Abstract
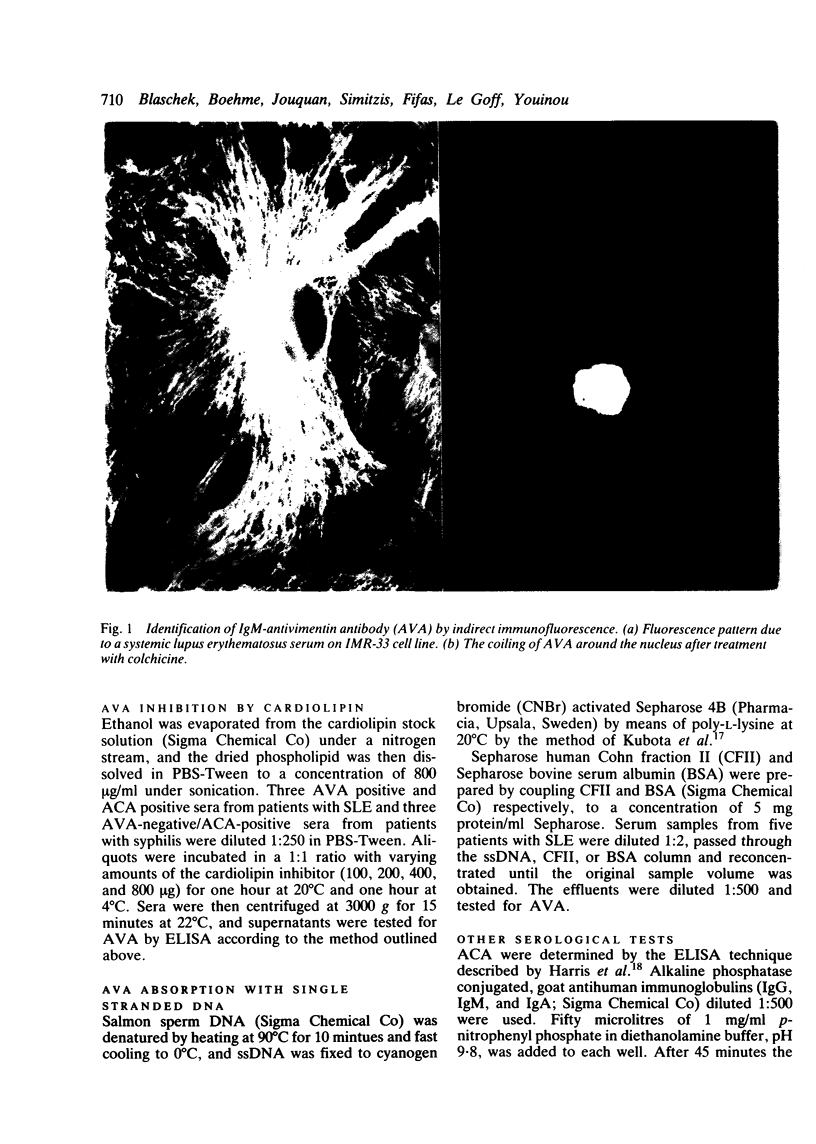
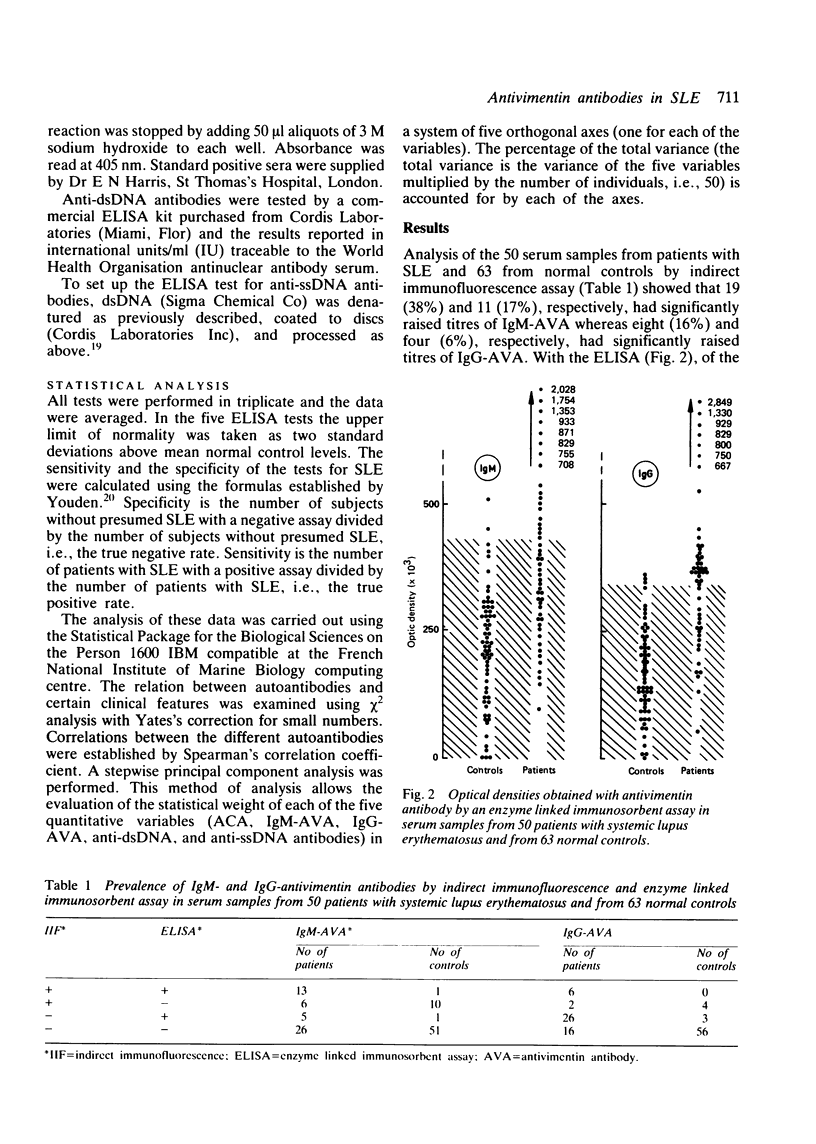
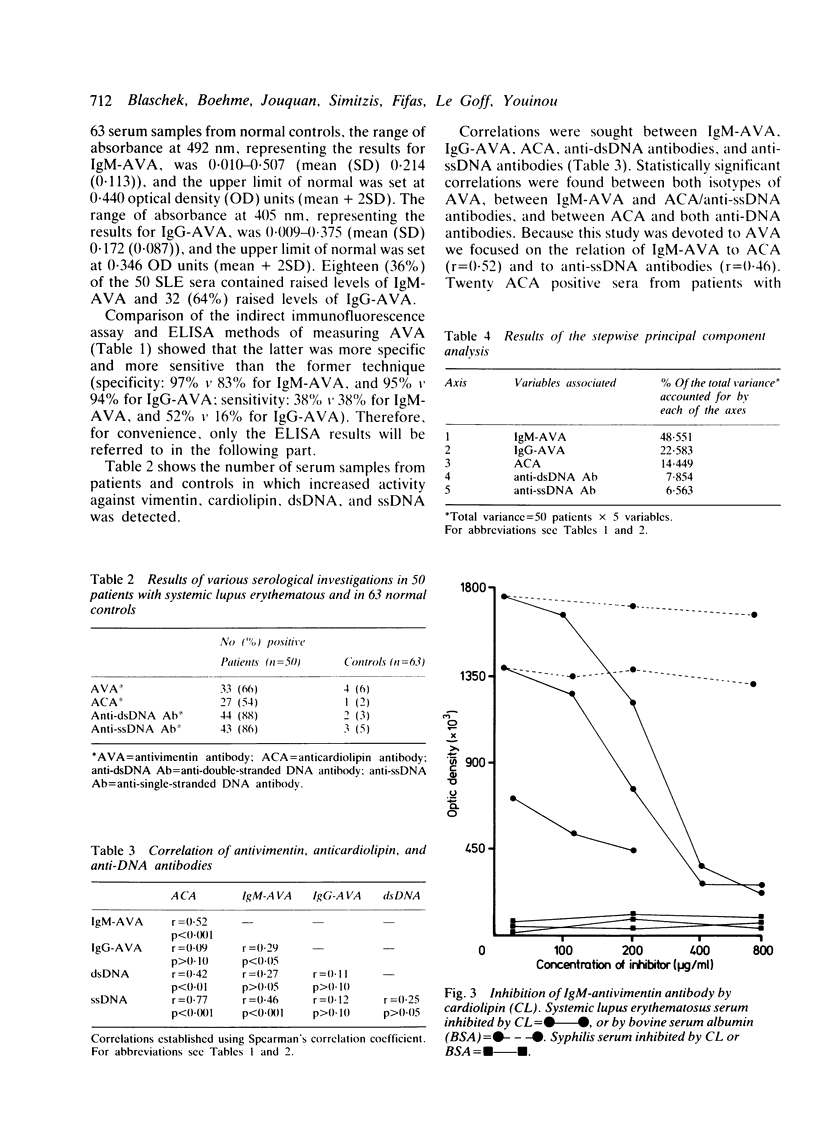
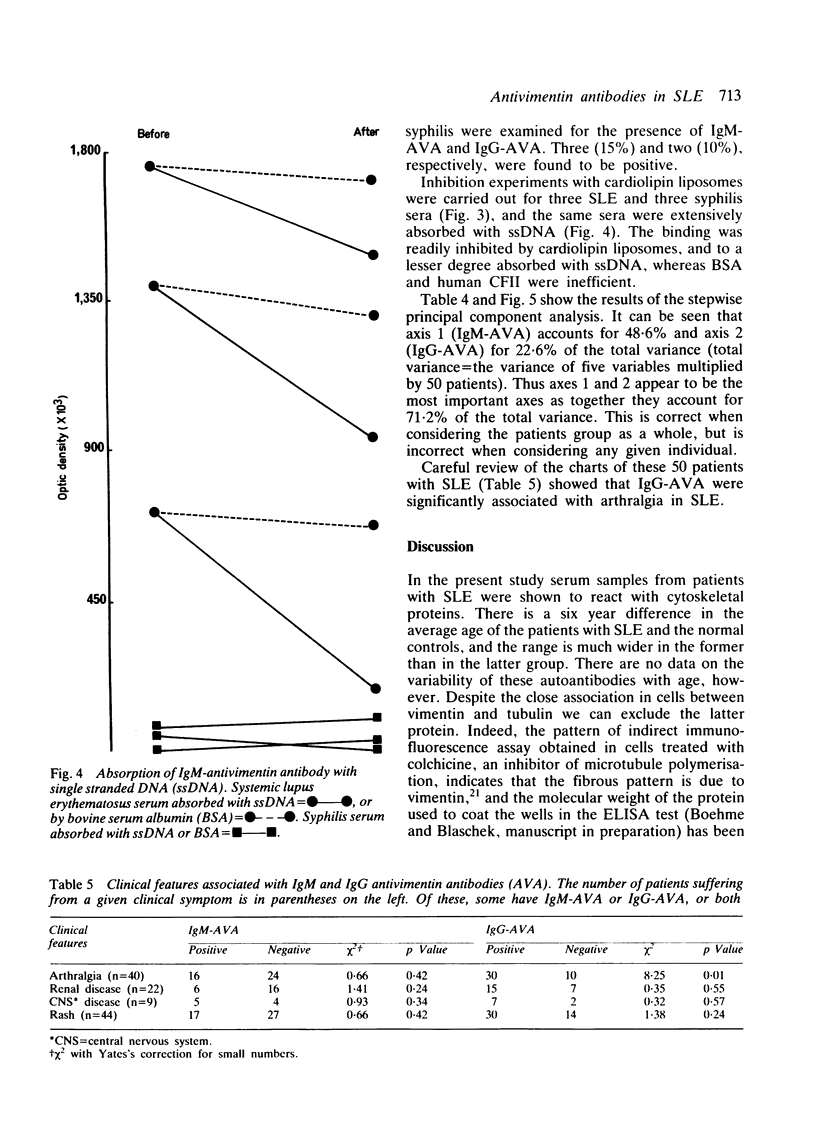
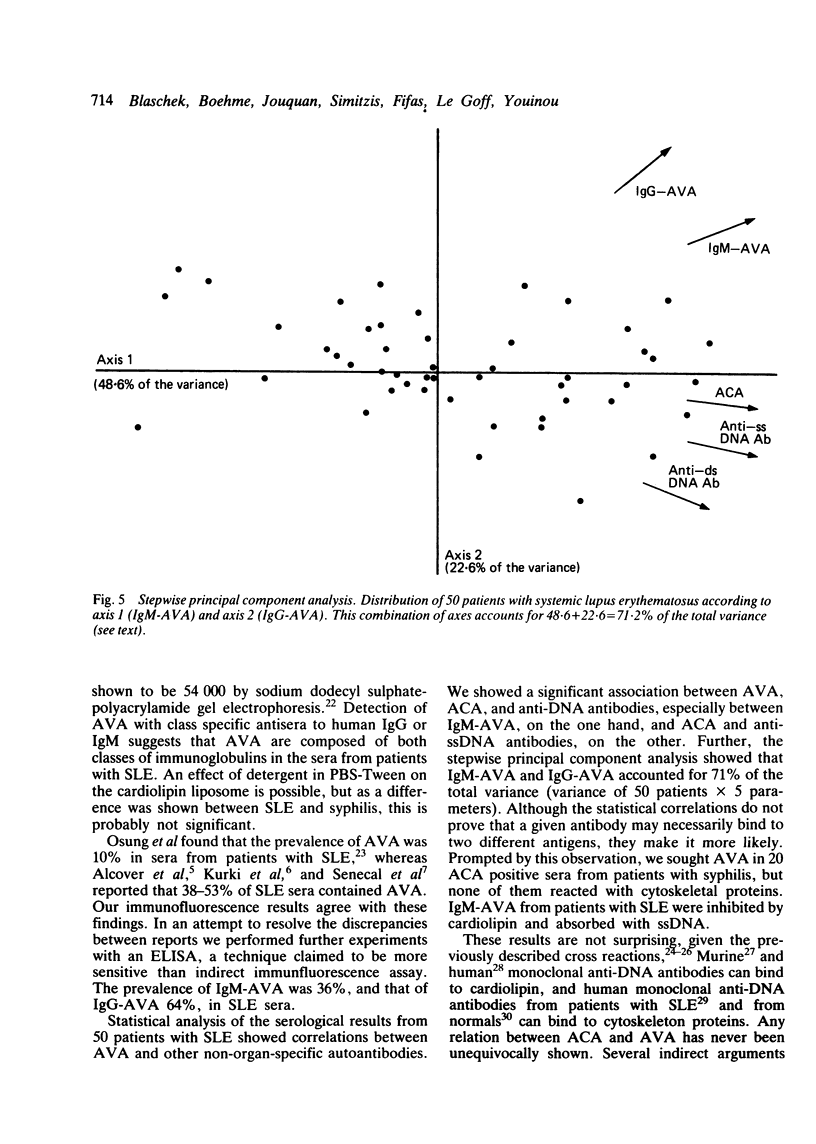
Tests for antivimentin antibodies (AVA) were performed on 50 systemic lupus erythematosus (SLE) and 63 control sera by indirect immunofluorescence and enzyme linked immunosorbent assay (ELISA). The prevalence was significantly raised in SLE (38% and 50% of sera positive for IgM-AVA and IgG-AVA, respectively, by immunofluorescence; 36% and 64% of sera positive for IgM-AVA and IgG-AVA, respectively, by ELISA) in comparison with the control sera. A significant correlation existed between IgM-AVA, on the one hand, and anticardiolipin antibodies (ACA) and anti-single-stranded DNA (ssDNA), on the other. A stepwise principal component analysis demonstrated that IgM-AVA and IgG-AVA accounted for 71% of the total variance in SLE (50 patients x 5 parameters = total variance). Twenty ACA positive serum samples from patients