Abstract
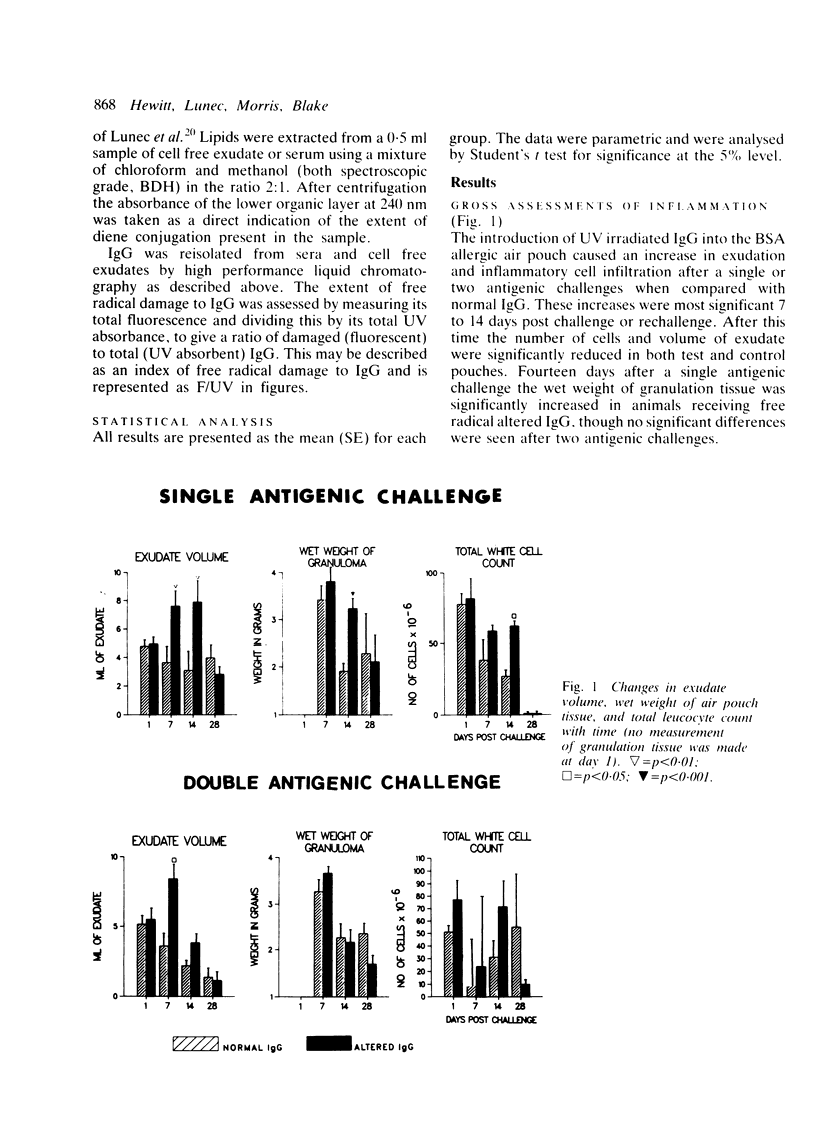
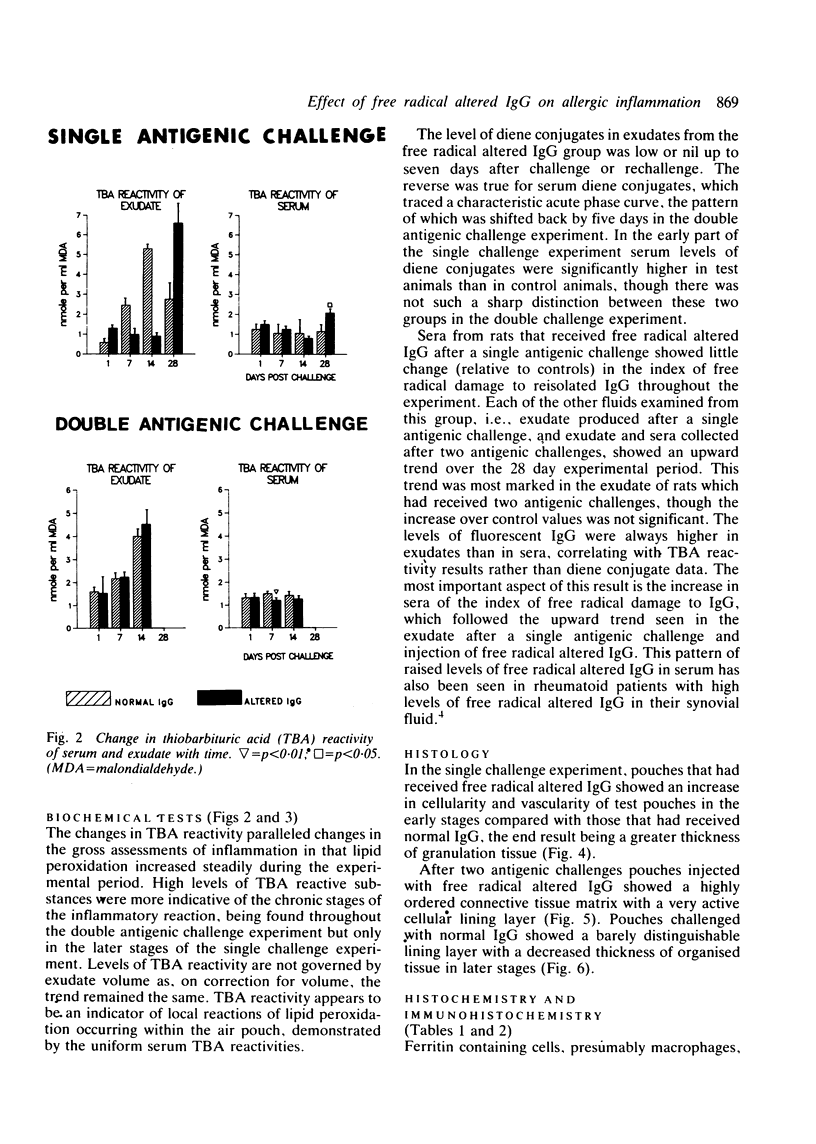
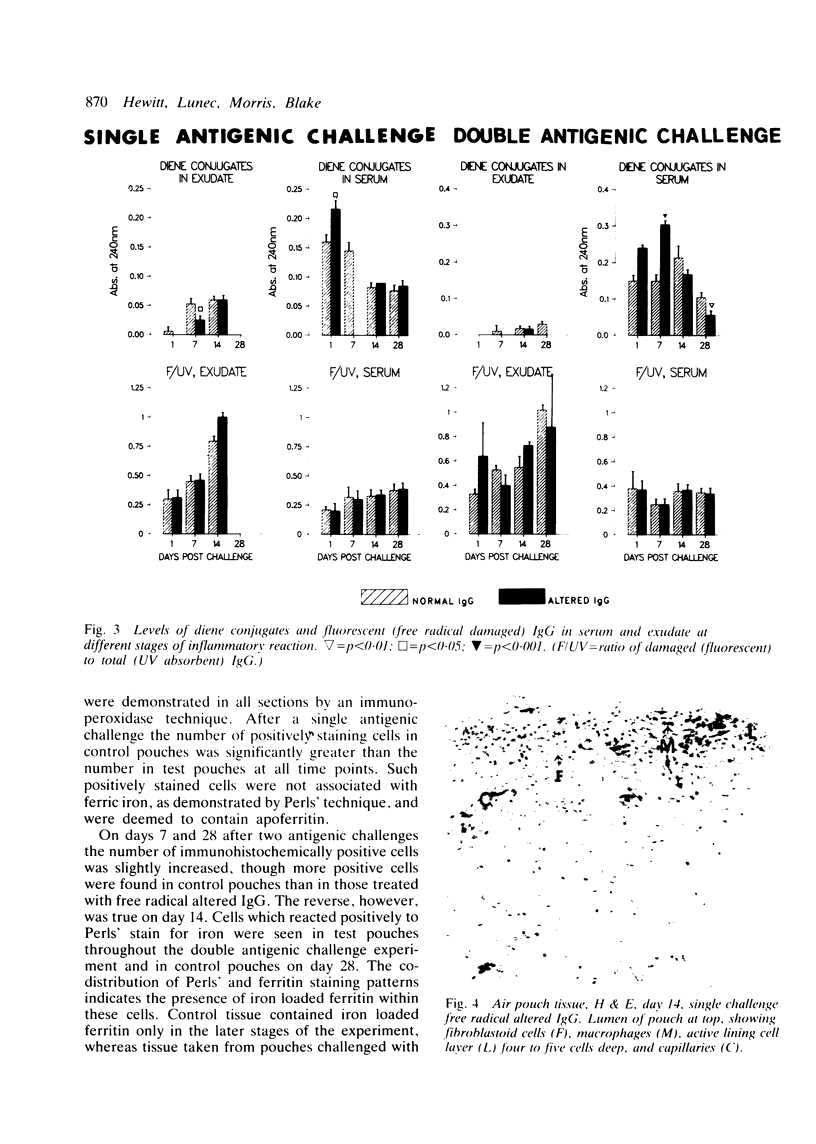
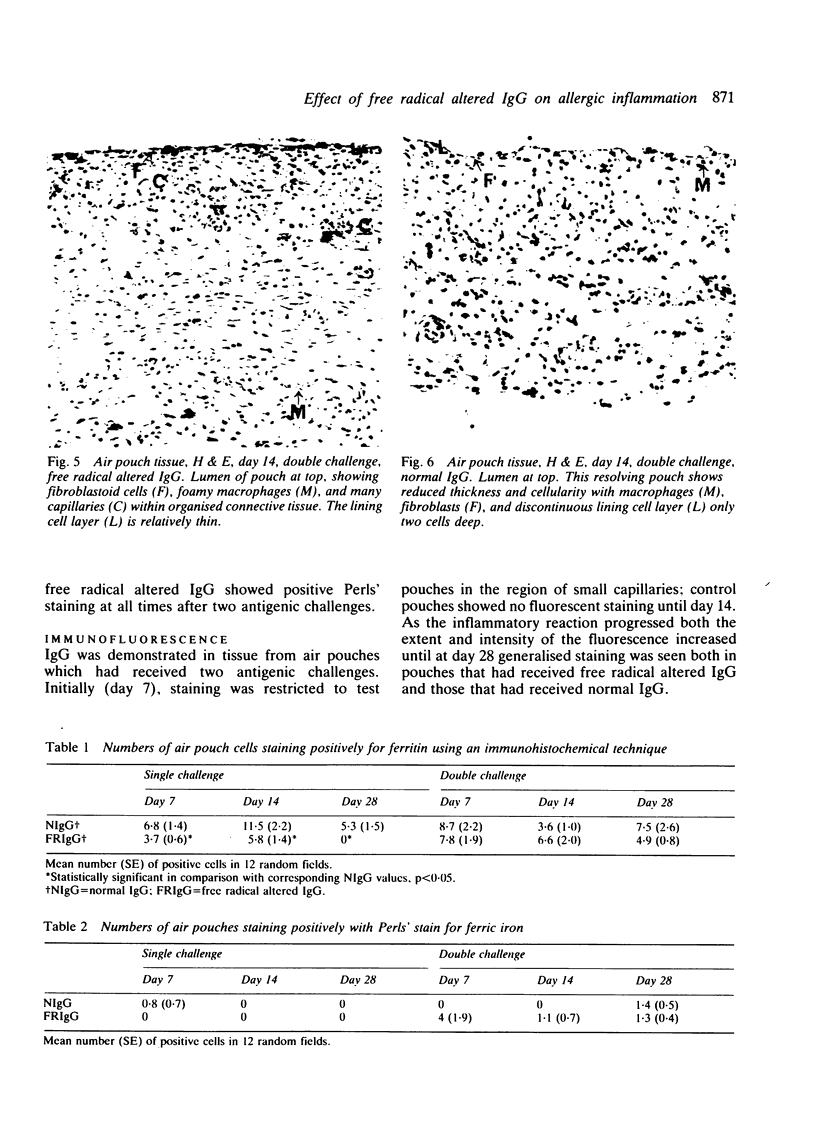
The rheumatoid synovium is capable of producing large amounts of IgG which may become modified by the actions of free radicals. A rat model of synovitis was established and challenged with both normal and free radical altered IgG. IgG was prepared from homologous pooled serum by high performance liquid chromatography, and free radical damage was induced by 15 minutes ultraviolet (UV) irradiation. The results showed a worsening in gross assessments of inflammation, increases in biochemical indices of lipid peroxidation, and also a rise in the proportion of IgG which, on reisolation, showed the characteristic fluorescence associated with free radical damage. This demonstrated how the presence of free radical altered IgG might convert an inflammatory insult to a more persistent stimulus, and the capacity of an environment subjected to continuing antigenic stimulation to induce further free radical