Abstract
Purpose
Approximately 95% of patients undergoing radiotherapy (RT) experience radiation dermatitis (RD). Evidence has suggested that photobiomodulation therapy (PBMT) can stimulate skin renewal and minimize RD. The aim of the present paper was to investigate the efficacy of PBMT in RD prevention through a comprehensive literature review.
Methods
A literature search of Ovid MEDLINE, Embase, and Cochrane databases was conducted from 1980 to March 2021 to identify RCT on the use of PBMT for RD prevention. Forest plots were developed using RevMan software to quantitatively compare data between studies.
Results
Five papers were identified: four in breast and one in head and neck cancer patients. Patients receiving PBMT experienced less severe RD than the control groups after 40 Gray (Gy) of RT (grade 3 toxicity: Odds Ratio (OR): 0.57, 95% CI 0.14–2.22, p = 0.42) and at the end of RT (grade 0 + 1 vs. 2 + 3 toxicity: OR: 0.28, 95% CI 0.15–0.53, p < 0.0001). RT interruptions due to RD severity were more frequent in the control group (OR: 0.81, 95% CI 0.10–6.58, p = 0.85).
Conclusion
Preventive PBMT may be protective against the development of severe grades of RD and reduce the frequency of RT interruptions. Larger sample sizes and other cancer sites at-risk of RD should be evaluated in future studies to confirm the true efficacy of PBMT, also in preventing the onset of RD and to finalize a standardized protocol to optimize the technique. At present, starting PBMT when RT starts is recommendable, as well as performing 2 to 3 laser sessions weekly.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-023-07673-y.
Keywords: Photobiomodulation therapy, Radiotherapy, Acute radiation dermatitis, Prevention, Laser therapy
Introduction
Radiation dermatitis (RD) is a prevalent reaction that arises as a side effect of radiotherapy (RT), affecting approximately 95% of patients undergoing RT [1, 2]. RD occurs when RT causes disruptions in normal skin regeneration and cell division, which consequently leads to cell damage or death [3]. Acute RD typically occurs between 30 and 90 days of radiation exposure and manifests as erythema, hyperpigmentation, dry desquamation, moist desquamation and ulceration [4].
In breast cancer (BC) patients, roughly 90% and 30% of patients have historically developed grade 1 and 2 reactions, respectively [5]. Recent advances in RT techniques have significantly lowered the severity of RD, with the incidence after hypo-fractionated breast RT being approximately 38% [6]. In head and neck cancer (HNC) patients, mild reactions develop in virtually all subjects, but more severe and dose-limiting toxicities happen in almost a fifth of irradiated subjects [7]. Nonetheless, RD can profoundly affect patients’ health-related quality of life (HRQoL) and limit the treatment duration and dose delivered, so it is important to manage or prevent RD and continue research.
Currently, there is no consensus on the optimal strategy to prevent or manage RD. Recommendations include skin cleansing, hydration, photoprotection, and general practices, such as wearing loose cotton clothing and avoiding actions that may irritate the skin [5]. The use of photobiomodulation therapy (PBMT), originally defined as “low-level laser therapy”, consists of the application of low-power light sources in the visible and infra-red spectrum to favour healing and pain decrease, to contrast inflammation and offer antimicrobial properties [8].
The use of PBMT in oncology patients has increased because it can be applied to different areas of the body and can be used for different side effects [9]. In addition to its high adherence and feasibility, symptom management through PBMT can improve nutritional status, HRQoL, and survivorship [10–12].
Given the potential benefits of PBMT in minimizing skin toxicity and associated symptoms, this systematic review and meta-analysis aimed to review randomized controlled trials (RCT) that examined the role of PBMT in preventing RD in patients undergoing RT treatment.
Materials and methods
A detailed description of the methodology for this paper will be described in a separate publication by Behroozian et al. To summarize, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct a literature search of the following databases: OVID MEDLINE, Embase, and Cochrane from 1946 to September 2020. An initial literature search was conducted from 1946 to September 2020 as part of the development of the Multinational Association of Supportive Care in Cancer (MASCC) Guidelines for Radiation Dermatitis Prevention and Management. This search was updated to identify additional studies published until March 2021. A PubMed search used keywords “PBMT” and “Radiodermatitis”. Studies met the criteria for inclusion in this review if they (1) consisted of RCTs that examined the efficacy of an intervention in RD prevention, and (2) investigated PBMT versus a placebo, standard of care, or no intervention. If the studies met these criteria and reported quantitative outcomes that could be compared between studies, they were included in the review and meta-analysis.
ReviewManager (RevMan) 5.4 software was used to quantitatively compare the studies through forest plots. To evaluate the overall quality of the studies in the meta-analysis, the Cochrane Risk of Bias (RoB) tool was used [13], and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria was used to assess the quality of evidence of each study [14]. Two independent reviewers assessed the RoB of each trial (V.R. and M.G.). Inconsistencies were solved by means of discussion and consensus, and in cases of disagreement, a third reviewer was consulted (G.N.M.). The methodological quality of evidence was assessed using the Hadorn criteria [15].
Results
Literature search results
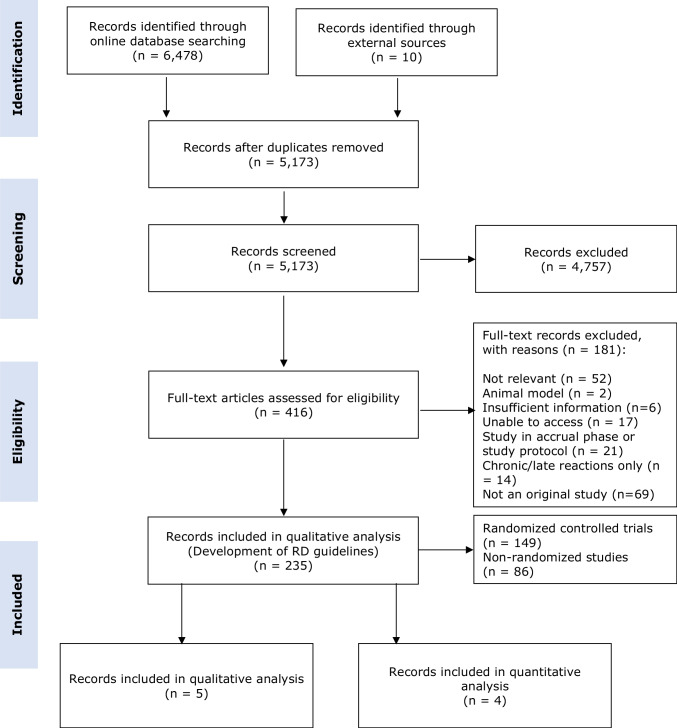
There were 5,173 articles identified in the initial literature search of the five databases. Of these, five studies were identified on PBMT [16–20], four of which were included in the quantitative analysis [16–19] (Fig. 1).
