Abstract
Basella alba, a green leafy vegetable with remarkable nutraceutical potential is widely used since ancient times to maintain a healthy colon. This plant has been investigated for its medicinal potential due to the increase in young adult cases of colorectal cancer each year. This study was accomplished to investigate Basella alba methanolic extract (BaME) antioxidant and anticancer properties. BaME consisted of a substantial amount of both phenolic and flavonoid compounds which exhibited significant antioxidant reactivity. Both colon cancer cell lines experienced a cell cycle arrest at the G0/G1 phase after receiving treatment with BaME, which inhibited pRb and cyclin D1 and raised p21 expression levels. This was associated with the survival pathway molecule inhibition and downregulation of E2F-1. The results of the current investigation confirm that BaME inhibits CRC cell survival and expansion. To conclude, the bioactive principles in the extract act as potential antioxidants and antiproliferative agents against colorectal cancer.
Keywords: Antioxidant, Antiproliferative, Basella alba, Colorectal cancer cell lines, E2F-1
1. Introduction
Cancer has become the deadliest disease growing beyond boundaries reckoning the death toll to nearly 10 million in 2020. The Global Cancer Incidence report of a cancer agency (International Agency for cancer research) of the world health organization (WHO) has designated colon cancer to be leading cause of mortality in the world (935000 deaths in 2020). Globally colon cancer is reported to be about 10.6% and 9.4% of all cancers type in men and women respectively (Sung et al., 2021). Colorectal cancer is considered to be a young person’s disease as every year 20% of people between the age group 20–54 are diagnosed with this cancer(Deen et al., 2016). This alarming data of incidence (1.93 million cases), low survival chance, and death rates of colon cancer cases have raised high concern over the efficiency of radio or chemotherapy. The chemotherapeutic drugs utilized currently for cancer treatment initiate several side effects and could lead to fatal conditions since they are not targeted drugs, as they fail to differentiate healthy cells and cancer cells(Sung et al., 2021). People worldwide have started adopting a traditional approach to medicine, an alternative for successful management of various diseases including main cancer. A survey has reported that nearly 31% of the world population’s cancer patients have preferred traditional medicines before choosing conventional medicine (Kumar et al., 2016, Sharma et al., 2022). Therefore, the investigation of plant agents that have natural therapeutic characteristics and a plant extract that shows cell cycle modulating properties for cancer patients is the high priority of the hour. (See Scheme 1).
Scheme 1.
A diagrammatic representation of general strategies applied for assaying methanolic extract from Basella alba for their anticancer activity.
Reactive oxygen species (ROS) are a group of different types of free radicals (superoxide anionic radicals, singlet oxygen, hydroxyl-radicals) and non-free-radical (hydrogen peroxide) species generated as byproducts of oxidation reactions. It is known to exert both beneficial (at low concentration) as well as deleterious (at high concentration) actions (Li et al., 2016). The cell has its own antioxidant system to remove the radicals produced internally but its efficiency is altered during pathological infections. Even oxidative stress conditions cause an imbalance where excess ROS are produced which causes damage to the vital components of the cells (biomolecules). This is mainly observed in degenerative diseases such as cancer (Perillo et al., 2020). Recently, the investigation of dietary natural antioxidants has been increasing to prevent the overproduction of ROS. Plant sources that decrease the cumulative effect of oxidative damage are the target of the researchers (Santos-Sánchez et al., 2019).
Basella alba L. which belongs to the Basellaceae family is a fast-growing green succulent mucilage leafy vegetable with great medicinal potential. Well known as Malabar or Indian spinach in India, Remayung in Malaysia or Pui shak in Bangladesh. This ethnomedicinally important plant has been used in the ancient Indian medicinal system to cure various diseases and ailments. The plant has been reported to be rich in vitamins and minerals. It exhibits androgenic potential, antiviral, antibacterial, antioxidant, antidiabetic, anti-inflammatory, antidepressant, antiulcer (as it cures aphtha- the mouth ulcers), wound healing, nephron- and hepatoprotective activity (Deshmukh and Gaikwad 2014). Despite having numerous biological benefits, there is a paucity of information about B. alba's effect on cancer in the literature. Also, in view of the increasing use of B. alba by the traditional healers or practioners among different communities, who claim it to have aphrodisiac, virilizing or anabolizing potentials and prospective to prevents catarrh and various diseases, it is highly necessary to scientifically investigate its biological activities especially its cytotoxic property which is less explored. Basella alba is used in the present study to investigate its methanolic extract role in rendering effective therapeutic properties against colorectal cancer (CRC) cell lines HT-29 and HCT-116.
2. Material and methods
2.1. Basella alba extract preparation
Fresh and healthy Basella alba, commonly called Indian spinach plant material was collected, shade dried, grounded into a coarse powder, and sieved. Stored at 4 °C in airtight containers for further analysis. The selected plant material was weighted and soaked in 10 L of anhydrous methanol and was left for 10 days at RT with intermittent stirring. The methanolic extract was filtered and concentrated at temperature 40 °C on a rotary-evaporator. The extract yield was 20.8%. The extract was collected and stored in brown bottle at −20 °C until used.
2.2. GC–MS spectral assessment
Gas chromatography-mass spectrophotometer (GC–MS) assessment was done using an Alltech EC-5 column in Agilent 6890 gas chromatography device.
2.3. Analysis of total phenol content (TPC) and total flavonoid content
Gao et al., 2019 procedure was followed with minor alterations to determine the total phenolic and flavonoid content in the extract (Gao et al., 2019).
2.4. Analysis of in vitro antioxidant activity
2.4.1. DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging activity
Applying a slightly modified protocol of DPPH assay (Müller et al., 2011), in vitro measurement of free radical scavenging activity of the extracts’ was done. The detailed protocol is given in supplementary information.
2.4.2. Superoxide (SO) radical scavenging activity
A tad-modified Chun et al (2003) method (Chun et al., 2003) was used for the analysis of the ability of plant extracts, quercetin, and BHT to quench the generation of superoxide radicals.
2.4.3. Phosphomolybdate assay
The little modified phosphomolybdate method (Benzie and Szeto 1999) was used to analyze the total antioxidant capacity of the extracts.
2.4.4. Hydroxyl radical scavenging (HRS) activity
Hydroxyl-radical is the most potent ROS that reacts with the cell membrane by abstracting hydrogen atoms from phospholipids (polyunsaturated fatty acid moieties) and leads to cellular damage by peroxidic reaction. Its scavenging activity was analyzed following a standard protocol (Chimi et al., 1991).
2.5. Analysis of anticancer activity
2.5.1. Antiproliferative activity of Basella alba methanol extract
HT-29 and HCT-116 are two CRC human cell lines (procured from ATCC, Manassas, VA) that were used. Cells were cultured according to standard protocol (Ganji et al., 2013). Using XTT assay (ATCC, Manassas, VA) cell viability was obtained. Absorbance at 475 nm with reference to 660 nm was read using a microplate reader.
