Abstract
Introduction
the identification and management of frailty occurs mostly in primary care. Several different models of care exist. This study aimed to assess the impact of a new General Practitioner (GP)-led modified Comprehensive Geriatric Assessment (CGA) on service delivery, healthcare utilisation and patient outcomes.
Method
patients with moderate–severe frailty (electronic Frailty Index score > 0.24) in Newbattle Medical Practice, Scotland, were eligible for a novel intervention (MidMed) in which an additional GP performed a modified CGA and was directly accessible for appointments. The recruits to the intervention (MidMed) group were compared with those waiting to be enrolled (non-MidMed). Outcomes included unscheduled hospital admissions, primary care consultations, continuity of care (Usual Provider of Care (UPC) index), outpatient attendances and mortality. Adjusted rate ratios (aRR), for MidMed compared to non-MidMed, were estimated using regression models adjusting for demographics and healthcare utilisation histories.
Results
510 patients were included: 290 MidMed (mean(SD) age 80.1(7.6)years; 59.6% female) and 220 non-MidMed (75.4(8.6)years; 57.7% female). Median follow-up was 396 days. aRR(95%CI) was 0.46(0.30–0.71) for >1 admission, 0.62(0.41–0.95) >1 Emergency Department (ED) attendance and 1.52(1.30–1.75) for use of primary care, with no difference in outpatient appointments or mortality. Continuity of care was better for the MidMed group (MidMed UPC 0.77(SD 0.19), non-MidMed 0.41(0.18), P < 0.001).
Conclusion
this GP-led service for frail patients was associated with lower risk of hospital readmission/ED reattendance, greater use of primary care and improved continuity of care. More detailed evaluation of novel primary care frailty services, over longer time-periods, including robust randomised controlled trials, are needed.
Keywords: frailty, general practice, Comprehensive Geriatric Assessment (CGA), older people
Key Points
MidMed was a novel primary care intervention for moderate-severe frailty (modified GP-led CGA and ongoing care).
Patients who received MidMed had reduction in recurrent hospital admissions, more primary care contacts and better continuity of care.
GP-led services for frail patients can impact outcomes.
Future studies need robust evaluation, including randomised controlled trials, and longer follow-ups.
Introduction
People living with frailty are at an increased risk of hospital admission, disability, and death [1–3]. Those living with moderate and severe frailty are among the highest users of healthcare [4, 5] comprising 15 and 5%, respectively, of people aged over 64 in Scotland, with a further 35% having mild frailty [6]. Therefore, the proactive identification and management of frailty is central to many guidelines and policies [7] aiming to improve care for older people. Around 90% of all NHS contacts take place in general practice [8], and with an ethos emphasising the importance of holistic care in the community [9], General Practitioners (GPs) are in an ideal position to identify people with frailty and deliver appropriate interventions.
Primary care services are, however, not generally configured to meet the needs of frail populations [10, 11] and GP workload is nearing ‘saturation point’ [12]. Care can be reactive and fragmented, and appointments are time limited, making it challenging to tackle the complex presentations of this vulnerable group with the available resource [13]. Several options have been explored to transition to a more proactive, integrated, person-centred model of primary care, but there is currently no consensus on a particular approach and evidence around models of care is lacking [14–17].
There is good evidence for the benefit of delivering Comprehensive Geriatric Assessment (CGA) in the hospital setting, however, evidence for its use in other settings, such as the community, is less strong [18, 19]. There is low-certainty evidence that a community-based full CGA may have a reduced risk of unplanned hospital admission [19]. Continuity of care is also important in high-quality care for frail older people [8]. Increased continuity of care is associated with improved patient outcomes, such as decreased hospital utilisation [20]. Conversely, low continuity of care is associated with a higher risk of mortality [21].
GP practices in Midlothian, Scotland, were allocated funding in 2019 by the Midlothian Health and Social Care Partnership (HSCP) to support the identification and management of frailty in their patients. The aim of this study is to evaluate the impact of a new GP-led modified CGA for people living with moderate to severe frailty on service delivery, healthcare utilisation and patient outcomes.
Method
An observational retrospective study of linked routinely collected healthcare data was used to evaluate the effectiveness of MidMed, a primary care intervention.
Study population
Newbattle Practice is a large urban GP practice in the county of Midlothian, on the outskirts of Edinburgh in Scotland, employing 21 GPs who provide primary care to 18,000 patients (13% aged ≥65). People with frailty were identified using the electronic Frailty Index (eFI) [22], which uses routinely collected primary care data, available through GP Read codes, to identify and grade frailty severity. Those categorised as moderately or severely frail using the eFI (score > 0.24) and living at home (i.e. not in a care home) were eligible for the new service called MidMed.
