Abstract
Matsutake mushrooms are among the best-known edible wild mushroom taxa worldwide. The representative Tricholoma matsutake is from East Asia and the northern and central regions of Europe. Here, we report the existence of T. matsutake under fir trees in Eastern Europe (i.e., Ukraine), as confirmed by phylogenetic analysis of nine loci on the nuclear and mitochondrial genomes. All specimens from Japan, Bhutan, China, North Korea, South Korea, Sweden, Finland, and Ukraine formed a T. matsutake clade according to the phylogeny of the internal transcribed spacer region. The European population of T. matsutake was clustered based on the β2 tubulin gene, with a moderate bootstrap value. In contrast, based on analyses of three loci, i.e., rpb2, tef1, and the β2 tubulin gene, T. matsutake specimens sampled from Bhutan and China belonged to a clade independent of the other specimens of this species, implying a genetically isolated population. As biologically available type specimens of T. matsutake have not been designated since its description as a new species from Japan in 1925, we established an epitype of this fungus, sampled in a Pinus densiflora forest in Nagano, Japan.
Keywords: bioresource conservation, edible mycorrhizal mushroom, lectotype, population analysis
1. Introduction
Matsutake mushrooms are gourmet foods in Japan and other Asian countries. Mushroom traders have been searching for new sources of matsutake mushrooms worldwide since the 1960s and have introduced new matsutake populations to the Japanese market (Hongo, 1971; Ogawa, 1978; Tsing, 2015). These matsutake mushrooms are identified as Tricholoma matsutake (S. Ito & S. Imai) Singer from eastern Asia and northern Europe, T. anatolicum H.H. Doğan & Intini from the Mediterranean region, T. magnivelare (Peck) Redhead and T. murrillianum Singer from North America, T. mesoamericanum Justo & Cifuentes from Mesoamerica, and T. bakamatsutake Hongo and T. fulvocastaneum Hongo from eastern and southeastern Asia (Hosford, Pilz, Molina, & Amaranthus, 1997; Ota et al., 2012; Endo et al., 2015; Trudell, Xu, Saar, Justo, & Cifuentes, 2017; Vaario, Yang, & Yamada, 2017; Yamanaka, Yamada, & Furukawa, 2020). All of these species are of high value in the mushroom economy, and Japanese imports of matsutake mushrooms amount to approximately 50 million dollars annually (Trade Statistics of Japan Ministry of Finance; https://www.customs.go.jp/toukei/info/tsdl.htm). In 2000, Swedish matsutake that had been identified as Tricholoma nauseosum (A. Blytt) Kytöv. (Kytövuori, 1988) was shown phylogenetically to be conspecific with Japanese T. matsutake (Bergius and Danell, 2000). This incurred a taxonomic issue, in that T. matsutake can be synonymized to T. nauseosum, based on the priority of the latter by the International Code of Nomenclature for algae, fungi, and plants. However, Ryman, Bergius, and Danell (2000) suggested retaining the name T. matsutake because of the established common name “matsutake mushroom” globally, and the historical and cultural importance of the mushroom in biology and the food industry. Recently, the scientific names of American matsutake mushrooms were revised, i.e., the eastern and pale tan color T. magnivelare, western and whitish T. murrillianum, and tan color Mesoamerican T. mesoamericanum, most of which had been identified as T. magnivelare, T. ponderosum (Armillaria ponderosa), T. murrillianum (A. arenicola) or merely “matsutake” (Smith, 1979; Redhead, 1984; Arora, 1986; Singer, 1986; Hosford et al., 1997; Trudell et al., 2017).
In this paper, we report a new T. matsutake from Eastern Europe (Ukraine). At present, the known geographic distribution of T. matsutake is restricted to Asia and northern and central Europe (Matsushita et al., 2005; Endo et al., 2015; Vaario et al., 2017). To fill the knowledge gap in T. matsutake ecology between Asia and Europe, exploration in the central region of the Eurasian continent has been desired. As we obtained a probable T. matsutake basidioma in 2020 from Ukraine (Fig. 1), we first clarified its species identity based on the internal transcribed spacer (ITS) sequence of nuclear ribosomal DNA (nuc rDNA). Then, we clarified how these two isolated populations of T. matsutake in Eurasia fit within the metapopulation based on phylogenetic analyses of multiple loci.
Fig. 1 - Basidiomata of Tricholoma matsutake in a fir-beech mixed forest in Ukraine (photographed by NB). Mature and open-veiled basidiomata (upper) and young basidiomata (lower).
We designate a type of T. matsutake sampled in Japan. The new species description as Armillaria matsutake (Ito and Imai, 1925) did not accompany a detailed morphological description but cited a picture of Matsutake, which was identified as Cortinellus edodes P. Henn. by Seiichi Kawamura (1913), sampled in Honshu Island, the main island of the Japanese Archipelago. Kawamura (1913) also did not designate any biological materials for the fungal description. Therefore, the drawing of C. edodes by Kawamura (1913) has been designated as the lectotype of T. matsutake (Ryman et al., 2000). Agaricus edodes Berk. (Berkeley, 1877), the original scientific name of C. edodes adopted by Kawamura (1913) to the lectotype of Matsutake, is now regarded as the basionym of Lentinula edodes (Berk.) Pegler. This taxonomic complication is clearly summarized in Ito and Imai (1925), but it is not necessarily sufficient in terms of the cause, so we again added a detailed account of this taxonomic history. Schröter (1886) obtained a dried specimen of Japanese Shiitake cultivated on log through Shinkichi Nagai and observed it, apart from the Shiitake specimen (No. 258) of Berkeley (1877), and described it as Collybia shiitake Sieboldt. Concurrently, Schröter (1886) observed a specimen of Matsutake (canned in salt water) obtained from Japan through S. Nagai and adopted the scientific name Agaricus (Arimillaria) edodes given to Shiitake by Berkeley (1877) for it. Schröter (1886), based on his own observation of the canned thinly-sliced basidiomata (the color and texture of the stipe surface was distinctly different above and below the annulus) with the aid of a drawing of Matsutake presented on a Japanese nature book focused on plant phenology (Baishiken, 1842), recognized a particularly strong relationship of Matsutake with the genus Armillaria, which might have led to the adoption of the Latin name. Hennings (1899) largely followed the identification of Schröter (1886) in these two species but took a slightly different position on Shiitake, changing the Latin name to Cortinellus shiitake (Schröter) P. Henn. On the other hand, for Matsutake, he followed Schröter's (1886) description and concluded that Berkeley's (1877) specimen (No. 258) is Matsutake. Hennings (1899) thought that Berkeley (1877) mistakenly labelled as Shiitake to the Matsutake specimen. Based on his own observation of a Japanese specimen of Matsutake (probably sampled in Tochigi Prefecture as described below) obtained through Mitsutaro Shirai, Hennings (1899) concluded that Matsutake is a species of the genus Armillaria, which is closely related to A. robusta (Alb. & Schwein.) Gillet. Hennings (1899) might have regarded Matsutake as Armillaria edodes but did not use the Latin name to Matsutake in the description. Hennings (1900) identified Matsutake as Cortinellus edodes (Berk.) P. Henn., which was largely dependent on the drawing of Matsutake sampled in Japan (probably in Kyoto) and drawn by M. Shirai (Shirai and Henning, 1899), and another drawing of Matsutake (originally sampled in Nagano Prefecture) by M. Shirai (Shirai and Henning, 1931) duplicated from Ichioka (1799). In Shirai and Henning (1931), which was basically compiled in 1899-1900 but released in 1931 by M. Shrai, Hennings annotated “Cortinellus matsutake” to the drawing of Matsutake. Unfortunately, Shirai and Henning (1899) lacks the drawing of Matsutake on the extant material stored in National Diet Library, Japan. Hennings (1901), based on his own observation of a Matsutake specimen immersed in alcohol from Japan sampled in Tochigi Prefecture by M. Shirai, described the species as Armillaria edodes Berk. Saccardo (1887) adopted Armillaria edodes (Berk.) Sacc. to Shiitake, based on the description by Berkeley (1877). We inferred that Kawamura (1913) did not adopt A. edodes to Matsutake but did provisionally C. edodes. Kawamura (1913) adopted C. shiitake (Schröter) P. Henn. to Shiitake.
Given the phylogenetic and other biological analyses of the T. matsutake population, an ideal type sampled in Japan should be designated. Currently, the Japanese T. matsutake population is endangered in its natural habitat, especially in western and lowland areas including Kyoto, a productive area (Kawamura, 1913) in the past, and surrounding provinces on Honshu Island due to depletion of the main host trees (i.e., Pinus densiflora Siebold et Zucc.) (Vaario et al., 2017; Brandrud, 2020; Yamanaka et al., 2020). Therefore, we set an epitype of T. matsutake sampled in a large P. densiflora forest in Nagano Prefecture, at a central and relatively high elevation on Honshu Island, which is currently the most productive area for this fungus in Japan.
Finally, we discuss the biogeography and taxonomy of T. matsutake, because its wide distribution in Eurasia implies diverse genetic variations among isolated geographic regions, underscoring the need for taxonomic reconsideration of this species.
2. Materials and Methods
2.1. Specimens examined
We used specimens of several matsutake mushrooms collected in Japan and other regions in the Northern Hemisphere (Table 1). All samples from abroad were brought to Japan legally and used for the following analyses as the collaborative research between Japan and each of these countries. Fresh materials were lyophilized and oven-dried at 60 °C overnight to inactivate DNases and other oxidative enzymes, and stored in the laboratory. Several specimens were obtained from mushroom traders or mushroom markets. Where necessary, specimens were deposited in the National Museum of Nature and Science (TNS), Japan. In addition, we used several cultured strains of T. matsutake for the phylogenetic characteristics and ectomycorrhizal properties of which have been reported.
Table 1. Samples of Tricholoma matsutake and related species examined in the present study.