2.5.2. Clonogenicity assay
The above-mentioned two CRC cell lines were seeded at density of 100 cells/well in 6 well plates. After 12 h. treatment with vehicle (DMSO), BaME at specified concentrations (12.5, 25 & 50 µg/ml) maintained at different times (24, 48, and 72 h) was done. The media was replenished every 3 days. After the 12th day, crystal violet was used to stain cell colonies. The number of colonies was analyzed visually and the colonies with > 50 cells were only counted. All experiments were performed.
2.5.3. Flow cytometry
As described above, Cells treated with vehicle and BaME were harvested after 24 h, fixed for 1 h at 4 °C in 70% ethanol. Then, using Propidium iodide (Sigma-Aldrich, US) the cells were stained for 15 min in the dark. Beckman Coulter Cytoflex flow cytometer was used for FACS analysis. Assays were performed in triplicates.
2.5.4. Western blotting
Treated CRC cell lines were harvested, processed, and lysed (protease inhibitors and RIPA buffer). Sample protein estimation was done using BCA protein assay kit. The western blotting technique was used to analyze extracted proteins (Ganji et al., 2013). The nitrocellulose membrane was used to blot the proteins that had been resolved using SDS-PAGE. Following primary antibody incubation, secondary antibody treatment was applied to the blotted membranes. Chemiluminescence enabled for the visualization of the attached antibodies. As a positive control and to confirm equal loading, β-actin housekeeping proteins were used. Flow-Jo software was used to analyze the results.
2.5.5. Quantitative real-time-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from CRC cell lines treated with BaME for 24 hrs using Trizol reagent. The reaction was performed with Applied Biosystems, USA. Using specific primers (Table S1) qRT-PCR was done by following the methodological steps. Initial denaturation (95 °C, 3 min), followed by 30 cycles of denaturation (95 °C, 1 min), primer annealing (57 °C, 30 sec), synthesis (72 °C, 1 min), then followed by final extension (72 °C, 7 min) step. Melting curve analysis was done. The relative quantities was compared to β-actin quantity (Ganji et al., 2013).
All the experimental data is presented as the means standard deviation for three replications.
3. Results
3.1. GC–MS spectral assessments
The chromatogram of GC–MS analysis of the BaME exhibited five peaks (Figure S1). The first peak showed to be 10-[Methoxycarbonyl]-N-acetylcolchinol. The second peak was determined to be Propanoic acid, 2- [3-acetoxy-4, 4, 14-trimethylandrost-8-en-17- yl]-. The next peaks were Hydroymethyl colchicines, Propanoic acid, 2- [3-acetoxy-4, 4, 14-trimethylandrost-8-en-17-yl]-, [22S]-21-Acetoy-6a, 11a-dihydroxy-16a, 17a-propylmethylenedioxy pregna −1, 4-diene-3, 20- dione. The five biomolecules identified were tabulated (Table S2) and their structures elucidated by mass spectrum were depicted in Figure S2 (a-f). Different phytochemicals which contribute to the medicinal and bioactivities of the extract are shown in Table S2.
3.2. Analysis of total phenol and flavonoid content
Particularly, the study is focused on the biological activities owing to the functions of phenolic and flavonoid compounds. The TPC of the BaME was determined using the FCR method (Folin – Ciocalteu reagent). The result of TPC was calculated from the regression equation of the standard plot (y = 0.0108x + 0.2665, r2 = 0.9948). TPC in BaME was 57.9 ± 2.66 µg gallic acid equivalent (GAE)/g of extract. The regression equation of the standard plot (y = 0.0009x + 0.0569, r2 = 0.9826) was used to calculate flavonoid content and it is expressed as quercetin equivalents (QE). Total flavonoid content in BaME was 118.1 ± 0.79 µg/g quercetin equivalent per extract.
3.3. Antioxidant activity assessments
The antioxidant activity of the BaME is shown in Table 1, Table 2. The radical scavenging activity and the total antioxidant capacity is evaluated. Since the complex makeup of the phytochemicals makes it impossible to test the antioxidant properties of plant extracts using just one method, four distinct approaches have been used.
Table 1.
Antioxidant activity of BaME and ascorbic acid in DPPH and Superoxide radical scavenging assay.
| Antioxidant assay | Sample |
Percentage inhibition at different concentration of sample (μg/ml) * |
IC50 (μg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| DPPH assay | Basella alba | 20.6 ± 1.9 | 39.4 ± 0.0 | 76.8 ± 0.02 | 82.6 ± 0.2 | 93.9 ± 1.2 | 46.6 ± 0.0 |
| Ascorbic acid | 28.24 ± 0.28 | 42.76 ± 0.43 | 62.27 ± 0.72 | 73.73 ± 0.37 | 94.81 ± 0.49 | 45.5 ± 0.4 | |
| Superoxide radical scavenging activity | Basella alba | 38.95 ± 0.39 | 47.21 ± 0.85 | 56.72 ± 0.64 | 68.90 ± 0.28 | 70.11 ± 0.80 | 47.2 ± 1.2 |
| Ascorbic acid | 38.46 ± 3.87 | 41.24 ± 0.02 | 53.59 ± 1.54 | 59.56 ± 2.11 | 63.77 ± 1.64 | 55.2 ± 2.10 | |
* values represent means ±.S.D, n = 3; Means not sharing the same letter are significantly different (LSD) at P < 0.01 probability level in last column.
Table 2.
Antioxidant activity of BaME and ascorbic acid in Phosphomolybdate and Hydroxyl radical scavenging assay.
| Antioxidant assay | Sample |
Percentage inhibition at different concentration of sample (μg/ml) * |
IC50 (μg/ml) | ||||
|---|---|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | |||
| Phosphomoly bdate assay | Basella alba | 19.97 ± 0.67 | 27.84 ± 0.86 | 37.83 ± 0.39 | 43.63 ± 0.58 | 53.95 ± 0.39 | 918.5 ± 0.1 |
| Ascorbic acid | 50.66 ± 8.4 | 63.66 ± 4.2 | 74.00 ± 7.2 | 84.00 ± 2.6 | 86.00 ± 5.3 | 124.3 ± 1.57 | |
| Hydroxyl radical scavenging activity | Basella alba | 24.8 ± 1.16 | 52.4 ± 2.06 | 68.5 ± 2.73 | 76.1 ± 2.36 | 79.3 ± 1.34 | 380.3 ± 0.9c |
| Ascorbic acid | 24.0 ± 7.21 | 48.0 ± 5.29 | 62.6 ± 8.38 | 77.0 ± 2.65 | 94.6 ± 4.16 | 430.4 ± 0.6 | |
* values represent means ±.S.D, n = 3; Means not sharing the same letter are significantly different (LSD) at P < 0.01 probability level in last column.
