Abstract
Paroxysmal nocturnal haemoglobinuria (PNH) is an ultra-orphan disease, which until 15 years ago had limited treatment options. Eculizumab, a monoclonal antibody that inhibits C5 in the terminal complement cascade, has revolutionised treatment for this disease, near normalising life expectancy and improving quality of life for patients. The treatment landscape of PNH is now evolving, with ravulizumab a second longer acting intravenous C5 inhibitor now licenced by the FDA and EMA. With different therapeutic targets in the complement cascade and difference modalities of treatment, including subcutaneous, oral and intravenous therapies being developed, increasing independence for patients and reducing healthcare requirements. This review discusses the current and future therapies for PNH.
Lay summary
Review of current and future treatments for patients with Paroxysmal Nocturnal Haemoglobinuria
What is Paroxysmal Nocturnal Haemoglobinuria?
Paroxysmal nocturnal haemoglobinuria (PNH) is a very rare disease. It arises from PNH stem cells in the bone marrow. In a normal bone marrow these are inactive; however, if there has been a problem in the bone marrow, the PNH stem cells can expand and make PNH red blood cells, white blood cells and platelets. The problem with these cells is that they lack the cell surface markers that usually protect them. Red blood cells are broken down in the circulation rather than the spleen, which gives rise to PNH symptoms such as abdominal pain, difficulty swallowing, erectile dysfunction and red or black urine (known as haemoglobinuria). The white blood cells and platelets are ‘stickier’ increasing the risk of blood clots. Previously life expectancy was reduced as there were limited treatment options available.
What was the aim of this review?
To provide an overview of current and future treatment options for PNH
Which treatments are available?
• Eculizumab is an treatment given through a vein (intravenous) every week for 5 weeks then every 2 weeks after this, and has been available for 13 years, improving life expectancy to near normal.
• Ravulizumab is a newer intravenous treatment similar to eculizumab but is given every 8 weeks instead of every 2 weeks. In clinical studies it was comparable with eculizumab.
• Future Treatments - There is new research looking at different methods of treatment delivery, including injections under the skin (subcutaneous) that patients can give themselves, treatments taken by mouth (oral) or a combination of an intravenous and oral treatment for those patients who are not optimally controlled on eculizumab or ravulizumab.
What does this mean?
PNH is now treatable. For years, the only drug available was eculizumab, but now different targets and drug trials are available. Ravulizumab is currently the only second licenced product available, in USA and Europe, there are other medications active in clinical trials.
Why is this important?
The benefit for patients, from treatment every 2 weeks to every 8 weeks is likely to be improved further with the development of these new treatments, providing patients with improved disease control and independence.
As we move into an era of more patient-friendly treatment options, the PNH community both physicians and patients look forward to new developments as discussed in this article.
Keywords: clinical trials, complement inhibition, drug development, Paroxysmal Nocturnal Haemoglobinuria
Introduction
Paroxysmal nocturnal haemoglobinuria (PNH), an ultra-orphan disease with a prevalence of 15.9 cases per million in Europe is a life-threatening disorder, characterised by haemolysis, bone marrow failure and thrombosis.1,2 Prior to the development of eculizumab, patients with PNH had a median survival of between 10 and 22 years.3,4
Clinical PNH arises from a stem cell mutation and subsequent expansion of these PNH stem cells in the bone marrow, often following an immunological ‘insult’, such as preceding aplastic anaemia, although this insult may be transient and without clinical symptoms.5 Somatic mutations in the phosphatidyl inositol glycan A (PIG-A) gene in bone marrow stem cells result in the loss of all glycophosphatidylinositol anchor proteins on haematopoietic cells.6,7 CD55 and CD59, the complement regulatory proteins on red blood cells are therefore deficient. CD55 regulates the formation of C3 and C5 convertases and CD59 the formation of the membrane attack complex (MAC). Thus in PNH, with a deficiency of one or both of these regulatory proteins there is unregulated activation of C3 and C5 convertase, formation of the MAC and alternative pathway self-amplification. This results in complement mediated intravascular haemolysis, free haemoglobin release and nitric oxide (NO) depletion.
NO depletion inhibits smooth muscle relaxation causing PNH symptoms such as abdominal pain, oesophageal spasm, erectile dysfunction and pulmonary hypertension.3,8,9 The complement and coagulation systems are closely interlinked rendering PNH patients at significantly increased risk of thrombosis, alleviated only partially by anticoagulation.10
Eculizumab, a humanized monoclonal (IgG2/4κ) antibody, produced by recombinant DNA technology, binds to the complement protein C5 preventing terminal complement activation and subsequent intravascular haemolysis. Licenced by the United States (US)Food and Drug Administration (FDA) and European Medicine Agency (EMA) in 2007,11,12 it has revolutionised treatment of patients with the disease in countries with access to this therapy. Life expectancy is now near that of the normal population.13 The risk of thrombosis has been significantly reduced with eculizumab, a relative risk reduction of 85%, overcoming the most severe life-threatening aspect of PNH.14 Eculizumab is however a high cost drug, and this has limited access to treatment for many patients with PNH throughout the world.
