Abstract
Chronic inflammation is associated with a decline in aging skeletal muscle health. Inflammation also appears to interfere with the beneficial skeletal muscle adaptations conferred by exercise training in older individuals. We hypothesize that the cyclooxygenase pathway is partially responsible for this negative inflammatory influence on aging skeletal muscle health and plasticity.
Keywords: Inflammation, Aging, Inflammaging, Skeletal Muscle, Exercise, Cyclooxygenase, Prostaglandin
SUMMARY for Table of Contents
Controlling cyclooxygenase pathway regulated inflammation may help protect the health and plasticity of aging skeletal muscle.
SYSTEMIC AND TISSUE SPECIFIC INFLAMMATION
The causes and consequences of inflammation have been of scientific interest since the ancient Greeks built the foundation of modern medicine. Inflammation can still be identified by the four classic signs (rubor, calor, dolor et tumor; redness, heat, pain and swelling) described by Celsus in the first century AD (1, 2). Two centuries later, Galen asserted that the inflammatory response was in fact a necessary part of the healing process. In the 1800’s, Virchow suggested that loss of function (functio laesa) be acknowledged as the fifth cardinal sign of inflammatory activity within a tissue (1, 2). Interest in understanding the role of inflammation in the loss of tissue function paired with technological advancements of the twentieth century led to the identification of numerous systemic and tissue specific inflammatory markers.
Systemic inflammation can be characterized by a large number of inflammatory biomarkers (3–6), and the most common ones include interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Severe systemic inflammation occurs in response to various clinical conditions (e.g., sepsis or SARS-CoV-2 infection) and is associated with pathologic concentrations of circulating inflammatory markers (7–10). In contrast, chronic low-grade systemic inflammation is characterized by the concentrations of inflammatory markers being only minimally elevated and is sometimes considered a normal part of the aging process (4, 11–13). This systemic elevation of pro-inflammatory markers can then outweigh the influence of anti-inflammatory compounds (14). Collectively, these effects create the net pro-inflammatory environment that develops with advanced age, otherwise referred to as “inflammaging.” In the twenty years since the term “inflammaging” was coined by Franceschi et al. (13), research involving the age-associated elevation in inflammation has experienced exponential growth (PubMed citations per year: 2000: 2; 2010: 13; 2015: 63, 2020: 251). As a result, chronic low-grade inflammation has been linked to numerous diseases such as cardiovascular disease, certain cancers, diabetes, certain gastrointestinal diseases, arthritis, dementia, osteoporosis, and sarcopenia (3, 4, 15).
Tissue-level inflammation is a highly complex and coordinated series of events involving inflammatory and immune cells resident to the tissue and recruited from the vasculature, various membrane-bound and intracellular receptors, and multiple intracellular signaling pathways and compounds (2, 16–18). These inflammatory-related events are yet to be fully elucidated and are further complicated by the various instigators of inflammation (e.g., overt tissue injury, acute or chronic exposure to endogenous or exogenous “toxic” compounds). While the goal is tissue protection and repair, unresolved inflammation can be associated with tissue damage and dysfunction, leading to chronic diseases (2, 16–19).
As the underlying cellular pathways that regulate inflammation at the systemic and tissue level are complex and continuing to be unraveled (16–18, 20–22), the cyclooxygenase (COX) pathway has emerged as a potential contributor to this process (19, 23–27). Several of the prostaglandins produced by the COX pathway are considered to be pro-inflammatory lipid mediators and have important biological roles in numerous tissues (3, 19, 23–25, 27–29). Much of the insight into the COX pathway has been discovered because of the readily available and commonly consumed COX inhibiting drugs that are taken acutely and chronically for inflammation and pain (e.g., ibuprofen, naproxen, indomethacin, celecoxib, aspirin, and acetaminophen). The goal of this review is to present the evidence for the association between inflammaging and the decline in skeletal muscle health, and then connect this information to the related COX pathway studies (epidemiological, randomized controlled trials, and pre-clinical). Collectively, this information provides the basis of the hypothesis that the COX pathway contributes, at least to some degree, to the chronic low-grade inflammation and resultant reduction in skeletal muscle health and plasticity associated with aging. A conceptual schematic of this hypothesis is presented in Figure 1.
Figure 1.