with syphilis were therefore tested for the presence of AVA, but hardly any were found to be positive. IgM-AVA from patients with SLE were inhibited by cardiolipin and absorbed with ssDNA. An association between AVA positivity and arthralgia was also shown in SLE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Molano J., Renart J., Gil-Aguado A., Nieto A., Avila J. Antibodies to vimentin intermediate filaments in sera from patients with systemic lupus erythematosus. Arthritis Rheum. 1984 Aug;27(8):922–928. doi: 10.1002/art.1780270812. [DOI] [PubMed] [Google Scholar]
- Blaschek M. A. Nicht-organspezifische antizytoplasmatische Autoantikörper. Immunserologischer Nachweis und diagnostische Relevanz. Wien Med Wochenschr. 1987 Jul 15;137(13):303–309. [PubMed] [Google Scholar]
- Bretherton L., Toh B. H., Jack I. IgM autoantibody to intermediate filaments in Mycoplasma pneumoniae infections. Clin Immunol Immunopathol. 1981 Mar;18(3):425–430. doi: 10.1016/0090-1229(81)90135-5. [DOI] [PubMed] [Google Scholar]
- Böhme M. W., Evans D. A., Miles M. A., Holborow E. J. Occurrence of autoantibodies to intermediate filament proteins in human visceral leishmaniasis and their induction by experimental polyclonal B-cell activation. Immunology. 1986 Dec;59(4):583–588. [PMC free article] [PubMed] [Google Scholar]
- Colaço C. B., Male D. K. Anti-phospholipid antibodies in syphilis and a thrombotic subset of SLE: distinct profiles of epitope specificity. Clin Exp Immunol. 1985 Feb;59(2):449–456. [PMC free article] [PubMed] [Google Scholar]
- Eilat D. Anti-DNA antibodies: problems in their study and interpretation. Clin Exp Immunol. 1986 Aug;65(2):215–222. [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Pisetsky D. S., Taylor R. P. Antibodies to DNA. A perspective. Arthritis Rheum. 1986 Dec;29(12):1417–1426. doi: 10.1002/art.1780291201. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Harris E. N., Gharavi A. E., Patel S. P., Hughes G. R. Evaluation of the anti-cardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol. 1987 Apr;68(1):215–222. [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Mortazavi-Milani S. M., Russell G., Holborow E. J. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985 Jul;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Minami R. M., Teplitz R. L. An enzyme-linked immunosorbent assay for antibodies to native and denatured DNA. J Immunol Methods. 1979;29(2):155–165. doi: 10.1016/0022-1759(79)90065-6. [DOI] [PubMed] [Google Scholar]
- Kubota T., Akatsuka T., Kanai Y. DNA affinity column chromatography: application in the isolation of distinct antibody populations from SLE sera. Clin Exp Immunol. 1985 Nov;62(2):321–328. [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Helve T., Virtanen I. Antibodies to cytoplasmic intermediate filaments in rheumatic diseases. J Rheumatol. 1983 Aug;10(4):558–562. [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Osung O. A., Chandra M., Holborow E. J. Antibody to intermediate filaments of the cytoskeleton. Ann Rheum Dis. 1982 Feb;41(1):69–73. doi: 10.1136/ard.41.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Senécal J. L., Oliver J. M., Rothfield N. Anticytoskeletal autoantibodies in the connective tissue diseases. Arthritis Rheum. 1985 Aug;28(8):889–898. doi: 10.1002/art.1780280808. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- YOUDEN W. J. Index for rating diagnostic tests. Cancer. 1950 Jan;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Youinou P., Le Goff P., Colaco C. B., Thivolet J., Tater D., Viac J., Shipley M. Antikeratin antibodies in serum and synovial fluid show specificity for rheumatoid arthritis in a study of connective tissue diseases. Ann Rheum Dis. 1985 Jul;44(7):450–454. doi: 10.1136/ard.44.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youinou P., Le Goff P., Miossec P., Moineau M. P., Ferec C. Untersuchungen zur Beziehung zwischen anti-perinukleären Faktoren, Anti-Keratin-Antikörpern und dem agglutinierenden und nichtagglutinierenden Rheumafaktor bei der chronischen Polyarthritis. Z Rheumatol. 1983 Jan-Feb;42(1):36–39. [PubMed] [Google Scholar]