Fig. 1.
Prisma diagram
Study characteristics
Detailed characteristics of all included studies have been summarized in Table 1. The studies included were published between 2010 and 2021. Four of the studies evaluated BC patients [16–18, 20] and one evaluated HNC patients [19]. All patients included in these studies were enrolled prior beginning RT [16–20].
Table 1.
Study characteristics
| Reference | Sample Size | Type of study | Experimental Arm (n) | Standard Arm (n) | Cancer Site | Timing of administration | Methods used to assess RD and HRQol | GRADE Certainty of Evidence | Methodological Quality of Evidence | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Fife et al. 2010 |
18 PBMT 15 SHAM |
RCT Double blinded |
PBMT + Topical treatment | Sham + Topical treatment | Breast | Immediately before and after each RT. Upon completion of RT, seven additional daily treatments for 2 weeks | NCI 5-point scale for grading skin reactions | II |
Minor flaws (statistical analysis) Major flaws (allocation to study groups) |
Some Concerns |
| Robijns et al. 2018 SCC |
60 PBMT 60 SHAM |
RCT Double blinded |
PBMT + Topical treatment | Sham + Topical treatment | Breast | 14 sessions in total, delivered biweekly from the first until the last day of RT over a period of 7 weeks |
RTOG/EORTC TEWL,HYDRATION, MELANIN, ERYTHEMA |
I | No flaws | Some Concerns |
| Robijns et al. 2019 TRANSDERMIS |
60 PBMT 60 SHAM |
RCT Double blinded |
PBMT + Topical treatment | Sham + Topical treatment | Breast | 14 sessions in total, delivered biweekly from the first until the last day of RT over a period of 7 weeks |
RTOG/EORTC RISRAS, SKINDEX (HRqol) |
I | No flaws | Some Concerns |
|
Robijns et al. 2022 LABRA |
39 PBMT 32 SHAM |
RCT Double blinded |
PBMT + Topical treatment | Placebo + Topical treatment | Breast | 8 sessions in total, delivered biweekly, from the first day of RT | RTOG/EORTC | II | Minor flaws | Some Concerns |
| Robijns et al. 2021 DERMISHEAD |
28 PBMT 18 SHAM |
RCT Double blinded |
PBMT + Topical treatment | Sham + Topical treatment | Head and Neck | 14 sessions in total, delivered biweekly from the first until the last day of RT over a period of 7 weeks |
NCI-CTCAE v4.03 RISRAS, SKINDEX (HRqol) |
II | Minor flaws | Some Concerns |
RCT Randomized Controlled Trial; PBMT photobiomodulation, RD Radiodermatitis; RT Radiotherapy; NCI National Cancer Institute, TEWL Transeoedermal Water Loss; HRQoL Health-Related Quality of Life
Risk of bias (Rob) and certainty of bias (GRADE) in individual studies
An overall value of “some concern”, due to 100% “low” rating on domains 1,3, and 4, and “some concern” rating on domains 2 and 5 for all the studies was obtained. As for GRADE, no flaws or minor flaws were detected, while major flaws were identified in the Fife et al. (2010) study regarding allocation concealment [18].
Individual study outcomes
All primary and secondary study outcomes have been listed in Table 2. Fife et al. (2010) compared the use of PBMT with sham therapy (i.e. using the same protocol in controls, but with an inactivated laser source) [18]. Patients in both groups were recommended to apply Aquaphor (Beirsdorf Inc, Wilton, CT) 3–4 times/day throughout the study. Skin reactions were monitored at baseline, weekly during RT, at the completion of RT, and two and six weeks after the completion of RT using the National Cancer Institute (NCI) 5-point scale for grading skin reactions by a blinded dermatologist. Authors observed no statistically significant differences in the incidence of RD, discomfort, pain, convenience, or satisfaction with treatment between the two groups (p > 0.05) [18].
Table 2.
Summary of outcomes
| Outcome | Fife et al. (2010) PBMT vs Sham (number of patients/whole sample) |
Robijns et al. (2018 SCC) PBMT vs SHAM (number of patients/whole sample) |
Robijns et al. (2019) TRANSDERMIS PBMT vs SHAM (number of patients/whole sample) |
Robijns et al. (2022) LABRA PBMT vs SHAM (number of patients/whole sample) |
Robijns et al. (2021) DERMISHEAD PBMT vs SHAM (number of patients/whole sample) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary Outcome: Incidence of RD | Grade 0 |
PBMT 0/18 SHAM 1/15 |
PBMT 0/60 (40Gy) SHAM 0/60 (40Gy) |
PBMT 0/60 (66Gy) SHAM 0/60 (66Gy) |
NA |
PBMT 34/39 (1 WEEK RT) SHAM 27/32 (1 WEEK RT) |
PBMT 19/39 (2 WEEKS RT) SHAM 10/32 (2 WEEKS RT) |
PBMT 0/39 (3 WEEKS RT) SHAM 0/32 (3 WEEKS RT) |
PBMT 1/28 (40Gy) SHAM 2/18 (40Gy) |
PBMT 0/28 (60Gy) SHAM 0/18 (66Gy) |
| Grade 1 |
PBMT 6/18 SHAM 4/15 |
PBMT 3/60 (40Gy) SHAM 1/60 (40Gy) |
PBMT 56/60 (66Gy) SHAM 42/60 (66Gy) |
NA |
PBMT 5/39 (1 WEEK RT) SHAM 5/32 (1 WEEK RT) |
PBMT 20/39 (2 WEEKS RT) SHAM 22/32 (2 WEEKS RT) |
PBMT 39/39 (3 WEEKS RT) SHAM 30/32 (3 WEEKS RT) |
PBMT 24/28 (40Gy) SHAM 12/18 (40Gy) |
