Abstract
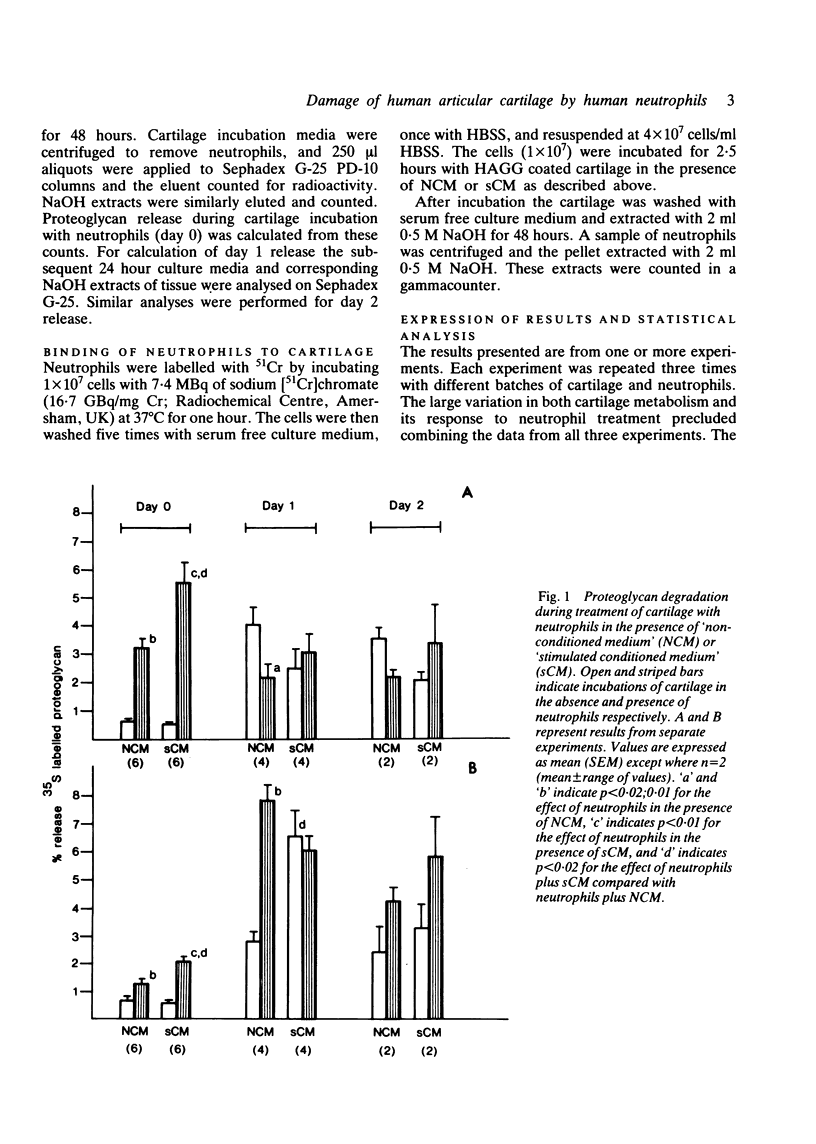
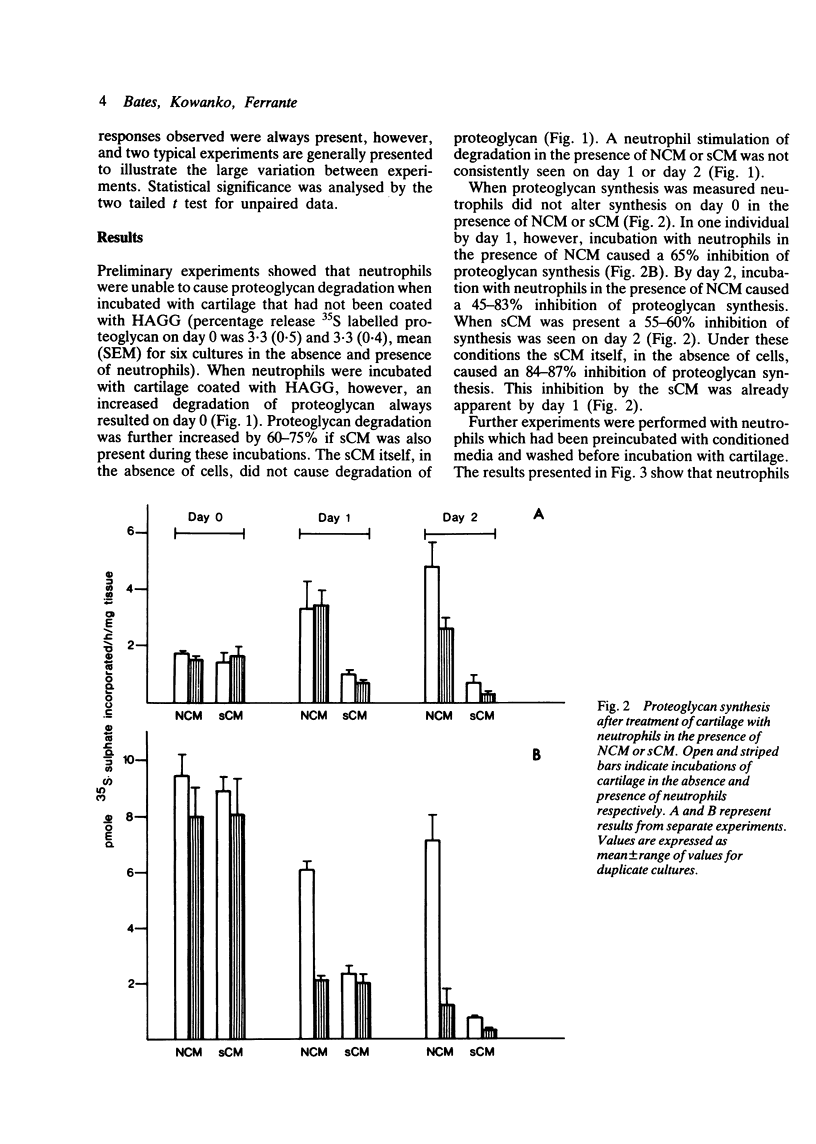
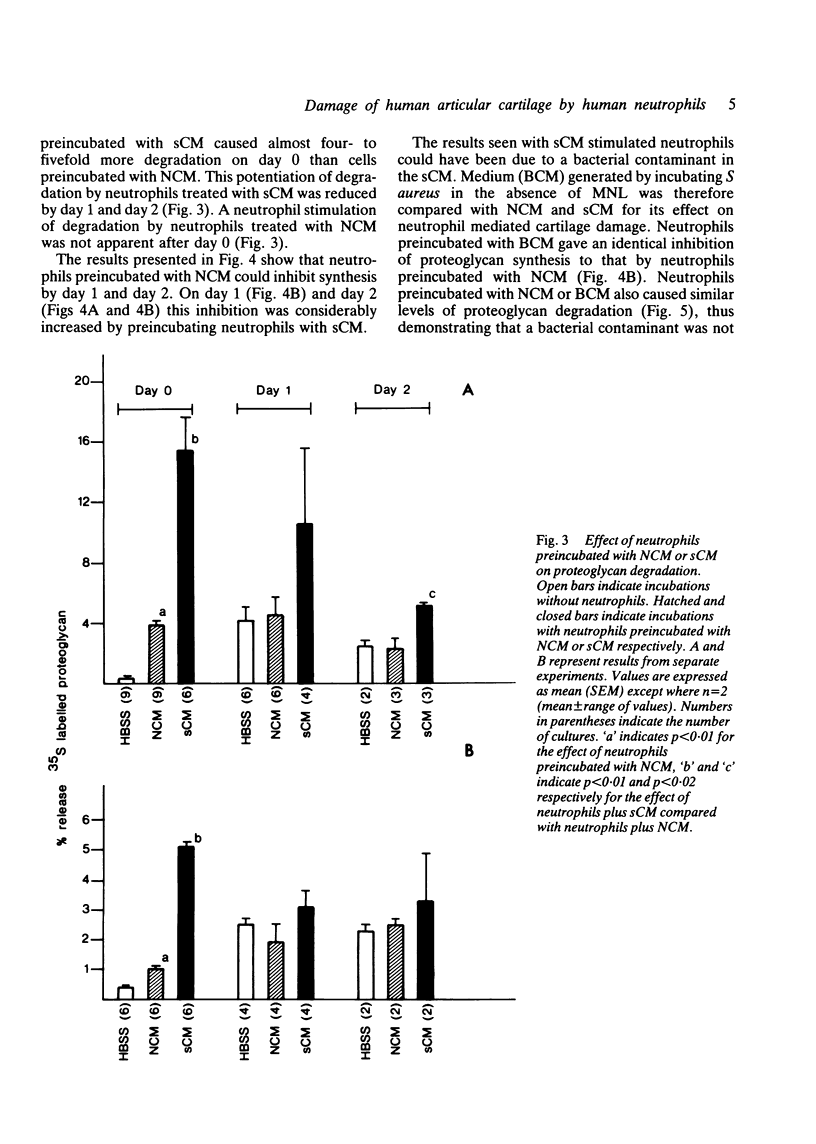
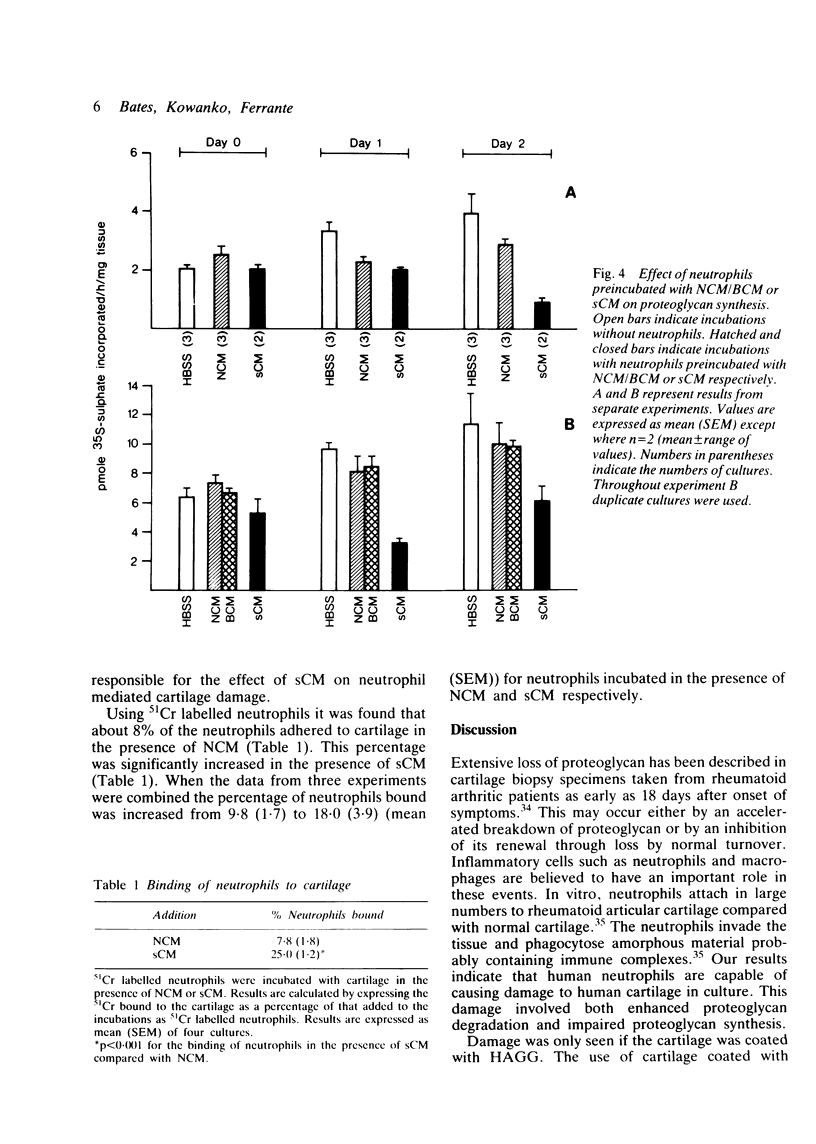
Human neutrophils were able to degrade proteoglycan and inhibit its synthesis when incubated with human articular cartilage coated with heat aggregated immunoglobulin G. These effects were potentiated when culture medium conditioned by mononuclear leucocytes stimulated with killed Staphylococcus aureus was also present during the incubations. Neutrophils preincubated with this conditioned medium and washed before incubation with cartilage also showed an increased ability to degrade proteoglycan and inhibit its synthesis. The percentage of neutrophils binding to cartilage was significantly increased in the presence of this conditioned medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew J. S., Lowther D. A., Handley C. J. Changes in proteoglycan biosynthesis following leukocyte elastase treatment of bovine articular cartilage in culture. Arthritis Rheum. 1984 Aug;27(8):905–912. doi: 10.1002/art.1780270810. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Harper G. S., Lowther D. A., Preston B. N. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984 May;8(5):629–637. [PubMed] [Google Scholar]
- Bates E. J., Johnson C. C., Lowther D. A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985 Feb 15;838(2):221–228. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Handley C. J. Oxygen free-radicals mediate an inhibition of proteoglycan synthesis in cultured articular cartilage. Ann Rheum Dis. 1984 Jun;43(3):462–469. doi: 10.1136/ard.43.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Burkhardt H., Schwingel M., Menninger H., Macartney H. W., Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. 1986 Mar;29(3):379–387. doi: 10.1002/art.1780290311. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario T. C., Andrews B. S., Martin D. A., Jason M., Treadwell T., Friou G., Tilles J. G. Interferon in synovial fluid and serum of patients with rheumatic disease. J Rheumatol. 1983 Aug;10(4):647–650. [PubMed] [Google Scholar]