The dramatic improvement in the management of PNH over the last decade is now enabling a move towards a more personalised patient-centred care with options to reduce frequency of treatment and the option to self-administer medication.
There is also an unmet clinical need in PNH for patients in whom control of intravascular haemolysis is suboptimal on eculizumab. As reported in the literature, 20% of patients require a higher dose of eculizumab to prevent haemolysis; these needs are not met in some countries where dose increases are not permitted.15 Breakthrough intravascular haemolysis can also occur at times of high stress such as infection or surgery, resulting in patients requiring careful timing of elective surgery and potentially an additional dose of eculizumab. Some patients also experience relatively high levels of extravascular haemolysis whilst receiving eculizumab. This is due to opsonisation of PNH red blood cells, leading to their increased clearance from the circulation. This means some patients remain transfusion dependent, despite eculizumab treatment.16
The therapeutic field of complement inhibition has become an area of increasing interest, as complement inhibitors are utilised not just in PNH but also in other diseases such as atypical haemolytic uremic syndrome, ophthalmology disorders and myasthenia gravis. With an increase in therapeutic options for patients, this should also lead to reductions in drug costs and wider access throughout the world.
To fully understand the evolution of PNH treatment, an understanding of the complement cascade is required.
Complement pathway
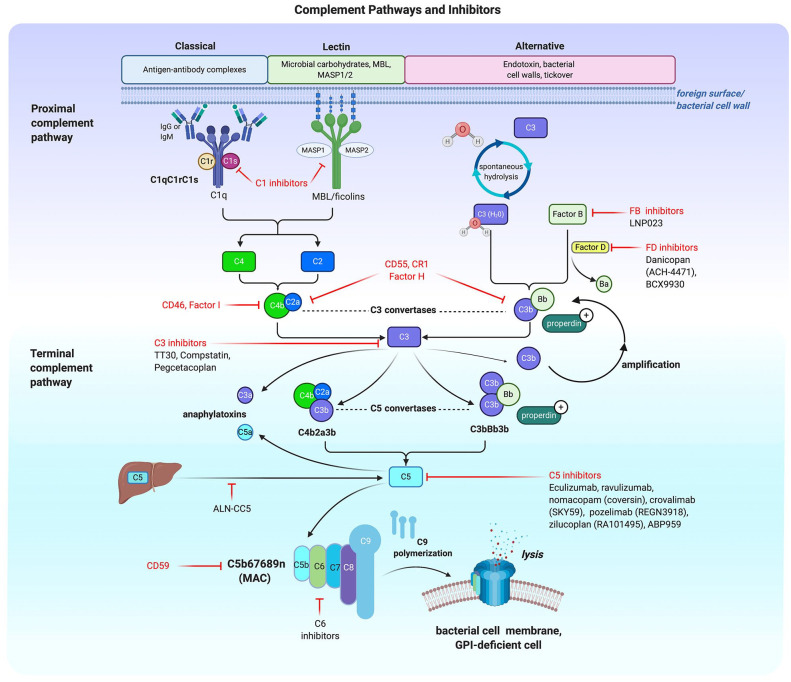
The complement system is complex, with the classical, lectin and alternative pathways converge at the complement protein C3. The classical pathway is triggered by antigen-antibody interactions activating the C1 complex; the mannose binding lectin (MBL) pathway is initiated through interaction between carbohydrates on the bacterial cell wall with either MBL or ficolin, associated with MBL/ficolin-associated serine proteases (MASPs).
In contrast the alternative pathway (AP) can be triggered through spontaneous hydrolysis of C3 with constant ‘tick over’.
All three pathways generate a C3 convertase, the CP and MBL generating a C2a4b molecule and the AP pathway producing C3bBb. Both the activated Cr1 and MBL molecules catalyse formation of C2a and C4b leading to the C3 convertase C2a4b. In the AP, C3 undergoes spontaneous hydrolysis and binds with activated Factor B. The active Factor B (Bb) is generated by Factor D and binds hydrolysed C3 forming the AP C3 convertase (C3bBb). Properdin helps to stabilise the C3 and C5 convertases. Once the AP C3 convertase is generated there is a potent amplification loop with multiple molecules of C3 being cleaved and further C3bBb generated. Both pathways generate their respective C5 convertases, which cleave C5 into C5b and potent anaphylatoxin C5a. C5b joins with C6, C7, C8 and multiple molecules of C9 to form the membrane attack complex (MAC). Each pathway has multiple regulators and targets for therapeutic inhibition.17,18
Deregulation of the three pathways give rise to a variety of disorders. Concentrating on the alternative pathway, inherited or acquired abnormalities of the proteins within the pathway can result in patients developing a wide range of disorders including PNH, atypical haemolytic uremic syndrome and glomerulonephritis.