3.3.1. DPPH radical scavenging activity
DPPH assay is the widely applied method to assess the ability of phytoconstituents to act as free radical scavengers or hydrogen donors. The concentration of plant extract needed to reduce the initial concentration of DPPH by 50% is known as the IC50 value of the plant extract. The results in Table 1 are showing the study of the scavenging property of methanolic plant extract on DPPH. To our knowledge, DPPH activity of methanolic extract of Basella alba have not been reported earlier. The present study showed the DPPH radical scavenging potential of BaME ranging from 20.06% to 93.9% at different concentrations (20–100 µg/ml) and ascorbic acid ranging from 28.24% to 94.81% at different concentrations (20 to 100 µg/ml) showed moderate to high scavenging abilities respectively. The extracts showed dose-dependent scavenging of DPPH radicals.
3.3.2. SO anion radical scavenging assay
The SO radical scavenging activity of BaME was evaluated using ascorbic acid as control. The extracts’ scavenging of radicals was in a dose dependent way. The highest scavenging effect towards SO was observed at concentration of 100 µg/mL (70.11 ± 0.80 µg/mL). We can rank the samples’ SO scavenging activity in the order of BaME (47.2 ± 1.2 µg/mL) and ascorbic acid (55.2 ± 2.10 µg/mL). When compared to ascorbic acid, BaME has significantly higher IC50 values (Table 1). The highest flavonoids and polyphenols content may probably would have contributed to the greater scavenging activity of the BaME. The findings thus strengthen BaME as a potential source of SO scavenging activity.
3.3.3. Phosphomolybdate assay (Total antioxidant capacity)
BaME examined demonstrated overall antioxidant property, as shown in Table 2. The decrease in phosphomolybdate antioxidant activity that we observed in this investigation suggests that it occurs in a dose-dependent manner. The lowest percentage reduction was exhibited by BaME (19.97%) at 200 µg/mL and highest (53.95%) at 1 mg/mL concentration. The lowest IC50 values were exhibited by BaME (918.5 µg/mL) when compared to ascorbic acid (124.3 ± 1.57 µg/mL) (Table 2). Compared to the extract the potent antioxidant ascorbic acid exhibited significant total antioxidant capacity. These results demonstrated that BaME has antioxidant activity, but very high concentration is required to exhibit its potential.
3.3.4. HRS activity
As shown in Table 2, the hydroxyl ion scavenging by BaME increased in a dose-dependent manner. The lowest percentage of reduction was exhibited by BaME compared to the commercial ascorbic acid. The IC50 values of BaME and ascorbic acid are 380.3 ± 0.9 µg/mL and 430.4 ± 0.6 µg/mL respectively, the HRS activity can be ranked. All results showed that the potency and scavenging capability of BaME increased with the amount of the sample at concentrations of 200–1000 g/mL (Table 2).
The antioxidant study shows remarkable findings that suggest that BaME could be a possible source of natural antioxidants with significant therapeutic properties to inhibit or regulate degenerative diseases related to oxidative stress.
3.4. Anticancer activity
3.4.1. Antiproliferative activity
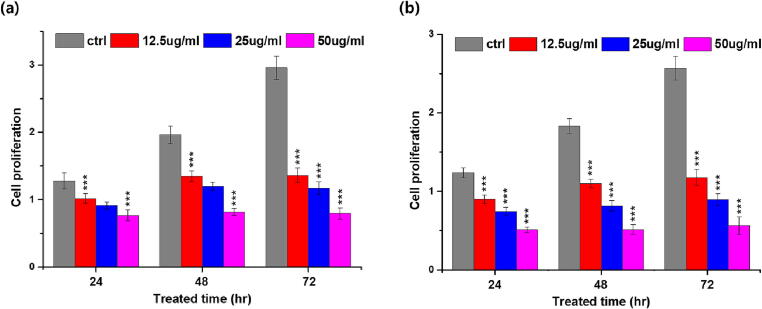
To investigate BaME anti-proliferative effects on HT-29 and HCT-116 CRC cancer cell lines, we performed a cell proliferation assay using XTT. The extract had the highest cytotoxicity against HT-29 at a concentration of 50 g/mL, inhibiting cell growth by 78.9% in 72 h. At 48 h, it was discovered that extract in HCT-116 and HT-29 had IC50 values of 51 µg/mL and 22 µg/mL, respectively, with 70% cell growth inhibition (Fig. 1 a and b respectively). These findings suggested that BaME, depending on dose and duration, had an anti-proliferative effect on CRC cells.
Fig. 1.
Determination of BaME cytotoxicity on CRC cell lines using the XTT assay. Cytotoxic effect on (a) HCT-116 cell lines and (b) HT- 29 cell lines. Each value in the figure is represented as mean ± SD (n = 6). *** are significantly different (P < 0.05).
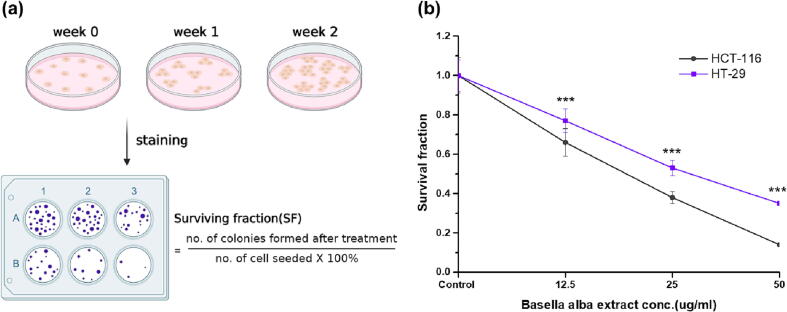
3.4.2. Clonogenicity assay
The descriptive images of the clonogenic assessment are represented in Fig. 2a. When a cell can proliferate indefinitely and make a big colony it is called clonogenic. Clonogenic assay is regarded an in vitro gold standard to assess the sensitivity of the cell towards drug therapy. In other words, it studies the drug’s effect on cell survival and proliferation (Willers et al., 2021). After treatment, a cell is deemed to be viable if it survives and can divide. Over time, in a drug-free environment, it forms clones (a small colony of genetically identical cells). Fig. 2b is showing the results of the clonogenic assay. In both cell lines HT-29 and HCT-116, the formation of the colony was significantly (***P < 0.001) inhibited in BaME compared to untreated cells. Therefore, the data reveals that after BaME treatment, the number of viable cells at IC50 values and the next higher doses reduced dramatically in both cell lines. It can be concluded that when BaME initiates an irreversible cell arrest process or else exerts ‘cidal’ and not ‘static’ activity.
Fig. 2.
Effect of BaME in colony formation in HCT116 and HT29 cell lines. (a) Descriptive images of the stained colonies and clonogenicity assessment. (b) Cell survival curves of both CRC cell lines with different concentration of BaME treatment. (***P < 0.001). Each bar represents mean ± standard deviation, n = 3.
