Abstract
A systematic review and meta-analysis of pertinent literature published from 2006 to January 2022 were conducted to study and compare vitrification and slow freezing, the two prominent methods of ovarian tissue cryopreservation. The primary outcome measures for this study were (1) proportion of intact primordial follicles, (2) proportion of intact stromal cells, (3) proportion of DNA fragmentation in primordial follicles, and (4) mean primordial follicle density. This meta-analysis of 19 studies revealed a significantly greater proportion of intact stromal cells in vitrified tissue versus slow-frozen tissue. No significant differences upon pooled analyses were observed between the two cryopreservation methods with respect to the proportion of intact primordial follicles, proportion of DNA fragmentation, or mean primordial follicle density. Due to differences seen in stromal cell viability, vitrification may be a preferred option to preserve histology of tissue. However, more work should be done to compare the two freezing techniques with less heterogeneity caused by patients, samples, and protocols.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-022-02692-w.
Keywords: Ovarian tissue cryopreservation, Fertility preservation, Slow freezing, Vitrification
Background
Following several advancements in technologies for ovarian tissue cryopreservation (OTC) [1–4], fertility preservation (FP) has been translated to diverse settings, including ovarian dysfunction [5], premature menopause [6], elective family planning, elective delay of menopause [7], and fertility preservation for non-malignant indications [8–10]. In the context of childhood cancer survivors and adult oncological patients with cancers too aggressive to postpone treatment in favor of assisted reproductive technology cycles, prospective fertility relies on gonadal tissue cryopreservation [11–14].
The two common methods of ovarian tissue cryopreservation include vitrification, which avoids ice crystal formation and intracellular mechanical damage by rapid conversion of cells from liquid to glass states, and slow freezing, which cools samples at controlled rates but can lead to extracellular injury as cells transform from aqueous to solid phases including ice crystal formation [15]. Vitrification is typically followed by rapid warming, whereas slow freezing accompanies a more gradual thawing protocol [16]. While both methods require a high level of technical skill in ovarian tissue preparation, the process of vitrification can be time-intensive, experience-demanding, and potentially cytotoxic due to the high concentrations of cryoprotective agents needed. Although slow freezing protocols require a freezing chamber and computer-controlled freezing programs which need routine maintenance and validation, this method utilizes lower levels of cryoprotective agent concentrations to safely preserve specimens [17].
Investigations into the histopathological and morphological differences in cryopreserved ovarian tissue based on the two protocols have led to conflicting conclusions. While some studies have found that vitrification results in superior stromal cell integrity, follicular quality and quantity, and less severe apoptosis than slow freezing [18, 19], others have concluded the opposite [20, 21]. Some studies found no significant difference in the rates of follicular growth or apoptosis between the two OTC methods [22], instead finding differences in the types of tissue damage caused by each [23]. Both slow freezing and vitrification typically result in more cytotoxicity and less follicular proliferation when compared to fresh controls [24]. Whereas anti-Müllerian hormone (AMH) mRNA expression, primordial follicle development, and overall morphology were significantly higher in slow-frozen tissue than in vitrified tissue in some studies [25, 26], vitrified tissue showed less follicular damage and improved oxidative function when compared to slow-frozen tissue in another study [23]. Estradiol levels were statistically comparable in slow-frozen and vitrified samples [20, 26]. Of note, the introduction of a novel needle immersed vitrification (NIV) method demonstrated superior follicular integrity, follicular density, and stromal morphology when compared to both slow-frozen and vitrified tissue [27]. OTC with NIV also resulted in a significantly greater amount of healthy primordial follicles and less apoptosis when compared to slow-frozen tissue [25].
Prior meta-analyses of the literature comparing vitrification to slow freezing protocols have been conducted; however, a consensus has not been reached regarding whether vitrification or slow freezing protocols result in superior morphology and physiology [28, 29]. Given that OTC is no longer considered an experimental means of FP for a range of indications [30], it is highly relevant at the present time to evaluate the two tissue cryopreservation methods and compare their respective efficacies in preserving human ovarian tissue. Thus, the objective of this meta-analysis is to systematically assess the histological outcomes of vitrification versus slow freezing of human ovarian tissue based on the relevant literature to date in order to provide pertinent information for laboratory and institutional practices in the setting of OTC.