Infection risk and complement inhibition
Inherited loss of complement proteins has resulted in an increased susceptibility to particular infections. This information is useful in informing the management of patients who are receiving specific anti-complement therapies targeted for disease management in conditions such as PNH. Neisseria infections are the only infections associated with loss of terminal complement proteins C5–C9. A retrospective analysis of nearly 10 years of patients with PNH treated with eculizumab demonstrated 67 cases of meningitis, representing 0.25 reports per 100 patient years (PY) of which eight were fatal (0.03 per 100 PY). However, the rate of meningococcal infection has decreased over time.20 Since terminal complement inhibitors eculizumab and latterly ravulizumab have become available, healthcare staff and patients are more familiar with risk, enabling them to mitigate it with vaccination, antibiotics and education of patients and healthcare professionals.
Whilst all patients as per treatment licence should receive meningitis vaccinations for subtypes ACWY and B, in the United Kingdom (UK), all patients are vaccinated at commencement of C5 complement inhibition (instead of 2 weeks leading up to treatment), due to concerns about increased haemolytic activity observed by the PNH national service when vaccinating prior to treatment (unpublished data). Patients also receive ciprofloxacin antibiotics for 2 weeks and long-term penicillin/erythromcyin.
This is standard practice in the UK, as advised by the Public Health England Meningococcal Reference Unit, the Centres for Disease Control (CDC) also recommended antibiotic prophylaxis in patients who are on complement inhibitors, both departments acknowledging the small risk of antibiotic resistant meningitis strains.21,22
Annual monitoring of AWCY antibody titres and revaccinating where immunity is suboptimal, as well as meningitis B booster every 5 years is also advised. It is not currently possible to assess antibody titres for meningitis B due to interference of C5 inhibition with the assay.
As newer therapeutic options target other proteins in the complement cascade, understanding and management of potential infection risks are essential. Targeting C3, factor B and factor D are likely to increase the risk of infections such as haemophilus influenzae, proteus and pseudomonas species, as well as meningitis. We must be careful to vaccinate where possible as above, in addition with haemophilus influenzae type B and pneumococcal vaccination, and to educate patients and healthcare staff of the risks.23 Prompt hospital admission for patients with pyrexia/concerns about meningococcal infection and treatment with intravenous antibiotics as per local hospital guidelines is essential to mitigate mortality risk.
Ravulizumab, mechanisms of action and clinical trials
Ravulizumab is a second-generation IgG mediated monoclonal antibody, similar to eculizumab, produced in a Chinese hamster ovary cell. Eculizumab when bound to C5 has increased clearance, thus reducing the half-life of the drug to 11 days and the need for treatment every 14 days. Eculizumab is transported into acidified endosomes (pH 6 environment), with or without bound C5 (see Figure 1). The eculizumab-C5 complex is thought to inefficiently dissociate at pH6, resulting in degradation of the complete C5-eculizumab complex and reducing the option for recycling of eculizumab. Lack of recycling of eculizumab results in loss of availability to bind to further newly generated C5, reducing the drug half-life.24
Figure 1.
Illustration of the three main pathways of complement activation and an overview of some of the targets of therapeutic complement inhibition. Figure created with BioRender.19
Modification of the eculizumab structure by four amino acids to form ravulizumab has resulted in increasing the half-life significantly. Mouse models demonstrate this process two-fold. Ravulizumab targets newly synthesized C5, binding to it and continuing cessation of the C5–C9 complement cascade (see Figure 1). There is a marked reduction in target mediated drug disposition, effected by altering the pH at which the monoclonal antibody-C5 complex (mAb-C5 complex) dissociates to pH 6. This allows it to dissociate in the endosome, enabling lysosomal degradation of C5 and releasing ravulizumab into the vascular space, recycling and allowing binding and neutralizing of newly generated C5.
This combination of reduction in target mediated drug deposition and recycling results in increased drug half-life. In vivo analysis of pharmacokinetics (PK) and pharmacodynamics (PD) data demonstrate a three- to four-fold increase in drug half-life compared with eculizumab in a single dose escalation study.24
Clinical trials with ravulizumab
The phase Ib (ALXN103 study) and phase II (ALXN201 study) multicenter trials included dose finding and PK studies in patients naive to complement inhibition. ALXN 103 was a dose escalation study of 13 patients with patients receiving treatment every 4 weeks once a loading dose had been completed. Dosing of cohorts included either 900 mg or 1800 mg every 4 weeks. Based on ALXN103 study dose selection for the ALXN201 study were selected with 26 patients enrolled, receiving either 1000 mg every 4 weeks, 1600 mg every 6 weeks, 2400 mg every 8 weeks, or 5400 mg every 12 weeks. The primary end point of reduction in lactate dehydrogenase (LDH) were reached with a mean LDH percent reduction from baseline ranging from 72.9% in study 201/cohort to 89.6% in study 201/cohort 4 [LDH inclusion criteria of three times upper limit of normal (ULN)]. Improved FACIT fatigue scores and transfusion requirements supported the effectiveness of ravulizumab leading into phase three studies.25
Phase III
Following the successful phase I/II trials, ravulizumab was trialed in a phase III non-inferiority multi-center study, ALXN301 [ClinicalTrials.gov identifier: NCT02946463] compared ravulizumab with eculizumab in treatment-naïve haemolytic PNH patients. Inclusion criteria included an LDH greater than 1.5 times of the ULN, a lower LDH threshold than for the phase I/II study which permitted increased recruitment and fewer screening failures.
