Abstract
Reelin, an extracellular matrix protein with putative antidepressant-like properties, becomes dysregulated by chronic stress. Improvement in cognitive dysfunction and depression-like behavior induced by chronic stress has been reported with both intrahippocampal and intravenous Reelin treatment but the mechanisms responsible are not clear. To determine if treatment with Reelin modifies chronic stress-induced dysfunction in immune organs and whether this relates to behavioral and/or neurochemical outcomes, spleens were collected from both male (n = 62) and female (n = 53) rats treated with daily corticosterone injections for three weeks that received Reelin or vehicle. Reelin was intravenously administered once on the final day of chronic stress, or repeatedly, with weekly treatments throughout chronic stress. Behavior was assessed during the forced swim test and the object-in-place test. Chronic corticosterone caused significant atrophy of the spleen white pulp, but treatment with a single shot of Reelin restored white pulp in both males and females. Repeated Reelin injections also resolved atrophy in females. Correlations were observed between recovery of white pulp atrophy and recovery of behavioral deficits and expression of both Reelin and glutamate receptor 1 in the hippocampus, supporting a role of the peripheral immune system in the recovery of chronic stress-induced behaviors following treatment with Reelin. Our data adds to research indicating Reelin could be a valuable therapeutic target for chronic stress-related disorders including major depression.
Keywords: depression, chronic stress, spleen, immunity, antidepressant, Reelin
Introduction
Immune function is regulated by glucocorticoids, rendering immunity susceptible to chronic stress. Glucocorticoids regulate HPA-axis negative feedback and lymphocyte survival1–5 and chronic stress impacts cortisol rhythms and sensitivity and dysregulates neurochemistry.6–9 Experimentally, rodents exposed to three weeks of corticosterone have neurobiological and behavioral alterations associated with major depression (MD) including unintended weight change, altered social behavior, learning and memory impairment, anxiety, increased despair-like behavior, anhedonia, loss of synapses, dendritic atrophy, and neurotransmitter dysregulation, particularly to GABAergic and glutamatergic systems.9–14 Peripheral inflammation can disrupt blood–brain barrier integrity and influence the transport of proinflammatory cytokines into the brain, affecting microglia.15,16 Microglia dysfunction can disrupt neuronal processes and increase the susceptibility to developing neuroinflammatory disorders including Alzheimer's dementia (AD) (reviewed by Huang et al.17). Although the resolution of mood disturbance is paramount for treating depression, targeting peripheral inflammation is an important therapeutic target to reduce the likelihood of developing psychiatric disorders of neuroinflammation known to present more frequently in individuals with a history of depression.18,19
Reelin, a large extracellular matrix glycoprotein that regulates synaptic plasticity, is dysregulated in numerous neuropsychiatric diseases including MD and AD.20–27 Altered expression and methylation of Reelin in peripheral blood is seen in various disease states.23,28,29 Reduced Reelin expression increases vulnerability to chronic stress-induced despair-like behavior, and chronic stress depletes hippocampal Reelin expression in otherwise healthy wild-type animals.9,30,31 Treatment with exogenous Reelin administered intrahippocampally or intravenously normalizes chronic stress-induced despair-like behavior.32,33 Various regulators of lymphocyte survival and proliferation are influenced by Reelin signaling, including phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin34–36 (reviewed by Zeng and Chi37). PI3K/Akt pathway activation selectively phosphorylates Bcl-2/Bcl-XL-associated death promoter, promoting cell survival by protecting differentiating neurons and T cells from apoptosis.38,39 Although Reelin-mediated regulation of cell survival and apoptosis in the periphery is less understood, homozygous Reeler mutants, which have a 126 kB deletion in both Reelin alleles resulting in absence of Reelin mRNA, show altered splenic T lymphocyte proliferation and down-regulation of immunity-related genes, consistent with a role of Reelin in immunity.40,41 Regulating cell survival and proliferation in lymphatic germinal centers could impact the resolution of chronic stress-induced inflammation to indirectly regulate neuroinflammation.
The spleen is a secondary lymphoid organ involved in innate and adaptive immunity, acting as a blood filter and lymphocyte germinal center, and is affected in various neuropsychiatric diseases (reviewed by Wei et al.42). The spleen is composed of two compartments: red pulp, where macrophages remove pathogens and aging erythrocytes from circulation, and white pulp, B- and T-lymphocyte germinal centers and surrounding marginal zone. Spleen alterations are reported in various models of chronic stress.43–47 Reelin is expressed in blood, is stored, transported, and released by platelets, and a large percentage of the body's total platelet supply are stored in the spleen.48–50 Direct functions of Reelin in the spleen are not understood, however, amyloid β (Aβ), one of the hallmark features of AD, is also stored in platelets and transported to the brain via platelets.51,52 Reelin reduces Aβ toxicity, leading to the hypothesis that stress-induced Reelin depletion affects platelet Aβ synthesis and dispersal into the brain.53,54 Additionally, splenectomy enhances amyloid pathology and cognitive dysfunction in transgenic animal models of AD.55
We hypothesized Reelin would recover glucocorticoid-induced atrophy of the spleen, which may have implications for future prognosis, given depression increases AD risk, both conditions are associated with both inflammation and Reelin dysregulation, and Reelin treatment recovers chronic stress-induced depression-like behavior, spatial memory deficits and neuronal dysregulation.18,19,32 We hypothesized three weeks of corticosterone exposure would induce splenic white pulp atrophy, as seen in various models of chronic stress, and that Reelin would reduce chronic stress-induced white pulp atrophy.43,45,56,57 We hypothesized behavioral and neurobiological outcomes would correlate to spleen morphology following chronic stress and Reelin treatment. To evaluate these hypotheses, rats were treated with corticosterone for three weeks before receiving Reelin or vehicle and performing the forced swim test and object-in-place task. Spleens were collected and stained to quantify white pulp and correlations were evaluated between white pulp, behavior, and hippocampal expression of Reelin and GluR1. Detailed group data of behavioral testing and hippocampal protein expression from these animals is reported in Allen et al.32
Materials and Methods
Animals
Young adult Long-Evans rats (N = 115), weighing approximately 200–250 g at the experiment start, were purchased from Charles River (Saint-Constant, QC). Animals were single housed in clear plastic cages in a colony room maintained at a constant temperature of 22±1 °C and a 12:12 h light/dark cycle (lights on at 0700 h and off at 1900 h). Rats were briefly handled once daily for 7 days prior to the experiment start. All experimental procedures were carried out during the light period of the light/dark cycle and were conducted in accordance with regulations outlined by the University of Victoria Committee on Animal Care and the Canadian Council on Animal Care.
Drugs
Experimental animals were randomly assigned to receive chronic corticosterone or vehicle subcutaneous injections for 21 days at a dosage of 40 mg/kg (1 mL/kg, s.c.; Steraloids, Newport, RI). Corticosterone was suspended in 0.9% (w/v) physiological saline (pH = 7.4) and 2% (v/v) Tween-80 (Sigma Aldrich, Germany). An a priori criterion involved removing animals losing > 25% free body weight, although no removal was necessary. A subset of rats also received 3 μg of recombinant Reelin (3820-MR-025/CR; R&D Systems) dissolved in 0.1% PBS (tail vein, 0.5 mL volume). Rats not receiving Reelin received a vehicle tail vein injection. Reelin was administered in two treatment schedules: a repeat schedule, administered on days 11 and 21 of corticosterone injections, and a single treatment, with Reelin injections on day 21 of corticosterone. Reelin was administered for approximately 1 h following corticosterone treatment. The dosage and duration of Reelin treatment were selected based upon past results showing 3 μg of Reelin to be most effective for resolving despair-like behavior.32 Groups sizes are as follows: Male Vehicle/Vehicle (MVV): n = 14; Female Vehicle/Vehicle (FVV): n = 11; Male Vehicle/Reelin (MVR): n = 10; Female Vehicle/Reelin (FVR): n = 10; Male Corticosterone/Vehicle (MCV): n = 19; Female Corticosterone/Vehicle (FCV): n = 14; Male Corticosterone/Reelin One-Shot (MCRO): n = 7; Female Corticosterone/Reelin One-Shot (FCRO): n = 8; Male Corticosterone/Reelin Repeated-doses (MCRR): n = 10; Female Corticosterone/Reelin Repeated-doses (FCRR): n = 10. Spleens derive from multiple experiments reported within32 resulting in unequal sample sizes here.