| Name | Basidioma specimen | Date | Country | Location | Canopy vegetationh |
| T. matsutake | AY-2200915-001 | Sep 15, 2020 | Ukraine | Kosiv, Ivano-Frankivsk | Aal, Fsy |
| T. matsutake | Ishida F-0247 | Sep 5, 2007 | Sweden | Åheden, Vasterbotten | Psy |
| T. matsutake | Ishida F-0315 | Aug 19, 2007 | Sweden | Hissjön, Umeå | Psy |
| T. matsutake | AT-0925* | 2000 | Sweden | n.d. | n.d. |
| T. matsutake | AY-2040800-001 | Aug, 2004 | Finland | Haukipudas, North Ostrobothnia | Psy |
| T. matsutake | AY-2070925-001 (= Tn-FIN1a) | Sep 25, 2007 | Finland | Kontiolahti, North Karelia (same sampling site of isolation origin EF b, *) | Psy |
| T. matsutake | AY-2040400-001 | April, 2004 | Bhutan | Thimphu | n.d. |
| T. matsutake | AY-1981000-002 (= BH1c) | Oct, 1998 | Bhutan | n.d. | n.d. |
| T. matsutake | Narimatsu S-1 | July 27, 2017 | Bhutan | Geneka | Pwa, Qsm |
| T. matsutake | Narimatsu S-2 | July 27, 2017 | Bhutan | Geneka | Qsm |
| T. matsutake | Narimatsu S-3-1 | July 27, 2017 | Bhutan | Geneka | Pwa, Qsm |
| T. matsutake | Narimatsu S-3-2 | July 27, 2017 | Bhutan | Geneka | Pwa, Qsm |
| T. matsutake | AY-1980831-001 | Aug 31, 1998 | China | n.d. | n.d. |
| T. matsutake | AY-1981000-001 (= CH1c) | Oct, 1998 | China | n.d. | n.d. |
| T. matsutake | Chi4* (= CH-HEc) | 2007 | China | Heilongjiang | n.d. |
| T. matsutake | Chi6* (= CH-YU1c) | 2007 | China | Yunnan | n.d. |
| T. matsutake | AY-1981000-003 (= NK1c) | Oct, 1998 | North Korea | n.d. | n.d. |
| T. matsutake | AT-0924* (= KFRI 432) | Sep 21, 1995 | South Korea | Hongcheon, Kangwon-do | Pde |
| T. matsutake | AY-2150919-001 | Sep 19, 2015 | Japan | Nishi-okoppe, Hokkaido | Asa |
| T. matsutake | AY-2101004-001 | Oct 4, 2010 | Japan | Nikko, Tochigi | n.d. |
| T. matsutake | AY-1981023-003 | Oct 23, 1998 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | AY-2051104-001 | Nov 4, 2005 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | AY-2051104-002 | Nov 4, 2005 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | AY-2051104-003 | Nov 4, 2005 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | AY-2071023-002 | Oct 23, 2007 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | Y1a, c, d, * (= NBRC 33136) | Oct, 1993 | Japan | Takaizuri, Hitachi-ohmiya, Ibaraki | Pde |
| T. matsutake | TUA-115 | Oct 10, 2019 | Japan | Mt. Norikuradake, Matsumoto, Nagano | Tdi |
| T. matsutake | AY-2131013-001 (= S-2131013-001e) | Oct 13, 2013 | Japan | Mt. Norikuradake, Matsumoto, Nagano | Ave |
| T. matsutake | TUA-84 | Oct 10, 2018 | Japan | Motoyama, Shiojiri, Nagano | Tsi |
| T. matsutake | AT-0740*, ** | June 24, 2001 | Japan | Nyu-yama, Ina, Nagano | Pde |
| T. matsutake | AT-0748*, ** | July 2, 2003 | Japan | Mt. Moriyasan, Ina, Nagano | Pde |
| T. matsutake | AY-2041007-002 (= TNS-F 82226, epitype) | Oct 7, 2004 | Japan | Kuwahara, Nakagawa, Nagano | Pde |
| T. matsutake | AY-2101020-001 | Oct 20, 2010 | Japan | Kuwahara, Nakagawa, Nagano | Pde |
| T. matsutake | AY-2181005-001 (= TNS-F 82227) | Oct 5, 2018 | Japan | Kuwahara, Nakagawa, Nagano | Pde |
| T. matsutake | AY-2021027-001 | Oct 27, 2002 | Japan | Matsukawa, Nagano | Pde |
| T. matsutake | AY-2041007-001 (= TNS-F 82228) | Oct 7, 2004 | Japan | Shobuzawa, Ooshika, Nagano | Tsi |
| T. matsutake | AY-2101021-002 | Oct 21, 2010 | Japan | Shobuzawa, Ooshika, Nagano | Tsi |
| T. matsutake | AY-2071101-001 (isolation origin of Tm#84f, *) | Nov 1, 2007 | Japan | Takagi, Nagano | Pde |
| T. matsutake | AT-0742*, **, g | Oct 12, 2001 | Japan | Kamihisakata, Iida, Nagano | Tsi |
| T. matsutake | AY-2191104-001 | Nov 4, 2019 | Japan | Mt. Misumiyama, Tottori, Tottori | Pde |
| T. magnivelare | AY-2080307-001 | Mar 7, 2008 | Canada | Quebec | n.d. |
| T. mesoamericanum | AY-1981000-004 (= MX1a) | Oct, 1998 | Mexico | n.d. | n.d. |
| T. murrillianum | AT-0913* (= Tp-C3a) | 1994 | Canada | n.d. | n.d. |
| T. murrillianum | AY-2080100-001 | Jan, 2008 | Canada | n.d. | n.d. |
| T. murrillianum | AY-2071018-001 | Oct 18, 2007 | Canada | n.d. | n.d. |
| T. anatolicum | AY-1981000-005 (= MC1a) | Oct, 1998 | Morocco | n.d. | n.d. |
| T. anatolicum | AY-1981000-006 (= MC1a) | Oct, 1998 | Morocco | n.d. | n.d. |
| T. anatolicum | AY-2061109-001 (= S-3-2-1a) | Nov 9, 2006 | Turkey | Babadağ, Denizli | Cli |
| T. anatolicum | AY-2061108-004 (= S-2-2-1a) | Nov 8, 2006 | Turkey | Mt. Çal, Fethiye | Cli |
| T. anatolicum | AY-2061109-002 (= S-2-3a) | Nov 9, 2006 | Turkey | Yayla Koru, Fethiye | Cli |
| T. fulvocastaneum | AY-2091112-001 | Nov 12, 2009 | Japan | Amami-ohshima Island, Kagoshima | Qmi, Plu |
| T. fulvocastaneum | AY-2110606 | Jun 6, 2011 | Laos | n.d. | n.d. |
| T. bakamatsutake | AY-2080800-001 | Aug, 2008 | China | Jilin Province | n.d. |
| T. bakamatsutake | AY-2050912-001 | Sep 12, 2005 | Japan | Nishinouchi, Hitachi-ohmiya, Ibaraki | Qsr |
| T. bakamatsutake | AT-0760* | Oct, 2003 | Japan | Kuwahara, Nakagawa, Nagaono | Qac |
| T. bakamatsutake | AY-2041007-003 | Oct 7, 2004 | Japan | Kuwahara, Nakagawa, Nagaono | Qsr |
| T. bakamatsutake | AY-2071001-002 | Oct 1, 2007 | Japan | Kuwahara, Nakagawa, Nagaono | Qsr |
a-g Please refer to Ota et al. (2012), Vaario et al. (2010), Murata et al. (2008), Yamada et al. (1999b), Endo et al. (2015), Yamada et al. (2019), and Saito et al. (2018), respectively.
h Aal: Abies alba, Asa: A. sachalinensis, Ave: A. veitchii, Cli: Cedrus libani, Fsy: Fagus sylvatica, Pde: Pinus deinsflora, Plu: P. luchuensis, Psy: P. sylvestris, Pwa: P. wallichiana, Qac: Quercus acutissima, Qmi: Q. miyagii, Qsm: Q. semecarpifolia, Qsr: Q. serrata, Tdi: Tsuga diversifolia, Tsi: T. sieboldii
* Cultured strain. ** These cultures were isolated from monotropoid mycorrhizal root tips of Monotropa hypopitys that grew on the colony of T. matsutake.
2.2. DNA analysis
DNA was extracted from dried basidioma specimens as described by Gardes and Bruns (1993) with minor modifications. For PCR, we focused on several loci: the ITS and intergenic spacer 1 (IGS1) regions of nuc rDNA, the translation elongation factor 1-alpha (tef-1), β2 tubulin, glyceraldehyde-3-phosphate dehydrogenase (gapdh), the DNA-directed RNA polymerase II subunit (rpb2), the small subunit (SSU) of the mitochondrial rRNA gene tandem repeat (mt rDNA), the mitochondrial ATP synthase membrane subunit 6 (atp6), and a macroevolutionary genomic marker specific to the phylum Basidiomycota (megB1) (Table 2). Although megB1 is located in the nuc rDNA IGS1 region in diverse Basidiomycota taxa, it is not associated with nuc rDNA in section Caligata of Tricholoma, including T. matsutake (Babasaki, Neda, & Murata, 2007). The primers used are listed in Table 2. PCR was conducted using the GeneAmp PCR System 2700 (Applied Biosystems, Foster City, CA, USA). The 25 µL reaction mixture consisted of 2.5 µL 10 × DreamTaq buffer, 2.5 µL dNTP mixture (0.2 mM), 2.5 µL each primer (0.5 µM), 0.125 µL DreamTaq DNA Polymerase (0.625 U; Thermo Fisher Scientific, Waltham, MA, USA), and 0.5 µL the extracted DNA as the template. The PCR parameters were initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 46-55 °C for 30 s, extension at 72 °C for 1.5 min, and a final extension step at 72 °C for 10 min. The PCR amplicons were electrophoresed (Mupid®-exU; TaKaRa Bio, Kusatsu, Japan) on 1.5% agarose gels for fragments ≥ 1 kb (01163-76; Nacalai Tesque, Kyoto, Japan) for 30 min, stained with 0.001% ethidium bromide solution, and visualized using an ultraviolet illuminator (NM-15; UVP, Upland, CA, USA). The PCR amplicons were purified using a QIAquick PCR Purification Kit (QIAGEN, Venlo, the Netherlands) and subjected to cycle sequencing. The ITS and IGS1 regions of nuc rDNA, gapdh, and the mt rDNA SSU were cloned manually before cycle sequencing using a Mighty TA-Cloning Kit (TaKaRa Bio) and then inserted into competent Escherichia coli JM109 cells (TaKaRa Bio).
Table 2. PCR primers used in the present study.
| Targeted locus | Primer name | Sequence (5'→3') | Tm value (°C) |
| nuc rDNA ITS (N*) | ITS1F (F)1 | CTTGGTCATTTAGAGGAAGTAA | 55 |
| ITS4B (R)1 | CAGGAGACTTGTACACGGTCCAG | 65 | |
| ITS4 (R) 2 | TCCTCCGCTTATTGATATGC | 60 | |
| nuc rDNA IGS1 (N) | CNL12 (F) 3 | CTGAACGCCTCTAAGTCAG | 56 |
| 5S-Anderson (R) 4 | CAGAGTCCTATGGCCGTGGAT | 66 | |
| RPB2 (N) | rpb2_tu_f1 (F) 5 | CTGTCGGYTCYTATTCTGC | 53 |
| rpb2_tu_r1 (R) 5 | GCTRGGATGAATCTCACAATG | 52 | |
| GAPDH (N) | gpd_tm_f1 (F) 6 | CTTGCCGACGGCCTTTG | 57 |
| gpd_tm_r1 (R) 6 | CCCTTCATCGATCTCGAATACATGG | 57 | |
| TEF1 (N) | tef1_tm_f1 (F) 6 | GTCAAACVCGAGAGCAYG | 54 |
| tef1_tm_r1 (R) 6 | CACAAGCTTGACRATRCAAGC | 55 | |
| tef1_tm_2f1 (F) 6 | CTCGAGSGATTTACCTGTCC | 55 | |
| tef1_tm_2r1 (R) 6 | GACCGATTCAACGAAATCGTG | 54 | |
| β2 tubulin gene (N) | b-tub1_tm_f1 (F) 6 | GCGTGAAATCGTCCACCTTC | 56 |
| b-tub1_tm_r1 (R) 6 | CAGGGAAYCGCAAGCAAG | 56 | |
| b-tub1_tm_2f1 (F) 6 | CCAGTTGGTGGACAGAAAG | 53 | |
| b-tub1_tm_2r1 (R) 6 | CTGGATCAGGTGCGAAGTT | 55 | |
| mt rDNA SSU (M*) | MS1 (F) 2 | CAGCAGTCAAGAATATTAGTCAATG | 52 |
| MS2 (R) 2 | GCGGATTATCGAATTAAATAAC | 48 | |
| ATP6 (M) | ATP-3f (F) 7 | TCTCCTTTAGAACAATTTGA | 46 |
| ATP-6r (R) 8 | AACTAATARAGGAACTAAAGCTA | 48 | |
| megB1 (N) | TS-1_fws2(F) 9 | CCCGTCTTAATCGACCATG | 52 |
| TS-2_rvc1(R) 9 | TCAGACATCCAAAGGAAGGT | 53 |
* N: nucleus; M: mitochondrion
1 Gardes & Bruns (1993); 2 White et al. (1990); 3 Anderson & Stasovski (1992); 4 Henrion et al. (1992); 5 Aoki et al. (2021); 6 This study; 7 Kretzer & Bruns (1999); 8 Binder & Hibbett (2003; http://www2.clarku.edu/faculty/dhibbett/Protocols_Folder/Primers/Primers.pdf); 9Babasaki et al. (2007).
