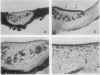
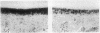Abstract
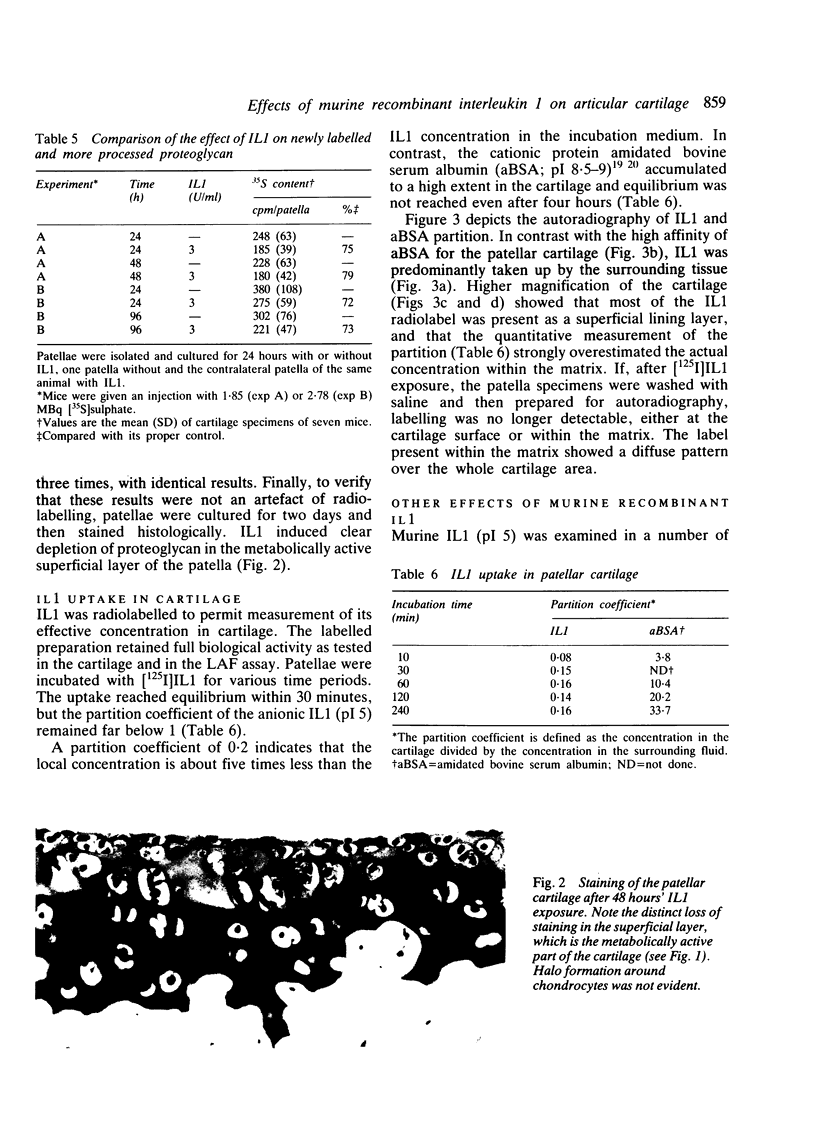
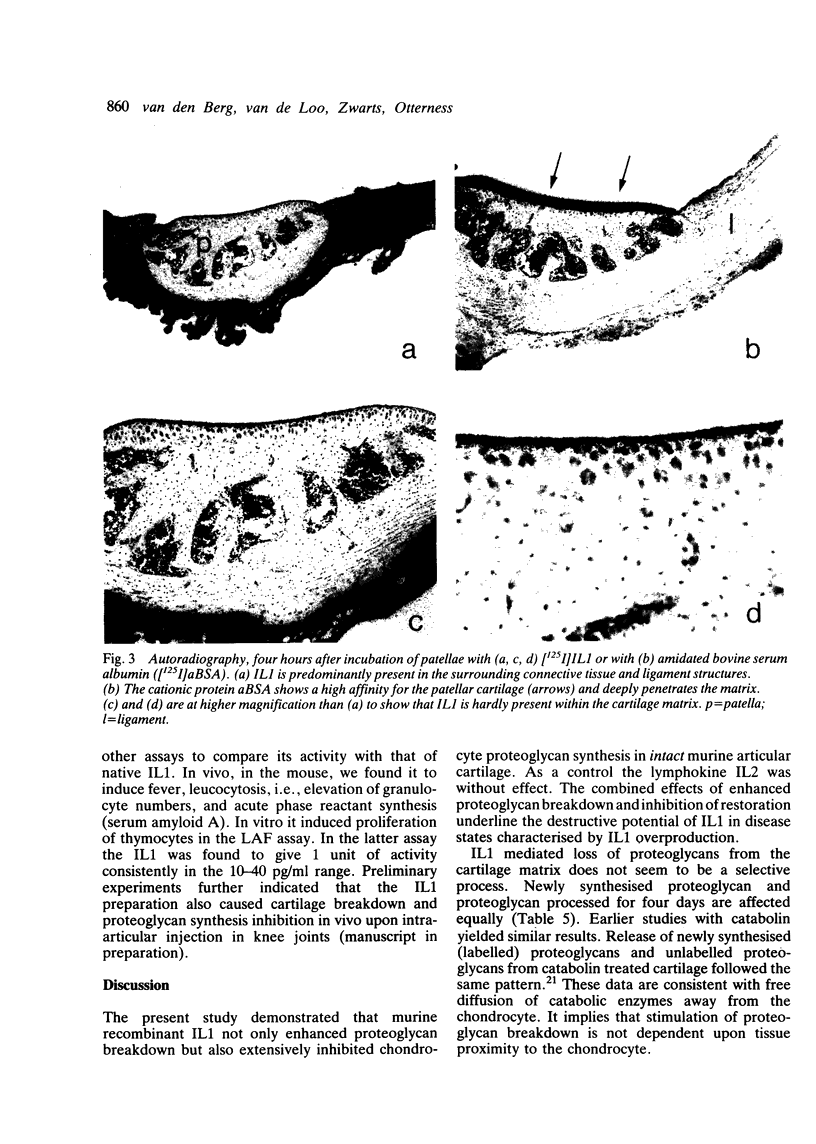
Murine recombinant interleukin 1 (IL1) was tested for its ability to affect intact murine articular cartilage. IL1 caused enhanced proteoglycan degradation and severe inhibition of chondrocyte synthetic function at a concentration of 3 U/ml (100 pg/ml). Inhibition of proteoglycan synthesis appeared to be delayed in onset but occurred consistently after 24 hours. Pulse chase experiments made it clear that proteoglycan degradation and inhibition of proteoglycan synthesis are two distinct actions of IL1. No indications were obtained for selective degradation of either newly synthesised or processed proteoglycan. Moreover, chondrocyte synthetic activity appeared to be inhibited uniformly throughout the cartilage matrix, i.e., no evidence was found for selective suppression of cells in certain regions. IL1 uptake measurement in the cartilage, using [125I]IL1, yielded a partition coefficient far below 1, and autoradiography demonstrated a faint but even distribution within the cartilage matrix. The coordinated induction of enhanced breakdown of proteoglycan and inhibition of proteoglycan synthesis, with such low concentrations of IL1 reaching the chondrocytes, underlines the impressive destructive potential of IL1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balavoine J. F., de Rochemonteix B., Williamson K., Seckinger P., Cruchaud A., Dayer J. M. Prostaglandin E2 and collagenase production by fibroblasts and synovial cells is regulated by urine-derived human interleukin 1 and inhibitor(s). J Clin Invest. 1986 Oct;78(4):1120–1124. doi: 10.1172/JCI112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew J. S., Lowther D. A., Handley C. J. Changes in proteoglycan biosynthesis following leukocyte elastase treatment of bovine articular cartilage in culture. Arthritis Rheum. 1984 Aug;27(8):905–912. doi: 10.1002/art.1780270810. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Petersen J., Halkjaer-Kristensen J., Ingemann-Hansen T. Interleukin-1-like activities in synovial fluids of patients with rheumatoid arthritis and traumatic synovitis. Rheumatol Int. 1985;5(2):79–82. doi: 10.1007/BF00270301. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. Identification of a common class of high affinity receptors for both types of porcine interleukin-1 on connective tissue cells. Nature. 1986 Nov 20;324(6094):263–266. doi: 10.1038/324263a0. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Golds E. E., Mort J. S., Roughley P. J. Human articular cartilage secretes characteristic metal dependent proteinases upon stimulation by mononuclear cell factor. J Rheumatol. 