Methods
Literature search strategy
A systematic review of the literature was conducted according to PRISMA guidelines [31]. Systematic searches were conducted in PubMed, Embase, Scopus, and Web of Science for studies published between January 2005 and January 2022. To identify relevant studies, the following search terms were used: ovarian/ovary tissue, vitrification, slow cooling, and slow freezing. The complete search strategy is available in the Supplementary material. A formal protocol for this meta-analysis was not prepared and was not registered.
Selection criteria
Selection criteria are summarized in Table 1. Studies were included in this systematic review if they met all of the following eligibility criteria: (1) examined both vitrification or slow freezing on human ovarian tissue; (2) presented original data through either a retrospective study, prospective study, or clinical trial; and (3) included at least one of the four outcomes to be extracted for this meta-analysis. Studies were excluded if they were conference abstracts, review articles, or animal model studies. Studies were also excluded if there was not enough data to conduct a meta-analysis with. For example, studies that reported a proportion without a standard deviation or the numerator and denominator were excluded. The screening of manuscripts was conducted by two reviewers (SB and MB), and a third reviewer was consulted to resolve discrepancies (VBJ).
Table 1.
Inclusion and exclusion criteria for studies included in this meta-analysis
| Inclusion criteria | Exclusion criteria |
|---|---|
| Examined both vitrification and slow freezing | Conference abstracts |
| Human ovarian tissue samples | Review articles |
| Used at least one of the four primary outcomes of this meta-analysis as a measure of comparison | Animal model studies |
| Original data (retrospective, prospective, or clinical trial) | No enough data reported to be included in meta-analysis |
Study quality measures
The National Institutes of Health Quality Assessment Tool for Case Series Studies [32], a nine-point quality assessment tool to evaluate internal validity, was used to assess quality of studies included in this meta-analysis. Study quality was assessed by two reviewers (SB and VBJ) independently and then together to resolve discrepancies in assessments.
Outcome measures
The following data were extracted by two researchers: study design and setting, patient demographics and diagnoses, surgical technique to remove ovarian tissue, and a brief overview of the vitrification and slow-freeze protocols. The primary outcome measures for this study were (1) proportion of intact primordial follicles (intact primordial follicles over total primordial follicles), (2) proportion of intact stromal cells (intact stromal cells over total stromal cells), (3) proportion of DNA fragmentation in primordial follicles (primordial follicles with evidence of DNA fragmentation over total primordial follicles), and (4) mean primordial follicle density. Secondary outcome measures included estradiol production and all primary outcome measures in fresh tissue versus slow-frozen or vitrified tissue. Data was reviewed by both researchers to ensure certainty in data accuracy.
Statistical analysis
Studies were only considered for inclusion if they had the necessary data elements to perform meta-analysis on the respective factors of interest. Meta-analysis of each primary outcome measure was performed under a random effects model using the R package “metafor.” To provide assessments of inter-study heterogeneity, we additionally reported Cochrane’s Q-statistic, corresponding p value, and I2 values. Heterogeneity across studies was measured using the I2 indicator, in which I2 > 50% suggests substantial heterogeneity.
For studies that reported a cell count denominator and a proportion, a cell count numerator was inferred based on the nearest whole integer. For studies that reported a mean and SD of observed proportions at the single-sample level, we applied a binomial distributional model to infer per sample counts (assuming uniform cell count per sample). To accommodate studies with multiple data points per preservation type, we aggregated observed/estimated counts stratified by preservation type. We then performed meta-analyses on the study-wise risk ratios from the derived 2 × 2 tables. For follicle density, we performed a meta-analysis of the log ratio of the means (log[RoM]), using the assumption of homogeneity of coefficient of variation within each group across studies (setting vtype = “AV” in escalc).
Results
Study characteristics and quality
A total of 5022 citations were identified among four databases (Fig. 1). After duplicates were removed, 3684 citations remained. After screening of abstracts for inclusion and exclusion criteria, 3665 citations were removed, resulting in 19 selected for quantitative analysis via meta-analysis.
Fig. 1.