For cycle sequencing, the 10 µL reaction mixture consisted of 2 µL distilled water (DW), 2 µL 10 × buffer, 1 µL 5 mM primer, 1 µL Ready Reaction Mix (BigDye Terminator v. 3.1 Cycle Sequencing Kit; Thermo Fisher Scientific), and 4 µL purified DNA solution. The reaction parameters for cycle sequencing were initial denaturation at 96 °C for 1 min, followed by 25 cycles of denaturation at 96 °C for 10 s, annealing at 46-55 °C (Table 2) for 5 s, and extension at 60 °C for 4 min. The amplicons were sequenced using an Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems). The obtained sequence data (chromatograms) were edited by Chromas 2.6.6 (http://technelysium.com.au/wp/chromas/) and checked the consensus between complementary sequences of each region in each sample by GeneStudio (http://genestudio.com/). The consensus sequences were deposited in DDBJ (Supplementary Table S1). Several sequences were downloaded from GenBank and UNITE (Supplementary Table S2). For phylogenetic analysis of each DNA region, sequences were aligned using ClustalW (Larkin et al., 2007) and MEGA7 (Kumar, Stecher, & Tamura, 2016). Alignment gaps were treated as missing data, and ambiguous positions were excluded from the analysis. Datasets for the ITS (ITS1-5.8S-ITS2; 651 bp) and IGS1 (420 bp) regions of nuc rDNA, rpb2 (461 bp), gapdh (629 bp), atp6 (347 bp), tef-1 (695 bp), SSUs of mt rDNA cluster (581 bp), β2 tubulin (710 bp), and megB1 (445 bp) were prepared. Maximum likelihood (ML) and Bayesian inference analyses were conducted to clarify the phylogenetic relationships among species and among specimens within T. matsutake. ML trees were constructed using RAxML version 8.2.4 with 1000 bootstrap replicates, which followed a general time-reversible model with a gamma distribution and invariant sites (Stamatakis, 2014). For Bayesian inference analysis, the SYM model of nucleotide substitution with a discrete gamma distribution was performed using MrBayes 3.2.1 (Ronquist et al., 2012). Two runs with four chains of Markov Chain Monte Carlo iterations were performed for 1,000,000 generations when the average standard deviation of split frequencies was below 0.01 (the first 25% of generations were treated as burn-in). Trees were kept for every 100 generations, and the remaining 75% of trees were used to calculate the 50% majority-rule consensus topology and to determine the posterior probabilities for individual branches.
2.3. Microscopy observations of selected specimens
In this study, we observed basidiospores and basidia and compared their sizes among the specimens distinguished by the phylogenetic analyses. Several gills were cut from each dried specimen, rehydrated in a few milliliters of 5% KOH solution for 30 min, transferred to several milliliters of DW, and incubated for several minutes. Fully rehydrated gills were sectioned with a razor, mounted on glass slides using lactic acid, and observed under a differential interference contrast (DIC) microscope with a 100 × oil-immersion objective lens (BX51N-33; Olympus, Tokyo, Japan or AXIO Scope 2; Carl Zeiss, Göttingen, Germany). When using Melzer's reagent (Clémençon, 2012), the dried material was rehydrated in 10 mL 70% ethanol solution for a few minutes, transferred to 20 mL DW for 1 h, and mounted in Melzer's reagent. The lengths and widths of cells were measured at a resolution of 0.1 µm on the recorded micrographs using ImageJ software (https://imagej.nih.gov/ij/). The Q-value (length/width) of each spore was calculated. At least 100 spores and basidia in each specimen were measured. As some samples had fewer spores and basidia, smaller numbers thereof were measured.
Measured numerical data were subjected to further statistical analyses. For most samples, the shortest 5% of all measured spore-length data (n ≥ 100) were excluded from the evaluation because such spores were most likely immature. In the case of samples with an insufficient number of measured spores (n < 100), 25% of the shortest samples were excluded from the evaluation. Because these specimens had much possibility to be measured the size of immature basidiospores. To compare numerical data, one-way analysis of variance was conducted using R (https://www.r-project.org/), and the significance of differences was determined by Tukey's post hoc test (p < 0.05). To indicate the range of cell size in the morphological description, we ordered the mean value of each specimen as minimum-average-maximum.
For the morphological definition of T. matsutake, we followed Kawamura (1930, 1955), Imazeki and Hongo (1957), and Christensen and Heilmann-Clausen (2013). In addition, as Kytövuori (1988) described sclerobasidia and sclerospores in T. nauseosum, we observed their presence/absence and measured their wall thickness. For the description of T. matsutake based on the epitype specimen, which was newly desiginated in this study, we conducted additional DIC microscopy observations and observed the sizes of the sterigma, pileipellis, and stipitipellis (Clémençon, 2012; Christensen and Heilmann-Clausen, 2013).
3. Results
3.1. Phylogenetic relationships among matsutake mushrooms
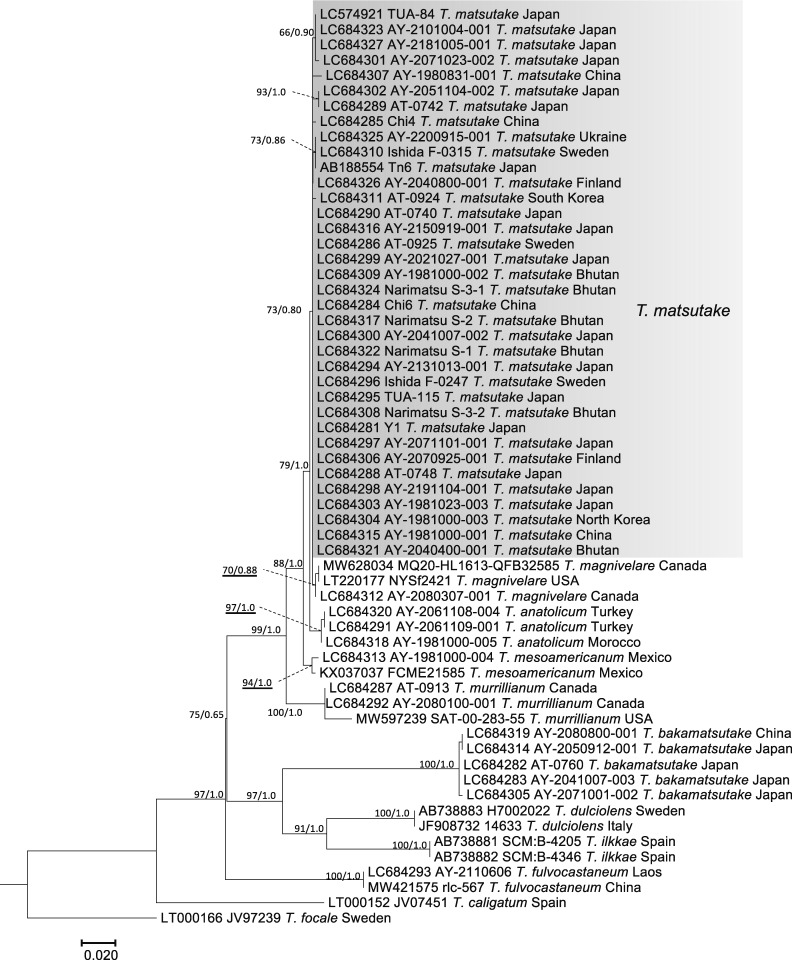
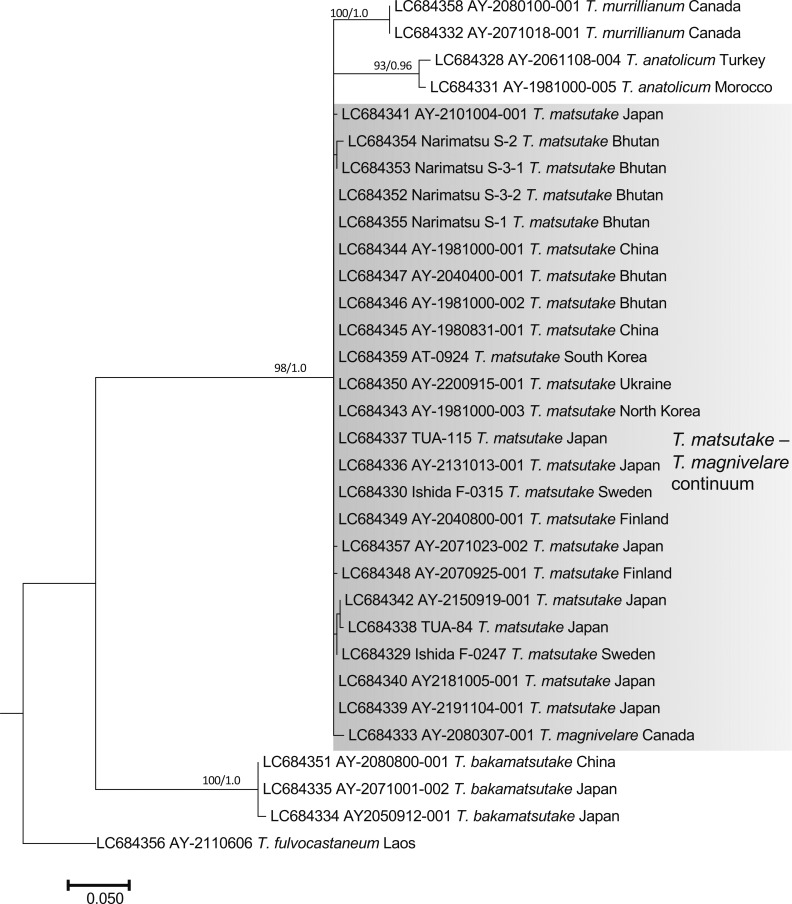
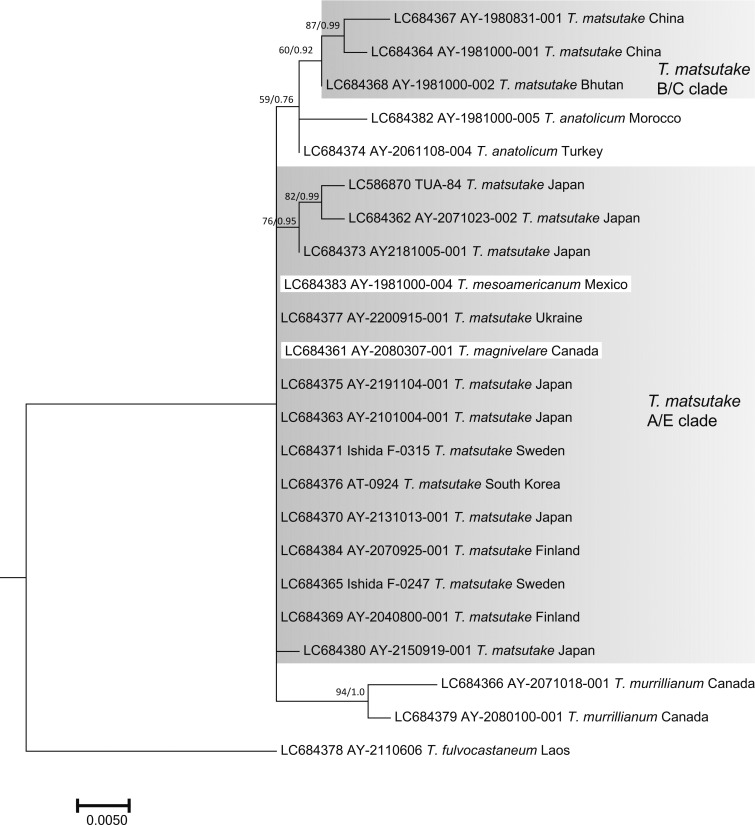
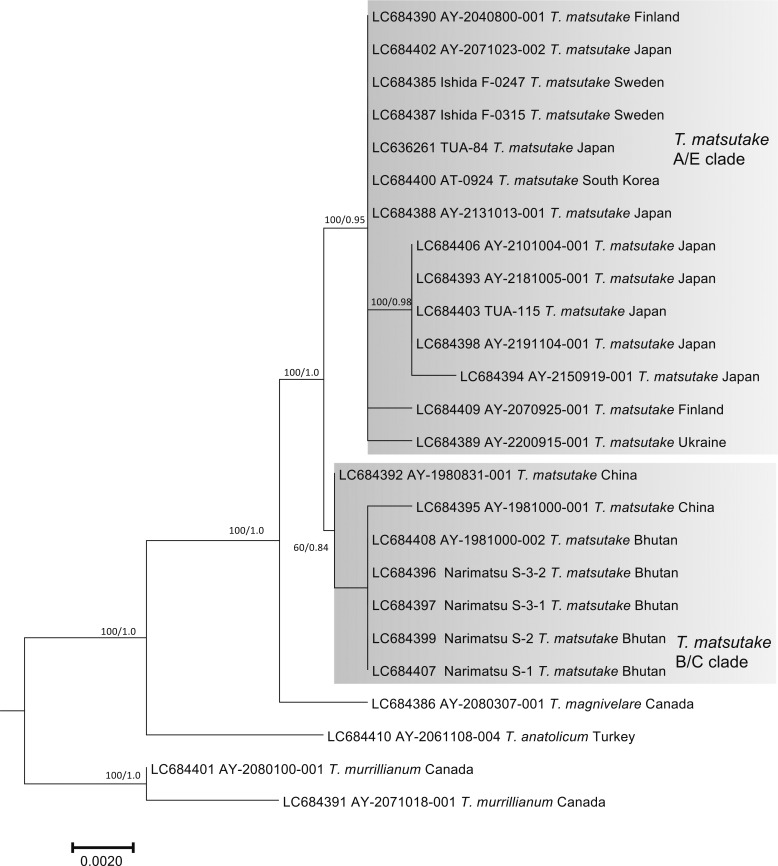
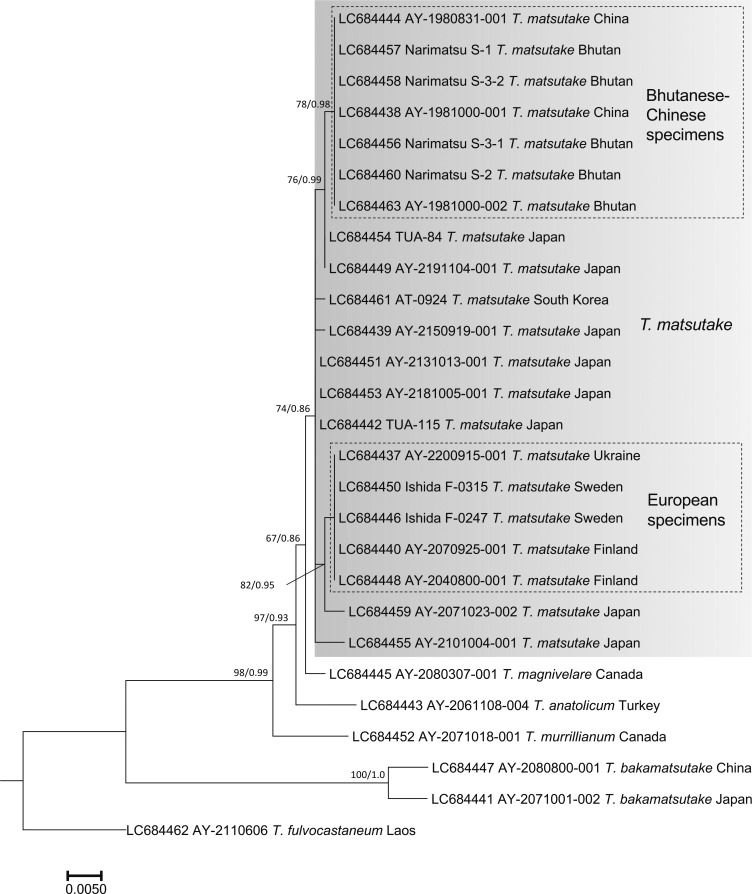
The nuc rDNA ITS phylogenetic tree included the Ukraine specimen in the T. matsutake clade that comprised all Japanese, Bhutanese, Chinese, North Korean, South Korean, Swedish, and Finnish specimens examined (Fig. 2). One Chinese T. bakamatsutake specimen, AY-2080800-001, sampled in Jilin Province and labeled as T. matsutake was included in the T. bakamatsutake clade. Other related species (T. magnivelare, T. anatolicum, T. mesoamericanum, T. murrillianum, and T. fulvocastaneum) formed an independent clade. The phylogenetic tree of nuc rDNA IGS1 (Fig. 3) showed a different topology from that of ITS. The T. magnivelare specimen AY-2080307-001 from Canada was included in the T. matsutake clade, demonstrating a T. matsutake-T. magnivelare continuum. In addition, T. anatolicum and T. murrillianum were regarded as parallel subclades to T. matsutake. The rpb2 phylogenetic tree (Fig. 4) showed unique topology; i.e., Bhutanese and Chinese T. matsutake specimens formed a subclade (B/C clade) independent from that of the other T. matsutake specimens (the A/E clade accompanying T. mesoamericanum and T. magnivelare). In addition, T. matsutake specimens in the A/E clade were found on branches that consisted of three Japanese T. matsutake specimens sampled in Nagano Prefecture. The tef-1 phylogenetic tree (Fig. 5) showed a similar topology to the rpb2 phylogenetic tree, i.e., separation of Bhutanese and Chinese T. matsutake specimens (B/C clade) from the other T. matsutake specimens (A/E clade). The β2 tubulin gene phylogenetic tree (Fig. 6) showed similar topology to that of ITS. In addition, European and Bhutanese-Chinese specimens consisted of subclades within the T. matsutake clade, with moderate support values.