Behavioral Testing
The spleens studied here are derived from a subset of animals tested to evaluate the dose–response of the antidepressant-like effects of Reelin treatment. Rats performed the forced swim test and object-in-place test to evaluate despair-like behavior and spatial memory—results of behavioral testing and hippocampal protein expression are reported elsewhere.32 The raw data was re-examined here for correlation analyses with the permission of the publisher.
Forced Swim Test
The forced swim test was conducted one day after the final corticosterone injection and Reelin treatment to evaluate the fast-acting antidepressant-like effects of Reelin. Animals were placed in a Plexiglas tank filled with 30 cm of water (27 ± 2 °C) and removed after 10 min. The duration of immobility, defined as swimming no more than that required to keep the nose above water, was manually scored to quantify despair-like behavior during the task while blinded to condition. This task has been the most reliable antidepressant screening tool in rodent research over recent decades. Scores in the forced swim test are represented as the percentage of change from the mean of vehicle/vehicles of the corresponding sex.
Object in Place Test
The object-in-place task was conducted to evaluate spatial memory 48 h after the forced swim test, and 24 h after a 10-minute exposure to the open field. In the training phase rats were placed in the arena for 5 min alongside one unique object 10 cm from each corner. After 5 min, animals were removed from the arena and returned to home cages for 1 h. Rats returned to the arena for a retention test during which two object positions were swapped. The duration exploring objects was scored while blind to condition to compare novel location exploration to exploration of objects in original positions during the first 2 min of the retention test. A discrimination ratio (DR) was calculated as follows; DR = (time exploring novel locations – time exploring familiar locations) ÷ (time exploring novel locations + time exploring familiar locations). A positive DR is interpreted as intact object-in-place recognition memory. Scores in the object-in-place test are represented as a percentage of change from the mean of the vehicle/vehicle group of the corresponding sex and are reported in Supplemental Tables 1 and 2.
Tissue Collection and Preparation
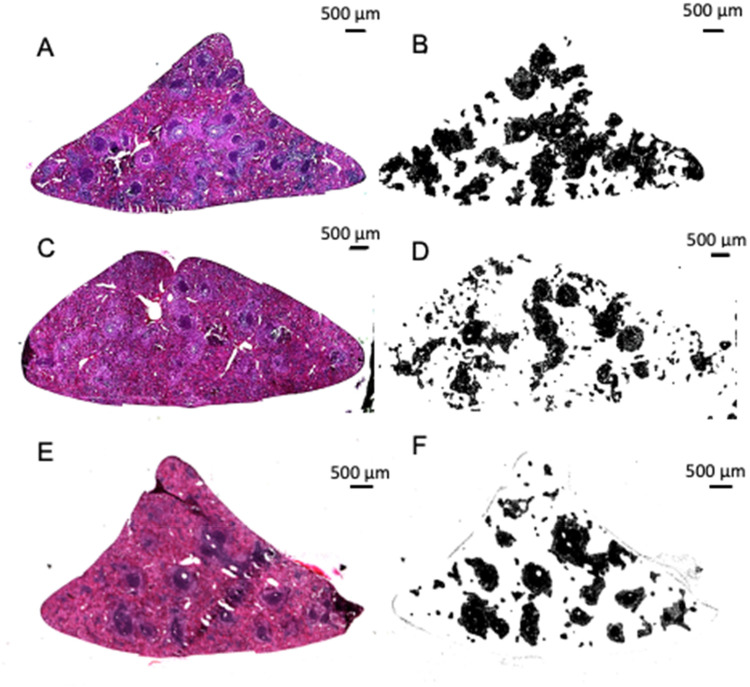
Following the conclusion of behavioral testing, animals were deeply anesthetized with 5% isoflurane before transcardial perfusion of 0.9% (w/v) physiological saline (pH of 7.4), followed by perfusion of 4% paraformaldehyde. Spleens and brains were collected and placed in 4% paraformaldehyde for 24 h, prior to transfer into 10% (w/v) sucrose in PBS, and finally into 30% (w/v) sucrose and 0.1% (w/v) sodium azide in PBS, where tissue remained until freezing. A portion of each spleen was frozen with liquid nitrogen while immersed in O.C.T. (Fischer Healthcare, USA), before sectioning at 15 µm using a Leica cryostat (CM 1850 UV, Germany). Following slide-mounted serial sectioning, spleens were stained with hematoxylin and eosin (H&E). White pulp staining heavily for hematoxylin (see Figure 1 for example). Tissue was fixed in 70% ethanol, followed by deionized water (dI) rinse before staining with hematoxylin (Gill No. 2, Sigma Aldrich, Germany) for 1 min, followed by dI rinse. The tissue was placed in a bluing reagent of 0.2% sodium bicarbonate (in dI) before brief placement in acidic ethanol. The tissue underwent another dI rinse before a 2-minute exposure to alcoholic Eosin Y (Sigma Aldrich, Germany). Eosin Y staining was followed by ethanol dehydration, xylene, and cover slipping. Immunohistochemistry was conducted on free-floating coronal sections through the hippocampus for quantification of Reelin expression and glutamate receptor 1 (GluR1) expression in the subgranular zone (SGZ). Sections were incubated with mouse anti-Reelin (MILLIPORE, MAB5364) or rabbit anti-GluR1 (MILLIPORE, AB1504) primary antibody at a concentration of 1:1000 for 48 h at 4 °C in a blocking solution of 5% (v/v) normal horse serum/normal goat serum, 0.5% (v/v) Triton X-100 and 1% (w/v) BSA in 0.1 M TBS. Sections were incubated for 1 h with either a biotinylated horse anti-mouse (1:500, Vector Laboratories BA2001) or goat anti-rabbit IgG (Vector laboratories, AB-2313606) secondary antibody before incubation in ABC (1:500, Vector Laboratories) for 1 h. Reelin immunolabeling was visualized with 0.02% (w/v) DAB and 0.0078% H2O2 and GluR1 with 0.05% (w/v) glucose oxidase DAB, 4.167% NiSO4 and 0.002% H2O2. Tissue was mounted on Super-Frost Plus Microscope glass slides before being dehydrated with ethanol and cover slipped with Permount (Fischer Scientific SP15-500).
Figure 1.
Representative images of spleen sections stained with hematoxylin and eosin. (A) Example section of spleen collected from a vehicle-treated rat. (B) Conversion of sample H&E-stained tissue image of spleen from vehicle-treated rat to binary. (C) Example section of spleen collected from a rat exposed to repeated corticosterone injections. (D) Conversion of sample H&E-stained tissue image of spleen from corticosterone-treated rat. (E) Example section of spleen collected from a corticosterone and Reelin-treated rat. (F) Conversion of sample H&E-stained tissue image of spleen from corticosterone and Reelin-treated rat.