- Cooke T. D., Hurd E. R., Ziff M., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. II. Preferential localization of antigen-antibody complexes to collagenous tissues. J Exp Med. 1972 Feb 1;135(2):323–338. doi: 10.1084/jem.135.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGré M., Mellbye O. J., Clarke-Jenssen O. Immune interferon in serum and synovial fluid in rheumatoid arthritis and related disorders. Ann Rheum Dis. 1983 Dec;42(6):672–676. doi: 10.1136/ard.42.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fay A. C., Trudgett A., McCrea J. D., Kirk F., Thompson J. M., Mitchell E. S., Boyd M. J., Roberts S. D., McNeill T. A. Detection and partial characterization of human B cell colony stimulating activity in synovial fluids of patients with rheumatoid arthritis. Clin Exp Immunol. 1985 May;60(2):316–322. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Abell T. J. Conditioned medium from stimulated mononuclear leukocytes augments human neutrophil-mediated killing of a virulent Acanthamoeba sp. Infect Immun. 1986 Feb;51(2):607–617. doi: 10.1128/iai.51.2.607-617.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Hill N. L., Abell T. J., Pruul H. Role of myeloperoxidase in the killing of Naegleria fowleri by lymphokine-altered human neutrophils. Infect Immun. 1987 May;55(5):1047–1050. doi: 10.1128/iai.55.5.1047-1050.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Mocatta T. J. Human neutrophils require activation by mononuclear leucocyte conditioned medium to kill the pathogenic free-living amoeba, Naegleria fowleri. Clin Exp Immunol. 1984 Jun;56(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Nandoskar M., Bates E. J., Goh D. H. Staphylococcus aureus-stimulated human mononuclear leucocyte-conditioned medium augments the basal and stimuli-induced neutrophil respiratory burst and degranulation. Immunology. 1987 Mar;60(3):431–438. