvdV, MDvdW and MHWAW reviewed and edited the manuscript. AFWvdS, MF, MDvdW and MHWAW supervised the preparation of this manuscript.
Funding: This work was supported by the Dutch Cancer Society (KWF), grant number 12617.
Disclaimer: KWF does and did not have a role in study design, collection, management, analysis, interpretation of data, writing of the report and the decision to submit the report for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Ullman AJ, Marsh N, Mihala G, et al. Complications of central venous access devices: a systematic review. Pediatrics 2015;136:e1331–44. 10.1542/peds.2015-1507 [DOI] [PubMed] [Google Scholar]
- 2. Schoot RA, van Dalen EC, van Ommen CH, et al. Antibiotic and other lock treatments for tunnelled central venous catheter-related infections in children with cancer. Cochrane Database Syst Rev 2013;2013:CD008975. 10.1002/14651858.CD008975.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Bosch CH, van der Bruggen JT, Frakking FNJ, et al. Incidence, severity and outcome of central line related complications in pediatric oncology patients; a single center study. J Pediatr Surg 2019;54:1894–900. 10.1016/j.jpedsurg.2018.10.054 [DOI] [PubMed] [Google Scholar]
- 4. Kavosi Z, Sarikhani Khorrami M, Keshavarz K, et al. Is taurolidine-citrate an effective and cost-effective hemodialysis catheter lock solution? A systematic review and cost- effectiveness analysis. Med J Islam Repub Iran 2016;30:347. [PMC free article] [PubMed] [Google Scholar]
- 5. Pittiruti M, Bertoglio S, Scoppettuolo G, et al. Evidence-based criteria for the choice and the clinical use of the most appropriate lock solutions for central venous catheters (excluding dialysis catheters): a gavecelt consensus. J Vasc Access 2016;17:453–64. 10.5301/jva.5000576 [DOI] [PubMed] [Google Scholar]
- 6. van den Bosch CH, Jeremiasse B, van der Bruggen JT, et al. The efficacy of taurolidine containing lock solutions for the prevention of central-venous-catheter-related bloodstream infections: a systematic review and meta-analysis. J Hosp Infect 2022;123:143–55. 10.1016/j.jhin.2021.10.022 [DOI] [PubMed] [Google Scholar]
- 7. Olthof ED, Rentenaar RJ, Rijs A, et al. Absence of microbial adaptation to taurolidine in patients on home parenteral nutrition who develop catheter related bloodstream infections and use taurolidine locks. Clin Nutr 2013;32:538–42. 10.1016/j.clnu.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 8. Caruso F, Darnowski JW, Opazo C, et al. Taurolidine antiadhesive properties on interaction with E. coli; its transformation in biological environment and interaction with bacteria cell wall. PLoS One 2010;5:e8927. 10.1371/journal.pone.0008927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorman SP, McCafferty DF, Woolfson AD, et al. Reduced adherence of micro-organisms to human mucosal epithelial cells following treatment with taurolin, a novel antimicrobial agent. J Appl Bacteriol 1987;62:315–20. 10.1111/j.1365-2672.1987.tb04926.x [DOI] [PubMed] [Google Scholar]
- 10. Olthof ED, Nijland R, Gülich AF, et al. Microbiocidal effects of various taurolidine containing catheter lock solutions. Clin Nutr 2015;34:309–14. 10.1016/j.clnu.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 11. Gorman SP, Mccafferty DF, Woolfson AD, et al. Electron and light microscopic observations of bacterial cell surface effects due to taurolidine treatment. Lett Appl Microbiol 1987;4:103–9. 10.1111/j.1472-765X.1987.tb01593.x [DOI] [Google Scholar]
