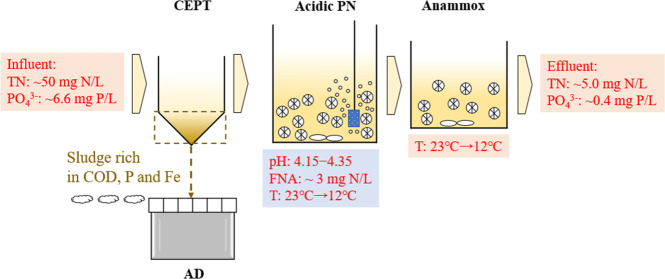
Abstract
Chemically enhanced primary treatment (CEPT) followed by partial nitritation and anammox (PN/A) and anaerobic digestion (AD) is a promising roadmap to achieve energy-neutral wastewater treatment. However, the acidification of wastewater caused by ferric hydrolysis in CEPT and how to achieve stable suppression of nitrite-oxidizing bacteria (NOB) in PN/A challenge this paradigm in practice. This study proposes a novel wastewater treatment scheme to overcome these challenges. Results showed that, by dosing FeCl3 at 50 mg Fe/L, the CEPT process removed 61.8% of COD and 90.1% of phosphate and reduced the alkalinity as well. Feeding by low alkalinity wastewater, stable nitrite accumulation was achieved in an aerobic reactor operated at pH 4.35 aided by a novel acid-tolerant ammonium-oxidizing bacteria (AOB), namely, Candidatus Nitrosoglobus. After polishing in a following anoxic reactor (anammox), a satisfactory effluent, containing COD at 41.9 ± 11.2 mg/L, total nitrogen at 5.1 ± 1.8 mg N/L, and phosphate at 0.3 ± 0.2 mg P/L, was achieved. Moreover, the stable performances of this integration were well maintained at an operating temperature of 12 °C, and 10 investigated micropollutants were removed from the wastewater. An energy balance assessment indicated that the integrated system could achieve energy self-sufficiency in domestic wastewater treatment.
Keywords: domestic wastewater treatment, CEPT, mainstream anammox, acidophilic ammonia oxidation, low temperature, energy neutrality
Short abstract
This study demonstrates a novel wastewater treatment scheme achieved by the newly discovered acidophilic ammonia oxidation, enabling domestic wastewater treatment plants to achieve energy neutrality in the future.
1. Introduction
Wastewater treatment plants (WWTPs) remove pollutants and protect water bodies while consuming considerable energy, i.e., the electricity consumed can account for about 3% of the total annual electricity demand.1 In recent years, WWTPs are pursuing technologies to maximize bioenergy recovery and to minimize energy consumption, enabling energy neutrality. It has been recognized worldwide that the widely applied chemically enhanced primary treatment (CEPT) together with anaerobic digestion (AD) is one of the easiest-to-use approaches for bioenergy recovery. In principle, CEPT can efficiently preconcentrate organic carbon from wastewater to primary sludge and subsequently fed to an anaerobic digester to produce bioenergy. This bioenergy can offset the energy demand of WWTPs and lower the energy requirement of aeration at the same time. Following the carbon removal, an innovative process, namely, partial nitritation and anammox (PN/A), has been proposed to autotrophically remove nitrogen for the carbon-deficient wastewater, ensuring effluent quality to meet the requirement, as does the conventional nitrification and denitrification process.2 Despite these advantages, the maximization of bioenergy recovery by CEPT and the energy-efficient PN/A in domestic sewage treatment are both limited by critical issues in practice, as elaborated below.
The CEPT usually uses iron salts (e.g., FeCl3) as an optimal flocculant. In comparison to other flocculants, it can efficiently reduce organic carbon and phosphorus in wastewater and simultaneously bring multiple benefits to the following sludge management process (e.g., improving sludge settleability and dewaterability and reducing notorious gas emission).3,4 However, the dosed FeCl3 can cause a secondary effect of decreasing alkalinity via the hydrolysis of metal ions (i.e., Fe3+),5,6 probably resulting in the molar ratio of alkalinity (calculated as CaCO3) to ammonium in mainstream wastewater to below 1.7 The decrease of wastewater alkalinity is generally neglectable for traditional denitrification but critical to the PN/A process. This is because denitrification can significantly regenerate alkalinity, as not does anammox. Moreover, microbial ammonia oxidation in the downstream nitrogen removal process further consumes alkalinity at 1 mol equiv CaCO3 per mol ammonia oxidized. Therefore, the alkalinity becomes insufficient to maintain the neutral pH of wastewater during the PN/A process, which can drop to below 6 when the alkalinity is depleted and no base is re-supplemented. Such low pH poses adverse effects on the traditional ammonia-oxidizing bacteria (AOB) and anammox bacteria that prefer neutral conditions.
Apart from the issue caused by low wastewater alkalinity, another key challenge for the application of mainstream PN/A is the stable suppression of nitrite-oxidizing bacteria (NOB).8 Many technologies have been developed to achieve this aim, including the use of low dissolved oxygen (DO),9−11 intermittent aeration,12,13 shortening sludge retention time (SRT),12,14,15 and sidestream sludge treatment using different strategies.16−20 Among all, free nitrous acid (FNA) is known as one of the most effective reagents for NOB inactivation, which is also one of the major reasons for the stable PN achieved in treating high-strength ammonium wastewater (∼1 g N/L).21 However, due to the significantly lower total nitrogen in domestic sewage (40–60 mg N/L) and the generally neutral pH level (>6) required by traditional AOB, it was deemed impossible to achieve an in situ FNA level sufficient for NOB suppression under mainstream conditions.
These two issues associated with CEPT and PN/A can be tactfully solved by a novel microbial process: acidophilic ammonia oxidation. This process can be catalyzed by the extremely acid-tolerant AOB, namely, Candidatus (Ca.) Nitrosoglobus, with the first member discovered from an acidic soil sample recently.22 Unlike the common AOB (e.g., Nitrosomonas) in wastewater treatment, Nitrosoglobus-like AOB can keep satisfactory activity at slightly acidic conditions (pH 4–5) and are still active at pH even low as 2.22−25 This capability to tolerate acidic condition indicates that a PN process can be operated under slightly acidic conditions. By performing the acidic ammonia oxidation, AOB can produce protons and nitrite to lower the wastewater pH and form a high FNA concentration at parts per million (ppm) level in situ.26 This technology fundamentally differs from all previous NOB inactivation strategies, which uses in situ self-sustained stress (i.e., FNA) instead of ex situ treatment of sludge and is proven energy-efficient and robust.27,28 To be noted, it is theoretically only possible to achieve such a low pH for domestic sewage with low alkalinity (i.e., CaCO3 alkalinity/ammonium molar ratio < 1), which perfectly fits the scenario using CEPT for carbon removal. Two birds with one stone, the acidic PN process has great potential not only to cope with the low alkalinity CEPT effluent but also to maintain stable PN for autotrophic nitrogen removal by anammox.
Collectively, this study aims to demonstrate a novel integration of a CEPT followed by a two-stage PN/A process in domestic wastewater treatment by incorporating acidophilic ammonia oxidation. To this end, the effects of FeCl3 dosage on the removal of organic carbon, phosphate, and alkalinity were first evaluated with batch tests, based on which an optimal FeCl3 dosage was selected for the long-term experiment. Afterward, a long-term experiment assessment was conducted in a laboratory system consisting of three units, including a CEPT, an acidic PN, and an anammox reactor, which were continuously fed with real wastewater for one year (Figure S1). After the long-term evaluation, the impacts of temperature variation mimicking the seasonal change (12–23 °C) and the system’s removal capacity on investigated organic micropollutants were further examined, which were reported, for the first time, for acidic AOB and would provide a comprehensive assessment of the technology. Throughout the 360-day operation, microbial communities were monitored by 16S rRNA amplicon sequencing. This novel integration of CEPT, acidic PN, and anammox provided a valuable solution to achieve energy neutrality in wastewater treatment.
2. Materials and Methods
2.1. Determination of an Optimal Iron Dosage for CEPT
Batch tests were first carried out to determine the optimal iron dosage for CEPT, based on the following standards: (i) decreasing the CaCO3 alkalinity/ammonium molar ratio to below 1 and (ii) achieving substantial removal (>70%) of total COD (TCOD) and total phosphorus (TP). Six identical 1-L beakers were used with different FeCl3 dosages (at 0, 10, 25, 50, 75, and 100 mg Fe/L). Initially, 500 mL of raw domestic wastewater was added to each beaker. Then, a concentrated FeCl3 solution (38% w/w) in the form of FeCl3·6H2O at an analytical grade was dosed in each system, giving different levels of Fe3+. The beaker without iron dosing served as a control. The CEPT was performed in batch mode. Each beaker was mixed with a magnetic stirrer at 300 rpm for 2 min and at 100 rpm for 10 min. After settling for 30 min, TCOD, soluble COD (SCOD), TP, phosphate (PO43–-P), organic nitrogen, NH4+-N, and alkalinity (in terms of CaCO3) were measured in the supernatant.