A total of 246 patients were randomized to receive either ravulizumab (single loading dose followed by a maintenance dose starting after 2 weeks, by weight-based dosing) or eculizumab for 6 months (600 mg weekly for 4 weeks followed by 900 mg every 2 weeks) with a 1:1 randomization. Ravulizumab was non-inferior to eculizumab for both the primary endpoints of LDH normalization and transfusion requirements (transfusion independence, 73.6 versus 66.1%; LDH normalization, 53.6 versus 49.4%, p < 0.001 (for non-inferiority). Secondary endpoints were also non-inferior which included breakthrough intravascular haemolysis and quality of life measures.26
The ALXN302 study [ClinicalTrials.gov identifier: NCT03056040] was a non-inferiority switch-study for PNH patients already on eculizumab. Inclusion criteria comprised ‘stable PNH’ on 900 mg of eculizumab every 14 days. A total of 195 patients were randomized switch to ravulizumab or continue eculizumab, with those on the standard arm offered a switch to ravulizumab at the end of the 26 week comparison period (1:1 randomisation). Similar to the phase III treatment naïve cohort, ravulizumab was non-inferior to eculizumab with minimal percentage change in LDH compared with the LDH at baseline (−0.82% for ravulizumab versus +8.39% for eculizumab).27 The 52-week combined data from the 301 and 302 study demonstrates ongoing effective management of PNH, with very few breakthrough events in the patients on ravulizumab.28 C5 levels in both the 301 and 302 studies patients on ravulizumab were shown to have C5 levels consistently below 0.5 µg/ml for the duration of the 8 week dosing schedule, compared with 22 or the 219 patients on eculizumab who had at least one C5 level rise above 0.5 µg/ml. This suggests ravulizumab provides a more effective, sustained C5 complement inhibition compared with eculizumab, due to a minority of patients in the eculizumab control arms not sustaining comparable C5 inhibition.29 Interestingly the five patients who experienced breakthrough events on ravulizumab (up to 26 weeks) did not have a rise in C5.30 The side effect and safety profile in all the above studies were comparable with that of eculizumab. Two patients across the four trials experienced meningitis, both had been vaccinated against meningitis and the meningitis strains isolated were penicillin resistant and of indeterminate penicillin sensitivity, respectively. Follow-up time is relatively short compared with data on eculizumab, and thus further cases of meningitis are likely to emerge in the future with use of ravulizumab.27
Headache was the most frequently reported adverse event (AE), which was also the case with eculizumab. The theory behind the headaches experienced by treatment naïve patients relates to an increase in NO (due to cessation of intravascular haemolysis and scavenging of NO by free haemoglobin) leading to a dilatation of cerebral blood vessels. Patients also reported headache when switching from eculizumab to ravulizumab (26.8% with ALXN1210 versus 17.3% with eculizumab). Several possible reasons for this include the longer infusion time, the requirement to be in a clinical care facility whereas patients may have been treated at home previously with eculizumab, or as speculated by authors of the trial, a deeper control of intravascular haemolysis; however, only well controlled patients on eculizumab were permitted to enter into the trial. Long term safety data are awaited.
The results of the phase III trials led to ravulizumab receiving marketing authorization by the Food and Drug Administration in 2018, and the European Medicines Agency in 2019 (Ultomiris®, Alexion Pharmaceuticals, New Haven, CT, USA). Approval in the UK and other countries is awaited at time of publication.31,32
The cost of ravulizumab is lower than eculizumab, stated to be approximately 10% lower in the US at $458,000; however, healthcare modelling suggest a more generous cost analysis. This is based on several factors, including improvements in patient’s quality of life and therefore productivity, and the reduction in breakthrough events reducing the need for healthcare visits and blood transfusions. The reduced frequency of infusions also reduces healthcare staff time. Whilst it remains relatively early post-ravulizumab-approval, dose increases as yet have not been required, unlike in eculizumab with 20% of patients requiring a higher dose, which adds to drug costs. It does, however, remain a very costly drug, with access likely remaining limited for patients worldwide.33,34
Whilst receiving licensed approval, there are areas for improvement with ravulizumab. Patients are still committed long term to lengthly 2-3 hour intravenous infusion every 8 weeks, requiring healthcare staff for administration and in some countries attendance at hospital.