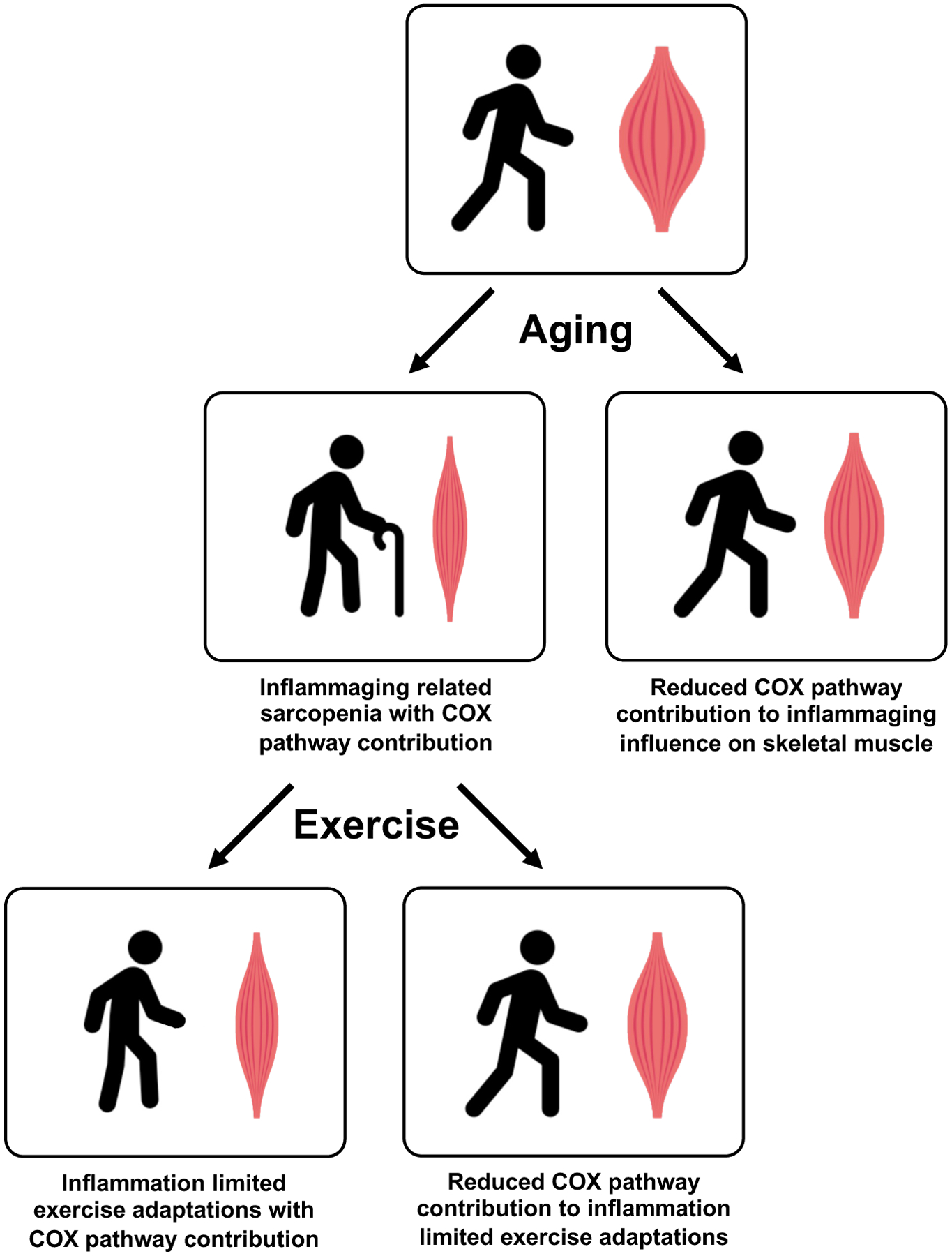
Proposed contribution of the COX pathway to the effect of inflammation on aging skeletal muscle. The authors hypothesize that the effect of age-related inflammation (i.e., “inflammaging”) on the development of sarcopenia can be mitigated by reducing the contribution of the COX pathway in aging skeletal muscle. Similarly, it appears the influence of age-related inflammation on limiting exercise adaptations can be mitigated by reducing the contribution of the COX pathway, ultimately promoting greater improvements in skeletal muscle health.
AGING, INFLAMMATION, AND SKELETAL MUSCLE HEALTH
It is well established, through several large cohort studies, that aging is associated with increases in circulating inflammatory markers, which in turn are associated with alterations in skeletal muscle mass and function and overall mobility. The Established Populations for Epidemiologic Studies of the Elderly (EPESE; n=1727) project reported in the late 1990’s that circulating IL-6 increased with age in individuals over 70 years old and higher levels of IL-6 were correlated with higher levels of functional disability (30). This project also showed that higher levels of circulating IL-6 predicted future onset of disability in older individuals (n=633) (31). Since these initial reports, several other studies have replicated and extended these findings. The Women’s Health and Aging Study (WHAS; n=620) showed that the severity of functional disability increased along with IL-6 concentrations and further connected this trend to declining muscle strength (32). The Health, Aging, and Body Composition (Health ABC; n=3075) study, using computed tomography measures of thigh muscle cross sectional area (CSA) and dual-energy x-ray absorptiometry measures of appendicular muscle mass, showed that higher levels of inflammatory markers (IL-6 and TNF-α) were associated with smaller muscle mass, along with lower knee extensor and handgrip strength (33). In a longitudinal follow up, Health ABC (n=2177) investigators also reported inflammation associated declines in thigh muscle size and knee extensor strength over five years (34). The Aging in the Chianti Area (InCHIANTI; n=1020) study found that circulating IL-6 and CRP levels were negatively correlated with overall physical performance (walking speed, chair stands, balance, handgrip strength) (35). The Longitudinal Aging Study Amsterdam (LASA; n=986), following older individuals over three years, reported that higher levels of circulating IL-6 and CRP were associated with a 2–3 fold greater risk of losing greater than 40% of muscle (handgrip) strength over this time period (15).