2.2. Long-Term Experimental Design and System Set-Up
The entire wastewater treatment process consisted of three units, i.e., a CEPT, an acidic PN, and an anammox unit. The CEPT of domestic wastewater was conducted in batch mode as described in Section 2.1. The domestic wastewater was fortnightly collected from a local pumping station located at St. Lucia, Queensland, Australia. After collection, the wastewater was immediately stored in a 1000 L storage tank in a temperature-controlled room (4 °C), minimizing biological transformation during storage. Prior to use, the wastewater was warmed up to room temperature (22 ± 1 °C) via a heater (IC-TH7100, RATEK). The key characteristics of collected raw wastewater are presented in Table S1. The supernatant of the CEPT process was fed to the downstream system to remove nitrogen via a two-stage PN/A process.
The acidic PN and anammox were both achieved in a moving bed biofilm reactor (MBBR). The working volume of the aerobic MBBR for acidic PN was 1.8 L, seeding with acidophilic AOB-enriched K5 carriers at a filling ratio of 30%. The enrichment process of acidophilic AOB in K5 carriers was detailed in our previous work.28 The anoxic MBBR of 1.0 L working volume was inoculated with anammox-containing K5 carriers at a filling ratio of 30%. The carriers were taken from a pilot-scale MBBR that treated AD liquor via the PN/A pathway in a local WWTP at Brisbane, Australia. About 5.0 L CEPT effluent was continuously pumped into the system each day, which was controlled by two peristaltic pumps (Figure S1). This resulted in hydraulic retention time (HRT) of 9.6 and 4.8 h for the aerobic and anoxic MBBRs, respectively. In specific, 4.5 L CEPT effluent was fed to the aerobic MBBR and flowed to the anoxic MBBR, while 0.5 L CEPT effluent was directly fed to the anoxic MBBR. The bypassing of part of CEPT effluent aimed at increasing the pH of anoxic MBBR to a neutral level, avoiding the negative effect of low pH on anammox activity.
The pH in each MBBR was monitored by a pH probe (miniCHEM, Labtek) and a transmitter (multiparameter transmitter M800, Mettler Toledo). In the aerobic MBBR, the pH was controlled by a programmable logic controller (PLC) via on/off control of the air pump, namely, pH-based aeration. Specifically, the air pump was turned off when the pH decreased to the setpoint (4.35) and vice versa. In the anoxic MBBR, the NaOH stock solution (0.1 M) was periodically added via a peristaltic pump that was operated intermittently at an on/off ratio of 1:90 min to maintain its pH value at around 7.0. In total, about 24 mL of NaOH stock solution (0.1 M) was dosed daily to the anoxic MBBR. The data was recorded for cost analysis. The two MBBRs were mixed with the magnetic stirrer at a rate of 200 rpm. The system was continuously operated for 360 days, which was divided into four phases, according to the operating temperature: 23 °C in phase I (day 1–275), 20 °C in phase II (day 276–295), 15 °C in phase III (day 296–315), and 12 °C in phase IV (day 316–360). The temperature was controlled by an immersion cooler (RC1, Ratek, Australia) and a precision immersion heater circulator (TH8000, Ratek, Australia).
2.3. Monitoring Plan for the Laboratory-Scale Treatment System
Samples were regularly taken (2–3 times per week) from the influent and the effluent of each MBBR and filtered through 0.22 μm poly(ethersulfone) disposable sterile Millipore filters (Merck) for the analysis of NH4+-N, NO2–-N, NO3–-N, and PO43–-P (Text S1 of the Supporting Information). The COD concentrations of influent and effluent of each MBBR were measured weekly. The maximal AOB, NOB, and anammox activities were assessed through batch tests (Text S2 of the Supporting Information). Liquid samples were collected at the end of phase I and then filtered through 0.22 μm poly(ethersulfone) disposable sterile Millipore filters (Merck) for the measurement of organic micropollutants (Text S1 of the Supporting Information). Amplicon sequencing (16S rRNA sequencing) was conducted at the end of phases I and IV to assess microbial communities of the two MBBRs (Text S3 of the Supporting Information). The mass and energy balance assessments of the proposed system were also conducted (Text S4 of the Supporting Information).
3. Results and Discussion
3.1. Performance of CEPT
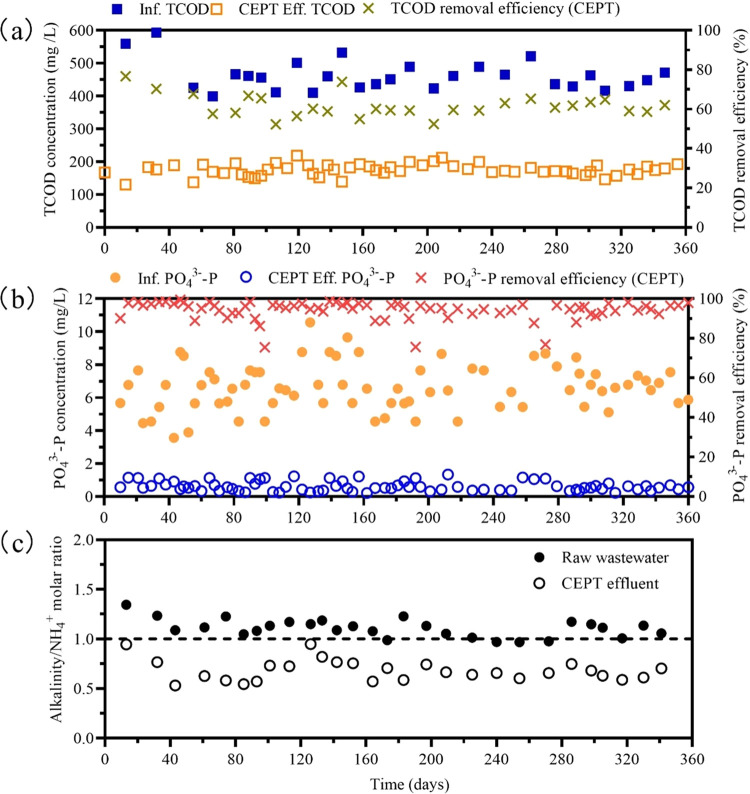
Five FeCl3 dosages (10, 25, 50, 75, and 100 mg Fe/L) were tested to evaluate changes of alkalinity, NH4+-N, pH, TCOD, SCOD, TP, and PO43–-P in raw domestic wastewater and CEPT effluent (Figure S2). The reduced alkalinity (measured as CaCO3) is linearly related to the levels of FeCl3 dosed (R2 = 0.991), as expected. The slope was estimated to be 1.15 mM CaCO3 alkalinity per mM FeCl3. This is consistent with the theoretical calculation that Fe(OH)2+ is the major iron(III) species (>95% in total Fe) in aqueous solutions at pH 4.8–7.0, 25 °C.29 The NH4+-N concentration was not influenced by iron dosages, while the pH of wastewater slightly decreased from 7.4 to 6.6 with the addition of FeCl3 increasing from 0 to 100 mg Fe/L. Consequently, the CaCO3 alkalinity/ammonium molar ratio decreased to <1 at dosages above 25 mg Fe/L, and this ratio was reduced to 0.62 by dosing FeCl3 at 100 mg Fe/L. Therefore, the FeCl3 dosage above 25 mg Fe/L would be needed to generate the low alkalinity wastewater. Meanwhile, the FeCl3 dosing significantly decreased TCOD, TP, and PO43–-P concentrations in domestic wastewater. Substantial (>70%) removal of TCOD and TP was achieved at 50 mg Fe/L and above, comparable to the results previously reported for CEPT.30,31 Taken together, the dosage of 50 mg Fe/L was chosen in the long-term experiment to maximize the recovery of organic carbon and phosphorus and to decrease the CaCO3 alkalinity/ammonium molar ratio to below 1.