The use of the eFI
In Scotland, the routine identification of frailty is not mandated and is currently not commonly embedded in routine practice. To optimise the use of the eFI, since 2018, staff used opportunistic contacts such as seasonal flu vaccination clinics to add information such as disability deficits to patient records. eFI reports used the subset of Read codes to capture the relevant deficits that contribute to the eFI score [4]. The eFI was only used to screen for eligibility to MidMed. Any subsequent formal diagnosis of frailty or changes to the patient record was only made following clinician assessment.
The MidMed intervention
MidMed was a new model of care introduced by the GP practice in partnership with Midlothian HSCP in June 2019, funding an additional GP (MidMed GP) for nine sessions with a special interest in frailty and multi-morbidity to provide modified CGA and direct patient care to MidMed patients only. The role did not include other GP work, such as being duty doctor. Patients were not randomised to MidMed. The order of enrolling patients to MidMed was determined by the MidMed GP and was mainly based on practicalities, such as ability to contact, or proximity to other MidMed patients.
Phase 1: started June 2019:—modified CGA
Patients received a proactive home visit from the MidMed GP who performed a CGA adapted for primary care. The detailed assessment used the framework and recommendations outlined in the Comprehensive Geriatric Assessment Toolkit for Primary Care Practitioners [23], focusing on the following assessments: physical, functional, social, psychological and medications aligned with the overall person-centred goals. A problem list was generated, resulting in a tailored management plan, including further investigations or referrals, and an anticipatory care plan (ACP), which helps patients consider what is important to them and plan for their future care [24]. The visit usually lasted between 60 and 90 minutes with additional time for administrative tasks afterwards, including updating the patient’s Key Information Summary (KIS) [25] (an ACP document accessible by all healthcare professionals).
Phase 2: started October 2019—Continued care
The MidMed GP also took over the ongoing care of MidMed patients. MidMed patients could book appointments directly with reception, bypassing the usual telephone and/or online triage system. Longer appointments and home visits were more readily available than for patients who were not part of MidMed.
Study participants
Patients who were enrolled into MidMed between 1 June 2019 and 30 September 2020 were included (MidMed group). The comparison group was those patients who were eligible for MidMed, but who had not yet been enrolled by the end of the study, i.e. were still receiving usual care (non-MidMed group). An individual’s enrolment date to the study was the first contact date with MidMed for the MidMed group and the date on the eFI report for the non-MidMed group. Each patient was followed up until the first of the following events: death, a long-term move to a care home, a move away from the practice area or the study end date. An independent Midlothian HSCP data analyst cleaned, linked and pseudonymised datasets from hospital and primary care administrative databases.
Outcomes
The primary outcome was unplanned hospital admission. Secondary outcomes included: emergency department (ED) attendance and outpatient attendance, primary care consultations, continuity of GP care and mortality. Continuity of care (how often the patient sees the same GP) was measured using the Usual Provider of Care (UPC) [26] index. This is the proportion of contacts with the most-seen GP for patients with ≥2 GP contacts within the study period, expressed as a fraction (number of contacts with most frequently seen GP divided by the total number of contacts) [26]. A score of 1 means all visits were to the same GP. High continuity of care has previously been defined as between 0.7 and 1 [27].
Statistical approach
Age, sex, socioeconomic deprivation scores (grouped into fifths based on local thresholds using the Scottish Index of Multiple Deprivation score), level of frailty at enrolment to the study and healthcare utilisation histories (number of emergency inpatient admissions, ED attendances, outpatient attendances and GP contacts) in the 12 months prior were extracted from the healthcare record for the MidMed group at enrolment and the non-MidMed group on the eFI report date. Baseline continuous data were presented as mean (with standard deviation (SD)) or median (with an interquartile range (IQR)) depending on data dispersion, and categorical counts as percentages. All analyses were performed using R software (version 4.0.0) [28].
The differences between groups were analysed by fitting multivariable regression models. Different models were used depending on the characteristics of the data. A hurdle model was used for unplanned hospital admissions and ED attendances. Most patients do not have an unplanned healthcare attendance so standard models perform poorly in summarising risk of repeated admissions. In this two-part method, modelling of the number of attendances first has to cross a hurdle (any attendance) and then a separate process assesses the count of attendances [29–31].
Negative binominal models were used for the number of primary care contacts and number of outpatient attendances [32]. The regression model assumptions for each model were checked and held true. An exposure term was incorporated to account for varying follow-up durations.