Fig. 2 - ML phylogenetic tree of the nuc rDNA ITS region of Tricholoma matsutake and closely related species in the section Caligata. Bootstrap (BS) values > 60% from ML trees (left) and Bayesian posterior probabilities (PP) > 0.60 (right) are shown near the nodes.
Fig. 3 - ML phylogenetic tree of the nuc rDNA IGS1 region of Tricholoma matsutake and closely related species in the section Caligata. Bootstrap (BS) values > 60% from ML trees (left) and Bayesian posterior probabilities (PP) > 0.60 (right) are shown near the nodes.
Fig. 4 - ML phylogenetic tree of the rpb2 gene of Tricholoma matsutake and closely related species in the section Caligata. Bootstrap (BS) values > 60% from ML trees (left) and Bayesian posterior probabilities (PP) > 0.60 (right) are shown near the nodes.
Fig. 5 - ML phylogenetic tree of the tef-1 gene of Tricholoma matsutake and closely related species in the section Caligata. Bootstrap (BS) values > 60% from ML trees (left) and Bayesian posterior probabilities (PP) > 0.60 (right) are shown near the nodes.
Fig. 6 - ML phylogenetic tree of the β2 tubulin gene of Tricholoma matsutake and closely related species in the section Caligata. Bootstrap (BS) values > 60% from ML trees (left) and Bayesian posterior probabilities (PP) > 0.60 (right) are shown near the nodes.
The gapdh phylogenetic tree (Supplementary Fig. S1), as well as the nuc rDNA ITS, tef-1 and β2 tubulin phylogenetic trees (Figs. 2, 5, 6), showed independency of T. matsutake from the other related species. The atp6 phylogenetic tree (Supplementary Fig. S2) showed independency of T. matsutake from the other related species, except for a Japanese specimen (AY-2101004-001). The megB1 phylogenetic tree (Supplementary Fig. S3) showed two isolated clades of T. matsutake, with the T. murrillianum clade positioned between them. The mt rDNA SSU phylogenetic tree (Supplementary Fig. S4) showed three isolated clades of T. matsutake, two of which included T. anatolicum. Notably, the Ukrainian specimen of T. matsutake was isolated from the other T. matsutake specimens.
3.2. Morphological characteristics of basidiospores and basidia in matsutakes
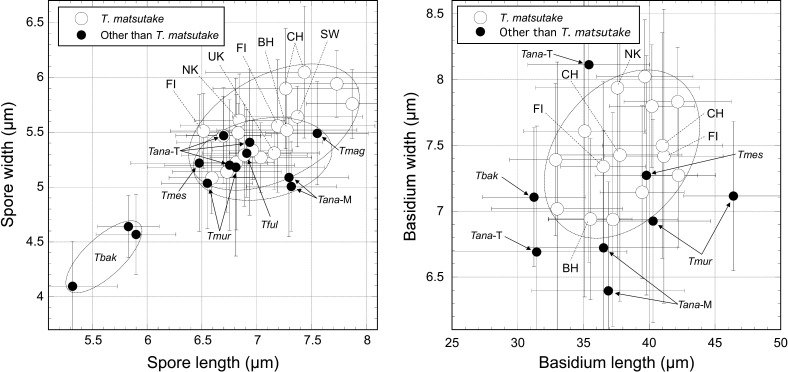
Based on the phylogenetic data (Figs. 2, 3, 4, 5, 6; Supplementary Figs. S1-S4), we compared the morphological characteristics of basidiospores and basidia among matsutake species, as well as among T. matsutake populations, i.e., the Bhutan-China group (B/C group; corresponding to specimens in the B/C clade of rpb2 and tef-1 phylogenies), and the other specimens sampled from far east Asia (the Japanese Archipelago and the Korean Peninsula) and Europe (A/E group; corresponding to specimens in the A/E clade of rpb2 and tef-1 phylogenies). Of the 31 specimens selected for microscopic observation (Supplementary Table S3), several had a limited number of measurements of basidium size. The sizes of about 3600 spores and 2100 basidia were measured.
The mean spore size of 10 Japanese T. matsutake specimens was 6.54-7.87 × 5.08-5.94 μm, the mean Q-value was 1.19-1.37, and the mean basidium size was 32.89-42.22 × 6.94-8.02 μm (Supplementary Table S3). Specimens in the A/E group other than the Japanese specimens (Ukraine, Sweden, North Korea, and Finland) had a mean spore size of 6.69-7.37 × 5.31-5.64 μm, a mean Q-value of 1.23-1.32, and a mean basidium size of 36.49-41.11 × 7.34-7.42 μm. Specimens in the B/C group (Bhutan and China) had a mean spore size of 6.84-7.43 × 5.61-6.05 μm, a mean Q-value of 1.22-1.24, and a mean basidium size of 37.59-41.00 × 6.94-7.50 μm. Mean spore lengths and mean Q-values did not differ significantly between the A/E and B/C groups according to t-tests (p = 0.210 and 0.379, respectively). However, mean spore width was significantly larger in the B/C group than in the A/E group (p = 0.024), although the limitation that only three specimens were evaluated in the B/C group should be noted. Mean basidium lengths and widths did not differ significantly between the A/E and B/C groups (p = 1.0 and 0.428, respectively).
The mean basidiospore sizes and shapes of specimens of T. anatolicum, T. magnivelare, T. mesoamericanum, T. murrillianum, and T. fulvocastaneum were similar to those of T. matsutake (Fig. 7; Supplementary Table S3). However, the spore Q-value of T. anatolicum sampled in Morocco was large (> 1.4). The spore size (length and width) of T. bakamatsutake was significantly smaller than that of T. matsutake (p < 0.01). The basidium lengths and shapes of T. anatolicum, T. mesoamericanum and T. murrillianum were within the range of those of T. matsutake. However, T. murrillianum had a larger length-to-width ratio and T. bakamatsutake had a smaller length-to-width ratio (due to a shorter length) compared with T. matsutake. The Chinese T. bakamatsutake specimen AY-2080800-001 (sliced segments and dried basidioma samples) labeled as T. matsutake but identified as T. bakamatsutake by the phylogenetic analyses and morphological observations had a few larger basidiospores on some sliced segments, implying spore contamination of T. matsutake. However, additional ITS analyses in each of the subsamples (n = 17) showed a single T. bakamatsutake signal (data not shown).
Fig. 7 - Morphological comparisons of basidiospores (left) and basidia (right) between Tricholoma matsutake and its closely related species. The open and closed circles show mean values in specimens from T. matsutake and other species, respectively. Bars in each plot show the standard deviations. Dotted circle lines show the mean values in T. matsutake and other species. Abbreviations: BH: Bhutan; CH: China; FI: Finland; NK: North Korea; SW: Sweden; UK: Ukraine; Tana-M: T. anatolicum Morocco; Tana-T: T. anatolicum Turkey; Tbak: T. bakamatsutake; Tful: T. fulvocastaneum; Tmag: T magnivelare; Tmes: T. mesoamericanum. The detailed data are presented in Supplementary Table S3.