In the long-term operation of CEPT (Figure 1), the COD concentration in CEPT effluent was consistently lower than that of raw wastewater, clearly showing the effect of FeCl3 dosing on COD removal. The average COD concentrations in the raw wastewater and CEPT effluent were 461.7 ± 46.1 and 174.9 ± 17.7 mg COD/L, respectively, representing an average removal efficiency of 61.8 ± 5.7% TCOD. The PO43–-P concentration in wastewater was reduced from 6.6 ± 1.4 to 0.6 ± 0.3 mg P/L, showing a reduction of 90.1 ± 5.4%. The protons produced in the Fe3+ hydrolytic process consumed alkalinity in the wastewater, and the NH4+-N concentrations in wastewater remained almost unchanged (46.6 ± 5.4 vs 45.7 ± 5.6 mg N/L). As such, the CaCO3 alkalinity/ammonium molar ratio decreased from 1.1 ± 0.1 in raw wastewater to 0.7 ± 0.1 in the CEPT effluent. Together, these results showed that dosing 50 mg of Fe to each liter of wastewater for CEPT effectively reduced the TCOD, PO43–-P, and alkalinity levels in raw wastewater.
Figure 1.
Performance of the CEPT process. (a) Total COD concentrations in the influent and effluent and the total COD removal efficiency. (b) Phosphate concentrations in the influent and effluent and the phosphate removal efficiency. (c) Molar ratio of CaCO3 alkalinity to ammonium in the raw wastewater and the chemically enhanced primary treatment (CEPT) effluent.
3.2. Performance of the Two-Stage PN/A Process
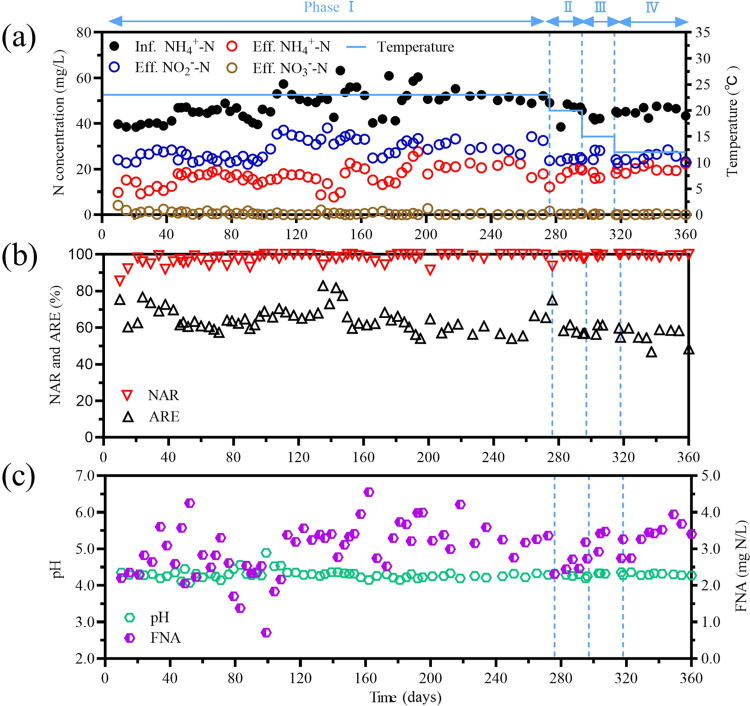
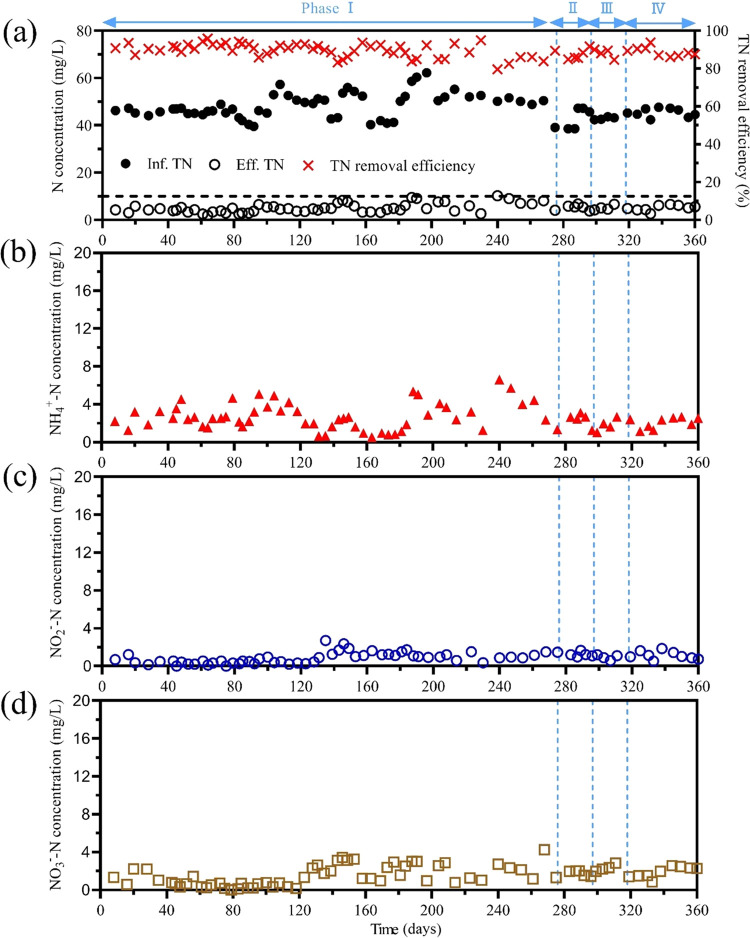
The stability of nitrogen removal performance of the proposed two-stage PN/A system was first investigated in phase I (days 1–275) at a temperature of 23 °C. After that, the operating temperature was stepwise decreased to 20 °C in phase II (days 276–295), to 15 °C in phase III (days 296–315), and to 12 °C in phase IV (days 316–360) to mimic the seasonal variations in temperature. In phase I, the average NO2–-N, NO3–-N, and NH4+-N concentrations in the effluent of the aerobic MBBR were 28.8 ± 4.2, 0.6 ± 0.8, and 16.9 ± 4.2 mg N/L (Figure 2a), respectively. These gave an average NH4+-N removal efficiency (ARE) of 64.7 ± 6.4% and a nitrite accumulation ratio (NAR) of 97.7 ± 2.9% (Figure 2b). Such high NAR means NOB in the aerobic MBBR was successfully suppressed. The aerobic MBBR was operated at acidic conditions (average pH of 4.3 ± 0.1), leading to the successful formation of high FNA concentration (3.0 ± 0.7 mg HNO2-N/L) under the condition of low NO2–-N concentration (about 30 mg N/L), which results in the stable inhibition of NOB (Figure 2c). The FNA concentration (i.e., 3.0 ± 0.7 mg HNO2-N/L) sustained in this aerobic MBBR was much higher than the reported threshold value (i.e., above 1 mg of HNO2-N/L) for NOB suppression.28 In the aerobic MBBR, TCOD of 36.0 ± 18.9 mg/L was removed (Figure S3), showing that heterotrophic bacteria survived in this acidic reactor and removed organic carbon to a certain extent. The feeding of anoxic MBBR was a mixture, consisting of aerobic MBBR effluent (90%, 4.5 L/days) and CEPT effluent (10%, 0.5 L/days). This mixture contains NO2–-N and NH4+-N at a ratio of 1.35 ± 0.35 (Figure 3), which is very close to the theoretical stoichiometric ratio (i.e., 1.32) for anammox bacteria. The average TN concentration in the effluent of the anoxic MBBR was 5.0 ± 2.0 mg N/L. Compared to 48.4 ± 5.3 mg N/L in the influent of the PN/A process (i.e., the CEPT effluent), this represents a decrease of 89.7 ± 3.6% in TN concentration (Figure 3a), which is also very close to the theoretical value of anammox reaction (i.e., 88.8%). The effluent NO3–-N concentration (1.5 ± 1.1 mg N/L) was lower than the theoretical value (about 5 mg N/L), implying the potential contribution of heterotrophic denitrification in the anoxic MBBR. The potential of denitrification was likely due to the organic carbon in the bypassed 10% of the CEPT effluent and corroborated by the observed reduction of COD in the anoxic MBBR from 77.3 ± 14.2 to 35.0 ± 9.1 mg/L (Figure S3). The average NH4+-N and NO2–-N concentrations in the effluent in the anoxic MBBR were 2.8 ± 1.4 and 0.9 ± 0.6 mg N/L, respectively. The pH of anoxic MBBR (i.e., the effluent of the system) was around 7.2 (Figure S4), which was significantly higher than that (i.e., 4.15–4.35) of aerobic MBBR. Three key factors elevating the pH of anoxic MBBR: (1) the bypassed 10% feeding from the CEPT effluent, (2) the alkalinity regenerated by denitrification in the anoxic process, and (3) the added NaOH solution. The recorded NaOH consumption of this process was only 25 g NaOH per ton of wastewater. This is actually a very low chemical consumption because of alkalinity from the other two pathways. In the real application, lime rather than NaOH can be used to elevate pH, which will further reduce the cost. The requirement of the base can also be reduced by increasing the ratio of CEPT effluent in the feed of an anammox tank.