The Mean Cumulative Function (MCF) was used to summarise the average number of primary care contacts per patient over time, taking into account censoring events such as loss to follow-up and death [33]. MCF was plotted for the MidMed and non-MidMed group, subdivided by severity of frailty. For continuity of care, the difference between the UPC index was calculated.
Based on a prior Cochrane review [34], which reported CGA had little or no difference in mortality, it was assumed MidMed was unlikely to have a large positive or negative impact on mortality. Therefore, mortality rates between the two groups were used to assess the effect of residual cofounding. A Cox proportional hazard model was used, and proportionality assumptions were tested using Schoenfeld Residuals and met.
The MidMed intervention and evaluation protocols were reviewed by the local NHS ethics committee. An ethics application waiver was approved as these formed part of a service improvement programme.
Results
Baseline characteristics
About 290 patients were enrolled into MidMed between June 2019 and September 2020, leaving 220 patients in the non-MidMed group (Supplementary S1). Table 1 shows baseline characteristics for both groups. The MidMed group were older and included a larger proportion of patients with severe frailty. The two groups were otherwise similar across all domains, although MidMed group had on average more primary care contacts and fewer outpatient attendances in the 12 months prior to baseline.
Table 1.
Baseline patient characteristics in MidMed and non-MidMed patients
| MidMed (n = 290) |
Non-MidMed (n = 220) |
P value | |
|---|---|---|---|
| Age (years)—mean (SD) | 80.1 (7.6) | 75.4 (8.6) | <0.001 |
| Frailty (eFI)a—median (IQR) | 0.33 [0.28,0.39] | 0.28 [0.25,0.31] | <0.001 |
| Sex - female, n (%) | 173 (59.6) | 127 (57.7) | 0.72 |
| Social Deprivation (SIMD)b,c most deprived quintile, n (%) | 150 (51.7) | 130 (59.1) | 0.12 |
| Comorbidities: Number of ICD 10 chapters where diagnoses are recordeda- mean (SD) | 3.66 (1.50) | 3.72 (1.37) | 0.64 |
| Healthcare contact in the 12 months prior to study inclusion | |||
| Hospital admissions One or more admissions, n (%) |
94 (32.4) | 66 (30.0) | |
| Total number of admissions | 182 | 110 | |
| Crude rate | 0.63 | 0.5 | 0.19 |
| ED attendances | |||
| One or more attendances, n (%) | 122 (42.1) | 91 (41.4) | |
| Total number of admissions | 251 | 172 | |
| Crude rate | 0.87 | 0.78 | 0.50 |
| Outpatient attendances | |||
| Mean (SD) | 5.7 (6.9) | 6.8 (8.0) | 0.08 |
| Primary care contacts | |||
| GP/nurse, mean (SD) | 14.2 (12.1) | 11.6(9.5) | 0.01 |
SD: standard deviation, eFI: electronic Frailty Index, IQR: Interquartile Range, ED: Emergency Department
a n = 14 excluded from eFI median as only frailty category known (rather than actual score)
bSIMD: Scottish Index of Multiple Deprivation quintile based on area of residence (postcode), relative to other places in Midlothian (lowest quintile most deprived)
cComorbidities grouped by the International Classification of Disease (ICD-10) chapter headings
The median follow-up time was 396 days (IQR 367 to 468 days). A total of 47 patients died and 8 moved to a care home. About, 273 (94%) MidMed patients had a modified CGA and care plan developed; non-completion was mainly due to moves into care homes.
Outcomes
Key primary and secondary outcomes for MidMed are summarised in Table 2, with the non-MidMed group used as reference.
Table 2.
Rates of hospital admissions, ED attendances and primary care contacts for MidMed patients, compared to non-MidMed
| Outcome | MidMed (n = 290) |
Non-MidMed (n = 220) |
Model 1 Adjusted only for length of follow-up |
Model 2 Fully adjusted for baseline differences |
||||
|---|---|---|---|---|---|---|---|---|
| Patients with any event n (%) |
Total number of events | Crude rate* | Patients with any event n (%) |
Total number of events | Crude rate* | Estimate (95% CI) |
Estimate (95% CI) |
|
| Hospital admissions† | 95 (32.8) | 148 | 0.51 | 75 (32.7) | 161 | 0.63 | OR (any admission or not) | |
| 1.19 (0.82–1.73) | 1.10 (0.72–1.69) | |||||||
| RR (one or more admission) | ||||||||
| 0.54 (0.33–0.87)** | 0.46 (0.30–0.71)*** | |||||||
| ED attendances† | 122 (42.1) | 203 | 0.70 | 100 (45.5) | 208 | 0.81 | OR (any attendance or not) | |
| 1.03 (0.72–1.48) | 0.92 (0.62–1.37) | |||||||
| RR (one or more attendance) | ||||||||
| 0.73 (0.48–1.12) | 0.62 (0.40–0.95)** | |||||||
| Primary care contacts | 276 (95.1) | 5,948 | 20.7 | 185 (84.1) | 3,279 | 12.8 | RR | |
| 1.62 (1.39–1.90)*** | 1.52 (1.30–1.75)*** | |||||||
| Outpatients | 232 (80.0) | 1,357 | 4.7 | 188 (85.4) | 1,553 | 6.2 | RR | |
| 0.80 (0.65–0.98)** | 0.88 (0.73–1.07) | |||||||
| Died | 32 (11.0) | - | - | 20 (9.1) | - | - | HR | |
| 1.36 (0.77–2.38) | 1.32 (0.72–2.45) | |||||||
Reference group: non-MidMed.