Alt text: (A, C, E) Low magnification image of a spleen section showing white pulp nodules for (A) vehicle/vehicle; (C) corticosterone/vehicle; (E) corticosterone/Reelin. (B, D, F) White and black binary conversion of the white pulp areas of figures (A, C, E) with white pulp represented by black.
Imaging and Spleen Analysis
Spleen sections were imaged with a Zeiss microscope (Imager.M2, Germany) at 2.5X magnification. Images were converted to binary using color thresholding in ImageJ Fiji free software (version 1.53c; WS Rasband, National Institute of Health, Bethesda, MD) using a semiquantitative method for isolating hematoxylin from images of H&E stains58 to quantify white pulp area (see Figure 1 for examples). To determine if binary recreations accurately represent white pulp, outlines were created using Fiji's wand tool from the binary image and overlayed onto the original. If outlines did not match, corrections are made prior to obtaining results. Image analysis was conducted while blind to the subject/condition. Three sections, separated by a minimum of 60 µm, were quantified for a percentage of spleen occupied by white pulp from each rat for statistical comparisons. Cell counts for Reelin and GluR1 immunolabeled cells were quantified as described previously.29 Estimates were obtained using the formula N=ΣQ–x1/ssfxA(x, ystep)a(frame)xt/h, where N is the cell count estimate; ΣQ is the number of counted cells, ssf is the section sampling fraction, A(x, ystep) is an area associated with each x, y movement; a(frame) is the counting frame area; t is the weighted average section thickness; and h is the height of the dissector. Analyses were conducted while blind to conditions.
Statistical Analysis
Statistical analyses were performed using Prism 9 software (version 9.3). Group differences in spleen mass and white pulp area were evaluated with factorial ANOVAs (2 × 2 × 2 design), with sex (male and female), corticosterone treatment (vehicle or CORT), and Reelin treatment (vehicle or Reelin) as factors, followed by t-tests where significant differences were observed using Dunnett's correction for multiple comparisons by comparing each group against CORT/vehicle. Those treated with either repeated or single-vehicle injections, and those treated with vehicle/Reelin were combined into control groups (i.e. Vehicle/Vehicle from the repeated Reelin study are grouped with Vehicle/Vehicle from the single-dose study). Corticosterone/Reelin treatment groups were similarly grouped for factorial ANOVAs but analyzed in isolation for post-hoc testing. Results are expressed as mean ± SEM. Pearson correlations of white pulp area against behavioral and neurobiological data were conducted where values are represented as the percentage differences from the control group mean of the corresponding sex to illustrate relationships between white pulp atrophy to either behavioral deficits or deficits to Reelin expression and/or GluR1 expression. All animals performed in the forced swim test, however not all animals performed in the object in place test or had protein expression quantified, as spleens analyzed here were derived from various experiments reported within,32 resulted in varying group sizes (n's reported in Tables 1 to 4). Group data regarding behavioral and neurobiological outcomes demonstrating deficits associated with chronic stress and subsequent recovery of behavioral deficits are reported in (Allen et al.).32 Raw data used for correlation analyses are reported in Supplemental Tables 1 and 2.
Table 1.
Pearson correlation coefficients, 95% confidence intervals, and sample sizes for correlations of white pulp area of spleen and behavior in the forced swim task, an object in place task, hippocampal sub-granular zone Reelin expression, and GluR1 expression for corticosterone treated males and females. Values are represented as the percentage differences from the mean of the vehicle/vehicle group of the corresponding sex.
| Treatment group | White pulp atrophy by forced swim test immobility | White pulp atrophy by object location discrimination ratio | White pulp atrophy by Reelin expression in SGZ | White pulp atrophy by GluR1 expression in SGZ |
|---|---|---|---|---|
| Male Corticosterone/Vehicle | 0.03 (−0.43 to 0.48) n = 19 | −0.28(−0.75 to 0.38) n = 11 | 0.05(−0.49 to 0.57) n = 14 | −0.10(−0.58 to 0.43) n = 15 |
| Female Corticosterone/Vehicle | −0.09(−0.59 to 0.47) n = 14 | 0.02(−0.66 to 0.67) n = 9 | −0.59(−0.97 to 0.61) n = 5 | 0.33(−0.43 to 0.82) n = 9 |
| All Corticosterone/Vehicle | 0.04(−0.31 to 0.38) n = 33 | −0.06(−0.44 to 0.34) n = 25 | −0.03(−0.48 to 0.43) n = 19 | −0.05(−0.45 to 0.36) n = 24 |
Note. GluR1: glutamate receptor 1; SGZ: subgranular zone. Values are represented as the percentage differences from the mean of the vehicle/vehicle group of the corresponding sex.
Table 4.
Pearson correlation coefficients, 95% confidence intervals, and sample sizes for correlations of white pulp area of spleen and behavior in the forced swim task, an object in place task, hippocampal sub-granular zone Reelin expression, and GluR1 expression for all rats grouped together, all males and all females.
| Treatment group | White pulp atrophy by forced swim test immobility | White pulp atrophy by object location discrimination ratio | White pulp atrophy by Reelin expression in SGZ | White pulp atrophy by GluR1 expression in SGZ |
|---|---|---|---|---|
| All rats | −0.31*** (−0.46 to −0.13) n = 115 | 0.09 (−0.11 to 0.29) n = 94 | 0.30* (0.06 to 0.50) n = 66 | 0.35*** (0.16 to 0.52) n = 91 |
| All males | −0.39** (−0.58 to −0.15) n = 62 | 0.23 (−0.09 to 0.50) n = 41 | 0.38* (.039 to 0.65) n = 32 | 0.32* (0.05 to 0.55) n = 52 |
| All females | −0.18 (−0.43 to 0.93) n = 53 | 0.02 (−0.25 to 0.29) n = 53 | 0.21 (−0.14 to 0.51) n = 34 | 0.35* (0.04 to 0.60) n = 39 |
Note. *Correlation is significant at the .05 level (two-tailed).
**Correlation is significant at the .01 level (two-tailed)
***Correlation is significant at the .001 level (two-tailed).
GluR1: glutamate receptor 1; SGZ: subgranular zone.
Values are represented as the percentage differences from the mean of the vehicle/vehicle group of the corresponding sex.
Results
Chronic Exposure to Corticosterone Reduces Spleen Mass
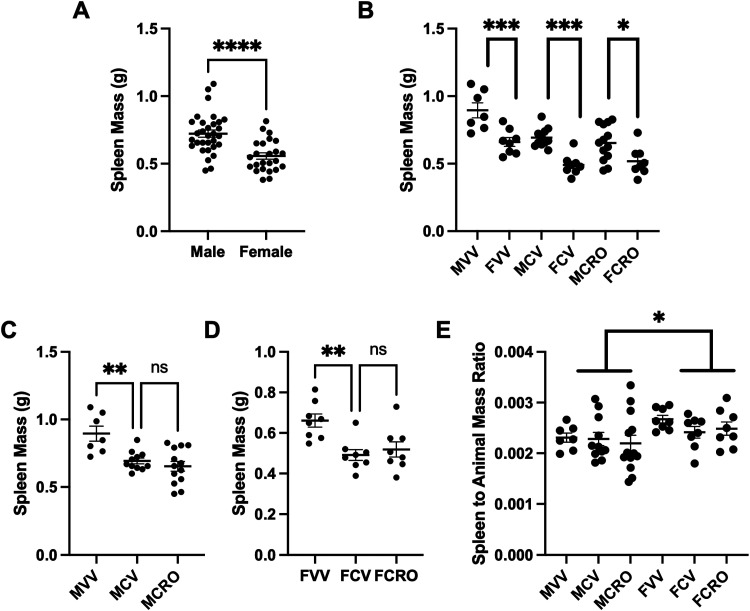
Results of factorial ANOVA of spleen masses found a significant main effect of sex [F(3,51) = 40.65, p<.0001] and CORT [F(1,54)= 22.09, p < .0001] but no main effect of Reelin treatment (p = .85). Additionally, no significant interaction was observed (p's > .33). We determined male spleens weighed significantly greater than female, both when treatment is not considered [(t(53) = 4.476, p < .0001)] (Figure 2A) and within each treatment (p's < .05) (Figure 2B). Post-hoc t-tests revealed reduced spleen mass in corticosterone-treated males (MCV = 0.694 ± .021) compared to those receiving vehicle [MVV = 0.895 ± .0551] [t(28)=3.555, p < .01]. Reductions were not recovered by Reelin [MCRO = 0.6535 ± .037; p > .05] (Figure 2C). In females, we observed significantly reduced spleen mass following corticosterone [FCV = 0.491 ± .030] relative to the vehicle [FVV = 0.661 ± .032] [t(21) = 3.763, p< .01], not recovered by Reelin [FCRO = 0.519 ± .038; p > .05] (Figure 2D). When comparing ratios of spleen mass to animal mass, factorial ANOVA found a main effect of sex [F(3,51) = 5.86; p = .019] but not for CORT or Reelin treatment (p's > .32). Post-hoc tests found no significant group differences (p's > .51) (Figure 2E).