Other C5 inhibitors
Currently the only licenced products for the treatment of PNH are C5 inhibitors available as intravenous preparations (see Figure 1). Alternative modalities of drug delivery are now being investigated, including daily and weekly subcutaneous (SC) therapies.
Pozelimab, a fully human monoclonal immunoglobulin G4P (IgG4P) antibody directed against C5, has been trialled in healthy volunteers has demonstrated complement inhibition with a reduction in the complement function assay CH50 and a favourable safety profile.35 Currently recruiting, an open-label single arm phase II study in treatment naïve patients, with initial IV then weekly SC dosing [ClinicalTrials.gov identifier: NCT03946748] with a target recruitment of 42 patients.
Crovalimab, a SC C5 inhibitor with sequential monoclonal antibody recycling technology (SMART), is another approach to treatment for PNH, developed by Roche Pharmaceuticals. It binds efficiently to C5, with enhanced uptake of bound C5 into endosomal cells and, similar to ravulizumab pH dependent dissociation, crovalimab dissociates from it antigen-antibody complex allowing degradation of C5 and the free antibody to be recycled back into plasma for further C5 inhibition. Optimal dosing frequency is suggested to be every 4 weeks following a phase I/II clinical trial which included treatment naïve (10 patients) and C5 inhibitor treated patients (19 patients) who switched to crovalimab, with a sustained response in LDH < 1.5 × upper limit of normal (ULN). Two patients on the higher dose (680 mg every 4 weeks), developed a skin rash which settled, otherwise reported AEs were similar to other C5 inhibitors [ClinicalTrials.gov identifier: NCT03157635].36
Zilucopan, a daily SC injection showed promising results; however, the company have been taken over and looks to not be pursing PNH as a disease area of interest.37 Similarly, nomacopan, a daily SC injection for PNH, presented at the 23rd European congress of haematology in 2018, showed a reduction in LDH and was well tolerated by eight patients, although it is no longer being developed by the company for PNH.38
ALN-CC5 represents an alternative approach. Developed by Alnylam Pharmaceuticals, it utilises RNAi technology to reduce the production of C5 in the hepatocytes and has been trialled in a small cohort of PNH patients [ClinicalTrials.gov identifier: NCT02352493].39
Biosimilars to eculizumab are also now either in clinical trials such as ABP959 (Amgen) [ClinicalTrials.gov identifier: NCT03818607] or being utilised for patients, with a significant cost saving to countries, increasing access to treatments for PNH patients.
Proximal complement inhibition
Proximal complement inhibition has developed rapidly over the last few years, with several complement protein targets being considered for or already in clinical trials including C3, factor B, factor D or combination treatments (see Figure 1).
Combination treatments offer an interesting perspective, proposed strategies include a combination of C3 and C5 inhibition, Factor D and C5 inhibition, or C5 inhibition using different modalities.
The combination of a licenced C5 inhibitor (eculizumab or ravulizumab), with an ‘add on’ proximal complement inhibitor may offer improved control for those patients with sub-optimal disease control on eculizumab, with several trials currently registered with ClincialTrials.gov.
Proximal complement inhibitor with C3b binding
Compstatin analogues target C3 by binding to C3 and C3b (active target) preventing cleavage of C3 to C3b, and binding to the activated C3–C3b complex. This prevents downstream activation of the complement cascade and intravascular haemolysis in PNH patients. Compstatin analogues also inhibit the amplification loop of complement activation. There are several different compstatin analogues in development, the most developed of these from a clinical trial perspective is Pegcetacoplan (Apellis pharmaceuticals), a pegylated compstatin analogue which binds to C3.