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Rencis V. O. Enhancement of base hexose-monophosphate shunt activity of human polymorphonuclear leucocytes by human beta-interferon. Immunol Lett. 1984;8(4):215–217. doi: 10.1016/0165-2478(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Separation of mononuclear and polymorphonuclear leucocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48(1):81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Gordon T. P., Bradley J., Roberts-Thomson P. J. Neutrophil activation by immune complexes and the role of rheumatoid factor. Ann Rheum Dis. 1984 Feb;43(1):34–39. doi: 10.1136/ard.43.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard G. C., Lowther D. A. Carrageenin-induced arthritis. II. Effect of intraarticular injection of carrageenin on the synthesis of proteoglycan in articular cartilage. Arthritis Rheum. 1976 Sep-Oct;19(5):918–922. doi: 10.1002/art.1780190513. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Herman J. H., Khosla R. C., Mowery C. S., Appel A. M. Modulation of chondrocyte synthesis by lymphokine-rich conditioned media. Arthritis Rheum. 1982 Jun;25(6):668–676. doi: 10.1002/art.1780250610. [DOI] [PubMed] [Google Scholar]
- Herman J. H., Musgrave D. S., Dennis M. V. Phytomitogen-induced, lymphokine-mediated cartilage proteoglycan degradation. Arthritis Rheum. 1977 May;20(4):922–932. doi: 10.1002/art.1780200404. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Release of neutral protease and beta-glucuronidase from human neutrophils in the presence of cartilage treated with various immunologic reactants. J Immunol. 1974 Jul;113(1):298–308. [PubMed] [Google Scholar]
- Ishikawa H., Smiley J. D., Ziff M. Electron microscopic demonstration of immunoglobulin deposition in rheumatoid cartilage. Arthritis Rheum. 1975 Nov-Dec;18(6):563–576. doi: 10.1002/art.1780180606. [DOI] [PubMed] [Google Scholar]
- Janis R., Hamerman D. Articular cartilage changes in early arthritis. Bull Hosp Joint Dis. 1969 Oct;30(2):136–152. [PubMed] [Google Scholar]
- Janoff A., Feinstein G., Malemud C. J., Elias J. M. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. I. Penetration of enzyme into rabbit articular cartilage and release of 35SO4-labeled material from the tissue. J Clin Invest. 1976 Mar;57(3):615–624. doi: 10.1172/JCI108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Dingle J. T. Human mononuclear cell factors mediate cartilage matrix degradation through chondrocyte activation. J Clin Invest. 