Figure 2.
(A) Spleen mass comparison between males and females. (B) Spleen mass comparison of sexes by treatment group. (C) Spleen mass comparisons for male treatment groups. (D) Spleen mass comparisons for female treatment groups. (E) The ratio of the spleen to animal mass for each treatment group.
M: male; F: female; VV: vehicle/vehicle; VR: vehicle/Reelin; CV: corticosterone/vehicle; CRR: corticosterone/repeated Reelin; CRO: corticosterone/Reelin – one shot Alt text: (A) Graph with spleen mass for each sex showing male spleens weigh more. (B) Graph of spleen mass with each condition split by the sexes showing males have larger spleens in each treatment. (C) Graph of spleen mass for males only demonstrates corticosterone reduces the mass of spleen and Reelin does not recover mass. (D) Graph of spleen mass for females only demonstrates corticosterone reduces the mass of spleen and Reelin does not recover mass. (E) Graph demonstrating the ratio of spleen mass to animal mass does not change after any treatment.
White Pulp Atrophy Following Corticosterone and Recovery Following Reelin
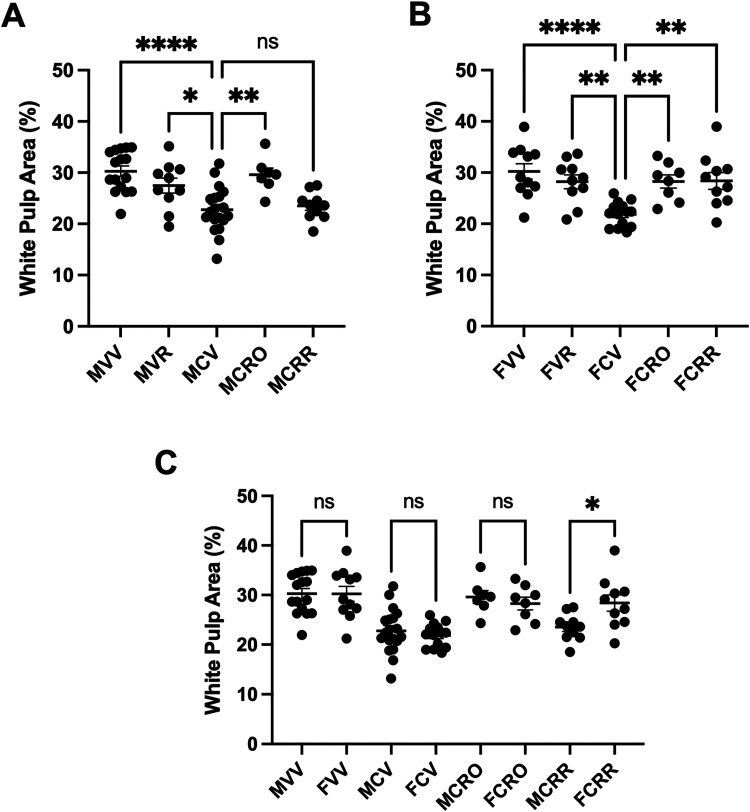
To evaluate if chronic stress reduced white pulp, we compared the percentage of surface area occupied by white pulp. Results of factorial ANOVA found a significant interaction between corticosterone and Reelin treatment (F(3,111) = 20.06, p < .0001), and a significant main effect of CORT [F(3,111) = 28.13, p < .0001]. No main effect of Sex or Reelin was observed (p's > .13). Post-hoc comparisons revealed a significant reduction in white pulp area in corticosterone-treated rats relative to controls [MCV = 22.8 ± 1.02; MVV = 30.3 ± 1.0] [t(57) = 5.492, p < .0001], that was recovered by one shot of Reelin [MCRO = 29.6 ± 1.29] [t(57) = 3.837, p < .01], but not repeated Reelin treatment [MCRR = 23.5 ± 0.86; p > .05] (Figure 3A). Post-hoc t-tests also revealed corticosterone-treated females [FCV = 21.87 ± 0.64] had reduced white pulp compared to the vehicle [FVV = 30.23 ± 1.51] [t(48) = 5.010, p < .0001]. In females, both one Reelin treatment [FCRO = 28.27 ± 1.294] [t(48) = 3.487, p < .01] and repeated reelin treatments [FCRR = 28.38 ± 1.642] [t(48) = 3.796, p < .01] resulted in significant recovery of white pulp atrophy (Figure 3B).
Figure 3.
Evaluation of H&E-stained spleen sections for a percentage of area occupied by white pulp. (A) White pulp areas in male treatment groups. (B) White pulp areas in female treatment groups. (C) White pulp comparison of sexes by treatment group.
M: male; F: female; VV: vehicle/vehicle; VR: vehicle/Reelin; CV: corticosterone/vehicle; CRR: corticosterone/repeated Reelin; CRO: corticosterone/Reelin – one shot Alt text: (A) Graph of spleen white pulp % area from the males in each condition showing C/V had less white pulp, whereas C/RO has a recovery of white pulp, C/RR does not. (B) Graph of spleen white pulp % area from the females in each condition showing C/V had less white pulp, whereas C/RO and C/RR have recovery of white pulp. (C) Graph comparing white pulp % area of males and females in each condition, showing only the repeated Reelin groups are significantly different, with females having more white pulp than males.
White Pulp Atrophy Correlates to Immobility in the Forced Swim Task, Spatial Memory in the Object in Place Task, and Hippocampal Expression of Reelin and GluR1
No significant correlations with white pulp atrophy and behavioral task performance, or either Reelin or GluR1 expression were observed in males or females treated with corticosterone (p's > .05) as shown in Table 1 (for individual data, see Supplemental Tables 1 and 2). In males exposed to repeated Reelin injections, a significant correlation was observed between change to white pulp and immobility during the forced swim test (p < .05), and discrimination ratio for spatial memory during the object in place task (p < .01) as shown in Table 2. No significant correlation was observed between white pulp atrophy in chronically stressed male rats treated with Reelin once or in female rats receiving Reelin in either treatment schedule. However, when pooling female treatment groups, a significant negative correlation was observed between white pulp areas and discrimination ratios during the object-in-place task (p < .05). No significant correlation was observed between white pulp and behavior in male or female rats treated with either vehicle/vehicle or in vehicle-treated rats receiving Reelin (p's > .05) as shown in Table 3. When aggregating groups, a significant positive correlation emerged between white pulp and GluR1 (p < .001), which was observed in males (p < .05) and females (p < .05). A significant positive correlation was also observed between SGZ Reelin expression and white pulp atrophy across all groups (p < .05) that was seen across males (p < .05) but not females (p > .05).