Figure 2.
Performance of aerobic MBBR (i.e., acidic PN). (a) Influent NH4+-N and effluent NH4+-N, NO2–-N, and NO3–-N concentrations and the temperature of the aerobic MBBR. (b) Nitrite accumulation ratio (NAR) and NH4+-N removal efficiency (ARE) of the aerobic MBBR. (c) The pH and free nitrous acid (FNA) concentration in the aerobic MBBR.
Figure 3.
Performance of anoxic MBBR (i.e., anammox). (a) Influent and effluent total nitrogen (TN) concentrations and the TN removal efficiency of the anoxic MBBR. Profiles of NH4+-N (b), NO2–-N (c), and NO3–-N (d) concentrations in the effluent of the anoxic MBBR.
With the temperature decreased to 20 °C in phase II, the NAR (97.8 ± 2.4%) remained stable, indicating robust NOB suppression, while the ARE slightly decreased to 62.0 ± 6.3%, implying the possible effect of temperature on AOB activity (detailed in Section 3.7). The relatively lower ARE has resulted in the lower NO2–-N to NH4+-N ratio (i.e., 1.22 ± 0.19) in the wastewater to the anoxic MBBR. However, the TN removal efficiency of anoxic MBBR appeared to be insusceptible, averaging at 87.3 ± 2.8% in phase II (Figure 2b). The ARE slowly decreased to 60.4 ± 4.7 and 56.0 ± 5.0% when the operating temperature decreased to 15 °C in phase III and 12 °C in phase IV, respectively. On the contrary, the NAR increased to 99.1 ± 0.7% in phase III and 99.4 ± 0.5% in phase IV. The possible reason is that the FNA concentration in the aerobic MBBR in phases III (3.1 ± 0.4 mg N/L) and IV (3.4 ± 0.3 mg N/L) became higher than that in phase I (3.0 ± 0.7 mg N/L) (Figure 2c). The FNA concentration is positively associated with NO2–-N concentration and negatively related to pH and temperature. Indeed, the pH in phases I, III, and IV was the same (4.3 ± 0.1), and phase I (28.8 ± 4.2 mg N/L) even had a higher NO2–-N concentration than that in phases III (25.9 ± 2.1 mg N/L) and IV (25.5 ± 1.9 mg N/L). This confirms that the higher FNA concentrations in phase III and IV were led by the lower operating temperature. In the anoxic MBBR, about 88.1 ± 2.4 and 88.9 ± 2.5% of TN in the wastewater were removed in phases III and IV (Figure 3a), which is comparable to that in phase I (89.7 ± 3.6%), indicating robust anammox activity at low temperature conditions.
3.3. Removal of Micropollutants
Ten micropollutants, which were dominated in the real wastewater used in the present study, were evaluated in different units of this system at the end of phase I (see Figure S5). These micropollutants are common pharmaceuticals and personal care products (PPCPs) found in domestic wastewater.32,33 For example, salicylic acid is widely used in skin care products; caffeine is often added to the drink; and ibuprofen is a widely used anti-inflammatory pain reliever. All of the investigated micropollutants were removed from wastewater in the proposed wastewater treatment system at different extents from 26.2 to 99.7%. The removal efficiency of four micropollutants exceeded 75%, including atenolol, acesulfame, caffeine, and salicylic acid. In comparison to the conventional nitrification/denitrification process and other PN/A processes, the proposed system achieved similar removal efficiencies for atenolol and acesulfame and significantly higher removal for carbamazepine (57.2% vs almost no removal).34,35 The average removal efficiencies of ten investigated micropollutants were 6.2 ± 7.1% in CEPT, 41.0 ± 25.2% in the aerobic MBBR, and 20.1 ± 26.1% in the anoxic MBBR. The FeCl3-induced flocculent process removed a small part of investigated micropollutants. In comparison, the majority of micropollutants was removed in the aerobic MBBR, possibly related to the co-metabolic pathway of AOB, as well as a high FNA concentration in acidic PN.36 For example, Cheng et al.37 reported that FNA at 1 mg N/L can chemically remove sulfamethoxazole.
3.4. Microbial Community Analysis
To shed light on the compositions and dynamics of the microbial community at different temperatures, microbial samples were taken from the aerobic and anoxic MBBRs in the steady state of phases I (23 °C) and IV (12 °C) for 16S rRNA gene amplicon sequencing. The Good’s coverage estimator on the OTUs showed that 99% of the species were captured in all samples. The top ten phyla and the top five genera in both MBBRs at different temperatures are shown in Figure S6, showing an overall stable microbial community from 23 to 12 °C. Proteobacteria, Actinobacteriota, and Planctomycetota consistently remained the top three bacterial populations in the aerobic MBBR, with average abundances of 63.3, 16.1, and 13.5%, respectively. Chloroflexi, Proteobacteria, and Planctomycetota had the highest relative abundance, accounting for 32.1, 24.3, and 17.1% of all of the detected bacteria in the anoxic MBBR, respectively.
The top five genera in the aerobic and anoxic MBBRs are also stable from 23 to 12 °C (Figure S6e). In the aerobic MBBR, only one nitrifying genus was detected, namely, Ca. Nitrosoglobus, with a relative abundance of 3.5% at 23 °C and 2.5% at 12 °C, while no NOB was detected. Ca. Nitrosoglobus, identified as the genus of AOB, belongs to the Proteobacteria phylum.22 This agrees with the nitrifying community reported for other acidic PN reactors23,27 and also explains the over 97% NAR achieved in the aerobic MBBR during the long-term operation (Figure 2b). The slight decrease in relative abundance of Ca. Nitrosoglobus may be associated with the negative impact of low temperature. Ca. Brocadia is a typical genus carrying out the anammox reaction, belonging to the Planctomycetes phylum.38 It is the only anammox bacteria detected in the anoxic MBBR and its relative abundance was stable at 23 °C (6.46%) and 12 °C (6.11%). Denitratisoma, which is a typical denitrifying genus, was consistently detected in the anoxic MBBR, with an average relative abundance of 3.66%.39 The results of microbial composition analysis also support the activity tests showing that the AOB activity in the aerobic MBBR was more sensitive to the decrease of temperature than the anammox activity in the anoxic MBBR (see more results in Section 3.6).
3.5. Development of a New Operation Strategy for Acidic PN
The acidic PN is a novel concept, which was recently discovered by Li et al.26 in treating diluted real urine that contained NH4+-N at about 200 mg N/L, followed by Wang et al.27 and Meng et al.,28 which both successfully maintained acidic PN in treating low-strength wastewater (i.e., <100 mg NH4+-N/L). These previous studies had focused on studying the feasibility and stability of acidic PN, while the research on applying the acidic PN process toward practical applications is still needed. This study, for the first time, proposed an integrated treatment process, using the acidic PN process to tactfully solve the issues of upfront CEPT (i.e., acidified wastewater by iron hydrolysis) and downstream autotrophic nitrogen removal via anammox (i.e., NOB suppression). This integrated system was comprehensively evaluated by treating real domestic sewage for 360 days at varying temperatures, during which satisfactory effluent quality was well maintained, with an average effluent TCOD concentration of 41.9 ± 11.2 mg/L, TN concentration of 5.1 ± 1.8 mg N/L, and phosphate concentration of 0.3 ± 0.2 mg/L (Table S2). The stable and superior performance provides evidence showing the applicability of the proposed integrated system, consisting of a CEPT, an acidic PN, and an anammox, to treat domestic wastewater in practice.
The acidic PN reactor should be operated at a reasonable pH range. At acidic conditions, nitrite can be chemically oxidized to nitrate, and the reaction rate increases with decreased pH.40 During chemical oxidation of nitrite, nitric oxide, a hazardous gas, can be produced,27,40 which may trigger occupational health and safety concerns.41 Thus, the acidic PN reactor cannot be operated at a very low pH condition (i.e., pH < 4) to avoid significant nitric oxide emission. Conversely, if the operating pH is higher than 5.5, the FNA concentration will be too low to suppress NOB. It is suggested that an adequate FNA concentration for NOB suppression should be above 1 mg N/L.28 Therefore, the previous study proposed using pH-based feeding to control the pH around the setpoint;28 however, this strategy is difficult to be achieved in the operation of real WWTPs. In the present study, a pH-based aeration strategy was applied for the first time. The aeration was turned off when the pH decreased to the setpoint, leading to the decrease in DO as well as the NH4+-N oxidation rate by AOB (Figure 4). Once the NH4+-N oxidation rate was lower than the NH4+-N loading rate, the pH will gradually increase to the setpoint triggering the aeration and the start of the next cycle. The DO reduction rate decreased when the DO concentration was below 1 mg O2/L (Figure 4), which agreed with previously reported oxygen affinity of Ca. Nitrosoglobus of 0.92 ± 0.37 mg O2/L.23 Overall, compared to the pH-based feeding method, this technology is more feasible and reliable in practice, as DO control has been widely used in many WWTPs.