CI: Confidence Interval. OR: Odds Ratio. RR: Rate Ratio. HR: Hazard Ratio. Green: reduced odds with MidMed. Red: increased odds with MidMed.
*Crude rate: estimated number of events per person per patient-year.
†Hurdle model for ED attendances and hospital admission. In this two-part method, modelling of the number of attendances first has to cross a hurdle (any attendance/admission) then a separate process assesses the count of attendances.
* * P value <0.05, ***P < 0.001.
Healthcare utilisation
There were similar rates of having at least one recorded hospital admission in both groups during the follow-up period (MidMed n = 95, 32.8%; non-MidMed n = 75, 32.7%, aOR1.10, 95%CI 0.72–1.69). However, patients in the MidMed group with at least one admission had a significantly lower risk of future admissions, compared to those in the non-MidMed group (aRR 0.46, 95%CI 0.30–0.71, P < 0.001). There was also a reduced risk of multiple ED attendances in the fully adjusted model (aRR0.62, 95%CI 0.40–0.95, P = 0.03). Prior hospital admissions/ED attendances and eFI scores were independent predictors in the hurdle models (Supplementary S2).
Patients in the MidMed group had more primary care contacts than those in the non-MidMed group (20.7 vs 12.8 per patient-year, respectively), even after adjustment for age, sex, social deprivation, frailty and previous primary care in the previous 12 months (aRR 1.52, 95%CI 1.30–1.75). The MidMed group received more home visits compared to the non-MidMed group, but more of the MidMed contact was by telephone (1.5 telephone appointments for every face-to–face appointment) (Supplementary S3).
The rate of GP contacts was generally constant over time, differing by frailty and higher in the MidMed group (Figure 1): the orange and red MidMed lines are approximately linear with a steady gradient. In the severely frail non-MidMed group, there is an appearance of a ‘catch up’ after 6 months, but this is based upon a small number of patients with this length of follow-up.
Figure 1.
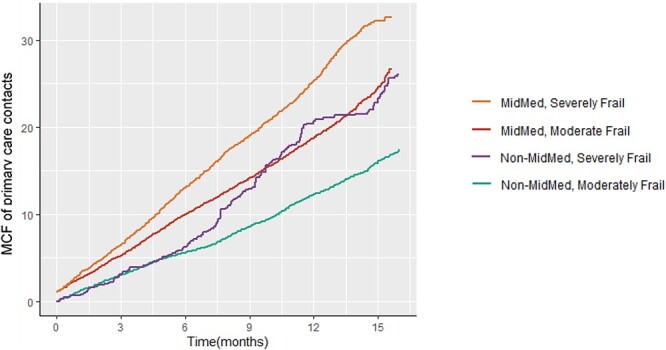
Comparison of numbers of primary care contacts over follow-up time for all patients expressed as a MCF (Mean Cumulative Function: average number of primary care contacts per patient over time, taking into account censoring events such as death), sub-grouped by frailty (unadjusted).
Continuity of care
The mean UPC was higher for the MidMed group (MidMed UPC 0.77 (SD 0.19), non-MidMed 0.41 (0.18), P < 0.001), as expected as the MidMed GP was aiming to provide all ongoing care to the MidMed group as part of phase 2 (Supplementary S4).
Anticipatory care planning and mortality
The proportion of patients with a KIS increased from 23 to 100%, and with a community ‘Do Not Attempt Cardio-Pulmonary Resuscitation’ form from 4 to 44%. About, 23% were added to the palliative care register. Unfortunately, this information was not available for the non-MidMed group. Mortality was slightly higher in the MidMed group (11%) compared to the non-MidMed group (9%), but this was not statistically significant when adjusted for demographics (aHR 1.32 (95%CI 0.72–2.45, P = 0.37)).