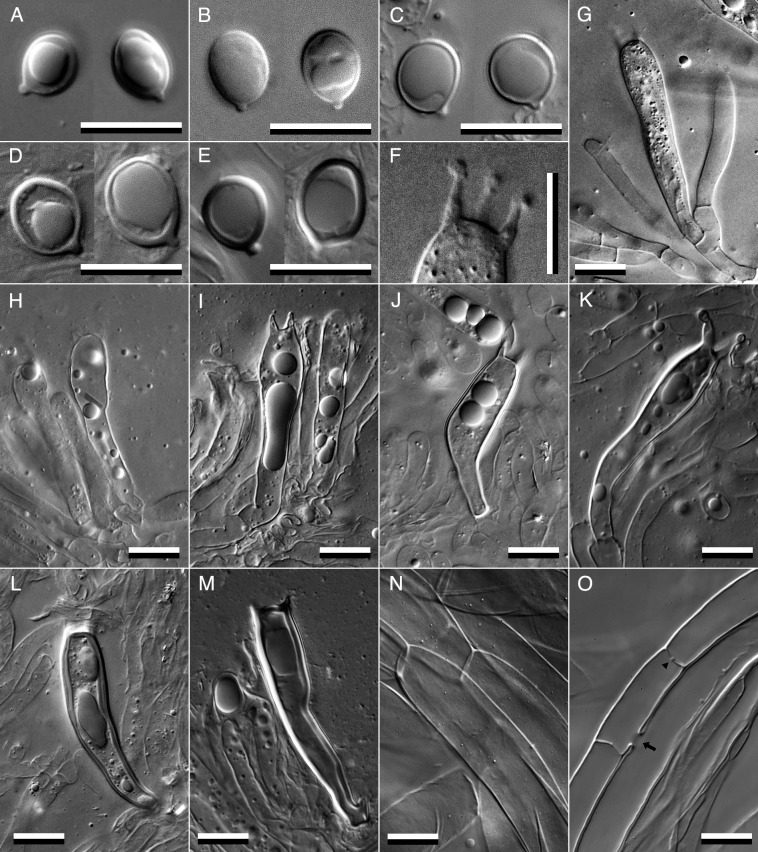
3.3. Sclerobasidia and sclerospores in matsutakes
Sclerobasidia and sclerospores, which are reportedly common in T. nauseosum (Kytövuori, 1988), are characteristic of matsutakes. Although distinct sclerobasidia and sclerospores were present in some specimens, their wall thickness varied. Therefore, we categorized wall thickness into three types: that of type A was 0.2-0.25 μm in spores and 0.25-0.3 μm in basidia, that of type B was 0.25-0.4 μm in spores and 0.3-0.7 μm in basidia, and that of type C was 0.4-0.6 μm in spores and 0.7-1.2 μm in basidia (Fig. 9). Types B and C fully correspond to sclerobasidia and sclerospores, respectively (Kytövuori, 1988). The type A sclerospores and sclerobasidia were sometimes difficult to distinguish from general basidiospores due to the slight differences in their wall thicknesses determined via DIC microscopy. Of the 10 Japanese T. matsutake specimens examined, 4 did not show a sclerobasidium or sclerospore, 6 showed type A sclerospores accounting for less than 30% of all spores, and 3 showed both type A sclerospores and sclerobasidia occupying less than 30% of all spores and basidia, respectively (Table 3). A limited number of type B basidia and a single type C basidium were observed on the AY-2051104-002 specimen. Specimens in the A/E group of T. matsutake other than the Japanese ones had type B or C sclerospores and sclerobasidia occupying approximately 5-30% of all spores and basidia, respectively. A Swedish specimen had type B sclerobasidia accounting for less than 1% of all basidia. The Finnish specimen AY-2070925-001 frequently had type C sclerospores and sclerobasidia, some with a wall thickness > 1 μm. However, AY-2200915-001 from Ukraine had only type A sclerospores and sclerobasidia. In the B/C group specimens of T. matsutake, AY-2040400-001 from Bhutan and AY-1980831-001 from China had both type A sclerospores and sclerobasidia occupying approximately 5-10% of all spores and basidia, respectively. However, AY-1981000-001 from China had both type A and type B sclerospores and sclerobasidia accounting for approximately 10-20% of all spores and basidia, respectively. Both sclerospores and sclerobasidia had inamyloid (indextrinoid) walls under Melzer's reagent staining, in contrast to the results of Kytövuori (1988) (data not shown). Specimens of T. anatolicum and T. fulvocastaneum had type C sclerospores and sclerobasidia, and T. bakamatsutake had type B sclerospores and type C sclerobasidia.
Table 3. Types of sclerospores and sclerobasidia .
| Species | Specimen | Phylogrop | Country | Sclerospores | Sclerobasidia | ||||
| State | Type | Frequency (%) | State | Type | Frequency (%) | ||||
| T. matsutake | AY-2200915-001 | A/E | Ukraine | +/− | A | < 1 | +/− | A | < 1 |
| Ishida F-0315 | A/E | Sweden | +/− | A | < 1 | +/− | A, B | < 1 | |
| AY-2040800-001 | A/E | Finland | + | A, B | < 5 | + | A, B | < 5 | |
| AY-2070925-001 | A/E | Finland | ++ | A-C | ~ 30 | ++ | A-C | ~ 30 | |
| AY-1981000-003 | A/E | North Korea | ++ | A, B | ~ 10 | ++ | A | ~ 20 | |
| AY-1981023-003 | A/E | Japan | + | A | < 5 | + | A | < 5 | |
| AY-2051104-001 | A/E | Japan | ++ | A | ~ 30 | - | |||
| AY-2051104-002 | A/E | Japan | ++ | A | ~ 20 | ++ | A (B, C) | ~ 10 | |
| AY-2051104-003 | A/E | Japan | + | A | < 5 | - | |||
| AY-2071023-002 | A/E | Japan | - | - | |||||
| AY-2131013-001 | A/E | Japan | +/− | A | < 1 | +/− | A | < 1 | |
| AY-2041007-002 | A/E | Japan | - | - | |||||
| AY-2041007-001 | A/E | Japan | - | - | |||||
| AY-2021027-001 | A/E | Japan | - | - | |||||
| AY-2071101-001 | A/E | Japan | + | A | < 5 | - | |||
| AY-2040400-001 | B/C | Bhutan | + | A | < 5 | - | |||
| AY-1981000-002 | B/C | Bhutan | ++ | A | ~ 10 | + | A | < 5 | |
| AY-1980831-001 | B/C | China | + | A | < 5 | + | A | < 5 | |
| AY-1981000-001 | B/C | China | ++ | A, B | ~ 20 | ++ | A, B | ~ 20 | |
| T. bakamatsutake | AY-2041007-003 | Japan | ++ | A, B | ~ 20 | ++ | A | < 5 | |
| AY-2071001-002 | Japan | ++ | A, B | ~ 10 | + | A, B | < 5 | ||
| AY-2080800-001 | China | +++ | A, B | ~ 50 | ++ | A-C | ~ 10 | ||
| T. fulvocastaneum | AY-2091112-001 | Japan | +++ | A-C | ~ 60 | + | B, C | < 5 | |
| T. magnivelare | AY-2080307-001 | Canada | +/− | A | < 1 | +/− | B | < 1 | |
| T. murrillianum | AY-2080100-001 | Canada | +/− | A | < 1 | - | |||
| AY-2071018-001 | Canada | +/− | A | < 1 | - | ||||
| T. mesoamericanum | AY-1981000-004 | Mexico | +/− | A | < 1 | - | |||
| T. anatolicum | AY-2061109-001 | Turkey | ++ | B, C | ~ 20 | +/− | B, C | < 1 | |
| AY-2061108-004 | Turkey | ++ | B, C | ~ 30 | +/− | B, C | < 1 | ||
| AY-2061109-002 | Turkey | ++ | A-C | ~ 10 | +/− | B, C | < 1 | ||
| AY-1981000-005 | Morocco | ++ | B, C | ~ 10 | ++ | B, C | ~ 20 | ||
| AY-1981000-006 | Morocco | ++ | B, C | ~ 30 | ++ | B, C | ~ 20 | ||
+++: Commonly Present, ++: Present, +: Present with low frequency, +/−: Rarely present, -: Absent.
3.4. Morphological definition of T. matsutake
Based on the descriptions of T. matsutake by Kawamura (1913, 1930, 1955), Ito and Imai (1925), Imazeki and Hongo (1957), Kytövuori (1988), and Christensen and Heilmann-Clausen (2013), we redescribed the morphological characteristics of this fungus. We cited the macroscopic characteristics from Kawamura (1955) and Imazeki and Hongo (1957), as well as our own observations, and revised the microscopic characteristics based on Japanese specimens, including the designated epitype, as follows.
4. Taxonomy
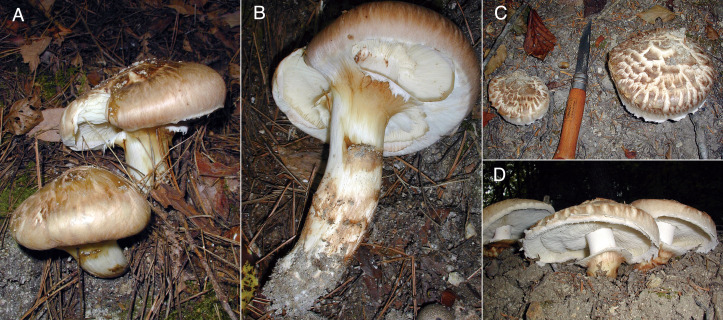
Tricholoma matsutake (S. Ito & S. Imai) Singer, Annls mycol. 41(1/3): 77, 1943. Fig. 8.
Fig. 8 - External morphology of Japanese Tricholoma matsutake. A, B: External morphology of basidiomata specimen AY-2101020-001 showing appressed squamulose on the pileus surface. This specimen may be the same as, or at least a sibling of, the epitype AY-2041007-002 and paratype AY-2181005-001 specimens (Table 1) because they were sampled from almost the same site in a P. densiflora stand. C, D: External morphology of basidiomata specimen AY-2101021-002, showing recurved squamulose on the pileus surface. This specimen may be the same as, or at least a sibling of, AY-2041007-001 (Table 1) and another specimen AY-2101021-001 (Trudell et al., 2017) because they were sampled from almost the same site in a T. sieboldii stand (Table 1).
≡ Armillaria matsutake S. Ito & S. Imai, Bot Mag 39: 327, 1925.
= Armillaria nauseosa A. Blytt, Norges Hymenomyceter: 22, 1905.
= Tricholoma nauseosum (A. Blytt) Kytöv., Karstenia 28: 69, 1988.
Misapplied synonyms: Agaricus edodes Berk., J. Schröter, Gartenflora 35: 135, 1886; Cortinellus edodes (Berk.) P. Henn., P. Hennings, Hedwigia 39: 156, 1900; Armillaria edodes Berk., P. Hennings, Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 28: 270, 1901; Armillaria caligata Viv., P. Hariot & N. Patouillard, Bull Mus Hist Nat 8: 132, 1902.
Description: Pileus 8-20(-30) cm diam, first convex, later plano-convex, finally plane, surface with pale yellowish brown to pale chestnut brown, appressed squamulose (Fig. 8A) but rarely recurved squamulose (Fig. 8C), lately darkish in color and sometimes radial cracks showing whitish flesh tissue, margin inrolled and connected to stipe with a cottony veil at first, incurved lately. Stipe 10-20(-30) × 1.5-3(-5) cm length and width, equal to subclavate, sometimes slightly curved, solid, surface white to pale cream on the upper area than apical annuls, pale yellowish brown or chestnut brown appressed or incurved squamulose below the annuls, annuls single, cottony with pale yellowish brown or chestnut brown in the outer side and whitish in the inner side, permanent (Fig. 8B, D). Gills white to pale cream color, medium spaced to crowded, smooth edge, sinuate. Flesh white, dense, with specific fragrance similar to cinnamon or the lumber of hinoki cypress. Basidiospores (5.8-)6.5-7.1-7.9(-10.3) × (4.1-)5.1-5.5-6.1(-9.6) µm length and width, Q = (1.02)1.19-1.28-1.37(-1.83), subglobose to broadly ellipsoidal, normally thin-walled, most specimens include type A sclerospores with low or moderate frequency, some specimens include type B and C sclerospores with low frequency (Fig. 9A-E). Basidia (22.8-)32.9-38.1-42.2(-59.7) × (4.9-)6.9-7.5-8.0(-11.9), clavate, curved at the one-third point from the base, basal clamp connection absent, normally thin-walled, but some Japanese and a North Korean and Bhutanese specimens included type A sclerobasidia, and Swedish, Finnish, and Chinese specimens included type B and type C sclerobasidia (Fig. 9G-M; Table 3). Sterigmata mostly 4 in number, (1.5-)1.9-3.3-5.4(-6.3) × (0.9-)1.0-1.5-2.0(-2.1) μm length and basal width, straight or incurved (Fig. 9F). Neither cheilocystidia nor pleurocystidia are observed. Pileipellis cutis, superpellis hyphae 23.8-58.8-119.4 × 4.2-9.0-18.2 μm, cylindrical, straight, or slightly curved, wall and intracellular space often show light brownish pigment, no clamp connections, subpellis hyphae are narrower than superpellis hyphae, often slightly curved, almost transparent, sometimes anastomosed with parallel hyphae and showing barrel-shaped swelling on the dolipore septum (Fig. 9N-O).