These larger aging cohort findings have received experimental support from smaller investigations. Lavin et al. (22) and Mikkelsen et al. (36) have reported strong negative associations between MRI determined muscle mass and circulating IL-6 and CRP, respectively, across young and older physically active and inactive individuals. These studies also support the notion that physical activity plays an important role in the regulation of the age-associated increase in inflammation and decline in muscle mass. Higher circulating inflammatory status has been shown to be associated with an impaired ability to respond to an exercise training intervention (e.g., increase muscle mass or improve muscle function) in hospitalized geriatric patients (37), mobility limited older adults (38), older women with knee osteoarthritis (39), and frail and pre-frail older adults (40). In support of these outcomes, myocellular studies have shown an increased susceptibility to pro-inflammatory signaling is likely linked to reduced skeletal muscle regenerative capacity in older individuals (20).
THE CYCLOOXYGENASE PATHWAY AND SKELETAL MUSCLE HEALTH
While the underpinnings of the inflammatory regulation of aging skeletal muscle health are complex and multifactorial, the COX pathway in skeletal muscle appears to play a role. Support for this is outlined below and summarized in Figure 2. The substrate for the COX pathway is the cell membrane derived lipid arachidonic acid, which funnels through the one of two main isoforms of COX (i.e., COX-1 or COX-2) and then through prostaglandin-specific synthases to produce one of several prostaglandins (PGE2, PGF2α, PGD2, and PGI2) (23, 25, 41). Each of these prostaglandins have one or more specific receptors that regulate downstream cellular events in an autocrine and paracrine fashion (23, 25, 29, 41).
Figure 2.
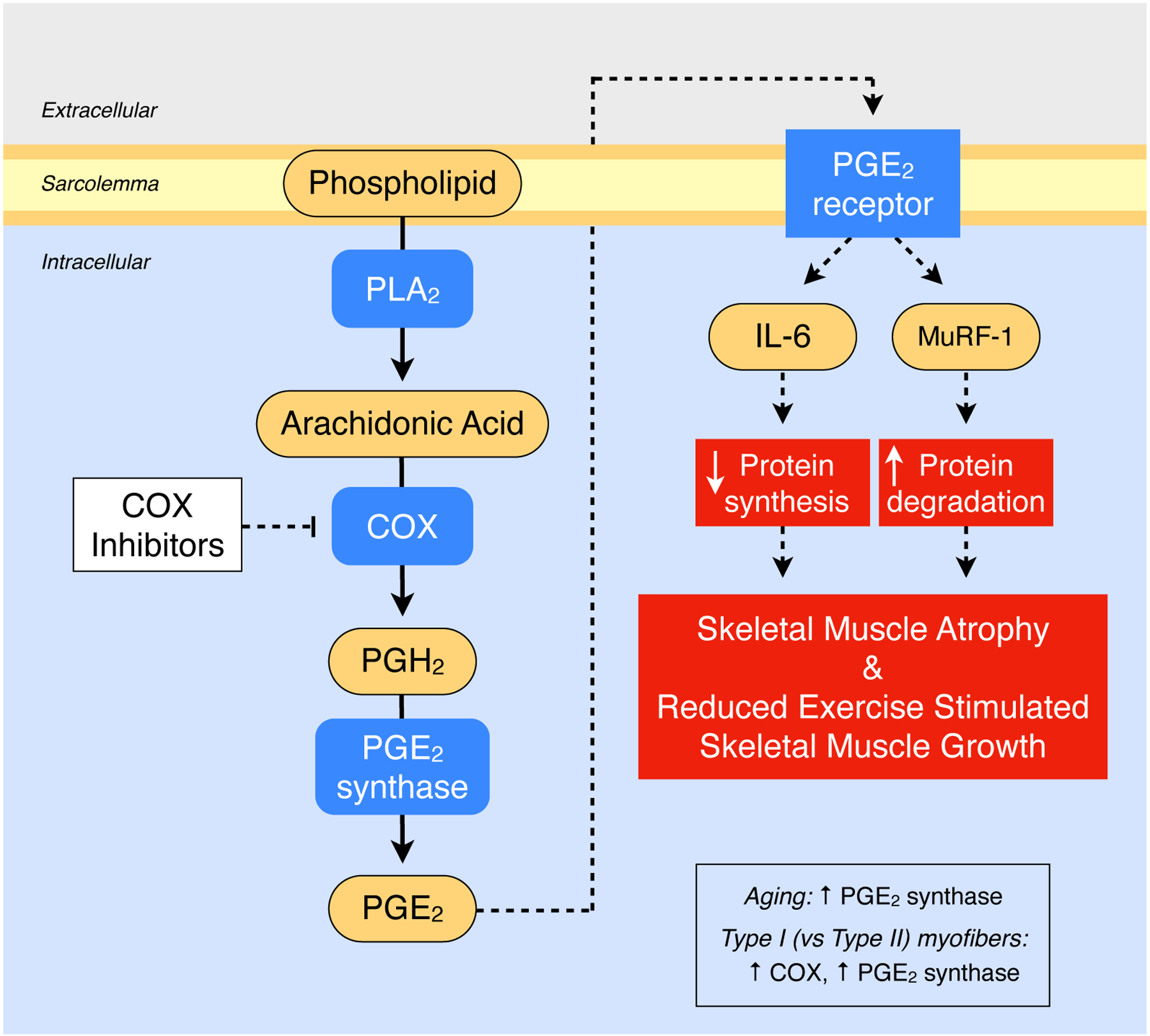
Overview of the PGE2 portion of the COX pathway (i.e., the PGE2/COX pathway) in skeletal muscle and the proposed series of reactions and downstream regulatory events that influence skeletal muscle mass. Aging and muscle fiber type alter the level of COX pathway components. See text for the studies and specific references that have delineated the items outlined in this schematic. PLA2, phospholipase A2; COX, cyclooxygenase; PGH2, prostaglandin H2; PGE2, prostaglandin E2; MuRF-1, muscle RING finger 1; IL-6, interleukin 6.