Figure 4.
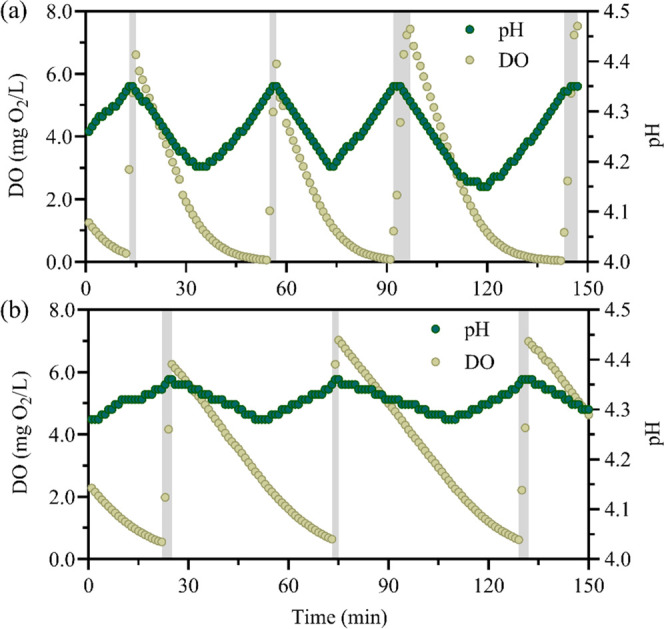
Profiles of pH and dissolved oxygen (DO) in the typical periods during stable operation of the aerobic MBBR at 23 °C (a) and 12 °C (b).
3.6. Robustness of Mainstream Anammox toward Temperature Variation
The low temperature of domestic wastewater is a major challenge for mainstream PN/A.42−44 Here, a high nitrogen removal efficiency (88.9 ± 2.5%) was stably achieved in treating domestic wastewater at temperatures as low as 12 °C. The nitrogen loading rate of the developed two-stage PN/A was ∼0.1 kg N/m3/days. This rate is comparable to that of conventional nitrification/denitrification process45 and is higher than most mainstream PN/A processes reported in the literature (Table S4).
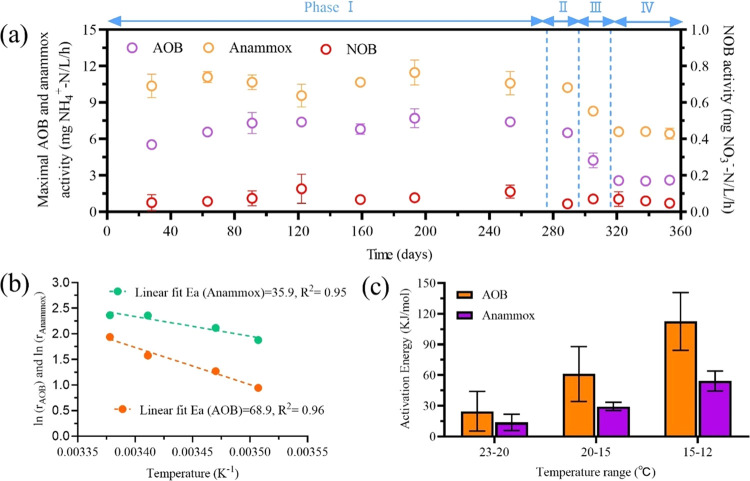
The maximal AOB and NOB activities of aerobic MBBR and the maximal anammox activity of anoxic MBBR were regularly monitored during the long-term operation (Figure 5a). Following the temperature decrease, the maximal AOB activity declined from 7.0 ± 0.8 mg NH4+-N/(L·h) at 23 °C to 6.5 ± 0.3 mg NH4+-N/(L·h) (20 °C), 4.2 ± 0.6 mg NH4+-N/(L·h) (15 °C), and 2.6 ± 0.2 mg NH4+-N/(L·h) (12 °C). This represents a reduction of the maximal AOB activity by 6.6, 39.2, and 63.1% when the temperature was reduced to 20, 15, and 12 °C, respectively, in comparison to that at 23 °C. Similarly, the maximal anammox activity decreased by 3.8% at 20 °C, 21.9% at 15 °C, and 38.5% at 12 °C, compared to that at 23 °C. The maximal NOB activity was detected at a very low level (<0.08 mg NO3–-N/(L·h)) at all operating temperatures. Overall, the maximal AOB and anammox activities were significantly impacted by the temperature.
Figure 5.
Impacts of temperature on the AOB, NOB, and anammox activity. (a) Profiles of maximal AOB, NOB (aerobic MBBR), and anammox (anoxic MBBR) activities during the studying period. (b) Arrhenius plots for the species-specific nitrogen conversion rates. (c) Apparent activation energy (Ea) values of AOB and anammox bacteria under different temperature intervals.
The natural logarithm of NH4+-N conversion rates of AOB and anammox was further plotted in the conventional Arrhenius plots (Figure 5b). The activation energy (Ea) values were then calculated, which can imply the impact of temperature on AOB and anammox activities. In general, a lower Ea value indicates more stable microbial activity, while a higher Ea value represents more sensitive microbial activity toward temperature change.46 The results showed that the Ea value of anammox bacteria was only half of that of AOB, indicating that anammox activity was more stable than AOB activity when the temperature decreased from 23 to 12 °C in the present study. The results of previous studies showed that the impact of temperature on microbial activity varies widely in different temperature ranges.46,47 Similarly, the Ea values of AOB and anammox at a temperature range of 23–20 °C were 24.7 ± 19.3 and 13.8 ± 8.1 KJ/mol, respectively (Figure 5c), which were increased by 3.6 and 2.9 times when the temperature was decreased from 15 to 12 °C. These results indicated that AOB and anammox activities were more sensitive to the decrease of temperature at lower temperature ranges.
It was often reported that the decrease in operating temperature significantly deteriorated the nitrogen removal performance of mainstream PN/A.48,49 For example, Gilbert et al.49 observed the decreased nitrogen removal rate by about 50% in a biofilm-based one-stage PN/A system when the operating temperature decreased from 20 to 10 °C. In contrast, the effluent quality of the present two-stage PN/A process at 12 °C is comparable to that at 23 °C (Table S2). A potential reason is that the present acidic PN and anammox processes both had overcapacity in nitrogen conversion. In specific, the maximal activities of AOB and anammox bacteria at 12 °C were still higher than the nitrogen loading rate applied in the long-term experiment, suggesting the overcapacity. Thus, a higher nitrogen removal efficiency (88.9 ± 2.5%) and satisfactory TN effluent concentration (5.1 ± 1.2 mg N/L) could be stably sustained in the present PN/A, even at a temperature of 12 °C. Apart from the overcapacity, another feature to ensure the superior nitrogen removal performance is that the suppression of NOB was well maintained at low temperatures. As suggested by many previous studies, the control of NOB became more critical at low temperatures.42 Here, with the in situ produced FNA, NOB were consistently and completely suppressed even in biofilms that were considered able to provide protection for NOB. Notably, FNA concentration is negatively correlated to temperature, suggesting that a lower temperature may even pose additional benefits for NOB suppression. This hypothesis was corroborated by the increase in NAR from 20 to 12 °C (Figure 2b). The robust nitrogen removal performance toward temperature change demonstrates that the two-stage biofilm-based acidic PN and anammox process is robust for domestic wastewater treatment with dynamic temperatures in practice.