Fig. 9 - Microscopic features of Tricholoma matsutake. A-E: basidiospores of AY-2200915-001 (Ukraine; A), epitype AY-2041007-002 (Japan; B), its thick-walled sclerospore type A of AY-2051104-002 (Japan; C), type B of AY-1981000-001 (China; D), and type C of AY-2070925-001 (Finland; E). F: sterigmata of the epitype AY-2041007-002, in which three of four sterigmata on a basidium can be seen in the same depth of field. G-M: Basidia of the epitype AY-2041007-002 (G), its thick-walled sclerobasidium type A of AY-2051104-002 (H) and AY-2070925-001 (I), type B of AY-2051104-002 (J) and AY-2070925-001 (K), and type C of AY-2051104-002 (L) and AY-2070925-001 (M). Superpellis (N) and subpellis (O) hyphae in the pileipellis layers of the epitype AY-2041007-002. The arrow indicates anastomosis between parallel hyphae, and the arrowhead indicates a barrel-shaped swelling on the dolipore septum.Bars: 10 μm.
Epitype examined: Japan, Nagano Prefecture: AY-2041007-002 (= TNS-F 82226, A. Yamada).
Other specimens examined: Japan, Nagano Prefecture: AY-2181005-001 ( = TNS-F 82227, W. Aoki), AY-2041007-001 (= TNS-F 82228, A. Yamada), AY-2021027-001 (T. Sawahata), AY-2071101-001 (A. Yamada), AY-2131013-001 (N. Endo); Ibaraki Prefecture: AY-1981023-003 (A. Yamada), AY-2051104-001 (H. Kobayashi), AY-2051104-002 (H. Kobayashi), AY-2051104-003 (H. Kobayashi), AY-2071023-002 (H. Kobayashi); North Korea: AY-1981000-003 (H. Murata); Finland: AY-2040800-001 (S. Anttila), AY-2070925-001 (L-M. Vaario); Sweden: Ishida F-0315 (T.A. Ishida); Ukraine: AY-2200915-001 (N. Bergius); Bhutan: AY-2040400-001(K. Matsushima), AY-1981000-002 (H. Murata); China: AY-1980831-001 (A. Yamada), AY-1981000-001(H. Murata).
GenBank accession numbers of DNA sequences: see Supplementary Table S1.
Ecology: Japanese T. matsutake is distributed in temperate to alpine zones on Honshu, Hokkaido, Shikoku, Kyushu, and Sadogashima Islands under various Pinaceae trees, including Pinus densiflora, P. thunbergii Parl., P. pumila (Pall.) Regel, Picea glehnii (F. Schmidt) Mast., Abies veitchii Lindl., A. sachalinensis (Fr. Schmidt) Mastersand, Tsuga diversifolia (Maxim.) Mast., T. sieboldii Carrière, forming ectomycorrhizal associations with these hosts, fruiting (Jul-)Aug-Nov(-Dec), occasionally in Mar-May in warmer coastal areas (Matsutake Research Association, 1964; Ogawa, 1978; Endo et al., 2015; Gisusi et al., 2019). Fruiting events at a stand level are, however, limited to within a few weeks. It is not clear whether five-needle pines other than P. pumila (P. parviflora var. parviflora Siebold et Zucc., P. parviflora var. pentaphylla (Mayr) A. Henry, and P. koraiensis Siebold et Zucc.) are hosts in Japan, although P. koraiensis is reportedly a harvest host of T. matsutake in northeastern China (Wang, Yu, Zhang, & Li, 2017). In the Nagano area, Japan, T. matsutake colonies are sometimes parasitized by the achlorophyllous Ericaceae plant Monotropa hypopitys L. (Kitamura, 2004; Fujiwara, 2011; Saito et al., 2018), which is a similar plant-fungus association to that between Allotropa virgata Torr. & Gray and T. murrillianum in the west Pacific region of North America (Bidartondo and Bruns, 2002; Hosford, 1997; Trudell et al., 2017). Geographic regions other than the Japanese Archipelago include Taiwan Island, the Korean Peninsula, southwestern and northeastern China, Bhutan, the Primorskaya Oblast, Sakhalin, the Kuril Islands in East Asia, and eastern, northern, and central Europe, under various coniferous trees. However, in southwestern China and Bhutan, oak trees are also associated with T. matsutake defined as the B/C group (e.g., Narimatsu, Terashima, Watanabe, Matsushita, & Penjor, 2019).
Comment: Our microscopic data on the Asian and European populations of T. matsutake matched the descriptions by Kawamura (1930, 1955) and Imazeki and Hongo (1957). In addition, our microscopic data largely matched the T. nauseosum description by Kytövuori (1988), except for the reaction of the sclerobasidium and sclerospore walls to Melzer's reagent. Although Kytövuori (1988) reported dextrinoid walls, our DIC microscopy observations revealed both inamyloid walls and intracellular contents. This difference might be attributed to the microscopy technique used, because Kytövuori (1988) conducted normal compound microscopy. We suggest that the dextrinoid walls (of probably thick-walled sclerospores) reported by Kytövuori (1988) might be an invalid interpretation. We did not find a distinct sclerospore or sclerobasidium in most Japanese T. matsutake specimens. Based on the phylogenetic data, the variations in sclerospores and paired sclerobasidia can be regarded as infraspecific characteristics of T. matsutake. Morphological distinction between the A/E and B/C groups of T. matsutake is almost impossible, although we did find a significant difference in spore width. As there were only a limited number of specimens in the B/C group, additional research is needed to validate such morphological differences. The very similar T. magnivelare species distributed in North America can be distinguished from T. matsutake by the external color of basidiomata and spore size (Trudell et al., 2017). However, these characteristics may not distinguish the species in some cases due to infraspecific variations. As indicated in Figure 7, the spores of our T. magnivelare specimen were larger than the size (5-7.5 × 3.5-5.5 μm) reported by Trudell et al. (2017).
5. Discussion
This is the first valid report of T. matsutake from eastern Europe. The finding that Scandinavian T. nauseosum is conspecific to the Japanese T. matsutake (Kytövuori, 1988) and their phylogenetic congruence (Bergius and Danell, 2000; Matsushita et al., 2005) revised our understanding of this fungal biogeography. The present findings of this mushroom from Ukraine imply that T. matsutake is probably widely distributed throughout the Eurasian region from East Asia to Europe via the central region of Eurasia, i.e., circumboreal area in Eurasia, as has already been suggested by Endo et al. (2015) and Vaario et al. (2017). At present, there is no valid report of T. matsutake in these areas, such as in the Ural Mountains and Central Siberian Plateau, although its presence has been suggested based on specimen and observation records (GBIF, 2021). The hosts of T. matsutake in central and northern Europe include Pinus sylvestris L. and Picea abies (L.) H. Karst. (Kytövuori, 1988; Vaario et al., 2017). However, the Abies association was first found in Ukraine. Even in Japan, the association between T. matsutake and Abies has not been accepted until recently (Endo et al., 2015; Gisusi et al., 2019), after root associations were confirmed by mycorrhizal analyses. Therefore, surveys of T. matsutake habitats under fir forests may be another valuable approach to elucidate their fungal biogeography.
The rpb2, tef-1, and β2 tubulin gene phylogenies included the Ukraine T. matsutake specimen in the A/E clade (Figs. 4, 5) or European population (Fig. 6), implying the significance of these loci for characterization of geographic origin. In contrast, the mt rDNA SSU phylogeny (Supplementary Fig. S4) revealed the unique and independent position of the Ukrainian specimen. At present, we cannot explain this. As the mt rDNA SSU phylogeny showed a mixed relationship among the four matsutake species T. matsutake, T. magnivelare, T. mesoamericanum, and T. anatolicum, this locus may not mirror the speciation and evolutionary processes of these taxa. In this study, the conspecificity of the Ukrainian specimen with Japanese T. matsutake was determined by phylogenetic analysis of eight loci (ITS, IGS1, rpb2, β2 tubulin, gapdh, tef-1 atp6, and megB1), strongly implying the taxonomic identity. These results also suggest that describing species based on the phylogeny of a single locus can be problematic for taxonomic study.
In contrast, our phylogenetic analyses showed divergence of the T. matsutake population within Asia, i.e., the A/E (far east Asia and Europe) and B/C (Bhutan and China) clades, based on the rpb2 and tef-1 phylogenetic analyses (Figs. 4, 5). It has been suggested that Asian T. matsutake has two distinct genetic groups, i.e., the far eastern population, including Japan (genotype A), and that at the foot of the Tibetan Plateau (genotype B), based on genomic analysis of the LTR retroelement DNA markers (Murata et al., 2008). Therefore, our phylogenetic data support genetic isolation between these two T. matsutake populations in Asia. Of the samples distinguished as the B/C clade in this study, specimens AY-1981000-002 and AY-1981000-001 from Bhutan (=BH1) and China (=CH1), respectively, were classified as genotype B by Murata et al. (2008). This implies that another Chinese specimen, AY-1980831-001, was sampled in a western region, such as Yunnan or Szechwan Province, not an eastern region, such as Heilongjiang or Jilin Province. In addition, specimen AY-1981000-003 from North Korea (=NK1), classified as genotype A (referred to as genotype A-2) by Murata et al. (2008), was grouped in the A/E clade (Figs. 4, 5). Most of the Japanese T. matsutake specimens analyzed by Murata et al. (2008) were sampled from lowland P. densiflora forests and classified as genotype A (referred to as genotype A-1 in the eastern population and as A-2 in several western populations). Therefore, our present T. matsutake samples harvested in subalpine Tsuga and Abies spp. stands as well as P. densiflora and sympatric T. sieboldii stands, all of which were grouped in the A/E clade, possibly correspond to genotype A-1 proposed by Murata et al. (2008). We suggest that these two genetically isolated T. matsutake groups in Asia correspond to two geographically isolated populations, or two incompatible populations, i.e., different biological species. Although we did not find a clear morphological difference between the two groups except in spore width, the reported ecological characteristics, i.e., host relationships, support their divergence from a geographic viewpoint. Japanese T. matsutake is associated solely with Pinaceae plants in nature (Ogawa, 1978; Yamada, Kanekawa, & Ohmasa,1999a; Endo et al., 2015; Gisusi et al., 2019), which is similar to European populations (Christensen and Heilmann-Clausen, 2013; Vaario et al., 2017) and has been largely confirmed by in vitro mycorrhizal synthesis experiments (Yamada, Maeda, & Ohmasa, 1999b; Yamada, Maeda, Kobayashi, & Murata, 2005; Vaario, Pennanen, Sarjala, Savonen, & Heinonsalo, 2010; Yamada et al., 2010; Saito et al., 2018). However, T. matsutake in Bhutan and southwestern China, such as in Yunnan and Sichuan Provinces, have been associated with both Pinaceae (e.g., Pinus wallichiana A. B. Jacks., P. yunnanensis Franch., Picea, and Tsuga) and Fagaceae (e.g., Quercus, Lithocarpus, and Castanopsis) (Cao, Yao, & Pegler, 2003; Yamanaka, Aimi, Wan, Cao, & Chen, 2011; Vaario et al., 2017; Wang et al., 2017; Narimatsu et al., 2019). Therefore, from a genetic viewpoint, the two diverged T. matsutake populations in Asia can be characterized based on host associations. In this respect, we need to consider another T. matsutake-related population (T. zangii Z. M. Cao, Y. J. Yao & Pegler, distributed at the foot of the Tibetan Plateau (Cao et al., 2003; Cao and Yao, 2004)). The geographic distribution of this fungus overlaps that of T. matsutake, but the habitats differ due to the higher elevation of T. matsutake. However, phylogenetic analysis of T. zangii has not been reported, which would allow verification of its position in relation to closely related species (Bao et al., 2007; Amend, Garbelotto, Fang, & Keeley, 2010; Zeng and Chen, 2015). As we did not analyze a T. zangii specimen in the present study, we did not conduct taxonomic treatment in the B/C clade of T. matsutake.