PBMT 20/28 (60Gy) SHAM 4/18 (66Gy) |
|
|
Grade 2 *2A+2B |
PBMT 12/18 SHAM 9/15 |
PBMT 54/60 (40Gy) SHAM 55/60 (40Gy) |
PBMT 4/60 (66Gy) SHAM 16/60 (66Gy) |
NA |
*PBMT 0/39 (1 WEEK RT) *SHAM 0/32 (1 WEEK RT) |
*PBMT 0/39 (2 WEEKS RT) *SHAM 0/32 (2 WEEKS RT) |
*PBMT 0/39 (3 WEEKS RT) *SHAM 2/32 (3 WEEKS RT) |
PBMT 3/28 (40Gy) SHAM 3/18 (40Gy) |
PBMT 8/28 (66Gy) SHAM 11/18 (66Gy) |
|
| Grade 3 |
PBMT 0/18 SHAM 1/15 |
PBMT 3/60 (40Gy) SHAM 4/60 (40Gy) |
PBMT 0/60 (66Gy) SHAM 2/60 (66Gy) |
NA |
PBMT 0/39 (1 WEEK RT) SHAM 0/32 (1 WEEK RT) |
PBMT 0/39 (2 WEEKS RT) SHAM 0/32 (2 WEEKS RT) |
PBMT 0/39 (3 WEEKS RT) SHAM 0/32 (3 WEEKS RT) |
PBMT 0/28 (40Gy) SHAM 1/18 (40Gy) |
PBMT 0/28 (66Gy) SHAM 3/18 (66Gy) |
|
| Secondary Outcome: Interruption RT |
PBMT 2/18 SHAM 1/15 |
PBMT 0/60 SHAM 0/60 |
NA |
PBMT 0/39 SHAM 0/32 |
PBMT 0/28 SHAM 0/18 |
|||||
| Secondary Outcome: Interruption of Study |
PBMT 0/18 SHAM 0/15 |
PBMT 9/69 SHAM 10/70 |
NA |
PBMT 5/44 SHAM 10/42 |
PBMT 5/33 SHAM 14/32 |
|||||
| Secondary Outcome: Adverse Events |
PBMT 0/18 SHAM 0/15 |
NA |
PBMT 0/60 SHAM 0/60 |
PBMT 0/39 SHAM 0/32 |
PBMT 0/28 SHAM 0/18 |
|||||
| Objective Measures | ERYTHEMA 40 Gy (mean ± SD) | NE |
PBMT: 81.05 ± 54.03 SHAM:81.42 ± 51.85 |
NA | NA | NA | NA | NA | NA | |
| ERYTHEMA 60 Gy (mean ± SD) | NA |
PBMT: 94.82 ± 53.97 SHAM:121.91 ± 63.12 |
NA | NA | NA | NA | NA | NA | ||
| TEWL 40GY (mean ± SD) | NA |
PBMT: -6.43 ± 28.73 SHAM: 5.35 ± 37.52 |
NA | NA | NA | NA | NA | NA | ||
| TEWL 66Gy (mean ± SD) | NA |
PBMT: -6.43 ± 28.73 SHAM: 5.35 ± 37.52 |
NA | NA | NA | NA | NA | NA | ||
| HYDRATION 40Gy (mean ± SD) | NA |
PBMT: -10.3 ± 17.89 SHAM: -3.21 ± 19.21 |
NA | NA | NA | NA | NA | NA | ||
| HYDRATION 66 Gy (mean ± SD) | NA |
PBMT: -23.61 ± 22.04 SHAM: -17.52 ± 18.69 |
NA | NA | NA | NA | NA | NA | ||
| MELANIN 40Gy (mean ± SD) | NA |
PBMT: 12.66 ± 30.88 SHAM: 9.73 ± 35.58 |
NA | NA | NA | NA | NA | NA | ||
| MELANIN 66Gy (mean ± SD) | NA |
PBMT: 76.96 ± 78.46 SHAM: 105.29 ± 99.71 |
NA | NA | NA | NA | NA | NA | ||
| RISRAS 40 Gy (mean ± SD) | NA | NA |
PBMT: 5.15 ± 3.86 SHAM:5.25 ± 3.53 |
NA | NA | NA |
PBMT(40Gy) 4,38 ± 2,67/28 SHAM (40Gy) 5,56 ± 4,11/18 |
PBMT (66Gy) 4,38 ± 2,67/28 SHAM: 5,56 ± 4,11/18 | ||
| RISRAS 60 Gy (mean ± SD) | NA | NA |
PBMT: 5.85 ± 3.80 SHAM:6.90 ± 3.70 |
NA | NA | NA | PBMT (40Gy) 6,81 ± 2,30/28 SHAM (40Gy) 8,36 ± 4,52/18 | PBMT (66Gy) 4,38 ± 2,67/28 SHAM (66Gy) 5,56 ± 4,11/18 | ||
| SKINDEX 40 Gy (mean ± SD) | NA | NA |
PBMT: 23.5 ± 19.7 SHAM:21.5 ± 19.6 |
NA | NA | NA | PBMT (40Gy) 4,38 ± 2,67/28 SHAM: 5,56 ± 4,11/18 | PBMT (66Gy) 4,38 ± 2,67/28 SHAM: 5,56 ± 4,11/18 | ||
| SKINDEX 60 Gy (mean ± SD) | NA | NA |
PBMT: 18.37 ± 18.3 SHAM:20.14 ± 24.0 |
NA | NA | NA | PBMT (40Gy) 4,38 ± 2,67/28 SHAM (40Gy) 5,56 ± 4,11/18 | PBMT (66Gy) 4,38 ± 2,67/28 SHAM: 5,56 ± 4,11/18 | ||
PBMT photobiomodulation, RD radiodermatitis; RT Radiotherapy; NA Not applicable (it refers to the fact that both studies by Robijns et al were on the same cohort of patients and data are reported for one of them or to study were the outcome was not evaluated)
Robijns et al. (2018) compared the combination of PBMT and topical skin care with sham and topical skin care alone [16]. In addition to general skin care practices (e.g., wearing loose fitting clothing, gentle washing with or without mild soap, patting dry with a soft towel instead of rubbing), patients were instructed to apply a hydroactive colloid gel (Flamigel®, Flen Pharma, Kontich, Belgium) on the irradiated zone (3 times/day), starting at the first day of RT, and a foam, absorbent, self-adhesive silicone dressing (Mepilex®, Mölnlycke Health Care, Gothenburg, Sweden) in case of painful skin reactions and/or moist desquamation. Authors used the RTOG/EORTC scales after 40 Gray (Gy) and 66 Gy, and evaluations were performed by two experienced nurses. The study was blinded, with only the laser operator aware of the patients’ allocation. Moreover, authors objectively assessed skin reactions by measuring transepidermal water loss (TEWL) and skin hydration levels after 40 Gy and 66 Gy RT using Tewameter® TM300 (Courage-Khazaka, Cologne, Germany) and the Corneometer® (Courage-Khazaka, Cologne, Germany). Reflectance spectrophotometry was used to measure skin erythema and melanin using the Mexameter® MX18 (Courage-Khazaka, Cologne, Germany). Erythema and melanin indexes were significantly higher in the control group (at baseline p = 0.016 and p = 0.019, respectively). The skin hydration level was significantly lower at the 40 Gy RT dose in the PBMT group (p = 0.036), but both groups had similar skin moisture index at the end of RT. The TEWL index decreased in both groups at 40 Gy and was significantly lower in the PBMT group than in the control group at the end of RT (p = 0.05) [16].