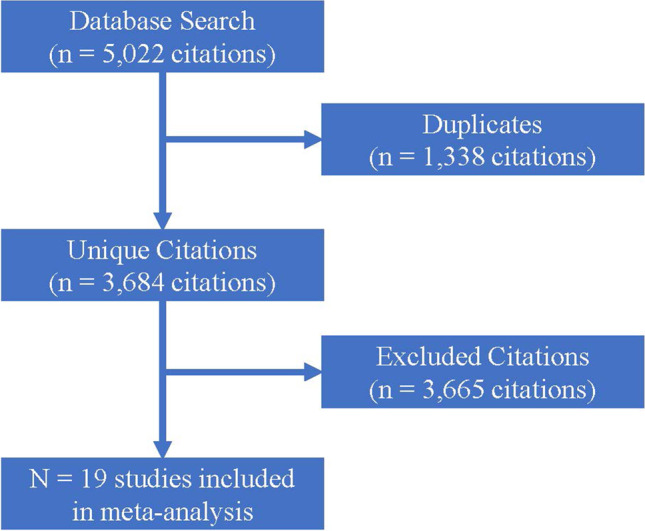
Study selection process for meta-analysis
Study characteristics are described in Table 2. The 19 studies included for meta-analysis were published from 2006 to January of 2022. The number of patients included ranged from 3 to 20, and patient ages ranged from 13 to 41 years old. A total of 201 patients were included across all studies.
Table 2.
Study characteristics of the 19 studies examined in this meta-analysis
| First author (publication year) | Study location | No. of Pts | Age range of Pts | Mean age (± SD) | Donor characteristics | Surgical technique |
|---|---|---|---|---|---|---|
| Abir (2017) | Tel Aviv, Israel | 9 | 13–31 | N/A | Postpubertal woman prior to anticancer therapy | Laparoscopy |
| Amorim (2012) | Brussels, Belgium | 7 | 30–41 | N/A | Benign gynecologic conditions | Laparoscopy |
| Borras (2022) | Barcelona, Spain | 18 | 20–40 | 26.6 ± 5.5 | Transgender | Gender affirming surgery including hysterectomy and bilateral adnexectomy |
| Chang (2011) | Seoul, South Korea | 11 | 20–41 | 31.9 | Benign cysts or cesarean section | Laparoscopy |
| Fabbri (2016) | Bologna, Italy | 6 | 14–34 | 24.5 ± 9.3 | Hodgkin lymphoma, breast cancer, brain tumor, and medulloblastoma | Laparoscopy |
| Galbinski (2022) | Brazil | 12 | N/A | 34.6 ± 3.2 | Not specified | Laparoscopy |
| Gandolfi (2006) | Milan, Italy | 3 | 26–33 | N/A | Monolateral endometrioma | Laparoscopy |
| Herraiz (2014) | Valencia, Spain | 8 | 18–37 | 27 | Breast cancer and Hodgkin lymphoma | Not specified |
| Keros (2009) | Stockholm, Sweden | 20 | 28–43 | 33.3 ± 4.0 | Planned cesarean sections | Not specified |
| Lee (2019) | Seoul, South Korea | 19 | 15–32 | N/A | Not specified | Not specified |
| Li (2007) | Guangzhou, China | 15 | 22–37 | 33.1 ± 2.9 | Benign ovarian cysts | Laparoscopy or open |
| Oktem (2011) | Turkey | 15 | N/A | 32.2 ± 2.8 | Benign ovarian cyst | Laparoscopy |
| Ramos (2021) | Brazil | 9 | 29–39 | 34.6 ± 3 | Unspecified gynecological conditions | Laparoscopy |
| Sanfillipo (2015) | Clermont-Ferrand, France | 5 | N/A | 28.0 ± 1.1 | Benign ovarian cyst | Laparoscopy |
| Sugishita (2021) | New Haven, Connecticut, USA | 5 | N/A | 31 ± 6.62 | Cadavers | Not specified |
| Wang (2008) | Sichuan, China | 5 | 21–37 | N/A | Endometrial cancer, cervical cancer, and breast cancer | Oophorectomy or ovarian biopsy |
| Xiao (2010) | Sichuan, China | 10 | 21–36 | 30 (median) | Not specified | Oophorectomy or ovarian cystectomy |
| Xiao (2013) | Sichuan, China | 6 | 21–36 | N/A | Not specified | Oophorectomy or ovarian cystectomy |
| Zhao (2019) | Zhengzhou City, China | 18 | N/A | 37.9 ± 6.4 | Not specified | Laparoscopy or open |
The average quality assessment score for the 19 studies was 6.65 (Supplementary Table 1). One of the nine questions in the Quality Assessment Tool for Case Series Studies was not applicable for the studies included in this analysis. Study scores ranged from 5 to a maximum of 8. All studies included clearly stated objectives, methods including statistics, and results.