3.4.3. FACS analysis
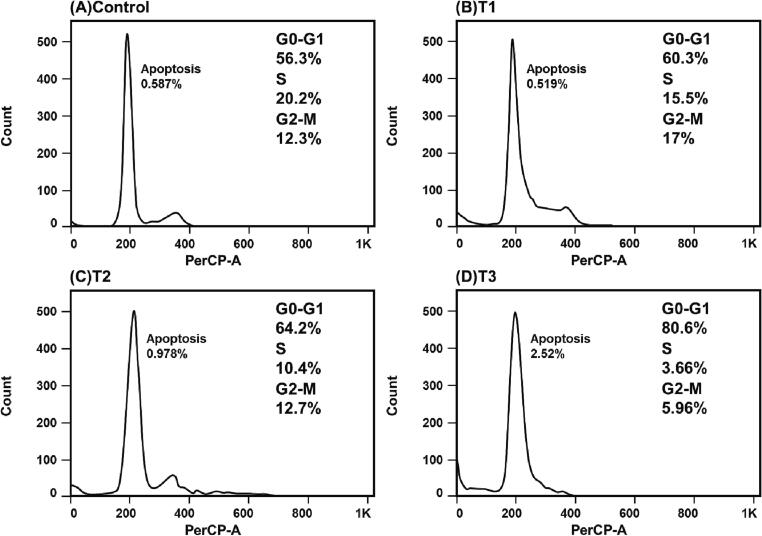
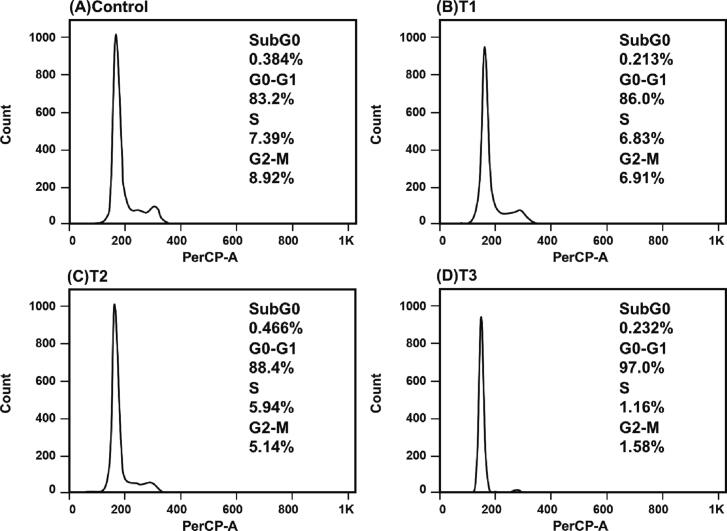
First, using FACS analysis we evaluated DNA content in treated and untreated CRC cells. The cells were stained with a DNA binding dye called propidium iodide which has an absorption maximum of 535 nm. The DNA content was depicted through fluorescence emission from the propidium iodide-DNA complex, which exhibits a maximum absorption at 617 nm. The results showed that CRC cells treated with BaME induced G0/G1 arrest (Fig. 3, Fig. 4). Nearly 56.3 to 80.6% of HCT-116 cells were arrested at G0/G1 stage, whereas 83.2 to 97% of HT-29 cells were arrested at the same stage. Fig. 3, Fig. 4 show that applying the IC50 concentration of BaME caused an accumulation of cells in the G0/G1 stages in both CRC cell lines, as seen by the existence of the sub G0 peak and a decline in the proportion of cells in the S phase as the extract concentration was raised. The cells were arrested within 24 h of treatment in the G0/G1 phase, indicating that BaME can arrest the cell cycle. These cells will eventually undergo apoptosis (a desirable form of cell death in cancer) if they are arrested at a specific stage of the cell cycle.
Fig. 3.
Cell cycle effects of BaME in colorectal cancer cell line HCT-116. T1, T2, T3 represent increase in concentration of the extract respectively.
Fig. 4.
Cell cycle effects of BaME in colorectal cancer cell line HT- 29. T1, T2, T3 represent increase in concentration of the extract respectively.
3.4.4. Western blotting
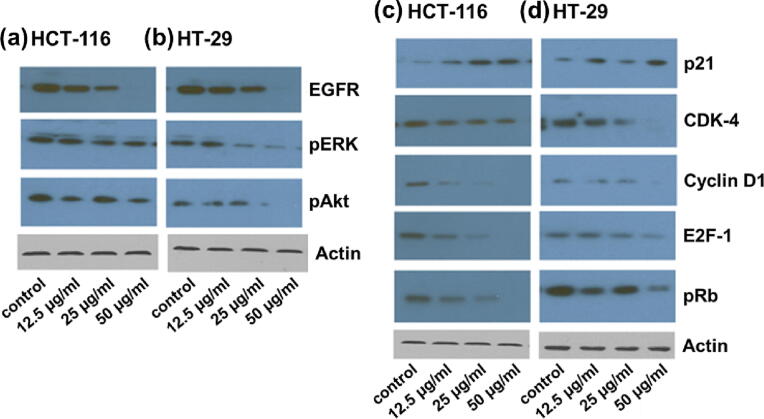
Further, we assessed the protein levels of cognate associates associated with the cell cycle in CRC cell lines (HT-29 and HCT-116) treated with BaME. Compared to controls, the level of two proteins cyclin D1 and Cdk4 in BaME-treated CRC cells were noted to be significantly decreased (Fig. 5). Gene p21 which is the tumor suppressor has regulated the expression levels of Cdk4 and cyclin D1. Then, in BaME-treated versions of both CRC cell lines, we tested the protein and messenger expression of the cyclin-dependent kinase inhibitor, p21 and the protein retinoblastoma (pRB). The amount of p21 protein expression in cells increased following BaME therapy. The increase in p21 protein and mRNA expression was accompanied by a reduction in pRb protein expression. Schematic representation of molecular action of BaME on cell cycle checkpoints and EGFR signaling pathway molecules is described in Fig. 6. pRb is a downstream molecule of Cdk4 and Cyclin D1 and the condition of Rb phosphorylation is found to control cell growth, proliferation, and different cellular activities. Overall, our findings suggest that in BaME-treated CRC cell lines, increased p21 expression is associated with decreased levels of Cdk4 and cyclinD1. These molecular effects clearly exhibit the arrest of cells at the G0/G1 stage. Because these pathways have been linked to survival and tumor resistance in previous research, the effects of BaME on the PI-3 k/Akt and MEK/ERK pathways were examined in the current investigation.
Fig. 5.
Treatment with BaME influences regulatory molecules involved in (a),(b) survival, and (c),(d) cell cycle molecular pathways in HCT-116 and HT-29 cell lines respectively representing the effect of the increase in the concentration of the extract on regulatory molecules expression.