1981 Sep;68(3):571–581. doi: 10.1172/JCI110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T., Oppenheim J. J., Jasin H. E. Human interleukin 1 mediates cartilage matrix degradation. Cell Immunol. 1985 Mar;91(1):92–99. doi: 10.1016/0008-8749(85)90034-6. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Gillard G. C. Carrageenin-induced arthritis. I. The effect of intraarticular carrageenin on the chemical composition of articular cartilage. Arthritis Rheum. 1976 Jul-Aug;19(4):769–776. doi: 10.1002/1529-0131(197607/08)19:4<769::aid-art1780190419>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Menninger H., Putzier R., Mohr W., Wessinghage D., Tillmann K. Granulocyte elastase at the site of cartilage erosion by rheumatoid synovial tissue. Z Rheumatol. 1980 May-Jun;39(5-6):145–156. [PubMed] [Google Scholar]
- Mohr W., Westerhellweg H., Wessinghage D. Polymorphonuclear granulocytes in rheumatic tissue destruction. III. an electron microscopic study of PMNs at the pannus-cartilage junction in rheumatoid arthritis. Ann Rheum Dis. 1981 Aug;40(4):396–399. doi: 10.1136/ard.40.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin. Interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci. 1975 Jun 13;256:409–419. doi: 10.1111/j.1749-6632.1975.tb36067.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. The role of the human neutrophil in the inflammatory reaction. Allergy. 1980 Jan;35(1):1–13. doi: 10.1111/j.1398-9995.1980.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Arthritogenic activity of interleukin 1. Agents Actions. 1986 Dec;19(5-6):337–338. doi: 10.1007/BF01971244. [DOI] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Braunsteiner H. Cationic proteins from human neutrophil granulocytes. Evidence for their chymotrypsin-like properties. Biochim Biophys Acta. 1975 Feb 27;379(2):606–617. doi: 10.1016/0005-2795(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Lowther D. A., Brown H. L. Antigen-induced arthritis. Studies on the inhibition of proteoglycan synthesis observed in articular cartilage during short-term joint inflammation. Arthritis Rheum. 