Table 2.
Pearson correlation coefficients, 95% confidence intervals, and sample sizes for correlations of white pulp area of spleen and behavior in the forced swim task, an object in place task, hippocampal sub-granular zone Reelin expression, and GluR1 expression for corticosterone treated males and females receiving Reelin.
| Treatment group | White pulp atrophy by forced swim test immobility | White pulp atrophy by object location discrimination ratio | White pulp atrophy by Reelin expression in SGZ | White pulp atrophy by GluR1 expression in SGZ |
|---|---|---|---|---|
| Male Corticosterone/Reelin One shot | 0.03 (−0.74 to 0.76) n = 7 | n = 0 | −0.45 (−0.90 to 0.46) n = 7 | −0.97* (−0.99 to −0.20) n = 4 |
| Female Corticosterone/Reelin One shot | −0.42 (−0.87 to 0.41) n = 8 | −0.36 (−0.85 to 0.46) n = 8 | 0.07 (−0.67 to 0.74) n = 8 | n = 0 |
| Male Corticosterone/Reelin Repeated | 0.64* (0.013 to 0.90) n = 10 | 0.84** (0.46 to 0.96) n = 10 | n = 0 | 0.35 (−0.36 to 0.80) n = 10 |
| Female Corticosterone/Reelin Repeated | 0.44 (−0.27 to 0.84) n = 10 | −0.61 (−0.90 to 0.03) n = 10 | 0.37 (−0.34 to 0.81) n = 10 | −0.06 (−0.67 to 0.59) n = 10 |
| Male Corticosterone/Reelin Both treatments | 0.25 (−0.27 to 0.65) n = 17 | 0.84** (0.46 to 0.96) n = 10 | −0.45 (−0.90 to 0.46) n = 7 | −0.05 (−0.56 to 0.49) n = 14 |
| Female Corticosterone/Reelin Both treatments | 0.17 (−0.32 to 0.59) n = 18 | −0.53* (−0.80 to −0.08) n = 18 | 0.24 (−0.25 to 0.64) n = 18 | −0.06 (−0.67 to 0.59) n = 10 |
| All Corticosterone/Reelin | 0.11 (−0.23 to 0.43) n = 35 | −0.31 (−0.61 to 0.07) n = 28 | 0.16 (−0.25 to 0.53) n = 25 | 0.04 (−0.37 to 0.43) n = 24 |
Note. *Correlation is significant at the .05 level (two-tailed).
**Correlation is significant at the .01 level (two-tailed).
GluR1: glutamate receptor 1; SGZ: subgranular zone.
Values are represented as the percentage differences from the mean of the vehicle/vehicle group of the corresponding sex.
Table 3.
Pearson correlation coefficients, 95% confidence intervals, and sample sizes for correlations of white pulp area of spleen and behavior in the forced swim task, an object in place task, hippocampal subgranular zone Reelin expression, and GluR1 expression for vehicle-treated males and females, including those that received Reelin.
| Treatment group | White pulp atrophy by forced swim test immobility | White pulp atrophy by object location discrimination ratio | White pulp atrophy by Reelin expression in SGZ | White pulp atrophy by GluR1 expression in SGZ |
|---|---|---|---|---|
| Male Vehicle/Vehicle | −0.43 (−0.76 to 0.086) n = 16 | −0.37 (−0.81 to 0.34) n = 10 | 0.34 (−0.33 to 0.78) n = 11 | −0.37 (−0.81 to 0.33) n = 10 |
| Female Vehicle/Vehicle | 0.54 (−0.09 to 0.86) n = 11 | 0.09 (−0.57 to 0.68) n = 10 | n = 1 | −0.05 (−0.66 to 0.60) n = 10 |
| Male Vehicle/Reelin | 0.49 (−0.20 to 0.86) n = 10 | −0.16 (−0.72 to 0.52) n = 10 | n = 0 | −0.18 (−0.73 to 0.50) n = 10 |
| Female Vehicle/Reelin | 0.29 (−0.42 to 0.78) n = 10 | 0.22 (−0.48 to 0.75) n = 10 | −0.15 (−0.71 to 0.53) n = 10 | 0.12 (−0.55 to 0.70) n = 10 |
| All Vehicle/Vehicle | 0.09 (−0.30 to 0.46) n = 27 | −0.001 (−0.43 to 0.43) n = 21 | 0.39 (−0.23 to 0.79) n = 12 | 0.28 (−0.14 to 0.62) n = 23 |
| All Vehicle/Reelin | 0.31 (−0.16 to 0.66) n = 20 | 0.01 (−0.44 to 0.45) n = 20 | −0.15 (−0.71 to 0.53) n = 10 | 0.04 (−0.41 to 0.48) n = 20 |
| All V/V & All V/R | 0.11 (−0.19 To 0.38) N = 47 | −0.03 (−0.33 to 0.28) n = 41 | 0.31 (−0.12 to 0.65) n = 22 | 0.21 (−0.10 to 0.48) n = 43 |
Note. GluR1: glutamate receptor 1; SGZ: subgranular zone.
Values are represented as the percentage differences from the mean of the vehicle/vehicle group of the corresponding sex.
Discussion
Corticosterone reduced spleen mass and induced white pulp atrophy in both males and females (Figures 2 and 3). This dosage and duration of corticosterone induces depression-like behavior and various neurobiological changes associated with MD59 and atrophy of spleen white pulp. The white pulp of corticosterone-treated rats frequently appears fragmented and dispersed, occupying significantly less surface area (see Figure 1C and D). The lack of homogeneity of atrophy within individual sections, as demonstrated in Figure 1C and E, led us to consider it valuable to conduct analysis of entire sections rather than limited visual fields, as conducted elsewhere.44,45 Results support research demonstrating experimental models of chronic stress, including chronic restraint stress or chronic unpredictable stress, alter the morphology of spleen germinal centers.43,45,56,57 Our results are consistent with previous results showing chronic stress disrupts spleen morphology.43–47,60 Although the spleen is a blood filter and fixation was conducted through the circulatory system, if altered spleen morphology affects filtration through the spleen to impact fixation quality, the result is the same—functional deficits contributing to fragmentation supports the determination that spleen dysfunction occurs following chronic stress.