3.7. Engineering Implications
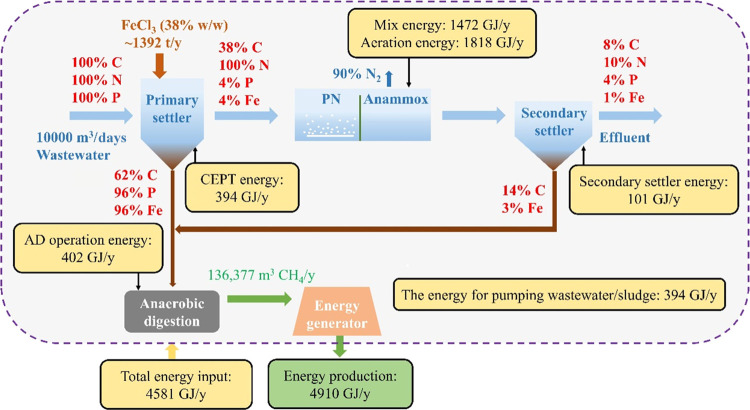
The PN/A process reduces the energy demand by 40% for nitrogen removal, compared to the conventional nitrification/denitrification process.50,51 This study not only achieved a stable PN/A process, showing that this system was not only robust to the temperature variation but also creatively incorporated the CEPT process with the PN/A process, achieving desirable COD, phosphate, and nitrogen removal performance and micropollutant abatement in the long-term experiment. Together, these enable a mass balance analysis of the proposed wastewater treatment, and the result indicates that 76% of organic carbon in wastewater recovered can be used for biogas production by AD (Figure 6). The mass balance analysis also indicates that above 95% of phosphate in wastewater and 99% of dosed iron are ended in AD. Iron(III) in feeding sludge can be reduced to iron(II) in AD (Figure 6 and Table S3), resulting in precipitation of sulfide during anaerobic sludge digestion, thereby mitigating odorous and corrosive issues.4,52 The biomass of the system was not intentionally wasted during the study period. The effluent SS concentration of this MBBR system was about 61 mg SS/L, which was much lower than that (i.e., 150–250 mg SS/L) of the typical MBBR process in treating domestic wastewater.53 A low biomass yield of this system was associated with the high COD removal efficiency of the CEPT process, and the high in situ FNA concentration in the aerobic MBBR had a strong biocidal effect on many microorganisms.21,54 The dosed iron can also increase sludge dewaterability and reduce sludge disposal costs.4,5 It is possible to further recover iron and phosphate in terms of a valuable product, i.e., vivianite, from anaerobically digested sludge.55−58 The produced biogas, if used for energy generation, can generate energy and meet the heat and electricity requirements of the treatment process, as evaluated previously.59 According to the energy balance, the proposed system could achieve energy self-sufficiency with a net energy production of 329 GJ/y in a WWTP with a treatment capacity of 10,000 m3/days (Figure 6). The achievement of net energy production was due to the significant reduction of energy consumption in aeration, which agreed with previous estimations for the mainstream PN/A process.60,61
Figure 6.
Assessments of mass balance (C, N, P, and Fe) and energy consumption by each unit of the proposed wastewater treatment process. The mass balance analysis was performed based on the measured data of the laboratory-scale treatment system. The ratio of organic carbon and Fe harvested from the secondary settler was assumed based on a solid concentration of ∼10 mg SS/L in the final effluent. The energy consumption assessment was conducted based on parameter values in Table S5.
This study achieved the mainstream PN/A process in a two-stage configuration. First, because low pH and high FNA are inhibitory to anammox bacteria, the acidic PN and anammox were separated in two tanks to avoid the inhibition of anammox activity. Second, one-stage PN/A used in treating low-strength wastewater usually requires a relatively high residual ammonium concentration control to maintain high anammox activity and suppress NOB activity.42,62 The residual ammonium concentration may be above 10 mg N/L and thus requires additional polishing before discharge. In the present two-stage configuration, the effluent TN concentration of the proposed novel two-stage process was about 5 mg N/L, which is even lower than the required residual ammonium concentration.42
Moreover, the operation of acidic PN is achieved by microbial ammonium oxidation, a microbially induced acid producing process. With the feeding of low alkalinity wastewater, the acid-tolerant AOB in the reactor can produce protons and nitrite to self-sustain FNA concentration at the ppm level and cause significant repression of NOB while the acid-tolerant AOB are scarcely inhibited; therefore, the PN performance can be stably sustained.26,27 The low alkalinity wastewater (i.e., CaCO3 alkalinity/ammonium molar ratio < 1) is thus a must for acidic PN. In real domestic wastewater, the CaCO3 alkalinity/ammonium molar ratio is a wide range of 0.3–1.4 (Table S6). For the wastewater with sufficient alkalinity (CaCO3 alkalinity/ammonium molar ratio > 1), the present study proposed to use FeCl3 to reduce the alkalinity. By dosing FeCl3 at a dosage of 50 mg Fe/L, the CaCO3 alkalinity/ammonium molar ratio of wastewater was reduced from 1.1 ± 0.1 to 0.7 ± 0.1 (Figure 1c). The iron dosage is comparable to the level (e.g., 20–60 mg Fe/L) applied in CEPT installed in many WWTPs.3,31 For the low alkalinity domestic wastewater, acidic PN can be directly achieved without iron dosing. As such, coagulants without affecting alkalinity can be used in CEPT to achieve the proposed novel wastewater treatment. Therefore, an iron dosage should be determined not only by removal effectiveness of organics and phosphate but also by effectiveness of alkalinity reduction when applied to different wastewater treatment plants. Overall, the demonstration of this novel, integrated, and cost-efficient wastewater treatment process, consisting of CEPT followed by the two-stage PN/A process, opens a new avenue for future domestic wastewater treatment.
Acknowledgments
This work was supported by the UQ Vice-Chancellor’s and Deputy Vice-Chancellor Research Strategic Initiatives Fund and District of Columbia Water and Sewerage Authority (DC Water). Z.H. thanks the support from the China Scholarship Council (CSC). Dr. M.Z. acknowledges the support of the special fund of State Key Joint Laboratory of Environmental Simulation and Pollution Control (19K10ESPCT) at Tsinghua University. Dr. T.L. is the recipient of an Australian Research Council (ARC) DECRA Fellowship (DE220101310). The authors thank Dr. Yijing Li for support with the analyses of micropollutant removal.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c06444.
Measurement of maximal activity for AOB, NOB, and anammox bacteria; DNA extraction, 16S rRNA gene amplicon sequencing, and data analyses; diagram of the laboratory-scale wastewater treatment system; changes of wastewater composition with the addition of FeCl3 at different concentrations; COD concentration in the effluent of aerobic and anoxic MBBRs; micropollutant removal in different units of the wastewater treatment system; microbial composition of aerobic and anoxic MBBRs; mass balance analysis of the proposed wastewater treatment; main characteristics of the used domestic wastewater; organic carbon and nutrient removal by the proposed wastewater treatment system; performance of the mainstream PN/A process in treating low-strength wastewater at low temperatures; and ammonium nitrogen and alkalinity concentrations in domestic wastewater (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wan J.; Gu J.; Zhao Q.; Liu Y. COD capture: a feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054 10.1038/srep25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten M. S. M.; Horn S. J.; van Loosdrecht M. C. M. Towards a more sustainable municipal wastewater treatment system. Water Sci. Technol. 1997, 35, 171–180. 10.2166/wst.1997.0341. [DOI] [Google Scholar]
- Shewa W. A.; Dagnew M. Revisiting Chemically Enhanced Primary Treatment of Wastewater: A Review. Sustainability 2020, 12, 5928 10.3390/su12155928. [DOI] [Google Scholar]