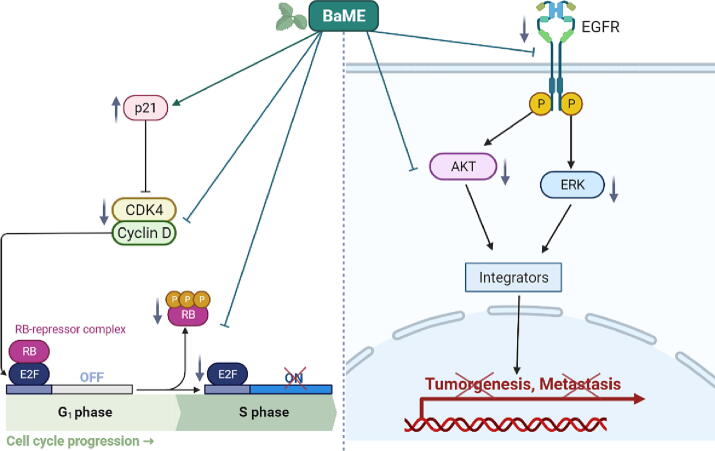
Fig. 6.
Schematic representation of molecular action of BaME on cell cycle checkpoints and EGFR signaling pathway molecules.
We found that active biomolecules in BaME decreased the signal transduction proteins pERK PI-3 K and pAKT expression (Fig. 5). BaME causes the G0/G1 cell cycle to be arrested by upregulating the expression of p21 and downregulating the expression of cyclin D1, Cdk4, and also pRb in human colorectal cancer (Fig. 6). Proliferative and cell cycle signaling pathways were markedly reduced after treatment with BaME (p < 0.001). Cell cycle molecules (E2F-1, pRb, cyclin D1, and Cdk4) as well as survival molecules (PI3K, pERK, and pAkt) are inhibited in both cell lines. The HT-29 cell line was significantly suppressed as compared to HCT-116. It is worth noting that Cyclin D1/Cdk4 complexes are studied extensively as cancer therapeutic targets since their role discovery in human cancers.
3.4.5. Quantitative RT-PCR.
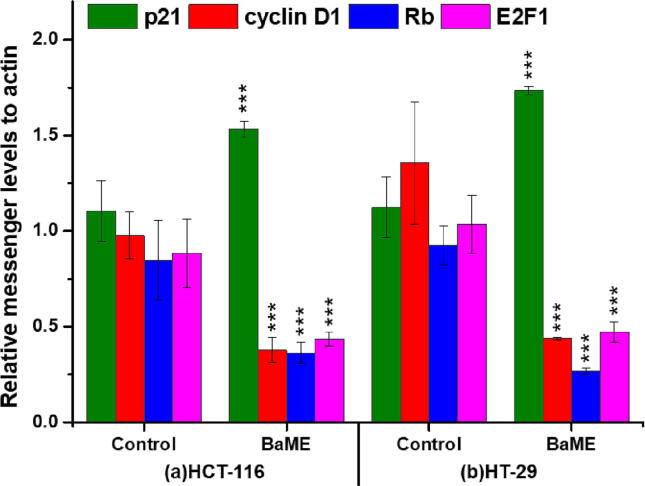
Additionally, qRT-PCR analysis was done to verify the protein expression observed by western blotting at the messenger RNA level, and similar results were obtained (Fig. 7). Cells treated for 24 hrs with DMSO and BaME (50 nM) were harvested, and mRNA extraction was done for qRT-PCR analysis. Results from qRT-PCR were compared with amplified β-actin (normalized). Results revealed a decrease in cyclin D1, pRb, and E2F-1 levels and a substantial (p < 0.001) increase in p21 mRNA expression levels. This expression pattern was shown in BaME-treated CRC cell lines compared to control. The results indicate that BaME inhibits cell survival and cell cycle factors controlling the development of colorectal cancer.
Fig. 7.
qRT- PCR analysis of signal molecules involved in survival and cell cycle. Each value represents the mean ± standard deviation, *** p < 0.001.
4. Discussion
There is a wealth of research to support the idea that consumption of fruits and vegetables regularly lowers the chance of developing chronic illnesses like cancer (Aune et al., 2017) (Hartley et al., 2013). Fruits and vegetables include a wide range of phytochemicals, such as phenols and flavonoids, which have been discovered to have anticancer activity and to provide a number of health advantages (Bøhn et al., 2010) (Boye et al., 1990). There have been reports of certain phytocompounds exhibiting both anti-diabetes mellitus (Venkatachalam et al., 2013) and anti-inflammatory activity (Albinjose et al., 2015). The green leafy vegetable Basella alba, an indigenous plant chosen for the present study, is not commercialized and less explored, should be encouraged for active farming as it exhibits good antioxidant and anticancer activity.
Plant-based compounds have enormous therapeutic potential with fewer side effects. Vimala and Keerthana, 2014 reported the presence of 10-Undecen-1-ol, Heptanoic acid 2-ethyl-, Phytol, 1, 4-Cyclohexanedimethanol, 1, 2-Benzenedicarboxylic acid (di isooctyl ester), and Squalene in ethanolic extract of B.alba leaves (Vimala and Keerthana 2014). Compounds like 10-[Methoxycarbonyl]-N-acetylcolchinol, Hydroymethyl colchicines, and [22S]-21-Acetoy-6a,11a-dihydroxy-16a,17a-propylmethylenedioxy pregna −1,4-diene-3,20-dione whose mass spectra is illustrated in Figure S1 a, b, and c are demonstrated to be the cause of the extract's anti-inflammatory properties. It has been reported that several main molecules that have a prominent role in CRC progression and development are related to inflammation. So, the molecules also play a role in anticancer activity (Madka and Rao 2013). The TPC of the methanolic extract of Arisaema jacquemontii Blume was reported to be 45.17 ± 1.70 GAE/g (Baba and Malik 2015). The results demonstrated that the investigated sample in the present work contains a considerable content of phenol phytoconstituent (57.9 ± 2.66 µg GAE/g). Many reported studies have related the TPC of plants with their antioxidant activity and demonstrated that extracts containing high amounts of phenolic exhibited higher antioxidant activity (scavenging and reducing capacity) (Dileep et al., 2012).
Flavonoids, the most widespread and extensively distributed class of plant phenolic chemicals, are distinguished by a benzo-γ-pyrone chemical structure. It is present in vegetables and fruits. Flavonoids that widely exist in plants are called natural biological modifiers and they belong polyphenolic compounds group. Flavonoids, as biological modifiers, modify the degradative effects of carcinogens allergens, and viruses (Shan et al., 2019). It exhibits anti-allergic, anti-inflammatory, anticancer, antioxidant, and antimicrobial properties. Previously it was reported that higher consumption of flavonoids, decreased the risk of cancer and cardiovascular diseases (Kabra et al., 2019). Total flavonoid content in BaME was 118.1 ± 0.79 µg/g quercetin equivalent per extract. While in the methanolic extract of Arisaema jacquemontii, it was reported to be 35 ± 2.20 rutin equivalent/g (Baba and Malik 2015). This study was able to point out the potential flavonoid content in BaME.