One of the key connections between the COX pathway, inflammatory regulation, and muscle mass can be traced through the connection to IL-6. The most abundant PG produced by the COX pathway in skeletal muscle is PGE2 (25) and when human skeletal muscle is incubated with PGE2 it stimulates transcription of IL-6 (42). Interestingly, this same effect has been shown in bone and nerve cells, along with delineation of the receptors and signaling pathways linked to IL-6 promotor activation in those tissues (43–46). Van Hall et al. (49) showed that infusion of IL-6 in healthy volunteers resulted in a 50% reduction in skeletal muscle protein turnover due to protein synthesis being blunted more than protein breakdown, which resulted in net amino acid loss from the muscle. These authors speculated this response was linked to an IL-6 directed increase in amino acid demand in other tissues (e.g., splanchnic tissues) and an associated systemic hypoaminoacidemia. Toth et al. (50) have shown a significant negative relationship between circulating IL-6 concentrations and skeletal muscle protein synthesis rates in young and old men and women. In addition, low level IL-6 infusion into the skeletal muscle of rats induced muscle atrophy (51) and reduced muscle growth (52). It is also noteworthy that PGE2 upregulates skeletal muscle transcription of ubiquitin ligase muscle RING finger-1 (MuRF-1), a central mediator of muscle proteolysis (42, 47, 48).
Larger observational study findings provide insight into the potential effects of COX inhibition on aging skeletal muscle health. Landi et al. (53), using the Aging and Longevity in the Sirente geographic area (ilSIRENTE; n=354) study cohort, have investigated the impact of regular consumption of COX inhibiting anti-inflammatory drugs on sarcopenia incidence in community dwelling individuals 80 years and older. Using the European Working Group on Sarcopenia in Older People criteria for sarcopenia, 29% of the cohort was diagnosed with sarcopenia, defined as having low physical function and low muscle mass. Individuals were considered COX inhibiting drug consumers if they took a COX inhibitor (celecoxib, diclofenac, indomethacin, ketoprofen, ketorolac, nimesulide, piroxicam, rofecoxib) at least 5 days per week during the study observation period, implying they were chronic consumers. These authors showed there was an inverse association between COX inhibiting drug use and sarcopenia. Sarcopenia was present in only 9% of the drug consuming group and 31% of the non-drug consuming group (P<0.05). Overall, they reported an ~80% reduction in risk of sarcopenia with COX inhibitor consumption in this cohort.
Controlling inflammatory burden through COX pathway inhibition also appears to impact the skeletal muscle health benefits derived by older individuals from resistance exercise. In a randomized controlled trial including a three-month resistance exercise training period, older individuals consuming a COX inhibitor (ibuprofen or acetaminophen) experienced significantly greater improvements in quadriceps muscle mass and muscle strength compared to the placebo consuming group (54). Follow up analysis on muscle samples from these trained individuals and separate investigations revealed some cellular explanations for the beneficial effects of COX inhibition (25, 42, 55–57). Intramuscular transcripts for IL-6 and MuRF-1 were elevated in response to exercise training in the placebo group but were unchanged in the COX inhibitor groups (55). This response is likely due to the COX inhibitor reduction in intramuscular production of PGE2 (25, 58), which in turn has been shown to upregulate IL-6 and MuRF-1 transcription in human skeletal muscle (42). Both of these key muscle regulators have been shown to have negative downstream effects on muscle protein turnover and growth (25, 42, 47–49, 51, 52), as discussed previously in this review, which could explain the additional muscle hypertrophy in the COX inhibitor consuming groups.