- Rebosura M. Jr; Salehin S.; Pikaar I.; Sun X.; Keller J.; Sharma K.; Yuan Z. A comprehensive laboratory assessment of the effects of sewer-dosed iron salts on wastewater treatment processes. Water Res. 2018, 146, 109–117. 10.1016/j.watres.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Calderon A. G.; Duan H.; Meng J.; Zhao J.; Song Y.; Yu W.; Hu Z.; Xu K.; Cheng X.; Hu S.; Yuan Z.; Zheng M. An integrated strategy to enhance performance of anaerobic digestion of waste activated sludge. Water Res. 2021, 195, 116977 10.1016/j.watres.2021.116977. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Duan H.; Wang Z.; Zhao J.; Ye L.; Yuan Z.; Zheng M.; Hu S. Centralized iron-dosing into returned sludge brings multifaceted benefits to wastewater management. Water Res. 2021, 203, 117536 10.1016/j.watres.2021.117536. [DOI] [PubMed] [Google Scholar]
- Aiyuk S.; Amoako J.; Raskin L.; van Haandel A.; Verstraete W. Removal of carbon and nutrients from domestic wastewater using a low investment, integrated treatment concept. Water Res. 2004, 38, 3031–3042. 10.1016/j.watres.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Li Z.; Hu Y.; Shi L.; Liu R.; Shi L.; Chen L.; Zhan X. Technology. What’s the best way to achieve successful mainstream partial nitritation-anammox application?. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1045–1077. 10.1080/10643389.2020.1745015. [DOI] [Google Scholar]
- Blackburne R.; Yuan Z.; Keller J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. 10.1007/s10532-007-9136-4. [DOI] [PubMed] [Google Scholar]
- Wu J.; Kong Z.; Luo Z.; Qin Y.; Rong C.; Wang T.; Hanaoka T.; Sakemi S.; Ito M.; Kobayashi S.; et al. A successful start-up of an anaerobic membrane bioreactor (AnMBR) coupled mainstream partial nitritation-anammox (PN/A) system: A pilot-scale study on in-situ NOB elimination, AnAOB growth kinetics, and mainstream treatment performance. Water Res. 2021, 207, 117783 10.1016/j.watres.2021.117783. [DOI] [PubMed] [Google Scholar]
- Li J.; Peng Y.; Yang S.; Li S.; Feng W.; Li X.; Zhang Q.; Zhang L. Successful Application of Anammox Using the Hybrid Autotrophic–Heterotrophic Denitrification Process for Low-Strength Wastewater Treatment. Environ. Sci. Technol. 2022, 56, 13964–13974. 10.1021/acs.est.2c02920. [DOI] [PubMed] [Google Scholar]
- Regmi P.; Miller M. W.; Holgate B.; Bunce R.; Park H.; Chandran K.; Wett B.; Murthy S.; Bott C. B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. 10.1016/j.watres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Hu Y.; Liu R.; Sheng X.; Chen L.; Wu G.; Hu H.; Zhan X. Start up of partial nitritation-anammox process using intermittently aerated sequencing batch reactor: Performance and microbial community dynamics. Sci. Total Environ. 2019, 647, 1188–1198. 10.1016/j.scitotenv.2018.08.098. [DOI] [PubMed] [Google Scholar]
- Han M.; Vlaeminck S. E.; Al-Omari A.; Wett B.; Bott C.; Murthy S.; De Clippeleir H. Uncoupling the solids retention times of flocs and granules in mainstream deammonification: A screen as effective out-selection tool for nitrite oxidizing bacteria. Bioresour. Technol. 2016, 221, 195–204. 10.1016/j.biortech.2016.08.115. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Peng Y.; Li J.; Liu J.; Zhang Q.; Li X.; Zhang L. Rapid initiation and stable maintenance of municipal wastewater nitritation during the continuous flow anaerobic/oxic process with an ultra-low sludge retention time. Water Res. 2021, 197, 117091 10.1016/j.watres.2021.117091. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Ye L.; Jiang G.; Hu S.; Yuan Z. Side-stream sludge treatment using free nitrous acid selectively eliminates nitrite oxidizing bacteria and achieves the nitrite pathway. Water Res. 2014, 55, 245–255. 10.1016/j.watres.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Liu Y.-C.; Xin J.; Zuo H.; Wang C.-W.; Wu W.-M. Ultrasonic Treatment Enhanced Ammonia-Oxidizing Bacterial (AOB) Activity for Nitritation Process. Environ. Sci. Technol. 2016, 50, 864–871. 10.1021/acs.est.5b04178. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Wu S.; Dong Q.; Huang X.; Yuan Z.; Liu Y. Achieving mainstream nitrogen removal via the nitrite pathway from real municipal wastewater using intermittent ultrasonic treatment. Ultrason. Sonochem. 2019, 51, 406–411. 10.1016/j.ultsonch.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zheng M.; Hu Z.; Duan H.; De Clippeleir H.; Al-Omari A.; Hu S.; Yuan Z. Unravelling adaptation of nitrite-oxidizing bacteria in mainstream PN/A process: Mechanisms and counter-strategies. Water Res. 2021, 200, 117239 10.1016/j.watres.2021.117239. [DOI] [PubMed] [Google Scholar]
- Cao S.; Koch K.; Du R.; Wells G. F.; Ye L.; Drewes J. r. E. Toward mainstream anammox by integrating sidestream treatment. Environ. Sci. Technol. 2022, 56, 10553–10556. 10.1021/acs.est.2c03256. [DOI] [PubMed] [Google Scholar]
- Duan H.; Gao S.; Li X.; Ab Hamid N. H.; Jiang G.; Zheng M.; Bai X.; Bond P. L.; Lu X.; Chislett M. M.; Hu S.; Ye L.; Yuan Z. Improving wastewater management using free nitrous acid (FNA). Water Res. 2020, 171, 115382 10.1016/j.watres.2019.115382. [DOI] [PubMed] [Google Scholar]
- Hayatsu M.; Tago K.; Uchiyama I.; Toyoda A.; Wang Y.; Shimomura Y.; Okubo T.; Kurisu F.; Hirono Y.; Nonaka K.; Akiyama H.; Itoh T.; Takami H. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 2017, 11, 1130–1141. 10.1038/ismej.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Ni G.; Maulani N.; Xia J.; De Clippeleir H.; Hu S.; Yuan Z.; Zheng M. Stoichiometric and kinetic characterization of an acid-tolerant ammonia oxidizer ‘Candidatus Nitrosoglobus’. Water Res. 2021, 196, 117026 10.1016/j.watres.2021.117026. [DOI] [PubMed] [Google Scholar]
- Faust V.; van Alen T. A.; den Camp H. J. O.; Vlaeminck S. E.; Ganigué R.; Boon N.; Udert K. M. Ammonia oxidation by novel “Candidatus Nitrosacidococcus urinae” is sensitive to process disturbances at low pH and to iron limitation at neutral pH. Water Res. X 2022, 17, 100157 10.1016/j.wroa.2022.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone N.; Pol A.; Mesman R.; van Kessel M. A.; Cremers G.; van Gelder A. H.; van Alen T. A.; Jetten M. S.; Lücker S.; Op den Camp H. J. Ammonia oxidation at pH 2.5 by a new gammaproteobacterial ammonia-oxidizing bacterium. ISME J. 2021, 15, 1150–1164. 10.1038/s41396-020-00840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Xu K.; Liu T.; Bai G.; Liu Y.; Wang C.; Zheng M. Achieving Stable Partial Nitritation in an Acidic Nitrifying Bioreactor. Environ. Sci. Technol. 2020, 54, 456–463. 10.1021/acs.est.9b04400. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zheng M.; Meng J.; Hu Z.; Ni G.; Guerrero Calderon A.; Li H.; De Clippeleir H.; Al-Omari A.; Hu S.; Yuan Z. Robust Nitritation Sustained by Acid-Tolerant Ammonia-Oxidizing Bacteria. Environ. Sci. Technol. 2021, 55, 2048–2056. 10.1021/acs.est.0c05181. [DOI] [PubMed] [Google Scholar]
- Meng J.; Hu Z.; Wang Z.; Hu S.; Liu Y.; Guo H.; Li J.; Yuan Z.; Zheng M. Determining Factors for Nitrite Accumulation in an Acidic Nitrifying System: Influent Ammonium Concentration, Operational pH, and Ammonia-Oxidizing Community. Environ. Sci. Technol. 2022, 56, 11578–11588. 10.1021/acs.est.1c07522. [DOI] [PubMed] [Google Scholar]
- Stefánsson A. Iron(III) Hydrolysis and Solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. 10.1021/es070174h. [DOI] [PubMed] [Google Scholar]
- Kazadi Mbamba C.; Lindblom E.; Flores-Alsina X.; Tait S.; Anderson S.; Saagi R.; Batstone D. J.; Gernaey K. V.; Jeppsson U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. 10.1016/j.watres.2019.01.048. [DOI] [PubMed] [Google Scholar]
- Taboada-Santos A.; Rivadulla E.; Paredes L.; Carballa M.; Romalde J.; Lema J. M. Comprehensive comparison of chemically enhanced primary treatment and high-rate activated sludge in novel wastewater treatment plant configurations. Water Res. 2020, 169, 115258 10.1016/j.watres.2019.115258. [DOI] [PubMed] [Google Scholar]
- Kulandaivelu J.; Gao J.; Song Y.; Shrestha S.; Li X.; Li J.; Doederer K.; Keller J.; Yuan Z.; Mueller J. F.; Jiang G. Removal of Pharmaceuticals and Illicit Drugs from Wastewater Due to Ferric Dosing in Sewers. Environ. Sci. Technol. 2019, 53, 6245–6254. 10.1021/acs.est.8b07155. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Ok Y. S.; Kim K.-H.; Kwon E. E.; Tsang Y. F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. 10.1016/j.scitotenv.2017.04.102. [DOI] [PubMed] [Google Scholar]