Aqueous extract of B.alba was evaluated for antioxidant activity while methanol extracts of B.alba aerial plants are reported to contain terpenoids, a class of psychoactive compounds, which has a central nervous system-suppressing effect that makes them effective for treating some stress-related issues, such as getting sound refreshing sleep in today’s hectic lifestyle (Bamidele et al., 2020). DPPH is a free radical nitrogen-centered molecule that reacts similarly to peroxyl radical. The rate of the reaction directly correlates with antioxidant activity. The odd electron in the DPPH free radical (purple) corresponds to a significant maximum absorption at 517 nm. The reduced DPPH-H is created when the odd electron from the DPPH radical combines with hydrogen from antioxidants that have the ability to scavenge free radicals. This results in a shift in color from purple to yellow and a reduction in the molar absorptivity of the DPPH radical at 517 nm. With regard to the quantity of electrons caught, the resulting decolorizing is stoichiometric (Sánchez-Moreno et al., 1999). The current results shows that BaME reacts with the hydrogen donors, reduces the radical to the corresponding hydrazine in the antioxidant principles. Biological reactions produce highly toxic radical species, a weak oxidant called superoxide anions. This ROS damages DNA and in turn damages cells leading to the generation of various diseases (Baba and Malik 2015). They are produced endogenously by flavoenzymes. They serve as precursors for strong and harmful radicals (single oxygen as well as hydroxyl radicals) that cause oxidative stress (Chanda and Dave 2009). The production of superoxide anion radicals by the PMS/NADH-NBT combination results in a decrease in the absorbance at 560 nm, which shows a dose-dependent rise in superoxide scavenging activity. These outcomes are equivalent to those of the TPC. The relativity between TPC and the antioxidative activity of fruits, plants, and vegetables is close which was reported by many researchers (Paz et al., 2015). Most importantly, for the antioxidant activity of an extract based on its phenolic content, it needs identification, further isolation, and characterization of each phenolic compound (Hossain and Shah 2015).
Total antioxidant capacity includes both water- and oil-soluble antioxidants that can neutralize ROS and guard against chronic illnesses like diabetes, cancer, and arthritis. The ability of the extracts to act as antioxidants has been regularly assessed using the phosphomolybdate method (Sahreen et al., 2010). The effectiveness of antioxidants is controlled by the structures and chemical composition of bioactive extract components (Valdés et al., 2015). In pathophysiological processes, the hydroxyl radical is referred to as a harmful ROS which can damage proteins, fatty acids, DNA, and practically every molecule in the biological system, and it promotes mutagenesis, cytotoxicity, and carcinogenesis (Sökmen and Khan 2016). The antioxidant activity of BaME is directly correlated with its HRS capacity. The maximum HRS activity was found in the seeds of C. occidentalis, followed by the stem and leaf extracts respectively (Arya et al., 2011). An earlier study reported the ethanolic extracts of Ixora coccinea to have an increase in NO scavenging activity that was dose-dependent (Banerjee et al., 2011). In another report, the methanolic extract of Limonia acidissima Linn. leaves showed greater NO scavenging compared to its petroleum ether and also chloroform extract (Attarde et al., 2011).
A previously study reported aqueous Basella alba extract with high content of phenolic compounds exhibited high antioxidant activity (Kumar et al., 2018) with significant cytotoxic activity on Jurkat cell lines (Sushila et al., 2010), Hep G2 (hepatocellular carcinoma), A431 (epidermoid carcinoma), and MG63 (osteosarcoma) (Kumar et al., 2018) cell lines. According to literature review, cell cycle molecules have been proved to regulate cell proliferation and survival (Maddika et al., 2007). Every type of cancer has distinct molecular alterations, such as the up- or down-regulation of biological molecules in cancer cells (Son et al., 2022). Studies on cell signaling have shown that cancer cells with faulty checkpoints are more susceptible to anticancer drugs. (Gabrielli et al., 2012). Thus, compounds that interfere with cell cycle checkpoints can be effective anticancer agents. In the current investigation, the effects of IC-50 value of BaME on the PI-3 k/Akt and MEK/ERK pathways were examined because previous research has reported the link of these pathways with survival and tumor resistance (Steelman et al., 2011, Ye and She, 2013). In preclinical studies reported on CRC models, the activation of Epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), or their downstream signaling pathways involving proteins like Akt, Ras, or Raf resulted in increasing cancer proliferation and resistance to therapy (Chen et al., 2010). While simultaneous suppression of both EGFR and IGFR-I receptors was linked to enhanced apoptosis and decreased proliferation in CRC cells (Kaulfuβ et al., 2009). The current work documents a decrease in pAkt and EGFR expression patterns, illuminating the function of BaME in regulating cell proliferation. Normally, Rb is found complex with E2F-1. Activation of c-myc and cyclin D1 is due to their response to growth-promoting signals (Butt et al., 2005, Chen et al., 2010). This results in Rb phosphorylation to form pRB, releasing E2F transcriptional factors (E2F –TFs) bound to Rb. E2F-1 plays a crucial role in cell proliferation and facilitates the transition of G1 phase cells into the S phase (Ramana et al., 2010, Garcia-Jove Navarro et al., 2013). Also, it transcribes genes involved in cancer cell resistance and growth (Stanelle et al., 2002). Impairment of the PI3K/Akt pathway causes a variety of human diseases, including diabetes, cardiovascular disease, neurological disorders, and cancer. While its elevated condition is considered to be a hallmark of cancer (Fruman et al., 2017). In cancer biology research, Akt (a Protein Kinase B) has emerged as a therapeutic target protein because of its major role in regulating various cellular functions like transcription, protein synthesis, metabolism, growth (influences TSC1/TSC2 complex and mTOR signaling) (Pham et al., 2021), cell migration, invasion, proliferation (via phosphorylation of p21 and p27, the CDK inhibitors), and survival (by inhibiting pro-apoptotic proteins). Receptors that cause PI3K (phosphoinositide 3-kinase) to produce PIP3 (phosphatidylinositol (3,4,5) triphosphates) activate the Akt signaling cascade. But the tumor suppressor phosphatase and PTEN (tensin homolog) inhibits Akt activity by dephosphorylating PIP3 (Carnero et al., 2008, Noorolyai et al., 2019). An activated Erk dimer phosphorylates a variety of transcription factors regulating gene expression by regulating targets in the cytosol and translocating them to the nucleus (Maik-Rachline et al., 2019). This study's showed that BaME decreased Rb and Erk expression levels, which reveals the extract's capacity to restrain the proliferation and growth of cancer cells. In conclusion, the current study demonstrates that BaME has targeted anticancer activity, however, the various molecular mechanisms of its action, as well as the network interaction of pathways, need to be investigated further. Studies in in vivo models may shed further light on the effects and functions of BaME as a alternate therapeutic agent in the management and prevention of cancer.