Discussion
Summary of results
In this retrospective observational service evaluation of a GP-led modified CGA intervention for people living with moderate–severe frailty, MidMed care was associated with a reduction of approximately 50% in the rate of recurrent hospital admissions and ED attendances. However, there was no reduction in overall risk of any unplanned hospitalisation or any ED attendance, or mortality, in those receiving MidMed care. The intervention increased the rate of primary care contacts by 50%, but with improved continuity of care.
This highlights the importance of considering repeated admissions, as well as single admissions, as outcomes. Possible explanations include more comprehensive GP follow-up on hospital discharge, more thorough advanced care planning or improved continuity of care, all of which are associated with fewer admissions [27, 35, 36]. The lack of impact on the risk of having at least one ED attendance or hospital admission may be true lack of effect, or could be due to the timescale of the evaluation: those admitted in the first few weeks following enrolment in MidMed are unlikely to have had enough time to benefit meaningfully from the enhanced care it provided.
The increase in primary care usage in addition to the high rate of completed CGA and anticipatory care plans suggests successful implementation of MidMed. This may reflect identification of unmet need for frail patients; i.e. the increased activity in this group reflected appropriate care, rather than inappropriate attendance, but we did not collect data on this. The change to a steeper MCF gradient for the severely frail, non-MidMed group in Figure 1 indicates that reactive primary care appointments may, with time, become close to equivalent to those in the MidMed group, which also included proactive appointments. This suggests that longer evaluation is essential, and the burden of increased primary care contacts from MidMed may reduce with time. The COVID-19 pandemic may have also affected outcomes. The first UK lockdown occurred during the study period. As patients were enrolled at different time points, lockdown would have been at variable times after enrolment. MidMed continued throughout the pandemic except for the temporary suspension of routine elements of the CGA between March and June 2020.
Strengths and weaknesses
Strengths of this study include that the intervention was provided by a single GP, leading to a consistent approach in performing CGA. Both groups had the same access to all primary and secondary care services (except for the MidMed service being evaluated) and both would have been affected similarly by service changes unrelated to MidMed, such as the introduction of Red Cross welfare calls or the reconfiguration of primary care in response to the COVID-19 pandemic. The study population was identified by eFI, a routine data measure that is available to all GPs, strengthening its generalisability. There were no exclusion criteria for entry into MidMed (if patients were moderately-severely frail and living at home). Outcome data were extracted by an independent data analyst from routine data, thereby limiting bias.
Common to many service evaluation methodologies, a limitation of this study is the use of observational data. This means that unmeasured baseline variables and inadequate adjustment for differences in baseline characteristics between groups may have caused residual confounding.
The eFI is a recognised feasible and acceptable population risk stratification tool for identifying patients with frailty and is widely available to GP Practices across the UK [6, 22]. The eFI was chosen to select patients eligible for MidMed because this had been a previous area of development work for the GP Practices in Midlothian and also, on balance, it was felt that this was the most practical screening tool in a practice with over 18,000 patients [4]. A limitation of using the eFI is that, due to its relatively high sensitivity and low specificity, some patients may have been inappropriately targeted for MidMed [37]. The GP who conducted the MidMed CGAs found that none of those identified by eFI were not frail, and only 12 patients were identified by other sources.
As MidMed was an older and frailer group, this group was more likely to be at higher baseline risk of adverse outcomes meaning there is a possibility of selection bias, and an underestimation of the effects of MidMed. As MidMed was designed as a proactive service and enrolment was based on practical factors, for example, patients who lived close to each other were enrolled at the same time to limit travel between visits. Patient location could have also introduced selection bias for the intervention but greater than 95% of the GP practice live in an urban area and at most 10-minute drive from the practice [38].
MidMed was delivered by one GP only, so we cannot be certain whether the benefits seen in the study are truly a reflection of the service itself or whether they are determined by the skills of the specific MidMed GP. Contamination bias may have also been present if other staff were influenced in how they cared for non-MidMed patients, leading to the impact of MidMed being underestimated.
Context of existing literature
MidMed is a composite intervention and so we cannot identify which components have the most impact on outcomes.
MidMed identified those with moderate and severe frailty using the eFI. Other studies have included patients at all levels of frailty [39] or patients recruited using less defined criteria [40]. The nature, components and extent of the CGA intervention also differs between studies, with some being led by nurses rather than GPs [41], some having enhanced access to geriatricians and a multidisciplinary team [42]. MidMed’s approach was novel in the addition of a named GP and increased appointment availability. However, there was no routine input of other multidisciplinary team members or robust absence cover. A reduction in healthcare usage might be expected following interventions, which deliver more accessible, holistic, person-centred care. CGA and better continuity of care have each been shown to be associated independently with fewer hospital admissions [18, 27, 43]. Increased primary care usage has been observed in other studies with similar follow-up times where new models of care have been introduced [10, 44–46] but may not extend beyond one year [46].