The same authors, one year later, evaluated the efficacy of PBMT in the same cohort, using two separate patient-reported scales: Radiation Induced Skin Reaction Assessment Scale (RISRAS) (to assess RD severity) and the Skindex-16 (to assess HRQoL) [17]. All the data were collected at the beginning of RT, after 40 Gy, and after 66 Gy. The RISRAS score (patient component) and Skindex-16 symptoms and functioning subscales (higher score, lower HRQoL) decreased more prominently in the PBMT group. They increased or remained stable in the control group, giving an overall decrease in the total Skindex-16 score in the PBMT group and a constant trend among controls. The RISRAS score (researcher component) had a more pronounced increase in the control group (p = 0.04) over the course of RT, despite no significant differences between the two groups at 40 Gy (p = 0.0562). In both cases, evaluators were blinded [17].
In 2021, Robijns et al. published an additional prospective, multi-center RCT comparing PBMT with standard skin care alone (following the same protocol of previous trials) in post‐lumpectomy BC patients undergoing hypo-fractionated whole breast irradiation (HF‐WBI) (LABRA trial) [20]. Seventy-one patients were included in the final evaluation, of which 39 were in the control group and 32 were in the PBMT group. PBMT was applied from the first until the last day of RT (two sessions/week, eight in total) by a trained operator using the class IV MLS® M6 laser (ASA Srl). Patients’ skin reactions were evaluated by the modified version of the RTOG criteria by two blinded, experienced nurses, in the first, second, and third weeks, and at the end of RT. Overall, after the first two weeks of treatment, half of the PBMT subjects (n = 16) had not developed acute RD (ARD), while 30% (n = 10) of controls did (p = 0.13). At week three, 94% (n = 30) of controls and all PBM subjects experienced at least grade 1 RD; 6% (n = 2) of controls already experienced grade 2 reactions (p = 0.11). At the end of RT, less patients developed ≥ 2 grades of ARD in the PBMT group, although this difference was not statistically significant and the authors concluded that PBMT was not effective[20].
Robijns et al. (2021) also conducted a study on PBMT use in HNC patients, evaluating skin toxicity and HRQoL [19]. PBMT was given to patients from the first day of RT until the last day of RT, two times per week, in 14 sessions, by a trained operator using the class IV MLS M6 laser (ASA Srl, Vicenza, Italy) and compared to controls who received sham therapy. Standard skin care, as described in the previous studies, was provided to both groups. Two different grading systems were used to score the severity of the skin reactions. The NCI-CTCAE v4.03 and the RISRAS. All the measurements were collected on the first day of RT, at an RT dose of 40 Gy, and on the last day of RT (60–70 Gy). At a dose of 40 Gy, there was no significant difference in skin toxicity between the control and PBMT groups (p = 0.57). Towards the end of RT, the number of severe skin reactions (grade 2–3) increased significantly (p = 0.01) in the controls. On the contrary, in the PBMT group, the development of ARD remained stable (p = 0.21). As such, there was a significant difference in skin toxicity between the two groups at the end of RT. Significantly more patients (77.8%) had grade 2 or 3 toxicity in the control group versus the PBMT group (28.6%) (p = 0.002). The patients in the PBMT group reported non-statistically significant lower median RISRAS scores than the control group at 40 Gy and at the end of RT. No significant differences were detected in patients’ HRQoL between the two groups at 40 Gy and at the end of RT, although a trend toward lower HRQoL in controls was seen. Despite the limited power of the study due to scarce adherence to the protocol, the authors concluded that PBMT significantly reduces the severity of ARD. Thus, they hypothesized that an improvement in HRQoL may be demonstrated with an increased sample size [19]. Across all studies, adverse events other than RD were not reported.
Laser parameters
Characteristics of individual PBMT protocols have been reported in Table 3. With the exception of the study by Fife et al. (2010), all included studies originated from the same authors, so treatment protocols were highly comparable [16, 17, 19, 20].
Table 3.
Protocol characteristics
| Paper | Type brand | Laser protocol | Sessions | Control group |
|---|---|---|---|---|
|
Fife et al. (2010) PBMT vs Sham (number of patients/whole sample) |
Gentlewaves Select 590-nm high-energy LED array |
2 cm distance 35 seconds Pulses 250 ms on and 100 ms off for 100 pulses |
Whole duration of RT, plus seven additional daily treatments | Sham treatments, (the button was not pressed to deliver the light) |
|
Robijns et al. (2018 SCC) PBMT vs SHAM (number of patients/whole sample) |
Class IV Diode MLS.M6 laser (ASA Srl, Vicenza, Italy) |
Simultaneous 905 nm pulsed mode (peak radiant power 25W, duty cycle of 50 %) and continuous mode at 808 nm (average radiant power 3.3 W, aperture diameter 5 cm, beam spot size at target 19.625 cm2, power density at target 0.168 W/ cm2). Energy density 4 J/cm2. The treatment time varied according to the to-be-treated surface area | 14 sessions of PBMT (2/week), starting at the first day of RT | Sham treatments in which the laser device was switched off, but still made the same sound as an active laser |
|
Robijns et al. (2019) TRANSDERMIS PBMT vs SHAM (number of patients/whole sample) |
# | § | 14 sessions of PBMT (2/week), starting at the first day of RT | * |
|
Robijns et al (2021) LABRA PBMT vs SHAM (number of patients/whole sample) |
# | § | From the first until the last day of RT (2 sessions/week, 8 sessions in total) by a trained operator | |
|
Robijns et al (2022) DERMISHEAD PBMT vs SHAM (number of patients/whole sample) |
# | § | From the first until the last day of RT (2 sessions/week, 8 sessions in total) by a trained operator |
PBMT Photobiomodulation, #,§,* consider the same data as the row before
Meta-analysis
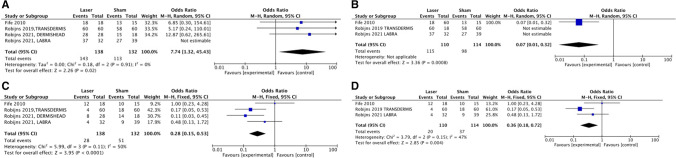
After the systematic review, a meta-analysis was performed on three studies to evaluate RD grades [16, 18, 20] and two studies in the evaluation of RISRAS and Skindex scores [17, 19].