Proportion of intact primordial follicles
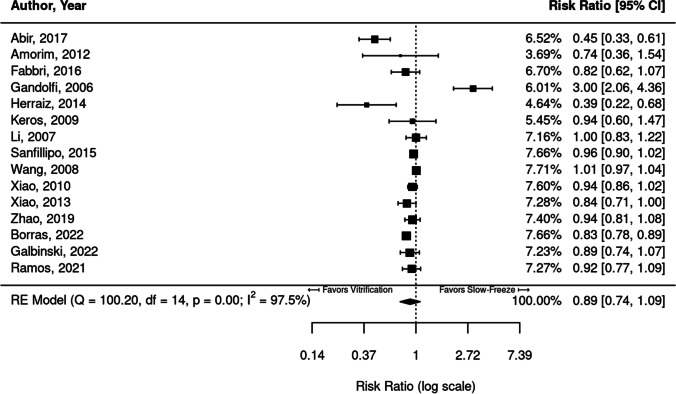
Fifteen studies were identified that reported the proportion (or number) of intact primordial follicles when comparing slow freezing to vitrification in ovarian tissue cells, shown in Fig. 2.
Fig. 2.
Meta-analysis of fifteen studies comparing the proportion of intact primordial follicles between vitrified and slow-frozen human ovarian tissue. Data leaning to the left of the dotted line are those that favor vitrification, i.e., have higher proportions of intact primordial follicles
Of the fifteen studies included in the meta-analysis for proportion of intact primordial follicles, two studies [33, 34] found significantly higher proportions of intact primordial follicles in vitrified tissue compared to slow-frozen tissue (RR = 0.45 [95% CI, 0.33, 0.61], 0.39 [95% CI, 0.22, 0.68]). One study [23] demonstrated higher proportions of intact primordial follicles in slow-frozen tissue (RR = 3.00; 95% CI, 2.06, 4.36). The remaining nine studies found no significant difference between freezing protocols.
Substantial heterogeneity was identified in this cohort of studies (I2 = 97.5%). When pooled together, there was no significant difference in proportion of primordial follicles between vitrification and slow freezing (RR = 0.89; 95% CI, 0.74,1.09).
Statistically greater proportions of intact primordial follicles were observed in fresh tissue compared to both vitrification (RR = 1.37; 95% CI, 1.07, 1.75) and slow-freeze (RR = 1.50; 95% CI, 1.22, 1.84) protocols.
Proportion of intact stromal cells
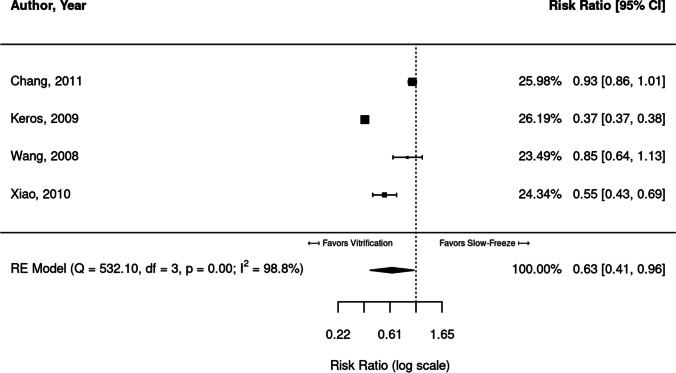
Four studies reported and compared proportions of intact stromal cells in slow freezing versus vitrification (Fig. 3).
Fig. 3.
Meta-analysis of four studies comparing the proportion of intact stromal cells in vitrified versus slow-frozen human ovarian tissue. Data leaning to the left of the dotted line are those that favor vitrification, i.e., have higher proportions of intact stromal cells
Of the four studies, two [19, 35] found significantly higher proportions of intact stromal cells in vitrified ovarian tissue (RR = 0.37 [CI, 0.37, 0.38] and 0.55 [CI, 0.43, 069]). The remaining two studies [18, 26] showed a similar trend, though not significant. Very high heterogeneity was identified between the four studies (I2 = 98.8%). The combined meta-analysis of the four studies found significantly higher proportions of intact stromal cells in vitrified tissue (RR = 0.63; 95% CI, 0.41, 0.96).
Statistically greater proportions of intact stromal cells were observed in fresh tissue compared to both vitrification (RR = 1.59; 95% CI, 1.19, 2.11) and slow-freeze (RR = 2.50; 95% CI, 2.08,3.00) protocols.