5. Conclusion
Basella alba, a variety of spinach, is used as a common green vegetable in Asian countries. The current investigation demonstrated BaME with good antioxidant and cytotoxic activity. BaME demonstrated an impressive antioxidant ability that was higher than ascorbic acid, a key antioxidant. It also showed antiproliferative potential by targeting the specific signaling pathway which leads to the arrest of the colon cancer cells at the G0/G1 stage. According to these findings, BaME is a promising source of phytochemicals and each compound identified in extracts has major biological importance, and further in silico docking studies can be done to prove its interaction with various small molecules in the signaling pathway which are the hallmark of cancer and open the doors for further drug development. This work will serve as a prospective reference for application of Basella alba as functional food.
CRediT authorship contribution statement
Aliya Sheik: Conceptualization, Methodology, Data curation, Validation, Formal analysis, Investigation, Writing – original draft. Eunsu Kim: Data curation, Validation, Formal analysis. Uma Adepelly: Resources, Supervision, Data curation, Conceptualization, Methodology, Formal analysis. Munirah Alhammadi: Software, Formal analysis. Yun Suk Huh: Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by INHA UNIVERSITY Research Grant.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103609.
Contributor Information
Aliya Sheik, Email: aliya@inha.ac.kr.
Eunsu Kim, Email: esk22212103@inha.edu.
Uma Adepelly, Email: vedavathi1@jntuh.ac.in.
Munirah Alhammadi, Email: munirah@inha.edu.
Yun Suk Huh, Email: yunsuk.huh@inha.ac.kr.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Albinjose J., Jasmine E., Selvankumar T., Srinivasakumar K. Bioactive compounds of tinospora cordifolia by gas chromatography-mass spectrometry (gc-ms) Int. J. Multidiscip. Res. Dev. 2015 [Google Scholar]
- Arya, V., Yadav, S., Kumar, S., Yadav, J.P., 2011. Antioxidant activity of organic and aqueous leaf extracts of cassia occidentalis l. In relation to their phenolic content. Natural product research [DOI] [PubMed]
- Attarde D., Chaudhari B., Bhambar R. Phytochemical investigation and in vitro antioxidant activity of extracts from leaves of limonia acidissima linn. (rutaceae) J. Pharm. Res. 2011 [Google Scholar]
- Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T., Greenwood D.C., Riboli E., Vatten L.J., Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017 doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, S.A., Malik, S.A., 2015. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of arisaema jacquemontii blume. Journal of Taibah University for Science. https://doi.org/https://doi.org/10.1016/j.jtusci.2014.11.001.
- Bamidele O., Okeke N.C., Adedeji T.G., Adedayo L.D., Akinnuga A.M. Methanol extracts of Basella alba leaves alleviate stress in rats. Chinese Herb. Med. 2020 doi: 10.1016/j.chmed.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Chanda A., Ghoshal A., Debnath R., Chakraborty S., Saha R., Das A. Nitric oxide scavenging activity study of ethanolic extracts of from two different areas of kolkata. Asian J. Exp. Biol. Sci. 2011 [Google Scholar]
- Benzie I.F.F., Szeto Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999 doi: 10.1021/jf9807768. [DOI] [PubMed] [Google Scholar]
- Bøhn S.K., Myhrstad M.C., Thoresen M., Holden M., Karlsen A., Tunheim S.H., Erlund I., Svendsen M., Seljeflot I., Moskaug J.Ø. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med. 2010 doi: 10.1186/1741-7015-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye, O., Lin, C., Hamel, E., Brossi, A., 1990. Antitubulin effects of congeners of n-acetylcolchinyl methyl-ether-synthesis of optically-active 5-iso-colchinyl methyl-ether and of demethoxy analogs of deacetamidocolchinyl methyl-ether. Abstracts Of Papers Of The American Chemical Society, Amer Chemical Soc 1155 16th St, NW, Washington, DC 20036
- Butt A.J., McNeil C.M., Musgrove E.A., Sutherland R.L. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-myc, cyclin d1 and cyclin e. Endocr. Relat. Cancer. 2005 doi: 10.1677/erc.1.00993. [DOI] [PubMed] [Google Scholar]
- Carnero A., Blanco-Aparicio C., Renner O., Link W., Leal J.F. The pten/pi3k/akt signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets. 2008 doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Chanda, S., Dave, R., 2009. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research.
- Chen J., Huang X.F., Katsifis A. Activation of signal pathways and the resistance to anti-egfr treatment in colorectal cancer. J. Cell. Biochem. 2010 doi: 10.1002/jcb.22905. [DOI] [PubMed] [Google Scholar]
- Chimi, H., Cillard, J., Cillard, P., Rahmani, M., 1991. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. Journal of the American Oil Chemists' Society. https://doi.org/https://doi.org/10.1007/BF02657682.