damage to IgG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco N. E., Dobkin L. W., Schur P. H. Immunological properties of isolated IgG and IgM anti-gamma-globulins (rheumatoid factors). Clin Exp Immunol. 1974 May;17(1):91–101. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad K. Presence of aggregated gamma-G-globulin in certain rheumatoid synovial effusions. Clin Exp Immunol. 1967 Jul;2(4):511–529. [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M., Watkins J., Wolborow E. J. Antiglobulin production to altered IgG in rheumatoid arthritis. Lancet. 1975 Mar 15;1(7907):611–614. doi: 10.1016/s0140-6736(75)91888-7. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNKEL H. G., MULLER-EBERHARD H. J., FUDENBERG H. H., TOMASI T. B. Gamma globulin complexes in rheumatoid arthritis and certain other conditions. J Clin Invest. 1961 Jan;40:117–129. doi: 10.1172/JCI104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Morris C. J., Wainwright A. C., Steven M. M., Blake D. R. The nature of iron deposits in haemophilic synovitis. An immunohistochemical, ultrastructural and X-ray microanalytical study. Virchows Arch A Pathol Anat Histopathol. 1984;404(1):75–85. doi: 10.1007/BF00704252. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Yoshino S., Kurihara A., Yoshimura H., Ishiguro M., Kiso S., Tsurufuji S. Delayed-type hypersensitivity as revealed on the footpads of mice to azobenzenearsonate-acetyl bovine serum albumin. Int Arch Allergy Appl Immunol. 1981;66(4):391–403. doi: 10.1159/000232848. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson A. J., Hollander J. L., Quismorio F. P., Abelson N. M. Experimental arthritis in man and rabbit dependent upon serum anti-immunoglobulin factors. Ann N Y Acad Sci. 1969 Dec 10;168(1):188–194. doi: 10.1111/j.1749-6632.1969.tb43107.x. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978 Nov 15;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaka K., O'Brien P. J. Generation of activated oxygen species by polymorphonuclear leukocytes. FEBS Lett. 1980 Feb 11;110(2):283–286. doi: 10.1016/0014-5793(80)80093-7. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Gabig T. G., Babior B. M. Evidence for production of oxidizing radicals by the particulate O-2-forming system from human neutrophils. Blood. 1979 Apr;53(4):666–676. [PubMed] [Google Scholar]
- Tsurufuji S., Yoshino S., Ohuchi K. Induction of an allergic air-pouch inflammation in rats. Int Arch Allergy Appl Immunol. 1982;69(3):189–198. doi: 10.1159/000233170. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Agnello V., Kunkel H. G. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970 May;6(5):689–706. [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Bacon P. A., Blake D. R., Scott D. L., Wainwright A. C., Walton K. W. A model of persistent antigen-induced chronic inflammation in the rat air pouch. Br J Exp Pathol. 1984 Apr;65(2):201–214. [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Blake D. R., Hewitt S., Morris C., Bacon P. A. Effect of blood on the activity and persistence of antigen induced inflammation in the rat air pouch. Ann Rheum Dis. 1985 Jul;44(7):485–490. doi: 10.1136/ard.44.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]