Relevance and future studies
Robust evaluation designs including randomised trials are needed to determine whether GP-run CGA interventions improve outcomes for frail patients. Many UK primary care guidelines and policies for the management of frail patients now include such interventions among their key recommendations [7]. To our knowledge though, there are as yet no randomised studies published in the UK assessing impact. A randomised feasibility trial of CGA implementation in primary care has recently been completed [47]. Trials should also be complemented by a process evaluation to examine whether the intervention was implemented and delivered as planned. Future studies would benefit from conducting an economic evaluation and from measuring patient-related outcomes such as function and quality of life.
The findings are relevant to other practices in the UK, and beyond frailty prevalence, and outcomes for the non-MidMed group are similar to elsewhere [22]. The model of employing a single additional GP can be replicated elsewhere with appropriate funding and training, though other models have been developed e.g. ‘micro-teams’ within a large GP practice [48], or GPs in the practice running a service alongside their usual work [49]. Evaluation should consider the use of eFI to identify frailty, the ability of healthcare data to determine outcomes, and integration into the healthcare system. Developments of the eFI, such as the eFI+, will better identify people with moderate to severe frailty who would most benefit from community interventions [50].
Conclusion
MidMed was successfully introduced and resulted in some reduction to secondary care use at the potential cost of more primary care contacts. This study suggests a possible model of care for meeting the needs of an increasingly frail population. We now need rigorous randomised controlled trials across a broader multi-practice population. These should include not only service level outcomes, but also patient/carer experiences and satisfaction with care.
Supplementary Material
Acknowledgements
Thanks to Jamie Megaw, strategic programme manager and Katy Simpson, assistance programme manager at Midlothian Health and Social Care Partnership for their support with the project, and James McKerrow for the provision of outcome data.
Contributor Information
Helen E Jones, NHS Lothian, Scotland, UK.
Atul Anand, Centre for Cardiovascular Science, University of Edinburgh, Scotland, UK; Ageing and Health Research Group, Usher Institute, University of Edinburgh, Scotland, UK.
Iain Morrison, Newbattle Medical Practice, Dalkeith, Scotland, UK.
Simon Hurding, Newbattle Medical Practice, Dalkeith, Scotland, UK.
Sarah H Wild, Centre for Population Health Sciences, Usher Institute, University of Edinburgh, Scotland, UK.
Stewart W Mercer, Centre for Population Health Sciences, Usher Institute, University of Edinburgh, Scotland, UK; Advanced Care Research Centre, Usher Institute University of Edinburgh, Scotland, UK.
Susan D Shenkin, Advanced Care Research Centre, Usher Institute University of Edinburgh, Scotland, UK; Ageing and Health Research Group, Usher Institute, University of Edinburgh, Scotland, UK.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The intervention was funded by Newbattle Medical Practice and in part by Midlothian Health and Social Care Partnership. This study was funded by Edinburgh Lothian Health Foundation (S76500). In addition, Stewart Mercer’s role in this research was funded by the Legal & General Group (research grant to establish the independent Advanced Care Research Centre at University of Edinburgh). The funders had no role in conduct of the study, interpretation or the decision to submit for publication. The views expressed are those of the authors and not necessarily those of Legal & General PLC or Edinburgh Lothian Health Foundation.
Data Availability Statement
Data is available from the authors on request.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006; 166: 418–23. [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 2006; 54: 975–9. [DOI] [PubMed] [Google Scholar]
- 4. Hub I. Frailty and the Electronic Frailty Index. Scotland, 2019. [Google Scholar]
- 5.Improvement Hub. Living and Dying Well with Frailty Collaborative: Health Improvement Scotland 2019; (accessed 12 October 2020). https://ihub.scot/improvement-programmes/community-care/electronic-frailty-index-efi/.