Pegcetacoplan was trialed as monotherapy in treatment naïve PNH patients, in the phase Ib PADDOCK study [ClinicalTrials.gov identifier: NCT02588833]. Inclusion criteria including LDH > 2 times ULN, a Hb < 10.5 g/dl and received at least one blood transfusion in the last 12 months. Pegcetacoplan was given SC as a daily injection with 95% of patients achieving sustained normalization of LDH levels, and an increase in haemoglobin from a baseline median of 8.0 g/dl to a median of 10.8 g/dl at day 29 of treatment. One potential advantage of C3 inhibition is the reduction in extravascular haemolysis, as shown in this trial by the increase in the proportion of PNH erythrocytes from 32% at baseline to 80% at day 85, the reduction of absolute reticulocyte counts and normalization of bilirubin levels.40
Due to anticipated control of extravascular haemolysis, pegcetacoplan was also trialed in a phase Ib dose escalation study [ClinicalTrials.gov identifier: NCT02264639] as an ‘add on’ therapy for those with a haemoglobin of <10 g/dl or had received a blood transfusion within the last 12 months whilst established on eculizumab. Mean Hb increased from 8.78 × g/dl to 11.5 × g/dl, with 2 years of trial completed, this confirms a combination treatment is also a considered approach.41
PNH disease control, both intravascular and extravascular haemolysis as demonstrated in these small phase Ib study led to a large phase III multi-centre trial [ClinicalTrials.gov identifier: NCT03500549] in PNH patients experiencing a suboptimal haematological response to eculizumab, defined as a Hb level <10.5 g/dl. In this study, after a short period of concomitant treatment (to reach pegcetacoplan steady state), patients were randomised to continue either eculizumab or pegcetacoplan monotherapy, with crossover for those in the standard arm of treatment. Preliminary 16-week data show superiority to eculizumab in this selected patient group, with a significant improvement in haemoglobin with an adjusted treatment difference of 3.84 g/l for patients on pegcetacoplan; 85.4% patients achieved transfusion avoidance and FACIT fatigue scores improved. Four patients experienced breakthrough haemolysis, compared with nine patients on eculizumab; however, it should be noted that three of the four patients discontinued pegcetacoplan. Whilst these results are impressive, follow up is short and longer term data is awaited.42
Factor D inhibitors
Factor D are the lowest levels of the complement proteins in the blood. A serine protease, it catalyzes cleavage of factor B leading to the formation of C3 convertase. Factor D controls this rate limiting step in the alternative pathway activation, and thus is an attractive target for complement inhibition and disease control in complement mediated disorders. Utilising an ‘upstream’ target in the complement cascade, the theory of inhibiting not only intravascular haemolysis but also reducing extravascular haemolysis caused by C3 opsonisation on red blood cells. There is also the significant additional benefit that the currently proposed factor D inhibitors are oral, providing patients with independence. It should however be remembered as discussed above; the risk of infections is likely to be higher with more proximal complement inhibition.43
Several companies are developing oral factor D molecules. Danicopan (ACH-4471) – an Achillion/Alexion pharmaceuticals product – has been trialed initially as first in human study as a single and multiple dose ascending study in healthy volunteers, for safety and PK data as the product ACH-5228.44 Proof of concept in 12 PNH patients suboptimally controlled on eculizumab, was presented in December 2019, with combined treatment with Danicopan and eculizumab.45 Mean improvements in Hb of 2.6 g/l led to follow-on trials running in parallel, with both treatment naïve PNH patients, and those suboptimally controlled on eculizumab and ravulizumab as an ‘ad on’ therapy to see whether this improves outcome [ClinicalTrials.gov identifier: NCT04170023].
Also developing an oral factor D inhibitor are Biocryst, with BCX9930. Combining a phase I/II study design, with healthy volunteers in part I and II, with PNH patients both treatment naïve, and those on complement inhibitors. The trial is open in South Africa with imminent opening in Europe [ClinicalTrials.gov identifier: NCT04330534].46
Factor B inhibition
LNP023 is another orally available drug, binding to factor B, developed by Novartis pharmaceuticals. Factor B drives the amplification loop of the alternative pathway, binding to C3B. Cleavage by factor D results in formation of C3bBb containing the catalytic substrate Bb. Thus targeting factor B removes the substrate for this amplification loop. The inhibitors are highly selective, targeting factor B alone. In vitro, there was also no evidence of C3 deposition on red blood cells, supporting the theory of reducing the risk of extravascular haemolysis.47
LNP023 is currently under investigation in a phase II trial in treatment naïve PNH patients [ClinicalTrials.gov identifier: NCT03439839].
Other complement targets
In addition to the development of proximal complement inhibition, there is the development of ‘downstream’ complement inhibition, with early development of C6 inhibitors, with studies in vitro, and in mouse and monkey models.48
Summary
As we move into an era of drug development for PNH, targeting the complement pathway ‘upstream’ from C5, with the potential benefits of inhibiting extravascular haemolysis, ‘downstream’ of C5 and the option of oral, SC or longer acting IV therapies mean treatment options and quality of life for patients are likely to be improved. It must be remembered that these new drugs whilst offering benefit, also increase the risk of infection to wider organisms other than Neisseria as more of the complement pathway is inhibited. The difficulty arises in conducting large multi-centre trials in a rare disease, with many different interested pharmaceutical companies and therapies. PNH patients have also benefitted from the recognition that complement inhibition as a target, is translatable across other disease fields, thus providing a wider ‘market’ for the pharmaceutical industry driving further drug development.
There remains a disparity throughout the world, in terms of access to treatments for PNH. Expense of treatment is usually a prohibiting factor, not only in developing countries but also in some developed countries. With the development of biosimilars and an increasingly competitive market for therapeutic targets and different methods of medication administration, there is hope for future reductions in cost pricing of drugs, but also of increased patient access schemes to support those in countries without access to these lifesaving treatments.
Patient compliance with self-administered treatments may be a concern. As a consequence of the short half-life of some of the newer treatments and the immediate loss of complement inhibition will render patients at risk of ‘rebound’ PNH with significant haemolysis and thrombotic events.
There also remains a wider concern about the risk of breakthrough haemolysis. If patients lose their complement inhibition control, there will be a high proportion of PNH red blood cells present thus risk of brisk and frank haemolysis if doses are missed or patients have overwhelming infection resulting in activation of the complement cascade. A ‘rescue’ strategy could be proposed in countries where C5 inhibition is available as a temporary management of these episodes.