Proportion of DNA fragmentation
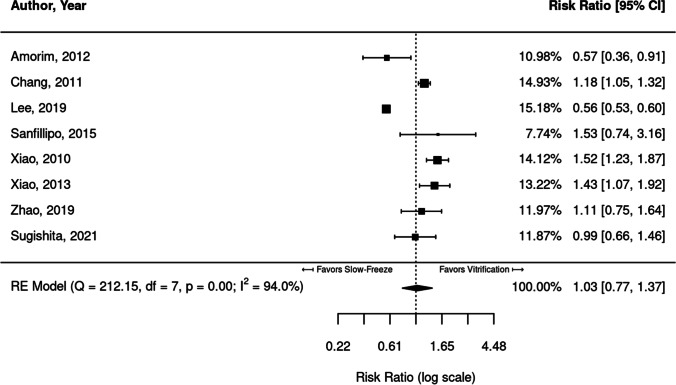
Eight studies reported proportions of DNA fragmentation as an outcome in the comparison of slow freezing versus vitrification. These eight studies are compared in Fig. 4.
Fig. 4.
Meta-analysis of eight studies comparing DNA fragmentation in vitrified versus slow-frozen human ovarian tissue. Data leaning to the right of the dotted line are those that favor vitrification, i.e., have lower proportions of DNA fragmentation
Three studies [18, 27, 35] showed significantly lower DNA fragmentation in vitrified cells (RR = 1.18, 1.52, 1.43), which is more favorable for vitrification. Two studies [36, 37] favored slow freezing and had significantly lower DNA fragmentation in slow-frozen cells (RR = 0.57; 95% CI, 0.36, 0.91). The remaining three studies [38–40] trended were not significantly different between slow-frozen and vitrified cells. Substantial heterogeneity was identified between studies (I2 = 94.0%), and DNA fragmentation did not significantly differ between vitrification and slow freezing methods when the studies were pooled (RR = 1.03; 95% CI, 0.77, 1.37).
In seven of the eight studies, DNA fragmentation data in fresh tissue were available. Fresh tissue demonstrated statistically less DNA fragmentation than both vitrification (RR = 0.41; 95% CI, 0.21, 0.82) and slow-freeze (RR = 0.36; 95% CI, 0.20, 0.66) protocols.
Mean primordial follicle density
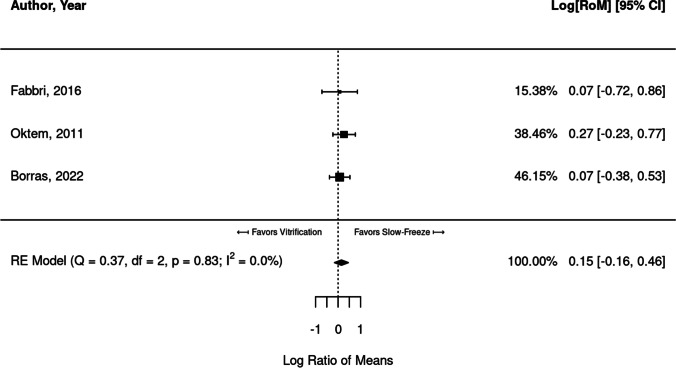
Six studies reported mean primordial follicle density when comparing vitrification and slow freezing. Of these, three studies [23, 24, 41] provided enough data to compare studies through a meta-analysis. When both freezing protocols were compared, a significant difference in follicular density was not identified upon pooling the three studies (RR = 0.15; 95% CI, − 0.16, 0.46) (Fig. 5).
Fig. 5.
Meta-analysis of three studies comparing follicular density between vitrified and slow-frozen human ovarian tissue. Data leaning to the left of the dotted line are those that favor vitrification, i.e., have higher proportions of follicular density
Note that Oktem et al. purports to report mean + / − SD, from which we extracted the corresponding summary statistics for analysis. However, they also note “The mean number of primordial follicles (number/mm2) in fresh and slow-frozen strips were comparable (1.95 ± 0.2 vs. 1.27 ± 0.1, P > 0.05).” Thus, it is highly suspicious that the SD is being reported, since these would yield very narrow distributions that would almost assuredly yield a significant result (easy to demonstrate via simulation). It is rather very likely the authors are actually reporting the standard error (SE) of the mean, which would be SE = SD/N − − √. We adjusted the reported SD values from Oktem et al. accordingly. This did not appreciably change the results.