- 6. Devereux NE, Graham E, Laura D, Paul B, Thomas M. Testing a proactive approach to frailty identification: the electronic frailty index. Quality BMJ Open 2019; 8: e000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner G, Clegg A. Best practice guidelines for the management of frailty: a British geriatrics society, age UK and Royal College of general practitioners report. Age Ageing 2014; 43: 744–7. [DOI] [PubMed] [Google Scholar]
- 8. Fund. TKs . General practice in England: an overview. Ideas that Change Health. London; 2009. Available from:https://www.kingsfund.org.uk/sites/default/files/general-practice-in-england-overview-sarah-gregory-kings-fund-september-2009.pdf. [Google Scholar]
- 9. Reeves D, Pye S, Ashcroft DMet al. The challenge of ageing populations and patient frailty: can primary care adapt? BMJ 2018; 362: k3349. [DOI] [PubMed] [Google Scholar]
- 10. Bleijenberg N, Drubbel I, Neslo REet al. Cost-effectiveness of a proactive primary care program for frail older people: a cluster-randomized controlled trial. J Am Med Dir Assoc 2017; 18: 1029–36.e3. [DOI] [PubMed] [Google Scholar]
- 11. Loenen T, VDBM M, Heinemann S, Baker R, Faber MJ, Westert GP. Trends towards stronger primary care in three western European countries; 2006-2012. BMC Fam Pract 2016; 17: 59. 10.1186/s12875-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hobbs FDR, Bankhead C, Mukhtar Tet al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007-14. Lancet 2016; 387: 2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero-Ortuno R. Frailty in primary care. Interdiscip Top Gerontol Geriatr 2015; 41: 85–94. [DOI] [PubMed] [Google Scholar]
- 14. Blom JW, Van den Hout WB, Den Elzen WPJet al. Effectiveness and cost-effectiveness of proactive and multidisciplinary integrated care for older people with complex problems in general practice: an individual participant data meta-analysis. Age Ageing 2018; 47: 705–14. [Google Scholar]
- 15. Looman WM, Huijsman R, Fabbricotti IN. The (cost-)effectiveness of preventive, integrated care for community-dwelling frail older people: a systematic review. Health Soc Care Community 2019; 27: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garrard JW, Cox NJ, Dodds RM, Roberts HC, Sayer AA. Comprehensive geriatric assessment in primary care: a systematic review. Aging Clin Exp Res 2020; 32: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hendry A, Vanhecke E, Carriazo AMet al. Integrated care models for managing and preventing frailty: a systematic review for the European joint action on frailty prevention (ADVANTAGE JA). Transl Med UniSa 2019; 19: 5–10. [PMC free article] [PubMed] [Google Scholar]
- 18. Briggs R, McDonough A, Ellis G, Bennett K, O'Neill D, Robinson D. Comprehensive geriatric assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst Rev 2022; 5: CD012705. 10.1002/14651858.CD012705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veronese N, Custodero C, Demurtas Jet al. Special Interest Group in Systematic Reviews of the European Geriatric Medicine Society (EuGMS); Special Interest Group in Meta-analyses and Comprehensive Geriatric Assessment of the European Geriatric Medicine Society (EuGMS). Comprehensive geriatric assessment in older people: an umbrella review of health outcomes. Age Ageing 2022; 51: afac104. 10.1093/ageing/afac104. [DOI] [PubMed] [Google Scholar]
- 20. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med 2015; 13: 123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maarsingh OR, Henry Y, Ven PM, Deeg DJ. Continuity of care in primary care and association with survival in older people: a 17-year prospective cohort study. British Journal of General Practice 2016; 66: e531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clegg A, Bates C, Young Jet al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The British Geriatrics Society . Comprehensive Geriatric Assessment Toolkit for Primary Care London, 2018.
- 24. Improvement SH. Health and Social Care Improvement in Scotland - Anticipatory Care Planning Toolkit.
- 25. iHub . Best Practice Statement for Key Information Summary (KIS) from the Scottish Governmen Scotland: Healthcare Improvement Scotland 2017(accessed 14 July 2022). Available from: https://ihub.scot/media/1910/kis-best-practice-statement-from-the-scottish-government.pdf.