Conclusion
PNH, an ultra-orphan life threatening disease, is now treatable. For years, the only licenced drug was eculizumab; however, as this fast-moving field now evolves, different targets and drug trials are available. Whilst ravulizumab is currently the only second licenced product available, in the US and Europe, there are other medications active in clinical trials. The benefit for patients, from treatment every 2 weeks to every 8 weeks is likely to be improved further with the development of SC, oral or a combination treatment, thus providing patients with more autonomy and independence. The issue of drug price is also addressed, one hopes, with a more competitive market. We can only aspire to enable worldwide, patients with PNH to receive the care and treatment availability that is afforded those in countries such as the US, Europe and UK.
Footnotes
Contributions: MG: Wrote, edited and reviewed the manuscript
AP: Contributed to the writing of the manuscript, reviewed the manuscript
RK: Reviewed and edited the manuscript
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Morag Griffin: Honoraria and travel support Alexion pharmaceuticals; Ad Board Biocryst pharmaceutics.
Richard Kelly: Honoraria, travel support and Advisory Board Alexion pharmaceuticals; lecture fees and Advisory Board Amgen pharmaceuticals (for ALL not PNH).
Alexandra Pike: PhD partly funded by Apellis Pharmaceuticals.
ORCID iD: Morag Griffin  https://orcid.org/0000-0002-4287-0595
https://orcid.org/0000-0002-4287-0595
Contributor Information
Morag Griffin, Department of Haematology, Bexley Wing, St James University Hospital, Leeds, LS9 7TF, UK.
Richard Kelly, Department of Haematology, Bexley Wing, St James University Hospital, Leeds, UK.
Alexandra Pike, University of Leeds, Leeds, UK.
References
- 1. Hoffbrand AV, Catovsky D, Tuddenahm EGD, et al. Postgraduate haematology. 6th ed. Chichester, West Sussex: Wiley-Blackwell, 2010. [Google Scholar]
- 2. Kelly R, Richards S, Hillmen P, et al. The pathophysiology of paroxysmal nocturnal hemoglobinuria and treatment with eculizumab. Ther Clin Risk Manag 2009; 5: 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillmen P, Lewis SM, Bressler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 1995; 333: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 4. de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood 2008; 112: 3099–3106. [DOI] [PubMed] [Google Scholar]
- 5. Young N, Maciejewski J, Sloand E, et al. The relationship of aplastic anemia and PNH. Int J Hematol 2002; 76(Suppl. 2): 168–172. [DOI] [PubMed] [Google Scholar]
- 6. Bessler M, Mason PJ, Hillmen P, et al. Paroxysmal nocturnal haemoglobinuria (PNH) is caused by somatic mutations in the PIG-A gene. EMBO J 1994; 13: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A trancripts in granuocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 1994; 330: 249–255. [DOI] [PubMed] [Google Scholar]
- 8. Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005; 293: 1653–1662. [DOI] [PubMed] [Google Scholar]
- 9. Hill A, Rother RP, Wang X, et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2010; 149: 414–425. [DOI] [PubMed] [Google Scholar]
- 10. Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003; 102: 3587–3591. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration. Drug approval package, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000TOC.cfm (accessed May 2020).
- 12. European Medicines Agency. Soliris, https://www.ema.europa.eu/en/medicines/human/EPAR/soliris (accessed May 2020).
- 13. Hill A, Kelly RJ, Kulasekararaj A, et al. Eculizumab in paroxysmal nocturnal hemoglobinuria (PNH): a report of all 153 patients treated in the UK. Blood 2012; 120: 3472. [Google Scholar]
- 14. Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007; 110: 4123–4128. [DOI] [PubMed] [Google Scholar]
- 15. Hillmen P, Muus P, Röth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2013; 162: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKinley CE, Richards S, Munir T, et al. Extravascular hemolysis due to C3-loading in patients with PNH treated with eculizumab: defining the clinical syndrome. Blood 2017; 130(Suppl. 1): 3471. [Google Scholar]
- 17. Murphy KM, Wevaer C. Janeway’s immunobiology. 9th ed. New York and London: Garland Science, 2017. [Google Scholar]
- 18. Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20: 34–50. [DOI] [PubMed] [Google Scholar]
- 19. BioRender, https://biorender.com/ (accessed 30 May 2020)
- 20. Socié G, Caby-Tasi M-P, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol 2019; 185: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Managing the risk of meningococcal disease among patients who receive complement inhibitor therapy, https://www.cdc.gov/meningococcal/clinical/eculizumab.html (accessed August 2020).