Reelin recovered chronic stress-induced white pulp atrophy, consistent with the role of Reelin in regulating immunity.40,41 Both treatment schedules resolved atrophy in females (Figure 3B) and males treated once had significant white pulp recovery (Figure 3A). As we observed recovery in rats receiving a single Reelin treatment on the final day of chronic stress, results suggest Reelin recovered atrophy of white pulp rather than prevent atrophy. When comparing treatment outcomes across males and females, only the repeated Reelin treatment showed sex differences, with males having significantly less white pulp than females (Figure 3C). Females generally have less endogenous Reelin than males and males may have transiently increased Reelin expression in acute phases of stress—although this transient phenomenon could be unique to early life stress.32,61,62 Absence of recovery in the treatment group expected to have the highest Reelin levels (i.e. males receiving multiple doses) is consistent with behavioral observations of diminishing antidepressant-like effects of Reelin at high doses32 suggesting “fine tuning” of Reelin signaling is more beneficial than providing excess. Although Reelin signaling is involved in lymphocyte apoptosis, proliferation, and differentiation, future studies are necessary to determine the downstream target(s) of Reelin responsible for the recovery of stress-induced white pulp atrophy.34–36,63,64 Reelin also influences leukocyte adhesion, which could impact the fragmentation of white pulp following chronic stress and recovery with Reelin treatment.65 Although Reelin did not normalize spleen mass (Figure 2C and D), which might be inferred as evidence of sustained immune dysfunction, no changes were observed for the spleen to body mass ratios (Figure 2E). This could indicate spleen mass may normalize with animal mass following cessation of chronic stress, potentially with or without Reelin treatment. Reduced spleen mass itself does not indicate spleen composition is altered, as this could be related to the global effects of corticosterone. However, concomitantly reduced mass and germinal center areas following chronic stress supports the conclusion that chronic corticosterone alters spleen composition, as seen in other rodent models of chronic stress.42,44,55,56 As we observed recovery of white pulp atrophy following Reelin treatment in chronically stressed rats and evidence shows splenectomy in transgenic AD mice exacerbates AD pathology,55 future experimentation is warranted to better understand if Reelin treatment impacts Aβ levels following chronic stress or amyloid aggregation later in life. In a model of chronic unpredictable stress, animals receiving minocycline (an antibiotic with anti-inflammatory properties) after the conclusion of the stress period had improved cognitive function relative to animals receiving vehicle injections after one week of treatment, consistent with a role of inflammation in the recovery of chronic stress-related deficits.66 It will be valuable to evaluate the impact of chronic stress with and without Reelin treatment on spleen white pulp morphology after longer periods following removal of stressors to determine whether the antidepressant and anti-inflammatory effects of Reelin will translate into improved prognosis following future stress exposures.
To evaluate if white pulp atrophy relates to behavioral or neurobiological outcomes relevant to depression, we correlated white pulp deficits with immobility during the forced swim test, discrimination ratios from the object-in-place task, and expression of Reelin and GluR1 in the SGZ. We correlated results from the object-in-place task for behavioral assessment of hippocampal function as research shows the object-in-place task is a hippocampus-dependent spatial memory task and deficits in the task have been reported following chronic corticosterone exposure.33 As past assessments identify alterations to hippocampal Reelin expression following chronic corticosterone, we chose to focus on the hippocampus for behavioral assessment and analysis of protein expression.32 Although Reelin is expressed in the entorhinal cortex and contributes to spatial memory, much less is known of the effects of chronic stress on entorhinal cortex Reelin expression67–69 Hippocampal glutamatergic signaling following chronic stress and Reelin treatment is of interest as both Reelin and inflammatory processes regulate NMDA receptor activity and trafficking.70,71 GluR1 data was selected from 32 following both Reelin treatment schedules to determine if recovery of peripheral immunity relates to the recovery of hippocampal glutamatergic signaling following Reelin treatment. Although alterations to these factors present at the group level following chronic corticosterone exposure, no significant correlations were seen between the severity of white pulp atrophy and behavioral or neurobiological outcomes in the absence of Reelin treatment (Table 1). This could suggest secondary immune dysfunction associated with chronic glucocorticoid exposure occurs independently from glucocorticoid-induced behavioral or neurobiological deficits, however, limited outcome variability across small sample sizes reduces the likelihood of meaningful correlations emerging. Therefore, an expanded assessment to identify potential relationships between peripheral inflammation and the level of behavioral and neurobiological deficit is required. In contrast to our results, past studies found that maintained spleen weight amidst chronic stress is associated with resilience to evoke despair-like behavior.46 The lack of relationships between despair-like behavior and white pulp in animals exposed to corticosterone is consistent with studies in which chronic stress exposure lasts at least three weeks that suggest increased spleen mass following stress relating to resilience is transient, and not seen when extending stress exposure.43,45,60
When evaluating correlations in rats treated with Reelin, correlations are observed between white pulp atrophy and behavioral task performance in chronically stressed rats receiving Reelin, although directions were not all accurately predicted. Our observation of correlations following Reelin treatment supports our hypothesis that peripheral immune recovery is related to the recovery of behavioral despair and spatial memory following Reelin treatment. More white pulp was associated with better spatial memory for Reelin-treated males, although the opposite was found for females (see Table 2). A positive correlation with forced swim task immobility indicates male rats treated with repeated Reelin injections with more white pulp showed more despair-like behavior—contrary to our hypothesis. Although these correlations emerge with limited sample sizes, which can lead to spurious correlations, that relationships emerge in multiple treatment groups across multiple behavioral assessments supports our hypothesis that Reelin-induced recovery of behavioral and neurobiological deficits is related to peripheral immune recovery. Although this exploratory analysis finds relationships between peripheral immunity and recovery of behavioral despair and spatial memory, further investigation into the immune-related effects of Reelin treatment following chronic stress is warranted with larger samples to better understand relationships between immunity and recovery of stress-induced behavioral deficits.
Relationships between white pulp and SGZ Reelin expression or GluR1 didn’t emerge in any treatment group. However, relationships emerged when aggregating treatments, as shown in Table 4. Although we observed recovery of white pulp atrophy following Reelin-treatment in females, and behavioral assessments found the recovery of despair-like behavior and SGZ Reelin expression in these animals,32 we did not observe a relationship between white pulp and SGZ Reelin expression or FST immobility in females (see Table 4). This could suggest sex differences exist between immunity, Reelin, and behavioral outcomes following chronic stress, consistent with numerous reports of sex differences in immune function (as reviewed by Klein and Flanagan72) and HPA axis sensitivity (as reviewed by Heck and Handa73). An unaccounted-for effect of estrus cycles could potentially mask relationships between white pulp and hippocampal protein expression or behavior in females, as Reelin expression fluctuates with the estrus cycle.74 Unfortunately, estrus cycles were not recorded or considered by Allen et al.32 Endogenous Reelin expression can’t be ascertained following recombinant Reelin treatment through assessment of Reelin in the SGZ as Reelin may be trafficked across the blood–brain barrier.75 Plasma Reelin is susceptible to proteolysis and breakage, making archival blood sample analyses unreliable.76 Future assessments should consider endogenous Reelin expression prior to Reelin treatment to consider the impact of endogenous expression on outcomes. Additionally, future evaluations should determine whether the anti-inflammatory effects of Reelin treatment are limited to peripheral immune structures, or if neuroinflammation is similarly affected. Given the numerous links between peripheral and CNS immunity, recovery in the periphery should translate into improved regulation of neuroinflammation and this should therefore be evaluated in future studies.
Although preliminary analysis conducted via hand-drawn white pulp outlines found there might exist white pulp deficits following chronic corticosterone exposure, it was considered a failure to include all fragmented white pulp in corticosterone-treated rats could be responsible for deficits to total white pulp area, and perhaps although disorganized morphologically, total area might be underrepresented by hand-drawn methods. This led to the use of color thresholding methods available with ImageJ for quantifying hematoxylin and eosin staining across whole sections.57 Spleens were only weighed after this preliminary analysis, therefore group sizes are reduced for mass analysis. When drawing conclusions pertaining to relationships between immunity and spatial memory, it is worth considering limitations of the task, and group size limitations for within-group correlations. Discrimination ratios might be an insufficiently sensitive measurement for quantifying relative spatial memory performance among those performing above chance, as group results are more meaningful than individual scores in novelty-based recognition tasks.77 Within-group relationships should be re-evaluated across larger datasets to better understand how immunity-related recovery following Reelin treatment impacts behavioral and neurobiological outcomes of chronic stress.