Estradiol levels
Three studies reported estradiol levels, and the data reported were not sufficient for a meta-analysis. Klocke et al. [22] did not find a significant difference in estradiol secretion between vitrified and slow-frozen ovarian tissue. Similarly, Oktem et al. [24] did not find a significant difference in estradiol levels between fresh, slow-frozen, and vitrified tissue, though they found the highest levels in fresh tissue after 3 days in culture. However, Herraiz et al. [42] found higher estradiol secretion in slow-frozen ovarian tissue than in vitrified tissue.
Discussion
The objective of this meta-analysis was to evaluate slow freezing versus vitrification as cryopreservation methods for ovarian tissue. We evaluated the two conventional ovarian tissue cryopreservation methods by proxy of four primary tissue-specific histological and biochemical outcome measures, including (1) proportion of intact primordial follicles, (2) proportion of intact stromal cells, (3) proportion of DNA fragmentation in primordial follicles, and (4) mean primordial follicle density. In addition, we also reported on estradiol production as a secondary outcome measure. The present meta-analysis of 19 studies revealed a significantly greater proportion of intact stromal cells in vitrified tissue versus slow-frozen tissue. However, no significant differences upon pooled analyses were observed between the two cryopreservation methods with respect to the proportion of intact primordial follicles, proportion of DNA fragmentation, or mean primordial follicle density. Both cryopreservation protocols led to significantly inferior histological outcomes compared to those observed in fresh tissue. Pooled analyses of studies reporting on estradiol production were not possible due to statistical considerations.
While primordial follicles (defined as the first class of follicles, which includes a flattened layer of granulosa cells) have been shown to be the most prevalent follicle type (> 90%) [43], their integrity may not fully reflect the efficacy of cryopreservation in maintaining the morphology of ovarian tissue, considering that the ovary is composed of multiple cell types that may play a supportive role in fertilization. For example, the ovarian stroma contains cellular components of the immune system, extracellular matrix, and blood vessels and has been shown to promote growth of immature follicles [44, 45]. Thus, the integrity of stromal cells following ovarian tissue cryopreservation has emerged as a key primary outcome. Interestingly, the ovarian stroma has been shown to be more prone to cryopreservation-mediated damage in comparison to primordial follicles [46]. Due to the use of cryoprotectants and dehydration of cells, vitrification prevents ice crystals from forming [28, 47]. Both penetrating and non-penetrating cryoprotectants are used during vitrification, therefore minimizing overall physical damage to cells. This may help to explain the results of this meta-analysis, which identified higher stromal cell viability in vitrified ovarian tissue. Additional modifications can be made to vitrification protocols to increase cell viability, which are recommended to be explored further. Ovarian follicular apoptosis has also been observed in response to OTC via evaluation by measuring DNA fragmentation using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) assay [48]. Therefore, a comprehensive approach utilizing multiple primary outcomes was used in this meta-analysis to compare the effects of slow freezing versus vitrification on ovarian tissue. While this study has found some benefit to using vitrification over slow freezing, there are several key practical differences between the two methods that may prove to be beneficial in a lab. For instance, slow freezing has a low potential of contamination with pathogenic agents and takes less skill to conduct and manipulate [15]. Further, while slow freezing uses less cryoprotectant agents and therefore is more susceptible to ice crystal formation, this characteristic provides less risk of toxicity due to cryoprotectant agents [15]. However, slow freezing inherently is a much longer process than vitrification (3 h or more vs. less than 10 min) and is more expensive due to the freezing machine required for the method [15]. Ultimately, while vitrification has some benefit in stromal cell viability, labs may choose to use one method over another due to any number of logistical reasons.
While individual studies reported significant differences between slow freezing and vitrification, substantial heterogeneity (I2 > 50) between studies hindered the pooled statistical analyses. For example, while 2/11 studies looking at the proportion of intact primordial follicles reported a significantly greater proportion of intact primordial follicles in vitrified ovarian tissue compared to slow-frozen tissue, and 7/11 studies showed a similar trend, the pooled analysis did not reveal a significant difference between the two cryopreservation methods (Fig. 2). Of note, there was substantial heterogeneity in this pooled analysis (I2 = 98.0%). Similarly, with respect to proportion of DNA fragmentation, 3/6 studies showed significantly lower DNA fragmentation in vitrified tissue, and 2/6 studies showed a similar but insignificant trend. However, upon pooled analysis, no significant difference between slow freezing and vitrification was observed (Fig. 4) (I2 = 79.8%).