In terms of myofiber growth in the trained individuals, additional follow-up analysis demonstrated the placebo group experienced hypertrophy of only the type II muscle fibers (+26%), while COX inhibition resulted in hypertrophy of both type I (+28%) and type II (+37%) muscle fibers (54, 56). This additional hypertrophy of the type I fibers in the COX inhibitor group corresponded to increases in phenotypic markers commonly more abundant with this fiber type (the mitochondrial enzyme citrate synthase and capillarization), which were not observed in the placebo group. These fiber type specific hypertrophy differences between the placebo and COX inhibitor groups also correspond with findings that human type I muscle fibers (vs. type II) have higher levels of the COX enzyme (COX-1) and PGE2 synthase (mPGES-2), both of which would support higher levels of PGE2 production in type I muscle fibers (57). Higher levels of PGE2 production could lead to increased PGE2 directed transcription of IL-6 and MuRF-1 in these same type I fibers (42). In addition, human type I muscle fibers (vs. type II) have been shown to have higher levels of the pro-inflammatory PGE2 receptor (EP3) and lower levels of the anti-inflammatory PGE2 receptor (EP4) (22, 29, 57). Overall, these findings provide insight into the mechanism of the COX inhibitor benefits on myofiber and whole muscle size, and ultimately on muscle function.
There is also support for these human COX inhibitor findings from pre-clinical studies. Rieu et al. (59) showed five months of COX inhibitor treatment in old rats with low grade inflammation had significant improvements in circulating inflammatory biomarkers, including a 60% reduction in IL-6. COX inhibition also improved albumin synthesis rate and blunted the decline in circulating albumin levels. In addition, in response to feeding muscle protein synthesis was increased and muscle proteolysis was reduced. These improvements in circulating inflammatory status and muscle protein turnover with chronic COX inhibition translated into increased muscle mass, measured in several muscles of varying fiber type.
The aforementioned effects of COX inhibiting drugs on skeletal muscle health and adaptations may be specific to older individuals. Skeletal muscle protein synthesis, satellite cell activity, intramuscular signaling, and growth have been shown to be negatively impacted by COX inhibiting drug consumption in younger individuals (60–65), although negative effects are not universally reported (66–69). The basis for these age-related differences have not been delineated, but may be related to differences in metabolic and molecular control of skeletal muscle adaptations between young and old individuals. To this end, aging alters the level of some components of the COX pathway that may contribute to these differences (57). The findings in younger individuals do underscore the involvement of the COX pathway, prostaglandins, and associated mechanisms in the control of skeletal muscle health related adaptations.
It should be noted that not all studies of aging and COX inhibition report an influence on skeletal muscle health (70–77). One apparent explanation is related to the dose of COX inhibitor used in some of these investigations. Several studies that have not shown any COX inhibitor influence have examined relatively low doses (Ibuprofen: 1.2 grams per week or Acetaminophen: 3 grams per week) (74–77) compared to those that have shown an influence (Ibuprofen: 8.4 grams per week or Acetaminophen: 28 grams per week) (54–56). For those studies that have used a higher dose and not shown an influence (70, 73), specific reasons for the differences across studies are not apparent. While speculative, the inflammatory burden in the subject population with knee osteoarthritis (73) or the relatively short exercise training program (6 weeks) after two weeks of casting-induced atrophy (70) may have played a role. It is also possible that the COX pathway was not substantially involved in regulating the adaptive responses to exercise in these study populations under the specific study conditions.
SUMMARY
Chronic inflammation often associated with advanced age (i.e., “inflammaging”) has been identified as a hallmark of numerous chronic diseases, including sarcopenia. As a result, many investigations have attempted to identify the mechanistic underpinnings that drive inflammaging-associated health decline. Chronic inflammation is inherently complex with many highly integrated response mechanisms, making the existence of a single causative molecular target unlikely. Yet, the COX pathway has emerged as a potential regulator of the health and function of aging skeletal muscle. It is noteworthy that this pathway appears to be involved in the inflammatory regulated health of several other tissues, which provides indirect support for the proposed hypothesis in skeletal muscle (78–84). Pre-clinical investigations combined with observations from prospective randomized controlled trials may provide further insight into the complex nature of regulating age-associated COX inhibition in skeletal muscle tissue. Because of potential side effects associated with COX inhibiting drugs, other ways to alter COX pathway activity either directly or through upstream or downstream mechanistic targets should be explored. Overall, this information could be useful for the development of guidelines for the prevention and treatment of sarcopenia, with the goal of helping older individuals maintain a healthy, independent lifestyle later in life.
KEY POINTS.
Aging skeletal muscle health is negatively associated with chronic low-grade inflammation, which human and animal studies suggest is at least partially regulated by the cyclooxygenase (COX) pathway.
Controlling COX-related inflammation may improve the adaptability of aging skeletal muscle to exercise.
This regulatory information should be integrated into the mosaic of inflammatory control of aging skeletal muscle health and considered in related prevention and treatment strategies.
Acknowledgements:
This review was based, in part, on work supported by NIH grants AG020532, AG00831, and AG038576. Conflicts of Interest: none.