- Chun O.K., Kim D.-O., Lee C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003 doi: 10.1021/jf034740d. [DOI] [PubMed] [Google Scholar]
- Deen, K.I., Silva, H., Deen, R., Chandrasinghe, P.C., 2016. Colorectal cancer in the young, many questions, few answers. World journal of gastrointestinal oncology. [DOI] [PMC free article] [PubMed]
- Deshmukh S., Gaikwad D. A review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella alba (basellaceae) J. Appl. Pharma. Sci. 2014 [Google Scholar]
- Dileep, N., KN, R., Junaid, S., Poornima, G., Swarnalatha, S., Kekuda, T., 2012. In vitro antioxidant activity of ripe pericarp of polyalthia longifolia thw. Research Journal of Pharmacy and Technology
- Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The pi3k pathway in human disease. Cell. 2017 doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli B., Brooks K., Pavey S. Defective cell cycle checkpoints as targets for anti-cancer therapies. Front. Pharmacol. 2012 doi: 10.3389/fphar.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji P.N., Park W., Wen J., Mahaseth H., Landry J., Farris A.B., Willingham F., Sullivan P.S., Proia D.A., El-Hariry I. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of hif-1α and stat-3. Angiogenesis. 2013 doi: 10.1007/s10456-013-9364-7. [DOI] [PubMed] [Google Scholar]
- Gao M.-R., Xu Q.-D., He Q., Sun Q., Zeng W.-C. A theoretical and experimental study: the influence of different standards on the determination of total phenol content in the folin–ciocalteu assay. J. Food Meas. Charact. 2019 doi: 10.1007/s11694-019-00050-6. [DOI] [Google Scholar]
- Garcia-Jove Navarro M., Basset C., Arcondéguy T., Touriol C., Perez G., Prats H., Lacazette E. Api5 contributes to e2f1 control of the g1/s cell cycle phase transition. PLoS One. 2013 doi: 10.1371/journal.pone.0071443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley L., Igbinedion E., Holmes J., Flowers N., Thorogood M., Clarke A., Stranges S., Hooper L., Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD009874.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.A., Shah M.D. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant merremia borneensis. Arab. J. Chem. 2015 [Google Scholar]
- Kabra, A., Sharma, R., Hano, C., Kabra, R., Martins, N., Baghel, U.S., 2019. Phytochemical composition, antioxidant, and antimicrobial attributes of different solvent extracts from myrica esculenta buch.-ham. Ex. D. Don leaves. Biomolecules. [DOI] [PMC free article] [PubMed]
- Kaulfuβ, S., Burfeind, P., Gaedcke, J., Scharf, J.-G., 2009. Dual silencing of insulin-like growth factor-i receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Molecular cancer therapeutics [DOI] [PubMed]
- Kumar, B.R., Anupam, A., Manchikanti, P., Rameshbabu, A.P., Dasgupta, S. and Dhara, S., 2018. Identification and characterization of bioactive phenolic constituents, anti-proliferative, and anti-angiogenic activity of stem extracts of Basella alba and rubra. Journal of food science and technology [DOI] [PMC free article] [PubMed]
- Kumar D., Goel N.K., Pandey A.K., Sarpal S.S. Complementary and alternative medicine use among the cancer patients in northern india. South Asian J. Cancer. 2016 doi: 10.4103/2278-330X.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., Jia, Z., Trush, M.A., 2016. Defining ros in biology and medicine. Reactive oxygen species (Apex, N.C.). https://doi.org/10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed]
- Maddika S., Ande S.R., Panigrahi S., Paranjothy T., Weglarczyk K., Zuse A., Eshraghi M., Manda K.D., Wiechec E., Los M. Drug Resistance Updates; 2007. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. [DOI] [PubMed] [Google Scholar]
- Madka V., Rao C.V. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr. Cancer Drug Targets. 2013 doi: 10.2174/15680096113139990036. [DOI] [PubMed] [Google Scholar]
- Maik-Rachline G., Hacohen-Lev-Ran A., Seger R. Nuclear erk: mechanism of translocation, substrates, and role in cancer. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, L., Fröhlich, K., Böhm, V., 2011. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (frap), abts bleaching assay (αteac), dpph assay and peroxyl radical scavenging assay. Food Chemistry. https://doi.org/https://doi.org/10.1016/j.foodchem.2011.04.045.
- Noorolyai, S., Shajari, N., Baghbani, E., Sadreddini, S., Baradaran, B., 2019. The relation between pi3k/akt signalling pathway and cancer. Gene. https://doi.org/https://doi.org/10.1016/j.gene.2019.02.076. [DOI] [PubMed]
- Paz M., Gúllon P., Barroso M.F., Carvalho A.P., Domingues V.F., Gomes A.M., Becker H., Longhinotti E., Delerue-Matos C. Food Chemistry; 2015. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. [DOI] [PubMed] [Google Scholar]
- Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. Ros in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020 doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.N.A., Le B., Yang S.H. Anticancer activity of the potential Pyropia yezoensis galactan fractionated in human prostate cancer cells. Biotechnol. Bioprocess Eng. 2021 [Google Scholar]
- Ramana, K.V., Tammali, R., Srivastava, S.K., 2010. Inhibition of aldose reductase prevents growth factor–induced g1-s phase transition through the akt/phosphoinositide 3-kinase/e2f-1 pathway in human colon cancer cells. Molecular cancer therapeutics [DOI] [PMC free article] [PubMed]
- Sahreen S., Khan M.R., Khan R.A. Evaluation of antioxidant activities of various solvent extracts of carissa opaca fruits. Food Chem. 2010 doi: 10.1186/s13065-017-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Moreno, C., Larrauri, J.A., Saura-Calixto, F., 1999. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food research international
- Santos-Sánchez, N.F., Salas-Coronado, R., Villanueva-Cañongo, C., Hernández-Carlos, B., 2019. Antioxidant compounds and their antioxidant mechanism, IntechOpen London, UK
- Shan S., Huang X., Shah M.H., Abbasi A.M. Evaluation of polyphenolics content and antioxidant activity in edible wild fruits. Biomed. Res. Int. 2019 doi: 10.1155/2019/1381989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Bhatia S.K., Banyal A., Chanana I., Kumar A., Chand D., Kulshrestha S., Kumar P. An overview on taxol production technology and its applications as anticancer agent. Biotechnol. Bioprocess Eng. 2022 [Google Scholar]
- Sökmen M., Khan M.A. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology. 2016 doi: 10.1007/s10787-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M.H., Park S.W., Sagong H.Y., Jung Y.K. Recent advances in electrochemical and optical biosensors for cancer biomarker detection. BioChip J. 2022 [Google Scholar]
- Stanelle J., Stiewe T., Theseling C.C., Peter M., Pützer B.M. Gene expression changes in response to e2f1 activation. Nucleic Acids Res. 2002 doi: 10.1093/nar/30.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman, L.S., Chappell, W.H., Abrams, S.L., Kempf, C.R., Long, J., Laidler, P., Mijatovic, S., Maksimovic-Ivanic, D., Stivala, F., Mazzarino, M.C., 2011. Roles of the raf/mek/erk and pi3k/pten/akt/mtor pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY). [DOI] [PMC free article] [PubMed]
- Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., Bray, F., 2021. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. [DOI] [PubMed]
- Sushila R., Deepti A., Permender R., Madhavi T., Dharmender R., Rathee D. Cytotoxic and antibacterial activity of Basella alba whole plant: a relatively unexplored plant. Pharmacologyonline. 2010 [Google Scholar]
- Valdés, A., Mellinas, A., Ramos, M., Burgos, N., Jiménez, A., Garrigós, M.d.C., 2015. Use of herbs, spices and their bioactive compounds in active food packaging. RSC advances.
- Venkatachalam M., Singaravelu G., Govindaraju K., Ahn J.S. Ptp 1b inhibitory action of a phytochemical propanoic acid, 2-(3-acetoxy-4, 4, 14-trimethylandrost-8-en-17-yl) Curr. Sci. 2013 [Google Scholar]
- Vimala J.R., Keerthana S. Preliminary phytochemical screening and antibacterial activity on Basella alba L. Int. J. Res. Develop. Pharm. Life Sci. 2014 [Google Scholar]
- Willers H., Pan X., Borgeaud N., Korovina I., Koi L., Egan R., Greninger P., Rosenkranz A., Kung J., Liss A.S., Parsels L.A. Screening and validation of molecular targeted radiosensitizers. Int. J. Radiat. Oncol.* Biol.* Phys. 2021 doi: 10.1016/j.ijrobp.2021.07.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., She Q. Integration of akt and erk signaling pathways in cancer: biological and therapeutic implications. J. Pharmacol. Clin. Toxicol. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.