- 22. Ladhani SN, Campbell H, Lucidarme J, et al. Invasive meningococcal disease in patients with complement deficiencies: a case series (2008-2017). BMC Infect Dis 2019; 19: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev 2010; 23: 740–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheridan D, Yu Z-X, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One 2018; 13: e0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Röth A, Rottinghaus ST, Hill A, et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv 2018; 2: 2176–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JW, de Fontbrune FS, Lee LWL, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood 2019; 133: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor–experienced adult patients with PNH: the 302 study; Blood 2019; 133: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill A, Piatek CI, de Latour RP, et al. Breakthrough hemolysis in adult patients with paroxysmal nocturnal hemoglobinuria treated with ravulizumab: results of a 52-week extension from two phase 3 studies. Blood 2019; 134(Suppl. 1): 952. [Google Scholar]
- 29. de Latour RP, Brodsky RA, Ortiz S, et al. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. Epub ahead of print 24 May 2020. DOI: 10.1111/bjh.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brodksy R, de Latour R, Rottinghaus S, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. Epub ahead of print 16 January 2020. DOI: 10.3324/haematol.2019.236877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Medicines Agency. Ultomiris, https://www.ema.europa.eu/en/medicines/human/EPAR/ultomiris (accessed May 2020).
- 32. U.S. Food and Drug Administration. FDA approves ravulizumab-cwvz for paroxysmal nocturnal hemoglobinuria, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ravulizumab-cwvz-paroxysmal-nocturnal-hemoglobinuria (accessed May 2020).
- 33. OptumRx. Ultomiris (ravulizumab-cwvz) – new orphan drug approval, https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-approvals/drugapproval_ultomiris_2018-1224.pdf (accessed July 2020).
- 34. O’Connell T, Buessing M, Johnson S, et al. Cost-Utility analysis of ravulizumab compared with eculizumab in adult patients with paroxysmal nocturnal hemoglobinuria. Pharmacoeconomics 2020; 38: 981–994. [DOI] [PubMed] [Google Scholar]
- 35. Devalaraja-Narashimha K, Ni YG, Huang C, et al. Pozelimab, a human antibody against complement factor C5, demonstrates robust inhibition of alternative complement activity both in normal human serum and in phase I normal healthy volunteers. Blood 2019; 134 (Suppl. 1): 2278. [Google Scholar]
- 36. Röth A, Nishimura J-I, Nagy Z, et al. The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood 2020; 135: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ra Pharmaceuticals. https://rapharma.com/ (accessed May 2020).
- 38. Hill A, Schrezenmeier H, Hillmen P, et al. RRA101495, a subcutaneously administered peptide inhibitor of complement component C5, for the treatment of paroxysmal nocturnal hemoglobinuria: phase 2 results. EHA Library 2018; abstract PF305. [Google Scholar]
- 39. Hill A, Valls AG, Griffin M, et al. A subcutaneously administered investigational RNAi therapeutic (ALN-CC5) targeting complement C5 for treatment of PNH and complement-mediated diseases: preliminary phase 1/2 study results in patients with PNH. Blood 2016; 128: 3891. [Google Scholar]
- 40. Wong RSM, Pullon HWH, Deschatelets P, et al. Inhibition of C3 with APL-2 results in normalisation of markers of intravascular and extravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH). Blood 2018; 132(Suppl. 1): 2314.30467190 [Google Scholar]
- 41. de Castro C, Grossi F, Maciejewski J, et al. C3 inhibition with pegcetacoplan in the treatment of subjects with paroxysmal nocturnal hemoglobinuria. Br J Haematol 2020; 189: (Suppl. 1): 137. [Google Scholar]
- 42. Hillmen P, Szer J, Weitz I, et al. Results of the pegasus phase III randomized trial demonstrating superiority of the C3 inhibitor pegcetacoplan, compared to eculizumab in patients with paroxysmal nocturnal hemoglobinuria. EHA Library 2020; abstract S192. [Google Scholar]
- 43. Lesavres PH, Müller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med 1978; 48: 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ellis-Pegler RB, Schwabe C, Ajari I, et al. An orally administered small molecule factor D inhibitor (ACH-4471) for treatment of PNH and complement diseases: preliminary phase I results in healthy volunteers. Haematologica 2016; 101: 416. [Google Scholar]
- 45. Kulasekararaj A, Risitano A, Jaciejewski J, et al. A phase 2 open-label study of danicopan (ACH-0144471) in patients with paroxysmal nocturnal hemoglobinuria (PNH) who have an inadequate response to eculizumab monotherapy. Blood 2019; 134(Suppl. 1): 3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. ClinicalTrials.gov. First-in-Human study of BCX9930 in healthy volunteers and patients with PNH. BioCryst pharmaceuticals, https://clinicaltrials.gov/ct2/show/NCT04330534?term=biocryst&draw=3&rank=9 (accessed May 2020).
- 47. Schubarta A, Anderson K, Mainolfib N, et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc Natl Acad Sci U S A 2019; 116: 7926–7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin K, Zhang L, Kong M, et al. Development of an anti-human complement C6 monoclonal antibody that inhibits the assembly of membrane attack complexes. Blood Adv 2020; 4: 2049–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]