The pooled analysis demonstrates that the incidence of severe RD (grade 3) was significantly lower in the PBMT groups at the end of RT (at 40 Gy OR: 0.57, 95% CI 0.14–2.22, p = 0.42 and at the end of RT OR: 0.15, 95% CI 0.03–0.93, p = 0.04). No significant differences emerged among studies that exclusively evaluated BC patients (Fig. 2). For BC and HNC together, at the end of RT (Fig. 3), results were similar and PBMT was protective against RD in all grades, except for grade 1 (OR: 3.40, 95% CI (1.52–7.53), p = 0.003) (Fig. 2). When considering erythema and edema together with desquamation (grade 2–3), which are the most frequent causes of patients’ discomfort and risk of RT interruption, the protective effect of PBMT over sham was more evident at the end of RT (OR: 0.28, 95% CI 0.15–0.53, p < 0.0001). When comparing studies on BC subjects with studies including both breast and HNC, it was found that PBMT is protective against minor grades or the absence of RD (OR: 0.07, 95% CI 0.01–0.32, p = 0.0008). Study interruptions occurred more frequently in PBMT groups (OR:2.01, 95% CI 0.78–5.19, p = 0.15), with the main reasons for interruptions being cited as “incomplete evaluations” and the COVID-19 pandemic (Appendix 1). PBMT appeared protective against RT interruption, although cases of interruption were limited and no significant difference could be established (OR:0.81, 95% CI 0.10–6.58, p = 0.85) (Appendix 1). The meta-analysis for RISRAS and Skindex-16 scores (Appendix 2) did not provide significant results, but the trend favored PBMT both at 40 Gy and at the end of RT.
Fig. 2.
Forest plots showing comparison for all grades of RD at 40 Gys and at the end of RT. A Grade 0,1,2,3 after 40 Gys including all cancer sites. B Grade 0,1,2,3 after 40 Gys considering only Breast Cancer patients. C Grade 0,1,2,3 at the end of RT including all cancer sites. D Grade 0,1,2,3 at the end of RT considering only Breast Cancer patients
Fig. 3.
Forest plots showing comparison for lower grades (0–1) versus higher grades (2–3) at the end of RT. A Grade 0,1 at the end of RT considering all cancer sites. B Grade 0,1 at the end of RT considering only Breast Cancer patients. C Grade 2,3 at the end of RT considering all cancer sites. D Grade 2,3 at the end of RT considering only Breast Cancer patients
Discussion
The present systematic review showed there is still insufficient support for a comprehensive consensus for the treatment of RD [21].
With the exception of the study by Fife et al. in 2010 [18], all other studies included in the present review were conducted recently [16, 17, 19, 20]. Since the majority of studies used the same laser parameters, protocols, and devices, this allowed for comparison across trials. A recently published meta-analysis on the same topic, including both RCT and non-RCTs, concluded that PBMT may reduce grade 3 RD and pain, although with weak evidence due to methodologically scarce power and low certainty of evidence [22]. We similarly found a protective role of PBMT for higher grades at 40 Gy, while such differences were not evident amongst low grades (0–1). This implies that starting PBMT early during RT may be advantageous for later phases. This is made more evident when we explored results from studies that exclusively examined BC patients, for whom the sample was much bigger than for HNC subjects. As such, increasing the sample size might strengthen our results.
Notably, no patients developed grade 3 RD in the LABRA Trial, which is the only trial that analyzes patients receiving HF‐WBI [20]. Since the standard skin care was the same in other studies by the same authors, and because patients were selected randomly, we hypothesize that the RT protocol played a role in this. In fact, authors declare that using HF‐WBI decreases the number of fractions and overall treatment time but can also decrease treatment direct and indirect costs[20]. Other authors [23, 24] confirm that HF‐WBI is associated with a considerably lower incidence of severe acute skin reactions compared to conventionally fractionated whole breast irradiation. Definitely, RT regimen and its association with other treatments such as chemotherapy should be considered as predictive factors for side effects severity [25]. In the studies included in the present systematic review, authors did not consider the role of exclusive RT versus combined RT/chemotherapy. In fact, Fife et al. and Robijns et al. included only patients receiving RT alone [16–18], whereas patients in the LABRA and DERMISHEAD trials receiving RT and chemotherapy regimens were included [19, 20]. In addition, Fife et al. [18] did not specify the randomization technique, whereas the cohort analyzed in Robijns et al. (2018 & 2019) was stratified by breast volume (small, medium, large) and the LABRA and DERMISHEAD trial used the oncological treatment as criterion to randomize patients [19, 20].
In accordance with previous studies, moist desquamation was more frequent among patients with large breast volume. This is relevant for clinical practice when when deciding whether to apply PBMT with a preventive strategy or when RD is already present. One of the major limitations of PBMT protocols, especially if preventive, is the need for multiple sessions for a long time, which may be demanding for the patients and the operators. Accordingly, given its association with RD, breast size could be could be used as a criterion for the application of PBMT.
Another interesting aspect was the report on the HRQoL of patients. RT frequently causes impaired HRQoL and, especially in HNC patients, hindered functional ability [26]. The evaluation of patient-reported outcomes is becoming increasingly considered by physicians [27]. HRQoL in patients affected by RD following breast RT has been shown to be worse in severe grades and during advanced phases of RT [28]. Some authors even demonstrated that there is a seasonal trend towards minor to major HRQoL impairment due, for example, to reduced covering clothing during summer or spotting due to moist desquamation [29, 30]. Other authors demonstrated that shame or concern regarding the appearance of the irradiated area and the influence of symptoms such as pruritus, increased sensitivity, pain, and burning could heavily affect HRQoL [28]. Therefore, preventing/managing RD is a relevant part of patient care.
In accordance with the DERMISHEAD trial, Song et al.[31] demonstrated that patients receiving RT experience physical and mental stress that may even lead to refuse treatment [19]. Authors recognize that HNC patients have poor physical and psychosocial health even at baseline and that the PBMT protocol put additional demand on an already burdensome period. If compared to the other studies, the HNC patients reported the lowest adherence to the treatment protocol [28, 32]. Another hypothesis put forward by Robijns et al. [19] and corroborated by other authors [33], is that low adherence may also be related to the absence of skin problems at the start of the trial. This is mainly of importance if in future studies prevention of mild to moderate RD is proven. Until then, it seems that PBMT can be initiated at the onset of RD.