REFERENCES
- 1.Scott A, Khan KM, Cook JL, and V D. What is “inflammation”? Are we ready to move beyond Celsus? Brit J Sport Med 38: 248–249, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallach D, Kang T-B, and Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol 14: 51–59, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Elisia I, Lam V, Hofs E, Li M, Hay M, Cho B, Brooks-Wilson A, Rosin M, Bu L, Jia W, and Krystal G. Effect of age on chronic inflammation and responsiveness to bacterial and viral challenges. PLoS One 12: e0188881, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy D, Fasano A, Miller G, Miller A, Mantovani A, Weyand C, Barzilai N, Goronzy J, Rando T, Effros R, Lucia A, Kleinstreuer N, and Slavich G. Chronic inflammation in the etiology of disease across the life span. Nat Med 25: 1822–1832, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germolec D, Shipowski K, Frawley R, and Evans E. Markers of inflammation. In: Immunotoxicity Testing Methods in Molecular Biology. New York, NY: Humana Press, 2018, p. 57–79. [DOI] [PubMed] [Google Scholar]
- 6.Walker K, Walston J, Gottesman R, Kucharska-Newton A, Palta P, and Windham B. Midlife systemic inflammation is associated with frailty in later life: The ARIC Study. J Gerontol 74: 343–349, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey L, Balk R, and Bone R. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med 119: 771–778, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Lavillegrand J, Garnier M, Spaeth A, Mario N, Hariri G, Pilon A, Berti E, Fieux F, Thietart S, Urbina T, Turpin M, Darrivere L, Fartoukh M, Verdonk F, Dumas G, Tedgui A, Guidet B, Maury E, Chantran Y, Voiriot G, and Ait-Oufella H. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensive Care 11: 1–10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chousterman B, Swirski F, and Weber G. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39: 517–528, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Valle D, Kim-Schulze S, Huang H, Beckmann N, Nirenberg S, Wang B, Lavin Y, Swartz T, Madduri D, Stock A, Marron T, Xie H, Patel M, Tuballes K, Oekelen O, Rahman A, Kovatch P, Aberg J, Schadt E, Jagannath S, Mazumdar M, Charney A, Firpo-Betancourt A, Mendu D, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, and Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26: 1636–1643, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Rodriguez L, Lopez-Hoyos M, Munoz-Cacho P, and Martinez-Taboada VM. Aging is associated with circulating cytokine dysregulation. Cell Immunol 273: 124–132, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, and Longo DL. The origins of age-related proinflammatory state. Blood 105: 2294–2299, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, and De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908: 244–254, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Minciullo P, Catalano A, Mandriffino G, Casciaro M, Crucitt A, Maltese G, Morabito N, Lasco A, Gangemi S, and Basile G. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp 64: 111–126, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Schaap LA, Pluijm SMF, Deeg DJH, and Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119: 526.e529–517, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Nathan C. Points of control in inflammation. Nature 420: 846–852, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C, and Ding A. Nonresolving inflammation. Cell 140: 871–882, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Feehan K, and Gilroy D. Is resolution the end of inflammation? Trends Mol Med 25: 198–214, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Korotkova M, and Lundberg IE. The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat Rev Rheumatol 10: 295–303, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Merritt E, Stec M, Thalacker-Mercer A, Windham S, Cross J, Shelley D, Tuggle S, Kosek D, Kim J, and Bamman M. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol 115: 937–948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peake J, Gatta P, and Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, and Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol 128: 87–99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons DL, Botting RM, and Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387–437, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Korotkova M, and Jakobsson P-J. Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol 10: 229–241, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Trappe TA, and Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol 115: 909–919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lone AM, Giansanti P, Jørgensen MJ, Gjerga E, Dugourd A, Scholten A, Saez-Rodriguez J, Heck AJR, and Taskén K. Systems approach reveals distinct and shared signaling networks of the four PGE2 receptors in T cells. Science Signaling 14: eabc8579, 2021. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, and DuBois R. Role of prostanoids in gastrointestinal cancer. J Clin Invest 128: 2732–2742, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth EM, Grosser T, Wang M, Yu Y, and FitzGerald GA. Prostanoids in health and disease. J Lipid Res 50 Suppl: S423–428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norel X, Sugimoto Y, Ozen G, Abdelazeem H, Amgoud Y, Bouhadoun A, Bassiouni W, Goepp M, Mani S, Manikpurage H, Senbel A, Longrois D, Heinemann A, Yao C, and Clapp L. International union of basic and clinical pharmacology. CIX. Differences and similarities between human and rodent prostaglandin E2 receptors (EP1–4) and prostacyclin receptor (IP): specific roles in pathophysiologic conditions. Pharmacol Rev 72: 910–968, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen H, Pieper C, Harris T, Rao K, and Currie M. The association of plasma IL-6 levels with functional disability in community dwelling elderly. J Gerontol 52A: M201–208, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Ferrucci L, Harris T, Guralnik J, Tracy R, Corti M, Cohen H, Pennix B, Pahor M, Wallace R, and Havlik R. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ferrucci L, Penninx BWJH, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, and Guralnik J. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 50: 1947–1954, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Visser M, Pahor M, Taaffe D, Goodpaster B, Simonsick E, Newman A, Nevitt M, and Harris T. Relationship of Interleukin-6 and Tumor Necrosis Factor-α With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J Gerontol 57: M326–332, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Schaap LA, Pluijm SMF, Deeg DJH, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M, and Study HA. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol 64: 1183–1189, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cesari M, Pennix B, Pahor M, Lauretani F, Corsi A, Williams G, Guralnik J, and Ferrucci L. Inflammatory Markers and Physical Performance in Older Persons: The InCHIANTI Study. J Gerontol 59: 242–248, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, and Kjær M. Life-long endurance exercise in humans: Circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev 134: 531–540, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Norheim K, Cullum C, Andersen J, Kjaer M, and Karlsen A. Inflammation relates to resistance training-induced hypertrophy in elderly patients. Med Sci Sports Exerc 49: 1079–1085, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Grosicki GJ, Barrett BB, Englund DA, Liu C, Travison TG, Cederholm T, Koochek A, Berens v, Gustafsson T, Benard T, Reid KF, and Fielding RA. Circulating interleukin-6 is associated with skeletal muscle strength, quality, and functional adaptation with exercise training in mobility-limited older adults. J Frailty Aging 9: 57–63, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Marriott K, Chopp-Hurley J, Loukov D, Karampatos S, Kuntz A, Wiebenga E, Stratford P, Noseworthy M, Bowdish D, and Maly M. Muscle strength gains after strengthening exercise explained by reductions in serum inflammation in women with knee osteoarthritis. Clin Biochem 86: 105381, 2021. [DOI] [PubMed] [Google Scholar]
- 40.Hangelbroek R, Knuiman P, Tieland M, and Groot Ld. Attenuated strength gains during prolonged resistance exercise training in older adults with high inflammatory status. Exp Gerontol 106: 154–158, 2018. [DOI] [PubMed] [Google Scholar]
- 41.Smith WL, Urade Y, and Jakobsson P-J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111: 5821–5865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Standley RA, Liu SZ, Jemiolo B, Trappe SW, and Trappe TA. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids 88: 361–364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Zhu F, and Konstantopoulos K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-D12,14-PGJ2. PLoS One 6: e27630, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wang P, Zhu F, and Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol 298: C1445–1456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiebich B, Schleicher S, Spleiss O, Czygan M, and Hüll M. Mechanisms of prostaglandin E2‐induced interleukin‐6 release in astrocytes: possible involvement of EP4‐like receptors, p38 mitogen‐activated protein kinase and protein kinase C. J Neurochem 79: 950–958, 2001. [DOI] [PubMed] [Google Scholar]
- 46.St-Jacques B, and Ma W. Role of prostaglandin E2 in the synthesis of the pro-inflammatory cytokine interleukin-6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem 118: 841–854, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, and Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology 23: 160–170, 2008. [DOI] [PubMed] [Google Scholar]
- 49.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, and Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Toth M, Matthews D, Tracy R, and Previs M. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288: E883–E891, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Haddad F, Zalvidar F, Cooper D, and Adams G. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Bodell P, Kodesh E, Haddad F, Zaldivar F, Cooper D, and Adams G. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol 106: 443–453, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landi F, Marzetti E, Liperoti R, Pahor M, Russo A, Martone AM, Colloca G, Capoluongo E, and Bernabei R. Nonsteroidal anti-inflammatory drug (NSAID) use and sarcopenia in older people: results from the ilSIRENTE study. J Am Med Dir Assoc 14: 626.e629–613, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, and Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trappe TA, Standley RA, Jemiolo B, Carroll CC, and Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 304: R198–205, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trappe TA, Ratchford SM, Brower BE, Liu SZ, Lavin KM, Carroll CC, Jemiolo B, and Trappe SW. COX inhibitor influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol 71: 1289–1294, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu SZ, Jemiolo B, Lavin KM, Lester BE, Trappe SW, and Trappe TA. Prostaglandin E2/cyclooxygenase pathway in human skeletal muscle: influence of muscle fiber type and age. J Appl Physiol 120: 546–551, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trappe TA, Fluckey JD, White F, Lambert CP, and Evans WJ. Skeletal Muscle PGF2α and PGE2 in Response to Eccentric Resistance Exercise: Influence of Ibuprofen and Acetaminophen. J Clin Endocrinol Metab 2001. [DOI] [PubMed] [Google Scholar]