In summary, results support our hypothesis that chronic corticosterone induces white pulp atrophy, and recovery of spleen morphology occurs following Reelin treatment. Relationships were observed between recovery of white pulp and behavioral and neurobiological outcomes relevant to depression following Reelin treatment. What remains unclear are the mechanism(s) through which Reelin restores secondary immune dysfunction and whether immune recovery with Reelin treatment impacts susceptibility to relapse upon future stress exposure or susceptibility to develop disorders of inflammation.
Supplemental Material
Supplemental material, sj-docx-1-css-10.1177_24705470231164920 for Intravenous Reelin Treatment Rescues Atrophy of Spleen White Pulp and Correlates to Rescue of Forced Swim Test Immobility and Neurochemical Alterations Induced by Chronic Stress by B.S. Reive, Jenessa N. Johnston, Carla L. Sánchez-Lafuente, Lucy Zhang, Aland Chang, Jasmine Zhang, Josh Allen, Raquel Romay-Tallon, Lisa E. Kalynchuk and Hector J. Caruncho in Chronic Stress
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to thank NSERC and CIHR for their financial support, which helps make this research possible.
ORCID iD: B.S. Reive https://orcid.org/0000-0001-5208-6051
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Jamieson CA, Yamamoto KR. Crosstalk pathway for inhibition of glucocorticoid-induced apoptosis by T cell receptor signalling. Proc Natl Acad Sci. 2000; 97(13): 7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002; 169(4): 1837–1843. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi H, Medina KL, Yokota T, et al. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int Immunol. 2005; 17(5): 501–511. [DOI] [PubMed] [Google Scholar]
- 4.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006; 63:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan SG, Gao S, Shao L, et al. A factor in lymph node and spleen induced by restraint stress in mice and rats suppresses lymphocyte proliferation. Neuroimmunomodulation 1995; 2(5): 2740281. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala and prefrontal cortex. Neuropsychopharmacology 2016; 41(1): 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005; 53(2): 129–139. [DOI] [PubMed] [Google Scholar]
- 8.Duan H, Yuan Y, Zhang L, et al. Chronic stress exposure decreases the cortisol awakening response in healthy young men. Int J Biol Stress 2013; 16(6): 630–637. [DOI] [PubMed] [Google Scholar]
- 9.Lussier AL, Lebedeva K, Fenton EY, et al. The progressive development of depression-like behaviour in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased Reelin expression in the adult dentate gyrus. Neuropharmacology 2013; 71:174–183. [DOI] [PubMed] [Google Scholar]
- 10.Kalynchuk LE, Gregus A, Boudeau D, Perrot-Sinal TS. Corticosterone increases depression-like behaviour, with some effects on predator odor-induced defensive behaviour, in male and female rats. Behav Neurosci 2004; 118(6): 1365–1377. [DOI] [PubMed] [Google Scholar]
- 11.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behaviour in male rats. Behav Brain Res. 2005; 156(1): 105–114. [DOI] [PubMed] [Google Scholar]
- 12.Fenton E, Fournier NM, Lussier AL, et al. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behaviour, hippocampal Reelin expression and neuronal maturation. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015; 60:52–59. [DOI] [PubMed] [Google Scholar]
- 13.Marks WN, Fenton EY, Guskjolen AJ, Kalynchuk LE. The effect of chronic corticosterone on fear learning and memory depends on dose and the testing protocol. Neuroscience 2015; 289:324–333. [DOI] [PubMed] [Google Scholar]
- 14.Lussier A, Romay-Tallon R, Caruncho HJ, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience 2013; 231:38–48. [DOI] [PubMed] [Google Scholar]
- 15.Goldman DH, Dykstra T, Smirnov I, et al. Age-associated suppression of exploratory activity during sickness is linked to meningeal lymphatic dysfunction and microglia activation. Nat Aging 2022; 2:704–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowyer JF, Sarkar S, Burks SM, et al. Microglial activation and responses to vasculature that result from an acute LPS exposure. Neurotoxicology 2020; 77:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Hussain B, Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2020; 27(1): 36–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richmond-Rakerd LS, D’Souza S, Milne BJ, Caspi A, Moffitt TE. Longitudinal associations of mental disorders with dementia 30-year analysis of 1.7 million New Zealand citizens. JAMA Psychiatry 2022; 79(4): 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song H, Sieurin J, Wirdefeldt K, et al. Association of stress-related disorders with subsequent neurodegenerative diseases. JAMA Neurol. 2020; 77(6): 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of Reelin expression as a putative vulnerability factor for schizophrenia. Proc Natl Acad Sci. 1998; 95(26): 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti A, Auta J, Davis JM, et al. Decrease in Reelin and glutamic acid decarboxylase (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000; 57(11): 1061–1069. [DOI] [PubMed] [Google Scholar]
- 22.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000; 5:654–663. [DOI] [PubMed] [Google Scholar]
- 23.Fatemi SH, Kroll JL, Stary JM. Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. NeuroReport 2001; 12(15): 3209–3215. [DOI] [PubMed] [Google Scholar]
- 24.Haas CA, Dudeck O, Kirsch M, et al. Role for Reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002; 22(14): 5797–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knuesel I, Nyffeler M, Mormède C, et al. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging. 2009; 30(5): 697–716. [DOI] [PubMed] [Google Scholar]
- 26.Ventrutti A, Kazdoba TM, Niu S, D’Arcangelo G. Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience 2011; 189:32–42. [DOI] [PubMed] [Google Scholar]
- 27.Herring A, Donath A, Steiner KM, et al. Reelin depletion is an early phenomenon of Alzheimer’s pathology. J Alzheimer’s Dis 2012; 30(4): 963–979. [DOI] [PubMed] [Google Scholar]
- 28.Hornig T, Sturm L, Fiebach B, Tebartz van Elst L. Increased blood-Reelin-levels in first episode schizophrenia. PLoS ONE 2015; 10(8): e0134671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfimova MV, Kondratiev NV, Golov AK, Golimbet VE. Methylation of the Reelin gene promoter in peripheral blood and its relationship with the cognitive function of schizophrenia patients. Mol Biol. 2018; 52(5): 676–685. [DOI] [PubMed] [Google Scholar]
- 30.Lussier AL, Caruncho HJ, Kalynchuk LE. Repeated exposure to corticosterone, but not restraint, decreases the number of Reelin-positive cells in the adult rat hippocampus. Neurosci Lett. 2009; 460(2): 170–174. [DOI] [PubMed] [Google Scholar]
- 31.Lussier AL, Romay-Talon R, Kalynchuk LE, Caruncho HE. Reelin as a putative vulnerability factor for depression: examining the depressogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology 2011; 60:1064–1074. [DOI] [PubMed] [Google Scholar]
- 32.Allen JA, Romay-Tallon R, Mitchell MA, et al. Reelin has antidepressant-like effects after repeated or singular peripheral injections. Neuropharmacology 2022; 15:109043. [DOI] [PubMed] [Google Scholar]
- 33.Brymer KJ, Johnston J, Botterill J, et al. Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm. Neuropsychopharmacology 2020; 45:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beffert U, Morfini G, Bock HH, et al. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem. 2002; 277(5): 49958–49964. [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Gudapati P, Dragovic S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 2010; 32(6): 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim EH, Sullivan JA, Plisch EH, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012; 188(9): 4305–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013; 25(3): 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkubo N, Vitek M, Morishima A, et al. Reelin signals survival through Src-family kinases that inactivate BAD activity. J Neurochem. 2007; 103(2): 820–830. [DOI] [PubMed] [Google Scholar]