- 26. Salisbury C, Sampson F, Ridd M, Montgomery AA. How should continuity of care in primary health care be assessed? The British journal of general practice : the journal of the Royal College of General Practitioners 2009; 59: e134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ 2017; 356: j84. 10.1136/bmj.j84. [DOI] [PubMed] [Google Scholar]
- 28. Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 29. Bethell J, Rhodes AE, Bondy SJ, Lou WYW, Guttmann A. Repeat self-harm: application of hurdle models. Br J Psychiatry 2010; 196: 243–4. [DOI] [PubMed] [Google Scholar]
- 30. Moineddin R, Meaney C, Agha M, Zagorski B, Glazier RH. Modeling factors influencing the demand for emergency department services in Ontario: a comparison of methods. BMC Emerg Med 2011; 11: 13. 10.1186/1471-227X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rose CE, Martin SW, Wannemuehler KA, Plikaytis BD. On the use of zero-inflated and hurdle models for Modeling vaccine adverse event count data. J Biopharm Stat 2006; 16: 463–81. [DOI] [PubMed] [Google Scholar]
- 32. Schober P, Vetter TR. Count data in medical research: Poisson regression and negative binomial regression. Anesthesia & Analgesia 2021; 132: 1378–9. [DOI] [PubMed] [Google Scholar]
- 33. Donaldson MG, Sobolev B, Kuramoto L, Cook WL, Khan KM, Janssen PA. Utility of the mean cumulative function in the analysis of fall events. J Gerontol A Biol Sci Med Sci 2007; 62: 415–9. [DOI] [PubMed] [Google Scholar]
- 34. Ellis G, Gardner M, Tsiachristas Aet al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017; 9: Cd006211. 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rytter L, Jakobsen HN, Rønholt Fet al. Comprehensive discharge follow-up in patients' homes by GPs and district nurses of elderly patients. A randomized controlled trial. Scand J Prim Health Care 2010; 28: 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes N, Kalsi T, Steves Cet al. Advance care planning (PEACE) for care home residents in an acute hospital setting: impact on ongoing advance care planning and readmissions. BMJ Supportive Palliative Care 2011; 1: 99. 10.1136/bmjspcare-2011-000053.114. [DOI] [Google Scholar]
- 37. Boyd PJ, Nevard M, Ford JA, Khondoker M, Cross JL, Fox C. The electronic frailty index as an indicator of community healthcare service utilisation in the older population. Age Ageing 2019; 48: 273–7. [DOI] [PubMed] [Google Scholar]
- 38. ISD-Scotland. General Practice - GP Workforce and Practice Populations: Public Health Scotland 2022. (accessed 10 November 2022). Available at: https://www.isdscotland.org/health-topics/general-practice/workforce-and-practice-populations/
- 39. Vestjens L, Cramm JM, Nieboer AP. Quality of primary care delivery and productive interactions among community-living frail older persons and their general practitioners and practice nurses. BMC Health Serv Res 2019; 19: 496. 10.1186/s12913-019-4255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGregor MJ, Cox MB, Slater JMet al. A before-after study of hospital use in two frail populations receiving different home-based services over the same time in Vancouver, Canada. BMC Health Serv Res 2018; 18: 248. 10.1186/s12913-018-3040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bleijenberg N, Boeije HR, Onderwater AT, Schuurmans MJ. Frail older adults' experiences with a proactive, nurse-led primary care program: a qualitative study. J Gerontol Nurs 2015; 41: 20–9; quiz 30-1. 10.3928/00989134-20150814-03. [DOI] [PubMed] [Google Scholar]
- 42. Looman WM, Fabbricotti IN, Kuyper R, Huijsman R. The effects of a pro-active integrated care intervention for frail community-dwelling older people: a quasi-experimental study with the GP-practice as single entry point. BMC Geriatr 2016; 16: 43. 10.1186/s12877-016-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beswick AD, Rees K, Dieppe Pet al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008; 371: 725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dalby DM, Sellors JW, Fraser FD, Fraser C, van Ineveld C, Howard M. Effect of preventive home visits by a nurse on the outcomes of frail elderly people in the community: a randomized controlled trial. Canadian Medical Association Journal 2000; 162: 497–500. [PMC free article] [PubMed] [Google Scholar]
- 45. Robinson TE, Boyd ML, North Det al. Proactive primary care model for frail older people in New Zealand delays aged-residential care: a quasi-experiment. J Am Geriatr Soc 2021; 69: 1617–26. [DOI] [PubMed] [Google Scholar]
- 46. Metzelthin SF, Rossum E, Hendriks MRet al. Reducing disability in community-dwelling frail older people: cost-effectiveness study alongside a cluster randomised controlled trial. Age Ageing 2015; 44: 390–6. [DOI] [PubMed] [Google Scholar]
- 47. Safari R. ClinicalTrials.Gov: Comprehensive Geriatric Assessment in Primary Care: A Randomised Feasibility Trial 2018. (accessed 11 June 2022)Available from:https://clinicaltrials.gov/ct2/show/NCT03394534.
- 48. Prestatyn Iach. Prestatyn Iach North Wales 2020(accessed 18 January 2022). Available from:https://healthyprestatyniach.co.uk/.
- 49. Megaw J. End of Project Report. The Health Foundation, 2018. (accessed 21 January 2022). Available from: https://www.health.org.uk/improvement-projects/building-an-analytical-framework-around-the-electronic-frailty-index-to. [Google Scholar]
- 50. Clegg A. ClinicalTrials.Gov: Development and Evaluation of the Electronic Frailty Index+ (eFI+). (accessed 10 November 2022). Available fromhttps://clinicaltrials.gov/ct2/show/NCT04113174.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the authors on request.