1986 Feb;13(1):20–27. [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., Hazleman B. The role of cytokines in arthritic diseases: in vitro and in vivo measurements of cartilage degradation. Int J Tissue React. 1987;9(4):349–354. [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., King B., Bard D. R. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis. 1987 Jul;46(7):527–533. doi: 10.1136/ard.46.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Saklatvala J., Hembry R., Tyler J., Fell H. B., Jubb R. A cartilage catabolic factor from synovium. Biochem J. 1979 Oct 15;184(1):177–180. doi: 10.1042/bj1840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Dingle J. T. Human mononuclear cell factors mediate cartilage matrix degradation through chondrocyte activation. J Clin Invest. 1981 Sep;68(3):571–581. doi: 10.1172/JCI110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T., Oppenheim J. J., Jasin H. E. Human interleukin 1 mediates cartilage matrix degradation. Cell Immunol. 1985 Mar;91(1):92–99. doi: 10.1016/0008-8749(85)90034-6. [DOI] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B. Influence of the severity and duration of murine antigen-induced arthritis on cartilage proteoglycan synthesis and chondrocyte death. Arthritis Rheum. 1985 Jul;28(7):813–819. doi: 10.1002/art.1780280713. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P., Cloutier J. M., Howell D. S., Ghandur-Mnaymneh L., Woessner J. F., Jr Neutral proteases capable of proteoglycan digesting activity in osteoarthritic and normal human articular cartilage. Arthritis Rheum. 1984 Mar;27(3):305–312. doi: 10.1002/art.1780270310. [DOI] [PubMed] [Google Scholar]
- Miossec P., Dinarello C. A., Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986 Apr;29(4):461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985 Aug 2;229(4712):479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- Pasternak R. D., Hubbs S. J., Caccese R. G., Marks R. L., Conaty J. M., DiPasquale G. Interleukin-1 stimulates the secretion of proteoglycan- and collagen-degrading proteases by rabbit articular chondrocytes. Clin Immunol Immunopathol. 1986 Dec;41(3):351–367. doi: 10.1016/0090-1229(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Howell D. S., Ghandur-Mnaymneh L., Enis J. E., Woessner J. F., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983 Jan;26(1):63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A. An experimental model for hydrogen peroxide-induced tissue damage. Effects of a single inflammatory mediator on (peri)articular tissues. Arthritis Rheum. 1986 Apr;29(4):532–538. doi: 10.1002/art.1780290411. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A. Elastase secreted by activated polymorphonuclear leucocytes causes chondrocyte damage and matrix degradation in intact articular cartilage: escape from inactivation by alpha-1-proteinase inhibitor. Br J Exp Pathol. 1987 Feb;68(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A., van den Bersselaar L. Cationization of catalase, peroxidase, and superoxide dismutase. Effect of improved intraarticular retention on experimental arthritis in mice. J Clin Invest. 1985 Jul;76(1):198–205. doi: 10.1172/JCI111946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Payne T., Dinarello C. A. Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol. 1987 Jan 15;138(2):496–503. [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Quantitation of glycosaminoglycan metabolism in anatomically intact articular cartilage of the mouse patella: in vitro and in vivo studies with 35S-sulfate, 3H-glucosamine, and 3H-acetate. Rheumatol Int. 1986;6(6):273–281. doi: 10.1007/BF00541319. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., van de Putte L. B. Salicylate-induced depletion of endogenous inorganic sulfate. Potential role in the suppression of sulfated glycosaminoglycan synthesis in murine articular cartilage. Arthritis Rheum. 1985 Aug;28(8):922–929. doi: 10.1002/art.1780280812. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., van den Berg W. B., Schalkwijk J., van de Putte L. B., van den Bersselaar L. The impact of protein size and charge on its retention in articular cartilage. J Rheumatol. 1987 Aug;14(4):798–805. [PubMed] [Google Scholar]
- van den Berg W. B., Kruijsen M. W., van de Putte L. B., van Beusekom H. J., van der Sluis-van der Pol M., Zwarts W. A. Antigen-induced and zymosan-induced arthritis in mice: studies on in vivo cartilage proteoglycan synthesis and chondrocyte death. Br J Exp Pathol. 1981 Jun;62(3):308–316. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van Lent P. L., van de Putte L. B., Zwarts W. A. Electrical charge of hyaline articular cartilage: its role in the retention of anionic and cationic proteins. Clin Immunol Immunopathol. 1986 May;39(2):187–197. doi: 10.1016/0090-1229(86)90083-8. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van de Putte L. B. Electrical charge of the antigen determines its localization in the mouse knee joint. Deep penetration of cationic BSA in hyaline articular cartilage. Am J Pathol. 1985 Nov;121(2):224–234. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van de Putte L. B., Zwarts W. A., Joosten L. A. Electrical charge of the antigen determines intraarticular antigen handling and chronicity of arthritis in mice. J Clin Invest. 1984 Nov;74(5):1850–1859. doi: 10.1172/JCI111604. [DOI] [PMC free article] [PubMed] [Google Scholar]