- 39.Mok CL, Gil-Gomez G, Williams O, et al. Bad can act as a key regulator of T cell apoptosis and development. J Exp Med. 1999; 189(3): 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenjohnson JM, Zalcman S, Vriend C, et al. Suppressed T cell function and macrophage function in the “Reeler” (rl/rl) mutant, a murine strain with elevated cerebellar norepinephrine concentration. Brain Behav Immunity. 1995; 9(1): 47–60. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Miranda P, Vazquez-Carretero MD, Gutierrez G, et al. Lack of reelin modifies the genes expression in the small intestine of mice. J Physiol Biochem. 2012; 68(2): 205–218. [DOI] [PubMed] [Google Scholar]
- 42.Wei Y, Wang T, Liao L, et al. Brain–spleen axis in health and diseases: a review and future perspective. Brain Res Bull. 2022; 182:130–140. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez ME, Martinez-Mota L, Salinas C, et al. Chronic stress induces structural alterations in splenic lymphoid tissue that are associated with changes in corticosterone levels in Wistar-Kyoto rats. Biomed Res Int. 2013; 2013:868742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vásquez B, Sandoval C, Smith RL, del Sol M. Effects of early and late adverse experiences on morpho-quantitative characteristics of Sprague-Dawley rat spleen subjected to stress during adulthood. Int J Clin Exp Pathol. 2015; 8(4): 3624–3635. [PMC free article] [PubMed] [Google Scholar]
- 45.Tahawy NE, Ali AH. Swimming exercise ameliorates the chronic immobilization stress-induced alterations in spleen and splenic T-cell population in adult male albino rats: histological and immunohistochemical study. Egypt J Histol. 2021; 44(1): 83–95. [Google Scholar]
- 46.Zhang K, Sakamoto A, Chang L, et al. Splenic NKG2D confers resilience versus susceptibility in mice after chronic social defeat stress: beneficial effects of (R)-ketamine. Eur Arch Psychiatry Clin Neurosci. 2021; 271:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002; 132(1–2): 66–71. [DOI] [PubMed] [Google Scholar]
- 48.Krueger I, Gremer L, Mangels L, et al. Reelin amplifies glycoprotein VI activation and alphaIIb beta3 integrin outside-in signaling via PLC Gamma 2 and Rho GTPases. Atheroscler Thromb Vasc Biol 2020; 40:2391–2403. [DOI] [PubMed] [Google Scholar]
- 49.Tseng WL, Huang CL, Chong KY, et al. Reelin is a platelet protein and functions as a positive regulator of platelet spreading on fibrinogen. Cell Mol Life Sci. 2010; 67:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of hypersplenic thrombocytopenia. J Clin Invest. 1966; 45(5): 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuchillo-Ibanez I, Mata-Balaguer T, Balmaceda V, et al. The β-amyloid peptide compromises Reelin signaling in Alzheimer’s disease. Sci Rep. 2016; 6:31646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu T, Chen L, Zhou L, Xu J, Guo K. Platelets transport β-amyloid from the peripheral blood into the brain by destroying the blood–brain barrier to accelerate the process of Alzheimer’s disease in mouse models. Aging (Albany NY). 2021; 13(5): 7644–7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoe H-S, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J Biol Chem. 2006; 281(46): 35176–35185. [DOI] [PubMed] [Google Scholar]
- 54.Lane-Donovan C, Philips GT, Wasser CR, et al. Reelin protects against amyloid β toxicity in vivo. Sci Signal. 2015; 8(384): ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu ZY, Chen DW, Tan CR, et al. Physiological clearance of Aβ by spleen and splenectomy aggravates Alzheimer-type pathogenesis. Aging Cell 2022; 21(1): e13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurfein BT, Hasdemir B, Milush JM, et al. Enriched environment and stress exposure influence splenic B lymphocyte composition. PloS ONE 2017; 12(7): e0180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Jiang W, Li ZZ, et al. Repetitive restraint stress changes spleen immune cell subsets through glucocorticoid receptor or β-adrenergic receptor in a stage dependent manner. Biochem Biophys Res Commun. 2018; 495(1): 1108–1114. [DOI] [PubMed] [Google Scholar]
- 58.Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019; 9(24): e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behaviour and HPA axis responses to a novel stressors. Behav Brain Res. 2006; 168(2): 380–285. [DOI] [PubMed] [Google Scholar]
- 60.Zhan H, Huang F, Yan F, et al. Alterations in splenic function and gene expression in mice with depressive-like behaviour induced by exposure to corticosterone. Int J Mol Med. 2017; 39(2): 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gross CM, Flubacher A, Tinnes S, et al. Early life stress stimulates hippocampal reelin gene expression in a sex-specific manner: evidence for corticosterone-mediated action. Hippocampus 2012; 22(3): 409–420. [DOI] [PubMed] [Google Scholar]
- 62.Kolaka R, Chotwiwatthanakun C, Chutabhakdikul N. Fetal exposure to high levels of maternal glucocorticoids alters Reelin signaling in the prefrontal cortex of rat pups. Int J Dev Neurosci. 2019; 78(1): 185–190. [DOI] [PubMed] [Google Scholar]
- 63.Sinclair LV, Finlay D, Feijoo C, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008; 9(5): 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011; 12(4): 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calvier L, Demuth G, Manouchehri N, et al. Reelin depletion protects against autoimmune encephalomyelitis by decreasing vascular adhesion of leukocytes. Sci Transl Med. 2020; 12(556): eaay7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poggini S, Lopez MB, Albanese NC, et al. Minocycline treatment improves cognitive and functional plasticity in a preclinical mouse model of major depressive disorder. Behav Brain Res. 2023; 441:114295. [DOI] [PubMed] [Google Scholar]
- 67.Vandrey B, Garden DL, Ambrozova V, et al. Fan cells in layer 2 of the lateral entorhinal cortex are critical for episodic-like memory. Curr Biol. 2020; 30(1): 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowland DC, Obenhaus HA, Skytoen ER, et al. Functional properties of stellate cells in entorhinal cortex layer II. eLife 2018; 7:e36664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winterer J, Maier N, Wozny C, et al. Excitatory microcircuits within superficial layers of the medial entorhinal cortex. Cell Rep. 2017; 19(6): 1110–1116. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Beffert U, Ertunc M, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005; 25(36): 8209–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wigerblad G, Huie JR, Yin HZ, et al. Inflammation-induced GluA1 trafficking and membrane insertion of Ca2 + permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A. Exp Neurol. 2017; 293:144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016; 16:626–638. [DOI] [PubMed] [Google Scholar]
- 73.Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis response to stress: an important role for gonadal hormones. Neuropsychopharmacology 2019; 44:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meseke M, Prols F, Schmahl C, et al. Reelin and aromatase cooperate in ovarian follicle development. Sci Rep. 2018; 8:8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Costas E, Fenton EY, Caruncho HJ. Reelin expression in brain endothelial cells: an electron microscopy study. BMC Neurosci. 2015; 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lugli G, Krueger JM, Davis JM, et al. Methodological factors influencing measurement and processing of plasma reelin in humans. BMC Biochem. 2003; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cole E, Simundic A, Mossa FP, Mumby DG. Assessing object-recognition memory in rats: pitfalls of the existent tasks and the advantages of a new test. Learn Behav 2019; 47(2): 141–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-css-10.1177_24705470231164920 for Intravenous Reelin Treatment Rescues Atrophy of Spleen White Pulp and Correlates to Rescue of Forced Swim Test Immobility and Neurochemical Alterations Induced by Chronic Stress by B.S. Reive, Jenessa N. Johnston, Carla L. Sánchez-Lafuente, Lucy Zhang, Aland Chang, Jasmine Zhang, Josh Allen, Raquel Romay-Tallon, Lisa E. Kalynchuk and Hector J. Caruncho in Chronic Stress