In the first RCT trial by Robijns et al., they demonstrated through an objective approach that the preventive application of PBMT is effective in reducing the incidence of moist desquamation in BC patients [16]. They innovatively used biophysical skin measurements to objectively demonstrate that PBMT is able to stabilize the degree of pigmentation (both erythema and melanin) and improve the skin barrier function during RT. Objective skin measurement was further investigated by Robijns et al., who evaluated erythema, dehydration, and TEWL, and concluded that PBMT can reduce moist desquamation and severe grades of RD, but not completely prevent it [19].Other authors have applied a similar approach demonstrating that biophysical quantitative measurements could detect the fine effects of irradiation even at a very early stage. This process seems to be mediated by the presence of mild inflammation, as in the case of surgical therapies prior to RT, leading to the conclusion that skin temperature is the most sensitive index for acute RD [34]. This type of evaluation aims at combining qualitative and quantitative aspects of RD grading and prevention [35]. Although our meta-analysis did not provide significant results in objective/subjective RD evaluation, the trend is in favor of PBMT, suggesting that including studies with larger samples would strengthen the power of the results. The introduction of a comprehensive evaluation of patients affected by or at risk of RD is helpful and recommended for future care.
In past decades [36], the use of PBMT has been used in various medical fields and is currently widely applied in oncological patients with side effects [37], oral mucositis in primis. [38]. Additionally, xerostomia, dysgeusia, lymphedema, pain, trismus, alopecia, neuropathy and RD can be managed with PBMT, sharing the same mechanism of action [39–43]. In general, molecular and cellular research has suggested that PBMT has biostimulating properties that allow tissue to regenerate and heal faster [44].
The use of PBMT as a tool to prevent and manage RD has been primarily investigated in BC patients [16–18, 20], while few observational studies have investigated the use of PBMT in the cervical and facial sites, despite these cancers being associated with significant pain, disfigurement, risk of RT interruption, and poor cancer prognosis [45]. Animal and clinical studies suggest that PBMT has analgesic properties, reduces inflammation, reduces fibrosis and appears to not cause side effects [5].
Another important aspect to discuss about PBMT protocols is the parameters used. In the included studies, red or infra-red wavelength were employed, whose efficacy is supported in animal studies [46]. The most promising results obtained with 905 nm protocols, as were seen in the studies by Robijns et al., had insufficient statistical power to suggest one parameter over the other, and this may be seen as a limitation of the present review. One of the goals of recent studies on PBMT in supportive care of cancer patients is the quality assessment of laser protocols and dosimetry for specific conditions, since different parameters can determine different light-tissue interaction effects [47]. Of note, none of the studies disclosed the ethnic backgrounds of the patient population. Skin pigmentation may severely affect the biological effect of PBMT since decreased chromophores’ adsorption may result in insufficient or inhibitory effects; as such, the therapy should be modulated according to skin color [48]. An interesting advantage over the studies conducted by Robijns et al. is the use of PBMT to specifically treat RD, whereas many studies on PBMT describe a single technique to be used for various side effects [49]. The correct timing, number of repetitions, and specific protocols for each therapeutic indication are fundamental for obtaining the effective PBMT dose window [50].
Regarding the frequency and timing of PBMT sessions for RD, recent literature confirmed the appropriateness of two to four sessions per week in preventive protocols and that LED treatments immediately after RT reduces the incidence of dermatitis in BC patients. As such, this timeframe should be considered and corroborates the approach employed in the included studies [51, 52].
With regards to other parameters, a recent position paper from the World Association for Photobiomodulation Therapy (WALT) suggested a power density (treatment surface irradiance) of 10–150 mW/cm2 for a total dose of 1 Einstein (photon fluence at 810 nm = 4.5pJ/cm2) treatment field performed within 30 to 120 min prior to oncotherapy to treat RD and a dose 2 Einstein (photon fluence at 810 nm = 9pJ/cm2) per treatment field three to four times per week, for at least five to six weeks, in case of subcutaneous inflammation associated with RD [53]. Although these parameters are in line with the studies analyzed in our review, data are still insufficient to transform these suggestions into guidelines.
One notable advantage of PBMT is the absence of side effects and its role in reducing the necessity of RT interruptions, which may indirectly affect prognosis. We have demonstrated a reduced number of RT interruptions in patients receiving PBMT, although the number of subjects who withdrew from RT due to RD severity was minimal and our confidence intervals were wide. Nonetheless, other studies have demonstrated that RT interruption due to RD severity may be reduced by PBMT administration. In fact, more than 65% of controls interrupted RT, while only 5.3% of PBMT treated subjects interrupted RT in a study by De Land et al. [54]. Similarly, significantly more interruptions were detected in controls rather in PBMT groups by Gouvêa de Lima et al. [55], declaring six versus zero interruptions, respectively (p = 0.02).
This work is not free of limitations, like the exiguous number of patients, further contributed by a loss to follow up or consent withdrawal. Moreover, only two body districts have been considered and only one paper dealt with HNC patients, making it difficult to draw conclusions on this specific area.
Conclusion
The results suggest that PBMT may be effective in preventing grades 2 and 3 RD. The optimal protocol cannot yet be established since most of the studies were performed by the same authors, and while this can ensure comparability, it cannot guarantee widespread consensus. There are benefits to the incorporation of both subjective and objective tools for RD assessment, especially when considering HRQoL, but studies with larger cohorts are needed to confirm efficacy.
Supplementary Information
Below is the link to the electronic supplementary material.
(PNG 216 kb)
(PNG 325 kb)
Acknowledgements
The authors would like to thank the Oncodermatology Study Group of the Multinational Association of Supportive Care in Cancer for their interest and support of this study.