Broadly speaking, heterogeneity in these outcomes between studies can be attributed to variations in slow freezing and vitrification protocols. These differences can include the method by which ovarian tissue is sectioned, the size of ovarian tissue section, the cryoprotectant(s) used for vitrification, the order in which these cryoprotectants are added, the cooling rates and temperature gradient for slow freezing, and the thawing methods utilized [49, 50]. In addition, some studies in this meta-analysis evaluated outcomes using thawed tissue following cryopreservation, while others evaluated outcomes using tissue that was thawed and grafted into mice; this further limited our ability to accurately compare outcomes between studies. Of note, the comparison between vitrification and slow freezing protocols in preserving the ovarian tissue was not the primary aim of several studies examined in this meta-analysis, which further contributed to the substantial heterogeneity observed. Finally, heterogeneity exists on both the patient-level and cellular-level, which may contribute to the heterogeneity seen in this meta-analysis. Thus, while the meta-analysis comparing the proportion of intact stromal cells statistically favored vitrification versus slow freezing, the other characteristics such as the proportion of intact primordial follicles, proportion of DNA fragmentation in primordial follicles, and mean primordial follicle density did not reveal any statistical difference between the two protocols, likely due in part to the substantial heterogeneity observed.
In comparison to previous meta-analyses by Shi et al. and Zhou et al., the present meta-analysis is the largest meta-analyses to date with 201 patients across 19 studies [28, 29]. The meta-analysis by Zhou et al. only evaluated the proportion of intact primordial follicles and included six studies — of which five are included in the present study. Our pooled analysis led to the same finding as Zhou et al. that slow freezing and vitrification do not significantly differ in outcomes. These findings are also concordant with the findings of Shi et al. However, while Shi et al. reported significantly greater DNA fragmentation in slow-frozen tissue on pooled analysis across six studies, we did not see a significant difference between slow freezing and vitrification upon pooled analysis across six studies — of which three are included in the meta-analysis by Shi et al. With respect to the proportion of intact stromal cells, we evaluated the same set of four studies discussed by Shi et al., which led to the same finding that vitrified tissue contains a significantly greater proportion of intact stromal cells.
Overall, while this meta-analysis is the most comprehensive meta-analysis to date in this subject with respect to the number of studies included and the primary outcomes discussed, the analysis was limited by substantial heterogeneity between studies, the absence of one or more primary outcomes in each of the studies examined, and statistical considerations including the lack of availability of data required for pooled analysis. Future studies comparing vitrification versus slow freezing of human tissue as a primary aim should use standardized vitrification and slow freezing protocols to reduce heterogeneity and confounding variables. Additionally, given the complexity of human ovarian tissue and the multiple cell types present in this tissue, future studies should evaluate tissue using all four outcome measures discussed in this meta-analysis. This will enable larger meta-analyses with a greater number of studies and patients to be conducted and will further reduce heterogeneity.
Supplementary information
Below is the link to the electronic supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supriya Behl and Vidhu B. Joshi are co-first authors in the making of this manuscript.
References
- 1.Marin L, et al. History, evolution and current state of ovarian tissue auto-transplantation with cryopreserved tissue: a successful translational research journey from 1999 to 2020. Reprod Sci. 2020;27(4):955–962. doi: 10.1007/s43032-019-00066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J. Introduction: fertility preservation, from cancer to benign disease to social reasons: the challenge of the present decade. Fertil Steril. 2013;99(6):1467–1468. doi: 10.1016/j.fertnstert.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 6.Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: a review. Transfus Med Hemother. 2019;46(3):173–181. doi: 10.1159/000499054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yding-Andersen C, Mamsen LS, Kristensen SG. Fertility preservation: freezing of ovarian tissue and clinical opportunities. Reproduction. 2019;158(5):F27–F34. doi: 10.1530/REP-18-0635. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth AJ, Allyse M, Khan Z. Fertility preservation for transgender individuals: a review. Mayo Clin Proc. 2020;95(4):784–792. doi: 10.1016/j.mayocp.2019.10.040. [DOI] [PubMed] [Google Scholar]
- 9.De Roo C, et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online. 2017;34(6):557–566. doi: 10.1016/j.rbmo.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Schneider F, et al. Andrology of male-to-female transsexuals: influence of cross-sex hormone therapy on testicular function. Andrology. 2017;5(5):873–880. doi: 10.1111/andr.12405. [DOI] [PubMed] [Google Scholar]