In this study, we set the epitype of T. matsutake because biologically available type specimens have not been designated in Japan since Kawamura (1913) identified “Matsutake” as Cortinellus edodes P. Henn. and described it with color drawings and a brief description. A detailed description of this fungus with microscopic features was provided by Seiichi Kawamura based on basidiomata sampled under a P. densiflora stand in the Kitayama area of Kyoto in November 1909 (Kawamura, 1930, 1955), which might have been the motif of the color drawings of C. edodes by Kawamura (1913). The basidioma specimens of Armillaria matsutake by Kawamura (1930, 1955) are also not extant. As the color drawings by Kawamura (1930, 1955) show the basidioma pileus as a reddish tan color, the specimen can be regarded as not fresh; Kawamura (1955) commented that the specimens consisted of “one young and two overmatured basidiomata.” Fresh basidiomata of T. matsutake are lighter in color, as reported by Imazeki and Hongo (1957), Imazeki, Otani, and Hongo (1988), Kytövuori (1988), and Christensen and Heilmann-Clausen (2013). Currently, T. matsutake habitats in Japan are endangered in western and lowland areas, including Kyoto, due to the decline of Pinus densiflora, the main host, in lowland areas caused by nematode disease and ongoing global warming (Vaario et al., 2017; Brandrud, 2020; Yamanaka et al., 2020). Therefore, we decided to designate an epitype of T. matsutake as that sampled in Nagano Prefecture, where the mountain ranges still harbor large forest areas of P. densiflora at mid-elevations, as well as the other hosts, Tsuga sieboldii, T. diversifolia, and Abies veitchii, at higher elevations. The designated epitype, AY-2041007-002 (= TNS-F 82226), was sampled from a P. densiflora forest associated with many productive sites of this fungus (Furukawa, Masuno, & Takeuchi, 2016). The specimen is thought to be a typical genetic resource in the diverse genetic pool of this fungus in Japan. The specimen AY-2181005-001 (= TNS-F 82227) is probably a sibling of (or of the same clone as) the epitype, AY-2041007-002, because the two specimens were sampled from the same site in a P. densiflora stand.
The T. bakamatsutake specimen AY-2080800-001 from Jilin Province, China, labeled as T. matsutake, was identified as T. bakamatsutake based on phylogenetic data. In fact, similar cases were found in traceability tests of T. matsutake imported from abroad (Murata et al., 2008), from which large and typical basidiomata were selected for DNA analysis, but small or atypical basidiomata were excluded. In fact, some samples corresponding to the latter case were identified as T. bakamatsutake by ITS sequence analysis (unpublished data). Tricholoma bakamatsutake has been identified in northeastern China and the neighboring Primorskaya Oblast in Russia under Quercus mongolica (Ogawa, 1978) trees, as well as in Japan under various Fagaceae trees (Hongo, 1971; Terashima, 1996). Therefore, if basidiomata of T. matsutake and T. bakamatsutake occur sympatrically in the same forest, such as in mixed Pinus-Quercus stands, mushroom harvesters may collect the two together. Our field research in Nagano Prefecture showed such coexistence in P. densiflora-Q. serrata and T. sieboldii-Q. serrata mixed stands, where basidiomata of both species occurred only a few meters apart (unpublished data). Although we did not analyze T. bakamatsutake specimens collected in southwestern China, these regions potentially have at least four matsutake mushroom species associated with Fagaceae: T. matsutake (B/C clade), T. zangii, T. bakamatsutake, and T. fulvocastaneum (Liu, Yuan, Wang, Sun, & Yang, 1999; Cao et al., 2003; Cao and Yang, 2004; Sanmee, Lumyong, Dell, & Lumyong, 2007; Yamada et al., 2010; Yamanaka et al., 2011) or more diverse taxa (Wang et al., 2017). Matsutake may be difficult to identify in the market, even in North America. At present, three species (T. murrillianum, T. magnivelare, and T. mesoamericanum) are exported to Japan. In addition, the presence of North American T. dulciolens, T. focale, and “T. caligatum” has been reported (Chapela and Garbelotto, 2004; Bessette, Bessette, Roody, & Trudell, 2013; Murata et al., 2013; Benazza-Bouregba, Savoie, Fortas, & Billette, 2016; Heilmann-Clausen, Christensen, Frøslev, & Kjøller, 2017). This suggests that these species may be mixed in the exported American matsutake, as is the case of T. bakamatsutake presence in exported Chinese matsutake.
Disclosure
The authors declare no conflicts of interest. All experiments in this study were performed in compliance with the current laws of Japan.
Supplementary Material
Acknowledgement
We thank Susanne Anttila, Ichiro Yokota, and Naoki Endo for providing us specimens and the information in the habitat of fungal materials.
References
- Amend, A., Garbelotto, M., Fang, Z., & Keeley, S. (2010) .Isolation by landscape in populations of a prized edible mushroom Tricholoma matsutake. Conservation Genetics, 11, 795–802. https://doi.org/10.1007/s10592-009-9894-0 [Google Scholar]
- Anderson, J. B., & Stasovski, E. (1992) .Molecular phylogeny of northern hemisphere species of Armillaria. Mycologia, 84, 505–516. https://doi.org/10.1080/00275514.1992.12026170 [Google Scholar]
- Aoki, W., Endo, N., Ushijima, S., Nagai, H., Ito, T., Fukuda, M., & Yamada, A. (2021) .Taxonomic revision of the Japanese Tricholoma ustale and closely related species based on molecular phylogenetic and morphological data. Mycoscience, 62, 307–321. https://doi.org/10.47371/mycosci.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, W., Archambault, R., Bérubé, J., Lamoureux, Y., Fukuda, M., & Yamada, A. (2022) .Tricholoma alpinum, sp. nov., under five-needle pines in alpine and subalpine zones in Japan. Mycologia. https://doi.org/10.1080/00275514.2021.2018889 [DOI] [PubMed] [Google Scholar]
- Arora, D. (1986) .Mushrooms demystified, 2nd edn. Berkeley: Ten Speed Press. [Google Scholar]
- Babasaki, K., Neda, H., & Murata, H. (2007) .megB1, a novel macroevolutionary genomic marker of the fungal phylum Basidiomycota. Bioscience, Biotechnology, and Biochemistry, 71, 1927–1939. https://doi.org/10.1271/bbb.70144 [DOI] [PubMed] [Google Scholar]
- Baishiken, R. (1842) .Haikai-kiyose-zukou-soumoku, vol. 2. https://dl.ndl.go.jp/info:ndljp/pid/2558025?tocOpened=1 [Google Scholar]
- Berkeley, M. J. (1877) .Enumeration of the fungi collected during the expedition of H. M. S. “Challenger” 1874-1875, third notice. Journal of Linnean Society, Botany, 16, 38–54. https://www.biodiversitylibrary.org/part/246355 [Google Scholar]
- Bao, D., Koike, A., Yao, F., Yamanaka, K., Aimi, T., & Kitamoto, Y. (2007) .Analyses of the genetic diversity of matsutake isolates collected from different ecological environments in Asia. Journal of Wood Science, 53, 344–350. https://doi.org/10.1007/s10086-006-0859-3 [Google Scholar]
- Benazza-Bouregba, M., Savoie, J.-M., Fortas, Z., & Billette, C. (2016) .A new record of Tricholoma caligatum (Tricholomataceae) from North Africa with a discussion of related species. Phytotaxa, 282, 119–128. https://doi.org/10.11646/phytotaxa.282.2.3 [Google Scholar]
- Bergius, N., & Danell, E. (2000) .The Swedish matsutake (Tricholoma nauseosum syn. T. matsutake): distribution, abundance and ecology. Scandinavian Journal of Forest Research, 15, 318–325. https://doi.org/10.1080/028275800447940 [Google Scholar]
- Bessette, A. E., Bessette, A. E., Roody, C. W., & Trudell, S. A. (2013) .Tricholoma of North America: A mushroom field guide. Austin: University of Texas Press. [Google Scholar]
- Bidartondo, M. I., & Bruns, T. D. (2002) .Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Molecular Ecology, 11, 557–569. https://doi.org/10.1046/j.0962-1083.2001.01443.x [DOI] [PubMed] [Google Scholar]
- Blytt, A. (1905) .Norges Hymenomyceter (Videnskabs-Seleskabets Skrifter. I. Math.-Naturv. Kl. 1904. No. 6). Christiania (Oslo): Fridtjof Nansens Fond. [Google Scholar]
- Brandrud, T.E. (2020) . “Tricholoma matsutake”. The IUCN Red List of Threatened Species, IUCN; 2020: T76267712A177054645. [Google Scholar]
- Cao, Z. M., Yao, Y. J., & Pegler, D. N. (2003) .Tricholoma zangii, a new name for T. quercicola M. Zang (Basidiomycetes: Tricholomataceae). Mycotaxon, 85, 159–164. [Google Scholar]
- Cao, Z.M., & Yao, Y.J. (2004) .Morphological and biogeographic study on Tricholoma matsutake-complex (in Chinese). Mycosystema, 23, 43–55. [Google Scholar]
- Chapela, I.H., & Garbelotto, M. (2004) .Phylogeography and evolution in matsutake and close allies inferred by analyses of ITS sequences and AFLPs. Mycologia, 96, 730–741. https://doi.org/10.1080/15572536.2005.11832921 [DOI] [PubMed] [Google Scholar]
- Christensen, M., & Heilmann-Clausen, J. (2013) .The genus Tricholoma - Fungi of Northern Europe - vol. 4. Tilst, Denmark: Svampetryk. [Google Scholar]
- Clémençon, H. (2012) .Cytology and plectology of the Hymenomycetes, 2nd edn. Stuttgart: J. Cramer. [Google Scholar]
- Endo, N., Dokmai, P., Suwannasai, N., Phosri, C., Horimai, Y., Hirai, N., Fukuda, M., & Yamada, A. (2015) .Ectomycorrhization of Tricholoma matsutake with Abies veitchii and Tsuga diversifolia in the subalpine forests of Japan. Mycoscience, 56, 402–412. https://doi.org/10.1016/j.myc.2014.12.004 [Google Scholar]
- Fujiwara, G. (2011) .All about the forest management for matsutake mushroom (In Japanese). Tokyo: National Forestry Extension Association in Japan. [Google Scholar]
- Furukawa, H., Masuno, K., & Takeuchi, Y. (2016) .Forest management of matsutake productive sites for the optimization to global warming. Annual Reports of Nagano Prefecture Forestry Research Center, 30, 87–100. https://agriknowledge.affrc.go.jp/RN/2010902230.pdf [Google Scholar]
- Gardes, M., & Bruns, T. D. (1993) .ITS primer with enhanced of specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- GBIF (2021) .Tricholoma matsutake (S.Ito & S.Imai) Singer in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei accessed via GBIF.org on 2022-04-18. [Google Scholar]
- Gisusi, S., Azuma, T., Yoshida, S., Yoneyama, S., Harada, A., Tsuda, M., & Tamai, Y. (2019) .Investigation of soil environments in the vicinity of Tricholoma matsutake mycelium in Abies sachalinensis stands (in Japanese).Japanese Journal of Mycology, 60, 43–48. https://agriknowledge.affrc.go.jp/RN/2010930783.pdf [Google Scholar]
- Hariot, P., & Patouillard, N. (1902) .Liste des Champignons récoltés au Japon par M. le Dr. Harmand. Bulletin du Muséum national d'histoire naturelle, 8, 129–133. https://www.biodiversitylibrary.org/item/137051#page/141/mode/1up [Google Scholar]
- Heilmann-Clausen, J., Christensen, M., Frøslev, T. G., & Kjøller, R. (2017) .Taxonomy of Tricholoma in northern Europe based on ITS sequence data and morphological characters. Persoonia, 38, 38–57. https://doi.org/10.3767/003158517X693174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings (1899) .Über essbare japanische Pilze. Notizblatt des Königl botanischen Gartens und Museums zu Berlin, 2, 385–386. https://ur.booksc.me/book/28034798/f13118 [Google Scholar]