- 59.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron M, Combaret L, and Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587: 5483–5492, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, and Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endo Metab 282: E551–556, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen L, Kjaer M, and Mackey A. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107: 1600–1611, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackey A, Kjaer M, Dandanell S, Mikkelsen K, Holm L, Døssing S, Kadi F, Koskinen S, Jensen C, Schrøder H, and Langberg H. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol 103: 425–431, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Markworth J, Vella L, Lingard B, Tull D, Rupasinghe T, Sinclair A, Maddipati K, and Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol 305: R1281–R1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markworth J, Vella L, Figueiredo V, and Cameron-Smith D. Ibuprofen treatment blunts early translational signaling responses in human skeletal muscle following resistance exercise. J Appl Physiol 117: 20–28, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Lilja M, Mandić M, Apro W, Melin M, Olsson K, Rosenborg S, Gustafsson T, and Lundberg T. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol 222: 1–16, 2017. [DOI] [PubMed] [Google Scholar]
- 66.Vella L, Markworth JF, Peake JM, Snow RJ, Cameron-Smith D, and Russell AP. Ibuprofen supplementation and its effects on NF-κB activation in skeletal muscle following resistance exercise. Physiological Reports 2: 1–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vella L, Markworth J, Paulsen G, Raastad T, Peake J, Snow R, Cameron-Smith D, and Russell A. Ibuprofen ingestion does not affect markers of post-exercise muscle inflammation. Front Physiol 7: 1–9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krentz J, Quest B, Farthing J, Quest D, and Chilibeck P. The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training. Appl Physiol Nutr Metab 33: 470–475, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Cardinale DA, Lilja M, Mandić M, Gustafsson T, Larsen FJ, and Lundberg TR. Resistance training with co-ingestion of anti-inflammatory drugs attenuates mitochondrial function. Front Physiol 8: 1074, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dideriksen K, Boesen A, Kristiansen J, Magnusson S, Schjerling P, Holm L, and Kjaer M. Skeletal muscle adaptation to immobilization and subsequent retraining in elderly men: No effect of anti-inflammatory medication. Exp Gerontol 82: 8–18, 2016. [DOI] [PubMed] [Google Scholar]
- 71.Dideriksen K, Reitelseder S, Malmgaard-Clausen N, Bechshoeft R, Petersen R, Mikkelsen U, and Holm L. No effect of anti-inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Exp Gerontol 83: 120–129, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Petersen S, Miller B, Hansen M, Kjaer M, and Holm L. Exercise and NSAIDs: effect on muscle protein synthesis in patients with knee osteoarthritis. Med Sci Sports Exerc 43: 425–431, 2011. [DOI] [PubMed] [Google Scholar]
- 73.Petersen SG, Beyer N, Hansen M, Holm L, Aagaard P, Mackey AL, and Kjaer M. Nonsteroidal anti-inflammatory drug or glucosamine reduced pain and improved muscle strength with resistance training in a randomized controlled trial of knee osteoarthritis patients. Arch Phys Med Rehabil 92: 1185–1193, 2011. [DOI] [PubMed] [Google Scholar]
- 74.Jankowski CM, Gozansky WS, MacLean PS, Shulman B, Wolfe P, Schwartz RS, and Kohrt WM. N-acetyl-4-aminophenol and musculoskeletal adaptations to resistance exercise training. Eur J Appl Physiol 113: 1127–1136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jankowski C, Shea K, Barry D, Linnebur S, Wolfe P, Kittelson J, Schwartz R, and Kohrt W. Timing of ibuprofen use and musculoskeletal adaptations to exercise training in older adults. Bone Reports 1: 1–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Candow D, Chilibeck P, Weisgarber K, Vogt E, and Baxter-Jones A. Ingestion of low-dose ibuprofen following resistance exercise in postmenopausal women. J Cachexia Sarcopenia Muscle 4: 41–46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duff W, Chilibeck P, Candow D, Gordon J, Mason R, Taylor-Gjevre R, Nair B, Szafron M, Baxter-Jones A, Zello G, and Kontulainen S. Effects of ibuprofen and resistance training on bone and muscle: a randomized controlled trial in older women. Med Sci Sports Exerc 49: 633–640, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Carbone L, Tylavsky F, Cauley J, Harris T, Lang T, Bauer D, Barrow K, and Kritchevsky S. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: Impact of cyclooxygenase selectivity. J Bone Miner Res 18: 1795–1802, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Fountain WA, Naruse M, Claiborne A, Gries KJ, Jones A, Minchev K, Lester BE, Raue U, Trappe S, and Trappe T. Low-dose aspirin and COX inhibition in human skeletal muscle. J Appl Physiol 129: 1477–1482, 2020. [DOI] [PubMed] [Google Scholar]
- 80.Naruse M, Fountain W, Claiborne A, Chambers T, Jones A, Stroh A, Montenegro C, Lynch C, Minchev K, Trappe S, and Trappe T. Influence of low dose aspirin, resistance exercise, and sex on human skeletal muscle PGE2/COX pathway activity. Physiol Rep 9: e14790, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ratchford SM, Lavin KM, Perkins RK, Jemiolo B, Trappe SW, and Trappe TA. Aspirin as a COX inhibitor and anti-inflammatory drug in human skeletal muscle. J Appl Physiol 123: 1610–1616, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothwell P, Fowkes F, Belch J, Ogawa H, Warlow C, and Meade T. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31–41, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Rothwell PM, Price JF, Fowkes F, Zanchetti A, Roncaglioni M, Tognoni G, Lee R, Belch J, Wilson M, Mehta Z, and Meade T. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. The Lancet 379: 1602–1612, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Kern S, Skoog I, Östling S, Kern J, and Börjesson-Hanson A. Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open 2: e001288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