- 12. Dümichen MJ, Seeger K, Lode HN, et al. Randomized controlled trial of taurolidine citrate versus heparin as catheter lock solution in paediatric patients with haematological malignancies. J Hosp Infect 2012;80:304–9. 10.1016/j.jhin.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 13. Handrup MM, Møller JK, Schrøder H. Central venous catheters and catheter locks in children with cancer: a prospective randomized trial of taurolidine versus heparin. Pediatr Blood Cancer 2013;60:1292–8. 10.1002/pbc.24482 [DOI] [PubMed] [Google Scholar]
- 14. Ince E, Oğuzkurt P, Temiz A, et al. Complications of total implantable access ports and efficacy of taurolidine-citrate lock solution against catheter-related infections. Afr J Paediatr Surg 2014;11:138–42. 10.4103/0189-6725.132806 [DOI] [PubMed] [Google Scholar]
- 15. Simon A, Ammann RA, Wiszniewsky G, et al. Taurolidine-citrate lock solution (taurolock) significantly reduces CVAD-associated grampositive infections in pediatric cancer patients. BMC Infect Dis 2008;8:102. 10.1186/1471-2334-8-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark JE, Graham N, Kleidon T, et al. Taurolidine-citrate line locks prevent recurrent central line-associated bloodstream infection in pediatric patients. Pediatr Infect Dis J 2019;38:e16–8. 10.1097/INF.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 17. Chong C-Y, Ong RY-L, Seah VX-F, et al. Taurolidine-citrate lock solution for the prevention of central line-associated bloodstream infection in paediatric haematology-oncology and gastrointestinal failure patients with high baseline central-line associated bloodstream infection rates. J Paediatr Child Health 2020;56:123–9. 10.1111/jpc.14506 [DOI] [PubMed] [Google Scholar]
- 18. National healthcare safety network (NHSN) device associated module. 2022. Available: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf
- 19. Chow S-C, Shao J, Wang H, et al. Sample size calculations in clinical research: third edition. 3rd edn. Boca Raton, Florida: Chapman and Hall/CRC, 2017. 10.1201/9781315183084 [DOI] [Google Scholar]
- 20. D’agostino RB, Chase W, Belanger A. The appropriateness of some common procedures for testing the equality of two independent binomial populations. Am Stat 1988;42:198–202. 10.1080/00031305.1988.10475563 [DOI] [Google Scholar]
- 21. Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. New York: John Wiley & Sons, 2003. 10.1002/0471445428 [DOI] [Google Scholar]
- 22. Lachin JM. Biostatistical methods. 1st ed. New York: John Wiley & Sons, 2000. 10.1002/9780470317051 [DOI] [Google Scholar]
- 23. Machin D, Campbell M, Fayers P, et al. Sample size tables for clinical studies. 2nd ed. Malden, Mass: Blackwell Science, 1997. [Google Scholar]
- 24. Ryan TP. Sample size determination and power. 1st ed. Hoboken, New Jersey: John Wiley & Sons, 2013. 10.1002/9781118439241 [DOI] [Google Scholar]
- 25. Martin D, Austin H. An efficient program for computing conditional maximum likelihood estimates and exact confidence limits for a common odds ratio. Epidemiology 1991;2:359–62. 10.1097/00001648-199109000-00008 [DOI] [PubMed] [Google Scholar]
- 26. Martin DO, Austin H. Exact estimates for a rate ratio. Epidemiology 1996;7:29–33. 10.1097/00001648-199601000-00006 [DOI] [PubMed] [Google Scholar]
- 27. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 28. Gray RJ. A class of $ K $ -sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 1988;16:1141–54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 29. Van Houwelingen HC. Dynamic prediction by landmarking in event history analysis. Scand J Stat 2007;34:70–85. 10.1111/j.1467-9469.2006.00529.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069760supp001.pdf (58.6KB, pdf)
bmjopen-2022-069760supp002.pdf (5MB, pdf)
bmjopen-2022-069760supp003.pdf (50.3KB, pdf)
bmjopen-2022-069760supp004.pdf (17.3MB, pdf)
bmjopen-2022-069760supp005.pdf (4.3MB, pdf)
bmjopen-2022-069760supp006.pdf (45.2MB, pdf)