- Hennings, P. (1900) .Fleischige Pilz aus Japan. Hedwigia, 39, 155–157. https://www.zobodat.at/pdf/Hedwigia_Beiblatt_39_1900_0155-0157.pdf [Google Scholar]
- Hennings, P. (1901) .Fungi japonici. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie, 28, 259–280. [Google Scholar]
- Henrion, B., Le Tacon, F., & Martin, F. (1992) .Rapid identification of genetic variation of ectomycorrhizal fungi by amplification of ribosomal RNA genes. New Phytologist, 122, 289–298. [DOI] [PubMed] [Google Scholar]
- Hongo, T. (1971) .Higher fungi from Mt. Chréa of the Atlas Mountains. Transactions of the Mycological Society of Japan, 12, 136–141. [Google Scholar]
- Hosford, D., Pilz, D., Molina, R., & Amaranthus, M. (1997) .Ecology and management of the commercially harvested American matsutake mushroom. Gen. Tech. Rep. PNW-GTR-412. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 68 p. [Google Scholar]
- Ichioka, T. (1799) .Shin-you-kin-pu. Iida: Harunobu Nakayama. https://dl.ndl.go.jp/info:ndljp/pid/2537202 [Google Scholar]
- Imazeki, R., & Hongo, T. (1957) .Colored illustrations of fungi of Japan (In Japanese). Osaka: Hoikusha. [Google Scholar]
- Imazeki, R., Otani, Y., & Hongo, T. (1988) .Fungi of Japan (In Japanese). Tokyo: Yama-kei Publishers. [Google Scholar]
- Ito, S., & Imai, S. (1925) .On the taxonomy of shii-take and matsu-take. The botanical magazine, Tokyo, 39, 319-328. [Google Scholar]
- Kawamura, S. (1913) .Illustrated Japanese fungi, 2nd delivery (In Japanese). Tokyo: The Forest Experiment Station of the Department of Agriculture and Forestry. [Google Scholar]
- Kawamura, S. (1930) .The Japanese fungi (In Japanese). Tokyo: Daichi-shoin. [Google Scholar]
- Kawamura, S. (1955) .Icons of Japanese fungi, vol.4 (in Japanese). Kazamashobo: Tokyo. [Google Scholar]
- Kitamura, D. (2004) .Studies in the mycorrhizae of Monotropaceae (In Japanese). Master Thesis Report, Shinshu University. [Google Scholar]
- Kretzer, A. M., & Bruns, T. D. (1999) .Use of atp6 in fungal phylogenetics: an example from the Boletales. Molecular Phylogenetics and Evolution, 13, 483–492. https://doi.org/10.1006/mpev.1999.0680 [DOI] [PubMed] [Google Scholar]
- Kumar, S., Stecher, G., & Tamura, K. (2016) .MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kytövuori, I. (1988) .The Tricholoma caligatum group in Europe and North Africa. Karstenia, 28, 67–77. https://doi.org/10.29203/ka.1988.266 [Google Scholar]
- Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007) .ClustalW and ClustalX version 2.0. Bioinformatics Applications Note, 23, 2947–2948. https://doi.org/10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Liu, P., Yuan, M., Wang, X., Sun, P., & Yang, X. (1999) .Notes on the resources of matsutake group and their reasonable utilization as well as effective conservation in China (in Chinese). Journal of Natural Resources, 14, 245–252. [Google Scholar]
- Matsushita, N., Kikuchi, K., Sasaki, Y., Guerin-Laguette, A., Vaario, L.-M., Suzuki, K., Lapeyrie, F., & Intini, M. (2005) .Genetic relationship of Tricholoma matsutake and T. nauseosum from the Northern Hemisphere based on analyses of ribosomal DNA spacer regions. Mycoscience, 46, 90–96. https://doi.org/10.1007/S10267-004-0220-X [Google Scholar]
- Matsutake Research Association (1964) .Matsutake (Tricholoma matsutake Singer) - Its fundamental studies and economic production of the fruit body- (In Japanese). Matsutake Research Association: Kyoto. [Google Scholar]
- Murata, H., Babasaki, K., Saegusa, T., Takemoto, K., Yamada, A., & Ohta, A. (2008) .Traceability of Asian matsutake, specialty mushrooms produced by the ectomycorrhizal basidiomycete Tricholoma matsutake, on the basis of retroelement-based DNA markers. Applied and Environmental Microbiology, 74, 2023–2031. https://doi.org/10.1128/AEM.02411-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, H., Ota, Y., Yamada, A., Ohta, A., Yamanaka, T., & Neda, H. (2013) .Phylogenetic position of the ectomycorrhizal basidiomycete Tricholoma dulciolens in relation to species of Tricholoma that produce “matsutake” mushrooms. Mycoscience, 54, 438–443. https://doi.org/10.1016/j.myc.2013.02.003 [Google Scholar]
- Narimatsu, M., Terashima, Y., Watanabe, K., Matsushita, N., & Penjor, D. (2019) .Host tree of Tricholoma matsutake in western Bhutan. Abstract of papers presented at the 63rd annual meeting of the Mycological Society of Japan, Akita, pp. 72. https://doi.org/10.11556/msj7abst.63.0_72a [Google Scholar]
- Ogawa, M. (1978) .The Biology of Matsutake (In Japanese). Tsukiji-shokan: Tokyo. [Google Scholar]
- Ota, Y., Yamanaka, T., Murata, H., Neda, H., Ohta, A., Kawai, M., Yamada, A., Konno, M., & Tanaka, C. (2012) .Phylogenetic relationship and species delimitation of matsutake and allied species based on multilocus phylogeny and haplotype analyses. Mycologia, 104, 1369–1380. https://doi.org/10.3852/12-068 [DOI] [PubMed] [Google Scholar]
- Redhead, S. A. (1984) .Mycological observations 13-14: on Hypsizygus and Tricholoma. Transaction of the Mycological Society of Japan, 25, 1–9. [Google Scholar]
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012) .MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman, S., Bergius, N., & Danell, E. (2000). (1459) Proposal to conserve the name Armillaria matsutake against Armillaria nauseosa (fungi, Basidiomycotina, Tricholomataceae). Taxon, 49, 555–556. [Google Scholar]
- Saccardo, P. A. (1887) .Sylloge fungorum omnium hucusque cognitorum, vol. 5part 7. Berlin: R. Friedländer & Sohn. https://www.biodiversitylibrary.org/bibliography/5371 [Google Scholar]
- Saito, C., Ogawa, W., Kobayashi, H., Yamanaka, T., Fukuda, M., & Yamada, A. (2018) .In vitro ectomycorrhization of Tricholoma matsutake strains is differentially affected by soil type. Mycoscience, 59, 89–97. https://doi.org/10.1016/j.myc.2017.09.002 [Google Scholar]
- Sanmee, R., Lumyong, S., Dell, B., & Lumyong, P. (2007) .First record of Tricholoma fulvocastaneum from Thailand. Mycoscience, 48, 131–133. https://doi.org/10.1007/S10267-006-0341-5 [Google Scholar]
- Schröter, J. (1886) .Essbare Pilze und Pilzkulturen in Japan. Gartenflora, 35, 101–107, 134-139. https://www.biodiversitylibrary.org/item/133306#page/146/mode/1up [Google Scholar]
- Shirai, M., & Henning, P. (1899) .Berurin-kinpu. Berlin, own publication. https://dl.ndl.go.jp/info:ndljp/pid/2541316 [Google Scholar]
- Shirai, M., & Henning, P. (1931) .Kinjin-fuzu. Own publication. https://dl.ndl.go.jp/info:ndljp/pid/2541314 [Google Scholar]
- Singer, R. (1943) .Das system der Agaricales, III. Annales Mycoligici, 41, 1-189. [Google Scholar]
- Singer, R. (1986) .The Agaricales in modern taxonomy, 4th edn. Keonigstein: Koeltz Scientific Books. [Google Scholar]
- Smith, A. H. (1979) .The stirps Caligata of Armillaria in North America. Sydowia Beihefte, 8, 368–377. [Google Scholar]
- Stamatakis, A. (2014) .RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics Applications Note 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima, Y. (1996) .Ecology of Tricholoma bakamatsutake in a evergreen Fagaceae forest. Mushroom Science and Biotechonology, 3, 101–108. https://doi.org/10.24465/kinoko.3.3_101 [Google Scholar]
- Tsing, A. L. (2015) .The mushroom at the end of the world: On the possibility of life in capitalist ruins. Oxford: Princeton University Press. [Google Scholar]
- Trudell, S. A., Xu, J., Saar, I., Justo, A., & Cifuentes, J. (2017) .North American matsutake: names clarified and a new species described. Mycologia, 109, 379–390. https://doi.org/10.1080/00275514.2017.1326780 [DOI] [PubMed] [Google Scholar]
- Vaario, L.-M., Pennanen, T., Sarjala, T., Savonen, E.-M., & Heinonsalo, J. (2010) .Ectomycorrhization of Tricholoma matsutake and two major conifers in Finland-an assessment of in vitro mycorrhiza formation. Mycorrhiza, 20, 511–518. https://doi.org/10.1007/s00572-010-0304-8 [DOI] [PubMed] [Google Scholar]
- Vaario, L.-M., Yang, X., & Yamada, A. (2017) .Biogeography of the Japanese gourmet fungus, Tricholoma matsutake: a review of the distribution and functional ecology of Matsutake. In: Tedersoo, L. (Ed.), Biogeography of Mycorrhizal Symbiosis. Ecological Studies (Analysis and Synthesis), vol. 230. Springer, Cham, pp. 319-344. https://doi.org/10.1007/978e3-319e56363e3_15. [Google Scholar]
- Wang, Y., Yu, F., Zhang, C., & Li, S. (2017) .Tricholoma matsutake: an edible mycorrhizal mushroom of high socioeconomic relevance in China. Scientia Fungorum, 46, 55–61. [Google Scholar]
- White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990) .Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J., & White, T. J. (Eds), PCR Protocols: A guide to methods and applications (pp. 315–322). San Diego: Academic Press. [Google Scholar]
- Yamada, A., Kanekawa, S., & Ohmasa, M. (1999. a) .Ectomycorrhiza formation of Tricholoma matsutake on Pinus densiflora. Mycoscience, 40, 193–198. https://doi.org/10.1007/BF02464298 [Google Scholar]
- Yamada, A., Maeda, S., & Ohmasa, M. (1999. b) .Ectomycorrhiza formation of Tricholoma matsutake isolates on Pinus densiflora in vitro. Mycoscience, 40, 455–463. https://doi.org/10.1007/BF02461022 [Google Scholar]
- Yamada, A., Maeda, K., Kobayashi, H., & Murata, H. (2005) .Ectomycorrhizal symbiosis in vitro between Tricholoma matsutake and Pinus densiflora seedlings that resembles naturally occurring ‘shiro’. Mycorrhiza, 16, 111–116. https://doi.org/10.1007/s00572-005-0021-x [DOI] [PubMed] [Google Scholar]
- Yamada, A., Kobayashi, H., Murata, H., Kalmiş, E., Kalyoncu, F., & Fukuda, M. (2010) .In vitro ectomycorrhizal specificity between the Asian red pine Pinus densiflora and Tricholoma matsutake and allied species from worldwide Pinaceae and Fagaceae forests. Mycorrhiza, 20, 333–339. https://doi.org/10.1007/s00572-009-0286-6 [DOI] [PubMed] [Google Scholar]
- Yamada, A., Hayakawa, N., Saito, C., Horimai, Y., Misawa, H., Yamanaka, T., & Fukuda, M. (2019) .Physiological variation among Tricholoma matsutake isolates generated from basidiospores obtained from one basidioma. Mycoscience, 60, 102–109. https://doi.org/10.1016/j.myc.2018.12.001 [Google Scholar]
- Yamanaka, K., Aimi, T., Wan, J., Cao, H., & Chen, M. (2011) .Species of host trees associated with Tricholoma matsutake and allies in Asia. Mushroom. Science and Biotechnology, 19, 79–87. https://doi.org/10.24465/msb.19.2_79 [Google Scholar]
- Yamanaka, T., Yamada, A., & Furukawa, H. (2020) .Advances in the cultivation of the highly-prized ectomycorrhizal mushroom Tricholoma matsutake. Mycoscience, 61, 49–57. https://doi.org/10.1016/j.myc.2020.01.001 [Google Scholar]
- Zeng, D. F., & Chen, B. (2015) .Genetic variability and bottleneck detection of four Tricholoma matsutake populations from northeastern and southwestern China. Environmental Microbiology, 17, 2870–2881. https://doi.org/10.1111/1462-2920.12809 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.