- 11.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnez J, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, et al. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33(6):741–746. doi: 10.1007/s10815-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oktay K, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 15.Jang TH, et al. Cryopreservation and its clinical applications. Integr Med Res. 2017;6(1):12–18. doi: 10.1016/j.imr.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rienzi L, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AbdelHafez FF, et al. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20(2):209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Chang HJ, et al. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011;37(8):1092–1101. doi: 10.1111/j.1447-0756.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 19.Keros V, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi G, et al. Apoptosis in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Cryo Lett. 2009;30(4):300–309. [PubMed] [Google Scholar]
- 21.Dolmans MM, et al. In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? Front Endocrinol (Lausanne) 2019;10:520. doi: 10.3389/fendo.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klocke S, et al. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291(2):419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 23.Fabbri R, et al. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod. 2016;31(8):1838–1849. doi: 10.1093/humrep/dew134. [DOI] [PubMed] [Google Scholar]
- 24.Oktem O, et al. Vitrified human ovaries have fewer primordial follicles and produce less antimullerian hormone than slow-frozen ovaries. Fertil Steril. 2011;95(8):2661–2664.e1. doi: 10.1016/j.fertnstert.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 25.Wang TR, et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum Reprod. 2016;31(4):763–773. doi: 10.1093/humrep/dew005. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23(10):2256–2265. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Z, et al. In vitro culture thawed human ovarian tissue: NIV versus slow freezing method. Cryo Lett. 2013;34(5):520–526. [PubMed] [Google Scholar]
- 28.Shi Q, et al. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep. 2017;7(1):8538. doi: 10.1038/s41598-017-09005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou XH, et al. Comparison of vitrification and conventional slow freezing for cryopreservation of ovarian tissue with respect to the number of intact primordial follicles: a meta-analysis. Medicine (Baltimore) 2016;95(39):e4095. doi: 10.1097/MD.0000000000004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Practice Committee of the American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institutes of Health: National Heart, L., and Blood Institute. Quality assessment tool for case series studies. [cited 2021; Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed June 2021
- 33.Herraiz S, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101(3):775–784.e1. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Abir R, et al. Attempts to improve human ovarian transplantation outcomes of needle-immersed vitrification and slow-freezing by host and graft treatments. J Assist Reprod Genet. 2017;34(5):633–644. doi: 10.1007/s10815-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Z, et al. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94(6):2323–2328. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Amorim CA, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98(5):1291–8. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, et al. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci. 2019;20(13):3346. doi: 10.3390/ijms20133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanfilippo S, et al. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing. Reprod Biol Endocrinol. 2015;13:67. doi: 10.1186/s12958-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, et al. Vitrification freezing of large ovarian tissue in the human body. J Ovarian Res. 2019;12(1):77. doi: 10.1186/s13048-019-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugishita Y, et al. Comparison of open and a novel closed vitrification system with slow freezing for human ovarian tissue cryopreservation. J Assist Reprod Genet. 2021;38(10):2723–2733. doi: 10.1007/s10815-021-02297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borras A, et al. Comparison between slow freezing and vitrification of ovarian tissue cryopreservation in assigned female at birth transgender people receiving testosterone therapy: data on histological and viability parameters. J Assist Reprod Genet. 2022;39(2):527–541. doi: 10.1007/s10815-021-02386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herraiz S, et al. Optimizing ovarian tissue quality before cryopreservation: comparing outcomes of three decortication methods on stromal and follicular viability. Fertil Steril. 2020;113(3):609–617.e3. doi: 10.1016/j.fertnstert.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt KL, et al. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18(6):1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 44.Tingen CM, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141(6):809–820. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, et al. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62(5):1218–1223. doi: 10.1095/biolreprod62.5.1218. [DOI] [PubMed] [Google Scholar]
- 46.Kim SS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82(3):679–685. doi: 10.1016/j.fertnstert.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Chang CC, Shapiro DB, Nagy ZP. The effects of vitrification on oocyte quality. Biol Reprod. 2022;106(2):316–327. doi: 10.1093/biolre/ioab239. [DOI] [PubMed] [Google Scholar]
- 48.Rimon E, et al. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27(2):345–353. doi: 10.3892/ijo.27.2.345. [DOI] [PubMed] [Google Scholar]
- 49.Amorim CA, et al. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23(2):160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Wallberg KA, Oktay K. Recent advances in oocyte and ovarian tissue cryopreservation and transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):391–405. doi: 10.1016/j.bpobgyn.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.