M.G. and V.R. are joint first authors. P.B., E.C., and T.B. are joint senior authors.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M.G., V.R., S.C., and T.B. H.L. performed the literature search. The first draft of the manuscript was written by V.R. and M.G., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan RJ, Webster J, Chung B, et al. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:1–19. doi: 10.1186/1471-2407-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porock D. Factors influencing the severity of radiation skin and oral mucosal reactions: Development of a conceptual framework. Eur J Cancer Care (Engl) 2002;11:33–43. doi: 10.1046/j.1365-2354.2002.00287.x. [DOI] [PubMed] [Google Scholar]
- 3.Seité S, Bensadoun RJ, Mazer JM. Prevention and treatment of acute and chronic radiodermatitis. Breast Cancer Targets Ther. 2017;9:551–557. doi: 10.2147/BCTT.S149752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kole AJ, Kole L, Moran MS. Acute radiation dermatitis in breast cancer patients: Challenges and solutions. Breast Cancer Targets Ther. 2017;9:313–323. doi: 10.2147/BCTT.S109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng G, Cassileth BR. Skin injury: acute dermatitis and chronic skin changes supportive care and quality of life. 5. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 6.de Jesus Martelletti LBS, de Aguiar BRL, Vieira LAC, et al. Incidence of acute radiodermatitis in women with breast cancer undergoing hypofractionated radiotherapy. Rev Bras Enferm. 2021;75:e20210118. doi: 10.1590/0034-7167-2021-0118. [DOI] [PubMed] [Google Scholar]
- 7.Bonomo P, Talamonti C, Desideri I, et al. Analysis of skin dose distribution for the prediction of severe radiation dermatitis in head and neck squamous cell carcinoma patients treated with concurrent chemo-radiotherapy. Head Neck. 2020;42:244–253. doi: 10.1002/hed.25997. [DOI] [PubMed] [Google Scholar]
- 8.Bensadoun RJ. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol. 2018;30:226–232. doi: 10.1097/CCO.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 9.Gobbo M, Ottaviani G, Rupel K, et al. Same strategy for pitfalls of radiotherapy in different anatomical districts. Lasers Med Sci. 2016;31:471–479. doi: 10.1007/s10103-015-1857-8. [DOI] [PubMed] [Google Scholar]
- 10.Gobbo M, Ottaviani G, Perinetti G, et al. Evaluation of nutritional status in head and neck radio-treated patients affected by oral mucositis: Efficacy of class IV laser therapy. Support Care Cancer. 2014;22:1851–1856. doi: 10.1007/s00520-014-2155-x. [DOI] [PubMed] [Google Scholar]
- 11.Morais MO, Martins AFL, de Jesus APG, et al. A prospective study on oral adverse effects in head and neck cancer patients submitted to a preventive oral care protocol. Support Care Cancer. 2020;28:4263–4273. doi: 10.1007/s00520-019-05283-1. [DOI] [PubMed] [Google Scholar]
- 12.Legouté F, Bensadoun RJ, Seegers V, et al. Low-level laser therapy in treatment of chemoradiotherapy-induced mucositis in head and neck cancer: Results of a randomised, triple blind, multicentre phase III trial. Radiat Oncol. 2019;14:83. doi: 10.1186/s13014-019-1292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed]
- 14.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ Clin Res. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadorn DC, Baker D, Hodges JS, Hicks N. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol. 1996;49:749–754. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 16.Robijns J, Censabella S, Claes S, et al. Prevention of acute radiodermatitis by photobiomodulation: A randomized, placebo-controlled trial in breast cancer patients (TRANSDERMIS trial) Lasers Surg Med. 2018;50:763–771. doi: 10.1002/lsm.22804. [DOI] [PubMed] [Google Scholar]
- 17.Robijns J, Censabella S, Claes S, et al. Biophysical skin measurements to evaluate the effectiveness of photobiomodulation therapy in the prevention of acute radiation dermatitis in breast cancer patients. Support Care Cancer. 2019;27:1245–1254. doi: 10.1007/s00520-018-4487-4. [DOI] [PubMed] [Google Scholar]
- 18.Fife D, Rayhan DJ, Behnam S, et al. A randomized, controlled, double-blind study of light emitting diode photomodulation for the prevention of radiation dermatitis in patients with breast cancer. Dermatol Surg. 2010;36:1921–1927. doi: 10.1111/j.1524-4725.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- 19.Robijns J, Lodewijckx J, Claes S, et al. Photobiomodulation therapy for the prevention of acute radiation dermatitis in head and neck cancer patients (DERMISHEAD trial) Radiother Oncol. 2021;158:268–275. doi: 10.1016/j.radonc.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Robijns J, Lodewijckx J, Puts S, et al. Photobiomodulation therapy for the prevention of acute radiation dermatitis in breast cancer patients undergoing hypofractioned whole-breast irradiation (LABRA trial) Lasers Surg Med. 2021;54:374–383. doi: 10.1002/lsm.23475. [DOI] [PubMed] [Google Scholar]
- 21.Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2002;15:216–224. doi: 10.1097/00129334-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 22.de Aguiar BRL, Guerra ENS, Normando AGC, et al. Effectiveness of photobiomodulation therapy in radiation dermatitis: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;162:103349. doi: 10.1016/j.critrevonc.2021.103349. [DOI] [PubMed] [Google Scholar]
- 23.Jagsi R, Griffith KA, Boike TP, et al. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015;1:918–930. doi: 10.1001/jamaoncol.2015.2590. [DOI] [PubMed] [Google Scholar]
- 24.Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20:352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 25.Koutcher LD, Wolden S, Lee N. Severe radiation dermatitis in patients with locally advanced head and neck cancer treated with concurrent radiation and cetuximab. Am J Clin Oncol Cancer Clin Trials. 2009;32:472–476. doi: 10.1097/COC.0b013e318193125c. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Bruner DW, Paul S, et al. A network analysis of self-reported psychoneurological symptoms in patients with head and neck cancer undergoing intensity-modulated radiotherapy. Cancer. 2022 doi: 10.1002/cncr.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingargiola R, De Santis MC, Iacovelli NA, et al. A monocentric, open-label randomized standard-of-care controlled study of XONRID®, a medical device for the prevention and treatment of radiation-induced dermatitis in breast and head and neck cancer patients. Radiat Oncol. 2020;15:193. doi: 10.1186/s13014-020-01633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Andrade Fuzissaki M, Paiva CE, de Oliveira MA, et al. The impact of radiodermatitis on breast cancer patients’ quality of life during radiotherapy: A prospective cohort study. J Pain Symptom Manage. 2019;58:92–99.e1. doi: 10.1016/j.jpainsymman.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Borm KJ, Vennekate JK, Vagedes J, et al. A comprehensive prospective comparison of acute skin toxicity after hypofractionated and normofractionated radiation therapy in breast cancer. Cancers Basel. 2021;13:5826. doi: 10.3390/cancers13225826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rzepecki A, Birnbaum M, Ohri N, et al. Characterizing the effects of radiation dermatitis on quality of life: A prospective survey-based study. J Am Acad Dermatol. 2022;86:161–163. doi: 10.1016/j.jaad.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Zhang R, Yao T, et al. Effect of team health education on radiodermatitis in patients with head and neck tumor radiotherapy under the joint committee international standards. Semin Oncol Nurs. 2021;37:151148. doi: 10.1016/j.soncn.2021.151148. [DOI] [PubMed] [Google Scholar]