1980 Apr;23(4):433–447. doi: 10.1002/art.1780230406. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Sriratana A., Brown H. L., Lowther D. A. Evidence for polymorphonuclear-leucocyte-derived proteinases in arthritic cartilage. Biochem J. 1981 Jan 1;193(1):193–202. doi: 10.1042/bj1930193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer V., Sriratana A., Lowther D. A. Carrageenin-induced arthritis: V. A morphologic study of the development of inflammation in acute arthritis. Semin Arthritis Rheum. 1983 Nov;13(2):160–168. doi: 10.1016/0049-0172(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Smith R. J., Bowman B. J., Speziale S. C. Interleukin-1 stimulates granule exocytosis from human neutrophils. Int J Immunopharmacol. 1986;8(1):33–40. doi: 10.1016/0192-0561(86)90070-6. [DOI] [PubMed] [Google Scholar]
- Stastny P., Rosenthal M., Andreis M., Ziff M. Lymphokines in the rheumatoid joint. Arthritis Rheum. 1975 May-Jun;18(3):237–243. doi: 10.1002/art.1780180307. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugai K., Ishikawa H., Hirohata K., Shirane H. Interaction of polymorphonuclear leukocytes with immune complexes trapped in rheumatoid articular cartilage. Arthritis Rheum. 1983 Dec;26(12):1434–1441. doi: 10.1002/art.1780261204. [DOI] [PubMed] [Google Scholar]
- Velvart M., Fehr K., Baici A., Sommermeyer G., Knöpfel M., Cancer M., Salgam P., Böni A. Degradation in vivo of articular cartilage in rheumatoid arthritis by leucocyte elastase from polymorphonuclear leucocytes. Rheumatol Int. 1981;1(3):121–130. doi: 10.1007/BF00541256. [DOI] [PubMed] [Google Scholar]
- Werb Z., Dingle J. T., Reynolds J. J., Barrett A. J. Proteoglycan-degrading enzymes of rabbit fibroblasts and granulocytes. Biochem J. 1978 Sep 1;173(3):949–958. doi: 10.1042/bj1730949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J. A., Warrington R. J., Sigurdson S. L., Rutherford W. J. The demonstration of an interleukin-2 like activity in the synovial fluids of rheumatoid arthritis patients. J Rheumatol. 1983 Feb;10(1):109–113. [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]