- Falås P.; Wick A.; Castronovo S.; Habermacher J.; Ternes T. A.; Joss A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. 10.1016/j.watres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureni M.; Falås P.; Robin O.; Wick A.; Weissbrodt D. G.; Nielsen J. L.; Ternes T. A.; Morgenroth E.; Joss A. Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures. Water Res. 2016, 101, 628–639. 10.1016/j.watres.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Li H.; Liu T.; Guo J. Enhanced removal of cephalexin and sulfadiazine in nitrifying membrane-aerated biofilm reactors. Chemosphere 2020, 263, 128224 10.1016/j.chemosphere.2020.128224. [DOI] [PubMed] [Google Scholar]
- Cheng Z.; Zuo Z.; Yang S.; Yuan Z.; Huang X.; Liu Y. Study of free nitrous acid (FNA)-based elimination of sulfamethoxazole: Kinetics, transformation pathways, and toxicity assessment. Water Res. 2021, 189, 116629 10.1016/j.watres.2020.116629. [DOI] [PubMed] [Google Scholar]
- Jetten M. S. M.; Niftrik Lv.; Strous M.; Kartal B.; Keltjens J. T.; Op den Camp H. J. M. Biochemistry and molecular biology of anammox bacteria. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 65–84. 10.1080/10409230902722783. [DOI] [PubMed] [Google Scholar]
- Du R.; Cao S.; Zhang H.; Li X.; Peng Y. Flexible Nitrite Supply Alternative for Mainstream Anammox: Advances in Enhancing Process Stability. Environ. Sci. Technol. 2020, 54, 6353–6364. 10.1021/acs.est.9b06265. [DOI] [PubMed] [Google Scholar]
- Udert K. M.; Larsen T. A.; Gujer W. Chemical Nitrite Oxidation in Acid Solutions as a Consequence of Microbial Ammonium Oxidation. Environ. Sci. Technol. 2005, 39, 4066–4075. 10.1021/es048422m. [DOI] [PubMed] [Google Scholar]
- WERF . Minimization of Odors and Corrosion in Collection Systems, Phase I; Water Environment Research Foundation Alexandria: Alexandria, VA, 2007. [Google Scholar]
- Wang Z.; Zheng M.; Duan H.; Yuan Z.; Hu S. A 20-Year Journey of Partial Nitritation and Anammox (PN/A): from Sidestream toward Mainstream. Environ. Sci. Technol. 2022, 56, 7522–7531. 10.1021/acs.est.1c06107. [DOI] [PubMed] [Google Scholar]
- Rong C.; Luo Z.; Wang T.; Qin Y.; Wu J.; Guo Y.; Hu Y.; Kong Z.; Hanaoka T.; Sakemi S.; et al. Biomass retention and microbial segregation to offset the impacts of seasonal temperatures for a pilot-scale integrated fixed-film activated sludge partial nitritation-anammox (IFAS-PN/A) treating anaerobically pretreated municipal wastewater. Water Res. 2022, 225, 119194 10.1016/j.watres.2022.119194. [DOI] [PubMed] [Google Scholar]
- Gilbert E. M.; Agrawal S.; Schwartz T.; Horn H.; Lackner S. Comparing different reactor configurations for Partial Nitritation/Anammox at low temperatures. Water Res. 2015, 81, 92–100. 10.1016/j.watres.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Phanwilai S.; Noophan P.; Li C.-W.; Choo K.-H. Effect of COD: N ratio on biological nitrogen removal using full-scale step-feed in municipal wastewater treatment plants. Sustainable Environ. Res. 2020, 30, 24 10.1186/s42834-020-00064-6. [DOI] [Google Scholar]
- Lotti T.; Kleerebezem R.; van Loosdrecht M. C. M. Effect of temperature change on anammox activity. Biotechnol. Bioeng. 2015, 112, 98–103. 10.1002/bit.25333. [DOI] [PubMed] [Google Scholar]
- Liu T.; Khai Lim Z.; Chen H.; Hu S.; Yuan Z.; Guo J. Temperature-Tolerated Mainstream Nitrogen Removal by Anammox and Nitrite/Nitrate-Dependent Anaerobic Methane Oxidation in a Membrane Biofilm Reactor. Environ. Sci. Technol. 2020, 54, 3012–3021. 10.1021/acs.est.9b05650. [DOI] [PubMed] [Google Scholar]
- Lotti T.; Kleerebezem R.; van Erp Taalman Kip C.; Hendrickx T. L. G.; Kruit J.; Hoekstra M.; van Loosdrecht M. C. M. Anammox Growth on Pretreated Municipal Wastewater. Environ. Sci. Technol. 2014, 48, 7874–7880. 10.1021/es500632k. [DOI] [PubMed] [Google Scholar]
- Gilbert E. M.; Agrawal S.; Karst S. M.; Horn H.; Nielsen P. H.; Lackner S. Low Temperature Partial Nitritation/Anammox in a Moving Bed Biofilm Reactor Treating Low Strength Wastewater. Environ. Sci. Technol. 2014, 48, 8784–8792. 10.1021/es501649m. [DOI] [PubMed] [Google Scholar]
- Schaubroeck T.; De Clippeleir H.; Weissenbacher N.; Dewulf J.; Boeckx P.; Vlaeminck S. E.; Wett B. Environmental sustainability of an energy self-sufficient sewage treatment plant: Improvements through DEMON and co-digestion. Water Res. 2015, 74, 166–179. 10.1016/j.watres.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Duan H.; Wei W.; Ni B.-J.; Laloo A.; Yuan Z. Achieving Stable Mainstream Nitrogen Removal via the Nitrite Pathway by Sludge Treatment Using Free Ammonia. Environ. Sci. Technol. 2017, 51, 9800–9807. 10.1021/acs.est.7b02776. [DOI] [PubMed] [Google Scholar]
- Kulandaivelu J.; Shrestha S.; khan W.; Dwyer J.; Steward A.; Bell L.; McPhee P.; Smith P.; Hu S.; Yuan Z.; Jiang G. Full-scale investigation of ferrous dosing in sewers and a wastewater treatment plant for multiple benefits. Chemosphere 2020, 250, 126221 10.1016/j.chemosphere.2020.126221. [DOI] [PubMed] [Google Scholar]
- Kängsepp P.; Sjölin M.; Mutlu A.; Teil B.; Pellicer-Nàcher C. First full-scale combined MBBR, coagulation, flocculation, Discfilter plant with phosphorus removal in France. Water Pract. Technol. 2020, 15, 19–27. 10.2166/wpt.2019.081. [DOI] [Google Scholar]
- Wang Q.; Ye L.; Jiang G.; Yuan Z. A free nitrous acid (FNA)-based technology for reducing sludge production. Water Res. 2013, 47, 3663–3672. 10.1016/j.watres.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Wijdeveld W. K.; Prot T.; Sudintas G.; Kuntke P.; Korving L.; van Loosdrecht M. C. M. Pilot-scale magnetic recovery of vivianite from digested sewage sludge. Water Res. 2022, 212, 118131 10.1016/j.watres.2022.118131. [DOI] [PubMed] [Google Scholar]
- Hao X.; Yu W.; Yuan T.; Wu Y.; van Loosdrecht M. C. Unravelling key factors controlling vivianite formation during anaerobic digestion of waste activated sludge. Water Res. 2022, 223, 118976 10.1016/j.watres.2022.118976. [DOI] [PubMed] [Google Scholar]
- Salehin S.; Rebosura M.; Keller J.; Gernjak W.; Donose B. C.; Yuan Z.; Pikaar I. Recovery of in-sewer dosed iron from digested sludge at downstream treatment plants and its reuse potential. Water Res. 2020, 174, 115627 10.1016/j.watres.2020.115627. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Raju C. S.; Almind-Jørgensen N.; Laustrup M.; Reitzel K.; Nielsen U. G. Variation in Phosphorus Speciation of Sewage Sludge throughout Three Wastewater Treatment Plants: Determined by Sequential Extraction Combined with Microscopy, NMR Spectroscopy, and Powder X-ray Diffraction. Environ. Sci. Technol. 2022, 56, 8975–8983. 10.1021/acs.est.2c01815. [DOI] [PubMed] [Google Scholar]
- Szarka N.; Scholwin F.; Trommler M.; Fabian Jacobi H.; Eichhorn M.; Ortwein A.; Thrän D. A novel role for bioenergy: A flexible, demand-oriented power supply. Energy 2013, 61, 18–26. 10.1016/j.energy.2012.12.053. [DOI] [Google Scholar]
- Gu J.; Zhang M.; Liu Y. A review on mainstream deammonification of municipal wastewater: Novel dual step process. Bioresour. Technol. 2020, 299, 122674 10.1016/j.biortech.2019.122674. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Ab Hamid N. H.; Reddy N.; Zheng M.; Yuan Z.; Duan H.; Ye L. Wastewater Primary Treatment Using Forward Osmosis Introduces Inhibition to Achieve Stable Mainstream Partial Nitrification. Environ. Sci. Technol. 2022, 56, 8663–8672. 10.1021/acs.est.1c05672. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Li H.; Duan H.; Liu T.; Wang Z.; Zhao J.; Hu Z.; Watts S.; Meng J.; Liu P.; et al. One-year stable pilot-scale operation demonstrates high flexibility of mainstream anammox application. Water Res. X 2023, 19, 100166 10.1016/j.wroa.2023.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.