- 32.Ringash J, Bernstein LJ, Devins G, et al. Head and neck cancer survivorship: learning the needs, meeting the needs. Semin Radiat Oncol. 2018;28:64–74. doi: 10.1016/j.semradonc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Perlow HK, Ramey SJ, Kwon D, et al. Disparities in adherence to head and neck cancer follow-up guidelines. Laryngoscope. 2019;129:2303–2308. doi: 10.1002/lary.27676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekine H, Kijima Y, Kobayashi M, et al. Non-invasive quantitative measures of qualitative grading effectiveness as the indices of acute radiation dermatitis in breast cancer patients. Breast Cancer. 2020;27:861–870. doi: 10.1007/s12282-020-01082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi K, Nozawa K, Yamazaki N, et al. Instrumental evaluation sensitively detects subclinical skin changes by the epidermal growth factor receptor inhibitors and risk factors for severe acneiform eruption. J Dermatol. 2019;46:18–25. doi: 10.1111/1346-8138.14691. [DOI] [PubMed] [Google Scholar]
- 36.Mester E, Juhász J, Varga P, Karika G. Lasers in clinical practice. Acta Chir Acad Sci Hung. 1968;9:349–357. [PubMed] [Google Scholar]
- 37.Zecha J, Raber-Durlacher JE, Nair RG, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24:2781–2792. doi: 10.1007/s00520-016-3152-z.Low. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guedes CDCFV, De Freitas Filho SAJ, De FPR, et al. Variation of energy in photobiomodulation for the control of radiotherapy-induced oral mucositis: A clinical study in head and neck cancer patients. Int J Dent. 2018;2018:4579279. doi: 10.1155/2018/4579279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palma LF, Gonnelli FAS, Marcucci M, et al. Impact of low-level laser therapy on hyposalivation, salivary pH, and quality of life in head and neck cancer patients post-radiotherapy. Lasers Med Sci. 2017;32:827–832. doi: 10.1007/s10103-017-2180-3. [DOI] [PubMed] [Google Scholar]
- 40.Elgohary HM, Eladl HM, Soliman AH, Soliman ES. Effects of ultrasound, laser and exercises on temporomandibular joint pain and trismus following head and neck cancer. Ann Rehabil Med. 2018;42:846–853. doi: 10.5535/arm.2018.42.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Mobadder M, Farhat F, El Mobadder W, Nammour S. Photobiomodulation therapy in the treatment of oral mucositis, dysgeusia and oral dryness as side-effects of head and neck radiotherapy in a cancer patient: A case report. Dent J. 2018;6:64. doi: 10.3390/dj6040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin H, Zou Z, Chang H, et al. Photobiomodulation therapy for hair regeneration: A synergetic activation of β-CATENIN in hair follicle stem cells by ROS and paracrine WNTs. Stem Cell Rep. 2021;16:1568–1583. doi: 10.1016/j.stemcr.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodewijckx J, Robijns J, Bensadoun R-J, Mebis J. Photobiomodulation therapy for the management of chemotherapy-induced peripheral neuropathy: An overview. Photobiomodulation Photomed Laser Surg. 2020;38:348–354. doi: 10.1089/photob.2019.4771. [DOI] [PubMed] [Google Scholar]
- 44.Khan I, Tang E, Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep. 2015;5:10581. doi: 10.1038/srep10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensadoun RJ, Bollet MA, Liem X, et al. New photobiomodulation device for prevention and cure of radiotherapy-induced oral mucositis and dermatitis: results of the prospective Safe PBM study. Support Care Cancer. 2022;30:1569–1577. doi: 10.1007/s00520-021-06574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, Byun HJ, Kim HJ, et al. Effect of photobiomodulation therapy on radiodermatitis in a mouse model: an experimental animal study. Lasers Med Sci. 2021;36:843–853. doi: 10.1007/s10103-020-03123-x. [DOI] [PubMed] [Google Scholar]
- 47.Khan I, Arany PR. Dosimetry for photobiomodulation therapy: Response to Sommers et al. Ann Transl Med. 2016;4:1–5. doi: 10.21037/atm.2016.05.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louzeiro GC, da Silveira Teixeira D, Cherubini K, et al. Does laser photobiomodulation prevent hyposalivation in patients undergoing head and neck radiotherapy? A systematic review and meta-analysis of controlled trials. Crit Rev Oncol Hematol. 2020;156:103115. doi: 10.1016/j.critrevonc.2020.103115. [DOI] [PubMed] [Google Scholar]
- 49.Abramoff MMF, Lopes NNF, Lopes LA, et al. Low-level laser therapy in the prevention and treatment of chemotherapy-induced oral mucositis in young patients. Photomed Laser Surg. 2008;26:393–400. doi: 10.1089/pho.2007.2144. [DOI] [PubMed] [Google Scholar]
- 50.Zecha JAEM, Raber-Durlacher JE, Nair RG, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24:271–292. doi: 10.1007/s00520-016-3152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gobbo M, Mergio E, Arany PR, et al. Quality assessment of PBM protocols for oral complications in head and neck cancer patients. Front Oral Health. 2022;3:945718. doi: 10.3389/froh.2022.945718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robijns J, Lodewijckx J, Bensadoun RJ, et al. A narrative review on the use of photobiomodulation therapy for the prevention and management of acute radiodermatitis: proposed mechanisms, current clinical outcomes, and preliminary guidance for clinical studies. Photobiomodulation Photomed Laser Surg. 2020;38:332–339. doi: 10.1089/photob.2019.4761. [DOI] [PubMed] [Google Scholar]
- 53.Robijns J, Nair RG, Lodewijckx J, et al. Photobiomodulation therapy in management of cancer therapy-induced side effects: WALT position paper. Front Oncol. 2022 doi: 10.3389/fonc.2022.927685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLand MM, Weiss RA, McDaniel DH, Geronemus RG. Treatment of radiation-induced dermatitis with light-emitting diode (LED) photomodulation. Lasers Surg Med. 2007;39:164–168. doi: 10.1002/lsm.20455. [DOI] [PubMed] [Google Scholar]
- 55.Gouvêa De Lima A, Villar RC, De Castro G, et al. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: A phase III randomized study. Int J Radiat Oncol Biol Phys. 2012;82:270–275. doi: 10.1016/j.ijrobp.2010.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 216 kb)
(PNG 325 kb)
Data Availability Statement
Not applicable.
Not applicable.